Mammalian diversity: gametes, embryos and reproduction
Richard R. Behringer A D , Guy S. Eakin A C and Marilyn B. Renfree BA Department of Molecular Genetics, University of Texas M. D. Anderson Cancer Center, Houston, TX 77030, USA.
B Department of Zoology, University of Melbourne, Vic. 3010, Australia.
C Present address: Program in Developmental Biology, Memorial Sloan-Kettering Cancer Center, New York, NY 10021, USA.
D Corresponding author. Email: rrb@mdanderson.org
Reproduction, Fertility and Development 18(2) 99-107 https://doi.org/10.1071/RD05137
Submitted: 14 October 2005 Accepted: 14 October 2005 Published: 14 December 2006
Abstract
The class Mammalia is composed of approximately 4800 extant species. These mammalian species are divided into three subclasses that include the monotremes, marsupials and eutherians. Monotremes are remarkable because these mammals are born from eggs laid outside of the mother’s body. Marsupial mammals have relatively short gestation periods and give birth to highly altricial young that continue a significant amount of ‘fetal’ development after birth, supported by a highly sophisticated lactation. Less than 10% of mammalian species are monotremes or marsupials, so the great majority of mammals are grouped into the subclass Eutheria, including mouse and human. Mammals exhibit great variety in morphology, physiology and reproduction. In the present article, we highlight some of this remarkable diversity relative to the mouse, one of the most widely used mammalian model organisms, and human. This diversity creates challenges and opportunities for gamete and embryo collection, culture and transfer technologies.
Introduction
Mammals comprise a fascinating class of animals that exhibit tremendous morphological, physiological and reproductive diversity. They live on land, fly in the air and swim in the sea. There are 4809 different species of mammals currently in existence (Nowak 1999). However, more than 60% of all mammalian species derive from only two orders. There are 2059 species in the order Rodentia (squirrels, chipmunks, gophers, mice, rats, beaver, porcupines and others) and 977 species in the order Chiroptera (bats; Nowak 1999). Thus, >40% of mammalian species are some type of rodent. Likewise, one in five mammalian species is a bat. Nevertheless, even within these two orders there is tremendous diversity. Consider rodents like flying squirrels that live in and glide between trees, the desert jerboa (Jaculus jaculus), with its long legs, that hops like a kangaroo and the quills of the American porcupine (Erethizon dorsatum). Likewise, consider flying foxes, large bats that eat fruit and vampire bats that feed on blood.
Mammals range in size from those that are very small to the largest animal known to have existed. The smallest mammal is believed to be Thailand’s Kitti’s hog-nosed bat (Craseonycteris thonglongyai), which has a head and body length of approximately 3 cm and weighs 1.5–2.0 g. Other tiny mammals include the smallest marsupials, desert-living dasyurids, the Ningauis (Fig. 1a), weighing approximately 2 g, and the Etruscan shrew (Suncus estruscus), an insectivore, weighing 1.5–2.5 g. The largest mammal (and animal) is the blue whale (Balaenoptera musculus), which can be up to 27 m long and weigh 150 tons. On land, the largest mammal is the African elephant (Loxodonta africanus), which can be 3.2 m tall at the shoulder and weigh 5.5 tons.

|
Monotreme, marsupial and eutherian mammals can be distinguished by a variety of characteristics, but it is their different modes of reproduction that most clearly lead to their classification. The monotremes include the platypus (Ornithorhynchus anatinus) and two species of echidnas (Tachyglossus aculeatus and Zaglossus bruijni; Fig. 1b). Monotremes are exceptional because their young are born from eggs laid and incubated outside of the mother’s body (Griffiths 1978, 1984). These eggs have a pliable, leathery shell that is 16.5 × 15 mm for the platypus and 14–15 mm in diameter for the echidnas (Flynn and Hill 1947). When the monotreme egg is laid, it already contains an embryo with many pairs of somites. In contrast with the monotremes, all marsupial and eutherian mammals are viviparous; they give birth to live young. Marsupials include familiar animals like kangaroos, koalas and opossums, but also many others, such as dasyurids (marsupial ‘mice’ and ‘cats’), bandicoots and wombats (Fig. 1c). The gestation periods of marsupials are short relative to the length of lactation and they give birth to highly immature (or altricial) young that finish a significant amount of their development after birth (Tyndale-Biscoe and Renfree 1987; Fig. 1d). These marsupial neonates can be considered ‘fetal’ relative to their eutherian counterparts. Thus, marsupial reproduction puts little investment into gestation, but a significant amount of energy into postnatal nurturing in the form of lactation: they have exchanged the placenta for the teat (Renfree 1980a). In most cases, this postnatal nurturing occurs with the young initially attached to a teat in a pouch or marsupium located on the ventral side of the mother’s body. However, not all marsupial species have pouches. In pouch-less marsupials, the young are also initially attached to the mother’s teats, but are directly exposed to the surrounding environment. To cope with this ‘premature’ birth, the neonates of marsupials are also precocial for certain aspects of their development. For example, they have sufficient neuromuscular development in the arms and shoulder girdle to move unaided by the mother to the teat. They can also breathe and digest milk.
