Superoxide dismutase affects the viability of thawed European mouflon (Ovis g. musimon) semen and the heterologous fertilization using both IVF and intracytoplasmatic sperm injection
Fiammetta Berlinguer A C , Sergio Ledda A , Irma Rosati A , Luisa Bogliolo B , Giovanni Leoni A and Salvatore Naitana AA Department of Animal Biology, University of Sassari, 07100 Italy.
B Institute of General Pathology, Pathological Anatomy and Veterinary Obstetrics-Surgery Clinic, University of Sassari, 07100 Italy.
C To whom correspondence should be addressed. email: vetfis@ssmain.uniss.it
Reproduction, Fertility and Development 15(1) 19-25 https://doi.org/10.1071/RD02048
Submitted: 11 June 2002 Accepted: 11 December 2002 Published: 11 December 2002
Abstract
This study evaluated the effects of superoxide dismutase (SOD) on viability and acrosome integrity of European mouflon spermatozoa after cryopreservation and on the fertilization rates of sheep oocytes after IVF or intracytoplasmatic sperm injection (ICSI). Frozen semen was thawed and washed with synthetic oviduct fluid supplemented with 0.6% bovine serum albumin. After centrifugation, the spermatozoa pellet was split into two culture systems: (i) without SOD; and (ii) in the presence of 1500 IU mL−1 SOD. Sperm viability and acrosome integrity were evaluated simultaneously, immediately after thawing and after 3, 6 and 9 h of culture (5% CO2, 39°C, 90% humidity), by incubating sperm with propidium iodide and fluorescein isothiocyanate-labelled Pisum sativum agglutinin. At the same time, sperm were assessed for motility using a standard scoring system (independent operators’ observation of sperm) that graded degree of motility (i.e. 1 = immotile to 10 = maximum motility, as observed at the moment of thawing). For IVF, frozen–thawed semen derived from the two culture systems was placed in culture together with in vitro-matured sheep oocytes. For ICSI, semen derived from the same culture systems as that for IVF was used, and incubated for 1 h under standard conditions. The results showed a marked difference (P < 0.01) between the percentages of live spermatozoa in medium with SOD and those obtained in medium alone, after 3, 6 and 9 h of culture. The percentages of intact acrosome spermatozoa were higher in medium with SOD after 6 h (P = 0.05) of culture. Spermatozoa motility decreased significantly in SOD containing medium at 3 and 6 h of culture compared with motility in control medium. Fertilization rates were significantly lower in medium with SOD than in medium alone, whereas in the ICSI system fertilization rates were significantly higher in the presence of SOD. The results indicate that the addition of SOD to the culture media enhances the viability rates and the acrosome integrity of cryopreserved mouflon spermatozoa.
Extra keywords: acrosome reaction
Introduction
The European mouflon (Ovis g. musimon) is now found in large numbers in several countries, although it is often interbred with the domestic sheep (Cugnasse 1994). These populations derive from Corsican and Sardinian mouflons, which live on the islands in small and isolated groups. To maintain resources of the pure breed, it is very important to study and preserve the natural populations of Corsican and Sardinian mouflons (Ptak et al. 2002), also considered ancestors of the modern sheep breeds (Naitana et al. 1990; Hiendleder et al. 1998).
To ensure that the creation of a genetic resource cryo-bank is useful, much effort must be made to increase the functional capacity of European mouflon spermatozoa after cryopreservation (Naitana et al. 1998).
Cold shock can injure spermatozoa at different levels of structures such as mitochondria (Windsor 1997) or plasma and acrosome membranes (Drobnis et al. 1993; Watson 1995), and can alter spermatozoon functional integrity (Gillan et al. 1999), hence reducing its fertilizing capacity. These modifications can also be determined by the accumulation of toxic catabolic products, including the reactive oxygen species (ROS) derived from the peroxidation of membrane unsaturated lipid (Mazzili et al. 1995). Many antioxidant molecules are physiologically secreted in the ram genital tract to protect sperm cells from peroxidative damage (Abu-Erreish et al. 1978). However, a significant reduction in the level of spermatozoa antioxidant has been reported as one of the causes of the enhanced susceptibility of these cells to peroxidative injuries after cryopreservation (Bilodeau et al. 2000). It has been found that the addition of superoxide dismutase (SOD), cytocrome c, catalase and glutathione peroxidase have positive effects in maintaining the motility and acrosome integrity of ram spermatozoa during liquid storage (Maxwell and Stojanov 1996).
In particular, SOD is an enzyme that detoxifies the superoxide anion (O2 −) catalysing a reaction, known as dismutation, in which O2 − reacts with itself, generating H2O2 and O2 (Aitken 1995).
Alvarez and Storey (1992) have suggested that the cause of the enhanced susceptibility of spermatozoa to peroxidative damage after cryopreservation might be the loss of SOD activity from spermatozoa following cryopreservation as a result of O2 − forming cytotoxic complexes in the presence of iron chelates. The addition of SOD for the protection of spermatozoa has been reported in several species (human: Kobayashi et al. 1991; rabbit: Holland et al. 1982; bull: Magnes and Li 1980).
