The Southern Angle-headed Dragon (Lophosaurus spinipes): a systematic review of the literature
Bradley J. Traynor A * , Heike Schütze
A * , Heike Schütze  B , Darryl L. Houston
B , Darryl L. Houston  C , Harold Heatwole C D and Eric J. Nordberg
C , Harold Heatwole C D and Eric J. Nordberg  A
A
A
B
C
D
Abstract
The Southern Angle-headed Dragon (Lophosaurus spinipes) is a cryptic agamid endemic to the warm-temperate and subtropical rainforests of south-eastern Australia. The aim of this review was to synthesise available information on L. spinipes relevant to its potential conservation in the face of predicted climatic change. A systematic literature review was conducted following the PRISMA Guidelines. Five databases (UNE library ‘PRIMO’ search, Academic Search Complete, GreenFILE, Scopus, and Web of Science) and Google Scholar were searched for publications up to November 2023 and supplemented by handsearching. Results were synthesised narratively using thematic analysis. Eighteen publications were included in the final review and categorised into six themes: Biology; Reproduction; Phylogeny and Taxonomy; Morphology and Morphometry; Ecology; and Vulnerability. Most publications discussed general morphology, distribution, basic descriptions of reproduction, or generalised diet; eight outlined predation and nesting behaviours; and one detailed an investigation into the thermal behaviour of L. spinipes. There is limited empirical data on L. spinipes. More research, particularly on population sizes, trends over time, thermal properties of the microclimate, thermal limits and metabolism with a focus on rising environmental temperatures, is required to underpin decisions relative to the conservation of this species.
Keywords: agamid, Australia, climatic change, dragon, ecology, endemic, lizard, systematic review, temperate rainforest.
Introduction
Climatic change is expected to impact ecosystems across the globe (Buckley and Huey 2016). Lizards are particularly at risk from extreme climate variability (Buckley and Huey 2016; Doan et al. 2022) because, as ectotherms, their body temperature is closely linked to the environment (Heatwole and Taylor 1987). While some species can thermoregulate to an extent through microclimate selection and behavioural adjustments, many rainforest specialists are thermoconformers, meaning their body temperature closely tracks ambient temperatures (Pough et al. 1998). This tight relationship with environmental temperature makes forest specialists more susceptible to increasing future climate variability (Huey et al. 2009).
A common method that species’ employ for adjusting to increased environmental temperatures is to alter their spatial distribution and follow their climate niche (Pörtner 2001; Rubenstein et al. 2023). Terrestrially, these relocations tend to see populations move to higher elevations, or to higher latitudes where thermal ranges are more suitable (Chen et al. 2011; Doan et al. 2022; Chan et al. 2024). However, for species that already occur at the higher elevations, upward shifts may not be possible, and species with fragmented distributions often have geographical barriers preventing latitudinal moves (Forero-Medina et al. 2011; Doan et al. 2022).
Australia was a part of the Gondwanan supercontinent (~100 mya), which was covered by rainforests (Riddington 2014), but many climatic shifts later, rainforests are now restricted to isolated, disjunct patches (Peel 1999). A combination of climatic shifts and human interference (logging, land use, pollution, and introduction of invasive species) are the main causes of fragmentation and loss of rainforest (Riddington 2014). As endemism has also been flagged as an indicator of possible vulnerability to climatic change (Manes et al. 2021), lizard species deemed to be most at risk are the endemic, range-restricted species with low fecundity (Todd et al. 2010). Thermoconforming lizards that are endemic to temperate rainforests, and are range-restricted, are even more at risk.
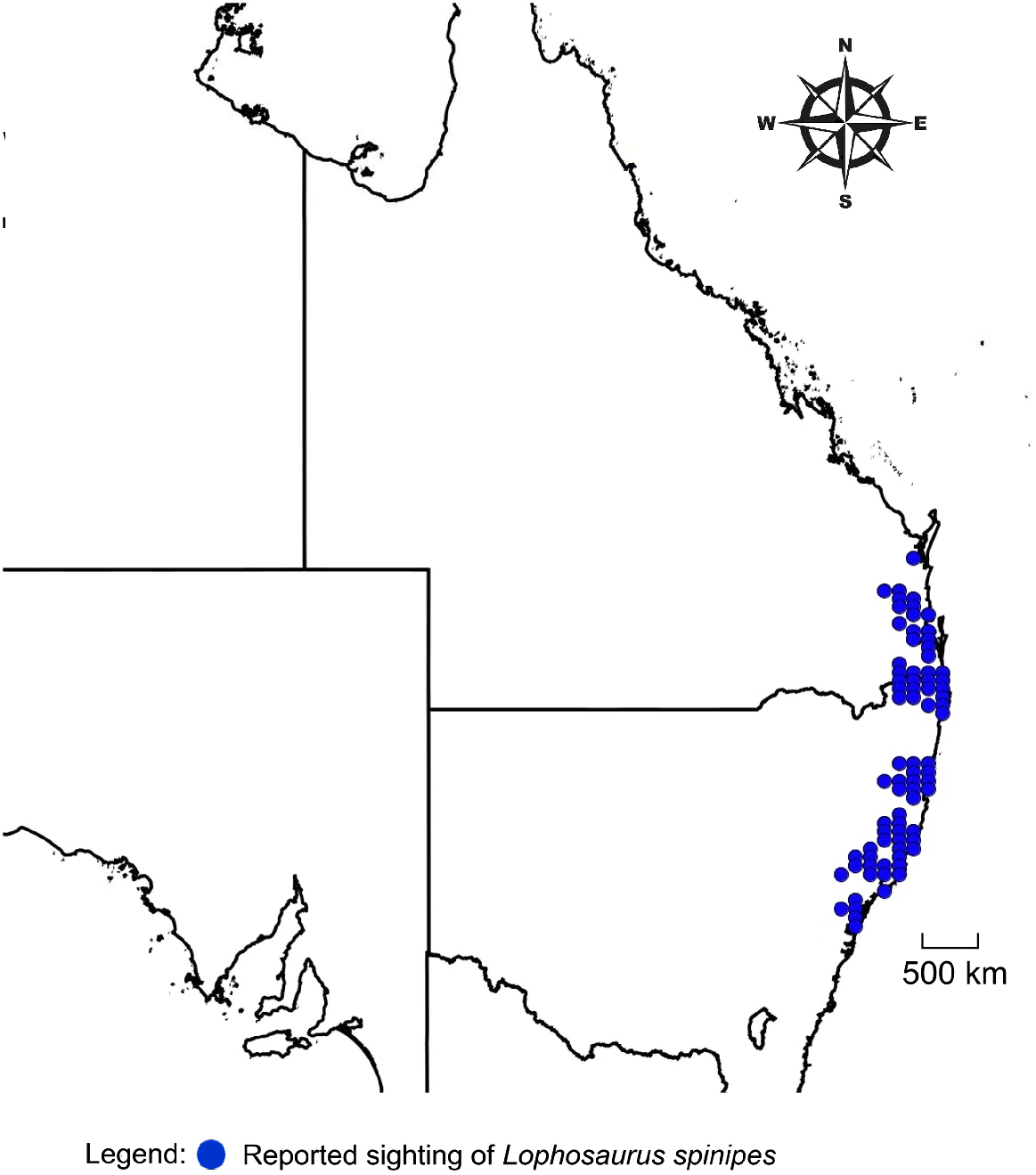
The Southern Angle-headed Dragon, Lophosaurus spinipes (previously classified in the genus Gonocephalus Kaup, 1825 and then Hypsilurus Peters, 1867), is a cryptic thermoconforming agamid endemic to the disjunct warm-temperate and subtropical rainforests of south-eastern Australia, east of the Great Dividing Range (Fig. 1). Lophosaurus spinipes have a small range, from southern Queensland (latitude 26°S) to the vicinity of Gosford (latitude 32°S) on the central coast of New South Wales (Webb 1984; Manning and Ehmann 1991; Cogger 2014) (Fig. 1). Much of this range lies within the World Heritage-listed Gondwana Rainforests of Australia, which is an important hotspot both for floral and faunal endemic species (Gregorio 2021).
