Is there any isotopic fractionation of nitrate associated with diffusion and advection?
Priscillia Semaoune A B , Mathieu Sebilo A , Joëlle Templier A and Sylvie Derenne AA Laboratoire de Biogéochimie et écologie des milieux continentaux, UMR CNRS/UPMC 7618, 4 place Jussieu, F-75252, Paris, France.
B Corresponding author. Email: semaoune.priscillia@gmail.com
Environmental Chemistry 9(2) 158-162 https://doi.org/10.1071/EN11143
Submitted: 18 November 2011 Accepted: 2 April 2012 Published: 4 May 2012
Journal Compilation © CSIRO Publishing 2012 Open Access CC BY-NC-ND
Environmental context. Anthropogenic nitrogen inputs have significant effects on terrestrial and aquatic ecosystems, the extent of which can be traced by using the natural stable isotopic composition of nitrate to integrate the sources of nitrogen and the biological processes of their production. In ecosystems, nitrates are transported by diffusion in water and advection of water masses, but these physical processes have not been characterised in terms of isotopic fractionation. We report experiments demonstrating that physical transport processes have a negligible effect on the isotopic composition of dissolved nitrate.
Abstract. We experimentally investigated the effect of the physical process of transport (diffusion and advection) on the isotopic composition of nitrate (δ15N and δ18O). Strict diffusion of nitrate in water was studied using a modified Richter apparatus. The combination of diffusion and advection processes was followed by elution of nitrate solution onto silica gel column. No significant isotopic fractionation was observed.
Throughout the last decades, increasing anthropogenic nitrogen inputs have significantly affected the nitrogen cycle in continental ecosystems. As a consequence, increasing nitrate contamination of surface waters and groundwaters has emerged as a major problem in many agricultural areas of the world.[1] Moreover, the effect and the fate of the excess nitrogen in the environment become a critical question. The use of isotopic biogeochemistry is a potentially powerful tool to allow a better characterisation of nitrate–nitrogen cycling dynamics in the environment.[2–4] The measurement of the naturally occurring stable isotopic composition of nitrate (i.e. δ15N and δ18O) integrates the sources of nitrogen and the processes affecting nitrate concentrations (mineralisation, nitrification and denitrification).[2] In ecosystems, complex systems involve generally sequential processes. The reaction between substrate and product can be limited by one step of transformation that has a lower reaction rate than the other steps. Isotopic fractionation (ϵ) cannot therefore be totally expressed; there are ‘apparent’ isotopic effects (ϵapp) that depend on the limiting process. However, only denitrification and nitrification processes are considered to generate isotopic fractionation, affecting the isotopic composition of nitrate, by enriching the substrate. As the soil is negatively charged, nitrates move freely through the soil environment by diffusion and advection, therefore diffusive fractionation could occur as demonstrated for other solute molecules.[5] Nevertheless, when the transport of nitrate is strictly limiting and its availability of nitrate is low, the biological fractioning-specific processes do not occur. In that case, the strong biological isotopic fractionation related to the higher level of energy needed to break or form chemical bonds involving 15N rather than 14N (primary isotope effects) is replaced by the isotopic effect due to diffusion depending on the mass difference between the isotopes. In spite of their major role, the influence of physical processes, such as diffusion and advection, on nitrate isotopic compositions have not been directly measured. More generally, the isotopic fractionation associated with the transport of nitrate is considered negligible and the influence of the nitrogen transport has not been experimentally studied.
The aim of this study was to determine experimentally the range of isotopic fractionation associated to these processes. First, we determined isotopic composition of nitrogen and oxygen of nitrate during diffusion in water. Then, we conducted the experiment through silica gel column where diffusion and advection participate simultaneously. The possible repercussion of nitrate transport on the isotopic fractionation associated with processes at the water column–sediment and plant–soil solution interfaces was discussed, where the transport is often limiting.
