193 Melatonin implants in spring improve embryo production of aged ewes after superovulation regardless of endometrial progesterone receptor expression
J. A. Abecia A , A. Meikle B , M. I. Vázquez C , A. Casao A , F. Forcada A and C. Sosa DA Instituto Universitario de Investigación en Ciencias Ambientales de Aragón (IUCA), Universidad de Zaragoza, Zaragoza, Spain;
B Facultad de Veterinaria, Universidad de la República, Montevideo, Uruguay;
C Facultad de Veterinaria, Universidad Nacional de Río Cuarto, Río Cuarto, Argentina;
D Dept. Anatomía Patológica, Medicina Legal y Forense y Toxicología, Universidad de Zaragoza, Zaragoza, Spain
Reproduction, Fertility and Development 31(1) 221-221 https://doi.org/10.1071/RDv31n1Ab193
Published online: 3 December 2018
Abstract
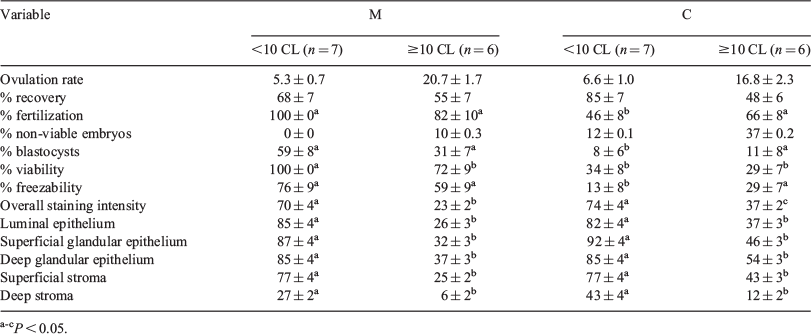
Twenty-three Rasa Aragonesa aged ewes (average age: 10.3 ± 0.3 years) were used to determine the effect of melatonin on ovulatory response, embryo production, and endometrial expression of progesterone receptors (PR) after superovulation. Ewes were treated (M, n = 13) or not (control, C, n = 10) with melatonin implants in March (Day 0, Northern Hemisphere autumn), and received intravaginal progestogen sponges for 14 days on Day 77. Superovulatory treatments consisted of 8 doses in decreasing concentrations (2 mL × 2 and 1 mL × 6) of 176 NIH-FSH-S1 units of NIADDK-oFSH-17 (Ovagen, ICPbio Reproduction, Auckland, New Zealand) administered twice daily starting 72 h before sponge removal. Seven days after oestrus, embryos were recovered by laparotomy, ewes were killed, and uterine horns were processed to study PR expression by immunohistochemistry. The amount of PR was estimated subjectively by 2 independent observers in 5 endometrial compartments: luminal epithelium (LE), superficial (sGE) and deep (dGE) glandular epithelia, and superficial (sS) and deep (dS) stroma. The extent of staining was expressed on a scale from 0 to 100. Data were analysed with a 2 × 2 factorial ANOVA. Melatonin implants improved fertilization (92 v. 57%, for M and C groups, respectively; P < 0.01), blastocyst (47 v. 9%; P < 0.01), viability (88 v. 31%; P < 0.0001), and freezability (69 v. 21%; P < 0.001) rates. Specifically, melatonin induced a significant reduction of the number of non-viable (degenerate and retarded) embryos (0.3 v. 1.5; P < 0.05) and increased blastocysts (2.8 v. 0.8; P < 0.05) per ewe. Melatonin treatment decreased PR staining intensity (47 v. 55%; P < 0.05), but this effect was not observed when the individual cell types were compared (Table 1). Because the number of corpora lutea (CL) was responsible for different PR expression in both groups (P < 0.0001), animals were divided into 2 ovulation rate categories: <10 CL and ≥10 CL, with lesser PR expression in the ≥10 CL group (P < 0.0001); this lower PR immunostaining in ≥10 CL is consistent with progesterone down-regulation of its own receptor. An interaction among number of CL and treatment was found for embryo quality (P < 0.05); thus, the positive effect of melatonin on this parameter was particularly effective in the low-ovulation-rate group. These results demonstrate that melatonin treatment in the autumn improves embryo quality in aged ewes, and that this effect is not explained by a differential endometrial sensitivity to progesterone.