Influence of pathogenic bacteria species present in the postpartum bovine uterus on proteome profiles
A. M. Ledgard A B , G. A. Smolenski A , H. Henderson A and R. S.-F. Lee AA AgResearch, Ruakura Research Centre, East Street, Hamilton 3240, New Zealand.
B Corresponding author. Email: anita.ledgard@agresearch.co.nz
Reproduction, Fertility and Development 27(2) 395-406 https://doi.org/10.1071/RD13144
Submitted: 9 May 2013 Accepted: 31 October 2013 Published: 12 December 2013
Journal Compilation © CSIRO Publishing 2015 Open Access CC BY-NC-ND
Abstract
In the first 2–3 weeks after parturition >90% of dairy cows will have some form of uterine infection. Uterine contamination with pathogens, such as Trueperella (formerly Arcanobacterium) pyogenes increases the risk of developing more severe endometritis, which can reduce conception rates. In this study, we compared the uterine proteome of cows infected with Trueperella pyogenes with that of uninfected cows, using 2D gel electrophoresis, and identified annexins A1 and A2 (ANXA1 and ANXA2), apolipoprotein A-1, calprotectin (S100A9), cathelicidin, enolase 1 (ENO1), peptidoglycan recognition protein 1 (PGLYRP1), phosphoglycerate mutase 1 (PGAM1), serine dehydratase (SDS) and serine protease inhibitors (SERPIN) B1, B3 and B4 proteins as differing in abundance in endometritis. Subsequently, levels of ten of these proteins were monitored in uterine samples collected from a herd of lactating, dairy cows at 15 and 42 days post-partum (DPP). The levels were compared with the cytology scores of the samples and the bacterial species isolated from the uterus. Cathelicidin, PGLYRP1, SERPINB1 and S100A9 levels at 15DPP showed strong positive correlations (r = 0.78, 0.80, 0.79, and 0.68 respectively; P < 0.001) with % of polymorphonuclear neutrophils (PMN). When compared with other bacterial pathogens identified, Streptococcus agalactiae and Truperella pyogenes induced increased expression of the indicator proteins, suggesting that these organisms may adversely affect the subsequent ability of the cow to conceive. Interestingly, there was no difference in the proportion of cows pregnant at 6 and 17 weeks after start of mating between the cows with high or low %PMN.
Additional keywords: Arcanobacterium pyogenes, endometritis, postpartum, pregnancy, Streptococcus agalactiae, uterine.
Introduction
After parturition it is well recognised that in the majority of cows, uterine bacterial contamination is an inevitable component of the natural involution process (Williams et al. 2005). These infections are non-specific and during the following 6 weeks the composition of uterine flora changes, with repeated cycles of contamination and clearance, as bacterial species are spontaneously cleared, allowing others to establish which, in turn, are eventually cleared in the majority of cases (Griffin et al. 1974; Santos and Bicalho 2012). However, the risk of developing clinical uterine infection is increased in cows that have twins or stillbirth, dystocia at calving or retained fetal membranes and those with metabolic disorders (LeBlanc 2008; Gautam et al. 2010). Clinical endometritis, characterised by purulent uterine discharge (Sheldon et al. 2006), is histologically associated with a loss or disruption of the epithelium with infiltration of inflammatory cells (Bondurant 1999).
Several bacterial species found in the uterus are associated with increased endometrial inflammation and purulent vaginal discharge. Sheldon et al. (2009) identified the most prevalent bacteria as Arcanobacterium pyogenes, Escherichia coli, Fusobacterium necrophorum, Prevotella melaninogenica and Proteus species but found no interaction between the pathogenic group present and the severity of intrauterine infection. Arcanobacterium pyogenes, now reclassified as Trueperella pyogenes (Yassin et al. 2011), is an opportunistic pathogen of domestic ruminants and, either alone or with other bacteria, is commonly associated with bovine uterine infections (Griffin et al. 1974; Lewis 1997). Repeated postpartum uterine sampling showed that T. pyogenes was the most common bacterial species isolated between 8 and 42 days postpartum (DPP) and was the most prevalent organism isolated in cows with severe endometritis (69% of severe cases); however, it was extremely rare after 21 DPP in cows that conceived to first service (Williams et al. 2005). Like most of the other microorganisms T. pyogenes did not persist and was eventually cleared. Several studies, where the postpartum uterine health status was monitored with uterine cytology or biopsies, also showed that a high percentage of cows (70–80%) with mild or subclinical endometritis, whatever the organisms, self-resolved the infection by 6–8 weeks postpartum (Griffin et al. 1974; Mateus et al. 2002; Gautam et al. 2010; Green et al. 2011). On the other hand, the isolation of a significant pathogen, such as T. pyogenes, at 42 DPP has been shown to negatively affect the final pregnancy rate (McDougall et al. 2011).
The presence of bacterial antigens elicits an immune response that results in the recruitment of polymorphonuclear neutrophils (PMN) into the uterus as a first line of defence against invading pathogens (Sheldon et al. 2009). These immune cells secrete cytokines and chemokines at the site of infection as part of the inflammatory response to assist in clearance of the infection. A mediator of these pro-inflammatory events, prostaglandin E2 (PGE2; Bos et al. 2004), is also one of the central hormones in reproductive processes (Asselin et al. 1997), playing a key role in maternal recognition of pregnancy. PGE2 is primarily synthesised by three forms of prostaglandin E synthase (PGES; Parent and Fortier 2005) with the mRNA expression of cytosolic PGES reported as being 2-fold lower in cows with subclinical endometritis compared with healthy cows (Gabler et al. 2009). Changes in the balance between endometrial production of prostaglandins PGE2 and PGF2α can alter the interaction between the endometrium and the ovaries, as PGE2 promotes the persistence of the corpus luteum, resulting in disruption of the ovarian cycles (Sheldon et al. 2009). Cows with severe endometritis tend to have abnormal ovarian activity, such as extended anoestrus, prolonged luteal phases and cystic ovaries (Mateus et al. 2002; Sheldon and Dobson 2004). A meta-analysis of 23 studies on the effect of diseases on dairy cattle reproduction found that endometritis was associated with a 20% decrease in conception to first service and an increase of 19 days to conception (Fourichon et al. 2000). It is also reported that cows with prolonged sub-clinical uterine infection (cytological definition of >10% PMN among nucleated cells at both 28 DPP and 42 DPP) have lower pregnancy rates and take longer to conceive than cows in lower percentage PMN categories (McDougall et al. 2011). As yet, the precise association between bacteria in the uterus and endometrial inflammation is not well understood and uterine inflammation may be present in cows with no bacteria isolated (Sheldon et al. 2009; LeBlanc et al. 2011).
The aim of this study was to increase our understanding of the effect of endometritis on the uterine environment by examining the proteome of the postpartum uterus and the influence of inflammation and of different bacterial species on the proteome. We postulated that the presence of pathogenic bacteria, such as T. pyogenes, may alter the protein profiles in the postpartum uterine environment in ways that negatively impact on fertility. An initial investigation using two-dimensional gel electrophoresis comparisons of samples taken around 21 DPP was followed by a larger study to investigate the influence of bacterial populations on the uterine environment in a normal milking herd. We examined the effects of elevated uterine PMN and the influence of different bacterial isolates on the levels of a subset of proteins, identified by mass spectrometry from the initial study. The herd was monitored at 6 and 17 weeks after start of mating for the proportion of cows pregnant to determine the effects of endometritis on fertility.
Materials and methods
Animals and experimental design
Experiments were undertaken in accordance with the regulations of the New Zealand Animal Welfare Act of 1999 under Ruakura Animal Ethics Committee approval RAEC12294. A mixed-age, mixed-breed herd of 225 cows, which did not include first-time-calving cows, were kept under the same normal pastoral ‘best farm practice’ grazing herd management at the DairyNZ Scott Farm. A monitor group of cows (n = 20 randomly selected across all age groups from the herd) were tested for mineral status (calcium, magnesium, selenium, urea and zinc; analysed by Gribbles Veterinary Pathology Ltd, Hamilton, New Zealand) at two different stages during the study (2–3 weeks pre- and post-calving).
