The value of second polar body detection 4 hours after insemination and early rescue ICSI in preventing complete fertilisation failure in patients with borderline semen
Haixia Jin A , Yimin Shu B , Shanjun Dai A , Zhaofeng Peng A , Senlin Shi A and Yingpu Sun A CA Reproductive Medicine Center, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450001, China.
B Department of Obstetrics and Gynecology, Stanford University School of Medicine, Stanford, CA 94305, USA.
C Corresponding author. Email: syp2008@vip.sina.com
Reproduction, Fertility and Development 26(2) 346-350 https://doi.org/10.1071/RD12369
Submitted: 14 November 2012 Accepted: 21 January 2013 Published: 28 March 2013
Abstract
In this study we evaluated the value of short-time insemination and early rescue intra-cytoplasmic sperm injection (ICSI) in preventing the occurrence of complete fertilisation failure for mild or moderate male infertility patients. A total of 866 couples with borderline semen who underwent in vitro fertilisation treatment in 2010 were included. Regular insemination was performed between January and June of 2010 and short-term insemination was performed from July through December 2010, where, as early as 4 h after insemination, oocytes were denuded from cumulus cells and extrusion of the second polar body was evaluated. Of the 4153 mature oocytes with a detectable second polar body 4 h after insemination, 3874 (93.3%) showed signs of fertilisation on Day 1. Where no second polar body was present in any of the retrieved oocytes for a given patient, rescue ICSI was performed immediately. Similar rates of normal fertilisation and percentage of good-quality embryos were obtained between early rescue ICSI and regular ICSI. Clinical pregnancy occurred in 16 of 43 patients (37.2%) receiving early rescue ICSI. Our results showed early rescue ICSI in combination with evaluation of the second polar body 4 h following insemination is an effective method to prevent complete fertilisation failure for patients with mild or moderate male infertility.
Additional keywords: borderline semen, clinical outcome, early rescue ICSI, failed fertilization, second polar body.
Introduction
Although rescue intra-cytoplasmic sperm injection (ICSI) on 1-day-old oocytes is a backup procedure for complete fertilisation failure or poor fertilisation following conventional IVF, developmental potential of the ensuing embryos is poor due to oocyte ageing (Morton et al. 1997; Yuzpe et al. 2000; Kuczyński et al. 2002). Currently rescue ICSI on 1-day-old oocytes is not recommended for patients with total failure of fertilisation. To avoid complete fertilisation failure, split fertilisation (conventional IVF and ICSI) on sibling oocytes was proposed for patients with mild male factor or unexplained infertility. Plachot et al. (2002) compared the fertilisation outcome of 58 first IVF couples with mild male infertility, among which 19 (32.8%) resulted in fertilisation after ICSI only and 39 (67.2%) with fertilisation in both IVF and ICSI. In another study performed by Hershlag et al. (2002), complete fertilisation failure after conventional IVF occurred in 10 of 60 couples (16.7%) with unexplained infertility and in 2 of 50 couples (4%) with borderline semen characteristics. Results from the above reports suggest that fertilisation could be successfully achieved in the large majority of patients with mild male infertility or borderline semen. Although very successful, ICSI is still undergoing safety evaluation and is not currently recommended for routine use in all in vitro fertilisation patients. If there is a method to predict fertilisation before an oocyte loses its developmental potential, then the time-consuming and unnecessary ICSI procedure could be avoided for most patients with borderline semen.
Several studies have investigated the potential role of the presence of the second polar body in predicting fertilisation. Results from Van den Bergh et al. (1995) demonstrated that second polar body extrusion was highly predictive for oocyte fertilisation as soon as 3 h after the ICSI procedure. According to their data, 29.4%, 56.6% and 78.3% of fertilised oocytes extruded the second polar body by the time points of 1 h, 2 h and 3 h after sperm injection, respectively. In another study by Suppinyopong et al. (2000), 84.4% of fertilised oocytes extruded the second polar body 6 h after ICSI. Based on these observations, Chen and Kattera (2003) removed the cumulus cells of cumulus–oocyte complexes (COCs) exposed to spermatozoa for 6 h and evaluated the presence of the second polar body. Oocytes with the second polar body were cultured and those without the second polar body were injected by rescue ICSI. Their results showed rescue ICSI at 6 h after insemination resulted in better fertilisation, pregnancy and implantation rates as compared with rescue ICSI 22 h after insemination when oocytes have become aged. Their findings demonstrated that early rescue ICSI based on the presence of the second polar body at 6 h after insemination provided an efficient back-up option for those unfertilised oocytes. Considering that patients with mild or moderate male infertility have more possibility of suffering complete fertilisation failure, detection of second polar body extrusion followed by early rescue ICSI is an alternative to ICSI in preventing total failure of fertilisation.
