Trends of polyfluoroalkyl compounds in marine biota and in humans
Renate Sturm A C and Lutz Ahrens BA Helmholtz-Zentrum Geesthacht, Centre for Materials and Coastal Research, Institute of Coastal Research, Department for Environmental Chemistry, D-21502 Geesthacht, Germany.
B Environment Canada, 4905 Dufferin Street, Toronto, ON, M3H 5T4, Canada.
C Corresponding author. Email: renate.sturm@hzg.de
Environmental Chemistry 7(6) 457-484 https://doi.org/10.1071/EN10072
Submitted: 6 July 2010 Accepted: 12 October 2010 Published: 21 December 2010
Environmental context. Polyfluoroalkyl compounds are used in a variety of industrial and consumer applications, including polymer production and for surface treatment of textiles and paper. Research over the last 10 years has shown that these compounds are ubiquitous environmental contaminants – they are extremely persistent, show toxic effects and accumulate in the food chain. We evaluate global, temporal and spatial trends of these important emerging contaminants.
Abstract. This review gives an overview of existing knowledge of polyfluoroalkyl compounds (PFCs) in humans and in marine biota. Temporal trends and spatial distribution of PFCs were globally compared in humans, marine mammals, seabirds and fish. In general, PFC concentrations in the environment have increased significantly from the beginning of the production up to the 1990s. After the phase-out of perfluorooctane sulfonyl fluoride (POSF) production starting in 2000, PFC concentrations in humans generally decreased. In marine biota no clear temporal trends were observed. The temporal trends depended on the species, their trophic levels and the geographical locations. PFC patterns in humans and in marine wildlife species were compared regarding perfluorooctane sulfonate (PFOS), perfluorooctanoate (PFOA), their shorter and longer chain homologues (C4–C15) and precursor compounds. Finally knowledge gaps were identified and recommendations for future work were presented.
Introduction
Polyfluoroalkyl compounds (PFCs) comprise a large group of industrial chemicals, consisting of a hydrophobic alkyl chain with a hydrophilic functional group. The alkyl chain is partly or fully fluorinated and typically contains between 4 and 18 carbon atoms. As a result PFCs are surface active substances which repel water, grease and dirt and are therefore used as detergents or impregnating agents for surface treatment in carpets, textiles, leather and paper, in polymer production, in fire-fighting foams, in cosmetics and cleaning agents as well as in numerous other industrial and consumer applications. PFCs have been industrially manufactured for over 50 years. The global production increased from about a few hundreds of tonnes in the beginning of the 1970s up to several thousands of tonnes in the 1990s.[1] Nowadays PFCs are detected everywhere in the environment even in remote regions like the Arctic and the Antarctic. They are regarded as a new and emerging class of environmental contaminants because of their persistence, their toxic properties and their bioaccumulative potential.
The major manufacturer of perfluorooctyl sulfonyl fluoride (POSF), the 3M Co., phased out the production of perfluorooctane sulfonate (PFOS), perfluorooctanoate (PFOA) and PFOS related products between 2000 and 2002 (see http://www.pops.int/documents/meetings/poprc/submissions/comments_2006/3M.doc, accessed 10 December 2010). Nevertheless, PFOS, PFOA and a variety of related PFCs are still being produced by other manufacturers in several countries of Europe and Asia and in the USA.[1,2] In the following years stewardship programs and statutory regulations have been discussed and announced to achieve reduction in facility emissions and product contents (see http://www.epa.gov/oppt/pfoa/pubs/stewardship/index.html, accessed 10 December 2010).[3]
Regarding the environmental impact of PFCs, the OSPAR commission (an international cooperation on environmental protection in the North-East Atlantic) published a list containing seventeen PFC substances that potentially fulfil the OSPAR criteria.[4] Those criteria include that the substances are a threat to the aquatic environment, show strong indications of risks for the marine environment, have been found widespread in one or more compartments of the maritime area and may endanger human health via consumption of food from the marine environment (http://www.ospar.org/content/content.asp?menu=00120000000070_000000_00#hazardous, accessed 10 December 2010). PFOS is considered to be a PBT substance (P, persistent; B, bioaccumulative; T, toxic) according to the criteria of the European Commission Technical Guidance Document on risk assessment.[4] Furthermore, PFOS fulfils the criteria for persistent organic pollutants (POPs) of the Stockholm Convention. Thus in 2009, PFOS and its salts were classified as POPs in terms of the Stockholm Convention.[5]
In 2006, an extensive review was published about biological monitoring of PFCs.[6] This data compilation confirmed that PFCs were globally distributed and their concentrations in biota were higher close to industrialised and urbanised regions than in remote regions. Longer chain PFCs (with more than eight C-atoms) can biomagnify through the food webs, reaching elevated values in higher trophic species. A recently published review of levels and trends of PFCs confirmed and upgraded those studies with special regard to the arctic environment.[7]
During the 1990s, PFOS concentrations in wildlife increased. Contrary to the expectation, the temporal trends in humans did not correlate with the trends in wildlife, but PFOS concentrations in humans reached a plateau in industrialised countries at the end of the 1980s. As a consequence of the conclusions by Houde et al.[6] it could be theorised that the phase-out of POSF-based products ca. 2001 should lead to a decrease in PFC concentration in the environment, but there had not been any evidence.
Based on these findings it was the aim of this review to investigate spatial and temporal trends of PFC concentrations in marine biota with particular attention to the time since the initial phase-out of POSF in 2000. In addition, trend data of biota were compared to PFC concentrations in human blood.
It is known that manufacturers replaced PFOS, PFOA and related compounds by shorter chain PFCs with four or six C-atoms instead of eight. Therefore the PFC pattern in biota should have changed since 2000. Furthermore, the occurrence and trends of PFOS precursor substances as well as longer chain PFCs with more than eight C-atoms were reviewed. Overall, in this study we have summarised trend data for PFCs and their pattern in biota from articles published up to March 2010.
Analytical challenges
It is important to note that analyses of PFCs in biota at low concentration levels pose a significant analytical challenge. Over the time analytical methods and quality assurance were improved. High performance liquid chromatography tandem mass spectrometry (HPLC-MS/MS) combined with electrospray ionisation is the most common method to analyse PFCs in biota. However, it can lead to signal enhancement or signal suppression due to matrix effects.[8–10] In 2009, it was reported that routine analytical methods are prone to overestimate perfluorohexane sulfonate (PFHxS) concentrations in human blood because of co-eluting endogenous steroid sulfates that share common fragmentation pathways.[11] Matrix-matched calibration curves regardless of extraction procedure lead to acceptable PFC quantitation in biota.[12] Based on the results of two worldwide interlaboratory method evaluation studies on water, fish and human blood carried out in 2005 and in 2007, it is recommended to use mass-labelled internal standards and well defined native standards for quantification of PFCs in biota and to minimise matrix effects by an appropriate clean-up.[13–15] Interlaboratory camparison studies of the determination of PFCs in human blood carried out in 2005 and 2006 led to the conclusion that laboratories, which possess appropriate instrumentation, use a well adapted sample preparation, have access to native and labelled standard compounds and the necessary experience, are capable of determining the most prevalent PFCs in human blood with sufficient accuracy and precision.[16] However, because of the problems in analytical determination data for comparison and evaluation of PFC concentrations in biota should be interpreted with caution in this overview.
There are a lot of reports regarding the concentrations of PFCs in biota, most of them concerning mammals, fish, birds, humans and their related food webs. Comparison of these data is difficult because of possible analytical issues and different biological tissues used for PFC analysis. For small animals (e.g. invertebrates or fish) the whole body tissue is usually homogenised before analysis, for larger animals (e.g. birds or marine mammals) only their organs, especially liver, were analysed. Thus, a comparison of the PFC concentrations in the whole body or different organs is difficult, because the tissue distribution was investigated only in a few species.[17,18]
Some authors published data about PFC concentrations in blood. They reported that PFOS concentrations in blood were lower than those in liver.[18–20] Already in 1979 studies in rats showed that PFOS is well absorbed orally and accumulates primarily in the liver and, to a lesser extent, in the serum or plasma.[21] In contrast, some studies resulted in highest values in blood and lower values in liver.[22] A study on 78 fish samples from different regions of Japan showed large variations in the ratios of PFOS concentrations between liver and blood samples.[23] The reason may be a disequilibrium of PFOS between liver and blood, possibly indicating an ongoing exposure of fish to PFOS. However, in spite of this inconsistency blood is a useful matrix for monitoring PFCs in living animals or humans. Another important aspect is that three different types of blood were chosen for PFC analysis: whole blood, blood serum obtained from whole blood after clotting and blood plasma, which comprises 55% of the blood fluid. In general, PFOS concentrations were higher in plasma or in serum than in whole blood.[24,25] For the purpose of comparison some authors converted whole-blood data to a serum basis by multiplying the whole-blood concentration by a factor of 2[26] or by a factor of 2.5.[23] Another study found serum to plasma ratios for PFOS, PFHxS and PFOA of 1 : 1 and serum or plasma to whole blood ratios of 2 : 1.[27] Thus, PFC concentrations determined in serum or plasma are directly comparable, whereas the whole blood data have to be multiplied by a minimum factor of 2, if they have to be compared to serum or plasma data. Different results were published by Kärrman et al.[24] They found plasma to whole blood ratios of less than 2. In this survey a factor of 2 was applied, if conversion was necessary.
Levels and trends in humans
Temporal trends and spatial distribution of PFCs in human blood
In most reports blood was used to study temporal trends in humans. In 2009, a review summarised human biomonitoring data of PFC levels in human blood, breast milk and human tissues as well as in air, dust, drinking water and food with regard to relevance for human exposure.[28] Highest concentrations of PFCs were found in blood of occupationally exposed workers at four production facilities, documented by the two major producers, the 3M Co. and DuPont, for the years 1995–2004. Over a period of 5 years (1995–2000), PFOS concentration in serum decreased from 2440 µg L–1 (n = 90) to ∼1290 µg L–1 (n = 188) in the factory in Decatur (USA) and from 1930 µg L–1 (n = 93) to 950 µg L–1 (n = 196) in Antwerp (Belgium). For PFOA the highest value was 6800 µg L–1 (n = 80) in 1995 in Cottage Grove (USA). It decreased to 4300 µg L–1 (n = 38) in 2002. In contrast, in Antwerp the PFOA concentration in serum increased from 1130 µg L–1 (n = 93) in 1995 to 2630 µg L–1 (n = 30) in 2003.[28,29]
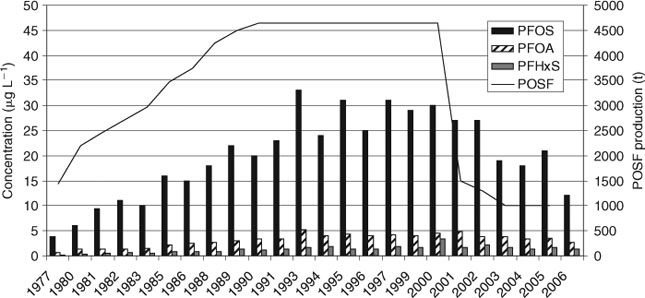
The temporal trend of PFCs in blood of the general population in the USA was analysed on the basis of blood samples from Washington County, Maryland, collected in the years 1974 (n = 178), 1989 (n = 178) and 2001 (n = 104).[30,31] PFOS data showed an increase during this period of time (from 29.5 to 35.7 µg L–1). PFOA, PFHxS and perfluorooctane sulfonamidoacetate (PFOSAA) increased by up to 3 µg L–1 between 1974 and 1989, whereas a further increase in 2001 could not be observed. The temporal trend since 2000 was investigated in more than 600 blood samples, obtained from six American Red Cross blood banks representing six metropolitan areas across the USA in 2000–01[30] and in 2006.[32] In addition the data of the two CDC NHANES studies were included to the trend considerations (see Fig. 1).[33,34]
|
However, this data compilation shows that since 2000 PFC concentrations have not continued increasing. From 2000–01 to 2006, the PFOS, PFOA and PFHxS concentrations decreased by 60, 25 and 30% respectively. The reasons for the decreasing trend of the PFC concentrations in human blood are probably the phase-out of POSF-based materials in 2000–01 and the PFC elimination half-lives in blood of a few years (i.e. 4.8 years for PFOS, 3.5 years for PFOA, 7.3 years for PFHxS[35]).
Temporal trend studies on PFC levels in blood of New York State infants 1–2 days after birth (Newborn Screening Program) showed maximum PFOS, PFOSA, PFOA and PFHxS concentrations between 1997 and 2001 followed by declining values until 2007 (Fig. 2) suggesting decreases in perinatal exposure.[36]
|
The observed declining concentrations after the year 2000 are coinciding with the phase-out in POSF production in the USA. Thus, the response in PFC exposure appeared directly after the beginning of production change-over for POSF and ammonium perfluorooctanoate (APFO). However, PFHxS decreased slower over the years than PFOS and PFOA, possibly due to its longer half-life and due to the continuing production of perfluorohexane sulfonyl fluoride (PHSF). No temporal trend was found for perfluorononanoate (PFNA), which could be due to the continued production of PFNA or its precursor compounds.
There are only a few other reports about trends of PFC concentrations in human blood from other countries besides USA. In Germany the PFC concentrations in blood of young students from several German cities decreased from 40 to 15 ng L–1 for PFOS (1985–2004) and from 13 to 7 ng L–1 for PFOA (1985–2004).[37] In another German study plasma samples of young adults from an area with PFOA-contaminated drinking water (Hochsauerland) were analysed. Samples were taken between 1974 and 2004. PFOA and PFOS levels were relatively constant with a small maximum between 1986 and 1990. In contrast, PFHxS levels steadily increased since 1977.[38]
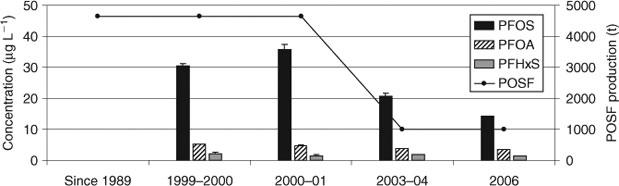
A comprehensive study was carried out in Norway, covering a time period of ∼30 years (1976–2007).[39] 57 pooled serum samples from patients of Norwegian hospitals (one pool per year) were analysed for 19 PFCs. Serum concentrations of PFOS, PFOA and PFHxS increased from 1977 to the mid 1990s, reached a plateau with maximum PFOS concentration of 31 µg L–1 and then started decreasing ca. 2001 (Fig. 3).
This declining trend in Norwegian blood (60% for PFOS, 40% for PFOA, 55% for PFHxS) was similar to that in American blood from six metropolitan areas in the USA (see above[32]) in the same time period (2000–06). Compared to estimated total global POSF production volumes,[1] the PFC decline in human blood started at nearly the same time as the phase-out in 2000.
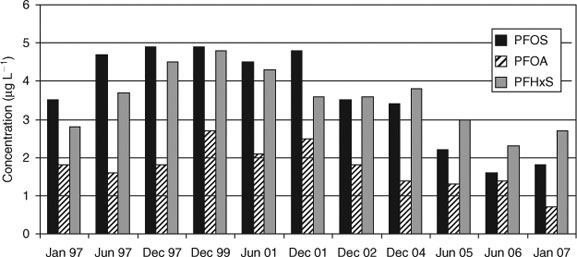
In China, a very high increase of the PFC concentrations in human blood was reported due to the rapid industrial progress in the past 30 years.[40] Serum samples collected from Chinese people in Shenyang in the years 1987, 1990, 1999 and 2002 showed extremely low PFC levels of <1 µg L–1 in 1987 and 1990 (Fig. 4). In the following years, the PFOS levels increased but still remained in the range of a few micrograms per litre. In the year 2004, Yeung et al. found very high values for PFOS in human blood from Shenyang (PFOS: 140 µg L–1, converted to a serum basis by multiplying by a factor of two).[41] Four years later, in 2008, the highest PFOS concentration in blood samples from several Chinese cities was 15.7 µg L–1 for Shenyang (converted to serum basis by multiplying by a factor of two).[42] A potential source for the high PFOS concentration in Shenyang could be a fluorochemical manufacturing plant, which is one of the biggest fluorochemical plants in China. The temporal trend of PFOS concentration in Chinese blood is shown in Fig. 4. Interestingly, the maximum PFOS concentration was not observed 2000–01 like in the USA or in Europe but later ca. 2004. In contrast to PFOS, the PFOA and PFHxS levels in human blood were in the same order of magnitude as those from USA or Europe. But it is noticeable that PFHxS continuously increased over the years in Shenyang.
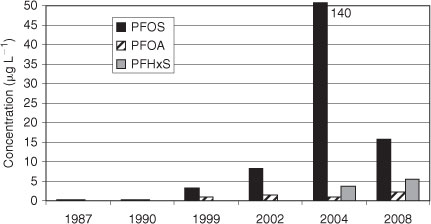
|
In Japan, significant geographical differences in serum concentrations of PFOS and PFOA were found.[43] Time trend studies using historical samples collected in Kyoto from 1983–99 demonstrated a 4.4-fold increase of the PFOA concentration in males coinciding with an increase in fluoropolymer production in Japan between 1983 and 1999. Uptake from drinking water might be one of the reasons for the high PFOA levels in serum from Kyoto. During this time period (1983–99), there was no obvious trend of increasing PFOS concentrations, but further measurements showed that PFOS in male serum had increased in 2003.[44]
In Australia two studies included PFCs in human blood. The first one was done in the years 2002–03 with pooled serum samples from 3802 Australian residents[45] and the other one in the years 2006–07 with pooled serum samples from 2420 donors.[46] Samples were taken from men and women of several different age groups from urban regions. A comparison of the two Australian studies showed no temporal trend between the years 2002–03 and 2006–07 (Fig. 5).
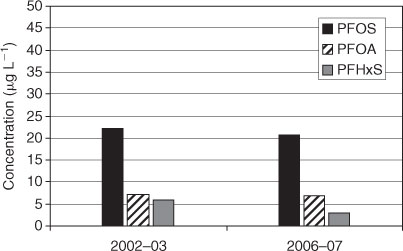
|
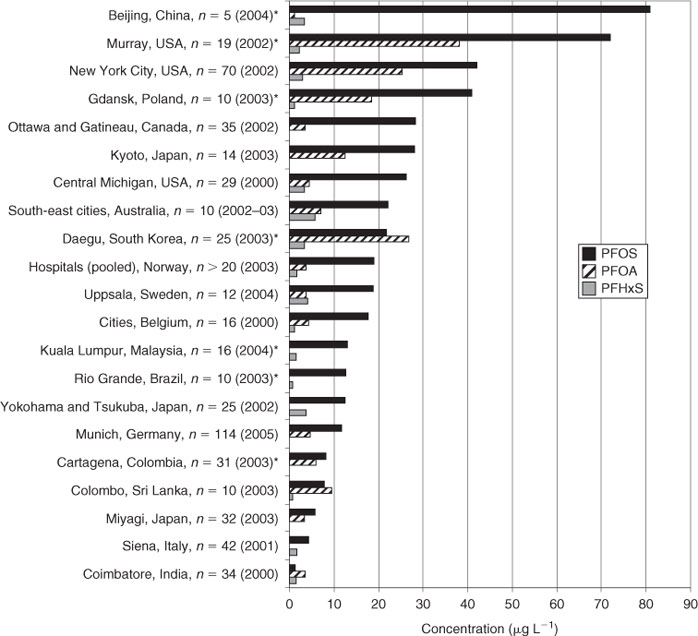
In general, PFOS and PFOA concentration levels were comparable to those from urban regions of Europe, USA, Canada or Japan, whereas the PFHxS levels in blood of Australians seem to be slightly higher than those from other countries (Fig. 6).
