Practising organometallic chemistry in nineteenth century Australia: David Orme Masson and diethyl magnesium
Ian D. Rae A *
A *
A School of Chemistry, University of Melbourne, Melbourne, Vic. 3010, Australia.
Historical Records of Australian Science 33(2) 122-132 https://doi.org/10.1071/HR22001
Published: 7 June 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of the Australian Academy of Science. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
By the late 1880s, the existence of alkyl derivatives of metals such as zinc and mercury was well established but diethyl magnesium had been poorly characterised and obtaining proof of its existence was a reasonable aim for chemists. Professor David Orme Masson and his student, Norman Wilsmore, at the university in the British colonial capital, Melbourne, accepted the challenge despite their distance from northern hemisphere centres of chemical research. The ‘tyranny of distance’ was tempered by their access to chemical journals and textbooks and by Masson’s connections at the ‘centre’, notably with William Ramsay. Wilsmore repeated the earlier experiments and also used methods that had been successful with other metals, but was unable to prepare diethyl magnesium. Masson rationalised this failure on the basis of the element’s position in the periodic classification of the elements that Mendeleev and Lothar Meyer had published, and on magnesium’s position on the atomic volume curve of Meyer, and concluded that diethyl magnesium could not exist. The weakness of these arguments was revealed when, near-coincidentally with Masson’s and Wilsmore’s publication of the results of their experiments, Philippe Löhr, working in Meyer’s laboratory, published successful syntheses of several alkyl magnesium derivatives by methods that had been unsuccessful in Wilsmore’s hands. Masson’s heuristic use of Meyer’s curve was unusual, and a notable feature of his approach to chemistry.
Keywords: alkyl, atomic volume, Frankland, Lohr, Meyer, organometallic, Wilsmore.
Introduction
David Orme Masson (1858–1937)1 arrived in Melbourne in late 1886 to take up the chair of chemistry at the University of Melbourne, following the death a year earlier of the first professor of chemistry, John Drummond Kirkland. Masson was young and, although well-connected in the world of chemistry, he was comparatively inexperienced. Studying in Edinburgh he had graduated MA (1877) and BSc (1880) before spending a year at Göttingen working with professors Friedrich Wöhler (1800–82) and Hans Hübner (1837–84),2 and a further year as a junior staff member with William Ramsay (1852–1916) at Bristol. He then returned to Edinburgh where he undertook research with Alexander Crum Brown and graduated DSc in 1884. Candidates for the Melbourne chair had been assessed in England by a committee where strong support for Masson’s candidacy by William Ramsay secured the appointment for his protégé. Masson occupied the chair until 1923 and, although he began with an active research program, he was known more in his later years for work on science policy and the foundation of organisations such as the Australian Chemical Institute and the Australian National Research Council.
Masson brought with him from Britain the intention of conducting chemical research in Australia in the way he might have done had he remained in Britain. There were difficulties in his way because the education systems in the Australian colonies were only in the early stages of development, despite nearly a century of European colonisation, and he had to cope with separation from the mainstreams of northern hemisphere research. The damage that this situation wrought on colonial society has been described by leading Australian historian Geoffrey Blainey (1930–) as ‘the tyranny of distance’,3 but simplistic interpretations of this term have been challenged by David Wade Chambers.4 Conceding that simple distance and geographical isolation may have impeded the establishment of European science in the Australian colonies, Chambers wrote about how difficult it was for those at distance from the centre to participate in networks where ideas could be developed through frequent communication and personal contact. Such isolation meant ‘exclusion from the latest ideas, at least from the very latest’ he wrote, but qualified this view by adding ‘unless the colonial research was closely integrated in professional networks’. Masson was able to mitigate some of the effects of isolation through a continuing relationship with William Ramsay up until the latter’s death in 1916. The two men shared a research interest in the arrangement of elements in the periodic table,5 and they spent time together when Masson went on study leave to Britain in the mid-1890s.6 Masson could never be ‘up with the latest’ because of the time it took for a letter to travel between London and Melbourne,7 but overseas journals (including those in German) and textbooks were available to him in Melbourne.
Organometallics at the University of Melbourne
When Norman Wilsmore (1868–1940)8 commenced research under Masson’s direction in the late 1880s there existed a substantial body of literature on organometallic chemistry and the preparation of alkyl compounds of a dozen metals and metalloids.9 This information was available to Australian researchers through libraries and private subscriptions to scientific journals. For example, the major products of the reaction between potassium acetate and arsenic trioxide, reported in 1760 by Louis Cadet de Gassicourt (1731–99), had been identified in the late 1830s by Robert Bunsen (1811–99) as tetramethyl diarsine (cacodyl) and bis(tetramethyldiarsine (cacodyl oxide) oxide).10 A period of intense activity in the field began with the work of Edward Frankland (1825–99) who prepared dimethyl- and diethyl zinc in 1849,11 and used the latter to prepare alkyl derivatives of tin, and boron and antimony by transmetalation.12 Reactions with diethyl zinc were used by George Buckton (1818–1905) to prepare alkyl derivatives of several metals;13 by Charles Friedel (1832–99) and James Crafts (1839–1917) to prepare tetraethyl silane from silicon tetrachloride;14 and by Clemens Winkler (1838–1904) to prepare tetraethylgermane from germanium tetrachloride.15 Dialkyl mercury compounds could also be employed as transmetallating agents, and in the early 1860s August Cahours (1813–91) had reported preparations of alkyl derivatives of tin, lead, tungsten, magnesium, aluminium, beryllium and arsenic although not all of the products were fully characterised using techniques of the day.16
Masson set Wilsmore the task of synthesising diethyl magnesium. Alkyl and aryl derivatives of metals and metalloids had been reported but there were none of alkali metals—those in Group 1 of the periodic classification of the elements—and the existence of those of the Group 2 elements beryllium and magnesium were not firmly established until much later.17 The synthesis of organometallic compounds of magnesium had produced inconclusive results leaving them uncharacterised and their existence open to reasonable doubt. Given that chemical experiments of such complexity as the synthesis of organometallics required had never before been attempted in Australia, Masson and Wilsmore were accepting a significant challenge.
