Helox suggests advective ventilation of the insect tracheal system during discontinuous gas exchange
Waseem Abbas A B * , Philip C. Withers A and Theodore A. Evans A
A B * , Philip C. Withers A and Theodore A. Evans A
A
B
Abstract
About a century ago, Krogh theorised that gas exchange by insects between spiracles and tissues occurs by diffusion without an advective (convective) component but physiological data supporting the relative roles of diffusion and advection are unclear, especially for small, flightless insects that lack air sacs. We examined the role of diffusion and advection in gas exchange for the small red flour beetle (Tribolium castaneum; 1.5 mg; no air sacs) and the larger speckled cockroach (Nauphoeta cinerea; 700 mg; air sacs) by comparing spiracular cycle durations during discontinuous gas exchange in air and helox (21% oxygen in helium), which should increase diffusive but not advective exchange. Helox reduced the burst-phase duration of beetles by 13%, suggesting a substantial role for advection and a minor role for diffusion, challenging the theory that small insects rely on diffusion and consistent with evidence for advective gas exchange mediated by tracheal compression. For cockroaches, burst phase duration was unaffected, supporting a primary role of advection and unmeasurable diffusion. We conclude that helox may provide a novel and useful experimental approach to discriminate between tracheal diffusion and advection. A mechanistic understanding of tracheal gas exchange for the red flour beetle could facilitate development of physiologically informed fumigation regimes for pest management.
Keywords: controlled atmospheres, convection, DGE, diffusion, gas exchange, insects, Nauphoeta cinerea, Tribolium castaneum, stored grain insect pests.
Introduction
Gas exchange by insects typically occurs through an air-filled tracheal system. The atmosphere is connected through spiracles with large tracheal trunks, then tracheal tubes, and finally many small, blind-ended tracheoles with diameter less than 2 μm (Quinlan and Gibbs 2006; Harrison et al. 2012; Wasserthal and Fröhlich 2017; Khan and Staples 2025). The terminal tracheoles are thin-walled, providing gas exchange with tissues. Movement of gases between the spiracles and tracheoles was originally suggested to occur only by diffusion, particularly for small insects (Krogh 1920, 1941), which has been supported by measurements for small ants (Lighton 1994). However, many large insects have been shown to meet their gas exchange requirements mainly through advection (velocity/pressure-induced bulk air flow) and rely on diffusion only in the thin-walled terminal tracheoles (Kestler 1985).
Advection occurs in many large insects by active ventilation of the tracheal system, to provide convective air movement and maximise gas exchange during activity (e.g. locomotion and feeding), using abdominal pumping movements, and haemolymph pressure oscillations that compress/expand tracheal tubes and air sacs if present (Harrison et al. 2012, 2013, 2023; Matthews and Terblanche 2015). Some large insects, such as locusts and cockroaches, couple spiracular opening and closing with ventilatory movements to achieve unidirectional bulk advective flow (Matthews and Terblanche 2015; Snelling et al. 2017; Harrison et al. 2023). Sláma (1999) and coauthors (Sláma and Coquillaud 1992; Sláma et al. 2007; Sláma and Santiago-Blay 2017) suggest that even small insects convectively ventilate their tracheal system for gas exchange, and expansion/collapse of tracheal tubes have been observed for resting Drosophila (Hale et al. 2006) and various other insects (Westneat et al. 2003).
Movement of gases between the tracheal system and ambient atmosphere is controlled by various patterns of spiracular opening and closing (diffusive gas exchange across the cuticle is negligible: Buck and Keister 1955; Buck 1962). Continuous gas exchange (CGE) occurs when spiracles are constantly open, cyclic gas exchange occurs when some spiracles open and close but not synchronously with others, and discontinuous gas exchange (DGE) is when there are three synchronised spiracular phases: Close (C), Flutter (F) and Open (O) (Terblanche and Woods 2018).
Insects might use DGE to survive desiccation (Buck et al. 1953; Lighton 1996) or suboptimal CO2/O2 levels or a combination of both the water scarcity and suboptimal CO2/O2 levels (Lighton and Berrigan 1995; Chown et al. 2006), avoid oxidative tissue damage by free radicals at rest (Hetz and Bradley 2005), or exclude ectoparasites/dust from entering the tracheal system (Miller 1974; Chown et al. 2006). Besides these adaptive explanations for DGE, the non-adaptive emergent property hypothesis (Chown and Holter 2000) proposes the display of DGE as an outcome of two interactive feedback systems (O2 and CO2) that regulate the opening and closing of spiracles and the neural hypothesis (Matthews and White 2011a) suggests the regulation of gas exchange by thoracic and abdominal ganglia during periods of brain inactivity as the reason for the use of DGE by insects.
The red flour beetle (Tribolium castaneum) is a stored grain pest, which encounters harsh desiccating conditions exacerbated by severe hypoxia/hypercarbia (controlled atmospheres) or phosphine fumigation (Hoback and Stanley 2001; Rajendran 2020). The remarkable survival of this cosmopolitan beetle and a few others (including, lesser grain borer and rice weevil) under such harsh conditions is widely reported (Chaudhry 2000; Nayak et al. 2015, 2020). Donahaye (1990) found more than half of the experimental population of red flour beetles tolerant to severe hypoxia (0.5% O2) for an exposure longer than eight days.
Few studies have investigated physiological adaptations linked to gas exchange as the tracheal system is the primary conduit for penetration of gaseous fumigants. Pimentel et al. (2007, 2008) recorded reduced metabolic rate in phosphine resistant strains of red flour beetle and lesser grain borer, suggesting that tracheal gas exchange is related to phosphine tolerance. Similarly, Malekpour et al. (2020) reported a reduced metabolic rate for phosphine resistant populations of red flour beetle. The American cockroach (Periplaneta americana), on exposure to sublethal concentrations of phosphine, reduced its metabolic rate by disruption of DGE and cessation of active ventilation, suggesting a physiological response influenced by tracheal movement (diffusion/advection) for tolerance to phosphine (Woodman et al. 2008). However, the role of tracheal gas movement has not been investigated for stored grain insects such as red flour beetle, let alone in response to modified gas mixtures to provide insight into physiological responses.
Quantifying the relative roles of diffusive and advective gas exchange for insects remains somewhat problematic (Socha et al. 2010), but we suggest that using a helox atmosphere could be a useful approach for this. Helox replaces the nitrogen in air with helium, while keeping the fractional O2 level constant (i.e. helox is 21% O2 + 79% He whereas normal air is 21% O2 + 79% N2). Helox has a lower density than air and 2–3 times higher rate of diffusion due to the lower molecular weight of He compared to N2 (Cook 1950; Wang and Peter 1975; Parkhurst and Mott 1990; Mott and Parkhurst 1991; Birchard 2000; Cooper and Withers 2014; Huang et al. 2014; Snelling et al. 2017). Importantly, helox does not affect metabolic rate (Holloway and Geiser 2001; Huang et al. 2014), making comparison of its effects on ventilatory patterns straightforward. Consequently, we predict that DGE characteristics should be affected for an insect breathing helox compared to air, e.g. substantial reduction of the O phase if there is a diffusive component of gas exchange, but no change if exchange is advective.