In this study, we evaluated the value of short-time insemination and early rescue ICSI in preventing the occurrence of complete fertilisation failure. Four hours after insemination cumulus cells were removed from COCs and the presence of the second polar body was evaluated. Early rescue ICSI was immediately performed when there was no second polar body present in any of the mature oocytes from the same patient. Fertilisation and ensuing embryonic development were observed. Clinical outcome following transfer of embryos derived from early rescue ICSI was also evaluated.
Materials and methods
Patients
This study was approved by the medical ethical committee of the First Affiliated Hospital of Zhengzhou University, Henan, China. Our retrospective analysis included 866 patients with borderline semen who underwent their first cycle of IVF treatment and had more than six retrieved oocytes between January 2010 and December 2010. The controlled ovarian hyperstimulation protocol consisted of gonadotrophin-releasing hormone (GnRH) agonist downregulation followed by follicle-stimulating hormone/human menopausal gonadotropins (FSH/HMG), microdose flare or antagonist protocols. Transvaginal ultrasound-guided oocyte retrieval was performed 36 h after 10 000 IU human chorionic gonadotrophin (hCG) administration. Retrieved COCs were washed free of follicular fluid and blood and placed in groups of four in 0.5 mL of equilibrated sage fertilisation medium (Cooper Surgical, Inc., Trumbull, CT, USA) with 5% human serum albumin (HSA; Irvine Scientific, Santa Ana, CA, USA) or G1.3 (Vitrolife, Englewood, CO, USA) in four-well Nunc dishes (BD Falcon, Franklin Lakes, NJ, USA) in a 37°C, 6% CO2 incubator.
Sperm analysis and insemination
According to our procedure manual, ICSI is performed only when there is a medical indication for doing so, such as a severe abnormal semen analysis or previous failed or poor fertilisation with conventional IVF (<20%), and in conjunction with testicular and epididymis sperm extraction. Semen analysis was performed according to manual of the World Health Organization (WHO) (1999). Liquefied ejaculates were processed over a two-layer discontinuous density gradient, formed by a top layer of 40% (v/v) PureSperm (NidaCon Laboratories AB, Gothenburg, Sweden) and a lower layer of 80% (v/v) PureSperm, by centrifugation at 500g for 15 min at room temperature. The pellet was resuspended in 3 mL sage fertilisation medium with 5% HSA and spun down at 200g for 10 min at room temperature. The resulting pellet was then resuspended in 2 mL of sage fertilisation medium and stored in a 37°C, 6% CO2 humidified air incubator.
Conventional IVF was performed for those with a post-wash total motile sperm count (TMSC) of over 20 million and ICSI was performed for those with a TMSC of lower than 2 million. For male subfertility, the decision to perform ICSI was made based on the post-wash TMSC. Patients defined as having mild (a TMSC of 10–20 million) or moderate (a TMSC of 2–10 million) male infertility were assigned to conventional IVF.
During the first half of 2010 (January to June, 2010), oocytes were inseminated 4–6 h after retrieval and a fertilisation evaluation was performed 14–18 h after insemination. From July through December 2010, we performed short-term insemination on patients with mild or moderate male infertility. For short-term insemination, COCs were exposed to spermatozoa at a concentration of 100 000 motile spermatozoa mL–1 in a 4-well Nunc dish three to four hours after oocyte retrieval (~39–40 h after hCG injection). Four hours after insemination (~43–44 h after hCG injection), cumulus cells were removed from COCs by using a pulled Pasteur pipette with a diameter of 150 µm. The presence of the second polar body was then evaluated under an inverted microscope (Nikon Eclipse TE 2000S; Nikon, Tokyo, Japan) at a magnification of 400× with Hoffman modulation optics. To ensure consistency of identification of extrusion of the second polar body, all participating embryologists were fully trained and a second embryologist witnessed the observation in case there were fragmentations in the perivitelline space. Patients without any mature oocytes at the evaluation of second polar body extrusion were excluded from this study.
