Genomic selection in beef cattle creates additional opportunities for embryo technologies to meet industry needs
Stephen Miller A *A Animal Genetics and Breeding Unit, a joint venture of NSW Department of Primary Industries and the University of New England, University of New England, Armidale, NSW 2351, Australia.
Reproduction, Fertility and Development 35(2) 98-105 https://doi.org/10.1071/RD22233
Published online: 9 November 2022
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of the IETS. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
The use of genotype information to improve the predictability of Expected Progeny Difference was first implemented in American Angus cattle in 2009 and has now grown to where over 50% of all registered calves are genotyped. Animals with only a genotype now have genetic prediction accuracy equivalent to eight or more progeny records across all traits. Reproductive technologies have also been widely adopted with approximately 50% of all calves born being the result of artificial insemination. Non-surgical embryo transfer started increasing in the mid 1990s with just over 10% of calves born being the result of embryo transfer since 2005. The number of embryos created with in vitro technologies has risen sharply since 2015 and now accounts for close to 30% of all ET calves. Genomics has enabled embryo technologies to be more impactful, as females can be selected with greater accuracy and sires can be used at earlier ages with moderate accuracy. Large numbers of females genotyped each year also increases the number of selection candidates, increasing the selection intensity. Genomics, combined with increased recording, also provides more information on females. This increases the spread in the estimated index values of current dams, identifying more elite dams for selection as embryo donors. The greater scope of female selection also contributes to better inbreeding management. Commercial animals genotyped could be targeted for oocyte harvesting at slaughter, creating opportunities for low cost high value beef embryos to be used in the beef on dairy segment of the industry.
Keywords: adoption, artificial insemination, commercial, embryo transfer, genetic, in vitro embryo production, reproduction and breeding strategy.
Introduction
Genomic selection has significantly changed the landscape of genetic improvement in beef cattle breeding. Some herald the technology as the most significant advancement to influence the industry since the introduction of artificial insemination over half a century ago. Genomic selection has been reviewed with reference to embryo technologies in the past (Seidel 2010). The potential of genomics to increase the rate of genetic improvement for economically relevant traits in beef cattle has also been reviewed (Johnston et al. 2012).
This paper will describe the influence of genomics in the context of the genetic evaluation of the American Angus Association, the world’s largest beef breed association and corresponding performance database. The genetic evaluation has been described in the past by Miller et al. (2018) and the implementation of genomics, including increases in genetic trends for measured traits since the implementation of genomics, has been recently described by Retallick et al. (2022). Increases in genetic trends since the implementation of genomic selection provides evidence that genomics has changed the way American Angus cattle are selected and evaluated.
Similar to genomics, embryo technologies, are a tool that can be exploited to increase genetic gains by reducing the generation interval and increasing the selection intensity for dams, where more calves are produced from the best dams. Genomic selection and embryo technologies are not additive but indeed complementary such that genomics provides the opportunity for embryo technologies to be more effective but also embryo technologies provide an opportunity for genomics to be more effective. This paper will highlight some of these opportunities.
Genomic selection
Genomic selection was first proposed as a technology by Meuwissen et al. (2001). Implementation of genomic selection was made possible through the advent of moderate density genotyping technologies while at relatively low cost (Matukumalli et al. 2009). It has resulted in a boon for breeders as they have made additional genetic progress in all major livestock species, especially dairy cattle (Scott et al. 2021). At the time, Schaeffer (2006) predicted a doubling in the rate of genetic gain with the implementation of genomic selection in dairy cattle, although it would not be implemented fully for another 4 years. This was accomplished primarily through the use of young bulls with moderately accurate proofs instead of traditional progeny proven sires that have more accuracy but greatly increased costs and generation intervals. This prediction of increased genetic gain has proven to be true and has even been surpassed in some instances (Fleming and Van Doormaal 2022).