Our hypothesis of helox-induced reduction in O phase duration has support from substantial experimental evidence (Förster and Hetz 2010; Matthews and White 2011b; Grieshaber and Terblanche 2015; Talal et al. 2018) that the levels are a more sensitive stimulus than in triggering the chemoreceptors responsible for modulating the beginning and termination of O phase. We investigate here whether helox affects a diffusion limitation for two insects differing in absence/presence of air sacs, the red flour beetle (1.5 mg, no air sacs; Kaiser et al. 2007; Socha et al. 2010) whose gas exchange is expected to be diffusion limited (Krogh 1920, 1941) and the larger speckled cockroach (Nauphoeeta cinerea, 700 mg; with air sacs) whose gas exchange has been measured to be advective (Matthews and White 2011b). The red flour beetle is a relatively small insect, considerably larger than miniature insects (e.g. feather-wing beetle, Paratuposa placentis, 0.0024 mg: Sun 2023) and smaller than the largest insects (e.g. giant weta, Deinacrida heteracantha, 45 g: Richards 1973).
Materials and methods
Insects
A phosphine-susceptible strain (MUWTC-5000) of the red flour beetle, T. castaneum, was kindly provided by Professor Yonglin Ren (Murdoch University, Perth, Western Australia). Beetles were provided with an artificial diet of flour and yeast (12:1) in a plastic jar with vents in the lid for ventilation (following Alnajim et al. 2019). Speckled cockroaches were purchased from Livefoods Unlimited (Adelaide, South Australia). They were maintained in a plastic container with a mesh lid, with the sides of the container lined with vaseline to avoid escape. Cockroaches were provided with dry cat food (Purina Fancy Feast, Rhodes, NSW, Australia) and empty cardboard egg trays for shelter. Water-filled glass vials with cotton wool in the open end provided drinking water.
Beetles and cockroaches were both maintained in a constant temperature room at 25 ± 1s.e. °C, 12L:12D photoperiod, ambient gaseous conditions of 21% O2, 0.03% CO2 and ambient RH range 60–80%.
Flow-through respirometry
Carbon dioxide emission was recorded for only the adult stage of the healthy red flour beetles, using flow-through respirometry at 25°C in a 1 mL glass syringe barrel. To increase the likelihood of recording DGE, only male beetles were measured (Nicholls et al. 2017) and each beetle was starved for 48 h, then placed in the chamber for at least 5 h before measurement (see Abbas et al. 2023). Each individual beetle (~1.5 mg) was then measured in air for 4 h, then in helox for 4 h, and finally in air for 4 h. A 30 min baseline with air was measured at the start and end of the 12 h respirometry measurement. The ventilation pattern was clear throughout the 12 h measurement period, but the CO2 baseline drifted too much to allow a meaningful calculation of absolute metabolic rate .
Carbon dioxide release and evaporative water loss were measured simultaneously in helox (BOC Gases, Canning Vale, WA, Australia) for comparison with equivalent results for cockroaches measured in air using flow-through respirometry at 23°C ± 0.1 s.e. (see Abbas et al. 2020). The respirometry chamber was a 10 mL plastic disposable syringe barrel cut to reduce its volume to about 5 mL. Adult cockroaches were selected randomly and irrespective of sex for measurement. Each respirometry measurement lasted for 2 h, and chambers were empty for 30 min at the start and end of a trial to obtain baselines. Chambers were darkened to reduce activity of insects. There was no significant difference in body mass (t22 = 0.372, P = 0.714) for cockroaches measured in helox (698 mg: this study) and air (675 mg: Abbas et al. 2020).
Air entering the chamber was regulated at 25 mL min−1 STPD using a mass flow controller (AFC 2600, Aalborg, Orangeburg, NY, USA; or C22208, Sierra Instruments, Monterey, CA, USA). Excurrent air from each respirometry system entered a brass housing containing a probe (HMP113, Vaisala Corporation, Helsinki, Finland) to measure relative humidity (RH) and temperature of excurrent air. Probes were calibrated using a DG-4 DewPoint Generator (Sable Systems International, Las Vegas, NV, USA) and a calibration-traceable mercury glass thermometer (Australian Calibrating Services, Melbourne, Vic, Australia). The CO2 concentration of excurrent air was then measured with an S151 analyser (Qubit systems, Kingston, Ontario, CA). The CO2 analyser was calibrated using nitrogen and a certified span gas (0.153% CO2, BOC Gases, Canning Vale, WA, Australia). Mass flow controllers were calibrated for air and helox flow rate using a Gilian Gilibrator 2 (Sensidyne, St Petersburg, FL, USA).
Data acquisition and analysis
Digital multimeters (Protek 506, Seoul, Korea and Thurlby 1905a, Thurlby Electronics Ltd, Huntingdon Cambridgeshire, UK), were used to measure analogue voltage signals from the humidity probes and CO2 analysers, respectively. These multimeters were connected via a USB port hub (UC2324, ATEN, North Ryde, NSW, Australia) to a desktop PC. Voltage signals were sampled every 0.2 s for beetles and every 10 s for cockroaches, and converted to ppm for CO2 concentration, percentage relative humidity for water vapour, and degrees Celsius for temperature using a custom written Visual Basic 6 program (PC Withers). Data were stored continuously in an Excel file during each respirometry trial. Raw values of CO2, H2O and temperature were calibration corrected and then analysed using an Excel spreadsheet (PC Withers and W Abbas). For the red flour beetle, the rate of CO2 emission could not be accurately calculated due to baseline drift over time, and the very small water loss signal was indistinct from the baseline EWL. For cockroaches, the rate of CO2 emission (, mL g−1 h−1) and rate of water loss (, mg g−1 h−1) were calculated using equations and calculations described in Abbas et al. (2020).
Gas exchange patterns
The CO2 emission trace of each beetle was examined at different time intervals to characterise the respiratory pattern. For calculations, both closed (C) and flutter (F) were combined as the interburst phase (IB) as there was no clear distinction between them (Wobschall and Hetz 2004). A beetle was considered to have DGE if its CO2 emission trace was constant for a sufficient time to clearly represent a burst (B, open phase is referred hereafter as burst or B) phase. DGE cycles (n = 2–8) were analysed for each individual beetle to calculate durations (sec) for B and IB phases and the entire cycle (IB + B). For an individual beetle, mean durations were calculated at different time intervals during the first 4 h in air, for the next 4 h in helox and for the final 4 h in air. The first 2 h in helox were not included for calculations due to unstable emission trace from the shift between air and helox.
