The role of the male flower spike as a cue for selective grazing in bladder saltbush
D. Walsh A D , R. Sinclair B , M. H. Andrew C and D. Coleman BA Centralian Land Management Association, PO Box 2534, Alice Springs, NT 0871, Australia.
B University of Adelaide, Adelaide, SA 5005, Australia.
C URS Australia – NRM Group, 25 North Tce, Hackney, SA 5069, Australia.
D Corresponding author. Email: ems@clma.com.au
The Rangeland Journal 27(2) 97-103 https://doi.org/10.1071/RJ05008
Submitted: 1 August 2005 Accepted: 24 August 2005 Published: 21 November 2005
Abstract
This paper reports the results of three cafeteria trials used to study palatability variation between the sex phenotypes of bladder saltbush (Atriplex vesicaria Heward ex Benth.). The results of the first trial show that Merino sheep preferentially grazed female samples compared to male ones, which supported earlier paddock-scale grazing trials and observations. In the second trial, the removal of male flower spikes led to increased consumption of male samples, suggesting that male flower spikes contain a grazing deterrent. The third trial showed that sheep were able to detect male material with or without spikes even when it was completely hidden within female plant material. In combination with observations made during the trials, these results suggest that there is a grazing deterrent present in male plants and that sheep use the male flower spike primarily as a visual cue when making grazing decisions.
Additional keywords: Atriplex vesicaria, Australian merino, female, palatability.
Introduction
Atriplex vesicaria (bladder saltbush) is a low-growing shrub with a lifespan of 25 to 30 years (Crisp 1978). The species is subdioecious having male, female and bisexual phenotypes (Bisalputra 1960). Flowering is opportunistic and can occur more than once a year (Wood 1936; Williams 1979). Bladder saltbush has long been recognised as an important species for ecosystem stability and pastoral production in the rangelands of southern Australia (Wood 1936; Wilson and Graetz 1979; Rhodes 1986). Due to its relative palatability and susceptibility to grazing and trampling, bladder saltbush has also assumed importance as an indicator of range condition in chenopod shrublands (Lange et al. 1994).
Since the 1970s, there has been a growing body of evidence to indicate that variation in palatability occurs between the sex phenotypes of bladder saltbush. Both Williams et al. (1978) and Graetz (1978) found that the sex ratio of grazed saltbush populations in western New South Wales was strongly biased in favour of the male phenotype and Graetz (1978) reported a 5-fold increase in the ratio of male to female plants with decreasing proximity from water. Subsequently, Graetz and Wilson (1979) found that female shrubs were more likely to be grazed than male shrubs at all distances from water. Beyond the sacrifice zone (where both sexes are trampled and grazed heavily), very few male plants are grazed, suggesting that when sheep are at their most selective, they prefer to graze females (Graetz 1978).
No further investigations of palatability variation between the sex phenotypes has been published since the 1970s until a recent study by Maywald (1998) 1 . The results of small-plot grazing trials from that study confirmed that female shrubs were preferentially grazed over male shrubs, which was the first confirmation of the phenomenon in the rangelands of South Australia (Walsh et al. 2005).
While Williams (1972) had previously argued that sheep seek out female shrubs due to a preference for the succulent unripe fruits, Graetz (1978) suggested that sheep avoid male shrubs because the terminally placed male flowering spikes act as a mechanical obstruction to the preferred foliage below. Walsh et al. (2005) found evidence to support the idea that it is the male flower spike that is a deterrent to grazing rather than fruits being an attractant.
