An investigation of phyllode variation in Acacia verniciflua and A. leprosa (Mimosaceae), and implications for taxonomy
Stuart K. Gardner A , Daniel J. Murphy B C , Edward Newbigin A , Andrew N. Drinnan A and Pauline Y. Ladiges AA School of Botany, The University of Melbourne, Vic. 3010, Australia.
B Royal Botanic Gardens Melbourne, Private Bag 2000, South Yarra, Vic. 3141, Australia.
C Corresponding author. Email: Daniel.Murphy@rbg.vic.gov.au
Australian Systematic Botany 18(4) 383-398 https://doi.org/10.1071/SB04052
Submitted: 20 December 2004 Accepted: 19 July 2005 Published: 31 August 2005
Abstract
Acacia verniciflua A.Cunn. and A. leprosa Sieber ex DC. are believed to be closely related, although strict interpretation of the current sectional classification of subgenus Phyllodineae places them in separate sections based on main nerve number. Six populations, comprised of the common and the southern variants of A. verniciflua and the large phyllode variant of A. leprosa, were sampled to test the value of nerve number as a taxonomic character and the current delimitation of these geographically variable species. Morphometrics, microscopy and the AFLP technique were used to compare and contrast populations. Phyllode nerve development was investigated and the abaxial nerve was found to be homologous with the mid-rib of a simple leaf. Three taxa were differentiated, two that are consistently two-nerved and one taxon that is variably one-nerved, two-nerved or both within a single plant. The first two-nerved taxon, characterised by smaller phyllodes, matches the type specimen of A. verniciflua. The second two-nerved taxon, characterised by large phyllodes, is apparently endemic to Mt William. The third taxon, with variable main nerve number, also has large phyllodes, and combines large phyllode variant A. leprosa (Wilhelmina Falls) and southern variant A. verniciflua (Kinglake) individuals. The number of main nerves per face in phyllodes is not a useful taxonomic character for sectional classification of Acacia. Although it has clearly proved functional in some instances, the character appears too variable to reliably define natural monophyletic groups. Anatomical features such as cell number of resinous glands and staining patterns proved to be informative.
Introduction
Acacia subgenus Phyllodineae (c. 960 species) is typified by mature phyllodinous foliage in the majority of species, although 69 taxa have bipinnate mature foliage (Maslin et al. 2003). Phyllodes have a broad morphological range, from which many taxonomically significant characters have been investigated (e.g. Vassal 1972; Pedley 1978).
Seven sections, largely based on phyllode nervature and inflorescence arrangement, are recognised within subgenus Phyllodineae (Pedley 1978). However, recent phylogenetic analysis has found that only one of these sections, Lycopodiifoliae (c. 17 species), is monophyletic (Murphy et al. 2000, 2003). Two of the largest sections are Phyllodineae (c. 408 species; Maslin 2001a ), also known as Uninerves (Bentham 1875), and Plurinerves (c. 212 species; Maslin 2001a ). Taxa in section Phyllodineae have phyllodes with one longitudinal nerve per face, while those ascribed to section Plurinerves have phyllodes with more than one longitudinal nerve per face.
A phyllode usually consists of a pulvinus and photosynthetic region, although it can be sessile, decurrent with the stem, or reduced to scales. The photosynthetic region is highly variable and ranges from vertically flattened, through terete, quadrangular and triquetrous to horizontally flattened. Phyllodes usually possess at least one extra-floral nectary on the adaxial nerve, and sometimes up to five. Boughton (1981, 1985) observed three types of extra-floral nectaries. She also investigated the indumentum and found almost all species have two kinds of trichomes, one glandular and one non-glandular (Boughton 1989). According to Arber (1918), the chief anatomical feature by which phyllodes differ from true leaf laminae is the occurrence of two opposing series of vascular bundles.
