CASA: tracking the past and plotting the future
M. T. Gallagher A B C , D. J. Smith A B C and J. C. Kirkman-Brown B C DA School of Mathematics, University of Birmingham, Birmingham, B15 2TT, UK.
B Institute for Metabolism and Systems Research, University of Birmingham, Birmingham, B15 2TT, UK.
C Centre for Human Reproductive Science, Birmingham Women’s and Children’s NHS Foundation Trust, Birmingham, B15 2TG, UK.
D Corresponding author. Email: j.kirkmanbrown@bham.ac.uk
Reproduction, Fertility and Development 30(6) 867-874 https://doi.org/10.1071/RD17420
Submitted: 12 October 2017 Accepted: 6 April 2018 Published: 29 May 2018
Journal Compilation © CSIRO 2018 CC BY
Abstract
The human semen sample carries a wealth of information of varying degrees of accessibility ranging from the traditional visual measures of count and motility to those that need a more computational approach, such as tracking the flagellar waveform. Although computer-aided sperm analysis (CASA) options are becoming more widespread, the gold standard for clinical semen analysis requires trained laboratory staff. In this review we characterise the key attitudes towards the use of CASA and set out areas in which CASA should, and should not, be used and improved. We provide an overview of the current CASA landscape, discussing clinical uses as well as potential areas for the clinical translation of existing research technologies. Finally, we discuss where we see potential for the future of CASA, and how the integration of mathematical modelling and new technologies, such as automated flagellar tracking, may open new doors in clinical semen analysis.
Additional keywords: clinical diagnostics, flagellar analysis, glyphs, image analysis, machine learning, mathematical modelling, sperm, viscosity.
Introduction
A human semen sample is a biologically complex entity, containing a wealth of information regarding reproductive potential and general health. Under magnification we see a wide range of cells, in particular spermatozoa: some moving rapidly through the field of view, others thrashing vigorously but not progressing and still more barely moving or stationary. Beyond motility characteristics, there are morphological variations in spermatozoa, from gross features down to molecular damage to the chromosomal cargo. It is simply unreasonable to expect to integrate this complexity through visual assessment. Nevertheless, the integration of computer-aided sperm analysis (CASA) into the world of clinical diagnostics has been limited, and in many cases non-existent. Although there has been an amount of healthy suspicion surrounding the use of ‘black-box’ semen analysing systems, by not developing and integrating some form of CASA into clinical analyses we are throwing away huge quantities of latent information with wide-ranging potential diagnostic impact.
Even before the introduction of computers in semen analysis, the kinematic motion of spermatozoa has been characterised by tracking the progression of the sperm head (Katz and Overstreet 1981; Holt et al. 1985). Although the presence of the flagellum has been noted, it has often been treated as though it is merely the ‘wheels’ of the cell, allowing movement but providing little else. This is far from true; in fact, until the spermatozoon reaches the site of fertilisation, the head can be thought of as just causing drag, with the flagellum acting as a motor through the axoneme (Machin 1958), as a sensing apparatus (Brokaw 1991), responding to the presence of viscosity (Smith et al. 2009b), enabling rheotactic behaviour (Miki and Clapham 2013) and guiding migration through boundary sensing (Denissenko et al. 2012). With all this in mind, we believe that the introduction of flagellar tracking, as pioneered by Hiramoto and Baba (1978), is fundamental to the development of CASA-Mot, and the progress towards a greater use of computer-aided analyses for clinical diagnostics. Throughout this review, we aim to not only give a view of what the community has been achieving in recent years, but also to suggest areas where CASA can be used and updated in order to give a more developed understanding of the health of a semen sample, paying particular attention to what has been the clinically underused area of flagellar analysis.
In the discussion surrounding CASA, and its potential for use in human clinical laboratories, there are three overarching voices: (1) those who are sceptical about CASA’s ability to surpass trained human semen analysts; (2) those who think future CASA systems will have a role to play in diagnostics, although more work needs to be done; and (3) those who believe that the only way to do accurate, unbiased and reproducible semen analysis is through the use of CASA. Each of these voices have aspects of their view that will be crucial in forming a consensus of need going forward.
The more sceptical voices with regard to CASA’s involvement in clinical semen analysis are typified by the position taken by the Association of Biomedical Andrologists (UK), believing that ‘no [CASA] is alternative to skilled and experienced andrology staff’ (Sanders et al. 2017), and that it is essential that CASA is, at a minimum, as accurate as manual methods of semen analysis. This group is focused on developing semen analysis to the level of an ISO standard, and therefore correctly point out that you cannot use CASA to replace World Health Organization (WHO)-trained staff; it is unlikely that a CASA-Conc count will ever be as accurate as the conventional European Society of Human Reproduction and Embryology (ESHRE) count using a 50-µL semen sample and killing cells so they can all be carefully observed. However, we believe that concentrating on this runs the risk of missing all the potential positive contributions that can be made through the use of CASA. However, this sceptical voice is not constrained to issues of count. A recent study by Talarczyk-Desole et al. (2017) suggests that although there are positives to using CASA, the time CASA-Morph takes to perform a sperm morphology assessment, as well as the differences shown between CASA-Mot results and trained laboratory staff, mean that there are still improvements needed before CASA is routinely used in clinical practice.
The second, more moderate voice is that which cautions against CASA being used as the be-all and end-all of clinical semen analysis while suggesting that CASA could be useful for a strict subset of analyses (often for categorising kinematic measures of semen quality). Advocates of this position have stated that CASA can be useful for a subset of analyses, but that ‘we should stop trying to use CASA for applications that are inherently problematic for the underlying technology’ (Mortimer et al. 2015). However, what differentiates this view from the sceptical position is the belief that ‘robust method[s] of automated semen analysis [are] clearly desirable and could provide unparalleled levels of intra- and interlaboratory consistency’ (Tomlinson et al. 2010).
Finally, the third voice is that of those who not only believe that CASA can improve upon manual semen analyses, but also that ‘[sperm functional tests] can only be done accurately and objectively with CASA systems’.1
Taking these voices into account, we believe that one should not view CASA as a replacement for WHO-trained laboratory staff in performing a sperm count. However, that does not mean that new and innovative tests, the likes of which can only be performed by a computer, should be ignored. Tomlinson (2016) warns that unless testing procedures and good practice guidelines have a strong evidence base, ‘the progress towards the development of more innovative methods for investigating male infertility will be slow’. In this review, we discuss areas where development of CASA can provide such evidence, particularly through the use of cutting-edge technology and modelling.