The respiratory pattern of cockroaches in helox was characterised from the CO2 emission trace. A cockroach was considered to have DGE if its CO2 emission trace had a clear closed phase (Schimpf et al. 2012). Closed (C) and flutter (F) phases were combined as the interburst phase (IB), as for flour beetles. DGE cycles (n = 2–8) were analysed for each cockroach to calculate (mL g−1 h−1) and the duration (s) for each phase, burst (B) and interburst (IB), and the entire cycle (IB + B). DGE cycles of cockroaches were also analysed for evaporative water loss rate (; water loss peaks corresponded with simultaneous CO2 emission) as for . Total evaporative water (TEWL) was partitioned into respiratory (REWL) and cutaneous (CEWL) components by assuming that interburst EWL reflected CEWL, and REWL was then calculated by subtraction of CEWL from TEWL calculated for an entire DGE cycle duration.
Statistical analyses
For red flour beetles, linear models were used to compare cycle and phase durations of DGE for air and helox over the 12 h experiment using the nlme package (v3.1–140: Pinheiro et al. 2019) in the R environment (v3.6.1: R Development Core Team 2020). Time was included as a fixed variable to account for its effect, and individual was included as a random effect to account for repeated measurements over time for individuals. Means are presented with standard error (s.e.) and sample size (n).
Physiological variables for speckled cockroaches measured in helox during DGE of cycle and phase durations (seconds), IB and B (μL g−1 h−1), metabolic rate (, μL g−1 h−1), total evaporative water loss (, μg g−1 h−1), IB and B (μg g−1 h−1), cuticular water loss (CWL, μg g−1 h−1), respiratory water loss (RWL, μg g−1 h−1), %CWL (percentage fraction of total evaporative water loss) and %RWL (percentage fraction of total evaporative water loss) were compared with those measured in air (Abbas et al. 2020) using StatistiXL (v2.0, www.statistixl.com) using a two tailed t-test.
Results
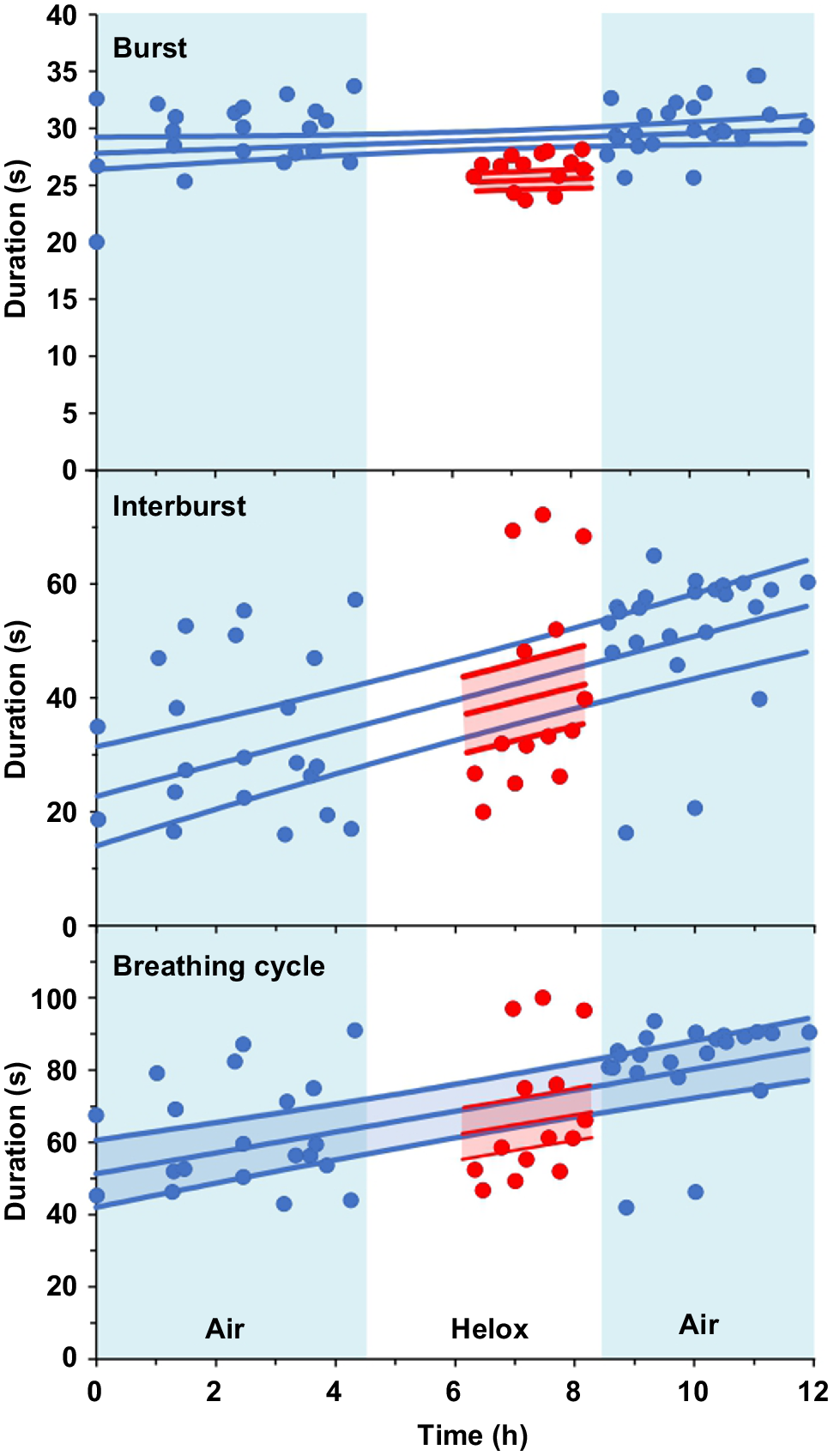
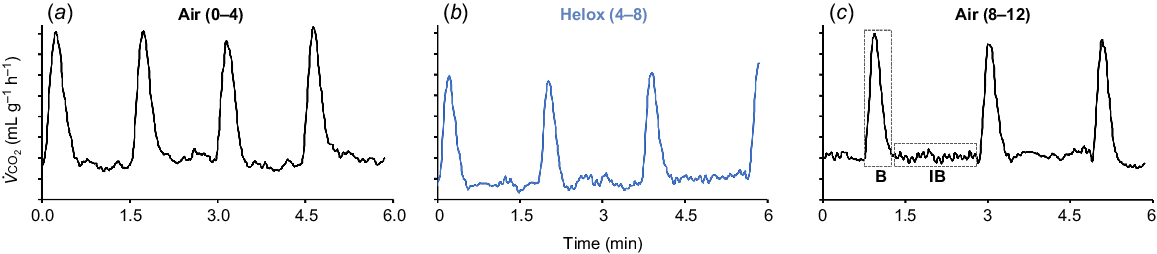
For red flour beetles, there was no effect of measurement time for the DGE burst phase duration in air or in helox (Fig. 1, Table 1) but the burst phase duration was reduced significantly (P < 0.001) by 12.7% in helox (25.8 ± 0.665 s) compared to air (29.5 ± 0.633 s). There was no significant individual variability for burst duration (P = 0.900). In contrast, the interburst duration increased significantly with measurement time (P < 0.001) but there was no effect of helox (P = 0.359). There was significant individual variability for interburst duration (P ˂ 0.001). The overall DGE cycle duration similarly increased significantly with time (t46 = 6.80, P < 0.001) and was reduced slightly (P = 0.0499) in helox, and there was significant individual variability (P < 0.001). These changes over time suggest a decrease in reflecting progressive effects of dehydration and starvation, but this requires confirmation by measurement of absolute , which was not possible in this study due to long-term baseline drift of the CO2 analyser. The patterns of DGE for the red flour beetle in air, helox and in air again were not noticeably different, except for the apparent effect of time on cycle and phase durations (Fig. 2).