In general, there was considerable variation by country in the PFC concentrations from severals cities all over the world (Fig. 6). In most cities PFOS was the major compound, but in South Korea (Republic of Korea), Sri Lanka and India PFOA exceeded the level of PFOS, suggesting the possibility of the presence of specific sources of exposure to PFOA.
Influence of age and sex on PFCs in human blood
As PFCs cannot be degraded in humans and show blood elimination half-lives of several years it can be expected that PFC concentrations increase with age as observed for other POPs (e.g. PCBs).[47] But in contrast to this assumption, in general in the US studies with a high sample number (i.e. NHANES[33,34] and American Red Cross study[32]) a correlation between the PFC concentration in human blood and the age of the blood donors could not be observed except for PFHxS. PFHxS concentrations were higher for adolescents than for adults.[34,48] This could be explained by the more frequent contact of children with carpeted floors and upholstered furniture, known to trap dust. PFHxS concentrations in house dust samples were higher than for other PFCs.[49] Similar results of high PFHxS levels in children’s blood were reported from Australia, where the highest mean concentrations of PFHxS and also of PFOA and PFNA were found in children <15 years old.[46] PFC concentrations appeared to increase from birth and to be stable after 10 years of age with the exception of PFOS, which increased steadily until >60 years.[45,46]
In China, PFC concentrations (i.e. PFOS, PFHxS, PFOA, PFNA) were found to be higher in blood of older people (>40 years) than of younger ones (<30 years).[42] However, in another study on blood samples collected from various Chinese cities no age-related differences were observed.[41] A plausible explanation for this inconsistency is not yet known.
A clearer trend was found for the differences in PFC levels between sexes. In the majority of the studies higher PFC levels have been found in males than in females. For example, both, the American Red Cross and the NHANES databases, have consistently observed higher PFOS and PFOA concentrations in males than in females, by ∼10–20%.[32] Similarly, in a German pilot study the PFOS concentrations in male plasma were >30% higher than in female plasma, whereas the difference in PFOA concentrations was <10%.[50] Investigations on Chinese blood samples also confirmed these observations.[41] Two main explanations for this trend were assumed. First, the excretion through menstrual bleeding might be a potential route for elimination in females.[43] Second, lactation and pregnancy may result in reduction of adult female PFC concentration. Both opinions are supported by the observation that there were no apparent sex differences for children less than 12 years of age.[46] In contrast, the time trend study on pooled serum samples of Norwegian adults collected during the period 1976–2007 could not confirm significant differences in PFC concentrations for men and women.[39]
Temporal trends and spatial distribution of PFCs in human breast milk
Comparing the PFC concentrations in milk and in blood of the same donors the PFOS milk levels were ∼1% of the corresponding serum levels.[51–53] Mean concentrations in breast milk from different countries were summarised by several authors.[54–56] Highest PFOS concentrations (medians) were found in breast milk from Hungary with ∼330 ng L–1 (n = 13; 1996–97)[57] followed by Japan with 196 ng L–1 (n = 24; 1999),[54] Sweden with 166 ng L–1 (n = 12; 2004),[51] Germany with 119 ng L–1 (n = 57; 2006),[57] Malaysia with 111 ng L–1 (n = 13; 2003),[54] USA with 106 ng L–1 (n = 43; 2004),[52] Philippines with 104 ng L–1 (n = 24; 2002–04),[54] China with 100 ng L–1 (n = 19; 2004),[58] whereas the PFOS concentrations in breast milk from Indonesia (n = 20; 2001), Vietnam (n = 40; 2000–01), Cambodia (n = 24; 2002) and India (n = 39; 2002–05) were below 100 ng L–1.[54] It is noticeable that the high PFOS values in milk from Hungary and Japan were found in samples taken before 2000, whereas the lower values were related to samples taken after 2000.
Temporal trends of PFCs in human milk, collected from Swedish women between 1996 and 2004, could not be observed.[51] But a subsequent study, covering the period from 1996 up to 2008, resulted in decreasing trends for PFOS and PFOA and an increasing trend for PFHxS in milk,[59] possibly indicating an increased exposure to PFHxS containing products.
Tissue distribution of PFCs in humans
Studies on tissue distribution or total body burden of PFCs in humans are rare. Pooled tissue samples from Italian donors had highest PFOA concentrations in lung (3.8 ng g–1), followed by kidney, liver and blood, whereas highest PFOS concentrations were reported in liver (13.6 ng g–1), followed by lung, hypophysis, kidney, blood and further tissues.[60] As expected, the PFOS burden in human liver was higher than in human blood. For PFOS the mean liver to serum ratio was 1.3 : 1 calculated from PFC analyses of liver and serum samples of 23 male and female US donors,[20] whereas the liver to serum ratio was 3.5 : 1 for 12 Spanish human tissue samples.[61]
PFC pattern in human tissues
PFOS, PFOA, PFHxS and PFOSA were detected most frequently in serum samples of the US population. Highest serum concentrations were found for PFOS followed by PFOA and PFHxS. PFNA, PFOSA, N-methylperfluorooctane sulfonamidoacetate (N-MeFOSAA) and N-ethylperfluorooctane sulfonamidoacetate (N-EtFOSAA) were detected in more than 90% of the samples in concentrations <1 µg L–1. Perfluorobutane sulfonate (PFBS), perfluoroheptanoate (PFHpA), perfluorodecanoate (PFDA), perfluoroundecanoate (PFUnDA) and perfluorododecanoate (PFDoDA) were detected less frequently.[32–34] PFOA was the most abundant perfluoroalkyl carboxylate (PFCA), followed by perfluorononanoate (PFNA), PFUnDA[41,42,62,63] and perfluorotridecanoate (PFTriDA).[39] The shorter chain acids PFHxS, PFBS[27,32,39,63] and perfluorohexanoate (PFHxA)[63,64] were found in human blood, whereas perfluorobutanoate (PFBA), N-methyl perfluorooctane sulfonamide (N-MeFOSA) and N-ethyl perfluorooctane sulfonamide (N-EtFOSA) were not detected.[39] Human blood serum predominantly consisted of linear isomers of the PFCAs compared to branched isomers.[65] This was explained by the biological isomer discrimination, which leads to an enrichment of n-PFOA in biological matrices.[66] In contrast to PFOA, n-PFOS was not enriched. Studies on PFOS in blood of the general population of Sweden, UK and Australia showed that the proportion of the linear PFOS isomer was only ∼58–70%, whereas the proportion of the linear isomer in the PFOS standard product is higher (76–79%).[67] Recently, polyfluoroalkyl phosphoric acids (PAPs) were detected in human blood.[68] PAPs are fluorinated surfactants used in human food contact paper products. In 2004–05 their concentrations in US human serum were ∼4.5 µg L–1 in total.
Only a few studies exist about the PFC pattern in human breast milk. In human breast milk samples, collected in 2004 in USA and from 1999 to 2005 in Asian countries, PFOS was the predominant PFC followed by PFOA, PFHxS and PFNA, whereas PFBS was only detected in some single samples.[52,54] PFOS and PFOA were also the dominant PFCs in human milk samples from China, collected in 2004 (45–360 ng L–1 and 47–210 ng L–1 respectively), followed by PFHxS, PFNA and PFUnDA. Low amounts (<10 ng L–1) of PFBS, PFHxA, PFHpA, PFDA as well as 8 : 2 fluorotelomer carboxylate (8 : 2 FTCA) and 8 : 2 fluorotelomer unsaturated carboxylate (8 : 2 FTUCA) could also be detected.[58]
Summary of levels and trends in humans
Overall, PFCs were detected in humans in all parts of the world indicating the ubiquitous presence of PFCs in exposure sources (e.g. food, drinking water, air, dust). However, higher concentrations were found in humans living in industrialised countries than in developing countries. In addition, in most studies significantly higher PFC concentrations were found in males than in females. Temporal trend studies showed generally increasing PFOS concentrations until ∼2000–01 in the USA and Europe and ∼2004 in China. Afterwards in several countries the concentrations started to decrease.
This overview on PFCs in humans provides a basis for the evaluation of changes in the contamination of marine biota. In the following chapters the PFC concentrations and patterns in marine biota were compiled in order to find out similarities or differences in temporal and spatial trends between humans and marine biota.
Levels and trends in marine mammals
Temporal trends and spatial distribution of PFCs in marine mammals
Temporal trends of PFCs in marine mammals
The investigation of temporal and spatial trends of PFC contamination in humans was usually done on the basis of whole blood, serum or plasma. For the assessment of trends of PFCs in marine biota mostly protein-rich organs instead of blood were used.
Temporal trend studies of PFCs in marine mammals from different regions are shown in Fig. 7. Liver samples of ringed seals (Phoca hispida) were collected between 1982 and 2003 at the eastern and the western coast of Greenland.[69] At both locations PFOS was the major PFC compound followed by PFUnDA and PFNA. PFOS concentrations showed an increasing temporal trend at both sites with higher concentrations in seals from eastern Greenland, possibly caused by long range transport from continental sources.[70]
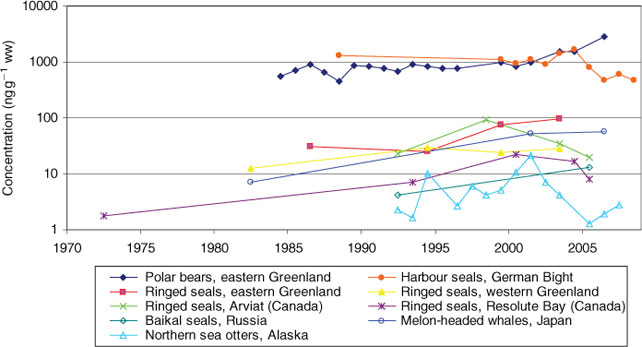
|
Ringed seal livers were also collected in the Canadian Arctic from Resolute Bay (1972–2005) and Arviat (1992–2005).[71] In agreement with the results from Greenland, at both Canadian locations PFOS was the major PFC compound followed by PFUnDA > PFNA ≥ PFTriDA. PFOA concentrations were similar to PFNA only in Resolute Bay. Similar to Greenland, the PFOS concentrations showed an increasing temporal trend until the end of the 1990s. But conversely to Greenland, maximum concentrations of PFOS and PFOSA were found during 1998 and 2000, followed by a significant decrease from 2000 to 2005.
A study on PFCs in livers of Baikal seals (Pusa sibirica) collected in Lake Baikal, Russia, showed higher concentrations of PFOS, PFDA and PFNA in 2005 than in 1992.[72] In contrast to the results for ringed seals from Greenland and Canada, the major PFC compound in Baikal seals livers was PFNA instead of PFOS.
In livers of melon-headed whales (Peponocephala electra) from Japan collected in 1982, 2001–02 and 2006, PFOSA concentrations were in the same order of magnitude as the PFOS concentrations. A decreasing concentration trend could only be observed for PFOSA between 2001–02 and 2006, but not for PFOS and the PFCAs, which increased over the whole time period.[73] Interestingly, decreasing concentrations were not observed for PFOS in blood of Japanese people either (see above).
Temporal trends of PFCs in wildlife were also reported in livers of northern sea otters (Enhydra lutris kenyoni) from south-central Alaska collected from 1992 to 2007, with PFOS, PFOSA and PFNA being the predominant PFCs.[74] PFOS and PFOSA concentrations increased from 1992 to 2001 and 1999 respectively and then decreased until 2007. In contrast, the PFNA concentration was constant from 2001 to 2003, and then increased from 2004 to 2007. A temporal trend study on southern sea otters (Enhydra lutris nereis) collected from the California coast during 1992–2002 confirmed an increase in PFOS concentration up to 1998 and a decrease after 2000.[75] Unusually high concentrations of PFOA in the sea otters suggest the existence of specific sources of this compound in the coastal area of California.
In a study about temporal trends of PFCs in livers of harbour seals (Phoca vitulina) from the German Bight during 1999–2008, the observation of an increasing trend in PFC concentrations up to the end of the 1990s was confirmed, followed by a maximum ca. 2000 and a subsequent significant decline until 2008.[76] This decreasing trend of PFC compounds was found for PFOS, the predominant PFC, as well as for C4–C7 perfluoroalkyl sulfonates (PFSAs), perfluorooctane sulfinate (PFOSi), PFOSA, and PFOA. For the longer chain PFCAs (C9–C13) no significant temporal trend could be observed. Overall, the concentrations in the liver samples of the harbour seals were high compared to other wildlife of similar trophic levels. In contrast to decreasing PFC concentrations in harbour seals from the German Bight, no significant temporal trend was observed in grey seals (Halichoerus grypus) from the Baltic Sea between 1999 and 2008. However, significantly increasing concentrations were found for C6–C8 PFSAs, PFOSi and C7–C14 PFCAs between 1974 and 1998 (J. Kratzer, L. Ahrens, A. Roos and R. Ebinghaus, unpubl. data).
Highest PFC values in wildlife were found in polar bears (Ursus maritimus), which are the top predators in the Arctic food chain (up to or exceeding 4000 ng g–1 ww).[77] Temporal trends for polar bear livers were reported from 1972 to 2002 from the North American Arctic,[78] and from 1984 to 2006 from central eastern Greenland.[79] Smithwick et al. found increasing PFOS and C9–C11 PFCAs concentrations between 1972 and 2002 at two locations (northern Baffin Island, Canada, and Barrow, Alaska), whereas the PFOSA concentration showed a decreasing tendency.[78] An increasing temporal trend was also observed for PFHxS in polar bear samples from Barrow and for PFOA in samples from Baffin Island. Dietz et al. found increasing concentrations of PFOS and C8–C13 PFCAs in livers of polar bears from central eastern Greenland from 1984 to 2006, whereas the temporal trend of PFOSA was not significant and PFHxS was not detected.[79]
As most of the polar bears’ diet consists of ringed and bearded seals it might be possible that similar trends exist for their prey. Indeed, similar increasing temporal trends were found between 1970s and 2000–02 in livers of ringed seals to those of polar bears from the same locations in Canada[71,78] and eastern Greenland.[69,79] But neither Smithwick et al. (data up to 2002 from the Canadian Arctic) nor Dietz et al. (data up to 2006 from eastern Greenland) could affirm declines of PFSAs or PFCAs in polar bears from 2000 to 2005 as reported for ringed seals from the Canadian Arctic.[71] However, the changes of the PFC concentrations in polar bears should be investigated in the future.
Spatial distribution of PFCs in marine mammals
An overview of the global distribution of PFCs in marine mammals is given in Table 1. For a comparative examination of spatial distribution trends ringed seals are an ideal species because of their widespread distribution and their relatively small home range.[80] Their PFC concentration is presumably representative of the regional marine food web contamination. Furthermore, ringed seals are an important prey source for polar bears that have been shown to be the highest contaminated marine mammals.[77,81,82] Butt et al. reported that mean concentrations were similar between ringed seal populations in the Canadian Arctic (11 locations, 10 liver samples per site). Concentrations of PFNA and PFUnDA generally ranged between 1 and 10 ng g–1 ww, whereas PFTriDA was less than 1 ng g–1 ww and PFOS concentrations were mainly between 10 and 40 ng g–1 ww.[80] Thus, PFOS concentrations of ringed seals from the Canadian Arctic were considerably lower than those reported in ringed seals from eastern Greenland (61–130 ng g–1 ww in 2003),[69] in harbour seals from the north-west Atlantic (US east coast) (8–1388 ng g–1 ww in 2000–07)[83] or the southern North Sea (45–488 ng g–1 ww in 2002[17]; 559–1665 ng g–1 ww in 2007),[18] or in grey seals from the Baltic Sea (156–1072 ng g–1 ww in 2006; J. Kratzer, L. Ahrens, A. Roos and R. Ebinghaus, unpubl. data). Remarkably higher concentrations of PFUnDA relative to PFNA were reported in livers of cetaceans like whales, dolphins and porpoises from Hong Kong and Japan[73,84] suggesting distinctive sources in the mid latitudes of eastern Asian countries and degradation of 10 : 2 fluorotelomer alcohol (10 : 2 FTOH).[73] In general, mammals feeding at higher trophic levels had higher concentrations of PFCs than mammals feeding at lower trophic levels, which indicate their high biomagnification potential.[77]
Tissue distribution of PFCs in marine mammals
The analysis of different tissues of marine mammals allows the investigation of tissue distribution of PFCs and the estimation of total body burdens. PFOS, PFBS, PFBA and some longer chain PFCAs were quantified in liver, kidney, spleen, blubber and muscle tissues of harbour seals (Phoca vitulina) from the Dutch Wadden Sea. Highest PFOS concentrations were found in liver and kidney.[17] In tissues of harbour porpoises (Phocoena phocoena relicta) from the Black Sea PFOS concentrations decreased from liver > kidney > muscle > brain > blubber.[85] Another study reported tissue distribution of PFCs in ringed seals (Phoca hispida) from Resolute Bay, Canadian Arctic, with highest PFOS concentrations in liver followed by lung, heart, spleen, blood, muscle and blubber.[86] An investigation of the total body burden and tissue distribution of 18 individual PFCs in harbour seals from the German Bight in 2007 resulted in decreasing PFOS concentrations in the following order: liver > lung > blood > thymus > kidney > heart > brain > muscle > thyroid > blubber.[18] Regarding the total body burden distribution (in percent of tissue mass) the highest ∑PFC amounts were found in blood.[18,86] As PFCs accumulate more in the liver than in other organs, liver tissue was chosen for most trend studies on PFCs.
PFC pattern in marine mammals
PFOA is not a predominant PFCA in marine mammal livers which is in contrast to human blood samples (see above). Longer chain PFCAs show relatively high amounts in polar bears with PFNA as the most abundant compound at concentrations often above 100 ng g–1 ww followed by PFUnDA and PFTriDA. In seals the concentrations of PFNA and PFUnDA were <100 ng g–1 ww. Levels of both compounds were in the same order of magnitude (Table 1).
In general, concentrations of odd-number chain length PFCAs in marine mammals were higher than those of even-number chain.[69,71,77,78,87,88] This specific odd–even PFCA pattern supports the hypothesis of atmospheric fluorotelomer alcohol (FTOH) degradation as a source of PFCAs. For example, 8 : 2 FTOH degrades to equal amounts of PFOA and PFNA, but PFNA has been shown to be more bioaccumulative than PFOA, resulting in higher PFNA levels in organs of biota compared to PFOA.[89] In the same way 10 : 2 FTOH possibly degrades to equal amounts of PFDA and PFUnDA, but levels of PFUnDA were higher in biota because of its higher bioaccumulation potential.[83]
A variety of branched isomers of longer chain PFCAs were found in liver samples of polar bears from Greenland.[90] The presence of branched isomers suggests contribution from electrochemical fluorination (ECF) that has been one of the two main production processes for over 50 years. ECF results in a mixture of even and odd numbered structural isomers (linear with up to 30% branched isomers), whereas production by telomerisation yields in even numbered, purely linear isomers.[2,10] However, in seals the branched/linear isomer distribution of PFOS (4% branched) was different from the expected isomer distribution that is found in technical grade PFOS (26% branched). This was explained by higher elimination rates of branched isomers than of linear isomers[10] as Loveless et al. have shown for APFO in rats and mice.[91]
The shorter chain PFCAs (C4, C6) were usually not detected in marine mammals, whereas the shorter chain PFSAs (C4, C6) were found in some animals in a few nanograms per gram range showing decreasing temporal trends in harbour seal livers from the German Bight.[76] In harbour seals from the Dutch Wadden Sea, PFBS was found only in spleen, but not in liver.[17] Polar bears showed the highest amounts of PFHxS among mammals, but PFBS was not analysed (Table 1).
