Exposure to atrazine during puberty reduces sperm viability, increases weight gain and alters the expression of key metabolic genes in the liver of male mice
Laura E. Cook A , Bethany J. Finger A , Mark P. Green A and Andrew J. Pask A B
A B
A School of BioSciences, The University of Melbourne, Melbourne, Vic. 3010, Australia.
B Corresponding author. Email: a.pask@unimelb.edu.au
Reproduction, Fertility and Development 31(5) 920-931 https://doi.org/10.1071/RD18505
Submitted: 20 July 2018 Accepted: 16 December 2018 Published: 14 January 2019
Journal Compilation © CSIRO 2019 Open Access CC BY-NC-ND
Abstract
Atrazine (ATZ) is one of the most widely used herbicides worldwide and is a common contaminant in human drinking water. It disrupts metabolic pathways in plants, and has metabolic and reproductive effects in vertebrates, including humans. Few studies have investigated the effects of exposure to low doses of ATZ, especially during sexual development in males. In this study, we exposed C57BL/6J male mice from weaning for 8 weeks to drinking water containing 0.5 mg kg−1 bodyweight (BW) day−1 ATZ, the ‘no observed effect’ level used by the Australian government, or a 10-fold higher dose (5 mg kg−1 BW day−1). Mice treated with the low dose of ATZ showed increased total and cumulative weight gain. At 12 weeks of age, there was a significant increase in the percentage of dead spermatozoa in both ATZ-exposed groups, as well as decreased epididymal sperm motility in the low-dose ATZ group. Significant changes in testis and liver gene expression were also observed following ATZ exposure. These data demonstrate that a low dose of ATZ can perturb metabolic and reproductive characteristics in male mice. A chronic reduction in sperm quality and increased weight gain could have negative consequences on the reproductive capacity of males, and further studies should consider the effects of long-term ATZ exposure on male reproductive health.
Additional keywords: bodyweight, endocrine disruptor, reproduction.
Introduction
Widespread exposure of humans to endocrine disrupting chemicals (EDCs) is thought to be contributing to the rapid decline in male reproductive health (Skakkebaek 2002; Gore et al. 2015). Western and industrialised countries are reporting decreased sperm counts (Swan et al. 2000; Jørgensen et al. 2012), an increased incidence of testicular cancer (Huyghe et al. 2007) and an increase in cryptorchidism and hypospadias, male congenital malformations (Toppari et al. 2001). Puberty is one of the critical windows of male sexual development regulated by coordinated hormonal signalling regimens and when spermatogenesis is established. Exposure to EDCs during this time could be affecting the reproductive outcomes of adult males in both wildlife and humans. It is known that embryonic exposure to EDCs can affect the timing of pubertal onset, testis development and later sperm health in F1 and F3 male mice (Doyle et al. 2013). Yet, few studies have focused on the effects of EDC exposure during the peripubertal period through to sexual maturity on reproductive outcomes in males.
One EDC of particular concern is atrazine (ATZ; 2-chloro-4-ethylamino-6-isopropylamino-1,3,5-triazine), which has been banned in the European Union (EU) since 2003 due to its consistent high levels in groundwater and potential effects on human health (European Commission Health and Consumer Protection Directorate General 2003). Despite the EU ban, ATZ remains one of the most widely used herbicides, with approximately 33 000 and 3000 tons used annually in the US (Farruggia et al. 2016) and Australia (Radcliffe 2002) respectively. Consequently, ATZ is one of the most commonly detected contaminants in human drinking water, with average concentrations of 5 μg L−1, although concentrations as high as 88 μg L−1 have been measured in the US (Graymore et al. 2001; Badach et al. 2007). In Australian river systems, ATZ concentrations have been measured as high as 2.4 mg L−1 (Radcliffe 2002). Because of its long half-life of >100 days in water (Australian Pesticides and Veterinary Medicines Authority 1997) and 240 days in the soil (US Environmental Protection Agency 2003), as well as its human health concerns, the Australian National Health and Medical Research Council (NHMRC) together with the National Resource Management Ministerial Council (NRMMC) has set the safe level guideline of ATZ in human drinking water at 0.02 mg L−1 (NHMRC and NRMMC 2011). This guideline was based on a 2-year no-observed-effect level (NOEL) study in female rats treated with 0.5 mg kg−1 bodyweight (BW) day−1 ATZ, with the end point based on the incidence of mammary tumours (National Health and Medical Research Council and National Resource Management Ministerial Council (NHMRC and NRMMC 2011). Regulation of ATZ in drinking water is more stringent in the US than in Australia, with a safe-level cut-off of 0.003 mg L−1 in the US (US Environmental Protection Agency 2003).
ATZ is capable of disrupting the endocrine, metabolic and nervous systems (Mizota and Ueda 2006; Hayes et al. 2011; Jin et al. 2015) and causes major abnormalities of the male reproductive system in vertebrates, including rats (Abarikwu et al. 2010; Victor-Costa et al. 2010), amphibians (Hayes et al. 2006), reptiles and fish (Spanò et al. 2004; Suzawa and Ingraham 2008). ATZ affects development of the reproductive system in several ways. Initially, ATZ was determined to be antiandrogenic (Hayes et al. 2011) by decreasing the expression of 5α-reductase, the enzyme responsible for the conversion of testosterone to the more potent androgen dihydrotestosterone (DHT; Kniewald et al. 1995). More recently, ATZ was also found to have oestrogenic effects via direct upregulation of the enzyme aromatase (encoded by the cytochrome P450 family 19 subfamily A member 1 (Cyp19a1) gene; Fan et al. 2007; Holloway et al. 2008), which converts testosterone to oestrogen. ATZ indirectly upregulates the expression of Cyp19a1 via its effect on steroidogenic factor-1 (Sf-1; also known as nuclear receptor subfamily 5 group A member 1 ( Nr5a1)) and by increasing cAMP production, as determined in both mammalian and fish cell lines (Roberge et al. 2004; Fan et al. 2007). These actions of ATZ result in disruption of steroid hormone concentrations.
ATZ can also affect male reproduction via secondary effects on metabolism. ATZ causes alterations in bodyweight, induces oxidative stress and changes the homeostasis of fatty acid and glucose metabolism in mature mice and rats (Lim et al. 2009; Jin et al. 2014, 2015). It is possible that ATZ exposure may manifest in more severe metabolic effects, based on research that has identified an association between exposure to EDCs in early life and non-alcoholic fatty liver disease (NAFLD) in adulthood (Foulds et al. 2017). Importantly, changes in metabolism are often associated with obesity, which affects male reproductive health by altering sperm quantity and quality, leading to decreased fertility and negative health outcomes for subsequent generations (Kasturi et al. 2008; Fullston et al. 2015).
Rodent studies of postnatal exposure to supraenvironmental ATZ levels report decreased bodyweights (Kniewald et al. 2000; Victor-Costa et al. 2010), decreased male sex organ weights (Kniewald et al. 1995; Stoker et al. 2000, 2002; Pogrmic et al. 2009; Abarikwu et al. 2010), changed expression of steroidogenic genes (Pogrmic et al. 2009; Jin et al. 2013; Riffle et al. 2014; Gely-Pernot et al. 2015), decreased testicular and epididymal sperm counts and sperm motility, with an increased percentage of dead and abnormal spermatozoa (Abarikwu et al. 2010; Song et al. 2014; Gely-Pernot et al. 2015). What is apparent is that although the effects of ATZ are concentration dependent, they do not adhere to a standard dose–response curve (Jin et al. 2013; Riffle et al. 2014). A non-linear dose–response curve has been observed for many EDCs (Vandenberg et al. 2012; Lagarde et al. 2015), highlighting the importance of studies into the effects of a range of doses on the reproductive system. To date, very few studies have investigated the effects of exposure to a more realistic ATZ dose during the male peripubertal period (Lim et al. 2009; Jin et al. 2014), a time when testosterone levels peak to cause masculinisation and the initiation of spermatogenesis (Osadchuk 2016). Human studies have reported an association between ATZ exposure during adulthood and poor semen quality of men in the US (Swan 2003, 2006).
In the present study, the low dose of ATZ selected (0.5 mg kg−1 BW day−1) was based on the NOEL in female rats determined by the Australian government and used to calculate the acceptable drinking water limit (0.02 mg L−1) in Australia (NHMRC and NRMMC 2011). A second reason for studying this dose of ATZ is that NOEL studies of endocrine disruptors commonly limit observations to only major developmental or disease manifestations. In the case of ATZ, the NOEL was based on the absence of mammary tumour development at 0.5 mg kg−1 BW day−1 (NHMRC and NRMMC 2011). However, this does not consider the subtler effects on reproductive parameters. At the low dose of ATZ chosen, only subtle effects on the reproductive parameters were anticipated; hence, a 10-fold higher dose (although still lower than exposures in most previous studies; Stoker et al. 2000; Stanko et al. 2010; Victor-Costa et al. 2010) was also examined to help determine end point effects that may otherwise have been missed in only the lowest-dose group. Thus, the purpose of this study was to test the hypothesis that exposure to a low dose of ATZ and a 10-fold higher dose during the window of puberty to sexual maturity (4–12 weeks postpartum) would negatively affect reproductive and metabolic function in male mice.