The adoption of genotyping by American Angus breeders is a very good indicator of the value of genotyping to their operations and is illustrated by increasing genotyping overtime (Fig. 1). Genomically enhanced expected progeny differences (EPD) were first implemented at the American Angus Association in 2009. The genotyping rates that are presented are the number of genotypes by birth year, which can be different from the number of genotypes in the evaluation each year as animals can always be genotyped later through access to stored semen and other sources of DNA. There are animals born before 2009 with genotypes in the evaluation but these tend to be more historic animals such as artificial insemination (AI) sires. Since then the number of genotypes has steadily increased and now represents just over half of all calves registered each year.
|
|
The genotyping rates in males and females is similar to the ratio of males and females registered each year. Although there are more females registered than males each year and there are more males than females genotyped, this discrepancy is not numerically significant. This demonstrates the importance of genotyping both males and females in a beef herd. It is also an indicator that genotyping is actively being used for selection and genetic improvement. If there was a very high percentage of only males being genotyped it could be an indication that genotyping was being used for marketing purposes, as the sale of young bulls can be the breeder’s largest source of income. However, this is clearly not the case in the American Angus herd, which is an indication of genotyping being used to select females. This is especially important when considered in the context of embryo technologies, as genomics could be influencing the selection of donor dams, as would be expected, given the increase in selection accuracy with genomics, providing more accurate EPD on all traits.
The premise of genomic selection is that animals measured for a trait can be used to train the genetic markers to draw relationships between the markers and the traits. With enough phenotypic records combined with enough genotyping, sufficient accuracy of genetic predictions for selection of animals not measured, including young animals, is possible. Phenotypic recording levels combined with sufficient genotyping in the early stages of genomic selection has allowed this threshold to be surpassed in American Angus cattle. The system is somewhat self-generating as once sufficient genomic prediction accuracy is obtained, breeders will begin to genotype their animals as standard practice, to take advantage of the accuracy of trait prediction early in life, across all traits. These genotyped animals later have phenotypic records on themselves and progeny allowing the database to grow in strength, fuelling more accurate genomic predictions and more adoption of genotyping. Phenotypes are critically important to enable this genomic prediction accuracy as it this combination of animals with phenotypes, or progeny phenotypes and genotypes that create the required reference set to enable the genotypes to predict genetic merit for traits. If a population was to only genotype animals and had no phenotypes, genomic selection would not be possible. Efforts in collecting phenotypic information is never done as the reference population of animals with phenotypes and genotypes must remain relevant to current selection candidates (Moore et al. 2022).
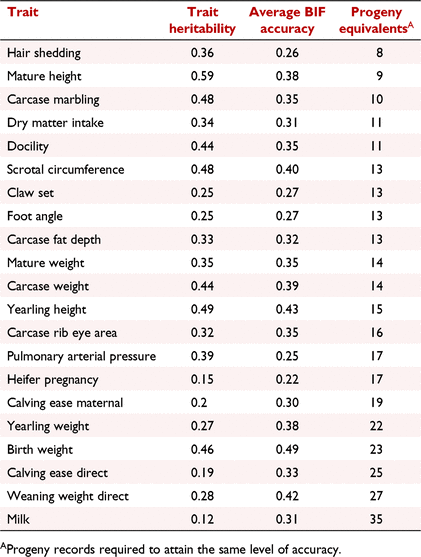
Retrospective analyses have shown that traits such as growth rate can be more accurately predicted with a genotype rather than a performance measurement (Angus University 2020). The accuracy now observed for all traits evaluated within American Angus for animals with a genotype, but no performance or progeny information, is summarised in Table 1. Two values are presented, the accuracy printed with the EPD (Beef Improvement Federation 2022) and the number of progeny records that would be required to attain that same accuracy given the heritability of the trait. Typically, the traits with more phenotypic records such as growth traits have greater accuracy based solely on a genotype. All traits have considerable accuracy with the minimum being equivalent to eight progeny records, which is Hair Shedding, the newest trait in the evaluation.
|
The accuracy outlined in Table 1 is important when considering selecting young animals as embryo donors. All animals will receive a prediction and although individual accuracies will differ depending on information on parents, this average accuracy across all traits provides a very useful selection tool. This is especially powerful when one considers that it would usually take a lifetime of recording for a female to attain such accuracy in a traditional phenotypic evaluation without genomics. Through trait recording alone, most females would never attain the level of accuracy that is possible with genomics. One of the reasons for this being that some traits are geographical region specific and others are sex specific. One example is heifer pregnancy in which a genomic-only prediction is equivalent to 17 progeny recorded. With 49.8% of females in the American Angus population being culled at age 6 or earlier (Oliveira et al. 2020), a female will not attain 17 progeny in total, let alone 17 female progeny all measured for the trait.
