New directions in assisted breeding techniques for fish conservation
Nicola Rivers A D , Jonathan Daly B C and Peter Temple-Smith A
A D , Jonathan Daly B C and Peter Temple-Smith A
A Department of Obstetrics and Gynaecology, School of Clinical Sciences, Monash University, Melbourne, Vic. 3168, Australia.
B Smithsonian Conservation Biology Institute, Front Royal, VA 22360, USA.
C Hawaii Institute of Marine Biology, 46-007 Lilipuna Road, Kaneohe, HI 96744, USA.
D Corresponding author. Email: nicola.rivers@monash.edu
Reproduction, Fertility and Development 32(9) 807-821 https://doi.org/10.1071/RD19457
Submitted: 13 December 2019 Accepted: 26 April 2020 Published: 2 June 2020
Journal Compilation © CSIRO 2020 Open Access CC BY
Abstract
Fish populations continue to decline globally, signalling the need for new initiatives to conserve endangered species. Over the past two decades, with advances in our understanding of fish germ line biology, new ex situ management strategies for fish genetics and reproduction have focused on the use of germ line cells. The development of germ cell transplantation techniques for the purposes of propagating fish species, most commonly farmed species such as salmonids, has been gaining interest among conservation scientists as a means of regenerating endangered species. Previously, ex situ conservation methods in fish have been restricted to the cryopreservation of gametes or maintaining captive breeding colonies, both of which face significant challenges that have restricted their widespread implementation. However, advances in germ cell transplantation techniques have made its application in endangered species tangible. Using this approach, it is possible to preserve the genetics of fish species at any stage in their reproductive cycle regardless of sexual maturity or the limitations of brief annual spawning periods. Combining cryopreservation and germ cell transplantation will greatly expand our ability to preserve functional genetic samples from threatened species, to secure fish biodiversity and to produce new individuals to enhance or restore native populations.
Additional keywords: cryoconservation, gonad cryopreservation, cryopreservation, germ cell transplantation, fish biology, sterilisation.
Introduction
Conservation status of fish in Australia
In 2015, the World Wildlife Fund (WWF) reported a 49% decline in global marine biomass, including a 50% decrease in fish harvested for local subsistence or commercial use (WWF 2015). In Australia, surveys of large fish numbers showed a 36% decrease in large marine fish biomass on fished reefs (Edgar et al. 2018). In inland waterways, Australia is home to 36 threatened freshwater fish (Cresswell and Murphy 2016), 26 of which reside only in the Murray–Darling Basin (MDB; Lintermans 2007). As for marine species, overfishing is a key contributor to declining freshwater fish numbers, but the MDB faces additional challenges, including poor water quality, cold water pollution due to damming and the introduction of invasive species (Lintermans 2007). Poor reproductive success, overfishing and competition with or predation by invasive species all contribute to declining fish populations, resulting in a predicted declined of 90% in MDB fish populations since European settlement (Lintermans 2007). The MDB also experienced several ‘mass death’ events in early 2019 due to toxic blue–green algae blooms related to droughts and the diversion of environmental water flows from the system, primarily for agriculture (Murray–Darling Basin Authority 2019). This sudden loss of biodiversity in the MDB may have ongoing implications for the genetic health of already vulnerable populations. Until issues in the environment can be addressed there is risk of further declines in fish populations, suggesting that ex situ conservation strategies are now required to provide an insurance policy for the conservation of these species.
Issues with current methods of ex situ conservation
Common ex situ conservation methods include captive breeding and cryobanking of animal cells and tissues. For many species, the circumstances under which reproduction occurs is not known or cannot be easily replicated in captivity. Therefore, cryobanking of samples from endangered fish species has become a more convenient method to store their genetics. Cryopreserved cells and tissues can be stored indefinitely as a source of genetic material to resurrect a population in case of a stochastic event causing sudden loss of biodiversity or extinction. Semen, or milt, has been successfully cryopreserved in many fish species, including Murray cod Maccullochella peelii peelii (Daly et al. 2008), brown trout Salmo trutta (Nynca et al. 2014), Atlantic salmon Salmo salar (Figueroa et al. 2015) and the zebrafish Danio rerio (Yang H et al. 2007). However, due to the large diversity in sperm morphology and function among fishes and the short reproductive periods in seasonal species, the optimisation process is often long and techniques may not be transferable across species, genera or families. Therefore, the time and resources required to preserve milt from a single endangered species are often unrealistic for use in conservation.
Even if spermatozoa can be stored, mature fish oocytes have not yet been successfully recovered after cryopreservation, limiting the use of cryopreserved semen samples to fresh fish eggs. Cryopreservation of fish oocytes and eggs presents more of a challenge because their large size limits the penetration of cryoprotectants, leading to fatal ice formation during cooling (Tsai 2009). Although cryopreservation of earlier-stage oocytes has been successful, subsequent in vitro culture and IVM failed to produce fertilisable eggs (Tsai 2009). When eggs of the same species are not available for fertilisation, androgenesis can produce offspring (androgenotes), using artificial techniques, in which the male is the only source of nuclear genetic material in the resulting individual (Schwander and Oldroyd 2016). While individuals produced in this way are usually homozygous and have low survivability (Pandian and Kirankumar 2003), androgenotes derived from preserved or cadaveric spermatozoa and surrogate eggs provide a foundation for the restoration of endangered fish species (Schwander and Oldroyd 2016). Although the combination of sperm cryopreservation and androgenesis is promising, it is limited in that it can only be used to reintroduce the genetic diversity of male individuals from which the genetic material is derived.
Embryos containing the genetic information from a male and a female make them an appropriate target for cryopreservation. However, like oocytes, embryos have a low surface to volume ratio, but they are also very complex, multicompartmented structures with a high lipid content and barriers to permeability that limit movement of the cryoprotectant and water across membranes (Hagedorn et al. 1997). Various strategies have been used to overcome these barriers, including direct injection of cryoprotectants into the embryo (Janik et al. 2000) and injection of gold nanoparticles that rapidly thaw the embryo when heated with a laser (Khosla et al. 2017). Despite this, a reliable cryopreservation method for fish embryos is yet to be established for most species.
New directions for the conservation of fish species
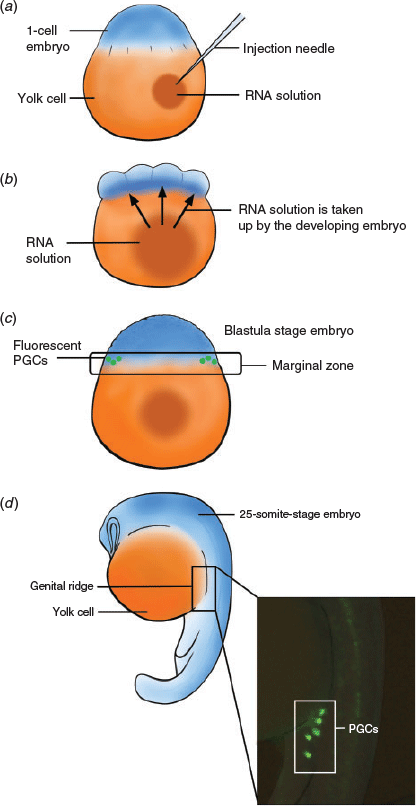
The lack of successful cryopreservation techniques for embryos and oocytes significantly reduces opportunities to bank genetics from endangered fish species and suggests the need for new directions to advance fish conservation. To develop these new directions, attention is turning to the use of earlier, undifferentiated cells, such as the primordial germ cells (PGCs), spermatogonia in males and oogonia in females. These cells have a relatively simple structure compared with their differentiated counterparts, namely spermatozoa and oocytes, which makes them an easier target for cryopreservation with the potential to standardise cryopreservation methods across species, and perhaps genera, reducing optimisation time and increasing opportunities to preserve endangered fish species (Yamaha et al. 2007). Because these early stage cells are undifferentiated, methods to produce viable gametes, spermatozoa and oocytes are now being investigated. The most promising method is germ cell surrogacy via germ cell transplantation (GCT). Germ line stem cells are extracted from cryopreserved tissue and injected into a surrogate species, where they differentiate into viable spermatozoa or oocytes (Kobayashi et al. 2007; Saito et al. 2010; Yoshizaki et al. 2010); an overview of this is shown in Fig. 1. The primary benefit of the combination of these two techniques is that, particularly when gonadal tissue is available, it is not necessary to spawn the threatened species in captivity, which is often challenging. The combination of cryopreservation and cell transplantation offers a unique and adaptable method for the storage and subsequent reintroduction of important genetics into a population, which could be hugely beneficial to the conservation of fish.
This review summarises the mechanisms underpinning teleost germ line development and shows how this has led to the development of methods that manipulate the germ line in a way that will improve current conservation strategies for endangered fish species both in Australia and globally.
Key aspects of germ line development in fish
A detailed understanding of the basic biological mechanisms of germ cell development is needed to successfully manipulate reproduction in fish species. The teleost group of fishes is remarkably diverse; however, many aspects of germ cell formation, migration and differentiation are highly conserved. Biomedical model fish species, such as the zebrafish, are well studied and provide the key knowledge of germ cell formation, migration and differentiation. Although the timing of these events is species specific, the mechanisms are similar and can therefore be broadly applied to many species.
Germ cell formation
PGCs are formed early in embryogenesis. In zebrafish, PGCs are specified during the cleavage stage in the first 30 min of development via a process termed ‘preformation’, in which maternal deposits of PGC-specific mRNA transcripts and proteins found in the germplasm determine germ cell fate (Clelland and Peng 2009; Eno and Pelegri 2016). Ablating or disturbing the germplasm results in sterility or subfertility, and transplantation of the germplasm can induce the germ line fate at ectopic sites, demonstrating the importance of accurate germplasm formation (Hartung and Marlow 2014). Two mRNA transcripts, DEAD-box helicase 4 (ddx4) also known as vasa, and Nanos C2HC-Type Zinc Finger 3 (nanos3 or nos3), found in the germplasm have been crucial to identifying primordial germ cells during development (Hartung et al. 2014). Maternal vasa mRNA, synthesised and deposited into the oocyte during oogenesis, localises to the cleavage furrows of the dividing embryo, creating four clusters that are eventually taken up by cells to become the first PGCs (Yoon et al. 1997; Extavour and Akam 2003). Nanos3, previously known as Nanos C2HC-Type Zinc Finger 1 (nanos1 or nos1), follows a similar expression pattern to vasa but, unlike vasa, nanos3 shows some expression in future somatic cells with post-transcriptional control mechanisms required to restrict expression to PGCs (Köprunner et al. 2001). When formed, PGCs are large, round cells with granular, light-staining cytoplasm, a large nucleus containing one or more dark-staining nucleoli, a distinct nuclear membrane and dense cytoplasmic inclusions, termed nuage, that contribute to the germplasm required for germ line specification in the next generation (Braat et al. 1999; Yoshizaki et al. 2002).
