Morphometric study of Euchiton traversii complex (Gnaphalieae: Asteraceae)
Christina Flann A B E F , Ilse Breitwieser C , Josephine M. Ward D , Neville G. Walsh B and Pauline Y. Ladiges AA School of Botany, The University of Melbourne, Vic. 3010, Australia.
B National Herbarium of Victoria, Royal Botanic Gardens Melbourne, Birdwood Avenue, South Yarra, Vic. 3141, Australia.
C Allan Herbarium, Landcare Research, Lincoln 8152, New Zealand.
D School of Biological Sciences, University of Canterbury, Christchurch, New Zealand.
E Present address: Nationaal Herbarium Nederland, Wageningen Branch, 6703 BL, Wageningen, The Netherlands.
F Corresponding author. Email: christinaflann@gmail.com
Australian Systematic Botany 21(3) 178-191 https://doi.org/10.1071/SB08005
Submitted: 23 January 2008 Accepted: 20 March 2008 Published: 20 August 2008
Abstract
A morphometric study was undertaken into alpine and subalpine species of Euchiton Cass. (Gnaphalieae: Asteraceae) in the Euchiton traversii species complex in south-eastern Australia and New Zealand. Phenetic analysis of both field-collected and herbarium specimens resolved the following six taxa included: Euchiton traversii (Hook.f.) Holub, Euchiton argentifolius (N.A.Wakef.) Anderb., Euchiton lateralis (C.J.Webb) Breitw. & J.M.Ward, Argyrotegium mackayi (Buchanan) J.M.Ward & Breitw., Argyrotegium fordianum (M.Gray) J.M.Ward & Breitw. and Argyrotegium poliochlorum (N.G.Walsh) J.M.Ward & Breitw. The results support the segregation of the genus Argyrotegium J.M.Ward & Breitw. from Euchiton. E. argentifolius is distinct from E. traversii, but conspecific with A. mackayi. E. lateralis is present in Tasmania as well as New Zealand. The distribution of Australian E. traversii is redefined to mainland alpine regions with a few rare occurrences in Tasmania. Australian E. traversii was shown to be similar to its New Zealand counterparts. Differences between E. lateralis and E. traversii were clarified. A. fordianum and A. poliochlorum are distinct species and their transfer to Argyrotegium is supported.
Introduction
Delimitation of several species of Euchiton is difficult. Several taxonomic treatments have made progress, notably Drury (1972) and P. G. Wilson (unpubl. data); however, the E. traversii complex of alpine taxa in Australia and New Zealand is unresolved. Six species are included in this complex, spanning a generic split (Ward et al. 2003). They are all small herbs with attractive silver foliage, often forming mats or clumps.
In New Zealand, the following three species from this complex are recognised in the Flora of New Zealand (Webb 1988a): E. traversii, E. lateralis and E. mackayi; the last species has recently been transferred to the new genus Argyrotegium as A. mackayi (Ward et al. 2003). In Australia, the following four species from this complex are currently recognised: E. traversii, E. argentifolius, E. poliochlorus and E. fordianus (Everett 1992; Buchanan 1999; Walsh 1999a); the last two have been transferred to Argyrotegium as A. poliochlorum and A. fordianum (Ward et al. 2003). The superficial similarities between these taxa have made identification difficult.
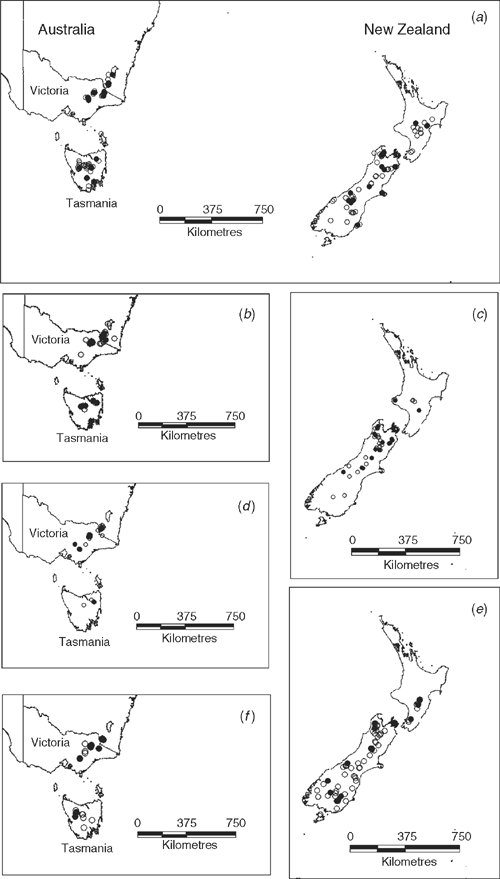
The most problematic taxa are E. argentifolius and E. traversii, the core species of the complex. Material from both taxa has been referred to the ‘E. traversii complex’ by Paul G. Wilson on herbarium determination slips. Gnaphalium traversii was published by J. D. Hooker (1867) on the basis of New Zealand material. This species is currently recorded as occurring in both New Zealand and Australia (Fig. 1a; Curtis 1963; Webb 1988a; Hnatiuk 1990; Everett 1992; Kirkpatrick 1997; Walsh 1999a). It is a stoloniferous plant with silver leaves in basal rosettes and with solitary capitula.