Current CASA-Mot technologies
Current CASA-Mot systems focus almost exclusively on analysing and tracking the sperm head, with some newer systems beginning to check for the presence of a tail in order to help exclude debris and other extraneous objects from analyses. However, mechanistically it is the flagellum that propels the spermatozoon and, as such, the behaviour of the flagellum is the fundamental characteristic governing the motility of spermatozoa. We believe that the introduction of high-throughput analysis of the flagellar waveform through CASA-Mot has the potential to dramatically increase the quality and quantity of clinically relevant information that can feed into diagnostics alongside more traditional semen analysis results (for a discussion of such flagellar parameters in fish, see Prokopchuk and Cosson 2017).
Not only is it common sense to discuss the flagellar waveform when analysing the kinematics of spermatozoa (after all, it is the flagellum that dictates the movement of the cell), but the movement of the flagellum also gives insight into new areas in which current analyses are lacking. The viscosity of a semen sample is one such area. It is well documented that the viscosity of a medium significantly affects aspects of sperm kinematics, including the flagellar waveform, trajectory and rate of rolling (Suarez et al. 1991; Smith et al. 2009b). As we discuss later, the WHO (2010) test for viscosity provides a binary outcome; samples are either normally or abnormally viscous. Although such a test provides some information about a sample’s viscosity, it precludes more quantitative investigations. An example of this is the statistically significant relationship between semen hyperviscosity and leucocytospermia demonstrated by Flint et al. (2014), a relationship that was not seen using the WHO (2010) manual guidelines. Although conventional head-based CASA-Mot systems may give some indication of the effect of viscosity on sperm motility (Hyun et al. 2012), it may be that an improved test for seminal viscosity can be built into CASA-Mot, and thus provide an additional diagnostic parameter.
Introducing high-throughput real-time flagellar capture into CASA-Mot would also enable investigation into the metabolic requirements of human spermatozoa (for a discussion of the metabolism of sea urchin spermatozoa, see Tyler and Rothschild 1951; for reviews into the metabolism of mammalian spermatozoa, see Storey 2008; Ford 2006). Mathematical modelling of flagellar mechanics and low Reynolds number fluid dynamics can be combined with live cell imaging (in the form of tracked flagellar tangent angle data) to model the energy requirements of a spermatozoon as it swims through a viscous medium (Ooi et al. 2014) in the case of tethered spermatozoon. For such investigations, it will be crucial to develop fast algorithms for tracking the flagellum accurately because current CASA-Mot systems that rely on head tracking do not provide the necessary information.
The newest additions to the CASA family have been in the form of smartphone attachments (Kanakasabapathy et al. 2017; YO Sperm Test (www.yospermtest.com, accessed 28 February 2018); TENGA Men’s Loupe (www.tenga.co.jp/, accessed 28 February 2018)), and other home tests (Björndahl et al. 2006). These applications provide access to indicative diagnostics to a wide range of peoples who may not have access to traditional fertility clinics. Although such devices are in their infancy, they offer a potential platform for improving access to services and information that previously would be out of reach.
Clinical translation of current research systems
Outside the clinical world of male reproductive health there has been a rapid rise in the number and quality of new and innovative experimental imaging systems and associated algorithms for analysis coming from the basic science communities. In order to envision the best possible translation of current research systems into a clinical environment, it is important to have an understanding of the development of these techniques and how they differ from current clinical technologies. Here we focus on improvements in the analysis of the three-dimensional (3D) motility of spermatozoa, from small-depth imaging using piezoelectric devices to more modern large-volume holographic imaging techniques.
Over the past few years there has been significant progress in the technology required for analysing freely swimming spermatozoa in an unconstrained 3D environment. The first such system was constructed by Crenshaw (1990), consisting of two microscopes, each with a camera, orientated perpendicular to each other in order to capture images of a subject in two orthogonal planes. The original work, involving large amounts of frame-by-frame analysis of single sea urchin spermatozoa, was time consuming and limited by the camera technology of the time. This led to a large body of theoretical investigation into helical swimming patterns (Cortez et al. 2005; Friedrich and Jülicher 2009).
The concept of multiplane imaging was improved by Corkidi et al. (2008), with the introduction of a piezoelectric device mounted to the objective (on a single microscope), the oscillation of which allowed images to be taken in 60 planes spanning a depth of 100 µm. This system, combined with a 4200-frames per second (f.p.s.) camera, had a depth-resolution of 3.2 µm, enabling the tracking of freely swimming sea urchin spermatozoa. Work in this field has been continued by Silva-Villalobos et al. (2014), where the flagellar movement of a single human spermatozoon has been captured and analysed in three dimensions. Such advances of technology bring with them other challenges: the huge amount of data (in the form of images) generated by a camera operating at approximately 4200 f.p.s. must be analysed. This has led to the conception of advanced algorithms for the processing of the sizeable datasets output by systems such as Corkidi’s (Corkidi et al. 2008), such as the algorithms of Pimentel et al. (2012), as well as the use of morphodynamic models (da Silva 2017) for quantitative image analysis of spermatozoa.
Alongside the development of the piezoelectric imaging setup of Corkidi et al. (2008), the scientific community were beginning to realise the possibility of digital holographic microscopy (DHM) in the 3D analysis of micro-organisms. First proposed as a tool for electron holography by Gabor (1948) and then extended for optical holography (Goodman and Lawrence 1967), DHM involves a coherent monochromatic light source split into two beams, the reference and object beams, the latter of which illuminates an object. The object causes the incoming light to scatter, and the wavefront caused by this scattering is captured. The reference beam and scattered object beam are then recombined and numerically back-propagated to produce an image of the object. The first use of DHM to record images of spermatozoa was by Micó et al. (2008), but it was not until the work of Di Caprio et al. (2010) that such imaging modalities were used to improve the understanding of sperm kinematics.
