Cyanobacteria produce arsenosugars
Shin-ichi Miyashita A C , Shoko Fujiwara A , Mikio Tsuzuki A B and Toshikazu Kaise A DA School of Life Sciences, Tokyo University of Pharmacy and Life Sciences, 1432-1 Horinouchi, Hachioji, Tokyo 192-0392, Japan.
B Japan Science and Technology Agency, CREST, 5, Sanbancho, Chiyoda-ku, Tokyo, 102-0075, Japan.
C Corresponding author. Present address: Environmental Standards Section, Inorganic Analytical Chemistry Division, National Metrology Institute of Japan, 1-1-1 Umezono, Tsukuba, Ibaraki 305-8563, Japan. Email: shinichi-miyashita@aist.go.jp
D Deceased November 2009.
Environmental Chemistry 9(5) 474-484 https://doi.org/10.1071/EN12061
Submitted: 24 January 2012 Accepted: 18 September 2012 Published: 9 November 2012
Journal Compilation © CSIRO Publishing 2012 Open Access CC BY-NC-ND
Environmental context. Although arsenic is known to accumulate in both marine and freshwater ecosystems, the pathways by which arsenic is accumulated and transferred in freshwater systems are reasonably unknown. This study revealed that freshwater cyanobacteria have the ability to produce arsenosugars from inorganic arsenic compounds. The findings suggest that not only algae, but cyanobacteria, play an important role in the arsenic cycle of aquatic ecosystems.
Abstract. Metabolic processes of incorporated arsenate in axenic cultures of the freshwater cyanobacteria Synechocystis sp. PCC 6803 and Nostoc (Anabaena) sp. PCC 7120 were examined. Analyses of arsenic compounds in cyanobacterial extracts using a high-performance liquid chromatography–inductively coupled plasma mass spectrometry system showed that both strains have an ability to biotransform arsenate into oxo-arsenosugar-glycerol within 20 min through (1) reduction of incorporated arsenate to arsenite and (2) methylation of produced arsenite to dimethylarsinic acid by methylarsonic acid as a possible intermediate product. In addition, Synechocystis sp. PCC 6803 cells are able to biosynthesise oxo-arsenosugar-phosphate from incorporated arsenate. These findings suggest that arsenosugar formation as well as arsenic methylation in cyanobacteria possibly play a significant role in the global arsenic cycle.
Additional keywords: arsenic biotransformation, arsenic metabolism, inductively coupled plasma mass spectrometry, Nostoc (Anabaena) sp. PCC 7120, photosynthetic prokaryote, Synechocystis sp. PCC 6803.
Introduction
Arsenic circulates widely in the lithosphere, hydrosphere and biosphere. It is a chemically reactive element and affects the physiological and biochemical activities of other elements. Living organisms have developed specific metabolic pathways to transform As encountered in the environment.[1,2]
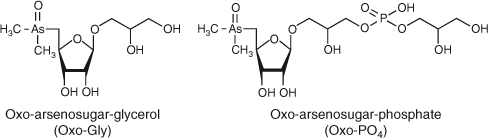
Many freshwater and marine microbes can accumulate inorganic As species and reduce arsenate (AsV, AsO43–) to arsenite (AsIII, AsO33–).[3–5] AsV inhibits oxidative phosphorylation and photophosphorylation, whereas AsIII is considered to block the SH sites of various proteins.[6,7] Some bacteria in freshwater and marine environments have the ability to methylate inorganic As species to soluble or volatile methylated forms of As.[8–10] Conversely, some microbes degrade organic As compounds. Trimethylarsine oxide (TMAO, (CH3)3AsO) has been reported as a product of the microbial breakdown of arsenobetaine (AB, (CH3)3As+CH2COOH), which is a major As species in marine fauna, under various conditions.[11] In addition, dimethylarsinoylribosides (usually termed arsenosugars) (Fig. 1), which are the predominant As species in marine algae, can be efficiently degraded to dimethylarsinoylethanol ((CH3)2AsOCH2CH2OH) by microbes cultured under anaerobic conditions.[12] Thus, microbes are involved in both the production and degradation of organic As compounds and play an important role in As circulation.
|
Arsenosugars are thought to play primary roles in the biotransformation and circulation of As in marine ecosystems. More than 15 types of arsenosugars have been isolated and characterised in the marine environment.[13] The most common types, the oxo-arsenosugars (Fig. 1), contain a dimethylarsinoyl group ((CH3)2AsO–) at the C5 position of D-ribose derivatives, which is readily reduced to a tertiary alkyl arsine structure.[14] The tertiary alkyl arsines are chemically active; for example, they could easily react with organic halogen compounds to form chemically stable quaternary alkyl arsonium compounds.[14,15] Thus, the oxo-arsenosugars might have physiological importance. Microbes living in marine environments are likely to have an ability to produce arsenosugars,[16,17] and several studies have centred on the role of marine microbes in the possible production and biodegradation of arsenosugars.[18–20] Thus, identification of As metabolites in microbes grown in pure culture, such as eukaryotic microalgae and prokaryotic cyanobacteria, has been performed to confirm biotransformation mechanisms of As after experimental exposure to inorganic As species. Previous reports generally indicate that microalgae (not only marine species (Dunaliella tertiolecta and Phaeodactylum tricornutum)[17] but also freshwater ones (Chlorella vulgaris,[21] Chlorella sp.,[22] Chlamydomonas reinhardtii[23,24] and Monoraphidium arcuatum[22]) can produce arsenosugars from incorporated AsV by reduction and methylation processes, and that cyanobacteria (freshwater species, Microcystis sp. PCC 7806, Nostoc sp. PCC 7120, Nostoc sp. and Synechocystis sp. PCC 6803) can methylate incorporated AsV by a reduction process but less positively metabolise it.[10,25,26] However, the production of arsenosugars by axenic cultures of prokaryotic microbes has not yet been proven.
Cyanobacteria are photosynthetic prokaryotes and their ecological diversity is noteworthy. They are important primary producers found in almost every conceivable habitat, from oceans to freshwater systems and from bare rock to soil. They are also found in extreme conditions of temperature, for example in thermal springs or in the desert.[27] Synechocystis sp. PCC 6803 (hereafter, Synechocystis) is a free-living freshwater unicellular cyanobacterium and one of the most extensively studied model organisms for the analysis of photosynthetic processes. The genus Nostoc (Anabaena) consists of nitrogen-fixing filamentous cyanobacteria, and one of the freshwater strains, Nostoc (Anabaena) sp. PCC 7120 (hereafter, Nostoc), has become a model organism for genetic studies of photosynthesis and nitrogen fixation studies. Although commercially available Nostoc sp. (Nostoc commune var. flagelliforme) powder was shown to contain an arsenosugar (oxo-arsenosugar-glycerol, Oxo-Gly),[26] this has not been demonstrated in isolated species[25] or axenic cultures of Nostoc. Although Yin et al.[10] demonstrated As methylation by an arsM gene product in cyanobacteria including Synechocystis and Nostoc, they have not yet investigated arsenosugar production (because their aim was to check methylated species, only samples prepared by HNO3 and microwave treatments, in which sugars might not be preserved,[28] were analysed). If it is substantiated that cyanobacteria have a mechanism to produce arsenosugars, their new role in the natural cycling of As in the environment as a source of arsenosugars will be elucidated. In the present study, the As toxicity, As uptake and metabolism of water-soluble As species after exposure to AsV during cyanobacterial growth were investigated for verification of the ability of cyanobacteria to produce arsenosugars.