The role of this enzyme has been studied also during oocyte in vitro maturation, fertilization and culture. The presence of SOD has been found to be beneficial during in vitro maturation and fertilization of porcine oocytes (Park et al. 1996), and it was also found to have beneficial effects on bovine embryonic development in vitro (Liu and Foote 1995). Moreover, several studies of mouse embryos (Noda et al. 1991; Umaoka et al. 1992; Chun et al. 1994) indicate that the two-cell block occurring in vitro could be alleviated by protection against oxidative stress mediated by SOD action. On the other hand, Luvoni et al. (1993) have observed that the addition of SOD from the time of oocyte collection to Day 8 of culture under 5% CO2 in air improved the cleavage rate of in vitro-inseminated bovine oocytes, although not during further stages of development.
In the present study, we compared the effect of SOD on acrosome integrity and viability rates of cryopreserved spermatozoa of European mouflon and on their potential to fertilize in vitro-matured (IVM) sheep oocytes using two different procedures: (i) intracytoplasmatic sperm injection (ICSI); and (ii) IVF.
Materials and methods
Reagents and media
When not specified, chemicals were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Source and preparation of spermatozoa
Semen was collected by artificial vagina from three adult mouflons during the breeding season and frozen in extender recommended for ram sperm. Fresh semen was diluted with a Tris-glucose–citric acid diluent (Evans 1988) containing 15% (v/v) egg yolk to a final concentration of 800 × 106 spermatozoa mL−1. The diluted semen was cooled to 4°C over a period of 2 h and a final dilution of up to 400 × 106 spermatozoa mL−1 was obtained by adding one volume of the same diluent supplemented with 10% glycerol (final concentration 5%) at the same temperature. Artificial insemination French straws (IMV, 0.25 mL) were filled with extended semen, frozen in liquid nitrogen (LN2) vapour (−70°C) for 7 min and held in LN2 tanks until required for use.
For assessment, we used a pool of semen obtained after thawing one straw of each mouflon semen. Frozen semen was thawed in a water bath (35°C) for 20 s and the contents of the three straws emptied into a conical tube containing 5 mL of warmed synthetic oviduct fluid supplemented with 50 μg mL−1 streptomycin and 50 IU mL−1 penicillin (SOF; Tervit et al. 1972) and 0.1% (w/v) polyvinyl alcohol (PVA).
Semen was centrifuged at 900g for 3 min and maintained at a constant temperature (35°C) to remove the freezing medium. The spermatozoa pellet was then resuspended at a concentration of 1 × 106 spermatozoa mL−1 in SOF and 0.6% (w/v) bovine serum albumin (BSA) and split between two culture systems: (i) without SOD; and (ii) in the presence of 1500 IU mL−1 SOD (Sigma S 5395; Sigma Chemical Co.). These two culture systems were used in all three experimental procedures.
Experiment 1. Assessment of motility, viability and acrosome integrity
Samples were evaluated for motility, viability and acrosome integrity of sperm immediately after thawing and after 3, 6 and 9 h of culture under standard conditions (5% CO2 in air, 39°C, 90% humidity).
Viability and acrosome integrity were assessed at the same time by incubating spermatozoa with two specific fluorochromes, as described by Naitana et al. (1998). The percentage of live spermatozoa was determined by incubating sperm cells with propidium iodide (PI; excluded from vital cells), and acrosome integrity was evaluated with fluorescein isothiocyanate-labelled Pisum sativum agglutinin (FITC-PSA), which is lecitin binding to glycoconjugates of the inner acrosome content.
The aliquots (100 μL) of sperm suspension, after the addition of 5 μL of a 0.1 mg mL−1 solution of FITC-PSA and 1.4 μL of a 1 mg mL−1 solution of PI, were incubated for 15 min at 39°C. To reduce background fluorescence, unbound FITC-PSA and PI were removed by adding 200 μL of phosphate-buffered saline (PBS) and by washing spermatozoa by centrifugation in a microcentrifuge for 2 min. The supernatant was aspirated and the pellet resuspended in 100 μL of PBS. After washing twice, in order to remove the two fluorochromes, a 10-μL sample was placed on a slide and coverslipped. For immobilization of sperm cells, the slide was dried immediately by incubating at 37°C for 10 min. To evaluate the stained sperm cells, at least 200 cells were counted in duplicate for each sample using a Diaphot epifluorescence microscope (Nikon, Tokyo, Japan).
Stained spermatozoa were classified according to the specific PI and FITC-PSA fluorescence exhibited. Dead sperm cells (plasmatic membrane-damaged spermatozoa) showed fluorescent red, whereas live spermatozoa did not show fluorescence. In addition, acrosome-damaged spermatozoa appeared green in the acrosomal region because the fluorescein isothiocyanate-labelled agglutinin of the P. sativum was able to gain access to the inner acrosome region and bind to the glycoconjugates of the inner acrosome content; whereas the spermatozoa with acrosome integrity showed no green fluorescence. Only viable sperm were evaluated for their acrosome status, considering that, in dead spermatozoa, acrosomal integrity, including cell membrane integrity, could be ‘damaged’ by other factors during the freezing and thawing procedures and thus mimic a physiological acrosome reaction (i.e. ice crystal formation (Watson 1995)).
Spermatozoa motility was evaluated by three independent operators observing semen samples under a stereomicroscope using a standard scoring system that graded degree of motility, (1 = immotile, 10 = maximum motility), which corresponded to the degree of motility that was observed at the moment of thawing (Woods and Garside 1996). All the procedures regarding the assessment of motility, viability and acrosome integrity, together with the previous spermatozoa preparation, were repeated three times under the same experimental conditions.
Experiment 2. In vitro fertilization
Recovery and maturation of oocytes