In this series of studies, the role of male spikes and female fruits was examined using cafeteria trials, which allowed sheep to make choices among forages presented to them in a strictly controlled environment. This approach has been used to investigate the dietary behaviour of many herbivores including impala (Frost 1981), deer (Schwartz et al. 1980; Gillingham and Bunnell 1989a , 1989b ), hares (Reichardt et al. 1984; Tahvanainen et al. 1985), horses (Houpt et al. 1990), camels (White and Robards 1997) and sheep (Kenney and Black 1984; Burritt and Provenza 1989a , 1989b ). By removing plants from the confounding influences of their environment, the plant cues that stimulate selective grazing can be investigated. Furthermore, the presentation of the plant material can be manipulated in ways not possible using small-plot or paddock trials (Schwartz et al. 1980).
Three trials were conducted in this study with a sequential development of hypotheses. The aims were:
-
to confirm whether sheep preferentially graze female over male plants in a cafeteria environment, and
-
to investigate the role of the male flower spike as a grazing deterrent.
Materials and methods
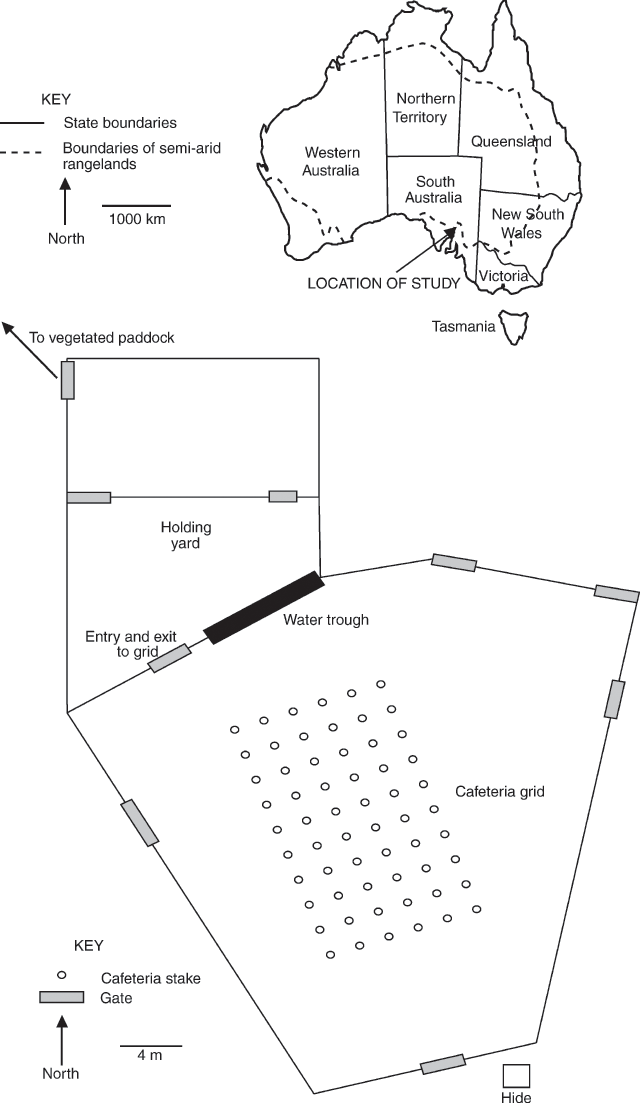
Cafeteria layout
The cafeteria was established in a yard at Middleback Station, South Australia and consisted of six rows of ten wooden stakes arranged in a grid pattern (Fig. 1). The 30-cm long stakes were driven 10 cm into the ground and were placed 2 m apart from each other. Plant samples were attached to these stakes during the trials using plastic cable ties. Replicate tests were conducted on different days because the yard only allowed space for one grid and several hours were required to prepare the plant samples for each test.
|
Experimental sheep
Ten mixed-sex hoggets were selected from a larger station flock for each trial. A different flock was used for each of the three trials. On the eve of each replicate the experimental sheep were mustered into a holding yard devoid of feed. Sheep had access to water at all times. Between replicates, the sheep were released into a fully vegetated paddock to resatiate.
Preparation of the samples
All plant material used in the trials was harvested from shrubs in Little One Mile Paddock, Middleback Station (see Walsh et al. 2005). This paddock has rarely been used by sheep (A. W. Nicolson, pers. comm.) and hence the saltbush community was expected to encompass a natural range of palatability variation.
On the eve of each replicate, six large plastic tubs were filled with rainwater to a depth of 5 cm. Bunches of plant material were cut from shrubs in Little One Mile Paddock and were stored in the tubs with their stems immersed in the water. Material was cut from heavily fruiting female and heavily flowering male shrubs to maximise visual differentiation. Three tubs of material were collected for each sex and the two sexes were kept separate. Loose-fitting lids were placed on the tubs to reduce evaporation and they were transported back to the on-site laboratory. Material from different shrubs was used to construct ‘composite’ samples in order to minimise potential strong palatability effects due to individual source shrubs. The samples were constructed on a digital balance and were tied at the base with unbleached string. The fresh weights of all samples were recorded (with all being between 40 and 50 g). The samples of a single treatment were prepared together and returned to the same tub. Loose-fitting lids were put on the tubs to reduce evaporation and the tubs were kept in the laboratory overnight.
Experimental treatments
October 1995 – male v. female trial
The design and treatments used in each trial are summarised in Table 1. Two treatments were compared in the first trial to test whether sheep would selectively graze the sex phenotypes in a cafeteria environment. Thirty female and 30 male samples were prepared for each of four replicate tests. The male source shrubs were at various stages of flower production and thus varied widely in the density, length and maturity of spikes. The female material used in this trial had mature dry fruits.