The simplest method of preparing alkyl derivatives of metals was the reaction between finely divided metal and an alkyl iodide. Frankland had used it to prepare diethyl zinc, and Wilhelm Hallwachs (1859–1922) and Vojtech Schafarik (1829–1902) at the University of Göttingen had prepared alkyl aluminium iodides (R2AlI) in that way, and in the same paper they reported experiments with magnesium.18 After heating the finely divided metal with dry ethyl iodide in a sealed tube for several days, much gas was released when the tube was opened and a white solid remained. Heating the solid produced a mobile liquid having a penetrating onion-like odour that did not spontaneously ignite on contact with air but produced a cloud of magnesium oxide when it encountered moisture. They judged that the mixture contained a hydrocarbon with traces of diethyl magnesium. The solid appeared to be magnesium iodide which, after drying, reacted with violence when water was added. Cahours, one of the leading researchers in the field of organometallic chemistry repeated this experiment with similar result, except that the volatile liquid when exposed to air, caught fire.19 Cahours regarded it as a mixture of diethyl magnesium and a hydrocarbon derived from two ethyl radicals to which he ascribed the formulae (based on atomic weight 6 for carbon) C4H5Mg and C8H10, respectively.
Masson and Wilsmore were unsuccessful in their attempts to prepare diethyl magnesium, but nonetheless they chose to present their work at the third meeting of the Australasian Association for the Advancement of Science (AAAS),20 held in Christchurch, New Zealand, in January 1891. The sophistication of Masson’s and Wilsmore’s chemistry was far in advance of work on Australian plants and minerals and associated analytical methods that AAAS chemists (Section B) had contributed to the inaugural meeting in Sydney in August and September of 1888 and the second, in Melbourne in January1900.21
The Christchurch meeting
Neither Masson nor Wilsmore was present at the Christchurch meeting: their papers were read by the secretary of Section B (Chemistry and Mineralogy), Mr George Gray, FCS, of Lincoln Agricultural School, near Christchurch. Masson was president of the section, and he contributed several papers, beginning with his presidential address, ‘The gaseous theory of solution’.22 It began with an apology: ‘It is a matter of great regret to myself that I am unable to visit Christchurch so as to take part in the meetings of the Chemistry Section, and personally discharge the duties of my office’, and he went on to write that he would not have accepted the office had he realised that he would be unable to attend. The subject of his address, the gaseous theory of solutions, signalled the beginning of an interest in the nature of solutions that he pursued over the next few decades. A second Masson paper read at Christchurch, on molecular volumes and boiling points,23 is evidence of his continuing interest in the concept of molecular (and atomic) volumes that I shall return to later in this article.
The experimental results
Although the work was undoubtedly collaborative, there were separate conference presentations by Wilsmore and Masson. Wilsmore’s paper described nine attempts to prepare diethyl magnesium from magnesium metal, during which he wrote that great care was taken to have both apparatus and materials thoroughly dry.24 Many years later the historian of the Melbourne department, Joan Radford, perhaps drawing on her own experience with preparation of Grignard reagents, wrote that his failure to exclude water from the reactions was probably the cause of his failure to produce the compound.25
The first four attempts were modifications of methods used by Hallwachs and Schafarik, and of Cahours, in which the reaction was expected to be:
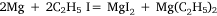
or:
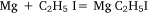
followed by:
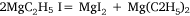
In the first attempt, ethyl iodide and a slight excess of magnesium filings were heated in a flask fitted with a condenser. The apparatus was filled with carbon dioxide26 and a mercury non-return valve to ensure the absence of air. The temperature was held between 75 and 100°C by means of an oil bath for 30 h by which time ethyl iodide had ceased to reflux. Throughout the heating there was a constant slow stream of gas passing through the valve and it burned with a luminous flame when ignited. The gas was not analysed but Wilsmore felt that it consisted of the products of the reaction:
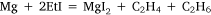
No volatiles were produced when the residue, identified as a mixture of anhydrous magnesium iodide, some unconsumed magnesium and ‘probably higher hydrocarbons’, was heated to 250°C although an ‘alliaceous’ (onion-like) smell was noticed.
Recognising that British chemists John Gladstone (1827–1902) and Alfred Tribe (1840–85) had found that zinc in the form of a zinc–copper couple was much more reactive than zinc metal alone and had successfully used it in the preparation of ethyl zinc iodide,27 Wilsmore tried such an approach to diethyl magnesium. When a metal combination prepared from magnesium metal and copper sulphate solution was reacted with ethyl iodide under a hydrogen atmosphere, there was no gaseous product and just a small amount of volatile kerosene-like material, in agreement with the observation of Hallwachs and Schafarik. In another attempt to increase the reactivity of magnesium, Wilsmore added a small amount of iodine to the ethyl iodide,28 the colour of iodine being only slowly discharged by the magnesium, as described by Alfred Wanklyn (1834–1906) and his student Ernest Chapman in their experiments with magnesium.29 Wilsmore also followed Gladstone and Tribe in the use of a ‘dry couple’30—that is, a mixture of magnesium filings and precipitated copper dried under a current of hydrogen—but without success, although the lack of gaseous emissions caused him to wonder if ‘success was at hand’.31 The final experiment in this series placed the reactants, ethyl iodide and the ‘wet’ copper magnesium couple, in a sealed tube as Cahours had done. When the tube was opened a great deal of gas was evolved and it burned with a luminous flame when ignited. As before, the residue was anhydrous magnesium iodide, and Wilsmore concluded that he was unable to confirm Cahours’s statement.
In experiment five, the concept of using a metal mixture was further applied and ethyl iodide was heated with a sodium–magnesium alloy, hoping for the reaction:
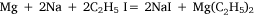
to take place. Heating on the water bath at 90–100°C for 2 h, under reflux, did not effect any change. To this point, the variations in experimental conditions that Wilsmore tried, like those of many earlier researchers, had concerned variations in the preparation of the metal, while continuing to provide the alkyl radical in the form of the alkyl iodide. However, Frankland and Frank Duppa (1828–73) had observed that ethyl acetate, which survived the reaction conditions unchanged, and was thus merely acting as a solvent, had a catalytic action on the formation of diethyl mercury.32 Wilsmore followed their example by repeating his experiment with the addition of this solvent. After heating on the water bath at 85°C for a further 60 h, the ethyl iodide had been consumed but an accident caused the flask to crack and water reacted violently with the contents. Events described below would show that this approach was likely to have been successful had it not been for the laboratory accident, and that Wilsmore would have been wise to have repeated the experiment, taking greater care to isolate the products.
The sixth and seventh experiments were attempts to prepare diethyl magnesium by metal exchange reactions with other organometallic compounds. The first of these involved diethyl mercury:
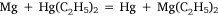
and was analogous to the formation of diethyl zinc reported by Frankland and Duppa.33 The reactants were heated in a sealed tube at 100–110°C for 4 h without result. When the temperature was raised to 130–140°C, after 4 h the tube exploded. The magnesium ribbon seemed to be mostly unaffected and Wilsmore attributed the increased gas pressure to the decomposition of diethyl mercury with formation of mercury and ‘paraffines’. The other attempt to achieve transmetalation was conducted with diethyl zinc, for which the simple equation above was joined by one attributed to Wanklyn (although no reference was cited):
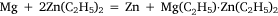
Once again, much gas was evolved when the tube was opened, and the residue reacted with water to produce hydrogen gas, which Wilsmore took to indicate that it consisted of a zinc–magnesium couple.