Eutherian mammals comprise most mammalian species (i.e. rodents and bats), including agriculturally important livestock and humans (Fig. 1e,f). Eutherian mammals are commonly referred to as ‘placental’ mammals, a misnomer because marsupials also have placentas (see below). Eutherian mammals give birth to progeny exhibiting varying amounts of altricial or precocial development. For example, the laboratory mouse gives birth to relatively altricial pups that lack hair, have their eyes and ears closed and are unable to move in a coordinated manner. In contrast, whales give birth to a calf that must immediately be able to swim.
Herein, we highlight some of the diversity found in mammalian reproduction. This diversity is found in gametes, embryos, female reproductive tracts, placentas and gestation. Describing morphological and physiological differences and determining the mechanisms that diversify these animals should lead to useful insights for embryo transfer technologies. This can only be achieved by studying many types of mammals, not just a restricted number of standard models.
Gamete diversity
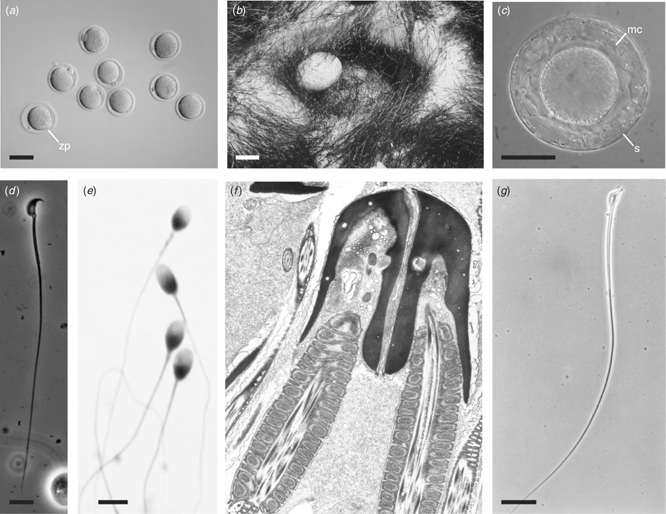
Oocytes and sperm are recognisable as such in different mammalian species, but there are many interesting variations in gametes between various types of mammals. The ovulated mouse oocyte is approximately 80 μm in diameter with a microscopically clear ooplasm (Fig. 2a). The oocyte is surrounded by the zone pellucida. The ovulated human oocyte has essentially the same appearance, except that it is somewhat bigger at approximately 120 μm (Gosden and Bownes 1995). Monotreme oocytes are, in contrast, 3–4 mm in diameter when ovulated, but the eggs with the shells are 15 mm when they are laid (Griffiths 1968, 1978; Fig. 2b). At fertilisation, a monotreme oocyte that is approximately 4000 μm in diameter is 50-fold larger in diameter and approximately 125 000-fold the volume of a mouse oocyte. Cleavage is meroblastic, like the chicken. Interestingly, the echidna egg at laying already has an embryo that has developed 19 pairs of somites (Griffiths 1968, 1978). Thus, in the echidna, a substantial amount of embryonic development occurs in utero nourished by an allantoic and vitelline placenta (Griffiths 1978, 1984). Once the monotreme egg is laid, the embryo completes the balance of its development outside the mother.