Traditional DHM setups require a traditional light microscopy setup as well as additional equipment for holography and, as such, can be expensive with a key cost being the objective lens of the microscope. To reduce this cost, as well as to simplify the imaging process, digital in-line holographic microscopy (DIHM) was introduced wherein the interference pattern is recorded directly onto a charge-coupled device (CCD) camera chip. The early work with DIHM was reviewed by Garcia-Sucerquia et al. (2006). This early work has been improved greatly and has, with some incredible engineering advances, been used to uncover new understanding about full 3D sperm navigation and behaviour (Crha et al. 2011; Su et al. 2012, 2013; Merola et al. 2013; Di Caprio et al. 2014; Jikeli et al. 2015; Daloglu and Ozcan 2017).
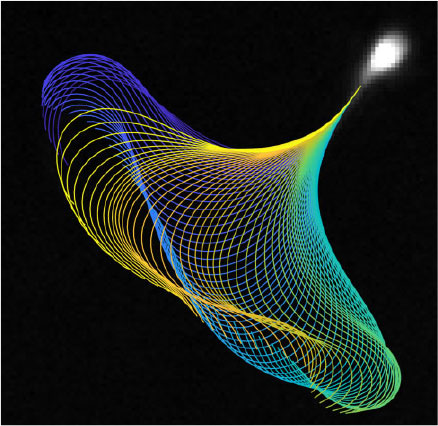
The importance of examining sperm swimming patterns in three dimensions was reviewed by Guerrero et al. (2011). Although full 3D sperm tracking (head + flagellum) has merit in aiding the scientific understanding of sperm behaviour, particularly regarding fertilisation in marine species, there are several practical downsides to the implementation of such analyses in a clinical human fertility environment, and more generally in internally fertilising species, the most obvious drawback being the need for additional equipment (although on-chip digital holography options are cheaper than traditional objective lens setups, most clinics will already have access to the latter) and training. These costs could be justified if it could be shown that there are significant relevant aspects of semen behaviour that can be seen in three dimensions but not in a conventional two-dimensional (2D) analysis. However, we believe that far from 3D analysis giving a clearer picture of how a human spermatozoon behaves in vivo, the behaviours seen in free-swimming human spermatozoa may have limited physiological relevance for reasons described below.
It is well documented in both experimental (Cosson et al. 2003) and theoretical (Rothschild 1963; Smith et al. 2009a; Denissenko et al. 2012) studies that free-swimming spermatozoa will accumulate at, and be guided by, surfaces present in their environments. For some externally fertilising marine species (e.g. sea urchins), the journey the spermatozoa must undergo to the site of fertilisation means that knowledge of the swimming patterns of such spermatozoa in a full 3D environment is essential. As such, looking at such spermatozoa with a traditional microscopy setup will not produce an accurate representation of their kinematic properties. In contrast, human spermatozoa swim in increasingly complex liquid film-like environments from the uterine cavity, which has a fluid volume of only 80–180 µL (Casslén 1986), to the labyrinth-like structure of the oviductal lumen (Suarez 2015). Environments like this make it unlikely that human spermatozoa will be freely swimming with no surface effects and, thus, it seems likely that the study of such free-swimming spermatozoa in a large-volume environment is less clinically relevant than conventional microscopy techniques. In addition, it has been shown that as spermatozoa accumulate at surfaces, the flagellar waveform becomes planar (Woolley 2003). Such planar beating reduces the need for full 3D analyses of the flagellar waveform, especially when considering the clinical case of human fertility.
Outside of the world of spermatozoa, the method of differential dynamic microscopy (DDM), has been developed for characterising the motility of micro-organisms in three dimensions, and has been suggested as a method to assess spermatozoa for biomedical purposes (Martinez et al. 2012). Instead of attempting to track individual cells directly, DDM measures characteristics analogous to the CASA motility parameters, such as the average speed and motile fraction of a sample. One of the advantages of this method is the ability to analyse a large sample (~104 cells) in a few minutes using a standard microscopy setup. Although this method would not be suitable for the analysis of single cells (e.g. to select a single cell in intracytoplasmic sperm injection (ICSI)), it could provide a useful method for the rapid screening of samples.
It is clear that there has been great technological progress in the 3D imaging of sperm swimming patterns in recent years. Although there is some evidence to suggest that investigations into the 3D swimming patterns of spermatozoa could have some diagnostic relevance, it seems that the relevance of 3D analyses in a clinical setting is yet to be established.
We suggest, then, that there is much to be gained from developing fast, efficient 2D algorithms for flagellar tracking.
The role of viscosity as a diagnostic tool
The importance of viscosity in the analysis of semen is largely overlooked in current practices. As already discussed, the WHO (2010) binary test for viscosity disregards the clinical relevance of the viscosity of a sample, except when it is considered to be very viscous. It has been demonstrated that it is straightforward to obtain a quantitative assessment of semen viscosity; the work of Rijnders et al. (2007), based on theory by Douglas-Hamilton et al. (2005), showed that accurate results can be calculated by timing how long it takes a sample to fill a 20-µm deep Leja chamber by capillary action. It may be possible to use slides with additional markings or chambers (such as the Proiser ISAS D4C) to increase the ease and accuracy by which the filling time can be measured, and thus, in the future, to make a more accurate viscosity measurement of a sample routine. The challenge for this, as for any change, is to generate the underpinning evidence that supports inclusion into standard diagnostic practice.
It is essential to note that, on the microscopic scales in which spermatozoa swim, the inertial forces in a fluid are minuscule when compared with viscous forces, corresponding mathematically to a very small Reynolds number. This viscous dominance is a consequence of the size of the spermatozoa rather than the value of the viscosity per se. Thus, one must be extremely careful in using intuition in thinking about such flows, because a major characteristic of low Reynolds number flows is the absence of any inertia. For excellent introductions to the field of small Reynolds number fluid dynamics, see Purcell (1977) and Lauga and Powers (2009).
There is clear evidence from both experimental studies and mathematical modelling and simulation that the presence of viscosity modulates sperm swimming behaviour (Brokaw 1966, 1975; Suarez et al. 1991; Smith et al. 2009b). However, it is not only viscous effects that are important; recent work by Tung et al. (2017) has demonstrated that bovine spermatozoa exhibit marked collective swimming behaviours when placed in viscoelastic fluids, behaviour that was not present in Newtonian fluids of low and high viscosity (Woolley et al. 2009). Although such behaviours have not yet been demonstrated in humans, it has been shown that there are significant variations in the viscoelasticity of cervical mucus during the ovulatory menstrual cycle (Wolf et al. 1977).
