Evaluation of parameters of health in Australian fur seal (Arctocephalus pusillus doriferus) pups during early and mid-lactation
Jessalyn J. Taylor A * , Rebecca R. McIntosh
A * , Rebecca R. McIntosh  B , Isabelle Charrier C and Rachael B. Gray A
B , Isabelle Charrier C and Rachael B. Gray A
A
B
C
Abstract
Seal Rocks, Victoria, is the largest Australian fur seal (Arctocephalus pusillus doriferus) breeding colony, but has one of the highest rates of decline in live pup abundance for the species. Establishing the current health status in the population is important as a point of comparison for future monitoring. This study aimed to develop haematological reference intervals and assess body condition in pups at Seal Rocks in early and mid-lactation. Pups from the 2021 and 2022 breeding seasons were sampled at 3–6 weeks of age (early lactation, n = 180) and 5–6 months of age (mid-lactation, n = 172). Haematological and morphometric parameters differed significantly (P < 0.05) between early and mid-lactation, but body condition did not. The differences in health parameters between early and mid-lactation relate to normal growth and ontogeny but highlight the increased vulnerability of pups in early lactation. These results provide an important reference point for future evaluations of health parameters in Australian fur seal pups.
Keywords: Australian fur seal, body condition, conservation management, haematology, marine conservation, pinniped, reference intervals, wildlife monitoring.
Introduction
Pinnipeds, like other marine megafauna, are important ocean sentinels (Bossart 2010), and changes in their health and population status can signal changes to the marine environment. Australian fur seal (Arctocephalus pusillus doriferus) live pup counts have been on a declining trend since 2007 (McIntosh et al. 2018, 2022). Seal Rocks, the species’ largest breeding colony, has the fourth highest rate of decline in live pup abundance with a 28% decrease between 2007 and 2013, and a further 6% between 2013 and 2017 (McIntosh et al. 2018, 2022). Further, adult female body condition at the species’ second largest breeding colony, Kanowna Island, has declined significantly in the period from 1998 to 2021, possibly caused by altered prey distribution and subsequent increased foraging effort (Geeson et al. 2023). Although there are no recent or longitudinal studies of adult female body condition at Seal Rocks, diet has changed over time (Kliska et al. 2022), but it is unknown whether this is contributing to the decline in pup abundance at the site. The population faces multiple threatening processes including impacts from climate change (McLean et al. 2018; Speakman et al. 2020), marine pollutants (Fulham et al. 2020, 2022; Taylor et al. 2021), low fecundity (Gibbens et al. 2010; Gardner et al. 2022), entanglement (McIntosh et al. 2015), and other external stressors, such as anthropogenic disturbance, particularly at sensitive sites like breeding colonies (Tripovich et al. 2012; Back et al. 2018), because disturbance to mother–pup pairs can result in reduced suckling and rest time (Tripovich et al. 2012; Martin et al. 2022, 2023; Taylor et al. 2024).
Monitoring parameters of health is important for conservation management and commonly used metrics in free-ranging populations include morphometric measurements and haematological analysis. Morphometric measurements such as weight and standard length are used to calculate body condition index (BCI), a robust metric of nutrition status that accounts for differences in pup age (Bradshaw et al. 2000). Standard length has also been used as a proxy for age in pinniped pups (Marcus et al. 2015; Lindsay et al. 2023), and weight and standard length are useful for measuring sexual dimorphism between male and female pups of the same age. Haematological parameters are commonly assessed against reference intervals (RIs) from comparable, ‘healthy’ individuals (reference population) to determine health status (Friedrichs et al. 2012), and can indicate nutritional status, presence of anaemia, inflammation, and other disease states. There are currently no established haematological RIs for Australian fur seals, necessitating their development to facilitate comparisons in future monitoring efforts. However, haematological and morphometric parameters change with age and fluctuate naturally according to season (e.g. breeding versus non-breeding) (Keogh et al. 2010; Kohyama et al. 2021). Further, RIs within the same species have been shown to differ between sites, for example in harbor seal (Phoca vitulina) pups (Trumble and Castellini 2002), highlighting the importance of age- and site-specific values.
Seal Rocks is located 1.8 km off the southwest tip of Phillip Island (Victoria, Australia) and represents approximately one quarter of the Australian fur seal population (McIntosh et al. 2022). Australian fur seals have a highly synchronous annual breeding cycle, which extends from late October through to January, with a median pupping date of 1 December (Warneke and Shaughnessy 1985). Pups are entirely maternally dependent until 3–4 months of age, at which time they can begin supplementing maternal provisioning with independent foraging; weaning occurs at approximately 11 months of age (Spence-Bailey et al. 2007). Females alternate periods of foraging at sea, during which pups fast, with time ashore to rest and nurse their pup (Arnould and Hindell 2001). In the absence of comprehensive historical or longitudinal data, little is known regarding changes to Australian fur seal health status at Seal Rocks (or across the species’ range in general), particularly beyond early lactation. Therefore, it is important to establish a contemporary baseline of health to provide a point of comparison for future monitoring and health evaluations.
The primary aim of this study was to develop haematological RIs in Australian fur seal pups at Seal Rocks during early (breeding season, austral summer) and mid-lactation (non-breeding season, austral late autumn). Additional aims were to assess BCI, to investigate factors affecting haematological and morphometric parameters, and to assess the effect of year of birth on BCI in pups sampled in early lactation in years outside the present study. It was hypothesised that a difference in parameters of health would be observed between early and mid-lactation, reflecting a change in physiology during a period of rapid growth for pups (i.e. from early neonatal to 6 months). In addition, given the range-wide decline in live pup abundance and declining female body condition at another site in central northern Bass Strait (approximately 150 km east of Seal Rocks) over the past 15 years, it was hypothesised that a declining trend in BCI would be observed in young neonatal pups. This study provides the first reference point for monitoring health in this population and can be combined with other parameters for future health assessments.
Materials and methods
Animals and study site
The highly synchronous breeding cycle of Australian fur seals enables estimation of pup age based on sampling date. Sample collection at Seal Rocks (38°31′34″S, 145°05′59″E) (Fig. 1) was undertaken over consecutive days during early lactation (pups approximately 3–6 weeks of age) in December 2021 (29 December to 2 January) and 2022 (31 December to 2 January) and in mid-lactation (pups approximately 5–6 months of age) the following May (9–13 May 2022, 11–14 May 2023). Sampling was conducted for up to 8 h per day of each sampling event. The four sampling events are hereafter referred to as 2021 early, 2021 mid-, 2022 early, and 2022 mid-lactation. With respect to the mid-lactation sampling events, the year (2021, 2022) refers to year of birth, not the year of sampling (2022 and 2023).
Study site. (a) Location of the Seal Rocks colony in relation to Phillip Island and the Australian mainland. (b) Aerial view of Seal Rocks with capture areas, West Cove (left) and Main Beach (right), outlined in red.
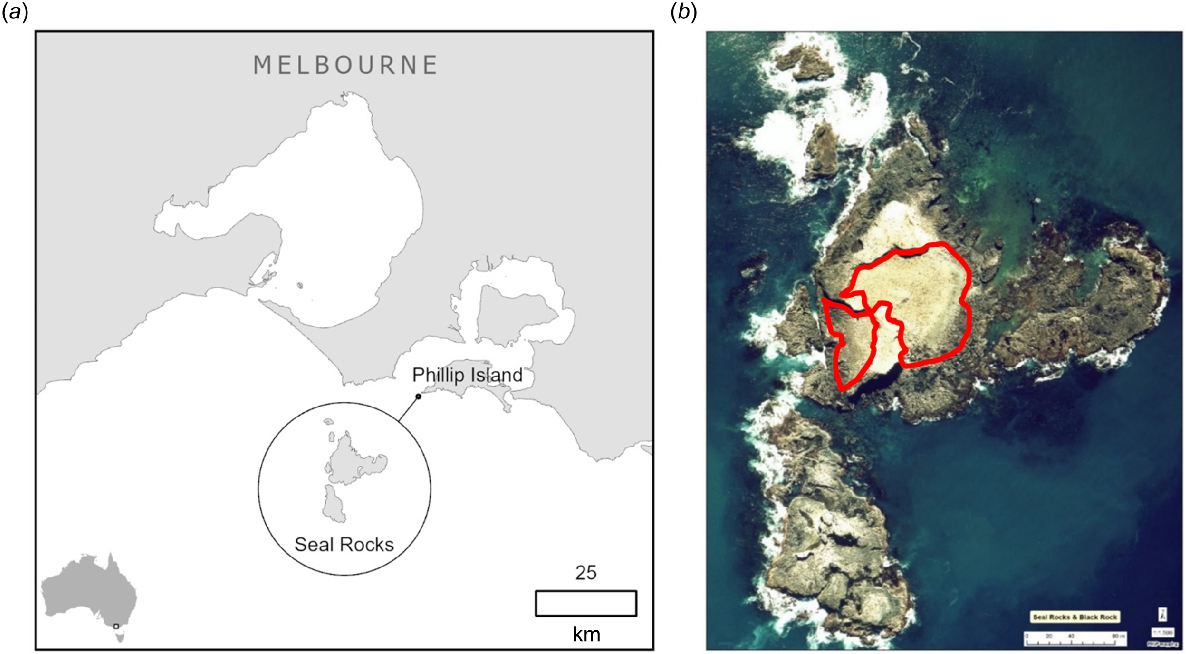
Pups were sampled from the two main accessible areas on Seal Rocks: West Cove and Main Beach (Fig. 1). In the 2021 early and mid-lactation sampling events, n = 100 pups were sampled per trip. In the 2022 early and mid-lactation sampling events, n = 80 and n = 72 pups were sampled, respectively.
All research was conducted in accordance with the Australian Code for the Care and Use of Animals for Scientific Purposes, as approved by the Phillip Island Nature Parks Animal Ethics Committee (2.2019 and 1.2022), and under Wildlife Research permit (10009034 and 10010268).
