Novel metabolomic method to assess the effect-based removal efficiency of advanced wastewater treatment techniques
Jana Späth A E , Malin Nording A , Richard Lindberg A , Tomas Brodin C D , Stina Jansson A , Jun Yang B , Debin Wan B , Bruce Hammock B and Jerker Fick A
A E , Malin Nording A , Richard Lindberg A , Tomas Brodin C D , Stina Jansson A , Jun Yang B , Debin Wan B , Bruce Hammock B and Jerker Fick A
A Department of Chemistry, Umeå University, SE 90187 Umeå, Sweden.
B Department of Entomology and Nematology, University of California at Davis, Davis, CA 95616, USA.
C Department of Ecology and Environmental Science, Umeå University, SE 90187 Umeå, Sweden.
D Department of Wildlife, Fish, and Environmental Studies, Swedish University of Agricultural Sciences, SE 90183 Umeå, Sweden.
E Corresponding author. Email: jana.spath@umu.se
Environmental Chemistry 17(1) 1-5 https://doi.org/10.1071/EN19270
Submitted: 25 September 2019 Accepted: 28 November 2019 Published: 8 January 2020
Journal Compilation © CSIRO 2020 Open Access CC BY-NC-ND
Environmental context. Advanced wastewater treatment is required to remove pharmaceuticals and many other consumer chemicals from wastewater effluent. There are conflicting findings, however, on the toxicity of treated effluent, and its effect on living organisms is often neglected. We show that the effect-based removal efficiency of wastewater treatment technologies can be assessed by metabolomic methods, and that this approach contributes to a safer and more controlled water quality.
Abstract. There are conflicting findings on the toxicity of effluent from wastewater treatment plants, and only limited possibilities for assessing the effect-based removal efficiency (EBRE) of different treatment techniques. We describe a metabolomics approach to detect perturbations in fatty acid catabolic pathways as a proxy for biological effects. Metabolites in three fatty acid pathways were analysed in a common damselfly larva (Coenagrion hastulatum) by liquid chromatography coupled to mass spectrometry. The larvae were exposed for one week to either conventionally treated effluent (activated sludge treatment), effluent additionally treated with ozone, or effluent additionally treated with biochar filtration, and results were compared with those from tap water control exposure. Five lipoxygenase-derived oxylipins (9,10,13-TriHOME, 9,12,13-TriHOME, 9-HODE, 9-HOTrE, and 13-HOTrE) decreased in response to conventionally treated effluent exposure. By using an additional treatment step, oxylipin levels were restored with exception of 9,10,13-TriHOME (ozonated effluent), and 9-HOTrE and 13-HOTrE (effluent filtered with biochar). Thus, exposure to wastewater effluent affected fatty acid metabolite levels in damselfly larvae, and a subset of the analysed metabolites may serve as indicators for biological effects in biota in response to effluent exposure. To that effect, our findings suggest a new metabolomics protocol for assessing EBRE.
Anthropogenic pollutants, which include pharmaceuticals such as lipid regulators, anticonvulsants and nonsteroidal anti-inflammatory drugs, have been found in aquatic environments worldwide (Heberer 2002; Luo et al. 2014; Ternes et al. 2015; Noguera-Oviedo and Aga 2016). Many of these pollutants are not removed in conventional wastewater treatment plants (WWTPs) and are therefore discharged into receiving waters (Eggen et al. 2014; Tran et al. 2018; Kümmerer et al. 2019). Pharmaceuticals are bioactive substances that are designed to cause physiological effects in living organisms, and the risks of their adverse effects on ecosystems have increasingly been recognised (German Environment Agency 2016; Ternes 1998). Such effects include feminisation of fish caused by endocrine-disrupting compounds (EDCs; Tyler and Jobling 2008) and behavioural alterations in fish exposed to anxiolytics (Brodin et al. 2013). To protect and improve the quality of receiving waters, removal of pharmaceuticals can be enhanced by implementing advanced wastewater treatment techniques, such as biochar or activated carbon adsorption and ozonation (Joss et al. 2008; Weidemann et al. 2018). The efficiency of such techniques has mostly been evaluated in terms of pollutant removal and there are many studies confirming that both techniques remove a broad range of pharmaceuticals from wastewater (Huber et al. 2005; Ternes et al. 2003). However, there is still dispute about the biological effects of ozonated effluents. For instance, some studies have reported reduced toxicity of ozonated WWTP effluents (Bundschuh et al. 2011), whereas others have shown that ozonation can affect gene expression, reproductive success and behavioural endpoints in exposed fish (Pohl et al. 2018).