Experimental
Materials
PlasmaCAL single-element standard solutions of 1000 µg mL–1 of As and zirconium were purchased from SCP SCIENCE (Montreal, Canada). Stock solutions containing 1000 µg As mL–1 of each of the following As species were prepared in water: AsIII from sodium arsenite (NaAsO2), AsV from disodium hydrogen arsenate (Na2HAsO4·7H2O), methylarsonic acid (MAV, (CH3)AsO(OH)2), dimethylarsinic acid (DMAV, (CH3)2AsO(OH)), trimethylarsine oxide (TMAO, (CH3)3AsO), arsenocholine (AC) from AC bromide ((CH3)3As+CH2CH2OH)Br–, arsenobetaine (AB, (CH3)3As+CH2COOH) and tetramethylarsonium (TMA) from TMA iodide ((CH3)4As+I–) (all the As-containing chemicals were purchased from Tri Chemical Laboratories Inc., Yamanashi, Japan). Oxo-arsenosugar-glycerol (Oxo-Gly) was synthesised following previously reported procedures.[29] A purified extract of the brown macroalga Fucus serratus containing four oxo-arsenosugars, Oxo-Gly, oxo-arsenosugar-phosphate (Oxo-PO4), oxo-arsenosugar-sulfonate (Oxo-SO3) and oxo-arsenosugar-sulfate (Oxo-SO4), was kindly donated by Prof. Kevin A. Francesconi (Karl-Franzens University, Graz, Austria).[30]
Cyanobacterial culture
Axenic cultures of Synechocystis[31] and Nostoc[32] were obtained from E. I. du Pont de Nemours and Co., Inc. (Wilmington, DE, USA) and Kazusa DNA Research Institute (Kazusa, Chiba, Japan). Synechocystis cells were grown axenically in sterilised BG-11 medium (nitrogen content, 18 mM; phosphorus content, 0.2 mM)[33] containing glucose (5 mM) with vigorous shaking at 120 rpm.[34] Nostoc cells were grown axenically in sterile BG-11 medium with orbital shaking, with or without air-bubbling. The cyanobacterial cells of each strain were incubated in 300 mL of medium in 500-mL Erlenmeyer flasks at 30 and 28 °C, with continuous illumination at 40 µmol photons m–2 s–1. No other organisms were observed microscopically, and no other organisms could be cultured.
As toxicity test
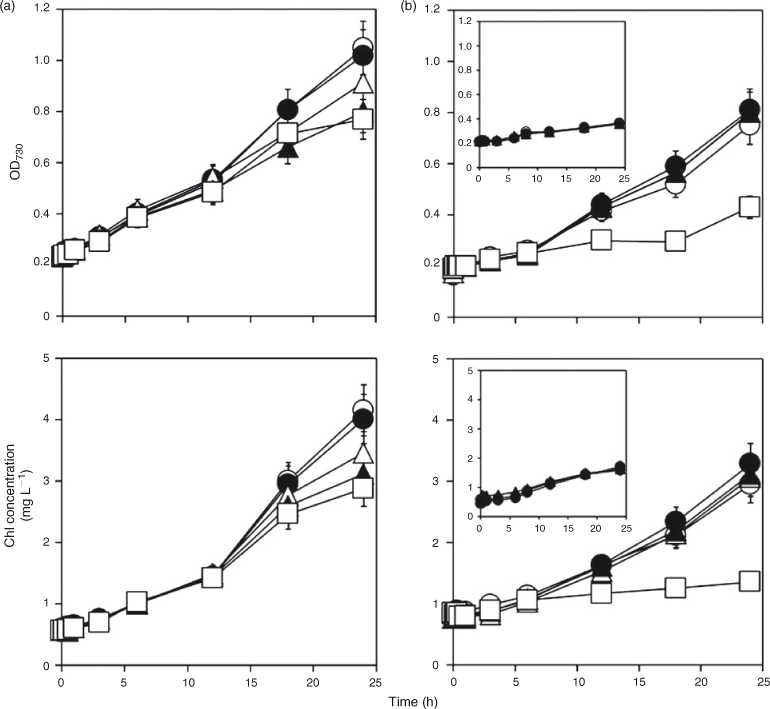
An As toxicity test based on turbidity and chlorophyll (Chl) measurements was performed to determine an appropriate AsV concentration for cyanobacterial cell exposure because excessive AsV levels may disrupt the process by which AsV is metabolised to arsenosugars. A filter-sterilised 100 mM AsV solution was added to each flask to final concentrations of 0–5 mM in the 300-mL cell suspensions, except for Nostoc cells to be grown without air-bubbling (in this case 0–1 mM AsV was applied). At appropriate intervals, turbidity (OD730) and Chl levels[35] were measured.
AsV uptake
A filter-sterilised 100 mM AsV solution was added to 300 mL of a cell suspension at the log phase at the selected concentrations. Cell suspensions (15 mL) were collected from the 500-mL Erlenmeyer flask into different polypropylene centrifuge tubes at appropriate intervals, and cells were harvested by centrifugation, and rinsed with 10 mL of As-free medium three times and once in 10 mL of water. Different subsamples were used for measurement of AsV uptake and identification of the As compounds (see below). The experiment was repeated three times. The following procedure was performed for total analysis of As in the cells. The cells were solubilised as described previously.[24] Briefly, the cells were predigested in the presence of conc. HNO3, and then completely solubilised in a closed-vessel microwave digestion system (speedwave MWS-3+; Berghof) after addition of hydrogen peroxide. Zirconium was added to the solutions as a single internal standard element, and the final HNO3 concentrations were adjusted to ~5 % (v/v).
Total As analysis was accomplished by inductively coupled plasma–mass spectrometry (ICP-MS, ELAN DRC-e; PerkinElmer SCIEX Inc., Concord, Canada) following previously reported procedures.[24] The following ICP-MS conditions were applied for specific determination of As (as AsO+ at m/z 91) and zirconium (as ZrO+ at m/z 106): RF power, 1600 W; nebuliser gas flow, 1.05 L min–1; auxiliary gas flow, 1.4 L min–1; plasma gas flow, 18 L min–1; cell gas flow, 0.7 mL min–1 oxygen (purity: 99.999 %); rejection parameter a (RPa), 0; rejection parameter q (RPq), 0.25 (ZrO+) and 0.55 (AsO+); axial field voltage, 250 V. Dynamic reaction cell (DRC) mode was used for the speciation analysis as well as total As analysis. Both analyses were performed, after optimisation of the oxygen gas flow rate into the cell. The analytical methods were validated through analyses of two certified reference materials (CRMs) (see below). The determined As amount was normalised with culture weight and Chl amount, to determine the total amount of As accumulated into cells in the whole culture and to infer the As content per cell respectively.