Sample collection
Pup sampling procedures were modified from the protocol described by Marcus et al. (2015) for Australian sea lion (Neophoca cinerea) pups. Pups were captured by hand or hoop-net (mid-lactation only) and physically restrained in a custom-made polyester pup bag with breathing holes for up to 8 min. Location of capture (West Cove or Main Beach), capture type (sleeping, awake, or mobile), time of capture, weight (measured to the nearest 0.1 kg on a hanging scale; Salter, Avery Weigh-Tronix, West Midlands, UK), standard length (measured to the nearest 0.5 cm), and pup sex were recorded. The presence of lesions, ocular discharge, and other signs of injury or illness were also noted as part of a physical examination.
Up to 10 mL of blood was collected from the brachial vein using a 1-inch 21-G needle and 5 mL syringe (Becton Dickinson, Franklin Lakes, USA). Blood was immediately transferred to 1.3 mL EDTA-anticoagulated tubes (Sarstedt, Numbrecht, Germany). All samples were stored on ice or refrigerated until processed. In some cases, blood could not be collected, resulting in n = 97 and n = 99 blood samples in the 2021 early and mid-lactation sampling events, respectively, and n = 80, and n = 69 blood samples in the 2022 early and mid-lactation sampling events, respectively.
As part of the 2021 early lactation sampling protocol, a unique ‘haircut’, representing a number from 1 to 100, was cut into the dorsosacral pelage of each pup. Hair dye (Schwarzkopf Nordic Blonde, Henkel Australia, Melbourne, Australia) was applied to the ‘haircut’. This mark was to prevent recapture in the same sampling event and facilitate recapture when the pup was older during the 2021 mid-lactation sampling event. However, too few uniquely marked pups were sighted during the 2021 mid-lactation sampling event for recapture, likely due to either moult or mortality, resulting in the capture of a random sample thereafter and the unique marks were not repeated. As part of the 2022 early and both mid-lactation sampling protocols, a single line of fur was removed from the dorsosacral pelage to ensure pups were not captured twice in the same sampling event. All pups were released as close as possible to where they were captured, and time of release was recorded.
Haematological processing and sample analysis
Blood samples were processed and analysed following the methodology reported by Marcus et al. (2015). All blood samples were processed in-field within 12 h of collection or, rarely, within 24 h of collection. EDTA anticoagulated whole blood samples were centrifuged (StatSpin MP, StatSpin Technologies, Norwood, USA) at 13,700g for 120 s in microhematocrit tubes (IRIS Sample Processing, Westwood, USA), packed cell volume (PCV; L/L) measured, and total plasma protein (TPP; g/L) estimated using a handheld refractometer (Reichert TS Meter, Cambridge Instruments, Buffalo, USA). Air-dried blood smears were prepared on glass microscope slides and fixed in 100% methanol. A 200 μL aliquot of EDTA anticoagulated whole blood was added to a microtube (Sarstedt, Numbrecht, Germany) with an equal volume of Streck cell preservative (Streck, Omaha, USA) and stored at 4°C until analysis.
Automated haematological analysis (Sysmex XT-2000iV, Sysmex, Kobe, Japan) was performed on Streck-preserved samples at Veterinary Pathology Diagnostic Service (VPDS, The University of Sydney) within 14 days of sample collection. Automated analysis provided total white blood cell counts (WBC; ×109/L), red blood cell counts (RBC; ×1012/L), haemoglobin concentration (HGB; g/L), mean cell volume (MCV; fL), mean cell haemoglobin (MCH; pg), mean cell haemoglobin concentration (MCHC; g/L), platelet counts (PLT; ×109/L), and nucleated red blood cell count (nRBC; ×109/L). With the exception of the three red cell (erythrocyte) indices (MCV, MCH, MCHC; these are index values and would not have been altered), all values were doubled to account for dilution with Streck cell preservative.
Prepared blood smears were stained with a Romanowsky-type rapid stain (Diff-Quik, Lab Aids, Sydney, Australia) at VPDS. Differential WBC counts were performed on stained blood smears using light microscopy 100× objective and oil immersion. One hundred leukocytes were counted for every 10 × 109/L WBC to determine the proportion of neutrophils (NEUT), lymphocytes (LYMPH), monocytes (MONO), eosinophils (EO), and basophils (BASO). Absolute counts were obtained by multiplying the percentage of each cell type by the automated WBC (×109/L).
Statistical analyses
All statistical analyses were performed in R Studio (ver. 4.3.1, R Core Team 2023), and statistical significance was determined at P < 0.05. Modelling was not performed on the red cell indices (MCV, MCH, MCHC) as they are derived values of RBC, PCV and HGB. Further, due to the presence of too many zero values, RIs were not developed for nRBC and BASO; these parameters were also excluded from modelling.
Reference intervals (RI) for pups at Seal Rocks were developed according to guidelines of the American Society for Veterinary Clinical Pathology (ASVCP) (Friedrichs et al. 2012). For the purpose of RI development, both early sampling events and both mid-lactation sampling events were combined, then data were partitioned based on results of preliminary generalised linear models (GLM), using the glm function in the stats package (R Core Team 2023), to assess the effect of pup age (early lactation, mid-lactation), year of birth (2021, 2022), and pup sex (male, female). For red cell indices (MCV, MCH, MCHC), a one-way ANOVA or non-parametric Kruskal–Wallis test was used to determine if there was a difference between year of birth to necessitate partitioning. Non-parametric Kruskal–Wallis tests were also performed on nRBC and BASO due to the presence of too many zero values.
Outliers were identified and removed prior to RI calculation after visual inspection of histograms and application of Horn’s algorithm, which calculates Tukey’s interquartile fences from Box–Cox transformed data (Horn and Pesce 2003). Using the methodology of Brandimarti et al. (2021), for variables with multiple zero values (nRBC, BASO), outliers were removed based on Cook’s distance. Non-parametric 95% reference intervals (RI) with 90% confidence intervals (CI) were then developed, using the referenceIntervals package (Finnegan 2022), for haematological parameters (PCV, TPP, RBC, HGB, MCV, MCH, MCHC, PLT, WBC, NEUT, LYMPH, MONO, EO, BASO). When partitioning of variables resulted in a smaller than recommended sample size (i.e. less than 120), 95% RIs were developed using robust methods, and 90% CI were developed using basic bootstrap estimation (5000 replicates) (Friedrichs et al. 2012).
Following RI development, the effects of factors on haematological parameters (PCV, TPP, RBC, HGB, PLT, WBC, NEUT, LYMPH, MONO, EO), were further evaluated using GLM fitted with identity link function. Factors initially included in the model were sex (male, female), presence of lesions (yes, no), body condition index (BCI), sampling event (2021 early lactation, 2021 mid-lactation, 2022 early lactation, 2022 mid-lactation), and capture location (Main Beach, West Cove). Body condition index was calculated based on the method outlined by Bradshaw et al. (2000) to account for the variation caused by body size or age. Least squares regression of loge(observed mass; kg) against loge(length; m) was used to obtain loge(predicted mass) with all sampling events combined. A likelihood ratio test was performed between a model containing pup sex (full model) and a model without (null model). No significant difference was found between the two, which justified pooling males and females for calculation of BCI. The relative BCI was then obtained from the ratio of loge(observed mass) to loge(predicted mass).
Generalised linear models were also performed to investigate variability in morphometric parameters (weight, standard length, BCI). Although BCI is a better indicator of nutritional status than weight or standard length alone, the latter two were modelled to determine differences with pup sex and age. Factors included in the model were sex (male, female) and sampling event (2021 early lactation, 2021 mid-lactation, 2022 early lactation, 2022 mid-lactation).
A stepwise method was used to select the final models with the step function from the stats package (R Core Team 2023), with the most parsimonious model considered to be the least parameterised with the lowest Akaike Information Criterion (AIC) (Harrison et al. 2018). The assumptions of homoscedasticity and normality were checked via visual assessment of residual plots, and variables were log- or square-root-transformed where necessary. Where transformations were applied, results are presented on the response scale. Significant differences between sampling events were investigated via pairwise comparisons of estimated marginal means using the emmeans package (Lenth 2024).
In addition, to compare BCI in neonatal pups sampled in early lactation from the present study with other years, morphometric data were obtained for 3–6-week-old pups born from 2015 to 2020 and 3–8-week-old pups born in 2023 (R. McIntosh, unpubl. data; R. Gray, unpubl. data). Body condition index was calculated as outlined above but was sex specific. Generalised linear models, fitted with identity link function, were used to investigate BCI variability across breeding years. Factors initially included in the BCI model were sex (male, female) and year of birth (2015–2023). In the BCI model, the reference level was set to 2023, as this was determined to be the most ‘normal’ year (i.e. median BCI = 1.00; Supplementary Material Table S2). As previously described, variables were transformed where necessary (results presented on response scale), the step function was used to determine final models, and significant differences between years were investigated via pairwise comparisons of estimated marginal means using the emmeans package.
Results
Male-biased sex ratios were observed in both early lactation sampling events and were determined to be significantly different from a 1:1 ratio (2021 early lactation: M/F = 1.56, χ21100 = 4.41, P = 0.036; 2022 early lactation: M/F = 2.33, χ2180 = 12.0, P < 0.001). Sex ratios in the mid-lactation sampling events were not significantly different from 1:1 (2021 mid-lactation: M/F = 1.04, χ21100 = 0.01, P = 0.920; 2022 mid-lactation: M/F = 1.06, χ2172 = 0.01, P = 0.906).