To address the lack of conclusive findings on the biological effects arising from WWTP effluent exposure, we hypothesised that metabolomics can be used to assess effect-based removal efficiencies (EBREs). To test this hypothesis, metabolites (oxylipins) in fatty acid catabolic pathways were analysed using a newly developed metabolomics protocol for damselfly larvae. Oxylipins, which include prostaglandins and other eicosanoids, are potent metabolites formed by oxidation of polyunsaturated fatty acids through three enzymatic pathways, i.e. the cyclooxygenase (COX), lipoxygenase (LOX) and cytochrome P450 (CYP) pathways. They regulate numerous metabolic functions, such as reproduction, inflammation and immune cell behaviour, in both vertebrates and invertebrates (Heckmann et al. 2008; Yang et al. 2009). Furthermore, oxylipins are responsive to environmental pollution. For example, elevated levels of three oxylipins were found in human lung lavage following air pollution exposure (Gouveia-Figueira et al. 2017) and prostaglandin levels were shown to be altered in fish exposed to WWTP effluent (David et al. 2017). As many common pharmaceuticals are designed to target enzymes responsible for the biosynthesis of oxylipins (Willenberg et al. 2015), they may serve as relevant metabolomic endpoints, in particular for the assessment of EBREs. The use of metabolomic endpoints may provide insights into the mode of action of a pollutant and the health and functional status of an organism at the molecular level. They are also likely to be affected by environmental stressors before changes in other endpoints occur (Bundy et al. 2009; Ekman et al. 2018; Viant 2007).
Northern damselfly larvae (Coenagrion hastulatum) were used as an invertebrate model species in this investigation because of their wide distribution and ecological importance. More specifically, damselfly larvae play an important role in aquatic food webs, preying on small invertebrates and serving as prey for larger invertebrates and vertebrates (Jonsson et al. 2014). Moreover, damselfly larvae have previously been used to detect the effects of exposure to pollutants (e.g. antihistamines, anxiolytics and pesticides) on a wide range of endpoints, such as life history traits, behaviour and physiology (Brodin et al. 2014; Finotello et al. 2017; Jonsson et al. 2014; Sniegula et al. 2017).
The aim of this study was to assess EBREs of different wastewater treatment technologies using a new metabolomics protocol. Damselfly larvae were exposed to conventionally-treated effluent (activated sludge treatment without N-removal), effluent additionally treated with ozone or effluent additionally treated with biochar filtration, and oxylipin profiles of exposed larvae were measured by liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS).
Northern damselfly larvae were collected in August 2017 at Lake Nydalasjön in Umeå, Sweden and taken to a laboratory at Umeå University. They were introduced into individual aquaria (10 × 10 cm), and subsequently exposed to WWTP effluents for seven days (see Supplementary Material). The exposure groups were as follows: (i) conventionally-treated WWTP effluent (E, n = 21), (ii) effluent treated with ozone (E+O, n = 21), (iii) effluent treated with biochar (E+B, n = 22), and (iv) tap water control (C, n = 18).
Final effluent was obtained at a WWTP and additionally treated with either ozone or a biochar adsorbent in-laboratory. Ozonation (5.5 mg L−1 ozone, 15 min residence time) was carried out in a column reactor using O3 generated from O2 gas by an EXT120-T Ultra ozone generator (Longevity Resources, Sidney, Canada). Biochar treatment was performed by adding 20 g of biochar to 2.5 L of effluent, followed by manually shaking for 5 min and a final filtration step. Biochar was produced from tomato residues by torrefaction, a slow pyrolysis operated under mild conditions (Nordin et al. 2013), at 260 °C for three hours in a rotating furnace (described by Weidemann and Lundin 2015).