The cows were grouped by calving date for uterine sampling between 14 and 17 days postpartum (average 15 DPP) for cytology, bacteriology and protein analysis. Sampling was repeated at 42 DPP. At the conclusion of the uterine sampling 12 cows were transferred to another trial and the remaining 199 cows were artificially inseminated (AI; up to three times) or naturally bred.
Uterine sampling
Prior to uterine sampling, the vulval area was cleaned. Collection of uterine samples was undertaken using a pap endocervical sample brush (cyto-brush; Ebos Group Ltd, Christchurch, New Zealand). The sterile cyto-brush was mounted on a styllette then protected by a cannula, which in turn was protected by a plastic sleeve. The protected cannula was inserted into the uterus through the cervix; once in the lumen of the uterus, the cyto-brush was passed through the plastic sleeve and rotated a quarter turn, while in contact with the endometrium. The cyto-brush was retracted back into the cannula tip before withdrawal from the uterus. Cell spreads were prepared and the cyto-brush end was cut off and dropped into a capped tube with 1 mL sterile saline for transport back to the laboratory.
Subsequent to the uterine sampling, the vaginal mucus from each cow was scored using a Metricheck (MC) device (Simcrotech, Hamilton, New Zealand) as an indication of uterine health. The device, a 40-mm diameter hemispherical cup made of silicon rubber attached to a 500-mm long stainless-steel rod, was inserted through the cleaned vulval lips, advanced to the anterior of the vagina and then raked caudally. Any mucoid material in the vagina was captured in the cup, which was then visually assessed. The scores ranged from MC1, being clear mucus, up to MC5, being smelly mucopurulent discharge (McDougall et al. 2007).
Cytology
Slides were dried and stained with Diff-Quik (Dade Behring, Newark, DE, USA) within 2 h of sample collection. Cytological examination (Kasimanickam et al. 2005) of slides was undertaken by a trained cytologist (Institute of Veterinary Animal Biomedical Sciences), with >100 nucleated cells assessed and the percentage that were PMN recorded (IVABS, Massey University, Palmerston North, New Zealand).
Bacteriology
Within 2 h of collection the cyto-brush end in 1 mL sterile saline was vortexed for 2 min then the cyto-brush was discarded. Washings were streaked with a sterile loop onto a Tryptic soy sheep blood with 3% salt agar plate (TSA) and onto a MacConkey agar plate (Fort Richard, Auckland, New Zealand). Protease inhibitors were then added to the remaining sample (1 mM ethylenediamine tetraacetic acid (EDTA) and 1 mM benzamidine) and samples were then centrifuged at 9000g for 5 min at 4°C and the supernatant and cellular debris stored separately at –20°C for analyses. The MacConkey plates were incubated at 37°C in normal atmosphere and the TSA plates were incubated in a CO2 atmosphere generated using GasPak EZ CO2 sachets (Becton Dickinson, Franklin Lakes, NJ, USA) placed in an air-tight container. Anaerobic bacteria were not examined. Bacterial growth on MacConkey agar was assessed after 24 h for characteristic Escherichia coli colony morphology, size, pigmentation, opacity and growth density (light, medium or heavy). Bacterial growth on TSA plates was assessed after four days for colony morphology, size and haemolysis patterns. Each colony type was Gram-stained and cell morphology examined. Further biochemical tests were undertaken as required, using established techniques (Bergey 1994). Gram-positive cocci were tested with catalase, coagulase and Chrisitie-Atkins-Munch-Peterson (CAMP) tests. Putative isolates of Trueperella pyogenes were confirmed by polymerase chain reaction (PCR) using primers, made to the Plo gene, which encodes pyolysin, the haemolytic exotoxin of T. pyogenes (5′-GGCCCGAATGTCACCGC-3′ and 5′-AACTCCGCCTCTAGCGC-3′; Billington et al. 1997).
Protein analysis
Two-dimensional (2D) gel electrophoresis
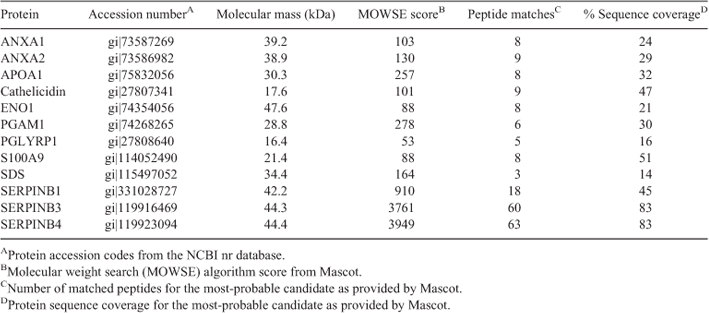
Cyto-brush supernatant samples collected from cows 21 DPP (Green et al. 2011) with bacterial endometritis (>50% PMN combined with a heavy growth of T. pyogenes cultured, n = 3) were compared with non-infected controls (<2% PMN and no bacteria cultured, n = 3) by 2D gel electrophoresis. Samples were processed essentially as previously described (Ledgard et al. 2012). Approximately 300 µg total protein was focussed in the first dimension in pre-cast immobilised pH gradient (IPG) strips (18 cm, pH 3–10; Amersham Biosciences, Uppsala, Sweden) using a flat-bed iso-electric focusing (IEF) cell system (Bio-Rad, Hercules, CA, USA) for 17 h at 20°C for a total of 63 kVh. Second-dimension separation was performed on discontinuous 12.5% (w/v) polyacrylamide sodium dodecyl sulfate gels. Protein spots were visualised with colloidal Coomassie G-250 stain and the gel images captured using a GS-800 calibrated densitometer (Bio-Rad). The non-infected control analysis set was visually compared with the infected analysis set for gross protein differences. Twelve prominent protein spots were excised, trypsin digested and tryptic peptides identified by either matrix-assisted laser desorption ionisation time-of-flight (MALDI-TOF) as previously described (Ledgard et al. 2012) or by liquid chromatography electrospray ionisation tandem mass spectrometry (LC-ESI MS/MS).
Liquid chromatography electrospray ionisation tandem mass spectrometry
Separation of peptides was carried out on a capillary column (C18, 20 cm, 75 µm ID, 5-µm particles, 300-Å pore size; Dionex, Sunnyvale, CA, USA) using a linear gradient of 2–60% acetonitrile : 0.2% formic acid over 60 min at a flow rate of 150 nL min–1. The analytical column outlet was directly connected to a Q-STAR Pulsar ionisation mass spectrometer (Applied Biosystems, Carlsbad, CA, USA) equipped with a stainless-steel nanospray needle (Proxeon Biosystems, Odense, Denmark). The mass spectrometer was programmed to acquire three simultaneous MS/MS traces of 1+, 2+, 3+, 4+ and 5+ charged peptides.
Mascot Daemon (Version 2.2.2; Matrix Science, London, UK) was used to convert the QSTAR data files to peak lists (Mascot Script for Analyst Version 1.6b25). Peak lists were searched against the National Center for Biotechnology Information (NCBI) non-redundant database (http://www.ncbi.nlm.nih.gov, accessed 1 March 2010) using an in-house Mascot server (Version 2.3.0.6; Matrix Science); taxonomy was restricted to Bos taurus.
Western blot analysis
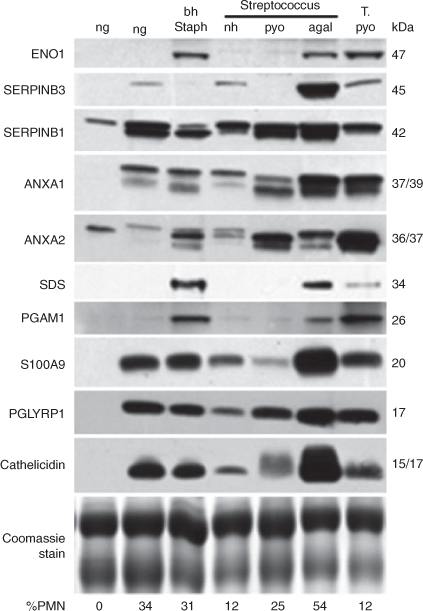
Western blotting, as described in Ledgard et al. (2011), was used to determine the relative levels of 10 proteins on a subset (n = 90) of samples. Samples were selected from the herd to include cows in the upper quartile (high) percentage of PMN (%PMN) as well as cows in the middle 50% (medium) and in the lower quartile (low) %PMN (n = 40, 25 and 25, respectively). To determine if there were any differences due to the bacterial species present, this subset also included cows from which selected potentially pathogenic bacteria were cultured: β-haemolytic, coagulase-negative Staphylococcus (bh Staph.); non-haemolytic (nh) Streptococcus (Strep.); Strep. agalactiae, Strep. pyogenes and T. pyogenes; n = 5, 9, 5, 1 and 8; respectively. These were compared with two groups from which there was no growth (ng) of organisms (n = 7 with high PMN, >13% and n = 14 with low PMN, <2%).