Extraction and identification of the As compounds formed by the two cyanobacteria
Biotransformation of incorporated AsV in the cyanobacterial cells exposed to AsV at the concentrations selected by the As toxicity test was confirmed by analyses of water-soluble As metabolites by using a combined ICP-MS and high-performance liquid chromatography (HPLC) system (HPLC-ICP-MS) after the following extraction procedures. The cells collected as described above in the AsV uptake section were freeze-dried,[24] because removing water completely was necessary for the following extraction carried out with a small precise amount (1 mL) of water. Each suspension in water was then homogenised on ice with a sonicator for 30 s at a power level of 50 W. This process was repeated six times with cooling intervals of 60 s between each sonication until almost all the cells were disrupted as observed by light microscopy. The homogenates were subsequently centrifuged at 1700g for 15 min at 4 °C. From these tubes, ~0.5 mL of each supernatant were filtered through a 0.45-µm membrane filter and placed in 0.6-mL polyethylene vials. The prepared samples were immediately frozen and stored in a deep freezer at –84 °C, and subjected to instrumental analysis within a few days. Extraction yields of water-soluble As metabolites were determined by comparing total As concentrations in the extracts and in the cells. The extraction yield was generally ~100 % of total As (Table S1), suggesting that extracts contained almost all the As compounds present in the cells. This high extractability might have been due to the method applied in this study, and the possibility that unstable macromolecules including As might have been broken down into soluble smaller forms by the vigorous sonication could not be ruled out.
Identification of water-soluble As compounds in the extracts was performed by HPLC-ICP-MS.[24] A reversed-phase (RP) or strong anion-exchange (SAX) HPLC column was used to separate As compounds. For the RP-HPLC method, the following operating conditions were applied: column, Shiseido CAPCELL PAK C18 MGII (4.6-mm internal diameter × 250-mm length, 5-µm particle size); guard column, Shiseido CAPCELL PAK C18 MGII (4.0-mm internal diameter × 10-mm length, 5-µm particle size); column temperature, 40 °C; mobile phase, 10-mM sodium 1-butansulfonate, 4-mM tetramethylammonium hydroxide, 4-mM malonic acid and 0.05 % methanol (pH 3.0 adjusted with HNO3); injection volume, 20 µL; flow rate, 1.0 mL min–1. For the SAX-HPLC method, the following operating conditions were applied: column, Hamilton PRP-X100 (4.1-mm internal diameter × 150-mm length, 10-µm particle size); guard column, Hamilton Steel PRP-X100 (2.3-mm internal diameter × 20-mm length, 12–20-µm particle size); column temperature, 40 °C; mobile phase, 20-mM ammonium dihydrogen phosphate (pH 6.0 adjusted with aqueous ammonia); injection volume, 50 µL; flow rate, 1.5 mL min–1.
For identification of As compounds in the extracts, chromatographic peaks with the retention times of eight As compounds (AsIII, AsV, MAV, DMAV, TMAO, AC, AB and TMA) in standard solutions and four oxo-arsenosugars (Oxo-Gly, Oxo-PO4, Oxo-SO3 and Oxo-SO4) in a purified extract of F. serratus (Table S2) were compared. Matrix effects seen in the retention times due to extracted cell components were evaluated by spiking standard As species into each of the samples. For quantification of As compounds in the extracts, external calibration curves of peak area versus concentration were employed in the range of 1 to 100 ng As mL–1 for each standard As compound. A calibration curve for DMAV was also used for quantification of oxo-arsenosugars.[36,37] When arsenosugars in a Fucus serratus extract were quantified with the calibration curve of DMAV, a good agreement with the reported values[30] was obtained (reported [Oxo-Gly] = 0.10 ± 0.004 µg As kg–1, obtained [Oxo-Gly] = 0.11 ± 0.008 µg As kg–1 (mean ± s.d.), 8.0 % relative standard deviation (RSD), n = 3; reported [Oxo-PO4] = 0.086 ± 0.002 µg As kg–1, obtained [Oxo-PO4] = 0.089 ± 0.007 µg As kg–1 (mean ± s.d.), 7.6 % RSD, n = 3; reported [Oxo-SO3] = 0.62 ± 0.02 µg As kg–1, obtained [Oxo-SO3] = 0.66 ± 0.06 µg As kg–1 (mean ± s.d.), 9.1 % RSD, n = 3; reported [Oxo-SO4] = 0.40 ± 0.01 µg As kg–1, obtained [Oxo-SO4] = 0.38 ± 0.03 µg As kg–1 (mean ± s.d.), 7.7 % RSD, n = 3).
The RP-HPLC column was used for both identification and quantification of the various As species, whereas the SAX-HPLC column was used only for identification because faster-eluting cations (TMAO, AC and TMA) and neutral ions (AsIII, AB and Oxo-Gly) cannot be correctly quantified by this column due to overlapping peak areas. Column recoveries of water-soluble As compounds (including unknown species, which were quantified by using standards with similar retention times) in extracts of Synechocystis and Nostoc cells exposed to AsV were reasonalby low, especially in Synechocystis (Table S1). This might be due to decomposition or retention of some extracted compounds on the column. Because the hidden As species as a fraction is potentially interesting for this study, the time course of this non-eluting As fraction in each cyanobacterial extract is shown in Fig. S1.
The analytical methods were validated through analyses of NMIJ CRM 7402-a codfish tissue (certified [total As] = 36.7 ± 1.8 mg As kg–1, found [total As] = 34.0 ± 0.34 mg As kg–1 (mean ± standard deviation (s.d.)), 0.99 % RSD, n = 3; certified [AB] = 33.1 ± 1.5 mg As kg–1, found [AB] = 30.9 ± 0.72 mg As kg–1, 2.3 % RSD, n = 3)[24] and NMIJ CRM 7901-a AB solution (certified [AB] = 10.27 ± 0.26 mg As kg–1, found [AB] = 10.12 ± 0.07 mg As kg–1, 0.7 % RSD, n = 3).
