Derivation of ecological standards for risk assessment of molybdate in soil
Koen Oorts A G , Erik Smolders B , Steve P. McGrath C , Cornelis A.M. van Gestel D , Michael J. McLaughlin E and Sandra Carey FA ARCHE, Liefkensstraat 35D, BE-9032 Ghent (Wondelgem), Belgium.
B Department of Earth and Environmental Sciences, Division of Soil and Water Management, Katholieke Universiteit Leuven, Kasteelpark Arenberg 20, BE-3001 Leuven, Belgium.
C Rothamsted Research, Harpenden, Hertfordshire, AL5 2JQ, UK.
D Department of Ecological Science, Faculty of Earth and Life Science, VU University Amsterdam, De Boelelaan 1085, NL-1081 HV Amsterdam, Netherlands.
E CSIRO Land and Water, Contaminant Chemistry and Ecotoxicology Program, Waite Campus, Waite Road, Urrbrae, SA 5064, Australia.
F International Molybdenum Association, 4 Heathfield Terrace, London, W4 4JE, UK.
G Corresponding author. Email: koen.oorts@arche-consulting.be
Environmental Chemistry 13(1) 168-180 https://doi.org/10.1071/EN15086
Submitted: 24 April 2015 Accepted: 31 July 2015 Published: 4 November 2015
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Environmental context. In order to assess the potential risks of elevated molybdenum concentrations in soil due to anthropogenic activities, toxicity thresholds must be known and environmental criteria defined. Setting such criteria for metals is not straightforward because of varying natural background concentrations and differences in toxicity between typical laboratory and field conditions and across soil types. Toxicity data and models were derived that account for these parameters so that soil quality criteria can be derived based on total molybdenum concentrations in soil.
Abstract. An extensive testing programme on the toxicity of sodium molybdate dihydrate in soil was initiated to comply with the European REACH Regulation. The molybdate toxicity was assayed with 11 different bioassays, 10 different soils, soil chemical studies on aging reactions, and toxicity tests before and after 1-year equilibration in field conditions. Differences in molybdate toxicity among soils were best explained by soil pH and clay content. A correction factor of 2.0 was selected to account for the difference in molybdate toxicity between laboratory and field conditions due to leaching and aging processes. Toxicity thresholds were determined as the HC5–50 (median hazardous concentration for 5 % of the species, i.e. median 95 % protection level) derived from the species sensitivity distribution of ecotoxicity data after bioavailability corrections. Uncertainty analysis illustrated that the HC5–50 provides a robust and ecologically relevant predicted no-effect concentration (PNEC) for risk characterisation. The 10th and 90th percentiles for site-specific PNEC values in European agricultural soil are 10.7 and 168 mg Mo kg–1 dry weight respectively based on a large survey of metal concentrations and soil properties in arable land soils. Total soil Mo concentrations in these soils are below corresponding PNEC values at most locations, suggesting no regional risks of molybdate to soil organisms at this scale. The information presented can be used in the EU risk-assessment framework as well as for national and international regulatory purposes for the setting of soil quality criteria based on total molybdenum concentrations, soil pH and clay content.
Additional keywords: bioavailability, soil ecotoxicity.
Introduction
Molybdenum (Mo) is a naturally occurring element, playing an essential role in biochemical processes in microorganisms, plants and animals.[1,2] The average concentration of Mo in the upper earth’s crust is 1.2 mg kg–1, making it 56th in order of crustal abundance.[3] Natural background concentrations in soil range between 1 and 5 mg kg–1, whereas Mo-rich soils may contain 10–100 mg Mo kg–1.[4] Molybdenum is used mainly in steel and alloys, with other applications in catalysts, pigments, lubricants, corrosion inhibitors, smoke suppressants and fertilisers (http://www.imoa.info/molybdenum_uses/molybdenum_uses.php, accessed September 2015). The main emission pathways to the environment are through mining activities, the application of biosolids and fertilisers, and atmospheric deposition from smelters.[5] Although the speciation of Mo in environmental emissions is unclear, it is most likely that aerosol-bound Mo is present as the sparingly soluble hexavalent Mo oxide (MoO3).
Molybdenum is present as MoVI in solutions at redox potential (Eh) >0 V and pH > 5.[6] The dominant soluble species found in oxygenated environments is the tetrahedral oxoanion molybdate (MoO42–). The dominant sorbents of molybdate in soils are oxides (e.g. iron(III) oxide, clay minerals and organic matter).[7–9] Molybdate sorption onto soils and minerals has been shown to decrease with increasing pH.[7,10]
Until recently, few data were available concerning MoO42– toxicity to soil organisms. However, according to the European Union (EU) Regulation number 1907/2006 concerning the Registration, Evaluation and Restriction of Chemical Substances (REACH), the registration dossier for high-volume chemicals (i.e. >1000 tonnes per year), such as MoO3, Mo metal and Na2MoO4, must comply with the minimum data requirements outlined in Annexes VII–X of the REACH legislation.[11] In practice, these requirements include data from chronic toxicity studies for at least one organism belonging to each of the three trophic levels of soil organisms, i.e. microorganisms, plants and invertebrates.[12]
The natural occurrence of metal compounds in all environmental compartments, including organisms, together with the chemical processes that affect the bioavailability of metals in soils have important implications for the effects assessment of metals to soil organisms.[13] The bioavailability and toxicity of metals or metalloids in soils is influenced by several abiotic factors such as (i) soil properties,[14–16] (ii) aging processes of the metals added,[17–19] and (iii) form of metal added to the soil.[20,21] It is generally accepted that the total metal concentration in soil is a poor predictor of its bioavailability and toxicity. However, there is not yet a generally accepted method for measurement of the bioavailable fraction of metals in soil, and data for (pseudo-) total metal concentrations, based on concentrated acid digestions, are most commonly available and used in setting soil quality criteria. Protocols have been developed to take bioavailability considerations into account for the derivation of ecological soil standard values based on total metal concentrations.[22] However, such information was not available for Mo and hence a research programme was initiated to collect data and develop bioavailability correction models to facilitate a sound risk assessment of Mo for soil organisms. The MoO42– ion is the prevailing form of Mo in the environment at relevant conditions of pH and redox potential[5,23] and hence is the relevant form for essentiality and potential toxicity to living organisms. The objective was therefore to evaluate the toxicity in soils for the MoO42– ion by assessing bioassays carried out with a soluble MoO42– salt (i.e. sodium molybdate, Na2MoO4). Results from toxicity tests with sodium molybdate dihydrate (Na2MoO4·2H2O) show that this form is the preferred Mo form for testing rather than MoO3, which is the dominant Mo compound in emissions to the environment. This is because large doses of MoO3 induce acidification and results are strongly confounded.[5]
The research programme consisted of testing the toxicity of Na2MoO4 in 11 different bioassays encompassing plants, invertebrates and microbial processes in 10 different natural soils covering a representative range in soil properties for Europe. In three soils, toxicity was also tested after 6 and 11 months’ equilibration after spiking with Na2MoO4 in order to assess changes in soil toxicity with time.[5,24–30]
The current paper presents the implementation of these data and bioavailability correction models in the effects assessment and the derivation of ecological standards for Mo in soil. This assessment covers direct toxicity only to soil organisms, i.e. microorganisms, plants and soil-dwelling invertebrates; the assessment of secondary poisoning to vertebrates is not discussed. The approach is illustrated by an environmental risk assessment for Mo in arable land soils at the European scale.
Materials and methods
Selection of ecotoxicological data
The ecotoxicological data in the present paper are derived from original papers in international peer-reviewed journals and from the International Molybdenum Association (IMOA) soils research programme. All data were thoroughly screened for their relevance and quality (reliability). Preference was given to standardised tests with standard species, as prescribed by organisations such as International Organization for Standardization (ISO), Organization for Economic Cooperation and Development (OECD) and United States Environmental Protection Agency (US EPA), but non-standard tests were also allowed when they met the relevance and reliability criteria. Tests that did not comply with the criteria below were rated as not relevant or reliable and were not used in the effects assessment.
Only data from observations in natural and artificial (OECD) soil media were selected. Tests performed in substrates that do not represent soils (e.g. nutrient solution, agar, pure quartz sand and farmyard manure) were not included in this effects assessment. The Mo compounds had to have been mixed homogeneously in the test soil and adequate time (i.e. ≥24 h) had to have elapsed between mixing metals or metal compounds into the test medium, introducing biota (plants or soil invertebrates) and the start of the test. In addition, the data used in the effects assessment had to be based on actual measured or analytically confirmed nominal soil Mo concentrations.