To assess EBRE, the effect of exposure to differently treated WWTP effluents on the relative concentrations of oxylipins in damselfly larvae was examined. Tissue extractions were performed according to Jonsson et al. (2014), followed by oxylipin analysis using the LC-MS/MS method (Yang et al. 2009). For the extraction of oxylipins, internal standards were added to the damselfly larvae and homogenisation was performed using 1.5 mL of acetonitrile and zirconium beads added to each sample with shaking for 4 min at 42 000 oscillations per minute using a Mini Beadbeater (Biospec Products Inc., Bartlesville, OK, USA). Samples were centrifuged at 15000 g for 10 min, the supernatant was withdrawn and then samples were re-extracted using 1.5 mL of acetonitrile following the above described procedure. The supernatants were combined, evaporated to dryness and reconstituted in 100 μL of methanol. A total of 87 oxylipins were analysed in the samples using LC-MS/MS (Yang et al. 2009).
Student’s t-test was used to detect significant differences (P < 0.05) in relative oxylipin concentrations between the exposure and control groups.
A total of 24 oxylipins derived from four fatty acids (arachidonic acid, linoleic acid [LA], α-linolenic acid [ALA] and eicosapentaenoic acid [EPA]) through two enzymatic pathways (LOX and CYP) were detected in the samples (Table S1 in the Supplementary Material). Among these, 16 were present in more than 75 % of samples of at least one exposure group, and hence included in the statistical analysis. Of these 16 oxylipins, 10 were not significantly different among the groups. Five LOX-derived oxylipins (9,10,13-TriHOME, 9,12,13-TriHOME, 9-HODE, 9-HOTrE and 13-HOTrE) were significantly lower in larvae exposed to conventionally-treated effluent compared with the control (Fig. 1a–e). Fewer oxylipins were affected by additional effluent treatments. One oxylipin, 9,10,13-TriHOME, was significantly lower in larvae exposed to ozonated effluent compared with the control. Two oxylipins, 9-HOTrE and 13-HOTrE, were significantly lower in larvae exposed to effluent treated with biochar. Additionally, 14,15-DiHETE (Fig. 1f), formed through the CYP pathway, was significantly lower in the biochar exposure group but was not affected by any other treatment. Overall, our results suggested that further treatment by ozonation or biochar filtration reduces the biological effects, as reported in other studies (Reungoat et al. 2010). In conclusion, in terms of EBREs, a lower effect was observed for both ozonation and biochar filtration compared with conventional WWTP effluent, i.e. these two treatment techniques had similar removal efficiencies of pollutants, such as pharmaceuticals affecting oxylipin pathways.
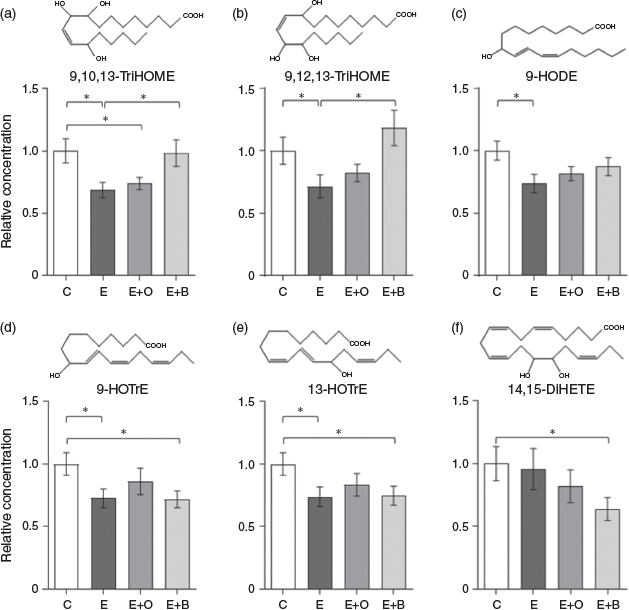
|
Oxylipins have previously not been detected in damselfly larvae. Hence, the biological role of these metabolites and their underlying biochemical pathways in this species remains to be investigated. However, in another invertebrate, Daphnia magna, several oxylipins, which include 9-HODE, 13-HODE, 9-HOTrE and 13-HOTrE, were shown to increase following a single exposure to different psychiatric drugs (Garreta-Lara et al. 2018), whereas in the present study, decreased levels were observed for the same compounds. We speculate that effluent exposure led to LOX inhibition by pharmaceuticals targeting LOX enzymes, which, in turn, resulted in decreased levels of the five oxylipins. Another explanation may be decreased precursor fatty acid levels owing to pollutant-induced stress. Finotello et al. (2017) showed that exposure to a pyrethroid pesticide led to lower fat content and reduced levels of two essential and two precursor fatty acids in damselfly larvae. Pollutant-induced stress requires more energy to maintain physiological integrity (Van Praet et al. 2014), which in turn may lead to lower fatty acid levels and supressed oxylipin synthesis.
