Something in the air: connections between global warming, ozone depletion, POPs and particulates
Ian D. RaeRoyal Australian Chemical Institute Inc., 1/21 Vale Street, North Melbourne, Vic. 3051, Australia. Email: idrae@unimelb.edu.au
Environmental Chemistry 5(1) 1-4 https://doi.org/10.1071/EN08012
Submitted: 3 October 2007 Accepted: 25 January 2008 Published: 22 February 2008
Environmental context. The release of chemical substances to the environment was long seen simply as a way to get rid of them from the immediate vicinity of their generation. Until recently there was little consideration that regional or global problems might result. Further, the various releases were studied by specialists who lacked the breadth of knowledge to understand that many of their specialties were linked and that chemical identity, toxicity, bio-accumulation, regional and global weather patterns and some arcane physical chemistry would need to be involved in a comprehensive analysis of the impact of chemicals on the environment.
Introduction
For thousands of years we have dumped our solid wastes in landfills of one kind or another. Most of what goes into a landfill stays there. Archaeologists rely on this fact to make deductions about societies that went out of existence long ago but left behind the evidence of their daily life and death. Although we still landfill much of our waste, and occasionally suffer from leachate contamination of surface and ground water, we have also learned to profit from the decay of vegetable matter, by making use of the methane that results from its anaerobic decay. Thus we gain an energy component and we cut back on the release of a potent greenhouse gas. Here we can see the beginning of my theme – the interconnectedness of what are treated as separate problems.
Energy recovery, although a worthy aim in itself, is linked with destruction of materials that are too dangerous to put into landfills. These are mostly organic materials and we have to be very careful when we burn them so as not to create even more dangerous substances, polychlorinated dibenzo-dioxins and -furans.[1] Although combustion releases to the atmosphere small quantities of overtly dangerous substances, it releases much larger quantities of carbon dioxide, which, until quite recently, was not regarded as a dangerous air pollutant.A Carbon dioxide has a lifetime in the atmosphere of ~70 years – not in the class of archaeological material but nonetheless long enough to be of great significance.
So, just as we bury solid wastes in landfills and say that we have ‘disposed’ of them, when all we have done is put them out of sight and often out of mind, our waste gases go to what the British engineer Robin Jeffrey has call the ‘skyfill’.[2] I plead guilty to dumping stuff in the skyfill when I was a laboratory chemist. At the beginning of my career in the 1960s, when I had waste solvents, I flushed them down the sink. My colleagues did likewise – I was not alone. When the regulations finally caught up with us, we put the volatile solvents in open dishes in the fume cupboard, and the draught did the rest. When that practice was outlawed, we segregated our waste solvents and stored them in large bottles that were taken away by a waste management company. Some were recycled for use as industrial solvents and no doubt found their way into the skyfill at the end of their second useful life. The solvents that could not be reused were burned in a more-or-less well-regulated incinerator.
The lifetimes of what we dump in the skyfill can range from days to decades, and because of the mobility of the wastes, their effects can be local, regional or global, whereas the impact of a landfill would be strictly local. This assumes, of course, that the landfill is not emitting gaseous material to its counterpart, the skyfill. There have been suspicions that this route to the sky could be more significant than we have recognised till now. When it comes to water bodies, large dams have been shown to emit large quantities of methane and nitrogen oxides.[3]
Both landfill and skyfill wastes are infinitesimal fractions of the lithosphere and the atmosphere, respectively, but I will argue that skyfill problems are more serious than landfill problems (which I do not propose to mention again).
What’s up there?
Four classes of substance make up the manifest of skyfilled material, and three of these classes are the subject of intergovernmental agreements:
-
those with global-warming potential (the Framework Convention on Climate Change, administered by the Intergovernmental Panel on Climate Change (IPCC), and its Kyoto Protocol);
-
ozone-depleting substances (the Montreal Protocol to the Vienna Convention);
-
persistent organic pollutants (POPs) (the Stockholm Convention); and
-
fine particles of a range of chemical compositions, which may constitute issues in their own right but are also involved in the science of the three conventions.