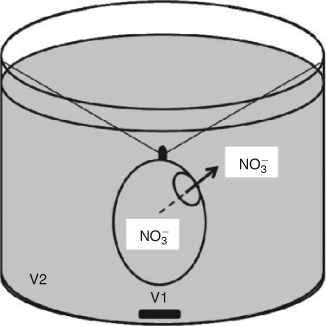
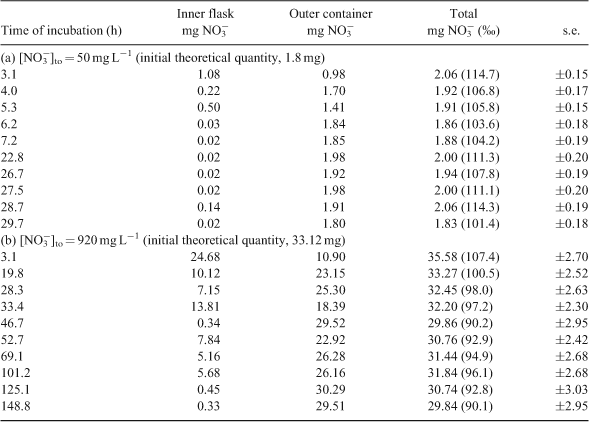
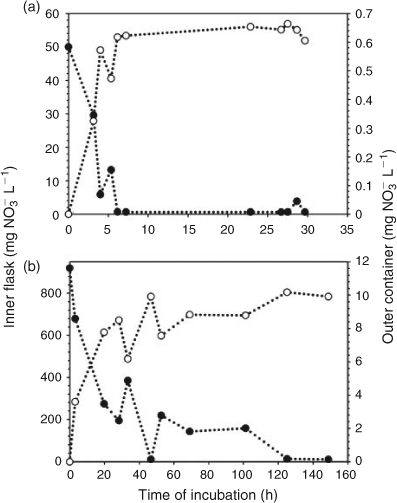
The strict diffusion process of nitrate in water was studied using previously designed experimental system (Fig. 1).[6] The dimensions of the containers were chosen in order to observe a slow diffusion. To test different kinetics, two nitrate concentrations (potassium nitrate in deionised water) were used: 50 mg L–1 corresponding to the World Health Organization drinking water standard and 920 mg L–1, an extreme concentration. The outer container was covered to minimise evaporation during the experiment. In a preliminary experiment, the profile of the kinetics was determined and the suitable durations for the measurements were chosen, ranging from 0 to 30 h for the lower and up to 150 h for the higher nitrate concentration. For each concentration, 10 batches were used and 1 batch was stopped by removing the inner flask at the times previously established from the preliminary experiment. For each batch, the nitrate concentration and δ15N and δ18O values of nitrate were measured for both inner flask and outer container. The mass balance was controlled.
|
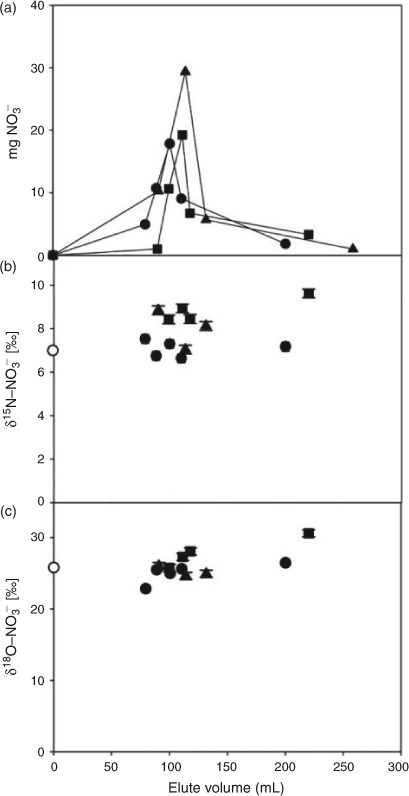
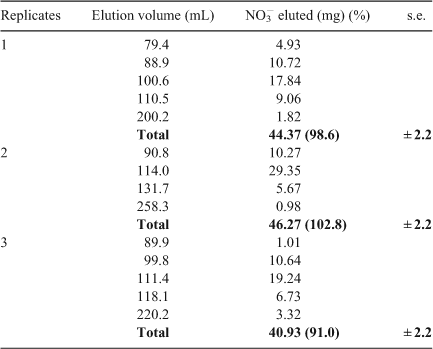
The combination of diffusion and advection processes was followed by elution of nitrate through a vertical column (height, 66 cm; diameter, 2.9 cm), filled with silica gel 60 (Sigma-Aldrich, Fallavier, France). A highly concentrated nitrate solution (45 mg of NO3– in 13 mL of deionised water) was pipetted on top of the column and deionised water was used for elution. Fractions of 1 mL were continuously collected. After elution of the dead volume of water and depending on the nitrate concentration, fractions had to be pooled to allow measurements. The experiment was performed in triplicate. Nitrate concentrations and δ15N and δ18O were measured. Mass balance was controlled.