Haematological RIs
All haematological parameters were partitioned by sampling event, and PCV, TPP, HGB, MCV, MCH, MCHC, NEUT, LYMPH and MONO were further partitioned by year of birth. No significant effect of pup sex was found for any haematological parameter. Variables with too many zero values (nRBC, 2021 mid-lactation MONO, BASO) are presented as range of observed values and median values only. Haematological RIs for Australian fur seal pups at Seal Rocks are presented for early (Table 1) and mid-lactation (Table 2) sampling events.
| Parameter | Unit | Year of birth | 95% RI (90% CI) | Median | n A | |
|---|---|---|---|---|---|---|
| PCV | L/L | 2021 | 0.29–0.45 (0.28–0.30; 0.44–0.46) | 0.37 | 95 | |
| 2022 | 0.32–0.50 (0.30–0.34; 0.49–0.52) | 0.42 | 78 | |||
| TPP | g/L | 2021 | 58.1–77.1 (56.7–59.8; 75.3–78.8) | 68.0 | 97 | |
| 2022 | 62.1–88.9 (59.3–64.3; 86.2–92.1) | 75.0 | 79 | |||
| RBC | ×1012/L | N/A | 3.35–4.64 (2.66–3.48; 4.52–4.92) | 4.02 | 175 | |
| HGB | g/L | 2021 | 114–162 (111–118; 158–166) | 138 | 96 | |
| 2022 | 105–158 (100–109; 154–163) | 132 | 78 | |||
| MCV | fL | N/A | 84.4–105 (77.6–86.1; 102–107) | 95.7 | 177 | |
| MCH | pg | 2021 | 30.7–38.3 (30.0–31.3; 37.7–38.9) | 34.4 | 97 | |
| 2022 | 29.1–37.1 (28.3–29.9; 36.4–37.8) | 33.0 | 78 | |||
| MCHC | g/L | 2021 | 330–389 (325–335; 385–395) | 359 | 97 | |
| 2022 | 312–379 (307–317; 385–386) | 344 | 76 | |||
| PLT | ×109/L | N/A | 247–728 (200–312; 696–758) | 516 | 168 | |
| nRBC | ×109/L | N/A | 0.00–1.84 | 0.04 | 177 | |
| WBC | ×109/L | N/A | 10.7–33.4 (10.0–11.6; 30.3–36.8 | 16.9 | 175 | |
| NEUT | ×109/L | N/A | 5.63–24.6 (5.23–6.37; 22.0–31.0) | 11.3 | 175 | |
| LYMPH | ×109/L | N/A | 1.50–8.02 (0.97–1.67; 7.05–8.92) | 3.42 | 177 | |
| MONO | ×109/L | N/A | 0.26–3.16 (0.16–0.36; 2.61–3.44) | 1.26 | 174 | |
| EO | ×109/L | N/A | 0.18–3.51 (0.15–0.26; 2.94–4.88) | 1.17 | 176 | |
| BASO | ×109/L | N/A | 0.00–0.34 | 0.00 | 176 |
| Parameter | Unit | Year of birth | 95% RI (90% CI) | Median | n A | |
|---|---|---|---|---|---|---|
| PCV | L/L | N/A | 0.38–0.54 (0.35–0.39; 0.52–0.55) | 0.46 | 155 | |
| TPP | g/L | 2021 | 63.1–80.7 (61.8–64.9; 79.7–82.2) | 72.0 | 92 | |
| 2022 | 63.2–84.8 (61.3–65.1; 82.3–87.3) | 74.0 | 68 | |||
| RBC | ×1012/L | N/A | 4.14–5.64 (4.10–4.34; 5.58–5.74) | 4.92 | 158 | |
| HGB | g/L | N/A | 138–190 (134–144; 188–196) | 163 | 158 | |
| MCV | fL | 2021 | 84.7–102 (83.4–85.9; 101–104) | 93.3 | 94 | |
| 2022 | 88.6–102 (87.4–89.8; 101–103) | 95.1 | 67 | |||
| MCH | pg | N/A | 30.9–36.9 (29.5–31.1; 35.9–38.0) | 32.9 | 161 | |
| MCHC | g/L | 2021 | 331–377 (327–335; 373–382) | 353 | 95 | |
| 2022 | 329–370 (324–333; 366–374) | 349 | 68 | |||
| PLT | ×109/L | N/A | 126–624 (72.0–156; 572–722) | 410 | 158 | |
| nRBC | ×109/L | N/A | 0.00–0.52 | 0.02 | 161 | |
| WBC | ×109/L | N/A | 6.91–18.1 (6.16–7.30;16.4–18.6) | 10.5 | 163 | |
| NEUT | ×109/L | 2021 | 1.58–9.17 (1.02–2.09; 8.49–9.84) | 5.36 | 95 | |
| 2022 | 0.85–5.43 (0.43–1.21; 5.05–5.88) | 3.01 | 68 | |||
| LYMPH | ×109/L | 2021 | 1.59–6.34 (1.28–1.90; 6.01–6.69) | 3.95 | 94 | |
| 2022 | 0.75–8.45 (0.09–1.44; 7.70–9.35) | 4.33 | 68 | |||
| MONO | ×109/L | 2021 | 0.07–1.25 | 0.31 | 91 | |
| 2022 | 0.31–2.22 (0.14–0.47; 2.04–2.40) | 1.24 | 68 | |||
| EO | ×109/L | N/A | 0.26–2.39 (0.17–0.38; 2.29–2.81) | 1.03 | 159 | |
| BASO | ×109/L | N/A | 0.00–0.07 | 0.00 | 151 |
For early lactation sampling events, pups born in 2021 had significantly lower PCV (P < 0.001) and TPP (P < 0.001), and significantly higher HGB (P = 0.003), MCH (P < 0.001), and MCHC (P < 0.001), than pups born in 2022. For mid-lactation sampling events, results were slightly more variable. Pups born in 2021 had significantly lower TPP (P < 0.001), MCV (P = 0.012) and MONO (P < 0.001), and significantly higher MCHC (P = 0.011), NEUT (P < 0.001) and LYMPH (P = 0.048) compared to pups born in 2022.
Factors affecting haematological parameters
Model results evaluating factors affecting haematological parameters are shown in Table 3. Sampling event was the only factor that significantly affected PCV, TPP, RBC, HGB, PLT, WBC, NEUT, LYMPH, and MONO. Pairwise comparisons over each sampling event for these nine haematological parameters are summarised below, with coefficients, t- and P-values available in Table S1. Sex, presence of lesions, and capture location did not significantly predict any of the haematological parameters measured and were removed from all models. Descriptive boxplots of haematological parameters across the four sampling events are shown in Fig. 2. Summary statistics for haematological parameters from each sampling event are available in Table S2.
| Parameter | BCI | Sampling event | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2021 Early lactation | 2022 Early lactation | 2022 Mid-lactation | |||||||||
| R2 (%) | d.f. | Coeff. (±s.e.) | t (P) | Coeff. (±s.e.) | t (P) | Coeff. (±s.e.) | t (P) | Coeff. (±s.e.) | t (P) | ||
| PCV | 49.2 | 324 | – | – | −0.084 (±0.006) | −14.2 (<0.001) | −0.045 (±0.006) | −7.12 (<0.001) | 0.007 (±0.007) | 1.06 (0.292) | |
| RBC | 65.1 | 329 | – | – | −0.852 (±0.049) | −17.4 (<0.001) | −0.908 (±0.052) | −17.5 (<0.001) | 0.085 (±0.054) | 1.57 (0.118) | |
| HGB | 57.5 | 328 | – | – | −23.01 (±1.809) | −12.7 (<0.001) | −29.93 (±1.908) | −15.7 (<0.001) | 4.758 (±2.000) | 2.38 (0.018) | |
| PLT | 19.7 | 322 | – | – | 105.8 (±16.52) | 6.40 (<0.001) | 95.57 (±17.16) | 5.57 (<0.001) | −20.51 (±18.16) | −1.13 (0.259) | |
| TPP A | 32.7 | 332 | – | – | 0.956 (±0.010) | −4.42 (<0.001) | 1.082 (±0.012) | 7.36 (<0.001) | 1.054 (±0.012) | 4.70 (<0.001) | |
| WBC A | 48.4 | 334 | – | – | 1.664 (±0.064) | 13.2 (<0.001) | 1.589 (±0.064) | 11.4 (<0.001) | 0.950 (±0.040) | −1.21 (0.229) | |
| NEUT A | 69.3 | 334 | – | – | 2.117 (±0.107) | 14.8 (<0.001) | 2.029 (±0.109) | 13.2 (<0.001) | 0.561 (±0.031) | −10.4 (<0.001) | |
| LYMPH A | 8.0 | 335 | – | – | 0.927 (±0.053) | −1.34 (0.182) | 0.854 (±0.051) | −2.64 (0.009) | 1.191 (±0.074) | 2.81 (0.005) | |
| MONO B | 46.8 | 329 | – | – | 0.284 (±0.002) | 13.9 (<0.001) | 0.330 (±0.002) | 14.3 (<0.001) | 0.282 (±0.002) | 12.7 (<0.001) | |
| EO B | 2.7 | 333 | 0.228 (±0.025) | 3.03 (0.003) | – | – | – | – | – | – | |
Model results are presented as percentage variance explained (R2), degrees of freedom (d.f.), estimated beta coefficients ± standard error (Coeff. ± s.e.), t-ratio (t), and P-values (P). Statistical significance (shown in bold) was set at P < 0.05. Reference level: 2021 Mid-lactation (Sampling event).
Descriptive boxplots of haematological parameters in Australian fur seal pups at Seal Rocks by sampling event (EL, early lactation; ML, mid-lactation). (a) PCV, packed cell volume. (b) RBC, red blood cell counts. (c) HGB, haemoglobin. (d) PLT, platelets. (e) TPP, total plasma protein. (f) WBC, total leukocytes. (g) NEUT, neutrophils. (h) LYMPH, lymphocytes. (i) MONO, monocytes. (j) EO, eosinophils. Horizontal line indicates the median. Lower and upper bounds indicate first and third quartiles, respectively. Whiskers extend to 1.5 times the interquartile range (IQR). Values greater than 1.5 times the IQR indicated by individual points. Letters at top of plots indicate results of pairwise comparisons; different letters indicate significant difference (P < 0.05) between groups. Note: tests on TPP, WBC, NEUT and LYMPH were performed on the log scale; tests on MONO and EO were performed on the square-root scale.