The disjunct nature and continued reduction of relevant habitat throughout its range due to ongoing clearing of land and a natural dwindling of rainforests (Webb 1984), may reduce the possibility of movements by localised populations to more suitable latitudes or elevations. As a thermoconformer with a very limited range, L. spinipes could possibly be highly susceptible to climatic change. The aim of this review was to determine ‘What data are available to determine the vulnerability of Lophosaurus spinipes to climatic change?’
Methods
This review was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Page et al. 2021) and the Transparency, Openness, and Reproducibility Checklist for Meta-Analyses and Systematic Reviews (Parker et al. 2018) (Supplementary File 1).
Search strategy
Five databases (University of New England library ‘PRIMO’ search, Academic Search Complete, GreenFILE, Scopus, and Web of Science) and Google Scholar were searched for peer reviewed, non-peer reviewed and grey literature published up to November 2023, and supplemented by handsearching and expert input. Search terms were developed using PIO (Population, Interest/Intervention, Outcome), a modified version of the PICO (Population, Interest/Intervention, Comparison and Outcome) framework (Richardson et al. 1995) that is used when there is no comparator. PIO and PICO are useful tools in evidence-based research for providing a framework to structure the research question and facilitate search terms. To capture all variations, alternative terms were combined with the Boolean operand ‘OR’ (Table 1); these searches then were combined using the ‘AND’ operand (Table 1).
| PIO* element | Search string | |
|---|---|---|
| Population | Lophosaurus spinipes OR L. spinipes OR Hypsilurus spinipes OR H. spinipes OR Gonocephalus spinipes OR G. spinipes OR angle-headed dragon OR forest dragon | |
| AND | ||
| Interest/Intervention | temperature OR heat OR climate | |
| AND | ||
| Outcome | metabolism OR reproduction OR growth OR survival OR fitness |
The above search yielded only two results. The search was then broadened by removing the ‘Interest’ and ‘Outcome’ search-strings one at a time to retrieve more results, but again few results were retrieved (seven and eight results respectively). Due to the limited results obtained, the decision was then made to conduct a very broad search using only the ‘Population’ search terms (Table 2).
| PIO element | Search string | |
|---|---|---|
| Population | Lophosaurus spinipes OR L. spinipes OR Hypsilurus spinipes OR H. spinipes OR Gonocephalus spinipes OR G. spinipes OR angle-headed dragon OR forest dragon |
The same search string was used in all selected databases and Google Scholar.
Inclusion/exclusion criteria
All peer reviewed, non-peer reviewed and grey literature (except commentaries, letters to the editor, and field guides) that mentioned Lophosaurus spinipes (or its previous classifications of Gonocephalus spinipes Kaup 1825 or Hypsilurus spinipes Peters, 1867 were included. This included mentions of their single Australian congener, Lophosaurus boydii, when there were data relevant to similarities in biology or behaviour. Publications needed to be available in full text.
Publications were excluded if they were commentaries, letters to the editor, field guides, or if the full text was not available.