Rescue ICSI and subsequent embryonic development
In case no second polar body was present in any of the retrieved oocytes from the same patient, rescue ICSI was performed immediately. The injected oocytes were cultured individually under mineral oil in 40-µL droplets of sage fertilisation medium or G1.3 at 37°C with a humidified atmosphere of 6% O2, 5% CO2 and 89% N2. A fertilisation check was performed 14–18 h after insemination or early rescue ICSI. Normally fertilised oocytes with two clear pronuclei (PN) were then cultured in sage cleavage medium (Cooper Surgical, Inc.) with 5% HSA or G1.3 for another 48 h before being transferred to the uterus on Day 3. The incidences of monopronuclear (1PN) and tripronucleate (3PN) zygotes were compared between mature oocytes with the presence of the second polar body at 4 h after insemination and those receiving rescue ICSI. To evaluate fertilisation and embryonic development of early rescue ICSI, comparable male infertility patients receiving regular ICSI on the same week was set as control.
Evaluation of embryo quality was performed at the pronuclear stage and on Days 2 and 3. Pronuclear morphology was recorded based on the size of the pronucleus and the number and polarisation of nucleolus precursor bodies (Scott 2003). On the morning of Days 2 and 3, embryo development was assessed, including blastomere number, size and regularity, and the presence and percentage of fragmentation. All embryos were graded on a scale of 1–5 with 1 being the best. Grade 1 embryos are the best embryos consisting of symmetrical blastomeres of equal size with no cytoplasmic fragmentation. Grade 2 embryos had blastomeres of equal size and minor cytoplasmic fragmentation covering <10% of the embryo surface. Grade 3 embryos had even or uneven blastomeres and minor cytoplasmic fragmentation covering 10–25% of the embryo surface. Grade 4 embryos had blastomeres of equal or unequal size and moderate to significant cytoplasmic fragmentation covering 25–50% of the embryo surface. Grade 5 embryos contained few blastomeres of any size and severe fragmentation covering >50% of the volume of the embryo. Good-quality embryos were defined as those with six to eight equal-sized cells and <10% fragmentation on Day 3. Selection of embryos for transfer was based on pronuclear scoring, first cleavage and Day 3 embryo morphology. Ultrasound-guided embryo transfer was performed with the use of an EchotipSoftpass catheter (Cook Ob/Gyn, Spencer, IN, USA), with a total transfer volume of 20–30 μL. Clinical pregnancies were defined by the presence of an intrauterine gestational sac with a heartbeat as seen on ultrasound, or by the diagnosis of an ectopic pregnancy at 5 weeks following embryo transfer.
Statistical analysis
Results were expressed as mean ± s.e.m. The chi-square test, Fisher’s exact test, one-way analysis of variance and Student’s t-test were used to compare groups as appropriate. P < 0.05 was considered to be statistically significant.
Results
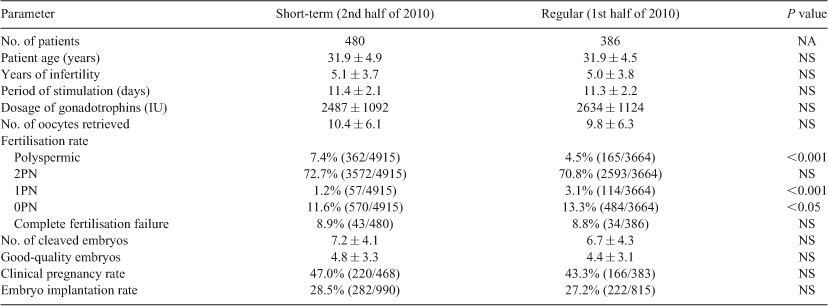
There were a total of 866 first IVF cycle patients with mild or moderate male infertility in 2010. Conventional IVF was performed on 386 of them during the first half of the year and 480 received short-term insemination during the second half of year. No significant differences were observed between the two insemination methods in patient age, period of infertility, dosage of gonadotrophins, number of oocytes retrieved and rate of normal fertilisation. Clinical pregnancy rate and embryo implantation were also similar between the two methods. Complete fertilisation failure did not differ significantly between the two insemination methods. Interestingly there was a slightly but significantly higher rate of polyspermic fertilisation following short-term insemination as compared with regular insemination (P < 0.05; Table 1).