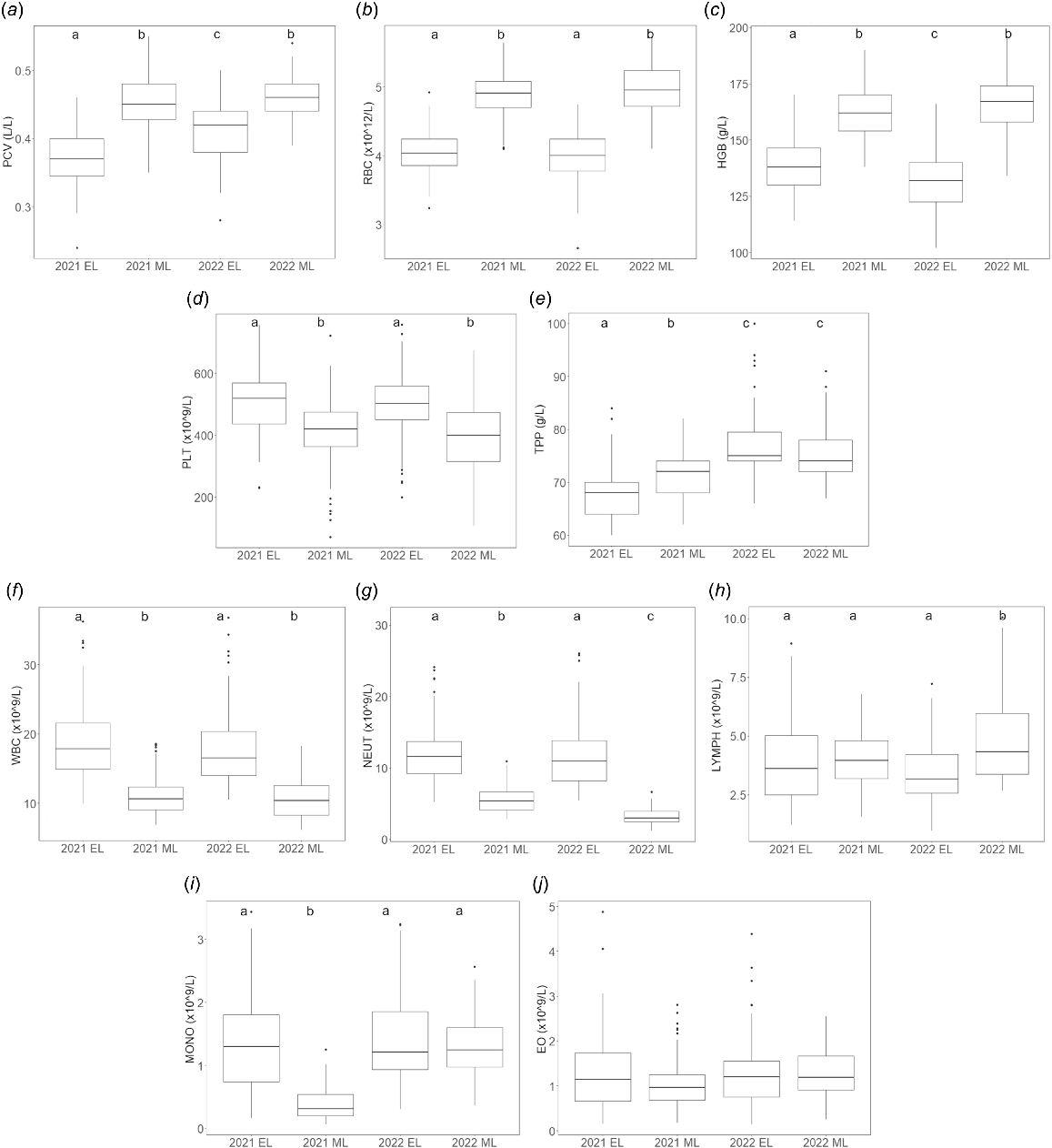
The three red cell parameters (PCV, RBC, HGB) were all significantly lower in early lactation than in mid-lactation. There was no significant difference in RBC between pups sampled in the two early lactation sampling events; however, pups sampled in 2022 early lactation had significantly higher PCV and lower HGB than pups sampled in 2021 early lactation. There were no significant differences between the two mid-lactation sampling events in PCV, RBC, or HGB.
Pups sampled in early lactation had significantly higher PLT than pups sampled in mid-lactation. There was no significant difference in PLT between the two early or mid-lactation sampling events.
While sampling event significantly predicted TPP, no consistent pattern was observed. Pups sampled in both 2021 events had significantly lower TPP than pups sampled in both 2022 events. Pups sampled in 2021 early lactation had significantly lower TPP than pups sampled in 2021 mid-lactation; however, there was no significant difference between the two 2022 sampling events.
Pups sampled in early lactation had significantly higher WBC than pups sampled in mid-lactation. There was no significant difference in WBC between the two early or mid-lactation sampling events.
Pups sampled in early lactation had significantly higher NEUT than pups sampled in mid-lactation. There was no significant difference in NEUT between the two early lactation sampling events; however, pups sampled in 2021 mid-lactation had significantly higher NEUT than pups sampled in 2022 mid-lactation.
Despite significant differences between sampling events, there was no consistent pattern in LYMPH. Pups sampled in 2022 mid-lactation had significantly higher LYMPH than pups sampled in the other three sampling events. There was no significant difference in LYMPH between pups sampled in 2021 early, 2021 mid-, or 2022 early lactation.
Similarly, despite significant differences between sampling events, there was no consistent pattern in MONO. Pups sampled in 2021 mid-lactation had significantly lower MONO than pups sampled in the other three sampling events. There was no significant difference in MONO between pups sampled in 2021 early, 2022 early, or 2022 mid-lactation.
Body condition index alone significantly predicted EO. Increases in BCI were associated with increased EO.
Morphometric parameters
Model results evaluating factors affecting morphometric parameters are shown in Table 4. Sex and sampling event significantly predicted body weight and standard length, but not BCI. Pairwise comparisons over each sampling event for body weight and standard length are summarised below; estimates, t- and P-values are available in Table S1. Descriptive boxplots of each morphometric parameter across the four sampling events are shown in Fig. 3. Summary statistics of morphometric parameters from each sampling event are available in Table S2.
| Parameter A | Sex | Sampling event | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2021 Early lactation | 2022 Early lactation | 2022 Mid-lactation | |||||||||
| R2 (%) | d.f. | Coeff. (±s.e.) | t (P) | Coeff. (±s.e.) | t (P) | Coeff. (±s.e.) | t (P) | Coeff. (±s.e.) | t (P) | ||
| Weight (kg) B | 74.5 | 335 | 1.136 (±0.024) | 6.13 (<0.001) | 0.514 (±0.014) | −24.5 (<0.001) | 0.496 (±0.014) | −24.4 (<0.001) | 0.899 (±0.027) | −3.56 (<0.001) | |
| Standard length (m) B | 77.7 | 335 | 1.043 ± 0.007 | 6.58 (<0.001) | 0.797 (±0.007) | −27.2 (<0.001) | 0.799 (±0.007) | −25.4 (<0.001) | 0.974 (±0.009) | −2.92 (0.004) | |
Model results are presented as percentage variance explained (R2), degrees of freedom (d.f.), estimated beta coefficients ± standard error (Coeff. ± s.e.), t-ratio (t), and P-values (P). Statistical significance (shown in bold) was set at P < 0.05. Reference level: Female (Sex), 2021 Mid-lactation (Sampling event).
Descriptive boxplots of morphometric parameters in Australian fur seal pups at Seal Rocks by sampling event (EL, early lactation; ML, mid-lactation). (a) Weight. (b) Standard length. (c) Body condition index (BCI). Horizontal line indicates the median. Lower and upper bounds indicate first and third quartiles, respectively. Whiskers extend to 1.5 times the interquartile range (IQR). Values greater than 1.5 times the IQR indicated by individual points. Letters at top of plots indicate results of pairwise comparisons; different letters indicate significant difference (P < 0.05) between groups. Note: tests on weight and standard length were performed on the log scale.
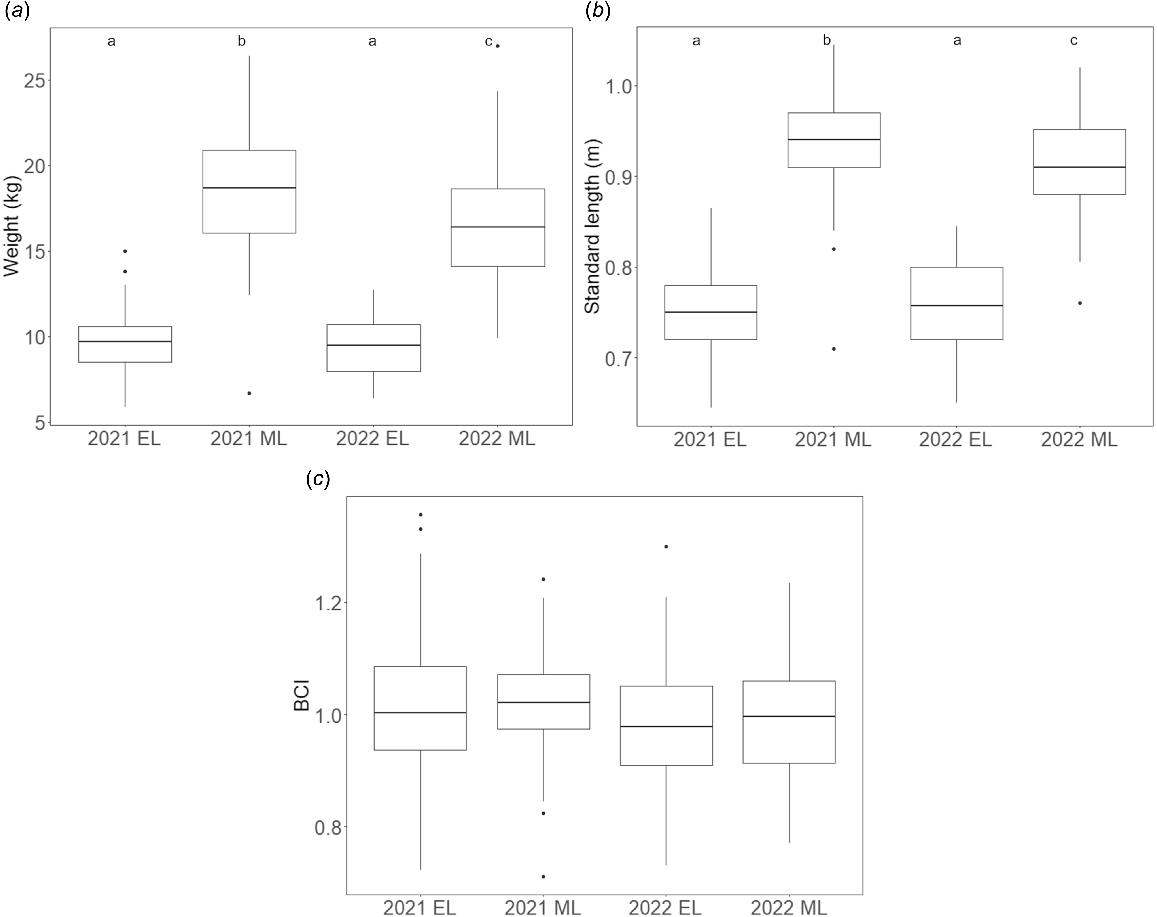
Male pups were shown to have significantly higher body weight and standard length than females. Pups of both sexes sampled in early lactation had significantly lower body weight and standard length than pups sampled in mid-lactation. There was no significant difference in pup body weight and standard length between the two early lactation sampling events; however, pups sampled in 2021 mid-lactation had significantly higher body weight and standard length than pups sampled in 2022 mid-lactation.