Oocytes used in this experiment were recovered from adult ovine ovaries collected at a local slaughterhouse and transported to the laboratory at 20–25°C within 1–2 h in Dulbecco’s PBS. Ovaries were dissected to isolate single follicles measuring between 2 and 7 mm in diameter. Only the cumulus oocyte complexes (COCs) that presented 4–10 layers of granulosa cells and a uniform cytoplasm were selected for this experiment. The COCs were allowed to mature in TCM 199 supplemented with 10% heat-treated fetal calf serum (FCS), 10 μL mL−1 of FSH/LH and 1 μg mL−1 estradiol. Culture conditions were 39°C, 5% CO2 in air, 2 mL of medium in 35 mm Petri dishes for 24 h.
At the end of the maturation period the COCs were denuded from the corona cells using glass micropipettes. The oocytes were then selected on the basis of the presence of the polar body and used for both fertilization systems (IVF and ICSI) in three replicated experiments performed under the same experimental conditions.
In vitro fertilization
The matured oocytes were divided into two groups and fertilized in vitro, as described by Walker et al. (1996) with some modifications. In vitro fertilization was performed in four-well Petri dishes (Nunclon; Nalge Nunc International, Kamstrup, Denmark) by depositing 20–30 oocytes into 500 μL of semen suspension (1 × 106 spermatozoa mL−1) derived from the two experimental culture systems (SOF and 0.6% (w/v) BSA), with or without SOD, as described earlier. Fertilization was performed at 39°C in an atmosphere containing 5% CO2 in air for 24 h.
After culture oocytes were placed on a microscope glass slide, cover-slipped and fixed in fixing solution (ethanol : acetic acid 3 : 1) at 4°C. After 48 h oocytes were stained with 1% lacmoid in fixing solution (Palomo et al. 1999) and assessed for pronuclei or decondensing sperm chromatin under an inverted microscope (Diaphot; Nikon).
Experiment 3. Intracytoplasmatic sperm injection
In vitro-matured oocytes, prepared as described earlier, were washed three times in handling medium (HEPES-buffered TCM 199 supplemented with 10% FCS) and then placed in the micromanipulation chamber. Spermatozoa derived from the two culture systems were placed in a 25-μL droplet of 9% polyvinyl pyrrolidone (PVP) with or without SOD after 1 h of culture under standard conditions. The spermatozoa from the culture system with SOD were placed in the droplet with SOD.
The micromanipulation chamber consisted of a 60-mm Petri dish containing several 50-μL drops of TCM 199 + PVA + HEPES containing five oocytes per drop arranged around a 25-μL central drop that contained spermatozoa. All drops were then covered with sterile mineral oil.
Intracytoplasmatic sperm injection was performed using a Leitz (Wetzlar, Heidelberg, Germany) micromanipulator and a Nikon (Tokyo, Japan) inverted microscope (Gomez et al. 1998). Injection and holding pipettes were made using a micropipette puller (Model P 87;Sutter Instruments, Novato, CA, USA) and a microforge (MD-900;Narishige Co., Setagaya-Ku, Tokyo, Japan). Sperm injection was carried out at room temperature. After ICSI, oocytes were cultured in SOF + 0.6% BSA under standard conditions for 24 h, as described earlier for IVF.
To evaluate the efficacy of ICSI, after 24 h of culture the oocytes were fixed and stained as described earlier in order to differentiate male from female pronuclei.
Statistical analysis
Analysis of viability and acrosome integrity of cryopreserved/thawed spermatozoa was repeated three times for each treatment. Significant effects of treatments and time were identified using two-way analysis of variance (ANOVA) for repeated measurements of arcsine-transformed data. ANOVA was followed by Fisher’s least significant multiple comparison test to identify any significant effects at each point, after first checking for normality. Motility data were obtained from observations made by three independent operators. Significant effects of treatments, times and operators were identified using two-way ANOVA for repeated measurements followed by Fisher’s least significant multiple comparison test. Mean results are presented ±SD.
The fertilization data obtained from the IVF and ICSI experimental procedures are expressed as a percentage of the treated oocytes used in each experiment. After arcsine transformation of the proportional data for penetreted oocytes (IVF) or for two pronuclei (ICSI), χ2-test was applied to assess statistical differences. Minitab 1.2 for Windows® was used to calculate statistical analysis.
Probability values of less than 0.05 were considered significant.
Results
Data analysis showed that SOD significantly influenced spermatozoa viability during the culture time (P < 0.01), as shown in Fig. 1. In the control medium, the percentages of live spermatozoa decreased progressively from 54 ± 4.6% to 4 ± 1.3% (mean ± SD) after 9 h of culture. In medium with SOD, its positive effects in maintaining sperm cell viability were evident after 3 h (P = 0.012), 6 h (P = 0.001) and 9 h (P = 0.001) of culture.
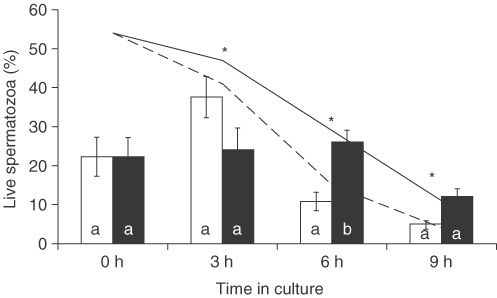
|
We also investigated the integrity of the acrosome membrane during these 9 h of culture, but only in viable spermatozoa. The results showed that the presence of SOD delays the onset of the acrosome reaction. In fact, after 6 h of culture the percentages of live spermatozoa that did not show an acrosome reaction were higher in medium with SOD than in medium alone (P = 0.05).
Results obtained from evaluation of sperm motility showed that SOD had a negative influence during the 9 h of culture (Table 1; P < 0.01). Spermatozoa motility decreased significantly in SOD-containing medium at 3 h (P = 0.024) and 6 h (P = 0.018) of culture compared with motility in control medium. No statistical difference was observed when operators were interpolated with times and treatments. These results seem to indicate that SOD has a detrimental effect on the maintenance of spermatozoa motility after thawing.