Global warming
Global warming, with its consequence, climate change, is the problem that looms largest in the public mind and, arguably, poses the greatest threat to human and environmental wellbeing. Action on greenhouse gases arose from the Earth Summit held in Rio in 1992 under the auspices of the United Nations Environment Program. The signatories to the Kyoto Protocol were almost all developed nations that felt they had the capacity to reduce their greenhouse emissions. Much emotion surrounds the decisions of two developed countries, the USA and until recently Australia, to decline to sign the Protocol on the grounds that to do so might do more damage to their industries than they were prepared to sustain. Both countries have moved away from their ‘greenhouse sceptic’ position, and they have begun to take action … but the Americans still have not signed up. The assessments published by the IPCC are built around fairly good modelling of climate change that might result from further increases in atmospheric carbon dioxide levels, and poorly managed data input into those models. Estimates of world development and likely greenhouse gas emissions vary widely, and the IPCC faithfully reports scenarios with widely varying atmospheric temperatures and sea levels. In addition to having to sort out realistic from unrealistic scenarios, the interested scientist or lay person has to make some assessment of what is long-term change in climate, and what is short-term variability (the weather). This is incredibly difficult. However, we can say that if long-term change is taking place, it is taking place inconsistently across the globe, and (in some regions) much faster than we had feared.
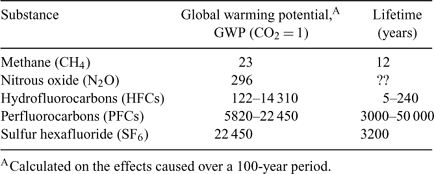
Whereas the concern of members of the public, and most governments, has been on carbon dioxide (CO2), five other gases or groups of gases have significant global warming potentials (GWPs):[4]
The greenhouse gas total is usually expressed as CO2-equivalent, which is the sum of the individual quantities each multiplied by its GWP. The breakdown for Australia (admittedly a few years old) is that 68% of our CO2-equivalent was actually CO2, 25% was methane and 6% was nitrous oxide, and there minor contributions from other gases.
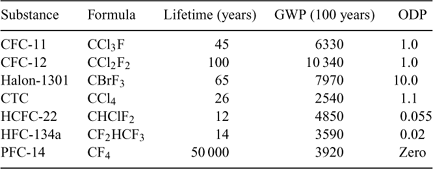
It is important to recognise that many substances other than the ‘big six’ (CO2 plus the five substances listed in Table 1) have global warming potentials. Even if the GWP is low, there can be significant impact if enough of the gas is released and finds it way to the stratosphere, and the same applies to relatively small emissions of substances with large GWP, as is the case with other substances that mainly come to our attention because of their ozone-depleting potential (ODP). See figures in Metz et al.[4] and the UNEP,[5] and Table 2.
|
|
Ozone depletion
The United Nations Environment Program (UNEP) celebrated the twentieth anniversary of the establishment of the Montreal Protocol[6] at a meeting of the Parties (signatories to the Protocol) and a series of functions in Montreal in September 2007. The major decision taken at the Meeting of Parties was to accelerate the phaseout for hydrofluorocarbons (HFCs), bringing back the deadlines from 2040 (developing countries) and 2030 (developed countries), to 2030 and 2020, respectively, and requiring a series of interim reduction steps.
The Protocol has had such publicity that most people overlook the fact that it is an implementation protocol (on substances that deplete the ozone layer) to the Vienna Convention for Protection of the Ozone Layer (1985). Over 95% of the production and consumption of substances controlled under the protocol have been phased out. Depletion of the ozone layer has all but ceased, but recovery will be very slow, owing to the atmospheric lifetimes of the substances responsible for depletion, so full recovery to 1980 levels is not expected until sometime after 2050.
An important feature of the Montreal Protocol, and one of the reasons for its success, is the differential treatment of developed and developing countries. Developing countries are given more time to comply with phaseout decisions, and in addition there is funding available to them from the Multilateral Fund provided by the developed countries. In addition, industries have been cooperative in developing replacements for the chemicals of concern. Through extensive consultation under the Protocol, targets have been set that industries could meet. One of the most difficult phaseouts is that of methyl bromide, a chemical widely used in fumigation of food crops and materials. Although use in field applications has declined and continues to decline, use for quarantine and pre-shipment purposes is permitted and so relatively large volumes of the gas are used in tropical countries from which goods are exported to cooler regions where there is continuing effort to keep out exotic insect and other pests.
Interestingly, one likely replacement for the use of chlorofluorocarbons (CFCs), hydrochlorofluorocarbons (HCFCs) and HFCs in refrigeration is carbon dioxide, the arch-villain of global warming.