An intercept-only model was returned for BCI, meaning no factors were retained in the final model, and it did not differ significantly with pup sex or sampling event.
Factors affecting BCI in early lactation
Year of birth was the only factor that significantly affected BCI (Table 5) in young neonatal pups sampled in early lactation. Sex was removed from the models. Pairwise comparisons over each year are summarised below, with estimates, t- and P-values available in Table S3. Summary statistics for BCI in each sampling year are also available in Table S4.
| 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | ||
|---|---|---|---|---|---|---|---|---|---|
| Coeff. (±s.e.) | 1.026 (±0.022) | 0.970 (±0.023) | 1.071 (±0.027) | 0.972 (±0.022) | 0.989 (±0.021) | 1.024 (±0.022) | 0.979 (±0.021) | 0.938 (±0.021) | |
| t (P) | 1.21 (0.229) | −1.28 (0.202) | 2.70 (0.007) | −1.26 (0.210) | −0.53 (0.596) | 1.13 (0.258) | −0.99 (0.325) | −2.94 (0.003) |
Model results are presented as estimated beta coefficients ± standard error (Coeff. ± s.e.), t-ratio (t), and P-values (P). Percent variance explained (R2) = 8.1%. Degrees of freedom (d.f.) = 661. Statistical significance (shown in bold) was set at P < 0.05. Reference level: 2023.
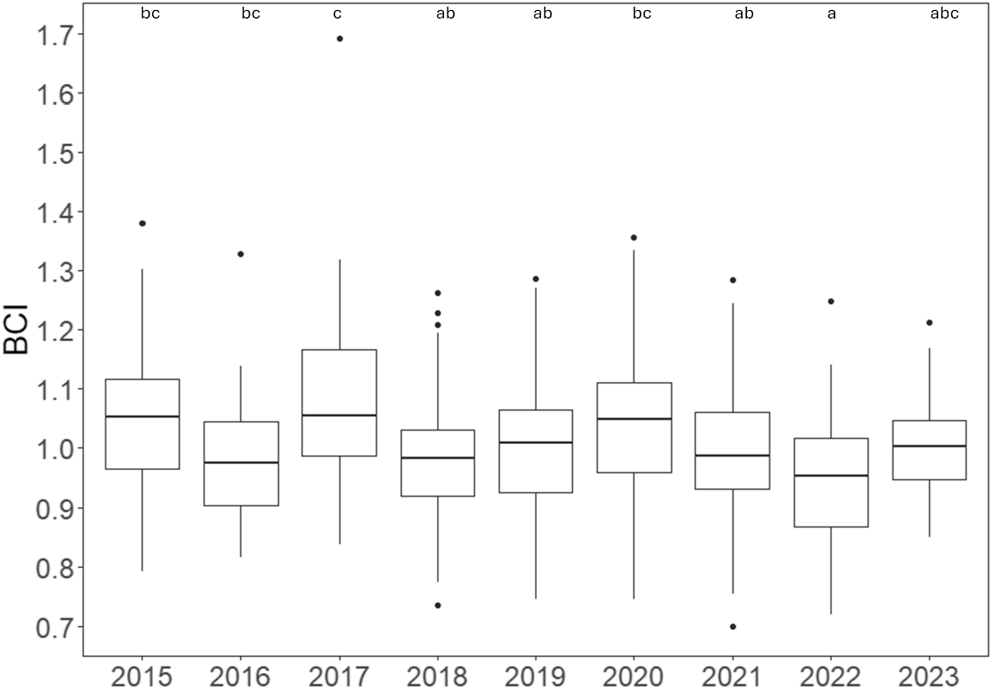
Descriptive boxplots of BCI in young neonatal pups born in 2015–2023 are shown in Fig. 4. When compared to those born in 2023, pups born in 2017 had significantly higher BCI, and pups born in 2022 had significantly lower BCI. Pairwise comparison over 2015–2023 showed multiple significant differences in pup condition between years, but no clear trend in BCI was evident. Further, results of the multiple comparison differed slightly from the single comparison in the model. Pups born in 2017 had the highest median BCI overall (Table S4) and had significantly higher BCI than pups born in 2016, 2018, 2019, 2021, and 2022. Pups born in 2015 and 2020 had significantly higher BCI than pups born in 2022. No other significant differences between year of birth were detected.
Descriptive boxplot of body condition index (BCI) in early lactation Australian fur seal pups at Seal Rocks by year of birth. Horizontal line indicates the median. Lower and upper bounds indicate first and third quartiles, respectively. Whiskers extend to 1.5 times the interquartile range (IQR). Values greater than 1.5 times the IQR indicated by individual points. Letters indicate results of pairwise comparisons; different letters indicate significant difference (P < 0.05) between groups. Note: tests were performed on the log scale.
Discussion
This study presents an evaluation of parameters of health in Australian fur seal pups at Seal Rocks and the developed RIs provide a reference point for future monitoring. These results also aid in the understanding of haematological and physiological changes after the early neonatal period. Sampling event was almost always the most important factor affecting haematological and morphometric parameters, with results varying depending on parameter. The differences in parameters of health between early and mid-lactation are most likely age-related and reflect normal ontogeny. Given that pup body condition in early lactation showed no obvious trend from 2015 to 2023, between-year differences in haematological and morphometric parameters are likely due to fluctuations in factors that were not explored in this study, such as disease states, maternal nutritional status, and environmental conditions.
Development of haematological reference intervals
This study established haematological RIs for Australian fur seal pups at Seal Rocks at 3–6 weeks (early lactation) and 5–6 months (mid-lactation) of age. The observed differences in red cell (PCV, RBC, HGB) and white blood cell (WBC, LYMPH, NEUT, MONO) parameters between early and mid-lactation reflect the growth and physiological changes that occur in the first 6 months of life, principally the development of the erythropoietic and immune systems (Halvorsen and Bechensteen 2002; Spence-Bailey et al. 2007; Brock et al. 2012). The three red cell indices (MCV, MCH, and MCHC) are derived from PCV, RBC, and HGB concentrations.
The lower PCV, HGB, and RBC counts observed in early compared to mid-lactation are likely related to physiological anaemia of infancy, which occurs in all infant mammals and involves suppression of erythropoiesis after entering an oxygen-rich environment at parturition (Halvorsen and Bechensteen 2002). Onset occurs at approximately 1 week of age, and red cell levels are lowest at approximately 8 weeks (Rea et al. 1998; Halvorsen and Bechensteen 2002; Spence-Bailey et al. 2007). Erythropoiesis is stimulated in response to increased demand for oxygen; however, based on analysis of oxygen-carrying capacity in Australian fur seals, rapid growth and expansion of blood volume exceeds the capacity of the erythropoietic system until approximately 4–5 months of age (Spence-Bailey et al. 2007).
There were also significantly higher WBC, NEUT, and MONO (2021 only), and significantly lower LYMPH (2022 only), in early versus mid-lactation. Parturition presents a sudden introduction to a pathogen-rich environment. At birth, the immune system of mammals consists primarily of innate immune cells (e.g. NEUT, MONO) and a low level of adaptive immune cells (e.g. LYMPH) with passively acquired maternal antibodies (Cavagnolo and Vedros 1979; Ross et al. 1994; Day 2007). Neutrophils are a critical component of the innate immune system, which is the first line of defence against pathogens, and an increase in peripheral WBC (driven by NEUT) is expected at this stage of development in response to pathogen exposure, as has been observed in other otariid pups (Keogh et al. 2010). Although development of the erythropoietic and immune systems are part of normal ontogeny, they are energetically demanding processes.
Haematological RIs are typically based on a sample of ‘healthy’ individuals, of known age and nutritional status, and without clinical signs of disease or injury (Friedrichs et al. 2012). While the approximate age of pups is known due to population-wide synchronicity of breeding and parturition, selection of individuals for inclusion in a reference population is challenging in free-ranging populations given that it is not possible to determine nutritional status or identify disease states based solely on physical examination findings, unless animals are obviously injured or emaciated. Sampling of injured or emaciated animals was avoided in this study; as such, the large sample size and removal of outliers ensures that this dataset is as representative as possible of a ‘healthy’ Seal Rocks population. Further, parameters of health can vary at different sites based on colony size and varying levels of exposure to pathogens and anthropogenic influences. For example, Galapagos sea lions (Zalophus wollebaeki) at two sites with differing density and proximity to human activity had significantly different haematological and immunological profiles (Brock et al. 2012, 2013). These differences highlight the importance of site-specific RIs, because what is considered ‘normal’ for one colony may not be for another.