|
Euchiton argentifolius, described as Gnaphalium argentifolium by Wakefield (1957), has been recorded only from Australia (Fig. 1b; Curtis 1963; Everett 1992; Walsh 1999a). It generally forms mats of silver foliage and has one to a few capitula. There is, at least superficially, a resemblance between this species and E. traversii. The generic positon of E. argentifolius with regard to the new genus Argyrotegium also requires clarification (Ward et al. 2003).
In New Zealand (Fig. 1c), E. lateralis, initially described as Gnaphalium laterale C.J.Webb (Webb 1988b), is a small stoloniferous plant with a solitary capitulum reminiscent of E. traversii. The boundary between large E. lateralis and small E. traversii has caused difficulties in identification. Webb noted, both in the description (Webb 1988b) and in the Flora of New Zealand (Webb 1988a), that some Australian material referred to G. traversii (= E. traversii) would be better placed in G. laterale (= E. lateralis).
In Australia, two species have been described that were previously referred to E. argentifolius, namely Argyrotegium fordianum and A. poliochlorum (Fig. 1d, f ). A. fordianum is the most robust of the species in this complex, with 5–15 capitula per inflorescence. Even though as a species it appears to be well delineated, is included in the present study on the basis of its previous inclusion under E. argentifolius. A. poliochlorum, the most recently described member of the complex (Walsh 1999b), differs from E. argentifolius in having larger capitula and from A. fordianum in having fewer (3–5), narrower capitula. It differs from both in having foliage that is grey-green rather than silvery-white as well as characters of the capitula.
Argyrotegium mackayi, recorded in the Flora of New Zealand (Webb 1988a) as a New Zealand endemic (Fig. 1e), was included because of its resemblance to E. argentifolius, being a small silvery-leaved herb with a solitary capitulum. Interestingly, A. mackayi has a historical connection to E. traversii. Initially, Buchanan (1882) described it as Raoulia m’kayi, although he noted that the large leaves allied it more closely to Gnaphalium. It was transferred to G. traversii as var. mackayi (Kirk 1899) where it remained until it was given specific status in Gnaphalium (Cockayne 1958). Webb (1988b), in the description of G. laterale (= E. lateralis), noted that specimens transferred to the new taxon were usually previously determined as G. mackayi (= A. mackayi) and he listed several characters for discriminating them. A. mackayi has terminal rather than lateral flowering stems and has glabrous cypselae as opposed to E. lateralis, which has cypsela hairs present.
Euchiton generally has the twin hairs characteristic of Gnaphalieae cypselae (Drury 1971; Anderberg 1991; Ward et al. 2003). The presence of paired papillae on the epidermal surface of the cypsela is restricted to Euchiton and it is one of the defining characters of the genus, clearly separating it from Argyrotegium, which has predominantly glabrous cypselae (Drury 1970, 1971, 1972; Anderberg 1991; Walsh 1999a; Ward et al. 2003; P. G. Wilson, unpubl. data). Previous work has illustrated cypselae of E. traversii (Drury 1970; Webb and Simpson 2001; Rozefelds 2001), A. mackayi (Webb and Simpson 2001) and A. poliochlorum (Rozefelds 2001), but not the other species of the E. traversii complex. Investigation of the cypsela surface of E. argentifolius should allow insight into the generic placement of the species.
This is the first study of the E. traversii complex across both Australia and New Zealand. Species delimitation of this complex across a broad geographic range is investigated by morphometric analysis, and light and scanning electron microscopy of micro-anatomical features of cypselae.
Materials and methods
Sampling
Field trips to the states of Victoria, New South Wales and Tasmania in Australia and to New Zealand were undertaken during the spring and summer of 2001–2002. Where possible, flowering material was collected for herbarium specimens, consisting of 10 rosettes for stoloniferous species, or branches for the non-stoloniferous species. Material was collected and fixed in 70% ethanol also for examination by light microscopy. More than 100 collections were made across the two countries. The collections are lodged at MEL, with duplicates at CHR. Herbarium abbreviations follow Holmgren et al. (1990).