The predictive value of second polar body extrusion in early assessment of fertilisation was evaluated in the 480 couples receiving short-term insemination during the second half of 2010 (Table 2). Of the 4153 mature oocytes with a detectable second polar body 4 h after insemination, 3874 (93.3%) showed signs of fertilisation, with 3521 (84.8%) being normally fertilised and 353 (8.5%) showing 1PN or 3PN on Day 1. Considering the poor embryonic development following rescue of aged oocytes on Day 1, rescue ICSI on the second day of oocyte retrieval has been discontinued for patients with total failed fertilisation following regular insemination in our program since 2007. Instead, early rescue ICSI was implemented on 43 patients where no second polar body was present in any of the retrieved oocytes 4 h after insemination (Table 3). Similar rates of fertilisation were obtained following early rescue ICSI as compared with regular ICSI on comparable patients on the retrieval day. No significant differences were observed in abnormal fertilisation and the ratio of good-quality embryos. Clinical pregnancy occurred in 16 patients, with a clinical pregnancy rate of 37.2%. Implantation rate of transferred embryos derived from rescue ICSI was lower than regular ICSI, but the difference did not reach significance.

|
Discussion
We performed early rescue ICSI for those patients in whom the second polar body was not evident in any of the retrieved oocytes 4 h after insemination. Fertilisation occurred in all 43 patients receiving rescue ICSI. Our results demonstrated that early rescue ICSI in combination with the second polar body evaluation as early as 4 h after insemination was an effective way to reduce the occurrence of complete fertilisation failure for mild or moderate male infertility patients. Transfer of cleavage-stage embryos from early rescue ICSI resulted in an acceptable rate of embryo implantation and live birth.
A prerequisite for early rescue ICSI is the detection of oocytes with fertilisation at an early time. Shortened exposure of oocytes to spermatozoa has been implemented in IVF practice for different purposes. It was reported that shortened exposure of oocytes to spermatozoa improved embryo quality by preventing the negative effects of oxygen free radicals produced by the spermatozoa during the insemination process (Dirnfeld et al. 1999; Kattera and Chen 2003). Our previous observations demonstrated that the duration of spermatozoon–oocyte co-incubation did not affect fertilisation, embryo quality, clinical pregnancy or implantation rates (Dai et al. 2012). In this study, we stripped the oocytes 4 h after insemination to evaluate second polar body extrusion. Our results further confirmed that short-time insemination and early ICSI would not affect oocyte fertilisation. Based on our observations, 93.3% of oocytes with the second polar body 4 h after insemination showed normal fertilisation 14–18 h after insemination. Short spermatozoon–oocyte incubation provides an approach to evaluate the occurrence of fertilisation at an early stage and therefore allows us an opportunity to perform rescue ICSI as early as possible.
A high rate of polyspermic fertilisation was observed following the early removal of cumulus cells from inseminated COCs, suggesting that there might be a failure of the block to polyspermy. Accumulated data indicate that oocyte, sperm and insemination conditions are all related to the occurrence of polyspermic fertilisation (Wang et al. 2003; Gardner and Evans 2006). The first obstacle to regulate the number of spermatozoa reaching or binding to the zona pellucida, and thus reducing multiple sperm penetration, is the cumulus cells surrounding the oocytes. Loss of cumulus cells during the insemination process could cause more spermatozoa to attach to the zona pellucida. Cumulus cell removal could also cause significant metabolic changes to the oocytes, which further affects the oocyte maturation and fertilisation process. As reported by Downs et al. (2006), denuded oocytes cultured alone consumed glucose and pyruvate, but with cumulus cells attached more glucose was consumed and significant amounts of pyruvate were accumulated in the medium, leading to an increase in the maturation of denuded oocytes. However, most of these in vitro-matured oocytes are cytoplasmically immature and appear to undergo higher sperm penetration of the zona consistent with their failure to undergo complete cortical granule loss (Van Blerkom et al. 1994).