April 1996 – spike removal trial
Four treatments were compared in the second trial (Table 1). As in the first trial, there was a female and a male treatment (with 15 samples of each in each replicate). The third treatment consisted of male samples from which the male spikes had been removed by fine dissection scissors. This treatment was included to test whether the removal of spikes would increase consumption. To control for any effects due to the physical cutting by the scissors, a fourth treatment was included. A strictly perfect clipping control was not possible because all male branches had spikes and clipping could not be done without removing some of these (and thus confounding the control). The fourth treatment consisted of female samples which had the tips of nearly all shoots removed with the scissors. About 1 cm was cut from the tip of each female shoot to simulate the removal of spikes on the males. Cuts were made diagonally to prevent a hedged-look and the weights of all completed samples were between 40 and 50 g. The male material used in this trial had well developed spikes that were releasing pollen, whilst all female material had ripe fruits.
September 1996 – hidden male trial
Three treatments were compared in the final trial, with 20 samples of each in each replicate (Table 1). The first treatment consisted of pure female samples that were not manipulated any further. The other two treatments consisted of female-dominated samples which had male material hidden inside them. The first of these had intact male material hidden inside whilst the other included male material with all spikes removed. For the hidden male treatments, a small bunch of male material comprising 20–25% of the total biomass of each sample was entirely enclosed by female material. Care was taken to ensure that the male material was completely hidden from view. The hidden male treatments were used to test whether the sheep could still detect the presence of male material in the absence of a visual cue. Clipped and non-clipped male material was used to determine whether there was any difference in the detectability of material with or without spikes. The male source material used in this trial had a mixture of old and new spikes and the female material had unripe fruits.
Replicate procedure
A random-number table was used to assign the samples to the 60 stakes before each replicate (Zar 1984). The tubs containing the samples were transported to the cafeteria yard about 1 hour before sunrise. Trials were conducted at dawn for two reasons. First, the samples were less likely to wilt because maximum air humidity occurs around sunrise and second, sheep in chenopod shrublands normally commence grazing around this time (Squires 1974, 1981). The samples were attached to the stakes using plastic cable ties and five microdots were applied to each sample using a quick-drying marker pen (Lange 1984). After all dots were applied, the ten sheep were let into the yard. Once grazing began, the sheep were observed from a hide south of the yard for the duration of all replicates. Notes were made on their behaviour and grazing patterns at intervals not exceeding 10 min. When sheep were actively grazing or moving, notes were made continuously. Each replicate was stopped as soon as all stakes had been visited at least twice, with a visit considered to have occurred if a sheep passed within 50 cm of a stake. These visits were recorded on a diagram of the grid. Naturally some stakes were visited more often than others, however, a trial did not end until the last stake had been visited twice. This method of determining the endpoint was used to ensure that all samples had the potential to be grazed or rejected by the sheep.
At the end of each replicate, the sheep were quietly mustered out of the cafeteria and into the large vegetated paddock near the yard. The microdots remaining on the samples were then recounted. Any microdots that had been broken off but not eaten were recorded as damaged. All material was then cleared from the grid and discarded. The percentage of microdots eaten from each sample was multiplied by the initial biomass to determine the amount of biomass eaten (after Lange 1984).