Actions taken under the Montreal Protocol have helped the global community to avoid millions of cases of fatal skin cancers and tens of millions of cases of non-fatal skin cancers and cataracts.
The Protocol has also delivered substantial climate benefits, because the ozone-depleting substances being phased out have substantial GWPs. Between 1990 and 2000, the reduction in releases of these substances has been equivalent to a reduction of 25 billion tonnes of carbon dioxide – substantially more than reductions achieved under the Kyoto Protocol.[7]
For industry, the combination of criteria for lifetime, ODP and GWP make choosing a chemical for a particular purpose anything but easy.
Persistent organic pollutants (POPs)
The POPs are a different kettle of fish – an apt metaphor, as they are known to migrate from warm and temperate regions of the globe to the Arctic, where they are found in the bodies of animals there, including fish, polar bears, and seals.
Twelve substances or groups of substances are listed under the Stockholm Convention, which came into effect in 2004 after years of negotiation. They are all organochlorine substances, cleverly dubbed by the Pesticide Action NetworkB the ‘dirty dozen’. There has been good progress in phasing out the insecticides Aldrin, Chlordane, Dieldrin, Endrin, Heptachlor, Hexachlorobenzene, Mirex and Toxaphene, and in reducing emissions of unintentionally produced polychlorodibenzo-dioxins and -furans. Programs are in place, but they are slow, for the withdrawal of polychlorobiphenyls, and there are restrictions on the use of DDT. Some countries, supported by the World Health Organization, have found they just cannot do without DDT, but it is restricted to indoor use so as to minimise releases to the environment.
Further substances have been nominated for listing under the Stockholm Convention, and recommendations will come from a committee of experts to the decision-making body, the Conference of the Parties (signatory countries to the Convention):
-
flame retardants: penta- and octabromodiphenyl ether, and hexabromobiphenyl;
-
insectides and their co-products: Chlordecone, hexachlorocyclohexanes (including the δ-isomer, Lindane);
-
industrial chemicals pentachlorobenzene and short-chained chlorinated paraffins;
-
perfluorooctane sulfonic acid, a component and degradation product of a large number of surface-active agents; and
-
Endosulfan, a pesticide widely used in Australia, was nominated by the European Union in 2007 for consideration by the expert committee.
The detection of these substances in the environment and consideration of their movement into the Arctic region has given rise to an extensive and growing research literature. A global distillation process emerges as the major distribution pathway, involving successive volatilisation and deposition.[8] Blais et al.[9] have described this ‘hopping’ behaviour as ‘a large chromatographic system with moving phases (air and water currents), stationary phases (soils, vegetation), and sinks (burial, degradation)’ and it has focussed attention on the little-used physical chemistry concept of fugacity[10]C as an indicator of the likelihood of gaseous diffusion. It is likely that there is particulate transport, too, because these organic substances bind well to many kinds of particles. In particular, in the case of the flame retardants, they may be released to the environment in the form of dust derived from materials into which they have been incorporated. Birds and fish can carry the contaminants over long distances, too,[9,11] and transfer of some pollutants by ocean currents is an established mechanism.[12]
Particulates
Fine particles in urban air pose health risks. These are dependent to some extent on the nature of the particle – crystalline quartz is especially dangerous, as are particles composed of some other inorganics, and particles carrying polycyclic aromatic hydrocarbons can carry these carcinogens deep in the airways. Particles of a range of sizes act at different sites within the body, the smaller ones being drawn deeper in. Most western jurisdictions monitor for particles with sizes up to 10 microns (PM10) as well as their smaller cousins, PM2.5 and PM1.0. Most particles in urban areas derive from combustion sources, often petrol- and diesel-engine vehicles. Recent studies in rural cities in Victoria have detected fine particles in their air, coming from nearby desert country and following local storm events, but their health effects are lower than those of particles found in the air of major cities. These are local or regional effects, but some particles exert their effects at the global level.
In the case of Antarctic ozone depletion, chlorine species form on the surface of stratospheric ice particles and when spring arrives and the particles are warmed by the sun, the chlorine species are let loose and quickly reduce the concentration of ozone molecules. This is the cause of the seasonal variation of Antarctic stratospheric ozone concentrations.D Although there are concentrations of the ozone-depleting substances over the Arctic, their impacts are lower because of the higher temperatures there and consequent lesser ice concentrations. The particles do not have to be ice crystals, either, as ozone concentrations were reduced following the eruption of Mt Pinatubo, in the Philippines, in June 1991.