Materials and methods
Animals and experimental design
Animal treatments were performed in accordance with NHMRC guidelines (NHMRC 2013) under approvals from The University of Melbourne Science Animal Ethics Committee (AEC 1513481.1). Six-week-old C57BL/6J female mice were mated, allowed to give birth and their pups weaned at 4 weeks of age. Male pups from each litter were randomly assigned to either the control or treatment groups. Male mice were housed individually and given either vehicle in distilled drinking water (<0.5% dimethylsulfoxide in distilled water; control group, n = 9), 0.5 mg kg−1 BW day−1 ATZ (Sigma-Aldrich; low-dose ATZ group, n = 10; dose based on the approved NOEL used to calculate the Australian safe drinking water level; NHMRC and NRMMC 2011) or 5 mg kg−1 BW day−1 ATZ (high-dose ATZ group, n = 14) in distilled drinking water. The concentration of ATZ in the water was altered weekly to reflect bodyweight and water consumption. All mice were maintained under a 12-h light–dark cycle and fed a soy-free diet (Speciality Feeds) ad libitum throughout their gestation, lactation and after weaning to avoid the effects of phytoestrogens. This is an essential component of the study design for EDCs that have potentially subtle effects. Once a week, drinking water was changed and mice were weighed. Food and water consumption were monitored weekly until the end of the study (Week 12) for five mice in each group by weighing the food and water bottles at the beginning and end of each week.
The 0.5 mg kg−1 BW day−1 ATZ-treated group was used to determine any potential effects from the ‘safe’ NOEL based on a 2-year dietary female rat study, in which the end point measure was increased incidence of mammary tumours (NHMRC and NRMMC 2011). This dose is accepted by the NHMRC and NRMMC as being the lowest ‘safe’ dose in rodents and in humans (NHMRC and NRMMC 2011).
The calculation for determining the acceptable water concentration (of 0.02 mg L−1) incorporates the acceptable daily intake (ADI), NOEL and a safety multiplication factor, as well as an interspecies extrapolation and intraspecies variation multiplication factor according to standard toxicological assessment methods (NHMRC and NRMMC 2011). The figure of 0.02 mg L−1 is derived as follows:

where 0.5 mg kg−1 (BW) day−1 is the NOEL based on a long-term (2-year) study in rats, 70 kg is taken as the average weight of an adult, 0.1 is a proportionality factor based on the assumption that 10% of the ADI will come from the consumption of drinking water, 2 L day−1 is the estimated maximum amount of water consumed by an adult and 100 is the safety factor applied to the NOEL derived from animal studies. This safety factor incorporates a factor of 10 for interspecies extrapolation and 10 for intraspecies variation (NHMRC and NRMMC 2011).
Fertility and embryo culture studies
At 11 weeks of age, males from the low-ATZ, high-ATZ and control groups (n = 9 males per group) were subjected to fertility studies. Three-week-old C57BL/6J female mice (n = 27) were superovulated with an intraperitoneal injection of 5 IU pregnant mare’s serum gonadotrophin (PMSG; Folligon; Intervet) followed 48 h later by 5 IU human chorionic gonadotrophin (hCG; Chorulon; Intervet). Individual superovulated females were then mated overnight with a male from the low-ATZ, high-ATZ or control treatment group. Twenty-two hours after hCG injection, mice were killed and pronucleate oocytes collected from the female tract in handling medium supplemented with 5 mg mL−1 human serum albumin (GMOPS+; Vitrolife, Göteberg, Sweden). Pronucleate oocytes were denuded of cumulus cells via incubation in GMOPS containing 300 IU mL−1 hyaluronidase (bovine testes, type IV; Sigma-Aldrich) for 20 s, followed by washing in GMOPS+. Denuded pronucleate oocytes were immediately washed in GMOPS+ and cultured in groups of 10 embryos per 20-µL drop of G1 medium (Vitrolife) under 3.5 mL paraffin oil (Ovoil; Vitrolife) for 48 h under 6% CO2, 5% O2 and 89% N2 at 37°C in a Sanyo 19M multigas incubator, as described previously (Gardner and Lane 2014; Finger et al. 2015). Embryos were assessed for development and transferred to pre-equilibrated G2 medium (Vitrolife) for a further 48 h. After a total of 96 h culture, embryos were assessed for blastocyst stage development rates from the number of fertilised pronucleate oocytes. Three independent biological replicate cultures were undertaken.
Postmortem analysis and tissue collection
After 8 weeks of exposure, the 12-week-old mice were anaesthetised with isoflurane (Forane; Abbott), blood samples were collected via cardiac puncture using a 1-mL syringe and 23-G needle (Becton Dickinson) and transferred to a 4-mL EDTA Vacutainer (Becton Dickinson) and mice were killed via cervical dislocation. Blood samples were centrifuged at 1500g for 15 min at 4°C, and the plasma was recovered and stored at −20°C for hormone analysis. At postmortem, bodyweight was recorded, and the testes, seminal vesicles, liver, kidneys, intraperitoneal fat, renal fat and testicular fat were collected, weighed and assessed further for any gross abnormalities. From the liver, a 100-mg sample from the left lobe was collected and snap frozen in liquid nitrogen for mRNA analyses. Similarly, one testis was snap frozen in liquid nitrogen for mRNA analyses and daily sperm production (DSP) calculations. Immediately after mice had been killed, each epididymis was transferred into an organ well culture dish (Thermo-Scientific) containing 500 μL GMOPS+ culture medium (Vitrolife, Göteborg, Sweden) at 37°C. The epididymides were pierced 10 times with a 23-G needle (Becton Dickinson) and spermatozoa were allowed to swim out for 10 min.
Epididymal sperm concentration
For each sample, a 10-μL aliquot of the 500-μL pool of epididymal spermatozoa in culture medium (described above) was added to 90 μL water. A 10-μL aliquot of this solution was pipetted onto each side of a haemocytometer (Neubauer). Spermatozoa were counted using light microscopy on a Nikon Eclipse TS100 at a magnification of ×200 according to previously defined methods (WHO 2010a). Duplicate counts were undertaken and averaged for each sample before calculating the sperm concentration.
Epididymal sperm live/dead stain
Live/dead staining of spermatozoa was undertaken based on methods modified from Pintado et al. (2000). Briefly, propidium iodide (PI; Sigma-Aldrich; 0.5 mg mL−1) was diluted 1 : 10 (v/v) with GMOPS culture medium (Vitrolife). A 5-μL aliquot of the PI working solution and one drop of H33342 (NucBlue; Thermo Fisher) were combined. An aliquot (40 μL) of epididymal spermatozoa in culture medium was added to this stain and incubated for 5 min at 37°C. Subsequently, 5 μL of 0.1% neutral buffered formalin (NBF; Sigma-Aldrich) in phosphate-buffered saline (PBS; Sigma-Aldrich) was added to stop the reaction and 2 μL bovine serum albumin (100 mg mL−1; MP Biomedical) was added to stop the spermatozoa adhering to one another. In total, 10 μL sperm suspension was placed on a Superfrost microscope slide (Platinum Pro; Thermo Fisher) and visualised on a fluorescent microscope (Nikon Eclipse Ti-U microscope) with the appropriate filters and imaged using a Nikon Digital Sight Camera. Captured images (~30 per sample) were processed in ImageJ (Schneider et al. 2012). In accordance with Lu et al. (2010), a minimum of 200 spermatozoa per slide was counted and the researcher was blinded to the treatment group when counting.
Epididymal sperm motility and acrosome integrity
An aliquot (250 μL) of epididymal spermatozoa was diluted 1 : 2 with GMOPS culture medium (Vitrolife) in a 5-mL tube (Falcon, Thermo Fisher) and placed in a 37°C incubator (MCO-19M; Panasonic) under 6% CO2 and 20% O2 for 90 min to allow capacitation to occur. An aliquot (10 μL) of the capacitated sperm suspension was placed on a warmed (37°C) glass slide (Grale; Thermo Fisher) and assessed by two independent researchers, blinded to the treatment group, using light microscopy (Nikon Eclipse TS100) at a magnification of ×200 according to previous defined methods (WHO 2010b). The mean of two estimations was used as the final motility score.