Nitrate concentrations were determined by High-performance Liquid Chromatography (HPLC) (Dionex, AS12 column).
The isotopic composition of the nitrates was determined using the gas-bench method – a head space method allowing the measurement of δ15N and δ18O at low concentrations after chemical transformation into nitrous oxide (N2O). We adapted the two-step cadmium-azide method.[7] The main modification was the use of a granular cadmium-filled column instead of spongy cadmium to convert nitrate to nitrite, to simplify and accelerate the step and to ensure a complete conversion.[8]
The stable isotope composition of N2O was determined using the purge-and-trap and continuous-flow isotope ratio mass spectrometry system (DeltaVplus Thermo coupled with Gas Bench II). The method was calibrated with nitrate standards (USGS32, δ15N = +180 ‰, δ18O = +25.7 ‰; USGS34, δ15N = –1.8 ‰, δ18O = –27.9 ‰ and USGS35, δ15N = +2.7 ‰, δ18O = +57.5 ‰). The linearity of the analysis was checked with internal standard nitrate (KNO3, δ15N = +4.5 ‰, δ18O = +23 ‰). The precision was 0.5 ‰ for δ15N and 1 ‰ for δ18O.
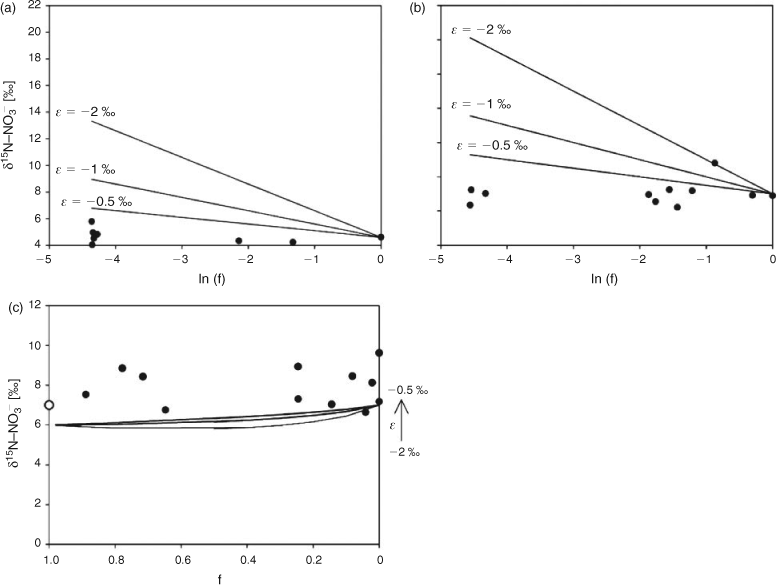
Nitrate diffusion in water from the inner flask to the outer container was observed for both initial nitrate concentrations. The kinetics of the nitrate diffusion (Table 1; Fig. 2a, b) were slightly different depending on the initial concentration. For 920 mg L–1 (Fig. 2b), a first step of fast diffusion was followed by a stationary phase before reaching the equilibrium concentration, whereas the equilibrium was directly and rapidly obtained for 50 mg L–1 (Fig. 2a). The discrepancy noticed for one or two measurements, related to the individuality of the batches, did not affect the general trend. For each batch, the nitrogen mass balance was quite satisfactory, due to the experimental difficulty to deliver exactly the same initial amount of nitrate and the accuracy of the HPLC measurement (±5 %) (Table 1). Thus, isotopic variations resulting from pollution or nitrate losses could be eliminated. The isotopic composition of nitrate along the diffusion process is reported as a function of nitrate flux (Fig. 3). For both concentrations, the isotopic composition of nitrate in inner-flask and outer-container solutions remained stable. For the experiment with 920 mg L–1 (δ15N, 6.9 ‰; δ18O, 25.8 ‰), the average values of δ15N and δ18O were 7.0 ‰ (standard deviation, 0.7 ‰) and 22.0 ‰ (1.1 ‰) in the flasks and 7.2 ‰ (0.3 ‰) and 24.5 ‰ (0.7 ‰) in the containers (Fig. 3c, d). In the same way, from the 50 mg L–1 nitrate solution (δ15N, 4.6 ‰; δ18O, 21.0 ‰) the average values of δ15N and δ18O were 4.3 ‰ (0.7 ‰) and 22.9 ‰ (2 ‰) in the flasks and 5.2 ‰ (1.4 ‰) and 25.4 ‰ (1.9 ‰) in the containers (Fig. 3a, b). No tendency to depletion or enrichment of δ15N and δ18O–NO3– was observed during the diffusion of nitrate. The theoretical isotopic enrichment of residual NO3– along kinetics diffusion was simulated with different amplitudes of ϵ (Fig. 4a, b), following Rayleigh equation:
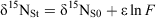
where δ15NS0 is the isotopic composition of the substrate at initial time 0, F = Ct/C0 with C0 and Ct the concentrations of substrate at time 0 and t. For both concentrations, experimental δ15N values were always lower than the simulated δ15N values even for ϵ = –0.5 ‰. According to the range of precision, no isotopic effect could be related to the nitrate flux. No significant fractionating process could therefore be associated to the strict diffusion of nitrate in water.