Experiments eight and nine involved attempts to react magnesium iodide with diethyl zinc, and magnesium metal with diethyl ether, but were similarly unsuccessful, leading Wilsmore to conclude that ‘the nett result of all my experiments is that I have not found it possible to prepare magnesium ethide’.
Wilsmore complemented his attempts to prepare diethyl magnesium (magnesium ethide) with an investigation of magnesium iodide that he prepared as described in the first of his attempts to prepare diethyl magnesium, yielding a product which gave the correct analytical figures for magnesium and for iodine.34 It reacted vigorously with water, so that it ‘might easily be mistaken for an ethide, as was done by Hallwachs and Schafarik; but no gas was given off, as would be the case with an ethide’. The molten salt could also be formed by the vigorous reaction of magnesium and iodine at high temperature. It dissolved in boiling anhydrous diethyl ether, from which it crystallised on cooling as colourless crystals probably having the formula MgI2·2(C2H5)2O. No analytical figures were provided and Wilsmore noted that ‘the nature of the crystals requires further investigation’.
Masson’s comments
In a commentary that was also read at the Christchurch meeting, Masson expressed his confidence in Wilsmore’s work and stated his view that neither Cahours nor Hallwachs and Schafarik had prepared diethyl magnesium, although the experiments of the former may have produced an impure sample and he conceded that Wanklyn may have shown that ‘magnesium can form an unstable double ethide with zinc’.35
The preparation of diethyl magnesium by Cahours was commonly included in textbooks, he observed, but ‘Mr. Wilsmore’s complete failure to obtain even a trace of the compound appears to me to necessitate an erasure from the text-books’. It is certainly true that Henry Roscoe (1833–1915) and Carl Schorlemmer (1834–92) in their 1881 textbook attributed the preparation of magnesium methyl and magnesium ethide to Cahours.36 Someone in Melbourne had taken Masson’s words to heart, because in a copy of the textbook accessioned in 1884 by Trinity College, a residential college of the University of Melbourne and held in the college library, beside the entry for the methyl derivative in which it is described as ‘strongly smelling mobile liquid’, they had pencilled: ‘Incorrect. First prepared by Löhr in 1899/1900 & is a solid’. The date given in the annotation is incorrect but the essence of the marginalia is correct: its significance will be evident later in this account.
Viewing their lack of success against the background of what was known about the formation of organometallic compounds, Masson wrote that Dmitrii Mendeleev (1834–1907)37 had observed that only certain groups of elements formed organometallic compounds. He did not cite a reference but Mendeleev’s views are expressed in his article ‘The periodic regularity of the chemical elements’.38 Assigning hydrogen to the first period, Mendeleev assigned the next fourteen elements in order of atomic weights—Li, Be, B, C, N, O, F, and Na, Mg, Al, Si, P, S, Cl—to two short series (‘kleine periode oder Reihe’), in which the vertical relationships between the elements were evident. Proceeding further along the atomic weight series, it was not possible to preserve the obvious vertical relationships—Li, Na, K, Rb and F, Cl, Br, I, for example—in seven-member series so he proposed the following arrangement in Table 1:
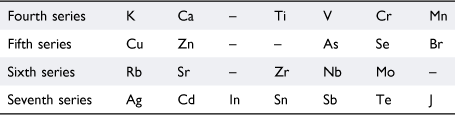
|
Even-plus-odd pairs of these were referred to as large periods (‘grosse Periode’). Drawing on the known chemistry of the time, Mendeleev commented that the members of the even-numbered (‘paaren’) series do not yield organometallic compounds, whereas those of odd-numbered (‘unpaaren’) series such as Zn, Cd, As, Sb, Se, Te, Br, J, Sn, Pb, Hg and Bi, do. Presumably because Mendeleev did not include the short periods in this assessment of chemical properties, Masson did not feel it necessary to point out that magnesium was in the third (another odd-numbered) series together with Na, Al, Si, P, S, and Cl, all of which, except sodium, had been reported to bond to alkyl groups.
Instead, Masson turned to another arrangement of the elements in which he found support for his contention that magnesium did not form organometallic compounds—the plot of atomic volume against atomic weight (Fig. 1) drawn up by Julius Lothar Meyer (1830–95).39 The formation of organometallic compounds, Masson wrote:40
Masson’s interpretation of the propensity of metals to form organometallics in terms of Meyer’s plot of atomic volumes is a rare example of its use as a heuristic device. His conclusion was that magnesium should be classed with calcium, strontium, and barium, which are positioned in similar places in Meyer’s curve. These elements shared the majority of chemical properties and many physical properties and do not form alkyl derivatives like those of cadmium, and mercury, all of which are positioned on rising parts of the curve. It was still possible, however, ‘as indicated by Wanklyn’s solitary qualitative experiment’ that magnesium could form an unstable double ethide with zinc.44 Magnesium resembled sodium more closely than any other element, he felt, which is not unexpected, considering that sodium is placed immediately before it in Mendeleev’s third series.characterises those elements which occur on the ascending portions of Lothar Meyer’s curve of atomic volumes, but not those on the descending portions. This is true at least of the long period, but the two short periods have been reckoned exceptional.41 In these short periods, however, a glance at the curve shows that the only elements undoubtedly on descending portions are beryllium and magnesium.42 The evidence for the existence of alkyl compounds in either case is so slight that one may be pardoned for doubting whether the short periods are exceptional after all. The only evidence of beryllium alkyl compounds comes also from Cahours, and his observations were of a purely qualitative character.43 About the alkyl compounds of boron and aluminium there is, of course, no uncertainty whatever: but these elements occur at the minima of the curve, and they naturally differ in their properties from the metals of the eighth group, which are at the minima of the long periods.
