Reproduction, Fertility and Development
Volume 33
Numbers 8 & 9 2021
Special Issue
Amphibian and Reptile Assisted Reproductive Technologies Part I
Guest Editors
Natalie Calatayud (Taronga Western Plains Zoo, Beckman Center for Conservation Research and Conservation Science Network)
John Clulow (The University of Newcastle and FAUNA Research Alliance)
Simon Clulow (University of Canberra and FAUNA Research Alliance)
Gina Della Togna (Universidad Interamericana de Panama and Smithsonian Tropical Research Institute)
Ruth Marcec (Detroit Zoological Society)
Assisted breeding technology (ABT) offers benefits for the genetic and reproductive management of farmed and endangered Crocodylia. This review examines the current status of ABT in the Crocodylia and future requirements for establishing a reliable AI program. We propose the saltwater crocodile as a research model for the development of reptile ABT based on their commercial use and ease of semen collection; the latter of which has allowed an empirical and molecular approach to understanding sperm preservation and physiology.
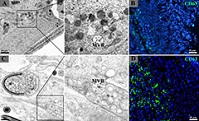
Most reptilian species have a dissociated reproductive pattern in which spermatogenesis is dissociated from male mating behaviour, and so are different to mammals and birds. In the present study, extracellular vesicles in the male reproductive tract of the Chinese softshell turtle were found to be associated with sperm maturation, transition and storage. Overall, extracellular vesicles play an indispensable role in the normal reproductive function of turtles, and can be used as an excellent biomarker for understanding male fertility.
Almost half of all crocodilian species are endangered or vulnerable. Working towards the development of artificial breeding technologies to secure the long-term survival of these species, our research has focused on the saltwater crocodile as a tractable model for understanding the functional regulation of crocodilian sperm physiology. Here, we demonstrate the efficacy of simple interventions in being able to suppress and reactivate the function of saltwater crocodile spermatozoa compatible with their storage, transport and subsequent use in AI protocols.
Although scarce, information on the morphology and histology of the male reproductive system of the Crocodylia species is necessary to determine the role of these tissues in the production of spermatozoa and seminal fluids. Accordingly, here we describe the gross and microanatomy of the male reproductive system (comprising the testis, rete testis, ductuli efferentes, ductuli epididymides, ductus epididymidis and ductus deferens) of the saltwater crocodile Crocodylus porosus, revealing conserved structural characteristics similar to those reported in Aves.
Assisted reproductive technologies (ARTs; e.g. storing gametes and reviving them through AI) have a significant role to play in wildlife conservation, yet are severely lacking for reptiles. Successful sperm cryopreservation was recently reported in a threatened lizard species, but short-term storage at temperatures above freezing have not been tested. We developed a technique for cold-storing lizard spermatozoa for up to 8 days before freezing, contributing to the development of valuable ARTs for lizards and other reptiles.
The development of assisted reproductive technologies, such as sperm cryopreservation and IVF, has the potential to play an important role in the conservation of amphibians. We report successful sperm cryopreservation in the threatened green and golden bell frog, with strong recovery of motility and vitality following cryopreservation with 1.4–2.1 M dimethylsulfoxide. We demonstrate the production of mature adults following fertilisation using cryopreserved spermatozoa. These results suggest that the use of these technologies can be implemented into existing breeding programs for threatened amphibians.
Captive breeding is often the last line of defence for many species, but faces challenges of high costs and genetic issues. We propose that integrating biobanking into the captive breeding of Australian amphibians could minimise rates of inbreeding, reduce colony sizes and lower program costs. Using biobanking, captive breeding institutions could produce output animals better suited for reintroduction into the wild that have cost less to maintain, and meet ambitious captive genetic diversity targets (90% heterozygosity retention for a minimum of 100 years).
Amphibian species are considered to be at very high risk of extinction. One of the reliable methods to save endangered amphibian species is to preserve spermatozoa treated with cryoprotectants, such as dimethylsulfoxide and dimethylformamide, in cryobanks at an ultra-low temperature (–196°C) in liquid nitrogen. When necessary, modern technologies enable offspring to be obtained from frozen–thawed individual reproductive cells.
This study is the first to document the lipid content of the sperm plasma and acrosomal membranes in a reptile. Lipid analysis of the saltwater crocodile revealed the plasma membrane had a higher ratio of unsaturated to saturated fatty acids than the acrosomal membrane. The crocodile sperm plasma membrane also contained higher levels of cholesterol than that recorded in other amniote species. This analysis will contribute to our understanding of reptile sperm preservation and physiology.
Spermatozoa were collected from 12 snakes representing four squamate families. Post-thaw viability was assessed after freezing in 12% dimethylsulfoxide (DMSO) or glycerol. This preliminary study suggests that glycerol is the preferred cryoprotectant for colubrid snake spermatozoa, whereas DMSO provides greater sperm cryoprotection for members of the Elapidae, Viperidae, and Pythonidae families. Photograph courtesy of San Diego Zoo Wildlife Alliance.
Conservation and reintroduction programs are increasingly reliant on establishing ex situ assurance colonies. However, reproductive anomalies and the overall health of the breeding population is not always documented or addressed. Here we examine the onset of male sexual characteristics and hermaphroditism in adult female mountain yellow-legged frogs (Rana muscosa) by using assisted reproductive technologies, life history and post-mortem findings.
Reproductive technologies can assist with the propagation and genetic management of threatened amphibian species. Here we report on the egg release response and artificial fertilisation success of females administered exogenous reproductive hormones.