Coupling between dimethylsulfide emissions and the ocean–atmosphere exchange of ammonia
M. T. Johnson A B and T. G. Bell AA School of Environmental Sciences, University of East Anglia, Norwich, NR4 7TJ, UK.
B Corresponding author. Email: martin.johnson@uea.ac.uk
Environmental Chemistry 5(4) 259-267 https://doi.org/10.1071/EN08030
Submitted: 22 May 2008 Accepted: 15 July 2008 Published: 19 August 2008
Environmental context. Dimethylsulfide (DMS) is recognised as a potentially significant climate-forcing gas, owing to its role in particle and cloud formation in the marine atmosphere, where it is the dominant source of acidity. Ammonia, the dominant naturally occurring base in the atmosphere, plays an important role in neutralising particles formed from DMS oxidation products and may even enhance the formation rate of new particles. A biogeochemical coupling has previously been proposed between DMS and ammonia fluxes from the ocean to the atmosphere, in the form of coproduction of the two gases in seawater. We revise this suggestion by introducing the concept of ‘co-emission’ of the gases, where DMS emission controls the rate of emission of ammonia from the ocean by acidifying the atmosphere.
Abstract. A strong correlation between aerosol ammonium and non-sea salt sulfate is commonly observed in the remote marine boundary layer. It has been suggested that this relationship implies a biogeochemical linkage between the nitrogen (N) and sulfur (S) cycles at the cellular biochemical level in phytoplankton in the ocean, or a linkage in the atmosphere (see P. S. Liss and J. N. Galloway, Interactions of C, N, P and S biogeochemical cycles and global change (Springer, 1993), and P. K. Quinn et al. in J. Geophys. Res. – Atmos. 1990, 95). We argue that an oceanic linkage is unlikely and draw on mechanistic and observational evidence to make the argument that the atmospheric connection is based on simple physical chemistry. Drawing on an established analogous concept in terrestrial trace gas biogeochemistry, we propose that any emission of dimethylsulfide (DMS) from the ocean will indirectly influence the flux of NH3 from the ocean, through the neutralisation of acidic DMS oxidation products and consequent lowering of the partial pressure of NH3 in the atmosphere. We present a simple numerical model to investigate this hypothesised phenomenon, using a parameterisation of the rate and thermodynamics of gas-to-particle conversion of NHx and explicitly modelled ocean–atmosphere NH3 exchange. The model indicates that emission of acidic sulfur to the atmosphere (e.g. as a product of DMS oxidation) may enhance the marine emission of NH3. It also suggests that the ratio of ammonium to non-sea salt sulfate in the aerosol phase is strongly dependent on seawater pH, temperature and wind speed – factors that control the ocean–atmosphere ammonia flux. Therefore, it is not necessary to invoke a stoichiometric link between production rates of DMS and ammonia in the ocean to explain a given ammonium to non-sea salt sulfate ratio in the aerosol. We speculate that this mechanism, which can provide a continuous resupply of ammonia to the atmosphere, may be involved in a series of biogeochemical-climate feedbacks.
Acknowledgements
We are indebted to Peter Liss, Tim Jickells, Roland von Glasow, Tim Lenton and Simon Clegg for useful and insightful discussions, help and advice and to the three reviewers of this manuscript, who provided positive and extremely constructive criticism.
[1]
R. J. Charlson ,
J. E. Lovelock ,
M. O. Andreae ,
S. G. Warren ,
Oceanic phytoplankton, atmospheric sulfur, cloud albedo and climate.
Nature 1987
, 326, 655.
| Crossref | GoogleScholarGoogle Scholar |
[Verified 30 July 2008]
[9]
S. Kloster ,
J. Feichter ,
E. M. Reimer ,
K. D. Six ,
P. Stier ,
P. Wetzel ,
DMS cycle in the marine ocean–atmosphere system – a global model study.
Biogeosciences 2006
, 3, 29.

[10]
I. Barnes ,
J. Hjorth ,
N. Mihalopoulos ,
Dimethyl sulfide and dimethyl sulfoxide and their oxidation in the atmosphere.
Chem. Rev. 2006
, 106, 940.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[11]
R. von Glasow ,
P. J. Crutzen ,
Model study of multiphase DMS oxidation with a focus on halogens.
Atmos. Chem. Phys. 2004
, 4, 589.