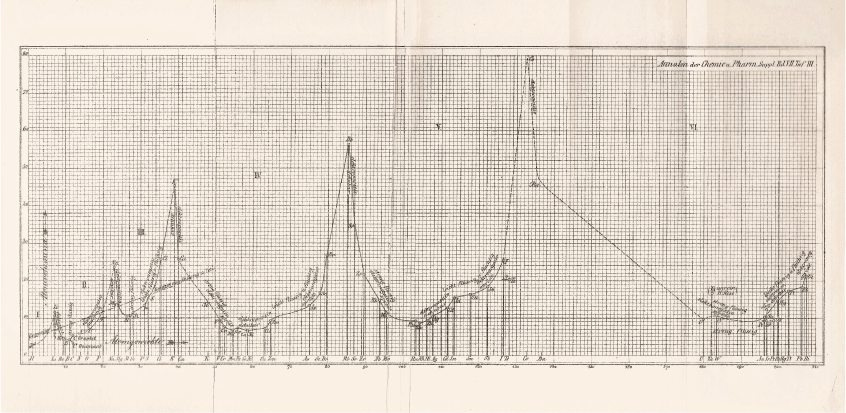
|
Chemical Society Proceedings
As well as their conference papers at the Christchurch meeting, on 29 December 1890, Masson sent a paper with a similar title—‘Does magnesium form compounds with hydrocarbon radicles’—to the Chemical Society in London. It was read, presumably by the Secretary, Henry Edward Armstrong (1848–1937), before a meeting of the society on 5 February 1891, and published in the Chemical Society Proceedings a fortnight later. It began with summaries of the results published by Hallwachs and Schafarik, Cahours, and Wanklyn, and described the experiments conducted at Melbourne and reported by Wilsmore to the AAAS meeting in Christchurch, including his investigation of magnesium iodide. In view of their lack of success, despite positive reports by others, they wrote:
They also raised the question of whether magnesium belonged in the part of the ‘second natural group’ of the periodic table, with zinc, mercury and cadmium, all of which had been shown to form compounds with ‘organic radicles’, or in that containing calcium, strontium and barium, which had not. Reading along the series, the authors observed that sodium, which preceded magnesium, did not form such compounds whereas aluminium, which followed it, did (as did beryllium in the same group as aluminium). They admitted the possibility that sodium might form ‘an unstable double compound with zinc and ethyl’, which they saw as analogous to magnesium’s ‘double ethide with zinc’, the existence of which Wanklyn had suggested.The authors are hence inclined to conclude that previous experimenters have used magnesium containing some impurity which facilitated its action on the iodide; but they do not see reason for assuming that the result was the production of magnesium ethide. They also suggest that possibly the magnesium previously used may have contained zinc, and that small quantities of zinc ethide mixed with hydrocarbons may have been mistaken for magnesium ethide.45
Without going into the detail of the Christchurch paper or referencing Meyer’s work, they wrote:
In the discussion that followed the presentation, Armstrong said Masson and Wilsmore’s paper had been dispatched on 29 December and that while it was in transit the question they posed, and to which they had given a negative answer, had been answered in the affirmative by the author of a paper that had appeared in the latest issue of the Annalen der Chemie. The author was Philipp Löhr who was working in Lothar Meyer’s laboratory at the University of Tübingen. His paper, based on his dissertation, concerned the synthesis of alkyl derivatives of cadmium and magnesium,47 during which he had found that reaction between magnesium and methyl or ethyl iodide was facile only in the presence of ethyl acetate, and that both dimethyl- and diethyl-magnesium were solids.It is exceptional for elements on the descending portions of the curve of atomic volumes to unite with hydrocarbon radicles, and a glance at the curve shows that beryllium and magnesium are the only elements undoubtedly occupying such a position that are believed to do so.46
However, Löhr pointed out that elemental analyses of the product of reaction with methyl iodide meant that it could be either methyl magnesium iodide (CH3MgI; but note that the Grignard reagents were yet unknown)48 or a mixture of magnesium iodide and dimethyl magnesium. To distinguish between these alternatives he performed alternative syntheses by reacting magnesium with dimethyl- and diethyl mercury. The products were grey solids that reacted with water to produce, respectively, methane and ethane. They were also spontaneously flammable in air and in carbon dioxide. Shortly afterwards, another of Meyer’s students at Tübingen, Hermann Fleck, used magnesium alkyls, produced by Löhr’s method from magnesium metal and the corresponding mercury compounds, as starting material in his research that included reactions of dimethyl mercury and the preparation of aryl magnesium compounds.49 The chemistry of these systems was more complicated than nineteenth-century European researchers had suspected. It was many years before American researchers later found that the transmetalation reactions were facilitated by the presence of mercuric chloride in catalytic amounts,50 that Grignard reagents RMgX could disproportionate as described by the equation 2RMgX ⇌ R2Mg + MgX2,51 and that complexing of the dialkyl magnesium with dioxane displaces the equilibrium to the right, enabling the isolation of solvates.52
Frankland and Wanklyn had found that the use of diethyl ether solvent facilitated the reactions between alkyl iodides and zinc metal,53 and Wanklyn had identified the formation of a solvate Zn(CH3)2·O(C2H5).54 Victor Grignard, in his Nobel Prize lecture of 1912, referred to the work of Philipp Löhr, Hermann Fleck and Fritz Waga (all of whom were students of Lothar Meyer at Tübingen), finding their results ‘discouraging’, but had succeeded in his own experiments when he followed the procedure of Frankland and Wanklyn and conducted his reaction in dry, deoxygenated diethyl ether.55 Mary Jo Nye has carefully examined the way that Grignard’s thinking about the use of ether solvent developed.56 Löhr’s ethyl acetate, Grignard’s diethyl ether and Johnson’s and Atkins’ dioxane are examples of the dipolar aprotic solvents that can act as Lewis bases and facilitate reactions with alkyl magnesium derivatives. The connection with Masson’s and Wilsmore’s work was noticed by another of Masson’s students, David Rivett, who succeeded him in the Melbourne chair, and wrote perhaps with some degree of exaggeration: ‘There is no doubt that (they) came very close to, but just missed, the formation of Grignard’s reagent which later played an important role in organic synthesis’.57 Another biographer thought that the work with Wilsmore ‘teetered on the verge of a valuable scientific advance without actually becoming one’.58
My review of Wilsmore’s experience raises questions about his experimental skills. On the one hand, he safely handled diethyl zinc, which is pyrophoric in air, and diethyl mercury, which is a dangerous neurotoxin.59 On the other hand, some of his experiments—that with the inclusion of ethyl acetate in experiment five and the transmetalation reactions60 in experiments six and seven—were conducted under conditions that been successful in the hands of Löhr, but not in his. Against this possibly harsh criticism of Wilsmore, I must allow that factors such as the purity of the reagents and the limited glassware likely to be available to him may have contributed to the failures.
Why did Masson turn to Lothar Meyer’s curve as an organising principle?