Results
As toxicity test
Cell growth curves of Synechocystis and Nostoc in the presence of AsV are shown in Fig. 2. In Synechocystis (Fig. 2a), cells exposed to 0.1 mM AsV grew at almost the same rate as the control cells cultivated in the absence of AsV (P = 0.21 at 24 h), whereas the cell growth was slightly depressed by 0.5–5 mM AsV after 18 h in a concentration-dependent manner (P < 0.001 at 18 h and <0.002 at 24 h). The 50 % inhibitory concentration (IC50) value for inhibition by AsV was >5 mM. In Nostoc grown with air-bubbling (Fig. 2b), cells exposed to 0.5 and 1 mM AsV grew at almost the same rate as the control cells (P > 0.08 at 24 h), whereas the cell growth was slightly stimulated by 0.1 mM AsV (P = 0.011 at 24 h). It was strongly suppressed by 5 mM AsV (P < 0.001 at 24 h), but the cells survived. Without air-bubbling (Fig. 2b, inset), cells exposed to 0.1 and 1 mM AsV grew at almost the same rate as the control cells. The IC50 values for inhibition by AsV were >5 mM for air-bubbled cells and >1 mM for non-air-bubbled cells. Accordingly, for monitoring As metabolites, AsV concentrations of ≤5 and ≤1 mM seemed to be appropriate for Synechocystis (without air-bubbling) and Nostoc (with or without air-bubbling) respectively. Thus, the cyanobacteria showed high resistance to AsV; the IC50 values for Synechocystis and Nostoc were beyond the concentrations tested (>5 or >1 mM), whereas that of the green alga Chlamydomonas reinhardtii CC-125 was 0.3 mM.[24]
AsV uptake and biotransformation of AsV
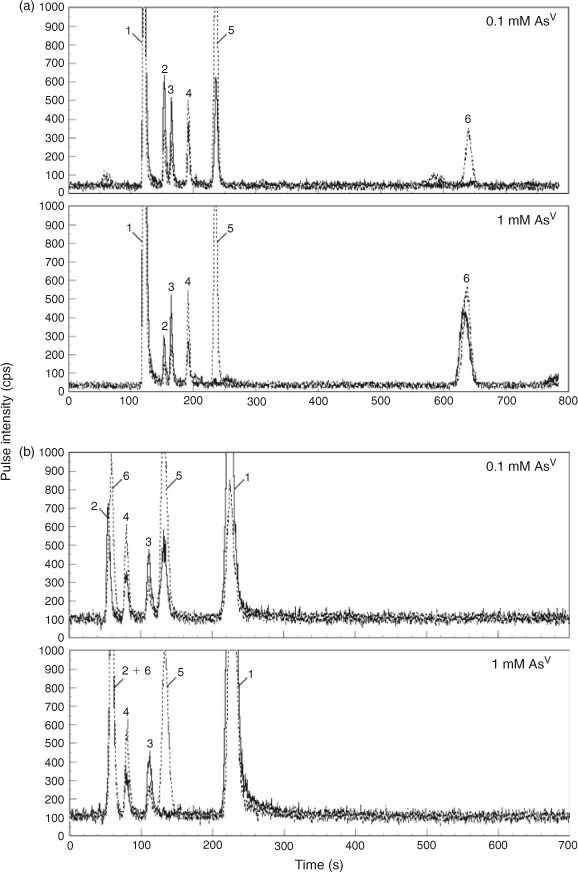
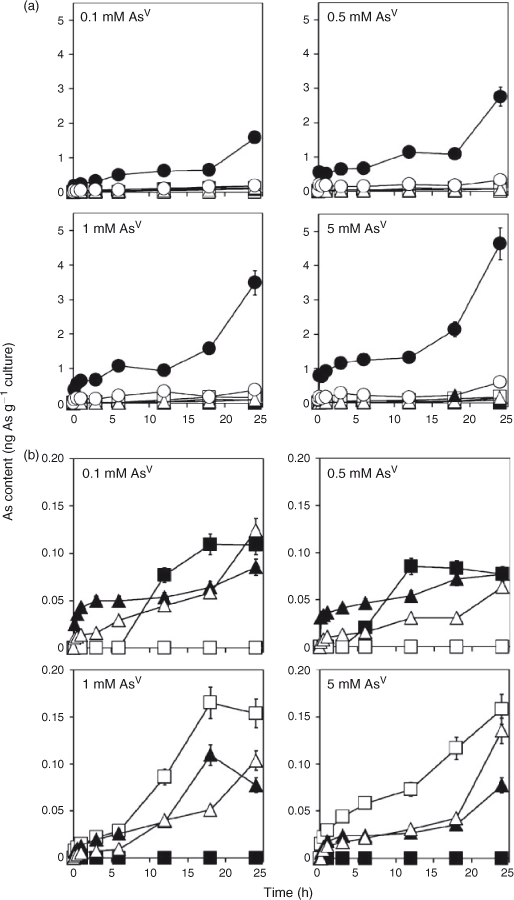
Although the incorporation of As into cells was repressed immediately after the addition of AsV in both strains (Fig. 3e, f, Table S1; the addition of AsV was done at time 0 in the graphs), the intracellular As content per gram of culture increased as the growth proceeded (Fig. 3a, b). Under these conditions, As molecules in the cyanobacterial extracts were analysed by HPLC-ICP-MS (Figs 4, 5, 6). The results demonstrated that the cells accumulated AsV as the most abundant As species, followed by AsIII (Figs 4, 5a, 6a, Table S1). In addition, arsenosugars Oxo-Gly and Oxo-PO4 were detected along with MAV, DMAV and relatively low levels of unknown As compounds (U1 (eluted at 3.5 min between DMAV and Oxo-PO4 in RP-HPLC, and most likely, 0.84 min with AsIII in SAX-HPLC) and U2 (eluted at 13 min after Oxo-Gly in RP-HPLC, and presumably, 8.1 min after AsV in SAX-HPLC)) (Figs 4, 5, 6, Table S1). These arsenosugars as well as other As metabolites were identified by HPLC-ICP-MS not only using the RP column (Fig. 4a) but also with the SAX column (Fig. 4b).

|
|
|
Discussion
The results demonstrated that the freshwater cyanobacteria Synechocystis sp. PCC 6803 and Nostoc sp. PCC 7120 incorporate As into sugars as Oxo-Gly and Oxo-PO4. This is the first report clearly demonstrating that cyanobacteria have an ability to produce arsenosugars, which could well have important implications for freshwater lakes and hot springs occurring in the vicinity of soils rich in As (there is in fact a report of arsenosugars in hotspring mats[38]).
The following metabolic pathway has been suggested for the formation of arsenosugars in algae.[4,39] Initially, AsV is reduced to AsIII by reductase(s) and stepwise methylated in an oxidative methyl transfer from S-adenosyl-l-methionine (SAM) to DMAV by methyltransferase(s).[40] In algae, the production of arsenosugars is a subsequent step from the methylation of AsIII.[4,39] DMAV is thought to be reduced to dimethylarsinous acid ((CH3)2As(OH)) by a reductase and then oxidised by the addition of the adenosyl group from SAM. This nucleoside, referred to as dimethylarsinyladenosine, then undergoes glycosidation to produce a range of arsenosugars. In cyanobacteria, AsV is reduced to AsIII and methylated to DMAV;[10] thereafter, DMAV is possibly biotransformed into Oxo-Gly and Oxo-PO4 by reduction, addition of a ribose group and glycosidation, as suggested by Lai et al.[26] and this study. Now that the arsenosugar formation has been verified in Synechocystis sp. PCC 6803 and Nostoc sp. PCC 7120, it is expected that the biosynthetic mechanism will be elucidated through well developed molecular biological analytical techniques. For example, once the enzymes can be purified, the genes could be cloned reasonably easily using the genome sequences which have been completely determined, and the functions of the gene products could be analysed by both in vivo and in vitro experiments.
