130 METABOLIC PROFILING OF MALE AND FEMALE BOVINE EMBRYOS USING NUCLEAR MAGNETIC RESONANCE (NMR) IMAGING
M. Rubessa A , A. Ambrosi C , K. M. Polkoff B , J. W. Stewart B , K. K. Herzog B , S. E. Denmark C and M. B. Wheeler CA Institute for Genomic Biology, University of Illinois at Urbana-Champaign, Urbana, IL, USA;
B Department of Animal Sciences, University of Illinois at Urbana-Champaign, Urbana, IL, USA;
C Department of Chemistry, University of Illinois at Urbana-Champaign, Urbana, IL, USA
Reproduction, Fertility and Development 27(1) 157-157 https://doi.org/10.1071/RDv27n1Ab130
Published: 4 December 2014
Abstract
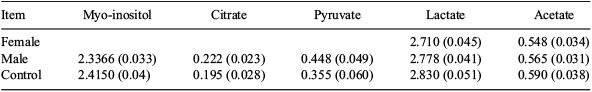
It has been previously shown that during the pre-implantation phase of embryo development, the pentose phosphate energy pathway is 4 times more active in female embryos when compared with male embryos (Tiffin et al. 1991 J. Reprod. Fertil. 93, 125–132). The different metabolic and growth rates can be attributed to the different expression of X-linked genes between the sexes during the early stages of pre-implantation development, in which both X chromosomes are still active in female embryos (Okamoto et al. 2004 Science 303, 644–649). The aim of this study was to evaluate, by proton magnetic resonance (H1-NMR) imaging, the different behaviour of female and male embryos. In this study we evaluated only energetic substrates or Krebs cycle's intermediates. Matured bovine cumulus-oocyte complexes were fertilized in vitro according to our standard procedures (Rubessa et al. 2011 Theriogenology 76, 1347–1355). Presumptive zygotes were placed in individual drops of 50 μL of SOF. Zygotes were incubated in a humidified mixture of 5% CO2, 6% O2, and 88% N2 in air at 39°C. After 48 h, the zygotes were placed into WOW culture, and the drops collected in tubes. The embryos were scored for quality on the basis of morphological criteria. In this experiment, we evaluated 10 embryos for each sex, at stage of tight morula, early blastocyst, blastocyst, and expanded; data were obtained from 2 replicates. The embryos had their sex determined according to our standard protocol (Alomar et al. 2008 Anim. Reprod. Sci. 107, 48–61). Samples of media (40 μL) were added to 660 μL of a stock solution prepared by dissolving 5.0 mg of sodium 3-(trimethylsilyl)-2,2′,3,3′-tetradeuteropropionate (TSP) in 50 mL of deuterium oxide. The TSP acted both as a chemical shift reference and as an internal standard for the purposes of quantitation. The resulting diluted samples were transferred to a 5-mm NMR tube. Samples were analysed on a Varian VNS-750 NB (750 MHz) spectrometer (Agilent Technologies, Santa Clara, CA, USA). Data were statistically analysed with ANOVA using the Generalized Linear Model (GLM) procedure (SAS, version 9, 1999, SAS Institute Inc., Cary, NC, USA), where the independent variable was the sample (female, male embryos and control media without embryos). Tukey's post-hoc test was used to perform multiple comparisons. The P-level was set at 0.05. All data were expressed as quadratic means and with standard error of the means. The results, reported in Table 1, indicate that there are no statistical differences between the sexes after 48 h of embryo culture. In conclusion, these results confirm that in the first phase of development, the embryos derive their energetic substrates from substrates contained within the embryos themselves.
|