The assessment was based on toxicity data to plants and soil-dwelling organisms (invertebrates and microorganisms) after chronic exposure only. The selected toxicity data on soil organisms were from ecotoxicity tests that studied relevant ecotoxicological parameters such as survival, growth, reproduction, litter breakdown, abundance. Relevant endpoints for soil microorganisms focussed on functional parameters such as respiration, nitrification and mineralisation. What comprises ‘chronic exposure’ is dependent on the life cycle of the test organisms. A priori fixed-exposure durations were therefore deemed not relevant. The duration had to be related to the typical life cycle and ideally encompass the entire life cycle or, for longer-lived species, the most sensitive life stage. Retained exposure durations were related to recommendations from standard ecotoxicity protocols (e.g. ISO, OECD, American Society for Testing and Materials (ASTM)). Typically, chronic test durations for the higher plants were within the range of 4 days (e.g. the barley root elongation test based on ISO 11269-1[31]) and 21 days (e.g. the tomato shoot yield test based on ISO 11269-2[32] or OECD 208[33]). Tests assessing the chronic effects of substances on sublethal endpoints of soil invertebrates had a typical exposure duration of 4 to 8 weeks for standard organisms.[34–36] Reported test durations using soil microorganisms varied between 24 h (glucose-induced respiration assay) and 28 days for a plant residue mineralisation assay and a nitrogen transformation test.[37,38]
If no statistical methodology was reported or if effects concentrations were derived ‘visually’, the data were considered unreliable. Effect levels derived from toxicity tests using a single test concentration always result in unbounded, unreliable data. Therefore, only the results from toxicity tests using a control and at least two Mo concentrations were used. EC10 (10 % effective concentrations) values as calculated from the concentration–effect relationship were preferred.[39] In some cases, no reliable EC10 could be derived because, for example. no significant dose–response curve could be fitted or the EC10 was outside the concentration range tested. When in these cases a bounded no observed effect concentration (NOEC) value could be derived, this NOEC value was used instead of the EC10. Unbounded NOEC (i.e. no effect at highest dose tested) or unbounded lowest observed effect concentration (LOEC) (i.e. significant effect at lowest dose tested) values or EC10 values extrapolated outside the concentration range tested were not used.
Bioavailability corrections
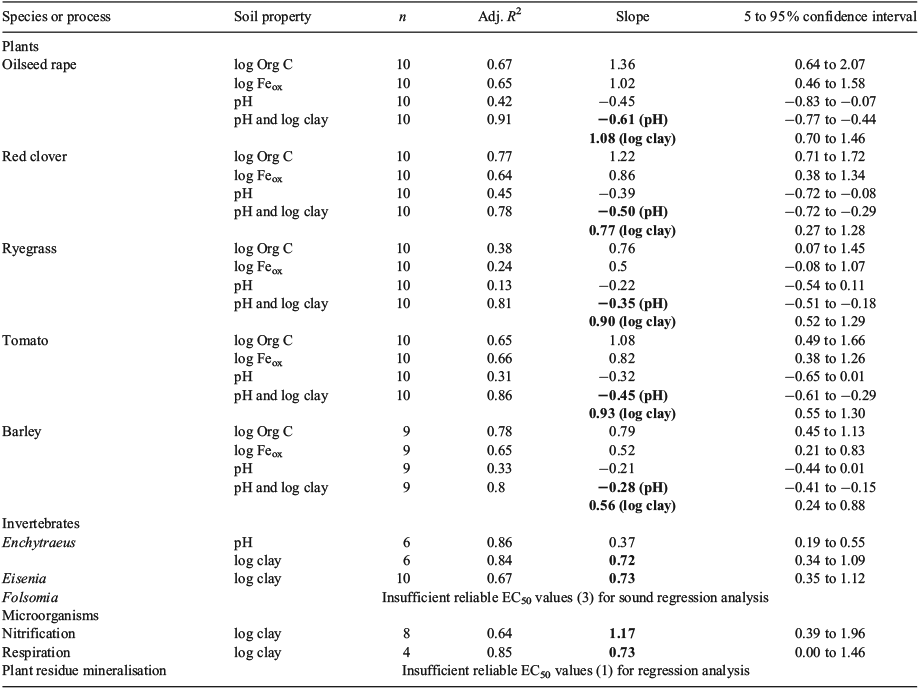
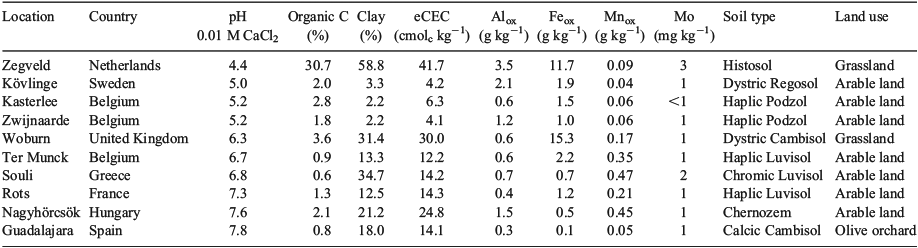
Bioavailability correction protocols were developed according to the procedures described by Smolders et al.[22] The toxicity of Na2MoO4 was tested in 10 uncontaminated topsoils collected throughout Europe comprising a representative range of key soil properties (Table 1). These soils were sampled in eight EU member states, covering arable and grassland soils and belonging to six major soil groupings (Cambisol, Chernozem, Histosol, Luvisol, Podzol, Regosol). The soils selected are representative of those in the EU, and sufficient to facilitate regression modelling of the toxicity of MoO42– v. soil properties. The toxicity of Na2MoO4 was tested in each soil for five plant species, three invertebrate species and three microbial processes (Table 2).
|
|
No suitable Mo-contaminated soils could be identified in the field to account for long-term effects that mitigate MoO42– toxicity. As an alternative, an experiment was conducted in which the change in MoO42– toxicity was monitored 0–11 months after spiking.[27] Briefly, three soils were spiked in the laboratory and aged outside under prevailing climatic conditions in Leuven, Belgium. After 6 and 11 months had elapsed, the soils were sampled and the toxicity was measured with 10 bioassays (the same as mentioned above, except for the barley root elongation assay). The changes in toxicity were quantified by the ‘laboratory-to-field’ factor (also called ‘leaching–aging factor’), which is calculated as the relative change in 10 or 50 % effective doses (ED10 or ED50, i.e. background-corrected effective concentrations) after leaching and aging. This factor was based on added or background-corrected Mo concentrations in soil because no changes in the bioavailability and toxicity of the natural Mo background concentration in soil were anticipated. The ECx values for aged soils were all based on actual, measured concentrations to correct for the decrease in total Mo concentration due to MoO42– leaching by percolating rainwater. During the aging period, the soils were incubated in pots allowing free drainage of percolating rainwater. Therefore, the laboratory-to-field factor covers both the effect of decreased ionic strength due to leaching of excess ions and the effect of long-term equilibration in soil of the added MoO42– ions (aging) on organism toxicity.
In addition to the toxicity assays on aged soils, the changes in lability of added soluble MoO42– ions in soil were also studied to measure differences in bioavailability over time of the added MoO42–.[40] In summary, 15 soils (ten soils selected for the development of bioavailability models, Table 1, supplemented with five other soils) were spiked with Na2MoO4 at the EC10 and EC90 of a plant assay (barley root elongation) and were equilibrated at 25 °C and at 60 % of their moisture content at pF 2.0 (suction of 100 cm of water). The isotopically exchangeable fraction (E value) was measured at 0, 0.5, 1, 3, 6, 12 and 18 months after spiking using the radioactive tracer, 99Mo (half-life t1/2 67 h). The change in the isotopically exchangeable fraction (%E) between 0.5 months (within a typical timeframe of a toxicity test) and 18 months (taken as a surrogate for long-term equilibration) was used as the basis to calculate the factor to explain MoO42– aging in soils (fixation factor = %E0.5 month/%E18 months).