Our results showed that 93.3% of mature oocytes with a detectable second polar body showed signs of fertilisation on Day 1, suggesting that the extrusion of the second polar body is a reliable predictor for subsequent fertilisation. One major concern regarding early rescue ICSI is the occurrence of polyspermic fertilisation. Although second polar body extrusion is highly predictive for oocyte fertilisation as early as 3 h after ICSI (Van den Bergh et al. 1995), it is still unclear whether those oocytes without a detectable second polar body at the time of cumulus removal have been penetrated by a spermatozoon or are actually undergoing a delayed fertilisation process. During the rescue ICSI, one more spermatozoon is injected into an oocyte, making it possible to generate bispermic triploid embryos. In this study, no increased rate of polyspermic fertilisation was observed following rescue ICSI, which is consistent with Chen and Kattera (2003). Due to the small number of oocytes receiving early rescue ICSI in both studies, further studies should be performed on a larger sample size to determine the effects of early rescue ICSI on polyspermic fertilisation.
Conflicting results of rescue ICSI have been reported, depending on the sources of oocytes and timing of rescue ICSI performed. As shown in several previously published reports, although 1-day-old oocytes could be normally fertilised by rescue ICSI (Nagy et al. 1995; Park et al. 2000), the quality of the ensuing embryos was poor and embryonic developmental potential was severely compromised (Morton et al. 1997; Yuzpe et al. 2000). However, when the timing of recue ICSI was moved from 22 h to 6 h following insemination, significantly higher rates of fertilisation and improved embryonic development were obtained (Chen and Kattera 2003; Nagy et al. 2006; Zhu et al. 2011), suggesting that rescue ICSI is still effective as long as oocytes have not lost their developmental competence at the time point when it is performed. By identifying the presence of second polar body as early as 4 h after insemination, we performed rescue ICSI on oocytes in whom the second polar body was not evident in any of the retrieved oocytes. Our results showed early rescue ICSI 4 h after insemination did not compromise fertilisation and subsequent embryonic development, suggesting that unfertilised mature oocytes 4 h after insemination still keep their further developmental potential. Although the difference was not significant, we realised that there was a trend to higher rates of embryo implantation and live birth in the regular ICSI group as compared with early rescue ICSI cycles. A larger sample size is required to accurately evaluate the clinical outcome following early rescue ICSI.
In conclusion, our results demonstrate that early rescue ICSI is an effective approach to prevent the occurrence of total failed fertilisation in patients with mild or moderate male infertility.
Acknowledgment
This study was support by National Natural Science Foundation of China (NSFC) No. 31271605 and Henan Province Programs for Science and Technology Development No.122300410077.
References
Chen, C., and Kattera, S. (2003). Rescue ICSI of oocytes that failed to extrude the second polar body 6 h post-insemination in conventional IVF. Hum. Reprod. 18, 2118–2121.| Rescue ICSI of oocytes that failed to extrude the second polar body 6 h post-insemination in conventional IVF.Crossref | GoogleScholarGoogle Scholar | 14507831PubMed |
Dai, S. J., Qiao, Y. H., Jin, H. X., Xin, Z. M., Su, Y. C., Sun, Y. P., and Chian, R. C. (2012). Effect of co-incubation time of sperm–oocytes on fertilization, embryonic development and subsequent pregnancy outcome. Syst. Biol. Reprod. Med. 58, 348–353.
| Effect of co-incubation time of sperm–oocytes on fertilization, embryonic development and subsequent pregnancy outcome.Crossref | GoogleScholarGoogle Scholar | 22856526PubMed |
Dirnfeld, M., Bider, D., Koifman, M., Calderon, I., and Abramovici, H. (1999). Shortened exposure of oocytes to spermatozoa improves in vitro fertilization outcome: a prospective, randomized, controlled study. Hum. Reprod. 14, 2562–2564.