Germ cell migration
PGCs must migrate to the region where the gonad will eventually form, the genital ridge, for successful development of the gonad. In zebrafish, migration begins at the blastula stage around 4.5 h post fertilisation (h.p.f.) and ends around 24 h.p.f. Migration is guided by chemotaxis, specifically the chemokine stromal-cell derived factor-1a (SDF-1a) and its receptor chemokine receptor 4 (CXCR4b), which is found on the surface of PGCs. PGCs migrate dorsally from the marginal region of the blastodisc through intermediate targets between somite levels 1–3 at the 1–5 somite stage, and somite levels 5–7 at the 16 somite stage before localising to the gonadal region around somite level 8 at 24 h.p.f. (Weidinger et al. 1999). In the presence of SDF-1a, CXCR4b releases calcium, resulting in myosin contractions on the leading edge of the PGC; these contractions result in a flow of cytoplasm responsible for migration (Blaser et al. 2006). PGCs with reduced CXCR4b activity are still capable of migration, but lack direction, often ending up in ectopic locations, whereas reduced SDF-1a activity results in fewer PGCs arriving at the gonad (Raz and Reichman-Fried 2006). During migration, PGCs are protected by the RNA-binding protein Dead end (dnd). The dnd gene is specifically expressed in the germplasm and PGCs in zebrafish and shares a similar expression pattern to vasa (Weidinger et al. 2003). Gene knockouts or knockdowns of dnd are commonly sterile, and so dnd is considered a critical factor for germ cell development in fish (Fujimoto et al. 2010; Wong and Zohar 2015; Li et al. 2016; Yoshizaki et al. 2016).
Differentiation and sexual development
Once reaching the gonadal ridge, the PGCs proliferate. Females appear to have a much faster rate of PGC proliferation than males in some species such as medaka (Oryzias latipes), and this often marks the first distinction of sexual differentiation between males and females (Wootton and Smith 2014b). There is evidence to suggest that a threshold number of PGCs is required to induce female gonad formation (Dai et al. 2015). For example, knockout or knockdown of the dnd gene results in sterility but, in some species (e.g. zebrafish) treated embryos also develop to be phenotypically male, indicating that the loss of the germ line affects secondary sex characteristics (Dranow et al. 2013). In addition, in medaka, mutations to the male sex determining region, the DM domain gene on the Y chromosome, or dmy result in a ‘female-like’ PGC proliferation pattern in male medaka and, when dmy expression is extremely low, can result in male-to-female sex reversal (Matsuda 2003; Otake et al. 2006). However, although PGC number has been shown to affect sex ratios in zebrafish and medaka, gene knockout or knockdown experiments in other species, such as goldfish Carassius auratus (Goto et al. 2012) and loach Misgurnus anguillicaudatus (Fujimoto et al. 2010), have not shown a similar effect of PGCs on sexual development or sex ratios.
As the gonad develops, PGCs differentiate into spermatogonia in males and oogonia in females. These cells, although sex specific, are remarkably plastic in that they can reverse depending on the signals from surrounding tissue. For example, in rainbow trout, spermatogonia injected into female surrogate fish reverted and differentiated into oogonia capable of generating oocytes that could be fertilised and produce offspring (Lee et al. 2013). Spermatogonia and oogonia are distinguishable from other differentiating germ cells in the gonad because they are both large, round cells with a low nucleocytoplasmic ratio (Papah et al. 2013; Lacerda et al. 2014; Uribe et al. 2014, 2016; Viana et al. 2018). Because spermatogonia and oogonia in their primary undifferentiated stage are similar in structure and function, their ability to reverse cell type in response to changes in sex is most likely due to the effects of surrounding somatic cells on gametogenesis. Spermatogonia and oogonia undergo Type 1 mitotic divisions typical to stem cells and subsequent Type 2 divisions signify commitment to gametogenesis in which the cells divide and differentiate synchronously to eventually form mature spermatozoa or oocytes (Nishimura and Tanaka 2014).
Identification of germ line stem cells
Successful identification of PGCs, spermatogonia and oogonia in vivo is the first step in piecing together the reproductive development of a species and finding opportunities for ex vivo conservation methods. Although the use of transgenic species forms the basis of identification methods, non-transgenic methods, such as fluorescent tags or basic histological analysis, are currently the most accessible identification methods for endangered species.
Visualising PGCs during embryo development
Identifying PGCs and timing their migration during embryogenesis has been critical to the success of techniques such as GCT. Histologically, PGCs are big, circular cells with a large nucleus and electrodense germplasm (McMillan 2007). Previously, PGC identification relied on these features alone, but the development of fluorescent tagging methods has made the identification of PGCs in live embryos possible. Germ line-specific genes vasa and nanos3 have been used in transgenic and non-transgenic methods to identify PGCs in vivo. The production of transgenic strains, such as rainbow trout Oncorhynchus mykiss, that produce green fluorescence in the germ line has been used to track germ cell migration and development (Yoshizaki et al. 2000, 2010; Takeuchi et al. 2002, 2003; Kobayashi et al. 2007; Okutsu et al. 2007; Lee et al. 2015). Data collected using transgenic embryos has been key to understanding the early formation and migration of PGCs; however, generating transgenic fish is currently not feasible when working with endangered species because of the need for a deeper knowledge of their genetics and the time required to produce transgenic animals.
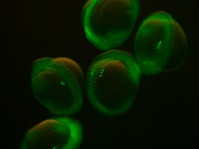
Chimeric RNA transcripts containing a portion of germ line-specific mRNA adhered to a fluorescent marker such as green fluorescent protein (GFP) can be injected into fish embryos and result in germ line-specific fluorescence (Yoshizaki et al. 2005), as shown in Fig. 2. Two non-transgenic methods have been described using the 3′ untranslated region (UTR) of vasa (Yoshizaki et al. 2005) and nanos3 (Saito et al. 2006). Injection of chimeric mRNA results in temporary PGC-specific fluorescence. Although this technique does not allow for permanent identification of PGCs across multiple generations, as in transgenic models, it enables the identification of PGCs for days after injection; for example, GFP-vasa 3′UTR mRNA identified PGCs in rainbow trout 50 days after injection (Yoshizaki et al. 2005). In addition, the vasa and nanos3 genes appear to be conserved between distantly related species, such that a chimeric RNA optimised in a common species could be used to tag PGCs in a distantly related endangered species. For example, vasa 3′UTR mRNA transcript originally isolated from Nibe croaker Nibea mitsukurii produced PGC-specific fluorescence in rainbow trout and zebrafish (Yoshizaki et al. 2005) and GFP-nos3 3′UTR RNA identified PGCs across many different species: medaka, zebrafish, pearl danio Danio albolineatus, goldfish, loach, herring Clupea pallasii and ice goby Leucopsarion petersii (Saito et al. 2006), sturgeon (genus Acipenser; Saito et al. 2014), smelt Hypomesus nipponensis (Takahashi et al. 2017), barfin flounder Verasper moseri (Goto et al. 2015) and eel Anguilla japonica (Saito et al. 2011).
|
The use of transcripts from other species as PGC probes in less-studied fish species allows for easy identification of PGCs without the initial step of vasa or nanos3 transcript isolation and sequencing. A disadvantage of using chimeric RNA is that inconsistencies in the injection technique between embryos may result in some PGCs not being identified, thereby lowering the number of cells available for transplant. For example, the number of PGCs identified in different species was extremely varied among embryos, ranging from 5 to 53 cells in zebrafish, 5 to 43 cells in pearl danio, 0 to 29 cells in loach, 6 to 90 cells in goldfish, 3 to 54 cells in medaka and 4 to 24 cells in the ice goby (Saito et al. 2006). Variation in PGC number is observed in transgenic species that presumably produce fluorescence in 100% of PGCs (Kobayashi et al. 2004), but observations of mRNA-injected embryos showed uneven fluorescence in the early blastodisc stages before PGC formation confirming uneven mRNA distribution (Saito et al. 2006). In the absence of alternative non-transgenic methods, injection of chimeric RNA is currently the most effective way of gathering information on germ cell migration in endangered species.
Visualising spermatogonia and oogonia in the gonad
Spermatogonia and oogonia are embedded deep in the body cavity and cannot be easily visualised in living animals. Histology is the primary method of identifying spermatogonia and oogonia during development. Spermatogonia can be identified by their large size, usually 10–15 µm (Kise et al. 2012), and multiple dark staining nucleoli (Wootton and Smith 2014a). Oogonia are similar in size to spermatogonia, but they are one of the smaller cells in the fish ovary because oocytes increase markedly in size during development. Similar to spermatogonia, oogonia are identified by their large nuclear to cytoplasmic ratio and nucleoli containing nucleus and more condensed chromatin compared with early stage oocytes (McMillan 2007). Using transgenic species of rainbow trout that produce fluorescent gonial cells can improve identification of spermatogonia and oogonia in juvenile fish during early stages of development when they are transparent. However, as fish develop, increased pigmentation of the skin and internal membranes can reduce visibility of the gonad. Although identification of spermatogonia and oogonia is challenging in live fish, there is an assumption that the cells are present in the gonad if the animal displays signs of sexual maturity, such as mature colouration and mating behaviour.
Reduced reproductive fitness in the wild often underpins the decline and eventual extinction of a species (Blomqvist et al. 2010). The identification and subsequent description of germ cell formation and differentiation mechanisms provide basic knowledge of reproductive development that can be manipulated to assist with species conservation management. New methods, such as the cryopreservation of early germ line cells in combination with cell surrogacy, mean that the genetic diversity of fish populations can be saved before they reach critical levels. This genetic diversity is then available for reintroduction, where needed, to alleviate the effects of inbreeding depression and to boost population numbers.
Cryopreservation
Collections of cryopreserved cells and tissues, often referred to as biobanks, cryobanks or ‘frozen zoos’, have been a popular method for safeguarding the genetics of endangered species for several decades (Clarke 2009). Because the physiological complexity of fish oocytes and embryos has prevented successful cryopreservation, targeting early stages of the germ line such as PGCs, oogonia and spermatogonia has become an alternative method of storing fish genetics.
Primordial germ cells
Despite problems recovering viable fish embryos after cryopreservation, extracting viable PGCs from cryopreserved embryos has been successful (Higaki et al. 2010, 2013). PGCs can also be cryopreserved as a single-cell suspension (Riesco et al. 2012) or extracted from cryopreserved genital ridge tissue from developing embryos (Kobayashi et al. 2007). Generally, extraction of PGCs from genital ridge tissue or whole embryos is preferable because it reduces the loss of cells and improves viability. PGCs cryopreserved as a single-cell suspension also have more DNA damage and a lower survival rate, around 25%, compared with 80% viability and less than 10% DNA damage in PGCs recovered from cryopreserved embryos or genital ridge tissue (Riesco et al. 2012).
In addition to post-thaw viability and low DNA damage, the migratory capacity of PGCs must be maintained after cryopreservation if these cells are to be used for GCT. During GCT, PGCs must be able to navigate and migrate through the surrogate embryo to the developing gonad to be able to colonise and differentiate into viable gametes. Pseudopodial activity is a critical indicator of the migratory ability of the PGCs. In fish embryos, cryopreservation of PGCs is known to be inhibited by the yolk sac, and partial removal of yolk before cryopreservation has been observed to improve pseudopodial movement in zebrafish PGCs after thawing (Higaki et al. 2013). Although depletion of the yolk did not result in viable embryos after thawing, yolk-depleted, 14- to 18-somite-stage embryos were twice as likely to have PGCs with intact membranes. In addition, post-thaw PGCs from seven of 12 embryos showed pseudopodial movement, indicating they retained migratory ability, which would be imperative for successful GCT (Higaki et al. 2010, 2013).
The combination of PGC cryopreservation and subsequent cell transplantation has produced viable gametes in transgenic (pvasa-Gfp) rainbow trout (Kobayashi et al. 2007), demonstrating the feasibility of combining cryopreservation and transplantation to regenerate fish populations. Although long-term post-thaw viability of fish embryos remains elusive, PGCs collected from cryopreserved embryos or the genital ridge present an achievable target for fish germplasm banking in endangered species.
Spermatogonia and oogonia
Access to embryos from endangered species may be limited if only one sex of the species is available or if breeding is not possible due to age or declining reproductive fitness. In these cases, gonadal tissue from juvenile or adult fish can be collected and cryopreserved. This tissue contains sex-specific derivatives of PGCs, spermatogonia in males and oogonia in females. Spermatogonia and oogonia have been isolated from cryopreserved tissues in various species, as indicated in Table 1. Past literature is skewed towards the cryopreservation of spermatogonia, although in the past 5 years more evidence of successful oogonial cell cryopreservation has been published (Lujić et al. 2017).