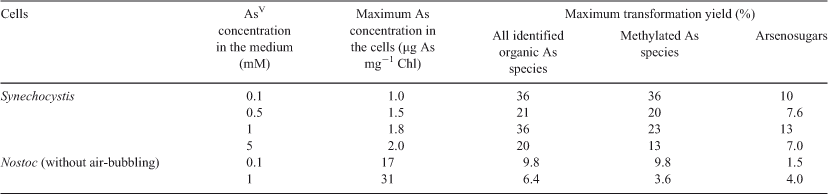
Interestingly, the types of oxo-arsenosugars changed, depending on the cyanobacterial species and the AsV concentrations (Figs 4, 5, 6, Table S1). In Nostoc sp. PCC 7120, Oxo-Gly was the only oxo-arsenosugar detected (Fig. 6, Table S1), as in N. commune var. flagelliforme.[26] Thus, the genus Nostoc generally may not have the ability to produce Oxo-PO4. Because Oxo-PO4 has been shown to be degraded to Oxo-Gly by an in vitro enzyme (glycerophosphorylcholine diesterase) reaction,[40,41] Oxo-PO4 might be biosynthesised from Oxo-Gly by some oppositely oriented reaction in cyanobacteria, and Nostoc may not have the responsible enzyme(s). Alternatively, AsV concentrations (≥0.1 mM) in the medium in this study might have been too high for Nostoc sp. PCC 7120 to produce Oxo-PO4; AsV would competitively inhibit the biosynthesis of phosphate-containing compounds such as Oxo-PO4. In Synechocystis, Oxo-PO4 was detected at 0.1–0.5 mM, whereas Oxo-Gly was found at 1–5 mM (Figs 4, 5, Table S1). At higher concentrations of AsV, AsV possibly inhibits the biosynthesis of a phosphate-containing compound, Oxo-PO4, as described above. At lower concentrations of AsV, Oxo-Gly may not be detected because the conversion from the possible precursor Oxo-Gly into Oxo-PO4 is not a limiting step under these conditions.
Concerning the time course of the arsenosugar production, the amount of Oxo-Gly, normalised to Chl content, continued to increase in Nostoc, whereas those of Oxo-Gly and Oxo-PO4 reached a peak quickly relative to Oxo-Gly in Nostoc and then decreased in Synechocystis. This may be due to the difference in time taken for the intracellular As concentration to change; the total As amount in Nostoc increased gradually, whereas that in Synechocystis reached a plateau quickly (Fig. 3c–f). The cause of this is still unclear. However, it might be due to a difference in activities of phosphate and AsV transport and As efflux systems. Or it might be simply due to the difference in cellular morphology and accessibility of AsV to each cell surface. Nostoc is filamentous and aggregates easily; thus, a lower exposed cell surface area may delay over-all influx of AsV into the cells, and thereby the subsequent repression of AsV uptake, which is normally seen immediately after the incorporation of AsV, may be retarded.
In Nostoc, the As accumulation levels relative to Chl levels in cells grown without air-bubbling were much higher than those in cells grown with air-bubbling (Fig. 3d, f). Cells grown without air-bubbling divide more slowly than air-bubbled cells (Fig. 2b). Therefore, each single cell in a non-air-bubbled culture may be exposed to more As than in the air-bubbled cultures. In addition the possibility that cells in a non-air-bubbled culture might be smaller and have more As due to a larger surface area of the cells cannot be ruled out, although cellular size did not seem to differ between the cultures microscopically.
When compared with another study[25] using a culture of Nostoc sp., their results showed a similar AsV concentration-dependency in AsV uptake to the current work. In our study, the As accumulation levels relative to Chl levels in cells cultured for 24 h in 0.1-, 0.5- and 1-mM AsV-containing media were 27, 47 and 47 %, shown as values relative to that in a 5-mM AsV-containing medium. In the study of Maeda et al. the As levels per dry weight in cells grown for 2 weeks in 0.14, 0.67 and 1.4 mM AsV were 10, 36 and 50 %, shown as values relative to that in 6.7-mM AsV-containing medium. These findings suggest that the As accumulation is not proportionate to AsV concentration in the medium.
Biotransformation of intracellular AsV and AsIII into simply methylated As compounds and further arsenosugars may prevent AsV from replacing the phosphate in ATP and AsIII from reacting with the –SH group of an enzyme. In Synechocystis and Nostoc, however, the percentage of organic As compounds was reasonably low (≤36 and ≤9.8 % in total As in the extracts (the percentages were relatively higher at lower As concentrations in the medium); Figs 5, 6, Table 1), as in Phormidium sp.,[42] suggesting that the major detoxification process in cyanobacteria is not the production of organic As compounds but reduction to AsIII and expulsion from cells. Thus, the methylation of As and the production of arsenosugars seem to be mainly performed for a purpose(s) other than detoxification (e.g. production of lipid-soluble As compounds for membrane lipid bilayers). Many marine algae have arsenolipids including phosphatidyldimethylarsinylribose (usually termed phosphatidylarsenosugar, an arsenoribosyl derivative of phosphatidyl glycerol and a derivative of Oxo-PO4),[43–45] and the arsenolipids are speculated to be included in the lipid bilayer structures of the algal outer membrane.[44] If cyanobacteria produce similar arsenolipids, they may be located at the cell membrane or thylakoid membrane, as well as phosphatidyl glycerol.
There may be a possibility that some part of the water-soluble As compounds present in extracts are not present in intact forms, due to the vigorous sonication. The stability of As species during sonication has been investigated in our previous study.[24] The study demonstrated that our sonication procedure induced few structural changes in water-soluble As compounds, but both oxidation of AsIII and Thio-DMAV ((CH3)2AsS(OH)) and degradation of Oxo-PO4 should be taken into account in considering the original forms of As metabolites in AsV-exposed cells. Furthermore, Oxo-Gly and Oxo-PO4 might be partially derived from the degradation of ribose-containing, lipid-soluble As compounds, such as phosphatidylarsenosugar (its precursors possibly include Oxo-Gly or Oxo-PO4).[43] Freshwater organisms, including the green algae C. reinhardtii and Cladophora glomerata, possess lipid-soluble or water-unextractable As species to some extent.[23] Therefore, analysis of such kinds of compounds should be required for understanding As behaviour in living organisms in freshwater ecosystems. In addition, the identification of other water-soluble compounds including unknown compounds U1 and U2 would be desirable. Based on their retention times in a similar chromatographic system to ours,[18] U1 might be cationic or neutral species, such as dimethylarsinoylethanol (degradation product of arsenosugars[40]) or trimethylated arsenosugar,[46] and U2 might be an anionic species, such as dimethylarsinoylacetate and dimethylated arsenosugar.[47]
Acknowledgements
The authors thank Dr Kenji Kinoshita (Tokyo Metropolitan Industrial Technology Research Institute, Japan) for helpful discussions, and PerkinElmer Japan Co., Ltd for kind support. This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Agriculture, Forestry and Fisheries (task number 2028, Japan). The first author acknowledges a Grant-in-Aid for a Research Fellow (DC2) from the Japan Society for the Promotion of Science.