Turning to the greenhouse effect, one of the factors that has proved most difficult to include in scenario building has been the effect of clouds of water and other particles. Water is a powerful greenhouse gas in its own right, and water vapour concentrations in the atmosphere are expected to rise as global warming proceeds. Clouds, however, reflect some sunlight but the extent of the negative feedback mechanism is still uncertain. There is also uncertainty about future volcanic eruptions, which, if past instances are a good guide, would produce global cooling because the clouds of dust and sulfate particles would likewise restrict the amount of infrared radiation reaching into the lower atmosphere.
More specific details are emerging about the fine particles in the ‘brown clouds’ that arise from biomass burning in tropical regions and can affect climate over significant regions of the globe.[13]
Concluding remarks
The present overview of four types of emission to the atmosphere, mostly coming from human activity (although there are natural sources), shows how interconnected they are. And so, although the sky may not be filled by the ‘skyfill’, any more than the land is filled by what we put in landfills, there is certainly something in the air.

|
[1]
[2]
R. Jeffrey ,
Lessons from UK nuclear experience.
Power Eng. J. 2002
, 16, 186.
| Crossref | GoogleScholarGoogle Scholar |

[3]
[4]
[5]
[6]
[7]
G. J. M. Velders ,
S. O. Andersen ,
J. S. Daniel ,
D. W. Faye ,
M. McFarland ,
The importance of the Montreal protocol in protecting climate.
Proc. Natl. Acad. Sci. USA 2007
, 104, 4814.
| Crossref | GoogleScholarGoogle Scholar |

[8]
F. Wania ,
D. Mackay ,
Global fractionation and cold condensation of low-volatility organochlorine compounds in polar regions.
Ambio 1993
, 22, 10.

[9]
J. M. Blais ,
R. W. Macdonald ,
D. Mackay ,
E. Webster ,
C. Harvey ,
J. P. Smol ,
Biologically mediated transport of contaminants to aquatic systems.
Environ. Sci. Technol. 2007
, 41, 1075.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[10]
G. N. Lewis ,
The osmotic pressure of concentrated solutions, and the laws of the perfect solution.
J. Am. Chem. Soc. 1908
, 30, 668.
| Crossref | GoogleScholarGoogle Scholar |

[11]
A. Evenset ,
J. Carroll ,
G. N. Christensen ,
R. Kallenborn ,
D. Gregor ,
G. W. Gabrielsen ,
Seabird guano is an efficient conveyer of persistent organic pollutants (POPs) to arctic lake ecosystems.
Environ. Sci. Technol. 2007
, 41, 1173.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[12]
F. Wania ,
A global mass balance analysis of the source of perfluorocarboxylic acids in the Arctic Ocean.
Environ. Sci. Technol. 2007
, 41, 4529.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[13]
S. W. Wei ,
R. Balasubramanian ,
E. Rianawati ,
S. Karthikeyan ,
D. G. Street ,
Characterization and source apportionment of particulate matter ≤2.5 μm in Sumatra, Indonesia, during a recent peat fire episode.
Environ. Sci. Technol. 2007
, 47, 3488.
and references therein.
| Crossref | GoogleScholarGoogle Scholar |

A This is an important, although seemingly semantic point. The US Supreme Court recently ruled that the Environmental Protection Agency had the power to regulate emissions of CO2 as a pollutant, and Australia has added greenhouse gases, including CO2, to the National Pollutant Inventory, although there was a strong push from non-believers to name it, in consequence, the National Emissions Inventory.
B The Dirty Dozen Campaign was launched in June 1985 although only eight persistent pesticides were targeted at that time. Frequent repetition of the ‘Dirty Dozen’ tag, notably by Greenpeace, has entrenched it in the lexicon of environmental taunts.
C Fugacity is a measure of the chemical potential of a substance in a particular phase, so a difference in fugacity between two phases indicates the tendency of a substance to escape to the phase in which it has lower fugacity – in the case of a POP, escaping from water to air. The measure was first proposed by G.N. Lewis.[10] There is also a useful on-line guide to fugacity on Wikipedia. Available at http://en.wikipedia.org/wiki/Fugacity, accessed July 2007.
D Up-to-date information on Antarctic stratospheric ozone concentrations, including graphics, is available at http://ozonewatch.gsfc.nasa.gov/, accessed July 2007.