The marsupial oocyte at fertilisation is generally larger than eutherian counterparts. For example, the tammar wallaby (Macropus eugenii) oocyte is 120 μm in diameter in the follicle (Tyndale-Biscoe and Renfree 1987). After ovulation, a thick mucoid coat and outer shell coat is added to the zona pellucida as the oocyte transits through the oviduct into the uterus (Fig. 2c). In eutherians, horses, rabbits and the elephant shrews (Elephantulus) all have mucoid coats surrounding their oocytes (Denker 2000). The shell of the marsupial oocyte has a consistency like cellophane. Recently, a gene encoding a shell coat protein has been cloned from the brushtail possum (Trichosurus vulpecula; Cui and Selwood 2003). These proteins are being considered as targets for marsupial immunocontraception for non-indigenous pest species (Roberts et al. 1997). A unique type of oocyte develops in dasyurids (Selwood 2000). Like the wallaby and other marsupials, there is a zona pellucida, thick mucoid coat and shell. However, inside the oocyte is a large yolk mass that is surrounded by an actin and microtubule network (Merry et al. 1995). After fertilisation, the yolk mass is expelled at cleavage as the two blastomeres are formed.
Mouse spermatozoa are approximately 120 μm long with a characteristically shaped head that curves and has a pointed tip (Fig. 2d). The length of time for a spermatogonium to produce functional spermatozoa is 35 days (Clermont 1972). Reliable protocols to routinely freeze and thaw functional mouse sperm have only recently been devised (Tada et al. 1990). However, cryopreservation of sperm from some strains of laboratory mice is still not efficient. Human spermatozoa are half the length of those of mice, being approximately 60 μm long. In contrast with the mouse, human sperm heads have a bulbous shape (Fig. 2e). In human, it takes 74 days to generate functional sperm from spermatogonia (Clermont 1972). Techniques to routinely freeze and thaw functional human sperm have been in existence for over 50 years (Bunge and Sherman 1953). The success with human sperm was presaged by studies in livestock. Cryopreservation of sperm from chicken had been reported a few years earlier by Polge et al. (1949). Cryopreservation of the sperm from cattle followed soon after (Stewart 1951). Although there are differences between the sperm of mouse, livestock and human, they fulfill our expectations of what spermatozoa should look like and how they should behave. Now consider the odd behaviour of the spermatozoa of American marsupials. In all but one of the American marsupial species, when the spermatozoa leave the testes, they form pairs in the epididymis, coupling as a unit by an intimate association of the sperm heads (Fig. 2f; Biggers and DeLamater 1965; Temple-Smith and Bedford 1980; Tyndale-Biscoe and Renfree 1987). In the oviduct, these sperm pairs uncouple before fertilisation. It has been shown that sperm pairs have a significantly higher motility in viscous media compared with single spermatozoa (Moore and Taggart 1995). Another unique distinction possessed by a marsupial is found in the honey possum (Tarsipes rostratus), with the longest (360 μm) spermatozoa described for any mammal (Fig. 2g; Russell and Renfree 1989).
Embryo variation before implantation
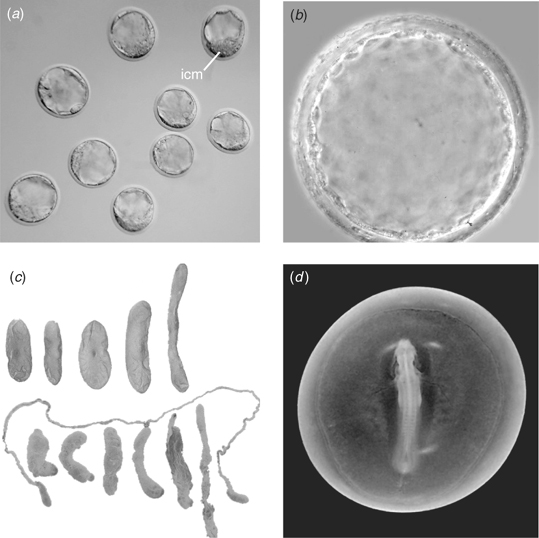
The blastocyst that is so familiar to mouse and human embryologists is composed of an outer layer of trophoblast cells and a clump of cells inside the trophoblast layer located on one side of the embryo called the inner cell mass (ICM; Fig. 3a). In mouse and human, the ICM is the source of embryonic stem cells (Evans and Kaufman 1981; Martin 1981; Thomson et al. 1998). However, a blastocyst with an ICM is not the only type of preimplantation embryo found in mammals. Marsupials form a unilaminar blastocyst (i.e. a blastocyst lacking an ICM; Fig. 3b; Tyndale-Biscoe and Renfree 1987). Subsequently, endodermal cells form beneath the outer cell layer to create an inner cell layer, producing a bilaminar blastocyst. A distinct region called the medullary plate forms at one region on the surface of the blastocyst and, subsequently, a primitive streak is initiated (Renfree 1994). The mechanisms leading to the formation of the medullary plate in the marsupial blastocyst are still unknown.
