Sperm cryopreservation in the Burmese python Python bivittatus as a model for endangered snakes
Carly Young A D , Nicole Ravida A , Michael Rochford B , Frank Mazzotti C , Michelle Curtis A and Barbara Durrant
A D , Nicole Ravida A , Michael Rochford B , Frank Mazzotti C , Michelle Curtis A and Barbara Durrant  A
A
A San Diego Zoo Wildlife Alliance, Beckman Center for Conservation Research, 15600 San Pasqual Valley Road, Escondido, CA 92027, USA.
B Transcon Environmental, Inc., 2455 Bennett Valley Road, Santa Rosa, CA 95404, USA.
C University of Florida, 3205 College Avenue, Davie, FL 33314, USA.
D Corresponding author. Email: cyoung@sdzwa.org
Reproduction, Fertility and Development 34(5) 401-409 https://doi.org/10.1071/RD21023
Submitted: 30 January 2021 Accepted: 2 July 2021 Published: 20 August 2021
Journal Compilation © CSIRO 2022 Open Access CC BY-NC
Abstract
Burmese pythons Python bivittatus captured in the Florida Everglades as part of an invasive species monitoring program served as a model for the development of sperm cryopreservation protocols for endangered snakes. Spermatozoa were collected from the vas deferens and initial motility, plasma membrane integrity and acrosome integrity were recorded before cryopreservation. Spermatozoa were extended in TES and Tris (TEST) yolk buffer with glycerol (GLY) or dimethyl sulfoxide (DMSO) concentrations of 8%, 12% or 16%, or combinations of GLY and DMSO with final concentrations of 4%:4%, 6%:6% or 8%:8%, and frozen at a rate of 0.3°C min−1. Sperm frozen in combinations of GLY and DMSO exhibited greater post-thaw motility and plasma membrane integrity than those frozen in GLY or DMSO alone. All DMSO and GLY:DMSO treatments preserved a greater proportion of intact acrosomes than GLY alone. To determine the best overall cryopreservation protocol for this species, a sperm quality index was calculated, giving equal weight to each of the three measured indicators of cryosurvival. This analysis revealed that Burmese python spermatozoa frozen in 6% GLY:6% DMSO or 4% GLY:4% DMSO exhibited the highest post-thaw viability. This study represents the first comparative, comprehensive attempt to develop a sperm cryopreservation protocol for any snake species.
Keywords: endangered species, freezing, reptiles, semen, squamates.
Introduction
There are nearly 3500 described species of snakes and, of the 2367 listed on the International Union for Conservation of Nature (IUCN) Red List of Threatened Species, 385 are categorised as threatened with extinction. However, 22% of IUCN-listed snake species lack sufficient data to categorise, leaving their status unknown. Although widely distributed in tropical and subtropical regions of the world, boas and pythons are globally threatened by illegal harvesting for food and the pet trade, ecosystem conversion, increasing human population and climate change (IUCN, https://www.iucn.org/commissions/ssc-groups/amphibians-reptiles/boa-and-python). Twenty-six percent of boa and python species assessed by IUCN are threatened with extinction, including the Burmese python Python bivittatus. This species is native to South-east Asia, but, since the end of the 20th century, intentionally or accidentally released pet Burmese pythons have become an established breeding population in south Florida in the US and are classified as an invasive species. As opportunistic, generalist predators, Burmese pythons disrupt the Everglades ecosystem by preying on native species, transmitting parasites to native species and potentially outcompeting some native species for food or other resources (Dorcas et al. 2012; Miller et al. 2018). A multi-agency eradication program initiated in 2008 monitors the python population, removing specimens regularly and conducting research on population control measures.
There are limited published reports of the collection and characterisation of snake spermatozoa (Zacariotti et al. 2007; Tourmente et al. 2007, 2011; Mozafari et al. 2012; Friesen et al. 2014; Moshiri et al. 2014), cooling of snake spermatozoa (Fahrig et al. 2007) and AI with fresh or cooled spermatozoa (Quinn et al. 1989; Mattson et al. 2007; Oliveri et al. 2018), and there is a dearth of literature on the development of cryopreservation protocols for snakes (Mengden et al. 1980; Mattson et al. 2009; Zacariotti et al. 2012; Young et al. 2019). In the absence of published snake literature, the protocols used in this study are based on those developed for the tegu (Young et al. 2017).