The acrosome reaction was induced for the remaining spermatozoa (490 μL) in the last 15 min of capacitation by adding 15 μL progesterone (1 mM; Sigma-Aldrich). Aliquots of resuspended spermatozoa (5 μL) were pipetted onto Superfrost glass slides (Thermo Fisher) and allowed to dry overnight at in the dark at room temperature. Acrosomal status was assessed using fluorescein isothiocyanate–peanut agglutinin conjugate (FITC-PNA; Sigma-Aldrich) using a method adapted from Cheng et al. (1996). Briefly, spermatozoa were fixed in 4% NBF and stained with FITC-PNA (1 mg mL−1) and counterstained with H33342 (NucBlue; Thermo Fisher). Images (10 per sample) were taken using an Olympus BX51 Microscope, captured using an Olympus DP70 Camera and overlayed in ImageJ (Schneider et al. 2012). The percentage of reacted, partially reacted and non-reacted spermatozoa was counted for each treatment group, as described previously (Fazeli et al. 1997).
Daily sperm production
For each male, DSP was determined using previously described methods (Robb et al. 1978). Briefly, snap-frozen testes of known weight were sectioned with a sterile razor blade. Sections were weighed and sonicated for 30 s at 22.5 kHz using a VirSonic ultra cell disruptor 100 (VirTis) in 500 µL DSP buffer, consisting of 0.9% NaCl and 0.05% Triton-X-100 (v/v; Chem Supply), which lyses all cells, except nuclei of elongated spermatids. The homogenate was stained with Trypan blue (Thermo Fisher), and diluted 1 : 1 (v/v) with water. The number of elongated spermatids was determined using a haemocytometer (Neubauer). DSP was calculated by dividing the total number of elongated spermatids per testis by 4.84, the number of days elongated spermatids spend in Stage 14–16 during spermatogenesis in the mouse (Oakberg 1956).
RNA extraction and quantitative reverse transcription–polymerase chain reaction
Four testis samples per group were individually homogenised and total RNA extracted using the GenElute mRNA kit (Sigma-Aldrich) according to the manufacturer’s instructions. Liver samples from four males per group were individually homogenised and total RNA extracted using TRIzol reagent (Thermo Fisher) according to the manufacturer’s instructions. The resulting total RNA concentration was quantified using a NanoDrop ND-1000 Spectrophotometer (Thermo Fisher). Samples were DNase treated using Ambion TURBO DNA-free according to the manufacturer’s instructions. The cDNA was generated from 100 ng RNA with SuperScript III First-Strand Synthesis System for reverse transcription–polymerase chain reaction (RT-PCR; Thermo Fisher) according to the manufacturer’s instructions using random hexamer primers. Thermal gradient polymerase chain reactions (PCRs) were performed to assess optimal primer annealing temperatures for each primer set (IDT). Quantitative RT-PCR (RT-qPCR) using SYBR Green Supermix (Bio-Rad Laboratories) was performed in triplicate on a Stratagene MX3000P (Thermo Fisher) for the Sry-box 9 (Sox9), low-density lipoprotein receptor (Ldlr), cytochrome P450 family 19 subfamily A member 1 (Cyp19a1) and steroidogenic factor-1 (SF-1) genes in testis samples and nuclear respiratory factor 1 (Nrf1), sirtuin 1 (Sirt1), succinate dehydrogenase complex flavoprotein subunit A (Sdha), thioredoxin-2 (Txn2), sterol regulatory element-binding protein 1c (Srebp1c), ATP citrate lyase (Acl), acetyl-CoA carboxylase (Acc) and stearoyl-CoA desaturase-1 (Scd1) genes in liver samples; data were normalised against the TATA-binding protein (Tbp) and β-actin (Actb) genes (for primer sequences, see Table 1). The cycling conditions were as follows: one cycle of 95°C for 15 s, followed by 40 cycles of 95°C for 15 s, 57°C for 30 s and 72°C for 30 s. A dissociation curve was also generated for each gene. The amplification efficiency of each gene was calculated using the standard curve. No-template controls were included in triplicate on each plate as a negative control. Tbp was selected as the most appropriate housekeeper gene based on Ct values. Gene expression from RT-qPCR was quantified relative to that of the housekeeper genes and is expressed as fold changes using Pfaffl’s equation (Pfaffl 2001).

|
Free testosterone hormone assay
Plasma samples collected from animals at postmortem were pooled (two animals per sample within group) to determine the free testosterone concentration using an ELISA method (Mouse F-TESTO Kit, E-EL-M0518; ElabScience) according to the manufacturer’s instructions. Samples (100 µL) were run in duplicate and read at 450 nm on a Multiskan EX plate reader (Thermo Fisher). Free testosterone concentrations in samples were calculated using a six-point standard curve, with a minimum detection limit of 0.1 ng mL−1.
Statistical analyses
Tissue weights are expressed relative to bodyweight. Embryo cleavage and blastocyst rates were arc-sin transformed. All data were tested for normality using the Shapiro–Wilks test before analysis. Cumulative bodyweight gain, total bodyweight and food and water consumption were compared between groups using repeated-measures in SAS version 9.2 (SAS Institute). All other data were analysed in RStudio 1.0.143 (R Core Team 2017) using one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test to compare between treatment groups for relative tissue weight, plasma free testosterone concentration, embryo cleavage and blastocyst rates, as well as gene expression data. Non-normally distributed sperm parameter data (sperm concentration, motility, live/dead ratio, acrosome reaction and DSP) were analysed using the non-parametric Kruskal–Wallis test followed by Dunn’s test for multiple comparisons using the PMCMR package in R Studio (Pohlert 2014). Significance was set at two-tailed P < 0.05. Data are presented as the mean ± s.e.m. unless stated otherwise.
Results
Mortality rates, food and water intake
No mortality occurred throughout the treatment period in any of the groups. Food and water intake was monitored and no differences were observed between the treatment groups throughout the study (P > 0.1). Mean water intake per week in the control, low-ATZ and high-ATZ groups was 36.2 ± 2.5, 37.8 ± 1.1 and 31.7 ± 2.0 mL respectively. Mean food intake per week in the control, low-ATZ and high-ATZ groups was 25.5 ± 0.8, 27.6 ± 1.0 and 26.6 ± 0.8 g respectively.
Total bodyweight and cumulative bodyweight gain
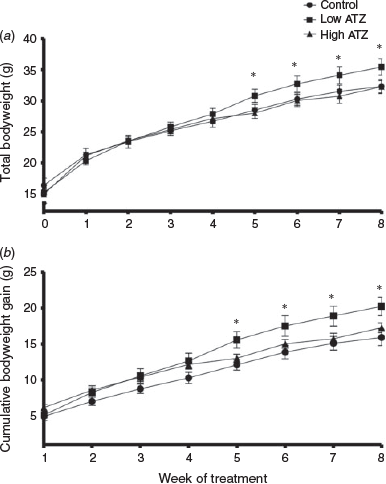
The effects of low and high doses of ATZ (0.5 and 5 mg kg−1 BW day−1 respectively) on weight gain and total bodyweight are shown in Fig. 1. Total bodyweight was significantly increased in the low-ATZ group at the end of the experiment compared with the control and high-ATZ groups (P = 0.03 and P = 0.01 respectively; Fig. 1a). There was no difference in total bodyweight between the high-ATZ and control groups at Week 8 (P > 0.1). There was a significant increase in total bodyweight in the low-ATZ group compared with the high-ATZ group between Week 5 and Week 8 (P < 0.05; Fig. 1a). At Week 5 of the treatment period, mice treated with the low dose of ATZ had a significantly greater mean cumulative bodyweight gain than mice in the control group (P = 0.009; Fig. 1b). Mice treated with the low dose of ATZ continued to have a greater mean cumulative bodyweight gain than mice in the control group through to Week 8 (P = 0.001; Fig. 1b). Mean cumulative bodyweight gain in mice treated with the high dose of ATZ did not differ significantly from that of mice in the control or low-ATZ groups throughout the entire treatment period (P > 0.1).
|
Relative tissue weight and gross morphology
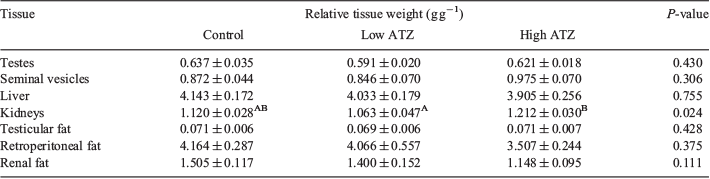
Relative weights of the liver, testis, seminal vesicles, retroperitoneal fat, renal fat and testicular fat were not significantly different between the control and low- or high-ATZ treatment groups (P > 0.1; Table 2). Relative kidney weight was significantly lower in the low- compared with high-ATZ group (P = 0.02), but not the control group. No difference in relative kidney weight was evident between the control and high-ATZ group (P > 0.1). No gross abnormalities, such as lipid droplets in the liver or differences in the size of paired testes, were observed in any males.
Plasma testosterone concentrations
Plasma free testosterone concentrations did not differ between the low- and high-ATZ groups compared with the control group (4.60 ± 0.75 and 4.38 ± 0.56 vs 3.46 ± 0.36 ng mL−1 respectively; P > 0.1). The intraassay CV was calculated to be 7.61%, the average of the %CV for each sample.
