Pharmaceutical pollution in marine waters and benthic flora of the southern Australian coastline
Benjamin M. Long A * , Samantha Harriage A , Nick L. Schultz
A * , Samantha Harriage A , Nick L. Schultz  A , Craig D. H. Sherman B and Michael Thomas C
A , Craig D. H. Sherman B and Michael Thomas C
A Future Regions Research Centre, Federation University Australia, Mt Helen, Vic. 3350, Australia.
B School of Life and Environmental Sciences, Deakin University, Waurn Ponds, Vic. 3219, Australia.
C Research and Development, Barwon Water, Geelong, Vic. 3220, Australia.
Environmental Chemistry 19(6) 375-384 https://doi.org/10.1071/EN22054
Submitted: 1 June 2022 Accepted: 14 November 2022 Published: 6 January 2023
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Environmental context. Most human pharmaceutical waste is discharged to the environment. While the presence of pharmaceuticals in freshwater systems is well documented globally, little is known of the impact on marine ecosystems. We measured pharmaceuticals in a marine environment in south-eastern Australia and found pharmaceutical concentrations around 24 000 times higher in benthic flora than in the marine surface waters. We discuss the potential use of seaweeds as biological indicators of pharmaceutical pollution.
Rationale. Pharmaceuticals are emerging pollutants of concern with a range of adverse consequences for organisms and ecosystems. Their presence in freshwater and estuarine systems has been well documented, but less is known about their prevalence in open ocean, or their uptake by benthic flora. This preliminary survey of the southern Australian coastline sought to measure the concentrations of key pharmaceuticals in both surface waters and benthic flora.
Methodology. This study used LC-MS/MS to measure the concentration carbamazepine, tramadol and venlafaxine in (1) samples from wastewater treatment plants, (2) ocean surface waters and (3) several species of benthic flora. Surface waters and benthic flora were sampled at two sites near waste water treatment plant (WWTP) discharges, and one site away from any discharge.
Results. All three pharmaceuticals were detected in surface water samples with their risk assessed (via risk quotient) as medium risk (carbamazepine) or low risk (venlafaxine, tramadol). All three pharmaceuticals were also detected in benthic flora, particularly in brown macroalgae; Tramadol was measured at a maximum of 34.7 ng g−1 in Hormosira banksii, and Venlafaxine was recorded at a maximum of 17.3 ng g−1 in Caulocystis cephalornithos.
Discussion. The calculated bioconcentration factors suggest the pharmaceutical concentrations in benthic flora were up to ~24 000 times higher than in surrounding surface water. There was also evidence that proximity to WWTP outfalls influenced the levels of pharmaceuticals in benthic flora. The results suggest that the benthic flora may be suitable bioindicators of pharmaceutical contamination and that the potential impacts of pharmaceutical pollutants in marine ecosystems demand further investigation.
Keywords: benthic flora, bioindicators, emerging contaminant, macroalgae, marine, pharmaceutical pollution, risk assessment, risk quotient.
Introduction
Pharmaceuticals are often classed as emerging contaminants of concern (Madikizela et al. 2020; Branchet et al. 2021), though they have been documented since the 1970s (Hignite and Azarnoff 1977) and throughout the late 20th century (Halling-Sørensen et al. 1998). The observed effects of pharmaceutical contaminants in aquatic systems include additive acute and chronic toxicity (mostly in fish) with a range of physiological consequences (see Mostofa et al. 2013 for a review), and ecosystem-level effects can include reduced biodiversity at different trophic levels. Human usage of pharmaceuticals is the key source of these contaminants, and wastewater treatment plants (WWTPs) are considered the primary vector to the environment (Madikizela et al. 2020). The sewerage system and wastewater treatment plants are not designed to remove trace organic contaminants and thus they are passed to the environment via discharge points (e.g. ocean outfalls) (Meyer et al. 2019).
The presence of pharmaceuticals in river systems is increasingly well characterised worldwide (Katsikaros and Chrysikopoulos 2021; Adeleye et al. 2022) and in Australia (Scott et al. 2014; Roberts et al. 2016; South Australian Environmental Protection Agency SA-EPA 2019). Pharmaceutical concentrations have been characterised in marine surface water and sediments (Fabbri and Franzellitti 2016; Madikizela et al. 2020; Branchet et al. 2021), though the presence and fate of pharmaceuticals in the marine environment is less well known. Discharge into the open ocean is a common way to eliminate treated wastewater where large dilution volumes aid in reducing potentially hazardous concentrations of contaminants. A recent review by Madikizela et al. (2020) summarised the results of marine and estuary pharmaceutical monitoring of 42 studies, with up to 151 pharmaceuticals detected in the ng L−1 to µg L−1 range worldwide. In Australia, there have been reports of pharmaceutical monitoring in large estuaries, including Darling Harbour, Sydney (Birch et al. 2015; Anim et al. 2020), Brisbane Harbour (Anim et al. 2020), the Yarra River, Melbourne (Anim et al. 2020) and Darwin Harbour (French et al. 2015). To date there have been no published studies of pharmaceuticals in open ocean surrounding Australia despite Australia having 140 monitored ocean outfalls (Rohmana et al. 2021).
Pharmaceuticals have been quantified in commercially farmed bivalves, including mussels (Álvarez-Muñoz et al. 2015; Świacka et al. 2019) and clams (Maranho et al. 2015), and in marine macroalgae such as Saccharina latissima and Laminaria digitata (Álvarez-Muñoz et al. 2015), and Enteromorpha sp., Turbinaria conoides and Ulva lactuca (Ali et al. 2018). The mechanisms of pharmaceutical uptake by marine macroalgae are poorly understood, as highlighted by Ali et al. (2018), with few data to facilitate our understanding.
This study sought to undertake a preliminary survey in the southern Australian ocean for concentrations of three commonly detected pharmaceuticals that have low removal from wastewater and are persistent in natural ecosystems (carbamazepine, tramadol and venlafaxine). These three neuroactive pharmaceuticals have been frequently detected in Australian baseline studies and hence were chosen as suitable markers of pharmaceutical contamination (Scott et al. 2014; Birch et al. 2015; French et al. 2015; Roberts et al. 2016; South Australian Environmental Protection Agency SA-EPA 2019; Anim et al. 2020). Carbamazepine is a psychiatric drug with minimal biotic and abiotic degradation in natural ecosystems. As such, it is a recalcitrant and ubiquitous pollutant to which many aquatic ecosystems are exposed. Exposure can alter behaviour, immune response, growth, fecundity, and community structure (Quinn et al. 2008; Lamichhane et al. 2013; Jarvis et al. 2014). Tramadol is a synthetic opioid that can influence animal behaviour even at low concentrations (Santos et al. 2021). Venlafaxine is an antidepressant that can influence embryonic development in fish (Painter et al. 2009) and is potentially toxic to higher plants (Feito et al. 2013). Carbamazepine, venlafaxine and tramadol are an issue globally as they are consistently found above or close to minimum response concentrations and are hence likely to have adverse effects on flora and fauna (Duarte et al. 2022; Wilkinson et al. 2022).