Regional risk assessment at European scale (GEMAS)
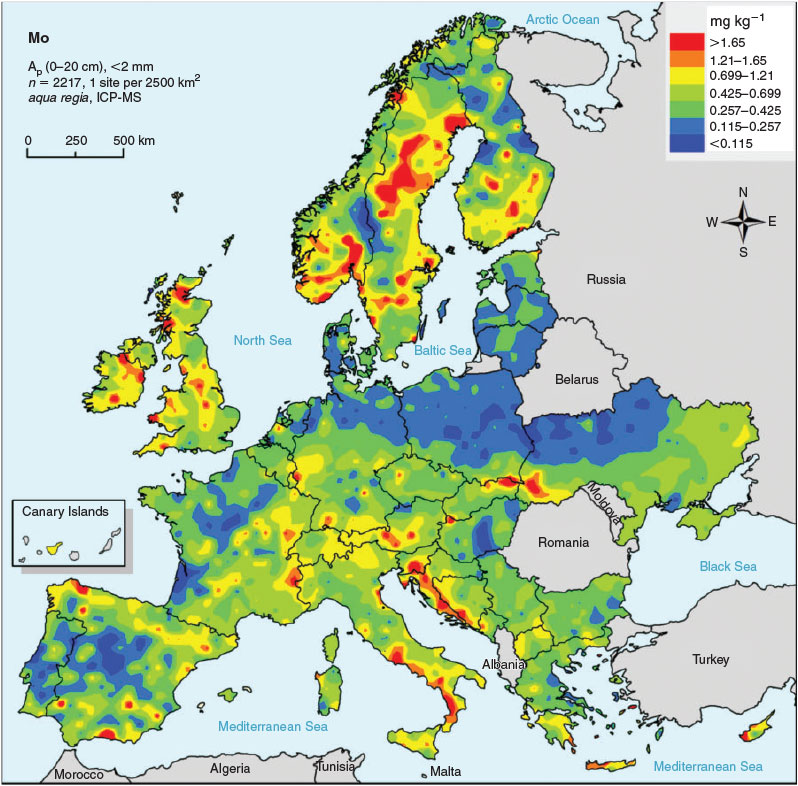
The predicted no-effect concentrations (PNECs or ecological soil standards) can be compared with prevailing exposure concentrations in soil to identify areas with potential risk. The GEMAS (Geochemical Mapping of Agricultural and Grazing Land Soils; http://gemas.geolba.ac.at/, accessed September 2015) project provides high quality and comparable data for both metal concentrations and soil properties known to influence metal bioavailability (pH, organic carbon content, clay content and effective Cation Exchange Capacity (eCEC)) in arable and grazing land at the European scale.[41] In total, 2108 samples of agricultural (arable) soil and 2024 samples of grazing land soil were collected at an average sampling density of 1 site per 2500 km2 (grid of 50 × 50 km). The measured aqua regia (3 parts HCl + 1 part HNO3)-soluble metal concentrations from the GEMAS project can be considered as ambient background concentrations, i.e. the sum of the natural background of a metal with diffuse anthropogenic input in the past or present (due to, for example, agricultural inputs, combustion of fossil fuels or traffic). The influence of point sources (e.g. from local industrial activities) was avoided owing to the sampling strategy adopted. The consistent land-use and sampling depth (0–20 and 0–10 cm for agricultural (arable) land and grazing land respectively) ensured a comparable level of exposure for all samples within the same land-use.
In order to assess the potential risk to soil organisms at the prevailing Mo concentrations, the GEMAS results for aqua regia-soluble Mo concentrations for arable land and grassland soils were compared with the site-specific ecological soil standards expressed as total Mo concentrations and normalised for the soil properties measured for the individual sampling sites. The risk characterisation ratio (RCR) for each site was calculated as the ratio of measured aqua regia-extractable Mo concentration to the corresponding PNEC. Distributions of PNEC and RCR values were calculated non-parametrically. Because sites were sampled in a regular grid over Europe, there was no bias due to spatial heterogeneity of sampling density, and it was appropriate to derive the distributions of PNEC and RCR values from the measured observations without the need for spatial interpolation to derive area-based distributions.
Results and discussion
Toxicity data
In total, 82 relevant and reliable EC10 values and four additional NOEC values passed the selection criteria for the effects assessment of MoO42– to soil organisms (Table 2). All selected data were derived from toxicity tests carried out according to international guidelines in the framework of the research programme initiated by IMOA.[25,28,30] Although some relevant studies with toxicity data for plants and microorganisms were identified in the scientific literature published before 2010, none of these studies contained reliable toxicity data useful for the effects assessment (see Supplementary material).
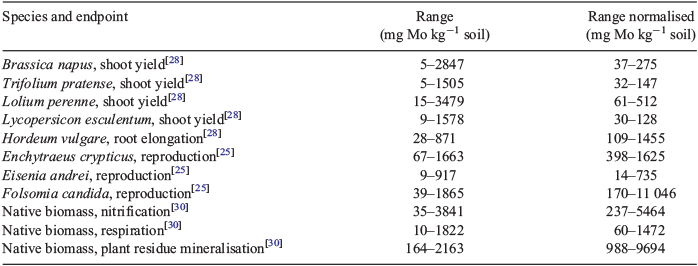
For plants, in total, 45 individual high-quality EC10 values were selected for the derivation of a soil threshold concentration for MoO42–. These EC10 values covered five different plant species and ranged from 5 mg Mo kg–1 dry weight (DW) (for oilseed rape and red clover) to 3479 mg Mo kg–1 DW (for ryegrass).[28] For five dose–response curves, no reliable EC10 or NOEC could be derived because a significant effect was already observed at the lowest dose tested.
The invertebrate toxicity assays resulted in 23 individual high quality NOEC or EC10 values (for three different species), ranging from 8.9 mg Mo kg–1 DW for Eisenia andrei to 1865 mg Mo kg–1 DW for the springtail Folsomia candida.[25] No significant inhibition of reproduction was observed at the largest dose tested in eight and two soils for the Folsomia candida and Enchytraeus crypticus assays respectively (unbounded NOEC values between 2628 and 3396 mg Mo kg–1 DW).
For microbial assays, 18 individual high-quality NOEC or EC10 values, derived for three different processes, were selected as reliable. These values ranged from 10 mg Mo kg–1 DW for glucose-induced respiration to 3841 mg Mo kg–1 DW for substrate-induced nitrification.[30] No toxic effect was observed at the largest dose tested (10 000 mg Mo kg–1 DW) in two, four and six soils for the substrate-induced nitrification, glucose-induced respiration and plant residue mineralisation assays respectively.
All results are expressed based on total or aqua regia-extractable elemental Mo concentrations and can be applied to other Mo compounds (‘read-across’). The justification for the read-across is that the concentrations of MoO42– in soils and the outcome of the tests conducted on a soluble Mo compound reflect the concentrations and toxicity levels for all soluble Mo compounds and the thresholds are a conservative estimate for sparingly soluble Mo compounds.
Bioavailability corrections – influence of soil properties on molybdate toxicity to soil organisms
The selected EC10 values for an endpoint varied from 13-fold to more than 500-fold among the soils tested (Table 2). A regression analysis (log–log basis) revealed that the variation in EC50 values among soils was, for all endpoints studied, significantly correlated with soil properties such as clay, ammonium oxalate-extractable iron oxides, pH (0.01 M CaCl2), eCEC and organic carbon (Table 3).[25,29,30] Regressions with soil properties were preferentially based on EC50 values, because EC50 is a more robust estimate (smaller confidence interval) for effects and is less affected by experimental error compared with the NOEC or EC10 values.
For plants, soil organic carbon content was for the various species tested the best single regressor. However, these regressions were strongly affected by one soil with high organic carbon content (30.7 %). Except for ryegrass (Lolium perenne), MoO42– toxicity was also significantly correlated with the oxalate-extractable iron (Feox) content of the soils. This is consistent with observations for arsenate, which also prevails in soil as an oxoanion.[15] Multiple regressions with pH and clay content or pH and eCEC significantly improved the regression fit compared with single linear regressions. In these regressions, clay content was most likely a good surrogate for the actual binding surfaces present in the soil, including clays, oxides and organic matter. A larger clay content results in more binding surfaces and therefore in lower availability and toxicity of the MoO42– anion in soil. Similarly, in the multiple regressions with pH and eCEC (not shown), the eCEC, which is theoretically a measure for binding of cations, is probably a measurement that integrates clay and organic matter in soil, which both have binding sites for molybdate. The negative regression of EC50 with pH (higher EC50 values and lower toxicity at low pH) is explained by the larger number of positively charged sorption sites for anions on the soil constituents (clay, oxides, organic matter) at low pH, resulting in a lower bioavailability. This regression with pH is consistent with common agricultural experience: Mo deficiency in acidic soils due to low MoO42– bioavailability can be overcome by liming to make the soil less acidic, thereby releasing MoO42– from the sorption sites and increasing its bioavailability.[42] Because of the large R2 values (0.78–0.91) for all five plant species, and the sound mechanistic explanation, the regression models with pH and clay content were selected for normalisation of the plant data.
For invertebrates, only models for Enchytraeus crypticus and Eisenia andrei could be developed. For both species, the clay content was the best single regressor for the EC50 values and the slopes of both regression equations were very consistent. Multiple linear regression models, using stepwise addition, did not consistently improve the regression fit for invertebrates. Bounded EC50 values for MoO42– toxicity to Folsomia candida were only obtained for three sandy soils with comparable soil properties. Therefore, no sound regression analysis with soil properties could be performed.
