62 DIFFERENT EFFECT OF TRICHOSTATIN A ON IN VITRO DEVELOPMENT OF PORCINE TRANSGENIC SOMATIC CELL NUCLEAR TRANSFER AND PARTHENOGENETICALLY ACTIVATED EMBRYOS
H. X. Wei A , K. Zhang A , Y. F. Ma A , Y. Li B , Q. Y. Li B , Y. P. Dai B and N. Li AA State Key Laboratory for Agrobiotechnology, College of Biological Science, China Agricultural University, Beijing, China;
B Jipulin Biotech Ltd., Beijing, China
Reproduction, Fertility and Development 20(1) 112-112 https://doi.org/10.1071/RDv20n1Ab62
Published: 12 December 2007
Abstract
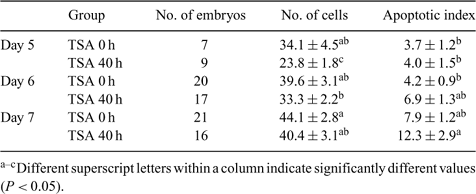
Accumulating evidence suggests that trichostatin A (TSA), a histone deacetylase inhibitor, can increase the success rate of somatic cloning. The objective of this study was to investigate the effect of 50 nm TSA treatment on the development of porcine somatic cell nuclear transfer (SCNT) and parthenogenically activated (PA) embryos. Cumulus-oocyte complexes were matured in vitro. The oocytes with the first polar body (PB1) were chosen for SCNT, and the rest with PB1 or good morphology were selected for PA by a single 100-μs direct current pulse of 1.6 kV cm–1, the same parameter as for electrical fusion. GFP transgenic fetal fibroblast cells were used as nuclear donors. Data were analyzed using SPSS (13.0; SPSS, Inc., Chicago, IL, USA) with one-way ANOVA. In Experiment 1, immediately after electrical fusion and activation, the reconstructed embryos were randomly cultured in porcine zygote medium 3 (PZM3) with 10 μg mL–1 cytochalasin B (CB) and 10 μg mL–1 cycloheximide (CHX), with either 0 nm (control) or 50 nm TSA for the first 4 h, before being cultured for another 20 h in PZM3 without CB and CHX. After being washed, the embryos were cultured in PZM3 medium without TSA until Day 6 at 39.0°C, 5% CO2, 5%O2, 90% N2, and 100% humidity. The same experimental design was used for PA embryos concurrently. The results showed that there were no significant differences in blastocyst rates for SCNT or PA between control and TSA groups (23.0 ± 6.1% v. 27.9 ± 6.3%; 21.0 ± 1.0% v. 17.5 ± 3.2%, respectively). Neither were there differences in the cell numbers of blastocysts (38.3 ± 5.7 v. 32.2 ± 3.4; 42.2 ± 3.5 v. 39.0 ± 1.9, respectively). In Experiment 2, TSA treatment was prolonged to either 36 or 40 h. The blastocyst rates of SCNT were increased (7.3 ± 1.2% (0 h), 13.3 ± 2.6% (36 h), and 20.0 ± 3.3% (40 h)), whereas those of PA were decreased (46.7 ± 5.0% (0 h), 27.7 ± 6.5% (36 h), and 30.8 ± 6.3% (40 h)). The cell numbers of blastocysts from either SCNT or PA were also decreased (SCNT: 47.5 ± 3.8, 37.5 ± 2.0, and 37.1 ± 3.3; PA: 46.1 ± 1.9, 37.5 ± 1.9, and 39.3 ± 2.2; P < 0.05). In Experiment 3, the cell number and the apoptotic index of Day 5, 6, and 7 PA blastocysts treated with 0 or 50 nm TSA were determined by the terminal deoxynucleotide-mediated nick end labeling (TUNEL) assay (Table 1). The results suggested that TSA treatment probably delayed embryo development, which may be one of the reasons for the lower cell numbers in the TSA-treated group.
|