Epididymal sperm parameters
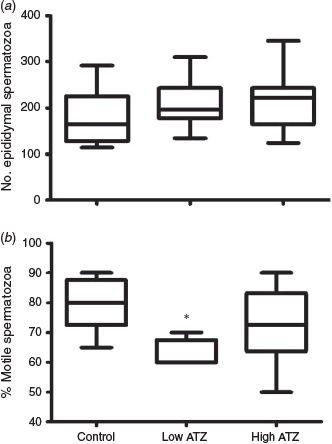
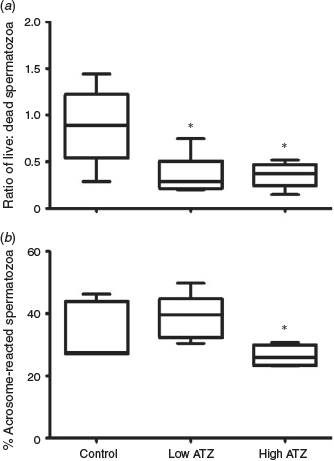
Epididymal sperm concentration was not affected by ATZ treatments compared with control (P > 0.1; Fig. 2a). However, exposure to ATZ resulted in a decrease in the percentage of motile spermatozoa estimated after capacitation, with a significant difference between the low-ATZ and control groups (P = 0.04; Fig. 2b). No differences between any other groups were evident (P > 0.1). There was an effect of ATZ treatment on the ratio of live to dead spermatozoa recovered from the epididymis (P = 0.007), with an increase in the number of dead spermatozoa compared with the number of live spermatozoa evident for both the low- and high-ATZ groups relative to the control group (P = 0.02 and P = 0.01 respectively; Fig. 3a). The percentage of spermatozoa that had undergone the acrosome reaction was significantly less for the males in the high- versus low-ATZ group (P = 0.05; Fig. 3b), with no change between any other groups, or for the percentage of partially or non-reacted spermatozoa (P > 0.1). There were also no differences in DSP between the low-ATZ, high-ATZ and control groups (3.04 ± 0.40, 3.64 ± 0.52, 3.29 ± 0.50 million spermatozoa produced per day respectively; P > 0.1).
|
|
RT-qPCR of testis and liver gene expression
In the testes, expression of Cyp19a1, which encodes for aromatase, Nr5a1 and Sox9 was not affected by treatment with the low or high dose of ATZ compared with expression in control testes (P > 0.1; Fig. 4a). However, Ldlr expression was significantly downregulated in the testes of mice in the high-ATZ compared with control group (P = 0.03; Fig. 4a). There was no difference in Ldlr expression between the low-ATZ and control groups (P < 0.1).

|
Significant changes were also determined in the liver for the expression of genes involved in mitochondrial function and fatty acid production (Fig. 4b). Expression of Nrf1 (P = 0.04), Sdha (P = 0.05) and Acl (P = 0.06) was higher in mice in the high-ATZ than control group. Mice in the low-ATZ group had increased expression of Scd1 (P = 0.02), Acl (P = 0.03), Sdha (P = 0.05) and Nrf1 (P = 0.07) relative to that in the control group. No differences in gene expression levels were evident between the low- and high-ATZ groups, or for Sirt1, Srepb1c, Txn2 and Acc between any groups (P > 0.1; Fig. 4b).
Embryo development and blastocyst rates
Embryos derived from spermatozoa of males exposed to high or low doses of ATZ did not differ compared with those derived from control males in terms of embryo cleavage (72.2 ± 7.5%, 78.9 ± 5.0% and 76.6 ± 8.0% respectively) or blastocyst rates (69.5 ± 5.6%, 73.8 ± 4.2% and 74.5 ± 6.1% respectively; P > 0.1; n = 177).
Discussion
The present study was designed to assess how exposure to a low dose of ATZ (used to calculate the approved safe concentration in Australian drinking water), as well as at a 10-fold higher dose, affected reproductive and metabolic function in male mice. Exposure of male mice to the low dose of ATZ during the peripubertal period and sexual maturity (i.e. a key window of androgen signalling and the initiation of spermatogenesis) resulted in an increase in total and cumulative bodyweight gain, as well as changes in the expression of metabolic genes in the liver. In addition, the low dose of ATZ increased the number of dead spermatozoa and decreased the number of motile spermatozoa recovered from the epididymis. The 10-fold higher dose of ATZ altered sperm viability and gene expression in the testis and liver, but did not affect bodyweight. Thus, our results demonstrate that exposure to the low dose of ATZ, used to determine the Australian drinking water guidelines (NHMRC and NRMMC 2011), can result in effects on the metabolic and reproductive systems of peripubertal males.
In this study, short-term exposure during the peripubertal period and sexual maturity to the low dose of ATZ resulted in a significant increase in bodyweight, regardless of food and water consumption. Interestingly, the 10-fold higher dose of ATZ did not alter the mean total or cumulative weight gain. Although not statistically significant, rats exposed to 30 or 300 µg kg−1 BW day−1 ATZ had a 5.5% increase in bodyweight without any changes to food intake or physical activity (Lim et al. 2009). Although consistent with the present study, the rats in that study were chronically exposed for 5 months and dietary phytoestrogens were not controlled for (Lim et al. 2009). The present study observed changes in bodyweight indicative of metabolic perturbations, after a short-term exposure, while eliminating phytoestrogens in the diet. It is postulated that long-term exposure to low concentrations of ATZ cause mitochondrial damage that resembles an insulin-resistant state (Lim et al. 2009). Interestingly, supraenvironmental ATZ exposure in the present and other studies either had no effect or decreased the bodyweight of rats and mice (Stanko et al. 2010; Victor-Costa et al. 2010; Riffle et al. 2014). This highlights the importance of investigating short exposure to lower doses, which may have different physiological effects, and affirms that the effects of EDCs, such as ATZ, are not always monotonic.
To investigate the metabolic causes of increased bodyweight evident in the low-ATZ group, we examined several key metabolism genes in the liver. Srebp1c, the transcriptional regulator of lipogenic genes, was not affected by ATZ, but the free fatty acid synthesis genes Acl and Scd1 were upregulated in the liver of males in the low-ATZ group. This finding is supported by a previous study, in which 5-week-old male mice chronically treated for 20 weeks with an even lower dose of ATZ (100 µg kg−1 BW day−1) had increased hepatic expression of Scd1 and Acl when ATZ was combined with a high-energy diet (Jin et al. 2014). Upregulation of lipogenic genes in the liver can result in increased free fatty acids and promote the production of triglycerides, leading to hepatic lipid accumulation. Furthermore, rodents exposed to supraenvironmental ATZ levels show fatty liver disease (Lim et al. 2009; Jin et al. 2014). In the present study, visual assessments of the liver from ATZ-treated males did not show overt hepatic lipid accumulation.
Metabolic disorders that contribute to perturbed fatty acid synthesis and obesity are linked to mitochondrial dysfunction and oxidative stress (Stepien et al. 2017). ATZ is able to disrupt mitochondrial function in mammalian cells (Sagarkar et al. 2016) and could be acting to perturb metabolism and weight gain. Although disrupted mitochondrial function and perturbed metabolism can be directly associated, these could also be due to independent effects of ATZ on each. The present study investigated three of the genes required for normal mitochondrial function, namely Nrf1, Sdha and Txn2. Interestingly, two of these genes were upregulated in the liver of male mice treated with the high and low doses of ATZ. The role of the SDHA protein is as an electron transporter associated with Complex II of the mitochondrial electron transport chain, part of the tricarboxylic acid (TCA) cycle, which releases ATP through the oxidation of fats (Kay and Weitzman 1987). Because the action of ATZ is to disrupt analogous complexes in the electron transport chain of plants, specifically Photosystem II (Bai et al. 2015), it is not surprising that ATZ can affects Sdha expression in mammals. Equally, Nrf1 functions as a transcription factor that activates the expression of metabolic genes and those critical in mitochondrial DNA replication and function (Scarpulla et al. 2012). Thus, upregulation of the Nrf1 and Sdha genes suggest increased mitochondrial activity in the liver, supporting a potential link between perturbed liver fatty acid metabolism and increased weight gain.