In addition to partitioning by pup age, it was necessary to further partition some parameters by year because of significant variability (see next section ‘Factors affecting haematological parameters’). Only further monitoring may identify longitudinal trends in parameters of health. These RIs also provide important contextual information when assessing the potential impacts of natural and anthropogenic threats on the colony because they demonstrate the physiological differences between pups in early (3–6 weeks) and mid-lactation (5–6 months). Pups are the most vulnerable age cohort, particularly during the early neonatal period, coinciding with the peak in human visitation at Seal Rocks (J. Taylor, unpubl. data).
These haematological RIs also provide important reference points for pups at Seal Rocks at these two life stages. Seal Rocks is the site with the highest pup production but has one of the highest rates of decline in live pup abundance in the Australian fur seal population (McIntosh et al. 2022), therefore the development of these RIs provides a valuable contribution to the ongoing monitoring of this species and interpretation of trends.
Factors affecting haematological parameters
With the exception of EO, the primary factor affecting pup haematological parameters was sampling event. Since sampling event accounts for the year of birth (2021, 2022) and pup age (early lactation, mid-lactation), this indicates that ontogenic and yearly influences were the most important drivers of variability.
A significant increase from early (3–6-week-old pups) to mid-lactation (5–6-month-old pups) was seen in PCV, RBC, and HGB and is likely due to normal ontogeny of the hematopoietic system. These three parameters typically follow the same pattern, increasing with age in young pinnipeds (Roletto 1993; Lander et al. 2003; Kohyama et al. 2021). Packed cell volume is also linked to body size, hydration status, and can also increase via splenic contraction in adrenalin-mediated physiological responses in mammals (Lander et al. 2003; Reif et al. 2006). Although RBC did not significantly differ between years in early and mid-lactation, there was an inexplicable divergence between PCV and HGB in the two early lactation sampling events; PCV in 2021 early lactation was lower than the 2022 early lactation, and HGB in 2021 early lactation was higher than 2022 early lactation. Red cell parameters have been reported to change independently of one another in pinnipeds that are emaciated or afflicted with specific disease states, such as pneumonia, parasitic infections, or enteritis and liver failure (Roletto 1993). However, without specific diagnostic investigations of disease states in the present study, the cause of the difference between the two early lactation sampling events is unknown.
A significant decrease from early to mid-lactation was seen in PLT. In contrast to the results of the present study, platelets are reported to increase with age in Steller sea lions (Eumetopias jubatus) (Gerlinsky et al. 2018) and humans (Jacob 2016). However, PLT in Australian sea lion pups was found to be lower in older pups, suggesting decreased PLT in the short term could reflect normal haematological ontogenesis (Lindsay et al. 2023). Increased PLT (thrombocytosis) has been associated with iron-deficiency anaemia and inflammatory conditions in mammals (McMichael et al. 2015; Brandimarti et al. 2021; McCartney 2022). Although young neonatal pups exhibit physiological anaemia and likely have a higher incidence of inflammatory demand than older pups due to increased exposure to pathogens, the most likely reason for the observed decrease from early to mid-lactation is normal ontogeny.
A significant increase from early to mid-lactation was observed in TPP, but only between the 2021 early and both mid-lactation sampling events. Total plasma protein is associated with nutritional status, inflammation, and organ function, and typically increases with age (Brock et al. 2012; Gerlinsky et al. 2018; Lindsay et al. 2023), but this was not apparent in 2022. Total plasma protein was also significantly higher in 2022 early, compared with 2021 early lactation. The reason for this is difficult to explain in the absence of relative albumin and globulin concentrations but could reflect differences in nutritional status, patterns of maternal attendance, or inflammatory status between the two sampling years (Brock et al. 2012), and could be ascertained in future using serum protein electrophoresis (e.g. Brock et al. 2013; Meza Cerda et al. 2022). Further, it is difficult to interpret differences based on only two time point comparisons across only 2 years. Comprehensive longitudinal sampling of pups at approximately one (early neonatal), five (mid-lactation, moult), eight (peak milk intake), and 10 (pre-weaning) months (e.g. Atkinson et al. 2011; Brock et al. 2012), concurrent with evaluation of maternal nutritional status, would aid the interpretation of these seasonal changes. Such a study should ideally be performed over at least 10 consecutive years and include a comparison colony to account for variability in maternal provisioning and influential environmental conditions that change over long timescales, often with lagged effects (Speakman et al. 2020; Kliska et al. 2022).
A significant increase from early to mid-lactation was observed in LYMPH between the two early lactation sampling events and 2022 mid-lactation. Lymphocytes are the primary component of the adaptive immune system, which takes time to develop due to its high specificity (Desforges et al. 2016). Neonatal pups in early lactation would therefore be expected to have lower LYMPH than older pups in mid-lactation. This was observed in pups sampled in 2022, but not 2021. Release of glucocorticoid (GC) stress hormones and exposure to environmental contaminants can also result in lymphopenia in vertebrates (Aguirre et al. 1995; Desforges et al. 2016; McCourt and Rizzi 2022); however, this is difficult to separate from the effects of neonatal immune development. The reason for the lack of difference between early and mid-lactation for LYMPH in pups born in 2021 is unclear. It should be noted that this model had very low explanatory power (R2 = 8.0%) (Table 3), which suggests other factors not explored in the current study could be contributing to variability in LYMPH.
The decrease in WBC and NEUT from early to mid-lactation follows the expected age-related pattern and appears to be driven by NEUT, as has been observed in other otariid pups (Keogh et al. 2010). Neutrophils are the primary cell type involved in innate immunity and are the most numerous WBC in marine mammals (Desforges et al. 2016). Young neonatal pups have reduced immunocompetence, resulting in increased innate immune system activity and thus higher NEUT counts in the blood when naïve individuals are exposed to pathogens. This pronounced neutrophilia decreases as animals age and develop adaptive immunity. Neutrophilia can also be a function of adrenalin- and GC-mediated stress responses (McCartney 2022) but, once again, without specific disease investigations or inflammatory markers, such as haptoglobin (Desforges et al. 2016), these effects are difficult to separate from those of immunodevelopment in young animals.
A significant decrease from early to mid-lactation was also observed in MONO, but only in 2021. Monocytes are another component of innate immunity and are generally low in number compared to NEUT and LYMPH (McCartney 2022). High MONO counts (monocytosis) can be seen during GC-mediated stress responses, in infections with increased demand for macromolecular phagocytosis, or in chronic inflammatory responses (Souza and Eren 2022). The decrease from 2021 early to 2021 mid-lactation may be due to normal ontogeny as pups age and develop adaptive immune responses; MONO has been shown to decrease with age in Steller sea lions (Gerlinsky et al. 2018). However, in other otariid pups, MONO has been shown to have no clear temporal trend (Keogh et al. 2010; Lindsay et al. 2023), which aligns with the lack of significant difference between 2022 early and mid-lactation. This disparity between the two sampling years doesn’t align with differences in the other differential WBC, making interpretation difficult, but could relate to other factors not measured in the present study.
The only WBC to not differ significantly between sampling events was EO, which was solely predicted by BCI. This finding is consistent with those previously reported in otariids, where no seasonal or age-related changes in EO were observed (Keogh et al. 2010; Kohyama et al. 2021). Eosinophils are involved in parasitic and inflammatory (particularly allergic) responses, increasing in response to parasite load and exposure to environmental pollutants (Davis et al. 2008; Young and Layne 2022). In some species, EO will vary diurnally and can decrease in response to GC secretion (Young and Layne 2022). Normal ranges will also vary between species and region (Young and Layne 2022). In harbor seals (Phoca vitulina) EO was shown to increase with age in pups and with BCI in subadult and adult animals, possibly in response to increased parasite or pollutant exposure from prey (Greig et al. 2010). Although no effect was observed, this model had very low explanatory power (R2 = 2.7%), so variability in EO is likely caused by another factor not accounted for in this study.
Factors affecting morphometric parameters
Variability in weight and standard length was driven by sampling event and pup sex, with male pups shown to have significantly greater standard length and body weight compared to females. Otariids are sexually dimorphic and male pups are, on average, heavier at birth than females in Australian fur seals (Arnould and Hindell 2002; Wall et al. 2023), long-nosed fur seals (Arctocephalus forsteri) (Bradshaw et al. 2003), Antarctic fur seals (Arctocephalus gazella) (Costa et al. 1988), and California sea lions (Zalophus californianus) (Ono and Boness 1996). Male-biased sex ratios may also have affected this, but these were observed only in early lactation and did not affect haematological parameters or BCI. The reason behind the male-biased sex ratios in neonatal pups is unclear but may be related to maternal provisioning or environmental conditions and has also been observed in long-nosed fur seals (Bradshaw et al. 2003). Little is known of sex ratios beyond the first 3 months of life; however, in northern elephant seals (Mirounga angustirostris), sex ratios were significantly male-biased at birth, but equal at weaning, suggesting lower rearing success in males (Le Bœuf et al. 1989). Whether or not this is the case in Australian fur seals is unknown and is beyond the scope of the current study.
The significant increase in weight and standard length between early and mid-lactation reflects pup growth between these two periods. While there was no significant difference between the two early lactation sampling events, pup body weight and length in 2022 mid-lactation was lower than in 2021 mid-lactation, which could indicate lower nutritional plane in 2022, impacting pup growth. Possible causes of this include reduced feed availability (or quality) and/or increased maternal absence (Lea et al. 2006). Body condition index was also lower in 2022, although this was not statistically significant, and BCI showed no significant changes between the four sampling events. There were no sex-related differences in BCI between males and females, indicating that while males are larger, pups of both sexes grew at approximately the same rate.