Fluorotelomer saturated and unsaturated carboxylates (FTCAs and FTUCAs) were found in very low concentrations in several marine mammal species such as ringed seals (<4.6 ng g–1 ww)[71] and dolphins from the US Atlantic Ocean coast (<1.4 ng g–1 ww),[92] but they were not detected in polar bears from the Canadian Arctic[78] and in dolphins and porpoises from South China and India.[84,88] The presence of those precursor compounds supports the hypothesis that atmospheric FTOHs are a source of PFCAs as FTOH can degrade via atmospheric oxidation to FTCAs and FTUCAs. But FTCAs and FTUCAs are also known degradation products of FTOHs in microbial processes and in rat metabolism and can be detected in sewage sludge.[71] Thus, it is not clear whether direct exposure of FTCAs and FTUCAs or uptake and subsequent metabolism of FTOH is the reason for these compounds in marine mammals.
PFOSA is known to be an intermediate in the production process of several PFCs. PFOSA was also reported in mammals as a metabolic product of N-EtFOSA, used as an insecticide, named Sulfluramid, for agricultural control of leaf-cutting ants in Brazil as well as for the domestic control of cockroaches and termites.[93–95] In mammals, PFOSA can rapidly be metabolised to PFOS.[93] Remarkably high PFOSA concentrations (in the same order as PFOS) were reported in liver of whales from the Canadian Arctic, from Iceland and the Faroe Islands and from Japan as well as in dolphins from Brazil (Table 1). It was reported that PFOSA concentrations in cetaceans were often higher than those of PFOS.[69,94–96] However, the reason for this pattern just in whales is unknown. The PFOS precursor N-EtFOSA itself was found in dolphins and porpoises from South China,[84] in whales from the Eastern Arctic[96] and in harbour seals from the German Bight.[18]
Influence of age and sex on PFCs in marine mammals
In general, in marine mammals no sex related differences in PFC concentrations were found (in seals,[71,72,80,83,97] in harbour porpoises,[85] in beluga whales,[98] in polar bears[87,99,100]). In contrast, significant differences between males and females were reported in grey seals from the Baltic Sea, males having higher concentrations of PFOS than females.[94]
No significant age trends were identified in marine mammals,[87,94,101] in ringed seals[71,80,102] and in harbour porpoises.[85] Contrarily, Smithwick et al. found increasing PFOS and C9–C14 PFCA concentrations in male polar bears up to an age of six.[99] Higher concentrations of PFOS in pups than in adults were also found in sea otters from Alaska,[74] Baikal seals from Russia,[72] bottlenose dolphins from the Atlantic Ocean,[92] harbour seals from the north-west Atlantic Ocean[83] and the German Bight,[76] harbour porpoises from the southern North Sea[97] and elephant seals from the Antarctic.[103] This was explained by higher rates of elimination in adults,[73] different consumption of different contaminated food,[76] and PFC transfer from mothers to their offspring through milk or placenta after parturition.[75,104]
Hence, a comparison of PFC concentrations is only meaningful within the same age group for males or females.[97]
Summary of levels and trends in marine mammals
Overall, high PFCs concentrations were detected in marine mammals of higher trophic levels, even when living in remote regions like the Arctic. In most of the studies no sex related differences in PFC concentrations were reported, but age related differences were observed. PFOS was the predominant compound in marine mammals liver. Among the PFCAs, PFNA and PFUnDA were the most abundant compounds, whereas the PFOA concentration was negligible. In marine mammals PFOS concentrations increased over time depending on the species and the sampling locations. Several species like seals from the German Bight, the Baltic Sea and from the Canadian Arctic showed maximum levels for single PFCs ca. 2000 followed by declines, for other mammals like polar bears concentrations have continued to increase.
Levels and trends in seabirds
Temporal trends and spatial distribution of PFCs in seabirds
Temporal trends of PFCs in liver of seabirds
There is only a limited number of temporal trend studies on PFCs in seabirds. Over a period of ∼20 years (1979–2000) liver samples of white-tailed sea eagles (Haliaeetus albicilla) were collected from Eastern Germany and Poland and analysed for PFOS, PFHxS, PFOA and PFOSA by Kannan et al.[94] Although the PFOS concentration had a high variation within the sampling year, increasing concentrations were indicated.
In another study liver samples of northern fulmars (Fulmaris glacialis) and thick-billed murres (Uria lomvia) from Canada, collected over a period of ∼30 years (1975, 1987, 1993, 2003–04), were analysed for long chain PFCAs including FTCAs and PFOS by Butt et al.[105] The PFOS contamination of the birds from the Canadian Arctic was low with levels usually <1 ng g–1 ww. In contrast to other wildlife animals, in which PFOS typically dominated the PFC pattern, in these seabirds PFCAs dominated with PFTriDA being the predominant compound (3.8–7.1 ng g–1 ww in 2003–04). Similar results were obtained in glaucous gull livers (Larus hyperboreus) from Norway with PFTriDA as the dominant PFCA as well.[106] This is in contrast to other wildlife animals in which the predominant PFCA typically was PFNA or PFUnDA but not PFTriDA[6,105] (see also below). Concerning temporal variations Butt et al. found increasing PFC concentrations in the Canadian birds between 1975 and 2004.[105] The PFCA concentrations increased between 1975 and 2004 in thick-billed murres and between 1975 and 1993 in northern fulmars, however, the PFCA concentration in northern fulmars remained constant between 1993 and 2003. The PFOS concentration was relatively constant between 1993 and 2003–04 in both bird species which is consistent with temporal trends in ringed seals (Fig. 8).[71]
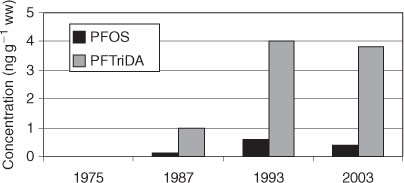
|
Temporal trends of PFCs in eggs of seabirds
Because of their relatively high PFC concentrations some scientists use birds’ eggs instead of liver for studying time trends. Moreover, seabird eggs are part of the traditional human diet in northern Norway and therefore a potential source of contaminant exposure. Verreault et al. reported on PFC pattern and temporal trends in eggs of herring gulls (Larus argentatus) from two colonies in northern Norway over a period of 20 years (1983, 1993, 2003).[107] Within the group of PFSAs, highest concentrations were found for PFOS with maximum values of up to 42 ng g–1 ww, whereas PFHxS and PFDS were found at much lower concentrations (<1 ng g–1 ww). PFBS was not detected, PFOSA only in low concentrations (<0.5 ng g–1 ww). Within the group of PFCAs, the PFUnDA and PFTriDA were the most dominant compounds with concentrations up to 4.2 and 2.8 ng g–1 ww respectively. Similar results were obtained for herring gull eggs collected in 2007 from the Laurentian Great Lakes of North America with PFUnDA and PFTriDA values both between 10 and 29 ng g–1 ww.[108] Regarding the temporal variation, a significant increase of PFOS, PFHxS and PFDS concentrations in Norwegian herring gull eggs was observed between 1983 and 1993.[107] From 1993 to 2003, the PFOS levels were levelling off, whereas PFDS continued increasing. For PFOS this observation agreed with the results of Haug et al., who reported that the PFOS concentrations in human blood of the Norwegian inhabitants reached a plateau in the mid 1990s (see above and Fig. 3).[39]
In 2005, a temporal trend study of PFOS in guillemot eggs (Uria aalge) from the Baltic Sea collected between 1968 and 2003 was published by Holmström et al.[109] PFOS increased between 1968 and 1997 followed by a decline (Fig. 9).
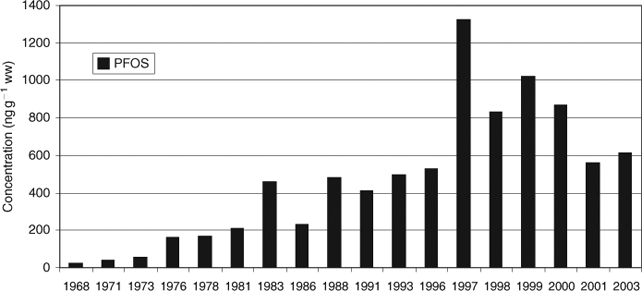
|
PFOA was not detected in any of the samples (<3 ng g–1 ww) and other PFCAs were not analysed. However, a peak for PFOS in 1997 as shown for Swedish guillemot eggs in Fig. 9 could not be confirmed by other authors,[105,107] possibly caused by a lack of samples during this period of time. The peak ca. 1997 followed by a decline in PFOS concentration occurred too early to be related to the phase-out which started in the year 2000. In comparison to the concentration levels in bird eggs from Norway (20–42 ng g–1 ww)[107] or bird livers from Canada (∼1 ng g–1 ww or lower),[105] the PFOS concentrations in bird eggs from Sweden were higher (several hundred nanograms per gram (wet weight)) but in the same order as the PFOS concentrations of birds from USA,[25] Japan and South Korea.[110] One of the reasons for the high PFOS contamination in guillemot eggs could be the relative high PFC contamination of the Baltic Sea region due to the high anthropogenic influence and the slow rate of water exchange with the North Sea.[70]
Spatial distribution of PFCs in seabirds
Regarding the spatial distribution of PFCs, in general the PFOS concentrations in livers of white-tailed sea eagles (Haliaeetus albicilla) from Europe (<3.9–127 ng g–1 ww) were severalfold less than those found in bald eagles (H. leucocephalus) from the United States (24–467 ng g–1 ww),[25] whereas PFHxS, PFOA and PFOSA were not detected. In contrast, PFOS concentrations in cormorant (Phalacrocorax carbo) livers from Southern Italy were in the same order of magnitude (32–150 ng g–1 ww) as those from North American Great Lakes.[94] Also contrary to sea eagles, the mean PFOA concentration in cormorant livers was, on average, 1.7-fold higher (95 ng g–1 ww) than the mean PFOS concentration (61 ng g–1 ww).
Overall, PFOS concentrations in seabirds were lower than those in other marine animals of similar trophic levels.[105] For example, PFOS concentrations in seabirds were ∼10-fold lower than in ringed seals and 1000-fold lower than in polar bears. On the other hand, ΣPFCAs in seabirds were in the same range as in ringed seals and 100-fold higher than in polar bears. Therefore Butt et al. concluded that seabirds have a higher biomagnification potential for PFCAs than mammals.[105]
The spatial distribution of PFC in birds is shown on global scale in Tables 2 and 3. In general, in remote areas like the Antarctica PFOS levels in seabirds were in the range of only a few nanograms per gram (wet weight), not only in the 1990s but even up to 2005, whereas the levels were up to several hundreds of nanograms per gram in more populated regions like the Swedish coast of the Baltic Sea, the Great Lakes of North America or the coastal areas of China, Japan and South Korea. Individual PFCs (e.g. PFOS, PFCAs and PFOSA) have different spatial distribution possibly due to long-range atmospheric transport and ocean current transport. Thus, according to Löfstrand et al. PFCs should not be treated as one group of chemicals.[111] Additionally, the assessment of the global distribution is difficult because PFC concentrations in birds depend on the diet (significant higher PFOS concentrations were found in the livers of piscivorous birds than in non-piscivorous birds)[112] and therefore on their trophic levels, their specific bioaccumulation and elimination rates and on their migration pattern. Thus, the comparison of different species is problematic, as a consequence further investigations are necessary.

|

|
Tissue distribution of PFCs in seabirds
The tissue distribution of PFCs was investigated in common guillemot (Uria aalge) from the Baltic Sea, which showed highest PFOS concentrations in eggs, followed by chick liver, kidney, adult liver and muscle.[113] Similar results were obtained for glaucous gulls (Larus hyperboreus) with highest PFOS concentration in plasma, followed by egg and liver, and brain.[106] Considerable amounts of PFOS were reported in spleen and in feathers (31–247 ng g–1 dw) compared to birds’ livers (12–476 ng g–1 ww).[114] It was concluded that the deposition of PFOS in the feathers might be an important elimination route for PFOS in birds. In another study the authors reported higher PFOS levels in the spleen (6.2–131.5 ng g–1 ww) than in the liver (4.0–55.7 ng g–1 ww) of brown pelicans (Pelecanus occidentalis) followed by moderate concentrations in lung, kidney and brain (1.2–17.3 ng g–1 ww) and lowest concentrations in heart and muscle (<7 ng g–1 ww).[115] As high concentrations of PFOS were found in bile of pelicans (17–100 ng mL–1) it was assumed that biliary excretion might be a major pathway of elimination of PFOS from biota tissues. In the future more research into the tissue distribution of PFCs and the total body burden in birds is needed.
PFC pattern in seabirds
Regarding the PFC pattern in seabirds, PFUnDA and PFTriDA were the most abundant compounds within the PFCAs. The dominance of odd-numbered PFCAs over the adjacent even numbered chain lengths had also been observed in other species (e.g. mammals, see above). Comparatively high amounts of even carbon-chain PFCAs (PFDA and PFDoDA) were found in birds from Hong Kong, where different exposure sources were assumed.[116]
Shorter chain PFCAs and PFSAs, like PFBS and PFBA, were mostly not analysed or not detected in seabirds. PFHxA was found in birds at low concentrations (several nanograms per gram (wet weight)). Higher concentrations of PFHxS were reported in birds from several locations in Europe (Tables 2, 3). Those birds were also high contaminated with PFOS and in one case with PFOA, indicating several sources of exposure.[94,114]
FTCAs were not found in seabirds.[106] But the corresponding unsaturated acids, 8 : 2 and 10 : 2 FTUCAs, were detected in some individuals at very low concentrations.[105,108] Those compounds can be generated from atmospheric oxidation or biodegradation of FTOHs.[89,105]
PFOSA occurred at different levels depending on the species and locations. In general, PFOSA concentrations in seabirds were low. High amounts were found in liver of Japanese cormorants (up to 362 ng g–1 ww) probably indicating a source of exposure.[117] Further PFOS metabolites like N-MeFOSA or N-EtFOSA were rarely analysed. N-EtFOSA was only detected in guillemot eggs from North-Western Europe at low concentrations (<10 ng g–1 ww).[111]
Influence of age and sex on PFCs in seabirds
Age and sex specific differences in PFC concentrations in seabirds have not been observed in most of the studies.[25,94,110,113] Analyses of blood of breeding lesser black-backed gulls (Larus fuscus) from northern Norway resulted in lower concentrations of PFCs in females than in males,[118] which is probably caused by excretion of PFCs through laying eggs as discussed earlier.[112,119,120] Additionally, high concentrations of long chain PFCAs in eggs support the assumption of an oviparous PFC transfer even though the extent might vary between species and different PFC compounds.[103,118,121] Lower PFC levels in females were also reported for C14- in fulmars and C15-PFCAs in murres[105] and for PFOS in mergansers.[112]
Summary of levels and trends in seabirds
Overall, PFOS concentrations in seabirds were lower than those in other marine animals of high trophic levels, whereas PFCA concentrations were of the same size or higher than in marine mammals. PFUnDA and PFTriDA were the most abundant PFCAs. A higher biomagnification potential for PFCAs in seabirds than in mammals was assumed.
Regarding the total body burden of PFCs, PFOS concentration in birds was higher in eggs than in liver. In remote regions like the Antarctica PFOS contamination of seabirds was low compared to more populated coastal areas of Europe, USA or Asia. Temporal trend studies of seabirds were rare. However, in one study PFOS levels increased between 1983 and 1993, then reached a plateau (1993–2003), in another study a maximum concentration was found in 1997 and in two studies increasing trends were obvious up to 2004.
Levels and trends in fish
Temporal trends and spatial distribution of PFCs in fish
For temporal and spatial trend studies in fish the whole fish tissue was mostly used and therefore no trend studies of PFCs in fish liver or blood are available. Thus, the comparison of fish with humans, marine mammals and seabirds is difficult. There were two trend publications about whole body PFOS concentration of archived lake trout samples (Salvelinus namaycush) from Lake Ontario.[122,123] According to Martin et al., the PFOS burden increased from 43 to 180 ng g–1 ww between 1980 and 2001. But the increase was not linear, decreasing concentrations in the mid-1990s were observed, followed by another increase until 2001.[123] The authors supposed this might be caused by an indirect influence of the invasion of zebra mussels being a new, not contaminated diet for the trouts. Concerning PFOS their results were confirmed by Furdui et al., who analysed fish samples between 1979 and 2004.[122] Initially PFOS increased and then declined between 1993 and 1998, followed by another increase until 2004. The authors additionally analysed longer chain PFCAs and PFOSA. These compounds showed similar temporal variations to that observed for PFOS. PFCA concentrations were generally low (up to 3 ng g–1 ww) with PFUnDA, PFDoDA and PFTriDA as the dominant compounds within this group. Maximum PFC concentrations were found between 1988 and 1993 followed by a minimum in 1998 which could not be explained satisfactorily.