Selection of studies
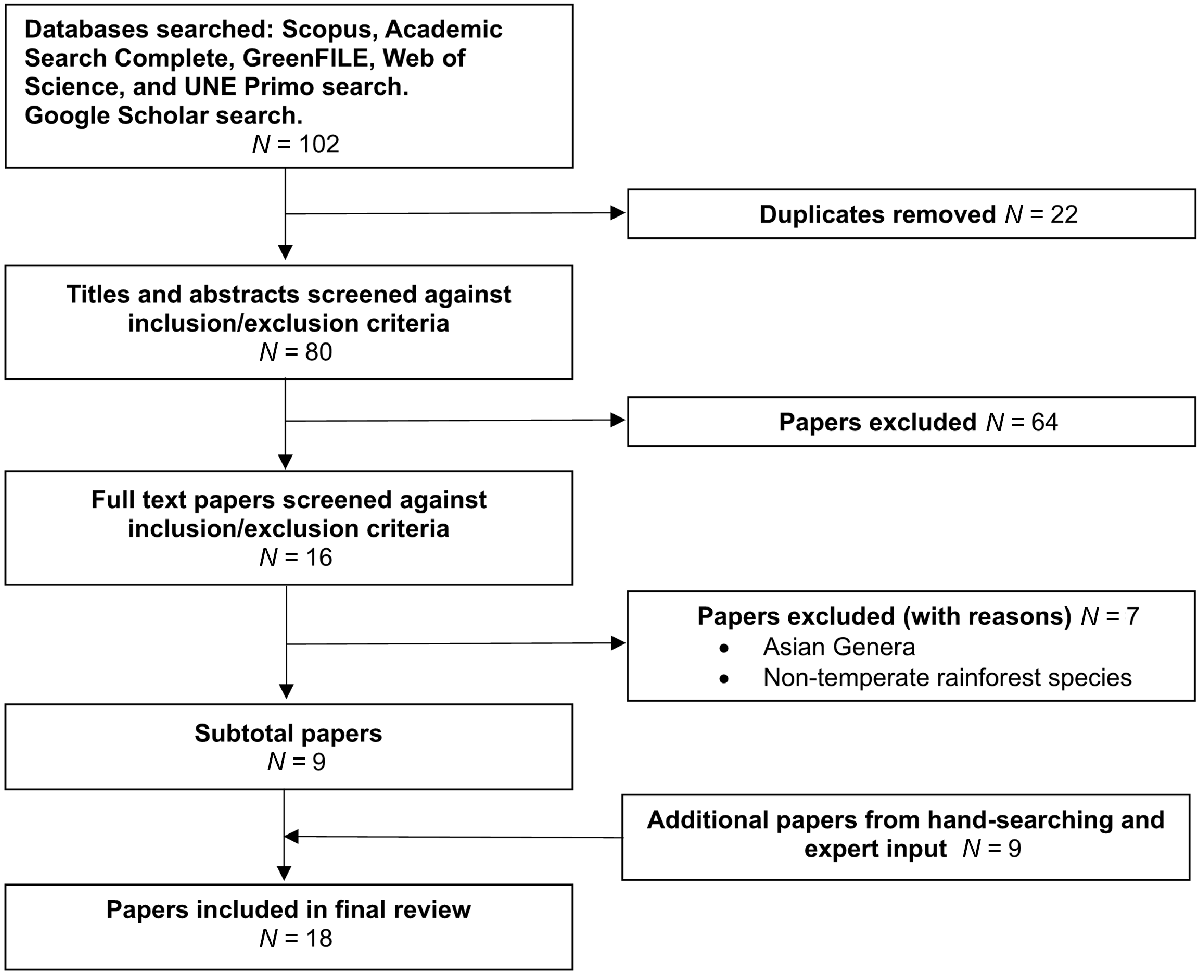
The process of selecting studies and the extraction of data were conducted according to the 2020 PRISMA framework (Page et al. 2021) (Fig. 2). The results were downloaded to Microsoft Excel for review and duplicates manually removed. The first and second authors independently screened the titles and abstracts against the criteria for inclusion/exclusion. Results were compared and disagreements were settled by discussion and consensus. The reviewers then screened the full text of the remaining articles in parallel. Reasons for exclusion were documented at each stage. The lead author then searched the reference lists of the included publications to find any that may not have been captured in the search, and expert input was also sought.
Synthesis of data
Using a standardised form for extraction, the lead author extracted the following from all publications: title, author, date, aims, type of study, methods, results, and conclusions. Meta-analysis was not possible due to the heterogeneity of the types of study. Data were analysed narratively into themes using Braun and Clarke’s (2006) six-step thematic analysis framework, which is a popular framework to help identify patterns (or themes) in data sets. Appraisal of quality was not conducted on the included articles due to the limited results.
Results
The search resulted in 102 publications (80 after duplicates were removed). Screening of titles and abstracts resulted in 64 publications being removed, leaving 16 for full text screening (Fig. 2). Seven more were excluded at the full text screening stage, leaving a subtotal of nine publications (Fig. 2). Another nine were included through handsearching and expert input, bringing the final number to 18 (Fig. 2).
Of the 18 publications included in the review (Table 3), most were observational studies, secondary analysis of existing data, or the sequencing of DNA. There were 12 peer-reviewed journal articles (Shea et al. 1991; Manning 1991, 1992; Manning and Ehmann 1991; Ota et al. 1992; Rummery et al. 1995; Alibardi 1999; Manthey and Denzer 2006; Hugall et al. 2008; Hagger et al. 2013; Pyron et al. 2013; Denzer and Manthey 2016), two peer-reviewed conference articles (Houston and Traynor 2018; Traynor and Houston 2018), one doctoral thesis (Harlow 2001), two governmental reports (Webb 1984; Kavanagh et al. 2022), and one popular science article (Torr 2003). Three had experimental components (Manning 1991; Rummery et al. 1995; Harlow 2001). Six main themes emerged and are discussed further below: Biology; Reproduction; Phylogeny and Taxonomy; Morphology and Morphometry; Ecology; Vulnerability.
| Author, date | Aim and methods | Results and conclusions | Theme(s) | |
|---|---|---|---|---|
| Alibardi (1999) | To describe the characteristics of the development of scales and epidermal differentiation in Hypsilurus (=Lophosaurus) spinipes and Physignathus (=Intellagama) lesueurii. Histological examination of the development of micro-ornamentation in scales of agamid lizards | Embryonic scales of agamids form from undulations in epidermis with later keratinisation. Epidermal layers initially flat, then dome-like nearer hatching, becoming asymmetric and differentiated toward last stages of development. | 4 | |
| Denzer and Manthey (2016) | To re-examine the taxonomy and nomenclature of the genus Hypsilurus (Lophosaurus). Examination of voucher specimens relating to GenBank sequences. | Parsimony analysis of existing data. Parsimonious and Maximum Likelihood trees designed. Clade containing Hypsilurus dilophus, H. boydii, and H. spinipes formed and contain sister group of Moloch horridus and Chelosania brunnea. Hypsilurus paraphyletic with two distinct clades. Differences between dentition of Australian/New Guinean Hypsilurus species and Asian ones. All Melanesian species are monophyletic with H. modestus. Australian species of Hypsilurus changed to Lophosaurus (Fitzinger, 1843 resurrected), with L. dilophus as type-species. | 3 | |
| Hagger et al. (2013) | To analyse existing published data to estimate/evaluate their vulnerability to climatic change in 38 subtropical rainforest species with a focus on endemic and/or threatened species using two aspects of vulnerability: (1) resistance, defined by indicators of rarity and (2) resilience, defined by indicators of a species’ potential to recover. | Montane subtropical rainforest deserves highest protection status as habitat for vulnerable taxa. Lophosaurus spinipes in study, listed as likely to recolonise; their vulnerability was high, but resilience also high, thereby offsetting vulnerability. | 6 | |
| Harlow (2001) | To determine the existence of temperature-based sex-determination of 20 agamids. Experimental. Laboratory-based incubation of agamid eggs at different temperatures, and field notes of nest temperatures. | No temperature-based sex determination in Hypsilurus, developmental stage at oviposition = 29–30 (Dufaure and Hubert 1961), Incubation at constant temperature above 26°C resulted in >50% mortality; no significant differences noted between sexes snout-vent length, and mass for Lophosaurus spinipes. | 1, 2, 4 | |
| Houston and Traynor (2018) | To determine any sexual dimorphism in the genus Lophosaurus. Morphological measurements of the three congeners of Lophosaurus from museum specimens (Queensland and Australian Museums). | No apparent significant differences between sexes except for adults’ colouration. Sexual characteristics (gonads, oviducal eggs, etc.) measured when possible. | 4 | |
| Hugall et al. (2008) | To resolve incongruence between existing views on morphology and phylogenies.based on mitochondrial DNA. Sequencing of two nuclear gene regions and comparison with phylogeny based on mitochondrial DNA. | Water dragons and forest dragons make paraphyletic basal assemblage to Amphiboluroids. Exclusively endemic Australian radiation. Lophosaurus spinipes suspected to be most ancient of Lophosaurus species. | 3 | |