Effect of year of birth on early lactation BCI
Contrary to expectations, BCI in early lactation (pups 3–8 weeks of age) showed no clear declining trend from 2015 to 2023, but fluctuated between years. This model also had low explanatory power (R2 = 8.1%), indicating that year of birth alone was a poor predictor of variability in BCI. While beyond the scope of the present study, changes in climate and oceanographic conditions, such as sea surface temperature (SST), at local and regional scales affect prey frequency of occurrence in the diet of Australian fur seals at Seal Rocks (Kliska et al. 2022), which may impact maternal foraging success and increase maternal absence (Lea et al. 2006). Further, little penguins (Eudyptula minor) at Phillip Island, close to Seal Rocks, have exhibited lower breeding success as SST increases (Pulvirenti et al. 2023) and, in recent years, heat stress has resulted in penguin mortality on Phillip Island (Reinhold et al. 2022). Extreme weather and climate variability are predicted to continue increasing as a result of climate change (IPCC 2023). Extreme ambient heat may result in pup mortality when there is no available shelter (R McIntosh and I Charrier, pers. obs.). Further, in years with higher rainfall and lower air temperatures, neonatal pup condition could be affected by increased thermoregulatory demand, as the natal coat of fur seals (lanugo) is not waterproof and is better adapted for insulation against heat, rather than cold (Erdsack et al. 2013). Increased ocean inundation and storm surge at Seal Rocks is predicted under climate change (McLean et al. 2018), which could also affect pup thermoregulation and energetic demands if they are more often wet while young.
In contrast to BCI calculated over the two study years, in which males and females could be pooled, BCI from 2015 to 2023 was sufficiently different in some years between males and females to necessitate sex-dependent calculation. It is possible that in some years, male and female pups were affected disproportionately by environmental variables or differences in maternal provisioning. Exploration of oceanographic and climatic measurements could be incorporated into future studies of parameters of health in Australian fur seal pups to determine whether they have a role in influencing pup body condition and other indicators of health status. Additional parameters of health, infectious disease, nutrition, and behaviour should also be incorporated in future studies of population health.
Conclusions
The haematological RIs developed in this study provide a valuable point of reference for future monitoring in Australian fur seal pups at Seal Rocks and highlight the importance of age-specific haematological RIs. Most changes in haematological and morphometric parameters from early to mid-lactation are due to normal hematopoietic ontogeny and growth. Future studies would benefit from including additional measures, such as serum protein electrophoresis and acute phase proteins, to better understand the role of nutritional and disease status on haematological parameters. Further, concurrent longitudinal monitoring of pup health (i.e. at 1, 5, 8 and 10 months of age) with pup production indices over the next decade is recommended to determine whether health status is a driving factor in declining live pup abundance. An examination of local and regional scale climatic events on pup health could explain variability observed in health metrics and assist population forecasts under climate change scenarios.
Declaration of funding
This research was funded by the Holsworth Wildlife Research Endowment & Ecological Society of Australia, the Foundation for Australia’s Most Endangered Species (FAME), The Royal Zoological Society of NSW & Paddy Pallin Science Grant, The University of Sydney, and Phillip Island Nature Parks. JJT was funded by a PhD stipend (Jean Walker Trust Fellowship) and Postgraduate Research Support Scheme from the University of Sydney.
Author contributions
Jessalyn Taylor: Methodology, Formal Analysis, Investigation, Writing – original draft, Visualisation, Project administration, Funding Acquisition. Rebecca McIntosh: Conceptualisation, Methodology, Investigation, Resources, Supervision, Writing – review and editing, Project Administration, Funding Acquisition. Isabelle Charrier: Supervision, Writing – review and editing, Funding Acquisition. Rachael Gray: Conceptualisation, Methodology, Investigation, Resources, Supervision, Writing – review and editing, Project Administration, Funding Acquisition.
Acknowledgements
The authors acknowledge the Bunurong people of the Eastern Kulin nation, upon whose land and sea Country this research took place. The authors also thank the Victorian Fisheries Authority (Cowes), KinaDiving, and Dive Phillip Island for transport to and from Seal Rocks, the many field volunteers that assisted with sample collection and processing, H. Schinagl for generating maps for publication, and Dr E. Hall for advice on statistical analysis. Pup data from 2023 was collected by A. Yaney-Keller. R code for calculation of BCI was provided by Dr M. Terkildsen.
References
Aguirre AA, Balazs GH, Spraker TR, Gross TS (1995) Adrenal and hematological responses to stress in juvenile green turtles (Chelonia mydas) with and without Fibropapillomas. Physiological Zoology 68(5), 831-854.
| Crossref | Google Scholar |
Arnould JPY, Hindell MA (2001) Dive behaviour, foraging locations, and maternal-attendance patterns of Australian fur seals (Arctocephalus pusillus doriferus). Canadian Journal of Zoology 79(1), 35-48.
| Crossref | Google Scholar |
Arnould JPY, Hindell MA (2002) Milk consumption, body composition and pre-weaning growth rates of Australian fur seal (Arctocephalus pusillus doriferus) pups. Journal of Zoology 256(3), 351-359.
| Crossref | Google Scholar |
Atkinson S, Arnould JPY, Mashburn KL (2011) Plasma cortisol and thyroid hormone concentrations in pre-weaning Australian fur seal pups. General and Comparative Endocrinology 172(2), 277-281.
| Crossref | Google Scholar | PubMed |
Back JJ, Hoskins AJ, Kirkwood R, Arnould JPY (2018) Behavioral responses of Australian fur seals to boat approaches at a breeding colony. Nature Conservation 31, 35-52.
| Crossref | Google Scholar |
Bossart GD (2010) Marine mammals as sentinel species for oceans and human health. Veterinary Pathology 48(3), 676-690.
| Crossref | Google Scholar | PubMed |
Bradshaw CJA, Davis LS, Lalas C, Harcourt RG (2000) Geographic and temporal variation in the condition of pups of the New Zealand fur seal (Arctocephalus forsteri): evidence for density dependence and differences in the marine environment. Journal of Zoology 252(1), 41-51.
| Crossref | Google Scholar |
Bradshaw CJA, Harcourt RG, Davis LS (2003) Male-Biased sex ratios in New Zealand fur seal pups relative to environmental variation. Behavioral Ecology and Sociobiology 53(5), 297-307.
| Crossref | Google Scholar |
Brandimarti ME, Gray R, Silva FRO, Herbert CA (2021) Kangaroos at maximum capacity: health assessment of free-ranging eastern grey kangaroos on a coastal headland. Journal of Mammalogy 102(3), 837-851.
| Crossref | Google Scholar | PubMed |
Brock PM, Hall AJ, Goodman SJ, Cruz M, Acevedo-Whitehouse K (2012) Applying the tools of ecological immunology to conservation: a test case in the Galapagos sea lion. Animal Conservation 16(1), 19-31.
| Crossref | Google Scholar |
Brock PM, Hall AJ, Goodman SJ, Cruz M, Acevedo-Whitehouse K (2013) Immune activity, body condition and human-associated environmental impacts in a wild marine mammal. PLoS ONE 8(6), e67132.
| Crossref | Google Scholar | PubMed |
Cavagnolo RZ, Vedros NA (1979) Serum and colostrum immunoglobulin levels in the northern fur seal Callorhinus ursinus. Developmental & Comparative Immunology 3, 139-146.
| Crossref | Google Scholar | PubMed |
Costa DP, Trillmich F, Croxall JP (1988) Intraspecific allometry of neonatal size in the Antarctic fur seal (Arctocephalus galapagoensis). Behavioral Ecology and Sociobiology 22, 361-364.
| Crossref | Google Scholar |
Davis AK, Maney DL, Maerz JC (2008) The use of leukocyte profiles to measure stress in vertebrates: a review for ecologists. Functional Ecology 22(5), 760-772.
| Crossref | Google Scholar |
Day MJ (2007) Immune system development in the dog and cat. Journal of Comparative Pathology 137, S10-S15.
| Crossref | Google Scholar | PubMed |
Desforges J-PW, Sonne C, Levin M, Siebert U, De Guise S, Dietz R (2016) Immunotoxic effects of environmental pollutants in marine mammals. Environment International 86, 126-139.
| Crossref | Google Scholar | PubMed |
Erdsack N, Dehnhardt G, Hanke W (2013) Coping with heat: function of the natal coat of cape fur seal (Arctocephalus pusillus pusillus) pups in maintaining core body temperature. PLoS ONE 8(8), e72081.
| Crossref | Google Scholar | PubMed |
Finnegan D (2022) ‘Package ‘referenceIntervals’, Vol. 2023.’ (CRAN) Available at https://cran.r-project.org/web/packages/referenceIntervals/index.html
Friedrichs KR, Harr KE, Freeman KP, Szladovits B, Walton RM, Barnhart KF, Blanco-Chavez J (2012) ASVCP reference interval guidelines: determination of de novo reference intervals in veterinary species and other related topics. Veterinary Clinical Pathology 41(4), 441-453.
| Crossref | Google Scholar | PubMed |
Fulham M, Power M, Gray R (2020) Diversity and distribution of Escherichia coli in three species of free-ranging Australian pinniped pups. Frontiers in Marine Science 7, 571171.
| Crossref | Google Scholar |
Fulham M, McDougall F, Power M, McIntosh RR, Gray R (2022) Carriage of antibiotic resistant bacteria in endangered and declining Australian pinniped pups. PLoS ONE 17(1), e0258978.
| Crossref | Google Scholar | PubMed |
Gardner BR, Stenos J, Hufschmid J, Arnould JPY, McIntosh RR, Tadepalli M, Tolpinrud A, Marenda M, Lynch M, Stent A (2022) An old pathogen in a new environment–implications of Coxiella burnetii in Australian fur seals (Arctocephalus pusillus doriferus). Frontiers in Marine Science 9, 809075.
| Crossref | Google Scholar |
Geeson JJ, Hindell MA, Hobday AJ, Speakman CN, Arnould JPY (2023) Long-term decline in body condition of female Australian fur seals: potential causes and implications. Frontiers in Marine Science 10, 1231337.
| Crossref | Google Scholar |
Gerlinsky CD, Haulena M, Trites AW, Rosen DAS (2018) Reference ranges and age-related and diving exercise effects on hematology and serum chemistry of female Steller sea lions (Eumetopias jubatus). Journal of Zoo and Wildlife Medicine 49(1), 18-29.