This survey was conducted through direct measurement of pharmaceuticals in surface waters and benthic flora. It was envisioned that as benthic flora are in constant contact with potentially contaminated water they could act as effective bioindicators. To the author’s knowledge this study is the first in Australia to survey pharmaceutical pollution in the open ocean and the first to use marine benthic flora as a bioindicator for pharmaceutical pollution.
Experimental
Sample sites
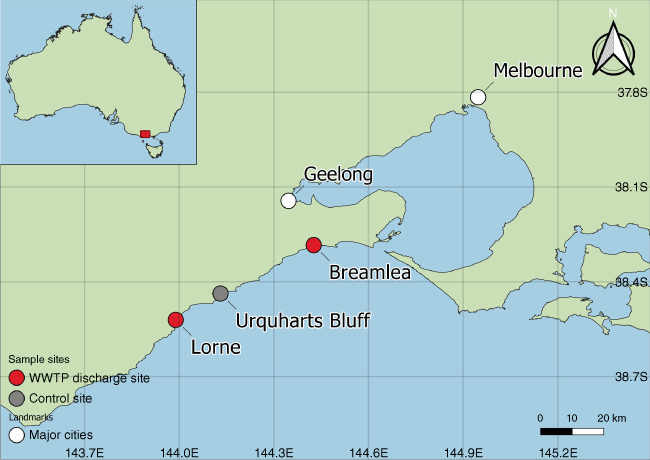
Three sample sites were chosen along the southern Australian coastline in the state of Victoria (Fig. 1). The coastline is within the Bass Strait region and is typically affected by swell, currents and winds from the Southern Ocean.
|
Sampling occurred on the 9th of March 2021 where the locations have an average temperature of 16.8°C, average rainfall of 37.4 mm and average wind speed of 16.5 km h−1 (tending from SSW) based on Australian Bureau of Meteorology weather statistics collected at an Airey’s Inlet weather station (−38.46°, 144.09°). For the month of March the average significant wave height is 1.50 m with a period of 7.1 s and a swell direction of 182° (S) based on data collected by the Apollo Bay Wave Buoy (−38.75°, 143.72°). Wave data was provided by the Victorian Coastal Monitoring Program with funding through Department of Environment, Land, Water and Planning, University of Melbourne and Deakin University.
The Breamlea sample site (Fig. 1) was chosen for its proximity to a large WWTP with an ocean outfall. The treatment plant services the City of Greater Geelong (Fig. 1) and its surrounding area. The WWTP services approximately 270 000 people (median age 40) (Australian Bureau of Statistics 2016) and discharges 51.6 ML day−1 only at programmed times (T. Murphy pers. comm. 2021) treating a population equivalent of 350 000 (W. McCance pers. comm. 2022). The discharge point is an underwater diffuser 800–1500 m offshore. The beach is a recreational surf beach.
The Lorne sample site (Fig. 1) was chosen for its proximity to a small WWTP and an economically important coastal town for the tourism industry. The WWTP services approximately 1000 people (Median age 54) (Australian Bureau of Statistics 2016) and discharges 0.7 ML day−1 only at programmed times (T. Murphy pers. comm. 2021) treating a population equivalent of 3100 (W. McCance pers. comm. 2022). The discharge point is directly onto a sandy beach and to the ocean via a manmade cleavage in the rock shelf.
Urquhart’s Bluff sample site was chosen as a control site as it is in a region with no known WWTP discharges. The nearest WWTP with discharge (Anglesea WWTP) is 7 km to the northeast or 6 km to the west in an overflow event (Airey’s Inlet WWTP). The site is a recreational surf beach with facilities for boating and fishing. The beach has toilet amenities with a septic tank not connected to water authority sewer infrastructure.
Sample collection
Samples were collected in March 2021 during a low tidal event (~0.5 m). Triplicate water samples were collected as 500 mL grab samples in polypropylene containers from rockpools open to the ocean water low in the intertidal zone (i.e. submerged under high tide conditions). We sampled benthic flora from the same rockpools, based on local availability, including attached macroalgae and seagrass. Five species of benthic flora were sampled across the three sites, including three species of brown algae (Hormosira banksii, Caulocystis cephalornithos and Cystophora subfarcinata), one seaweed (Codium sp.) and one seagrass (Amphibolis antarctica), though not all species were present at each site. Both surface water and flora samples were held at 0°C before storing at −20°C. WWTP water samples were collected by ALS Limited as grab samples of final effluent.
Sample preparation – surface water
Water samples were defrosted from −20 to 4°C and pH-adjusted to a pH between 3 and 4. Phenomenex Strata octadecylsilane (C18) cartridges were then pre-conditioned with 10 mL of methanol before the addition of 1 mL of 4 mM hydrochloric acid. A 100 mL aliquot was taken from the samples and drawn through the cartridges at a flow of less than 2 mL min−1. Once processed, 2.5 mL of methanol, followed by 2.5 mL of acetonitrile was passed through the cartridges to elute the sample into a 6 mL test tube before being placed under a flow of nitrogen gas to evaporate the solvent. The residue was reconstituted in a 1 mL solution containing 3% acetonitrile/water and 10 ng mL−1 deuterated isotope standards of carbamazepine-d10, tramadol-d3-13C and venlafaxine-d6. The solutions were filtered using 0.45 µm filters directly into HPLC vials for analysis.
Sample preparation – benthic flora
Flora samples were defrosted and dried at 40°C, 0.2 g of the dried, ground flora material was weighed out and 10 mL of methanol was introduced. The sample was vortexed for 1 min, then sonicated for 10 min. The sample was then centrifuged for 10 min at 5000 rpm and the supernatant decanted. The process was repeated two more times with acetonitrile, and the supernatants were combined. The subsequent 30 mL of methanol/acetonitrile was placed under a flow of nitrogen gas at 40°C until evaporated. The residue was then dissolved in 20 mL of a 10 ng mL−1 deuterated isotope solution containing carbamazepine-d10, tramadol-d3-13C and venlafaxine-d6. A 1 mL aliquot of the solutions was filtered using 0.45 µm nylon filters directly into HPLC vials for analysis.
Analysis and LC/MS parameters
The analysis of the surface water and flora species was based on the method by Birch et al. (2015). The analysis was conducted using a Shimazdu LCMS-8030 with a kinetex C18 column. The eluent strength gradient was increased from 3% acetonitrile in H2O with 0.1% formic acid to 100% acetonitrile with 0.1% formic acid over 11 min. The run was then held at 100% acetonitrile with 0.1% formic acid for a further 4 min. The column was re-equilibrated for 5 min before the next injection. The needle wash solution was a 1/1 ratio of methanol/MilliQ water followed by pure MilliQ water. In terms of the mass spectrometer (MS), the nebulising gas flow was at a rate of 2 L min−1, the heating block temperature was 400°C, the DL temperature was at 250°C and the drying gas flow was at 15 L min−1.