| Kavanagh et al. (2022) | To set species baseline data from existing datasets, and to predict trends and vulnerabilities. Multiple statistical methods and models employed. | Baseline data addressed for 520 native and 11 introduced species of fauna, and over 2800 native and 300 introduced species vascular plants. Species distribution models (SOM) carried out on over 450 species of fauna from over 5700 systematic survey sites, over 170 floral species from nearly 5250 systematic survey sites. Species occupation modelling was carried out, but many were limited by available data. Climatic change and fire noted as key drivers of change and threat to forest-dependant biodiversity. More data are required on reptiles and certain other species for modelling purposes. Data available for L spinipes are insufficient. | 6 | |
| Manning (1991) | To observe nesting, incubation-temperatures, and hatching-times in Hypsilurus (=Lophosaurus) spinipes. Spool-tracking, duration of incubation, and measurements of nest temperature. | Nesting was observed and nests were protected from predation. Trial nests dug initially, then final nest as a shallow depression. Hatching occurred 73–75 days later. Hatching success rate of 66%. Temperatures of nests between 17.5°C and 19.2°C. Reactions to threats were noted. | 1, 2 | |
| Manning (1992) | To document the diet of Hypsilurus (=Lophosaurus) spinipes. Observation in situ, food trials, and analysis of scats. | High proportion of ants, arthropod larvae, and other invertebrates. Ambush (sit-and-wait) hunting observed. Feeding and drinking described. Scats contain parts of ants, beetles, and cockroaches. | 1 | |
| Manning and Ehmann (1991) | To document behaviour of Hypsilirus (=Lophosaurus) spinipes. Spool-tracking and field observations in situ covering feeding, basking, activity, preferences of type and width of perch. Experimentation of territoriality. | Data on morphology, habitat and feeding were documented. Low activity-temperatures compared to other agamids (17.8°C−25.1°C; mean 21.7°C). | 1 | |
| Manthey and Denzer (2006) | To revise the Melanesian and Australian genus Hypsilurus (=Lophosaurus). Reviewing type specimens, museum material, and publications. | Several species and subspecies are described as new, Hypsilurus given nomen protectum, and Lophosaurus becomes nomen oblitum. | 3 | |
| Ota et al. (1992) | To investigate karyotypic differences across the genus Gonocephalus (=Lophosaurus). Karyotyping of two Bornean species of Gonocephalus and comparison to Australian G. spinipes. | Karyologically extremely specific and different from other agamids and compared to one Australian congener, Gonocephalus spinipes. Presence of a zoogeographic dichotomy between Asian and Australian species. | 3 | |
| Pyron et al. (2013) | To reconstruct a phylogeny for extant squamates. Large-scale sampling of taxa using nuclear and mitochondrial sequencing of DNA. | New phylogeny shows Australian radiation of Hypsilurus (Lophosaurus) species northward, rather than southward as previously postulated. Lophosaurus spinipes as earliest congener. Moloch and Chelosania make Hypsilurus (Lophosaurus) paraphyletic. Lophosaurus spinipes suspected to be most ancient of Lophosaurus species. | 3 | |
| Rummery et al. (1995) | To determine the thermal biology of Hypsilurus (=Lophosaurus) spinipes in situ and in the laboratory. Use of temperature-sensitive radio trackers for observations in the field and laboratory thermal gradient (experimental). | Thermoconformers with accuracy and precision of thermoregulation low. High degree of correlation between ambient temperatures and specimens’ temperatures. Little to no overt thermoregulatory behaviours. Reliance on camouflage combined with ambush-predation. Set-point ranges between 16.6°C and 31°C in situ, with no preference in gradient. | 1 | |
| Shea et al. (1991) | To observe nesting behaviours of Hypsilurus (=Lophosaurus) spinipes. Observations in situ. | Nest positions and descriptions given. Shallow nests in hard-packed open areas. Only light covering over eggs. | 1, 2 | |
| Torr (2003) | To broadly discuss forest dragons’ ecology and behaviour; comparison between Boyd’s forest dragons and southern angle-headed dragons (Hypsilurus (=Lophosaurus) spinipes). | Scant data available. Morphology of both species listed. Thermoconformity noted in Boyd’s forest dragon, Hypsilurus (Lophosaurus) boydii, and nesting behaviours in Hypsilurus (Lophosaurus) spinipes noted. | 1, 4 | |
| Traynor and Houston (2018) | To examine the diet of genus Lophosaurus. Gut-content analysis in three congeners of Lophosaurus from specimens in the Queensland and Australian Museums. | High proportion of ants in diets of all three species despite the differences in size of the lizards. Invertebrate diet most obvious. | 1 | |
| Webb (1984) | To review the distribution, ecology, and status of Gonocephalus (=Lophosaurus) spinipes. Review of anecdotal, published, and unpublished data sources. | Descriptions of distribution and related issues. Description of habitat: temperate rainforest and associated wet sclerophyll ecotones and understorey. Activity includes perching, feeding, and breeding on ground. Camouflage and defence described. Probable predators listed as unknown. Scant information on reproduction and clutch-sizes available. Status of diet and conservation described. | 1, 2 |
Key: 1, Biology; 2, Reproduction; 3, Phylogeny and Taxonomy; 4, Morphology and Morphometry; 5, Ecology; 6, Vulnerability.
Biology
Nine publications (50%) were categorised in the biology theme. These included publications on behaviour (Webb 1984; Manning and Ehmann 1991; Rummery et al. 1995; Torr 2003), threat displays (Manning and Ehmann 1991), diet (Webb 1984; Manning 1991, 1992; Traynor and Houston 2018), nesting behaviours and duration of incubation (Manning 1991; Shea et al. 1991; Harlow 2001), and thermoregulation (Manning 1991; Manning and Ehmann 1991; Rummery et al. 1995; Torr 2003).