To gain a better understanding of the affected metabolic pathways in damselfly larvae, analyses of other physiological endpoints, such as fatty acids and other metabolites related to available energy reserves, are needed. Inhibitor experiments, similar to the studies performed on other species (Knight et al. 1999), may provide valuable insights into oxylipin biosynthesis in damselfly larvae. To verify enzymatic activity, chiral LC-MS/MS analysis of monohydroxy metabolites, such as 9-HODE and 13-HODE, can also be used since it enables identification of (S) (enzymatic) and (R) (non-enzymatic) enantiomers (Garreta-Lara et al. 2018). In the present study, control group exposure was carried out in tap water instead of lake water to minimise the uncertainty arising from the potential presence of pollutants in the lake water. Future studies should address the effect of tap water in comparison to lake water. Additionally, metabolite levels following laboratory-exposure should be compared with levels of naïve individuals.
Because little is known about the fatty acid metabolism of damselfly larvae, the effects of altered oxylipin levels on individuals and populations are difficult to predict. However, owing to their important physiological functions in other invertebrates, disruption of oxylipin biosynthesis is likely to have severe consequences (Heckmann et al. 2008). Considering the current knowledge gap, this study represents the first important step towards using oxylipins as a proxy for biological effects, a method that can be used to address mixture effects of WWTP effluent and EBREs of various wastewater treatment techniques.
In conclusion, oxylipins belonging to the LOX and COX pathways were detected in the larvae of the Northern damselfly, an organism considered as a suitable target species for EBRE assessment owing to their ecological importance. We showed that exposure of damselfly larvae to WWTP effluent led to disruption of the biosynthesis of a subset of these fatty acid metabolites. Additional effluent clean-up by either ozonation or biochar filtration resulted in restored levels of some metabolites. The results suggested that metabolomics, in particular the fatty acid cascade as physiological endpoints, may be a useful tool for evaluating the effect-based removal efficiencies of wastewater treatment technologies.
Supplementary material
The supplementary material contains further information on the exposure set-up, normalised peak areas of all oxylipins detected in the samples (Table S1) and a list of oxylipins included in the analysis but not detected in any of the samples.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
Financial support from the Superfund Research Program Award P42 ES04699, NIEHS River Award R35 ES030443, the Swedish Research Councils FORMAS (2018–00823) and VR (2014–6354), and Marie Sklodowska Curie Actions, Cofund, Project INCA 600398 are gratefully acknowledged. We thank Alexandra Charlson at Umeå University for providing biochar.
References
Brodin T, Fick J, Jonsson M, Klaminder J (2013). Dilute Concentrations of a Psychiatric Drug Alter Behavior of Fish from Natural Populations. Science 339, 814–815.| Dilute Concentrations of a Psychiatric Drug Alter Behavior of Fish from Natural PopulationsCrossref | GoogleScholarGoogle Scholar | 23413353PubMed |
Brodin T, Piovano S, Fick J, Klaminder J, Heynen M, Jonsson M (2014). Ecological effects of pharmaceuticals in aquatic systems—impacts through behavioural alterations. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 369, 20130580
| Ecological effects of pharmaceuticals in aquatic systems—impacts through behavioural alterationsCrossref | GoogleScholarGoogle Scholar | 25405968PubMed |
Bundschuh M, Pierstorf R, Schreiber WH, Schulz R (2011). Positive Effects of Wastewater Ozonation Displayed by in Situ Bioassays in the Receiving Stream. Environmental Science & Technology 45, 3774–3780.