Multiple reaction mode (MRM) optimisation
The MRM was optimised for the following pharmaceuticals using 100 ng mL−1 standards: tramadol, tramadol-d3-13C, carbamazepine, carbamazepine-d10, venlafaxine and venlafaxine-d6. One ion was used for quantification and a second ion used to confirm the identity of the compound.
Pharmaceutical standards and quantification
The pharmaceutical standards were prepared from ampule sealed samples purchased from Novachem™. The samples were dissolved in ethanol or methanol depending on solubility. Mixed pharmaceutical standards were prepared to cover a range of 0.05–50 ng mL−1. Deuterated isotopes of carbamazepine, tramadol and venlafaxine were included in both the standards and the samples in an isotope dilution protocol to reduce matrix effects and to characterise those pharmaceuticals with greater accuracy (Du et al. 2012). Calibration curves were constructed using linear regression with r values of >0.999.
QA/QC
Throughout the analysis, a MilliQ water instrument blank and field blanks were analysed to ensure there was no carry over and standards were re-run to ensure precision and accuracy. The limit of quantification (LOQ) for pharmaceuticals was 0.5 ng L−1 in surface water and 5 ng g−1 in benthic flora. The limit of detection (LOD) was evaluated as a signal to noise ratio >3. Method recoveries measured for carbamazepine and venlafaxine were found to be 103% (±6%) and 56% (±8%), respectively. Birch et al. has shown that tramadol has a 54% recovery using similar methodology (Birch et al. 2015).
Statistical analysis
Shapiro–Wilk tests were used to check for the normal distribution of pharmaceutical concentrations from the WWTPs, surface waters and the benthic flora samples. The data from the WWTP were normally distributed and t-tests were used to test for differences in pharmaceutical concentrations between the two WWTPs. The data for surface waters and benthic flora were not normally distributed and so non-parametric tests were applied. To test the difference in the levels of pharmaceuticals of surface water among the three sites, Kruskal–Wallis tests were used with a post hoc Dunn test and a Bonferroni P-value adjustment for pairwise comparisons. Similarly, Dunn tests were used to test the difference in pharmaceutical concentrations in benthic flora for any species for which a pharmaceutical was recorded at multiple sites. All tests were performed using R (R Core Team 2021) and the packages dplyr (Wickham et al. 2020) and FSA (Ogle et al. 2021). Statistical analysis included values extrapolated below the LOQ for comparison of means. Samples where the detected concentration was below the LOD were included as zeros. Reported concentration ranges reflect absolute values measured.
Risk quotients
Risk quotients (RQ) were calculated using the protocol outlined by Zhou et al. (2019) (Eqn 1), however the concentrations involved in this study do not allow for an optimised risk quotient.

The measured environmental concentrations (MEC) were as calculated in this study and the predicted no effect concentrations (PNEC) were used as described by Zhou et al. (2019). Zhou et al. (2019) defined the PNEC values through a literature screening approach using chronic no-observed effect concentrations for crustaceans (carbamazepine) and fish (venlafaxine), or EC50 values for algae (tramadol). The PNEC values included in this study were: carbamazepine (PNEC = 10 ng L−1), tramadol (PNEC = 959 ng L−1) and venlafaxine (PNEC = 91.9 ng L−1). Thresholds for high risk (RQ > 1), moderate risk (1 < RQ < 0.1) and low risk (RQ < 0.1) were defined as per a recent study by Gani et al. (2021).
Results
WWTP discharge
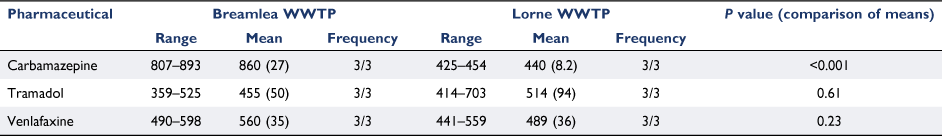
Pharmaceutical concentrations in the WWTP discharge are presented in Table 1. The highest recorded concentration was carbamazepine at Breamlea WWTP (860 ng L−1) and the lowest was carbamazepine at Lorne WWTP (440 ng L−1). The concentration of carbamazepine was significantly higher at Breamlea WWTP than at Lorne (P < 0.001).
|
Surface water
Across all three sample sites there was a total of 15 detections of pharmaceuticals from nine samples (Table 2). Carbamazepine was detected in three samples across two sites. The highest concentration of carbamazepine (0.99 ng L−1) was detected at Urquhart’s Bluff and there were no detections at Lorne. Tramadol was detected in seven samples across all sites. The highest concentration of tramadol (3.68 ng L−1) was noted at the Urquhart’s Bluff site and lowest measurable concentration (0.9 ng L−1) at the Breamlea site. Venlafaxine was detected in six samples across all sites. The highest concentration of venlafaxine (1.44 ng L−1) was at the Breamlea site and lowest concentration (0.52 ng L−1) at the Urquhart’s Bluff site. There were no significant differences in pharmaceutical concentrations among sites for any of the three pharmaceuticals (P > 0.05 for all pairwise comparisons).

|
Based on the maximum surface water concentrations measured, risk quotients were calculated according to the method by Zhou et al. (2019). Carbamazepine was classified as a moderate risk (RQ = 0.1) and both tramadol (RQ = 0.006) and venlafaxine (RQ = 0.02) were classified as low risk.
Benthic flora
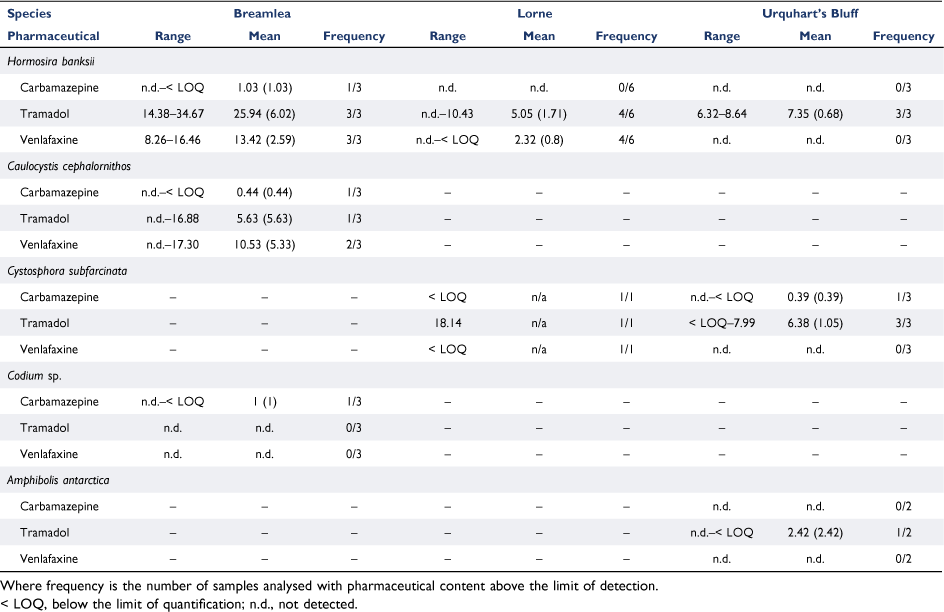
There were 24 detections of pharmaceuticals in the benthic flora from 27 samples (Table 3). There were 16 detections of tramadol, 10 detections of venlafaxine, and 5 detections of carbamazepine. However, no detections of carbamazepine were above the limit of quantification. Tramadol was detected at all three sites. The highest detected concentration of tramadol (34.67 ng g−1) was in Hormosira banksii at the Breamlea site. The lowest quantifiable measurement (6.21 ng g−1) was in Hormosira banksii at the Lorne site. The concentration of tramadol in Hormosira banksii was significantly greater at Breamlea than at Lorne (P = 0.026). Venlafaxine was quantifiable at both the Breamlea and Lorne sites but was not detected at Urquhart’s Bluff. The highest detected concentration of venlafaxine (17.3 ng g−1) was in a Caulocystis cephalornithos at the Breamlea site while the lowest quantifiable measurement (8.2 ng g−1) was in Hormosira banksii at the Urquhart’s Bluff site.