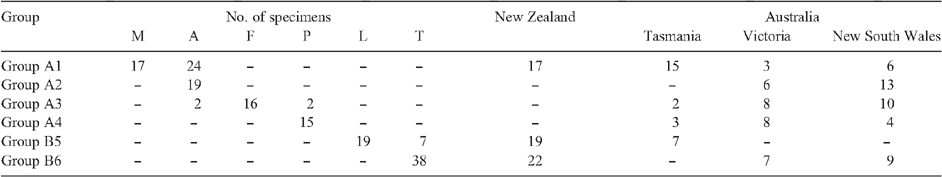
Herbarium specimens for the species in the E. traversii complex were obtained on loan to MEL from six herbaria (AD, CANB, CANU, CHR, HO, NSW). In all, 159 specimens from this herbarium material and field collections were chosen to cover the geographic range and morphological variation of the six species in the E. traversii complex (Table 1, Fig. 1). The number of specimens included per species was 45 for E. argentifolius, 23 for Australian E. traversii, 22 for New Zealand E. traversii, 19 for E. lateralis, 17 for A. mackayi, 16 for A. fordianum and 17 for A. poliochlorum.

|
Scanning electron microscopy was based on at least one specimen of each species, with duplicates for geographic regions; the inclusion of one cypsela per specimen included in the study was not possible owing to the destructive nature of the sampling. Cypselae were chosen from dried herbarium material included in the morphometric analysis. Air-dried cypselae were mounted on stubs with double-sided carbon tape. Prepared specimens were sputter-coated with gold and examined with a JEOL 840 scanning electron microscope.
Morphometric measurements
In all, 64 characters were measured for this analysis (Table 2, dataset available as an Accessory Publication on the web). Of the characters measured, 13 were binary, 35 continuous quantitative, eight multi-state and eight counts. The dataset incorporated both characters that were expected to differ between species and those that are variable within as well as between species. It is important to compare specimens at similar stages of development; hence, fruiting specimens were preferentially chosen for measurements. The peduncle elongates between flowering and fruiting; therefore, peduncle length at fruiting stage provides comparable measurements. Specimens with at least three flowering plants, preferably fruiting, were collected. For each herbarium sheet, three ramets were chosen as replicates to cover the variation and scored for all quantitative characters. Only one capitulum from each sheet was dissected because of the destructive nature of this sampling. Bracts and florets were separated individually for measurement.

|
Analysis
After averaging replicates, morphometric data were analysed phenetically with the PATN computer package (Belbin 1987). Analysis was undertaken of the whole complex as well as subsets. The Gower metric was used to create a dissimilarity matrix (Gower 1971), with the default setting in PATN. This metric was chosen because it has been shown to be the best association measure for mixed datasets and it also includes 0–0 matches (Crisp and Weston 1993; Ward 1993). Individuals were clustered into groups by hierarchical agglomerative clustering, based on the space-conserving unweighted pair-group method of arithmetic averages (UPGMA). Kruskal–Wallis values were calculated by PATN for groups defined in the dendrogram. The same association matrix was also used in an ordination analysis by hybrid multi-dimensional scaling (HMDS; Faith et al. 1987). The ordination was run 20 times from different random starting configurations and the result with the lowest stress value was used. Three-dimensional ordinations gave acceptable stress values, whereas two-dimensional plots had unacceptably high stress values of > 0.2 (Faith et al. 1987).