Interestingly, perturbed fatty acid metabolism may also affect reproduction, because Ldlr expression was decreased in the testis of males in the high-ATZ group, suggesting a possible effect on steroidogenesis. Ldlr is involved with the uptake of extracellular cholesterol in the testis (Eacker et al. 2008), an essential precursor to testosterone synthesis. In contrast with previous studies, Cyp19a1 and Sf-1 mRNA levels were not altered in testes of ATZ-treated males in the present study. Despite variation in experimental design, previous in vivo and in vitro studies have consistently reported an effect of ATZ on aromatase, but these have been a result of exposure to supraenvironmental ATZ concentrations. For example, Cyp19a1 expression was increased in the testes of male mice after treatment with 25 mg kg−1 BW day−1 ATZ (Gely-Pernot et al. 2015) and in adult zebrafish gonads (Suzawa and Ingraham 2008), whereas in vitro ATZ-exposure studies report increased Cyp19a1 expression in human choriocarcinoma cells, mouse adrenal gland cells and human liver cells (Suzawa and Ingraham 2008) and increased aromatase activity in human granulosa cells (Holloway et al. 2008). One possible explanation for the lack of effect on Cyp19a1 in the present study is the timing and duration of ATZ exposure, with potentially more pronounced effects if exposure was undertaken over a lifetime, or during early fetal development when gonadal differentiation occurs, rather than postnatally, once germ cells are established (McLaren 2000).
Previous studies have identified ATZ to affect mammalian spermatozoa, which is thought to result from alterations in steroidogenesis (Abarikwu et al. 2011), increased oxidative stress (Pogrmic et al. 2009; Abarikwu et al. 2010), inhibition of mitochondrial function in spermatozoa (Hase et al. 2008) and epigenetic modifications (Gely-Pernot et al. 2015; Hao et al. 2016). However, these studies investigated supraenvironmental doses of ATZ, and the affected pathways are likely to vary with concentration. Effects on spermatogenesis are nevertheless evident at low concentrations of ATZ in other vertebrates, including amphibians (Xenopus laevis; Hayes et al. 2010), fish (Salmo salar; Moore and Waring 1998) and reptiles (Caiman latirostris; Rey et al. 2009). In cattle, environmentally relevant concentrations of ATZ and its metabolite diaminechlorotriazine were found to decrease in vitro sperm viability (Komsky-Elbaz and Roth 2017). Despite this, in the present study no changes in DSP or sperm concentration in the epididymis were observed for either ATZ treatment group. Furthermore, we did not observe any change in free testosterone concentrations in the plasma between the treated and control groups. However, ATZ at both the low and high doses increased the number of dead spermatozoa and, at the low dose, reduced the number of motile spermatozoa recovered from the epididymis. Notably, the present study is the first to investigate fertility outcomes of short-term in vivo exposure to ATZ on sperm viability (i.e. embryo development rates) in mammals. Despite decreased motility and an increase in the percentage of dead spermatozoa, we found no effect on cleavage or blastocyst rates in embryos fertilised by spermatozoa from the ATZ-exposed mice. This does not preclude there being subtle effects on embryo quality, cell number, cell lineage allocation or metabolism, as well as on the ability to conceive and fetal development, frequently reported after EDC exposure (Greenlee et al. 2004; Gore et al. 2015; Choi et al. 2016), although further studies would be required to investigate this. It is well established that changes in these early life parameters can negatively affect later life health or the health of subsequent generations (Lane et al. 2014; Fleming et al. 2015; Susiarjo et al. 2015). This includes exposure to ATZ, with negative effects on body composition and reproduction (testis dysfunction and early puberty) increasing in severity over three generations in mice (McBirney et al. 2017).
In summary, the present study demonstrated that ATZ negatively affects male development, even after a short exposure during the peripubertal period, to a low dose of ATZ that was determined in standard toxicological studies as having no observed effect and used to calculate the safe limit of ATZ concentrations in Australian drinking water (NHMRC and NRMMC 2011). The effect of ATZ on male reproductive parameters was most likely caused by alterations to metabolism, rather than by altered hormone signalling. The combined effects of weight gain and decreased sperm viability are known to have major effects on human male reproductive success and offspring health (Palmer et al. 2012). Thus, our finding suggests that exposure to low levels of ATZ may be contributing to decreasing fertility rates in men and that methods for determining ‘safe’ concentrations in the drinking water should be reassessed.
Conflicts of interest
Mark P. Green currently holds the position of Merck Serono Senior Lecturer in Reproductive Biology at The University of Melbourne. The other authors declare no conflicts of interest.
Acknowledgements
The authors thank Tania Long and Darren Cipolla for their technical contributions and help with animal husbandry. These studies were supported by an Australian Research Council Future Fellowship (FT140100964), held by Andrew J. Pask.
References
Abarikwu, S. O., Adesiyan, A. C., Oyeloja, T. O., Oyeyemi, M. O., and Farombi, E. O. (2010). Changes in sperm characteristics and induction of oxidative stress in the testis and epididymis of experimental rats by a herbicide, atrazine. Arch. Environ. Contam. Toxicol. 58, 874–882.| Changes in sperm characteristics and induction of oxidative stress in the testis and epididymis of experimental rats by a herbicide, atrazine.Crossref | GoogleScholarGoogle Scholar | 19672647PubMed |
Abarikwu, S. O., Farombi, E. O., Kashyap, M. P., and Pant, A. B. (2011). Atrazine induces transcriptional changes in marker genes associated with steroidogenesis in primary cultures of rat Leydig cells. Toxicol. In Vitro 25, 1588–1595.
| Atrazine induces transcriptional changes in marker genes associated with steroidogenesis in primary cultures of rat Leydig cells.Crossref | GoogleScholarGoogle Scholar | 21693180PubMed |
Australian Pesticides and Veterinary Medicines Authority (1997). Technical report: Environmental assessment. Available at https://apvma.gov.au/sites/default/files/publication/14356-atrazine-env.pdf [verified 20 Dec 2018].
Badach, H., Nazimek, T., and Kaminska, I. A. (2007). Pesticide content in drinking water samples collected from orchard areas in central Poland. Ann. Agric. Environ. Med. 14, 109–114.
| 17655187PubMed |
Bai, X., Sun, C., Xie, J., Song, H., Zhu, Q., Su, Y., Qian, H., and Fu, Z. (2015). Effects of atrazine on photosynthesis and defense response and the underlying mechanisms in Phaeodactylum tricornutum. Environ. Sci. Pollut. Res. Int. 22, 17499–17507.
| Effects of atrazine on photosynthesis and defense response and the underlying mechanisms in Phaeodactylum tricornutum.Crossref | GoogleScholarGoogle Scholar | 26139402PubMed |
Cheng, F. P., Fazeli, A., Voorhout, W. F., Marks, A., Bevers, M. M., and Colendbrander, B. (1996). Use of peanut agglutinin to assess the acrosomal status and the zona pellucida-induced acrosome reaction in stallion spermatozoa. J. Androl. 17, 674–682.
| 9016398PubMed |
Choi, B. I., Harvey, A. J., and Green, M. P. (2016). Bisphenol A affects early bovine embryo development and metabolism that is negated by an oestrogen receptor inhibitor. Sci. Rep. 6, 29318.
| Bisphenol A affects early bovine embryo development and metabolism that is negated by an oestrogen receptor inhibitor.Crossref | GoogleScholarGoogle Scholar | 27384909PubMed |
Doyle, T. J., Bowman, J. L., Windell, V. L., McLean, D. J., and Kim, K. H. (2013). Transgenerational effects of di-(2-ethylhexyl) phthalate on testicular germ cell associations and spermatogonial stem cells in mice. Biol. Reprod. 88, 112.
| Transgenerational effects of di-(2-ethylhexyl) phthalate on testicular germ cell associations and spermatogonial stem cells in mice.Crossref | GoogleScholarGoogle Scholar | 23536373PubMed |
Eacker, S. M., Agrawal, N., Qian, K., Dichek, H. L., Gong, E. Y., Lee, K., and Braun, R. E. (2008). Hormonal regulation of testicular steroid and cholesterol homeostasis. Mol. Endocrinol. 22, 623–635.
| Hormonal regulation of testicular steroid and cholesterol homeostasis.Crossref | GoogleScholarGoogle Scholar | 18032697PubMed |
European Commission Health and Consumer Protection Directorate General (2003). Atrazine SANCO/10496/2003-final. Review report for the active substance atrazine; Finalized in the Standing Committee on the Food Chain and Animal Health at its meeting on 3 October 2003 in support of a decision concerning the non-inclusion of atrazine in Annex I of Directive 91/414/EEC and the withdrawal of authorisation for plant protection products containing this active substance. European Commission Health and Consumer Protection Directorate-General, 2003. Available at http://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=activesubstance.ViewReview&id=108
Fan, W., Yanase, T., Morinaga, H., Gondo, S., Okabe, T., Nomura, M., Komatsu, T., Morohashi, K., Hayes, T. B., Takayanagi, R., and Nawata, H. (2007). Atrazine-induced aromatase expression is SF-1 dependent: implications for endocrine disruption in wildlife and reproductive cancers in humans. Environ. Health Perspect. 115, 720–727.
| Atrazine-induced aromatase expression is SF-1 dependent: implications for endocrine disruption in wildlife and reproductive cancers in humans.Crossref | GoogleScholarGoogle Scholar | 17520059PubMed |
Farruggia, F. T., Rossmeisl, C. M., Hetrick, J. A., Biscoe, M., and Branch, M. E. R., III (2016). ‘Refined Ecological Risk Assessment for Atrazine.’ (US Environmental Protection Agency, Office of Pesticide Programs: Washington, DC.)