[12]
Y. J. Yoon ,
P. Brimblecombe ,
Modelling the contribution of sea salt and dimethyl sulfide-derived aerosol to marine CCN.
Atmos. Chem. Phys. 2002
, 2, 17.

[13]
H. Sievering ,
J. M. Cainey ,
M. Harvey ,
J. McGregor ,
S. Nichol ,
P. K. Quinn ,
Aerosol non-sea-salt sulfate in the remote marine boundary layer under clear-sky and normal cloudiness conditions: ocean-derived biogenic alkalinity enhances sea-salt sulfate production by ozone oxidation.
J. Geophys. Res. 2004
, 109, D19317.
| Crossref | GoogleScholarGoogle Scholar |

[14]
N. S. Holmes ,
A review of particle formation events and growth in the atmosphere in the various environments and discussion of mechanistic implications.
Atmos. Environ. 2007
, 41, 2183.
| Crossref | GoogleScholarGoogle Scholar |

[15]
M. Kulmala ,
How particles nucleate and grow.
Science 2003
, 302, 1000.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[16]
[17]
F. J. Dentener ,
P. J. Crutzen ,
A three-dimensional model of the global ammonia cycle.
J. Atmos. Chem. 1994
, 19, 331.
| Crossref | GoogleScholarGoogle Scholar |

[18]
F. Dentener ,
J. Drevet ,
J. F. Lamarque ,
I. Bey ,
B. Eickhout ,
A. M. Fiore ,
D. Hauglustaine ,
L. W. Horowitz ,
M. Krol ,
U. C. Kulshrestha ,
M. Lawrence ,
C. Galy-Lacaux ,
S. Rast ,
D. Shindell ,
D. Stevenson ,
T. Van Noije ,
C. Atherton ,
N. Bell ,
D. Bergman ,
T. Butler ,
J. Cofala ,
B. Collins ,
R. Doherty ,
K. Ellingsen ,
J. Galloway ,
M. Gauss ,
V. Montanaro ,
J. F. Muller ,
G. Pitari ,
J. Rodriguez ,
M. Sanderson ,
F. Solmon ,
S. Strahan ,
M. Schultz ,
K. Sudo ,
S. Szopa ,
O. Wild ,
Nitrogen and sulfur deposition on regional and global scales: a multimodel evaluation.
Global Biogeochem. Cy. 2006
, 20, GB4003.
| Crossref | GoogleScholarGoogle Scholar |

[19]
N. Gruber ,
J. N. Galloway ,
An Earth-system perspective of the global nitrogen cycle.
Nature 2008
, 451, 293.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[20]
E. A. Holland ,
F. J. Dentener ,
B. H. Braswell ,
J. M. Sulzman ,
Contemporary and pre-industrial global reactive nitrogen budgets.
Biogeochemistry 1999
, 46, 7.

[21]
[22]
P. K. Quinn ,
W. E. Asher ,
R. J. Charlson ,
Equilibria of the marine multiphase ammonia system.
J. Atmos. Chem. 1992
, 14, 11.
| Crossref | GoogleScholarGoogle Scholar |

[23]
T. G. Bell ,
M. T. Johnson ,
T. D. Jickells ,
P. S. Liss ,
Ammonia/ammonium dissociation coefficient in seawater: a significant numerical correction.
Environ. Chem. 2007
, 4, 183.
| Crossref | GoogleScholarGoogle Scholar |

[24]
M. T. Johnson ,
P. S. Liss ,
T. G. Bell ,
T. J. Lesworth ,
A. R. Baker ,
A. J. Hind ,
T. D. Jickells ,
K. F. Biswas ,
E. M. S. Woodward ,
S. W. Gibb ,
Field observations of the ocean–atmosphere exchange of ammonia: fundamental importance of temperature as revealed by a comparison of high and low latitudes.
Global Biogeochem. Cy. 2008
, 22, GB1019.
| Crossref | GoogleScholarGoogle Scholar |