Burst, interburst and total durations for the red flour beetle during discontinuous gas exchange in air (0–4 h), then helox (4–8 h) and again in air (8–12 h). Points are individual beetles (N = 9), central lines are the linear regression for air and helox, with standard error bands. Blue = air, red = helox.
| Variables | Estimate ± s.e. | Statistics | |
|---|---|---|---|
| Burst duration (s) | |||
| Intercept | 29.5 ± 0.633 | t46 = 46.5, P < 0.001 | |
| Time | 0.084 ± 0.083 | t46 = 1.00, P = 0.320 | |
| Helox | −3.74 ± 0.665 | t46 = 5.62, P < 0.001 | |
| Individual | – | LLR5 = 0.159, P = 0.900 | |
| Interburst duration (s) | |||
| Intercept | 23.5 ± 4.29 | t46 = 5.49, P < 0.001 | |
| Time | 2.74 ± 0.38 | t46 = 7.18, P < 0.001 | |
| Helox | −2.85 ± 3.07 | t46 = 0.93, P = 0.359 | |
| Individual | – | LLR5 = 22.2, P < 0.001 | |
| Cycle duration (s) | |||
| Intercept | 53.0 ± 4.58 | t46 = 11.6, P < 0.001 | |
| Time | 2.84 ± 0.417 | t46 = 6.80, P < 0.001 | |
| Helox | −6.78 ± 3.36 | t46 = 2.01, P = 0.0499 | |
| Individual | – | LLR5 = 20.2, P < 0.001 | |
Example of CO2 emission traces for a red flour beetle with sequential measurement in air (a), helox (b) and then in air (c) as for Fig. 1. DGE cycles (n = 2–8) were analysed for an individual beetle to calculate duration (s) for each phase and the entire cycle (IB + B). The phase durations calculated for Burst (B) and Interburst (IB) are indicated as dotted areas in 2c. The scale for is arbitrary as the baseline drifted over time. The tick marks on the vertical axis indicate a difference of 0.5 mL g−1 h−1 for .
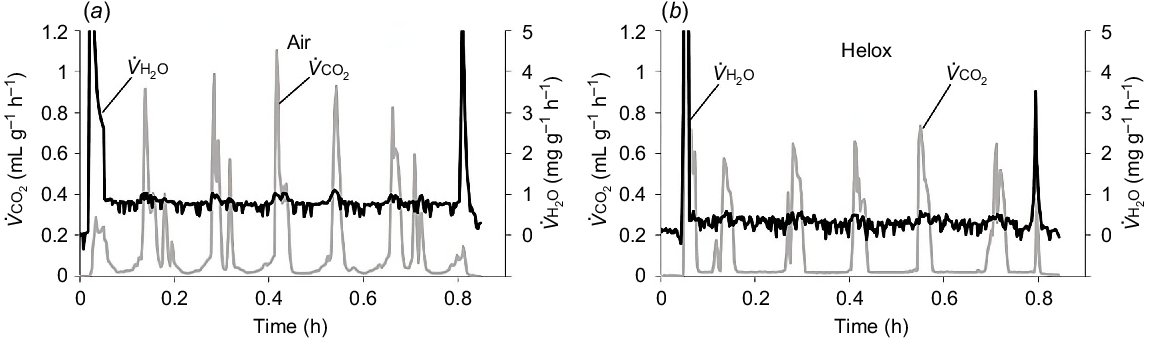
Cockroaches had similar DGE patterns for the 2 h periods measured in separate experiments in air (n = 15) (Fig. 3a) and helox (n = 9) (Fig. 3b). There was no difference in air or helox for any of the DGE pattern characteristics, burst, interburst or total durations. Burst phase duration of 200 ± 21.3 s in air and 249 ± 59.9 s in helox were not significantly different (t10 = 0.733, P = 0.480), interburst duration of 177 ± 35.0 s in air and 173 ± 31.7 s in helox did not differ (t22 = 0.078, P = 0.938), and cycle duration of 377 ± 42.6 s in air and 422 ± 62.3 s in helox did not differ (t22 = 0.590, P = 0.561). Likewise, there were no significant differences for (μL g−1 h−1) for interburst of 42.1 ± 4.46 in air and 47.0 ± 5.96 in helox (t22 = 0.632, P = 0.534), for burst of 341 ± 26.5 in air and 322 ± 23.4 in helox (t22 = 0.484, P = 0.633) and total (metabolic rate) of 195 ± 8.17 in air and 200 ± 18.2 in helox (t11 = 0.212, P = 0.836). There were also no significant differences for evaporative water loss variables of cockroaches measured in air (n = 15) and helox (n = 9); total evaporative water loss (TEWL; μg g−1 h−1) of 943 ± 93.9 in air and 926 ± 170 in helox (t22 = 0.090, P = 0.929), interburst of 849 ± 91.6 in air and 816 ± 152 in helox (t22 = 0.191, P = 0.851), and burst of 1049 ± 104 in air and 1013 ± 187 in helox (t22 = 0.174, P = 0.863). In absolute terms (μg g−1 h−1), respiratory evaporative water loss did not differ for 93.7 ± 35.2 in air and 110 ± 25.2 in helox (t10 = 0.581, P = 0.574), nor did cutaneous evaporative water loss (air = 849 ± 91.6, helox = 816 ± 152; t22 = 0.191, P = 0.851). REWL expressed as a percentage of TEWL was 10.8 ± 1.31% in air and 12.0 ± 1.98% in helox; CEWL was 89.2 ± 1.31% in air and 88.0 ± 1.98% in helox; there were no significant differences between air and helox (REWL, t22 = 0.490, P = 0.629; CEWL, t22 = 0.490, P = 0.629).
Examples of respirometry traces for metabolic rate () and total evaporative water loss () for discontinuous gas exchange by a speckled cockroach measured in air (a) and another individual measured in helox (b). The cockroaches were placed in the respirometry system at the start of the trace and removed at the end of the trace.
Discussion
The main objective of this study was to quantify the relative roles of diffusion and advection for insect gas exchange using exposure to air and helox atmospheres for two insects differing in absence/presence of air sacs, and to contribute to our understanding of the potential for physiological mechanisms to influence management of stored grain insect pests. It is difficult to predict the diffusion coefficient for a helox mixture, since it is a quaternary mix of He, O2, CO2 and water vapour, but empirical measurements suggest that helox should approximately double the diffusion rate (2.18×: Erasmus and Rahn 1976; Chang 1987; 2.3×: Parkhurst and Mott 1990; Mott and Parkhurst 1991; 2.1×: Birchard 2000; Huang et al. 2014; Snelling et al. 2017), hence halve the burst phase duration (for constant due to its lower density. Insects and vertebrates alike are widely reported to have no effect of helox on metabolic rate (Cook 1950; Wang and Peter 1975; Holloway and Geiser 2001; Cooper and Withers 2014; Huang et al. 2014; but see Cook 1950).