Results
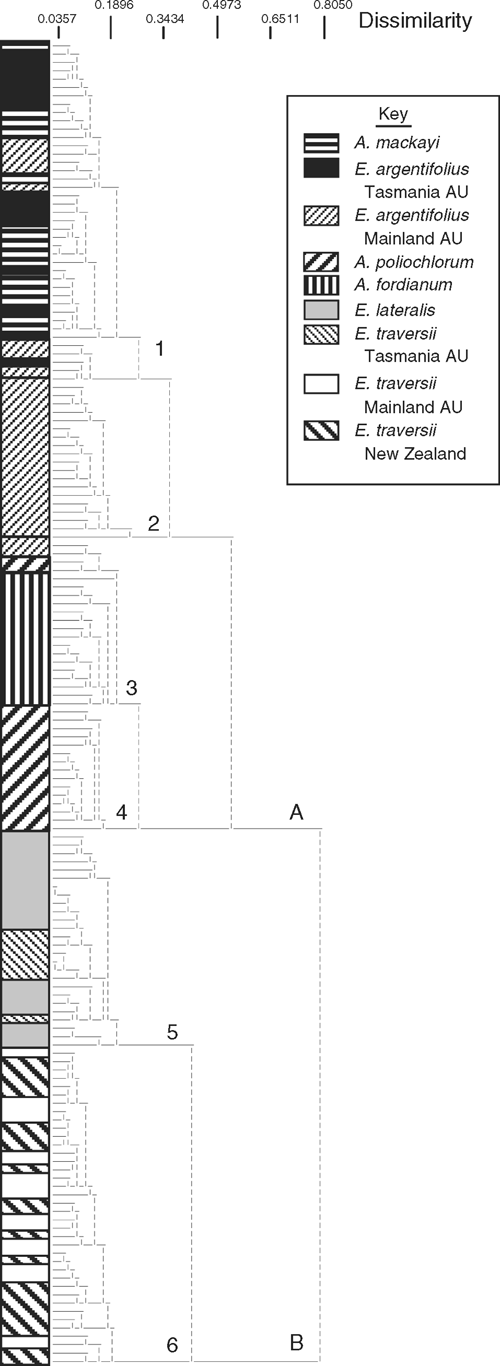
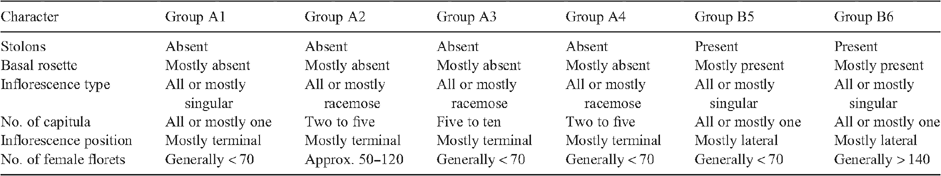
Figure 2 shows the dendrogram, with all 159 specimens coded by original identification and geographic location (see key). The dendrogram shows two main groups, A and B. Group A includes specimens of E. argentifolius, A. mackayi, A. poliochlorum and A. fordianum, and Group B those of E. lateralis and E. traversii (Table 3). Group A is further divided into four subgroups (1–4) and Group B into two subgroups (5, 6).
|
|
Group 1 consists of specimens originally referred to A. mackayi from New Zealand, mixed with specimens from both Tasmania and mainland Australia originally referred to E. argentifolius. Group 2 comprises only mainland Australian specimens of E. argentifolius. Group 3 consists predominantly of Australian specimens of A. fordianum from New South Wales, Victoria and Tasmania, along with two specimens of A. poliochlorum and two of E. argentifolius, all four from New South Wales. Group 4 is exclusively A. poliochlorum. Group 5 includes all of the New Zealand specimens of E. lateralis and all of the Tasmanian specimens of E. traversii. Group 6 consists of all of the specimens of E. traversii from both New Zealand and mainland Australia.
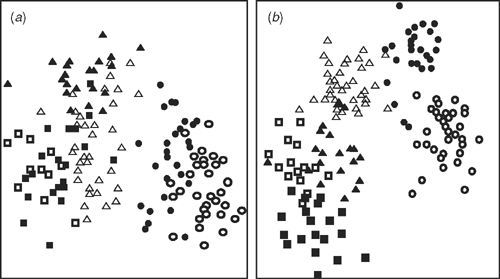
The ordination in three dimensions comparing the same specimens as in Fig. 2 provides a test of the cohesion of the groups shown in the dendrogram (Fig. 3). The six groups identified by the cluster analysis formed clusters in the ordination, with varying degrees of separation. No significant subclustering based solely on geographic groupings was apparent. To compare these results across a wider sample, the remaining specimens on loan were examined. The groups were confirmed across this wider range of specimens, except for Groups 1 and 2. These were not obviously morphologically distinct from each other, although together they were distinct from the other four groups.
|
Characters associated with the six groups that had high Kruskal–Wallis values are given in Table 4. Three characters that are particularly important for separating Groups A and B are the presence or absence of stolons and basal rosette as well as inflorescence position.
|
Argyrotegium fordianum (Group 3) and A. poliochlorum (Group 4) both formed tight clusters, with few other specimens included (Figs 2, 3). Given this result, combined with their ease of identification, no further analysis was performed on these species. The two groups that included specimens of A. mackayi and E. argentifolius (Groups 1 and 2) were analysed separately from the whole dataset as were the groups that included E. lateralis and E. traversii (Groups 5 and 6). This was done to check that the influence of the other groups had not obscured further patterns and to run comparative statistics (Kruskal–Wallis test).