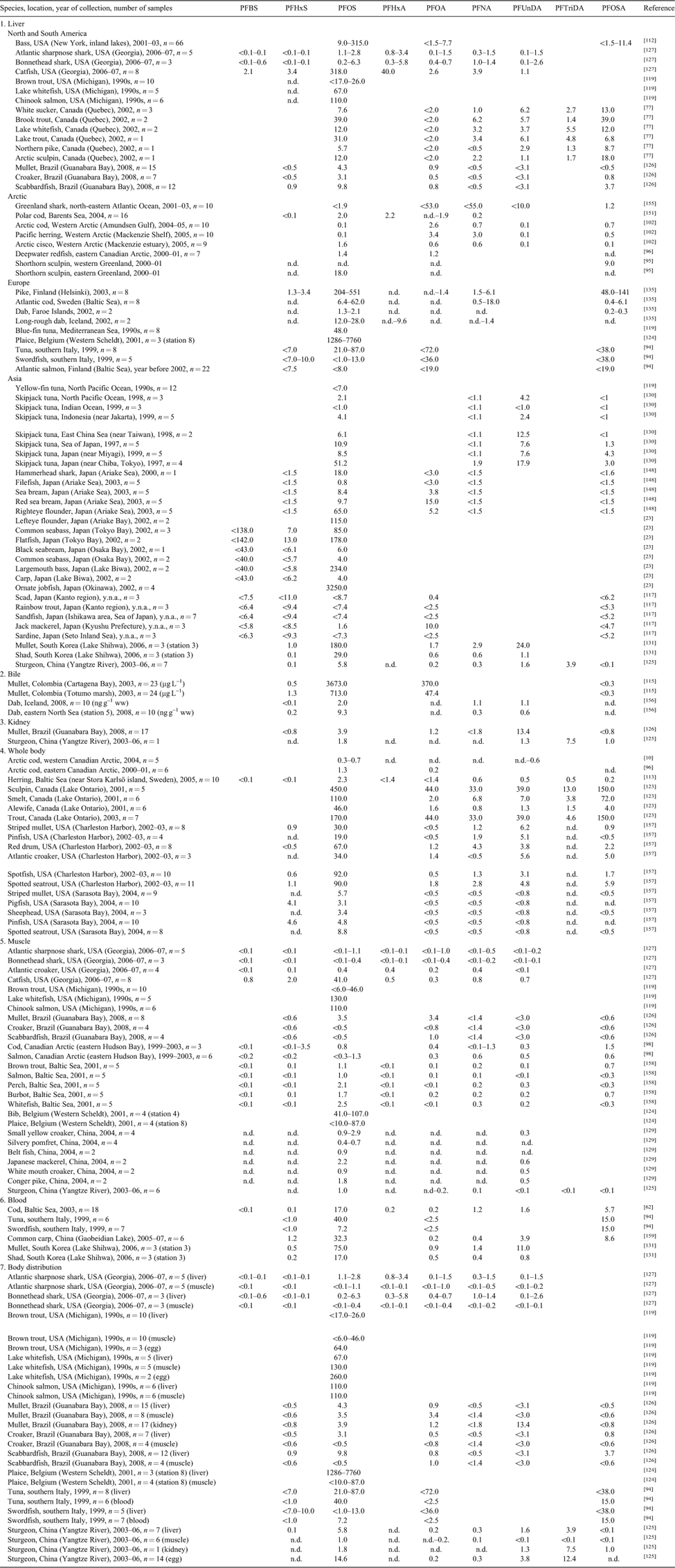
Geographic variations of PFCs and PFC profiles of different fish species are given in Table 4. Regarding fish liver, highest PFOS concentrations were found in plaice (Pleuronectes platessa) of the Western Scheldt, Belgium (up to 7760 ng g–1 ww),[124] which might originate from paper mill factories using PFOS for surface treatment. High PFOS contamination was also obvious in fish livers from other industrialised regions like Canada and USA (up to 318 ng g–1 ww for Georgia), Japan (up to 3250 ng g–1 ww) and Europe (up to 551 ng g–1 ww for Finland), whereas lowest PFOS concentrations were detected in fish livers from remote regions like the Arctic, the North Atlantic and North Pacific Oceans (up to 18 ng g–1 ww for eastern Greenland).
|
Tissue distribution of PFCs in fish
Investigations on tissue distribution of PFCs in fish from Brazil, Georgia (USA) and China indicate higher amounts of PFOS in liver than in muscle (Table 4).[125–127] PFOS contamination of fish from Michigan waters showed varying results when comparing liver and muscle, but highest values for fish eggs (260 ng g–1 ww).[119] High levels of PFOS and PFCAs were also reported in fish eggs from Chinese sturgeon (Acipenser sinensis).[125] PFOSA, PFOSAA and N-EtFOSAA were only detected in absorptive organs.[125] PFC concentrations in blood of bluefin tuna (Thunnus thynnus) and swordfish (Xiphias gladius) from Italy were in a similar order of magnitude as in the corresponding livers.[94] When rainbow trout (Oncorhynchus mykiss) were exposed to PFCs for 12 days to determine the tissue distribution, PFCs accumulated to the highest extent in blood > kidney > liver > gall bladder and to the lowest extent in gonads > adipose > muscle.[22]
PFC pattern in fish
Within the PFCAs, in fish the longer chain compounds like PFNA, PFUnDA and PFTriDA were most abundant. In general, the concentrations of the three compounds were in a similar order of magnitude (a few nanograms per litre) and often found in higher concentrations than PFOA (Table 4). PFUnDA was the most dominant PFCA which is probably caused by the high bioaccumulation and biomagnification potential.[123,128–131] The high concentrations of PFUnDA in Asian skipjack tuna livers (Katsuwonus pelamis) indicate distinctive sources of PFCs arising from East Asian countries probably originating from the degradation of 10 : 2 FTOH.[130] In contrast, in Chinese sturgeon (Acipenser sinensis) PFTriDA was the most abundant PFCA in all tissues analysed except for muscle.[125]
Even in remote mountain lakes (n = 6) of the Qinghai–Tibetan Plateau of China (average altitude exceed 4000 m)[132] as well as in remote mountain lakes (n = 4) of the French Alpes (altitude 1600–2000 m)[133] PFOS and PFCAs could be detected in fish samples. In both locations PFOS and PFCAs concentrations in fish were in the range of a few ng per g ww. Also at both locations, PFUnDA was the most abundant PFCA in fish. As there were no known point sources such as factories, wastewater effluents or polluted rivers near those remote lakes, volatile PFOS and PFCA precursors must have been deposited into the lake by the atmosphere. This hypothesis has been supported by several studies assuming long-range atmospheric transport of PFOS and PFOA precursor substances.[89,134]
Branched C11 and C13 PFCA isomers were detected in lake trout from Lake Ontario probably caused by PFC production processes.[122] High amounts of branched chain PFOS isomers (up to 75 ng g–1 ww) were found in fish from Georgia waters which was probably due to the metabolism of precursor compounds.[127]
PFOSA occurred at different levels in certain species and locations (Table 4). It was found in fish from the Baltic Sea and in American fish liver samples in a similar range as PFOS and in lower concentrations in skipjack tuna from Japan.[130] In general, PFOSA concentrations in marine fish were low, whereas high amounts in fish of Lake Ontario (up to 150 ng g–1 ww; whole fish)[123] or fish from the coast near Helsinki (up to 141 ng g–1 ww; liver)[135] probably indicate different sources of exposure.[77] PFOSAA was found in fish from China.[125] Additionally N-EtFOSAA, another precursor of PFOS, was found in fish from a lake in South Korea.[131] Those FOSAAs are known to be oxidation products of perfluorochemical mixtures in paper-protectants and surface treatment applications[31] and often found in the effluents of wastewater treatment plants and in rivers.[131] Further PFOS metabolites, like N-MeFOSA or N-EtFOSA, were rarely analysed. In general, low concentrations of shorter chain PFCAs and PFSAs (e.g. PFBA, PFHxA, PFBS, PFHxS) were reported except for some fishes e.g. from Georgia that were strongly influenced by industrial and commercial discharges in Georgia waters.[127] These findings were explained by the low bioaccumulation potential of the C4-and C6-compounds (see http://www2.dupont.com/Capstone/en_US/assets/downloads/final_final_capstone_gen_summary_09_05_2008.pdf, accessed 10 December 2010).
Influence of age and sex on PFCs in fish
Age and sex specific differences in PFC contamination of fish have rarely been investigated. In livers of skipjack tuna (Katsuwonus pelamis) from the Sea of Japan no significant difference existed in PFOS concentrations between the sexes,[130] whereas in livers of bass (Micropterus salmoides, Micropterus dolomieu) from remote New York State inland lakes, PFOS values were lower in females than in males.[112] Because of the high PFOS concentrations in fish eggs,[119,120] oviparous elimination in female fish was assumed. Increasing concentrations of longer chain PFCAs with fish age were found for Chinese sturgeon (Acipenser sinensis) probably due to a specific accumulation of the longer chain compounds.[125] Thus, there are many open questions concerning the spatial distribution and the temporal trends of PFCs in fish and further investigations are needed.
Summary of levels and trends in fish
Overall, temporal trend studies of PFCs in marine fish were rare. However, increasing trends of PFOS concentrations in whole fish tissues were obvious up to 2004. Fish was contaminated with PFCs globally, and PFOS was detected even in fish of remote mountain lakes.
In marine fish, PFNA, PFUnDA and PFTriDA were the most abundant PFCAs with mostly higher amounts of PFUnDA.
Comparison to temporal trends in sediments
Accumulation of PFOS in animals of higher trophic levels is controlled by a dynamic equilibrium between uptake and elimination or by protein turnover, if PFOS covalently binds to proteins in liver and blood. Therefore, abiotic matrices such as dated sediments might be better indicators for temporal trend analysis of PFOS.[94]
Recently two temporal reconstructions of PFC pollution from dated sediment core samples collected in Tokyo Bay with sediment depositing during the 1950s up to 2009 were reported.[136,137] Most of the PFCs increased especially after the early 1970s. Over the years the concentration of PFOS gradually decreased from the early 1990s, the PFOS precursors FOSAAs decreased rapidly in the late 1990s, whereas PFOA increased rapidly, due to the shift from POSF based products to telomer based products after the phase-out time. Reconstruction of PFC pollution with shorter chain PFCs (<C8) was not possible because of their low sorption to the sediment. Longer chain PFCAs (C9–C13) showed a similar trend as PFOA. The increasing time trend of PFOA in sediment of Tokyo Bay was in agreement with the results of trend studies on PFOA levels in human blood serum from Kyoto from 1983 up to 2004, although considerable regional differences in Japanese human serum concentrations were reported.[44,138,139] In contrast, the decrease of PFOS concentration in sediment from Tokyo from the early 1990s could not be confirmed in human blood from Kyoto.
Among the PFCAs, PFTriDA was the most dominant compound in sediment from Tokyo Bay. This result is very interesting because PFTriDA has been shown to accumulate in birds to a high extent and also in fish and mammals as mentioned above. As toxicity of PFCAs increases with increasing chain length,[140] future monitoring should be aware of the importance of those compounds.
Summary and conclusions
PFCs in humans
Most trend studies on PFCs focussed on human blood. In USA and Europe PFOS levels increased from the 1970s to the mid 1990s, reached a plateau, and then started decreasing ca. 2000–01. In China the decrease began ca. 2004, in Japan no subsequent decline could be observed, and for Australia not enough data exist to evaluate the temporal trend. The observed declines after the year 2000 are coinciding with the phase-out in POSF production in the USA. Thus, the response in PFC exposure appeared contemporary after the beginning of production change-over for POSF and APFO. Besides the phase-out, lower emissions due to optimisation of production processes might be a reason for the declining temporal trend, too. However, the temporal decrease for PFHxS is slower than that for PFOS and PFOA, possibly due to its longer half-life or due to continuing production of PHSF.
Thus, the initially mentioned hypothesis that the phase-out of POSF based products ca. 2001 should be reflected by a decrease in PFC concentrations in the environment can be confirmed for humans from industrialised regions in the case of PFOS and PFOA and to some extent also in the case of PFHxS. Shorter chain (e.g. PFBS, PFBA, PFHxA) and longer chain compounds (e.g. PFNA, PFUnDA, PFTriDA) as well as PFOS precursors (e.g. PFOSA, PFOSAA) were often detected in concentrations below 1 µg L–1 or below the limit of quantification making a comparison of trends difficult.
In general, no correlations between PFC concentrations in human blood and age of the blood donors could be observed with one exception, for which PFHxS levels were reported to be higher in children than in adults. This could be related to an increased contact of children with carpeted floors containing PFHxS. Obvious higher PFC contamination were found in males than in females possibly due to elimination by lactation, pregnancy and menstrual bleeding of women.
PFCs in marine biota
The comparability of PFC trends in marine animals is limited, due to the low number of samples in most studies. Second, seasonal variations of the PFC contamination in the animals due to different diet and third, differences in age and sex of the animals were not considered in most studies. Fourth, the PFC contamination often varied between different parts of the body depending on the time period of exposure. As PFC bind to proteins in liver and blood, accumulation in biota of higher trophic levels is controlled by a dynamic equilibrium between uptake and elimination.[94] Thus, exposure experiments over a period of only a few days often lead to higher PFC amounts in blood than in the accumulating organs resulting in the assumption that blood is higher contaminated than liver or other organs. Fifth, marine organisms are often also contaminated with PFOS precursor compounds (e.g. PFOSA, N-MeFOSA, N-EtFOSA)[18,96] that can metabolise to PFOS,[123] and with branched chain PFOS isomers which were not quantified.[127] Thus, incorrect conclusions concerning PFOS burden could be done. Finally, the PFC concentrations increase significantly with increasing trophic levels[98] and species-specific differences were observed.[103] Therefore only animals of the same species can be compared directly although this precondition is difficult to fulfil because of limited numbers of samples and limited studies for one species. Therefore, abiotic matrices like sediments or lower trophic organisms might be better indicators for temporal trends of PFCs than animals of higher trophic levels.[94] However, temporal trend studies in different marine mammal species showed similar trends.
PFOS concentrations of ringed seal livers collected from the Canadian Arctic initially showed an increasing temporal trend until the end of the 1990s, followed by a decline from ∼2000–05. A similar concentration gradient was found for sea otters from Alaska and from California and for harbour seals from the German Bight. In contrast, continued increasing PFOS trends without subsequent declines were observed in ringed seals from Greenland (up to 2003), in polar bears from Greenland (up to 2006) and in melon-headed whales from Japan (up to 2006). One explanation for the differences might be the remoteness of Greenland Sea and Arctic Ocean from industrialised regions. Thus, in these regions the PFC declines possibly might occur a few years later than in industrialised regions caused by the very slow transportation of PFCs via ocean currents that were assumed to be one of the possible sources for PFCs in Arctic regions.[141] The missing decline for PFOS concentrations in Japanese melon-headed whales could not be explained. Interestingly the PFOS values in human blood of Japanese people did not show a decline, either.
Overall, the PFOS contamination of seabirds was lower than that of other marine animals of similar trophic levels. In some seabirds PFCAs dominated the PFC pattern instead of PFOS with PFTriDA being the predominant acid in liver. This observation is in contrast to other wildlife animals, in which the prominent PFCA typically is PFNA or PFUnDA. Thus, seabirds probably have a higher biomagnification potential for PFCAs than marine mammals. The few temporal trend studies on seabirds indicate an increase of PFOS and PFTriDA concentrations over the years possibly reaching a plateau during the 1990s. As there were only a few data available until 2003–04, a decline, as it was seen for humans and marine mammals, could not be observed.
Trend studies on fish were available only for whole fish tissues. An increase of PFOS burden of lake trout from Lake Ontario was found between 1980 and 2001–04. The longer chain PFCAs (e.g. PFNA, PFUnDA, PFTriDA) and PFOS were the most abundant acids, whereas PFUnDA often had the highest values.
Regarding the overall PFC pattern, for most of the biota species PFOS was the predominant PFC. Branched PFOS isomers were often not calculated because these isomers were usually not completely chromatographically separated and standards were lacking.[127,142] A study on the distribution of isomers in biota, water and sediment from the North American environment supported the hypothesis that long-range transport of linear volatile precursors, subsequent degradation and deposition contribute to the presence of linear PFCAs in remote regions.[66]
PFOA and PFNA are the predominant PFCAs that have been produced and applied.[2] The environmental presence of longer chain PFCAs (C10–C15) may partly be a result of impurities of longer carbon chain compounds in the technical products and partly due to atmospheric degradation of other long chain PFCs such as FTOHs. In general, PFOA is only a minor contributor to the overall burden of PFCAs in all marine biota samples. In marine mammals the dominant PFCA is PFNA, whereas the concentrations of longer chain PFCAs generally decreased with increasing chain length (from C10 to C15). This is contrary to their bioaccumulation potential, which normally increases with increasing chain length[22] but in accordance with the observed decrease of atmospheric FTOH concentration with increasing chain length.[89]
For seabirds and fish the PFC pattern was different from that in marine mammals. In fish PFNA, PFUnDA and PFTriDA were the most abundant PFCAs with PFUnDA as the predominant acid. In seabirds PFUnDA and PFTriDA are the most abundant PFCAs.[108,113] Shorter chain PFCAs and PFSAs (e.g. PFBA, PFHxA, PFBS, PFHxS) were mostly not analysed or not detected. PFHxA was found in seabirds at low concentrations (several nanograms per gram (wet weight)). Remarkable amounts of PFHxS were reported in birds from several locations in Europe (up to 120 ng g–1 ww). In marine mammals the C4- and C6-PFCAs were mostly not detected, whereas the PFSAs (e.g. PFHxS, PFBS) were detected in some animals at a low range of nanograms per gram (wet weight). In 2005, it was the first time that PFBS was reported in wildlife found in harbour seals from the Dutch Wadden Sea collected in 2002.[17] After the voluntary phase-out of PFOS by the main manufacturer, this compound was announced as the official successor for PFOS-related products[17] as PFBS was not supposed to be bioaccumulative (see http://www.3m.com). Polar bears showed the highest amounts of PFHxS among mammals, but unfortunately PFBS was not analysed.
The comparison of the odd and even-chain-pattern showed that in all three animal groups (i.e. marine mammals, seabirds, fish) the odd chain PFCA concentration exceeded the corresponding shorter even PFCA.[77,90,103] Odd-chain length PFCAs are supposed to be more bioaccumulative than even-chain length PFCAs.[89] However, the reason for the different profiles in mammals, fish and birds is unknown.[77]
PFOSA is known to be an intermediate in the production process of several PFCs and it also appears in mammals as a metabolic product of N-EtFOSA.[93,95] In mammals, PFOSA can rapidly be metabolised to PFOS.[93] PFOSA concentrations in cetaceans were often higher than those of PFOS, whereas concentrations in seabirds and in marine fish were generally low. High amounts in fish probably indicate different sources of exposure.[77] However, degradation of PFOSA, N-EtFOSA and other precursor substances of PFOS may contribute to PFOS levels in marine biota and also may inflate estimated biomagnification values within marine food webs.[143]
In conclusion, the recent replacement of POSF based compounds by shorter chain length compounds reduced the direct emissions of PFOS, but the continued production of its precursor compounds (e.g. FTOHs, FOSAs, FOSEs) and biodegradation might result in further accumulation of PFOS.[98] PFHxS contamination of humans and marine biota was relatively high. Thus, further studies on PFHxS contamination are necessary. In the future, other shorter chain PFCs should be observed thoroughly as their production and use might increase. Longer chain PFCAs (e.g. PFNA, PFUnDA, PFTriDA) should be included in monitoring programs as some biota species show relative high accumulation of these compounds. Further trend studies on fish and birds including their food webs in connection with an assessment of human exposure on PFCs are recommended.
References
[1] A. G. Paul, K. C. Jones, A. J. Sweetman, A first global production, emission, and environmental inventory for perfluorooctane sulfonate. Environ. Sci. Technol. 2009, 43, 386.| A first global production, emission, and environmental inventory for perfluorooctane sulfonate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVyks7%2FJ&md5=0ee7522ab4391fa49e201e7f3870a6e9CAS | 19238969PubMed |
[2] K. Prevedouros, I. T. Cousins, R. C. Buck, S. H. Korzeniowski, Sources, fate and transport of perfluorocarboxylates. Environ. Sci. Technol. 2006, 40, 32.
| Sources, fate and transport of perfluorocarboxylates.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXht1Gru7zK&md5=462f8fe241ab6289b0735fe1fa36c029CAS | 16433330PubMed |
[3] European Parliament and Council, Directive 2006/122/ECOF 2006.
[4] OSPAR Background Document on Perfluorooctane Sulphonate (PFOS), Publication Number: 269/2006 2005 [updated in 2006] (OSPAR Commission). Available at http://www.ospar.org/documents/dbase/publications/P00269_BD%20on%20PFOS%20_2006%20version_.pdf [verified 10 December 2010].
[5] The 9 new POPs under the Stockholm Convention, in Stockholm Convention on persistent organic pollutants (POPs), Conference of the Parties (COP4), Geneva, 4–8 May 2009. Available at http://chm.pops.int/Programmes/New%20POPs/The%209%20new%20POPs/tabid/672/language/en-US/Default.aspx [verified 10 December 2010].