For the microbial processes studied, only models for substrate-induced nitrification and respiration could be developed. No valid model could be derived for the microbial plant residue mineralisation (PRM) assay because only one bounded EC50 value was observed for this assay. Although only four bounded EC50 values were available for glucose-induced respiration, the results of the regression analysis were considered useful because the range in soil properties covered by these four soils was still sufficiently wide (pH: 5.2–7.3; organic carbon: 0.9–2.8 %; clay: 2–12 %; eCEC: 4.1–14.3 cmolc (charge) kg–1; Feox: 1–2.2 g kg–1) and can be considered representative for the soils in Europe with the highest bioavailability and toxicity of Mo (i.e. high pH, low organic carbon content, clay content and eCEC). For both the nitrification and respiration processes, a consistent significant linear regression of EC50 values with the soil clay content was observed (R2 0.64 and 0.85). Similarly to the invertebrates, multiple linear regression models did not consistently improve the regression fit for the microbial endpoints studied.
In summary, the key soil properties identified as governing MoO42– toxicity in soils were the pH and clay content for plants and the clay content for invertebrates and microorganisms. Molybdate toxicity to soil organisms generally decreased (i.e. increasing EC50 values) with decreasing pH and increasing clay content. The best linear regression models (log–log basis) accounted for 64 to 91 % of the variability of the EC50 for the soil organisms studied (Table 3).
Bioavailability corrections – effect of leaching and aging processes
All toxicity data discussed above referred to soils tested 7 days after spiking with soluble Na2MoO4 without removal of excess salts by leaching. The threshold values for MoO42– in soil derived from these data, expressed as total soil Mo, could overestimate the toxicity of Mo in a field with soil contaminated over a longer time period at the same total soil Mo concentration. Previous findings reported the solubility or availability of MoO42– to decrease in soils or mineral phases with increasing incubation time.[42–46]
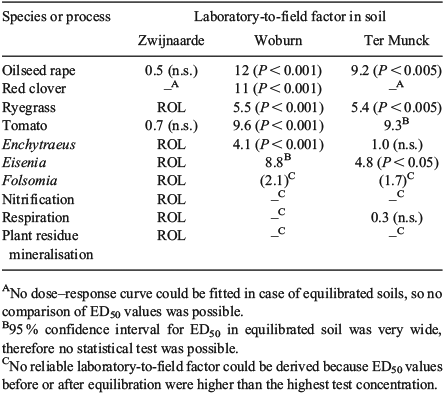
Comparison of MoO42– toxicity between freshly spiked soils and soils aged for 11 months indicated that long-term equilibration of MoO42– in soils under field conditions generally decreased its toxicity to soil organisms. Laboratory-to-field factors were calculated as the ratio of ED50 values before and after 11-month equilibration (Table 4). In total, eight ED50-based laboratory-to-field factors were significantly larger than one (i.e. significant decrease in toxicity with aging), whereas no laboratory-to-field factor was significantly smaller than one (i.e. increase in toxicity with aging). The median decrease in toxicity after leaching and aging processes was a factor of 5.4.
|
The MoO42– toxicity data for aged soils did not allow strong conclusions to be drawn on the potential effect of soil properties on the laboratory-to-field factor. The sandy Zwijnaarde soil (pH 5.2) seemed to have limited aging (Table 4), but results for this soil were strongly biased because of the high degree of MoO42– leaching (up to 99 % at the largest dose) and the low MoO42– concentration remaining in the soil after long-term equilibration under field conditions (<80 mg Mo kg–1 after 11 months with initial spiked concentrations up to 10 000 mg kg–1). Molybdate loss from the Woburn and Ter Munck soils was more limited, with on average 62 and 53 % of the added Mo lost from the soils respectively after 11 months of equilibration. Both soils still contained >4000 mg Mo kg–1 at the end of the 11-month equilibration period.
Van Gestel et al.[27] concluded that natural attenuation of MoO42– ecotoxicity under field conditions is related to leaching of excess MoO42– and other ions, as well as to slow aging reactions. The loss of MoO42– through leaching during the equilibration period was taken into account by calculating all ED50 values based on actual measured Mo concentrations in soil, corrected for the background Mo concentration in the control soils. The laboratory-to-field factor is hence the result of combined effects of (i) decreased ionic strength after leaching and the corresponding alleviation of salt- or pH-related stress to soil organisms, and the change in MoO42– availability through the decrease in concentration of competing ions in the soil solution; and (ii) stronger binding or fixation of MoO42– onto or into soil solid phases (aging). Studies including NaCl testing with plants and microorganisms suggested direct salt stress to be of limited importance at a Na2MoO4 dose below 1000 mg added Mo kg–1.[5,29] No literature or test data were available on change in molybdate availability and toxicity through the decrease in concentration of competing ions in the soil solution after leaching excess ions from the soil. However, data for Cu, Ni and Pb show a median leaching factor (i.e. (EDx, leached)/(EDx, spiked)) between 1.3 and 2.0 across a broad range of soils.[18,47–49] As a first approximation, a similar level of effect may be anticipated for the influence of leaching of excess ions after application of Na2MoO4 on MoO42– toxicity. The isotopically exchangeable fraction (E value) for MoO42– (expressed as percentage of total Mo added) was assessed with an isotopic dilution technique and was found to decrease with increasing incubation time.[40] The chemical fixation factor, calculated as the change in isotopically exchangeable Mo between 0.5 and 18 months, ranges between 1.0 and 2.8, with a median fixation factor of 1.4 for the 15 soils tested. The isotopically exchangeable fraction of Mo not only depended on time after spiking, but was also correlated with both clay content and pH of the soil. However, no significant correlation between the fixation factor (i.e. change of E value with time) and soil properties was found.
Because there were no significant correlations between the laboratory-to-field factors or fixation factors with the soil type or endpoint type, a generic constant correction factor was selected for the discrepancy in MoO42– toxicity between laboratory and field conditions. A constant factor of 2.0 was chosen for the derivation of ecological soil thresholds and risk characterisation of MoO42– in soil. This factor corresponds to the 32nd percentile of the individual laboratory-to-field factors values based on ED50 values (Table 4). The factor of 2.0 is approximately equal to the product of the median factor found for chemical fixation of MoO42– in several soils (factor 1.4) and the median factor for the effects of leaching on the toxicity thresholds for other metals (factor 1.3–2.0). The factor 2.0 further corresponds to the 2.1- and 2.0-fold decrease in solution Mo concentrations between freshly spiked and 11-month equilibration observed for two of the three test soils respectively, at a total soil concentration of ~50 mg Mo kg–1 dry soil.[27]
Derivation of ecological soil standards
The available ecotoxicity database for the effect of MoO42– on soil organisms covers five plants species, three invertebrate species and three microbial processes and therefore fulfils the requirements of the REACH regulation for the use of the species sensitivity distribution (SSD) approach for derivation of ecological soil standards.[39]
The general framework for implementation of bioavailability into derivation of ecological threshold concentrations is reported by Smolders et al.[22] In summary, the following steps were followed. After selection of the reliable EC10 (or NOEC) values, the added EC10 values (=ED10 values) were derived by subtracting the background concentrations of Mo in the tested control soils from the EC10 values expressed as total measured concentrations. In a second step, toxicity thresholds derived from soils tested within 120 days after spiking with a soluble Mo salt were corrected for the discrepancy in toxicity between freshly spiked soils in laboratory conditions and field-contaminated soils by multiplying all individual added EC10 or NOEC values by the laboratory-to-field factor of 2.0 for MoO42–. The background Mo concentration from each individual test soil was then added again to calculate the total ‘aged’ EC10 or NOEC values. Because of the negligible contribution of the background concentration of Mo to the total NOEC or EC10 values, application of the laboratory-to-field factor has an almost proportional effect on the final toxicity thresholds.
In the following step, the toxicity data were corrected for differences in metal availability among soils. Normalising for the effect of soil properties allows the calculation of a specific threshold concentration for the effect of MoO42– to soil organisms in the soil under investigation. Each total aged EC10 or NOEC value is normalised to the soil properties of a specific target soil, using the slope of the respective regression function (log–log based, Table 3) and the following equation:

where ‘reference’ is the soil for which the soil standard must be derived, ‘test’ is the tested soil, and ‘abiotic factor’ is the soil property in the selected regression model. Both pH and clay content of the tested soils and target soil must be known to normalise toxicity data for MoO42– for specific soil conditions.
In case no regression model is available for a specific species or endpoint, the bioavailability model of another similar species or endpoint within the same trophic level can be used. The model with the smallest slope observed for the invertebrate assays, i.e. the model for Enchytraeus crypticus, was selected for normalisation of Folsomia candida EC10 values for site-specific ecological soil standard derivation. The selection of the model with the smallest slope can be considered as a conservative approach because it minimises the risk of overcorrection. The slope derived for the substrate-induced respiration assay was selected for normalisation of EC10 values for the PRM assay because both assays are related to the carbon mineralisation process.
Normalisation of the individual EC10 or NOEC data towards specific soil properties strongly reduces the within species-variation in EC10 or NOEC values for most organisms covered by the database (Table 2, Fig. 1). This illustrates the adequacy of these models and the significance of soil properties in controlling MoO42– bioavailability and toxicity to soil organisms, and further demonstrates the importance of normalising toxicity data and separating the biological variation from the variation in MoO42– availability due to varying soil properties.