Similar to PGCs, cell viability is improved when spermatogonia or oogonia are cryopreserved within gonadal tissues as opposed to in a single-cell suspension (Hagedorn et al. 2018). Cryomedia are often composed of a mixture of permeating and non-permeating cryoprotectants, as well as protective additives, including egg yolk, bovine serum albumin and fetal bovine serum. Varying concentrations of dimethyl sulfoxide, trehalose and egg yolk tend to outperform other cryoprotectants across multiple species, including rainbow trout (Lee et al. 2013, 2016), Nile tilapia Oreochromis niloticus (Lacerda et al. 2010) and tiger puffer Takifugu rubripes (Yoshikawa et al. 2018). Because spermatogonia and oogonia are more differentiated when they are collected, they do not possess the migratory ability of PGCs; however, these cell types are usually transplanted directly into the gonad of sterile adult fish or into the intraperitoneal cavity of juvenile fish. For this reason, it is not necessary for these cells to retain any migratory ability, so these samples can be collected at any stage of adult fish life. In addition, because these cells are present from the beginning of gonadal development, there is more flexibility for sample collection compared with spermatozoa and oocytes, which may only be available during seasonal spawning periods.
The cryopreservation of early germ cells gives conservation biologists a feasible means of storing genetic material from endangered species with relatively high viability compared with targeting differentiated cells types, such as spermatozoa and especially oocytes. Because these cells are already committed to the germ cell fate, they are easier to differentiate than cells collected from somatic tissue, such as fin clips, which have been used in somatic cell nuclear transfer in zebrafish but did not result in live adult fish (Siripattarapravat et al. 2009).
Sterilisation of the surrogate
Prior to injection of donor germ cells, a recipient must be sterilised to ensure the exclusive production of donor gametes. Although there are many different methods of sterilisation, all have their drawbacks and must be carefully considered on a species-by-species basis. The most effective method of sterilisation usually depends on germ cell formation and the age at which the sterilisation occurs; for example, sterilisation at the embryonic stage before germ cell development will likely require a different approach to sterilisation in mature adult fish that have established gametogenesis. Sterilisation at the embryo stage is often focused on genetic manipulation, such as gene knockout or knockdown therapies, that prevent the germ line from forming, whereas sterilising adult fish requires treatment with chemotherapeutic drugs that target germ cells based on their rapid proliferation.
Transgenic and non-transgenic gene expression manipulation
During embryogenesis, key genes are activated that are associated with germ cell development and survival. These genes include vasa, nanos3, dnd and critical elements of the migration pathway. Genes can be knocked out in transgenic strains or knocked down by the injection of antisense morpholino oligonucleotides (AMO), such as dnd-AMO, which results in disruption to the signalling pathways, leading to PGC death or ectopic migration, leaving the future gonad devoid of germ cells and the adult fish unable to reproduce.
The use of dnd-AMO for the knockdown of dnd expression has resulted in sterility in many species, including medaka (Li et al. 2016), zebrafish (Slanchev et al. 2005; Siegfried and Nusslein-Volhard 2008), loach (Fujimoto et al. 2010), rainbow trout (Yoshizaki et al. 2016) and goldfish (Goto et al. 2012). Generally, dnd-AMO is injected directly into the yolk or cytoplasm of the early stage embryo, where it is absorbed and can result in 100% germ cell depletion. However, injecting individual embryos can be time consuming. Embryos immersed in media containing dnd-AMO have shown similar results to direct injection of dnd-AMO but, to achieve 100% ablation in this case, the embryos must be produced by IVF, not natural spawning, indicating that the timing of treatment affects success (Wong and Zohar 2015). As mentioned previously, knockdown of dnd can result in phenotypically male populations in some fish species (Dai et al. 2015), but sex-reversal can be induced by treatment with oestrogen to produce sterile, phenotypically female fish (Slanchev et al. 2005).
Although the use of dnd-AMO has been successful in many fish species, preparing a host embryo using this method requires the development of additional techniques, such as artificial fertilisation or specifically timed mating, to ensure embryos are available for injection within a specific time frame. The method of microinjection is also determined by the egg structure and early embryogenesis of the selected host species. For species conservation, it is possible that the biology of host species selected as surrogates based on their relatedness to the donor species may be relatively undescribed. Such hosts will require substantial optimisation before the successful application of this method of sterilisation.
Altering chromosome number
Sterility can also be induced by altering the chromosome number, or ploidy, after fertilisation by preventing extrusion of the second polar body, resulting in the retention of a third set of chromosomes by the embryo (Hammed et al. 2010). By inducing sterility this way, many embryos can be treated together with relatively high success. Heat shock, cold shock and pressure shock can be used to induce polyploidy, specifically triploidy. Although triploidy in other animal groups is often fatal, fish tolerate the triploid state but are sterile (Pearson 2001).
Applying immense mechanical pressure to embryos early after fertilisation prevents exclusion of the polar body. This method has been used in large farmed species such as rainbow trout (Chourrout 1984; Couture et al. 2007). Pressure shock in the range of 34 000–76 000 kPa, depending on the species (Benfey and Sutterlin 1984; Cassani and Caton 1986; Malison et al. 1993; Peruzzi and Chatain 2000; Huergo and Zaniboni-Filho 2006), is often used by large aquaculture facilities to induce triploidy and prevent sexual maturation in food fish. However, the equipment needed to apply hydrostatic pressure is not always available for smaller operations, and alternative methods, such as thermal shock, are used. The use of cold shock or heat shock can depend on the natural rearing temperature of the species. For example, cold-water species such as the Atlantic salmon responded poorly (0–4% triploidy) to cold shock but respond favourably to heat shock (66–100% triploidy; Peruzzi et al. 2007). However, this may only apply to some species. In the blue tilapia Oreochromis aureus, a warm-water species, heat shocking at a mean (±s.d.) temperature of 39.5 ± 0.2°C for 3.5–4 min resulted in 100% triploidy with 61% survival (Don and Avtalion 1986). In contrast, the red tilapia, a hybrid of blue tilapia and Mozambique tilapia (Oreochromis mossambicus) and a close relative of O. aureus, responded to cold shock for 30 min at 9°C with a 98.7% triploidy rate and 75.8% survival to the yolk sac stage (Pradeep et al. 2014).
Inducing DNA damage in the germ line
Irradiation with ultraviolet (UV) light can result in DNA damage that prevents germ cell formation but must be germ cell specific to prevent genetic abnormalities and high mortality in the embryos. In many species this may not be possible because the germplasm is embedded within the embryo. However, it is possible in sterlet Acipenser ruthenus because, in this species, the germplasm localises at the vegetal pole in the early stages of embryogenesis. Dechorionated sterlet embryos suspended in water naturally orientate animal pole up, with the germplasm on the opposite pole underneath. Because of this orientation, UV irradiation underneath the embryos results in disrupted germplasm and subsequent sterilisation without damage to the animal pole (Saito et al. 2018). Other species with similar germplasm localisation could also be sterilised using this method.
Treatment with chemotherapeutic agents
In some species, spermatogonia and oogonia proliferate in response to increasing temperature and can be targeted by cytotoxic drugs such as busulfan. Treatment with multiple injections of busulfan combined with increased tank temperature has induced sterility in Nile tilapia (Lacerda et al. 2010), Patagonian pejerrey Odontesthes hatcheri (Majhi et al. 2014) and yellow tail tetra Alestopetersius caudalis (de Siqueira-Silva et al. 2015). Although sterilisation using this method has been successful, some species had higher female mortality due to increased ulceration after injection, which was not present in males (Majhi et al. 2014). Not all species respond in this way to changes in temperature, so this method is usually restricted to warm-water species (Saito et al. 2018). Regardless of its potential toxicity, busulfan is the only method of sterilisation for adult fish and is therefore a commonly used method of sterilisation because intervention at the embryo stage is often not feasible for species that take many years to reach sexual maturity.
Isolating germ cells for downstream applications
To improve germ cell transplant success, the suspension of germ cells to be injected into a surrogate should be enriched with a high concentration of the target cells (spermatogonia, oogonia or PGCs). Therefore, successful GCT requires the development of robust methods to isolate these cells from surrounding gonadal tissue.
Visual selection of cells
Isolation and identification of PGCs from the surrounding cells in a developing embryo is relatively simple if they produce fluorescence, such as in embryos injected with GFP-nos3 3′UTR or transgenic embryos. Embryos are crushed or enzymatically digested using a mixture of collagenase, trypsin and sodium citrate, and the resulting single-cell suspension is then assessed under a fluorescent microscope and the fluorescing PGCs are selected (Saito et al. 2008). However, this process can be time consuming because only a few PGCs are present in the embryo, and these must be identified and visually isolated from a suspension of cells. In contrast, spermatogonia and oogonia are often present in large numbers, so more high-throughput techniques of isolating these cells are required.
Density gradient-based separation of cells
Separation of cell suspensions by density gradient centrifugation is a basic but effective method of size-based cell separation. Gradients consisting of layers of increasing densities of Percoll act as a ‘molecular sieve’, allowing smaller cells to pass through while capturing larger cells in the top layers. Successful separation of cells produces multiple ‘bands’ within the gradient, with each band corresponding to a different population of cells. Because spermatogonia and oogonia are large, density gradient-based sorting has been used successfully to isolate large germ cell populations in several fish species, including sturgeon Acipenser baerii (Pšenička et al. 2015), Nile tilapia (Lacerda et al. 2010), rainbow trout (Bellaiche et al. 2014), common carp (Franěk et al. 2019), Patagonian pejerry (Majhi et al. 2014), tench Tinca tinca (Linhartová et al. 2014) and catfish Ictalurus furcatus (Shang et al. 2018). Isolation of gonial cells by gradient centrifugation is cost effective, but lacks specificity because it sorts only on size and cannot exclude somatic cells, such as Sertoli cells and Leydig cells in the testis. It also requires a large number of cells to be successful, with protocols requiring tissue from many animals. This limits its application in smaller endangered species, which may not have enough cellular content to be visible in the gradient.
Cell sorting by flow cytometry
Flow cytometry allows for the analysis of individual cells in a suspension based on their light-scattering properties and, if available, fluorescence. By analysing each individual cell in a suspension, populations of rare cells can be easily detected and separated. Isolation is relatively straightforward when using transgenic species because gonial cells can be separated based on fluorescence alone (Takeuchi et al. 2002; Kobayashi et al. 2004; Okutsu et al. 2006a; Yoshizaki et al. 2010). However, the forward scatter (FSC) and side scatter (SSC) properties of spermatogonia from transgenic species can be extrapolated across cell suspensions from non-transgenic animals. For example, by gating events based on the FSC and SSC profiles of transgenic rainbow trout spermatogonia, cells from wild-type rainbow trout, Japanese char Salvelinus leucomaenis and masu salmon Oncorhynchus masou were isolated (Kise et al. 2012). Subsequent transplantation of these cells into surrogates resulted in significantly higher colonisation rates (mean (±s.d.) 78.3 ± 8.0%, 80.0 ± 19.0% and 48.8 ± 1.0% for cells from rainbow trout, masu salmon and Japanese char respectively) compared with unsorted cells, for which colonisation rates ranged from 0% to 29.0 ± 4.0%, demonstrating that the gate successfully captured an enriched sample of spermatogonial cells (Kise et al. 2012). Gating events in a high FSC region also successfully isolated spermatogonia in Pacific blue fin tuna Thunnus orientalis (Ichida et al. 2017). Given that FSC correlates with the size of the cell, size-specific beads have been used to model the scatter properties of certain cell sizes. In species where the size of the spermatogonial cells is known, a size-specific gate is set based on the FSC of the beads with the assumption that events captured by this gate will contain the target cells. This method has been used successfully to isolate spermatogonia from the starry goby Asterropteryx semipunctata (Hagedorn et al. 2018); however, aside from injecting these isolated cells into a recipient fish to produce mature spermatozoa, validation of the presence of the target germ cells in the isolated population is difficult. Therefore, the isolated cell suspensions are often referred to only as ‘large cells’ with a presumption that spermatogonia are present.