[6] M. Houde, J. W. Martin, R. J. Letcher, K. R. Solomon, D. C. G. Muir, Biological monitoring of polyfluoroalkyl substances: a review. Environ. Sci. Technol. 2006, 40, 3463.
| Biological monitoring of polyfluoroalkyl substances: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XktVers7w%3D&md5=eb93be85fce35b6422b2879576886d65CAS | 16786681PubMed |
[7] C. M. Butt, U. Berger, R. Bossi, G. T. Tomy, Levels and trends of poly- and perfluorinated compounds in the arctic environment. Sci. Total Environ. 2010, 408, 2936.
| Levels and trends of poly- and perfluorinated compounds in the arctic environment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXntV2isL0%3D&md5=abe5a2f71428acba4f89a5fed5973790CAS | 20493516PubMed |
[8] J. W. Martin, K. Kannan, U. Berger, P. de Voogt, J. Field, J. Franklin, P. Giesy John, T. Harner, C. G. Muir Derek, B. Scott, M. Kaiser, U. Jarnberg, C. Jones Kevin, A. Mabury Scott, H. Schroeder, M. Simcik, C. Sottani, B. van Bavel, A. Karrman, G. Lindström, S. van Leeuwen, Analytical challenges hamper perfluoroalkyl research. Environ. Sci. Technol. 2004, 38, 248A.
| Analytical challenges hamper perfluoroalkyl research.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXltlOitbk%3D&md5=f15e12e086f6f4278b06e68951a06f34CAS | 15296292PubMed |
[9] C. R. Powley, S. W. George, T. W. Ryan, R. C. Buck, Matrix effect-free analytical methods for determination of perfluorinated carboxylic acids in environmental matrixes. Anal. Chem. 2005, 77, 6353.
| Matrix effect-free analytical methods for determination of perfluorinated carboxylic acids in environmental matrixes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXps1erurk%3D&md5=b27ad812e238dcfd12e56825f41cd8e8CAS | 16194099PubMed |
[10] C. R. Powley, S. W. George, M. H. Russell, R. A. Hoke, R. C. Buck, Polyfluorinated chemicals in a spatially and temporally integrated food web in the Western Arctic. Chemosphere 2008, 70, 664.
| Polyfluorinated chemicals in a spatially and temporally integrated food web in the Western Arctic.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtlGgtbvK&md5=7e6d8ffa3de2fbc30e80cc9a9d2a03bfCAS | 17698166PubMed |
[11] E. Chan, M. Sandhu, J. P. Benskin, M. Ralitsch, N. Thibault, D. Birkholz, J. W. Martin, Endogenous high-performance liquid chromatography/tandem mass spectrometry interferences and the case of perfluorohexane sulfonate (PFHxS) in human serum; are we overestimating exposure? Rapid Commun. Mass Spectrom. 2009, 23, 1405.
| Endogenous high-performance liquid chromatography/tandem mass spectrometry interferences and the case of perfluorohexane sulfonate (PFHxS) in human serum; are we overestimating exposure?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXltl2ls7s%3D&md5=261d66e15f57bb419d508a8877328991CAS | 19347969PubMed |
[12] W. K. Reagen, M. E. Ellefson, K. Kannan, J. P. Giesy, Comparison of extraction and quantification methods of perfluorinated compounds in human plasma, serum, and whole blood. Anal. Chim. Acta 2008, 628, 214.
| Comparison of extraction and quantification methods of perfluorinated compounds in human plasma, serum, and whole blood.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXht1elsL%2FP&md5=554dcac15409d6d62bca5026cd35fe9bCAS | 18929010PubMed |
[13] S. P. J. van Leeuwen, A. Kärrman, B. van Bavel, J. de Boer, G. Lindström, Struggle for quality in determination of perfluorinated contaminants in environmental and human samples. Environ. Sci. Technol. 2006, 40, 7854.
| Struggle for quality in determination of perfluorinated contaminants in environmental and human samples.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtFynur3I&md5=5853407156d1db91e162fd0f2c36e7b3CAS | 17256538PubMed |
[14] S. P. J. van Leeuwen, C. P. Swart, I. van der Veen, J. de Boer, Significant improvements in the analysis of perfluorinated compounds in water and fish: results from an interlaboratory method evaluation study. J. Chromatogr. A 2009, 1216, 401.
| Significant improvements in the analysis of perfluorinated compounds in water and fish: results from an interlaboratory method evaluation study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsFCls7%2FF&md5=058dcdb886967fe44c26e6d2fb7bc57aCAS | 19041981PubMed |
[15] Water quality – Determination of perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) – Method for unfiltered samples using solid phase extraction and liquid chromatography/mass spectrometry. ISO 25101 2009.
[16] G. Lindström, A. Kärrman, B. van Bavel, Accuracy and precision in the determination of perfluorinated chemicals in human blood verified by interlaboratory comparisons. J. Chromatogr. A 2009, 1216, 394.
| Accuracy and precision in the determination of perfluorinated chemicals in human blood verified by interlaboratory comparisons.Crossref | GoogleScholarGoogle Scholar | 19026417PubMed |
[17] K. I. Van de Vijver, P. Hoff, K. Das, S. Brasseur, W. Van Dongen, E. Esmans, P. Reijnders, R. Blust, W. De Coen, Tissue distribution of perfluorinated chemicals in harbor seals (Phoca vitulina) from the Dutch Wadden Sea. Environ. Sci. Technol. 2005, 39, 6978.
| Tissue distribution of perfluorinated chemicals in harbor seals (Phoca vitulina) from the Dutch Wadden Sea.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXotVSlsL4%3D&md5=67c6d616eb5447a847e47205608827acCAS | 16201619PubMed |
[18] L. Ahrens, U. Siebert, R. Ebinghaus, Total body burden and tissue distribution of polyfluorinated compounds in harbor seals (Phoca vitulina) from the German Bight. Mar. Pollut. Bull. 2009, 58, 520.
| Total body burden and tissue distribution of polyfluorinated compounds in harbor seals (Phoca vitulina) from the German Bight.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXktVGqsbs%3D&md5=f432375e0d873303f42357c9a5954b69CAS | 19121527PubMed |
[19] T. Dauwe, K. Van de Vijver, W. De Coen, M. Eens, PFOS levels in the blood and liver of a small insectivorous songbird near a fluorochemical plant. Environ. Int. 2007, 33, 357.
| PFOS levels in the blood and liver of a small insectivorous songbird near a fluorochemical plant.Crossref | GoogleScholarGoogle Scholar | 17188355PubMed |
[20] G. W. Olsen, K. J. Hansen, L. A. Stevenson, J. M. Burris, J. H. Mandel, Human donor liver and serum concentrations of perfluorooctanesulfonate and other perfluorochemicals. Environ. Sci. Technol. 2003, 37, 888.
| Human donor liver and serum concentrations of perfluorooctanesulfonate and other perfluorochemicals.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXnsl2isQ%3D%3D&md5=69214b5548450c089c5ff5fee6eea6d9CAS | 12666917PubMed |
[21] J. D. Johnson, S. J. Gibson, R. F. Ober, Absorption of FC-95–14C in rats after a single oral dose, docket AR-226–0007 1979 (US EPA: Washington, DC).
[22] J. W. Martin, S. A. Mabury, K. R. Solomon, D. C. G. Muir, Bioconcentration and tissue distribution of perfluorinated acids in rainbow trout (Oncorhynchus mykiss). Environ. Toxicol. Chem. 2003, 22, 196.
| 1:CAS:528:DC%2BD38XpslCmtrc%3D&md5=e65c671b222815c5f4863dafa566be6fCAS | 12503765PubMed |
[23] S. Taniyasu, K. Kannan, Y. Horii, N. Hanari, N. Yamashita, A survey of perfluorooctane sulfonate and related perfluorinated organic compounds in water, fish, birds, and humans from Japan. Environ. Sci. Technol. 2003, 37, 2634.
| A survey of perfluorooctane sulfonate and related perfluorinated organic compounds in water, fish, birds, and humans from Japan.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXjs1GgtLo%3D&md5=793fcef6dc9d206217a1af95059f6136CAS | 12854699PubMed |
[24] A. Kärrman, B. van Bavel, U. Jarnberg, L. Hardell, G. Lindström, Perfluorinated chemicals in relation to other persistent organic pollutants in human blood. Chemosphere 2006, 64, 1582.
| Perfluorinated chemicals in relation to other persistent organic pollutants in human blood.Crossref | GoogleScholarGoogle Scholar | 16403420PubMed |
[25] K. Kannan, J. C. Franson, W. W. Bowerman, K. J. Hansen, P. D. Jones, J. P. Giesy, Perfluorooctane sulfonate in fish-eating water birds including bald eagles and albatrosses. Environ. Sci. Technol. 2001, 35, 3065.
| Perfluorooctane sulfonate in fish-eating water birds including bald eagles and albatrosses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXksVaku7k%3D&md5=dfa1f73c9821ca249111ab5a2f4cd713CAS | 11505980PubMed |
[26] K. Kannan, S. Corsolini, J. Falandysz, G. Fillmann, K. S. Kumar, B. G. Loganathan, M. A. Mohd, J. Olivero, N. Van Wouwe, J. H. Yang, K. M. Aldous, Perfluorooctanesulfonate and related fluorochemicals in human blood from several countries. Environ. Sci. Technol. 2004, 38, 4489.
| Perfluorooctanesulfonate and related fluorochemicals in human blood from several countries.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXlvV2qsrY%3D&md5=f374583a83a3aa406b18ef186828769eCAS | 15461154PubMed |
[27] D. J. Ehresman, J. W. Froehlich, G. W. Olsen, S.-C. Chang, J. L. Butenhoff, Comparison of human whole blood, plasma, and serum matrices for the determination of perfluorooctanesulfonate (PFOS), perfluorooctanoate (PFOA), and other fluorochemicals. Environ. Res. 2007, 103, 176.
| Comparison of human whole blood, plasma, and serum matrices for the determination of perfluorooctanesulfonate (PFOS), perfluorooctanoate (PFOA), and other fluorochemicals.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtVKnsLw%3D&md5=f0004c45f84ef2e02b73f3de9b95bbddCAS | 16893538PubMed |
[28] H. Fromme, S. A. Tittlemier, W. Völkel, M. Wilhelm, D. Twardella, Perfluorinated compounds – Exposure assessment for the general population in western countries. Int. J. Hyg. Environ. Health 2009, 212, 239.
| Perfluorinated compounds – Exposure assessment for the general population in western countries.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXlt1GgtLc%3D&md5=e69afc65a512069937639e93d10bdaedCAS | 18565792PubMed |
[29] G. W. Olsen, J. M. Burris, M. M. Burlew, J. H. Mandel, Epidemiologic assessment of worker serum perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) concentrations and medical surveillance examinations. J. Occup. Environ. Med. 2003, 45, 260.
| Epidemiologic assessment of worker serum perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) concentrations and medical surveillance examinations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXhs1elt7g%3D&md5=1c7b2f59cc806ed2034d2a68d4e7b672CAS | 12661183PubMed |
[30] G. W. Olsen, T. R. Church, J. P. Miller, J. M. Burris, K. J. Hansen, J. K. Lundberg, J. B. Armitage, R. M. Herron, Z. Medhdizadehkashi, J. B. Nobiletti, E. M. O’Neill, J. H. Mandel, L. R. Zobel, Perfluorooctanesulfonate and other fluorochemicals in the serum of American Red Cross adult blood donors. Environ. Health Perspect. 2003, 111, 1892.
| Perfluorooctanesulfonate and other fluorochemicals in the serum of American Red Cross adult blood donors.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXit1Oquw%3D%3D&md5=56682973fe04710e10a8ec85cf2c2e9bCAS | 14644663PubMed |
[31] G. W. Olsen, H. Y. Huang, K. J. Helzlsouer, K. J. Hansen, J. L. Butenhoff, J. H. Mandel, Historical comparison of perfluorooctanesulfonate, perfluorooctanoate, and other fluorochemicals in human blood. Environ. Health Perspect. 2005, 113, 539.
| Historical comparison of perfluorooctanesulfonate, perfluorooctanoate, and other fluorochemicals in human blood.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXkvFCjtL8%3D&md5=262851283f8dbaa19806bc2c2da9510dCAS | 15866760PubMed |
[32] G. W. Olsen, D. C. Mair, T. R. Church, M. E. Ellefson, W. K. Reagen, T. M. Boyd, R. M. Herron, Z. Medhdizadehkashi, J. B. Nobiletti, J. A. Rios, J. L. Butenhoff, L. R. Zobel, Decline in perfluorooctanesulfonate and other polyfluoroalkyl chemicals in American red cross adult blood donors, 2000–2006. Environ. Sci. Technol. 2008, 42, 4989.
| Decline in perfluorooctanesulfonate and other polyfluoroalkyl chemicals in American red cross adult blood donors, 2000–2006.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXmtVOns78%3D&md5=50cf60be8f8e65193801a47bd303e86fCAS | 18678038PubMed |
[33] A. M. Calafat, Z. Kuklenyik, J. A. Reidy, S. P. Caudill, J. S. Tully, L. L. Needham, Serum concentrations of 11 polyfluoroalkyl compounds in the US population: data from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ. Sci. Technol. 2007, 41, 2237.
| Serum concentrations of 11 polyfluoroalkyl compounds in the US population: data from the National Health and Nutrition Examination Survey (NHANES) 1999–2000.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXisVGlsLY%3D&md5=428203c88001f60586e58ef78cd4125aCAS | 17438769PubMed |
[34] A. M. Calafat, L. Y. Wong, Z. Kuklenyik, J. A. Reidy, L. L. Needham, Polyfluoroalkyl chemicals in the US population: data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Environ. Health Perspect. 2007, 115, 1596.
| Polyfluoroalkyl chemicals in the US population: data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtl2ns7%2FK&md5=573ec9fe11a86da505107009684f2e78CAS | 18007991PubMed |
[35] G. W. Olsen, J. M. Burris, D. J. Ehresman, J. W. Froehlich, A. M. Seacat, J. L. Butenhoff, L. R. Zobel, Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ. Health Perspect. 2007, 115, 1298.
| Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtFamsr7J&md5=a82b02d19377490c829ca62e6f2247c2CAS | 17805419PubMed |
[36] H. M. Spliethoff, L. Tao, S. M. Shaver, K. M. Aldous, K. A. Pass, K. Kannan, G. A. Eadon, Use of newborn screening program blood spots for exposure assessment: declining levels of perfluorinated compounds in New York State Infants. Environ. Sci. Technol. 2008, 42, 5361.
| Use of newborn screening program blood spots for exposure assessment: declining levels of perfluorinated compounds in New York State Infants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXmvFOqsrw%3D&md5=f9ea0ec0f7296d137430e8b42e6b6b60CAS | 18754394PubMed |
[37] G. A. Wiesmüller, R. Eckard, L. Dobler, A. Günsel, M. Oganowski, C. Schröter-Kermani, C. Schlüter, A. Gies, F. H. Kemper, The environmental specimen bank for human tissues as part of the German environmental specimen bank. Int. J. Hyg. Environ. Health 2007, 210, 299.
| The environmental specimen bank for human tissues as part of the German environmental specimen bank.Crossref | GoogleScholarGoogle Scholar | 17369091PubMed |
[38] M. Wilhelm, J. Hölzer, L. Dobler, K. Rauchfuss, O. Midasch, M. Kraft, J. Angerer, G. Wiesmüller, Preliminary observations on perfluorinated compounds in plasma samples (1977–2004) of young German adults from an area with perfluorooctanoate-contaminated drinking water. Int. J. Hyg. Environ. Health 2009, 212, 142.
| Preliminary observations on perfluorinated compounds in plasma samples (1977–2004) of young German adults from an area with perfluorooctanoate-contaminated drinking water.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXjsVKltb8%3D&md5=cdfe90cdbe3bfcd05cddff5e0ab288e9CAS | 18550432PubMed |
[39] L. S. Haug, C. Thomsen, G. Becher, Time trends and the influence of age and gender on serum concentrations of perfluorinated compounds in archived human samples. Environ. Sci. Technol. 2009, 43, 2131.
| Time trends and the influence of age and gender on serum concentrations of perfluorinated compounds in archived human samples.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFKit70%3D&md5=75cdfc56f64a10f1ac00ee3421e79661CAS | 19368225PubMed |
[40] Y. H. Jin, N. Saito, K. H. Harada, K. Inoue, A. Koizumi, Historical trends in human serum levels of perfluorooctanoate and perfluorooctane sulfonate in Shenyang, China. Tohoku J. Exp. Med. 2007, 212, 63.
| Historical trends in human serum levels of perfluorooctanoate and perfluorooctane sulfonate in Shenyang, China.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXmtVyktr4%3D&md5=deb1f15615234e6e620b427ca6b812a5CAS | 17464105PubMed |
[41] L. W. Y. Yeung, M. K. So, G. Jiang, S. Taniyasu, N. Yamashita, M. Song, Y. Wu, J. Li, J. P. Giesy, K. S. Guruge, P. K. S. Lam, Perfluorooctanesulfonate and related fluorochemicals in human blood samples from China. Environ. Sci. Technol. 2006, 40, 715.
| Perfluorooctanesulfonate and related fluorochemicals in human blood samples from China.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtlekurbP&md5=0a7d096047c070c61d2b39a91ee20c9dCAS | 16509308PubMed |
[42] J. Liu, J. Li, Y. Luan, Y. Zhao, Y. Wu, Geographical distribution of perfluorinated compounds in human blood from Liaoning Province, China. Environ. Sci. Technol. 2009, 43, 4044.
| Geographical distribution of perfluorinated compounds in human blood from Liaoning Province, China.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXlt1Sgt7Y%3D&md5=5abfff8c7439b097879656aa1ac2d15dCAS | 19569328PubMed |
[43] K. Harada, A. Koizumi, N. Saito, K. Inoue, T. Yoshinaga, C. Date, S. Fujii, N. Hachiya, I. Hirosawa, S. Koda, Y. Kusaka, K. Murata, K. Omae, S. Shimbo, K. Takenaka, T. Takeshita, H. Todoriki, Y. Wada, T. Watanabe, M. Ikeda, Historical and geographical aspects of the increasing perfluorooctanoate and perfluorooctane sulfonate contamination in human serum in Japan. Chemosphere 2007, 66, 293.
| Historical and geographical aspects of the increasing perfluorooctanoate and perfluorooctane sulfonate contamination in human serum in Japan.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1Cqt7rF&md5=2579597b33fe6f33cd24de4676c5fb70CAS | 16793116PubMed |
[44] K. H. Harada, A. Koizumi, Environmental and biological monitoring of persistent fluorinated compounds in Japan and their toxicities. Environ. Health Prev. Med. 2009, 14, 7.
| Environmental and biological monitoring of persistent fluorinated compounds in Japan and their toxicities.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXjt1Sjt7k%3D&md5=5ee151d3c7c60a726c84c61e9be6e3e8CAS | 19568863PubMed |
[45] A. Kärrman, J. F. Mueller, B. Van Bavel, F. Harden, L. M. L. Toms, G. Lindström, Levels of 12 perfluorinated chemicals in pooled Australian serum, collected 2002–2003, in relation to age, gender, and region. Environ. Sci. Technol. 2006, 40, 3742.
| Levels of 12 perfluorinated chemicals in pooled Australian serum, collected 2002–2003, in relation to age, gender, and region.Crossref | GoogleScholarGoogle Scholar | 16830536PubMed |
[46] L.-M. L. Toms, A. M. Calafat, K. Kato, J. Thompson, F. Harden, P. Hobson, A. Sjodin, J. F. Mueller, Polyfluoroalkyl chemicals in pooled blood serum from infants, children, and adults in Australia. Environ. Sci. Technol. 2009, 43, 4194.
| Polyfluoroalkyl chemicals in pooled blood serum from infants, children, and adults in Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXltVKktL4%3D&md5=a8e0c5b15ec9f4727b58b767e0e00908CAS | 19569351PubMed |
[47] O. Päpke, P. Fürst, Background contamination of humans with dioxins, dioxin-like PCBs and other POPs. Handb. Environ. Chem. 2003, 3, 271.