Obtaining sufficient samples from snake species for the development of cryopreservation protocols is problematic, necessitating the use of model species for scientific study. As part of an invasive species monitoring and eradication program, Burmese pythons were captured in southern Miami-Dade County, Florida. Following humane euthanasia and necropsy, reproductive tracts from healthy male pythons were donated to San Diego Zoo Wildlife Alliance from the University of Florida for this sperm cryopreservation study. This collaboration allowed the ethical sourcing of snakes, obviating the need to collect specimens from their native range or to kill captive animals. As a member of the Pythonidae family, Burmese pythons may be an important model for closely related endangered boas and pythons. This study represents the first comparative, comprehensive attempt to develop a sperm cryopreservation protocol for any snake species.
Materials and methods
Animals
This study was conducted with the approval of the Zoological Society of San Diego’s Institutional Animal Care and Use Committee. University of Florida Invasive Species Program personnel obtained free-ranging Burmese pythons from the southern Miami-Dade population of the Southern Glades Wildlife and Environmental Area, as well as Homestead and Florida City residential areas. Fifty males were collected during five annual breeding seasons from December to April 2013–17. Each male was weighed (kg) and measured for total length (cm). Spermatozoa from 20 males did not meet our minimum criterion for cryopreservation (i.e. an initial motility score (IMS) <144; see below) and were eliminated from further study. Of the remaining 30 males, 24 were used in preliminary studies designed to identify the best cryoprotectants and freeze rates. Based on preliminary study results and our previous work with the Argentine black and white tegu Tupinambis merianae (Young et al. 2017), the treatments described in this study were conducted with the remaining six males.
Sperm collection and evaluation
After postmortem removal, python vas deferens were shipped overnight at 4°C in 0.9% saline with each end of the vas deferens ligated to prevent the leakage of spermatozoa. Following slicing of the vas deferens, spermatozoa were diluted in M199 with HEPES (Sigma-Aldrich) and analysed at 22°C. For each male, the sperm IMS (calculated as % motile spermatozoa × speed of progression2 × 100, where speed of progression (SOP) is graded on a scale of 1–5, with 1 = non-progressive movement and 5 = fastest forward progression), plasma membrane integrity (IPL; eosin–nigrosin stain; Williams and Pollak 1950) and acrosome integrity (IAC; Pope stain modified by removal of sodium citrate; Pope et al. 1991) were recorded before freezing. Each analysis was performed on 100 spermatozoa. Spermatozoa with an IMS <144 were not cryopreserved, resulting in the elimination of 20 males. Detached heads were counted as non-motile spermatozoa without intact plasma membranes. All assessments were conducted using a Nikon Eclipse 55i at a magnification of ×400 (motility, SOP and IPL) or ×1000 under oil (IAC).
Sperm cryopreservation and thawing
In preliminary experiments, cryoprotectants (dimethyl sulfoxide (DMSO), glycerol, ethylene glycol, dimethylacetamide (DMA), dimethylformamide (DMFA)) in TES and Tris (TEST) yolk buffer with various freeze rates (0.3, 1.0, 6.3, 15°C min−1) were compared. Based on the results of those experiments, the spermatozoa for this study were diluted in 4°C TEST yolk buffer (Jaskey and Cohen 1981) to approximately 100 × 106 spermatozoa in 500 µL with one of nine cryoprotectant treatments: 8%, 12% or 16% final glycerol (GLY) concentration; 8%, 12% or 16% final DMSO concentration; 4%:4%, 6%:6%, or 8%:8% GLY:DMSO for final cryoprotectant concentrations of 8%, 12% or 16%. Extended spermatozoa were frozen in cryovials (Nunc, Thermo Fisher Scientific) in a controlled-rate freezer (CryoMed; Thermo Fisher Scientific) at a rate of 0.3°C min−1 to –40°C before being plunged into liquid nitrogen. Vials were thawed in a 37°C water bath for 90 s. Cryoprotectant was removed by centrifugation at 60g for 10 min at 22°C and the sperm pellet was resuspended in 500 µL M199 with HEPES. At this time (T0), all variables were assessed for each vial and expressed as the percentage of initial (%IMS, %IPL, %IAC) to account for differences between males at the time of sperm collection. Following preliminary experiments comparing post-thaw holding temperatures of 4°C, 22°C and 37°C, spermatozoa were maintained at 22°C for 60 min (T60) and re-evaluated for %IMS and %IPL as described above. In preliminary studies, IAC did not decline appreciably over the post-thaw incubation period of 60 min. Therefore, %IAC was not recorded at T60 in this study.