Euchiton argentifolius–A. mackayi
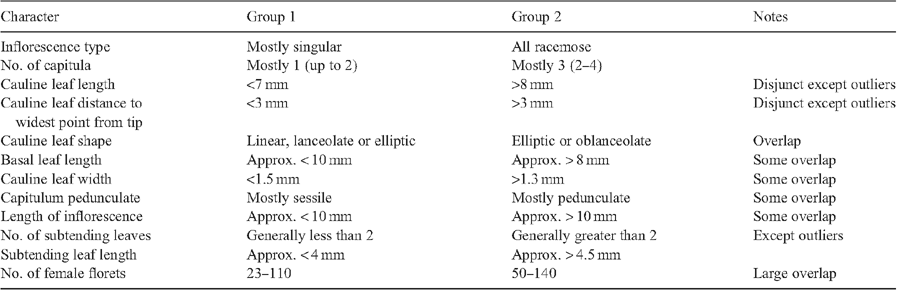
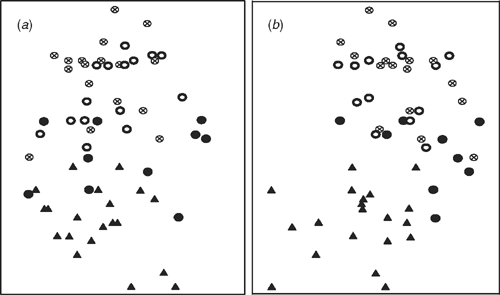
All specimens of A. mackayi and E. argentifolius were included in the re-analysis of this subset. Two characters, stolons and basal midvein, were removed as invariant. The same overall groups were obtained in the dendrogram (not shown) for this subset, although there was some rearrangement of individual specimens (see Table 5 for important characters). The ordination (Fig. 4) showed the specimens clustering loosely in Groups 1 and 2, but there was no clear disjunction between the groups.
|
|
Euchiton lateralis–E. traversii
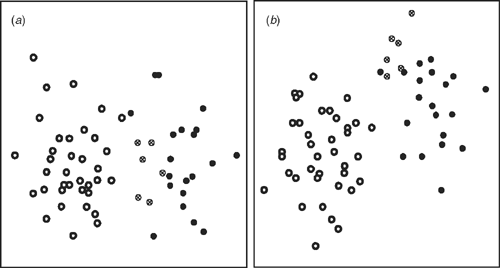
All specimens of E. lateralis and E. traversii were included in the re-analysis of this subset, with two invariant characters, stolons and subtending leaf shape, removed. The re-analysed dendrogram (not shown) of E. lateralis and E. traversii showed the same groups with slight rearrangement of specimens, but within the same two clusters (Groups 5, 6, Fig. 2). The ordination (Fig. 5) showed clear groupings, with an obvious disjunction. The characters that were important in separating the groups are shown in Table 6.
|

|
Scanning electron microscopy
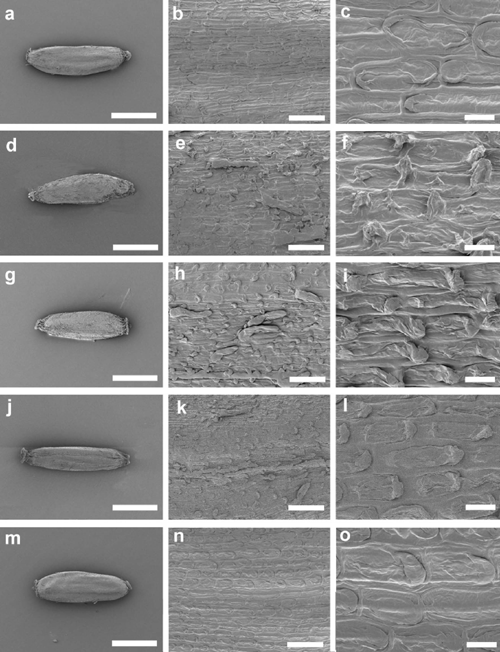
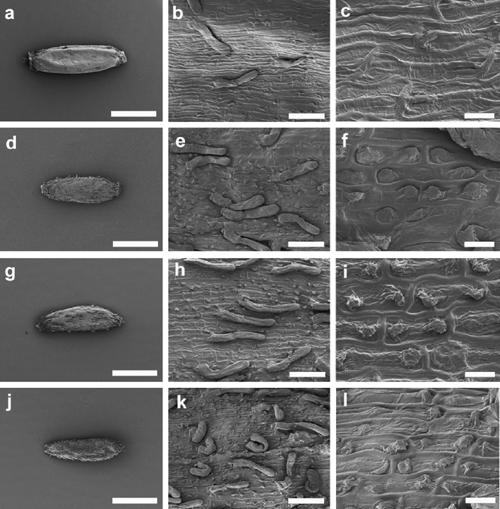
Figures 6–8 illustrate cypsela ornamentation (hairs and papillae) in the six taxa included in the analysis of E. traversii complex. E. argentifolius (Tasmanian specimen, Fig. 6a–c) and A. mackayi (New Zealand specimen, Fig. 6m–o) usually lacked hairs on the cypselae (Fig. 6b, n); however, a specimen of A. mackayi from New Zealand (Fig. 6d–f), and two of E. argentifolius from Victoria (Fig. 6g–l), showed very few clavate twin hairs (Fig. 6e, k) or sparse clavate twin hairs (Fig. 6h). Neither Group 1 nor 2 shown in the dendrogram (Fig. 2) was internally consistent regarding the presence of hairs. A higher percentage (68%) of specimens lacked hairs on the cypsela in Group 1 than had them (20%, 12% missing data; Character 59, Table 2). Group 1 included all of the A. mackayi from New Zealand and E. argentifolius from Tasmania plus a few from the mainland. A slightly higher percentage (47%) had cypsela hairs than did not (42%, 11% missing data; Character 59, Table 2) in Group 2. Presence or absence of cypsela hairs did not allow discrimination of two clear groups, one corresponding to A. mackayi and one to E. argentifolius, regardless of geographic distribution.