A 50% reduction in burst phase duration, expected for a diffusive effect of helox, was not observed for either the flour beetle or the cockroach. Our finding of a significant ~13% reduction in burst phase duration for the flour beetle, mediated by helox, suggests this small beetle does not rely on diffusion alone but has a substantial advective exchange component of ~74% (100 – [2 × 13]). Birchard (2000), using mass loss measurement rather than respirometric gas exchange, similarly observed a significant (68%) reduction in burst phase gas exchange in helox for developing moth pupae consistent with advective exchange or lower spiracular resistance in helox. These effects of helox challenge Krogh’s century old diffusional theory of insect respiration (Krogh 1920, 1941), as does reconsideration of Krogh’s calculations (Buck 1962). Rather, our results suggesting advective flow support the microrespirography studies of Sláma and colleagues (Sláma and Coquillaud 1992; Sláma 1999) showing complex patterns of O2 and CO2 exchange for small as well as large insects, in humid and dry conditions (Sláma et al. 2007), which reflect a combination of active ventilation by haemolymph pressure pulsations and sudden bursts of dissolved CO2 from the tissue buffer. The diapausing adult beetle (Bruchus affinis, 5 mg), has a continuous O2 uptake but periodic bursts of CO2 from tissue buffers associated with haemocoelic pressure pulsations (Sláma and Coquillaud 1992). The pea aphid (Acyrthosiphon pisum, 3 mg) and various life stages of the termite (Prorhinotermes simplex, 0.6–4.5 mg) use CGE in humid conditions but in drier conditions have various patterns of DGE with large bursts of CO2 elimination by ventilation, diffusion or by a combination of both (Sláma and Jedlička 2012). X-ray imaging of Drosophila showing compression/expansion cycles of trachea provides evidence for advective gas exchange in small insects (Westneat et al. 2003; Hale et al. 2006). In addition, many pupae ranging in size from 1.5 mg (Phyllonorycter strigulatella) to 11.5 g (Pseudosphinx tetrio) also have active ventilation during the burst phase reflecting extracardiac pressure pulsations (Sláma 2008; Sláma and Santiago-Blay 2017). Tenney (1985) used hypobaria to manipulate the diffusion coefficient but was unable to exclude a role of advection for tracheal gas exchange by the larva of the Mexican jumping bean moth (Carpocapsa saltitans).
The mechanism for tracheal advection by red flour beetles needs elaboration. Given that these small beetles do not have air sacs (Kaiser et al. 2007), it is unlikely that rhythmic inflation and deflation of tracheae by abdominal or thoracic pumping is responsible (Westneat et al. 2008; Socha et al. 2010). The unidirectional air flow that provides substantial advective exchange for large, active insects (Socha et al. 2010; Harrison et al. 2013) has not been shown for small insects. Pressure pulsations caused by local flow of haemolymph, as reported for a similar sized beetle (Sláma and Coquillaud 1992) and other insects (Sláma and Jedlička 2012; Sláma and Santiago-Blay 2017) might provide advective flow of air within the tracheae and even the small blind-ended tracheoles in flour beetles. Understanding the dynamics of tracheal air flow in a diversity of small insects could further be elucidated by X-ray imaging technology, which has demonstrated tracheal compression/expansion cycles for Drosophila (Westneat et al. 2003; Harrison et al. 2023). Adopting an interdisciplinary approach for a comprehensive understanding of the mechanisms of insect respiration could help in developing accurate tracheal airflow models of gas exchange apart from providing bases for bioengineering (Khan and Staples 2025).
The speckled cockroach showed no effect of helox on burst phase duration, consistent with our expectation of their having a substantial advective component for gas exchange even at rest (Matthews and White 2011b; Matthews and Terblanche 2015). Talal et al. (2018) quoted unpublished data that helox did not affect burst-phase duration for Schistocerca gregaria, and many other studies have shown advective gas exchange for various beetles, grasshoppers, moths, etc. (Buck et al. 1953; Kestler 1985; Harrison 1997; Westneat et al. 2003; Greenlee and Harrison 2004a, 2004b; Westneat et al. 2008). Our results not only reaffirm that gas exchange is driven by advection in these cockroaches, consistent with evidence for larger insects such as locusts and cockroaches (Greenlee and Harrison 2004a, 2004b; Matthews and White 2011b), but also suggest that there is an insubstantial diffusion limitation of the blind-ended terminal tracheoles. Tracheole diffusion limitation for CO2 and H2O is presumably minimised by a high at the tracheole head due to abdominal pumping and advective exchange (Matthews and White 2011b; Matthews and Terblanche 2015; Snelling et al. 2017). The effect of helox on metabolic rate was insignificant, consistent with observations for a similar sized locust (Huang et al. 2014). Surprisingly, there was also no effect of helox on total EWL or cuticular or respiratory components of EWL, for speckled cockroaches in helox relative to air. No effect of helox on cuticular EWL suggests that evaporation of water across the boundary layer is primarily limited by diffusion through the cuticle (which would be unaffected by helox) rather than diffusion through the air boundary layer (which would be affected by helox). For both large and small insects, the burst phase of DGE begins with spiracles opening and a large outburst of CO2. The spiracles remain open even when the haemolymph drops to a level less than the threshold (Sláma and Santiago-Blay 2017; Talal et al. 2018). The few studies (Förster and Hetz 2010; Matthews and White 2011b; Grieshaber and Terblanche 2015; Talal et al. 2018) that have examined the relative roles of intratracheal and in transition of DGE phases concluded that chemoreceptors are more sensitive to threshold level such that initiation and termination of burst phase is triggered by high and low levels respectively, with Rowe et al. (2022) reporting that this transition of phases is not around fixed threshold levels. The roles of production and excretion of CO2 from an insect’s body in determining burst phase duration of DGE is not straightforward. Insects during DGE can maintain acid–base homeostasis when spiracles are closed by correcting respiratory acidosis through retention of CO2/HCO3− in haemolymph (Förster and Hetz 2010; Sláma and Santiago-Blay 2017). A two-step process first liberates dissolved CO2/HCO3− from haemolymph and tissues into tracheoles at the gas/liquid interphase followed by excretion of gaseous CO2 from the tracheal system into the air. Transport of CO2 into the haemolymph has different kinetics than its excretion via spiracles. The many-fold slower release of CO2 from haemolymph into the tracheal system is likely the rate limiting step determining the B (open) duration, for which spiracles need to remain open (Sláma and Jedlička 2012; Sláma and Santiago-Blay 2017).