Statistical analysis
All six male pythons contributed spermatozoa to the study, with a minimum of three individuals randomly assigned to each of the nine cryopreservation treatments. To account for possible differences between vials within male and treatment, three vials of spermatozoa were thawed for each male in each treatment. A single data point for one male at T60 was identified as an outlier by Robust Fit Outliers (JMP), and was therefore omitted from data analysis. Normal distribution was confirmed before analysis. Differences between cryoprotectant and cryoprotectant concentration were analysed by analysis of variance (ANOVA) and the Tukey–Kramer honestly significant difference test. Differences between time points for each treatment were analysed with repeated-measures ANOVA. All analyses were performed with JMP (SAS Institute). All data are expressed as the mean ± s.e.m. Differences were considered significant at two-sided P < 0.05.
To determine the best overall cryopreservation protocol, a sperm quality index (SQI) was calculated giving equal weight to each of the three measured indicators of cryosurvival (SQI = %IMS × %IPL × %IAC/100).
Results
Animals
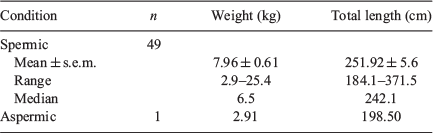
Python weights and total lengths are presented in Table 1. Of the 50 male pythons examined, 49 had spermatozoa in the vas deferens. The single aspermic male was at the bottom of the weight range and was nearly the shortest in length, perhaps indicating immaturity.
|
Preliminary experiments
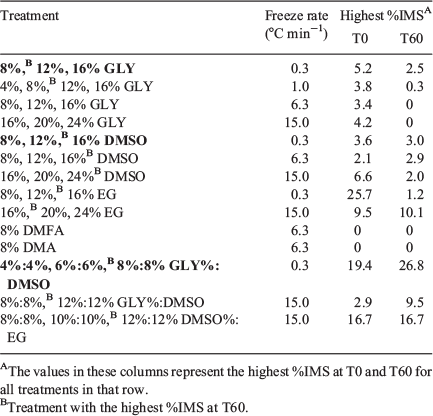
To determine the best freeze rate and cryoprotectants for python spermatozoa, preliminary experiments were conducted. Table 2 lists the cryoprotectants and their concentrations, as well as freeze rates for 29 experimental treatments and the nine treatments in the present study. Although ethylene glycol alone or in combination with DMSO resulted in the highest %IMS at both T0 and T60 respectively, it was eliminated as a cryoprotectant in subsequent studies because it caused most sperm tails to assume an abnormal helical morphology. Of the other treatments, all GLY:DMSO combinations supported the highest %IMS at T60, the post-thaw assessment that more accurately represents sperm quality at the time of a potential AI.
|
Sperm evaluation
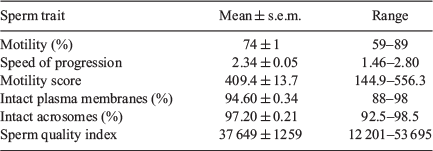
Mean pre-freeze sperm parameters of six male pythons in this study at the time of collection (~24 h postmortem) are summarised in Table 3. Motility ranged from 59% to 89%, with an SOP range of 1.46–2.8, yielding a mean IMS of 409.42. IPL and IAC were high, averaging 94–97%.
|
Sperm cryosurvival
Motility score
Regardless of GLY, DMSO or GLY:DMSO concentrations, cryoprotectant significantly affected %IMS at T0 and T60 (each P < 0.0001). At both time points, spermatozoa frozen in all GLY:DMSO combinations maintained a significantly greater %IMS than those frozen in GLY or DMSO alone.
Treatment significantly affected %IMS at T0 (P = 0.0004) and T60 (P < 0.0001). Although not statistically significant, GLY:DMSO treatments maintained higher %IMS at both time points than all treatments with GLY or DMSO alone. The 6%:6% GLY:DMSO treatment was significantly better than all DMSO treatments and the 16% GLY treatment at T0. At T60, the 6%:6% GLY:DMSO treatment exhibited significantly higher %IMS than all other treatments except 4%:4% GLY:DMSO (Fig. 1).
Within cryoprotectant treatment groups, %IMS was not affected by concentration for spermatozoa frozen in GLY or DMSO alone at T0 (P = 0.1191 and P = 0.5482 respectively). However, at T60, GLY concentration affected %IMS (P = 0.0027) with 8% GLY being superior to 12% and 16% GLY. Also at T60, 12% DMSO was significantly better than 16% DMSO (P = 0.0166). For spermatozoa frozen in GLY:DMSO, concentration did not affect %IMS at T0 (P = 0.6497) or T60 (P = 0.1546).