Nerve number is of doubtful merit for classification, both because of contradictory results from molecular phylogenies, and observations of variable nerve numbers within taxa, populations, and even individuals. To date there has been little research on the development of vasculature in phyllodes for any species of subgenus Phyllodineae, except those species with verticillate phyllodes in sections Lycopodiifoliae and Phyllodineae (Rutishauser and Sattler 1986). Despite this lack, several apparent trends have been revealed through morphometric taxonomic investigations. There appears to be a relationship between phyllode breadth and longitudinal nerve number in plurinerved species such as Acacia longifolia Andrews (Willd.) (Boke 1940; Pedley 1978). The venation of uninerved species is also affected by increasing phyllode breadth in species such as A. decora Rchb., which has more lateral nerves in broader phyllodes (Pedley 1977). This variation has been observed within and between populations and within individuals. During development, juveniles of A. stricta Andrews (Willd.) produce broader phyllodes with two nerves per face in the transition to adult, narrower, uninerved phyllodes (SK Gardner, unpubl. data). Acacia bivenosa DC. and A. verniciflua A.Cunn. also develop a second longitudinal nerve, although both are placed in section Phyllodineae and are recognised as belonging to predominately uninerved groups of taxa (Entwisle et al. 1996; Maslin 2001a ).
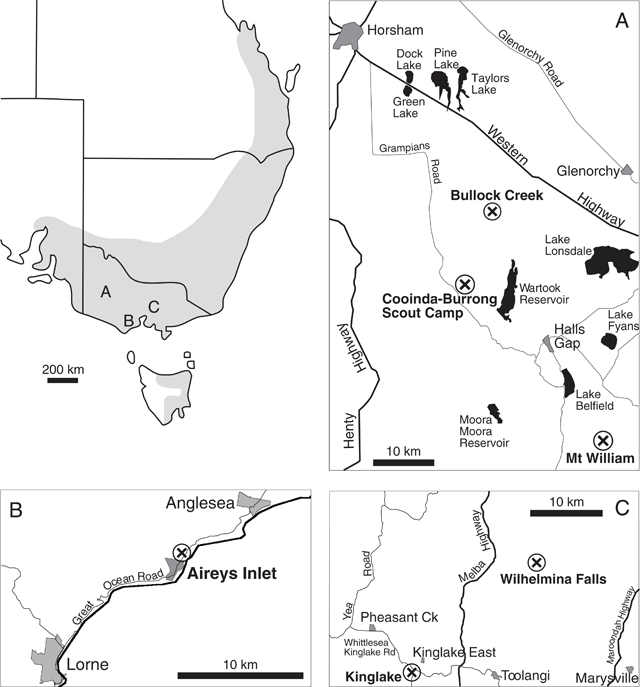
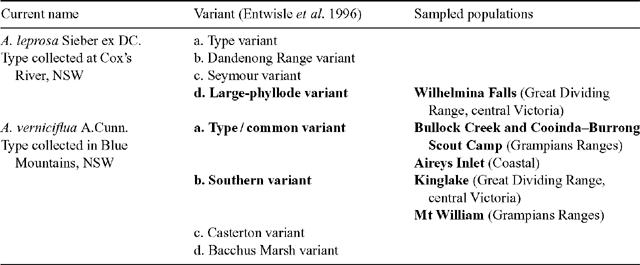
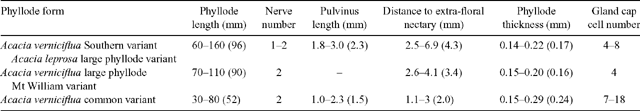
A complex of viscid Acacia taxa, including A. verniciflua (Varnish Wattle, two-nerved) and A. leprosa Sieber ex DC. (Cinnamon Wattle, primarily one-nerved), is geographically highly variable. It is a group of species believed to be closely related due to several distinctive characters (Maslin 2001a ). Acacia verniciflua and A. leprosa occur across a broadly similar range in south-eastern Australia (Fig. 1). The Flora of Victoria (Entwisle et al. 1996) and Flora of Australia (Maslin 2001b , 2001c ) treatments of A. verniciflua and A. leprosa recognise four informal variants within each species, based largely upon vegetative variation (Table 1). Murphy (1996) analysed all eight variants of A. leprosa and A. verniciflua, and their close relative A. ausfeldii Regel. Across the geographic range of the group Murphy suggested that the southern variant of A. verniciflua, with large phyllodes and two mid-nerves, was more similar to the large phyllode variant of A. leprosa with one mid-nerve, than to the other forms of two-nerved A. verniciflua. Murphy also suggested that a two-nerved, large phyllode population on Mt William in the Grampians, western Victoria, may be a distinct taxon. He recommended that the A. verniciflua and A. leprosa complex, in particular, needed further study.
|
|
Our primary aim was to use A. verniciflua and A. leprosa to test the hypothesis that nerve number is not a useful taxonomic character for sectional classification of these species, and is instead related to the growth and development of individual phyllodes. The secondary aim was to test the relationship between the large phyllode forms (A. verniciflua southern variant and A. leprosa large phyllode variant sensu Flora of Victoria; Entwisle et al. 1996) found within each taxon. To do this, populations were compared on the basis of morphological measurements and AFLP analysis. Phyllode anatomy and development were studied by light and scanning electron microscopy.
Materials and methods
Plant materials
Six populations (Fig. 1) were selected to best represent the variation in large-phyllode forms, size and nervature (Table 1), as identified by Murphy (1996) and Entwisle et al. (1996). Generally, ten individuals were sampled from each population, with additional samples collected to obtain sufficient developmental stages of foliage for microscopy. Stages of plant material included from vegetative buds to mature phyllodes. A voucher specimen was collected for each population and lodged at the University of Melbourne Herbarium (MELU). The Mt William population, where plants had suffered from heavy herbivory, was augmented by herbarium specimens and pickled material available at MELU.
Morphometrics
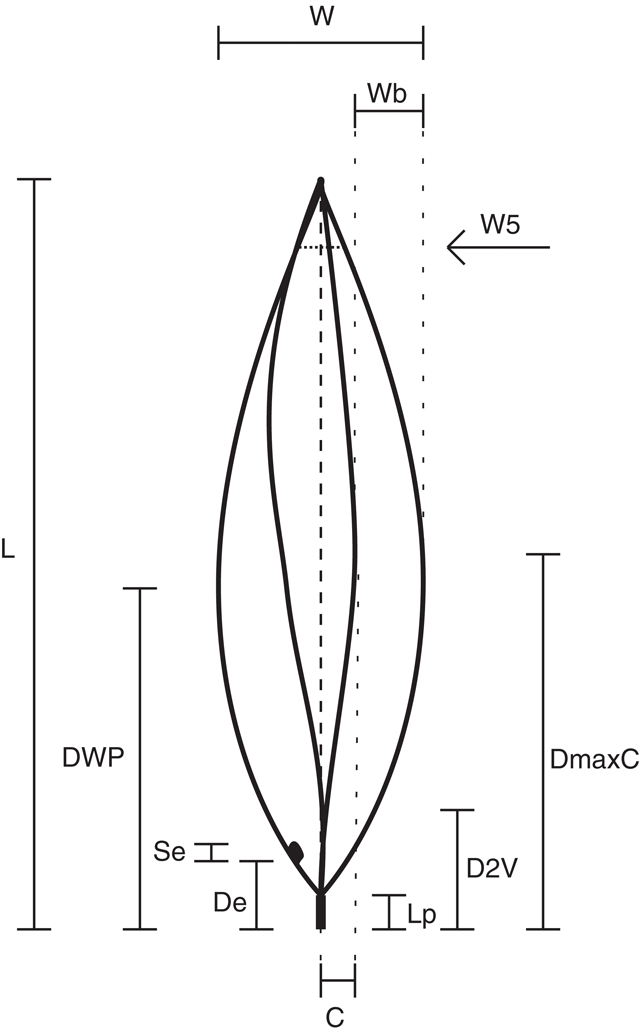
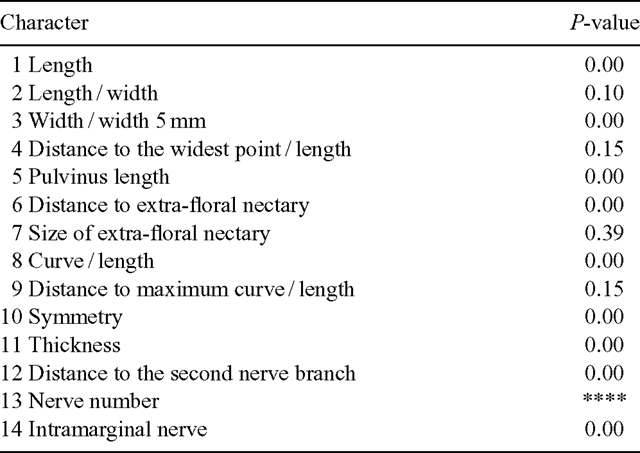
Morphological measurements were made of phyllodes to characterise and compare each population. To standardise sampling the last five phyllodes of the previous year’s growth on a single stem were selected from each plant. These were pressed and dried before being measured. Twelve characters were recorded for each phyllode (Fig. 2, Table 2). Characters 2, 3, 4, 8, 9 and 10 are ratios, which give measures of the shape and relative position of phyllode features as described. Lamina thickness was measured at the widest point, on the abaxial side of the mid-nerve. Where necessary, a portion of the abaxial nerve was removed with a razor blade to ensure that only the lamina thickness was measured. A smaller character set was used to incorporate the data from the previous investigation of Murphy (1996), in which characters 5, 7, 8, 9, 10 and 14 (Table 2) were not scored; it was not possible to measure these from herbarium material (e.g. herbivory, size of extra-floral nectary reduced by severe drying).
|

