107 Follicular wave synchronization and FSH stimulation prior to ovum pickup for in vitro embryo production
L. Ferré A , M. Kjelland B , T. Stroud C and P. Ross DA National Institute of Agricultural Technology, Tres Arroyos, Buenos Aires, Argentina;
B Conservation, Genetics and Biotech, Valley City, ND, USA;
C Hoofstock Genetics, Ranger, TX, USA;
D Department of Animal Science, University of California, Davis, CA, USA
Reproduction, Fertility and Development 32(2) 180-180 https://doi.org/10.1071/RDv32n2Ab107
Published: 2 December 2019
Abstract
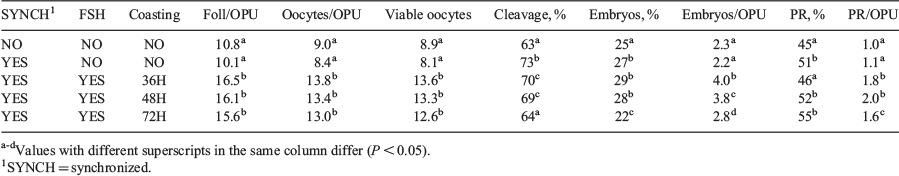
In vitro embryo production (IVP) has become a reliable alternative for genetic improvement in beef and dairy herds. Maximizing embryo yield and pregnancy per donor are key factors. The aim of this study was to compare ovum pickup (OPU) yields, developmental competence of cumulus-oocyte complexes (COCs), and pregnancy rates from Angus donors in a commercial IVP setting. Donors (>4-year-old pluriparous open dry cows) were handled under the same feeding and environmental conditions. Treatment groups were organised as follows: Group 1: no synchronization (SYNCH; n = 5); Group 2: SYNCH with no superstimulation (SOV; n = 5); Group 3: SYNCH + SOV (n = 5) and OPU 36 h after last FSH injection; Group 4: SYNCH + SOV (n = 5) and OPU 48 h after last FSH injection; and Group 5: SYNCH + SOV (n = 5) and OPU 72 h after last FSH injection. Follicular waves in groups 2, 3, 4, and 5 were synched by gonadotrophin-releasing hormone (GnRH), prostaglandin F2α (PGF), and controlled internal drug release (CIDR). No pre-synch was used. Injections of FSH (pFSH = 180 mg, Folltropin) were performed IM twice a day, for three days. A minimum of three replicates were performed for each donor. A Mindray DP30V equipped with a micro-convex transducer 5.0-8.5 MHz probe, disposable 20-gauge needle, and a flow rate of 15 mL min−1 were used for OPU. All visible follicles (Foll) were punctured and retrieved into a 50-mL 36°C warmed tube with media (phosphate-buffered saline, bovine serum albumin (BSA), and heparin). Viable oocytes were classified according to IETS guidelines. The COCs were matured in 100 µL of M199 medium supplemented with ALA-glutamine (0.1 mM), Na pyruvate (0.2 mM), gentamicin (5 µg mL−1), epidermal growth factor (50 ng mL−1), oFSH (50 ng mL−1), bLH (3 μg mL−1), cysteamine (0.1 mM), and 10% fetal bovine serum (FBS) for 22 to 24 h. Fertilization (Day 0) was carried out using highly fertile sires selected by discontinuous 40%/80% layers (PureSperm) and diluted to a final concentration of 1 × 106 sperm mL−1. Matured oocytes were fertilized in 50 µL of modified synthetic oviductal fluid (SOF) media supplemented with fructose (90 µg mL−1), penicillamine (3 µg mL−1), hypotaurine (11 µg mL−1), and heparin (10 µg mL−1). After 18 h, presumptive zygotes were denuded and cultured under low oxygen tension in 50-µL drops of SOF-BSA for 7 days. On Day 3.5, 2% of FBS was added. On Day 7, fresh transferable (grade 1 and 2, IETS standards) blastocysts were implanted into synchronized recipient cows. Around Day 30, ultrasound diagnosis was performed to determined pregnancy rate (PR). We used ANOVA for comparisons of mean values and X2 test for proportions, α = 0.05 (Table 1). In conclusion, synchronization, FSH stimulation, and 48-h coasting before OPU in Angus cows increased the number of collected viable oocytes and embryo development rates. More transferrable embryos and higher rates of PR per OPU were obtained using 36- and 48-h coasting, respectively.
|