|
|
|
Argyrotegium mackayi clearly displayed imbricate single papillae (e.g. Fig. 6o). Four specimens of A. mackayi and E. argentifolius had some semblance of a second papilla, although generally very small (Fig. 6c, f, i, l). E. argentifolius material from Tasmania showed almost featureless cells, with one flat papilla not extending much over the next cell, with the hint of a second papilla on some cells (Fig. 6c). The A. mackayi specimen with a few clavate hairs present also had tiny projections at the end of each cell opposite the large imbricate papilla (Fig. 6l). The Victorian specimens of E. argentifolius differed again in having one large papilla and one very small one, but the large papilla extended at right angles from the cell surface (Fig. 6f, i). However, none of the papillae in these images was more similar to the true paired papillae than to the single imbricate papilla state.
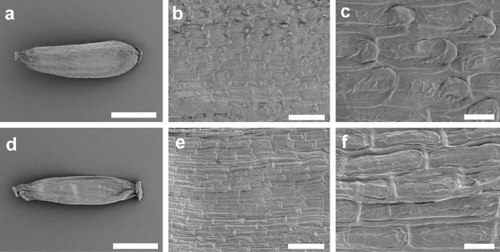
The cypselae of A. fordianum (Fig. 7a–c) lacked hairs but had minute imbricate papillae (Fig. 7c). In A. poliochlorum (Fig. 7d–f), the papillae were difficult to discern and appeared not, or barely, to overlap the adjoining cell (Fig. 7f).
The cypselae of both E. lateralis and E. traversii (Fig. 8a–l) had clavate twin hairs (Fig. 8b, e, h, k). These specimens all had the paired papillae of true Euchiton (Fig. 8c, f, i, l). Although the specimen of E. lateralis from New Zealand (Fig. 8c) appeared almost smooth at high magnification, some paired papillae were apparent at lower magnification (Fig. 8b). During examination of cypselae it became apparent that some parts of their surface retained clearer evidence of these papillae than others.
Discussion
Overall pattern
Clear patterns emerged in this investigation, allowing for a reassessment of the delimitation of the Euchiton traversii complex at both the specific and generic level. The clusters shown in the analyses did not correspond directly to the six species as currently defined (Subgroups 1–6, Figs 2, 3), but instead shed light on the confusion in this species complex and offer hypotheses for its resolution.
The two main groups, A and B, correspond on the one hand to species included in the genus Argyrotegium (Ward et al. 2003) plus E. argentifolius (Group A) and on the other hand to species remaining in Euchiton (Group B).
Characters important in separating Groups A and B in the present analysis were stolons, basal rosettes and inflorescence position (Table 4). The presence of stolons and rosettes also distinguishes Euchiton from Argyrotegium (Ward et al. 2003); however, inflorescence position varies within Euchiton (Drury 1972; Webb 1988a). A. nitidulum is the only species referred to Argyrotegium not included in the present analysis, since we do not consider it to be part of the E. traversii complex.
The usual presence of epidermal hairs on the cypselae of Euchiton and their absence in Argyrotegium was considered by Ward et al. (2003) to be an important distinguishing character, although A. mackayi sometimes has a few hairs and the present study showed that these hairs are sometimes also present, although sparse, in E. argentifolius (Fig. 6).
According to the literature there are two types of cypsela epidermal papillae relevant to these taxa; these are paired papillae, unique to Euchiton, and single imbricate papillae in Argyrotegium (Drury 1970, 1971, 1972; Anderberg 1991; Breitwieser and Ward 2003; Ward et al. 2003). The present study showed some evidence for the presence of a second, much-reduced papilla in two species with imbricate papillae (A. mackayi and E. argentifolius, Fig. 6), thus providing a link between two character states previously recorded as distinct.
Argyrotegium fordianum–A. poliochlorum
Specimens of A. fordianum and A. poliochlorum formed well defined groups. Specimens of A. poliochlorum (Group 4, Fig. 2, Table 3) formed the only cluster that does not include specimens originally identified as other species. Group 3 (Fig. 2, Table 3) contained predominantly specimens of A. fordianum along with two specimens of A. poliochlorum and two of E. argentifolius. In reviewing these four specimens, it was apparent that they should not be referred to A. fordianum, and that the original identifications were correct. They cluster with the A. fordianum group because they are unusually large-leaved plants. Floral characters showed them to be correctly determined.