Aliquots of cyto-brush supernatant protein samples (5 or 10 μg of total protein) were subjected to polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate (SDS–PAGE) and electroblotted onto nitrocellulose membrane while a slice of the top of the gel was Coomassie stained to confirm even loading of the gels. Membranes were Ponceau S stained to confirm equal protein transfer of the samples. Primary antibodies used were prepared in rabbits: annexin A1 (ANXA1) and ANXA2 (1 : 5000; gift from Dr R. B. Pepinsky, Biogen, Cambridge, MA, USA), enolase 1 (ENO1, 1 : 2000; GeneTex, San Antonio, TX, USA), cathelicidin (1 : 25 000; Smolenski et al. 2011), S100A9 (1 : 5000; gift from Dr A. Molenaar, Agresearch, Hamilton, New Zealand), phosphoglycerate mutase 1 (PGAM1, 1 : 2000; GeneTex), peptidoglycan recognition protein 1 (PGLYRP1, 1 : 2000; Abnova, Taipei, Taiwan), serine protease inhibitors (SERPINs) B1 and B3 (1 : 2000, 1 : 4000; Abcam, Cambridge, UK), serine dehydratase (SDS, 1 : 2000; Aviva, San Diego, CA, USA). Secondary antibody was horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (1 : 20 000; Sigma-Aldrich, St Louis, MO, USA). Immunoreactive proteins were detected and quantified by chemiluminescence (Luminol; Sigma-Aldrich, Auckland, New Zealand) using a charge-coupled device (CCD) camera-based instrument (ChemiDoc XRS; Bio-Rad). Bands were quantified using Quantity One software (Bio-Rad) and expressed as optical density (O.D.) units per µg of total cyto-brush protein.
Statistics
Correlation of 15 DPP %PMN with abundance of each protein investigated was calculated using Genstat 15 (2013; VSN International, Hemel Hampstead, UK). As the data had a large range of several orders of magnitude, and for some proteins many samples had none of that protein present (level of zero), protein levels were cube-root transformed to stabilise the variances and to avoid problems with zero values. Correlation graphs show O.D. units on a cube-root axis. Correlation with 42 DPP %PMN was not investigated as at this sampling point only 12 of 211 cows had >7%PMN.
Relative protein abundance differences due to particular bacterial pathogens isolated were analysed individually for each protein by analysis of variance (ANOVA) by sample category using GenStat 15 (2013), after log transformation of the data. Half the minimum non-zero value specific to the individual protein was added to all values for that protein to account for zero values. Sample category means were compared using Fisher’s unprotected least-significant-difference test. Bar charts represent means of back-transformed data ± standard error of means (s.e.m.) with different letters added above the bar for each sample category to indicate which means are significantly different (P < 0.05) on the log-transformed scale.
Results
Herd status (pre- and post-calving)
Fourteen animals were removed from the trial due to complications related to difficult calving requiring antibiotic treatment, lameness, late calving and one was empty. There were no concerns regarding the herd-level mineral status as results from the monitor group of cows sampled indicated normal concentrations for the analytes tested (data not shown). Seventeen of the cows had milk fever and were treated.
Uterine health
PMN status
The 211 cows sampled at 15 DPP were classified according to their %PMN score as: high (PMN >13%; upper 25%; n = 53), medium (PMN 2–13%; middle 50%; n = 104) and low (PMN <2%; lower 25%; n = 54). Of these, 19 (9%) had over 30% PMN with the highest being one cow with 61% PMN.
By 42 DPP only four cows had ≥13% PMN (13, 13, 25 and 30% PMN) with 49 in the medium %PMN range and the remainder with low %PMN.
Bacteriology
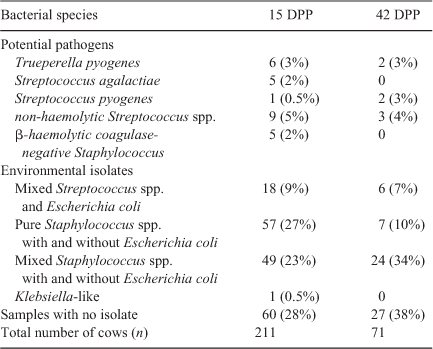
Aerobic and microaerophilic bacteria were recovered from 71% of cows at 15 DPP. Five potential pathogenic bacterial species were identified, either as a pure growth or occasionally with environmental bacterial species: β-haemolytic, coagulase-negative Staphylococcus (bh Staph.), non-haemolytic Streptococcus (nh Strep.), Strep. agalactiae, Strep. pyogenes and T. pyogenes (Table 1). In 50% of the cows, mixed populations of environmental bacterial species were isolated from the samples and they were categorised as three different populations (mixed Strep. species, pure Staph. species and mixed Staph. species, with and without E. coli). In addition, there was one cow with a pure, heavy growth of a Klebsiella-like species. At 15 DPP, E. coli was detected in 59 cows; its presence in uterine isolates diminished as the calving season progressed and E. coli was never present as the only species cultured.
|
Nine cows had clinical endometritis at 15 DPP with MC4 or 5 and PMN between 12 and 61%. Of these, one had a heavy pure growth of Strep. pyogenes, the other, nh Strep.; both had calved twins. Three cows with retained membranes had heavy growths of Strep. agalactiae (n = 2) or mixed Staph. species with E. coli (n = 1). The remaining four, who had uncomplicated calving, had heavy growths of either T. pyogenes (n = 2) or haemolytic Staph. species (n = 2). All but one of these nine cows spontaneously resolved their clinical endometritis by 42 DPP, as indicated by MC scores of 1 and PMN of <10%.
Seventy-one cows from the protein analysis subset of 90 were also sampled at 42 DPP for bacteriology (19 cows that had 0% PMN at 15 DPP were not analysed). Potential pathogens were recovered from only seven (10%) cows (Table 1). These included the four cows with high %PMN; the other three had 11 or 12% PMN.
Pregnancy status
The number of cows in calf, as determined by the presence of a viable fetus 4 months after start of mating, using transrectal ultrasonography, was 159/197 (81%, after discounting the 14 cows who had been sacrificed for another trial or died). Of these, 32/41 (78%) were in the high %PMN classification group at 15 DPP, 86/102 (84%) in the medium %PMN group and 41/54 (76%) in the low %PMN group. The proportion of cows in calf by 6 weeks after the start of mating (6-week in-calf rate), calculated from insemination dates for AI-bred cows and fetal age for naturally mated cows, was 27/41 (66%) when cows had high, 73/102 (72%) medium and 33/54 (61%) low %PMN at 15 DPP.
At 42 DPP there were only six cows with >13% PMN and when compared with those with <13% PMN, there was no difference in the proportion of cows in calf 4 months after the start of mating (83 and 81%, respectively). Of nine cows with a T. pyogenes infection, three were slaughtered for another trial, and the remaining six became pregnant whereas, of the five cows with a Strep. agalactiae infection (two with the highest %PMN but self-resolved by 42 DPP), only two became pregnant.