Nevertheless, the slight reduction for flour beetles of burst duration by helox (13% not a 50% reduction) presumably reflects a minor diffusive component of CO2 excretion. Moreover, multiple studies have documented advective excretion of CO2 facilitated by haemocoel pressure pulsations for insects both large and small in rather large outbursts followed by a decline in release over time during burst phase (Sláma and Jedlička 2012; Sláma and Santiago-Blay 2017; Snelling et al. 2017; Talal et al. 2018). This lends credence to our suggestion that small red flour beetles have dominantly advective gas exchange. Our suggested role of helox as a tool to quantitatively partition diffusive and advective exchange merits further study.
It has been long known that the susceptibility of an insect to the action of a fumigant is directly related to its rate of metabolism (Cotton 1932). This has been substantiated over the last two decades for a wide range of insects including cockroaches and stored grain insects such as the red flour beetle and lesser grain borer (Pimentel et al. 2007, 2008; Woodman et al. 2008; Malekpour et al. 2020). A reduction in metabolic rate coupled with loss of active ventilation as expressed by P. americana on exposure to phosphine posits that a change in the movement of tracheal gases (advection replaced by diffusion) could reduce the fumigant uptake rate (Woodman et al. 2008) as diffusion-based exchange is much slower than advective exchange (Matthews and Terblanche 2015). Although we have not examined red flour beetles for changes of these variables in response to phosphine, reliance of beetles on advection rather than diffusion in normal air and helox corresponds to the mechanism of tracheal gaseous movements in P. americana (under normal air: Woodman et al. 2008) and N. cinerea (from this study). Therefore, it is reasonable to assume that red flour beetles would show a response similar to P. americana on exposure to phosphine to reduce uptake of fumigant.
Resistance to phosphine has been widely reported in stored grain insect pests including red flour beetle (Nayak et al. 2017, 2020) with a few species having higher survival potential against anoxic/hypercapnic controlled atmospheres (Knipling et al. 1961; Donahaye 1990). Apart from empirical studies (Afful et al. 2020; Shan et al. 2023), research is extensively focused on potential biological pathways (Li et al. 2023), structural modifications (Kim et al. 2023) and visualisation of the tracheal system (Iwan et al. 2015) to elucidate the mechanisms of phosphine resistance. Exploring the respiratory physiology of stored grain insects (relative roles of tracheal diffusion and advection, and metabolic rate) could facilitate an understanding of their physiological responses to phosphine, controlled atmospheres and mechanisms for resistance to fumigants.
Data availability
The data that support this study are available in this article and raw values for means will be made available upon request to the corresponding author.
Author contributions
WA and PCW conceptualised the study and designed the experiments, WA performed the experiments, ran analyses, and wrote the manuscript. All authors contributed to editing the manuscript.
Acknowledgements
The first author gratefully acknowledges the support of UWA and UAF for his PhD scholarship. We thank Professor Yonglin Ren at Murdoch University for supplying flour beetles. We extend our gratitude to two anonymous reviewers for their constructive inputs on an earlier version of this manuscript.
References
Abbas W, Withers PC, Evans TA (2020) Water costs of gas exchange by a speckled cockroach and a darkling beetle. Insects 11(9), 632.
| Crossref | Google Scholar |
Abbas W, Withers PC, Evans TA (2023) Gas exchange patterns for a small, stored-grain insect pest, Tribolium castaneum. Bulletin of Entomological Research 113(3), 361-367.
| Crossref | Google Scholar | PubMed |
Afful E, Tadesse TM, Nayak MK, Phillips TW (2020) High-dose strategies for managing phosphine-resistant populations of Rhyzopertha dominica (F.) (Coleoptera: Bostrichidae). Pest Management Science 76(5), 1683-1690.
| Crossref | Google Scholar | PubMed |
Alnajim I, Du X, Lee B, Agarwal M, Liu T, Ren Y (2019) New method of analysis of lipids in Tribolium castaneum (Herbst) and Rhyzopertha dominica (Fabricius) insects by direct immersion solid-phase microextraction (DI-SPME) coupled with GC–MS. Insects 10(10), 363.
| Crossref | Google Scholar |
Birchard GF (2000) The effect of changing the gaseous diffusion coefficient on the mass loss pattern of Hyalophora cecropia pupae. Physiological and Biochemical Zoology 73(4), 488-493.
| Crossref | Google Scholar | PubMed |
Buck J (1962) Some physical aspects of insect respiration. Annual Review of Entomology 7, 27-56.
| Crossref | Google Scholar |
Buck J, Keister M (1955) Cyclic CO2 release in diapausing Agapema pupae. Biology Bulletin 109(1), 144-163.
| Crossref | Google Scholar |
Buck J, Keister M, Specht H (1953) Discontinuous respiration in diapausing Agapema pupae. Anatomical Record 117, 541.
| Google Scholar |
Chaudhry MQ (2000) Phosphine resistance. Pesticide Outlook 11(3), 88-91.
| Crossref | Google Scholar |
Chown SL, Holter P (2000) Discontinuous gas exchange cycles in Aphodius fossor (Scarabaeidae): a test of hypotheses concerning origins and mechanisms. Journal of Experimental Biology 203(2), 397-403.
| Crossref | Google Scholar |
Chown SL, Gibbs AG, Hetz SK, Klok CJ, Lighton JRB, Marais E (2006) Discontinuous gas exchange in insects: a clarification of hypotheses and approaches. Physiological and Biochemical Zoology 79(2), 333-343.
| Crossref | Google Scholar | PubMed |
Cook SF (1950) The effect of helium and argon on metabolism and metamorphosis. Journal of Cellular and Comparative Physiology 36(1), 115-127.
| Crossref | Google Scholar | PubMed |
Cooper CE, Withers PC (2014) Physiological responses of a rodent to heliox reveal constancy of evaporative water loss under perturbing environmental conditions. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 307(8), R1042-R1048.
| Crossref | Google Scholar | PubMed |
Cotton RT (1932) The relation of respiratory metabolism of insects to their susceptibility to fumigants. Journal of Economic Entomology 25(5), 1088-1103.
| Crossref | Google Scholar |
Donahaye E (1990) Laboratory selection of resistance by the red flour beetle, Tribolium castaneum (Herbst), to an atmosphere of low oxygen concentration. Phytoparasitica 18(3), 189-202.
| Crossref | Google Scholar |
Erasmus BD, Rahn H (1976) Effects of ambient pressures, He and SF6 on O2 and CO2, transport in the avian egg. Respiration Physiology 27(1), 53-64.
| Crossref | Google Scholar | PubMed |
Förster TD, Hetz SK (2010) Spiracle activity in moth pupae – the role of oxygen and carbon dioxide revisited. Journal of Insect Physiology 56(5), 492-501.
| Crossref | Google Scholar | PubMed |
Greenlee KJ, Harrison JF (2004a) Development of respiratory function in the American locust Schistocerca americana I. Across-instar effects. Journal of Experimental Biology 207(3), 497-508.
| Crossref | Google Scholar |
Greenlee KJ, Harrison JF (2004b) Development of respiratory function in the American locust Schistocerca Americana. II. Within-instar effects. Journal of Experimental Biology 207(3), 509-517.