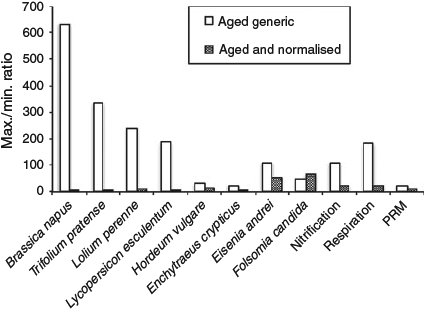
|
Where multiple data are available for the same species or microbial process, a species or process mean value is calculated as the geometric mean from all data for the most sensitive endpoint for each species or process. This species or process mean approach is preferred for normalised data, where the remaining variation among data for a given species or process can be mainly attributed to intraspecies variation in sensitivity. This is, however, not the case for non-normalised data, where variation between toxicity data is also caused by differences in bioavailability among soils.
Finally, a SSD was fitted to the normalised, aged species or process mean EC10 values, and the median hazardous concentration for 5 % of the species (HC5–50), equivalent to a median 95 % protection level, was derived as the median 5th percentile of this distribution (Fig. 2). The SSD for the effect of MoO42– on soil organisms illustrates that plants are generally the most sensitive to MoO42– toxicity in soil and that microbial endpoints are the most tolerant.
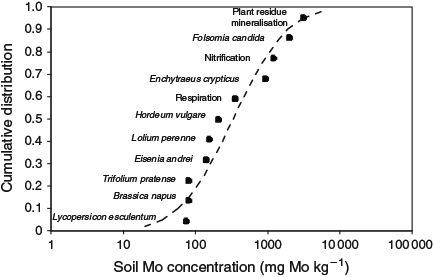
|
Once an HC5–50 value is derived, its robustness and degree of protection for direct molybdate toxicity to soil organisms under field conditions should be evaluated. Therefore, the following criteria are usually used: the quality and representativeness of the data set, the statistical uncertainty on the HC5–50 value, the evaluation of chronic toxicity data below this HC5–50 value and a comparison with field data.[39]
Chronic toxicity data are available for MoO42– for several plant species, invertebrate species and microbial processes. The selected endpoints were all relevant for potential effects at the population level: yield based on root elongation and shoot yield for the terrestrial plants, reproduction for the invertebrates, and nitrogen and carbon transformation for microbial processes. Data were either from tests focussing on sensitive life stages (e.g. root elongation, reproduction) or from ‘chronic exposure’ (e.g. growth, reproduction). All reliable chronic EC10 and NOEC data were extracted from tests performed in natural and artificial soils, covering a wide range of the soil characteristics in Europe (pH, organic carbon, clay and oxalate-extractable iron content, Table 1). The soils covered by the toxicity data properly reflected the variability in physicochemical conditions encountered in European soils (Table 5).
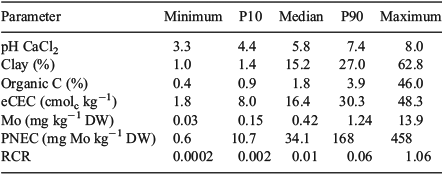
|
The toxicity data set was composed of plant, invertebrate and microbial data and includes the major taxonomic groups. In total, 86 individual chronic EC10 or NOEC values were selected covering 11 different species or microbial processes. Data were available for five different agricultural plant species belonging to five different families and covering both monocotyledonous and dicotyledonous plants. Toxicity data for invertebrates covered arthropods and Annelida with different exposure routes and feeding strategies, belonging to three different species from three different families. The data set further included three different microbial endpoints representing the C and N cycles. This largely fulfils the requirement of 10–15 different EC10 or NOEC values (preferably more than 15) from chronic studies for different species covering at least eight different taxonomic groups from three trophic levels for the use of the statistical extrapolation approach.[39] The overall quality, diversity and representativeness of the taxonomic groups covered by the data were therefore considered adequate.
Different distributions were evaluated for fitting the species sensitivity distributions. The final distribution function was selected on the basis of the Anderson–Darling goodness-of-fit test because this test highlights differences between the tail of the distribution (lower tail is the region of interest) and the input data. There was no consistent best-fitting distribution for the various soil scenarios tested. The log-normal distribution was accepted for all soil scenarios according to the Anderson–Darling test. Comparison of the uncertainty around the HC5–50 showed that there was no consistent difference between results of the log-normal and best-fitting distributions. Therefore, the uniform application of the log-normal distribution was preferred for derivation of the HC5–50 values.
A comparison of the HC5–50 values with the corresponding normalised species or process mean EC10 values for several EU soil scenarios shows that no species or process mean values fall below the HC5–50 derived by the log-normal distribution, except for acidic soils (pH < 4.5), where the normalised species-mean value for Eisenia andrei reproduction can be lower than the HC5–50 value fitted with the log-normal distribution.
The HC5–50 value was finally validated by MoO42– toxicity data from field or microcosm studies. Only one field study was identified where the effect of MoO42– applications at 0, 90, 270 and 810 kg Mo ha–1 (as (NH4)6Mo7O24·4H2O), corresponding to an added dose of ~0, 30, 90 and 270 mg Mo kg–1 in the plough layer (0–20 cm), on crop yield was studied.[50–53] The same Nagyhörcsök soil (Hungary) was used in the present research programme (Table 1): a calcareous Chernozem soil with pH 7.3, 3 % organic matter, 20 % clay and 5 % CaCO3. Molybdate was applied in the spring of 1991 with two-fold replication and each plot had a total area of 21 m2. Maize, carrot, potato, pea, red beet, spinach and wheat were grown in the first, second, third, fourth, fifth, sixth and seventh year respectively. Only in the first cropping season was a significant effect on maize dry matter yield (82 % effect) and maize grain yield (44 % effect) observed at the highest dose (Table 6). In subsequent seasons, no effects on carrot, potato, pea, red beet, spinach or wheat yield were observed. Measured total soil Mo concentrations are only reported for samples taken in 1994 and 22–42 % of the MoO42– added in 1991 was recovered. It can be expected that actual Mo concentrations during the first cropping season in 1991 were still significantly higher. Measured ammonium acetate–EDTA-extractable Mo-concentrations in soil showed a clear decrease in extractability of Mo with time after application.
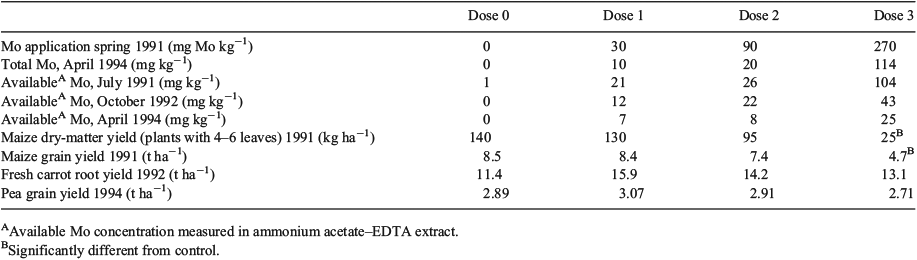
|
This field study in Hungary suggests that the toxicity in the first year after application (NOEC = 26 mg ammonium acetate–EDTA-extractable Mo kg–1) is more than a factor 2 above the HC5–50 value calculated for this soil (11.4 mg total Mo kg–1) and hence the HC5–50 can be considered as conservative. Moreover, the ammonium acetate–EDTA-extractable soil concentration underestimates the actual total Mo concentrations during the maize cropping season and therefore actual total effect concentrations were likely still higher. In this field study, maize yield was tested almost immediately after MoO42– application to the soil.[50,53] Therefore, significant aging may not have already taken place before the start of the maize growth and hence the data for maize yield can be considered as freshly spiked data, overestimating MoO42– toxicity after aging. Based on this field study, it is concluded that no negative effects are predicted at concentrations below the HC5–50 derived from the laboratory toxicity data, and this value is therefore considered a protective threshold for effects of MoO42– under field conditions.
Based on the above evaluation, and in particular the availability of normalisation models and field validation, it can be concluded that the available toxicity data and models allow the derivation of an HC5–50 that is protective against direct MoO42– toxicity in the soil. This provides a robust and ecologically relevant PNEC to be retained for use in risk characterisation.