|
Discussion
The data presented in this study demonstrate that emerging pharmaceutical pollutants are detectible in ocean surface waters and benthic flora near the source of WWTP effluent. While a direct link from the source to the water and flora sampled cannot be confirmed, the sites closest to the WWTP outfalls demonstrated higher concentrations of pharmaceuticals in macroalgae than the sites situated away from large WWTP outfalls. The data suggest that these are emerging pollutants of concern for the southern Australian benthic environment that require monitoring and further research into the potential ecological impacts of these pollutants.
WWTP discharge
The Breamlea WWTP discharges 51.6 ML day−1 of treated wastewater (containing 860 ng L−1 carbamazepine, 455 ng L−1 tramadol and 560 ng L−1 venlafaxine) and the Lorne WWTP discharges 0.7 ML day−1 (containing 440 ng L−1 carbamazepine, 514 ng L−1 tramadol and 489 ng L−1 venlafaxine). The concentrations of tramadol and venlafaxine are similar at both sites however there was a significantly lower carbamazepine concentration at the Lorne WWTP. This may be due to population demographics, WWTP design or simply an artifact of grab sampling. Carbamazepine levels in WWTP effluent have been recorded at lower levels than presented here (studies in China (Ben et al. 2018) and Colombia (Botero-Coy et al. 2018) found maximum concentrations of 55 and 78 ng L−1, respectively), though the levels reported here are within the range reported from a study in Spain (Jelic et al. 2011) of 400–1400 ng L−1 in which the WWTP was designed to treat equivalent population sizes.
Surface water
Despite significant dilution in the marine environment, the focal pharmaceuticals are still detectable in surface water through standard grab sampling techniques. The maximum measured value for carbamazepine was measured at 0.99 ng L−1 at Urquhart’s Bluff and the lowest detected concentration (0.09 ng L−1) at Lorne. In Australian estuaries, carbamazepine ranges from 2.7 ng L−1 in Sydney estuary (Birch et al. 2015) to as high as 550 ng L−1 in Darwin Harbour (French et al. 2015). Globally, carbamazepine in the open ocean has been previously characterised (Madikizela et al. 2020), with recent detections off the coasts of Tunisia (max. 0.5 ng L−1; Afsa et al. 2020), Spain (max. 0.1 ng L−1; Biel-Maeso et al. 2018) and France (max. 0.140 ng L−1; Martínez Bueno et al. 2016). The concentrations of carbamazepine presented in this study are generally lower than the ranges reported in Australian estuary studies but are similar to the ranges reported in global oceanic studies.
Tramadol was measured at a maximum concentration of 5.72 ng L−1 at Urquhart’s Bluff, but was not detected in some samples at both Lorne and Urquhart’s Bluff. Darwin Harbour and Brisbane estuaries noted high concentrations of tramadol (270 and 81.9 ng L−1 respectively; French et al. 2015; Anim et al. 2020), while the Yarra river estuary (8.9 ng L−1; Anim et al. 2020) and Sydney estuary (1.3 ng L−1; Birch et al. 2015; Anim et al. 2020) concentrations are similar to concentrations reported in this study, despite there being no known wastewater inputs directly to Sydney estuary (Birch et al. 2015). Tramadol has not been well reported globally, with Alygizakis et al. (2016) reporting a maximum concentration of 1 ng L−1 in the Saronic Gulf, Greece and Wahlberg et al. (2011) reporting an average value of 14 ng L−1 surrounding Stockholm, Sweden.
The range of venlafaxine detected was 0.31–1.6 ng L−1. These values were low in comparison to all Australian estuary detections; 260 ng L−1 in Darwin Harbour (French et al. 2015), 86.2 ng L−1 in Brisbane Estuary (Anim et al. 2020), 10.0 ng L−1 in Yarra River Estuary (Anim et al. 2020) and 5.2 ng L−1 in Sydney Estuary (Birch et al. 2015; Anim et al. 2020). Globally, venlafaxine has been poorly represented but has been detected as high as 291 ng L−1 in coastal waters in North-Western Spain (Fernández-Rubio et al. 2019). There was a noted high variability between the three triplicates in all water samples collected, and in many cases for venlafaxine and tramadol the concentrations were below the limit of detection for our methodology. The grab sampling technique employed may account for the high variability, as samples were collected from individual rockpools with potential for microenvironments to form (i.e. concentration through evaporation or degradation due to higher temperature). However, these effects were likely minimised as samples were collected on a cool day and from a location with low exposure time above the intertidal mark. This variability highlights the need for suitable bioindicators that reflect longer-term pharmaceutical concentrations in marine environments.
Urquhart’s bluff site included high individual measurements for all three pharmaceuticals. This site was chosen as a control site as there was no wastewater discharge within 7 km. However, it is possible that these pharmaceuticals are present due to a prevailing southerly swell which would carry any discharge from Lorne. There is also a septic tank system at the site which could leech into the nearby water. Further testing of sites away from outfalls is needed to determine how proximity to outfalls influences pharmaceutical concentrations in marine surface waters.
At each site, the risk quotient for each pharmaceutical in surface waters was less than one for the maximum noted concentrations. The risk quotients suggest a moderate risk of harm from carbamazepine exposure and low risk of harm from tramadol and venlafaxine exposure to marine organisms based on PNEC values. Nevertheless, as emerging contaminants, more research is needed on the potential ecological impacts of long-term low-level exposure to such pharmaceuticals (Khan et al. 2021), their mixtures (Srain et al. 2021) and the role that these low-level concentrations might have at transferring pharmaceuticals into food webs (Richmond et al. 2018).