Regardless of cryoprotectant concentration, time after thaw significantly affected %IMS in GLY treatments, with declines from T0 to T60 (P < 0.0001). However, %IMS in spermatozoa frozen in DMSO (P = 0.0848) or GLY:DMSO (P = 0.9339) did not significantly decline from T0 to T60.
Plasma membrane integrity
Regardless of GLY, DMSO or GLY:DMSO concentration, cryoprotectant significantly affected %IPL at T0 and T60 (each P < 0.0001). Spermatozoa frozen in GLY:DMSO and GLY maintained a greater %IPL than spermatozoa frozen in DMSO at T0. At T60, spermatozoa frozen in GLY:DMSO maintained a greater %IPL than spermatozoa frozen in GLY or DMSO.
Treatment significantly affected %IPL at T0 (P < 0.0001) and T60 (P = 0.0266). The 6%:6% and 8%:8% GLY:DMSO treatments maintained higher %IPL at both time points than all other treatments, although the differences were not significant. Spermatozoa frozen in 6%:6% GLY:DMSO and 8%:8% GLY:DMSO exhibited significantly greater retention of %IPL than the 4%:4% GLY:DMSO and all DMSO treatments at T0. However, at T60 there were no significant differences between treatments (Fig. 2).
Within cryoprotectant, concentration significantly affected %IPL for spermatozoa frozen in DMSO or GLY:DMSO at T0 (P = 0.0014 and P = 0.0005 respectively) but did not affect %IPL at T60 (P = 0.0578 and P = 0.1574 respectively). At T0, the lowest DMSO concentration (8%) was significantly less effective in protecting sperm plasma membranes than higher DMSO concentrations of 12% and 16%. Similarly, the lowest concentration of GLY:DMSO (4%:4%) was significantly less protective at T0 than higher concentrations of 6%:6% and 8%:8%. GLY concentration did not affect %IPL at T0 (P = 0.5371) or T60 (P = 0.6592).
Regardless of cryoprotectant concentration, time after thaw significantly affected %IPL in GLY:DMSO and GLY treatments, with declines from T0 to T60 (P = 0.0003 and P < 0.0001 respectively). However, %IPL in spermatozoa frozen in DMSO did not decline significantly from T0 to T60 (P = 0.1626).
Acrosome integrity
Regardless of GLY, DMSO or GLY:DMSO concentration, cryoprotectant significantly affected %IAC (P < 0.0001). DMSO and GLY:DMSO were more efficient than GLY at preserving IAC.
Treatment significantly affected %IAC (P < 0.0001), with all GLY concentrations resulting in greater loss of IAC than all other treatments, although some differences were not significant (Fig. 3).
Within cryoprotectant, the %IAC was significantly affected by concentration (P = 0.0043 for GLY; P = 0.0001 for DMSO; P = 0.0107 for GLY:DMSO). For each cryoprotectant, the lowest concentration preserved IAC significantly better than the highest concentration.
Sperm quality index
Regardless of concentration, cryoprotectant significantly affected the SQI at T0 (P < 0.0001) and T60 (P < 0.0001). Spermatozoa frozen in GLY:DMSO retained a significantly higher SQI at both time points than those frozen in GLY or DMSO.
The SQI was significantly affected by treatment at T0 (P < 0.0001) and T60 (P < 0.0001). The 4%:4% and 6%:6% GLY:DMSO treatments maintained significantly higher SQI scores than all other treatments at T0 and T60 (Fig. 4).
Within cryoprotectants, SQI was not affected by concentration in spermatozoa frozen in DMSO at T0 (P = 0.1605), but SQI was affected by concentration at T60 (P = 0.0089). At T60, 12% DMSO yielded a significantly higher SQI than 8% and 16% DMSO. SQI was affected by cryoprotectant concentration for spermatozoa frozen in GLY at T0 (P = 0.0034) and T60 (P < 0.0001), with 8% GLY yielding the highest SQI at T0 and T60. Interestingly, SQI was not significantly affected by cryoprotectant concentration for spermatozoa frozen in GLY:DMSO at T0 (P = 0.1389) or T60 (P = 0.1600), but 6%:6% GLY:DMSO yielded the highest SQI at both time points.
Discussion
The development and optimisation of sperm cryopreservation protocols for snakes is crucial to preserving genetic diversity and conserving this important reptile taxon (Clulow and Clulow 2016; Strand et al. 2020). However, just a single journal article (Mengden et al. 1980) and three published abstracts (Mattson et al. 2009; Zacariotti et al. 2012; Young et al. 2019) describe freezing of snake spermatozoa, indicating the urgent need for comprehensive studies leading to successful cryobanking of these critical resources.