Influencing genetic change
The formula for genetic progress in a breeding programme, often called the breeders equation, was outlined by Lush (1937) and involves four elements, some of which can be influenced by genomics and are indeed complementary. The formula is (selection intensity × accuracy of selection × genetic standard deviation) divided by the generation interval. As outlined in Table 1, clearly the accuracy of selection is influenced by genomics, especially when all traits are considered and selection is at a young age. High accuracy can of course be obtained for all traits with dedicated progeny recording, but this then extends the generation interval, as parents are significantly older before they have an accurate proof. This then highlights the first such interaction between accuracy and generation interval. Genomics offers moderate accuracy at the lowest possible generation interval.
To investigate if the application of genomics could be changing the generation interval in American Angus, the average age of the parents of calves born in each year was determined (Fig. 2). The application of genomics, genetic progress, application of reproductive technologies and the average age of parents are all intertwined. Prior to genomics, accuracy, especially for sires, was the result of many progeny. This was often attained in AI sires who could have progeny across a number of herds as well as participate in progeny proving programs. It is expected that as the use of AI sires increases (Fig. 3), the average age of sires would also increase. Contrarily, dams are a little bit different as they simply cannot attain the large numbers of progeny sires can. However, EPD technology provides more genetic information on dams and has supported the use of younger dams throughout time (Fig. 2). If genomics is being employed to enable selection of younger parents without the need for extensive progeny recording, a decrease in the age of the parents is expected. This trend is initially recognised in sires in 2010 shortly after the implementation of genomically enhanced EPD. Since 2010, there’s been a steady decrease in the average age of sires suggesting that producers are using younger sires. This further exemplifies that producers are taking advantage of the accuracy available through genomic predictions that historically would have only been available through an extensive use of a single sire to generate enough progeny to gain such accuracy. These observational trends are not a direct link for the cause of this change, therefore, speculation of these relationships need to be considered with caution.
|
|
Genomic prediction also provides the opportunity to increase the selection intensity. Selection intensity is related to the proportion of animals selected. As selection intensity increases, the selection differential and progress obtained also increases. When considering the use of embryo technologies, the selection of elite females becomes important. The initial challenge of selecting donor dams before genomics, was the accurate identification of these elite females. The identification was often limited to dams of elite AI sires, or certain cow families from popular herds. This provided a fairly narrow pool of candidates. Currently with genomics, there are over 70 000 females genotyped each year, increasing the pool of selection candidates with moderately accurate genetic estimates of merit across all traits. Given an increasing investment in embryo production along with a larger pool of selection candidates as a result of genomic selection, the intensity and contribution to genetic change has increased. The further upside of this larger scope for selection is an improvement in the ability to manage long-term inbreeding, as the selection pressure is taken away from some historically popular cow families, as was more common before genomics. Differences in inbreeding accumulation in American Angus before and after the implementation of genomic selection has been studied and no detrimental impacts of the implementation of genomics on inbreeding was found. The accumulated inbreeding in females was found to be reduced with genomic selection (Lozada-Soto et al. 2021).
The genetic standard deviation is one aspect of the breeder’s equation that is generally considered to be somewhat fixed in the short-term. Fig. 4 shows the change in American Angus self-replacing index $ Combined ($C (USD)), the change in mean as well as standard deviation for current dams. The underlying standard deviation of $C (USD) is constant, but the increasing s.d. of the estimates of $C (USD) for individual animals is important for selection. The $C (USD) index includes both terminal traits such as growth and carcass traits along with maternal traits such as milk and heifer pregnancy. This index was newly released in 2019 and comprehensively includes the traits of economic importance for selection in a commercial production system in Angus cattle, each weighted by their impact on system profitability. Selection of females with the entire value chain in mind is most appropriately done with this $C (USD) index. The graph clearly shows how the average value of $C (USD) has been steadily increasing overtime in these current females. In regards to identification of elite females, the consideration of the standard deviation of the $C (USD) index within these females is of equal importance. The standard deviation indicates the identified variation that exists within the females. The greater the standard deviation, the greater the variability in genetics being identified. This is somewhat related to the accuracy of the information being used. With little accuracy there is little information to base the prediction of differences between animals. As genomic predictions are more accurate over time, for more traits and as more females are genotyped and measured for contributing traits, we see a steady increase in the ability to identify differences between females in $C (USD) as an increase in the standard deviation. This increased accuracy and increase spread in the underlying EPD’s contributing to the index is also contributing to more spread amongst the $C (USD) predicted on the females. This increased standard deviation of the index will allow more elite females to be identified at the very top of the bell curve and these females will be the candidates for advanced reproductive technologies like embryo transfer.