| Shortened exposure of oocytes to spermatozoa improves in vitro fertilization outcome: a prospective, randomized, controlled study.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1MvlvFemsg%3D%3D&md5=dab7314a1b8785eab4069504814870efCAS | 10527987PubMed |
Downs, S. M., Gilles, R., Vanderhoef, C., Humpherson, P. G., and Leese, H. J. (2006). Differential response of cumulus cell-enclosed and denuded mouse oocytes in a meiotic induction model system. Mol. Reprod. Dev. 73, 379–389.
| Differential response of cumulus cell-enclosed and denuded mouse oocytes in a meiotic induction model system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhsVKhtLo%3D&md5=4f3bbdb0c569f92abe5a4b71723478dbCAS | 16362973PubMed |
Gardner, A. J., and Evans, J. P. (2006). Mammalian membrane block to polyspermy: new insights into how mammalian eggs prevent fertilisation by multiple sperm. Reprod. Fertil. Dev. 18, 53–61.
| Mammalian membrane block to polyspermy: new insights into how mammalian eggs prevent fertilisation by multiple sperm.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtlanu7vI&md5=5bc53f18a8529f4be5467d6bd7b85bafCAS | 16478602PubMed |
Hershlag, A., Paine, T., Kvapil, G., and Feng, H. (2002). Napolitano B. In vitro fertilization-intracytoplasmic sperm injection split: an insemination method to prevent fertilization failure. Fertil. Steril. 77, 229–232.
| Napolitano B. In vitro fertilization-intracytoplasmic sperm injection split: an insemination method to prevent fertilization failure.Crossref | GoogleScholarGoogle Scholar | 11821076PubMed |
Kattera, S., and Chen, C. (2003). Short co-incubation of gametes in in vitro fertilization improves implantation and pregnancy rates: a prospective, randomized, controlled study. Fertil. Steril. 80, 1017–1021.
| Short co-incubation of gametes in in vitro fertilization improves implantation and pregnancy rates: a prospective, randomized, controlled study.Crossref | GoogleScholarGoogle Scholar | 14556826PubMed |
Kuczyński, W., Dhont, M., Grygoruk, C., Pietrewicz, P., Redzko, S., and Szamatowicz, M. (2002). Rescue ICSI of unfertilized oocytes after IVF. Hum. Reprod. 17, 2423–2427.
| Rescue ICSI of unfertilized oocytes after IVF.Crossref | GoogleScholarGoogle Scholar | 12202435PubMed |
Morton, P. C., Yoder, C. S., Tucker, M. J., Wright, G., Brockman, W. D., and Kort, H. I. (1997). Reinsemination by intracytoplasmic sperm injection of 1-day-old oocytes after complete conventional fertilization failure. Fertil. Steril. 68, 488–491.
| Reinsemination by intracytoplasmic sperm injection of 1-day-old oocytes after complete conventional fertilization failure.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2svmtVGgug%3D%3D&md5=12be8c82ec4ebb9ee29e6f9c4c4ffdd7CAS | 9314920PubMed |
Nagy, Z. P., Staessen, C., Liu, J., Joris, H., Devroey, P., and Van Steirteghem, A. C. (1995). Prospective, auto-controlled study on reinsemination of failed-fertilized oocytes by intracytoplasmic sperm injection. Fertil. Steril. 64, 1130–1135.
| 1:STN:280:DyaK28%2FmvFGmuw%3D%3D&md5=3698abc11aa87e4f0ed5a8cded253dd7CAS | 7589665PubMed |
Nagy, Z. P., Rienzi, L. F., Ubaldi, F. M., Greco, E., Massey, J. B., Kort, H. I., Nagy, Z. P., Rienzi, L. F., Ubaldi, F. M., Greco, E., Massey, J. B., and Kort, H. I. (2006). Effect of reduced oocyte aging on the outcome of rescue intracytoplasmic sperm injection. Fertil. Steril. 85, 901–906.