References
[1] B. P. Rosen, Biochemistry of arsenic detoxification. FEBS Lett. 2002, 529, 86.| Biochemistry of arsenic detoxification.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xnt1KksbY%3D&md5=2356933a012f956e4f960f1d11f75508CAS |
[2] F. J. Zhao, S. P. McGrath, A. A. Meharg, Arsenic as a food chain contaminant: mechanisms of plant uptake and metabolism and mitigation strategies. Annu. Rev. Plant Biol. 2010, 61, 535.
| Arsenic as a food chain contaminant: mechanisms of plant uptake and metabolism and mitigation strategies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXnslSjsb0%3D&md5=ce0232b3fd327dc19b9ec5c1af8cc6cbCAS |
[3] R. Mukhopadhyay, B. P. Rosen, L. T. Phung, S. Silver, Microbial arsenic: from geocycles to genes and enzymes. FEMS Microbiol. Rev. 2002, 26, 311.
| Microbial arsenic: from geocycles to genes and enzymes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XmtVamsrg%3D&md5=bb038f4146f21579dbdef6b34050166cCAS |
[4] A. Raab, J. Feldmann, Microbial transformation of metals and metalloids. Sci. Prog. 2003, 86, 179.
| Microbial transformation of metals and metalloids.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXlslamtro%3D&md5=3e1b56c114cf1139ad94ed5b6baf97a2CAS |
[5] J. Messens, S. Silver, Arsenate reduction: thiol cascade chemistry with convergent evolution. J. Mol. Biol. 2006, 362, 1.
| Arsenate reduction: thiol cascade chemistry with convergent evolution.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XovVSrtL8%3D&md5=a54ab5b101ecad2bd6fb2dba2d88fa6bCAS |
[6] M. Avron, A. T. Jagendorf, Evidence concerning the mechanism of adenosine triphosphate formation by spinach chloroplasts. J. Biol. Chem. 1959, 234, 967.
| 1:CAS:528:DyaG1MXnvVChtQ%3D%3D&md5=4d4f50fa5f1a2adba0722433f0377037CAS |
[7] M. F. Hughes, Arsenic toxicity and potential mechanisms of action. Toxicol. Lett. 2002, 133, 1.
| Arsenic toxicity and potential mechanisms of action.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XksFKqsLs%3D&md5=a2fb86034319c63f41a5ea361624794aCAS |
[8] M. Shariatpanahi, A. C. Anderson, A. A. Abdelghani, Uptake and distribution of sodium arsenate by bacterial cells. Trace Subst. Environ. Health 1982, 16, 170.
| 1:CAS:528:DyaL3sXltlGju7g%3D&md5=b869499f0475f8d487e46223acc798c0CAS |
[9] F. V. Vidal, V. M. V. Vidal, Arsenic metabolism in marine bacteria and yeast. Mar. Biol. 1980, 60, 1.
| Arsenic metabolism in marine bacteria and yeast.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3MXpsleitA%3D%3D&md5=4bb0d4b5eaf404528e8cba0bc3f4b3e6CAS |
[10] X. X. Yin, J. Chen, J. Qin, G.-X. Sun, B. P. Rosen, Y.-G. Zhu, Biotransformation and volatilization of arsenic by three photosynthetic cyanobacteria. Plant Physiol. 2011, 156, 1631.
| Biotransformation and volatilization of arsenic by three photosynthetic cyanobacteria.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXptFWksbY%3D&md5=477b3ffa776589c0f1f60f799fbbf380CAS |
[11] K. Hanaoka, S. Tagawa, T. Kaise, The fate of organoarsenic compounds in marine ecosystems. Appl. Organomet. Chem. 1992, 6, 139.
| The fate of organoarsenic compounds in marine ecosystems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38Xis1ajurY%3D&md5=96888ff243b4b9e2ea73185a3bf351fbCAS |
[12] J. S. Edmonds, K. A. Francesconi, J. A. Hansen, Dimethyloxarsylethanol from anaerobic decomposition of brown kelp (Ecklonia radiata): a likely precursor of arsenobetaine in marine fauna. Experientia 1982, 38, 643.
| Dimethyloxarsylethanol from anaerobic decomposition of brown kelp (Ecklonia radiata): a likely precursor of arsenobetaine in marine fauna.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL38Xks12js7s%3D&md5=2f853e6c7f8640b333dfe3ab262ca56cCAS |
[13] J. S. Edmonds, K. A. Francesconi, Organoarsenic compounds in the marine environment, in Organometallic Compounds in the Environment (Ed. P. J. Craig) 2003, pp. 195–222 (Wiley: Chichester, UK).
[14] Y. Shibata, M. Morita, A novel trimethylated arseno-sugar isolated from the brown alga Sargassum thunbergii. Agric. Biol. Chem. 1988, 52, 1087.
| A novel trimethylated arseno-sugar isolated from the brown alga Sargassum thunbergii.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1cXksFWksLg%3D&md5=e4f07f28d4b65d960cc2ce4603052d05CAS |
[15] K. A. Francesconi, J. S. Edmonds, R. V. Stick, Arsenocholine from anaerobic decomposition of a trimethylarsonioriboside. Appl. Organomet. Chem. 1992, 6, 247.
| Arsenocholine from anaerobic decomposition of a trimethylarsonioriboside.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XksV2jt7w%3D&md5=0137623f24be090d44f19ee4bed6cba0CAS |
[16] J. S. Edmonds, Y. Shibata, K. A. Francesconi, R. J. Rippingale, M. Morita, Arsenic transformations in short marine food chains studied by HPLC-ICP MS. Appl. Organomet. Chem. 1997, 11, 281.
| Arsenic transformations in short marine food chains studied by HPLC-ICP MS.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXis12qsr8%3D&md5=6630d5291e333edadb512c1bf5da976bCAS |
[17] S. Foster, D. Thomson, W. Maher, Uptake and metabolism of arsenate by axenic cultures of the microalgae Dunaliella tertiolecta and Phaeodactylum tricornutum. Mar. Chem. 2008, 108, 172.
| Uptake and metabolism of arsenate by axenic cultures of the microalgae Dunaliella tertiolecta and Phaeodactylum tricornutum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXnvVyrtQ%3D%3D&md5=7af0582d1114fb1c57b8d2eff493eda8CAS |
[18] S. Khokiattiwong, W. Gössler, S. N. Pedersen, R. Cox, K. A. Francesconi, Dimethylarsinoylacetate from microbial demethylation of arsenobetaine in seawater. Appl. Organomet. Chem. 2001, 15, 481.