2D gel electrophoresis
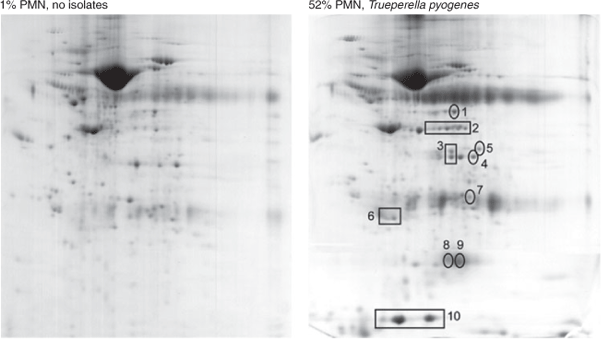
The proteome of three control non-infected animals differed from that of three cows with T. pyogenes uterine infections, as determined by 2D gels (Fig. 1). Twelve prominent proteins that were increased in the profiles of cows with T. pyogenes uterine infections compared with controls were identified with either MALDI-TOF or LC-ESI MS/MS mass spectrometry. The identity of these proteins is shown in Table 2. Representative immunoblots showing the relative abundances of some of these proteins in different sample types are shown in Fig 2.
|
|
Relationship of specific protein levels with %PMN
A subset of 90 of the 211 (43%) cows was examined for uterine protein levels at 15 DPP and correlation with %PMN was calculated (Fig. 3). These included cows with high, medium and low %PMN (n = 40, 25 and 25, respectively). There was a strong positive correlation between %PMN and cathelicidin, PGLYRP1, SERPINB1 and S100A9 levels (r = 0.78, 0.80, 0.79 and 0.68, respectively; P < 0.001) and a moderate correlation with ANXA1 and ENO1 (r = 0.68 and 0.64, respectively; P < 0.001). A relationship with %PMN was evident with PGAM1, ANXA2 and SERPINB3 (r = 0.46, 0.44 and 0.55, respectively; P < 0.01); however, many cows had no detectable PGAM1 or SERPINB3 in the samples (59% and 74%, respectively), regardless of the %PMN. A significantly greater percentage of the cows that were positive for PGAM1 had ≥13% PMN, compared with those that were negative for that protein (68 vs 38%, P < 0.01). This was not the case for SERPINB3. Similarly, of the 90 samples analysed, only 13 (14%) had SDS present and all but two of these had >13% PMN. For those where the SDS protein was undetectable, 25 (28%) had >13% PMN (data not shown).
By 42 DPP only six cows of this subset of 90 had >13% PMN (for five animals, the %PMN increased between 15 and 42 DPP; for the other animal, %PMN decreased from 31 to 13% over this period). These were the only animals with PGLYRP1 present in their 42 DPP cyto-brush protein samples, and relatively high levels of cathelicidin and S100A9 (except for one). Only one other cow had S100A9 present; this cow had a heavy growth of Strep. agalactiae at 15 DPP. In addition to these six cows, a further 12 cows had very low levels of cathelicidin present in their 42 DPP cyto-brush protein samples, all with PMN <13%. PGAM1 and ENO1 were present in 30% and 10%, respectively, of this subset of samples at 42 DPP, but levels were not correlated with %PMN. Most cows at 42 DPP had ANXA1 (98%), ANXA2 (90%) and SERPINB1 (75%) present in the samples but their levels, also, were not correlated with %PMN. Only two cows had SDS present at 42 DPP; T. pyogenes had been cultured from both of these at 15 DPP. These were also the only two of four cows that had SERPINB3 present at 42 DPP.
Relationship between levels of specific protein and bacterial species isolated
Uteri infected with the identified potential pathogens had significantly (P < 0.05) elevated mean levels of ANAX1, cathelicidin, ENO1, PGLYRP1, SERPINB1, PGAM1 and S100A9 proteins compared with the low %PMN, no growth of organisms samples (Fig. 4). Similarly, the high %PMN, no growth (ng) group also had significantly elevated levels of these proteins compared with the low %PMN ng group. Protein level of SERPINB3 was significantly (P < 0.05) elevated in the presence of Strep. agalactiae and T. pyogenes compared with the other groups. Strep. agalactiae was the only bacterial group where levels of ANXA2 and PGAM1 differed from the high %PMN ng group.
Discussion
In this study, we have identified 12 proteins whose abundance increased in the presence of T. pyogenes and elevated %PMN. We have subsequently demonstrated that the abundance of six of these proteins showed good correlation with %PMN in uterine cytology samples taken from a herd of dairy cows at 15 DPP, irrespective of the species of bacteria present. Five potential pathogens were identified from uteri in this trial as well as a mix of environmental bacteria. For simplicity, we employed a selective culture method that did not support growth of anaerobic, mycobacterial or fungal uterine contaminants or those unculturable organisms, which would also be present (Santos and Bicalho 2012). The indicator protein levels present in the high %PMN no growth (ng) group of this study may well be due to uterine inflammation as a consequence of the presence of unidentified organisms or, possibly, persistence of the inflammatory response after an earlier bacterial contamination had been cleared. We chose to sample the uterine environment earlier than usually recommended in the postpartum period in order to get a snapshot of bacteria present before they were eliminated from the uterus, as has been shown by Griffin et al. (1974). In this way, we could assess the impact of infection by various microorganisms on the ability of the cow to recover from these infections and on her ability to conceive thereafter.
The presence of T. pyogenes and Strep. agalactiae in the postpartum uterus elicited the greatest difference in protein response when compared with uninfected uteri. In the bovine udder, the response of the host immune defence mechanism depends on the type of bacterial infection (Petzl et al. 2008; Schukken et al. 2011) and it would appear from the protein response differences seen in this trial that this could be the case in the uterus as well. Although T. pyogenes is recognised as a major pathogen of endometritis, its effect appears to be confined to the uterus. Pyolysin, the toxin produced by T. pyogenes, stimulated PGF2α and PGE2 production in cultured endometrial cells but when it was infused into uteri, ovarian function was not disrupted (Miller et al. 2007). Although Strep. agalactiae has not been cited as a significant cause of bovine endometritis, it is considered an obligate intramammary pathogen that is highly contagious and is a major cause of subclinical mastitis in dairy cattle (Keefe 1997). Strep. agalactiae is also well known in human neonatal infections due to its colonisation of the maternal genitourinary tract (Spellerberg 2000). All of those cows infected with either of these two pathogens at 15 DPP had %PMN in the uppermost quartile, and this is reflected in the strength of the protein response. Many of the indicator proteins identified in the preliminary trial can be attributed to the presence of PMN in the uterine environment, as they are produced by PMN. Indeed, the whole-herd study showed that the levels of many these proteins did correlate to %PMN in the cytology samples. The main function of PMN is to engulf and destroy pathogens; however, the activities of PMN can also potentially harm tissues through the excessive production of proteolytic granular enzymes or generation of reactive oxygen species (Paape et al. 2002). Tissue damage to the endometrium could potentially lead to disruption of fertility.
One of the indicator proteins, cathelicidin, is an anti-microbial polypeptide found in macrophage lysosomes and secondary granules of PMN and acts as part of the innate host defence in sites such as the human vaginal and cervical mucosa (Cole 2006). Following secretion, cathelicidin proteins are cleaved by proteases to release C-terminal peptides with potent antimicrobial activity. The peptides bind to bacterial cell membranes and cause perforation of the membrane. There are at least seven distinct cathelicidin proteins known to be expressed in cattle (Tomasinsig and Zanetti 2005); the antibody that we used here detects all cathelicidins. Our data showed that the presence and levels of cathelicidin in the uterine samples was clearly correlated with %PMN, indicating the PMN were the prime source of the cathelicidin.
Another of the indicator proteins is PGLYRP1, which is from a family of pattern-recognition molecules that are part of the innate immune system. They recognise bacteria and bind to the unique bacterial cell-wall component, peptidoglycan (Dziarski and Gupta 2010). The extracellular protein PGLYRP1 is expressed primarily in PMN granules and has higher bactericidal activity against Gram-positive bacteria but becomes highly bactericidal for Gram-negative bacteria in the presence Zn2+ (Wang et al. 2007). There have been no reports of PGLYRP1 being expressed in endometrial tissues; therefore we assume that the protein from the cyto-brush sampling was due to the presence of PMN. The good correlation between PGLYRP1 levels in the uterine samples and %PMN supports this assumption.
Calprotectin, a heterodimer of the two calcium-binding proteins S100A8 and S100A9, was originally discovered as an immunogenic protein secreted by neutrophils and is important as a pro-inflammatory mediator in acute and chronic inflammation (Foell et al. 2004; Gebhardt et al. 2006). In this study, the amount of S100A9 in uterine samples was linked to the abundance of PMN. Both S100 proteins were present in the endometrial epithelial and stromal cells of early postpartum cows experiencing severe negative energy balance, as well as in the increased numbers of infiltrating leucocytes present (Swangchan-Uthai et al. 2013).