An interesting, but as yet unexplored, question is how the ratio of viscosity between cervical mucus and semen affects penetration. Unpublished experiments by our group (J. C. Kirkman-Brown, D. J. Smith, T. J. Connolly and R. Frettsome, unpubl. data) indicate that there is a significant difference in the results of a capillary penetration assay (for an explanation of the methods used, see Ivic et al. 2002), with prepared spermatozoa performing much worse. Traditionally, poor penetration is viewed as a deficiency in kinematic behaviour of spermatozoa. In the situation where the ratio in viscosities is large, we should potentially be considering this as a viscosity problem rather than a swimming problem. However, more data need to be gathered to investigate this.
An in-depth review into the need for understanding the effect of viscosity was presented by Kirkman-Brown and Smith (2011). We feel that this is an area where more progress is needed, and one that could have a large effect on clinical diagnostics.
Mathematical modelling for mechanical and metabolic insight from imaging
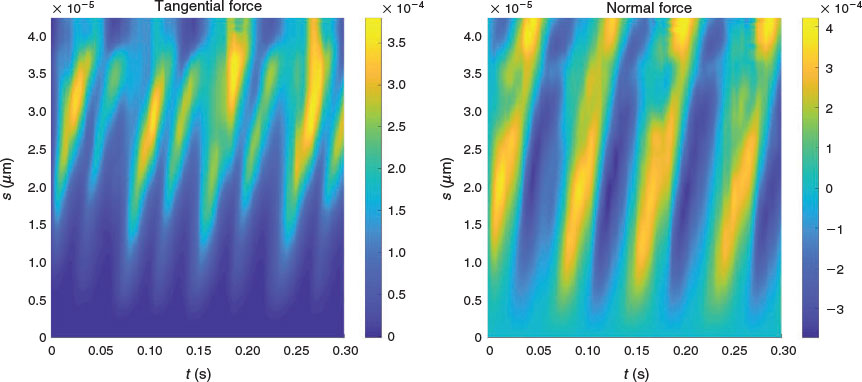
There is an increasingly large body of work in the mathematical fluid mechanics of motile cells in low Reynolds number flows, and particularly relating to spermatozoa (Gaffney et al. 2011). The simplest model is that of Gray and Hancock (1955), who established the resistive force theory for sperm propulsion, stating that the thrust exerted by the propagation of a flagellar wave can be approximated by calculation of the tangential and normal components of the flagellar velocity, multiplied by drag coefficients associated with motion in each direction (Cn and Ct respectively, with Cn/Ct ≈ 2). Given tracking data of the flagellar waveform (see Fig. 1), it is straightforward to parameterise the waveform by tangent angle as a function of the arc length along the flagellum and time, from which one can calculate the velocities of the flagellum in the tangential and normal directions. Such velocity data can then be multiplied by the drag coefficients to give the resistive force theory approximation to the tangential and normal force exerted by the flagellum on the surrounding fluid (for an example of this from tracked data, see Fig. 2 and Ooi et al. 2014). In order to exploit the information available from coupling efficient flagellar tracking algorithms with live cell imaging data, the sliding filament model for flagellar bend propagation proposed by Brokaw (1971) can be extended to couple the total force produced by a flagellum with a model for the relative contributions of the constituent components of force in the flagellum to investigate the internal activity and rate of working of interfilament active forces, and answer questions regarding the energy transport along the flagellum. More details regarding the calculation of such aspects of the flagellum are provided by Gaffney et al. (2011). There are also more accurate methods for fluid mechanics, such as the regularised Stokeslet methods of Gillies et al. (2009) and the use of boundary element and principal component analysis by Ishimoto et al. (2017).
|
Existing tracking techniques in CASA-Mot, as well as the majority of wider image processing, is performed through the use of algorithmic manipulation of images in order to generate useful data, for example the thresholding of pixel data and fitting of curves. Although this approach has been very successful, and often provides useful information, there is an untapped opportunity to analyse data through modelling the biology and physics that produced the image. To this end, we developed the concept of model-based image analysis, discussed in the context of imaging nanowire sensors by Gallagher et al. (2017). Through knowledge of the physics of image formation, including models of optical effects, such as diffusion, and understanding of how the imaging subject behaves, we can construct a mathematical framework for the inverse problem of image formation; that is, given an experimental image, how do we measure the subject without introducing extra errors into the results through ad hoc manipulations? The principles of using a mathematical basis for analysing spermatozoa can be seen in the work of da Silva (2017) and van der Horst and Sanchez (2016).
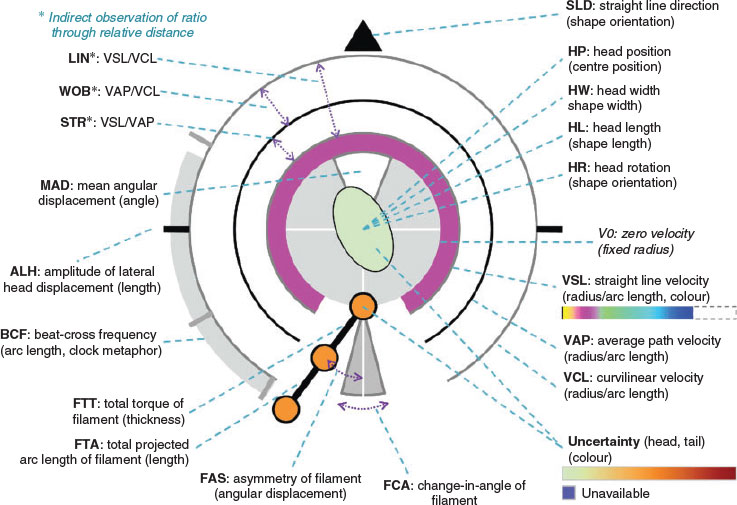
Although the focus of improving CASA-Mot has historically been on improving the accuracy of CASA-Mot results, it is also imperative to consider how those results are displayed. The increasingly large amounts of data generated from CASA-Mot analyses need to be visualised for diagnostic use. To this end, van der Horst et al. (1999) introduced the use of star glyphs for displaying five kinematic parameters of spermatozoa, namely curvilinear velocity (VCL), straight line velocity (VSL), average path velocity (VAP), linearity (LIN) and dance (DNC). Subsequently, Duffy et al. (2015) developed a way of displaying the multitude of data from a semen analysis, as well as additional mechanical parameters, in a glyph schematic (see Fig. 3), thus reducing the need for repetitive watching of videos or digesting endless tables of data.