|
In experiments 2 and 3 we tested the fertilizing capacity of mouflon spermatozoa by IVF or ICSI using IVM ovine oocytes. Maturation rate in these experiments ranged between 85% and 91%, as is usually obtained in our laboratory. We selected mature oocytes on the basis of the presence of the polar body and used only these oocytes in both experiments. In all cases, final lacmoid staining confirmed that only MII oocytes were used. Data from the IVF system showed that the fertilization rates were significantly lower (P < 0.001) in medium with SOD than in medium alone, as shown in Table 2. Although we obtained 83.3% of penetrated oocytes in the control group, this percentage decreased significantly (44.1%; P < 0.001) after adding SOD to the culture medium. In both culture systems the rate of polyspermic oocytes was superimposable (P > 0.05).
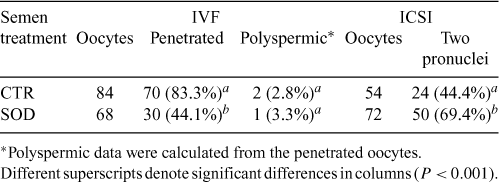
|
Conversely, in the ICSI system, the fertilization rates were significantly higher (P < 0.001) in the presence of SOD. The percentage of oocytes with two pronuclei was found to be significantly higher in medium with SOD than in medium alone (69.4% v. 44.4%; Table 2.).
Discussion
The results obtained in the present study demonstrate the effects of SOD on prolonging in vitro viability and on delaying the onset of acrosome reaction of cryopreserved European mouflon spermatozoa after thawing. This delay is essential for maintaining their functional properties as sperm membrane must remain undamaged to be capable of binding to the zona pellucida and of responding with the acrosome reaction to the appropriate signals of oocytes (Wassarman 1999).
Sperm viability can be supported for extended periods in an environment in which extracellular oxidative stress is minimized by reducing the oxygen tension or by the addition of antioxidant and chelating agents (Vishwanath and Shannon 1997). Superoxide dismutase, together with glutathione (GSH), is one of the most important scavenger systems that the spermatozoa have; it reduces the superoxide anion (O2 −) to hydrogen peroxide (H2O2). Mitochondrial SOD converts O2 − to H2O2, which is catalysed either by catalase or glutathione peroxidase to oxygen and water (Raineri et al. 2001). Spermatozoa have SOD activity (Aitken 1995), and Alvarez et al. (1987) have calculated that the activity of this enzyme can account for all of the H2O2 generated by these cells.
Miesel et al. (1993) have shown that the in vitro inhibition of SOD by diethyl dithiocarbamate causes the rapid oxidation of seminal plasma sulfhydryls, suggesting a pivotal role for SOD in maintaining the antioxidant defence system and in protecting spermatozoa against oxidant-induced injury. In fact, the addition of antioxidants to the culture medium during the liquid storage of ram semen delayed membrane destabilization of spermatozoa (Maxwell and Stojanov 1996). Using laparoscopic insemination in in vivo fertility tests, the same investigators showed that the presence of SOD increased the fertility rate.
Conversely, in the present study, a lower percentage of fertilization was obtained when SOD was added to the IVF medium. As already shown by other investigators (O’Flaherty et al. 1997; De Lamirande and Gagnon 1998; O’Flaherty et al. 1999), one of the most frequently studied effects of SOD is the delay of the onset of sperm capacitation and, therefore, of the physiological acrosome reaction.
De Lamirande and Gagnon (1998) have investigated the possibility that this process in human sperm involves oxido-reduction reactions of the sulfhydryl–disulfide pair and have shown that capacitation induced by some sulfhydryl-targeted agents was associated with increased sperm production of O2 −. Therefore, it is likely that SOD inhibits capacitation. The same results have been obtained by O’Flaherty et al. (1997) using frozen–thawed bull spermatozoa; they found that the addition of SOD or H2O2 to the incubation medium decreased the percentage of capacitated spermatozoa, thus supporting the theory that the presence of superoxide anions would be necessary for sperm capacitation.
The effect of SOD in preventing the capacitation process might explain why the fertilization rates decreased significantly in comparison with the control group in the present study’s system with SOD added. Although we could not find any specific references for the mouflon in this regard, other studies (Park et al. 1996; Luvoni et al. 1996) have shown that the addition of SOD during IVF has a detrimental effect on the fertilization rates in porcine and bovine species.