The scanning electron microscopic images of the cypsela of A. fordianum and A. poliochlorum (Fig. 7) confirmed an absence of twin hairs and paired papillae. There is no dissent within the literature regarding the presence of imbricate papillae on A. fordianum and A. poliochlorum cypselae (Walsh 1999b; Ward et al. 2003; P. G. Wilson, unpubl. data). Previous images of A. poliochlorum have shown minute imbricate papillae (Rozefelds 2001); however, the specimen illustrated here had very reduced papillae, single if discernable, but hardly imbricate, indicating that this character is also more variable than originally thought. A. fordianum was shown to have epidermal papillae clearly of the imbricate type (Fig. 7) as recorded in Ward et al. (2003). The transfer of these species to the genus Argyrotegium (Ward et al. 2003) was supported by this additional evidence.
Euchiton argentifolius–A. mackayi
Through the analysis of this broad range of specimens an unexpected association has arisen between E. argentifolius, an Australian entity, and A. mackayi, believed until recently to be a New Zealand endemic. In 1994, one of us (J. W.) noted a resemblance to A. mackayi of a specimen of Tasmanian E. argentifolius (HO 520058, dupl. of CANU 38112) and after further collecting in Tasmania, deemed A. mackayi to be present in Australia (Ward et al. 2003). The possibility that Australia shared some alpine species of the E. traversii complex with New Zealand was suggested by Short (1987) as well as Webb (1988a, 1988b). In the analysis presented here, Group 1 (Fig. 2) clustered all of the Tasmanian E. argentifolius specimens and some of the mainland Australian specimens with all specimens of A. mackayi. Group 2 contained the remaining E. argentifolius specimens, all from mainland Australia.
Argyrotegium mackayi is unequivocally present in Australia and includes specimens previously referred to E. argentifolius (Group 1, Figs 2, 4, Table 3). The overall similarity observed in the field is reflected in the similarity shown by morphometric character analysis. A. mackayi is found in the same habitats in the two countries, i.e. predominantly in subalpine herbfields. The new question is whether E. argentifolius exists in its own right or simply encompasses larger forms of A. mackayi, with a tendency to a higher number of capitula but with no clear disjunct characters. Analysis of the subset of the data including only these taxa did not alter the pattern, there being no clear disjunction to warrant recognition of two species (Fig. 4).
There are two character differences shown in Table 4 that are relevant to the comparison of Groups 1 and 2; the first is indicated by both the type of inflorescence and number of capitula, and the second by the number of female florets. The latter character showed overlap with generally fewer than 70 female florets in Group 1 and ~50–120 in Group 2.
When these groups were re-analysed separately, these same two characters appeared as important (with number of female florets less so); however, all showed overlap and the two groups consisted of a continuum with a seemingly arbitrary break (Table 5). Other characters that were important in the subset analysis are cauline, basal and subtending leaf-size characters, whether the capitula are pedunculate, length of inflorescence and number of subtending leaves (Table 5). In all cases, Group 1 had the lower number of counts and the smaller measurements and specimens in Group 2 seemed to be generally larger and have more capitula. None of these characters showed a clear disjunction as there was some overlap and the measurements could easily be interpreted as being part of a continuum across the two groups (Table 5).
A comparison of field-collected plants with plants grown in controlled conditions from seed of the same population showed that capitulum number varied phenotypically (data not included). Deletion of the characters inflorescence type and number of capitula, however, did not alter group composition, although groups were slightly less well defined. In all three analyses, including the whole E. traversii complex and the E. argentifolius–A. mackayi subset, both with and without the two influential inflorescence characters, two groups were shown but they were not discontinuous, and no characters clearly separated them.
In terms of geographic patterns within E. argentifolius, Group 1 includes all of the Tasmanian specimens plus some from mainland Australia, whereas all of the specimens in Group 2 are from mainland Australia. There is significant variation in plant size among members of Group 2 and, although the number of capitula is generally greater than in Group 1, there is no clear division to recognise E. argentifolius as a taxon separate from A. mackayi. All specimens on loan for these taxa were examined to check that those included in the analysis were not a biased sample. The existence of larger, multiheaded specimens of A. mackayi from New Zealand was confirmed across the larger sample but the existence of the two separate taxa was not apparent.