Transplantation
GCT is the primary method for differentiating cryopreserved gonial cells into functional gametes that can give rise to donor-related offspring. Compared with other methods, such as in vitro germ cell differentiation, using a surrogate has the benefit of germ cell production throughout a reproductive lifespan. Although testicular cell cultures from the cyprinid honmoroko Gnathopogon caerulescens have been able to produce motile spermatozoa, production declined within months (Higaki et al. 2017). For endangered species with a proven regression in reproductive success, the ability to ‘offload’ reproductive function onto a more fecund species is a unique method to propagate new individuals and improve population numbers. Although many publications have described successful migration and gametogenesis, GCT resulting in the production of viable donor offspring has only been reported in a few species, namely zebrafish, pearl danio, loach, goldfish (Saito et al. 2008) and rainbow trout (Takeuchi et al. 2003; Kobayashi et al. 2007). The available literature indicates that several factors are important for transplantation success, including the appropriate timing of GCT in the donor and surrogate species, the transplantation method used and the effect of recipient sex on germ cell differentiation.
Timing of PGC migration affects transplantation success
The mechanisms behind PGC migration are highly conserved, as demonstrated by the successful migration of zebrafish PGCs in the distantly related Japanese eel (Saito et al. 2011). However, the migratory period of PGCs is species specific and must be carefully considered to ensure adequate migration and colonisation of the gonad. For example, PGCs collected from 10- to 15-somite-stage pearl danio embryos colonised the genital ridge in 50% of recipient zebrafish embryos when injected at the blastula stage (Saito et al. 2008). However, in transplants where PGCs were isolated later at the 25 somite stage, no colonisation occurred but some embryos did have PGCs localised along the normal migration route, indicating some migratory capacity was maintained at this stage but not sustained long enough for complete migration (Saito et al. 2008). Similar findings were reported in rainbow trout, with a migratory period occurring around 20–35 days post fertilisation (d.p.f.; Takeuchi et al. 2003). Cells collected from the blastula (2.5 d.p.f.) and gastrula (6 d.p.f.) stages and injected into 35-d.p.f. hatchlings were unable to colonise the genital ridge, whereas cells transplanted from 35-d.p.f. hatchlings into the same age had a colonisation rate of 21.6%, indicating that earlier stage cells where not yet receptive to migratory cues in the host. The host environment plays an equally important role because cells from 35-d.p.f. trout had declining colonisation rates when transplanted into older hosts, with 10% success in 40-d.p.f. hosts and 0% in 45-d.p.f. hosts (Takeuchi et al. 2003).
Because these mechanisms are so highly conserved, the efficiency of migration is not related to the phylogenetic distance between the host and donor. In some cases, transplants between distantly related species result in higher colonisation success. For example, in a study comparing PGC colonisation rates between phylogenetically diverse groups, zebrafish to zebrafish PGC transplants had a colonisation rate of 28.9%, whereas PGCs from goldfish localised to the gonadal region in 63.5% of zebrafish hosts (Saito et al. 2010). This may be attributed to differences in the length of migration in different species. PGCs collected from species with a relatively long migratory period may have more opportunity to respond to the cues in the recipient embryo, thereby increasing the chance of colonisation (Saito et al. 2010; Goto and Saito 2019).
Currently the evidence suggests that knowledge of the commencement, conclusion and length of PGC migration in both the donor and host is critical to transplantation success. The use of GFP-nos3 3′UTR mRNA and GFP-vasa 3′UTR mRNA transcripts has made the mapping of PGC migration in different species more easy and accessible for endangered species. Although troubleshooting of poor colonisation rates often focuses on migration, many other aspects of germ cell development may affect transplant success, such as the effects of genetic or hormonal cues that are not conserved across species. These mechanisms are rarely studied in the context of GCT and represent a significant gap in our knowledge of how introduced germ cells may navigate an unknown environment.
Transplantation techniques after the embryo stage
Because spermatogonia and oogonia are more differentiated than PGCs, they have reduced migratory ability and must be injected directly into the gonad or the region where the gonad will form in juvenile or adult fish. Cells can be tagged with fluorescent markers before injection to assist with visualisation during and after injection. For example, PKH26 is a red-fluorescing cell membrane label that allows cell tracking in vivo for over 100 days. PKH26 has been used to tag cells in various fish species, including tiger puffer T. rubripes (Hamasaki et al. 2017), Chinese sturgeon Acipenser sinensis (Ye et al. 2017) and Nile tilapia (Farlora et al. 2014). Spermatogonia and oogonia can be transplanted into the peritoneal cavity of juvenile fish, where they will migrate towards the genital ridge, which lies along the dorsal wall (Yoshizaki and Lee 2018). Embryo and larval-stage surrogates lack a developed immune response (Yoshizaki et al. 2012). The fish immune system is similar to that of mammals, including a cell-specific immune rejection driven by the thymus and T cell-like cells (Chilmonczyk 1992). It is therefore possible that adult surrogates could produce an immune response to the transplanted cells, resulting in poor transplantation efficiency. Thus, the use of immunosuppressed or immunodeficient surrogates may avoid low success rates due to immune rejection (Okutsu et al. 2006b), but generating these animals may be difficult for the purposes of working with endangered species. However, there is evidence that regions of the gonad are immune privileged (i.e. they are isolated from the blood supply and therefore unable to initiate an immune response; de Siqueira-Silva et al. 2018). Other than poor cell colonisation rates, there are few published data relating to the immune response in cell transplant recipients. This suggests that further investigation into the immunological physiology of surrogates is warranted to determine specific mechanisms that could affect transplant efficiency.
In adults, cells can be introduced directly into the gonad by surgical or non-surgical methods. Pejerrey Odontesthes bonariensis spermatogonia injected into Patagonian pejerrey O. hatcheri testes via surgical intervention colonised the gonad in two of the three fish assessed 4 weeks after transplantation, and 20% of recipients produced donor-derived spermatozoa 8 months after transplantation (Majhi et al. 2009). Immune rejection and reabsorption of the transplanted cells may have accounted for the lack of gamete production in a further 16 treated fish but, as mentioned previously, there is little evidence to confirm this. A non-surgical method using a catheter to inject Jundia catfish spermatogonia into Nile tilapia testes via the spermatic duct resulted in donor-derived sperm production in all surrogates (Silva et al. 2016). Although surgical transplantation methods have shown success, they do pose an increased risk to the surrogate because of the higher anaesthetic dose required and the greater risk of infection after surgery; because of this, non-surgical interventions are more popular.
Species relatedness and the importance of host sex in transplantation success
GCT is possible between distantly related species, but there appears to be a strong effect of the sex of the host on the success of GCT, with more successful results from male surrogates. PGCs isolated from goldfish and loach differentiated into spermatozoa after transplantation into a sterile zebrafish embryo. Despite divergence between goldfish and zebrafish approximately 50 mya and divergence between loach and zebrafish 133.4 mya, the mechanism of spermatogenesis is conserved (Saito et al. 2008). However, this was not observed in female chimeras. Goldfish or loach PGCs transplanted into female zebrafish did not undergo oogenesis (Saito et al. 2008). Because oogenesis requires more maternal input from surrogates, in particular the synthesis and deposition of lipids to form yolk, it is possible that the lack of success of these GCTs reflects interspecies differences in oogenesis (Lubzens et al. 2010). However, in the goldfish and loach transplants, no germ cells were found even in the early stages. This was also noted in zebrafish PGC transplants into hybridised zebrafish and pearl danio recipients. Female chimeras had no identifiable germ cells and the gonads were underdeveloped and threadlike (Wong et al. 2011). However, it is important to indicate that in that study control hybridised zebrafish and pearl danio females also had poor ovary structure compared with hybridised males (Wong et al. 2011). Hybridisations in carp have also resulted in poor female outcomes; in common carp and goldfish, oogenesis was observed in hybrids but not beyond the yolk globule stage (Yamaha et al. 2003).
Although less reported, there have been some transplant successes in female fish. For example interspecies oogonial cell transplants between Patagonian pejerrey and pejerrey resulted in oocytes capable of producing viable offspring (Majhi et al. 2014), as did allogenic transplants in rainbow trout, (Yoshizaki et al. 2010; Lee et al. 2016). In Chinese sturgeon, oogonial cells successfully colonised the gonad in 40% of recipients (n = 10) compared with 70% for spermatogonia (n = 10), but assessment of oogenesis was not performed (Ye et al. 2017). There is a skew in the literature regarding GCT in males versus females. Although it is difficult to ascertain whether this is due to low success rates using female recipients or a lack of research, what is evident from the available publications is that oogenesis is more sensitive to genetic abnormalities and interspecies differences.
Next-generation effects of GCT
Few data are available on the downstream effects of GCT on future generations.
Although the gametes that result from PGC transplantation represent the donor species, the gonadal environment in which those gametes develop and mature is controlled by the surrogate, so there is potential for interspecies differences to arise. For example, pearl danio PGCs transplanted into a zebrafish resulted in oocytes the same size as zebrafish eggs and embryos that were smaller than average pearl danio embryos. However, these embryos had pigmentation that was similar to pearl danios and matured into fertile pearl danio adults (Saito et al. 2008). Although these differences in size are small and did not affect the fertility of the offspring, they clearly show that the surrogate can influence gamete production in ways that alter normal embryo development. Further investigation into the extent of the effect of the surrogate on offspring outcomes is paramount if these techniques are to be considered for mass application in endangered species.
Practical applications for conservation and concluding remarks
Targeting early germ line cells as a means of securing endangered fish genetics has greater application for long-term conservation strategies. Compared with conservation methods that aim to store gametes such as spermatozoa, cryopreservation of PGCs, spermatogonia and oogonia has a clear advantage in that each individual cell has the potential, when transplanted into the surrogate, to generate gametes for the lifetime of that individual. As broodstock, these surrogates can be used to establish breeding programs for threatened species and potentially produce millions of larvae for restocking programs. The collection of these early germ line cells is therefore far more efficient than the cryopreservation of spermatozoa or milt, because these samples are limited by the sperm population in a single ejaculate. In the context of conservation, the use of germ cell surrogacy is better suited to producing large numbers of offspring because, by selecting highly fecund surrogates, the number of gametes available can be greatly increased compared with the reduced reproductive capabilities of the threatened donor species. Considering that conservation programs often require thousands of new individuals to be produced over many years to successfully support dwindling populations, the application of germ cell surrogacy is arguably better suited to support large-scale fish conservation efforts than the cryopreservation of spermatozoa, eggs and embryos.
A great challenge in applying these methods to threatened species is that their reproductive biology is often poorly understood. Most of the previous research is based on farmed species, such as salmonids, and although many aspects of germ cell development in fish are conserved, the translation of these methods into endangered species will require optimisation. Australia in particular is home to many endemic species that, due to the dry climate, have evolved in isolation in restricted freshwater river systems. Although previous studies suggest that spermatogenesis is well conserved in the bony fishes, the production of donor eggs via germ cell surrogacy will most likely require the use of host species that are closely related to the donor. Because no germ cell surrogacy has yet been attempted in an Australian fish species, the development of techniques, particularly in relation to host sterilisation and cell transplantation, will require a significant research effort to determine which species will make suitable hosts for early germ cells from Australian fish.