Risk assessment of arable and grazing land soils in Europe
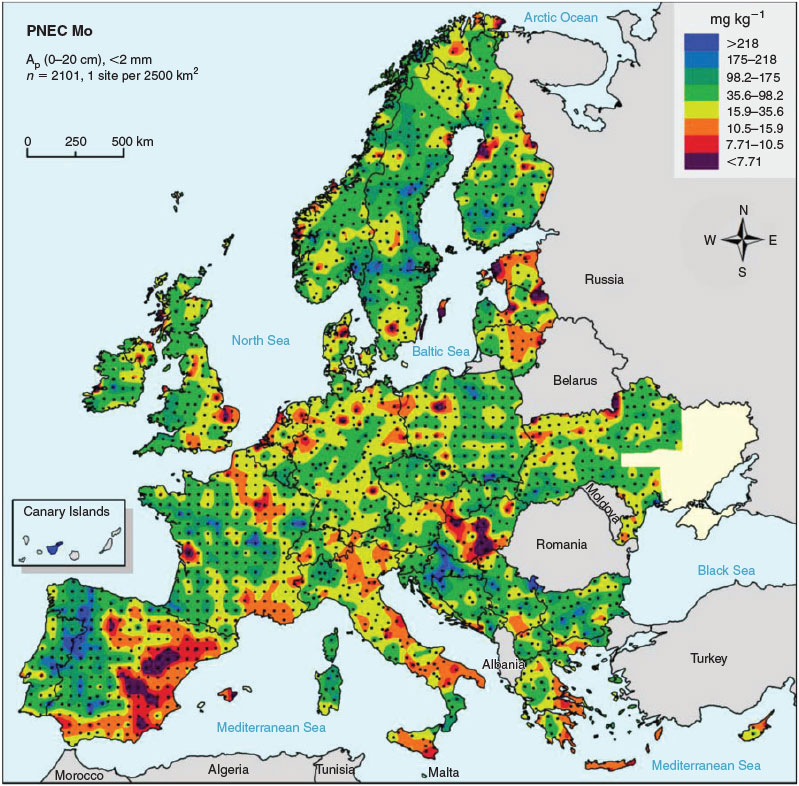
The results from the GEMAS survey demonstrate the large range in ambient background concentrations of Mo and soil properties affecting MoO42– toxicity in soil across Europe (Table 5, Fig. 3). PNEC values for all 2108 arable land soils sampled in the GEMAS project vary by three orders of magnitude (0.6–458 mg Mo kg–1 DW), with a median value of 34.1 mg Mo kg–1 DW (Table 5, Fig. 4). The large spatial variation in both exposure and PNECs of Mo in soil stresses the need to take into account this variation in ambient background concentrations and soil properties when assessing effects and risks of metals in soil. Similar results were obtained for grassland soils in Europe (data not shown). The 10th percentile of all individual PNEC values, being 10.7 mg Mo kg–1 DW, can be suggested as a reasonable worst-case PNEC value for a generic risk assessment in the absence of information on pH and clay content of the soil.
|
|
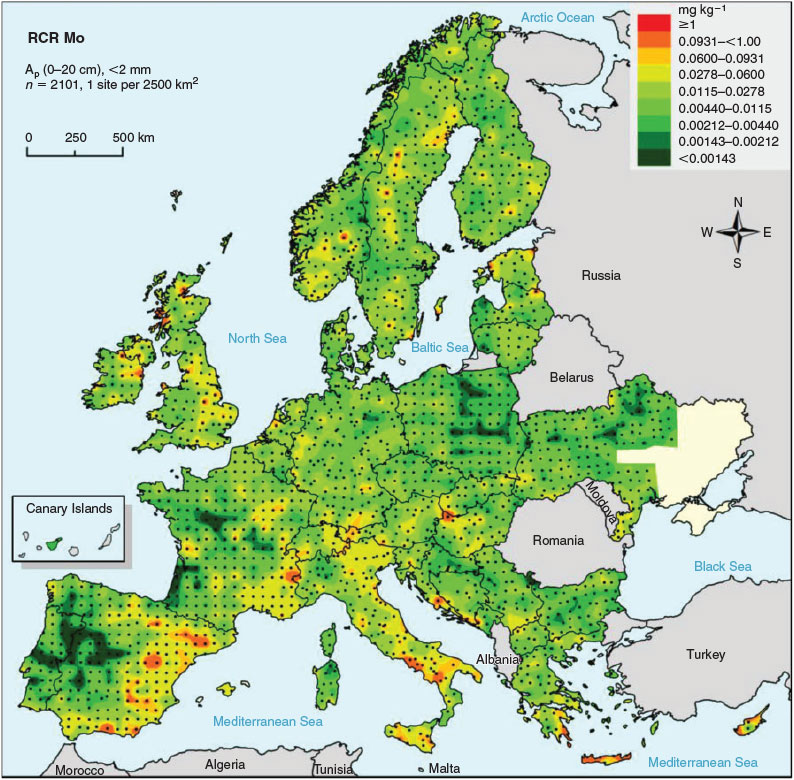
Risk characterisation ratios (RCRs), calculated as the ratio of measured aqua regia-extractable molybdenum concentration over the corresponding PNEC, range from 0.0002 to a maximum of 1.1 with 50th and 90th percentiles of 0.01 and 0.06 respectively (Table 5, Fig. 5). Because the 90th percentile of the RCR values is well below 1, i.e. the exposure concentration is well below the PNEC, it can be concluded that prevailing Mo concentrations in arable soils do not pose a risk for MoO42– toxicity at the regional scale in Europe. The largest RCR values are generally observed for countries in southern Europe (France, Greece, Italy and Spain), mainly owing the combination of high background concentrations of Mo and high pH and hence low PNEC values. Only one site, with the highest aqua regia-extractable Mo concentration observed and a rather low PNEC (<20th percentile for Europe), has an RCR value larger than 1, which is an indication for a potential risk of MoO42– toxicity to soil organisms. A reasonable worst-case value for RCR in a regional risk assessment for Europe can be calculated as the median of the 90th percentiles of the RCR for all regions (= countries) covered. This approach results in a reasonable worst-case RCR of 0.05 for arable land and this value can be used as a generic estimate for risk due to regional background concentrations in a risk assessment of sites with additional local point emissions (e.g. from smelters).
|
As a reference, a generic PNEC derived as the HC5–50 of a species sensitivity distribution with the raw EC10 data without corrections for aging or normalisation to reference soil properties is 5.1 mg Mo kg–1 soil. Applied to the European data set for arable soils, this yields an RCR ranging from 0.03 to 0.24 (10th–90th percentile) and predicts risk at ambient (mostly natural) concentration in 16 sites instead of in one. Such risk at background concentrations is difficult to defend because these background concentrations are also the reference in the toxicity tests. This illustrates that bioavailability correction does remove prediction of risk at natural background concentrations while still ensuring adequate protection of soil organisms against Mo toxicity.
Conclusions
Over the last few years, a comprehensive data set describing MoO42– toxicity to soil organisms (plants, invertebrates and microorganisms) has been generated. Molybdate toxicity data for the same endpoint, but tested in various soils, varied from 13-fold to more than 500-fold and this illustrates the importance of soil properties for MoO42– bioavailability and toxicity in soils. Furthermore, the bioavailability and toxicity of MoO42– in soil decrease with longer equilibration time after incorporation. A correction factor of 2.0 was selected to account for the difference in MoO42– toxicity between typical laboratory conditions (no leaching, no aging) and field conditions (leaching of excess ions with percolating rainwater and long-term equilibration). Molybdate toxicity for the various endpoints studied was best correlated with soil pH and clay content and regression models were developed to normalise toxicity data for variation of these properties among soils. Toxicity thresholds were determined as the HC5–50 (i.e. median 95 % protection level) as derived from the SSD of ecotoxicity data after correction for leaching and aging processes as well as normalised to clay content and pH. An uncertainty analysis of this HC5–50 showed that it was protective against direct toxicity of Mo and Mo compounds in the terrestrial environment and that it provides a robust and ecologically relevant PNEC to be determined for risk characterisation purposes. PNEC values in European arable land soils varied commonly between 10.7 and 168 mg Mo kg–1 DW (10th and 90th percentile respectively) and no risk of Mo toxicity to soil organisms was expected at the European regional scale. The information presented in the present paper can be used for national and international regulatory purposes for the setting of soil quality criteria for Mo and Mo compounds.
Acknowledgements
This research was funded by the International Molybdenum Association (IMOA).
