197 EFFECTS OF PRE-IN VITRO MATURATION WITH CAFFEINE ON BOVINE OOCYTE DEVELOPMENTAL CAPACITY
S. M. B. Ulloa A B , J. Heinzmann A , D. Herrmann A , U. Baulain A , K.-G. Hadeler A , P. Aldag A , A. Lucas-Hahn A and H. Niemann AA Institute of Farm Animal Genetics, Biotechnology, Friedrich-Loeffler-Institut (FLI), Mariensee, Germany;
B Facultad de Ciencias Agropecuarias, Universidad de Ciencias Aplicadas y Ambientales–UDCA, Bogotá, Colombia
Reproduction, Fertility and Development 28(2) 229-230 https://doi.org/10.1071/RDv28n2Ab197
Published: 3 December 2015
Abstract
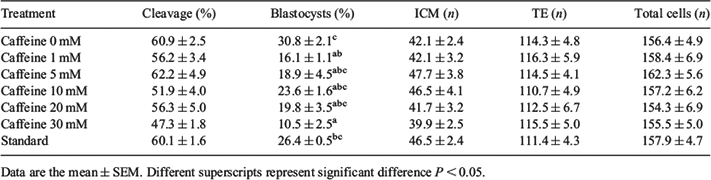
High cyclic adenosine monophosphate (cAMP) concentrations are critical for maintaining oocyte meiotic arrest in vivo. For in vitro maturation (IVM), the oocyte is released mechanically from the follicle, which induces a significant drop in intra-oocyte cAMP levels, triggering non-physiological meiotic resumption. It has been proposed that modulation of cAMP before IVM can increase bovine blastocyst rates in vitro. Caffeine is a nonspecific competitive phosphodiesterases (PDE) inhibitor and can inhibit meiotic resumption of oocytes due to maintenance of cAMP levels. It has been reported that gamete treatment with caffeine can increase developmental potential. The current study evaluated the effects of pre-in vitro maturation culture with different concentrations of caffeine on meiotic progress, developmental rates and blastocyst cell numbers. Bovine ovaries were collected from a local abattoir. A total of 6648 cumulus-oocyte complexes were obtained by slicing. Caffeine was used in 5 different concentrations (1, 5, 10, 20, and 30 mM) during slicing, searching, and 2 h pre-IVM culture. A control group, with 2 h pre-IVM without caffeine (0 mM) and a standard control were also included. Oocytes were washed either after standard or pre-IVM treatments and cultured for 24 h in vitro without caffeine. After IVM, oocytes were fertilised in vitro for 19 h, and zygotes were cultured in vitro for 8 days until the blastocyst stage. Subsets of oocytes were fixed in 2% glutaraldehyde at 9, 20, and 24 h after IVM. Hoechst staining was performed to evaluate nuclear status of matured oocytes. Cleavage and blastocyst formation rates were evaluated at Days 3 and 8 after IVF. Expanded blastocysts from all treatments were submitted to differential staining. One-way ANOVA from R software was applied to evaluate differences in cleavage and blastocysts rates and blastocyst cell numbers. Fisher’s exact test complemented by Bonferroni correction was used to determine meiotic progress. Caffeine maintained oocytes in meiotic arrest after 9 h of IVM in a concentration-dependent manner (germinal vesicle: 79.0%, 92.2%, 66.7%, 55.1%, 56.9%, 43.9%, 30.2%, respectively, for 30, 20, 10, 5, 1, 0 mM and standard; P < 0.016). Cleavage rates were similar in all treatments; however 30 mM caffeine decreased blastocyst rates (Table 1; P < 0.05). The number of cells did not differ significantly among in vitro treatments (Table 1; P > 0.05). Developmental competence was not affected by 2 h pre-IVM culture. Caffeine supplementation before IVM delayed resumption of meiosis and affected embryo development.
|