Park and colleagues (1996) have compared the effects of different antioxidants (catalase (CAT), SOD, mercapto-ethanol (ME)) during IVM and IVF of porcine oocytes. Superoxide dismutase was used at a concentration of 0.01, 0.1 or 1 mg mL−1 during both IVM and IVF; it did not influence nuclear maturation, but was found to inhibit penetration rates in a dose-dependent manner if added during IVF. The same result was obtained by Luvoni et al. (1996). They studied the effect of adding 1500 or 3000 IU mL−1 of SOD during IVM, IVF and IVC on bovine oocytes and presumptive zygotes. Their results showed a significant depressing effect of SOD (both 1500 and 3000 IU mL−1) on the percentage of fertilized oocytes during the insemination interval. The investigators suggested that the negative effect of SOD compromised the positive role of active oxygen species during fertilization. An alternative explanation might include toxicity of H2O2 (rather than superoxide radical), which reduces human sperm movement and the capacity of human sperm cells to react acrosomally and fuse with egg membrane (as reported by Aitken 1995).
Other investigators have demonstrated that H2O2, while not affecting sperm viability, causes a loss in sperm motility in egg yolk tris extender (Bilodeau et al. 2001). A ROS such as H2O2 has been shown to decrease sperm motility in various species, such as mouse, human, bull and rabbit (Alvarez and Storey 1982; O’Flaherty et al. 1997). Because SOD catalyses the reaction O2 − to H2O2, it improves the rate at which H2O2 is produced and, therefore, accelerates the rate at which sperm motility is lost (Aitken 1995).
Results of the present study seem to confirm these data because in the study’s culture system we observed a decrease in sperm motility when SOD was added. Therefore, the lower percentage of fertilization that we reported in the IVF system in the presence of SOD could also be a result of its detrimental influence on sperm motility, as the fertilizing capacity of spermatozoa has been shown to be related to sperm motility (Yanagimachi 1981).
In any case, SOD action during IVF is not completely understood. Other reports have shown that the addition of an antioxidant (SOD or GSH) during IVF of oocytes did not influence sperm penetration (Blondin et al. 1997) but increased pronucleus formation and blastocyst production (Park et al. 1997; Earl et al. 1997).
In the ICSI system, on the other hand, the pronucleus formation rates were significantly higher in the presence of SOD (69.4% v. 44.4% in the absence of SOD). Other investigators have demonstrated that, in pigs, the supplementation of media with SOD during IVF enhanced pronucleus formation after oocyte penetration by spermatozoa (Park et al. 1997). In their experiments, IVF was performed after adding different SOD concentrations (0, 1, 10, 100, 1000 units mL−1) and then sperm–oocytes were cultured in fertilization medium with (1 unit mL−1) or without SOD after insemination. Park and colleagues (1997) did not find any differences in penetration rates with or without SOD supplementation, but pronuclear formation rates were higher in medium with SOD. They have suggested that pronuclear formation under physiological conditions is protected from oxidative stress in SOD-rich oviduct fluid. The effectiveness of SOD on pronucleus formation in mouse pronuclear stage embryos has also been suggested by Noda et al. (1991), who demonstrated that SOD attenuates the two-cell block in mouse embryos cultured in vitro when added to culture medium, allowing the embryos to undergo cleavage past the two-cell block.
The positive role played by the antioxidant compounds after spermatozoa oocyte penetration has been described by many investigators. Perreault and Zuelke (1996) have shown that diamide-induced oxidative stress inhibits sperm nucleus decondensation and disrupts spindle microtubules, thus providing evidence that the sperm cell scavenger systems play a role in meiotic spindle organization and pronuclear development. In addition, the reduction of disulfide bonds in the sperm after incorporation is important for the formation of the male pronucleus, as well as for the disassembly of the sperm tail-connecting piece and pronuclear apposition (Sutovsky and Schatten 1997).
The different results in the fertilization rates obtained from the IVF and ICSI techniques in media with SOD can be explained by its effect on sperm cell capacitation. Intra-cytoplasmatic sperm injection seems to be influenced less by capacitation status as spermatozoa are injected directly into the cytoplasm of oocytes. It has been reported that it is not necessary to induce membrane changes or a physiological acrosome reaction before ICSI in sheep in order to improve fertilization rates (Gomez et al. 1997). The results of the present study seem to indicate that membrane modifications, such as capacitation, are not required in mouflon spermatozoa before ICSI. In fact, in the present study, higher fertilization rates were obtained in medium with SOD.
In conclusion, the study demonstrates that the addition of SOD to the culture media can definitely enhance viability rates as well as maintaining the acrosome integrity of cryopreserved mouflon spermatozoa after thawing, but its beneficial effects in IVF programmes are advantageous only under specific conditions.
Acknowledgments
This study was supported by MURST special project Cofinlab.
Abu-Erreish, G. , Magnes, L. , and Li, T. K. (1978). lIsolation and properties of superoxide dismutase from ram spermatozoa and erytrocytes Biol. Reprod. 18, 554–60.
| PubMed |