The detection and capture of L. spinipes is difficult throughout the year due to its effective camouflage and arboreal lifestyle (Shea et al. 1991). Most sightings occur during spring and early summer (late November and early December) when gravid females are seeking sites for nesting along roadside verges (Shea et al. 1991; Manning 1991; Manning and Ehmann 1991; Rummery et al. 1995).
Webb (1984) originally suggested an omnivorous diet for L. spinipes. However, observational studies (Manning and Ehmann 1991; Manning 1992), trials offering various sources of foods (Manning 1992), and direct inspection of the gut contents of museum specimens (Traynor and Houston 2018), found Insecta (insects), Arachnida (spiders), and unidentified unsegmented roundworms assumed to be gut-parasites. These confirmed the species as a predator of invertebrates. Although Chilopoda (centipedes) and Diplopoda (millipedes) were not observed in any of these studies of L. spinipes, both taxa were found in the guts of the related species L. boydii and L. dilophus. Most soft-bodied prey are usually broken down very rapidly, thereby making them indistinguishable in the gut contents, but Clitellata (earthworms) and Gastropoda (slugs and snails) have been identified in the field being ingested in some cases (Webb 1984; Manning and Ehmann 1991). Many species of ants, or their parts, have been noted in most guts of Lophosaurus species (Traynor and Houston 2018).
Manning (1991) and Shea et al. (1991) observed nesting behaviours in the field, where the females were seen digging test nests along the dirt roads in the area. Several days later the females were seen digging shallow (2–4 cm) nests in which they proceeded to deposit the eggs. Upon completion of deposition, they covered (or partially covered) the eggs with soil. The postoviposition duration of incubation has been reported with variable results: 74–75 days in situ (N = 1 nest) (Manning 1991), and between 68 and 125 days in the laboratory (temperature-dependent) (Harlow 2001). Harlow (2001) trialled a range of temperatures on developing eggs, and found incubation to last 125 days at 20°C, about 68 days at 26°C, and mortality increased substantially when incubated at a constant 27°C.
Observational studies on the movements of L. spinipes using the spool tracking technique indicated that they move in short bursts, over short distances, relying on crypsis as their defensive strategy (Manning and Ehmann 1991; Manning 1992). An experimental study showed that males were territorial with clear separation of their defended areas, with territories of between 300 m2 and 700 m2 (Manning and Ehmann 1991). Males frequently move over the ground rather than arboreally, whereas females move substantially shorter distances on the ground, and tend to remain on the abundant saplings and vines of different species within the forest (Manning and Ehmann 1991). Females show no preference for particular species of tree or vine (Rummery et al. 1995). Females are not harassed when crossing males’ territories, but males are actively attacked or threatened upon entering another male’s territory (Manning and Ehmann 1991; Rummery et al. 1995).
Males and females tend to perch above the ground at an average of 2.6 m and 3 m respectively (Manning and Ehmann 1991). The diameters of perches were variable: Manning and Ehmann (1991) noted a range of 20–60 mm, whereas Rummery et al. (1995) indicated <20 mm even though a greater range of perch-diameters was available. Both studies suggested that the perch was chosen to provide a better grip for climbing, to increase camouflage, and to widen the animal’s field of vision (Manning and Ehmann 1991; Rummery et al. 1995).
Lophosaurus spinipes was originally thought to exhibit some heliothermy (that is, utilising sunlight as a source of heat), as apparent basking was observed (Manning 1991; Manning and Ehmann 1991). However, a later study demonstrated that this species does not actively shuttle to maintain a precise range of temperature, and the species is considered to be a thermoconformer (Rummery et al. 1995). Its habitat of closed-forest offers fewer basking sites than those of many other agamids, and constitutes an area of prohibitive costs either for heliothermy or thigmothermy (that is, absorbing heat from the substrate) (Rummery et al. 1995). It was noted that females may increase their thermoregulatory effort during gravidity (Rummery et al. 1995), but this topic requires further investigation. In contrast to most other Australian agamids, L. spinipes is active over a wider range of temperatures: 11−26°C (Rummery et al. 1995); 17.8−25.1°C (Manning and Ehmann 1991). Although this species is reported to have a broad set-point range, it was seen to actively avoid temperatures outside of that range whenever and wherever possible (Rummery et al. 1995). The lower set-points and temperatures that the lizard voluntarily accepts during its activity period can be compared with those of most other agamids (Manning and Ehmann 1991; Rummery et al. 1995) that inhabit more arid and open environments. It was suggested that choice of microhabitat (the height and diameter of perches and the openness of their location), may result in an imprecise level of thermoregulation (Rummery et al. 1995). It is important to note that the study by Rummery et al (1995) was carried out late in the seasonal activity cycle of L. spinipes (April of 1992), and may not have entirely captured temporal changes in thermoregulatory effort.
Reproduction
Four publications discussed reproductive processes of related species of dragons. Three discussed observations and anecdotes (Webb 1984; Manning 1991; Shea et al. 1991), and one was an experimental study (Harlow 2001).
Observations in the field of L. spinipes’ on nest site selection, nesting behaviours, clutch sizes, and hatching success, in two different latitudinal areas of New South Wales, Australia, appeared in the early 1990s (Manning 1991; Shea et al. 1991). Lophosaurus spinipes selects more open areas such as roads and tracks for oviposition in very shallow nests (Manning 1991; Shea et al. 1991; Harlow 2001), making them more easily detected by predators such as brush turkeys (Alectura lathami) and lace monitors (Varanus varius) (Webb 1984; Manning 1991). An experimental study (Harlow 2001) recorded the fates of embryos of L. spinipes incubated at different temperatures, and showed that mortality of developing embryos during incubation at temperatures above 26°C was greater than for those incubated at lower temperatures.
Phylogeny and taxonomy
Five publications discussed the phylogeny and taxonomy of L. spinipes (Ota et al. 1992; Manthey and Denzer 2006; Hugall et al. 2008; Pyron et al. 2013; Denzer and Manthey 2016). The classification of the genus Lophosaurus and its phylogenetic placement have changed considerably over the years, with some revisions based solely on morphology, others on both morphology and molecular analysis, and some on molecular analysis alone (Manthey and Denzer 2006; Hugall et al. 2008; Pyron et al. 2013; Denzer and Manthey 2016). A comparison of the karyology of two Bornean species of Gonocephalus with that of Australian Gonocephalus (Lophosaurus) spinipes, demonstrated a clear difference and a possible zoogeographic dichotomy between Asian and Australian species (Ota et al. 1992). This finding would seem to add further weight to the argument for an early, endemic agamid radiation in Australia (Ota et al. 1992).