Benthic flora
The potential of benthic flora as bioindicators for environmental contaminants has been recognised globally (Álvarez-Muñoz et al. 2015; Maranho et al. 2015; Ali et al. 2018; Świacka et al. 2019), though few suitable candidates from the southern Australian coastline have been identified. While globally distributed species such as Ulva lactuca grow in southern Australian waters (Womersley 1984) and were studied by Ali et al. (2018) it was not found at any of our field sites.
Our only detection of carbamazepine in benthic flora was below the limit of quantification. This is consistent with previous studies; Ali et al. (2018) detected carbamazepine at low levels (max. 1.7 ng g−1 dry weight) in macroalgae in the Saudi Red Sea, and Álvarez-Muñoz et al. (2015) did not detect carbamazepine in macroalgae in European coastal waters. In both those studies, carbamazepine was detected in bivalves and fish. Álvarez-Muñoz et al. (2015) also did not detect venlafaxine in their samples, again despite its detection in fish and bivalves. Conversely, our results suggest tramadol is readily uptaken by the macroalgae species studied. To the authors knowledge tramadol uptake by macroalgae has not been reported elsewhere.
The maximum concentration of any pharmaceutical found in the benthic flora was tramadol in Hormosira Banksii at Breamlea (34.7 ng g−1). Using a bioconcentration factor (BCF) model outlined by Arnot and Gobas (2006), this equates to a BCF of ~24 000 L kg−1 and hence a 24 000 times increase in concentration. This accumulation was also noted in other species where pharmaceuticals were detected, most notably venlafaxine and tramadol in Caulocystis sp. at Breamlea. These high concentrations also indicate that these algae bioaccumulate tramadol and the other pharmaceuticals by the European Union definition of bioaccumulation (European Commision 2006). The benthic flora accumulate the pharmaceuticals to higher levels than found in the surface water, and while the RQ calculated for the surface water indicate that there is likely to be no negative effects on marine organisms, this may not be true for marine species who feed on benthic flora.
Hormosira banksii has previously been suggested as a bioindicator of wastewater influence as the species is sensitive to wastewater components (e.g. ammonia), such that its recruitment and growth is inhibited in the presence of wastewater (Doblin and Clayton 1995). Our data suggest that this species is a suitable indicator of pharmaceutical presence, and it would be useful to test the sensitivity of the species to a broad range of pharmaceutical concentrations.
Hormosira Banksii at the Breamlea site showed significantly higher concentrations of tramadol than the Lorne site, and this may reflect the higher concentration of pharmaceuticals in the surface waters over a long timescale and be related to higher WWTP discharge volumes at Breamlea. This strengthens the assumption that WWTP discharge impacts the benthic environment that is close in proximity to discharge sites. Nevertheless, the detection of pharmaceuticals in both surface water and flora at Urquhart’s Bluff, away from known discharge sites, suggests that the zone of influence of pharmaceuticals requires further investigation.
The benthic flora results also highlight the potential variability in pharmaceutical uptake, both among different pharmaceuticals and among macroalgae species. A such, the analysis of a broader range of macroalgae species is warranted, both in the search for suitable bioindicators, and to observe the potential ecological impacts of pharmaceuticals in marine environments.
Conclusion
The survey suggested through both surface water and benthic flora samples that all sites were contaminated with pharmaceutical pollution. The measurement of pharmaceutical concentration in benthic flora suggested that the Breamlea site near a large Victorian WWTP has higher levels of pharmaceutical pollution than other sites along the coastline on a longer timescale. As such, our study highlights that benthic flora such as Hormosira banksii can be utilised as bioindicator tools for the marine environment. Benthic flora are often perennial (Schiel and Taylor 1999), they are well adapted to survive in the rough seas and constantly changing conditions of the southern Victorian coastline, and we have shown they readily uptake pharmaceuticals.
The potential impacts of pharmaceuticals in the marine environment warrant future investigations, including the combined effects of pharmaceutical pollution and other environmental stressors, such as ocean acidification (Freitas et al. 2016) and ocean temperatures. It is the intent of the authors to expand upon this initial investigation to investigate seasonality and fluctuating ocean conditions though passive/composite sampling.
Data availability
Final measured values, detector responses, recoveries, calibration curves and R markdown files are available via figshare. DOI: 10.25955/20506704.
Conflicts of interest
This work was supported by Barwon Water and reports concentrations of pharmaceuticals within their remit.
Declaration of funding
This work was funded by Barwon Water and Central Highlands Water.
Acknowledgements
The authors acknowledge the traditional owners of the lands where this research was conducted, the Wathaurong, Gadubanud and Gunditjmara peoples and wish to pay respect to the Elders past, present and emerging, while also acknowledging that Indigenous Australians were Australia’s first scientists.
References
Adeleye AS, Xue J, Zhao Y, Taylor AA, Zenobio JE, Sun Y, Han Z, Salawu OA, Zhu Y (2022). Abundance, fate, and effects of pharmaceuticals and personal care products in aquatic environments. Journal of Hazardous Materials 424, 127284| Abundance, fate, and effects of pharmaceuticals and personal care products in aquatic environments.Crossref | GoogleScholarGoogle Scholar |
Afsa S, Hamden K, Lara Martin PA, Mansour HB (2020). Occurrence of 40 pharmaceutically active compounds in hospital and urban wastewaters and their contribution to Mahdia coastal seawater contamination. Environmental Science and Pollution Research 27, 1941–1955.
| Occurrence of 40 pharmaceutically active compounds in hospital and urban wastewaters and their contribution to Mahdia coastal seawater contamination.Crossref | GoogleScholarGoogle Scholar |
Ali AM, Rønning HT, Sydnes LK, Alarif WM, Kallenborn R, Al-Lihaibi SS (2018). Detection of PPCPs in marine organisms from contaminated coastal waters of the Saudi Red Sea. Science of The Total Environment 621, 654–662.
| Detection of PPCPs in marine organisms from contaminated coastal waters of the Saudi Red Sea.Crossref | GoogleScholarGoogle Scholar |
Álvarez-Muñoz D, Rodríguez-Mozaz S, Maulvault AL, Tediosi A, Fernández-Tejedor M, Van den Heuvel F, Kotterman M, Marques A, Barceló D (2015). Occurrence of pharmaceuticals and endocrine disrupting compounds in macroalgaes, bivalves, and fish from coastal areas in Europe. Environmental Research 143, 56–64.
| Occurrence of pharmaceuticals and endocrine disrupting compounds in macroalgaes, bivalves, and fish from coastal areas in Europe.Crossref | GoogleScholarGoogle Scholar |
Alygizakis NA, Gago-Ferrero P, Borova VL, Pavlidou A, Hatzianestis I, Thomaidis NS (2016). Occurrence and spatial distribution of 158 pharmaceuticals, drugs of abuse and related metabolites in offshore seawater. Science of The Total Environment 541, 1097–1105.