Gregory Girolami and Vera Mainz translated Mendeleev’s 1869 paper on atomic volume61 that was published in 1870 in the Proceedings of the Second Congress of Russian Scientists that had been held in Moscow in the previous year,62 citing Theron M. Cole Jr.63 and asserting that ‘atomic volumes played an important role in the development of chemistry in the 19th century’. Both Mendeleev and Meyer saw the variation in atomic volumes as an expression of periodicity of the elements, but comparing the work of the two, Girolami and Mainz noted that the chart published by Lothar Meyer makes evident ‘at a single glance’ what Mendeleev described in ten pages of ‘dense text’. Alan Rocke made the same point when he referred to the heuristic value of Meyer’s curve.64
As the nineteenth century was drawing to a close, the use of a periodic table, to which Mendeleev and Lothar Meyer had made major contributions,65 had become common in teaching and learning situations, and was widely available from suppliers of chemicals and apparatus.66 Masson offered no explanation of why he turned to Meyer’s curve to depict the placement of elements that formed organometallic compounds. The simplicity of doing so, as described above, was no doubt one reason, but there was something in his past that might have influenced the choice. Masson had spent a year working with William Ramsay (1852–1916) at Bristol and contributed to Ramsay’s work on atomic and molecular volumes, on which a suite of papers was published by the Chemical Society.67 In one of those communications, Ramsay reported research on the volumes of sodium and bromine at their boiling points (pp. 49–50 and p. 50, respectively), and shared with Masson (p. 50) another on the volume of phosphorus at its boiling point. In the discussion of their results (pp. 51–53), Ramsay outlined how the value determined for phosphorus allowed them to assign to phosphorus oxychloride the structure O═PCl3 rather than the alternative P(OCl)Cl2. So we can see that Masson had experience with the measurement and use of atomic and molecular volumes, which might have inclined him to explanations based on such properties.
Masson’s Christchurch paper on molecular volumes and boiling points revealed his continuing interest,68 and he would also been aware of the work of a British chemist who studied the properties of the elements, and the compounds they formed, and the placement of elements on Meyer’s curve and in Mendeleev’s ‘large periods’, the even- and odd-numbered series discussed earlier. This was Thomas Carnelly (1854–90),69 whose work on the physical properties of chemical compounds is most often associated with the melting point, but who also made significant contributions to the study of periodicity, first in 1884 in a paper about the physical properties of inorganic halogen compounds,70 and then a year later about organometallics.71 As only limited physical data were available for compounds of metals with organic groups (methyl, ethyl, propyl, butyl, and phenyl), Carnelley concentrated on the boiling points and specific gravities (densities), finding that they varied in the same way as those of the halogen derivatives he had previously studied. His Table I (including the names of the discoverers but no literature references) provided a useful summary of the organometallics that were known at the time. Alkyl derivatives of magnesium were conspicuously absent from the lists, as were others such as those of cadmium, possibly because no physical data were available for them, or that their existence (perhaps as a consequence of the lack of data) could not be taken as certain. Carnelly cited Mendeleev’s conclusion that ‘elements belonging to even series (except for Series 2) do not combine with alcohol radicals to form methides, ethides &c., whilst those belonging to odd series generally do so combine’. Just as was the case with the halogen derivatives, Carnelley found some exceptions to the patterns of boiling points, ‘either at or near the maxima or minima (i.e. at the turning points) of Meyer’s curve of the elements’. In the same vein, Mendeleev had remarked that the last members of an even series resemble in many respects (in their low oxidation states) the first members of the following odd series, but that there are sharp differences between the last members of the odd series (halides) and the first members (metals and alkalies) of the following even series.
Concluding remarks
The ‘tyranny of distance’ did not prevent Masson and Wilsmore from undertaking research of a kind they might have expected to see conducted in British or European laboratories. Research in the field of organometallics was in a quiet phase following a burst of activity in the 1850s and 1860s, but the results of the earlier research were readily accessible in Melbourne in chemistry journals and textbooks. The experiments conducted by Wilsmore followed closely the experience of Cahours and of Hallwachs and Schafarik with magnesium, and of research leaders like Frankland, Duppa, Wanklyn and Cahours with other metals. It is not clear how closely Masson was involved with Wilsmore’s experiments, but the failure to follow up what should have been even then (and certainly so in retrospect) the most promising of them—that involving the addition of ethyl acetate to the mixture that was never worked-up due to the breaking of the glass vessel—is a beginner’s mistake. A more attentive research director might have recommended repetition of the experiment.
Nor can the near-coincidence of publication by Masson and Wilsmore, on the one hand, and Philipp Löhr on the other, be attributed to distance. Coincident or near-coincident reporting of research findings are not uncommon—the most notable in the field of chemistry being that of Mendeleev’s and Lothar Meyer’s work on the periodic classification of the elements. The only unusual feature of the diethyl magnesium findings is their stark contradiction.
Masson’s explanation for Wilsmore’s negative findings, by reference to Mendeleev and Lothar Meyer, is perhaps the most interesting aspect of this story. In the two decades after their publications, it was the search for elements with appropriate atomic weights and chemical properties to fill the gaps in the tables that drew most attention to them, leading to the discovery of gallium (1875), scandium (1879) and germanium (1886). Those tempting gaps were not so evident in the atomic volume curves, and in any case it was molecular volume rather than atomic volume that attracted the attention of chemists interested in decisions between isomeric molecular structures and the systematic variation in molecular volume in homologous series. The atomic volume curve received much less attention.
Neither Masson nor Wilsmore continued work in the field of organometallic chemistry, confining themselves to physical chemistry—Masson with solution chemistry and Wilsmore with electrode potentials. There was no organometallic chemistry in Australia until 1920 when George Burrows (1888–1950) and Eustace Turner (1893–1966), recent appointees at the University of Sydney, published the first of a series of papers on organo-arsenic compounds.72
Data availability
All data used in the creation of this article are publicly available.
Conflicts of interest
The author has no conflicts of interest. He is co-editor of Historical Records of Australian Science but was blinded from the peer-review process for this paper.
Declaration of funding
No funding was required for the preparation of this article.
References
Aitken, R. A., and Gil, M. P. (2019) The periodic table and other wallcharts in the teaching of chemistry in St Andrews, 1884–1919, Philosophical Transactions of the Royal Society of London A, 378, 1–17.Anonymous (1891) Thomas Carnelley, Journal of the Chemical Society Transactions, 59, 455–61.
Apjohn, J. (1864) Manual of the Metalloids, London.
Blainey, G. (1966) The Tyranny of Distance: How Distance Shaped Australian History, Melbourne.