|
|
Embryo technologies
Fig. 3 shows adoption of reproductive technologies in American Angus cattle overtime. The influence that AI has played in the breed is quite clear. Starting from the early 60s, there has been a steady increase in the proportion of calves born from AI matings. Proportion of calves from AI matings has been steady at close to 50% in recent years. The influence of AI on genetic progress is likely considerably higher than this when the proportion of calves whose sire was the result of an AI mating is also considered. The proportion of calves whose sire is the result of an AI mating has been over 80% in recent years.
Unlike AI where adoption rates have been increasing steadily from the early 60s, the adoption of embryo transfer (ET) technology did not occur until later. The proportion of calves produced through ET noticeably starting to increase in the mid-90s and has stayed fairly steady at just over 10% of calves registered since 2005. Similar to AI, the proportion of calves where at least one parent was the result of ET is remarkably higher. Going back to the late 80s the proportion of animals who have an ET parent has been steadily increasing and is over 40% in recent time. This is most likely the result of AI sires being ET calves. This highlights the impact ET technologies are having in the most influential category of animals when it comes to genetic progress. The use of in vitro embryo production is more recent and shows that the portion of calves resulting from in vitro embryo production started to increase in 2015 considerably and now represents close to 30% of all ET calves born. This recent proliferation of the use of in vitro embryo production is consistent with the trend illustrated for North America by Mueller and Van Eenennaam (2022).
One reproductive technology that has intersected with genomics that is also worth mentioning is embryo genotyping. By genotyping an embryo through biopsy, the same selection accuracy (Table 1), is possible. This allows selection pressure on embryos pre-implantation which can be significant when recipients are a limiting factor. The potential application of embryo genotyping to increase genetic progress was reviewed by Humblot et al. (2010) and more recently by Mueller and Van Eenennaam (2022), and challenges with genotyping embryos after a DNA amplification step has been addressed by Shojaei Saadi et al. (2014). The genotyping of embryos is already available to American Angus breeders and results from use of this technology have been recently presented (Barten 2018), with genomically enhanced EPDs calculated and used for selection. The application, in practice, is influenced by a number of factors including the cost of embryo production, the value differential between selected and unselected embryos, along with the cost of raising calves through recipients. The value of embryo genotyping is highest when the cost of embryo production is low and the value differential between selected and unselected embryos is high and when the cost of recipients is high. Although available to Angus breeders, the technology has seen limited application to date.
This paper focusses on technologies that are currently available and implemented. A technology that has been discussed for some time but has yet to be put into practice on a commercial scale is in vitro breeding (IVB). This was touched on as a possible way to increase progress in the future by Mueller and Van Eenennaam (2022). New enabling breakthroughs to support this approach were recently reviewed by Goszczynski et al. (2019). The approach leverages genomic selection as an important part of the system. Briefly, embryos from elite parents are used to create multiple embryonic stem cell lines (ESC) which can be genotyped and selected. The selected ESC are used to create gametes to create hundreds of embryos through IVF, which are used to create the next generation of ESC and the cycle repeats. The time to complete one cycle is 3–4 months, which greatly reduces the generation interval. The authors predict that 100 generations would be possible in a 25-year span compared to 10 with regular genomic selection in a dairy cattle breeding program. This greatly reduced generation interval contributes directly to increased genetic progress. Beyond the scope of this paper that is focussed on current implemented technologies, it is worth noting these emerging technologies that could provide another step change in the future.