In the absence of alternative ex situ conservation methods, cryopreservation of gonadal tissue combined with GCT is a unique, effective and adaptable strategy for the conservation of endangered fish. Unlike fish oocytes and embryos, early stage germ cells have relatively high viability rates after cryopreservation and offer a tangible and simple method of storing valuable fish genetics for future use. Germ cell transplantation has proven to be a feasible method for the production of gametes from donor cells in some species (Takeuchi et al. 2003; Kobayashi et al. 2007; Saito et al. 2008) and the introduction of non-transgenic methods make it more accessible than ever before for endangered species (Saito et al. 2006). However, there are many gaps in our understanding of fish reproductive biology, such as the effect of endocrine and paracrine signalling during gametogenesis, which may account for low reporting of oocyte production in female surrogates. Further investigation into the underlying mechanisms of oogenesis and how this process differs among species may improve gamete production in female surrogates. Nevertheless, optimisation of the GCT method does not negate cryopreservation of gonadal tissue as an achievable means of storing the genetics of important fish species now before species and genetic diversity are lost forever. A changing climate and increasing environmental pressures make ex situ conservation methods an essential approach for safeguarding endangered species from potentially toxic and unsustainable environments. With fish populations in decline, it is imperative that all possible conservation strategies are investigated to ensure a continuing global biodiversity of fish populations.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgement
Nicola Rivers is the recipient of a Research Training Program Stipend provided by the Australian Government.
References
Bellaiche, J., Lareyre, J. J., Cauty, C., Yano, A., Allemand, I., and Le Gac, F. (2014). Spermatogonial stem cell quest: nanos2, marker of a subpopulation of undifferentiated A spermatogonia in trout testis. Biol. Reprod. 90, 79.| Spermatogonial stem cell quest: nanos2, marker of a subpopulation of undifferentiated A spermatogonia in trout testis.Crossref | GoogleScholarGoogle Scholar | 24554733PubMed |
Benfey, T. J., and Sutterlin, A. M. (1984). Triploidy induced by heat shock and hydrostatic pressure in landlocked Atlantic salmon (Salmo salar L.). Aquaculture 36, 359–367.
| Triploidy induced by heat shock and hydrostatic pressure in landlocked Atlantic salmon (Salmo salar L.).Crossref | GoogleScholarGoogle Scholar |
Blaser, H., Reichman-Fried, M., Castanon, I., Dumstrei, K., Marlow, F. L., Kawakami, K., Solnica-Krezel, L., Heisenberg, C. P., and Raz, E. (2006). Migration of zebrafish primordial germ cells: a role for myosin contraction and cytoplasmic flow. Dev. Cell 11, 613–627.
| Migration of zebrafish primordial germ cells: a role for myosin contraction and cytoplasmic flow.Crossref | GoogleScholarGoogle Scholar | 17084355PubMed |
Blomqvist, D., Pauliny, A., Larsson, M., and Flodin, L.-A. (2010). Trapped in the extinction vortex? Strong genetic effects in a declining vertebrate population. BMC Evol. Biol. 10, 33.
| Trapped in the extinction vortex? Strong genetic effects in a declining vertebrate population.Crossref | GoogleScholarGoogle Scholar | 20122269PubMed |
Braat, A. K., Zandbergen, T., Van De Water, S., Goos, H. J., and Zivkovic, D. (1999). Characterization of zebrafish primordial germ cells: morphology and early distribution of vasa RNA. Dev. Dyn. 216, 153–167.
| Characterization of zebrafish primordial germ cells: morphology and early distribution of vasa RNA.Crossref | GoogleScholarGoogle Scholar | 10536055PubMed |
Cassani, J. R., and Caton, W. E. (1986). Efficient production of triploid grass carp (Ctenopharyngodon idella) utilizing hydrostatic pressure. Aquaculture 55, 43–50.
| Efficient production of triploid grass carp (Ctenopharyngodon idella) utilizing hydrostatic pressure.Crossref | GoogleScholarGoogle Scholar |
Chilmonczyk, S. (1992). The thymus in fish: development and possible function in the immune response. Annu. Rev. Fish Dis. 2, 181–200.
| The thymus in fish: development and possible function in the immune response.Crossref | GoogleScholarGoogle Scholar |
Chourrout, D. (1984). Pressure-induced retention of second polar body and suppression of first cleavage in rainbow trout: production of all-triploids, all-tetraploids, and heterozygous and homozygous diploid gynogenetics. Aquaculture 36, 111–126.
| Pressure-induced retention of second polar body and suppression of first cleavage in rainbow trout: production of all-triploids, all-tetraploids, and heterozygous and homozygous diploid gynogenetics.Crossref | GoogleScholarGoogle Scholar |
Clarke, A. G. (2009). The Frozen Ark Project: the role of zoos and aquariums in preserving the genetic material of threatened animals. Int. Zoo Yearb. 43, 222–230.
| The Frozen Ark Project: the role of zoos and aquariums in preserving the genetic material of threatened animals.Crossref | GoogleScholarGoogle Scholar |
Clelland, E., and Peng, C. (2009). Endocrine/paracrine control of zebrafish ovarian development. Mol. Cell. Endocrinol. 312, 42–52.
| Endocrine/paracrine control of zebrafish ovarian development.Crossref | GoogleScholarGoogle Scholar | 19406202PubMed |
Couture, R., Schamber, T., Firmenich, A., and Banner, C. (2007). Pressure shock induction of triploid rainbow trout. (Oregon Department of Fish and Wildlife Oregon: Corvallis.) Available at https://www.dfw.state.or.us/fish/OHRC/docs/2013/pubs/Pressure_Shock_Induction_of_Triploid_Rainbow_Trout.pdf [verified 21 May 2020].
Dai, X., Jin, X., Chen, X., He, J., and Yin, Z. (2015). Sufficient numbers of early germ cells are essential for female sex development in zebrafish. PLoS One 10, e0117824.
| Sufficient numbers of early germ cells are essential for female sex development in zebrafish.Crossref | GoogleScholarGoogle Scholar | 26660527PubMed |
Daly, J., Galloway, D., Bravington, W., Holland, M., and Ingram, B. (2008). Cryopreservation of sperm from Murray cod, Maccullochella peelii peelii. Aquaculture 285, 117–122.
| Cryopreservation of sperm from Murray cod, Maccullochella peelii peelii.Crossref | GoogleScholarGoogle Scholar |
de Siqueira-Silva, D. H., dos Santos Silva, A. P., Ninhaus-Silveira, A., and Veríssimo-Silveira, R. (2015). The effects of temperature and busulfan (Myleran) on the yellowtail tetra Astyanax altiparanae (Pisces, Characiformes) spermatogenesis. Theriogenology 84, 1033–1042.
| The effects of temperature and busulfan (Myleran) on the yellowtail tetra Astyanax altiparanae (Pisces, Characiformes) spermatogenesis.Crossref | GoogleScholarGoogle Scholar | 26164805PubMed |
de Siqueira-Silva, D. H., Saito, T., Dos Santos-Silva, A. P., da Silva Costa, R., Psenicka, M., and Yasui, G. S. (2018). Biotechnology applied to fish reproduction: tools for conservation. Fish Physiol. Biochem. 44, 1469–1485.
| Biotechnology applied to fish reproduction: tools for conservation.Crossref | GoogleScholarGoogle Scholar | 29707740PubMed |
Don, J., and Avtalion, R. R. (1986). The induction of triploidy in Oreochromis aureus by heat shock. Theor. Appl. Genet. 72, 186–192.
| The induction of triploidy in Oreochromis aureus by heat shock.Crossref | GoogleScholarGoogle Scholar | 24247833PubMed |
Dranow, D. B., Tucker, R. P., and Draper, B. W. (2013). Germ cells are required to maintain a stable sexual phenotype in adult zebrafish. Dev. Biol. 376, 43–50.
| Germ cells are required to maintain a stable sexual phenotype in adult zebrafish.Crossref | GoogleScholarGoogle Scholar | 23348677PubMed |
Edgar, G. J., Ward, T. J., and Stuart-Smith, R. D. (2018). Rapid declines across Australian fishery stocks indicate global sustainability targets will not be achieved without an expanded network of ‘no-fishing’ reserves. Aquat. Conserv. 28, 1337–1350.
| Rapid declines across Australian fishery stocks indicate global sustainability targets will not be achieved without an expanded network of ‘no-fishing’ reserves.Crossref | GoogleScholarGoogle Scholar |
Eno, C., and Pelegri, F. (2016). Germ cell determinant transmission, segregation, and function in the zebrafish embryo. In ‘Insights from Animal Reproduction’. (Ed. R. Payan-Carreira.) pp. 115–142. (InTech: Rijeka, Croatia.)
Extavour, C. G., and Akam, M. (2003). Mechanisms of germ cell specification across the metazoans: epigenesis and preformation. Development 130, 5869–5884.
| Mechanisms of germ cell specification across the metazoans: epigenesis and preformation.Crossref | GoogleScholarGoogle Scholar | 14597570PubMed |
Farlora, R., Hattori-Ihara, S., Takeuchi, Y., Hayashi, M., Octavera, A., Alimuddin, , and Yoshizaki, G. (2014). Intraperitoneal germ cell transplantation in the Nile tilapia Oreochromis niloticus. Mar. Biotechnol. (NY) 16, 309–320.
| Intraperitoneal germ cell transplantation in the Nile tilapia Oreochromis niloticus.Crossref | GoogleScholarGoogle Scholar | 24096828PubMed |
Figueroa, E., Merino, O., Risopatrón, J., Isachenko, V., Sánchez, R., Effer, B., Isachenko, E., Farias, J. G., and Valdebenito, I. (2015). Effect of seminal plasma on Atlantic salmon (Salmo salar) sperm vitrification. Theriogenology 83, 238–245.e2.
| Effect of seminal plasma on Atlantic salmon (Salmo salar) sperm vitrification.Crossref | GoogleScholarGoogle Scholar | 25442390PubMed |
Franěk, R., Marinovic, Z., Lujic, J., Urbanyi, B., Fucikova, M., Kaspar, V., Psenicka, M., and Horvath, A. (2019). Cryopreservation and transplantation of common carp spermatogonia. PLoS One 14, e0205481.
| Cryopreservation and transplantation of common carp spermatogonia.Crossref | GoogleScholarGoogle Scholar | 30998742PubMed |
Fujimoto, T., Nishimura, T., Goto-Kazeto, R., Kawakami, Y., Yamaha, E., and Arai, K. (2010). Sexual dimorphism of gonadal structure and gene expression in germ cell-deficient loach, a teleost fish. Proc. Natl Acad. Sci. USA 107, 17211–17216.
| Sexual dimorphism of gonadal structure and gene expression in germ cell-deficient loach, a teleost fish.Crossref | GoogleScholarGoogle Scholar | 20855617PubMed |
Golpour, A., Siddique, M. A. M., Rodina, M., and Pšenička, M. (2016). Short-term storage of sterlet Acipenser ruthenus testicular cells at –80 °C. Cryobiology 72, 154–156.
| Short-term storage of sterlet Acipenser ruthenus testicular cells at –80 °C.Crossref | GoogleScholarGoogle Scholar | 26964775PubMed |
Goto, R., and Saito, T. (2019). A state-of-the-art review of surrogate propagation in fish. Theriogenology 133, 216–227.