|
Microscopy
Fresh material from each population was fixed in FAA (70% ethanol, 10% formalin, 5% acetic acid, 15% distilled water v / v) for microscopy. Prior to use, fixed phyllodes were washed and stored in 70% ethanol.
The basic fuchsin technique (Fuchs 1963), which stains only lignified elements, was used to investigate the branching patterns of vascular bundles. Samples were rehydrated, stained, and unstained tissues were cleared with a concentrated NaOH solution. Samples were treated using a graded ethanol series to remove excess stain and for dehydration. Dehydration and destaining usually required two weeks to complete, although buds often required more than a month. A final bleaching step in hydrochloric acid was unnecessary because the cleared tissue was translucent.
Dehydrated material was infiltrated with a graded London Resin (LR) white series. Samples were embedded in gelatin capsules and sectioned on a heavy-duty motorised microtome with Ralph-type glass knives. Sections cut at 2 μm thick provided sufficient tissue for strong staining with 0.01% toluidine blue in 0.05% acetate buffer (pH 4.4).
To remove resin from the viscid phyllodes of A. verniciflua and A. leprosa for scanning electron microscopy, samples were rotated gently in 100% ethanol, replaced every two days, for three weeks. Samples were critical-point dried, mounted on stubs and coated with gold with an Edwards S150B sputter coater. Specimens were photographed with a Philips XL 30 Field Emission Scanning Microscope (FEG SEM).
DNA extraction
Several phyllodes from each sampled plant were collected in the field at all six localities and stored on silica gel. Genomic DNA was isolated from 20 mg of dried phyllode tissue with the DNeasy Plant Minikit (Qiagen, Valencia, CA) following the manufacturer’s protocol. Concentrations of DNA in the resulting samples were quantified by agarose gel electrophoresis with a quantitative ladder (Low DNA Mass Ladder; Invitrogen, Carlsbad, CA). DNA quality was tested on a subset of samples by restriction enzyme digestion (EcoRI). The completeness of the digestion was assessed by agarose gel electrophoresis.
AFLP procedure
Samples of ∼100 ng of DNA were used for AFLP analysis, which was performed with the AFLP Analysis System I kit and the protocol recommended by the manufacturer (Invitrogen). Genomic DNA was digested to completion with EcoRI and MseI and ligated to kit-supplied adapters. Two rounds of selective PCR were performed. The first round used the primers supplied by the manufacturer, EcoRI+A and MseI+C. The second round of amplification was performed with primers (not supplied in the kit) that had a greater number of selective bases but whose first selective base matched those of the preamplification.
Preliminary screens of various selective primers found four selective bases to be necessary for both primers to reduce the number of fragments to a level that enabled scoring. Two primer combinations were used, EcoRI+ACGT with either MseI+CTAT or MseI+CTAG. The EcoRI+4 primers used in the selective step were radioactively end-labelled with [γ-33P]-ATP (Amersham, Piscataway, NJ) and T4 polynucleotide kinase (Promega, Madison, WI). PCR products from the second amplification were mixed with an equal volume of sequencing loading buffer [98% (v / v) formamide, 10 mm EDTA, 0.025% (w / v) xylene cyanol, 0.025% (w / v) bromophenol blue]. This also acted as a ‘stop’ buffer, halting the action