| Occurrence and spatial distribution of 158 pharmaceuticals, drugs of abuse and related metabolites in offshore seawater.Crossref | GoogleScholarGoogle Scholar |
Anim AK, Thompson K, Duodu GO, Tscharke B, Birch G, Goonetilleke A, Ayoko GA, Mueller JF (2020). Pharmaceuticals, personal care products, food additive and pesticides in surface waters from three Australian east coast estuaries (Sydney, Yarra and Brisbane). Marine Pollution Bulletin 153, 111014
| Pharmaceuticals, personal care products, food additive and pesticides in surface waters from three Australian east coast estuaries (Sydney, Yarra and Brisbane).Crossref | GoogleScholarGoogle Scholar |
Arnot JA, Gobas FAPC (2006). A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms. Environmental Reviews 14, 257–297.
| A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms.Crossref | GoogleScholarGoogle Scholar |
Australian Bureau of Statistics (2016) 2016 Census QuickStats. Retrieved from Australian Bureau of Statistics. Available at https://www.abs.gov.au/census/find‐census‐data/quickstats/2016/CED801
Australian Bureau of Statistics (2021) Australian Statistical Geography Standard (ASGS) Edition 3. Available at https://www.abs.gov.au/statistics/standards/australian-statistical-geography-standard-asgs-edition-3/jul2021-jun2026 [verified 18 August 2022]
Ben W, Zhu B, Yuan X, Zhang Y, Yang M, Qiang Z (2018). Occurrence, removal and risk of organic micropollutants in wastewater treatment plants across China: Comparison of wastewater treatment processes. Water Research 130, 38–46.
| Occurrence, removal and risk of organic micropollutants in wastewater treatment plants across China: Comparison of wastewater treatment processes.Crossref | GoogleScholarGoogle Scholar |
Biel-Maeso M, Baena-Nogueras RM, Corada-Fernández C, Lara-Martín PA (2018). Occurrence, distribution and environmental risk of pharmaceutically active compounds (PhACs) in coastal and ocean waters from the Gulf of Cadiz (SW Spain). Science of the Total Environment 612, 649–659.
| Occurrence, distribution and environmental risk of pharmaceutically active compounds (PhACs) in coastal and ocean waters from the Gulf of Cadiz (SW Spain).Crossref | GoogleScholarGoogle Scholar |
Birch GF, Drage DS, Thompson K, Eaglesham G, Mueller JF (2015). Emerging contaminants (pharmaceuticals, personal care products, a food additive and pesticides) in waters of Sydney estuary, Australia. Marine Pollution Bulletin 97, 56–66.
| Emerging contaminants (pharmaceuticals, personal care products, a food additive and pesticides) in waters of Sydney estuary, Australia.Crossref | GoogleScholarGoogle Scholar |
Botero-Coy AM, Martínez-Pachón D, Boix C, Rincón RJ, Castillo N, Arias-Marín LP, Manrique-Losada L, Torres-Palma R, Moncayo-Lasso A, Hernández F (2018). ‘An investigation into the occurrence and removal of pharmaceuticals in Colombian wastewater’. Science of the Total Environment 642, 842–853.
| ‘An investigation into the occurrence and removal of pharmaceuticals in Colombian wastewater’.Crossref | GoogleScholarGoogle Scholar |
Branchet P, Arpin-Pont L, Piram A, Boissery P, Wong-Wah-Chung P, Doumenq P (2021). Pharmaceuticals in the marine environment: What are the present challenges in their monitoring. Science of the Total Environment 766, 142644
| Pharmaceuticals in the marine environment: What are the present challenges in their monitoring.Crossref | GoogleScholarGoogle Scholar |
Doblin MA, Clayton MN (1995). Effects of secondarily-treated sewage effluent on the early life-history stages of two species of brown macroalgae: Hormosira banksii and Durvillaea potatorum. Marine Biology 122, 689–698.
| Effects of secondarily-treated sewage effluent on the early life-history stages of two species of brown macroalgae: Hormosira banksii and Durvillaea potatorum.Crossref | GoogleScholarGoogle Scholar |
Du B, Perez‐Hurtado P, Brooks BW, Chambliss CK (2012). Evaluation of an isotope dilution liquid chromatography tandem mass spectrometry method for pharmaceuticals in fish. Journal of Chromatography A 1253, 177–183.
| Evaluation of an isotope dilution liquid chromatography tandem mass spectrometry method for pharmaceuticals in fish.Crossref | GoogleScholarGoogle Scholar |
Duarte IA, Fick J, Cabral HN, Fonseca VF (2022). Bioconcentration of neuroactive pharmaceuticals in fish: Relation to lipophilicity, experimental design and toxicity in the aquatic environment. Science of the Total Environment 812, 152543
| Bioconcentration of neuroactive pharmaceuticals in fish: Relation to lipophilicity, experimental design and toxicity in the aquatic environment.Crossref | GoogleScholarGoogle Scholar |
European Commission (2006) Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the registration, evaluation, authorisation and restriction of chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC. Official Journal of the European Union 396, 1–849.
Fabbri E, Franzellitti S (2016). Human pharmaceuticals in the marine environment: Focus on exposure and biological effects in animal species. Environmental Toxicology and Chemistry 35, 799–812.
| Human pharmaceuticals in the marine environment: Focus on exposure and biological effects in animal species.Crossref | GoogleScholarGoogle Scholar |
Feito R, Valcárcel Y, Catalá M (2013). Preliminary data suggest that venlafaxine environmental concentrations could be toxic to plants. Chemosphere 90, 2065–2069.
| Preliminary data suggest that venlafaxine environmental concentrations could be toxic to plants.Crossref | GoogleScholarGoogle Scholar |
Fernández-Rubio J, Rodríguez-Gil JL, Postigo C, Mastroianni N, López de Alda M, Barceló D, Valcárcel Y (2019). Psychoactive pharmaceuticals and illicit drugs in coastal waters of North-Western Spain: Environmental exposure and risk assessment. Chemosphere 224, 379–389.
| Psychoactive pharmaceuticals and illicit drugs in coastal waters of North-Western Spain: Environmental exposure and risk assessment.Crossref | GoogleScholarGoogle Scholar |
Freitas R, Almeida Â, Calisto V, Velez C, Moreira A, Schneider RJ, Esteves VI, Wrona FJ, Figueira E, Soares AMVM (2016). The impacts of pharmaceutical drugs under ocean acidification: New data on single and combined long-term effects of carbamazepine on Scrobicularia plana. Science of the Total Environment 541, 977–985.
| The impacts of pharmaceutical drugs under ocean acidification: New data on single and combined long-term effects of carbamazepine on Scrobicularia plana.Crossref | GoogleScholarGoogle Scholar |
French VA, Codi King S, Kumar A, Northcott G, McGuinness K, Parry D (2015). Characterisation of microcontaminants in Darwin Harbour, a tropical estuary of northern Australia undergoing rapid development. Science of the Total Environment 536, 639–647.