| Crossref | Google Scholar | PubMed |
Gibbens J, Parry LJ, Arnould JPY (2010) Influences on fecundity in Australian fur seals (Arctocephalus pusillus doriferus). Journal of Mammalogy 91(2), 510-518.
| Crossref | Google Scholar |
Greig DJ, Gulland FMD, Rios CA, Hall AJ (2010) Hematology and serum chemistry in stranded and wild-caught harbor seals in central California: reference intervals, predictors of survival, and parameters affecting blood variables. Journal of Wildlife Diseases 46(4), 1172-1184.
| Crossref | Google Scholar | PubMed |
Halvorsen S, Bechensteen AG (2002) Physiology of erythropoietin during mammalian development. Acta Paediatrica 91(438), 17-26.
| Crossref | Google Scholar |
Harrison XA, Donaldson L, Correa-Cano ME, Evans J, Fisher DN, Goodwin CED, Robinson BS, Hodgson DJ, Inger R (2018) A brief introduction to mixed effects modelling and multi-model inference in ecology. PeerJ 6, e4794.
| Crossref | Google Scholar | PubMed |
Horn PS, Pesce AJ (2003) Reference intervals: an update. Clinica Chimica Acta 334(1–2), 5-23.
| Crossref | Google Scholar |
Jacob EA (2016) Hematological differences in newborn and aging: a review study. Hematology & Transfusion International Journal 3(3), 178-190.
| Crossref | Google Scholar |
Keogh MJ, Maniscalco JM, Atkinson S (2010) Steller sea lion (Eumetopias jubatus) pups undergo a decrease in circulating white blood cells and the ability of T cells to proliferate during early postnatal development. Veterinary Immunology and Immunopathology 137(3–4), 298-304.
| Crossref | Google Scholar | PubMed |
Kliska K, McIntosh RR, Jonsen I, Hume F, Dann P, Kirkwood R, Harcourt R (2022) Environmental correlates of temporal variation in the prey species of Australian fur seals inferred from scat analysis. Royal Society of Open Science 9(10), 211723.
| Crossref | Google Scholar |
Kohyama K, Kiyota M, Inoshima Y (2021) Longitudinal study of northern fur seal (Callorhinus ursinus) hematology. Journal of Veterinary Medical Science 83(7), 1128-1137.
| Crossref | Google Scholar | PubMed |
Lander ME, Harvey JT, Gulland FMD (2003) Hematology and serum chemistry comparisons between free-ranging and rehabilitated harbor seal (Phoca vitulina richardsi) pups. Journal of Wildlife Diseases 39(3), 600-609.
| Crossref | Google Scholar | PubMed |
Le Bœuf BJ, Condit R, Reiter J (1989) Parental investment and the secondary sex ratio in northern elephant seals. Behavioral Ecology and Sociobiology 25, 109-117.
| Crossref | Google Scholar |
Lea M-A, Guinet C, Cherel Y, Duhamel G, Dubroca L, Pruvost P, Hindell M (2006) Impacts of climatic anomalies on provisioning strategies of a Southern Ocean predator. Marine Ecology Progress Series 310, 77-94.
| Crossref | Google Scholar |
Lenth RV (2024) ‘Emmeans: estimated marginal means, aka least-squares means.’ (CRAN) Available at https://cran.r-project.org/web/packages/emmeans/index.html
Lindsay SA, Fulham M, Caraguel CGB, Gray R (2023) Mitigating disease risk in an endangered pinniped: early hookworm elimination optimizes the growth and health of Australian sea lion pups. Frontiers in Veterinary Science 10, 1161185.
| Crossref | Google Scholar | PubMed |
Marcus AD, Higgins DP, Gray R (2015) Health assessment of free-ranging endangered Australian sea lion (Neophoca cinerea) pups: effect of haematophagous parasites on haematological parameters. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 184, 132-143.
| Crossref | Google Scholar |
Martin M, Gridley T, Elwen SH, Charrier I (2022) Assessment of the impact of anthropogenic airborne noise on the behaviour of Cape fur seals during the breeding season in Namibia. Journal of Experimental Marine Biology and Ecology 550, 151721.
| Crossref | Google Scholar |
Martin M, Gridley T, Elwen S, Charrier I (2023) Inter-site variability in the Cape fur seal’s behavioural response to boat noise exposure. Marine Pollution Bulletin 196, 115589.
| Crossref | Google Scholar | PubMed |
McIntosh RR, Kirkwood R, Sutherland DR, Dann P (2015) Drivers and annual estimates of marine wildlife entanglement rates: a long-term case study with Australian fur seals. Marine Pollution Bulletin 101(2), 716-725.
| Crossref | Google Scholar | PubMed |
McIntosh RR, Kirkman SP, Thalmann S, Sutherland DR, Mitchell A, Arnould JPY, Salton M, Slip DJ, Dann P, Kirkwood R (2018) Understanding meta-population trends of the Australian fur seal, with insights for adaptive monitoring. PLoS ONE 13(9), e0200253.
| Crossref | Google Scholar | PubMed |
McIntosh RR, Sorrell KJ, Thalmann S, Mitchell A, Gray R, Schinagl H, Arnould JPY, Dann P, Kirkwood R (2022) Sustained reduction in numbers of Australian fur seal pups: implications for future population monitoring. PLoS ONE 17(3), e0265610.
| Crossref | Google Scholar | PubMed |
McLean LJ, George S, Ierodiaconou D, Kirkwood RJ, Arnould JPY (2018) Impact of rising sea levels on Australian fur seals. PeerJ 6, e5786.
| Crossref | Google Scholar | PubMed |
McMichael L, Edson D, McLaughlin A, Mayer D, Kopp S, Meers J, Field H (2015) Haematology and plasma biochemistry of wild black flying-foxes, (Pteropus alecto) in Queensland, Australia. PLoS ONE 10(5), e0125741.
| Crossref | Google Scholar |
Meza Cerda M-I, Gray R, Thomson PC, Butcher L, Simpson K, Cameron A, Marcus AD, Higgins DP (2022) Developing immune profiles of endangered Australian sea lion (Neophoca cinerea) pups within the context of endemic hookworm (Uncinaria sanguinis) infection. Frontiers in Veterinary Science 9, 824584.
| Crossref | Google Scholar | PubMed |
Ono KA, Boness DJ (1996) Sexual dimorphism in sea lion pups: differential maternal investment, or sex-specific differences in energy allocation? Behavioral Ecology and Sociobiology 38, 31-41.
| Google Scholar |
Pulvirenti J, Reina RD, Chiaradia A (2023) Exploring subcolony differences in foraging and reproductive success: the influence of environmental conditions on a central place foraging seabird. Royal Society Open Science 10(6), 220362.
| Crossref | Google Scholar |
Rea LD, Castellini MA, Fadely BS, Loughlin TR (1998) Health status of young Alaska Steller sea lion pups (Eumetopias jubatus) as indicated by blood chemistry and hematology. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 120(4), 617-623.
| Crossref | Google Scholar |
R Core Team (2023) ‘R: A Language and Environment for Statistical Computing’. (R Foundation for Statistical Computing: Vienna, Austria). Available at https://www.R-project.org/
Reif JS, Bachand A, Aguirre AA, Borjesson DL, Kashinsky L, Braun R, Antonelis G (2006) Morphometry, hematology, and serum chemistry in the Hawaiian monk seal (Monachus Schauinslandi). Marine Mammal Science 20(4), 851-860.
| Crossref | Google Scholar |
Reinhold S-L, Goldsworthy SD, Arnould JPY, Gillanders BM, Connell SD, McIntosh RR (2022) Tracing seal predation back to the source colony of their penguin prey: a trace element and stable isotope analysis. Frontiers in Marine Science 9, 813106.
| Crossref | Google Scholar |
Roletto J (1993) Hematology and serum chemistry values for clinically healthy and sick pinnipeds. Journal of Zoo and Wildlife Medicine 24(2), 145-157.
| Google Scholar |
Ross PS, De Swart RL, Visser IKG, Vedder LJ, Murk W, Bowen WD, Osterhaus ADME (1994) Relative immunocompetence of the newborn harbour seal, Phoca vitulina. Veterinary Immunology and Immunopathology 42(3–4), 331-348.
| Crossref | Google Scholar | PubMed |
Speakman CN, Hoskins AJ, Hindell MA, Costa DP, Hartog JR, Hobday AJ, Arnould JPY (2020) Environmental influences on foraging effort, success and efficiency in female Australian fur seals. Scientific Reports 10(1), 17710.
| Crossref | Google Scholar | PubMed |
Spence-Bailey LM, Verrier D, Arnould JPY (2007) The physiological and behavioural development of diving in Australian fur seal (Arctocephalus pusillus doriferus) pups. Journal of Comparative Physiology B 177(4), 483-494.
| Crossref | Google Scholar |
Taylor S, Terkildsen M, Stevenson G, de Araujo J, Yu C, Yates A, McIntosh RR, Gray R (2021) Per and polyfluoroalkyl substances (PFAS) at high concentrations in neonatal Australian pinnipeds. Science of the Total Environment 786, 147446.
| Crossref | Google Scholar | PubMed |
Taylor JJ, McIntosh RR, Gray RB, Charrier I (2024) Behavioural response of Australian fur seals (Arctocephalus pusillus doriferus) to vessel noise during peak and off-peak human visitation. Marine Pollution Bulletin 208, 116947.
| Crossref | Google Scholar |
Tripovich JS, Hall-Aspland S, Charrier I, Arnould JP (2012) The behavioural response of Australian fur seals to motor boat noise. PLoS ONE 7(5), e37228.
| Crossref | Google Scholar | PubMed |
Trumble SJ, Castellini MA (2002) Blood chemistry, hematology, and morphology of wild harbor seal pups in Alaska. The Journal of Wildlife Management 66(4), 1197-1207.
| Crossref | Google Scholar |
Wall D, Thalmann S, Wotherspoon S, Lea M-A (2023) Is regional variability in environmental conditions driving differences in the early body condition of endemic Australian fur seal pups? Wildlife Research 50(12), 993-1007.
| Crossref | Google Scholar |