| Background contamination of humans with dioxins, dioxin-like PCBs and other POPs.Crossref | GoogleScholarGoogle Scholar |
[48] K. Kato, A. M. Calafat, L.-Y. Wong, A. A. Wanigatunga, S. P. Caudill, L. L. Needham, Polyfluoroalkyl compounds in pooled sera from children participating in the National Health and Nutrition Examination Survey 2001–2002. Environ. Sci. Technol. 2009, 43, 2641.
| Polyfluoroalkyl compounds in pooled sera from children participating in the National Health and Nutrition Examination Survey 2001–2002.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXitVOnurw%3D&md5=d919f5021e78c4d6ec250941684b6636CAS | 19452929PubMed |
[49] M. J. Strynar, A. B. Lindstrom, Perfluorinated compounds in house dust from Ohio and North Carolina, USA. Environ. Sci. Technol. 2008, 42, 3751.
| Perfluorinated compounds in house dust from Ohio and North Carolina, USA.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXkvVyhtLw%3D&md5=16d3b87efc2cf51c7c5cb2c56f3d2dc8CAS | 18546718PubMed |
[50] O. Midasch, T. Schettgen, J. Angerer, Pilot study on the perfluorooctanesulfonate and perfluorooctanoate exposure of the German general population. Int. J. Hyg. Environ. Health 2006, 209, 489.
| Pilot study on the perfluorooctanesulfonate and perfluorooctanoate exposure of the German general population.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXktlWmsg%3D%3D&md5=e6fad93aebf365e7d2d19e0ce19f981eCAS | 16872899PubMed |
[51] A. Kärrman, I. Ericson, B. van Bavel, P. O. Darnerud, M. Aune, A. Glynn, S. Lignell, G. Lindström, Exposure of perfluorinated chemicals through lactation: levels of matched human milk and serum and a temporal trend, 1996–2004, in Sweden. Environ. Health Perspect. 2007, 115, 226.[Published online ahead of print 28 November 2006]
| Exposure of perfluorinated chemicals through lactation: levels of matched human milk and serum and a temporal trend, 1996–2004, in Sweden.Crossref | GoogleScholarGoogle Scholar | 17384769PubMed |
[52] L. Tao, K. Kannan, C. M. Wong, K. F. Arcaro, J. L. Butenhoff, Perfluorinated compounds in human milk from Massachusetts, US. Environ. Sci. Technol. 2008, 42, 3096.
| Perfluorinated compounds in human milk from Massachusetts, US.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXivFOmsLw%3D&md5=468dd3a7e4ccb040de18561f0989b46fCAS | 18497172PubMed |
[53] T. Bernsmann, P. Fürst, Determination of perfluorinated compounds in human milk. Organohalogen Compd. 2008, 70, 718.
| 1:CAS:528:DC%2BD1MXjtlSru7o%3D&md5=2aee75dc9cdb5dd0ff23a3d5315272efCAS |
[54] L. Tao, J. Ma, T. Kunisue, E. L. Libelo, S. Tanabe, K. Kannan, Perfluorinated compounds in human breast milk from several Asian countries, and in infant formula and dairy milk from the United States. Environ. Sci. Technol. 2008, 42, 8597.
| Perfluorinated compounds in human breast milk from several Asian countries, and in infant formula and dairy milk from the United States.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXht1ehsb%2FN&md5=7f823a2994d7d3b17adc93a5840ab8f7CAS | 19068854PubMed |
[55] N. Kishikawa, N. Kuroda, Evaluation of organic environmental pollutants detected in human milk. J. Health Sci. 2009, 55, 1.
| Evaluation of organic environmental pollutants detected in human milk.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXitFWjtrs%3D&md5=eaa0af7aa3e1f8189e8d8b9f0b0e25f3CAS |
[56] A. Kärrman, J. L. Domingo, X. Llebaria, M. Nadal, E. Bigas, B. Bavel, G. Lindström, Biomonitoring perfluorinated compounds in Catalonia, Spain: concentrations and trends in human liver and milk samples. Environ. Sci. Pollut. Res. 2010, 17, 750.
| Biomonitoring perfluorinated compounds in Catalonia, Spain: concentrations and trends in human liver and milk samples.Crossref | GoogleScholarGoogle Scholar |
[57] W. Völkel, O. Genzel-Boroviczeny, H. Demmelmair, C. Gebauer, B. Koletzko, D. Twardella, U. Raab, H. Fromme, Perfluorooctane sulphonate (PFOS) and perfluorooctanoic acid (PFOA) in human breast milk: results of a pilot study. Int. J. Hyg. Environ. Health 2008, 211, 440.
| Perfluorooctane sulphonate (PFOS) and perfluorooctanoic acid (PFOA) in human breast milk: results of a pilot study.Crossref | GoogleScholarGoogle Scholar | 17870667PubMed |
[58] M. K. So, N. Yamashita, S. Taniyasu, Q. Jiang, J. P. Giesy, K. Chen, P. K. S. Lam, Health risks in infants associated with exposure to perfluorinated compounds in human breast milk from Zhoushan, China. Environ. Sci. Technol. 2006, 40, 2924.
| Health risks in infants associated with exposure to perfluorinated compounds in human breast milk from Zhoushan, China.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XjtFSqsrY%3D&md5=8e43e656e5b1ddcbb8c342f7305a6680CAS | 16719092PubMed |
[59] A. Kärrman, U. Eriksson, B. Van Bavel, L. Hardell, M. Aune, G. Lindström, Temporal trends of perfluorinated compounds in Swedish blood and breast milk 1996–2009, Poster presentation at NECC, Longyearbyen, Norway, 2–5 March 2010. Available at www.unis.no/50_INTERNAL/5040_Conference/NECC_2010/NECC2010_completeprogram_220210.pdf [Verified 23 November 2010].
[60] L. Maestri, S. Negri, M. Ferrari, S. Ghittori, F. Fabris, P. Danesino, M. Imbriani, Determination of perfluorooctanoic acid and perfluorooctanesulfonate in human tissues by liquid chromatography/single quadrupole mass spectrometry. Rapid Commun. Mass Spectrom. 2006, 20, 2728.
| Determination of perfluorooctanoic acid and perfluorooctanesulfonate in human tissues by liquid chromatography/single quadrupole mass spectrometry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtVCgurnE&md5=c25702c4cc58dc108e104a3fcab6d667CAS | 16915561PubMed |
[61] A. Kärrman, M. Nadal, F. Garcia, J. L. Domingo, B. van Bavel, G. Lindström, Levels of perfluorinated compounds in human liver samples from Catalonia, Spain. Organohalogen Compd. 2008, 70, 1063.
[62] J. Falandysz, S. Taniyasu, A. Gulkowska, N. Yamashita, U. Schulte-Oehlmann, Is fish a major source of fluorinated surfactants and repellents in humans living on the Baltic coast? Environ. Sci. Technol. 2006, 40, 748.
| Is fish a major source of fluorinated surfactants and repellents in humans living on the Baltic coast?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XlsFei&md5=5599837db368ee3f6afa8f5fdf2953c1CAS | 16509313PubMed |
[63] A. Kärrman, Perfluorinated chemicals in relation to other persistent organic pollutants in human blood. Chemosphere 2006, 64, 1582.
| Perfluorinated chemicals in relation to other persistent organic pollutants in human blood.Crossref | GoogleScholarGoogle Scholar | 16403420PubMed |
[64] L. W. Y. Yeung, Y. Miyake, S. Taniyasu, Y. Wang, H. Yu, M. K. So, G. Jiang, Y. Wu, J. Li, J. P. Giesy, N. Yamashita, P. K. S. Lam, Perfluorinated compounds and total and extractable organic fluorine in human blood samples from China. Environ. Sci. Technol. 2008, 42, 8140.
| Perfluorinated compounds and total and extractable organic fluorine in human blood samples from China.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtFOqt7nF&md5=b8963fe0893d329b43e62eb5129606ecCAS | 19031915PubMed |
[65] A. O. M. De Silva, S. A. Mabury, Isomer distribution of perfluorocarboxylates in human blood: potential correlation to source. Environ. Sci. Technol. 2006, 40, 2903.
| Isomer distribution of perfluorocarboxylates in human blood: potential correlation to source.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XislCjt7k%3D&md5=4bcf7d2e866dcdc1b1da0bc8afb0badfCAS | 16719089PubMed |
[66] A. O. De Silva, D. C. G. Muir, S. A. Mabury, Distribution of perfluorocarboxylate isomers in select samples from the North American environment. Environ. Toxicol. Chem. 2009, 28, 1801.
| Distribution of perfluorocarboxylate isomers in select samples from the North American environment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtVKmt7rF&md5=ca5448c5d1127b63bb823d470005005cCAS | 19358623PubMed |
[67] A. Kärrman, I. Langlois, B. van Bavel, G. Lindström, M. Ohme, Identification and pattern of perfluorooctane sulfonate (PFOS) isomers in human serum and plasma. Environ. Int. 2007, 33, 782.
| Identification and pattern of perfluorooctane sulfonate (PFOS) isomers in human serum and plasma.Crossref | GoogleScholarGoogle Scholar | 17442394PubMed |
[68] J. C. D’eon, P. W. Crozier, V. I. Furdui, E. J. Reiner, E. L. Libelo, S. A. Mabury, Observation of a commercial fluorinated material, the polyfluoroalkyl phosphoric acid diesters, in human sera, wastewater treatment plant sludge, and paper fibers. Environ. Sci. Technol. 2009, 43, 4589.
| Observation of a commercial fluorinated material, the polyfluoroalkyl phosphoric acid diesters, in human sera, wastewater treatment plant sludge, and paper fibers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXltFOlsLw%3D&md5=19eab90a3fb315a2c02f2eb3d9b4e8f9CAS | 19603681PubMed |
[69] R. Bossi, F. F. Riget, R. Dietz, Temporal and spatial trends of perfluorinated compounds in ringed seal (Phoca hispida) from Greenland. Environ. Sci. Technol. 2005, 39, 7416.
| Temporal and spatial trends of perfluorinated compounds in ringed seal (Phoca hispida) from Greenland.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXps12ktrY%3D&md5=7c2f458143b155b2bb5bb6a6d27f3a89CAS | 16245810PubMed |
[70] L. Ahrens, W. Gerwinski, N. Theobald, R. Ebinghaus, Sources of polyfluoroalkyl compounds in the North Sea, Baltic Sea and Norwegian Sea: evidence from their spatial distribution in surface water. Mar. Pollut. Bull. 2010, 60, 255.
| Sources of polyfluoroalkyl compounds in the North Sea, Baltic Sea and Norwegian Sea: evidence from their spatial distribution in surface water.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhvFKitrY%3D&md5=3dbd0ac7945ffb523dc65d58222b53dfCAS | 19818459PubMed |
[71] C. M. M. Butt, D. C. G. Muir, I. Stirling, M. Kwan, S. A. Mabury, Rapid response of Arctic ringed seals to changes in perfluoroalkyl production. Environ. Sci. Technol. 2007, 41, 42.
| Rapid response of Arctic ringed seals to changes in perfluoroalkyl production.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1ClurvE&md5=680f7787cda9baf35f64ee49bb6bb90fCAS | 17265925PubMed |
[72] H. Ishibashi, H. Iwata, E. Y. Kim, L. Tao, K. Kannan, M. Amano, N. Miyazaki, S. Tanabe, V. B. Batoev, E. A. Petrov, Contamination and effects of perfluorochemicals in Baikal Seal (Pusa sibirica). 1. Residue level, tissue distribution, and temporal trend. Environ. Sci. Technol. 2008, 42, 2295.
| Contamination and effects of perfluorochemicals in Baikal Seal (Pusa sibirica). 1. Residue level, tissue distribution, and temporal trend.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXis1Kgu70%3D&md5=5887a2d2aa4582e86770c549554237c8CAS | 18504956PubMed |
[73] K. Hart, K. Kannan, T. Isobe, S. Takahashi, T. K. Yamada, N. Miyazaki, S. Tanabe, Time trends and transplacental transfer of perfluorinated compounds in melon-headed whales stranded along the Japanese coast in 1982, 2001/2002, and 2006. Environ. Sci. Technol. 2008, 42, 7132.
| Time trends and transplacental transfer of perfluorinated compounds in melon-headed whales stranded along the Japanese coast in 1982, 2001/2002, and 2006.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtVGntbbP&md5=d39d7a123c4a071585808044d1a1c1f1CAS | 18939537PubMed |
[74] K. Hart, V. A. Gill, K. Kannan, Temporal trends (1992–2007) of perfluorinated chemicals in northern sea otters (Enhydra lutris kenyoni) from south-central Alaska. Arch. Environ. Contam. Toxicol. 2009, 56, 607.
| Temporal trends (1992–2007) of perfluorinated chemicals in northern sea otters (Enhydra lutris kenyoni) from south-central Alaska.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXjsVWnurc%3D&md5=2bfcc5cf192d4c0de1798bb6cfe6074fCAS | 18839236PubMed |
[75] K. Kannan, E. Perrotta, N. J. Thomas, Association between perfluorinated compounds and pathological conditions in southern sea otters. Environ. Sci. Technol. 2006, 40, 4943.
| Association between perfluorinated compounds and pathological conditions in southern sea otters.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XmvVGhurw%3D&md5=59125a2d106c6df372b5ca670d5433b5CAS | 16955890PubMed |
[76] L. Ahrens, U. Siebert, R. Ebinghaus, Temporal trends of polyfluoroalkyl compounds in harbor seals (Phoca vitulina) from the German Bight, 1999–2008. Chemosphere 2009, 76, 151.
| Temporal trends of polyfluoroalkyl compounds in harbor seals (Phoca vitulina) from the German Bight, 1999–2008.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXmtFSis7w%3D&md5=6205f42fbb0f548d8f6158d90b4ff966CAS | 19394671PubMed |
[77] J. W. Martin, M. M. Smithwick, B. M. Braune, P. F. Hoekstra, D. C. G. Muir, S. A. Mabury, Identification of long-chain perfluorinated acids in biota from the Canadian Arctic. Environ. Sci. Technol. 2004, 38, 373.
| Identification of long-chain perfluorinated acids in biota from the Canadian Arctic.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXptlOgsr8%3D&md5=50c81b0bc98c77f8b62f12d3393f3acfCAS | 14750710PubMed |
[78] M. Smithwick, R. J. Norstrom, S. A. Mabury, K. Solomon, T. J. Evans, I. Stirling, M. K. Taylor, D. C. G. Muir, Temporal trends of perfluoroalkyl contaminants in polar bears (Ursus maritimus) from two locations in the North American Arctic, 1972–2002. Environ. Sci. Technol. 2006, 40, 1139.
| Temporal trends of perfluoroalkyl contaminants in polar bears (Ursus maritimus) from two locations in the North American Arctic, 1972–2002.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XitVGhsg%3D%3D&md5=25b9754de82b3d704d3de509ae0e0606CAS | 16572767PubMed |
[79] R. Dietz, R. Bossi, F. F. Riget, C. Sonne, E. W. Born, Increasing perfluoroalkyl contaminants in east Greenland polar bears (Ursus maritimus): a new toxic threat to the Arctic bears. Environ. Sci. Technol. 2008, 42, 2701.
| Increasing perfluoroalkyl contaminants in east Greenland polar bears (Ursus maritimus): a new toxic threat to the Arctic bears.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXisFyisLg%3D&md5=062b23cbd521a5ead40c55a9dee2f092CAS | 18505019PubMed |
[80] C. M. Butt, S. A. Mabury, M. Kwan, X. W. Wang, D. C. G. Muir, Spatial trends of perfluoroalkyl compounds in ringed seals (Phoca hispida) from the Canadian Arctic. Environ. Toxicol. Chem. 2008, 27, 542.
| Spatial trends of perfluoroalkyl compounds in ringed seals (Phoca hispida) from the Canadian Arctic.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXisFKmu7s%3D&md5=38008aa0626b31cb1e10c9fdd33b515aCAS | 17988182PubMed |
[81] A. E. Derocher, H. Wolkers, T. Colborn, M. Schlabach, T. S. Larsen, O. Wiig, Contaminants in Svalbard polar bear samples archived since 1967 and possible population level effects. Sci. Total Environ. 2003, 301, 163.
| Contaminants in Svalbard polar bear samples archived since 1967 and possible population level effects.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XpsFSksLc%3D&md5=7183040e44a6785a083db7a408c4c1b6CAS | 12493194PubMed |
[82] R. J. Norstrom, S. E. Belikov, E. W. Born, G. W. Garner, B. Malone, S. Olpinski, M. A. Ramsay, S. Schliebe, I. Stirling, M. S. Stishov, M. K. Taylor, O. Wiig, Chlorinated hydrocarbon contaminants in polar bears from Eastern Russia, North America, Greenland, and Svalbard: biomonitoring of Arctic pollution. Arch. Environ. Contam. Toxicol. 1998, 35, 354.
| Chlorinated hydrocarbon contaminants in polar bears from Eastern Russia, North America, Greenland, and Svalbard: biomonitoring of Arctic pollution.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXltVWqsbs%3D&md5=078f1be9ebc5b9e06e608d848f1e4342CAS | 9680529PubMed |
[83] S. Shaw, M. L. Berger, D. Brenner, L. Tao, Q. Wu, K. Kannan, Specific accumulation of perfluorochemicals in harbor seals (Phoca vitulina concolor) from the northwest Atlantic. Chemosphere 2009, 74, 1037.
| Specific accumulation of perfluorochemicals in harbor seals (Phoca vitulina concolor) from the northwest Atlantic.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhs1ejur8%3D&md5=d563b2a4207641b2935ac3b7d269d5deCAS | 19101009PubMed |
[84] L. W. Y. Yeung, Y. Miyake, Y. Wang, S. Taniyasu, N. Yamashita, P. K. S. Lam, Total fluorine, extractable organic fluorine, perfluorooctane sulfonate and other related fluorochemicals in liver of Indo-Pacific humpback dolphins (Sousa chinensis) and finless porpoises (Neophocaena phocaenoides) from South China. Environ. Pollut. 2009, 157, 17.
| Total fluorine, extractable organic fluorine, perfluorooctane sulfonate and other related fluorochemicals in liver of Indo-Pacific humpback dolphins (Sousa chinensis) and finless porpoises (Neophocaena phocaenoides) from South China.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVGgsb3J&md5=c6341213a10a05446e9fc16ba29660dbCAS | 18805607PubMed |
[85] K. I. Van de Vijver, L. Hoslbeek, K. Das, R. Blust, C. Joiris, W. De Coen, Occurrence of perfluorooctane sulfonate and other perfluorinated alkylated substances in harbor porpoises from the Black Sea. Environ. Sci. Technol. 2007, 41, 315.
| Occurrence of perfluorooctane sulfonate and other perfluorinated alkylated substances in harbor porpoises from the Black Sea.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1SlsrzP&md5=64a8c39db6135f370cc0bbc3095a4d54CAS | 17265965PubMed |
[86] S. Sturman, J. Small, K. R. Solomon, D. C. G. Muir, Tissue distribution of polyfluorinated compounds from two ringed seal (Phoca hispida) populations in the Canadian Arctic, poster presentation at SETAC Europe 17th Annual Meeting, Porto, Portugal, 20–24 May 2007.