Aitken, R. J. (1995). Free radicals, lipid peroxidation and sperm function Reprod. Fertil. Dev. 7, 659–68.

Alvarez, J. G. , and Storey, B. T. (1982). Spontaneous lipid peroxidation in rabbit epididymal spermatozoa: its effect on sperm motility Biol. Reprod. 27, 1102–8.
| PubMed |

Alvarez, J. G. , and Storey, B. T. (1992). Evidence for increased lipid peroxidative damage and loss of superoxide dismutase activity as a mode of sublethal cryodamage to human sperm during crypreservation J. Androl. 13, 232–41.
| PubMed |

Alvarez, J. G. , Touchstone, J. C. , Blasco, L. , and Storey, B. T. (1987). Spontaneous lipid peroxidation and production of hydrogen peroxide and superoxide in human spermatozoa. Superoxide dismutase as major enzyme protectant against oxygen toxicity J. Androl. 8, 338–48.
| PubMed |

Ballou, J. D. (1992). Potential contribution of cryopreserved germ plasma to the preservation of genetic diversity and conservation of endangered species in captivity Cryobiology 29, 19–25.
| PubMed |

Bilodeau, J. F. , Chatterjee, S. , Sirard, M. A. , and Gagnon, C. (2000). Levels of antioxidant defenses are decreased in bovine spermatozoa after a cycle of freezing and thawing Mol. Reprod. Dev. 55, 282–8.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Bilodeau, J. F. , Blanchette, S. , Gagnon, C. , and Sirard, M. A. (2001). Thiols prevent H2O2-mediated loss of sperm motility in cryo-preserved bull semen Theriogenology 56, 275–86.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Blondin, P. , Coenen, K. , and Sirard, M. A. (1997). The impact of reactive oxygen species on bovine sperm fertilizing ability and oocyte maturation J. Androl. 18, 454–60.
| PubMed |

Chun, Y. S. , Kim, J. H. , Lee, H. T. , and Chung, K. S. (1994). Effect of superoxide dismutase on the development of preimplantation mouse embryos Theriogenology 41, 511–20.
| Crossref | GoogleScholarGoogle Scholar |