|
Marsupial blastocyst-stage embryos (i.e. those surrounded by a zona, mucoid coat and shell) have been transferred successfully into the uterus and allowed to develop until late gestation in the quokka (Setonix brachyurus) and in the tammar wallaby, but these experiments were not designed to achieve birth (Tyndale-Biscoe 1963, 1970; Renfree and Tyndale-Biscoe 1973). Thus, a live marsupial has yet to be born as a result of embryo transfer. Embryo transfer in the marsupial poses some interesting challenges. For example, in vitro fertilisation of oocytes would yield a zygote lacking a mucoid coat and shell. Thus, transfer of these types of embryos into the oviduct or upper uterus would seem appropriate to acquire these additional investments. It is not known whether a marsupial embryo lacking these two investments could develop normally if transferred directly into the uterus. However, there is evidence that some shell coat is deposited by the uterus (Shaw 1996). In addition, if an American marsupial is used as a sperm donor, then methods must be devised to deal with sperm pairs if isolated from the cauda epididymis.
Another interesting aspect of preimplantation development in mammals is found in cows, sheep, goats, pigs and others. After forming a blastocyst with an ICM, these embryos undergo a very dramatic morphological change, elongating extensively. In the pig, these filamentous preimplantation embryos can elongate up to 1 m, although their association with the uterus ultimately compacts them to approximately 10–15 cm. Yet, these embryos are predominantly trophoblast with a very small embryonic disc (Fig. 3c). Another interesting preimplantation mammalian embryo again is the marsupial. For example, the tammar wallaby develops a full neural axis, heart and many pairs of somites while still free-floating in the uterus (Fig. 3d). Ultimately, the marsupial embryo attaches to the uterine endometrium, quickly goes through limited organogenesis and is born. Thus, preimplantation-stage mammalian embryos are not limited to blastocysts, but can progress to significant stages of embryonic development.
Female reproductive tract and placental diversity
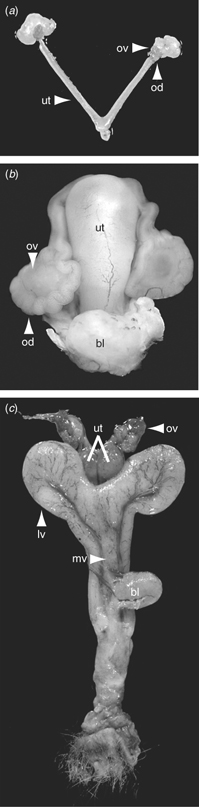
There are significant differences in the development and morphology of the female reproductive tracts of mammals (Renfree 1993; Kobayashi and Behringer 2003). Herein, we focus on three types of female reproductive tracts, exemplified by two eutherians (a rodent and a bat) and marsupials. The mouse has a bicornuate uterus (i.e. two uterine horns with single vagina; Fig. 4a). Oocytes ovulated simultaneously from both ovaries transit the oviduct, the site of fertilisation, to enter each uterine horn, which can accommodate multiple conceptuses. Surgical embryo transfer methods into the oviduct and uterus are routine in this species. The short-tailed fruit bat (Carollia perspicillata) has a simplex uterus with a single cervix and a single vagina, like human (Fig. 4b). Carollia is monovular, giving rise to a singleton, although in at least one case a twin embryo was observed (Rasweiler and Badwaik 1996; R. R. Behringer, C. J. Cretekos and J. J. Rasweiler, unpublished observations). The female marsupial reproductive tract appears quite foreign to researchers familiar with rodents or other types of eutherian species. The female reproductive tracts of the tammar wallaby and the Virginia opossum (Didelphis virginiana) exemplify the monovular and polyovular marsupial species, respectively. There are two completely separate uteri with two lateral vaginae and a median vagina (a transient structure in all but the kangaroos, wallabies and honey possum) that serves as the birth canal (Renfree 1993; Fig. 4c). The vaginae open into the urogenital sinus. In the wallaby, ovulation occurs from alternate ovaries; a single oocyte is ovulated and transits through the oviduct to enter one of the uteri and, if fertilised, continues development to term (Tyndale-Biscoe and Renfree 1987; Renfree 1994). In the Virginia opossum, up to 20–30 ovulations occur from the two ovaries, despite the fact that there are only 15 or so teats in the pouch to nurture the young after birth, so there is a large postnatal mortality (Tyndale-Biscoe and Renfree 1987).