| Dimethylarsinoylacetate from microbial demethylation of arsenobetaine in seawater.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXktFOqs7g%3D&md5=ffcd7dee87c8593ed438ff3ab505b800CAS |
[19] S. C. R. Granchinho, C. M. Franz, E. Polishchuk, W. R. Cullen, K. J. Reimer, Transformation of arsenic(V) by the fungus Fusarium oxysporum melonis isolated from the alga Fucus gardneri. Appl. Organomet. Chem. 2002, 16, 721.
| Transformation of arsenic(V) by the fungus Fusarium oxysporum melonis isolated from the alga Fucus gardneri.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XpslWmtrk%3D&md5=1fcbd7199562f43b269e45914ac1110bCAS |
[20] D. Thomson, W. Maher, S. Foster, Arsenic and selected elements in inter-tidal and estuarine marine algae, south-east coast, NSW, Australia. Appl. Organomet. Chem. 2007, 21, 396.
| Arsenic and selected elements in inter-tidal and estuarine marine algae, south-east coast, NSW, Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXms1yit7s%3D&md5=0493daa3e459512498fb87fc5539fabaCAS |
[21] L. A. Murray, A. Raab, I. L. Marr, J. Feldmann, Biotransformation of arsenate to arsenosugars by Chlorella vulgaris. Appl. Organomet. Chem. 2003, 17, 669.
| Biotransformation of arsenate to arsenosugars by Chlorella vulgaris.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXmvFOrtL4%3D&md5=00f756f5d5afad289c14fd149b72e26bCAS |
[22] J. L. Levy, J. L. Stauber, M. S. Adams, W. A. Maher, J. K. Kirby, D. F. Jolley, Toxicity, biotransformation, and mode of action of arsenic in two freshwater microalgae (Chlorella sp. and Monoraphidium arcuatum). Environ. Toxicol. Chem. 2005, 24, 2630.
| Toxicity, biotransformation, and mode of action of arsenic in two freshwater microalgae (Chlorella sp. and Monoraphidium arcuatum).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtVKqt7bP&md5=8a624afc288151934f2a0365b1030d2bCAS |
[23] S. Miyashita, M. Shimoya, Y. Kamidate, T. Kuroiwa, O. Shikino, S. Fujiwara, K. A. Francesconi, T. Kaise, Rapid determination of arsenic species in freshwater organisms from the arsenic-rich Hayakawa River in Japan using HPLC-ICP-MS. Chemosphere 2009, 75, 1065.
| Rapid determination of arsenic species in freshwater organisms from the arsenic-rich Hayakawa River in Japan using HPLC-ICP-MS.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXltFWltLk%3D&md5=4e386fe9ea1fd72695560d58ef440557CAS |
[24] S. Miyashita, S. Fujiwara, M. Tsuzuki, T. Kaise, Rapid biotransformation of arsenate into oxo-arsenosugars by a freshwater unicellular green alga, Chlamydomonas reinhardtii. Biosci. Biotechnol. Biochem. 2011, 75, 522.
| Rapid biotransformation of arsenate into oxo-arsenosugars by a freshwater unicellular green alga, Chlamydomonas reinhardtii.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXltF2lsL8%3D&md5=930fa995d2b9fbfcb8d438b354f4f4bcCAS |
[25] S. Maeda, K. Mawatari, A. Ohki, K. Naka, Arsenic metabolism in a freshwater food chain: blue-green alga (Nostoc sp.) → shrimp (Neocaridina denticulata) → carp (Cyprinus carpio). Appl. Organomet. Chem. 1993, 7, 467.
| Arsenic metabolism in a freshwater food chain: blue-green alga (Nostoc sp.) → shrimp (Neocaridina denticulata) → carp (Cyprinus carpio).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXht12qsbw%3D&md5=248367c3b71a7c86ac7702f65443f68dCAS |
[26] V. W.-M. Lai, W. R. Cullen, C. F. Harrington, K. J. Reimer, The characterization of arsenosugars in commercially available algal products including a Nostoc species of terrestrial origin. Appl. Organomet. Chem. 1997, 11, 797.
| The characterization of arsenosugars in commercially available algal products including a Nostoc species of terrestrial origin.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXmvFaqsrw%3D&md5=d162d75ed8fb62323890e4aca9e66ccbCAS |
[27] B. A. Whitton, M. Potts, Introduction to the cyanobacteria, in The Ecology of Cyanobacteria: Their Diversity in Time and Space (Eds B. A. Whitton, M. Potts) 2000, pp. 1–11 (Kluwer Academic Publishers: Dordrecht, the Netherlands).
[28] B. M. Gamble, P. A. Gallagher, J. A. Shoemaker, X. Wei, C. A. Schwegel, J. T. Creed, An investigation of the chemical stability of arsenosugars in simulated gastric juice and acidic environments using IC–ICP-MS and IC-ESI-MS/MS. Analyst (Lond.) 2002, 127, 781.
| An investigation of the chemical stability of arsenosugars in simulated gastric juice and acidic environments using IC–ICP-MS and IC-ESI-MS/MS.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XktVegsb8%3D&md5=77ebdb1ba3f6459c63ff1de96747a301CAS |
[29] D. P. McAdam, A. M. A. Perera, R. V. Stick, The synthesis of (R)-2′,3′-dihydroxypropyl 5-deoxy-5-dimethylarsinyl-β-D-riboside, a naturally occurring arsenic-containing carbohydrate. Aust. J. Chem. 1987, 40, 1901.
| The synthesis of (R)-2′,3′-dihydroxypropyl 5-deoxy-5-dimethylarsinyl-β-D-riboside, a naturally occurring arsenic-containing carbohydrate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1cXktlyjtbY%3D&md5=52a5885b7c22d0cfb5e76638e48a4738CAS |
[30] A. D. Madsen, W. Gössler, S. N. Pedersen, K. A. Francesconi, Characterization of an algal extract by HPLC-ICP-MS and LC-electrospray MS for use in arsenosugar speciation studies. J. Anal. At. Spectrom. 2000, 15, 657.
| Characterization of an algal extract by HPLC-ICP-MS and LC-electrospray MS for use in arsenosugar speciation studies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXjslWksLY%3D&md5=2fdb16ed253759d050a8ade19c0bf70aCAS |
[31] J. G. K. Williams, Construction of specific mutants in photosystem II photosynthetic reaction center by genetic engineering methods in Synechocystis 6803. Methods Enzymol. 1988, 167, 766.