The opportunity to collect spermatozoa from a large number of free-ranging Burmese pythons provided sufficient samples to analyse cryosurvival following 29 preliminary experiments and nine experimental treatments. The latter compared two cryoprotectants, GLY and DMSO, alone or in combination, across a range of concentrations. A slow freeze rate of 0.3°C min−1 was selected for the present study based on preliminary experiments and a previous study of Argentine black and white tegu spermatozoa (Young et al. 2017).
Spermatozoa were analysed during necropsy following capture in Florida from male pythons not included in the present study. Compared with the freshly collected spermatozoa from those males, the mean motility of spermatozoa from males in the present study was presumably unaffected by overnight shipment at 4°C (71.8% vs 74.0% respectively; P = 0.1189). However, SOP was reduced in shipped spermatozoa (2.87 vs 2.34; P < 0.0001). This reduction in sperm parameters was less than reported over a shorter time for fresh spermatozoa in two snake species (Tourmente et al. 2011). Incubation of fresh spermatozoa at near ambient (25°C) or higher (30–37°C) temperatures resulted in rapid, marked declines in motility (Tourmente et al. 2011).
The relatively cooler post-thaw incubation temperature in the present study (22°C) may positively affect python sperm motility. High temperatures negatively affect sperm motility in some species due to enzyme denaturation, such as in the golden hamster (Mahi and Yanagimachi 1973), or changes in sperm membrane order, fluidity, permeability and thickness, such as in ectotherms (Crockett 1998) and rainbow trout (Müller et al. 2008). In our previous work with tegu (Young et al. 2017), higher motility scores were maintained when thawed spermatozoa were incubated at 37°C. It is tempting to attribute these differences between the ectothermic tegu and python to body temperatures during their respective breeding seasons: in Florida, tegu breed in summer, whereas pythons breed during the cooler months of December–March. However, sperm physiology in reptiles is insufficiently understood and, to date, there is insufficient evidence in this field to draw a definitive conclusion about the effects of high temperatures on sperm function.
Mammalian semen samples are normally centrifuged after thawing at 400–600g (Cochran et al. 1984; Herold et al. 2004) to remove cryoprotectants. Our previous work indicated that tegu spermatozoa required centrifugation at 30g to prevent head detachment (Young et al. 2017). However, this slow centrifugation was unsuccessful in pelleting python spermatozoa for post-thaw recovery. A compromise of 60g was sufficient to pellet python spermatozoa, but head detachment was 7.2–19.4% at T0 after thawing and ranged from 12.1 to 46% at T60 depending on the cryopreservation treatment (data not shown). This observation confirms the importance of incorporating longevity studies into the development of cryopreservation protocols. One noteworthy finding was the increase of detached heads as the concentration of cryoprotectant increased. DMSO and GLY:DMSO provided some degree of structural protection compared with GLY treatments.
Mengden et al. (1980) reported a 30% post-thaw ‘recovery rate’ of snake semen extended in Triladyl, a diluent designed for preserving bull semen, followed by a fast freeze by pelleting on dry ice. The species of snake and pre- and post-freezing sperm parameters were not specified. Photomicrographs of spermatozoa from one of the species Mengden et al. (1980) studied, namely the Angolan python Python anchietae, showed swollen heads, similar to those we observed when extending post-thaw Burmese python spermatozoa in a related diluent (Biladyl). Corn snake semen frozen in Biladyl by Mattson et al. (2009) exhibited 27% post-thaw motility, but sperm morphology was not reported. It is possible that the citrate in both extenders caused the python sperm heads to swell. We also documented this morphological anomaly in many reptile species when attempting acrosome analysis with Pope stain, which contains citrate. When citrate is removed from the stain formula, the sperm heads maintain normal proportions. Interestingly, Sirinarumitr et al. (2010) extended semen from Olive Ridley and hawksbill turtles in eight different media, reporting greatly reduced motility in those containing citrate. In our preliminary experiments before the present study, no motility was recovered when python spermatozoa were extended after thawing in Biladyl.
Zacariotti et al. (2012) compared rattlesnake semen cryosurvival in TEST yolk buffer with DMSO and Lake’s extender with glycerol (Lake et al. 1960; commonly used to freeze avian semen). No sperm motility was recovered in semen frozen in Lake’s extender with either 2% or 4% DMSO, regardless of freeze rate (pelleted in liquid nitrogen vapour or 1°C min−1). TEST yolk buffer with 8% glycerol demonstrated between 1% and 48% non-progressive motility (SOP = 1 on a scale of 1–5) after thawing when spermatozoa were frozen at a rate of 1°C min−1. Mattson et al. (2009) used a fast freeze (1 inch over liquid nitrogen after slow cooling for 1 h) for corn snake semen extended in Biladyl with 17% glycerol. Although moderate motility was recovered at thawing, SOP was <1 on a scale 0–5. Other freezing methods and cryoprotectants were investigated, but not described in their abstract. In our preliminary studies with tegu and python, a very slow freeze rate of 0.3°C min−1 was superior to even moderately faster rates of 1, 6.3 and 15°C min−1.