Also upregulated in the presence of bacterial infection were the annexins. They constitute a superfamily of proteins with a characteristic repeating conserved calcium-binding domain able to bind to cellular membranes, each with a unique N-terminal tail (Raynal and Pollard 1994). One member of the family, ANXA1, was originally described as a phospholipase A2 (PLA2)-inhibitory protein. The liberation of arachidonic acid, which is a rate-limiting step in the generation of prostaglandins, is mainly mediated by the hydrolytic action of PLA2 (Kudo et al. 1993). As well as affecting the metabolism of arachidonic acid, ANXA1 inhibits the activity of other pro-inflammatory enzymes (nitric-oxide synthase in macrophages and cyclooxygenases in activated microglia) and has an inhibitory effect on both neutrophil and monocyte migration in inflammation (Parente and Solito 2004; Hutchinson et al. 2011). Expression of ANXA1 is widespread, including high levels in macrophages and PMN (Perretti and D’Acquisto 2009) and human endometrial glandular epithelial and stromal cells (Li et al. 2008). ANXA2 is a suppressor of cytosolic PLA2 and has been reported as being upregulated in the receptive human endometrium (Domínguez et al. 2009). In this study, the levels of ANXA1 were better correlated with %PMN than ANXA2.
The superfamily of serine proteinase inhibitors (SERPINs) is generally known for its ability to inhibit serine proteinases; however, some members do not possess proteinase-binding activity and perform other functions, including hormone transport, B-cell development and neurological development (Gatto et al. 2013). Serine proteinases are involved in the resolution of infections, functioning to kill bacteria and inducing inflammation, but there is a need to regulate their activity to avoid tissue damage; serpin-mediated inhibition of these proteinases is one such mechanism. One of the most effective inhibitors of PMN-derived proteinases (such as neutrophil elastase) is SERPINB1, which itself is abundant in the secretory granules of sheep PMN (Subramaniam et al. 2010). This would account for the strong correlation of SERPINB1 levels with %PMN evident in this trial. SERPINB3 and its homologue, SERPINB4, are widely co-expressed in human tissue, including in the uterus, but their function is not clearly defined. SERPINB3 is able to inhibit cysteine proteases, which may contribute to its anti-apoptotic behaviour and also play a role in immune homeostasis (Gatto et al. 2013). The levels of SERPINB3 were not so well correlated with %PMN as levels of SERPINB1; however, three out of the four highest levels of SERPINB3 were found in samples with Strep. agalactiae infection.
In this study, mean levels of ENO1 also tended to be higher in samples from Strep. agalactiae-infected cows. Enolase 1 (α) is a multifunctional protein implicated in the initiation of microbial and autoimmune disease processes through its expression on the surface of epithelial cells, neutrophils, T-cells, B-cells, monocytes and Gram-positive cocci (Pancholi 2001). Specific auto-reactive antibodies to ENO1 have been identified in the sera of patients with endometriosis (Walter et al. 1995) and of rheumatic fever patients, with the Group A streptococcal enolase acting as a cross-reactive antigen that may play an important role in the initiation of auto-immune disease symptoms (Fontán et al. 2000). Strep. agalactiae expresses a cell-surface enolase (Hughes et al. 2002) that has the potential to initiate auto-reactive antibodies in a manner similar to Group A Streptococcus.
The glycolytic enzyme, PGAM1, which catalyses the conversion of 3-phosphoglycerate into 2-phosphoglycerate (Fothergill-Gilmore and Watson 1989) and the gluconeogenic enzyme, SDS, which catalyses the deamination of serine to pyruvate (Snell 1984) showed little, if any, correlation with %PMN. Significantly more cows that were positive for PGAM1 had ≥13%PMN, when compared with those negative for PGAM1; furthermore, significantly more cows infected with pathogenic species were positive for PGAM1. Thus, the presence of PGAM1 in the samples is associated with more severe infections and with the presence of pathogenic species. PGAM1, along with annexins, is temporally regulated during the oestrous cycle (Ledgard et al. 2012) and like many other glycolytic enzymes, may have a multifunctional role, though its role in postpartum uterine infection is unclear. Neutrophil PGAM1 levels were significantly decreased in calves treated with dexamethasone, an immune-suppressive and anti-inflammatory drug, (Beveridge et al. 2008), suggesting that PGAM1 may be an inflammatory marker. There was an increased likelihood of PGAM1 being found in uteri with Strep. agalactiae infection and of both PGAM1 and SDS with T. pyogenes infection.
This study has identified several proteins that could be used as biomarkers of subclinical endometritis, particularly those that show good correlation with %PMN. While cytological examination of the postpartum uterus and the detection of PMN in uterine samples is currently regarded as the most reliable method for diagnosing uterine disease, the diagnosis is subject to operator and sampling inconsistencies. The use of some of these indicator proteins, particularly those produced by PMN, could provide an enhanced diagnostic test for subclinical endometritis, as failure to detect PMN on a cytological spread does not necessarily indicate an absence of inflammation.
Most of the indicator proteins identified in this study are innate immune defence proteins; just what effect their presence has on the uterine tissues and subsequently, on the fertility of the cow is unknown. It is interesting that Strep. agalactiae generally provoked a greater uterine response, as assessed by the levels of many of the indicator proteins and %PMN, when compared with T. pyogenes or other species, suggesting the outcome from infection by this organism may be more serious. Indeed, there was a suggestion that infection with this species reduced conception rate, although this needs to be verified with larger numbers of animals. We did not detect any apparent consequence of postpartum elevated PMN in the uterus on the proportion of cows in calf at 4 months after start of mating, concurring with a report from a similar pasture-based system (Plöntzke et al. 2010). This may have been due to the ability of the majority of cows to clear the infections by 42 DPP and thus avoid long-term uterine damage.
Acknowledgements
We thank staff of Scott Farm (DairyNZ, Hamilton, New Zealand), Dr S. Meier, S. Morgan and K. Schmidt for animal husbandry and sample collection and staff of AgResearch, M. Berg and Dr M. Green for sample collection, N. Steele for technical assistance and P. Hunt for graphic arts. This work was supported by the Ministry of Business, Innovation and Employment (formerly the Foundation for Research, Science and Technology, Wellington, New Zealand; grant UOAX0814) and funding from New Zealand Dairy Farmers through DairyNZ Inc. (Hamilton, New Zealand; AN808).
References
Asselin, E., Lacroix, D., and Fortier, M. A. (1997). IFN-tau increases PGE2 production and COX-2 gene expression in the bovine endometrium in vitro. Mol. Cell. Endocrinol. 132, 117–126.| IFN-tau increases PGE2 production and COX-2 gene expression in the bovine endometrium in vitro.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXlslCjt78%3D&md5=2699d0d53cd89f7afb77a1ee976a3403CAS | 1:CAS:528:DyaK2sXlslCjt78%3D&md5=2699d0d53cd89f7afb77a1ee976a3403CAS | 9324053PubMed |
Bergey, D. H. (1994). ‘Bergey’s Manual of Determinative Bacteriology’. 9th edn. (Eds J. G. Holt, N. R. Krieg, P. H. Sneath, J. T. Staley and S. T. Williams.) (Lippincott Williams & Wilkins: Philadelphia.)