Commercial opportunities
Genomics could also open up new opportunities in the commercial sector when combined with reproductive technologies. Genotyping of commercial animals through products such as GeneMax Advantage (Angus Genetics Inc. 2022) are being used to select and manage commercial animals. This includes the selection of replacement heifers but also the management and marketing of feeder cattle. These genotyped commercial animals essentially have the same level of information regarding genetic prediction accuracy as seedstock animals, as the genomic reference is the same. This technology allows the identification of superior commercial females prior to their slaughter as feeder cattle or cull females. With this level of genetic information on commercial females coming to packing plants, their oocytes could be harvested for in vitro embryo production. These oocytes, combined with sexed semen, could provide a low-cost embryo to go back into the commercial production system, if efficient methods for embryo production in this scenario were available. An obvious area where this added-value beef embryo could be used is in the creation of beef calves from dairy cows, commonly called the beef on dairy market. The use of beef semen on dairy cows has increased considerably in recent years due in part to sexed semen, combined with low prices of dairy cattle. Dairy calves intended for meat production are often highly discounted. This allows full beef embryos to have a greater value than a dairy and beef cross calf. Instead of breeding these dairy cattle to beef semen to offset poor beef qualities from the dairy cow, these same dairy cows could become pregnant through a high genetic merit beef embryo coming from this commercial production system.
Collecting oocytes from commercial slaughter facilities has been used in the past and is not a new technology. The benefit that genomics provides is that now females chosen are simply not a random sample of commercial animals, but a selected cohort of genetically superior animals. This is also a relatively low-cost enterprise as the cost of genotyping the females has already been incurred for another purpose. The challenge of continuing forward progress will be to maintain animal identification through the point of ovary collection. If this were to become a significant commercial enterprise it would be expected that efficiencies through improved processes, in terms of embryo yield and conception rates, would be gained, driving further efficiencies in the system.
Conclusions
Genomics has been a significant advancement in selection of American Angus cattle with moderate accuracies available across all measured traits. This provides the opportunity to use younger sires without the need for progeny testing and a trend to have a lower average age of sires in recent years supports this. Genomics supports effective use of embryo technologies as more genetic differences among young females are identified and with close to half of all females being genotyped each year, this large pool of selection candidates increases selection intensity and helps manage inbreeding. Genomics provides further opportunities for embryo technologies in the commercial sector as genotyped females could be targeted at slaughter for oocyte harvesting for use in an in vitro embryo production program. Genomics provides more accurate information for selection, which is complementary to embryo technologies, which capitalise on the identified elite female genetics.
Data availability
This paper being a review did not generate any new research data. Summary data from the American Angus Association has been presented. The raw data behind these summaries is confidential to the American Angus Association.
Conflicts of interest
The author declares no conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
Acknowledgements
Access to summary data from the American Angus Association is gratefully acknowledged. The author thanks University of New England Professor Emeritus Robert Banks for inspiring the idea of checking the average age of parents over time as evidence of effective selection with genomics.
References
Angus Genetics Inc. (2022) Understanding Genemax advantage results factsheet. Available at https://www.angus.org/AGI/understanding-gmx-advantage-results_final_102814.pdfAngus University (2020) “Diving into the data” webinar. Available at https://www.angus.org/University/Webinars
Barten M (2018) In ‘Beef Improvement Federation annual research symposium and convention’. June 20–23, Loveland, CO. Available at http://www.bifconference.com/bif2018/summaries/BIF2018NeogenMattBarten.htm
Beef Improvement Federation (2022) Guidelines Wiki. Available at https://guidelines.beefimprovement.org/index.php/Accuracy
Fleming A, Van Doormaal B (2022) The impact of genomics. Available at https://lactanet.ca/en/impact-genomics/
Goszczynski, DE, Cheng, H, Demyda-Peyrás, S, Medrano, JF, Wu, J, and Ross, PJ (2019). In vitro breeding: application of embryonic stem cells to animal production. Biology of Reproduction 100, 885–895.
| In vitro breeding: application of embryonic stem cells to animal production.Crossref | GoogleScholarGoogle Scholar |
Humblot, P, Le Bourhis, D, Fritz, S, Colleau, JJ, Gonzalez, C, Guyader Joly, C, Malafosse, A, Heyman, Y, Amigues, Y, Tissier, M, and Ponsart, C (2010). Reproductive technologies and genomic selection in cattle. Veterinary Medicine International 2010, 192787.
| Reproductive technologies and genomic selection in cattle.Crossref | GoogleScholarGoogle Scholar |
Johnston, DJ, Tier, B, and Graser, H-U (2012). Beef cattle breeding in Australia with genomics: opportunities and needs. Animal Production Science 52, 100–106.