In contrast to the tegu, python spermatozoa frozen with DMSO, alone or in combination with GLY, preserved greater IAC than spermatozoa frozen in GLY alone. However, python IPL was more efficiently retained in spermatozoa frozen in GLY. These opposing results prompted trials with combinations of the two cryoprotectants in an attempt to enhance both critical aspects of viability. The relative importance of motility, IPL and IAC in fertility prediction has not been determined for any reptile species. Therefore, we assigned equal weight to these parameters in the calculation of the SQI. The SQI provides a composite overview of the nine treatment groups in this study. The SQI analysis revealed that Burmese python spermatozoa frozen at 0.3°C min−1 in low percentages of GLY:DMSO exhibited higher post-thaw viability than spermatozoa in all other treatment groups. At higher concentrations of the cryoprotectant combination, motility and IAC declined such that the cryoprotectant combination was not advantageous.
Finally, this study demonstrates the advantage of freezing python spermatozoa at 0.3°C min−1 with a combination of GLY and DMSO. The dual cryoprotectants provided superior overall cryosurvival due to the protective effects of DMSO on IAC and GLY preservation of IPL. Although IPL and IAC were maintained at acceptable levels, even in the best treatment group (6%:6% GLY:DMSO), post-thaw motility scores averaged just 26.8% of initial values by T60. Optimisation of cryopreservation protocols for snakes is needed to ensure that banked spermatozoa retain sufficient viability to produce fertile eggs following AI.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This work was supported by San Diego Zoo Global.
Acknowledgements
The authors appreciate Michiko Squires, Sara Williams and Seth Farrisand of the University of Florida Croc Docs Program for their help in obtaining specimens, and Christopher Tubbs and Tom Jensen for critical review of the manuscript.
References
Clulow, J., and Clulow, S. (2016). Cryopreservation and other assisted reproductive technologies for the conservation of threatened amphibians and reptiles: bringing the ARTs up to speed. Reprod. Fertil. Dev. 28, 1116–1132.| Cryopreservation and other assisted reproductive technologies for the conservation of threatened amphibians and reptiles: bringing the ARTs up to speed.Crossref | GoogleScholarGoogle Scholar |
Cochran, J. D., Amann, R. P., Froman, D. P., and Pickett, B. W. (1984). Effects of centrifugation, glycerol level, cooling to 5°C, freezing rate and thawing rate on the post-thaw motility of equine sperm. Theriogenology 22, 25–38.
| Effects of centrifugation, glycerol level, cooling to 5°C, freezing rate and thawing rate on the post-thaw motility of equine sperm.Crossref | GoogleScholarGoogle Scholar | 16725933PubMed |
Crockett, E. L. (1998). Cholesterol function in plasma membranes from ectotherms: membrane-specific roles in adaptation to temperature. Am. Zool. 38, 291–304.
| Cholesterol function in plasma membranes from ectotherms: membrane-specific roles in adaptation to temperature.Crossref | GoogleScholarGoogle Scholar |
Dorcas, M. E., Willson, J. D., Reed, R. N., Snow, R. W., Rochford, M. R., Miller, M. A., Meshaka, W. E., Andreadis, P. T., Mazzotti, F. J., Romagosa, C. M., and Hart, K. M. (2012). Severe mammal declines coincide with proliferation of invasive Burmese pythons in Everglades National Park. Proc. Natl Acad. Sci. USA 109, 2418–2422.
| Severe mammal declines coincide with proliferation of invasive Burmese pythons in Everglades National Park.Crossref | GoogleScholarGoogle Scholar | 22308381PubMed |
Fahrig, B. M., Mitchell, M. A., Eilts, B. E., and Paccamonti, D. L. (2007). Characterization and cooled storage of semen from corn snakes (Elaphe guttata). J. Zoo Wildl. Med. 38, 7–12.
| Characterization and cooled storage of semen from corn snakes (Elaphe guttata).Crossref | GoogleScholarGoogle Scholar | 17469269PubMed |
Friesen, C. R., Squire, M. K., and Mason, R. T. (2014). Intrapopulational variation of ejaculate traits and sperm depletion in red-sided garter snakes. J. Zool. (Lond.) 292, 192–201.
