29 Cryopreservation by slow freezing of bovine in vitro embryos in different stages of development
L. R. Peixoto A , B. L. Cardoso A , N. J. Lopes A , B. A. P. Maiollo A , M. F. A. Borges A , J. H. Tannura A , R. L. Krisher B and M. Rubessa BA Genus plc, Mogi Mirim, São Paulo, Brazil
B Genus plc, DeForest, WI, USA
Reproduction, Fertility and Development 34(2) 249-249 https://doi.org/10.1071/RDv34n2Ab29
Published: 7 December 2021
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of the IETS
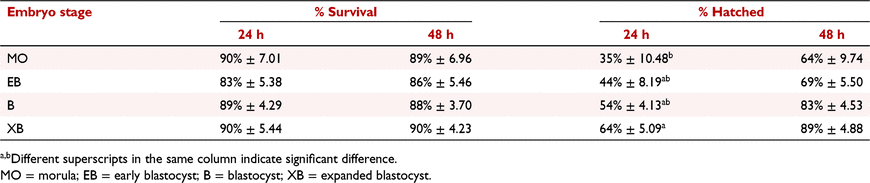
In vitro-produced embryos are an important tool for the multiplication of valuable genetically high-merit animals. Cryopreservation preserves the embryo and its genetic material, providing greater flexibility for commercialisation of embryo technology. The objective of this study was to evaluate the viability post-thaw of bovine in vitro-produced embryos, cryopreserved by slow freezing, at different embryo stages. Embryos were produced from oocytes of ovaries collected in a local slaughterhouse. The oocytes stayed in maturation medium for 24 h at 38.5°C in 5% CO2 in air. After fertilisation, the zygotes were cultured in medium without fetal bovine serum at 38.5°C in 5% CO2, 5% O2, and 90% nitrogen. On Day 7 of culture, the embryos were evaluated and classified by stage and quality grade, according to the fourth edition of the International Embryo Technology Society manual. After classification, the embryos were cryopreserved by slow freezing. For the study, seven replicates were performed, and 559 grade 1 embryos were distributed in four experimental groups according to their stage: morula (n = 68); early blastocyst (n = 89); blastocyst (n = 180), and expanded blastocyst (n = 222). The embryos were placed in freezing medium (1.5 M ethylene glycol, 0.1 M sucrose, and 0.4% bovine serum albumin) and loaded into 0.25-mL straws. Then the straws were placed in a freezing machine (TK5000; TK Technology) using the standard slow freezing protocol. For thaw, the embryos were removed from liquid nitrogen, exposed to the air for 5 s, and immersed in water at 30°C for 30 s. Post-thaw, the embryos were cultured and evaluated for survival and hatching at 24 and 48 h. Data were analysed by one-way ANOVA followed by Tukey test using JMP 14 software (SAS Institute Inc.). Results are described in Table 1. There was no difference in embryo survival post-thaw between treatments. After 24 h, significantly fewer morula hatched (35 ± 10%) compared with expanded blastocysts (64 ± 5%; P = 0.04), whereas at 48 h, there was no difference in hatching according to the stage at which the embryo was cryopreserved. In summary, embryos at early (morula and early blastocyst) as well advanced (blastocyst and expanded blastocyst) developmental stages are able to maintain their viability after cryopreservation by slow freezing, as demonstrated by continued development and hatching when cultured for 48 h post-thaw. These data suggest that early embryos on Day 7 can be reliably cryopreserved by slow freezing to provide more embryos for transfer.
|