| Effect of reduced oocyte aging on the outcome of rescue intracytoplasmic sperm injection.Crossref | GoogleScholarGoogle Scholar | 16580372PubMed |
Park, K. S., Song, H. B., and Chun, S. S. (2000). Late fertilization of unfertilized human oocytes in in vitro fertilization and intracytoplasmic sperm injection cycles: conventional insemination versus ICSI. J. Assist. Reprod. Genet. 17, 419–424.
| Late fertilization of unfertilized human oocytes in in vitro fertilization and intracytoplasmic sperm injection cycles: conventional insemination versus ICSI.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3crhtVelsA%3D%3D&md5=d07913bfa66f689b6b8289cbb4dc0251CAS | 11062851PubMed |
Plachot, M., Belaisch-Allart, J., Mayenga, J. M., Chouraqui, A., Tesquier, L., and Serkine, A. M. (2002). Outcome of conventional IVF and ICSI on sibling oocytes in mild male factor infertility. Hum. Reprod. 17, 362–369.
| Outcome of conventional IVF and ICSI on sibling oocytes in mild male factor infertility.Crossref | GoogleScholarGoogle Scholar | 11821279PubMed |
Scott, L. (2003). Pronuclear scoring as a predictor of embryo development. Reprod. Biomed. Online 6, 201–214.
| Pronuclear scoring as a predictor of embryo development.Crossref | GoogleScholarGoogle Scholar | 12676001PubMed |
Suppinyopong, S., Choavaratana, R., and Karavakul, C. (2000). Timing of second polar body extrusion and pronuclear formation after intracytoplasmic sperm injection (ICSI). J. Med. Assoc. Thai. 83, 500–503.
| 1:STN:280:DC%2BD3czit1agtg%3D%3D&md5=4e1fa38ed6bc2cd971f43f89f54ec54eCAS | 10863895PubMed |
Van Blerkom, J., Davis, P. W., and Merriam, J. (1994). The developmental ability of human oocytes penetrated at the germinal vesicle stage after insemination in vitro. Hum. Reprod. 9, 697–708.
| 1:STN:280:DyaK2czitFGgtQ%3D%3D&md5=5d94c788ddec9dc991725c6b0b498e76CAS | 8046026PubMed |
Van den Bergh, M., Bertrand, E., and Englert, Y. (1995). Second polar body extrusion is highly predictive for oocyte fertilization as soon as 3 hr after intracytoplasmic sperm injection (ICSI). J. Assist. Reprod. Genet. 12, 258–262.
| Second polar body extrusion is highly predictive for oocyte fertilization as soon as 3 hr after intracytoplasmic sperm injection (ICSI).Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK28%2FlvFKmtQ%3D%3D&md5=ca8851cbe13d576bb520baee2762470bCAS | 7580022PubMed |
Wang, W. H., Day, B. N., and Wu, G. M. (2003). How does polyspermy happen in mammalian oocytes? Microsc. Res. Tech. 61, 335–341.
| How does polyspermy happen in mammalian oocytes?Crossref | GoogleScholarGoogle Scholar | 12811738PubMed |
World Health Organization (WHO) (1999). ‘World Health Organization Laboratory Manual for Human Semen and Sperm Cervical–Mucus Interaction’. 4th edn. (Cambridge University Press: Cambridge, UK.)
Yuzpe, A. A., Liu, Z., and Fluker, M. R. (2000). Rescue intracytoplasmic sperm injection (ICSI) – salvaging in vitro fertilization (IVF) cycles after total or near total fertilization failure. Fertil. Steril. 73, 1115–1119.
| Rescue intracytoplasmic sperm injection (ICSI) – salvaging in vitro fertilization (IVF) cycles after total or near total fertilization failure.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3czhsF2hsw%3D%3D&md5=3bf6c1bf17c375b2d155d2ae579b3560CAS | 10856467PubMed |
Zhu, L., Xi, Q., Nie, R., Chen, W., Zhang, H., and Li, Y. (2011). Rescue intracytoplasmic sperm injection: a prospective randomized study. J. Reprod. Med. 56, 410–414.
| 22010525PubMed |