|
There is also tremendous variation in placental structure in mammals and the reader is referred to Mossman (1987) and Comparative Placentation (http://medicine.ucsd.edu/cpa, accessed 1 October 2005). The mammalian placenta is a transient organ that mediates exchange between the mother and the fetus through juxtaposition or fusion of the fetal membranes to the lining of the uterus (Mossman 1937). The term ‘placenta’ is commonly used loosely to mean specifically the chorioallantoic placenta. Most marsupial species have a choriovitelline (yolk sac) placenta rather than a chorioallantoic placenta. However, there are exceptions. A true chorioallantoic placenta has been described in marsupials for four species of bandicoots, as well as a mildly invasive connection of the allantois to the chorion in didelphids, dasyurids and vombatids (Tyndale-Biscoe and Renfree 1987; Freyer et al. 2003). A choriovitelline placenta is also important during early gestation for eutherian mammals. It serves as a site for physiological exchange before the chorioallantois forms but, in many species, remains functional later in pregnancy (Renfree 1982).
Gestation: days, months, years
There is a tremendous range in the length of gestation between mammalian species. Gestation in the mouse is approximately 20 days. The first 4 days (20% of gestation) result in the generation of the blastocyst, which is 0.1–0.2 mm in diameter (Kaufman 1992). Gastrulation occurs on Day 7 (approximately one-third of the way through gestation), with the balance of gestation allocated to organogenesis and growth. Most would consider this to be a relatively short length of gestation. Depending upon the strain and size of the litter, newborn mice weigh 1–1.5 g. Thus, for a female mouse weighing 20 g, giving birth to six pups represents approximately 25% of the mother’s initial bodyweight. In humans, gestation is generally 37–42 weeks. The blastocyst (Carnegie Stage 3) is 0.1–0.2 mm in diameter and is formed at 4 days (approximately 1.5% of gestation). Gastrulation initiates at Stage 6, which is evident on Day 13 (approximately 5% of gestation; O’Rahilly and Müller 1987). Closure of the anterior neural tube is complete at Stage 12 (26 days or approximately 10% of gestation). In contrast, completion of closure of the anterior neural tube in the mouse occurs on Day 10 (50%) of gestation.
Now consider gestation in other eutherian mammals. Carollia, the short-tailed fruit bat, weighs somewhat less than the laboratory mouse (approximately 19 v. 20–25 g) and gives birth to a singleton after a gestation of 113–120 days (Rasweiler and Badwaik 1997). Blastocyst formation occurs at Stage 3 at 10–12 days of gestation and anterior neural tube closure occurs at Stage 11 sometime before 40 days of gestation (De Bonilla and Rasweiler 1974; Cretekos et al. 2005). Thus, small mammals can have relatively long gestation periods. In the case of Carollia, the neonate is born with fur, eyes and ears open, and must be able to hold onto the mother, who will fly with her pup. The gestation of the blue whale (Balaenoptera musculus) is 11–12 months, only a few months longer than that of human, but it yields a single calf that is 7.32 m long and the gestation of the African elephant (Loxodonta africana) is 22–24 months whose single calf weighs 100 kg (Boitani and Bartoli 1982).