References
[1] D. I. Arnon, P. R. Stout, The essentiality of certain elements in minute quantity for plants with special reference to copper. Plant Physiol. 1939, 14, 371.| The essentiality of certain elements in minute quantity for plants with special reference to copper.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaA1MXmtVagtg%3D%3D&md5=f58a800ddea7d037950570463f9c8b2fCAS | 16653564PubMed |
[2] R. J. P. Williams, J. da Silva, The involvement of molybdenum in life. Biochem. Biophys. Res. Commun. 2002, 292, 293.
| The involvement of molybdenum in life.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XitFeisb8%3D&md5=746e8e1a964e0e00e506da2c8918549cCAS |
[3] D. R. Lide, Handbook of Chemistry and Physics, 90th edn 2009 (CRC Press LLC: Boca Raton, FL)
[4] Z. L. L. He, X. E. Yang, P. J. Stoffella, Trace elements in agroecosystems and impacts on the environment. J. Trace Elem. Med. Biol. 2005, 19, 125.
| Trace elements in agroecosystems and impacts on the environment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhslagtrY%3D&md5=173607d9d37c4602db6e11feaab5da70CAS |
[5] J. Buekers, J. Mertens, E. Smolders, Toxicity of the molybdate anion in soil is partially explained by effects of the accompanying cation or by soil pH. Environ. Toxicol. Chem. 2010, 29, 1274.
| 1:CAS:528:DC%2BC3cXpsFCqu70%3D&md5=3b0fcb0ee920277f2a91b7b76a092447CAS | 20821569PubMed |
[6] A. D. Anbar, Molybdenum stable isotopes: observations, interpretations and directions. Rev. Mineral. Geochem. 2004, 55, 429.
| Molybdenum stable isotopes: observations, interpretations and directions.Crossref | GoogleScholarGoogle Scholar |
[7] A. Bibak, O. K. Borggard, Molybdenum adsorption by aluminium and iron oxides and humic acid. Soil Sci. 1994, 158, 323.
| Molybdenum adsorption by aluminium and iron oxides and humic acid.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXis1ans7c%3D&md5=53cad20182961799e335386504c1bf9aCAS |
[8] S. Goldberg, H. S. Forster, C. L. Godfrey, Molybdenum adsorption on oxides, clay minerals, and soils. Soil Sci. Soc. Am. J. 1996, 60, 425.
| Molybdenum adsorption on oxides, clay minerals, and soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28Xitleqs74%3D&md5=0c47a4311a294d38200545422a639274CAS |
[9] S. Goldberg, S. M. Lesch, D. L. Suarez, Predicting molybdenum adsorption by soils using soil chemical parameters in the constant capacitance model. Soil Sci. Soc. Am. J. 2002, 66, 1836.
| Predicting molybdenum adsorption by soils using soil chemical parameters in the constant capacitance model.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XoslKhsLg%3D&md5=d39d58c040c9903ed62912798603b973CAS |
[10] S. Goldberg, H. S. Forster, Factors affecting molybdenum adsorption by soils and minerals. Soil Sci. 1998, 163, 109.
| Factors affecting molybdenum adsorption by soils and minerals.Crossref | GoogleScholarGoogle Scholar |
[11] Regulation (EC) Number 1907/2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemical substances (REACH) 2006 (European Commission: Brussels, Belgium).
[12] Guidance on Information Requirements and Chemical Safety Assessment. Chapter R.7.11. Effects on Terrestrial Organisms 2014 (European Chemicals Agency: Helsinki, Finland). Available at http://echa.europa.eu/documents/10162/13632/information_requirements_r7c_en.pdf [Verified 19 September 2015].
[13] Guidance on Information Requirements and Chemical Safety Assessment. Appendix R.7.13–2: Environmental Risk Assessment for Metals and Metal Compounds 2008 (European Chemicals Agency: Helsinki, Finland). Available at http://echa.europa.eu/documents/10162/13632/information_requirements_r7_13_2_en.pdf [Verified 19 September 2015].
[14] K. Oorts, U. Ghesquiere, K. Swinnen, E. Smolders, Soil properties affecting the toxicity of CuCl2 and NiCl2 for soil microbial processes in freshly spiked soils. Environ. Toxicol. Chem. 2006, 25, 836.
| Soil properties affecting the toxicity of CuCl2 and NiCl2 for soil microbial processes in freshly spiked soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XitVais74%3D&md5=cfc09d39d98863a192a287ab61ff29daCAS | 16566169PubMed |
[15] J. Song, F. J. Zhao, S. P. McGrath, Y. M. Luo, Influence of soil properties and aging on arsenic phytotoxicity. Environ. Toxicol. Chem. 2006, 25, 1663.
| Influence of soil properties and aging on arsenic phytotoxicity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xlt1Whsbw%3D&md5=4d6c0b7f47c4afd7037776b1a1514c59CAS | 16764487PubMed |
[16] P. Criel, K. Lock, H. Van Eeckhout, K. Oorts, E. Smolders, C. R. Janssen, Influence of soil properties on copper toxicity for two soil invertebrates. Environ. Toxicol. Chem. 2008, 27, 1748.
| Influence of soil properties on copper toxicity for two soil invertebrates.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXovFWjtb8%3D&md5=f84d3c43259c209e1cac1aee4ee2a14dCAS | 18290689PubMed |
[17] K. Lock, N. Waegeneers, E. Smolders, P. Criel, H. Van Eeckhout, C. R. Janssen, Effect of leaching and aging on the bioavailability of lead to the springtail Folsomia candida. Environ. Toxicol. Chem. 2006, 25, 2006.
| Effect of leaching and aging on the bioavailability of lead to the springtail Folsomia candida.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XnvFait74%3D&md5=f54c26d74e76f07ea7226ad0d1c15385CAS | 16916018PubMed |
[18] K. Oorts, H. Bronckaers, E. Smolders, Discrepancy of the microbial response to elevated copper between freshly spiked and long-term contaminated soils. Environ. Toxicol. Chem. 2006, 25, 845.
| Discrepancy of the microbial response to elevated copper between freshly spiked and long-term contaminated soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XitVais78%3D&md5=52531c0368a6926e5d07ee75800c95c9CAS | 16566170PubMed |
[19] L. A. Wendling, J. K. Kirby, M. J. McLaughlin, Aging effects on cobalt availability in soils. Environ. Toxicol. Chem. 2009, 28, 1609.
| Aging effects on cobalt availability in soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXovFersbk%3D&md5=94942b53d5bc7e3b777e2f73d497f015CAS | 19642829PubMed |
[20] F. Degryse, J. Buekers, E. Smolders, Radio-labile cadmium and zinc in soils as affected by pH and source of contamination. Eur. J. Soil Sci. 2004, 55, 113.
| Radio-labile cadmium and zinc in soils as affected by pH and source of contamination.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXisFCgtbk%3D&md5=a0795bcffcd2919d838aa6dc7a4adbe0CAS |
[21] Y. B. Ma, E. Lombi, M. J. McLaughlin, I. W. Oliver, A. L. Nolan, K. Oorts, E. Smolders, Aging of nickel added to soils as predicted by soil pH and time. Chemosphere 2013, 92, 962.
| Aging of nickel added to soils as predicted by soil pH and time.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXltFCrtLs%3D&md5=f155998faa096398c1dd0193a3e9655bCAS |
[22] E. Smolders, K. Oorts, P. van Sprang, I. Schoeters, C. R. Janssen, S. P. McGrath, M. J. McLaughlin, Toxicity of trace metals in soil as affected by soil type and aging after contamination: using calibrated bioavailability models to set ecological soil standards. Environ. Toxicol. Chem. 2009, 28, 1633.
| Toxicity of trace metals in soil as affected by soil type and aging after contamination: using calibrated bioavailability models to set ecological soil standards.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXovFertr4%3D&md5=5e3e2c3f9d5174628be5dab9c3024447CAS | 19301943PubMed |
[23] N. Xu, W. Braida, C. Christodoulatos, J. P. Chen, A review of molybdenum adsorption in soils/bed sediments: speciation, mechanism, and model applications. Soil Sediment Contam. 2013, 22, 912.
| A review of molybdenum adsorption in soils/bed sediments: speciation, mechanism, and model applications.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXktVOqt7s%3D&md5=3c75537c3e3ed4c0ef314967eacc628dCAS |
[24] M. Díez-Ortiz, I. Giska, M. Groot, E. M. Borgman, C. A. M. Van Gestel, Influence of soil properties on molybdenum uptake and elimination kinetics in the earthworm Eisenia andrei. Chemosphere 2010, 80, 1036.
| Influence of soil properties on molybdenum uptake and elimination kinetics in the earthworm Eisenia andrei.Crossref | GoogleScholarGoogle Scholar | 20674662PubMed |
[25] C. A. M. van Gestel, E. Borgman, R. A. Verweij, M. D. Ortiz, The influence of soil properties on the toxicity of molybdenum to three species of soil invertebrates. Ecotoxicol. Environ. Saf. 2011, 74, 1.
| The influence of soil properties on the toxicity of molybdenum to three species of soil invertebrates.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtlKrs7fK&md5=8a147af9eccfbd8bb816c77eefd7ca61CAS |
[26] C. A. M. Van Gestel, M. D. Ortiz, E. Borgman, R. A. Verweij, The bioaccumulation of molybdenum in the earthworm Eisenia andrei: influence of soil properties and ageing. Chemosphere 2011, 82, 1614.