| Characterisation of microcontaminants in Darwin Harbour, a tropical estuary of northern Australia undergoing rapid development.Crossref | GoogleScholarGoogle Scholar |
Gani KM, Hlongwa N, Abunama T, Kumari S, Bux F (2021). Emerging contaminants in South African water environment- a critical review of their occurrence, sources and ecotoxicological risks. Chemosphere 269, 128737
| Emerging contaminants in South African water environment- a critical review of their occurrence, sources and ecotoxicological risks.Crossref | GoogleScholarGoogle Scholar |
Halling-Sørensen B, Nors Nielsen S, Lanzky PF, Ingerslev F, Holten Lützhøft HC, Jørgensen SE (1998). Occurrence, fate and effects of pharmaceutical substances in the environment- A review. Chemosphere 36, 357–393.
| Occurrence, fate and effects of pharmaceutical substances in the environment- A review.Crossref | GoogleScholarGoogle Scholar |
Hignite C, Azarnoff DL (1977). Drugs and drug metabolites as environmental contaminants: Chlorophenoxyisobutyrate and salicylic acid in sewage water effluent. Life Sciences 20, 337–341.
| Drugs and drug metabolites as environmental contaminants: Chlorophenoxyisobutyrate and salicylic acid in sewage water effluent.Crossref | GoogleScholarGoogle Scholar |
Jarvis AL, Bernot MJ, Bernot RJ (2014). The effects of the psychiatric drug carbamazepine on freshwater invertebrate communities and ecosystem dynamics. Science of the Total Environment 496, 461–470.
| The effects of the psychiatric drug carbamazepine on freshwater invertebrate communities and ecosystem dynamics.Crossref | GoogleScholarGoogle Scholar |
Jelic A, Gros M, Ginebreda A, Cespedes-Sánchez R, Ventura F, Petrovic M, Barcelo D (2011). Occurrence, partition and removal of pharmaceuticals in sewage water and sludge during wastewater treatment. Water Research 45, 1165–1176.
| Occurrence, partition and removal of pharmaceuticals in sewage water and sludge during wastewater treatment.Crossref | GoogleScholarGoogle Scholar |
Katsikaros AG, Chrysikopoulos CV (2021). Occurrence and distribution of pharmaceuticals and personal care products (PPCPs) detected in lakes around the world - A review. Environmental Advances 6, 100131
| Occurrence and distribution of pharmaceuticals and personal care products (PPCPs) detected in lakes around the world - A review.Crossref | GoogleScholarGoogle Scholar |
Khan HK, Rehman MYA, Junaid M, Lv M, Yue L, Haq I-u, Xu N, Malik RN (2021). Occurrence, source apportionment and potential risks of selected PPCPs in groundwater used as a source of drinking water from key urban-rural settings of Pakistan. Science of the Total Environment 807, 151010
| Occurrence, source apportionment and potential risks of selected PPCPs in groundwater used as a source of drinking water from key urban-rural settings of Pakistan.Crossref | GoogleScholarGoogle Scholar |
Lamichhane K, Garcia SN, Huggett DB, DeAngelis DL, La Point TW (2013). Chronic Effects of Carbamazepine on Life-History Strategies of Ceriodaphnia dubia in Three Successive Generations. Archives of Environmental Contamination and Toxicology 64, 427–438.
| Chronic Effects of Carbamazepine on Life-History Strategies of Ceriodaphnia dubia in Three Successive Generations.Crossref | GoogleScholarGoogle Scholar |
Madikizela LM, Ncube S, Tutu H, Richards H, Newman B, Ndungu K, Chimuka L (2020). Pharmaceuticals and their metabolites in the marine environment: Sources, analytical methods and occurrence. Trends in Environmental Analytical Chemistry 28, e00104
| Pharmaceuticals and their metabolites in the marine environment: Sources, analytical methods and occurrence.Crossref | GoogleScholarGoogle Scholar |
Maranho LA, DelValls TA, Martín-Díaz ML (2015). Assessing potential risks of wastewater discharges to benthic biota: An integrated approach to biomarker responses in clams (Ruditapes philippinarum) exposed under controlled conditions. Marine Pollution Bulletin 92, 11–24.
| Assessing potential risks of wastewater discharges to benthic biota: An integrated approach to biomarker responses in clams (Ruditapes philippinarum) exposed under controlled conditions.Crossref | GoogleScholarGoogle Scholar |
Martínez Bueno MJ, Herrera S, Munaron D, Boillot C, Fenet H, Chiron S, Gómez E (2016). POCIS passive samplers as a monitoring tool for pharmaceutical residues and their transformation products in marine environment. Environmental Science and Pollution Research 23, 5019–5029.
| POCIS passive samplers as a monitoring tool for pharmaceutical residues and their transformation products in marine environment.Crossref | GoogleScholarGoogle Scholar |
Meyer MF, Powers SM, Hampton SE (2019). An Evidence Synthesis of Pharmaceuticals and Personal Care Products (PPCPs) in the Environment: Imbalances among Compounds, Sewage Treatment Techniques, and Ecosystem Types. Environmental Science & Technology 53, 12961–12973.
| An Evidence Synthesis of Pharmaceuticals and Personal Care Products (PPCPs) in the Environment: Imbalances among Compounds, Sewage Treatment Techniques, and Ecosystem Types.Crossref | GoogleScholarGoogle Scholar |
Mostofa KMG, Liu C-Q, Vione D, Gao K, Ogawa H (2013). Sources, factors, mechanisms and possible solutions to pollutants in marine ecosystems. Environmental Pollution 182, 461–478.
| Sources, factors, mechanisms and possible solutions to pollutants in marine ecosystems.Crossref | GoogleScholarGoogle Scholar |
Ogle DH, Wheeler P, Dinno A (2021) FSA: Fisheries Stock Analysis. R package version 0.8.32. Available at https://github.com/droglenc/FSA
Painter MM, Buerkley MA, Julius ML, Vajda AM, Norris DO, Barber LB, Furlong ET, Schultz MM, Schoenfuss HL (2009). Antidepressants at environmentally relevant concentrations affect predator avoidance behavior of larval fathead minnows (Pimephales promelas). Environmental Toxicology and Chemistry 28, 2677–2684.
| Antidepressants at environmentally relevant concentrations affect predator avoidance behavior of larval fathead minnows (Pimephales promelas).Crossref | GoogleScholarGoogle Scholar |
Quinn B, Gagné F, Blaise C (2008). An investigation into the acute and chronic toxicity of eleven pharmaceuticals (and their solvents) found in wastewater effluent on the cnidarian, Hydra attenuata. Science of the Total Environment 389, 306–314.