Buckton, G. B. (1858) VIII—On the isolation of the radical, mercuric methyl, Philosophical Transactions of the Royal Society of London, 148, 163–8.
| VIII—On the isolation of the radical, mercuric methylCrossref | GoogleScholarGoogle Scholar |
Buckton, G. B. (1859) XXI—On the isolation of the organo-metals, mercuric, stannic and plumbic ethyls; and observations on some of the derivatives: second memoir, Philosophical Transactions of the Royal Society of London, 149, 417–35.
| XXI—On the isolation of the organo-metals, mercuric, stannic and plumbic ethyls; and observations on some of the derivatives: second memoirCrossref | GoogleScholarGoogle Scholar |
Burrows, G. J., and Turner, E. E. (1920) A new type of compound containing arsenic, Journal of the Chemical Society, 117, 1373–83.
| A new type of compound containing arsenicCrossref | GoogleScholarGoogle Scholar |
Cahours, A. (1860a) Untersuchungen über die metallhaltigen organischen Radicale: erster Thiel, Annalen der Chemie und Pharmacie, 114, 227–55.
| Untersuchungen über die metallhaltigen organischen Radicale: erster ThielCrossref | GoogleScholarGoogle Scholar |
Cahours, A. (1860b) Recherches sur les Radicaux Organométalliques, Annales de Chimie et de Physique, 58, 5–82.
Cahours, A. (1862) Untersuchungen über die metallhaltigen organischen Radicale, Annalen der Chemie und Pharmacie, 122, 48–71.
| Untersuchungen über die metallhaltigen organischen RadicaleCrossref | GoogleScholarGoogle Scholar |
Carnelley, T. (1884) I—The periodic law, as illustrated by certain physical properties of inorganic compounds, The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science, 18, 1–22.
| I—The periodic law, as illustrated by certain physical properties of inorganic compoundsCrossref | GoogleScholarGoogle Scholar |
Carnelley, T. (1885) XXVIII—The periodic law, as illustrated by certain physical properties of organic compounds: part 1: the alkyl compounds of the elements, The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science, 20, 259–68.
| XXVIII—The periodic law, as illustrated by certain physical properties of organic compounds: part 1: the alkyl compounds of the elementsCrossref | GoogleScholarGoogle Scholar |
Chambers, D. W. (1991) ‘Does distance tyrannize science?’, in International Science and National Scientific Identity, eds R. W. Home and S. G. Kohlstedt, Dordrecht, Boston, pp. 19–38.
Cole Jr, T. M. (1975) Early atomic speculations of Marc Antoine Gaudin: Avogadro’s hypothesis and the periodic system, Isis, 66, 334–360.
| Early atomic speculations of Marc Antoine Gaudin: Avogadro’s hypothesis and the periodic systemCrossref | GoogleScholarGoogle Scholar |
De Garis, B. K. (1990) Wilsmore, Norman Thomas Mortimer (1868–1940), Australian Dictionary of Biography, 12, 517–518.
Edwards, G. N. (1865) Two cases of poisoning by mercuric methide, Saint Bartolomew’s Hospital Reports, i, 141–150.
Edwards, G. N. (1866) Note on the termination of the second case of poisoning by mercuric methide, Saint Bartolomew’s Hospital Reports, ii, 211–212.
Fleck, H. (1893) Ueber magnesium-Alkyle, Justus Liebig’s Annalen der Chemie, 276, 129–147.
| Ueber magnesium-AlkyleCrossref | GoogleScholarGoogle Scholar |
Fournier, J. (2013) Auguste Cahours (1813–1891), Les densités de vapeur, les organométalliques et la valence, l’actualité chimique, No. 373, avril, 27–30.
Frankland, E. (1849) Ueber die Isolirung der organischen Radicale, Annalen der Chemie und Pharmacie, 71, 171–213.
| Ueber die Isolirung der organischen RadicaleCrossref | GoogleScholarGoogle Scholar |
Frankland, E. (1850) On the isolation of the organic radicals, Journal of the Chemical Society, 2, 263–296.
Frankland, E. (1861) On organo-metallic bodies, Transactions of the Chemical Society, 13, 177–235.
Frankland, E., and Duppa, B. F. (1863) L—On a new method of producing the mercury compounds of the alcohol-radicles, Journal of the Chemical Society, 16, 415–425.
| L—On a new method of producing the mercury compounds of the alcohol-radiclesCrossref | GoogleScholarGoogle Scholar |
Frankland, E., and Duppa, B. F. (1864) III—On a new method on a new method of preparing the zinc-compounds of the alcohol-radicles, Journal of the Chemical Society, 17, 29–36.
| III—On a new method on a new method of preparing the zinc-compounds of the alcohol-radiclesCrossref | GoogleScholarGoogle Scholar |
Friedel, C., and Crafts, J. M. (1863) Sur quelques nouvelles Combinaisons organique du Silicium et sur le Poids atomique de cet élément, Comptes rendus hebdomadaires de l’Academie des Sciences, 56, 590–593.
Gilman, H., and Schulze, F. (1927a) CCCLIII—Beryllium Dialkyls, Journal of the Chemical Society, , 2663–2669.
| CCCLIII—Beryllium DialkylsCrossref | GoogleScholarGoogle Scholar |
Gilman, H., and Schulze, F. (1927b) Magnesium diethyl and its reaction with acetyl chloride, Journal of the American Chemical Society, 49, 2328–2330..
| Magnesium diethyl and its reaction with acetyl chlorideCrossref | GoogleScholarGoogle Scholar |
Girolami, G. S., and Mainz, V. V. (2019) Mendeleev, Meyer, and atomic volumes: an introduction to an English translation of Mendeleev’s 1869 article ‘On the atomic volume of simple bodies’, Bulletin for the History of Chemistry, 44, 100–108.
Gladstone, J. H., and Tribe, A. (1877) XX—Preparation of copper-zinc couples, Journal of the Chemical Society, 31, 561–570.
| XX—Preparation of copper-zinc couplesCrossref | GoogleScholarGoogle Scholar |
Gladstone, J. H., and Tribe, A. (1879) LXII—On dry copper-zinc couples and analogous reagents, Journal of the Chemical Society, 35, 567–578.
| LXII—On dry copper-zinc couples and analogous reagentsCrossref | GoogleScholarGoogle Scholar |
Grignard, V. (1912) ‘The use of organomagnesium compounds in preparative organic chemistry’, Nobel lecture, 11 December. Available at https://www.nobelprize.org/uploads/2018/06/grignard-lecture.pdf [viewed January 2021]
Hallwachs, W., and Schafarik, A. (1859) Ueber die Verbindungen der Erdmetalle mit organischen Radicalen, Justus Liebig’s Annalen der Chemie, 109, 206–209.
| Ueber die Verbindungen der Erdmetalle mit organischen RadicalenCrossref | GoogleScholarGoogle Scholar |
Home, R. W. (1991) ‘A world-wide scientific network and patronage system: Australian and other “colonial” fellows of the Royal Society of London’, in International Science and National Scientific Identity, eds R. W. Home and S. G. Kohlstedt, Dordrecht, Boston, pp. 151–179.