|
Conclusions
There has been a tendency to characterise CASA as solely a tool for performing current semen analyses through the use of a computer instead of the eyes of a technician. There is merit in the view that one should be sceptical about how accurately CASA, for example in a sperm count, can be when compared with a trained technician. However, where CASA can be revolutionary is in the areas where traditional semen analyses are unable to tread; a prime example of this is through in-depth flagellar tracking and analysis, the value of which we hope we have convinced the reader of. We believe that the development and inclusion of flagellar tracking into current CASA-Mot systems will greatly improve its use as a tool for clinical diagnostics.
There is certainly room for new tests involving a more holistic approach to semen analysis. As discussed, investigations into the effect of viscosity and the energy requirements of the cell are two areas where current analyses are lacking, but one could also consider the sensory abilities of the flagellum, response to ambient flow and biochemical stimuli, statistics on the metabolic state of clinical samples and many other aspects of spermatozoa. In the future, it may then be possible to combine all these data, together with knowledge of clinical outcomes, and apply the ideas of machine learning and pattern recognition to develop a complete model for the prediction of penetration, migration and fertilisation, and implement such a model in a clinically and diagnostically relevant manner.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgements
The ongoing support of patients and staff at the Birmingham Women’s and Children’s NHS Foundation Trust are fundamental to our research work. The authors gratefully acknowledge funding from the Engineering and Physical Sciences Research Council, Healthcare Technologies Challenge Award (EP/N021096/1). Jackson Kirkman-Brown is funded by a National Institute of Health Research (NIHR), and Health Education England, Senior Clinical Lectureship Grant: The role of the human sperm in healthy live birth (NIHRDH-HCS SCL-2014-05-001). This article presents independent research funded in part by the National Institute for Health Research NIHR and Health Education England. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
References
Björndahl, L., Kirkman-Brown, J., Hart, G., Rattle, S., and Barratt, C. L. R. (2006). Development of a novel home sperm test. Hum. Reprod. 21, 145–149.| Development of a novel home sperm test.Crossref | GoogleScholarGoogle Scholar |
Brokaw, C. J. (1966). Effects of increased viscosity on the movements of some invertebrate spermatozoa. J. Exp. Biol. 45, 113–139.
Brokaw, C. J. (1971). Bend propagation by a sliding filament model for flagella. J. Exp. Biol. 55, 289–304.
Brokaw, C. J. (1975). Effects of viscosity and ATP concentration on the movement of reactivated sea-urchin sperm flagella. J. Exp. Biol. 62, 701–719.
Brokaw, C. J. (1991). Calcium sensors in sea urchin sperm flagella. Cell Motil. Cytoskeleton 18, 123–130.
| Calcium sensors in sea urchin sperm flagella.Crossref | GoogleScholarGoogle Scholar |
Casslén, B. (1986). Uterine fluid volume. Cyclic variations and possible extrauterine contributions. J. Reprod. Med. 31, 506–510.
Corkidi, G., Taboada, B., Wood, C. D., Guerrero, A., and Darszon, A. (2008). Tracking sperm in three-dimensions. Biochem. Biophys. Res. Commun. 373, 125–129.
| Tracking sperm in three-dimensions.Crossref | GoogleScholarGoogle Scholar |
Cortez, R., Fauci, L., and Medovikov, A. (2005). The method of regularized Stokeslets in three dimensions: analysis, validation, and application to helical swimming. Phys. Fluids 17, 031504.
| The method of regularized Stokeslets in three dimensions: analysis, validation, and application to helical swimming.Crossref | GoogleScholarGoogle Scholar |
Cosson, J., Huitorel, P., and Gagnon, C. (2003). How spermatozoa come to be confined to surfaces. Cell Motil. Cytoskeleton 54, 56–63.
| How spermatozoa come to be confined to surfaces.Crossref | GoogleScholarGoogle Scholar |
Crenshaw, H. C. (1990). Helical orientation – a novel mechanism for the orientation of microorganisms. In ‘Biological Motion’. (Eds W. Alt and G. Hoffmann.) pp. 361–386. (Springer: Berlin, Heidelberg.)
Crha, I., Zakova, J., Huser, M., Ventruba, P., Lousova, E., and Pohanka, M. (2011). Digital holographic microscopy in human sperm imaging. J. Assist. Reprod. Genet. 28, 725–729.
| Digital holographic microscopy in human sperm imaging.Crossref | GoogleScholarGoogle Scholar |
da Silva, P. A. P. (2017). Quantitative image analysis of cells using morphodynamical models: sea urchin spermatozoa as case study. Ph.D. Thesis, Intituto de Technologia Quimica E Biologica Antonion Xavier (I.T.Q.B.) Universidade Nova de Lisboa.
Daloglu, M. U., and Ozcan, A. (2017). Computational imaging of sperm locomotion. Biol. Reprod. 97, 182–188.
| Computational imaging of sperm locomotion.Crossref | GoogleScholarGoogle Scholar |
Denissenko, P., Kantsler, V., Smith, D. J., and Kirkman-Brown, J. (2012). Human spermatozoa migration in microchannels reveals boundary-following navigation. Proc. Natl Acad. Sci. USA 109, 8007–8010.
| Human spermatozoa migration in microchannels reveals boundary-following navigation.Crossref | GoogleScholarGoogle Scholar |
Di Caprio, G., Gioffre, M. A., Saffioti, N., Grilli, S., Ferraro, P., Puglisi, R., Balduzzi, D., Galli, A., and Coppola, G. (2010). Quantitative label-free animal sperm imaging by means of digital holographic microscopy. IEEE J. Sel. Top. Quant. 16, 833–840.
| Quantitative label-free animal sperm imaging by means of digital holographic microscopy.Crossref | GoogleScholarGoogle Scholar |
Di Caprio, G., El Mallahi, A., Ferraro, P., Dale, R., Coppola, G., Dale, B., Coppola, G., and Dubois, F. (2014). 4D tracking of clinical seminal samples for quantitative characterization of motility parameters. Biomed. Opt. Express 5, 690–700.
| 4D tracking of clinical seminal samples for quantitative characterization of motility parameters.Crossref | GoogleScholarGoogle Scholar |
Douglas-Hamilton, D. H., Smith, N. G., Kuster, C. E., Vermeiden, J. P. W., and Althouse, G. C. (2005). Capillary-loaded particle fluid dynamics: effect on estimation of sperm concentration. J. Androl. 26, 115–122.