The inherent interrelatedness of three Hypsilurus (Lophosaurus) species from Australia and New Guinea (dilophus, boydii, and spinipes), along with the fact that they demonstrated few similarities with any other members of the genus, led to their placement within the dilophus subgroup (Manthey and Denzer 2006). A dichotomous key for the genus was developed. The rise of sequencing and analysis of DNA created incongruities within the morphologically-based phylogenies (Pyron et al. 2013). To reduce these conflicts, additional groupings and subcategories were added to taxonomic nomenclature. The use of both morphological and molecular data to revise the classification of squamate reptiles resulted in a new, large-scale phylogenetic revision (Pyron et al. 2013). Although reviews of phylogeny had been previously conducted at many taxonomic levels, a large-scale phylogeny had not been attempted. The familial and subfamilial classifications within squamate reptiles were reviewed and altered to match the newly presented phylogeny; Hypsilurus was retained, and the Australian–New Guinean species remained in the dilophus grouping (Pyron et al. 2013).
In 2008, the Australasian members of Hypsilurus (Lophosaurus) were once again revised as the newer molecular phylogenies were still found to be highly incongruent with the morphological scheme (Hugall et al. 2008). This revision, based upon mitochondrial deoxyribonucleic acid (mtDNA) sequences, provided acceptance for the molecular phylogeny, and added support to the idea that lizards from mesic-forest came onto the Australian plate from the north during the Miocene epoch. This theory has since been rescinded and the idea of an Australian radiation moving south to north has been adopted (Pyron et al. 2013; Denzer and Manthey 2016). The resultant phylogeny places the genera Physignathus (Intellagama) and Hypsilurus (Lophosaurus) into a “… paraphyletic basal assemblage to the more derived Australian forms such as Amphibolurus and Ctenophorus …” (Hugall et al. 2008, p. 343). Finally, the taxonomy and nomenclature of Hypsilurus was revisited after an examination of voucher specimens’ sequences held on GenBank (Denzer and Manthey 2016). Some confusion was noted in assignation of sequences to voucher specimens and with respect to some of the phylogenies. The resultant outcome was to place the Australian and New Guinean species (formerly in the dilophus group) into the resurrected genus Lophosaurus Fitzinger 1843, where they reside to this date.
Morphology and morphometry
Four papers discussed morphology and morphometry. These included an article of popular science (Torr 2003); a descriptive analysis of the development of the ornamentation of scales (Alibardi 1999); an experimental study on female agamids and their offspring (Harlow 2001); and an account of the gross morphometry of museum specimens (Houston and Traynor 2018).
The developmental stages and the layers involved in the micro-ornamentation on scales in Hypsilurus (Lophosaurus) spinipes and Physignathus (Intellagama) lesueurii were shown to follow the same process, and was also noted to be similar in process to that of other species of lizards (Alibardi 1999). Consensus on the morphometric details of L. spinipes was reached (Harlow 2001; Torr 2003; Houston and Traynor 2018). However, morphometry of the three members of the genus Lophosaurus taken from museum holdings at the Queensland Museum and the Australian Museum, yielded inconclusive results regarding morphometric differences between the sexes. There was a lack of any significant sexual dimorphism in size (but sexual dichromatism was noted in L. spinipes) (Manning and Ehmann 1991; Shea et al. 1991). Although Torr (2003) also included morphometric measurements of L. spinipes that demonstrated an overall difference in size between these two congeners, the article lacked correlative analysis and added little to the morphometry of L. spinipes.
Ecology
Only one publication reviewed the overall ecology of Lophosaurus spinipes. This was a government report (Webb 1984).
Concerns raised about the status of L. spinipes by the Forestry Commission of NSW and NSW National Parks and Wildlife Services, led to a comprehensive report being produced by the Forestry Commission of NSW (Webb 1984). Fragmentation of habitat due to heavy logging, clearing for houses and infrastructure, and natural dwindling of rainforests noted south of the Hunter River Valley (resulting in disjunct localised populations), caused a reduction in sightings of the species. Further concerns grew with the proposal for the extensive forest-operation in the Hastings River catchment at that time, the lack of records in the area, and the unknown status of this species in general. Webb (1984) noted the paucity of data on this species, and used these scant records along with anecdotal evidence to conclude that the northern populations were well represented, but numbers were of concern in the areas at lower latitudes.
Vulnerability
Two publications highlighted the vulnerability of L. spinipes. These were an analysis of existing data sets (Hagger et al. 2013), and a governmental report (Kavanagh et al. 2022).
Hagger et al. (2013) evaluated the vulnerabilities to climatic change in 38 species in subtropical rainforests, and focussed on endemic and/or threatened species, using two aspects of vulnerability to evaluate each species: (1) resistance, defined by indicators of rarity; and (2) resilience, defined by indicators of a species’ potential to recover (Hagger et al. 2013). This study deemed L. spinipes’ vulnerability to be high; however, its resilience was also measured as high, thus offsetting vulnerability. The species was therefore listed as less vulnerable than many others due to its suspected ability to recolonise after disturbance (Webb 1984). Hagger et al. (2013) also concluded that montane, subtropical rainforest (L. spinipes’ habitat) deserves the highest protection status as habitat for vulnerable taxa.
Kavanagh et al. (2022) concluded that climatic change and fire were the key drivers of change and threat to forest-dependant species, which included L. spinipes. There were too few records for L. spinipes to conduct any modelling on distribution and occupancy.
Discussion
This review sought to find out what data are available to determine the vulnerability of Lophosaurus spinipes to climatic change. We found 18 articles that were thematically classified as focussing on: biology (N = 9); reproduction (N = 4); phylogeny and taxonomy (N = 5); morphology (N = 4); ecology (N = 1); and vulnerability (N = 2). Lophosaurus spinipes is a cryptic, thermoconforming species with lower ranges of temperatures at which it is active than is true of most other agamids (Rummery et al. 1995), and with a limited and disjunct distribution (Webb 1984; Manning and Ehmann 1991). This review found that the only other information related to its vulnerability was on the mortality of developing embryos incubated at constant temperatures between 20°C and 28°C, with mortality increasing significantly above 26°C, and no eggs surviving at 28°C (Harlow 2001). This suggests that L. spinipes could be vulnerable to climatic warming if temperatures of nests are high enough to push the average incubation temperature above this threshold.