[87] K. Kannan, S. H. Yun, T. J. Evans, Chlorinated, brominated, and perfluorinated contaminants in livers of polar bears from Alaska. Environ. Sci. Technol. 2005, 39, 9057.
| Chlorinated, brominated, and perfluorinated contaminants in livers of polar bears from Alaska.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtFKhurzL&md5=184f479eafd13ccd0244fa84689b1993CAS | 16382925PubMed |
[88] L. W. Y. Yeung, N. Yamashita, S. Taniyasu, P. K. S. Lam, R. K. Sinha, D. V. Borole, K. Kannan, A survey of perfluorinated compounds in surface water and biota including dolphins from the Ganges River and in other waterbodies in India. Chemosphere 2009, 76, 55.
| A survey of perfluorinated compounds in surface water and biota including dolphins from the Ganges River and in other waterbodies in India.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXmtFSitrg%3D&md5=22f7e3e01f2605a6b47aec60d46e26b6CAS | 19328521PubMed |
[89] D. A. Ellis, J. W. Martin, A. O. De Silva, S. A. Mabury, M. D. Hurley, M. P. Sulbaek Andersen, T. J. Wallington, Degradation of fluorotelomer alcohols: a likely atmospheric source of perfluorinated carboxylic acids. Environ. Sci. Technol. 2004, 38, 3316.
| Degradation of fluorotelomer alcohols: a likely atmospheric source of perfluorinated carboxylic acids.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjvVShsb0%3D&md5=dd951d8e26efdf9b0eba3cdf75382d96CAS | 15260330PubMed |
[90] A. O. De Silva, S. A. Mabury, Isolating isomers of perfluorocarboxylates in polar bears (Ursus maritimus) from two geographical locations. Environ. Sci. Technol. 2004, 38, 6538.
| Isolating isomers of perfluorocarboxylates in polar bears (Ursus maritimus) from two geographical locations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXptF2lsr4%3D&md5=6a19de3c3dcfe3ab4e8a419122fd76a3CAS | 15669310PubMed |
[91] S. E. Loveless, C. Finlay, N. E. Everds, S. R. Frame, P. J. Gillies, J. C. O’Connor, C. R. Powley, G. L. Kennedy, Comparative responses of rats and mice exposed to linear/branched, linear, or branched ammonium perfluorooctanoate (APFO). Toxicology 2006, 220, 203.
| Comparative responses of rats and mice exposed to linear/branched, linear, or branched ammonium perfluorooctanoate (APFO).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhvVWksL8%3D&md5=68d4f93c144f0af22af61788e19811ddCAS | 16448737PubMed |
[92] M. Houde, R. S. Wells, P. A. Fair, G. D. Bossart, A. A. Hohn, T. K. Rowles, J. C. Sweeney, K. R. Solomon, D. C. G. Muir, Polyfluoroalkyl compounds in free-ranging bottlenose dolphins (Tursiops truncatus) from the Gulf of Mexico and the Atlantic Ocean. Environ. Sci. Technol. 2005, 39, 6591.
| Polyfluoroalkyl compounds in free-ranging bottlenose dolphins (Tursiops truncatus) from the Gulf of Mexico and the Atlantic Ocean.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXmsFWiu78%3D&md5=1ecf0d99530722b1a33def6ff6904dfeCAS | 16190216PubMed |
[93] P. R. Dorneles, J. Lailson-Brito, A. F. Azevedo, J. Meyer, L. G. Vidal, A. B. Fragoso, J. P. Torres, O. Malm, R. Blust, K. Das, High accumulation of perfluorooctane sulfonate (PFOS) in marine tucuxi dolphins (Sotalia guianensis) from the Brazilian coast. Environ. Sci. Technol. 2008, 42, 5368.
| High accumulation of perfluorooctane sulfonate (PFOS) in marine tucuxi dolphins (Sotalia guianensis) from the Brazilian coast.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXntF2rs7c%3D&md5=6c6d0c5e96380bdc7c3305a71eee3f1aCAS | 18754395PubMed |
[94] K. Kannan, S. Corsolini, J. Falandysz, G. Oehme, S. Focardi, J. P. Giesy, Perfluorooctanesulfonate and related fluorinated hydrocarbons in marine mammals, fishes, and birds from coasts of the Baltic and the Mediterranean Seas. Environ. Sci. Technol. 2002, 36, 3210.
| Perfluorooctanesulfonate and related fluorinated hydrocarbons in marine mammals, fishes, and birds from coasts of the Baltic and the Mediterranean Seas.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xks1Kktbk%3D&md5=87bc2e29cd4d8f2d98f2e6cf256cc720CAS | 12188342PubMed |
[95] R. Bossi, F. F. Riget, R. Dietz, C. Sonne, P. Fauser, M. Dam, K. Vorkamp, Preliminary screening of perfluorooctane sulfonate (PFOS) and other fluorochemicals in fish, birds and marine mammals from Greenland and the Faroe Islands. Environ. Pollut. 2005, 136, 323.
| Preliminary screening of perfluorooctane sulfonate (PFOS) and other fluorochemicals in fish, birds and marine mammals from Greenland and the Faroe Islands.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXjtlahu74%3D&md5=1118b07d226548b5de18d6be03547d95CAS | 15840540PubMed |
[96] G. T. Tomy, W. Budakowski, T. Halldorson, P. A. Helm, G. A. Stern, K. Friesen, K. Pepper, S. A. Tittlemier, A. T. Fisk, Fluorinated organic compounds in an eastern Arctic marine food web. Environ. Sci. Technol. 2004, 38, 6475.
| Fluorinated organic compounds in an eastern Arctic marine food web.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXovFSmtbY%3D&md5=b8eea344c625d6afd345cd2bc3dd83b7CAS | 15669302PubMed |
[97] K. I. Van de Vijver, P. T. Hoff, K. Das, W. Van Dongen, E. L. Esmans, T. Jauniaux, J.-M. Bouquegneau, R. Blust, W. De Coen, Perfluorinated chemicals infiltrate ocean waters: link between exposure levels and stable isotope ratios in marine mammals. Environ. Sci. Technol. 2003, 37, 5545.
| Perfluorinated chemicals infiltrate ocean waters: link between exposure levels and stable isotope ratios in marine mammals.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXos1SltrY%3D&md5=495efd8c9adb88cc2155eb35b257aa96CAS | 14717162PubMed |
[98] B. C. Kelly, M. G. Ikonomou, J. D. Blair, B. Surridge, D. Hoover, R. Grace, F. A. P. C. Gobas, Perfluoroalkyl contaminants in an Arctic marine food web: trophic magnification and wildlife exposure. Environ. Sci. Technol. 2009, 43, 4037.
| Perfluoroalkyl contaminants in an Arctic marine food web: trophic magnification and wildlife exposure.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXlt1Shu7k%3D&md5=2dab31854d303ccdf9c3048686d94613CAS | 19569327PubMed |
[99] M. Smithwick, D. C. G. Muir, S. A. Mabury, K. R. Solomon, J. W. Martin, C. Sonne, E. W. Born, R. J. Letcher, R. Dietz, Perfluoroalkyl contaminants in liver tissue from east Greenland polar bears (Ursus maritimus). Environ. Toxicol. Chem. 2005, 24, 981.
| Perfluoroalkyl contaminants in liver tissue from east Greenland polar bears (Ursus maritimus).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXivVGgsrc%3D&md5=8c04729def624996f472db2b7a2bad5aCAS | 15839574PubMed |
[100] M. Smithwick, S. A. Mabury, K. R. Solomon, C. Sonne, J. W. Martin, E. W. Born, R. Dietz, A. E. Derocher, R. J. Letcher, T. J. Evans, G. W. Gabrielsen, J. Nagy, I. Stirling, M. K. Taylor, D. C. G. Muir, Circumpolar study of perfluoroalkyl contaminants in polar bears (Ursus maritimus). Environ. Sci. Technol. 2005, 39, 5517.
| Circumpolar study of perfluoroalkyl contaminants in polar bears (Ursus maritimus).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXltlGns7o%3D&md5=3dd99a9726bf3811e641517447a79ab6CAS | 16124282PubMed |
[101] K. Kannan, Accumulation of perfluorooctane sulfonate in marine mammals. Environ. Sci. Technol. 2001, 35, 1593.
| Accumulation of perfluorooctane sulfonate in marine mammals.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXhvVSgsbk%3D&md5=c8b5dafc3f6a050a8f8e2199fd8393edCAS | 11329707PubMed |
[102] G. T. Tomy, K. Pleskach, S. H. Ferguson, J. Hare, G. Stern, G. MacInnis, C. H. Marvin, L. Loseto, Trophodynamics of some PFCs and BFRs in a western Canadian Arctic marine food web. Environ. Sci. Technol. 2009, 43, 4076.[Published online ahead of print 4 May 2009]
| Trophodynamics of some PFCs and BFRs in a western Canadian Arctic marine food web.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXltlKksbg%3D&md5=76f3b7e89bc796136670e77c3c7aac01CAS | 19569333PubMed |
[103] L. Tao, K. Kannan, N. Kajiwara, M. M. Costa, G. Fillmann, S. Takahashi, S. Tanabe, Perfluorooctanesulfonate and related fluorochemicals in albatrosses, elephant seals, penguins, and Polar Skuas from the Southern Ocean. Environ. Sci. Technol. 2006, 40, 7642.
| Perfluorooctanesulfonate and related fluorochemicals in albatrosses, elephant seals, penguins, and Polar Skuas from the Southern Ocean.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtFelt7%2FE&md5=d6c707e995b85728b1babdae6eb6ce60CAS | 17256507PubMed |
[104] M. Houde, B. C. Balmer, S. Brandsma, R. S. Wells, T. K. Rowles, K. R. Solomon, D. C. G. Muir, Perfluoroalkyl compounds in relation to life-history and reproductive parameters in bottlenose dolphins (Tursiops truncatus) from Sarasota Bay, Florida, USA. Environ. Toxicol. Chem. 2006, 25, 2405.
| Perfluoroalkyl compounds in relation to life-history and reproductive parameters in bottlenose dolphins (Tursiops truncatus) from Sarasota Bay, Florida, USA.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XovVOrt74%3D&md5=9098ca3038a3fbfe0e535e81b7c4917bCAS | 16986796PubMed |
[105] C. M. Butt, S. A. Mabury, D. C. G. Muir, B. M. Braune, Prevalence of long-chained perfluorinated carboxylates in seabirds from the canadian arctic between 1975 and 2004. Environ. Sci. Technol. 2007, 41, 3521.
| Prevalence of long-chained perfluorinated carboxylates in seabirds from the canadian arctic between 1975 and 2004.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXkt1OrsLc%3D&md5=916b78ca744632337e8f52e564b442a4CAS | 17547173PubMed |
[106] J. Verreault, M. Houde, G. W. Gabrielsen, U. Berger, M. Hauks, R. J. Letcher, D. C. G. Muir, Perfluorinated alkyl substances in plasma, liver, brain, and eggs of glaucous gulls (Larus hyperboreus) from the Norwegian Arctic. Environ. Sci. Technol. 2005, 39, 7439.
| Perfluorinated alkyl substances in plasma, liver, brain, and eggs of glaucous gulls (Larus hyperboreus) from the Norwegian Arctic.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXpsFOrtro%3D&md5=f144bcb6d06fd3b0b06c49e1c83c39d8CAS | 16245813PubMed |
[107] J. Verreault, U. Berger, G. W. Gabrielsen, Trends of perfluorinated alkyl substances in herring gull eggs from two coastal colonies in northern Norway: 1983–2003. Environ. Sci. Technol. 2007, 41, 6671.
| Trends of perfluorinated alkyl substances in herring gull eggs from two coastal colonies in northern Norway: 1983–2003.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtVSjsbnM&md5=467092453029254958de6e885e4f22d9CAS | 17969679PubMed |
[108] W. A. Gebbink, C. E. Hebert, R. J. Letcher, Perfluorinated carboxylates and sulfonates and precursor compounds in herring gull eggs from colonies spanning the Laurentian Great Lakes of North America. Environ. Sci. Technol. 2009, 43, 7443.
| Perfluorinated carboxylates and sulfonates and precursor compounds in herring gull eggs from colonies spanning the Laurentian Great Lakes of North America.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtVKmur3I&md5=28104fba44ced8256cc74c09ca6a922eCAS | 19848159PubMed |
[109] K. E. Holmström, U. Järnberg, A. Bignert, Temporal trends of PFOS and PFOA in guillemot eggs from the Baltic Sea, 1968–2003. Environ. Sci. Technol. 2005, 39, 80.
| Temporal trends of PFOS and PFOA in guillemot eggs from the Baltic Sea, 1968–2003.Crossref | GoogleScholarGoogle Scholar | 15667078PubMed |
[110] K. Kannan, J.-W. Choi, N. Iseki, K. Senthilkumar, D. H. Kim, S. Masunaga, J. P. Giesy, Concentrations of perfluorinated acids in livers of birds from Japan and Korea. Chemosphere 2002, 49, 225.
| Concentrations of perfluorinated acids in livers of birds from Japan and Korea.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xms12isLs%3D&md5=5235fdca6732d8a310272422078bdb9fCAS | 12363300PubMed |
[111] K. Löfstrand, H. Jorundsdottir, G. Tomy, J. Svavarsson, P. Weihe, T. Nygard, A. Bergman, Spatial trends of polyfluorinated compounds in guillemot (Uria aalge) eggs from North-Western Europe. Chemosphere 2008, 72, 1475.
| Spatial trends of polyfluorinated compounds in guillemot (Uria aalge) eggs from North-Western Europe.Crossref | GoogleScholarGoogle Scholar | 18573516PubMed |
[112] E. Sinclair, D. T. Mayack, K. Roblee, N. Yamashita, K. Kannan, Occurrence of perfluoroalkyl surfactants in water, fish, and birds from New York State. Arch. Environ. Contam. Toxicol. 2006, 50, 398.
| Occurrence of perfluoroalkyl surfactants in water, fish, and birds from New York State.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XjsV2hsrc%3D&md5=c655a056947b110dff2f8a7f168757baCAS | 16435086PubMed |
[113] K. E. Holmström, U. Berger, Tissue distribution of perfluorinated surfactants in common guillemot (Uria aalge) from the Baltic Sea. Environ. Sci. Technol. 2008, 42, 5879.
| Tissue distribution of perfluorinated surfactants in common guillemot (Uria aalge) from the Baltic Sea.Crossref | GoogleScholarGoogle Scholar | 18767639PubMed |
[114] J. Meyer, V. L. B. Jaspers, M. Eens, W. de Coen, The relationship between perfluorinated chemical levels in the feathers and livers of birds from different trophic levels. Sci. Total Environ. 2009, 407, 5894.
| The relationship between perfluorinated chemical levels in the feathers and livers of birds from different trophic levels.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFCqu77L&md5=7a23a436c8566351cb662493266fffdcCAS | 19716165PubMed |
[115] J. Olivero-Verbel, L. Tao, B. Johnson-Restrepo, J. Guette-Fernandez, R. Baldiris-Avila, I. O’Byrne-Hoyos, K. Kannan, Perfluorooctanesulfonate and related fluorochemicals in biological samples from the north coast of Colombia. Environ. Pollut. 2006, 142, 367.
| Perfluorooctanesulfonate and related fluorochemicals in biological samples from the north coast of Colombia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xjs1aitrk%3D&md5=3da5c5fe9bc928d5ce78f84168436511CAS | 16303219PubMed |
[116] Y. Wang, L. W. Y. Yeung, S. Taniyasu, N. Yamashita, J. C. W. Lam, P. K. S. Lam, Perfluorooctane sulfonate and other fluorochemicals in waterbird eggs from South China. Environ. Sci. Technol. 2008, 42, 8146.
| Perfluorooctane sulfonate and other fluorochemicals in waterbird eggs from South China.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtFOqt7nM&md5=a32ab307bb737c93f729ebb8af026dd7CAS | 19031916PubMed |
[117] K. Senthilkumar, E. Ohi, K. Sajwan, T. Takasuga, K. Kannan, Perfluorinated compounds in river water, river sediment, market fish, and wildlife samples from Japan. Bull. Environ. Contam. Toxicol. 2007, 79, 427.
| Perfluorinated compounds in river water, river sediment, market fish, and wildlife samples from Japan.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtFGgsL%2FF&md5=b6bc4cfd61fbfcafcd91d5daf94def21CAS | 17634852PubMed |
[118] J. O. Bustnes, K. Borga, K. E. Erikstad, S. H. Lorentsen, D. Herzke, Perfluorinated, brominated, and chlorinated contaminants in a population of lesser black-backed gulls (Larus fuscus). Environ. Toxicol. Chem. 2008, 27, 1383.
| Perfluorinated, brominated, and chlorinated contaminants in a population of lesser black-backed gulls (Larus fuscus).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXmsVGltbo%3D&md5=69ae4075b3d9f1aaafd0157dcc2b1881CAS | 18229973PubMed |
[119] J. P. Giesy, K. Kannan, Global distribution of perfluorooctane sulfonate in wildlife. Environ. Sci. Technol. 2001, 35, 1339.
| Global distribution of perfluorooctane sulfonate in wildlife.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXhsVGnurg%3D&md5=60c8a4e10f918a66182166bc8dce2430CAS | 11348064PubMed |
[120] K. Kannan, L. Tao, E. Sinclair, S. D. Pastva, D. J. Jude, J. P. Giesy, Perfluorinated compounds in aquatic organisms at various trophic levels in a Great Lakes food chain. Arch. Environ. Contam. Toxicol. 2005, 48, 559.
| Perfluorinated compounds in aquatic organisms at various trophic levels in a Great Lakes food chain.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXksVaisrY%3D&md5=ce1b88c0c019cd9d51f46fb37e834308CAS | 15883668PubMed |
[121] J. O. Bustnes, K. E. Erikstad, S.-H. Lorentsen, D. Herzke, Perfluorinated and chlorinated pollutants as predictors of demographic parameters in an endangered seabird. Environ. Pollut. 2008, 156, 417.
| Perfluorinated and chlorinated pollutants as predictors of demographic parameters in an endangered seabird.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXht12rtLnJ&md5=c225c177f3dc280348ce3cf19c89853eCAS | 18329768PubMed |
[122] V. I. Furdui, P. A. Helm, P. W. Crozier, C. Lucaciu, E. J. Reiner, C. H. Marvin, D. M. Whittle, S. A. Mabury, G. T. Tomy, Temporal trends of perfluoroalkyl compounds with isomer analysis in lake trout from Lake Ontario (1979–2004). Environ. Sci. Technol. 2008, 42, 4739.
| Temporal trends of perfluoroalkyl compounds with isomer analysis in lake trout from Lake Ontario (1979–2004).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXmsFOltbc%3D&md5=7ca472835b8b4e66fed3a221bea51c9aCAS | 18677999PubMed |
[123] J. W. Martin, D. M. Whittle, D. C. G. Muir, S. A. Mabury, Perfluoroalkyl contaminants in a food web from lake Ontario. Environ. Sci. Technol. 2004, 38, 5379.
| Perfluoroalkyl contaminants in a food web from lake Ontario.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXnsFOgt7Y%3D&md5=44ca8551ac7f919956ce655db7e383f0CAS | 15543740PubMed |
[124] P. T. Hoff, K. Van de Vijver, W. Van Dongen, E. L. Esmans, R. Blust, W. M. De Coen, Perfluorooctane sulfonic acid in bib (Trisopterus luscus) and plaice (Pleuronectes platessa) from the Western Scheldt and the Belgian North Sea: distribution and biochemical effects. Environ. Toxicol. Chem. 2003, 22, 608.
| 1:CAS:528:DC%2BD3sXhtlCkur0%3D&md5=222be1c991c1de66fe422e652e6dcb51CAS | 12627649PubMed |
[125] H. Peng, Q. Wei, Y. Wan, J. P. Giesy, L. Li, J. Hu, Tissue distribution and maternal transfer of poly- and perfluorinated compounds in Chinese sturgeon (Acipenser sinensis): implications for reproductive risk. Environ. Sci. Technol. 2010, 44, 1868.