Hunter, D., Bomford, R. B., and Russell, D. S. (1940) Poisoning by methyl mercury compounds, Quarterly Journal of Medicine, 9, 193–226.
Jensen, W. B. (2002) Mendeleev on the Periodic Law—Selected Readings 1869–1905, Cincinnati, Ohio.
Johnson, G. O., and Adkins, H. (1932) Certain factors influencing the yield of Grignard reagents and the ratio of R2Mg to RMgX, Journal of the American Chemical Society, 54, 1943–1947.
| Certain factors influencing the yield of Grignard reagents and the ratio of R2Mg to RMgXCrossref | GoogleScholarGoogle Scholar |
Löhr, P. (1891) Ueber Alkylverbindungen des Cadmiums und des Magnesiums, Justus Liebig’s Annalen der Chemie, 261, 48–87.
| Ueber Alkylverbindungen des Cadmiums und des MagnesiumsCrossref | GoogleScholarGoogle Scholar |
Macleod, R. (Ed.) (1988) The Commonwealth of Science: ANZAAS and the Scientific Enterprise in Australasia, 1888–1988, Melbourne.
Masson, O. (1891a) ‘The gaseous theory of solution’, in Report of the Third Meeting of the Australasian Association for the Advancement of Science, ed. J. Hector, Wellington, New Zealand, pp. 84–103.
Masson, O. (1891b) ‘On molecular volumes and boiling-points in relation to chemical character’, in Report of the Third Meeting of the Australasian Association for the Advancement of Science, ed. J. Hector, Wellington, New Zealand, pp. 103–106.
Masson, O. (1891c) ‘Does magnesium form alkyl compounds?’, in Report of the Third Meeting of the Australasian Association for the Advancement of Science, ed. J. Hector, Wellington, New Zealand, pp. 107–108.
Masson, O., and Wilsmore, N. T. M. (1891) Does magnesium form compounds with hydrocarbon radicles?, Proceedings of the Chemical Society, 7, 16–19.
Masters, A. F. (1990) A brief history of organometallic chemistry in Australia and New Zealand, Applied Organometallic Chemistry, 4, 389–418.
| A brief history of organometallic chemistry in Australia and New ZealandCrossref | GoogleScholarGoogle Scholar |
Mendeleev, D. I. (2019) On the atomic volume of simple bodies, Bulletin for the History of Chemistry, 44, 109–115.
Mendelejeff, D. (1872) Die periodische Gesetzmässigkeit der chemischen Elemente, Annalen der Chemie und Pharmacie, VIII: Supplmentbanden, 2, 133–229.
Meyer, L. (1870) Die Natur der chemischen Elemente als function ihrer Atomgewichte, Annalen der Chemie und Pharmacie Supplementband, 7, 354–364.
Nye, M. J. (1986) Science in the Provinces, Berkeley, California.
Radford, J. (1978) The Chemistry Department of the University of Melbourne: Its Contribution to Australian Science 1854–1959, Melbourne.
Rae, I. D. (1988) ‘Chemists at ANZAAS: cabbages or kings?’, in The Commonwealth of Science: ANZAAS and the Scientific Enterprise in Australasia, 1888–1988, ed. R. MacLeod, Melbourne, pp. 166–195.
Rae, I. D. (2013) David Orme Masson, the periodic classification of the elements and his ‘Flap’ model of the periodic table, Historical Records of Australian Science, 24, 40–52.
| David Orme Masson, the periodic classification of the elements and his ‘Flap’ model of the periodic tableCrossref | GoogleScholarGoogle Scholar |
Ramsay, W. (1881) Communications from the laboratory of the University College, Bristol, Journal of the Chemical Society, Transactions, 39, 49–53.
Rasmussen, S. C. (2021) Transmetalation: a fundamental organometallic reaction critical to synthesis and catalysis, ChemTexts, 7, 1.
| Transmetalation: a fundamental organometallic reaction critical to synthesis and catalysisCrossref | GoogleScholarGoogle Scholar |
Rivett, A. C. D. (1939) Sir David Orme Masson, 1858–1937, Obituary Notices of Fellows of the Royal Society, 2, 454–464.
Rocke, A. J. (2019) Lothar Meyer’s pathway to periodicity, Ambix, 66, 265–302.
| Lothar Meyer’s pathway to periodicityCrossref | GoogleScholarGoogle Scholar | 31645216PubMed |
Roscoe, H. E., and Schorlemmer, C. (1881) A Treatise on Chemistry, vol III, Organic Chemistry, Part I, the Chemistry of the Hydrocarbons and Their Derivates, London.
Russell, C. A. (1996) Edward Frankland: Chemistry, Controversy and Conspiracy in Victorian England, Cambridge, UK.
Selleck, R. J. W. (2013) Finding Home: the Masson Family, North Melbourne.
Seyferth, D. (2001a) Cadet’s Fuming Arsenical Liquid and the Cacodyl Compounds of Bunsen, Organometallics, 20, 1488–1498.
| Cadet’s Fuming Arsenical Liquid and the Cacodyl Compounds of BunsenCrossref | GoogleScholarGoogle Scholar |
Seyferth, D. (2001b) Zinc Alkyls, Edward Frankland, and the beginnings of main-group organometallic chemistry, Organometallics, 20, 2940–2955.
| Zinc Alkyls, Edward Frankland, and the beginnings of main-group organometallic chemistryCrossref | GoogleScholarGoogle Scholar |
Seyferth, D. (2009) The Grignard reagents, Organometallics, 28, 1598–1605.
| The Grignard reagentsCrossref | GoogleScholarGoogle Scholar |
Strohmeier, W., and Seifert, F. (1961) Notiz zur Isolierung von Dialkylmagnesiumverbindungen aus Grignard-Lösungen, Chemische Berichte, 94, 2356–2357.
| Notiz zur Isolierung von Dialkylmagnesiumverbindungen aus Grignard-LösungenCrossref | GoogleScholarGoogle Scholar |
Unidentified (H. E. R. and P. P. B.) (1890) Thomas Carnelley, Nature, 42, 522–523.
Wanklyn, J. A. (1861) XIV—On zinc-methyl, Quarterly Journal of the Chemical Society, 13, 124–129.
| XIV—On zinc-methylCrossref | GoogleScholarGoogle Scholar |
Wanklyn, J. A. (1866) XII—On a new method of forming organo-metallic Bodies, Journal of the Chemical Society, 19, 128–130.
| XII—On a new method of forming organo-metallic BodiesCrossref | GoogleScholarGoogle Scholar |
Wanklyn, J. A., and Chapman, E. T. (1866) XV.—On magnesium, Journal of the Chemical Society, 19, 141–144.
| XV.—On magnesiumCrossref | GoogleScholarGoogle Scholar |
Weickhardt, L. (1989) Masson of Melbourne, Melbourne.