| An investigation into the acute and chronic toxicity of eleven pharmaceuticals (and their solvents) found in wastewater effluent on the cnidarian, Hydra attenuata.Crossref | GoogleScholarGoogle Scholar |
R Core Team (2021) ‘R: A language and environment for statistical computing.’ (R Foundation for Statistical Computing: Vienna, Austria)
Richmond EK, Rosi EJ, Walters DM, Fick J, Hamilton SK, Brodin T, Sundelin A, Grace MR (2018). A diverse suite of pharmaceuticals contaminates stream and riparian food webs. Nature Communications 9, 4491
| A diverse suite of pharmaceuticals contaminates stream and riparian food webs.Crossref | GoogleScholarGoogle Scholar |
Roberts J, Kumar A, Du J, Hepplewhite C, Ellis DJ, Christy AG, Beavis SG (2016). Pharmaceuticals and personal care products (PPCPs) in Australia’s largest inland sewage treatment plant, and its contribution to a major Australian river during high and low flow. Science of The Total Environment 541, 1625–1637.
| Pharmaceuticals and personal care products (PPCPs) in Australia’s largest inland sewage treatment plant, and its contribution to a major Australian river during high and low flow.Crossref | GoogleScholarGoogle Scholar |
Rohmana QA, Fischer A, Gemmill J (2021) National Outfall Database: Outfall Ranking Report 2019/2020 Financial Year. Report to the Department of Agriculture, Water and Environment. Australia: Clean Ocean Foundation and University of Tasmania.
Santos MES, Horký P, Grabicová K, Hubená P, Slavík O, Grabic R, Douda K, Randák T (2021). Traces of tramadol in water impact behaviour in a native European fish. Ecotoxicology and Environmental Safety 212, 111999
| Traces of tramadol in water impact behaviour in a native European fish.Crossref | GoogleScholarGoogle Scholar |
Schiel DR, Taylor DI (1999). Effects of trampling on a rocky intertidal algal assemblage in southern New Zealand. Journal of Experimental Marine Biology and Ecology 235, 213–235.
| Effects of trampling on a rocky intertidal algal assemblage in southern New Zealand.Crossref | GoogleScholarGoogle Scholar |
Scott PD, Bartkow M, Blockwell SJ, Coleman HM, Khan SJ, Lim R, McDonald JA, Nice H, Nugegoda D, Pettigrove V, Tremblay LA, Warne MSJ, Leusch FDL (2014). A national survey of trace organic contaminants in Australian rivers. Journal of Environmental Quality 43, 1702–1712.
| A national survey of trace organic contaminants in Australian rivers.Crossref | GoogleScholarGoogle Scholar |
South Australian Environmental Protection Agency (2019) Pharmaceutical products and other human-sourced chemicals in creeks. Available at https://www.epa.sa.gov.au/files/14363_info_wq_pharma_study.pdf [verified 1 December 2020]
Srain HS, Beazley KF, Walker TR (2021). Pharmaceuticals and personal care products and their sublethal and lethal effects in aquatic organisms. Environmental Reviews 29, 142–181.
| Pharmaceuticals and personal care products and their sublethal and lethal effects in aquatic organisms.Crossref | GoogleScholarGoogle Scholar |
Świacka K, Maculewicz J, Smolarz K, Szaniawska A, Caban M (2019). Mytilidae as model organisms in the marine ecotoxicology of pharmaceuticals - A review. Environmental Pollution 254, 113082
| Mytilidae as model organisms in the marine ecotoxicology of pharmaceuticals - A review.Crossref | GoogleScholarGoogle Scholar |
Wahlberg C, Björlenius B, Paxéus N (2011). Fluxes of 13 selected pharmaceuticals in the water cycle of Stockholm, Sweden. Water Science and Technology 63, 1772–1780.
| Fluxes of 13 selected pharmaceuticals in the water cycle of Stockholm, Sweden.Crossref | GoogleScholarGoogle Scholar |
Wickham H, François R, Henry L, Müller K (2020) dplyr: A Grammar of Data Manipulation. R package version 1.0.2. Available at https://CRAN.R‐project.org/package=dplyr
Wilkinson JL, Boxall ABA, Kolpin DW, Leung KMY, Lai RWS, Galbán-Malagón C, Adell AD, Mondon J, Metian M, Marchant RA, Bouzas-Monroy A, Cuni-Sanchez A, Coors A, Carriquiriborde P, Rojo M, Gordon C, Cara M, Moermond M, Luarte T, Petrosyan V, Perikhanyan Y, Mahon CS, McGurk CJ, Hofmann T, Kormoker T, Iniguez V, Guzman-Otazo J, Tavares JL, De Figueiredo FG, Razzolini MTP, Dougnon V, Gbaguidi G, Traoré O, Blais JM, Kimpe LE, Wong M, Wong D, Ntchantcho R, Pizarro J, Ying G-G, Chen C-E, Páez M, Martínez-Lara J, Otamonga J-P, Poté J, Ifo SA, Wilson P, Echeverría-Sáenz S, Udikovic-Kolic N, Milakovic M, Fatta-Kassinos D, Ioannou-Ttofa L, Belušová V, Vymazal J, Cárdenas-Bustamante M, Kassa BA, Garric J, Chaumot A, Gibba P, Kunchulia I, Seidensticker S, Lyberatos G, Halldórsson HP, Melling M, Shashidhar T, Lamba M, Nastiti A, Supriatin A, Pourang N, Abedini A, Abdullah O, Gharbia SS, Pilla F, Chefetz B, Topaz T, Yao KM, Aubakirova B, Beisenova R, Olaka L, Mulu JK, Chatanga P, Ntuli V, Blama NT, Sherif S, Aris AZ, Looi LJ, Niang M, Traore ST, Oldenkamp R, Ogunbanwo O, Ashfaq M, Iqbal M, Abdeen Z, O’Dea A, Morales-Saldaña JM, Custodio M, de la Cruz H, Navarrete I, Carvalho F, Gogra AB, Koroma BM, Cerkvenik-Flajs V, Gombač M, Thwala M, Choi K, Kang H, Ladu JLC, Rico A, Amerasinghe P, Sobek A, Horlitz G, Zenker AK, King AC, Jiang J-J, Kariuki R, Tumbo M, Tezel U, Onay TT, Lejju JB, Vystavna Y, Vergeles Y, Heinzen H, Pérez-Parada A, Sims DB, Figy M, Good D, Teta C (2022). Pharmaceutical pollution of the world’s rivers. Proceedings of the National Academy of Sciences 119, e2113947119
| Pharmaceutical pollution of the world’s rivers.Crossref | GoogleScholarGoogle Scholar |
Womersley HBS (1984) The Marine Benthic Flora of Southern Australia Part I. Available at http://www.flora.sa.gov.au/efsa/Marine_Benthic_Flora_SA/Part_I/Ulva_lactuca.shtml [verified 10 December 2021]
Zhou S, Di Paolo C, Wu X, Shao Y, Seiler T-B, Hollert H (2019). Optimization of screening-level risk assessment and priority selection of emerging pollutants – The case of pharmaceuticals in European surface waters. Environment International 128, 1–10.
| Optimization of screening-level risk assessment and priority selection of emerging pollutants – The case of pharmaceuticals in European surface waters.Crossref | GoogleScholarGoogle Scholar |