| Intrapopulational variation of ejaculate traits and sperm depletion in red-sided garter snakes.Crossref | GoogleScholarGoogle Scholar |
Herold, F. C., De Haas, K., Cooper, D., Colenbrander, B., Nothling, J. O., Theunisen, W., et al. (2004). Comparison of three different media for freezing of epididymal sperm from the African buffalo (Syncerus caffer) and influence of equilibration time on the post-thaw sperm quality. Onderstepoort J. Vet. Res. 71, 203–210.
| Comparison of three different media for freezing of epididymal sperm from the African buffalo (Syncerus caffer) and influence of equilibration time on the post-thaw sperm quality.Crossref | GoogleScholarGoogle Scholar | 15580769PubMed |
Jaskey, D. G., and Cohen, M. R. (1981). Twenty-hour to ninety-six-hour storage of human spermatozoa in TEST-Yolk buffer. Fertil. Steril. 35, 205–208.
| Twenty-hour to ninety-six-hour storage of human spermatozoa in TEST-Yolk buffer.Crossref | GoogleScholarGoogle Scholar | 7202744PubMed |
Lake, P. E., Schindler, H., and Wilcox, F. H. (1960). Long distance transportation of fowl semen by air. World’s Poult. Sci. J. 16, 145–149.
| Long distance transportation of fowl semen by air.Crossref | GoogleScholarGoogle Scholar |
Mahi, C. A., and Yanagimachi, R. (1973). The effects of temperature, osmolality and hydrogen ion concentration on the activation and acrosome reaction of golden hamster spermatozoa. J. Reprod. Fertil. 35, 55–66.
| The effects of temperature, osmolality and hydrogen ion concentration on the activation and acrosome reaction of golden hamster spermatozoa.Crossref | GoogleScholarGoogle Scholar | 4126362PubMed |
Mattson, K. J., De Vries, A., McGuire, S. M., Krebs, J., Louis, E. E., and Loskutoff, N. M. (2007). Successful artificial insemination in the corn snake, Elaphe gutatta, using fresh and cooled semen. Zoo Biol. 26, 363–369.
| Successful artificial insemination in the corn snake, Elaphe gutatta, using fresh and cooled semen.Crossref | GoogleScholarGoogle Scholar | 19360586PubMed |
Mattson, K., DeVries, A., Krebs, J., and Loskutoff, N. (2009). Cryopreservation of corn snake, Elaphe gutatta, semen. Reprod. Fertil. Dev. 21, 179–180.
| Cryopreservation of corn snake, Elaphe gutatta, semen.Crossref | GoogleScholarGoogle Scholar |
Mengden, G. A., Platz, C. C., Hubbard, R., and Quinn, H. (1980). Semen collection, freezing and artificial insemination in snakes. In ‘Contributions to Herpetology. No. 1. Reproductive Biology and Diseases of Captive Reptiles’. (Eds J. B. Murphy and J. T. Collins.) pp. 71–78. (Society for the Study of Reptiles, St Louis University: St Louis, MO.)
Miller, M. A., Kinsella, J. M., Snow, R. W., Hayes, M. M., Falk, B. G., Reed, R. N., Mazzottie, F. J., Guyer, C., and Romagosa, C. M. (2018). Parasite spillover: indirect effects of invasive Burmese pythons. Ecol. Evol. 8, 830–840.
| Parasite spillover: indirect effects of invasive Burmese pythons.Crossref | GoogleScholarGoogle Scholar | 29375757PubMed |
Moshiri, M., Todehdehghan, F., and Shiravi, A. (2014). Study of sperm reproductive parameters in mature Zanjani viper. Cell J. 16, 385.
| 25383339PubMed |
Mozafari, S. Z., Shiravi, A., and Todehdehghan, F. (2012). Evaluation of reproductive parameters of vas deferens sperms in Caucasian snake (Gloydius halys caucasicus). Vet. Res. Forum 3, 119–123.
| 25653757PubMed |
Müller, K., Müller, P., Gwenaelle, P., Kurz, A., and Labbe, C. (2008). Characterization of sperm plasma membrane properties after cholesterol modification: consequences for cryopreservation of rainbow trout spermatozoa. Biol. Reprod. 78, 390–399.
| Characterization of sperm plasma membrane properties after cholesterol modification: consequences for cryopreservation of rainbow trout spermatozoa.Crossref | GoogleScholarGoogle Scholar | 18003944PubMed |
Oliveri, M., Bartoskova, A., Spadola, F., Morici, M., Giuseppe, M., and Knotek, Z. (2018). Method of semen collection and artificial insemination in snakes. J. Exot. Pet Med. 27, 75–80.
| Method of semen collection and artificial insemination in snakes.Crossref | GoogleScholarGoogle Scholar |
Pope, C., Zhang, Y., and Dresser, B. (1991). A simple staining method for evaluating acrosomal status of cat spermatozoa. J. Zoo Wildl. Med. 22, 87–95.