Duffy, B., Thiyagalingam, J., Walton, S., Smith, D. J., Trefethen, A., Kirkman-Brown, J. C., Gaffney, E. A., and Chen, M. (2015). Glyph-based video visualization for semen analysis. IEEE Trans. Vis. Comput. Graph. 21, 980–993.
| Glyph-based video visualization for semen analysis.Crossref | GoogleScholarGoogle Scholar |
Flint, M., Plessis, S. S., and Menkveld, R. (2014). Revisiting the assessment of semen viscosity and its relationship to leucocytospermia. Andrologia 46, 837–841.
| Revisiting the assessment of semen viscosity and its relationship to leucocytospermia.Crossref | GoogleScholarGoogle Scholar |
Ford, W. C. L. (2006). Glycolysis and sperm motility: does a spoonful of sugar help the flagellum go round? Hum. Reprod. Update 12, 269–274.
| Glycolysis and sperm motility: does a spoonful of sugar help the flagellum go round?Crossref | GoogleScholarGoogle Scholar |
Friedrich, B. M., and Jülicher, F. (2009). Steering chiral swimmers along noisy helical paths. Phys. Rev. Lett. 103, 068102.
| Steering chiral swimmers along noisy helical paths.Crossref | GoogleScholarGoogle Scholar |
Gabor, D. (1948). A new microscopic principle. Nature 161, 777–778.
| A new microscopic principle.Crossref | GoogleScholarGoogle Scholar |
Gaffney, E. A., Gadêlha, H., Smith, D. J., Blake, J. R., and Kirkman-Brown, J. C. (2011). Mammalian sperm motility: observation and theory. Annu. Rev. Fluid Mech. 43, 501–528.
| Mammalian sperm motility: observation and theory.Crossref | GoogleScholarGoogle Scholar |
Gallagher, M. T., Neal, C. V., Arkill, K. P., and Smith, D. J. (2017). Model-based image analysis of a tethered Brownian fibre for shear stress sensing. J. R. Soc. Interface 14, 20170564.
| Model-based image analysis of a tethered Brownian fibre for shear stress sensing.Crossref | GoogleScholarGoogle Scholar |
Garcia-Sucerquia, J., Xu, W., Jericho, S. K., Klages, P., Jericho, M. H., and Kreuzer, H. J. (2006). Digital in-line holographic microscopy. Appl. Opt. 45, 836–850.
| Digital in-line holographic microscopy.Crossref | GoogleScholarGoogle Scholar |
Gillies, E. A., Cannon, R. M., Green, R. B., and Pacey, A. A. (2009). Hydrodynamic propulsion of human sperm. J. Fluid Mech. 625, 445–474.
| Hydrodynamic propulsion of human sperm.Crossref | GoogleScholarGoogle Scholar |
Goodman, J. W., and Lawrence, R. W. (1967). Digital image formation from electronically detected holograms. Appl. Phys. Lett. 11, 77–79.
| Digital image formation from electronically detected holograms.Crossref | GoogleScholarGoogle Scholar |
Gray, J., and Hancock, G. J. (1955). The propulsion of sea-urchin spermatozoa. J. Exp. Biol. 32, 802–814.
Guerrero, A., Carneiro, J., Pimentel, A., Wood, C. D., Corkidi, G., and Darszon, A. (2011). Strategies for locating the female gamete: the importance of measuring sperm trajectories in three spatial dimensions. Mol. Hum. Reprod. 17, 511–523.
| Strategies for locating the female gamete: the importance of measuring sperm trajectories in three spatial dimensions.Crossref | GoogleScholarGoogle Scholar |
Hiramoto, Y., and Baba, S. A. (1978). A quantitative analysis of flagellar movement in echinoderm spermatozoa. J. Exp. Biol. 76, 85–104.
Holt, W. V., Moore, H. D. M., and Hillier, S. G. (1985). Computer-assisted measurement of sperm swimming speed in human semen: correlation of results with in vitro fertilization assays. Fertil. Steril. 44, 112–119.
| Computer-assisted measurement of sperm swimming speed in human semen: correlation of results with in vitro fertilization assays.Crossref | GoogleScholarGoogle Scholar |
Hyun, N., Chandsawangbhuwana, C., Yang-Wong, C., Berns, M. W., Zhu, Q., and Shi, L. Z. (2012). Effects of viscosity on sperm motility studied with optical tweezers. J. Biomed. Opt. 17, 025005.
| Effects of viscosity on sperm motility studied with optical tweezers.Crossref | GoogleScholarGoogle Scholar |
Ishimoto, K., Gadêlha, H., Gaffney, E. A., Smith, D. J., and Kirkman-Brown, J. (2017). Coarse-graining the fluid flow around a human sperm. Phys. Rev. Lett. 118, 124501.
| Coarse-graining the fluid flow around a human sperm.Crossref | GoogleScholarGoogle Scholar |
Ivic, A., Onyeaka, H., Girling, A., Brewis, I. A., Ola, B., Hammadieh, N., Papaioannou, S., and Barratt, C. L. R. (2002). Critical evaluation of methylcellulose as an alternative medium in sperm migration tests. Hum. Reprod. 17, 143–149.
| Critical evaluation of methylcellulose as an alternative medium in sperm migration tests.Crossref | GoogleScholarGoogle Scholar |
Jikeli, J. F., Alvarez, L., Friedrich, B. M., Wilson, L. G., Pascal, R., Colin, R., Pichlo, M., Rennhack, A., Brenker, C., and Kaupp, U. B. (2015). Sperm navigation along helical paths in 3D chemoattractant landscapes. Nat. Commun. 6, 7985–7994.