| Beef cattle breeding in Australia with genomics: opportunities and needs.Crossref | GoogleScholarGoogle Scholar |
Lozada-Soto, EA, Maltecca, C, Lu, D, Miller, S, Cole, JB, and Tiezzi, F (2021). Trends in genetic diversity and the effect of inbreeding in American Angus cattle under genomic selection. Genetics Selection Evolution 53, 50.
| Trends in genetic diversity and the effect of inbreeding in American Angus cattle under genomic selection.Crossref | GoogleScholarGoogle Scholar |
Lush JL (1937) ‘Animal breeding plans.’ (Iowa State Press: Ames, Iowa)
Matukumalli, LK, Lawley, CT, Schnabel, RD, Taylor, JF, Allan, MF, Heaton, MP, O’Connell, J, Moore, SS, Smith, TPL, Sonstegard, TS, and Van Tassell, CP (2009). Development and characterization of a high density SNP genotyping assay for cattle. PLoS ONE 4, e5350.
| Development and characterization of a high density SNP genotyping assay for cattle.Crossref | GoogleScholarGoogle Scholar |
Meuwissen, THE, Hayes, BJ, and Goddard, ME (2001). Prediction of total genetic value using genome-wide dense marker maps. Genetics 157, 1819–1829.
| Prediction of total genetic value using genome-wide dense marker maps.Crossref | GoogleScholarGoogle Scholar |
Miller SP, Wang L, Retallick KJ, Moser DW (2018) Developments in the genetic evaluation of American Angus cattle. In ‘Proceedings of the 11th world congress on genetics applied to livestock production’.
Moore KL, Ferdosi MH, Girard CG, Walkom SF, Johnston DJ (2022) A new metric to assess reference populations for genomic selection in Australian beef breeds. In ‘Proceedings of the 12th world congress on genetics applied to livestock production’.
Mueller, ML, and Van Eenennaam, AL (2022). Synergistic power of genomic selection, assisted reproductive technologies, and gene editing to drive genetic improvement of cattle. CABI Agriculture and Bioscience 3, 13.
| Synergistic power of genomic selection, assisted reproductive technologies, and gene editing to drive genetic improvement of cattle.Crossref | GoogleScholarGoogle Scholar |
Oliveira, HR, Brito, LF, Miller, SP, and Schenkel, FS (2020). Using random regression models to genetically evaluate functional longevity traits in North American Angus cattle. Animals 10, 2410.
| Using random regression models to genetically evaluate functional longevity traits in North American Angus cattle.Crossref | GoogleScholarGoogle Scholar |
Retallick KJ, Lu D, Garcia A, Miller SP (2022) Genomic selection in the US: where it has been and where it is going? In ‘Proceedings of the 12th world congress on genetics applied to livestock production’.
Schaeffer, LR (2006). Strategy for applying genome-wide selection in dairy cattle. Journal of Animal Breeding and Genetics 123, 218–223.
| Strategy for applying genome-wide selection in dairy cattle.Crossref | GoogleScholarGoogle Scholar |
Scott, BA, Haile-Mariam, M, Cocks, BG, and Pryce, JE (2021). How genomic selection has increased rates of genetic gain and inbreeding in the Australian national herd, genomic information nucleus, and bulls. Journal of Dairy Science 104, 11832–11849.
| How genomic selection has increased rates of genetic gain and inbreeding in the Australian national herd, genomic information nucleus, and bulls.Crossref | GoogleScholarGoogle Scholar |
Seidel, GE (2010). Brief introduction to whole-genome selection in cattle using single nucleotide polymorphisms. Reproduction, Fertility and Development 22, 138–144.
| Brief introduction to whole-genome selection in cattle using single nucleotide polymorphisms.Crossref | GoogleScholarGoogle Scholar |
Shojaei Saadi, HA, Vigneault, C, Sargolzaei, M, Gagné, D, Fournier, É, de Montera, B, Chesnais, J, Blondin, P, and Robert, C (2014). Impact of whole-genome amplification on the reliability of pre-transfer cattle embryo breeding value estimates. BMC Genomics 15, 889–904.
| Impact of whole-genome amplification on the reliability of pre-transfer cattle embryo breeding value estimates.Crossref | GoogleScholarGoogle Scholar |


