131 In vitro nuclear maturation and blastocyst developmental rates after intracytoplasmic sperm injection of equine oocytes held for 24 h at room temperature in Tyrode’s albumin lactate pyruvate-Hepes (TALP-h) or in a commercial embryo holding medium
A. F. Bragulat A B , A. Gambini B C , M. B. Rodriguez B C , O. Briski B C , C. Alonso A , C. Castañeira A , DF Salamone B C and L. Losinno AA Laboratorio de Producción Equina, FAV, UNRC, Córdoba, Argentina;
B CONICET, Buenos Aires, Argentina;
C Facultad de Agronomía, UBA, Buenos Aires, Argentina
Reproduction, Fertility and Development 33(2) 173-174 https://doi.org/10.1071/RDv33n2Ab131
Published: 8 January 2021
Abstract
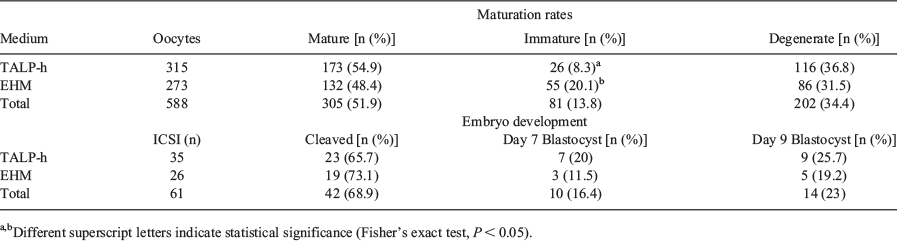
The interest in equine intracytoplasmic sperm injection (ICSI) for commercial and research applications has rapidly increased. Shipping immature oocytes at room temperature has been proven successful, and to identify the optimal conditions for holding oocytes, several mediums are being tested. The aim of this study was to compare the effect of holding equine oocytes in Tyrode’s albumin lactate pyruvate-Hepes (TALP-h, Bavister and Yanagimachi 1977 Biol. Reprod. 16, 228-237) medium or in commercial embryo holding medium (EHM, Syngro® Holding) on in vitro nuclear maturation rates and pre-implantation embryo development after ICSI. Cumulus–oocyte complexes (COCs) were recovered from ovaries of slaughtered mares and assigned randomly in 2-mL cryovials with TALP-h or EHM, with a maximum of 30 oocytes per cryovial. COCs were shipped to the ICSI laboratory at 20 to 25°C for 24 to 28 h followed by IVM for 24 h in a humidified atmosphere of 5% CO2 in air at 38.5°C. Maturation medium was TCM-199 with 10% fetal bovine serum, 1 μL mL−1 insulin-transferrin-selenium, 1 mM sodium pyruvate, 100 mM cysteamine, and 0.1 mg mL−1 FSH. After mechanical cumulus cell removal, nuclear maturation rate was assessed using a stereomicroscope. Oocytes with an intact oolemma and extrusion of the first polar body (PB) were classified as mature, oocytes without a visible PB were considered immature, and oocytes without an intact oolemma were considered degenerate. Matured oocytes were subjected to ICSI without piezo-drill system (one proved stallion) in 20-μL droplets of TALP-h with a 7-μm glass sharp micropipette in an inverted microscope (Nikon Eclipse TE-300 microscope) using hydraulic micromanipulators (Narishige, Medical Systems). Presumptive ICSI zygotes were cultured in DMEM F12/Global Total® with 6% fetal bovine serum for 9 days at 38.5°C in a humidified atmosphere of 5% O2 and 5% CO2 in air. On Day 5 of culture, cleavage was recorded and medium was refreshed. Blastocysts rates were recorded on Day 7 and 9 of culture. In vitro nuclear maturation rates are shown in Table 1. We observed a significantly higher proportion of immature oocytes in the EHM group compared with the TALP-h group. After ICSI of some matured oocytes of each group, no significant differences were observed in cleavage or blastocyst rate (Table 1). Our results suggest that either TALP-h or commercial embryo holding medium are suitable for oocyte shipping and to support blastocyst development after ICSI.
|