| A state-of-the-art review of surrogate propagation in fish.Crossref | GoogleScholarGoogle Scholar | 31155037PubMed |
Goto, R., Saito, T., Takeda, T., Fujimoto, T., Takagi, M., Arai, K., and Yamaha, E. (2012). Germ cells are not the primary factor for sexual fate determination in goldfish. Dev. Biol. 370, 98–109.
| Germ cells are not the primary factor for sexual fate determination in goldfish.Crossref | GoogleScholarGoogle Scholar | 22824426PubMed |
Goto, R., Saito, T., Kawakami, Y., Kitauchi, T., Takagi, M., Todo, T., Arai, K., and Yamaha, E. (2015). Visualization of primordial germ cells in the fertilized pelagic eggs of the barfin flounder Verasper moseri. Int. J. Dev. Biol. 59, 465–470.
| Visualization of primordial germ cells in the fertilized pelagic eggs of the barfin flounder Verasper moseri.Crossref | GoogleScholarGoogle Scholar | 26864487PubMed |
Hagedorn, M., Hsu, E., Kleinhans, F. W., and Wildt, D. E. (1997). New approaches for studying the permeability of fish embryos: toward successful cryopreservation. Cryobiology 34, 335–347.
| New approaches for studying the permeability of fish embryos: toward successful cryopreservation.Crossref | GoogleScholarGoogle Scholar | 9200820PubMed |
Hagedorn, M. M., Daly, J. P., Carter, V. L., Cole, K. S., Jaafar, Z., Lager, C. V. A., and Parenti, L. R. (2018). Cryopreservation of fish spermatogonial cells: the future of natural history collections. Sci. Rep. 8, 6149.
| Cryopreservation of fish spermatogonial cells: the future of natural history collections.Crossref | GoogleScholarGoogle Scholar | 29670253PubMed |
Hamasaki, M., Takeuchi, Y., Yazawa, R., Yoshikawa, S., Kadomura, K., Yamada, T., Miyaki, K., Kikuchi, K., and Yoshizaki, G. (2017). Production of tiger puffer Takifugu rubripes offspring from triploid grass puffer Takifugu niphobles parents. Mar. Biotechnol. (NY) 19, 579–591.
| Production of tiger puffer Takifugu rubripes offspring from triploid grass puffer Takifugu niphobles parents.Crossref | GoogleScholarGoogle Scholar | 28942506PubMed |
Hammed, A. M., Fashina-Bombata, H. A., and Osinaike, A. O. (2010). The use of cold shock in inducing triploidy in African mud catfish (Clarias gariepinus). Afr. J. Biotechnol. 9, 1844–1847.
| The use of cold shock in inducing triploidy in African mud catfish (Clarias gariepinus).Crossref | GoogleScholarGoogle Scholar |
Hartung, O., and Marlow, F. L. (2014). Get it together: how RNA-binding proteins assemble and regulate germ plasm in the oocyte and embryo. In ‘Zebrafish: Topics in Reproduction, Toxicology and Development’. (Eds C. A. Lessman and E. A. Carver.) pp. 65–106. (Nova Science Publishers, Inc.: New York, USA.)
Hartung, O., Forbes, M. M., and Marlow, F. L. (2014). Zebrafish vasa is required for germ-cell differentiation and maintenance. Mol. Reprod. Dev. 81, 946–961.
| Zebrafish vasa is required for germ-cell differentiation and maintenance.Crossref | GoogleScholarGoogle Scholar | 25257909PubMed |
Higaki, S., Eto, Y., Kawakami, Y., Yamaha, E., Kagawa, N., Kuwayama, M., Nagano, M., Katagiri, S., and Takahashi, Y. (2010). Production of fertile zebrafish (Danio rerio) possessing germ cells (gametes) originated from primordial germ cells recovered from vitrified embryos. Reproduction 139, 733–740.
| Production of fertile zebrafish (Danio rerio) possessing germ cells (gametes) originated from primordial germ cells recovered from vitrified embryos.Crossref | GoogleScholarGoogle Scholar | 20154175PubMed |
Higaki, S., Kawakami, Y., Eto, Y., Yamaha, E., Nagano, M., Katagiri, S., Takada, T., and Takahashi, Y. (2013). Cryopreservation of zebrafish (Danio rerio) primordial germ cells by vitrification of yolk-intact and yolk-depleted embryos using various cryoprotectant solutions. Cryobiology 67, 374–382.
| Cryopreservation of zebrafish (Danio rerio) primordial germ cells by vitrification of yolk-intact and yolk-depleted embryos using various cryoprotectant solutions.Crossref | GoogleScholarGoogle Scholar | 24383132PubMed |
Higaki, S., Shimada, M., Kawamoto, K., Todo, T., Kawasaki, T., Tooyama, I., Fujioka, Y., Sakai, N., and Takada, T. (2017). In vitro differentiation of fertile sperm from cryopreserved spermatogonia of the endangered endemic cyprinid honmoroko (Gnathopogon caerulescens). Sci. Rep. 7, 42852.
| In vitro differentiation of fertile sperm from cryopreserved spermatogonia of the endangered endemic cyprinid honmoroko (Gnathopogon caerulescens).Crossref | GoogleScholarGoogle Scholar | 28211534PubMed |
Huergo, G. M., and Zaniboni-Filho, E. (2006). Triploidy induction in Jundiá, Rhamdia quelen, through hydrostatic pressure shock. J. Appl. Aquacult. 18, 45–57.
| Triploidy induction in Jundiá, Rhamdia quelen, through hydrostatic pressure shock.Crossref | GoogleScholarGoogle Scholar |
Ichida, K., Kise, K., Morita, T., Yazawa, R., Takeuchi, Y., and Yoshizaki, G. (2017). Flow-cytometric enrichment of Pacific bluefin tuna type A spermatogonia based on light-scattering properties. Theriogenology 101, 91–98.
| Flow-cytometric enrichment of Pacific bluefin tuna type A spermatogonia based on light-scattering properties.Crossref | GoogleScholarGoogle Scholar | 28708521PubMed |
Cresswell, I. D., and Murphy, H. (2016). Biodiversity: Freshwater species and ecosystems. In ‘Australia State of the Environment 2016.’ (Australian Government Department of the Environment and Energy: Canberra.)
Janik, M., Kleinhans, F. W., and Hagedorn, M. (2000). Overcoming a permeability barrier by microinjecting cryoprotectants into zebrafish embryos (Brachydanio rerio). Cryobiology 41, 25–34.
| Overcoming a permeability barrier by microinjecting cryoprotectants into zebrafish embryos (Brachydanio rerio).Crossref | GoogleScholarGoogle Scholar | 11017758PubMed |
Khosla, K., Wang, Y., Hagedorn, M., Qin, Z., and Bischof, J. (2017). Gold nanorod induced warming of embryos from the cryogenic state enhances viability. ACS Nano 11, 7869–7878.
| Gold nanorod induced warming of embryos from the cryogenic state enhances viability.Crossref | GoogleScholarGoogle Scholar | 28702993PubMed |
Kise, K., Yoshikawa, H., Sato, M., Tashiro, M., Yazawa, R., Nagasaka, Y., Takeuchi, Y., and Yoshizaki, G. (2012). Flow-cytometric isolation and enrichment of teleost type A spermatogonia based on light-scattering properties. Biol. Reprod. 86, 107.
| Flow-cytometric isolation and enrichment of teleost type A spermatogonia based on light-scattering properties.Crossref | GoogleScholarGoogle Scholar | 22219211PubMed |
Kobayashi, T., Takeuchi, Y., Yoshizaki, G., and Takeuchi, T. (2003). Cryopreservation of trout primordial germ cells. Fish Physiol. Biochem. 28, 479–480.
| Cryopreservation of trout primordial germ cells.Crossref | GoogleScholarGoogle Scholar |
Kobayashi, T., Yoshizaki, G., Takeuchi, Y., and Takeuchi, T. (2004). Isolation of highly pure and viable primordial germ cells from rainbow trout by GFP-dependent flow cytometry. Mol. Reprod. Dev. 67, 91–100.
| Isolation of highly pure and viable primordial germ cells from rainbow trout by GFP-dependent flow cytometry.Crossref | GoogleScholarGoogle Scholar | 14648879PubMed |
Kobayashi, T., Takeuchi, Y., Takeuchi, T., and Yoshizaki, G. (2007). Generation of viable fish from cryopreserved primordial germ cells. Mol. Reprod. Dev. 74, 207–213.
| Generation of viable fish from cryopreserved primordial germ cells.Crossref | GoogleScholarGoogle Scholar | 16998845PubMed |
Köprunner, M., Thisse, C., Thisse, B., and Raz, E. (2001). A zebrafish nanos-related gene is essential for the development of primordial germ cells. Genes Dev. 15, 2877–2885.
| 11691838PubMed |
Lacerda, S. M. S. N., Batlouni, S. R., Costa, J. M. G., Segatelli, T. M., Quirino, B. R., Queiroz, B. M., Kalapothakis, E., and Franca, L. R. (2010). A new and fast technique to generate offspring after germ cells transplantation in adult fish: the Nile tilapia (Oreochromis niloticus) model. PLoS One 5, e10740.
| A new and fast technique to generate offspring after germ cells transplantation in adult fish: the Nile tilapia (Oreochromis niloticus) model.Crossref | GoogleScholarGoogle Scholar |
Lacerda, S. M. S. N., Costa, G. M. J., and de França, L. R. (2014). Biology and identity of fish spermatogonial stem cell. Gen. Comp. Endocrinol. 207, 56–65.
| Biology and identity of fish spermatogonial stem cell.Crossref | GoogleScholarGoogle Scholar |
Lee, S., and Yoshizaki, G. (2016). Successful cryopreservation of spermatogonia in critically endangered Manchurian trout (Brachymystax lenok). Cryobiology 72, 165–168.
| Successful cryopreservation of spermatogonia in critically endangered Manchurian trout (Brachymystax lenok).Crossref | GoogleScholarGoogle Scholar | 26827783PubMed |
Lee, S., Iwasaki, Y., Shikina, S., and Yoshizaki, G. (2013). Generation of functional eggs and sperm from cryopreserved whole testes. Proc. Natl Acad. Sci. USA 110, 1640–1645.
| Generation of functional eggs and sperm from cryopreserved whole testes.Crossref | GoogleScholarGoogle Scholar | 23319620PubMed |
Lee, S., Seki, S., Katayama, N., and Yoshizaki, G. (2015). Production of viable trout offspring derived from frozen whole fish. Sci. Rep. 5, 16045.
| Production of viable trout offspring derived from frozen whole fish.Crossref | GoogleScholarGoogle Scholar | 26522018PubMed |
Lee, S., Katayama, N., and Yoshizaki, G. (2016). Generation of juvenile rainbow trout derived from cryopreserved whole ovaries by intraperitoneal transplantation of ovarian germ cells. Biochem. Biophys. Res. Commun. 478, 1478–1483.
| Generation of juvenile rainbow trout derived from cryopreserved whole ovaries by intraperitoneal transplantation of ovarian germ cells.Crossref | GoogleScholarGoogle Scholar | 27581197PubMed |
Li, M., Hong, N., Xu, H., Song, J., and Hong, Y. (2016). Germline replacement by blastula cell transplantation in the fish medaka. Sci. Rep. 6, 29658.
| Germline replacement by blastula cell transplantation in the fish medaka.Crossref | GoogleScholarGoogle Scholar | 27406328PubMed |
Linhartová, Z., Rodina, M., Guralp, H., Gazo, I., Saito, T., and Pšenička, M. (2014). Isolation and cryopreservation of early stages of germ cells of tench (Tinca tinca). Czech J. Anim. Sci. 59, 381–390.