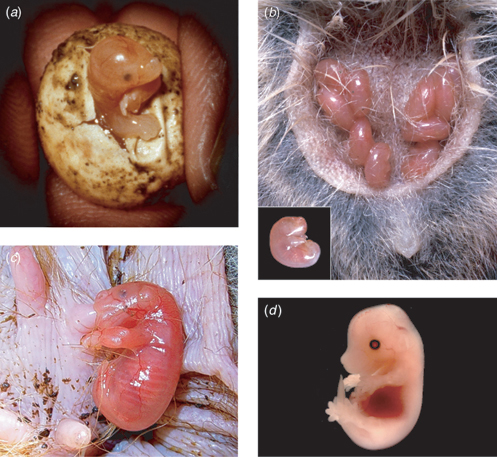
In monotremes, gestation results in the production of a somite-stage embryo that is subsequently encapsulated by a shell and laid. The mother must incubate the egg until hatching (Fig. 5a). As mentioned above, marsupial mammals invest much less energy in gestation. Much of marsupial gestation is used to generate the blastocyst. Once gastrulation occurs, there is a brief period of organogenesis followed by birth (Fig. 5b). The approximately 5 kg female tammar wallaby gives birth after a gestation period of 26–27 days to a single pouch young, approximately 16 mm in length and weighing approximately 440 mg (Fig. 5c). The newborn pouch young are roughly similar in developmental stage to a 15-day-old mouse embryo (Fig. 5d) or 40-day-old human embryo (Tyndale-Biscoe and Renfree 1987). The initial blastocyst stage is reached at 7 days (~25%) of gestation and closure of the anterior neural tube occurs by 21 days (~80%) of gestation (Tyndale-Biscoe and Renfree 1987). After birth, there is a 9- to 10-month period of lactation. As mentioned above, neonatal marsupials are born altricial with no fur and undifferentiated eyes and ears. The entire posterior region of the neonate is also highly immature, with poorly formed hindlimbs, undifferentiated gonads, functional mesonephroi and non-functional metanephroi that are still maturing. Yet, organ systems critical for neonatal survival are sufficiently mature at birth for movement to the teat and the ability to breathe, suckle and digest food. For comparison with other marsupials, the honey possum, with a head to tail length of approximately 80 mm and a bodyweight of 7–10 g, gives birth after a gestation estimated to be 21–28 days to pouch young that weigh less than 5 mg (Renfree 1980b; Russell and Renfree 1989). The stripe-faced dunnart (Sminthopsis macroura) has a gestation period of 10.7 days, giving birth to between one and eight pups that weigh approximately 10 mg (Godfrey 1969; Selwood and Woolley 1991). The laboratory opossum (Monodelphis domestica) has a gestation of 13–14 days, giving birth to eight to 12 pups that weigh 100 mg (Baggott et al. 1987; Mate et al. 1994). The genome of Monodelphis has now been sequenced and assembled (http://www.ensembl.org, accessed 1 October 2005) and the genome of the tammar wallaby is currently being sequenced (http://kangaroo.genomics.org.au/public, accessed 1 October 2005), promising to reveal new insights into mammalian biology. Knowledge of these variations in the length of gestation and timing of developmental processes could ultimately be useful for managing preterm infants.
|
Conclusions
Mammals have evolved and adapted over millions of years to exploit niches on the land, in the air and in the sea. In doing so, they have assumed many forms, physiologies and reproductive strategies. Studying mammalian diversity provides the opportunity for determining the ranges of gene expression, developmental processes and reproduction that lead to this variety. Results from descriptive and experimental studies of many types of mammals will likely be very useful for enhancing embryo transfer technology. Thus, it is important to study many species of mammals and not only the few standard model systems.
Acknowledgments
The authors thank Chris Cretekos, Marvin Meistrich, John Rasweiler and Geoff Shaw for helpful discussions. The authors also thank Peter Akinwunmi, Gordon Baker, Chris Cretekos, the late Mervyn Griffiths, Marvin Meistrich, David Parer, David Paul, Geoff Shaw and Peter Temple-Smith for the images. The authors’ work reported herein was supported by National Institutes of Health grant HD30284 and National Science Foundation grant IBN 0220458 to RRB.
Baggott, L. M. , Davis-Butler, S. , and Moore, H. D. (1987). Characterization of oestrus and timed collection of oocytes in the grey short-tailed opossum, Monodelphis domestica. J. Reprod. Fertil. 79, 105–114.
| PubMed |
Bunge, R. G. , and Sherman, J. K. (1953). Fertilizing capacity of frozen human spermatozoa. Nature 172, 767–768.
| PubMed |
Heuser, C. H. , and Streeter, G. L. (1929). Early stages in the development of pig embryos from the period of initial cleavage to the time of appearance of the limb-buds. Contr. Embryol. Carnegie. Inst. 20, 1–29.
Kobayashi, A. , and Behringer, R. R. (2003). Developmental genetics of the female reproductive tract in mammals. Nat. Rev. Genet. 4, 969–980.
| Crossref | GoogleScholarGoogle Scholar | PubMed |
Polge, C. , Smith, A. U. , and Parkes, A. S. (1949). Revival of spermatozoa after vitrification and dehydration at low temperatures. Nature 164, 666.
Renfree, M. B. (1980b). Embryonic diapause in the honey possum Tarsipes spencerae. Search 11, 81.
Renfree, M. B. , and Tyndale-Biscoe, C. H. (1973). Transferrin variation between mother and fetus in the marsupial, Macropus eugenii. J. Reprod. Fertil. 32, 114–116.
Selwood, L. (2000). Marsupial egg and embryo coats. Cells Tissues Organs 166, 208–219.
| Crossref | GoogleScholarGoogle Scholar | PubMed |