of any enzymes in the solution, protecting the replicated DNA fragments from degradation. Mixtures were heated for 3 min at 90°C, cooled on ice, and 4 μL of each sample was electrophoresed in a 5% denaturing polyacrylamide sequencing gel (Cambrex BioScience, Mt Waverly, Vic.) at 70 W for ∼3 h. Gels were attached to filter paper and heat dried under a vacuum in a Bio-Rad Model 483 slab dryer (Bio-Rad, Hercules, CA). Dried gels were exposed to X-ray film (X-OMAT AR, Kodak, Tokyo, Japan) for approximately 4 days at room temperature.
Preliminary screening of 12 primer combinations provided two primer pairs that gave clear and reproducible banding patterns for most samples. These pairs differed by one nucleotide base in one of the primers and yet resulted in different banding patterns.
|
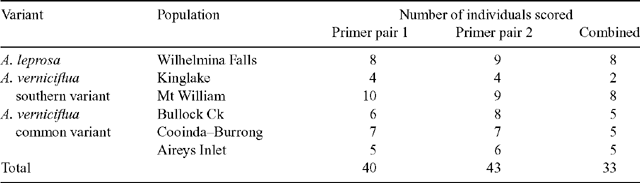
At least ten samples from each population were analysed, 67 in total. No bands were shared by all individuals from all populations. Individuals were omitted from the overall analysis if the banding pattern for either primer pair could not be scored. The number of individuals successfully scored for each population is shown in Table 3. Primer pair 2 had more than twice the number of scorable bands as primer pair 1 (151 v. 67). This discrepancy meant that in the combined analysis (data from both primer pairs), the overall pattern was dominated by primer pair 2.
Data analysis
Data matrices of individual plants scored for morphological or AFLP characters were subjected to multivariate pattern analysis with the software package PATN (Belbin 1995), for both agglomerative hierarchical classification and ordination. Dissimilarity matrices were constructed for both datasets, each using a different distance measure. Gower metric was used for the morphometric data because it allows binary characters to be used while remaining robust for continuous characters (Gower 1971). The Jaccard coefficient (Jaccard 1908 in Sneath and Sokal 1973) was used for the AFLP data, because it is designed for binary data and does not treat dual absence of a character as a similarity between the individuals.
The fusion strategy used for cluster analysis was the Unweighted Pair Group Method of arithmetic Averages (UPGMA). Ordination was performed by Non-metric Multidimensional Scaling (NMDS), which is an iterative process using a steepest descent algorithm to minimise stress, the deviation between the original distance matrix and the expression of those distances in the reduced number of dimensions (Quinn and Keough 2002).
Analysis of variance could not be used to test the significance of characters between clusters in the dendrograms as homogeneity of variance is one of the basic assumptions of ANOVA tests. Kruskal–Wallis was chosen because it is a non-parametric test and does not compare the variances (Quinn and Keough 2002). The results of this test are probability (P) values, or confidence values.
Results
Morphometrics comparison of populations