Fazeli, A., Hage, W. J., Cheng, F. P., Voorhout, W. F., Marks, A., Bevers, M. M., and Colenbrander, B. (1997). Acrosome-intact boar spermatozoa initiate binding to the homologous zona pellucida in vitro. Biol. Reprod. 56, 430–438.
| Acrosome-intact boar spermatozoa initiate binding to the homologous zona pellucida in vitro.Crossref | GoogleScholarGoogle Scholar | 9116143PubMed |
Finger, B. J., Harvey, A. J., Green, M. P., and Gardner, D. K. (2015). Combined parental obesity negatively impacts preimplantation mouse embryo development, kinetics, morphology and metabolism. Hum. Reprod. 30, 2084–2096.
| Combined parental obesity negatively impacts preimplantation mouse embryo development, kinetics, morphology and metabolism.Crossref | GoogleScholarGoogle Scholar | 26089300PubMed |
Fleming, T. P., Velazquez, M. A., and Eckert, J. J. (2015). Embryos, DOHaD and David Barker. J. Dev. Orig. Health Dis. 6, 377–383.
| Embryos, DOHaD and David Barker.Crossref | GoogleScholarGoogle Scholar | 25952250PubMed |
Foulds, C. E., Trevino, L. S., York, B., and Walker, C. L. (2017). Endocrine-disrupting chemicals and fatty liver disease. Nat. Rev. Endocrinol. 13, 445–457.
| Endocrine-disrupting chemicals and fatty liver disease.Crossref | GoogleScholarGoogle Scholar | 28524171PubMed |
Fullston, T., McPherson, N. O., Owens, J. A., Kang, W. X., Sandeman, L. Y., and Lane, M. (2015). Paternal obesity induces metabolic and sperm disturbances in male offspring that are exacerbated by their exposure to an ‘obesogenic’ diet. Physiol. Rep. 3, e12336.
| Paternal obesity induces metabolic and sperm disturbances in male offspring that are exacerbated by their exposure to an ‘obesogenic’ diet.Crossref | GoogleScholarGoogle Scholar | 25804263PubMed |
Gardner, D. K., and Lane, M. (2014). Mammalian preimplantation embryo culture. Methods Mol. Biol. 1092, 167–182.
| Mammalian preimplantation embryo culture.Crossref | GoogleScholarGoogle Scholar | 24318820PubMed |
Gely-Pernot, A., Hao, C., Becker, E., Stuparevic, I., Kervarrec, C., Chalmel, F., Primig, M., Jegou, B., and Smagulova, F. (2015). The epigenetic processes of meiosis in male mice are broadly affected by the widely used herbicide atrazine. BMC Genomics 16, 885.
| The epigenetic processes of meiosis in male mice are broadly affected by the widely used herbicide atrazine.Crossref | GoogleScholarGoogle Scholar | 26518232PubMed |
Gore, A. C., Chappell, V. A., Fenton, S. E., Flaws, J. A., Nadal, A., Prins, G. S., Toppari, J., and Zoeller, R. T. (2015). EDC-2: The Endocrine Society’s second scientific statement on endocrine-disrupting chemicals. Endocr. Rev. 36, E1–E150.
| EDC-2: The Endocrine Society’s second scientific statement on endocrine-disrupting chemicals.Crossref | GoogleScholarGoogle Scholar | 26544531PubMed |
Graymore, M., Stagnitti, F., and Allinson, G. (2001). Impacts of atrazine in aquatic ecosystems. Environ. Int. 26, 483–495.
| Impacts of atrazine in aquatic ecosystems.Crossref | GoogleScholarGoogle Scholar | 11485216PubMed |
Greenlee, A. R., Ellis, T. M., and Berg, R. L. (2004). Low-dose agrochemicals and lawn-care pesticides induce developmental toxicity in murine preimplantation embryos. Environ. Health Perspect. 112, 703–709.
| Low-dose agrochemicals and lawn-care pesticides induce developmental toxicity in murine preimplantation embryos.Crossref | GoogleScholarGoogle Scholar | 15121514PubMed |
Hao, C., Gely-Pernot, A., Kervarrec, C., Boudjema, M., Becker, E., Khil, P., Tevosian, S., Jegou, B., and Smagulova, F. (2016). Exposure to the widely used herbicide atrazine results in deregulation of global tissue-specific RNA transcription in the third generation and is associated with a global decrease of histone trimethylation in mice. Nucleic Acids Res. 44, 9784–9802.
| 27655631PubMed |
Hase, Y., Tatsuno, M., Nishi, T., Kataoka, K., Kabe, Y., Yamaguchi, Y., Ozawa, N., Natori, M., Handa, H., and Watanabe, H. (2008). Atrazine binds to F1F0-ATP synthase and inhibits mitochondrial function in sperm. Biochem. Biophys. Res. Commun. 366, 66–72.
| Atrazine binds to F1F0-ATP synthase and inhibits mitochondrial function in sperm.Crossref | GoogleScholarGoogle Scholar | 18060860PubMed |
Hayes, T. B., Stuart, A. A., Mendoza, M., Collins, A., Noriega, N., Vonk, A., Johnston, G., Liu, R., and Kpodzo, D. (2006). Characterization of atrazine-induced gonadal malformations in African clawed frogs (Xenopus laevis) and comparisons with effects of an androgen antagonist (cyproterone acetate) and exogenous estrogen (17 beta-estradiol): support for the demasculinization/feminization hypothesis. Environ. Health Perspect. 114, 134–141.
| Characterization of atrazine-induced gonadal malformations in African clawed frogs (Xenopus laevis) and comparisons with effects of an androgen antagonist (cyproterone acetate) and exogenous estrogen (17 beta-estradiol): support for the demasculinization/feminization hypothesis.Crossref | GoogleScholarGoogle Scholar | 16818259PubMed |
Hayes, T. B., Khoury, V., Narayan, A., Nazir, M., Park, A., Brown, T., Adame, L., Chan, E., Buchholz, D., Stueve, T., Gallipeau, S., and Wake, D. B. (2010). Atrazine induces complete feminization and chemical castration in male African clawed frogs (Xenopus laevis). Proc. Natl Acad. Sci. USA 107, 4612–4617.
| Atrazine induces complete feminization and chemical castration in male African clawed frogs (Xenopus laevis).Crossref | GoogleScholarGoogle Scholar | 20194757PubMed |
Hayes, T. B., Anderson, L. L., Beasley, V. R., de Solla, S. R., Iguchi, T., Ingraham, H., Kestemont, P., Kniewald, J., Kniewald, Z., Langlois, V. S., Luque, E. H., McCoy, K. A., Munoz-de-Toro, M., Oka, T., Oliveira, C. A., Orton, F., Ruby, S., Suzawa, M., Tavera-Mendoza, L. E., Trudeau, V. L., Victor-Costa, A. B., and Willingham, E. (2011). Demasculinization and feminization of male gonads by atrazine: consistent effects across vertebrate classes. J. Steroid Biochem. Mol. Biol. 127, 64–73.
| Demasculinization and feminization of male gonads by atrazine: consistent effects across vertebrate classes.Crossref | GoogleScholarGoogle Scholar | 21419222PubMed |
Holloway, A. C., Anger, D. A., Crankshaw, D. J., Wu, M., and Foster, W. G. (2008). Atrazine-induced changes in aromatase activity in estrogen sensitive target tissues. J. Appl. Toxicol. 28, 260–270.
| Atrazine-induced changes in aromatase activity in estrogen sensitive target tissues.Crossref | GoogleScholarGoogle Scholar | 17685393PubMed |
Huyghe, E., Plante, P., and Thonneau, P. F. (2007). Testicular cancer variations in time and space in Europe. Eur. Urol. 51, 621–628.
| Testicular cancer variations in time and space in Europe.Crossref | GoogleScholarGoogle Scholar | 16963178PubMed |
Jin, Y., Wang, L., and Fu, Z. (2013). Oral exposure to atrazine modulates hormone synthesis and the transcription of steroidogenic genes in male peripubertal mice. Gen. Comp. Endocrinol. 184, 120–127.
| Oral exposure to atrazine modulates hormone synthesis and the transcription of steroidogenic genes in male peripubertal mice.Crossref | GoogleScholarGoogle Scholar | 23376530PubMed |
Jin, Y., Lin, X., Miao, W., Wu, T., Shen, H., Chen, S., Li, Y., Pan, Q., and Fu, Z. (2014). Chronic exposure of mice to environmental endocrine-disrupting chemicals disturbs their energy metabolism. Toxicol. Lett. 225, 392–400.