| Isolation and cryopreservation of early stages of germ cells of tench (Tinca tinca).Crossref | GoogleScholarGoogle Scholar |
Lintermans, M. (2007). ‘Fishes of the Murray–Darling Basin: An Introductory Guide.’ (Murray-Darling Basin Authority: Canberra, Australia)
Lubzens, E., Young, G., Bobe, J., and Cerdà, J. (2010). Oogenesis in teleosts: how fish eggs are formed. Gen. Comp. Endocrinol. 165, 367–389.
| Oogenesis in teleosts: how fish eggs are formed.Crossref | GoogleScholarGoogle Scholar | 19505465PubMed |
Lujić, J., Marinović, Z., Sušnik Bajec, S., Djurdjevič, I., Kása, E., Urbányi, B., and Horváth, Á. (2017). First successful vitrification of salmonid ovarian tissue. Cryobiology 76, 154–157.
| First successful vitrification of salmonid ovarian tissue.Crossref | GoogleScholarGoogle Scholar | 28438562PubMed |
Majhi, S. K., Hattori, R. S., Yokota, M., Watanabe, S., and Strüssmann, C. A. (2009). Germ cell transplantation using sexually competent fish: an approach for rapid propagation of endangered and valuable germlines. PLoS One 4, e6132.
| Germ cell transplantation using sexually competent fish: an approach for rapid propagation of endangered and valuable germlines.Crossref | GoogleScholarGoogle Scholar | 19572014PubMed |
Majhi, S. K., Hattori, R. S., Rahman, S. M., and Strussmann, C. A. (2014). Surrogate production of eggs and sperm by intrapapillary transplantation of germ cells in cytoablated adult fish. PLoS One 9, e95294.
| Surrogate production of eggs and sperm by intrapapillary transplantation of germ cells in cytoablated adult fish.Crossref | GoogleScholarGoogle Scholar | 24748387PubMed |
Malison, J. A., Procarione, L. S., Held, J. A., Kayes, T. B., and Amundson, C. H. (1993). The influence of triploidy and heat and hydrostatic pressure shocks on the growth and reproductive development of juvenile yellow perch (Perca jlavescens). Aquaculture 116, 121–133.
| The influence of triploidy and heat and hydrostatic pressure shocks on the growth and reproductive development of juvenile yellow perch (Perca jlavescens).Crossref | GoogleScholarGoogle Scholar |
Matsuda, M. (2003). Sex determination in fish: lessons from the sex-determining gene of the teleost medaka, Oryzias latipes. Dev. Growth Differ. 45, 397–403.
| Sex determination in fish: lessons from the sex-determining gene of the teleost medaka, Oryzias latipes.Crossref | GoogleScholarGoogle Scholar | 14706065PubMed |
McMillan, D. B. (2007). ‘Fish Histology Female Reproductive Systems.’ (Springer Verlag: Dordrecht.)
Murray–Darling Basin Authority (MDBA) (2019). Response to recent fish deaths: recommended action plan. Available at https://www.mdba.gov.au/sites/default/files/pubs/Response-fish-death-events-recommended-action%20plan-2019_0.pdf [verified 21 May 2020].
Nishimura, T., and Tanaka, M. (2014). Gonadal development in fish. Sex Dev. 8, 252–261.
| Gonadal development in fish.Crossref | GoogleScholarGoogle Scholar | 25034975PubMed |
Nynca, J., Dietrich, G. J., Dobosz, S., Grudniewska, J., and Ciereszko, A. (2014). Effect of cryopreservation on sperm motility parameters and fertilizing ability of brown trout semen. Aquaculture 433, 62–65.
| Effect of cryopreservation on sperm motility parameters and fertilizing ability of brown trout semen.Crossref | GoogleScholarGoogle Scholar |
Okutsu, T., Suzuki, K., Takeuchi, Y., Takeuchi, T., and Yoshizaki, G. (2006a). Testicular germ cells can colonize sexually undifferentiated embryonic gonad and produce functional eggs in fish. Proc. Natl Acad. Sci. USA 103, 2725–2729.
| Testicular germ cells can colonize sexually undifferentiated embryonic gonad and produce functional eggs in fish.Crossref | GoogleScholarGoogle Scholar | 16473947PubMed |
Okutsu, T., Yano, A., Nagasawa, K., Shikina, S., Kobayashi, T., Takeuchi, Y., and Yoshizaki, G. (2006b). Manipulation of fish germ cell: visualization, cryopreservation and transplantation. J. Reprod. Dev. 52, 685–693.
| Manipulation of fish germ cell: visualization, cryopreservation and transplantation.Crossref | GoogleScholarGoogle Scholar | 17220596PubMed |
Okutsu, T., Shikina, S., Kanno, M., Takeuchi, Y., and Yoshizaki, G. (2007). Production of trout offspring from triploid salmon parents. Science 317, 1517.
| Production of trout offspring from triploid salmon parents.Crossref | GoogleScholarGoogle Scholar | 17872437PubMed |
Otake, H., Shinomiya, A., Matsuda, M., Hamaguchi, S., and Sakaizumi, M. (2006). Wild-derived XY sex-reversal mutants in the medaka, Oryzias latipes. Genetics 173, 2083–2090.
| Wild-derived XY sex-reversal mutants in the medaka, Oryzias latipes.Crossref | GoogleScholarGoogle Scholar | 16702419PubMed |
Pandian, T. J., and Kirankumar, S. (2003). Androgenesis and conservation of fishes. Curr. Sci. 85, 917–931.
Papah, M. B., Kisia, S. M., Ojoo, R. O., Makanya, A. N., Wood, C. M., Kavembe, G. D., Maina, J. N., Johannsson, O. E., Bergman, H. L., Laurent, P., Chevalier, C., Bianchini, A., Bianchini, L. F., and Onyango, D. W. (2013). Morphological evaluation of spermatogenesis in Lake Magadi tilapia (Alcolapia grahami): a fish living on the edge. Tissue Cell 45, 371–382.
| Morphological evaluation of spermatogenesis in Lake Magadi tilapia (Alcolapia grahami): a fish living on the edge.Crossref | GoogleScholarGoogle Scholar | 23916093PubMed |
Pearson, P. L. (2001). Triploidy. In ‘Encyclopedia of Genetics’. (Eds S. Brenner and J. H. Miller.) pp. 2055–2056. (Academic Press: New York.)
Peruzzi, S., and Chatain, B. (2000). Pressure and cold shock induction of meiotic gynogenesis and triploidy in the European sea bass, Dicentrarchus labrax L.: relative efficiency of methods and parental variability. Aquaculture 189, 23–37.
| Pressure and cold shock induction of meiotic gynogenesis and triploidy in the European sea bass, Dicentrarchus labrax L.: relative efficiency of methods and parental variability.Crossref | GoogleScholarGoogle Scholar |
Peruzzi, S., Kettunen, A., Primicerio, R., and Kaurić, G. (2007). Thermal shock induction of triploidy in Atlantic cod (Gadus morhua L.). Aquacult. Res. 38, 926–932.
| Thermal shock induction of triploidy in Atlantic cod (Gadus morhua L.).Crossref | GoogleScholarGoogle Scholar |
Pradeep, P. J., Srijaya, T. C., Hassan, A., Chatterji, A. K., Withyachumnarnkul, B., and Jeffs, A. (2014). Optimal conditions for cold-shock induction of triploidy in red tilapia. Aquacult. Int. 22, 1163–1174.
Pšenička, M., Saito, T., Linhartová, Z., and Gazo, I. (2015). Isolation and transplantation of sturgeon early-stage germ cells. Theriogenology 83, 1085–1092.
| Isolation and transplantation of sturgeon early-stage germ cells.Crossref | GoogleScholarGoogle Scholar | 25559841PubMed |
Raz, E., and Reichman-Fried, M. (2006). Attraction rules: germ cell migration in zebrafish. Curr. Opin. Genet. Dev. 16, 355–359.
| Attraction rules: germ cell migration in zebrafish.Crossref | GoogleScholarGoogle Scholar | 16806897PubMed |
Riesco, M. F., Martinez-Pastor, F., Chereguini, O., and Robles, V. (2012). Evaluation of zebrafish (Danio rerio) PGCs viability and DNA damage using different cryopreservation protocols. Theriogenology 77, 122–130.e2.
| Evaluation of zebrafish (Danio rerio) PGCs viability and DNA damage using different cryopreservation protocols.Crossref | GoogleScholarGoogle Scholar | 21872308PubMed |
Saito, T., Fujimoto, T., Maegawa, S., Inoue, K., Tanaka, M., Arai, K., and Yamaha, E. (2006). Visualization of primordial germ cells in vivo using GFP-nos1 3′UTR mRNA. Int. J. Dev. Biol. 50, 691–699.
| Visualization of primordial germ cells in vivo using GFP-nos1 3′UTR mRNA.Crossref | GoogleScholarGoogle Scholar | 17051479PubMed |
Saito, T., Goto-Kazeto, R., Arai, K., and Yamaha, E. (2008). Xenogenesis in teleost fish through generation of germ-line chimeras by single primordial germ cell transplantation. Biol. Reprod. 78, 159–166.
| Xenogenesis in teleost fish through generation of germ-line chimeras by single primordial germ cell transplantation.Crossref | GoogleScholarGoogle Scholar | 17901077PubMed |
Saito, T., Goto-Kazeto, R., Fujimoto, T., Kawakami, Y., Arai, K., and Yamaha, E. (2010). Inter-species transplantation and migration of primordial germ cells in cyprinid fish. Int. J. Dev. Biol. 54, 1481–1486.
| Inter-species transplantation and migration of primordial germ cells in cyprinid fish.Crossref | GoogleScholarGoogle Scholar | 20979025PubMed |
Saito, T., Goto-Kazeto, R., Kawakami, Y., Nomura, K., Tanaka, H., Adachi, S., Arai, K., and Yamaha, E. (2011). The mechanism for primordial germ-cell migration is conserved between Japanese eel and zebrafish. PLoS One 6, e24460.
| The mechanism for primordial germ-cell migration is conserved between Japanese eel and zebrafish.Crossref | GoogleScholarGoogle Scholar | 21931802PubMed |
Saito, T., Pšenička, M., Goto, R., Adachi, S., Inoue, K., Arai, K., and Yamaha, E. (2014). The origin and migration of primordial germ cells in sturgeons. PLoS One 9, e86861.
| The origin and migration of primordial germ cells in sturgeons.Crossref | GoogleScholarGoogle Scholar | 25546433PubMed |
Saito, T., Güralp, H., Iegorova, V., Rodina, M., and Pšenicka, M. (2018). Elimination of primordial germ cells in sturgeon embryos by ultraviolet irradiation. Biol. Reprod. 99, 556–564.
| Elimination of primordial germ cells in sturgeon embryos by ultraviolet irradiation.Crossref | GoogleScholarGoogle Scholar | 29635315PubMed |
Schwander, T., and Oldroyd, B. P. (2016). Androgenesis: where males hijack eggs to clone themselves. Philos. Trans. R. Soc. Lond. B Biol. Sci. 371, 20150534.
| Androgenesis: where males hijack eggs to clone themselves.Crossref | GoogleScholarGoogle Scholar | 27619698PubMed |
Shang, M., Su, B., Perera, D. A., Alsaqufi, A., Lipke, E. A., Cek, S., Dunn, D. A., Qin, Z., Peatman, E., and Dunham, R. A. (2018). Testicular germ line cell identification, isolation, and transplantation in two North American catfish species. Fish Physiol. Biochem. 44, 717–733.
| Testicular germ line cell identification, isolation, and transplantation in two North American catfish species.Crossref | GoogleScholarGoogle Scholar | 29357082PubMed |
Siegfried, K. R., and Nusslein-Volhard, C. (2008). Germ line control of female sex determination in zebrafish. Dev. Biol. 324, 277–287.