[25]
P. K. Quinn ,
R. J. Charlson ,
T. S. Bates ,
Simultaneous observations of ammonia in the atmosphere and ocean.
Nature 1988
, 335, 336.
| Crossref | GoogleScholarGoogle Scholar |

[26]
L. L. Sørensen ,
O. Hertel ,
C. A. Skjoth ,
M. Lund ,
B. Pedersen ,
Fluxes of ammonia in the coastal marine boundary layer.
Atmos. Environ. 2003
, 37, 167.
| Crossref | GoogleScholarGoogle Scholar |

[27]
S. W. Gibb ,
R. F. C. Mantoura ,
P. S. Liss ,
Ocean–atmosphere exchange and atmospheric speciation of ammonia and methylamines in the region of the NW Arabian Sea.
Global Biogeochem. Cy. 1999
, 13, 161.
| Crossref | GoogleScholarGoogle Scholar |

[28]
C. Perrino ,
M. Gherardi ,
Optimization of the coating layer for the measurement of ammonia by diffusion denuders.
Atmos. Environ. 1999
, 33, 4579.
| Crossref | GoogleScholarGoogle Scholar |

[29]
P. K. Quinn ,
T. S. Bates ,
J. E. Johnson ,
D. S. Covert ,
R. J. Charlson ,
Interactions between the sulfur and reduced nitrogen cycles over the central Pacific Ocean.
J. Geophys. Res. – Atmos. 1990
, 95, 16405.
| Crossref | GoogleScholarGoogle Scholar |

[30]
W. A. H. Asman ,
R. M. Harrison ,
C. J. Ottley ,
Estimation of the net air–sea flux of ammonia over the southern bight of the North Sea.
Atmos. Environ. 1994
, 28, 3647.
| Crossref | GoogleScholarGoogle Scholar |

[31]
D. I. Savoie ,
J. M. Prospero ,
R. J. Larsen ,
F. Huang ,
M. A. Izaguirre ,
T. Huang ,
T. H. Snowdon ,
L. Custals ,
C. G. Sanderson ,
Nitrogen and sulfur species in Antarctic aerosols at Mawson, Palmer Station, and Marsh (King George Island).
J. Atmos. Chem. 1993
, 17, 95.
| Crossref | GoogleScholarGoogle Scholar |

[32]
[33]
S. M. Turner ,
G. Malin ,
P. S. Liss ,
D. S. Harbour ,
P. M. Holligan ,
The seasonal variation of dimethyl sulfide and dimethylsulfoniopropionate concentrations in nearshore waters.
Limnol. Oceanogr. 1988
, 33, 364.

[34]
M. Johnson ,
R. Sanders ,
V. Avgoustidi ,
M. Lucas ,
L. Brown ,
D. Hansell ,
M. Moore ,
S. Gibb ,
P. Liss ,
T. Jickells ,
Ammonium accumulation during a silicate-limited diatom bloom indicates the potential for ammonia emission events.
Mar. Chem. 2007
, 106, 63.
| Crossref | GoogleScholarGoogle Scholar |

[35]
A. Bode ,
C. G. Castro ,
M. D. Doval ,
M. Varela ,
New and regenerated production and ammonium regeneration in the western Bransfield Strait region (Antarctica) during phytoplankton bloom conditions in summer.
Deep Sea Res. II 2002
, 49, 787.
| Crossref | GoogleScholarGoogle Scholar |

[36]
P. M. Glibert ,
J. J. McCarthy ,
Uptake and assimilation of ammonium and nitrate by phytoplankton: indices of nutritional status for natural assemblages.
J. Plankton Res. 1984
, 6, 677.
| Crossref | GoogleScholarGoogle Scholar |

[37]
R. Simo ,
Production of atmospheric sulfur by oceanic plankton: biogeochemical, ecological and evolutionary links.
Trends Ecol. Evol. 2001
, 16, 287.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[38]
M. A. Sutton ,
W. A. H. Asman ,
J. K. Schjorring ,
Dry deposition of reduced nitrogen.
Tellus B Chem. Phys. Meterol. 1994
, 46, 255.
| Crossref | GoogleScholarGoogle Scholar |

[39]
N. Van Breemen ,
P. A. Burrough ,
E. J. Velthorst ,
H. F. Vandobben ,
T. Dewit ,
T. B. Ridder ,
H. F. R. Reijnders ,
Soil acidification from atmospheric ammonium sulfate in forest canopy throughfall.
Nature 1982
, 299, 548.
| Crossref | GoogleScholarGoogle Scholar |

[40]
P. Brimblecombe ,
Dew as a sink for sulfur dioxide.
Tellus 1978
, 30, 151.