| Chronic exposure of mice to environmental endocrine-disrupting chemicals disturbs their energy metabolism.Crossref | GoogleScholarGoogle Scholar | 24440342PubMed |
Jin, Y., Lin, X., Miao, W., Wang, L., Wu, Y., and Fu, Z. (2015). Oral exposure of pubertal male mice to endocrine-disrupting chemicals alters fat metabolism in adult livers. Environ. Toxicol. 30, 1434–1444.
| Oral exposure of pubertal male mice to endocrine-disrupting chemicals alters fat metabolism in adult livers.Crossref | GoogleScholarGoogle Scholar | 24916741PubMed |
Jørgensen, N., Joensen, U. N., Jensen, T. K., Jensen, M. B., Almstrup, K., Olesen, I. A., Juul, A., Andersson, A. M., Carlsen, E., Petersen, J. H., Toppari, J., and Skakkebaek, N. E. (2012). Human semen quality in the new millennium: a prospective cross-sectional population-based study of 4867 men. BMJ Open 2, e000990.
| Human semen quality in the new millennium: a prospective cross-sectional population-based study of 4867 men.Crossref | GoogleScholarGoogle Scholar | 22761286PubMed |
Kasturi, S. S., Tannir, J., and Brannigan, R. E. (2008). The metabolic syndrome and male infertility. J. Androl. 29, 251–259.
| The metabolic syndrome and male infertility.Crossref | GoogleScholarGoogle Scholar | 18222914PubMed |
Kay, J., and Weitzman, P. D. J. (1987). Krebs citric acid cycle: half a century and still turning. (Biochem Soc Symp vol 54). The Biochemical Society, London.
Kniewald, J., Osredecki, V., Gojmerac, T., Zechner, V., and Kniewald, Z. (1995). Effect of s-triazine compounds on testosterone metabolism in the rat prostate J. Appl. Toxicol. 15, 215–218.
| Effect of s-triazine compounds on testosterone metabolism in the rat prostateCrossref | GoogleScholarGoogle Scholar | 7560742PubMed |
Kniewald, J., Jakominic, M., Tomljenovic, A., Simic, B., Romac, P., Vranesic, D., and Kniewald, Z. (2000). Disorders of male rat reproductive tract under the influence of atrazine. J. Appl. Toxicol. 20, 61–68.
| Disorders of male rat reproductive tract under the influence of atrazine.Crossref | GoogleScholarGoogle Scholar | 10641017PubMed |
Komsky-Elbaz, A., and Roth, Z. (2017). Effect of the herbicide atrazine and its metabolite DACT on bovine sperm quality. Reprod. Toxicol. 67, 15–25.
| Effect of the herbicide atrazine and its metabolite DACT on bovine sperm quality.Crossref | GoogleScholarGoogle Scholar | 27836535PubMed |
Lagarde, F., Beausoleil, C., Belcher, S. M., Belzunces, L. P., Emond, C., Guerbet, M., and Rousselle, C. (2015). Non-monotonic dose-response relationships and endocrine disruptors: a qualitative method of assessment. Environ. Health 14, 13.
| Non-monotonic dose-response relationships and endocrine disruptors: a qualitative method of assessment.Crossref | GoogleScholarGoogle Scholar | 25971433PubMed |
Lane, M., Robker, R. L., and Robertson, S. A. (2014). Parenting from before conception. Science 345, 756–760.
| Parenting from before conception.Crossref | GoogleScholarGoogle Scholar | 25124428PubMed |
Lim, S., Ahn, S. Y., Song, I. C., Chung, M. H., Jang, H. C., Park, K. S., Lee, K. U., Pak, Y. K., and Lee, H. K. (2009). Chronic exposure to the herbicide, atrazine, causes mitochondrial dysfunction and insulin resistance. PLoS One 4, e5186.
| Chronic exposure to the herbicide, atrazine, causes mitochondrial dysfunction and insulin resistance.Crossref | GoogleScholarGoogle Scholar | 19787048PubMed |
Lu, J. C., Huang, Y. F., and Lu, N. Q. (2010). WHO Laboratory Manual for the Examination and Processing of Human Semen: its applicability to andrology laboratories in China. Zhonghua Nan Ke Xue 16, 867–871.
| 21243747PubMed |
McBirney, M., King, S. E., Pappalardo, M., Houser, E., Unkefer, M., Nilsson, E., Sadler-Riggleman, I., Beck, D., Winchester, P., and Skinner, M. K. (2017). Atrazine induced epigenetic transgenerational inheritance of disease, lean phenotype and sperm epimutation pathology biomarkers. PLoS One 12, e0184306.
| Atrazine induced epigenetic transgenerational inheritance of disease, lean phenotype and sperm epimutation pathology biomarkers.Crossref | GoogleScholarGoogle Scholar | 28931070PubMed |
McLaren, A. (2000). Establishment of the germ cell lineage in mammals. J. Cell. Physiol. 182, 141–143.
| Establishment of the germ cell lineage in mammals.Crossref | GoogleScholarGoogle Scholar | 10623876PubMed |
Mizota, K., and Ueda, H. (2006). Endocrine disrupting chemical atrazine causes degranulation through Gq/11 protein-coupled neurosteroid receptor in mast cells. Toxicol. Sci. 90, 362–368.
| Endocrine disrupting chemical atrazine causes degranulation through Gq/11 protein-coupled neurosteroid receptor in mast cells.Crossref | GoogleScholarGoogle Scholar | 16381660PubMed |
Moore, A., and Waring, C. P. (1998). Mechanistic effects of a triazine pesticide on reproductive endocrine function in mature male Atlantic salmon (Salmo salar L.) Pestic. Biochem. Physiol. 62, 41–50.
| Mechanistic effects of a triazine pesticide on reproductive endocrine function in mature male Atlantic salmon (Salmo salar L.)Crossref | GoogleScholarGoogle Scholar |
National Health and Medical Research Council (NHMRC) (2013). ‘Australian Code for the Care and Use of Animals for Scientific Purposes.’ (Australia: NHMRC)
National Health and Medical Research Council (NHMRC) and National Resource Management Ministerial Council (NRMMC) (2011). Australian Drinking Water Guidelines Paper 6 National Water Quality Management Strategy. National Health and Medical Research Council, National Resource Management Ministerial Council, Commonwealth of Australia, Canberra. Available at https://nhmrc.gov.au/about-us/publications/australian-drinking-water-guidelines#block-views-block-file-attachments-content-block-1
Oakberg, E. F. (1956). Duration of spermatogenesis in the mouse and timing of stages of the cycle of the seminiferous epithelium. Am. J. Anat. 99, 507–516.
| Duration of spermatogenesis in the mouse and timing of stages of the cycle of the seminiferous epithelium.Crossref | GoogleScholarGoogle Scholar | 13402729PubMed |
Osadchuk, L. V. (2016). Spermatogenesis parameters and testosterone production at puberty as predictors of testicular functional activity in mice (Mus musculus). J. Evol. Biochem. Physiol. 52, 475–481.
| Spermatogenesis parameters and testosterone production at puberty as predictors of testicular functional activity in mice (Mus musculus).Crossref | GoogleScholarGoogle Scholar |
Palmer, N. O., Bakos, H. W., Fullston, T., and Lane, M. (2012). Impact of obesity on male fertility, sperm function and molecular composition. Spermatogenesis 2, 253–263.
| Impact of obesity on male fertility, sperm function and molecular composition.Crossref | GoogleScholarGoogle Scholar | 23248766PubMed |
Pfaffl, M. W. (2001). A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45.
| A new mathematical model for relative quantification in real-time RT-PCR.Crossref | GoogleScholarGoogle Scholar | 11328886PubMed |
Pintado, B., de la Fuente, J., and Roldan, E. R. (2000). Permeability of boar and bull spermatozoa to the nucleic acid stains propidium iodide or Hoechst 33258, or to eosin: accuracy in the assessment of cell viability. J. Reprod. Fertil. 118, 145–152.
| 10793636PubMed |
Pogrmic, K., Fa, S., Dakic, V., Kaisarevic, S., and Kovacevic, R. (2009). Atrazine oral exposure of peripubertal male rats downregulates steroidogenesis gene expression in Leydig cells. Toxicol. Sci. 111, 189–197.
| Atrazine oral exposure of peripubertal male rats downregulates steroidogenesis gene expression in Leydig cells.Crossref | GoogleScholarGoogle Scholar | 19541795PubMed |
Pohlert, T. (2014). ‘The Pairwise Multiple Comparison of Mean Ranks Package.’ Available at: https://cran.r-project.org/web/packages/PMCMR/index.html
R Core Team (2017). ‘R: A Language and Environment for Statistical Computing.’ (R Foundation for Statistical Computing: Vienna.)