| Crossref | Google Scholar |
Grieshaber BJ, Terblanche JS (2015) A computational model of insect discontinuous gas exchange: a two-sensor, control systems approach. Journal of Theoretical Biology 374, 138-151.
| Crossref | Google Scholar | PubMed |
Hale ME, Waters JS, Lee WK, Socha JJ, Fezza K, Westneat MW (2006) Drawing inspiration from insect breathing and heaving conventional wisdom: convective tracheal and air sac mechanisms in Drosophila visualized with x-ray imaging. Integrative and Comparative Biology 46, e53.
| Google Scholar |
Harrison JF (1997) Ventilatory mechanism and control in grasshoppers. American Zoologist 37(1), 73-81.
| Crossref | Google Scholar |
Harrison JF, Woods HA, Roberts SP (2012) ‘Ecological and environmental physiology of insects.’ (Oxford University Press: Oxford, UK) doi:10.1093/acprof:oso/9780199225941.001.0001
Harrison JF, Waters JS, Cease AJ, VandenBrooks JM, Callier V, Klok CJ, Shaffer K, Socha JJ (2013) How locusts breathe. Physiology 28(1), 18-27.
| Crossref | Google Scholar | PubMed |
Harrison JF, McKenzie EKG, Talal S, Socha JJ, Westneat MW, Matthews PGD (2023) Air sacs are a key adaptive trait of the insect respiratory system. Journal of Experimental Biology 226(10), jeb245712.
| Crossref | Google Scholar |
Hetz SK, Bradley TJ (2005) Insects breathe discontinuously to avoid oxygen toxicity. Nature 433(7025), 516-519.
| Crossref | Google Scholar | PubMed |
Hoback WW, Stanley DW (2001) Insects in hypoxia. Journal of Insect Physiology 47(6), 533-542.
| Crossref | Google Scholar | PubMed |
Holloway JC, Geiser F (2001) Effects of helium/oxygen and temperature on aerobic metabolism in the marsupial sugar glider, Petaurus breviceps. Physiological and Biochemical Zoology 74(2), 219-225.
| Crossref | Google Scholar | PubMed |
Huang S-P, Sender R, Gefen E (2014) Oxygen diffusion limitation triggers ventilatory movements during spiracle closure when insects breathe discontinuously. Journal of Experimental Biology 217(13), 2229-2231.
| Crossref | Google Scholar |
Iwan D, Kamiński MJ, Raś M (2015) The Last Breath: a μCT-based method for investigating the tracheal system in Hexapoda. Arthropod Structure & Development 44(3), 218-227.
| Crossref | Google Scholar | PubMed |
Kaiser A, Klok CJ, Socha JJ, Lee W-K, Quinlan MC, Harrison JF (2007) Increase in tracheal investment with beetle size supports hypothesis of oxygen limitation on insect gigantism. Proceedings of the National Academy of Sciences 104(32), 13198-13203.
| Crossref | Google Scholar |
Kestler P (1985) Respiration and respiratory water loss. In ‘Environmental physiology and biochemistry of insects’. (Ed. KH Hoffman) pp. 137–183. (Springer: Berlin) doi:10.1007/978-3-642-70020-0_6
Khan S, Staples AE (2025) Mechanisms of insect respiration. Nature Reviews Physics 7, 135-148.
| Crossref | Google Scholar |
Kim D, Kim K, Lee YH, Lee S-E (2023) Transcriptome and Micro-CT analysis unravels the cuticle modification in phosphine-resistant stored grain insect pest, Tribolium castaneum (Herbst). Chemical and Biological Technologies in Agriculture 10(1), 88.
| Crossref | Google Scholar |
Knipling GD, Sullivan WN, Fulton RA (1961) The survival of several species of insects in a nitrogen atmosphere. Journal of Economic Entomology 54(5), 1054-1055.
| Crossref | Google Scholar |
Krogh A (1920) Studien über tracheenrespiration. Pflüger’s Archiv für die gesamte Physiologie des Menschen und der Tiere 179(1), 95-112.
| Crossref | Google Scholar |
Li L, Shan C, Liu Q, Li B, Liu T (2023) Comparative analysis of the metabolic profiles of strains of Tribolium castaneum (Herbst) adults with different levels of phosphine resistance based on direct immersion solid-phase microextraction and gas chromatography-mass spectrometry. Molecules 28(23), 7721.
| Crossref | Google Scholar |
Lighton JRB (1994) Discontinuous ventilation in terrestrial insects. Physiological Zoology 67(1), 142-162.
| Crossref | Google Scholar |
Lighton JRB (1996) Discontinuous gas exchange in insects. Annual Review of Entomology 41(1), 309-324.
| Crossref | Google Scholar |
Lighton JRB, Berrigan D (1995) Questioning paradigms: caste-specific ventilation in harvester ants, Messor pergandei and M. julianus (Hymenoptera: Formicidae). Journal of Experimental Biology 198(2), 521-530.
| Crossref | Google Scholar |
Malekpour R, Arnold PA, Rafter MA, Daglish GJ, Walter GH (2020) Effects of sublethal phosphine exposure on respiration rate and dispersal propensity of adult females of Tribolium castaneum. Journal of Pest Science 93, 149-157.
| Crossref | Google Scholar |
Matthews PGD, Terblanche JS (2015) Evolution of the mechanisms underlying insect respiratory gas exchange. In ‘Advances in insect physiology. Vol. 49’. (Ed. R Jurenka) pp. 1–24. (Academic Press: New York, USA) doi:10.1016/bs.aiip.2015.06.004
Matthews PGD, White CR (2011a) Discontinuous gas exchange in insects: is it all in their heads? The American Naturalist 177(1), 130-134.
| Crossref | Google Scholar |
Matthews PGD, White CR (2011b) Regulation of gas exchange and haemolymph pH in the cockroach Nauphoeta cinerea. Journal of Experimental Biology 214(18), 3062-3073.
| Crossref | Google Scholar |
Miller PL (1974) Respiration – aerial gas transport. In ‘The physiology of insect. Vol. 6’. 2 edn. (Ed. M Rockstein) pp. 345–402. (Academic Press: New York, USA) doi:10.1016/b978-0-12-591606-6.50012-4
Mott KA, Parkhurst DF (1991) Stomatal responses to humidity in air and helox. Plant, Cell & Environment 14(5), 509-515.
| Crossref | Google Scholar |
Nayak MK, Daglish GJ, Phillips TW (2015) Managing resistance to chemical treatments in stored products pests. Stewart Postharvest Review 11(1), 1-6.
| Crossref | Google Scholar |
Nayak MK, Falk MG, Emery RN, Collins PJ, Holloway JC (2017) An analysis of trends, frequencies and factors influencing the development of resistance to phosphine in the red flour beetle Tribolium castaneum (Herbst) in Australia. Journal of Stored Products Research 72, 35-48.
| Crossref | Google Scholar |
Nayak MK, Daglish GJ, Phillips TW, Ebert PR (2020) Resistance to the fumigant phosphine and its management in insect pests of stored products: a global perspective. Annual Review of Entomology 65, 333-350.