Beveridge, J. D., Gordon, B. M., Brewer, D., Clark, M. E., and Caswell, J. L. (2008). Altered protein expression in neutrophils of calves treated with dexamethasone. Can. J. Vet. Res. 72, 249–252.
| 1:CAS:528:DC%2BD1cXmvFSjsrk%3D&md5=c6cc50b279c8db007d175e49ce3cd75eCAS | 18505188PubMed |
Billington, S. J., Jost, B. H., Cuevas, W. A., Bright, K. R., and Songer, J. G. (1997). The Arcanobacterium (Actinomyces) pyogenes haemolysin, pyolysin, is a novel member of the thiol-activated cytolysin family. J. Bacteriol. 179, 6100–6106.
| 1:CAS:528:DyaK2sXmsFCisLs%3D&md5=7651c23515ee8f616c076b033399e72fCAS | 9324258PubMed |
Bondurant, R. H. (1999). Inflammation in the bovine female reproductive tract. J. Anim. Sci. 77, 101–110.
| 1:CAS:528:DyaK1MXlvFWhtbc%3D&md5=18ceb3d26f07450bf21ceb07f40e6417CAS | 15526785PubMed |
Bos, C. L., Richel, D. J., Ritsema, T., Peppelenbosch, M. P., and Versteeg, H. H. (2004). Prostanoids and prostanoid receptors in signal transduction. Int. J. Biochem. Cell Biol. 36, 1187–1205.
| Prostanoids and prostanoid receptors in signal transduction.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjsVWnsLc%3D&md5=42d440c368ad6be5980b94dbb5528a11CAS | 15109566PubMed |
Cole, A. M. (2006). Innate host defence of human vaginal and cervical mucosae. Curr. Top. Microbiol. Immunol. 306, 199–230.
| Innate host defence of human vaginal and cervical mucosae.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xos1ymur0%3D&md5=a5865419c628dc8038426be0d8b5ff99CAS | 16909923PubMed |
Domínguez, F., Garrido-Gómez, T., López, J. A., Camafeita, E., Quiñonero, A., Pellicer, A., and Simón, C. (2009). Proteomic analysis of the human receptive versus non-receptive endometrium using differential in-gel electrophoresis and MALDI-MS unveils stathmin 1 and annexin A2 as differentially regulated. Hum. Reprod. 24, 2607–2617.
| Proteomic analysis of the human receptive versus non-receptive endometrium using differential in-gel electrophoresis and MALDI-MS unveils stathmin 1 and annexin A2 as differentially regulated.Crossref | GoogleScholarGoogle Scholar | 19556289PubMed |
Dziarski, R., and Gupta, D. (2010). Review: mammalian peptidoglycan recognition proteins (PGRPs) in innate immunity. Innate Immun. 16, 168–174.
| Review: mammalian peptidoglycan recognition proteins (PGRPs) in innate immunity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXpt1Cnsb0%3D&md5=7db835c8e3406de84a5f33d0d246ae40CAS | 20418257PubMed |
Foell, D., Frosch, M., Sorg, C., and Roth, J. (2004). Phagocyte-specific calcium-binding S100 proteins as clinical laboratory markers of inflammation. Clin. Chim. Acta 344, 37–51.
| Phagocyte-specific calcium-binding S100 proteins as clinical laboratory markers of inflammation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXktVOnsrg%3D&md5=55beb46790f3152e663acbefaa66c84dCAS | 15149869PubMed |
Fontán, P. A., Pancholi, V., Nociari, M. M., and Fischetti, V. A. (2000). Antibodies to streptococcal surface enolase react with human alpha-enolase: implications in poststreptococcal sequelae. J. Infect. Dis. 182, 1712–1721.
| Antibodies to streptococcal surface enolase react with human alpha-enolase: implications in poststreptococcal sequelae.Crossref | GoogleScholarGoogle Scholar | 11069244PubMed |
Fothergill-Gilmore, L. A., and Watson, H. C. (1989). The phosphoglycerate mutase. Adv. Enzymol. Relat.Areas Mol. Bio. 62, 227–313.
| 1:CAS:528:DyaL1MXlt12nsb4%3D&md5=1aa634a95ca36e6f93aa36d492817c3fCAS |
Fourichon, C., Seegers, H., and Malher, X. (2000). Effect of disease on reproduction in the dairy cow: a meta-analysis. Theriogenology 53, 1729–1759.
| Effect of disease on reproduction in the dairy cow: a meta-analysis.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3cvpsFensQ%3D%3D&md5=41c23c9e6d5a8251d11fec42c771f5e2CAS | 10968418PubMed |
Gabler, C., Drillich, M., Fischer, C., Holder, C., Heuwieser, W., and Einspanier, R. (2009). Endometrial expression of selected transcripts involved in prostaglandin synthesis in cows with endometritis. Theriogenology 71, 993–1004.
| Endometrial expression of selected transcripts involved in prostaglandin synthesis in cows with endometritis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXivVWltb4%3D&md5=a3b70f40d099814d4e18fac701671999CAS | 19162311PubMed |
Gatto, M., Iaccarino, L., Ghirardello, A., Bassi, N., Pontisso, P., Punzi, L., Shoenfeld, Y., and Doria, A. (2013). Serpins, immunity and autoimmunity: old molecules, new functions. Clin. Rev. Allergy Immunol. 45, 267–280.
| Serpins, immunity and autoimmunity: old molecules, new functions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsVymurbL&md5=fd1b84e4ce1880c4b74d96f100c2421aCAS | 23325331PubMed |
Gautam, G., Nakao, T., Koike, K., Long, S. T., Yusuf, M., Ranasinghe, R. M., and Hayashi, A. (2010). Spontaneous recovery or persistence of postpartum endometritis and risk factors for its persistence in Holstein cows. Theriogenology 73, 168–179.
| Spontaneous recovery or persistence of postpartum endometritis and risk factors for its persistence in Holstein cows.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1MfhslGruw%3D%3D&md5=b03fb44216799f2b357c5bb9b952cf8bCAS | 19837450PubMed |
Gebhardt, C., Németh, J., Angel, P., and Hess, J. (2006). S100A8 and S100A9 in inflammation and cancer. Biochem. Pharmacol. 72, 1622–1631.
| S100A8 and S100A9 in inflammation and cancer.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtFOjt77F&md5=a8af9bcc144ee8903b6c126f970ef983CAS | 16846592PubMed |
Green, M. P., Ledgard, A. M., Beaumont, S. E., Berg, M. C., McNatty, K. P., Peterson, A. J., and Back, P. J. (2011). Long-term alteration of follicular steroid concentrations in relation to subclinical endometritis in postpartum dairy cows. J. Anim. Sci. 89, 3551–3560.
| Long-term alteration of follicular steroid concentrations in relation to subclinical endometritis in postpartum dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsVSqsr3F&md5=895eff854620e4adf20210ea53dc1857CAS | 1:CAS:528:DC%2BC3MXhsVSqsr3F&md5=895eff854620e4adf20210ea53dc1857CAS | 21666004PubMed |
Griffin, J. F. T., Hartigan, P. J., and Nunn, W. R. (1974). Non-specific uterine infection and bovine fertility. I. Infection patterns and endometritis during the first seven weeks post-partum. Theriogenology 1, 91–106.
| Non-specific uterine infection and bovine fertility. I. Infection patterns and endometritis during the first seven weeks post-partum.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE28%2Fms1ynsA%3D%3D&md5=3d1aa10c744529759fb7d5420f442a36CAS |
Hughes, M. J., Moore, J. C., Lane, J. D., Wilson, R., Pribul, P. K., Younes, Z. N., Dobson, R. J., Everest, P., Reason, A. J., Redfern, J. M., Greer, F. M., Paxton, T., Panico, M., Morris, H. R., Feldman, R. G., and Santangelo, J. D. (2002). Identification of major outer surface proteins of Streptococcus agalactiae. Infect. Immun. 70, 1254–1259.
| Identification of major outer surface proteins of Streptococcus agalactiae.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XhsFSls7o%3D&md5=f560f08f74f7a52b916b637e9627427dCAS | 11854208PubMed |
Hutchinson, J. L., Rajagopal, S. P., Sales, K. J., and Jabbour, H. N. (2011). Molecular regulators of resolution of inflammation: potential therapeutic targets in the reproductive system. Reproduction 142, 15–28.
| Molecular regulators of resolution of inflammation: potential therapeutic targets in the reproductive system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtFSqu7%2FK&md5=e347c7678a13d9d814395b996a62b6b4CAS | 21490125PubMed |
Kasimanickam, R., Duffield, T. F., Foster, R. A., Gartley, C. J., Leslie, K. E., Walton, J. S., and Johnson, W. H. (2005). A comparison of the cytobrush and uterine lavage techniques to evaluate endometrial cytology in clinically normal postpartum dairy cows. Can. Vet. J. 46, 255–259.
| 15884649PubMed |
Keefe, G. P. (1997). Streptococcus agalactiae mastitis: a review Can. Vet. J. 38, 429–437.
| 1:STN:280:DyaK2szmtlynsg%3D%3D&md5=1ef335415ca4423a2f23312467dd06feCAS | 9220132PubMed |
Kudo, I., Murakami, M., Hara, S., and Inoue, K. (1993). Mammalian non-pancreatic phospholipases A2. Biochim. Biophys. Acta 1170, 217–231.