Analyses
Analysis of variance (ANOVA) was used to compare the biomass consumed from each treatment. Where the ‘day’ term was not significant (P>0.05), replicates were pooled and no interaction term was included in the ANOVA. Where the result of the ANOVA was significant, pairwise comparisons were made using the Tukey–Kramer HSD test. All analyses were performed using the JMP program (SAS Institute, Cary, NC).
Results
October 1995 – male v. female trial
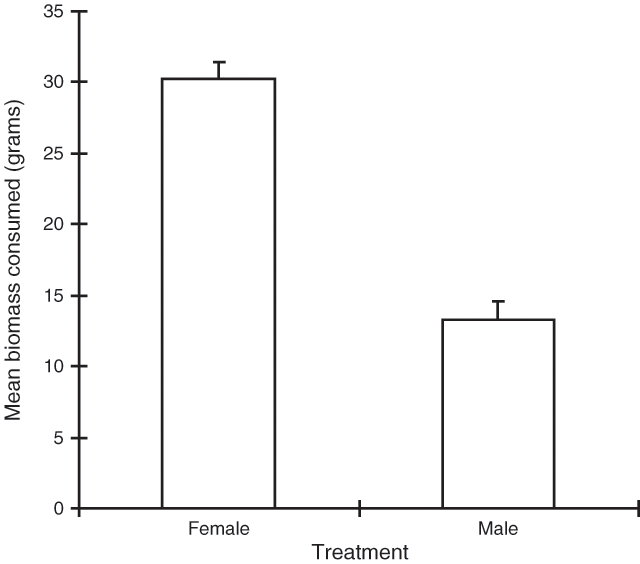
The analysis showed that both ‘day’ and ‘treatment’ were significant (P < 0.001), but there was no significant interaction between them (P > 0.5). Figure 2 shows that the female plant material was consumed significantly more than the male material.
|
April 1996 – spike removal trial
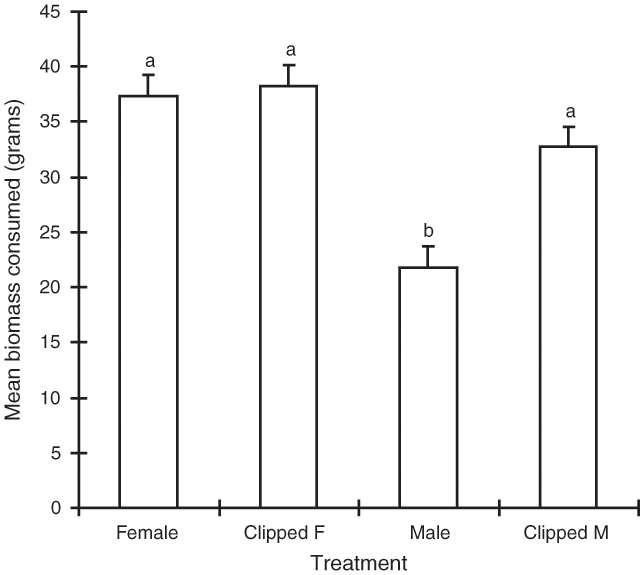
‘Treatment’ was the only significant term in the model (P < 0.001). The removal of male flower spikes was found to increase the consumption of male plant material relative to unclipped material (Fig. 3). The consumption of the non-clipped and clipped female treatments did not differ significantly (Fig. 3).
|
September 1996 – hidden male trial
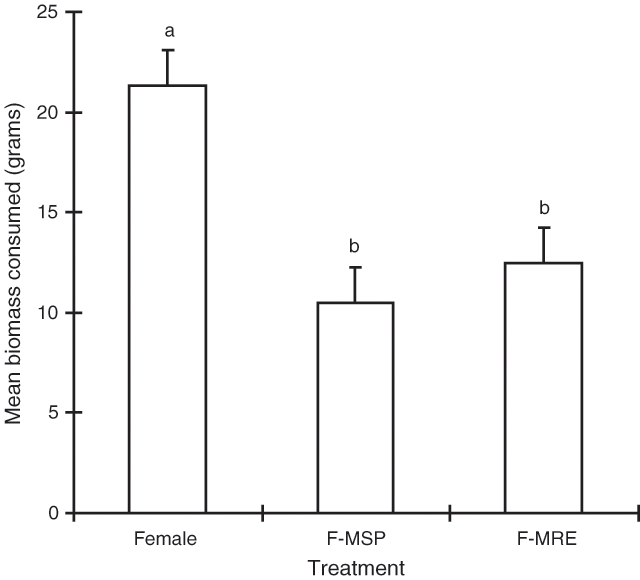
‘Treatment’ was the only significant term in the model (P < 0.001) and Fig. 4 shows that the pure female treatment was consumed significantly more than either of the two hidden male treatments.
|
Discussion
The use of the cafeteria method relied on the assumption that the cues underlying palatability variation were a feature of the plants rather than their environment. This was confirmed in the first trial, which showed that the female phenotype was preferentially grazed over the male phenotype. This pattern was consistent with all previous findings to date (Williams 1972; Williams et al. 1978; Graetz and Wilson 1979; Walsh et al. 2005).
The role of the male flower spike was then investigated in the second and third trials. Removal of male flower spikes in the second trial increased the consumption of male material. As clipping had no significant effect on the consumption of female samples, it can be argued that clipping per se was not the cause of increased consumption of clipped male samples. The use of clipped female samples as a control assumed that the sexes would not differ in their response to the physical treatment of clipping. At this time, there is no reason to doubt this assumption.
The second trial suggested the presence of a ‘deterrent’ in the male flower spike and the final trial demonstrated that sheep were able to detect and avoid both clipped and non-clipped male material in the absence of a visual cue. Behavioural observations made during the first two trials, when the visual cue was present, showed that sheep were often able to reject flowering male samples without having to sniff or taste them. However, when spikes were removed, sheep would sometimes take prolonged sniffs or bites before rejecting male samples. The ability of sheep to detect both clipped and unclipped hidden male material suggests that the olfactory cue may have been relative stronger in the final trial. Taken together, these data and observations suggest that sheep use the male flower spike as a visual cue and the strength of the olfactory cue might vary over time.