|
|
Nitrate transport through silica gel column allowed for us to follow diffusion and advection processes. The silica gel was inert, thus there was no chemical interaction with the solution. The nitrate was transported through the gel and recovered at the bottom of the column. A good reproducibility in elution kinetics was derived from the triplicate (Fig. 5a), with the maximum recovery of NO3– in very few fractions ~100 mL of elution. More than 90 % of total nitrate were eluted (Table 2). The δ15N–NO3– and δ18O–NO3– values of the different fractions, collected for each experiment, did not show any enrichment or depletion along with the elution (Fig. 5b, c). The mean values obtained from the three experiments were 7.9 ‰ for δ15N–NO3– (s.d., 1.0 ‰), 26.0 ‰ for δ18O–NO3– (s.d., 1.9 ‰), from initial values of δ15N–NO3– (7.0 ‰) and δ18O–NO3– (25.8 ‰). The theoretical isotopic composition of eluted NO3– was simulated with different amplitudes of ϵ, following Rayleigh equation (Fig. 4c):
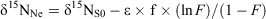
where δ15NS0 is the isotopic composition of the substrate at initial time, F = Nt/N0 with N0 and Nt the quantity of substrate at time 0 and t. In contrast to simulated data, which showed at first depletion in 15N, the experimental data showed no tendency to depletion or enrichment of δ15N. According to the range of precision, the combination of advection and diffusion processes thus did not involve ϵ < –0.5 ‰.
|
|
From these experimental results, the influence of the transport of nitrate on the isotopic fractionation associated either with denitrification or with nitrate uptake by plants in ecosystems can be reconsidered when diffusion is the limiting step. Indeed, in this case its isotopic fractionation will drive the apparent isotopic effect associated with biological processes. Although a large range of ϵapp has been reported for denitrification from both laboratory cultures and field measurements (0 to –40 ‰),[3] a much lower and more limited ϵapp (0 to –3.6 ‰) was observed in the case of benthic denitrification.[9] This was assumed to reflect a low nitrate concentration due to the fact that nitrate transport to the sediment is a rate-limiting step. We have here experimentally demonstrated the validity of this assumption. The magnitude of the isotopic values of nitrate cannot reflect either the transport or the progress of the denitrification reaction.[10] The low amount of available nitrate does not allow a full isotopic discrimination process and the ϵapp is lowered. Similarly, when nitrate concentration is low in comparison to plant needs, roots extension and advection of water are not sufficient to transfer the nutrients to the roots and the diffusion of nitrogen in soil becomes the major process. Under these conditions, isotopic fractionation related to nitrogen uptake by the plant is likely different to what is observed in experiments under hydroponic conditions,[11] where the diffusion and advection are not the limiting processes. In N-limiting conditions, the nitrogen uptake can be limited by diffusion and the isotopic effect associated with uptake cannot be expressed. The modification of the magnitude of isotopic effect associated with uptake should have affect plant δ15N. This could partly contribute to the variability of plant δ15N, which is difficult to interpret. In addition, the origin of nitrate pollution in groundwater could be more easily determined as it is ascertained that the isotopic signature of nitrate is not affected by diffusive transport through soils and sediments.
In environmental systems, nitrate can be transported by diffusion in water and advection of water masses to the place where the processes occur in soils, sediments and groundwater. Our laboratory experiments revealed that the transport of nitrate by diffusion and advection has no significant influence on nitrogen and oxygen isotopic composition of nitrate. Indeed, given analytical and experimental uncertainty, if there was an isotopic fractionation it would necessarily be higher than –0.5 ‰. The magnitude of the isotopic effect associated with different processes can be modified when the transport of nitrate is limiting. In this case, it is likely that apparent isotopic fractionation related to biological processes decreases. This may account for the large range of ϵapp reported in literature for denitrification.