| Mammalian non-pancreatic phospholipases A2.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXjtF2k&md5=0ca6bed3b72fb65108241486a9ae5e54CAS | 1:CAS:528:DyaK2cXjtF2k&md5=0ca6bed3b72fb65108241486a9ae5e54CAS | 8218339PubMed |
LeBlanc, S. J. (2008). Postpartum uterine disease and dairy-herd reproductive performance: a review. Vet. J. 176, 102–114.
| Postpartum uterine disease and dairy-herd reproductive performance: a review.Crossref | GoogleScholarGoogle Scholar | 18328749PubMed |
LeBlanc, S. J., Osawa, T., and Dubuc, J. (2011). Reproductive tract defence and disease in postpartum dairy cows. Theriogenology 76, 1610–1618.
| Reproductive tract defence and disease in postpartum dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsVanurzF&md5=d6ed029da7c68070dbd11a21d0209231CAS | 1:CAS:528:DC%2BC3MXhsVanurzF&md5=d6ed029da7c68070dbd11a21d0209231CAS | 21890187PubMed |
Ledgard, A. M., Meier, S., and Peterson, A. J. (2011). Evaluation of the uterine environment early in pregnancy establishment to characterise cows with a potentially superior ability to support conceptus survival. Reprod. Fertil. Dev. 23, 737–747.
| Evaluation of the uterine environment early in pregnancy establishment to characterise cows with a potentially superior ability to support conceptus survival.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MjhsVCktA%3D%3D&md5=33611b737d285d6fecbae7e4b5a5b2cbCAS | 1:STN:280:DC%2BC3MjhsVCktA%3D%3D&md5=33611b737d285d6fecbae7e4b5a5b2cbCAS | 21791175PubMed |
Ledgard, A. M., Berg, M. C., McMillan, W. H., Smolenski, G., and Peterson, A. J. (2012). Effect of asynchronous transfer on bovine embryonic development and relationship with early cycle uterine proteome profiles. Reprod. Fertil. Dev. 24, 962–972.
| Effect of asynchronous transfer on bovine embryonic development and relationship with early cycle uterine proteome profiles.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC38bisl2rtg%3D%3D&md5=1eadccc146d53205a835ebf29f18567dCAS | 1:STN:280:DC%2BC38bisl2rtg%3D%3D&md5=1eadccc146d53205a835ebf29f18567dCAS | 22935157PubMed |
Lewis, G. S. (1997). Uterine health and disorders. J. Dairy Sci. 80, 984–994.
| Uterine health and disorders.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXjsVOksbs%3D&md5=113f77a7e26b867dfc6b4df63068f9c1CAS | 1:CAS:528:DyaK2sXjsVOksbs%3D&md5=113f77a7e26b867dfc6b4df63068f9c1CAS | 9178140PubMed |
Li, C. Y., Lang, J. H., Liu, H. Y., and Zhou, H. M. (2008). Expression of annexin-1 in patients with endometriosis. Chin. Med. J. (Engl.) 121, 927–931.
| 1:CAS:528:DC%2BD1cXnslGqtrY%3D&md5=857711311ed09220b9b3a95508bcba41CAS |
| 1:CAS:528:DC%2BD1cXnslGqtrY%3D&md5=857711311ed09220b9b3a95508bcba41CAS | 18706208PubMed |
Mateus, L., da Costa, L. L., Bernardo, F., and Silva, J. R. (2002). Influence of puerperal uterine infection on uterine involution and postpartum ovarian activity in dairy cows. Reprod. Domest. Anim. 37, 31–35.
| Influence of puerperal uterine infection on uterine involution and postpartum ovarian activity in dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD387ltVGhtw%3D%3D&md5=f627510169bd194c9d1c0e2de13921a1CAS | 11882243PubMed |
McDougall, S., Macaulay, R., and Compton, C. (2007). Association between endometritis diagnosis using a novel intravaginal device and reproductive performance in dairy cattle. Anim. Reprod. Sci. 99, 9–23.
| Association between endometritis diagnosis using a novel intravaginal device and reproductive performance in dairy cattle.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2s7it12msg%3D%3D&md5=ec694d042d62b0b07269e3bff3d1f8a0CAS | 16630700PubMed |
McDougall, S., Hussien, H., Aberdein, D., Buckle, K., Roche, J. R., Burke, C. R., Mitchell, M. D., and Meier, S. (2011). Relationships between cytology, bacteriology and vaginal discharge scores and reproductive performance in dairy cows. Theriogenology 76, 229–240.
| Relationships between cytology, bacteriology and vaginal discharge scores and reproductive performance in dairy cows.Crossref | GoogleScholarGoogle Scholar | 21601918PubMed |
Miller, A. N., Williams, E. J., Sibley, K., Herath, S., Lane, E. A., Fishwick, J., Nash, D. M., Rycroft, A. N., Dobson, H., Bryant, C. E., and Sheldon, I. M. (2007). The effects of Arcanobacterium pyogenes on endometrial function in vitro, and on uterine and ovarian function in vivo. Theriogenology 68, 972–980.
| The effects of Arcanobacterium pyogenes on endometrial function in vitro, and on uterine and ovarian function in vivo.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtVOgsb7E&md5=074ea222e709748b1d266bab6728057fCAS | 17825901PubMed |
Paape, M., Mehrzad, J., Zhao, X., Detilleux, J., and Burvenich, C. (2002). Defence of the bovine mammary gland by polymorphonuclear neutrophil leukocytes. J. Mammary Gland Biol. Neoplasia 7, 109–121.
| Defence of the bovine mammary gland by polymorphonuclear neutrophil leukocytes.Crossref | GoogleScholarGoogle Scholar | 12463734PubMed |
Pancholi, V. (2001). Multifunctional alpha-enolase: its role in diseases. Cell. Mol. Life Sci. 58, 902–920.
| Multifunctional alpha-enolase: its role in diseases.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXlvFSmsrY%3D&md5=04ae1248af98766a08bc0165fe8180edCAS | 1:CAS:528:DC%2BD3MXlvFSmsrY%3D&md5=04ae1248af98766a08bc0165fe8180edCAS | 11497239PubMed |
Parent, J., and Fortier, M. A. (2005). Expression and contribution of three different isoforms of prostaglandin E synthase in the bovine endometrium. Biol. Reprod. 73, 36–44.
| Expression and contribution of three different isoforms of prostaglandin E synthase in the bovine endometrium.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXls1Srtrs%3D&md5=7c7ef6888af91daf74baeeabac9721d0CAS | 1:CAS:528:DC%2BD2MXls1Srtrs%3D&md5=7c7ef6888af91daf74baeeabac9721d0CAS | 15744024PubMed |
Parente, L., and Solito, E. (2004). Annexin 1: more than an anti-phospholipase protein. Inflamm. Res. 53, 125–132.
| Annexin 1: more than an anti-phospholipase protein.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjvVyht7k%3D&md5=6c0ae5a2d45c62afb85f906d8af09195CAS | 1:CAS:528:DC%2BD2cXjvVyht7k%3D&md5=6c0ae5a2d45c62afb85f906d8af09195CAS | 15060718PubMed |
Perretti, M., and D’Acquisto, F. (2009). Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat. Rev. Immunol. 9, 62–70.
| Annexin A1 and glucocorticoids as effectors of the resolution of inflammation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsFartbzP&md5=791089b728a2b49b44f8ee19dea982b9CAS | 19104500PubMed |
Petzl, W., Zerbe, H., Günther, J., Yang, W., Seyfert, H. M., Nürnberg, G., and Schuberth, H. J. (2008). Escherichia coli, but not Staphylococcus aureus triggers an early increased expression of factors contributing to the innate immune defence in the udder of the cow. Vet. Res. 39, 18.
| Escherichia coli, but not Staphylococcus aureus triggers an early increased expression of factors contributing to the innate immune defence in the udder of the cow.Crossref | GoogleScholarGoogle Scholar | 18258172PubMed |
Plöntzke, J., Madoz, L. V., De la Sota, R. L., Drillich, M., and Heuwiesera, W. (2010). Subclinical endometritis and its impact on reproductive performance in grazing dairy cattle in Argentina. Anim. Reprod. Sci. 122, 52–57.