The hypothesis that sheep selectively graze female shrubs because they are attracted to the succulent, unripe fruits (Williams 1972) is not supported by this study. If this were true, we would have expected plants within the three treatments in the final trial to be consumed equally. Instead, the inclusion of male material lead to significantly lower consumption of otherwise ‘female’ samples in this trial. Overall results of these cafeteria trials thus support Graetz’s (1978) hypothesis that the terminal male flower spike is a deterrent to consumption. However, we disagree with the notion that the male flower spikes act as a physical barrier to the foliage below. The soft male flower spike in A. vesicaria rarely exceeds 5 cm in length and 5 mm in diameter. It is difficult to imagine that an animal that can efficiently consume the leaves and stems of thorny plants such as Acacia victoriae, Maireana aphylla and Sclerolaena spp. (Belovsky et al. 1991) would find the soft male flower spike of A. vesicaria a physical obstruction. Rather, we believe that sheep use the spike primarily as a visual cue to a chemical deterrent contained in male plants. The nature of this chemical deterrent is not known at this time (see Walsh et al. 2005).
The lack of adaptations such as spines, thorns, stinging hairs, protected meristems and basal resprouting suggest that herbivory by vertebrate animals has not been an important selective pressure for A. vesicaria (Anderson 1982). This is despite the belief that before European settlement, much of the arid interior would have been grazed by transient populations of marsupials (Osborn et al. 1932; Beadle 1959; Moore 1959; Williams 1980; Lange et al. 1994; Moseby and Bice 2004). Furthermore, given the significant negative impact of preferential grazing on seed production in A. vesicaria (Maywald 1998; Hunt 2001), we would expect that any defence against herbivory would be invested in female shrubs. These observations suggest to us that the deterrent in male shrubs is coincidental rather than a direct evolutionary response to sheep grazing.
There are at least two alternative explanations for the presence of a deterrent in male saltbush. The first is that male bladder saltbushes may have been more susceptible to a defoliation-related evolutionary pressure than females. Harborne (1988) has suggested that chemical defences in plants usually arise as a result of herbivory by insects. Insect attacks on A. vesicaria and A. nummularia were first reported over 100 years ago (Olliff 1892). Olliff noted that the larvae of the saltbush scale (Pulvinaria maskelli) swarmed over the shrubs soon after hatching and ate the bark, stems, younger shoots and leaves. Froggatt (1910) recorded several species of caterpillars, beetles and weevils in saltbush specimens. The most destructive insects appear to be the caterpillars, which, at times, have reached plague proportions:
‘[caterpillars] appeared in such enormous numbers that they left hundreds of thousands of acres of rich saltbush plains absolutely bare, with all the old saltbush practically dead’ (Froggatt 1910, p. 465)
Even in the last 20 years, caterpillar plagues have been implicated in the decline of saltbush stands in the Riverine Plain area of New South Wales (Clift et al. 1987). Further research is required to determine whether the sexes of saltbush vary in their susceptibility to insect attack.
The second explanation for the deterrent is based on the differential physiological response of the sex phenotypes to stress. It is known that environmental stress influences the concentrations of metabolites in plants and that male plants tend to occupy more ‘stressful’ microsites (McArthur 1977; Freeman et al. 1980; Charnov 1982). For example, variation in the concentrations of metabolites has been shown to occur throughout the year and between the sex phenotypes in A. canescens (Tiedemann et al. 1987). It is, therefore, possible that due to the relatively stressful microsites they occupy, male bladder saltbushes produce concentrations of physiological metabolites that happen to repel sheep.
Preferential grazing of the female phenotype across the species’ range offers the opportunity to refine the use of bladder saltbush as an indicator species. To date, the reduction in density of bladder saltbush has been used as an indicator of declining land condition (Lange et al. 1994). Our results suggest that assessments of the size and reproductive vigour of female shrubs, and the measurement of population sex ratios, may provide an early warning system for the monitoring and management of Atriplex vesicaria.