UPGMA classification, based on all phyllode characters and 51 individuals excluding Mt William shows two major groups (Fig. 3) consisting of large v. small phyllode individuals. Group 1, the large phyllode group, has two subgroups that correlate to one-nerved (A) and two- or variable-nerved (B) individuals. Table 4 summarises the Kruskal–Wallis significance values between the groups for each character. Group 1A consists of individuals from the A. leprosa population at Wilhelmina Falls that have one nerve, although one of the individuals (SG5) had a single phyllode with two nerves. Group 1B includes all plants of the large phyllode form initially identified as A. verniciflua from Kinglake, as well as two two-nerved individuals (SG4, SG6) and a variable-nerved individual (SG8) from Wilhelmina Falls. Group 2 comprises all individuals of the common variant of A. verniciflua from the Grampians Ranges and Aireys Inlet, which have smaller phyllodes than groups 1A and B. The two main groups were distinguished by phyllode length (1), tip shape (3), pulvinus length (5), extra-floral nectary position (6), curvature (8), lamina symmetry (10) and thickness (11; Table 3). Ordination of the 51 individuals shown in three dimensions (Fig. 4; stress value 0.09) confirmed the groups found in the cluster analysis.
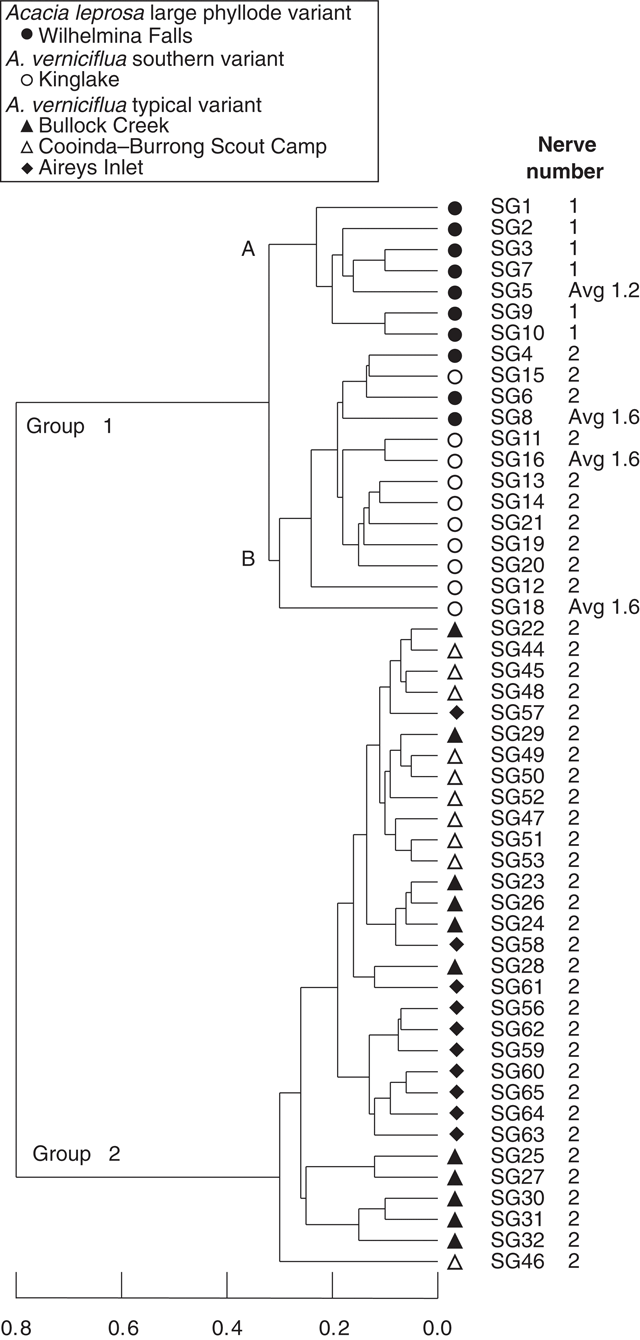
|
|
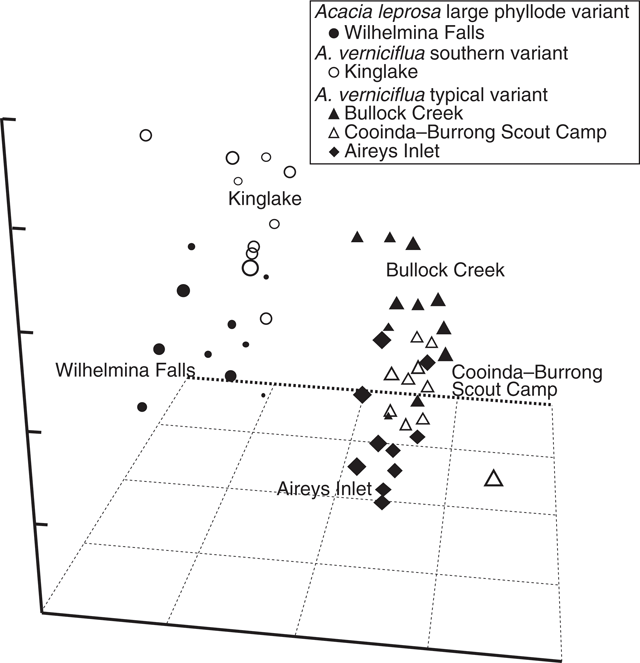
|
A second analysis of 56 individual plants included data for five herbarium specimens (MELU) from Mt William (Fig. 5), and excluded characters 5, 7, 8, 9, 10 and 14 (Table 2) as described in the methods section. The pattern of group composition in the dendrogram is the same as for the total character set (Fig. 3). The large phyllode, two-nerved individuals from Mt William cluster with the other large phyllode, two-nerved plants in group 1B from Kinglake and Wilhelmina Falls. Ordination (not shown) confirmed the three groups and overlap of Mt William with Kinglake individuals in Group 1B.
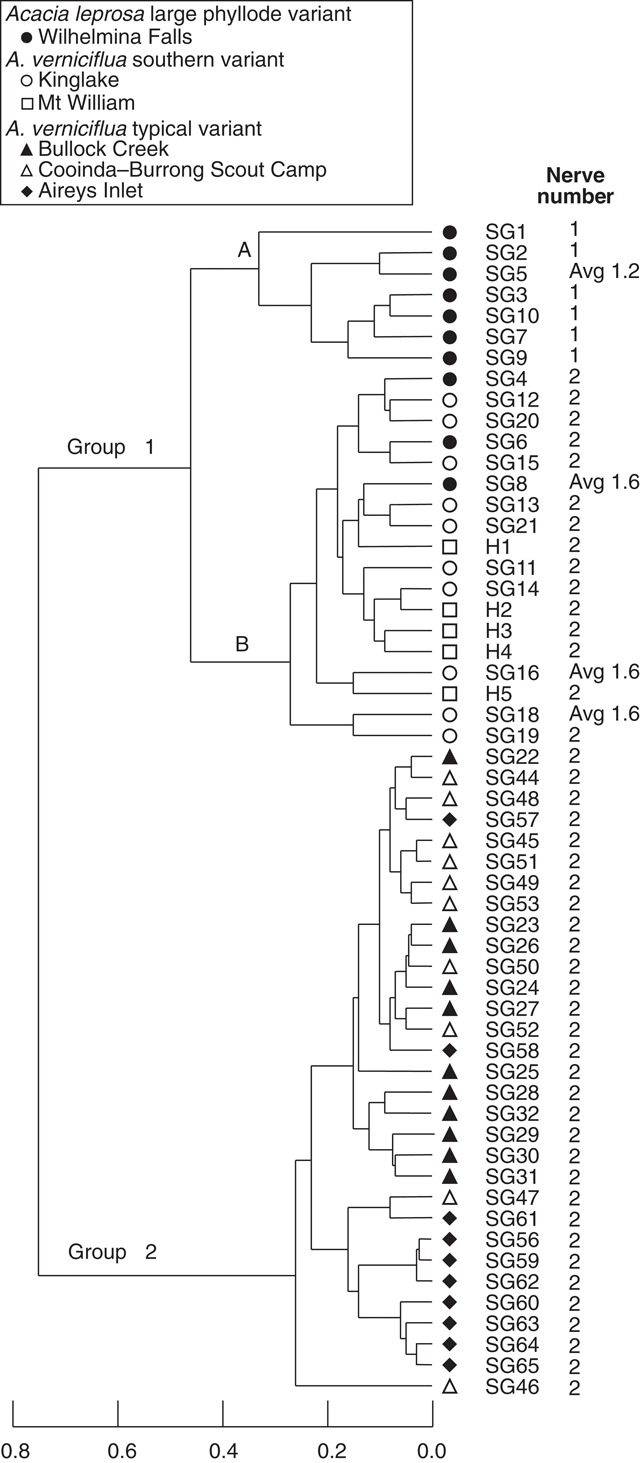
|
A third analysis (Fig. 6) excluded the three characters relating to nerve features (12, 13 and 14; Table 2). Their removal from the analysis resulted in the loss of distinction between groups 1A and 1B. As in Fig. 3, group 1 includes all individuals with large phyllodes irrespective of nerve number (Wilhelmina Falls, Kinglake and Mt William populations) and group 2 included all of the samples of the small phyllode, common variant of A. verniciflua (Bullock Creek, Cooinda–Burrong and Aireys Inlet). Characters 1, 3, 6 and 11 (Table 2) supported these clusters, as in the other analyses, but notably no characters supported the separation by nerve number except the nerve characters themselves.
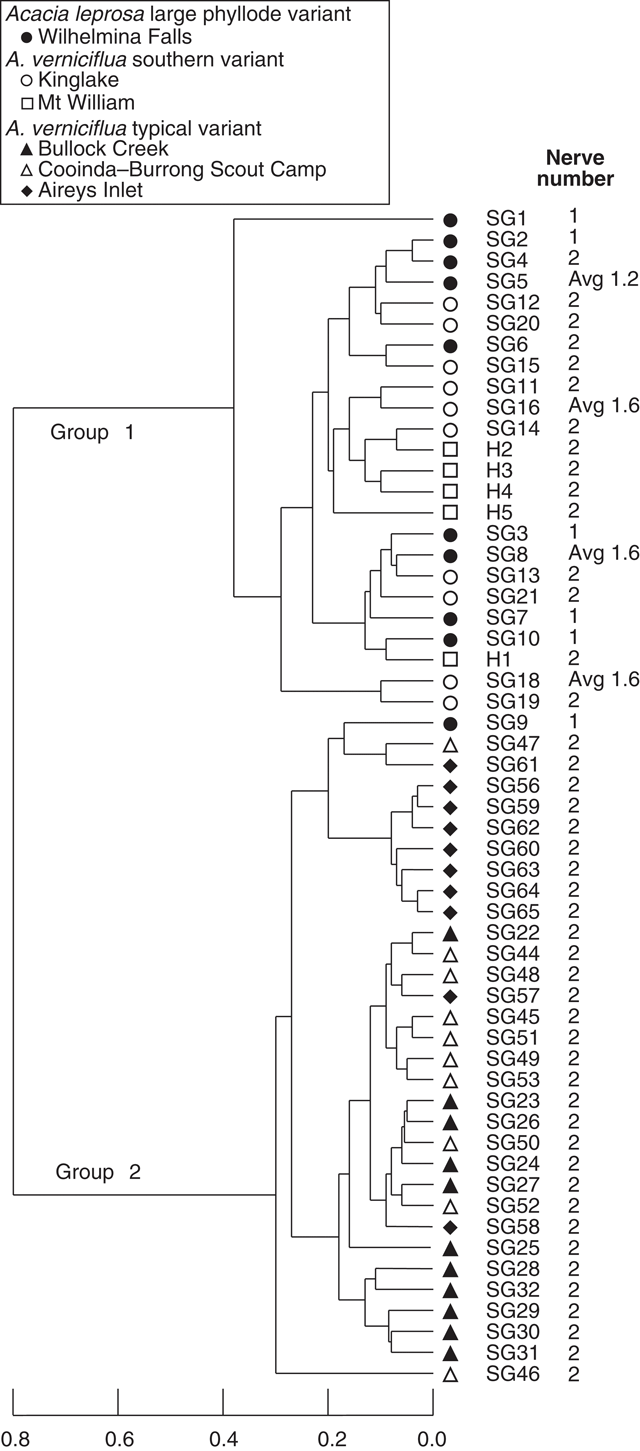
|
Phyllode anatomy
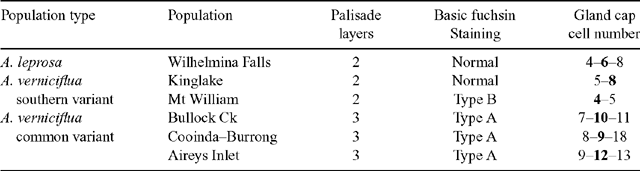
Table 5 is a summary of anatomical characters characterising the populations sampled. Phyllodes from the common variant of A. verniciflua were generally thicker but more variable than the others. Cross-sections of mature phyllodes show that this greater thickness can be attributed to an extra layer of palisade mesophyll (Fig. 7c, f ). Phyllodes typical of A. leprosa and the large phyllode form of A. verniciflua from Kinglake and Mt William have two layers of palisade mesophyll, whilst the common variant of A. verniciflua has three layers of palisade mesophyll.
|
|
All phyllode forms have relatively continuous palisade mesophyll, with only the major nerves and associated fibre caps interrupting the lamina completely to the epidermis (Fig. 7b, e ). In the large phyllode A. verniciflua, the secondary mid-nerve also totally interrupts the mesophyll. The second nerve is of similar size in the common variant of A. verniciflua but only partially interrupts the mesophyll owing to the greater thickness of the phyllode.
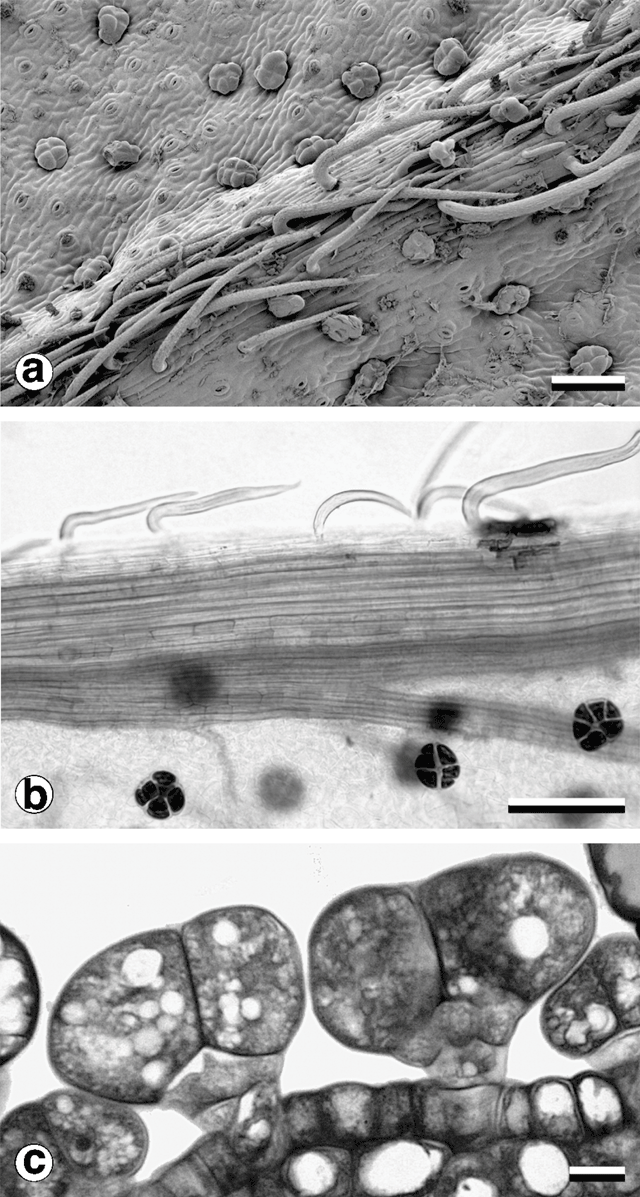