Wilsmore, N. T. M. (1891a) ‘Unsuccessful attempts to prepare magnesium ethyl’, in Report of the Third Meeting of the Australasian Association for the Advancement of Science, ed. J. Hector, Wellington, New Zealand, pp. 108–115.
Wilsmore, N. T. M. (1891b) ‘Note on magnesium iodide’, in Report of the Third Meeting of the Australasian Association for the Advancement of Science, ed. J. Hector, Wellington, New Zealand, p. 116.
Winkler, C. (1887) Mittheilungen über das germanium, Journal für praktische Chemie, 36, 177–209.
| Mittheilungen über das germaniumCrossref | GoogleScholarGoogle Scholar |
1 Weickhardt (1989). Selleck (2013). Rivett (1939).
2 It is likely that Masson was more closely associated with Hubner since Wohler was in his eighties by then. Masson was not associated with any publication from Göttingen.
3 Blainey (1966).
4 Chambers (1991).
5 Both were interested in the structure and the positioning of elements in the periodic table as described by Rae (2013).
6 Addressing in 1901 the election of Australian and other colonial scientists to fellowship of the Royal Society of London, Ramsay placed Masson first among scientists of ‘our Australian colonies’ but tempered his praise by adding that it was ‘well to elect a colonial now and then’ (cited in Home 1991). Although he did not have a significant record of publication, Ramsay said Masson had published some good work at least in some of his research.
7 The Massons’ journey to Australia in 1886 took 43 days; for comparison, the typical sailing time between UK and US was 7–10 days.
8 De Garis (1990). Wilsmore studied under Masson at the University of Melbourne, and was awarded BSc (1890) and MSc (1893) degrees before leaving to study with William Ramsay and Norman Collie in London. Subsequently he worked with Walther Nernst in Göttingen and Richard Lorenz in Zurich before taking up an appointment at University College London where, in 1907, he was co-disoverer of ketene. He returned to Australia in 1913 as foundation professor of chemistry at the University of Western Australia, in Perth.
9 The term ‘metalloid’ has been accepted as applicable to elements showing metallic and non-metallic properties following its introduction by Apjohn (1864).
10 Seyferth (2001a).
11 Frankland (1849). Frankland (1850).
12 Seyferth (2001b).
13 Buckton (1858). Buckton (1859).
14 Friedel and Crafts (1863).
15 Winkler (1887).
16 Cahours (1860a). Cahours (1860b). Cahours (1862).
17 Gilman and Schulze (1927a).
18 Hallwachs and Schafarik (1859).
19 Fournier 2013. Cahours (1860a) pp. 240–242.
20 Macleod (1988). The Australasian (later Australian and New Zealand) Association for the Advancement of Science (AAAS) was founded in 1888, modelled on the British Association for the Advancedment of Science that had been formed in 1831.
21 Rae (1988).
22 Masson (1891a).
23 Masson (1891b).
24 Wilsmore (1891a).
25 Radford (1978) p. 55.
26 This may not have been as inert as Wilsmore supposed, given the propensity (discovered later) of organometallics to react with carbon dioxide.
27 Gladstone and Tribe (1877).
28 The use of iodine in this way was not described by Frankland (1849), but Wilsmore noted that Henry Roscoe (1833–1915) and Carl Schorlemmer (1834–92) had written in 1881 that this improved method was now employed in Frankland’s laboratory in the preparation of diethyl zinc. Roscoe and Schorlemmer (1881) p. 460.
29 Wanklyn and Chapman (1866).
30 Gladstone and Tribe (1879).
31 There was no mention of gas emitted in the previous experiment.
32 Frankland and Duppa (1863).
33 Frankland and Duppa (1864).
34 Wilsmore (1891b).
35 Masson (1891c).
36 Roscoe and Schorlemmer (1881) pp. 245 and 455 respectively.
37 The Russian chemist’s name has been transliterated into English and German in various ways, but I will use Mendeleev unless quoting directly from an original.
38 Mendelejeff (1872). An English translation of this paper is included in Jensen (2002).
41 Masson was referring to the parts of Meyer’s curve that included elements in the short period, lithium to fluorine and sodium to chlorine, respectively, and the subsequent section (‘long period’) in which a greater number of elements were located.
42 Masson was reading the curve from left to right.
43 The probable formation of beryllium alkyls was reported by Cahours (1862) and by Frankland (1861) but the products were impure and it was many years before pure materials were obtained.
39 Meyer (1870).
40 Masson (1891c).
44 Masson did not give a reference, but it is likely that he was referring to Wanklyn’s claim to have prepared Mg(C2H5)2·Zn(C2H5)2 from magnesium metal and diethyl zinc. Wanklyn (1866).
45 Masson and Wilsmore (1891).
46 As above.
47 Löhr (1891).
48 Seyferth (2009).
49 Fleck (1893).
50 Gilman and Schulze (1927b).
51 Johnson and Atkins (1932).
52 Strohmeier and Seifert (1961).
53 Frankland (1861).
54 Wanklyn (1861).
55 Grignard (1912).
56 Nye (1986) pp. 164–187.
57 Rivett (1939).
58 Selleck (2013) p. 52.
59 Diethyl mercury is a homologue of dimethyl mercury that was reported to have been the cause of the deaths of two assistants working with Frankland and Duppa. Frankland and Duppa (1863). Edwards (1865). Edwards (1866). Hunter and others (1940). A full account of the incident and the extended discussion it provoked is provided by Russell (1996) pp. 251–252.
60 Rasmussen (2021).
61 Girolami and Mainz (2019).
62 Mendeleev (2019).
63 Cole (1975). Cole traced the concept of atomic volume to Jean Baptiste André Dumas (1800–1884).
64 Rocke (2019). Rocke also addressed the matter in his lecture ‘Lothar Meyer and Dmitrii Mendeleev: Parallel Paths to Periodicity, 1869–72’, to the Society for History of Alchemy and Chemistry, 22 April 2021. https://www.youtube.com/SocietyforHistoryofAlchemyandChemistry, viewed April 2021.
65 Mendeleev and Lothar Meyer were jointly awarded the Davy Prize of the Chemical Society of London in 1903.
66 Aitken and Gil (2019).
67 Ramsay (1881).
68 Masson (1891b).
69 Anonymous (1891). Unidentified (H. E. R. and P. P. B.) (1890). Although names were not given, one the authors (H. E. R.) could be identified as Henry Enfield Roscoe.
70 Carnelley (1884).
71 Carnelley (1885).
72 Masters (1990). Burrows Turner (1920).