|
Acknowledgments
We are indebted to the Nicolson families of Middleback and Roopena stations and the many people who assisted with the field work. We are grateful to Dr D. Carl Freeman and two anonymous referees for reviewing a draft of the manuscript. This research was funded by an International Wool Secretariat Postgraduate Scholarship and The University of Adelaide, and was conducted as part of the Middleback Field Centre research program. Animal ethics committee approval S/14/94.
Anderson, D. J. (1982). Environmentally adaptive traits in arid zone plants.
‘Evolution of the flora and fauna of arid Australia’. pp. 133–139. (Peacock Publications: Adelaide.)
Beadle, N. C. W. (1959). Some aspects of ecological research in semi-arid Australia.
‘Monographiae biologicae. VIII: Biogeography and ecology in Australia’. pp. 452–460. ( Dr. W. Junk: The Hague.)
Belovsky G. E.,
Schmitz O. J.,
Slade J. B., Dawson T. J.
(1991) Effects of spines and thorns on Australian arid zone herbivores of different body masses. Oecologia 88, 521–528.

Bisalputra T.
(1960) Anatomical and morphological studies in the Chenopodiaceae. I: Inflorescence of Atriplex and Bassia.
Australian Journal of Botany 8, 226–242.

Burritt E. A., Provenza F. D.
(1989a) Food aversion learning: conditioning lambs to avoid a palatable shrub (Cercocarpus montanus). Journal of Animal Science 67, 650–653.