| Germ line control of female sex determination in zebrafish.Crossref | GoogleScholarGoogle Scholar | 18930041PubMed |
Silva, M. A., Costa, G. M., Lacerda, S. M., Brandão-Dias, P. F., Kalapothakis, E., Silva Júnior, A. F., Alvarenga, E. R., and França, L. R. (2016). Successful xenogeneic germ cell transplantation from Jundia catfish (Rhamdia quelen) into adult Nile tilapia (Oreochromis niloticus) testes. Gen. Comp. Endocrinol. 230–231, 48–56.
| Successful xenogeneic germ cell transplantation from Jundia catfish (Rhamdia quelen) into adult Nile tilapia (Oreochromis niloticus) testes.Crossref | GoogleScholarGoogle Scholar | 26972155PubMed |
Siripattarapravat, K., Pinmee, B., Venta, P. J., Chang, C. C., and Cibelli, J. B. (2009). Somatic cell nuclear transfer in zebrafish. Nat. Methods 6, 733–735.
| Somatic cell nuclear transfer in zebrafish.Crossref | GoogleScholarGoogle Scholar | 19718031PubMed |
Slanchev, K., Stebler, J., de la Cueva-Mendez, G., and Raz, E. (2005). Development without germ cells: the role of the germ line in zebrafish sex differentiation. Proc. Natl Acad. Sci. USA 102, 4074–4079.
| Development without germ cells: the role of the germ line in zebrafish sex differentiation.Crossref | GoogleScholarGoogle Scholar | 15728735PubMed |
Takahashi, E., Shimizu, Y., Urushibata, H., Kawakami, Y., Arai, K., and Yamaha, E. (2017). Migration behavior of PGCs and asymmetrical gonad formation in pond smelt Hypomesus nipponensis. Int. J. Dev. Biol. 61, 397–405.
| Migration behavior of PGCs and asymmetrical gonad formation in pond smelt Hypomesus nipponensis.Crossref | GoogleScholarGoogle Scholar | 28695959PubMed |
Takeuchi, Y., Yoshizaki, G., Kobayashi, T., and Takeuchi, T. (2002). Mass isolation of primordial germ cells from transgenic rainbow trout carrying the green fluorescent protein gene driven by the vasa gene promoter. Biol. Reprod. 67, 1087–1092.
| Mass isolation of primordial germ cells from transgenic rainbow trout carrying the green fluorescent protein gene driven by the vasa gene promoter.Crossref | GoogleScholarGoogle Scholar | 12297522PubMed |
Takeuchi, Y., Yoshizaki, G., and Takeuchi, T. (2003). Generation of live fry from intraperitoneally transplanted primordial germ cells in rainbow trout. Biol. Reprod. 69, 1142–1149.
| Generation of live fry from intraperitoneally transplanted primordial germ cells in rainbow trout.Crossref | GoogleScholarGoogle Scholar | 12773413PubMed |
Tsai, S. (2009). Development of cryopreservation techniques for early stage zebrafish (Danio rerio) oocytes. Ph.D. Thesis, University of Bedfordshire, Luton.
Uribe, M. C., Grier, H. J., and Mejía-Roa, V. (2014). Comparative testicular structure and spermatogenesis in bony fishes. Spermatogenesis 4, e983400.
| Comparative testicular structure and spermatogenesis in bony fishes.Crossref | GoogleScholarGoogle Scholar | 26413406PubMed |
Uribe, M. C., Grier, H. J., García-Alarcón, A., and Parenti, L. R. (2016). Oogenesis: from oogonia to ovulation in the flagfish, Jordanella floridae Goode and Bean, 1879 (Teleostei: Cyprinodontidae. J. Morphol. 277, 1339–1354.
| Oogenesis: from oogonia to ovulation in the flagfish, Jordanella floridae Goode and Bean, 1879 (Teleostei: Cyprinodontidae.Crossref | GoogleScholarGoogle Scholar | 27418385PubMed |
Viana, I. K. S., Gonçalves, L. A. B., Ferreira, M. A. P., Mendes, Y. A., and Rocha, R. M. (2018). Oocyte growth, follicular complex formation and extracellular-matrix remodeling in ovarian maturation of the imperial zebra pleco fish Hypancistrus zebra. Sci. Rep. 8, 13760.
| Oocyte growth, follicular complex formation and extracellular-matrix remodeling in ovarian maturation of the imperial zebra pleco fish Hypancistrus zebra.Crossref | GoogleScholarGoogle Scholar |
Weidinger, G., Slanchev, J. K., Dumstrei, K., Wise, C., Lovell-Badge, R., Thisse, C., Thisse, B., and Raz1, E. (2003). dead end, a novel vertebrate germ plasm component, is required for zebrafish primordial germ cell migration and survival. Curr. Biol. 13, 1429–1434.
| dead end, a novel vertebrate germ plasm component, is required for zebrafish primordial germ cell migration and survival.Crossref | GoogleScholarGoogle Scholar | 12932328PubMed |
Weidinger, G., Wolke, U., Köprunner, M., Klinger, M., and Raz, E. (1999). Identification of tissues and patterning events required for distinct steps in early migration of zebrafish primordial germ cells. Development 126, 5295–5307.
| 10556055PubMed |
Wong, T.-T., and Zohar, Y. (2015). Production of reproductively sterile fish by a non-transgenic gene silencing technology. Sci. Rep. 5, 15822.
| Production of reproductively sterile fish by a non-transgenic gene silencing technology.Crossref | GoogleScholarGoogle Scholar | 26510515PubMed |
Wong, T.-T., Saito, T., Crodian, J., and Collodi, P. (2011). Zebrafish germline chimeras produced by transplantation of ovarian germ cells into sterile host larvae. Biol. Reprod. 84, 1190–1197.
| Zebrafish germline chimeras produced by transplantation of ovarian germ cells into sterile host larvae.Crossref | GoogleScholarGoogle Scholar | 21248287PubMed |
Wootton, R. J., and Smith, C. (2014a). Gametogenesis. In ‘Reproductive Biology of Teleost Fishes’ pp. 45–80. (John Wiley & Sons.)
Wootton, R. J., and Smith, C. (2014b). Sex differentiation. In ‘Reproductive Biology of Teleost Fishes’. pp. 31–43. (John Wiley & Sons.)
World Wildlife Fund (WWF) (2015). Living blue planet report. Species, habitats and human well-being. WWF, Gland, Switzerland.
Yamaha, E., Murakami, M., Hada, K., Otani, S., Fujimoto, T., Tanaka, M., Sakao, S., Kimura, S., Sato, S., and Arai, K. (2003). Recovery of fertility in male hybrids of a cross between goldfish and common carp by transplantation of PGC (primordial germ cell)-containing graft. Genetica 119, 121–131.
| 14620952PubMed |
Yamaha, E., Saito, T., Goto-Kazeto, R., and Arai, K. (2007). Developmental biotechnology for aquaculture, with special reference to surrogate production in teleost fishes. J. Sea Res. 58, 8–22.
| Developmental biotechnology for aquaculture, with special reference to surrogate production in teleost fishes.Crossref | GoogleScholarGoogle Scholar |
Yang, H., Carmichael, C., Varga, Z. M., and Tiersch, T. R. (2007). Development of a simplified and standardized protocol with potential for high-throughput for sperm cryopreservation in zebrafish Danio rerio. Theriogenology 68, 128–136.
| Development of a simplified and standardized protocol with potential for high-throughput for sperm cryopreservation in zebrafish Danio rerio.Crossref | GoogleScholarGoogle Scholar | 17544099PubMed |
Ye, H., Li, C. J., Yue, H. M., Du, H., Yang, X. G., Yoshino, T., Hayashida, T., Takeuchi, Y., and Wei, Q. W. (2017). Establishment of intraperitoneal germ cell transplantation for critically endangered Chinese sturgeon Acipenser sinensis. Theriogenology 94, 37–47.
| Establishment of intraperitoneal germ cell transplantation for critically endangered Chinese sturgeon Acipenser sinensis.Crossref | GoogleScholarGoogle Scholar | 28407859PubMed |
Yoon, C., Kawakami, K., and Hopkins, N. (1997). Zebrafish vasa homologue RNA is localized to the cleavage planes of 2- and 4-cell-stage embryos and is expressed in the primordial germ cells. Development 124, 3157–3165.
| 9272956PubMed |
Yoshikawa, H., Ino, Y., Shigenaga, K., Katayama, T., Kuroyanagi, M., and Yoshiura, Y. (2018). Production of tiger puffer Takifugu rubripes from cryopreserved testicular germ cells using surrogate broodstock technology. Aquaculture 493, 302–313.
| Production of tiger puffer Takifugu rubripes from cryopreserved testicular germ cells using surrogate broodstock technology.Crossref | GoogleScholarGoogle Scholar |
Yoshizaki, G., and Lee, S. (2018). Production of live fish derived from frozen germ cells via germ cell transplantation. Stem Cell Res. 29, 103–110.
| Production of live fish derived from frozen germ cells via germ cell transplantation.Crossref | GoogleScholarGoogle Scholar | 29649725PubMed |
Yoshizaki, G., Takeuchi, Y., Sakatani, S., and Takeuchi, T. (2000). Germ cell-specific expression of green fluorescent protein in transgenic rainbow trout under control of the rainbow trout vasa-like gene promoter. Int. J. Dev. Biol. 44, 323–326.
| 10853829PubMed |
Yoshizaki, G., Takeuchi, Y., Kobayashi, T., Ihara, S., and Takeuchi, T. (2002). Primordial germ cells: the blueprint for a piscine life. Fish Physiol. Biochem. 26, 3–12.
| Primordial germ cells: the blueprint for a piscine life.Crossref | GoogleScholarGoogle Scholar |
Yoshizaki, G., Tago, Y., Takeuchi, Y., Sawatari, E., Kobayashi, T., and Takeuchi, T. (2005). Green fluorescent protein labeling of primordial germ cells using a nontransgenic method and its application for germ cell transplantation in salmonidae. Biol. Reprod. 73, 88–93.
| Green fluorescent protein labeling of primordial germ cells using a nontransgenic method and its application for germ cell transplantation in salmonidae.Crossref | GoogleScholarGoogle Scholar | 15744027PubMed |
Yoshizaki, G., Ichikawa, M., Hayashi, M., Iwasaki, Y., Miwa, M., Shikina, S., and Okutsu, T. (2010). Sexual plasticity of ovarian germ cells in rainbow trout. Development 137, 1227–1230.
| Sexual plasticity of ovarian germ cells in rainbow trout.Crossref | GoogleScholarGoogle Scholar | 20223765PubMed |
Yoshizaki, G., Okutsu, T., Morita, T., Terasawa, M., Yazawa, R., and Takeuchi, Y. (2012). Biological characteristics of fish germ cells and their application to developmental biotechnology. Reprod. Domest. Anim. 47, 187–192.
| Biological characteristics of fish germ cells and their application to developmental biotechnology.Crossref | GoogleScholarGoogle Scholar | 22827369PubMed |
Yoshizaki, G., Takashiba, K., Shimamori, S., Fujinuma, K., Shikina, S., Okutsu, T., Kume, S., and Hayashi, M. (2016). Production of germ cell-deficient salmonids by dead end gene knockdown, and their use as recipients for germ cell transplantation. Mol. Reprod. Dev. 83, 298–311.
| Production of germ cell-deficient salmonids by dead end gene knockdown, and their use as recipients for germ cell transplantation.Crossref | GoogleScholarGoogle Scholar | 26860442PubMed |