Radcliffe, J. C. (2002). ‘Pesticide Use in Australia.’ (Australian Academy of Technological Sciences and Engineering). Available at: www.atse.org.au
Rey, F., Gonzalez, M., Zayas, M. A., Stoker, C., Durando, M., Luque, E. H., and Munoz-de-Toro, M. (2009). Prenatal exposure to pesticides disrupts testicular histoarchitecture and alters testosterone levels in male Caiman latirostris. Gen. Comp. Endocrinol. 162, 286–292.
| Prenatal exposure to pesticides disrupts testicular histoarchitecture and alters testosterone levels in male Caiman latirostris.Crossref | GoogleScholarGoogle Scholar | 19364509PubMed |
Riffle, B. W., Klinefelter, G. R., Cooper, R. L., Winnik, W. M., Swank, A., Jayaraman, S., Suarez, J., Best, D., and Laws, S. C. (2014). Novel molecular events associated with altered steroidogenesis induced by exposure to atrazine in the intact and castrate male rat. Reprod. Toxicol. 47, 59–69.
| Novel molecular events associated with altered steroidogenesis induced by exposure to atrazine in the intact and castrate male rat.Crossref | GoogleScholarGoogle Scholar | 24887032PubMed |
Robb, G. W., Amann, R. P., and Killian, G. J. (1978). Daily sperm production and epididymal sperm reserves of pubertal and adult rats. J. Reprod. Fertil. 54, 103–107.
| Daily sperm production and epididymal sperm reserves of pubertal and adult rats.Crossref | GoogleScholarGoogle Scholar | 712697PubMed |
Roberge, M., Hakk, H., and Larsen, G. (2004). Atrazine is a competitive inhibitor of phosphodiesterase but does not affect the estrogen receptor. Toxicol. Lett. 154, 61–68.
| Atrazine is a competitive inhibitor of phosphodiesterase but does not affect the estrogen receptor.Crossref | GoogleScholarGoogle Scholar | 15475179PubMed |
Sagarkar, S., Gandhi, D., Devi, S. S., Sakharkar, A., and Kapley, A. (2016). Atrazine exposure causes mitochondrial toxicity in liver and muscle cell lines. Indian J. Pharmacol. 48, 200–207.
| Atrazine exposure causes mitochondrial toxicity in liver and muscle cell lines.Crossref | GoogleScholarGoogle Scholar | 27114639PubMed |
Scarpulla, R. C., Vega, R. B., and Kelly, D. P. (2012). Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol. Metab. 23, 459–466.
| Transcriptional integration of mitochondrial biogenesis.Crossref | GoogleScholarGoogle Scholar | 22817841PubMed |
Schneider, C. A., Rasband, W. S., and Eliceiri, K. W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675.
| NIH Image to ImageJ: 25 years of image analysis.Crossref | GoogleScholarGoogle Scholar | 22930834PubMed |
Skakkebaek, N. E. (2002). Endocrine disrupters and testicular dysgenesis syndrome. Horm. Res. 57, 43.
| 12065926PubMed |
Song, Y., Jia, Z. C., Chen, J. Y., Hu, J. X., and Zhang, L. S. (2014). Toxic effects of atrazine on reproductive system of male rats. Biomed. Environ. Sci. 27, 281–288.
| 24758756PubMed |
Spanò, L., Tyler, C. R., van Aerle, R., Devos, P., Mandiki, S. N., Silvestre, F., Thomé, J. P., and Kestemont, P. (2004). Effects of atrazine on sex steroid dynamics, plasma vitellogenin concentration and gonad development in adult goldfish (Carassius auratus). Aquat. Toxicol. 66, 369–379.
| Effects of atrazine on sex steroid dynamics, plasma vitellogenin concentration and gonad development in adult goldfish (Carassius auratus).Crossref | GoogleScholarGoogle Scholar | 15168945PubMed |
Stanko, J. P., Enoch, R. R., Rayner, J. L., Davis, C. C., Wolf, D. C., Malarkey, D. E., and Fenton, S. E. (2010). Effects of prenatal exposure to a low dose atrazine metabolite mixture on pubertal timing and prostate development of male Long-Evans rats. Reprod. Toxicol. 30, 540–549.
| Effects of prenatal exposure to a low dose atrazine metabolite mixture on pubertal timing and prostate development of male Long-Evans rats.Crossref | GoogleScholarGoogle Scholar | 20727709PubMed |
Stepien, K. M., Heaton, R., Rankin, S., Murphy, A., Bentley, J., Sexton, D., and Hargreaves, I. P. (2017). Evidence of oxidative stress and secondary mitochondrial dysfunction in metabolic and non-metabolic disorders. J. Clin. Med. 6, 71.
| Evidence of oxidative stress and secondary mitochondrial dysfunction in metabolic and non-metabolic disorders.Crossref | GoogleScholarGoogle Scholar |
Stoker, T. E., Laws, S. C., Guidici, D. L., and Cooper, R. L. (2000). The effect of atrazine on puberty in male Wistar rats: an evaluation in the protocol for the assessment of pubertal development and thyroid function. Toxicol. Sci. 58, 50–59.
| The effect of atrazine on puberty in male Wistar rats: an evaluation in the protocol for the assessment of pubertal development and thyroid function.Crossref | GoogleScholarGoogle Scholar | 11053540PubMed |
Stoker, T. E., Guidici, D. L., Laws, S. C., and Cooper, R. L. (2002). The effects of atrazine metabolites on puberty and thyroid function in the male Wistar rat. Toxicol. Sci. 67, 198–206.
| The effects of atrazine metabolites on puberty and thyroid function in the male Wistar rat.Crossref | GoogleScholarGoogle Scholar | 12011479PubMed |
Susiarjo, M., Xin, F., Bansal, A., Stefaniak, M., Li, C., Simmons, R. A., and Bartolomei, M. S. (2015). Bisphenol A exposure disrupts metabolic health across multiple generations in the mouse. Endocrinology 156, 2049–2058.
| Bisphenol A exposure disrupts metabolic health across multiple generations in the mouse.Crossref | GoogleScholarGoogle Scholar | 25807043PubMed |
Suzawa, M., and Ingraham, H. A. (2008). The herbicide atrazine activates endocrine gene networks via non-steroidal NR5A nuclear receptors in fish and mammalian cells. PLoS One 3, e2117.
| The herbicide atrazine activates endocrine gene networks via non-steroidal NR5A nuclear receptors in fish and mammalian cells.Crossref | GoogleScholarGoogle Scholar | 18461179PubMed |
Swan, S. H. (2003). Semen quality in relation to pesticide exposure in Missouri males. Mo. Med. 100, 554.
| 14699813PubMed |
Swan, S. H. (2006). Semen quality in fertile US men in relation to geographical area and pesticide exposure. Int. J. Androl. 29, 62–68.
| Semen quality in fertile US men in relation to geographical area and pesticide exposure.Crossref | GoogleScholarGoogle Scholar | 16466525PubMed |
Swan, S. H., Elkin, E. P., and Fenster, L. (2000). The question of declining sperm density revisited: an analysis of 101 studies published 1934–1996. Environ. Health Perspect. 108, 961–966.
| The question of declining sperm density revisited: an analysis of 101 studies published 1934–1996.Crossref | GoogleScholarGoogle Scholar | 11049816PubMed |
Toppari, J., Kaleva, M., and Virtanen, H. E. (2001). Trends in the incidence of cryptorchidism and hypospadias, and methodological limitations of registry-based data. Hum. Reprod. Update 7, 282–286.
| Trends in the incidence of cryptorchidism and hypospadias, and methodological limitations of registry-based data.Crossref | GoogleScholarGoogle Scholar | 11392374PubMed |
US Environmental Protection Agency (EPA) (2003). ‘Interim Reregistration Eligibility Decision for Atrazine, 2005.’ (US EPA.)
Vandenberg, L. N., Colborn, T., Hayes, T. B., Heindel, J. J., Jacobs, D. R., Lee, D. H., Shioda, T., Soto, A. M., vom Saal, F. S., Welshons, W. V., Zoeller, R. T., and Myers, J. P. (2012). Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr. Rev. 33, 378–455.
| Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses.Crossref | GoogleScholarGoogle Scholar | 22419778PubMed |
Victor-Costa, A. B., Bandeira, S. M., Oliveira, A. G., Mahecha, G. A., and Oliveira, C. A. (2010). Changes in testicular morphology and steroidogenesis in adult rats exposed to atrazine. Reprod. Toxicol. 29, 323–331.
| Changes in testicular morphology and steroidogenesis in adult rats exposed to atrazine.Crossref | GoogleScholarGoogle Scholar | 20045047PubMed |
World Health Organization (WHO) (2010a). 2.7 Sperm numbers. In ‘WHO Laboratory Manual for the Examination and Processing of Human Semen’. 5th edn. (Ed. T. G. Cooper.) pp. 33–44. (WHO: Switzerland)
World Health Organization (WHO) (2010b). 2.5 Sperm motility. In ‘WHO Laboratory Manual for the Examination and Processing of Human Semen’. 5th edn. (Ed. T. G. Cooper.) pp. 21–26. (WHO: Switzerland)
* These authors contributed equally to this study.