Burritt E. A., Provenza F. D.
(1989b) Food aversion learning: ability of lambs to distinguish safe from harmful foods. Journal of Animal Science 67, 1732–1739.
| PubMed |

Charnov, E. L. (1982).
Clift D. K.,
Semple W. S., Prior J. C.
(1987) A survey of bladder saltbush (Atriplex vesicaria Heward ex Benth.) dieback on the Riverine Plain of south-eastern Australia from the late 1970s to 1983. Australian Rangeland Journal 9, 39–48.

Crisp M. D.
(1978) Demography and survival under grazing of three Australian semi-desert shrubs. Oikos 30, 520–528.

Freeman D. C.,
Harper K. T., Charnov E. L.
(1980) Sex change in plants: old and new observations and new hypotheses. Oecologia 47, 222–232.
| Crossref | GoogleScholarGoogle Scholar |

Froggatt W. W.
(1910) Insects which damage saltbush. Agricultural Gazette of New South Wales 21, 465–470.

Frost S. K.
(1981) Food selection in young naive impala Aepyceros melampus.
South African Journal of Zoology 16, 123–124.

Gillingham M. P., Bunnell F. L.
(1989a) Effects of learning on food selection and searching behaviour of deer. Canadian Journal of Zoology 67, 24–32.

Gillingham M. P., Bunnell F. L.
(1989b) Black-tailed deer feeding bouts: dynamic events. Canadian Journal of Zoology 67, 1353–1362.

Graetz R. D.
(1978) The influence of grazing by sheep on the structure of a saltbush (Atriplex vesicaria Hew. ex Benth.) population. Australian Rangeland Journal 1, 117–125.

Graetz, R. D. ,
and
Wilson, A. D. (1979). An assessment of herbivore diets in the chenopod shrublands.
‘Studies of the Australian arid zone. IV: Chenopod shrublands’. pp. 144–159. (CSIRO Publishing: Melbourne.)
Harborne, J. B. (1988).
Houpt K. A.,
Zahorik D. M., Swartzmann-Andert J. A.
(1990) Taste aversion learning in horses. Journal of Animal Science 68, 2340–2344.
| PubMed |

Hunt L. P.
(2001) Low seed availability may limit recruitment in grazed Atriplex vesicaria and contribute to its local extinction. Plant Ecology 157, 53–67.
| Crossref | GoogleScholarGoogle Scholar |

Kenney P. A., Black J. L.
(1984) Factors affecting diet selection by sheep. 1. Potential intake rate and acceptability of feed. Australian Journal of Agricultural Research 35, 551–563.
| Crossref | GoogleScholarGoogle Scholar |

Lange R. T.
(1984) Leaf marking in rangeland grazing studies. Transactions of the Royal Society of South Australia 108, 213–214.

Lange R. T.,
Lay B. G., Tynan R. W.
(1994) Evaluation of extensive arid rangelands: the Land Condition Index (LCI). Transactions of the Royal Society of South Australia 118, 125–131.

Maywald D. L.
(1998)
McArthur E. D.
(1977) Environmentally induced changes of sex expression in Atriplex canescens.
Heredity 38, 97–103.