Cugnasse, J. M. (1994). Revision taxonomique des mouflons des iles mediterraneennes Mammalia 58, 507–12.(in French)

De Lamirande, E. , and Gagnon, C. (1998). Paradoxical effect of reagents for sulfhydryl and disulfide groups on human sperm capacitation and superoxide production Free Radic. Biol. Med. 25, 803–17.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Drobnis, E. Z. , Crowe, L. M. , Berger, T. , Anchordoguy, T. J. , Overstreet, J. W. , and Crowe, F. (1993). Cold shock damage is due to lipid phase transitions in cell membranes: a demonstration using sperm as a model J. Exp. Zool. 265(4), 432–7.
| PubMed |

Earl, C. R. , Kelly, J. , Rowe, J. , and Armstrong, D. T. (1997). Glutathione treatment of bovine sperm enhances in vitro blastocysts production rates Theriogenology 47, 255.
| Crossref | GoogleScholarGoogle Scholar |

Evans, G. (1988). Current topics in artificial insemination of sheep Aust. J. Biol. Sci. 41, 103–16.
| PubMed |

Gillan, L. , and Maxwell, W. M. (1999). The functional integrity and fate of cryopreserved ram spermatozoa in the female tract J. Reprod. Fertil. Suppl. 54, 271–83.
| PubMed |

Gomez, M. C. , Catt, J. W. , Gillan, L. , Evans, G. , and Maxwell, W. M. C. (1997). Effect of culture, incubation and acrosome reaction of fresh and frozen-thawed ram spermatozoa for in vitro fertilization and intracytoplasmic sperm injection Reprod. Fertil. Dev. 9, 665–73.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Gomez, M. C. , Catt, J. W. , Evans, G. , and Maxwell, W. M. (1998). Cleavage development and competence of sheep embryos fertilized by intracytoplasmic sperm injection and in vitro fertilization Theriogenology 49, 1143–54.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Hiendleder, S. , Mainz, K. , Plante, Y. , and Lewalski, H. (1998). Analysis of mitochondrial DNA indicates that domestic sheep are derived from two different ancestral maternal sources: no evidence for contributions from Urial and Argali sheep J. Heredity 89, 113–20.
| Crossref | GoogleScholarGoogle Scholar |

Holland, M. K. , Alvarez, J. G. , and Storey, B. T. (1982). Production of superoxide and activity of superoxide dismutase in rabbit epididymal spermatozoa Biol. Reprod. 27, 1109–18.
| PubMed |

Kobayashi, T. , Miyazaki, T. , Natori, M. , and Nozawa, S. (1991). Protective of superoxide dismutase in human sperm motility: superoxide dismutase activity and lipid peroxide in human seminal plasma spermatozoa Hum. Reprod. 6, 987–91.
| PubMed |

Liu, Z. , and Foote, R. H. (1995). Development of bovine embryos in KSOM with added superoxide dismutase and taurine and with 5 and 20% O2 Biol. Reprod. 53, 786–90.
| PubMed |

Luvoni, G. C., Parravicini, E., Gandolfi, F., and Lauria, A. (1993). Oxidative stress and in vitro bovine embryogenesis: preliminary findings on the use of superoxide dismutase (SOD) In pp. 195–9. (Grafiche Scuderi s.a.s.: Messina, Italy.)
Luvoni, G. C. , Keskintepe, L. , and Brackett, B. G. (1996). Improvement in bovine embryo production in vitro by glutathione-containing culture media Mol. Reprod. Dev. 43, 437–43.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Magnes, L. J. , and Li, T. K. (1980). Isolation and properties of superoxide dismutase from bovine spermatozoa Biol. Reprod. 22, 965–9.
| PubMed |

Maxwell, W. M. , and Stojanov, T. (1996). Liquid storage of ram semen in the absence or presence of some antioxidant Reprod. Fertil. Dev. 8, 1013–20.
| PubMed |

Mazzili, F. , Rossi, T. , Sabatini, L. , Pulcinelli, F. M. , Rapone, S. , Pondero, F. , and Gazzaniga, P. P. (1995). Human spem cryopreservation and reactive oxygen species (ROS) production Acta Eur. Fertil. 26, 145–8.
| PubMed |

Miesel, R. , Drzejczak, P. J. , and Kurpisz, M. (1993). Oxidative stress during the interaction of gametes Biol Reprod 49, 918–23.
| PubMed |

Naitana, S. , Ledda, S. , Cocco, E. , Manca, L. , and Masala, B. (1990). Haemoglobin phenotypes of the wild European mouflon sheep living in the island of Sardinia Anim. Genetics 22, 67–75.