[41]
G. D. Farquhar ,
P. M. Firth ,
R. Wetselaar ,
B. Weir ,
On the gaseous exchange of ammonia between leaves and the environment: determination of the ammonia compensation point.
Plant Physiol. 1980
, 66, 710.
| PubMed |

[42]
D. Fowler ,
M. A. Sutton ,
C. Flechard ,
J. N. Cape ,
R. L. Storeton-West ,
M. Coyle ,
R. I. Smith ,
The control of SO2 dry deposition onto natural surfaces by NH3 and its effects on regional deposition.
Water Air Soil Pollut. Focus 2001
, 1, 39.
| Crossref | GoogleScholarGoogle Scholar |

[43]
S. C. Doney ,
N. Mahowald ,
I. Lima ,
R. A. Feely ,
F. T. Mackenzie ,
J. F. Lamarque ,
P. J. Rasch ,
Impact of anthropogenic atmospheric nitrogen and sulfur deposition on ocean acidification and the inorganic carbon system.
Proc. Natl. Acad. Sci. USA 2007
, 104, 14580.
| Crossref | GoogleScholarGoogle Scholar |

[44]
L. Zhuang ,
B. J. Huebert ,
Lagrangian analysis of the total ammonia budget during Atlantic Stratocumulus Transition Experiment/Marine Aerosol and Gas Exchange.
J. Geophys. Res. 1996
, 101, 4341.
| Crossref | GoogleScholarGoogle Scholar |

[45]
[46]
T. D. Blackall ,
L. J. Wilson ,
M. R. Theobald ,
C. Milford ,
E. Nemitz ,
J. Bull ,
P. J. Bacon ,
K. C. Hamer ,
S. Wanless ,
M. A. Sutton ,
Ammonia emissions from seabird colonies.
Geophys. Res. Lett. 2007
, 34, L10801.
| Crossref | GoogleScholarGoogle Scholar |

[47]
M. Legrand ,
F. Ducroz ,
D. Wagenbach ,
R. Mulvaney ,
J. Hall ,
Ammonium in coastal Antarctic aerosol and snow: role of polar ocean and penguin emissions.
J. Geophys. Res. – Atmos. 1998
, 103, 11043.
| Crossref | GoogleScholarGoogle Scholar |

[48]
P. S. Liss ,
P. G. Slater ,
Flux of gases across the air–sea interface.
Nature 1974
, 247, 181.
| Crossref | GoogleScholarGoogle Scholar |

[49]
S. L. Clegg ,
P. Brimblecombe ,
A. S. Wexler ,
Thermodynamic model of the system H+–NH4
+–SO4
2––NO3
––H2O at tropospheric temperatures.
J. Phys. Chem. A 1998
, 102, 2137.
| Crossref | GoogleScholarGoogle Scholar |

[50]
R. M. Harrison ,
A. M. N. Kitto ,
Estimation of the rate constant for the reaction of acid sulfate aerosol with NH3 gas from atmospheric measurements.
J. Atmos. Chem. 1992
, 15, 133.
| Crossref | GoogleScholarGoogle Scholar |

[51]
Z. H. Shon ,
D. Davis ,
G. Chen ,
G. Grodzinsky ,
A. Bandy ,
D. Thornton ,
S. Sandholm ,
J. Bradshaw ,
R. Stickel ,
W. Chameides ,
G. Kok ,
L. Russell ,
L. Mauldin ,
D. Tanner ,
F. Eisele ,
Evaluation of the DMS flux and its conversion to SO2 over the Southern Ocean.
Atmos. Environ. 2001
, 35, 159.
| Crossref | GoogleScholarGoogle Scholar |