| Tissue distribution and maternal transfer of poly- and perfluorinated compounds in Chinese sturgeon (Acipenser sinensis): implications for reproductive risk.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhslSit7o%3D&md5=70b5faa65da904a140e484d6e0dd9a03CAS | 20143820PubMed |
[126] N. Quinete, Q. Wu, T. Zhang, S. H. Yun, I. Moreira, K. Kannan, Specific profiles of perfluorinated compounds in surface and drinking waters and accumulation in mussels, fish, and dolphins from southeastern Brazil. Chemosphere 2009, 77, 863.
| Specific profiles of perfluorinated compounds in surface and drinking waters and accumulation in mussels, fish, and dolphins from southeastern Brazil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXht1Gnt77I&md5=3dbe9d43f6a3d3cf958d7a8a9e71c8adCAS | 19744696PubMed |
[127] K. Senthil Kumar, Y. Zushi, S. Masunaga, M. Gilligan, C. Pride, S. Sajwan Kenneth, Perfluorinated organic contaminants in sediment and aquatic wildlife, including sharks, from Georgia, USA. Mar. Pollut. Bull. 2009, 58, 621.
| Perfluorinated organic contaminants in sediment and aquatic wildlife, including sharks, from Georgia, USA.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXktVGqtrc%3D&md5=9e3225f41a6d9a5216023e8f613cd7caCAS | 19152950PubMed |
[128] J. M. Conder, R. A. Hoke, W. de Wolf, M. H. Russell, R. C. Buck, Are PFCAs bioaccumulative? A critical review and comparison with regulatory criteria and persistent lipophilic compounds. Environ. Sci. Technol. 2008, 42, 995.
| Are PFCAs bioaccumulative? A critical review and comparison with regulatory criteria and persistent lipophilic compounds.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXltFWmsw%3D%3D&md5=970ac0cbff0f1069d759ab5a78ec5b3cCAS | 18351063PubMed |
[129] A. Gulkowska, Q. Jiang, M. K. So, S. Taniyasu, P. K. S. Lam, N. Yamashita, Persistent perfluorinated acids in seafood collected from two cities of China. Environ. Sci. Technol. 2006, 40, 3736.
| Persistent perfluorinated acids in seafood collected from two cities of China.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XksValt7s%3D&md5=6155b5cd9b1192c54e0f1e6357be80f0CAS | 16830535PubMed |
[130] K. Hart, K. Kannan, L. Tao, S. Takahashi, S. Tanabe, Skipjack tuna as a bioindicator of contamination by perfluorinated compounds in the oceans. Sci. Total Environ. 2008, 403, 215.
| Skipjack tuna as a bioindicator of contamination by perfluorinated compounds in the oceans.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXptlemsLw%3D&md5=79609087437b8766678247a0d88077bcCAS | 18619650PubMed |
[131] H. Yoo, N. Yamashita, S. Taniyasu, T. Lee Kyu, D. Jones Paul, L. Newsted John, S. Khim Jong, P. Giesy John, Perfluoroalkyl acids in marine organisms from Lake Shihwa, Korea. Arch. Environ. Contam. Toxicol. 2009, 57, 552.
| Perfluoroalkyl acids in marine organisms from Lake Shihwa, Korea.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFens7jO&md5=d356b214d52a3c4b4947008b15c3a257CAS | 19152061PubMed |
[132] Y. Shi, Y. Pan, R. Yang, Y. Wang, Y. Cai, Occurrence of perfluorinated compounds in fish from Qinghai-Tibetan Plateau. Environ. Int. 2010, 36, 46.
| Occurrence of perfluorinated compounds in fish from Qinghai-Tibetan Plateau.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsV2mtL7I&md5=0514727e15456e15ed024b8ff7677821CAS | 19836079PubMed |
[133] L. Ahrens, N. Marusczak, J. Rubarth, A. Dommergue, R. Nedjai, C. Ferrari, R. Ebinghaus, Distribution of perfluoroalkyl compounds and mercury in fish liver from high-mountain lakes in France originating from atmospheric deposition. Environ. Chem. 2010, 7, 422.
| Distribution of perfluoroalkyl compounds and mercury in fish liver from high-mountain lakes in France originating from atmospheric deposition.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsFChsrfF&md5=76a86f1cc06b9cc78e13948843678281CAS |
[134] A. Dreyer, I. Weinberg, C. Temme, R. Ebinghaus, Polyfluorinated compounds in the atmosphere of the Atlantic and Southern Oceans: evidence for a global distribution. Environ. Sci. Technol. 2009, 43, 6507.
| Polyfluorinated compounds in the atmosphere of the Atlantic and Southern Oceans: evidence for a global distribution.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXptlSmsLc%3D&md5=cb76c8da5b46abf7a6757e8525c308cbCAS | 19764209PubMed |
[135] R. Kallenborn, U. Berger, U. Järnberg, Perfluorinated alkylated substances (PFAS) in the nordic environment 2004 (Nordic Council of Ministers: Copenhagen). Available at www.klif.no/nyheter/dokumenter/pfas_nmr2004.pdf [Verified 23 November 2010].
[136] Y. Zushi, M. Tamada, Y. Kanai, S. Masunaga, Time trends of perfluorinated compounds from the sediment core of Tokyo Bay, Japan (1950s–2004). Environ. Pollut. 2010, 158, 756.
| Time trends of perfluorinated compounds from the sediment core of Tokyo Bay, Japan (1950s–2004).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXot1Omtg%3D%3D&md5=c3af678cf7be649ae09bec7cb2dd137eCAS | 19883962PubMed |
[137] L. Ahrens, N. Yamashita, L. W. Y. Yeung, S. Taniyasu, Y. Horii, P. K. S. Lam, R. Ebinghaus, Partitioning behavior of per- and polyfluoroalkyl compounds between pore water and sediment in two sediment cores from Tokyo Bay, Japan. Environ. Sci. Technol. 2009, 43, 6969.
| Partitioning behavior of per- and polyfluoroalkyl compounds between pore water and sediment in two sediment cores from Tokyo Bay, Japan.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtVWnurrM&md5=d43f6b8f3ac5480a30d14af4e8963b48CAS | 19806729PubMed |
[138] K. H. Harada, The influence of time, sex and geographic factors on levels of perfluorooctane sulfonate und perfluorooctanoate in human serum over the last 25 years. J. Occup. Health 2004, 46, 141.
| The influence of time, sex and geographic factors on levels of perfluorooctane sulfonate und perfluorooctanoate in human serum over the last 25 years.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjslersbg%3D&md5=97e3a63fd662c91ab9e1e57d602d88f4CAS | 15090689PubMed |
[139] K. H. Harada, S. Hashida, T. Kaneko, K. Takenaka, M. Minata, K. Inoue, N. Saito, A. Koizumi, Biliary excretion and cerebrospinal fluid partition of perfluorooctanoate and perfluorooctane sulfonate in humans. Environ. Toxicol. Pharmacol. 2007, 24, 134.
| Biliary excretion and cerebrospinal fluid partition of perfluorooctanoate and perfluorooctane sulfonate in humans.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXot1yjsbw%3D&md5=fb03d59da85639104dd6bfc6164c727eCAS |
[140] A. A. Jensen, P. B. Poulsen, R. Bossi, Survey and environmental/health assessment of fluorinated substances in impregnated consumer products and impregnating agents, Survey of Chemical Substances in Consumer Products, No. 99 2008 (Danish Ministry of the Environment, EPA). Available at http://www.mst.dk/Publikationer/Publications/2008/10/978-87-7052-845-0.htm [Verified 23 November 2010].
[141] J. Armitage, I. T. Cousins, R. C. Buck, K. Prevedouros, M. H. Russell, M. MacLeod, S. H. Korzeniowski, Modeling global-scale fate and transport of perfluorooctanoate emitted from direct sources. Environ. Sci. Technol. 2006, 40, 6969.
| Modeling global-scale fate and transport of perfluorooctanoate emitted from direct sources.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtV2qs7%2FK&md5=2f4aead417454cd7630ac27fb0c54157CAS | 17154003PubMed |
[142] K. J. Hansen, L. A. Clemen, M. E. Ellefson, H. O. Johnson, Compound-specific, quantitative characterization of organic fluorochemicals in biological matrices. Environ. Sci. Technol. 2001, 35, 766.
| Compound-specific, quantitative characterization of organic fluorochemicals in biological matrices.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXivValtA%3D%3D&md5=f1859e6a251b8f03840feffbfb9d312dCAS | 11349290PubMed |
[143] G. T. Tomy, S. A. Tittlemier, V. P. Palace, W. R. Budakowski, E. Braekevelt, L. Brinkworth, K. Friesen, Biotransformation of N-ethyl perfluorooctanesulfonamide by rainbow trout (Onchorhynchus mykiss) liver microsomes. Environ. Sci. Technol. 2004, 38, 758.
| Biotransformation of N-ethyl perfluorooctanesulfonamide by rainbow trout (Onchorhynchus mykiss) liver microsomes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXptl2mtLY%3D&md5=6f5d978df482f7831fbb531b14b479ecCAS | 14968861PubMed |
[144] S. K. Ostertag, B. A. Tague, M. M. Humphries, S. A. Tittlemier, H. M. Chan, Estimated dietary exposure to fluorinated compounds from traditional foods among Inuit in Nunavut, Canada. Chemosphere 2009, 75, 1165.
| Estimated dietary exposure to fluorinated compounds from traditional foods among Inuit in Nunavut, Canada.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXlsVGqtrw%3D&md5=488a6b9202da946ed7068e537a88593aCAS | 19342075PubMed |
[145] J. Leonel, K. Kannan, L. Tao, G. Fillmann, R. C. Montone, A baseline study of perfluorochemicals in Franciscana dolphin and Subantarctic fur seal from coastal waters of Southern Brazil. Mar. Pollut. Bull. 2008, 56, 778.
| 1:CAS:528:DC%2BD1cXktV2ltbY%3D&md5=f1e8c00b7c7b57f4a1268840159a2814CAS | 18295806PubMed |
[146] L. T. Quakenbush, J. J. Citta, Perfluorinated contaminants in ringed, bearded, spotted, and ribbon seals from the Alaskan Bering and Chukchi Seas. Mar. Pollut. Bull. 2008, 56, 1809.
| Perfluorinated contaminants in ringed, bearded, spotted, and ribbon seals from the Alaskan Bering and Chukchi Seas.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtFOlsb3P&md5=df920ef98cbbb293285425ca18a113acCAS | 18656906PubMed |
[147] R. J. Law, P. Bersuder, L. K. Mead, P. D. Jepson, PFOS and PFOA in the livers of harbour porpoises (Phocoena phocoena) stranded or bycaught around the UK. Mar. Pollut. Bull. 2008, 56, 792.
| PFOS and PFOA in the livers of harbour porpoises (Phocoena phocoena) stranded or bycaught around the UK.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXktV2lur8%3D&md5=7382e3b9811ff043c42e8fbac807f4dbCAS | 18281063PubMed |
[148] H. Nakata, K. Kannan, T. Nasu, H. S. Cho, E. Sinclair, A. Takemura, Perfluorinated contaminants in sediments and aquatic organisms collected from shallow water and tidal flat areas of the Ariake Sea, Japan: environmental fate of perfluorooctane sulfonate in aquatic ecosystems. Environ. Sci. Technol. 2006, 40, 4916.
| Perfluorinated contaminants in sediments and aquatic organisms collected from shallow water and tidal flat areas of the Ariake Sea, Japan: environmental fate of perfluorooctane sulfonate in aquatic ecosystems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xmslaqsrc%3D&md5=38db4e0075ce86566eb12dacd5d97743CAS | 16955886PubMed |
[149] A. Schiavone, S. Corsolini, K. Kannan, L. Tao, W. Trivelpiece, D. Torres, S. Focardi, Perfluorinated contaminants in fur seal pups and penguin eggs from South Shetland, Antarctica. Sci. Total Environ. 2009, 407, 3899.
| Perfluorinated contaminants in fur seal pups and penguin eggs from South Shetland, Antarctica.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXltValsr0%3D&md5=ecdedf24d2e7af40f4dd2468cb735d49CAS | 19321191PubMed |
[150] L. B. Knudsen, K. Borga, E. H. Jorgensen, B. van Bavel, M. Schlabach, J. Verreault, G. W. Gabrielsen, Halogenated organic contaminants and mercury in northern fulmars (Fulmarus glacialis): levels, relationships to dietary descriptors and blood to liver comparison. Environ. Pollut. 2007, 146, 25.
| Halogenated organic contaminants and mercury in northern fulmars (Fulmarus glacialis): levels, relationships to dietary descriptors and blood to liver comparison.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtlart7k%3D&md5=1b017b0285886f1711c85e2a5c36f6bcCAS | 17000037PubMed |
[151] M. Haukås, U. Berger, H. Hop, B. Gulliksen, G. W. Gabrielsen, Bioaccumulation of per- and polyfluorinated alkyl substances (PFAS) in selected species from the Barents Sea food web. Environ. Pollut. 2007, 148, 360.
| Bioaccumulation of per- and polyfluorinated alkyl substances (PFAS) in selected species from the Barents Sea food web.Crossref | GoogleScholarGoogle Scholar | 17258363PubMed |
[152] D. Herzke, T. Nygard, U. Berger, S. Huber, N. Rov, Perfluorinated and other persistent halogenated organic compounds in European shag (Phalacrocorax aristotelis) and common eider (Somateria mollissima) from Norway: a suburban to remote pollutant gradient. Sci. Total Environ. 2009, 408, 340.
| Perfluorinated and other persistent halogenated organic compounds in European shag (Phalacrocorax aristotelis) and common eider (Somateria mollissima) from Norway: a suburban to remote pollutant gradient.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsVWqsbvL&md5=3a1754ba6ff0573532ed14f1fb7e0396CAS | 19836057PubMed |
[153] C. Miljeteig, H. Strom, M. V. Gavrilo, A. Volkov, B. M. Jenssen, G. W. Gabrielsen, High levels of contaminants in ivory gull (Pagophila eburnea) eggs from the Russian and Norwegian Arctic. Environ. Sci. Technol. 2009, 43, 5521.
| High levels of contaminants in ivory gull (Pagophila eburnea) eggs from the Russian and Norwegian Arctic.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXmvFOhsrw%3D&md5=40580ff83377a861852b4af1916de4d2CAS | 19708391PubMed |
[154] H. Yoo, K. Kannan, S. K. Kim, K. T. Lee, J. L. Newsted, J. P. Giesy, Perfluoroalkyl acids in the egg yolk of birds from Lake Shihwa, Korea. Environ. Sci. Technol. 2008, 42, 5821.
| Perfluoroalkyl acids in the egg yolk of birds from Lake Shihwa, Korea.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXnvFCqurc%3D&md5=17619ed3bb320e27aa4ce727b9ad8045CAS | 18754515PubMed |
[155] A. Strid, G. Tomy, N. Ismail, O. Päpke, J. Svavarsson, A. Bergman, Halogenated environmental pollutants in Greenland shark (Somniosus microcephalus) from the north-east Atlantic. Organohalogen Compd. 2006, 68, 2055.
| 1:CAS:528:DC%2BD1MXjsFCms7g%3D&md5=e02e8521ff35f9176d34fe6b053e705fCAS |
[156] L. Ahrens, R. Ebinghaus, Spatial distribution of polyfluoroalkyl compounds in dab (Limanda limanda) bile fluids from Iceland and the North Sea. Mar. Pollut. Bull. 2010, 60, 145.
| Spatial distribution of polyfluoroalkyl compounds in dab (Limanda limanda) bile fluids from Iceland and the North Sea.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXktlWjtA%3D%3D&md5=0bf922588e3e5182b33b0ea91e254652CAS | 19897214PubMed |
[157] M. Houde, T. A. D. Bujas, J. Small, R. S. Wells, P. A. Fair, G. D. Bossart, K. R. Solomon, D. C. G. Muir, Biomagnification of perfluoroalkyl compounds in the bottlenose dolphin (Tursiops truncatus) food web. Environ. Sci. Technol. 2006, 40, 4138.
| Biomagnification of perfluoroalkyl compounds in the bottlenose dolphin (Tursiops truncatus) food web.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XkvVGgt7s%3D&md5=854df743a58275a9b07b98be9f419c9aCAS | 16856728PubMed |
[158] U. Berger, A. Glynn, K. E. Holmström, M. Berglund, E. H. Ankarberg, A. Toernkvist, Fish consumption as a source of human exposure to perfluorinated alkyl substances in Sweden – analysis of edible fish from Lake Vaettern and the Baltic Sea. Chemosphere 2009, 76, 799.
| Fish consumption as a source of human exposure to perfluorinated alkyl substances in Sweden – analysis of edible fish from Lake Vaettern and the Baltic Sea.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXos12ksrk%3D&md5=a88f57af37852ccd8c1f465b20b716adCAS | 19457539PubMed |
[159] X. M. Li, L. W. Y. Yeung, M. Q. Xu, S. Taniyasu, P. K. S. Lam, N. Yamashita, J. Y. Dai, Perfluorooctane sulfonate (PFOS) and other fluorochemicals in fish blood collected near the outfall of wastewater treatment plant (WWTP) in Beijing. Environ. Pollut. 2008, 156, 1298.
| Perfluorooctane sulfonate (PFOS) and other fluorochemicals in fish blood collected near the outfall of wastewater treatment plant (WWTP) in Beijing.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVSgtLnF&md5=8b69d01a50efd92d71d43006954fdd32CAS | 18439735PubMed |
[160] C. Kubwabo, N. Vais, F. M. Benoit, A pilot study on the determination of perfluorooctanesulfonate and other perfluorinated compounds in blood of Canadians. J. Environ. Monit. 2004, 6, 540.
| A pilot study on the determination of perfluorooctanesulfonate and other perfluorinated compounds in blood of Canadians.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXksVCht7g%3D&md5=1972d20c5dc85a5b82b2b1e2a5111938CAS | 15173906PubMed |
[161] A. Kärrman, J. F. Mueller, B. Van Bavel, F. Harden, L.-M. L. Toms, G. Lindström, Levels of 12 perfluorinated chemicals in pooled Australian serum, collected 2002–2003, in relation to age, gender, and region. Environ. Sci. Technol. 2006, 40, 3742.
| Levels of 12 perfluorinated chemicals in pooled Australian serum, collected 2002–2003, in relation to age, gender, and region.Crossref | GoogleScholarGoogle Scholar | 16830536PubMed |
[162] H. Fromme, O. Midasch, D. Twardella, J. Angerer, S. Boehmer, B. Liebl, Occurrence of perfluorinated substances in an adult German population in southern Bavaria. Int. Arch. Occup. Environ. Health 2007, 80, 313.
| Occurrence of perfluorinated substances in an adult German population in southern Bavaria.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXnvVShsA%3D%3D&md5=eceb1c4c2dc35325f6d36bcba66c055aCAS | 16915390PubMed |
[163] K. S. Guruge, S. Taniyasu, N. Yamashita, S. Wijeratna, K. M. Mohotti, H. R. Seneviratne, K. Kannan, N. Yamanaka, S. Miyazaki, Perfluorinated organic compounds in human blood serum and seminal plasma: a study of urban and rural tea worker populations in Sri Lanka. J. Environ. Monit. 2005, 7, 371.
| Perfluorinated organic compounds in human blood serum and seminal plasma: a study of urban and rural tea worker populations in Sri Lanka.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXis1yis78%3D&md5=498200faa9359a76dc237b308be6b664CAS | 15798805PubMed |