Moore, R. M. (1959). Ecological observations on plant communities grazed by sheep in Australia.
‘Monographiae biologicae. VIII: Biogeography and ecology in Australia’ pp. 500–513. (Dr. W. Junk: The Hague.)
Moseby K. E., Bice J. K.
(2004) A trial re-introduction of the Greater Stick-nest Rat (Leporillus conditor) in arid South Australia. Ecological Management and Restoration 5, 118–124.
| Crossref | GoogleScholarGoogle Scholar |

Olliff A. S.
(1892) Entomological notes: further remarks on the saltbush scale (Pulvinaria maskelli Oll.). Agricultural Gazette of New South Wales 3, 176–180.

Osborn T. G. B.,
Wood J. G., Paltridge T. B.
(1932) On the growth and reaction to grazing of the perennial saltbush Atriplex vesicarium. An ecological study of the biotic factor. Proceedings of the Linnean Society of New South Wales 57, 377–402.

Reichardt P. B.,
Bryant J. B.,
Clausen T. P., Wieland G. D.
(1984) Defence of winter-dormant Alaska paper birch against snowshoe hares. Oecologia 65, 58–69.
| Crossref | GoogleScholarGoogle Scholar |

Rhodes, D. W. (1986). Fifty years’ grazing of bladder saltbush (Atriplex vesicaria) in central-northern New South Wales, Australia.
‘ Rangelands: a resource under siege. Proceedings IInd International Rangeland Congress’. pp. 139–140. (Australian Academy of Science: Canberra.)
Schwartz C. C.,
Regelin W. L., Nagy J. G.
(1980) Deer preference for juniper forage and volatile oil treated foods. Journal of Wildlife Management 44, 114–120.

Squires V. R.
(1974) Grazing distribution and activity patterns of Merino sheep on a saltbush community in south-east Australia. Applied Animal Ethology 1, 17–30.
| Crossref | GoogleScholarGoogle Scholar |

Squires, V. R. (1981).
Tahvanainen J.,
Helle E.,
Julkunen-Tiitto R., Lavola A.
(1985) Phenolic compounds of willow bark as deterrents against feeding by mountain hare. Oecologia 65, 319–323.
| Crossref | GoogleScholarGoogle Scholar |

Tiedemann A. R.,
McArthur E. D., Freeman D. C.
(1987) Variations in physiological metabolites and chlorophyll in sexual phenotypes of ‘Rincon’ fourwing saltbush. Journal of Range Management 40, 151–155.

Walsh D. L.,
Andrew M. H.,
Sinclair R., Coleman D.
(2005) Evidence for palatability variation between the sex phenotypes of bladder saltbush from small-plot grazing trials. The Rangeland Journal 27, 89–96.

White A., Robards G.
(1997) Grazing studies reveal camels have limited potential to control woody weeds in western New South Wales. Range Management Newsletter 97, 8–14.

Williams D. G.
(1972)
Williams, D. G. (1979). The comparative ecology of two perennial chenopods.
‘Studies of the Australian arid zone. IV: Chenopod shrublands’. pp. 29–40. (CSIRO Publishing: Melbourne.)
Williams D. G.,
Anderson D. J., Slater K. R.
(1978) The influence of sheep on pattern and process in Atriplex vesicaria populations from the Riverine Plain of New South Wales. Australian Journal of Botany 26, 381–392.

Williams, O. B. (1980). Evolution of grazing systems.
‘Grazing animals’. pp. 1–11. (Elsevier: Amsterdam.)
Wilson, A. D. ,
and
Graetz, R. D. (1979). Management of the semi-arid rangelands of Australia.
‘Management of semi-arid ecosystems’. pp. 83–111. (Elsevier
Wood J. G.
(1936) Regeneration of the vegetation on the Koonamore vegetation reserve. Transactions of the Royal Society of South Australia 60, 96–111.

Zar, J. H. (1984).
1Senior author’s maiden name.