References
[1] L. I. Wassenaar, Evaluation of the origin and fate of nitrate in the Abbotsford Aquifer using the isotopes of 15N and 180 in NO3– Appl. Geochem. 1995, 10, 391.| Evaluation of the origin and fate of nitrate in the Abbotsford Aquifer using the isotopes of 15N and 180 in NO3–Crossref | GoogleScholarGoogle Scholar |
[2] R. D. Evans, Soil nitrogen isotope composition, in Stable Isotopes in Ecology and Environmental Science 2nd Edition (Eds R. M. Michener and K. Lajtha) 2007, pp. 83–98 (Blackwell Scientific: Oxford, UK).
[3] M. F. Lehmann, P. Reichert, S. M. Bernasconi, A. Barbieri, J. A. McKenzie, Modelling nitrogen and oxygen isotope fractionation during denitrification in a lacustrine redox-transition zone Geochim. Cosmochim. Acta 2003, 67, 2529.
| Modelling nitrogen and oxygen isotope fractionation during denitrification in a lacustrine redox-transition zoneCrossref | GoogleScholarGoogle Scholar |
[4] M. Sebilo, G. Billen, B. Mayer, D. Billiou, M. Grably, J. Garnier, A. Mariotti, Assessing nitrification and denitrification in the seine river and estuary using chemical and isotopic techniques Ecosystems (N. Y.) 2006, 9, 564.
| Assessing nitrification and denitrification in the seine river and estuary using chemical and isotopic techniquesCrossref | GoogleScholarGoogle Scholar |
[5] E. M. LaBolle, G. E. Fogg, J. B. Eweis, J. Gravner, D. G. Leaist, Isotopic fractionation by diffusion in groundwater Water Resour. Res. 2008, 44, W07405.
| Isotopic fractionation by diffusion in groundwaterCrossref | GoogleScholarGoogle Scholar |
[6] F. M. Richter, R. A. Mendybaev, J. N. Christensen, I. D. Hutcheon, R. W. Williams, N. C. Sturchio, J. A. D. Beloso, Kinetic isotopic fractionation during diffusion of ionic species in water Geochim. Cosmochim. Acta 2006, 70, 277.
| Kinetic isotopic fractionation during diffusion of ionic species in waterCrossref | GoogleScholarGoogle Scholar |
[7] M. R. McIlvin, M. A. Altabet, Chemical conversion of nitrate and nitrite to nitrous oxide for nitrogen and oxygen isotopic analysis in freshwater and seawater Anal. Chem. 2005, 77, 5589.
| Chemical conversion of nitrate and nitrite to nitrous oxide for nitrogen and oxygen isotopic analysis in freshwater and seawaterCrossref | GoogleScholarGoogle Scholar |
[8] B. Shilman, N. Teplyakov, Detailed protocol for nitrate chemical reduction to nitrous oxide for δ15N and δ18O analysis of nitrate in fresh and marine waters. Annual Report Submitted to the Earth Science Research Administration, TR-GSI/15/2007 2007 (Ministry of National Infrastructures: Jerusalem).
[9] D. M. Sigman, R. Robinson, A. N. Knapp, A. van Geen, D. C. McCorkle, J. A. Brandes, R. C. Thunell, Distinguishing between water column and sedimentary denitrification in the Santa Barbara Basin using the stable isotopes of nitrate Geochem. Geophys. Geosyst. 2003, 4, 1040.
| Distinguishing between water column and sedimentary denitrification in the Santa Barbara Basin using the stable isotopes of nitrateCrossref | GoogleScholarGoogle Scholar |
[10] P. M. Groffman, M. A. Altabet, J. K. Bohlke, K. Butterbach-Bahl, M. B. David, M. K. Firestone, A. E. Giblin, T. M. Kana, L. P. Nielsen, M. A. Voytek, Methods for measuring denitrification: diverse approaches to a difficult problem Ecol. Appl. 2006, 16, 2091.
| Methods for measuring denitrification: diverse approaches to a difficult problemCrossref | GoogleScholarGoogle Scholar |
[11] T. Yoneyama, O. Ito, W. M. H. G. Engelaar, Uptake, metabolism and distribution of nitrogen in crop plants traced by enriched and natural 15N: progress over the last 30 years Phytochem. Rev. 2003, 2, 121.
| Uptake, metabolism and distribution of nitrogen in crop plants traced by enriched and natural 15N: progress over the last 30 yearsCrossref | GoogleScholarGoogle Scholar |