| Crossref | Google Scholar | PubMed |
Nicholls E, Fowler R, Niven JE, Gilbert JD, Goulson D (2017) Larval exposure to field-realistic concentrations of clothianidin has no effect on development rate, over-winter survival or adult metabolic rate in a solitary bee, Osmia bicornis. PeerJ 5, e3417.
| Crossref | Google Scholar |
Parkhurst DF, Mott KA (1990) Intercellular diffusion limits to CO2 uptake in leaves: studies in air and helox. Plant Physiology 94(3), 1024-1032.
| Crossref | Google Scholar | PubMed |
Pimentel MAG, Faroni LRDA, Tótola MR, Guedes RNC (2007) Phosphine resistance, respiration rate and fitness consequences in stored-product insects. Pest Management Science 63(9), 876-881.
| Crossref | Google Scholar | PubMed |
Pimentel MAG, Faroni LRDA, Batista MD, Silva FHd (2008) Resistance of stored-product insects to phosphine. Pesquisa Agropecuária Brasileira 43, 1671-1676.
| Crossref | Google Scholar |
Pinheiro J, Bates D, DebRoy S, Sarkar D (2019) nlme: linear and nonlinear mixed mffects models, R package version 3.1-140. Available at https://CRAN.R-project.org/package=nlme
Quinlan MC, Gibbs AG (2006) Discontinuous gas exchange in insects. Respiratory Physiology & Neurobiology 154(1–2), 18-29.
| Crossref | Google Scholar | PubMed |
Rajendran S (2020) Insect pest management in stored products. Outlooks on Pest Management 31(1), 24-35.
| Crossref | Google Scholar |
R Development Core Team (2020) R: a language and environment for statistical computing. (R Foundation for Statistical Computing: Vienna, Austria) Available at https://www.R-project.org/
Richards AM (1973) A comparative study of the biology of the giant wetas Deinacrida heteracantha and D. fallai (Orthoptera: Henicidae) from New Zealand. Journal of Zoology 169(2), 195-236.
| Crossref | Google Scholar |
Rowe TTC, Gutbrod MS, Matthews PGD (2022) Discontinuous gas exchange in Madagascar hissing cockroaches is not a consequence of hysteresis around a fixed PCO2 threshold. Journal of Experimental Biology 225(2), jeb242860.
| Crossref | Google Scholar |
Schimpf NG, Matthews PGD, White CR (2012) Cockroaches that exchange respiratory gases discontinuously survive food and water restriction. Evolution 66(2), 597-604.
| Crossref | Google Scholar | PubMed |
Shan C, Li B, Li L, Du X, Ren Y, McKirdy SJ, Liu T (2023) Comparison of fumigation efficacy of methyl bromide alone and phosphine applied either alone or simultaneously or sequentially against Bactrocera correcta in Selenicereus undatus (red pitaya) fruit. Pest Management Science 79(12), 4942-4951.
| Crossref | Google Scholar | PubMed |
Sláma K (1999) Active regulation of insect respiration. Annals of the Entomological Society of America 92(6), 916-929.
| Crossref | Google Scholar |
Sláma K (2008) Extracardiac haemocoelic pulsations and the autonomic neuroendocrine system (coelopulse) of terrestrial insects. Terrestrial Arthropod Reviews 1(1), 39-80.
| Crossref | Google Scholar |
Sláma K, Coquillaud M-S (1992) Homeostatic control of respiratory metabolism in beetles. Journal of Insect Physiology 38(10), 783-791.
| Crossref | Google Scholar |
Sláma K, Jedlička P (2012) Respiratory metabolism of the pea aphid, Acyrthosiphon pisum (Hemiptera: Aphididae). European Journal of Entomology 109(4), 491.
| Crossref | Google Scholar |
Sláma K, Santiago-Blay JA (2017) Terrestrial insects with tracheae breath by actively regulating ventilatory movements: physiological similarities to humans. Life: The Excitement of Biology 5, 4-70.
| Crossref | Google Scholar |
Sláma K, Šobotník J, Hanus R (2007) Respiratory concerts revealed by scanning microrespirography in a termite Prorhinotermes simplex (Isoptera: Rhinotermitidae). Journal of Insect Physiology 53(4), 295-311.
| Crossref | Google Scholar | PubMed |
Snelling EP, Duncker R, Jones KK, Fagan-Jeffries EP, Seymour RS (2017) Flight metabolic rate of Locusta migratoria in relation to oxygen partial pressure in atmospheres of varying diffusivity and density. Journal of Experimental Biology 220(23), 4432-4439.
| Crossref | Google Scholar |
Socha JJ, Förster TD, Greenlee KJ (2010) Issues of convection in insect respiration: insights from synchrotron X-ray imaging and beyond. Respiratory Physiology & Neurobiology 173, S65-S73.
| Crossref | Google Scholar | PubMed |
Sun M (2023) Colloquium: miniature insect flight. Reviews of Modern Physics 95(4), 041001.
| Crossref | Google Scholar |
Talal S, Gefen E, Ayali A (2018) Intricate but tight coupling of spiracular activity and abdominal ventilation during locust discontinuous gas exchange cycles. Journal of Experimental Biology 221(6), jeb174722.
| Crossref | Google Scholar |
Tenney SM (1985) Oxygen supply and limiting oxygen pressures in an effect larva. Respiration Physiology 60(1), 121-134.
| Crossref | Google Scholar | PubMed |
Terblanche JS, Woods HA (2018) Why do models of insect respiratory patterns fail? Journal of Experimental Biology 221(13), jeb130039.
| Crossref | Google Scholar |
Wang LC, Peter RE (1975) Metabolic and respiratory responses during Helox-induced hypothermia in the white rat. American Journal of Physiology 229(4), 890-895.
| Crossref | Google Scholar | PubMed |
Wasserthal LT, Fröhlich AS (2017) Structure of the thoracic spiracular valves and their contribution to unidirectional gas exchange in flying blowflies Calliphora vicina. Journal of Experimental Biology 220(2), 208-219.
| Crossref | Google Scholar |
Westneat MW, Betz O, Blob RW, Fezzaa K, Cooper WJ, Lee W-K (2003) Tracheal respiration in insects visualized with synchrotron X-ray imaging. Science 299(5606), 558-560.
| Crossref | Google Scholar | PubMed |
Westneat MW, Socha JJ, Lee W-K (2008) Advances in biological structure, function, and physiology using synchrotron X-ray imaging. Annual Review of Physiology 70, 119-142.
| Crossref | Google Scholar | PubMed |
Wobschall A, Hetz SK (2004) Oxygen uptake by convection and diffusion in diapausing moth pupae (Attacus atlas). International Congress Series 1275, 157-164.
| Crossref | Google Scholar |
Woodman JD, Haritos VS, Cooper PD (2008) Effects of phosphine on the neural regulation of gas exchange in Periplaneta americana. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 147(3), 271-277.
| Crossref | Google Scholar | PubMed |