| The bioaccumulation of molybdenum in the earthworm Eisenia andrei: influence of soil properties and ageing.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXit12jsrg%3D&md5=d8dd91d3839e81f70f2b8ef81a2fc39aCAS |
[27] C. A. M. van Gestel, S. P. McGrath, E. Smolders, M. D. Ortiz, E. Borgman, R. A. Verweij, J. Buekers, K. Oorts, Effect of long-term equilibration on the toxicity of molybdenum to soil organisms. Environ. Pollut. 2012, 162, 1.
| Effect of long-term equilibration on the toxicity of molybdenum to soil organisms.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xps1Skuw%3D%3D&md5=aa35bb51bc1d1d553e8f7803ab6c9ac9CAS |
[28] S. P. McGrath, C. Mico, F. J. Zhao, J. L. Stroud, H. Zhang, S. Fozard, Predicting molybdenum toxicity to higher plants: estimation of toxicity threshold values. Environ. Pollut. 2010, 158, 3085.
| Predicting molybdenum toxicity to higher plants: estimation of toxicity threshold values.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtFamu7nL&md5=a1e39f217ab60b41c29a829831511fc7CAS | 20656390PubMed |
[29] S. P. McGrath, C. Mico, R. Curdy, F. J. Zhao, Predicting molybdenum toxicity to higher plants: influence of soil properties. Environ. Pollut. 2010, 158, 3095.
| Predicting molybdenum toxicity to higher plants: influence of soil properties.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtFamu7nE&md5=15f2a68a535b7bf92266f400e1afde12CAS | 20656387PubMed |
[30] J. Buekers, E. Smolders, Toxicity and Bioavailability of Molybdenum in Terrestrial Environments: Micro-organisms. Final Report to the International Molybdenum Association (IMOA) 2009 (University of Leuven).
[31] ISO-11269–1, Soil Quality – Determination of the Effects of Pollutants on Soil Flora. Part 1. Method for the Measurement of Inhibition of Root Growth 1995 (International Organization for Standardization: Geneva).
[32] ISO-11269–2, Soil Quality – Determination of the Effects of Pollutants on Soil Flora. Part 2. Effects of Contaminated soil on the Emergence and Early Growth of Higher Plants 1995 (International Organization for Standardization: Geneva).
[33] OECD-208, Terrestrial Plant Test: Seedling Emergence and Seedling Growth Test 2006 (Organisation for Economic Co-operation and Development: Paris).
[34] OECD-222, Earthworm Reproduction Test (Eisenia fetida/Eisenia andrei) 2004 (Organisation for Economic Co-operation and Development: Paris).
[35] OECD-220, Enchytraeid Reproduction Test 2004 (Organisation for Economic Co-operation and Development: Paris).
[36] ISO-11267. Soil Quality – Inhibition of Reproduction of Collembola (Folsomia candida) by Soil Pollutants 1999 (International Organization for Standardization: Geneva).
[37] OECD-216, Soil Microorganisms: Nitrogen Transformation Test 2000 (Organisation for Economic Co-operation and Development: Paris).
[38] OECD-217, Soil Microorganisms: Carbon Transformation Test 2000 (Organisation for Economic Co-operation and Development: Paris).
[39] Guidance on Information Requirements and Chemical Safety Assessment. Chapter R.10: Characterisation of Dose [Concentration]–Response for Environment 2008 (European Chemicals Agency: Helsinki, Finland). Available at http://echa.europa.eu/documents/10162/13632/information_requirements_r10_en.pdf [Verified 19 September 2015].
[40] J. K. Kirby, M. J. McLaughlin, Y. B. Ma, B. Ajiboye, Aging effects on molybdate lability in soils. Chemosphere 2012, 89, 876.
| Aging effects on molybdate lability in soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XoslGqsrk%3D&md5=6c0371b748c7fb9b8e1b60673936cb30CAS | 22704209PubMed |
[41] C. Reimann, M. Birke, A. Demetriades, P. Filzmoser, P. O’Connor (Eds), Chemistry of Europe’s Agricultural Soils – Part A: Methodology and Interpretation of the GEMAS Data Set – Geologisches Jahrbuch, B 102 2014 (Schweizerbarth: Hanover).
[42] R. F. Brennan, Residual value of molybdenum for wheat production on naturally acidic soils of Western Australia. Aust. J. Exp. Agric. 2006, 46, 1333.
| Residual value of molybdenum for wheat production on naturally acidic soils of Western Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XpsFWltbk%3D&md5=37d38b55561401633ce40bd84cd0f950CAS |
[43] N. J. Barrow, T. C. Shaw, Factors affecting long-term effectiveness of phosphate and molybdate fertilizers. Commun. Soil Sci. Plant Anal. 1974, 5, 355.
| Factors affecting long-term effectiveness of phosphate and molybdate fertilizers.Crossref | GoogleScholarGoogle Scholar |
[44] N. J. Barrow, T. C. Shaw, Slow reactions between soil and anions. 4. Effect of time and temperature of contact between soil and molybdate on uptake of molybdenum by plants and on molybdate concentration in soil solution. Soil Sci. 1975, 119, 301.
| Slow reactions between soil and anions. 4. Effect of time and temperature of contact between soil and molybdate on uptake of molybdenum by plants and on molybdate concentration in soil solution.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE2MXhsFCnsL8%3D&md5=4cf3313f239b38a95e2342c56d31aeceCAS |
[45] N. J. Barrow, P. J. Leahy, I. N. Southey, D. B. Purser, Initial and residual effectiveness of molybdate fertilizer in two areas of south-western Australia. Aust. J. Agric. Res. 1985, 36, 579.
| Initial and residual effectiveness of molybdate fertilizer in two areas of south-western Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXlt1agtLc%3D&md5=8fdc4dc0108d4efb916aa3533d6c7d79CAS |
[46] F. Lang, M. Kaupenjohann, Immobilisation of molybdate by iron oxides: effects of organic coatings. Geoderma 2003, 113, 31.
| Immobilisation of molybdate by iron oxides: effects of organic coatings.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXos1Ohuw%3D%3D&md5=63f7ef3833489f29b871e27774f1aa60CAS |
[47] D. P. Stevens, M. J. McLaughlin, T. Heinrich, Determining toxicity of lead and zinc runoff in soils: salinity effects on metal partitioning and on phytotoxicity. Environ. Toxicol. Chem. 2003, 22, 3017.
| Determining toxicity of lead and zinc runoff in soils: salinity effects on metal partitioning and on phytotoxicity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXps1eltbs%3D&md5=4a67119f16968064f5f2dd5f667a9f37CAS | 14713044PubMed |
[48] M. Bongers, B. Rusch, C. A. M. Van Gestel, The effect of counterion and percolation on the toxicity of lead for the springtail Folsomia candida in soil. Environ. Toxicol. Chem. 2004, 23, 195.
| The effect of counterion and percolation on the toxicity of lead for the springtail Folsomia candida in soil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjvVKluw%3D%3D&md5=4695a40f8b6c178b047dafaf64cebe76CAS | 14768885PubMed |
[49] K. Oorts, U. Ghesquiere, E. Smolders, Leaching and aging decrease nickel toxicity to soil microbial processes in soils freshly spiked with nickel chloride. Environ. Toxicol. Chem. 2007, 26, 1130.
| Leaching and aging decrease nickel toxicity to soil microbial processes in soils freshly spiked with nickel chloride.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXls1yjsb4%3D&md5=0151c1508787abb8a86a569c0d2a6e44CAS | 17571677PubMed |
[50] I. Kádár, Effect of heavy metal load on soil and crop. Acta Agronomica Hungarica 1995, 43, 3.
[51] P. A. Biacs, H. G. Daood, I. Kadar, Effect of Mo, Se, Zn, and Cr treatments on the yield, element concentration, and carotenoid content of carrot. J. Agric. Food Chem. 1995, 43, 589.
| Effect of Mo, Se, Zn, and Cr treatments on the yield, element concentration, and carotenoid content of carrot.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXktFehtbw%3D&md5=2957189231170f70ba6d63ddea6c08a9CAS |
[52] F. Nyarai-Horvath, T. Szalai, I. Kadar, P. Csatho, Germination characteristics of pea seeds originating from a field trial treated with different levels of harmful elements. Acta Agronomica Hungarica 1997, 45, 147.
| 1:CAS:528:DyaK2sXls1WjtLg%3D&md5=a0beb6274b7822d4a8594681577cedd8CAS |
[53] I. Kádár, L. Szabo, J. Sarkadi, Contamination of food chains with heavy metals and harmful elements. Project report 1998 (Hungarian Academy of Sciences, Research Institute of Soil Science and Agrochemistry: Budapest) [in Hungarian].
[54] World Reference Base for Soil Resources. World Soil Resources Report 84 1998 (Food and Agriculture Organization, International Soil and Reference Information Center, International Society of Soil Science: Rome, Italy).