Consistent with other observations among Acacia species (Boughton 1986) and in the varnish wattle complex (Murphy 1996), two trichome types were observed (Fig. 8). Non-glandular trichomes in all populations sampled were unicellular, with a very short stalk segment bent sharply close to the location of attachment to the phyllode. Their distribution was restricted to stems, pulvinus and major longitudinal nerves. In two-nerved specimens, non-glandular trichomes were also found on the secondary mid-nerve. Non-glandular trichomes were not found on any other vascular bundles, even those that approached the mid-nerves in size, such as the intramarginal nerves found in some plants.
|
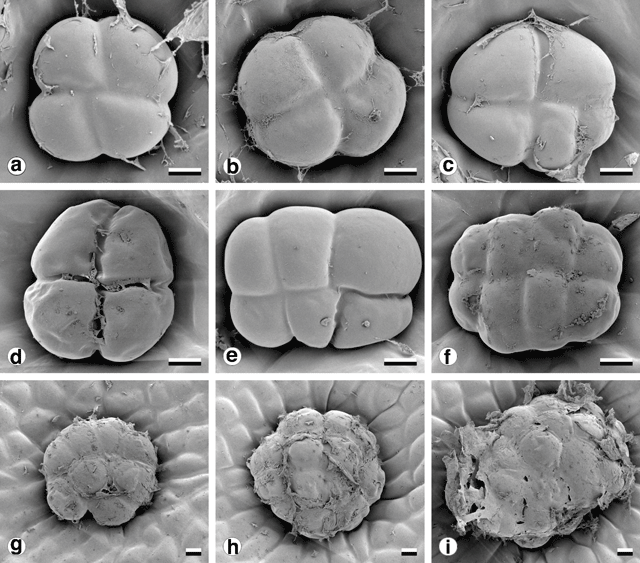
Glandular trichomes were found on all vegetative surfaces, and were fully developed very early in phyllode differentiation. They have a uniseriate stalk with a head consisting of four or more ‘cap’ cells divided vertically (Fig. 8). On mature phyllodes they occur in depressions on the phyllode surface. The number of cap cells on glandular trichomes was variable, but significantly different for each form (Fig. 9 and Table 5). There was also a notable change in the arrangement of cap cells as the number increased. Four cap cells were always arranged in a circle and were typical of the Mt William population (Fig. 9a, b ). In only one observed instance did a glandular trichome from the Mt William population have more than four cap cells (Fig. 9c ). Five to eight cap cells were arranged in two rows (Fig. 9e, f ) and were typical of both one- and two-nerved, large phyllode forms of A. leprosa (Wilhelmina Falls) and A. verniciflua (Kinglake), although they did range as low as four (Fig. 9d ). Nine or more cap cells were sometimes disorganised in appearance (Fig. 9g, i ) but were usually arranged in two tiers. The upper tier consisted of four evenly arranged cells, with a lower tier surrounding these that consisted of the remainder of the cap cells (Fig. 9h ). This latter type was found in the common variant populations of A. verniciflua, from Aireys Inlet and the Grampian Ranges.
|
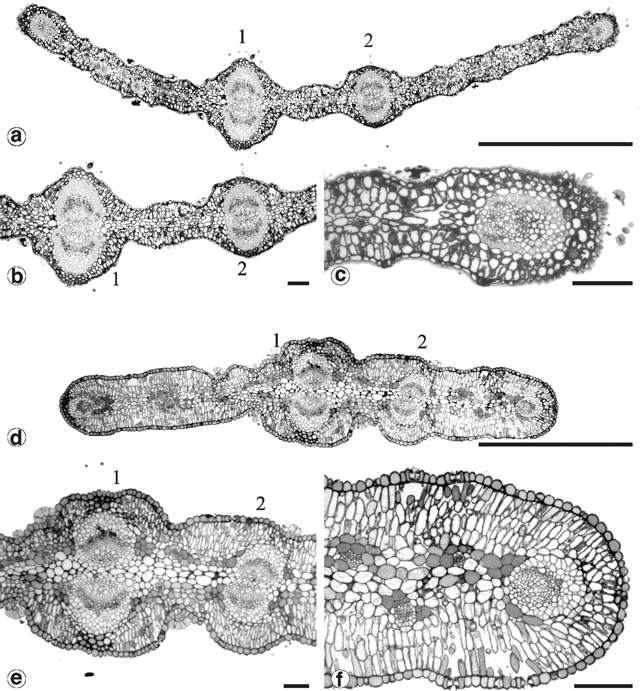
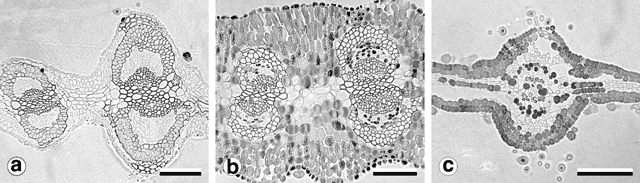
Phyllodes of the populations from Kinglake and Wilhelmina Falls stained and cleared as expected using the basic fuchsin technique (Fig. 10a ). In these phyllodes only the cell walls of the vascular bundle fibre caps and the contents of the cap cells of the glandular trichomes were stained (Fig. 11a ). Phyllodes from other populations stained throughout their entire lamina (Fig. 10b ). This prevented investigation and comparison of the vasculature, but was a significant result. Sections of these solidly-stained phyllodes elucidated two distinct patterns. In phyllodes from the common variant of A. verniciflua the contents of all cell types stained, except the phloem and the vascular cambium (Fig. 11b ). The darkest stained regions were the epidermal layer, the isodiametric cells between the epidermis and the vascular bundle fibre caps, and individual parenchyma cells. This staining pattern is termed type ‘A’. The large phyllode form of A. verniciflua from Mt William possessed a unique pattern of staining (Fig. 11c ). Two distinct layers of cells stained in the palisade mesophyll, as well as individual parenchyma cells. The parenchyma cells adjacent to the phloem stained darkest. All other cell types disintegrated, including the vascular bundle fibre caps.