| Positive Effects of Wastewater Ozonation Displayed by in Situ Bioassays in the Receiving StreamCrossref | GoogleScholarGoogle Scholar |
Bundy JG, Davey MP, Viant MR (2009). Environmental metabolomics: a critical review and future perspectives. Metabolomics 5, 3
| Environmental metabolomics: a critical review and future perspectivesCrossref | GoogleScholarGoogle Scholar |
David A, Lange A, Abdul-Sada A, Tyler CR, Hill EM (2017). Disruption of the Prostaglandin Metabolome and Characterization of the Pharmaceutical Exposome in Fish Exposed to Wastewater Treatment Works Effluent As Revealed by Nanoflow-Nanospray Mass Spectrometry-Based Metabolomics. Environmental Science & Technology 51, 616–624.
| Disruption of the Prostaglandin Metabolome and Characterization of the Pharmaceutical Exposome in Fish Exposed to Wastewater Treatment Works Effluent As Revealed by Nanoflow-Nanospray Mass Spectrometry-Based MetabolomicsCrossref | GoogleScholarGoogle Scholar |
Eggen RIL, Hollender J, Joss A, Schärer M, Stamm C (2014). Reducing the Discharge of Micropollutants in the Aquatic Environment: The Benefits of Upgrading Wastewater Treatment Plants. Environmental Science & Technology 48, 7683–7689.
| Reducing the Discharge of Micropollutants in the Aquatic Environment: The Benefits of Upgrading Wastewater Treatment PlantsCrossref | GoogleScholarGoogle Scholar |
Ekman DR, Keteles K, Beihoffer J, Cavallin JE, Dahlin K, Davis JM, Jastrow A, Lazorchak JM, Mills MA, Murphy M, Nguyen D, Vajda AM, Villeneuve DL, Winkelman DL, Collette TW (2018). Evaluation of targeted and untargeted effects-based monitoring tools to assess impacts of contaminants of emerging concern on fish in the South Platte River, CO. Environmental Pollution 239, 706–713.
| Evaluation of targeted and untargeted effects-based monitoring tools to assess impacts of contaminants of emerging concern on fish in the South Platte River, COCrossref | GoogleScholarGoogle Scholar | 29715690PubMed |
Finotello S, Feckler A, Bundschuh M, Johansson F (2017). Repeated pulse exposures to lambda-cyhalothrin affect the behavior, physiology, and survival of the damselfly larvae Ischnura graellsii (Insecta; Odonata). Ecotoxicology and Environmental Safety 144, 107–114.
| Repeated pulse exposures to lambda-cyhalothrin affect the behavior, physiology, and survival of the damselfly larvae Ischnura graellsii (Insecta; Odonata)Crossref | GoogleScholarGoogle Scholar | 28601515PubMed |
Garreta-Lara E, Checa A, Fuchs D, Tauler R, Lacorte S, Wheelock CE, Barata C (2018). Effect of psychiatric drugs on Daphnia magna oxylipin profiles. The Science of the Total Environment 644, 1101–1109.
| Effect of psychiatric drugs on Daphnia magna oxylipin profilesCrossref | GoogleScholarGoogle Scholar | 30743823PubMed |
German Environment Agency (2016). Pharmaceuticals in the environment – the global perspective. Available at https://www.umweltbundesamt.de/en/publikationen/pharmaceuticals-in-the-environment-the-global [verified 9 September 2019]
Gouveia-Figueira S, Karimpour M, Bosson JA, Blomberg A, Unosson J, Pourazar J, Sandström T, Behndig AF, Nording ML (2017). Mass spectrometry profiling of oxylipins, endocannabinoids, and N-acylethanolamines in human lung lavage fluids reveals responsiveness of prostaglandin E2 and associated lipid metabolites to biodiesel exhaust exposure. Analytical and Bioanalytical Chemistry 409, 2967–2980.
| Mass spectrometry profiling of oxylipins, endocannabinoids, and N-acylethanolamines in human lung lavage fluids reveals responsiveness of prostaglandin E2 and associated lipid metabolites to biodiesel exhaust exposureCrossref | GoogleScholarGoogle Scholar | 28235994PubMed |
Heberer T (2002). Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: a review of recent research data. Toxicology Letters 131, 5–17.
| Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: a review of recent research dataCrossref | GoogleScholarGoogle Scholar | 11988354PubMed |
Heckmann LH, Sibly RM, Timmermans MJ, Callaghan A (2008). Outlining eicosanoid biosynthesis in the crustacean Daphnia. Frontiers in Zoology 5, 11
| Outlining eicosanoid biosynthesis in the crustacean DaphniaCrossref | GoogleScholarGoogle Scholar | 18625039PubMed |
Huber MM, Göbel A, Joss A, Hermann N, Löffler D, McArdell CS, Ried A, Siegrist H, Ternes TA, von Gunten U (2005). Oxidation of Pharmaceuticals during Ozonation of Municipal Wastewater Effluents: A Pilot Study. Environmental Science & Technology 39, 4290–4299.
| Oxidation of Pharmaceuticals during Ozonation of Municipal Wastewater Effluents: A Pilot StudyCrossref | GoogleScholarGoogle Scholar |
Jonsson M, Fick J, Klaminder J, Brodin T (2014). Antihistamines and aquatic insects: Bioconcentration and impacts on behavior in damselfly larvae (Zygoptera). The Science of the Total Environment 472, 108–111.
| Antihistamines and aquatic insects: Bioconcentration and impacts on behavior in damselfly larvae (Zygoptera)Crossref | GoogleScholarGoogle Scholar | 24291135PubMed |
Joss A, Siegrist H, Ternes TA (2008). Are we about to upgrade wastewater treatment for removing organic micropollutants?. Water Science and Technology 57, 251–255.
| Are we about to upgrade wastewater treatment for removing organic micropollutants?Crossref | GoogleScholarGoogle Scholar | 18235179PubMed |
Knight J, Rowley AF, Yamazaki M, Clare AS (1999). Eicosanoids are modulators of larval settlement in the barnacle, Balanus amphitrite. Journal of the Marine Biological Association of the United Kingdom 80, 113–117.
| Eicosanoids are modulators of larval settlement in the barnacle, Balanus amphitriteCrossref | GoogleScholarGoogle Scholar |
Kümmerer K, Dionysiou DD, Olsson O, Fatta-Kassinos D (2019). Reducing aquatic micropollutants – Increasing the focus on input prevention and integrated emission management. The Science of the Total Environment 652, 836–850.
| Reducing aquatic micropollutants – Increasing the focus on input prevention and integrated emission managementCrossref | GoogleScholarGoogle Scholar | 30380490PubMed |
Luo Y, Guo W, Ngo HN, Nghiem LD, Hai FI, Zhang J, Liang S, Wang XC (2014). A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. The Science of the Total Environment 473–474, 619–641.
| A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatmentCrossref | GoogleScholarGoogle Scholar | 24394371PubMed |
Noguera-Oviedo K, Aga DS (2016). Lessons learned from more than two decades of research on emerging contaminants in the environment. Journal of Hazardous Materials 316, 242–251.
| Lessons learned from more than two decades of research on emerging contaminants in the environmentCrossref | GoogleScholarGoogle Scholar | 27241399PubMed |
Nordin A, Pommer L, Nordwaeger M, Olofsson I (2013). Biomass conversion through torrefaction. In ‘Technologies for converting biomass to useful energy: Combustion, gasification, pyrolysis, torrefaction and fermentation’. (Ed. E Dahlquist) pp. 217–244. (CRC Press: Boca Raton, FL)
Pohl J, Björlenius B, Brodin T, Carlsson G, Fick J, Larsson DGJ, Norrgren L, Örn S (2018). Effects of ozonated sewage effluent on reproduction and behavioral endpoints in zebrafish (Danio rerio). Aquatic Toxicology 200, 93–101.
| Effects of ozonated sewage effluent on reproduction and behavioral endpoints in zebrafish (Danio rerio)Crossref | GoogleScholarGoogle Scholar | 29729477PubMed |
Reungoat J, Macova M, Escher BI, Carswell S, Mueller JF, Keller J (2010). Removal of micropollutants and reduction of biological activity in a full scale reclamation plant using ozonation and activated carbon filtration. Water Research 44, 625–637.