Finding publications with information on L. spinipes was difficult: we performed several searches to yield any useful results, with the final search being a very broad search for the species name. In all, 50% of the included articles were added through expert input. Although expert input is an important part of a literature search, particularly for unpublished data, reliance on individual expertise is no substitute for information being available in perpetuity through searchable databases.
Half of the 18 articles included in this review fell under the theme of biology. General biological data, together with environmental data, are used in mechanistic modelling, an important tool in predicting vulnerability to climatic change (Kearney and Porter 2020). The above, in conjunction with existing and historical data sets, have been used successfully in biophysical modelling (Hagger et al. 2013; Visconti et al. 2013; Thurstan et al. 2015; Zubova et al. 2021). However, these models require specific information on the natural history of the species (such as growth rates, and thresholds for survival), detailed physiology (such as critical thermal limits, metabolic rates, and evaporative water loss), and characteristics of the habitat (such as microhabitat availability, competition, and predator–prey relationships); such data are scarce or not available for L. spinipes.
The articles contained in this review included mainly single observations or those of short duration; no long-term monitoring was performed to show trends over time. A 1984 Forestry Commission of NSW report (Webb 1984) stated that more data on trends in population were needed; almost 40 years later, another report found that there was insufficient data on the species, and that more fine-scale data were needed to properly assess distribution and trends in populations (Kavanagh et al. 2022). As such, this gap in knowledge remains.
The cryptic nature, and difficulty in location of specimens of L. spinipes may provide insight into why there is a dearth of literature available. However, a paucity of data itself should be a cause for concern about the status of any species, as the IUCN’s listing of conservation-status are based upon the data available at the time (IUCN 2025). These listings of status are generated based on known geographic range, estimates of the sizes of and declines of populations, conservation already in place, life-history traits, field surveys, museum records, remote sensing, and possibly citizen science (IUCN 2025).
Effective action in conservation relies upon understanding population dynamics which, in turn, relies on the collection and availability of relevant long-term data (Bayraktarov et al. 2019; Binley et al. 2024). As a thermoconforming endemic species that inhabits temperate and subtropical rainforests, with a very restricted range, it is imperative that focus for L. spinipes be directed at filling the gaps in knowledge. This will ensure that decisions on its vulnerability and need for conservation are not overlooked, and that decisions are well grounded in robust evidence (Bayraktarov et al. 2019; Binley et al. 2024). More research, particularly on population sizes, trends over time, thermal properties of the microclimate, experimental determination of thermal limits, and metabolism with a focus on rising environmental temperatures, is required to determine the vulnerability of L. spinipes due to climatic change.
Strengths and limitations
This review was undertaken using rigorous systematic methods. It includes a wide range of sources to comprehensively capture the available evidence. It is the first synthesis of information on L. spinipes to ascertain what data are available to help guide decisions on whether the species is vulnerable to climatic change, and to provide recommendations for future research.
This review has some limitations. The selected databases searched were chosen as they contained the most relevant and up-to-date information on the topic. However, it is possible that publications catalogued in other databases could have been missed. Due to the two-part PRISMA screening process, even if the inclusion criteria were present in the full text, it is possible that the publication was screened out in the Title/Abstract Screening stage because the criteria were not present there. Only published literature was included, and publication-bias may be present. Due to the limited number of results, appraisal of quality of the included publications was not conducted, and the results should be interpreted with caution.
Data availability
Data used for this paper are available from the corresponding author upon reasonable request.
Declaration of funding
BJT received University of New England Higher Degree by Research (HDR) funding. The research was further supported by an Australian Government Research Training Program (RTP) Scholarship.
Author contributions
BJT and HS conceptualised the study and conducted the database searches. BJT performed the hand search, synthesised the results and drafted the initial manuscript. HS substantially revised the manuscript. All authors reviewed and revised subsequent drafts and approved the final version.
Acknowledgements
We thank Harald Ehmann for his assistance in providing manuscripts that were otherwise difficult to obtain.
References
Alibardi L (1999) Formation of large micro-ornamentations in developing scales of agamine lizards. Journal of Morphology 240(3), 251-266.
| Crossref | Google Scholar | PubMed |
Atlas of Living Australia (n.d.) Occurrence records. Available at https://biocache.ala.org.au/occurrences/search?q=taxa%3A%22Lophosaurus+spinipes%22#tab_mapView [accessed 20 April 2025]
Bayraktarov E, Ehmke G, O’Connor J, et al. (2019) Do big unstructured biodiversity data mean more knowledge? Frontiers in Ecology and Evolution 6, 239.
| Crossref | Google Scholar |
Binley AD, Vincent JG, Rytwinski T, et al. (2024) Making the most of existing data in conservation research. Perspectives in Ecology and Conservation 22(2), 122-128.
| Crossref | Google Scholar |
Braun V, Clarke V (2006) Using thematic analysis in psychology. Qualitative Research in Psychology 3(2), 77-101.
| Crossref | Google Scholar |
Buckley LB, Huey RB (2016) Temperature extremes: geographic patterns, recent changes, and implications for organismal vulnerabilities. Global Change Biology 22(12), 3829-3842.
| Crossref | Google Scholar | PubMed |
Chan W-P, Lenoir J, Mai G-S, et al. (2024) Climate velocities and species tracking in global mountain regions. Nature 629, 114-120.
| Crossref | Google Scholar |
Chen I-C, Hill JK, Ohlemüller R, et al. (2011) Rapid range shifts of species associated with high levels of climate warming. Science Adviser 333(6045), 1024-1026.
| Crossref | Google Scholar | PubMed |
Denzer W, Manthey U (2016) Remarks on the taxonomy and nomenclature of the genus Hypsilurus Peters, 1867 (Reptilia, Agamidae, Amphibolurinae). Zoosystematics and Evolution 92(1), 103-110.
| Crossref | Google Scholar |
Doan TM, Markham S, Gregory A, et al. (2022) Hot lizards: testing the tolerance to climate warming of thermoconformers in the Andes (Squamata: Gymnophthalmidae). Ichthyology & Herpetology 110(1), 87-95.
| Crossref | Google Scholar |
Dufaure JP, Hubert J (1961) Table de dévelopment du lizard viviparae (Lacerta vivipara Jacquin). Archives D’anatomie Microscopique Et De Morphologie Expérimentale 50, 309-327.