|
|
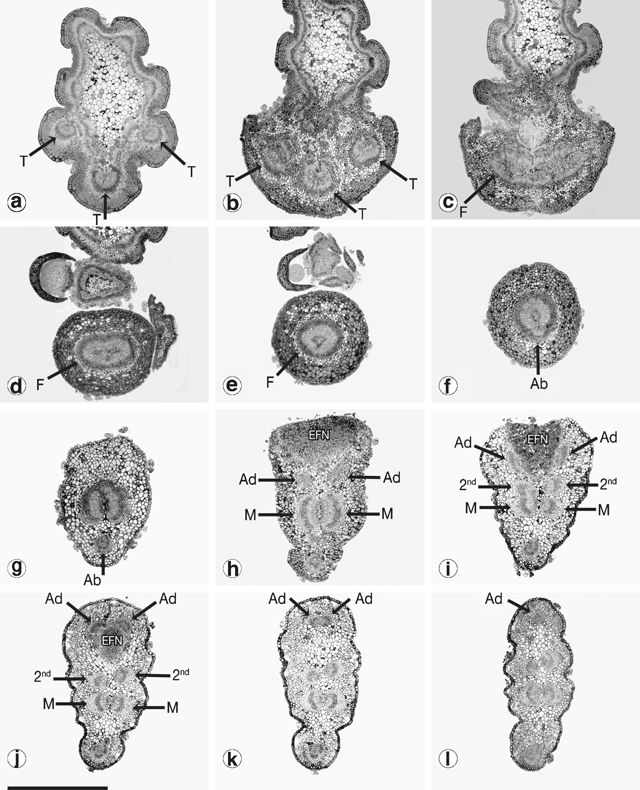
It was not possible to compare the development of the vasculature between phyllodes using Fuchs’ staining and clearing technique. Serial cross-sections for a selected number of populations were attempted instead. Figure 12 shows the sequence of vasculature changes in the common variant of A. verniciflua. Three vascular traces emerge from the stele at the initiation point of the phyllode (Fig. 12a, b ). The traces fuse into a ring as they enter the pulvinus (Fig. 12c, d, e ). At the distal end of the pulvinus, the adaxial marginal nerve branches off from the ring leaving a U-shaped bundle (Fig. 12f ). Moving away from the base of the phyllode, the large bundle splits into two opposing nerves, and a smaller bundle branches off from each of these on the adaxial edge, below the extra-floral nectary (Fig. 12g, h ). At this point there are five vascular bundles in the phyllode. The two new branches run separately along the adaxial margin, around either side of the extra-floral nectary, and then fuse to form the adaxial marginal nerve (Fig. 12h, l ). The two opposing bundles forming the major nerve in the middle of the phyllode each branches off a slightly smaller bundle that, opposing each other, become the secondary mid-nerve (Fig. 12i, j ). Therefore, as shown in Fig. 12l , just beyond the extra-floral nectary, all of the major nerves are already formed, including the secondary mid-nerve.
|
Genetic variation
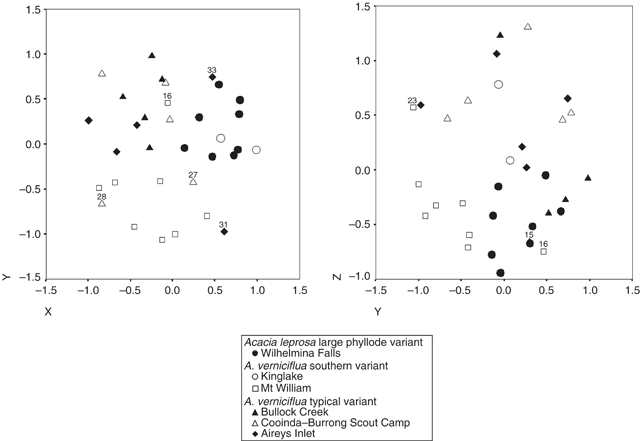
Ordination of the combined data of both primer pairs showed weak clustering into three groups (Fig. 13). The stress value for the ordination was high (0.24), indicating that the pattern is not a very reliable representation of the data. One group includes all of the individuals of the common variant of A. verniciflua (small phyllode, two-nerved) from Aireys Inlet, Bullock Creek and Cooinda–Burrong Scout Camp (Grampians Ranges). Another group includes all of the individuals of A. leprosa and A. verniciflua (large phyllodes, one- or two-nerved) from the populations at Wilhelmina Falls and King Lake. The final group is made up exclusively of large phyllode, A. verniciflua individuals from Mt William and is the most distinct.
|
Discussion
Phyllode variation
The morphometric analyses support the need for redefinition of the large phyllode taxa within the varnish wattle complex. The large phyllode variant of A. leprosa and the southern variant of A. verniciflua (Entwisle et al. 1996) are one taxon, regardless of nerve number. It is notable that the removal of qualitative nerve characters from the multivariate analysis resulted in the two subgroups of large phyllode individuals intergrading (see Fig. 6). The most significant phyllode characters shared by these forms are phyllode length, pulvinus length, acuteness of the phyllode tip, position of the extra-floral nectary, lamina symmetry around the mid-nerve, phyllode thickness and the distance to the branching point of the second nerve (where one is present). Populations of this taxon occur in similarly high rainfall, mesic habitats in the Great Dividing Range, north-east of Melbourne. These forms also had glandular trichomes with between four and eight cap cells in two rows, and stained as expected using the basic fuchsin technique (vascular bundles and cap cell contents only).
The common variant of A. verniciflua, sampled from several populations, ranging from inland in the Grampians Ranges to the coast at Aireys Inlet, have smaller phyllodes, always with two nerves. The common variant of A. verniciflua occurs in areas of lower rainfall than the large phyllode forms, and is the variant that most closely matches the holotype specimen of A. verniciflua held at KEW (Maslin 2001b ). Glandular trichomes of the common variant of A. verniciflua all had between 8 and 13 cap cells, either in the two-tier arrangement or disorganised. Following the basic fuchsin technique, phyllodes of the common variant of A. verniciflua all had stain in the contents of the palisade and spongy mesophyll.
The Mt William population has phyllodes of similar size to Kinglake and Wilhelmina Falls. It differs in having the lowest number of cap cells, four, as well as the smallest amount of variation in cap cell number, with only an occasional glandular trichome possessing five. The Mt William population was also distinct in its basic fuchsin staining pattern, in that only the contents of the palisade mesophyll stained. Cap cell number suggests affinity of the Mt William population to the large phyllode variant of A. leprosa. The staining pattern, in contrast, implies a closer affinity of the Mt William population to the common variant of A. verniciflua, including populations from sites within the Grampians.
The basic fuchsin staining demonstrated the same distinct groups as defined by the glandular trichome cap cells, notably distinguishing the Mt William population from all others investigated. The darkly stained substance was unusual, and had not been recorded previously. Histochemical analysis may provide information on the nature of this substance and screening other species and variants in the varnish wattle complex may further clarify taxa and their relationships.
Genetic analysis
The analysis of total genetic dissimilarity lends support to the results of both morphometric and anatomical investigations. Few of the large phyllode individuals of A. verniciflua from Kinglake could be scored accurately, but those that were clustered most closely to the A. leprosa individuals from Wilhelmina Falls. The large phyllode individuals of A. verniciflua from Mt William were confirmed as a discrete cluster in all genetic analyses.
The lack of any bands shared by all individuals in any of the AFLP analyses is atypical. In a similar study of genetic variation between three subspecies of Banksia integrifolia L.f., Evans et al. (2002) found that between 25% and 50% of scorable bands were monomorphic across all individuals, and confirmed the subspecies. The Grampians Ranges have a history of isolation, and a high level of endemism (Elliot 1975), which may explain the lack of any consistent AFLP markers across the populations. The high level of dissimilarity is consistent with both primer pairs, and supports the distinction of the Mt William form, and the remaining large phyllode individuals (Kinglake and Wilhelmina Falls) as two distinct taxa.
Phyllode development
The investigation into the branching of vascular bundles led to several conclusions concerning the homology of phyllodes. Previous approaches, such as basic anatomy and inferences from the sequence of heteroblastic leaf development in acacias, have led researchers to state that the phyllode is homologous with the petiole of a bipinnate leaf (e.g. Mann 1894; Goebel 1905; Troll 1939), or with the petiole and rachis (e.g. Bentham 1875; Reinke 1897), and make comparisons with the monocotyledonous leaf. Investigating the developmental morphology of phyllodes, Kaplan (1980) proposed a new model: that the phyllode is actually the positional homologue of the lamina of a bipinnate leaf. In essence, this suggests that the phyllode is directly comparable to a simple leaf. Kaplan’s theory does not, however, address the issue of the opposing vascular bundles found in phyllodes.
The pattern of branching observed in the vascular bundles of A. verniciflua phyllodes suggests that the abaxial marginal nerve is homologous to the mid-rib in a simple leaf. This implies that laminar expansion occurs on both sides of the ‘mid-rib’, but vertically, and fused together. The emergence of the adaxial marginal nerve as two separate bundles, originating on opposing sides that eventually fuse rather than directly from the vascular ring found in the pulvinus, supports our interpretation and has been observed (together with other patterns) in several other Acacia species (von Wartburg 1991).
An in-depth investigation of the vascular development in phyllodes is required to clarify these issues further, and to determine why major nerves align opposite each other while minor nerves generally do not. Other phyllode forms need investigating, such as terete and horizontally flattened phyllodes, characteristic of some species. A comparative developmental study between different types and degrees of flattening in phyllodes may answer such questions.
Conclusion
Although taxonomy is not the primary focus of this paper, the results clarify a problematic relationship not adequately investigated elsewhere. The full taxonomic treatment of the entire varnish wattle complex is being completed by Murphy (unpubl. data). Three taxa require recognition: A. verniciflua common variant; a taxon which amalgamates A. verniciflua southern variant and A. leprosa large phyllode variant, delimited to include one-nerved, two-nerved and variable individuals; and a new taxon so far confirmed from Mt William in the Grampians. The clearest defining characters of each taxon, as recognised in this study, are summarised in Table 6. The combination of morphological and anatomical characters in the Mt William population may indicate hybrid origin; however, the AFLP data can neither prove nor disprove this hypothesis. The Mt William population is also isolated both ecologically and geographically from all other populations of taxa in the varnish wattle complex.
|
Nerve number is not a useful character for classification of the species within the varnish wattle complex, despite its emphasis in defining sections in Acacia subgenus Phyllodineae (Bentham 1875; Vassal 1972; Pedley 1978, 1986; Entwisle et al. 1996). For the Wilhelmina Falls and Kinglake populations it proved to be variable within species as well as within populations. The population at Wilhelmina Falls, previously thought to be a large phyllode form of A. leprosa, displayed variation in nerve number: of ten plants collected two were two-nerved, and two more had one- and two-nerved phyllodes on the same branchlet. Kinglake was supposedly a two-nerved, large phyllode, A. verniciflua population yet two of the individuals there were also variable. The second mid-nerve is not, however, an irrelevant character from a developmental perspective. The occurrence of non-glandular trichomes upon the second mid-nerve and their absence from similar sized lateral nerves, strongly suggest that the development of the second mid-nerve is very similar to that of the mid-nerve and marginal nerves.
Three recent molecular phylogenies of Acacia (Murphy et al. 2000, 2003; Miller et al. 2003) have found no sections of subgenus Phyllodineae (except section Lycopodiifoliae) to be monophyletic. The majority of species ascribed to section Plurinerves are usually nested within members of both sections Juliflorae and Phyllodineae, while the members of section Phyllodineae are scattered across several clades. Clearer understanding of the morphological development of structures, such as phyllode vasculature, is essential to develop an accurate infra-generic classification of Acacia.
Arber A
(1918) The phyllode theory of the monocotyledonous leaf, with special reference to anatomical evidence. Annals of Botany 32, 465–501.