Sniegula S, Janssens L, Stoks R (2017). Integrating multiple stressors across life stages and latitudes: Combined and delayed effects of an egg heat wave and larval pesticide exposure in a damselfly. Aquatic Toxicology 186, 113–122.
| Integrating multiple stressors across life stages and latitudes: Combined and delayed effects of an egg heat wave and larval pesticide exposure in a damselflyCrossref | GoogleScholarGoogle Scholar | 28282618PubMed |
Ternes TA (1998). Occurrence of drugs in German sewage treatment plants and rivers. Water Research 32, 3245–3260.
| Occurrence of drugs in German sewage treatment plants and riversCrossref | GoogleScholarGoogle Scholar |
Ternes TA, Stüber J, Herrmann N, McDowell D, Ried A, Kampmann M, Teiser B (2003). Ozonation: a tool for removal of pharmaceuticals, contrast media and musk fragrances from wastewater?. Water Research 37, 1976–1982.
| Ozonation: a tool for removal of pharmaceuticals, contrast media and musk fragrances from wastewater?Crossref | GoogleScholarGoogle Scholar | 12697241PubMed |
Ternes T, Joss A, Oehlmann J (2015). Occurrence, fate, removal and assessment of emerging contaminants in water in the water cycle (from wastewater to drinking water). Water Research 72, 1–390.
| Occurrence, fate, removal and assessment of emerging contaminants in water in the water cycle (from wastewater to drinking water)Crossref | GoogleScholarGoogle Scholar | 25857678PubMed |
Tran NH, Reinhard M, Gin KYH (2018). Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions – a review. Water Research 133, 182–207.
| Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions – a reviewCrossref | GoogleScholarGoogle Scholar | 29407700PubMed |
Tyler CR, Jobling S (2008). Roach, Sex, and Gender-Bending Chemicals: The Feminization of Wild Fish in English Rivers. Bioscience 58, 1051–1059.
| Roach, Sex, and Gender-Bending Chemicals: The Feminization of Wild Fish in English RiversCrossref | GoogleScholarGoogle Scholar |
Van Praet N, De Bruyn L, De Jonge M, Vanhaecke L, Stoks R, Bervoets L (2014). Can damselfly larvae (Ischnura elegans) be used as bioindicators of sublethal effects of environmental contamination?. Aquatic Toxicology 154, 270–277.
| Can damselfly larvae (Ischnura elegans) be used as bioindicators of sublethal effects of environmental contamination?Crossref | GoogleScholarGoogle Scholar | 24974017PubMed |
Viant MR (2007). Metabolomics of aquatic organisms: the new ‘omics’ on the block. Marine Ecology Progress Series 332, 301–306.
| Metabolomics of aquatic organisms: the new ‘omics’ on the blockCrossref | GoogleScholarGoogle Scholar |
Weidemann E, Lundin L (2015). Behavior of PCDF, PCDD, PCN and PCB during low temperature thermal treatment of MSW incineration fly ash. Chemical Engineering Journal 279, 180–187.
| Behavior of PCDF, PCDD, PCN and PCB during low temperature thermal treatment of MSW incineration fly ashCrossref | GoogleScholarGoogle Scholar |
Weidemann E, Niinipuu M, Fick J, Jansson S (2018). Using carbonized low-cost materials for removal of chemicals of environmental concern from water. Environmental Science and Pollution Research International 25, 15793–15801.
| Using carbonized low-cost materials for removal of chemicals of environmental concern from waterCrossref | GoogleScholarGoogle Scholar | 29582326PubMed |
Willenberg I, Ostermann AI, Schebb NH (2015). Targeted metabolomics of the arachidonic acid cascade: current state and challenges of LC–MS analysis of oxylipins. Analytical and Bioanalytical Chemistry 407, 2675–2683.
| Targeted metabolomics of the arachidonic acid cascade: current state and challenges of LC–MS analysis of oxylipinsCrossref | GoogleScholarGoogle Scholar | 25577350PubMed |
Yang J, Schmelzer K, Georgi K, Hammock BD (2009). Quantitative Profiling Method for Oxylipin Metabolome by Liquid Chromatography Electrospray Ionization Tandem Mass Spectrometry. Analytical Chemistry 81, 8085–8093.
| Quantitative Profiling Method for Oxylipin Metabolome by Liquid Chromatography Electrospray Ionization Tandem Mass SpectrometryCrossref | GoogleScholarGoogle Scholar | 19715299PubMed |