| Sperm navigation along helical paths in 3D chemoattractant landscapes.Crossref | GoogleScholarGoogle Scholar |
Kanakasabapathy, M. K., Sadasivam, M., Singh, A., Preston, C., Thirumalaraju, P., Venkataraman, M., Bormann, C. L., Draz, M. S., Petrozza, J. C., and Shafiee, H. (2017). An automated smartphone-based diagnostic assay for point-of-care semen analysis. Sci. Transl. Med. 9, eaai7863.
| An automated smartphone-based diagnostic assay for point-of-care semen analysis.Crossref | GoogleScholarGoogle Scholar |
Katz, D. F., and Overstreet, J. W. (1981). Sperm motility assessment by videomicrography. Fertil. Steril. 35, 188–193.
| Sperm motility assessment by videomicrography.Crossref | GoogleScholarGoogle Scholar |
Kirkman-Brown, J. C., and Smith, D. J. (2011). Sperm motility: is viscosity fundamental to progress? Mol. Hum. Reprod. 17, 539–544.
| Sperm motility: is viscosity fundamental to progress?Crossref | GoogleScholarGoogle Scholar |
Lauga, E., and Powers, T. R. (2009). The hydrodynamics of swimming microorganisms. Rep. Prog. Phys. 72, 096601.
| The hydrodynamics of swimming microorganisms.Crossref | GoogleScholarGoogle Scholar |
Machin, K. E. (1958). Wave propagation along flagella. J. Exp. Biol. 35, 796–806.
Martinez, V. A., Besseling, R., Croze, O. A., Tailleur, J., Reufer, M., Schwarz-Linek, J., Wilson, L. G., Bees, M. A., and Poon, W. C. K. (2012). Differential dynamic microscopy: a high-throughput method for characterizing the motility of microorganisms. Biophys. J. 103, 1637–1647.
| Differential dynamic microscopy: a high-throughput method for characterizing the motility of microorganisms.Crossref | GoogleScholarGoogle Scholar |
Merola, F., Miccio, L., Memmolo, P., Di Caprio, G., Galli, A., Puglisi, R., Balduzzi, D., Coppola, G., Netti, P., and Ferraro, P. (2013). Digital holography as a method for 3D imaging and estimating the biovolume of motile cells. Lab Chip 13, 4512–4516.
| Digital holography as a method for 3D imaging and estimating the biovolume of motile cells.Crossref | GoogleScholarGoogle Scholar |
Micó, V., Zalevsky, Z., Ferreira, C., and García, J. (2008). Superresolution digital holographic microscopy for three-dimensional samples. Opt. Express 16, 19260–19270.
| Superresolution digital holographic microscopy for three-dimensional samples.Crossref | GoogleScholarGoogle Scholar |
Miki, K., and Clapham, D. E. (2013). Rheotaxis guides mammalian sperm. Curr. Biol. 23, 443–452.
| Rheotaxis guides mammalian sperm.Crossref | GoogleScholarGoogle Scholar |
Mortimer, S. T., van der Horst, G., and Mortimer, D. (2015). The future of computer-aided sperm analysis. Asian J. Androl. 17, 545–553.
| The future of computer-aided sperm analysis.Crossref | GoogleScholarGoogle Scholar |
Ooi, E. H., Smith, D. J., Gadêlha, H., Gaffney, E. A., and Kirkman-Brown, J. C. (2014). The mechanics of hyperactivation in adhered human sperm. R. Soc. Open Sci. 1, 140230.
| The mechanics of hyperactivation in adhered human sperm.Crossref | GoogleScholarGoogle Scholar |
Pimentel, J. A., Carneiro, J., Darszon, A., and Corkidi, G. (2012). A segmentation algorithm for automated tracking of fast swimming unlabelled cells in three dimensions. J. Microsc. 245, 72–81.
| A segmentation algorithm for automated tracking of fast swimming unlabelled cells in three dimensions.Crossref | GoogleScholarGoogle Scholar |
Prokopchuk, G., and Cosson, J. (2017). Biophysics of fish sperm flagellar movement: present knowledge and original directions. In ‘Cytoskeleton – Structure, Dynamics, Function and Disease’. (Ed. J. C. Jimenez-Lopez.) Chapter 7. (InTech.) Available from: https://mts.intechopen.com/books/cytoskeleton-structure-dynamics-function-and-disease/biophysics-of-fish-sperm-flagellar-movement-present-knowledge-and-original-directions [verified 28 February 2018].
Purcell, E. M. (1977). Life at low Reynolds number. Am. J. Phys. 45, 3–11.
| Life at low Reynolds number.Crossref | GoogleScholarGoogle Scholar |
Rijnders, S., Bolscher, J. G. M., McDonnell, J., and Vermeiden, J. P. W. (2007). Andrology lab corner: filling time of a lamellar capillary-filling semen analysis chamber is a rapid, precise, and accurate method to assess viscosity of seminal plasma. J. Androl. 28, 461–465.
| Andrology lab corner: filling time of a lamellar capillary-filling semen analysis chamber is a rapid, precise, and accurate method to assess viscosity of seminal plasma.Crossref | GoogleScholarGoogle Scholar |
Rothschild, L. (1963). Non-random distribution of bull spermatozoa in a drop of sperm suspension. Nature 198, 1221–1222.
| Non-random distribution of bull spermatozoa in a drop of sperm suspension.Crossref | GoogleScholarGoogle Scholar |
Sanders, D., Fensome-Rimmer, S., and Woodward, B. (2017). Uncertainty of measurement in andrology: UK best practice guideline from the Association of Biomedical Andrologists. Br. J. Biomed. Sci. 74, 157–162.
| Uncertainty of measurement in andrology: UK best practice guideline from the Association of Biomedical Andrologists.Crossref | GoogleScholarGoogle Scholar |
Silva-Villalobos, F., Pimentel, J. A., Darszon, A., and Corkidi, G. (2014). Imaging of the 3D dynamics of flagellar beating in human sperm. In ‘2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society’, 26–30 August 2014, Chicago, IL, USA. pp. 190–193. (IEEE: Chicago, IL, USA.)