Bentham G
(1875) Revision of the suborder Mimoseae. Transactions of the Linnean Society of London 30, 335–664.

Boke NH
(1940) Histogenesis and morphology of the phyllode in certain species of Acacia.
American Journal of Botany 27, 73–90.

Boughton VH
(1981) Extrafloral nectaries of some Australian phyllodinous acacias. Australian Journal of Botany 29, 663–664.

Boughton VH
(1985) Extrafloral nectaries of some Australian bipinnate acacias. Australian Journal of Botany 33, 175–184.

Boughton VH
(1986) Phyllode structure, taxonomy and distribution in some Australian acacias. Australian Journal of Botany 34, 663–674.

Boughton VH
(1989) Trichomes from the foliage of some Australian acacias. Australian Journal of Botany 37, 157–168.

Evans KM,
Newbigin E, Ladiges PY
(2002) An investigation of genetic variation in Banksia integrifolia (Proteaceae) by the use of the AFLP technique. Australian Systematic Botany 15, 9–17.
| Crossref | GoogleScholarGoogle Scholar |

Fuchs C
(1963) Fuchsin staining with NaOH clearing for lignified elements of whole plants or plants organs. Stain Technology 38, 141–144.

Gower JC
(1971) A general coefficient of similarity and some of its properties. Biometrics 27, 857–871.

Kaplan DR
(1980) Heteroblastic leaf development in Acacia: morphological and morphogenetic implications. La Cellule 73, 135–203.

Maslin BR,
Miller JT, Seigler DS
(2003) Overview of the generic status of Acacia (Leguminosae: Mimosoideae). Australian Systematic Botany 16, 1–18.
| Crossref | GoogleScholarGoogle Scholar |

Miller JT,
Andrew RL, Bayer RJ
(2003) Molecular phylogenetics of the Australian acacias of subg. Phyllodineae (Fabaceae: Mimosoideae) based on the trnK intron. Australian Journal of Botany 51, 167–177.
| Crossref | GoogleScholarGoogle Scholar |

Murphy DJ,
Miller JT,
Bayer RJ, Ladiges PY
(2003) Molecular phylogeny of Acacia subgenus Phyllodineae (Mimosoideae: Leguminosae) based on DNA sequences of the internal transcribed spacer region. Australian Systematic Botany 16, 19–26.
| Crossref | GoogleScholarGoogle Scholar |

Murphy DJ,
Udovicic F, Ladiges PY
(2000) Phylogenetic analysis of Australian Acacia (Leguminosae: Mimosoideae) by using sequence variations of an intron and two intergenic spacers of chloroplast DNA. Australian Systematic Botany 13, 745–754.
| Crossref | GoogleScholarGoogle Scholar |

Pedley L
(1977) Notes on Leguminosae, I. Austrobaileya 1, 25–42.

Pedley L
(1978) A revision of Acacia Mill. in Queensland. Austrobaileya 1, 75–234.

Pedley L
(1986) Derivation and dispersal of Acacia (Leguminosae), with particular reference to Australia, and the recognition of Senegalia and Racosperma.
Botanical Journal of the Linnean Society 92, 219–254.

Reinke J
(1897) Untersuchungen über die Assimilationsorgane der Leguminosen. Jahrbücher für wissenschaftliche Botanik 30,

Rutishauser R, Sattler R
(1986) Architecture and development of the phyllode–stipule whorls of Acacia longipedunculata: controversial interpretations and continuum approach. Canadian Journal of Botany 64, 1987–2019.

Vassal J
(1972) Apport des recherches ontogéniques et sé)minolgiques a l’étude morphologique, taxonomique et phylogénique du genre Acacia.
Bulletin de la société d’histoire naturelle de Toulouse 108, 1–115.

von Wartburg J
(1991) Phyllode venation and anatomy in Australian Acacias: preliminary results. Bulletin of the International Group for the Study of Mimosaceae 19, 96–127.