| Subclinical endometritis and its impact on reproductive performance in grazing dairy cattle in Argentina.Crossref | GoogleScholarGoogle Scholar | 20727691PubMed |
Raynal, P., and Pollard, H. B. (1994). Annexins: the problem of assessing the biological role for a gene family of multifunctional calcium- and phospholipid-binding proteins. Biochim. Biophys. Acta 1197, 63–93.
| Annexins: the problem of assessing the biological role for a gene family of multifunctional calcium- and phospholipid-binding proteins.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXivFahsro%3D&md5=7279eb1b3b000b69612e16bdf6697e75CAS | 8155692PubMed |
Santos, T. M., and Bicalho, R. C. (2012). Diversity and succession of bacterial communities in the uterine fluid of postpartum metritic, endometritic and healthy dairy cows. PLoS ONE 7, e53048.
| Diversity and succession of bacterial communities in the uterine fluid of postpartum metritic, endometritic and healthy dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXotVahsw%3D%3D&md5=eb2441531d547e12e4111f6588b09a5bCAS | 23300859PubMed |
Schukken, Y. H., Günther, J., Fitzpatrick, J., Fontaine, M. C., Goetze, L., Holst, O., Leigh, J., Petzl, W., Schuberth, H. J., Sipka, A., Smith, D. G., Quesnell, R., Watts, J., Yancey, R., Zerbe, H., Gurjar, A., Zadoks, R. N., and Seyfert, H. M. (2011). Host-response patterns of intramammary infections in dairy cows. Vet. Immunol. Immunopathol. 144, 270–289.
| Host-response patterns of intramammary infections in dairy cows.Crossref | GoogleScholarGoogle Scholar | 21955443PubMed |
Sheldon, I. M., and Dobson, H. (2004). Postpartum uterine health in cattle. Anim. Reprod. Sci. 82–83, 295–306.
| Postpartum uterine health in cattle.Crossref | GoogleScholarGoogle Scholar | 15271461PubMed |
Sheldon, I. M., Lewis, G. S., LeBlanc, S., and Gilbert, R. O. (2006). Defining postpartum uterine disease in cattle. Theriogenology 65, 1516–1530.
| Defining postpartum uterine disease in cattle.Crossref | GoogleScholarGoogle Scholar | 16226305PubMed |
Sheldon, I. M., Cronin, J., Goetze, L., Donofrio, G., and Schuberth, H. J. (2009). Defining postpartum uterine disease and the mechanisms of infection and immunity in the female reproductive tract in cattle. Biol. Reprod. 81, 1025–1032.
| Defining postpartum uterine disease and the mechanisms of infection and immunity in the female reproductive tract in cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsV2lt7vP&md5=1eb97bfdce6227dfbb27d396ccc1f21eCAS | 19439727PubMed |
Smolenski, G. A., Wieliczko, R. J., Pryor, S. M., Broadhurst, M. K., Wheeler, T. T., and Haigh, B. J. (2011). The abundance of milk cathelicidin proteins during bovine mastitis. Vet. Immunol. Immunopathol. 143, 125–130.
| The abundance of milk cathelicidin proteins during bovine mastitis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXpvFymurg%3D&md5=82390d763309ab1f2f06aae76005f2e8CAS | 21774993PubMed |
Snell, K. (1984). Enzymes of serine metabolism in normal, developing and neoplastic rat tissues. Adv. Enzyme Regul. 22, 325–400.
| Enzymes of serine metabolism in normal, developing and neoplastic rat tissues.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2cXkt1WgtLw%3D&md5=0cc8eb9c221340df4c3e3446b363c7a3CAS | 1:CAS:528:DyaL2cXkt1WgtLw%3D&md5=0cc8eb9c221340df4c3e3446b363c7a3CAS | 6089514PubMed |
Spellerberg, B. (2000). Pathogenesis of neonatal Streptoccoccus agalactiae infections. Microbes Infect. 2, 1733–1742.
| Pathogenesis of neonatal Streptoccoccus agalactiae infections.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3M7itFantQ%3D%3D&md5=1a07b84b1c4b46497356d404407243dfCAS | 11137046PubMed |
Subramaniam, R., Dassanayake, R. P., Norimine, J., Brown, W. C., Knowles, D. P., and Srikumaran, S. (2010). Molecular cloning, characterisation and in vitro expression of SERPIN B1 of bighorn sheep (Ovis canadensis) and domestic sheep (Ovis aries) and comparison with that of other species. Vet. Immunol. Immunopathol. 137, 327–331.
| Molecular cloning, characterisation and in vitro expression of SERPIN B1 of bighorn sheep (Ovis canadensis) and domestic sheep (Ovis aries) and comparison with that of other species.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtFWqu73E&md5=80fe2fced109463c190cbacc69fd7c70CAS | 1:CAS:528:DC%2BC3cXhtFWqu73E&md5=80fe2fced109463c190cbacc69fd7c70CAS | 20591503PubMed |
Swangchan-Uthai, T., Chen, Q., Kirton, S. E., Fenwick, M. A., Cheng, Z., Patton, J., Fouladi-Nashta, A. A., and Wathes, D. C. (2013). Influence of energy balance on the antimicrobial peptides S100A8 and S100A9 in the endometrium of the post-partum dairy cow. Reproduction 145, 527–539.
| Influence of energy balance on the antimicrobial peptides S100A8 and S100A9 in the endometrium of the post-partum dairy cow.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXpsVejsb0%3D&md5=cb47252e08b5cd939c696aa985bc2e0eCAS | 23533291PubMed |
Tomasinsig, L., and Zanetti, M. (2005). The cathelicidins – structure, function and evolution. Curr. Protein Pept. Sci. 6, 23–34.
| The cathelicidins – structure, function and evolution.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhsVWqtLg%3D&md5=39b2430430dd0b63c0e5f75500dff937CAS | 15638766PubMed |
Walter, M., Berg, H., Leidenberger, F. A., Schweppe, K.-W., and Northemann, W. (1995). Autoreactive epitopes within the human a-enolase and their recognition by sera from patients with endometriosis. J. Autoimmun. 8, 931–945.
| Autoreactive epitopes within the human a-enolase and their recognition by sera from patients with endometriosis.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK28vit1Squw%3D%3D&md5=f42c995a33255fb38a9b1ebaed0fcc09CAS | 8824716PubMed |
Wang, M., Liu, L. H., Wang, S., Li, X., Lu, X., Gupta, D., and Dziarski, R. (2007). Human peptidoglycan recognition proteins require zinc to kill both gram-positive and gram-negative bacteria and are synergistic with antibacterial peptides. J. Immunol. 178, 3116–3125.
| 1:CAS:528:DC%2BD2sXhvFSit78%3D&md5=19430cbc0545e103b449d136d3eff958CAS |
| 1:CAS:528:DC%2BD2sXhvFSit78%3D&md5=19430cbc0545e103b449d136d3eff958CAS | 17312159PubMed |
Williams, E. J., Fischer, D. P., Pfeiffer, D. U., England, G. C., Noakes, D. E., Dobson, H., and Sheldon, I. M. (2005). Clinical evaluation of postpartum vaginal mucus reflects uterine bacterial infection and the immune response in cattle. Theriogenology 63, 102–117.
| Clinical evaluation of postpartum vaginal mucus reflects uterine bacterial infection and the immune response in cattle.Crossref | GoogleScholarGoogle Scholar | 15589277PubMed |
Yassin, A. F., Hupfer, H., Siering, C., and Schumann, P. (2011). Comparative chemotaxonomic and phylogenetic studies on the genus Arcanobacterium Collins et al. 1982 emend. Lehnen et al. 2006: proposal for Trueperella gen. nov. and emended description of the genus Arcanobacterium. Int. J. Syst. Evol. Microbiol. 61, 1265–1274.
| Comparative chemotaxonomic and phylogenetic studies on the genus Arcanobacterium Collins et al. 1982 emend. Lehnen et al. 2006: proposal for Trueperella gen. nov. and emended description of the genus Arcanobacterium.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MrnvVemtg%3D%3D&md5=268fb34755e86d0620c8e661c81d1390CAS | 1:STN:280:DC%2BC3MrnvVemtg%3D%3D&md5=268fb34755e86d0620c8e661c81d1390CAS | 20622055PubMed |