Smith, D. J., Gaffney, E. A., Blake, J. R., and Kirkman-Brown, J. C. (2009a). Human sperm accumulation near surfaces: a simulation study. J. Fluid Mech. 621, 289–320.
| Human sperm accumulation near surfaces: a simulation study.Crossref | GoogleScholarGoogle Scholar |
Smith, D. J., Gaffney, E. A., Gadêlha, H., Kapur, N., and Kirkman-Brown, J. C. (2009b). Bend propagation in the flagella of migrating human sperm, and its modulation by viscosity. Cell Motil. Cytoskeleton 66, 220–236.
| Bend propagation in the flagella of migrating human sperm, and its modulation by viscosity.Crossref | GoogleScholarGoogle Scholar |
Storey, B. T. (2008). Mammalian sperm metabolism: oxygen and sugar, friend and foe. Int. J. Dev. Biol. 52, 427–437.
Su, T.-W., Xue, L., and Ozcan, A. (2012). High-throughput lensfree 3D tracking of human sperms reveals rare statistics of helical trajectories. Proc. Natl Acad. Sci. USA 109, 16018–16022.
| High-throughput lensfree 3D tracking of human sperms reveals rare statistics of helical trajectories.Crossref | GoogleScholarGoogle Scholar |
Su, T.-W., Choi, I., Feng, J., Huang, K., McLeod, E., and Ozcan, A. (2013). Sperm trajectories form chiral ribbons. Sci. Rep. 3, 1664–1671.
| Sperm trajectories form chiral ribbons.Crossref | GoogleScholarGoogle Scholar |
Suarez, S. S. (2015). Gamete and zygote transport. In ‘Kobil and Neill’s Physiology of Reproduction’. (Eds T. M. Plant and A. Zeleznik.) pp. 197–232. (Academic Press: New York.)
Suarez, S. S., Katz, D. F., Owen, D. H., Andrew, J. B., and Powell, R. L. (1991). Evidence for the function of hyperactivated motility in sperm. Biol. Reprod. 44, 375–381.
| Evidence for the function of hyperactivated motility in sperm.Crossref | GoogleScholarGoogle Scholar |
Talarczyk-Desole, J., Berger, A., Taszarek-Hauke, G., Hauke, J., Pawelczyk, L., and Jedrzejczak, P. (2017). Manual vs. computer-assisted sperm analysis: can CASA replace manual assessment of human semen in clinical practice? Ginekol. Pol. 88, 56–60.
| Manual vs. computer-assisted sperm analysis: can CASA replace manual assessment of human semen in clinical practice?Crossref | GoogleScholarGoogle Scholar |
Tomlinson, M. J. (2016). Uncertainty of measurement and clinical value of semen analysis: has standardisation through professional guidelines helped or hindered progress? Andrology 4, 763–770.
| Uncertainty of measurement and clinical value of semen analysis: has standardisation through professional guidelines helped or hindered progress?Crossref | GoogleScholarGoogle Scholar |
Tomlinson, M. J., Pooley, K., Simpson, T., Newton, T., Hopkisson, J., Jayaprakasan, K., Jayaprakasan, R., Naeem, A., and Pridmore, A. (2010). Validation of a novel computer-assisted sperm analysis (CASA) system using multitarget-tracking algorithms. Fertil. Steril. 93, 1911–1920.
| Validation of a novel computer-assisted sperm analysis (CASA) system using multitarget-tracking algorithms.Crossref | GoogleScholarGoogle Scholar |
Tung, C.-K., Lin, C., Harvey, B., Fiore, A. G., Ardon, F., Wu, M., and Suarez, S. S. (2017). Fluid viscoelasticity promotes collective swimming of sperm. Sci. Rep. 7, 3152–3160.
| Fluid viscoelasticity promotes collective swimming of sperm.Crossref | GoogleScholarGoogle Scholar |
Tyler, A., and Rothschild, L. (1951). Metabolism of sea urchin spermatozoa and induced anaerobic motility in solutions of amino acids. Proc. Soc. Exp. Biol. Med. 76, 52–58.
| Metabolism of sea urchin spermatozoa and induced anaerobic motility in solutions of amino acids.Crossref | GoogleScholarGoogle Scholar |
van der Horst, G., and Sanchez, E. (2016). Tracking sperm movement in four dimensions and models for sperm functionality. J. Polish Soc. Androl. 3, 72–73.
van der Horst, G., Seier, J. V., Spinks, A. C., and Hendricks, S. (1999). The maturation of sperm motility in the epididymis and vas deferens of the vervet monkey, Cercopithecus aethiops. Int. J. Androl. 22, 197–207.
| The maturation of sperm motility in the epididymis and vas deferens of the vervet monkey, Cercopithecus aethiops.Crossref | GoogleScholarGoogle Scholar |
World Health Organization (WHO) (2010). ‘WHO Laboratory Manual for the Examination and Processing of Human Semen.’ 5th edn. (WHO: Geneva, Switzerland.)
Wolf, D. P., Blasco, L., Khan, M. A., and Litt, M. (1977). Human cervical mucus. II. Changes in viscoelasticity during the ovulatory menstrual cycle. Fertil. Steril. 28, 47–52.
| Human cervical mucus. II. Changes in viscoelasticity during the ovulatory menstrual cycle.Crossref | GoogleScholarGoogle Scholar |
Woolley, D. M. (2003). Motility of spermatozoa at surfaces. Reproduction 126, 259–270.
| Motility of spermatozoa at surfaces.Crossref | GoogleScholarGoogle Scholar |
Woolley, D. M., Crockett, R. F., Groom, W. D. I., and Revell, S. G. (2009). A study of synchronisation between the flagella of bull spermatozoa, with related observations. J. Exp. Biol. 212, 2215–2223.
| A study of synchronisation between the flagella of bull spermatozoa, with related observations.Crossref | GoogleScholarGoogle Scholar |
1 van der Horst, G. (2016). Standardization of semen analysis for humans. Microptic S. L. Blog. Available at http://www.micropticsl.net/wordpress/standardization-of-semen-analysis-for-humans/, [verified accessed August 2017]. van der Horst, G. (2017). IVF including ICSI needs CASA sperm functionality more than ever before! Microptic S. L. Blog. Available at http://www.micropticsl.net/wordpress/ivf-including-icsi-needs-casa-sperm-functionality-more-than-ever-before-2/, [verified accessed August 2017].