[52]
D. J. Coffman ,
D. A. Hegg ,
A preliminary study of the effect of ammonia on particle nucleation in the marine boundary layer.
J. Geophys. Res. – Atmos. 1995
, 100, 7147.
| Crossref | GoogleScholarGoogle Scholar |

[53]
P. Korhonen ,
M. Kulmala ,
A. Laaksonen ,
Y. Viisanen ,
R. McGraw ,
J. H. Seinfeld ,
Ternary nucleation of H2SO4, NH3, and H2O in the atmosphere.
J. Geophys. Res. – Atmos. 1999
, 104, 26349.
| Crossref | GoogleScholarGoogle Scholar |

[54]
T. Anttila ,
H. Vehkamaki ,
I. Napari ,
M. Kulmala ,
Effect of ammonium bisulphate formation on atmospheric water–sulphuric acid–ammonia nucleation.
Boreal Environ. Res. 2005
, 10, 511.

[55]
W. D. Scott ,
F. C. R. Cattell ,
Vapor pressure of ammonium sulfates.
Atmos. Environ. 1979
, 13, 307.
| Crossref | GoogleScholarGoogle Scholar |

[56]
D. V. Spracklen ,
K. S. Carslaw ,
M. Kulmala ,
V.-M. Kerminen ,
G. W. Mann ,
S.-L. Sihto ,
The contribution of boundary layer nucleation events to total particle concentrations on regional and global scales.
Atmos. Chem. Phys. 2006
, 6, 5631.

A Harrison and Kitto[
50
] found kinetic control of aerosol sulfate neutralisation by NH3 during a ‘connected flow’ study over S.E. England. They observed that the pseudo-first order rate constant (with respect to NH3) for the reaction decreases with increasing neutralisation (Eqn 1).
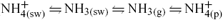 where K has units of s–1. Although these observations were made under a very different biogeochemical regime to that of the remote marine atmosphere, they strongly indicate a decrease in reaction rate towards aerosol neutralisation.
Quinn et al.[
22
] predict exponentially increasing pNH3(g) over aerosol tending towards neutralisation in their thermo-dynamic model of the atmospheric NHx system. The authors do not present the details of their model, but we have closely reproduced their findings using the Aerosol Inorganics Model (AIM) described in Clegg et al.[
49
] and in a related model, PITZ93, which is more reliable at near-neutral pH (S. Clegg, pers. comm.). Furthermore, the strong pH dependence of pNH3(g) over ammonium sulfate aerosols has been observed in laboratory studies.[
55
]
where K has units of s–1. Although these observations were made under a very different biogeochemical regime to that of the remote marine atmosphere, they strongly indicate a decrease in reaction rate towards aerosol neutralisation.
Quinn et al.[
22
] predict exponentially increasing pNH3(g) over aerosol tending towards neutralisation in their thermo-dynamic model of the atmospheric NHx system. The authors do not present the details of their model, but we have closely reproduced their findings using the Aerosol Inorganics Model (AIM) described in Clegg et al.[
49
] and in a related model, PITZ93, which is more reliable at near-neutral pH (S. Clegg, pers. comm.). Furthermore, the strong pH dependence of pNH3(g) over ammonium sulfate aerosols has been observed in laboratory studies.[
55
]
B In regions where seawater temperatures are low and ambient ammonia fluxes are likely to be from atmosphere to ocean (owing to advection from source regions), the coupling of the fluxes may in fact be via an inhibition of NH3 flux into the ocean, rather than enhanced emission of NH3 from the sea surface.
C Recent modelling studies have suggested that new particle formation is rare or non-existent in the MBL, owing to high temperatures inhibiting particle formation.[
56
] These modelling studies consider only binary homogeneous nucleation between sulfuric acid and water (probably not the only process in new particle formation in the marine atmosphere[
15
]) and thus may not be entirely correct. Either way, it has no bearing on our hypothesised process: the co-emitted DMS and ammonia are already spatially and temporally separated owing to the oxidation time for DMS and an extension to this separation while new particles sink back into the MBL is of little consequence at large scales of space and time.