| Construction of specific mutants in photosystem II photosynthetic reaction center by genetic engineering methods in Synechocystis 6803.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1MXhvVKqs7g%3D&md5=30e1cbbdacee7a899b65182ed035f911CAS |
[32] T. Kaneko, Y. Nakamura, C. P. Wolk, T. Kuritz, S. Sasamoto, A. Watanabe, M. Iriguchi, A. Ishikawa, K. Kawashima, T. Kimura, Y. Kishida, M. Kohara, M. Matsumoto, A. Matsuno, A. Muraki, N. Nakazaki, S. Shimpo, M. Sugimoto, M. Takazawa, M. Yamada, M. Yasuda, S. Tabata, Complete genomic sequence of the filamentous nitrogen-fixing cyanobacterium Anabaena sp. strain PCC 7120. DNA Res. 2001, 8, 205.
| Complete genomic sequence of the filamentous nitrogen-fixing cyanobacterium Anabaena sp. strain PCC 7120.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXotlCgu7k%3D&md5=1479bdc4b5b38129345014bbdb2456adCAS |
[33] R. Rippka, J. Deruelles, J. B. Waterbury, M. Herdman, R. Y. Stanier, Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 1979, 111, 1.
| Generic assignments, strain histories and properties of pure cultures of cyanobacteria.Crossref | GoogleScholarGoogle Scholar |
[34] M. Akai, K. Onai, M. Kusano, M. Sato, H. Redestig, K. Toyooka, M. Morishita, H. Miyake, A. Hazama, V. Checchetto, I. Szabò, K. Matsuoka, K. Saito, M. Yasui, M. Ishiura, N. Uozumi, Plasma membrane aquaporin AqpZ is essential for glucose metabolism during photomixotrophic growth of Synechocystis sp. PCC 6803. J. Biol. Chem. 2011, 286, 25224.
| Plasma membrane aquaporin AqpZ is essential for glucose metabolism during photomixotrophic growth of Synechocystis sp. PCC 6803.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXosFGmtLo%3D&md5=3e48d401942abe348b104f26cf505adaCAS |
[35] G. MacKinney, Absorption of light by chlorophyll solutions. J. Biol. Chem. 1941, 140, 315.
| 1:CAS:528:DyaH3MXkt1GgsQ%3D%3D&md5=1d91744059e5cbaa7d5361850a922a1eCAS |
[36] I. Koch, J. Feldmann, L. Wang, P. Andrewes, K. J. Reimer, W. R. Cullen, Arsenic in the Meager Creek hot springs environment, British Columbia, Canada. Sci. Total Environ. 1999, 236, 101.
| Arsenic in the Meager Creek hot springs environment, British Columbia, Canada.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXmt1Wrurg%3D&md5=36ac34919dc80894919aac25510a5d01CAS |
[37] Y. Shibata, M. Sekiguchi, A. Otsuki, M. Morita, Arsenic compounds in zoo- and phyto-plankton of marine origin. Appl. Organomet. Chem. 1996, 10, 713.
| Arsenic compounds in zoo- and phyto-plankton of marine origin.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XnsFynu7k%3D&md5=5495b218e90d3314149517d176d1427cCAS |
[38] J. Qin, C. R. Lehr, C. Yuan, X. C. Le, T. R. McDermott, B. P. Rosen, Biotransformation of arsenic by a Yellowstone thermoacidophilic eukaryotic alga. Proc. Natl. Acad. Sci. USA 2009, 106, 5213.
| Biotransformation of arsenic by a Yellowstone thermoacidophilic eukaryotic alga.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXksVert7Y%3D&md5=396bc10200fce3d6e24b19091697346dCAS |
[39] S. Maeda, Investigations in organoarsenic chemistry, in Arsenic and Old Mustard: Chemical Problems in the Destruction of Old Arsenical and ‘Mustard’ Munitions (Eds J. F. Bunnett, M. Mikolajczyk) 1998, pp. 135–148 (Kluwer Academic Publishers: Dordrecht, the Netherlands).
[40] J. S. Edmonds, K. A. Francesconi, Transformations of arsenic in the marine environment. Experientia 1987, 43, 553.
| Transformations of arsenic in the marine environment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2sXltFaqtrc%3D&md5=07a0368eec64d73f51881d58e52e74c8CAS |
[41] R. V. Cooney, R. O. Mumma, A. A. Benson, Arsoniumphospholipid in algae. Proc. Natl. Acad. Sci. USA 1978, 75, 4262.
| Arsoniumphospholipid in algae.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1MXhvFShtQ%3D%3D&md5=0f71fc9f2c9a87c3149032d0fc1f5a30CAS |
[42] S. Maeda, S. Fujita, A. Ohki, I. Yoshifuku, S. Higashi, T. Takeshita, Arsenic accumulation by arsenic-tolerant freshwater blue-green alga (Phormidium sp.). Appl. Organomet. Chem. 1988, 2, 353.
| Arsenic accumulation by arsenic-tolerant freshwater blue-green alga (Phormidium sp.).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1MXktlCn&md5=a479fda657cfa9060aa829b4954691a5CAS |
[43] M. Morita, Y. Shibata, Isolation and identification of arseno-lipid from a brown alga, Undaria pinnatifida (Wakame). Chemosphere 1988, 17, 1147.
| Isolation and identification of arseno-lipid from a brown alga, Undaria pinnatifida (Wakame).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1cXkvVOlt78%3D&md5=cd17541ee1fd9c0b7f3ecbad743a2e69CAS |
[44] A. A. Benson, Radiochromatographic exploration. J. Am. Oil Chem. Soc. 1987, 64, 1309.
| Radiochromatographic exploration.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2sXlsFSnur8%3D&md5=00f8a1695e841954e927079f29db03b8CAS |
[45] S. García-Salgado, G. Raber, R. Raml, C. Magnes, K. A. Francesconi, Arsenosugar phospholipids and arsenic hydrocarbons in two species of brown macroalgae. Environ. Chem. 2012, 9, 63.
| Arsenosugar phospholipids and arsenic hydrocarbons in two species of brown macroalgae.Crossref | GoogleScholarGoogle Scholar |
[46] K. A. Francesconi, J. S. Edmonds, R. V. Stick, Synthesis, NMR spectra and chromatographic properties of five trimethylarsonioribosides. Appl. Organomet. Chem. 1994, 8, 517.
| Synthesis, NMR spectra and chromatographic properties of five trimethylarsonioribosides.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXhtlensLg%3D&md5=37f4eb0a974ce1b05862ec79389cbd00CAS |
[47] K. A. Francesconi, J. S. Edmonds, R. V. Stick, B. W. Skelton, A. H. White, Arsenic-containing ribosides from the brown alga Sargassum lacerifolium: X-ray molecular structure of 2-amino-3-[5′-deoxy-5′-(dimethylarsinoyl)ribosyloxy]-propane-1-sulphonic acid. J. Chem. Soc., Perkin Trans. 1 1991, 1991, 2707.
| Arsenic-containing ribosides from the brown alga Sargassum lacerifolium: X-ray molecular structure of 2-amino-3-[5′-deoxy-5′-(dimethylarsinoyl)ribosyloxy]-propane-1-sulphonic acid.Crossref | GoogleScholarGoogle Scholar |