Quinn, H., Blasedel, T., and Platz, C. C. (1989). Successful artificial insemination in the checkered garter snake Thamnophis marcianus. Int. Zoo Yb 28, 177–183.
| Successful artificial insemination in the checkered garter snake Thamnophis marcianus.Crossref | GoogleScholarGoogle Scholar |
Sirinarumitr, K., Patthong, Y., Jaimjaturong, P., Woonwong, Y., Petchsamut, W., Limpasuntisin, P., Manawatthana, S., Sirinarumitr, T., Sanyathitiseree, P., Kornkaewrat, K., and Suithunmapinunta, P. (2010). Extender for sperm dilution in Olive Ridley turtle (Lepidochelys olivacea) and hawksbill turtle (Eretmochelys imbricata) semen. In ‘Proceedings of the 5th International Symposium on SEASTAR2000 and Asian Bio-logging Science (The 9th SEASTAR2000 Workshop)’, February 2010, Kyoto. (Ed. N. Arai.) pp. 7–10. (Graduate School of Informatics, Kyoto University.)
Strand, J., Thomsen, H., Jensen, J. B., Marcussen, C., Nicolajsen, T. B., Skriver, M. B., Søgaard, I. M., Ezaz, T., Purup, S., Callesen, H., and Pertoldi, C. (2020). Biobanking in amphibian and reptilian conservation and management: opportunities and challenges. Conserv. Genet. Resour. 12, 709–725.
| Biobanking in amphibian and reptilian conservation and management: opportunities and challenges.Crossref | GoogleScholarGoogle Scholar |
Tourmente, M., Cardozo, G. A., Guidobaldi, H. A., Giojalas, L. C., Bertona, M., and Chiaraviglio, M. (2007). Sperm motility parameters to evaluate the seminal quality of Boa constrictor occidentalis, a threatened snake species. Res. Vet. Sci. 82, 93–98.
| Sperm motility parameters to evaluate the seminal quality of Boa constrictor occidentalis, a threatened snake species.Crossref | GoogleScholarGoogle Scholar | 16857223PubMed |
Tourmente, M., Giojalas, L. C., and Chiaraviglio, M. (2011). Sperm parameters associated with reproductive ecology in two snake species. Herpetologica 67, 58–70.
| Sperm parameters associated with reproductive ecology in two snake species.Crossref | GoogleScholarGoogle Scholar |
Williams, W., and Pollak, O. (1950). Study of sperm vitality with the aid of eosin-nigrosin stain. Fertil. Steril. 1, 178–181.
| Study of sperm vitality with the aid of eosin-nigrosin stain.Crossref | GoogleScholarGoogle Scholar |
Young, C., Ravida, N., Curtis, M., Mazzotti, F., and Durrant, B. (2017). Development of a sperm cryopreservation protocol for the Argentine black and white tegu (Tupinambis merianae). Theriogenology 87, 55–63.
| Development of a sperm cryopreservation protocol for the Argentine black and white tegu (Tupinambis merianae).Crossref | GoogleScholarGoogle Scholar | 27639519PubMed |
Young, C., Ravida, N., and Durrant, B. (2019). Challenges in the development of semen cryopreservation protocols for snakes. Cryobiology 91, 190.
| Challenges in the development of semen cryopreservation protocols for snakes.Crossref | GoogleScholarGoogle Scholar |
Zacariotti, R. L., Grego, K. F., Fernandes, W., Sant’Anna, S. S., and de Barros Vaz Guima˜res, M. A. (2007). Semen collection and evaluation in free-ranging Brazilian rattlesnakes (Crotalus durissus terrificus). Zoo Biol. 26, 155–160.
| Semen collection and evaluation in free-ranging Brazilian rattlesnakes (Crotalus durissus terrificus).Crossref | GoogleScholarGoogle Scholar | 19360568PubMed |
Zacariotti, R., Guimaraes, M., Jensen, T., and Durrant, B. (2012). Cryopreservation of snake semen: are we frozen in time? Reprod. Fertil. Dev. 24, 171.
| Cryopreservation of snake semen: are we frozen in time?Crossref | GoogleScholarGoogle Scholar |