| Google Scholar |
Forero-Medina G, Joppa L, Pimm SL (2011) Constraints to species’ elevational range shifts as climate changes. Conservation Biology 25(1), 163-171.
| Crossref | Google Scholar | PubMed |
Gregorio J (2021) Gondwana rainforests. Reunion Technology Inc, Montreal, Canada. Available at https://www.worldatlas.com/forests/gondwana-rainforests.html [accessed 25 April 2025]
Hagger V, Fisher D, Schmidt S, et al. (2013) Assessing the vulnerability of an assemblage of subtropical rainforest vertebrate species to climate change in south-east Queensland. Austral Ecology 38(4), 465-475.
| Crossref | Google Scholar |
Houston DL, Traynor BJ (2018) The agamid genus Lophosaurus Fitzinger, 1843: preliminary comparative morphological data among the three species L. spinipes, L. boydii and L. dilophus from wet preserved museum specimens. In ‘2018 Combined ASH (Australian Societry of Herpetologists) & SRARNZ (Society for Research on Amphibians and Reptiles in New Zealand) Conference’, 10–13 December 2018, Kindilan, Redlands Bay, Queensland.
Huey RB, Deutsch CA, Tewksbury JJ, et al. (2009) Why tropical forest lizards are vulnerable to climate warming. Proceedings of the Royal Society B: Biological Sciences 276, 1939-1948.
| Crossref | Google Scholar | PubMed |
Hugall AF, Foster R, Hutchinson M, et al. (2008) Phylogeny of Australasian agamid lizards based on nuclear and mitochondrial genes: implications for morphological evolution and biogeography. Biological Journal of the Linnean Society 93(2), 343-358.
| Crossref | Google Scholar |
IUCN (2025) The IUCN Red List of Threatened Species. Version 2025-1: Raw data to Red List. Available at https://www.iucnredlist.org/assessment/process [accessed 25 April 2025]
Kearney MR, Porter WP (2020) NicheMapR – an R package for biophysical modelling: the ectotherm and Dynamic Energy Budget models. Ecography 43(1), 85-96.
| Crossref | Google Scholar |
Manes S, Costello MJ, Beckett H, Debnath A, Devenish-Nelson E, Grey K, et al. (2021) Endemism increases species’ climate change risk in areas of global biodiversity importance. Biological Conservation 257, 109070.
| Crossref | Google Scholar |
Manning A (1991) Notes on the nesting, incubation and hatching of the southern angle-headed dragon, Hypsilurus spinipes (Squamata: Agamidae). Herpetofauna 21(2), 15-19.
| Crossref | Google Scholar |
Manning A (1992) Diet of the southern angle-headed dragon (Hypsilurus spinipes). Herpetofauna 22(2), 18-20.
| Crossref | Google Scholar |
Manning A, Ehmann H (1991) A study of the activity and behaviour of the southern angle-headed dragon using the spool tracking technique. Herpetofauna 21(1), 5-16.
| Crossref | Google Scholar |
Manthey U, Denzer W (2006) A Revision of the Melanesian–Australian angle head lizards of the genus Hypsilurus (Sauria: Agamidae: Amphibolurinae), with description of four new species and one new subspecies. Hamadryad 30(1&2), 1-40.
| Google Scholar |
Ota H, Matsui M, Hikida T, et al. (1992) Extreme karyotypic divergence between species of the genus Gonocephalus (Reptilia: Squamata: Agamidae) from Borneo and Australia. Herpetologica 48, 120-124.
| Google Scholar |
Page MJ, McKenzie JE, Bossuyt PM, et al. (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71.
| Crossref | Google Scholar |
Parker TH, Griffith SC, Bronstein JL, et al. (2018) Empowering peer reviewers with a checklist to improve transparency. Nature Ecology & Evolution 2, 929-935.
| Crossref | Google Scholar | PubMed |
Pörtner HO (2001) Climate change and temperature-dependent biogeography: oxygen limitation of thermal tolerance in animals. Naturwissenschaften 88, 137-146.
| Crossref | Google Scholar | PubMed |
Pyron RA, Burbrink FT, Wiens JJ (2013) A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evolutionary Biology 13, 93.
| Crossref | Google Scholar | PubMed |
Richardson WS, Wilson MC, Nishikawa J, et al. (1995) The well-built clinical question: a key to evidence-based decisions. ACP Journal Club 123, A12.
| Crossref | Google Scholar | PubMed |
Riddington M (2014) Victoria’s rainforests and the potential impacts of a changing climate. Contributions 131(6), 209-218.
| Google Scholar |
Rubenstein MA, Weiskopf SR, Bertrand R, et al. (2023) Climate change and the global redistribution of biodiversity: substantial variation in empirical support for expected range shifts. Environmental Evidence 12, 7.
| Crossref | Google Scholar |
Rummery C, Shine R, Houston DL, et al. (1995) Thermal biology of the Australian forest dragon, Hypsilurus spinipes (Agamidae). Copeia 1995(4), 818-827.
| Crossref | Google Scholar |
Shea GM, Husband G, Weigel J (1991) Nesting of the southern rainforest dragon, Hypsilurus spinipes (Squamata: Agaminae). Herpetofauna 21(2), 7-10.
| Crossref | Google Scholar |
Thurstan RH, McClenachan L, Crowder LB, et al. (2015) Filling historical data gaps to foster solutions in marine conservation. Ocean & Coastal Management 115, 31-40.
| Crossref | Google Scholar |
Traynor BJ, Houston DL (2018) Genus Lophosaurus Fitzinger, 1843: Preliminary comparative data on the diet of the two northern-most species Lophosaurus boydii (Nth Qld) and L. dilophus (PNG). In ‘2018 Combined ASH (Australian Societry of Herpetologists) & SRARNZ (Society for Research on Amphibians and Reptiles in New Zealand) Conference’, 10–13 December 2018, Kindilan, Redlands Bay, Queensland.
Visconti P, Marco M, Álvarez-romero JG, et al. (2013) Effects of errors and gaps in spatial data sets on assessment of conservation progress. Conservation Biology 27(5), 1000-1010.
| Crossref | Google Scholar | PubMed |
Zubova E, Kashulin N, Terentyev P, et al. (2021) Occurrence of fish species in the inland water of Murmansk Region (Russia): research in 1972–2021. Biodiversity Data Journal 9, e68131.
| Crossref | Google Scholar | PubMed |