Naitana, S. , Ledda, S. , Leoni, G. , Bogliolo, L. , Loi, P. , and Cappai, P. (1998). Membrane integrity and fertilizing potential of cryopreserved spermatozoa in European mouflon Anim. Reprod. Sci. 52, 105–12.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Noda, Y. , Matsumoto, H. , Umaoka, Y. , Tatsumi, K. , Kishi, J. , and Mori, T. (1991). Involvement of superoxide radicals in the mouse two-cell block Mol. Reprod. Dev. 28, 356–60.
| PubMed |

O’Flaherty, C. , Beconi, M. , and Beorlegui, N. (1997). Effect of natural antioxidant, superoxide dismutase and hydrogen peroxide on capacitation of frozen-thawed bull spermatozoa Andrologia 29, 269–75.
| PubMed |

O’Flaherty, C. M. , Beorlegui, N. B. , and Beconi, M. T. (1999). Reactive oxygen species requirements for bovine sperm capacitation and acrosome reaction Theriogenology 52, 289–301.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Palomo, M. J. , Izquierdo, D. , Mogas, T. , and Paramio, M. T. (1999). Effect of semen preparation on IVF of prepubertal goat oocytes Theriogenology 51, 927–40.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Park, C. K. , Roy, F. , and Sirard, M. A. (1996). The effect of free radicals and anti-oxidant during in vitro maturation and fertilization of porcine oocytes (abstract) Theriogenology 45, 275.
| Crossref | GoogleScholarGoogle Scholar |

Park, C. K. , Lee, J. H. , Cheong, H. T. , Yang, B. K. , and Kim, C. I. (1997). Effect of superoxide dismutase (SOD) on pronucleus formation of porcine oocytes fertilized in vitro Theriogenology 48, 1137–46.
| Crossref | GoogleScholarGoogle Scholar |

Perrault, S. D. , and Zuelke, K. A. (1996). Diamide induced oxidative stress disrupts fertilization of mature hamster (Mesocricetus auratus) oocytes Theriogenology 45, 260.(Abstract.)
| Crossref | GoogleScholarGoogle Scholar |

Ptak, G. , Clinton, M. , Barboni, B. , Muzzeddu, M. , Cappai, P. , Tischner, M. , and Lio, P. (2002). Preservation of the wild European mouflon: the first example of genetic management using a complete program of reproductive biotechnologies Biol. Reprod. 66, 796–801.
| PubMed |

Raineri, I. , Carlson, E. J. , Gacayan, R. , Carra, S. , Oberley, T. D. , Huang, T. T. , and Epstein, C. G. (2001). Strain-dependent high-level expression of a transgene for manganese superoxide dismutase is associated with growth retardation and decreased fertility Free Radic. Biol. Med. 31, 1018–30.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Sutovsky, P. , and Schatten, G. (1997). Depletion of glutathione during bovine oocyte maturation reversibly blocks the decondensation of the male pronucleus and pronuclear apposition during fertilization Biol. Reprod. 56, 1503–12.
| PubMed |

Tervit, H. R. , Whittingham, D. G. , and Rowson, L. E. A. (1972). Successful culture in vitro of sheep and cattle ova J. Reprod. Fertil. 30, 487–93.

Umaoka, Y. , Noda, Y. , Narimoto, K. , and Mori, T. (1992). Effects of oxygen toxicity on early development of mouse embryos Mol. Reprod. Dev. 31, 28–33.
| PubMed |

Vishwanath, R. , and Shannon, P. (1997). Do sperm cell age? A review of the physiological changes in sperm during storage at ambient temperature Reprod. Fertil. Dev. 9, 321–31.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Walker, S. K. , Hill, J. L. , Kleemann, D. O. , and Nancarrow, C. D. (1996). Development of ovine embryos in synthetic oviductal fluid containing amino acids at oviductal fluid concentrations Biol. Reprod. 55, 703–8.
| PubMed |

Wassarman, P. M. (1999). Zona pellucida glycoprotein mZP3: a versatile player during mammalian fertilization J. Reprod. Fertil. 116, 211–16.
| PubMed |

Watson, P. F. (1995). Recent developments and concepts in the cryo-preservation of spermatozoa and the assessment of their post-thawing function Reprod. Fertil. Dev. 7, 871–91.
| PubMed |

Windsor, D. P. (1997). Mitochondrial function and ram sperm fertility Reprod. Fertil. Dev. 9, 279–84.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Woods, J. , and Garside, D. A. (1996). An in vivo and in vitro investigation into the effects of alpha-chlorohydrin on sperm motility and correlation with fertility in the Han Wistar rat Reprod. Toxicol. 10, 199–207.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Yanagimachi, R. (1981). Mechanisms of fertilization in mammals In