Scanning electron microscopic evidence also contributed towards elucidating the relationship between A. mackayi and E. argentifolius. Despite Argyrotegium species usually having glabrous cypselae, A. mackayi has been recorded as having few twin hairs present in some specimens (Webb and Simpson 2001; Breitwieser and Ward 2003; Ward et al. 2003). E. argentifolius was described as having glabrous cypselae, with a few specimens pubescent (Wakefield 1957). Since then, E. argentifolius has been recorded as having twin hairs present (Gray 1976; Everett 1992) or as glabrous or sparsely hairy (Walsh 1999a). One of the specimens of E. argentifolius shown here (Fig. 6b) was glabrous, the density of hairs on another (Fig. 6e) was very similar to that shown in Fig. 6k on a New Zealand A. mackayi specimen and the third (Fig. 6h) had a higher density of hairs although they were still sparse. Specimens studied here (Fig. 6) simply showed a gradation from glabrous to sparsely hairy, extending the variation slightly further than previously recorded. As the circumscription of A. mackayi has thus far encompassed the presence of a few hairs, the greater variation observed is able to be accommodated.
The cypselae in the three samples of E. argentifolius and the two of A. mackayi were not identical with regard to papillae (Fig. 6c, f, i, l, o). The specimens lacked the distinctive paired papillae of Euchiton; however, several had protrusions at the opposite end of the cell to the imbricate papilla. These were found on both A. mackayi and E. argentifolius specimens. In the same way that Webb and Simpson (2001) noted that cypselae of E. mackayi (= A. mackayi) often seem unornamented at lower magnifications because the papillae are so fine; perhaps these tiny secondary ‘vestigial’ papillae on these otherwise imbricate cypselae have been overlooked until now, owing to the unavailability of scanning electron microscopic images.
Variation is present in both E. argentifolius and A. mackayi, lending support to their recognition as the same species. Type material of E. argentifolius and A. mackayi was viewed at K and in collections from MEL and NSW. The material collected by Mueller from the Munyang Mountains (Snowy Mountains) that was designated as lectotype of Gnaphalium argentifolium by Wakefield (1957) was slightly larger than the A. mackayi material (as Raoulia m’kayi Buchanan 1882) but there were no conflicting characters between the type specimens. A comparison of the type descriptions of E. argentifolius (as Gnaphalium argentifolium, Wakefield 1957) and A. mackayi (as Raoulia m’kayi) showed few differences. In terms of habit, A. mackayi is described as prostrate (Buchanan 1882) whereas E. argentifolius is described as ascending (Wakefield 1957). Capitula are noted as solitary or a few together for E. argentifolius but capitulum number is not specified for A. mackayi, so inferred to be single.
On the basis of the phenetic analysis presented here, scanning electron microscopic evidence and examination of much herbarium material, including all of the relevant types, transfer of all E. argentifolius to A. mackayi is supported and this transfer will be effected in a future article summarising the taxonomy of the whole genus (C. Flann, unpubl. data). The close association between A. mackayi and E. argentifolius is not surprising, given the historical taxonomic confusion between E. argentifolius, E. traversii and A. mackayi. However, owing to the national nature of previous work, this overlap had not been observed. Examples such as this vindicate the importance of investigating taxa across their natural range and not according to political boundaries.
Euchiton lateralis–E. traversii
One of the other questions addressed by the present study was whether E. lateralis is present in Australia as well as New Zealand as suggested by Webb (1988a, 1988b) and Webb and Simpson (2001). The analyses of the whole E. traversii complex and the subset of only E. lateralis and E. traversii material clearly showed Tasmanian specimens identified as E. traversii clustering with E. lateralis (Group 5, Figs 2, 3, 5). These specimens also showed characters that delimit E. lateralis. As is discussed later, this does not indicate that the two are not well delimited, rather that most Tasmanian E. traversii material is better referred to E. lateralis. As the latter has not been recorded in Australia it was not an option in available keys. All of the mainland Australia and New Zealand specimens of E. traversii clustered together in Group 6 and thus the species occurs on both land masses (Figs 2, 3, 5).
The most important character in distinguishing E. lateralis from E. traversii is the number of female florets, which shows a strong disjunction between the two groups. E. lateralis (Group 5) has generally fewer than 70 (up to 80) female florets per capitulum whereas E. traversii (Group 6) has generally more than 140 (from as few as 100, up to 280 in the present study). This upper value can be even higher; e.g. Drury (1972) noted E. traversii as having 233–305 female florets, possibly on the basis of glasshouse-grown material.
Overall, E. lateralis has smaller capitula, inner bracts, bisexual florets and pappus bristles, fewer inner and outer bracts and narrower basal leaf width. Most of these characters showed a clear disjunction between taxa, with only a few outliers (Table 6).
Although the number of female florets is not enumerated in the type description of E. lateralis, being described as ‘many’, a difference in capitulum size from E. traversii was noted (Webb 1988b). The capitula of E. lateralis were recorded as 1.5–3.0 mm v. 4.0–6.0 mm in diameter in E. traversii and involucral bracts as 4.0–5.2 mm in E. lateralis v. 5.5–6.5 mm long in E. traversii (Webb 1988b). Capitulum diameter is a problematic character as it varies with stage of development and whether it is measured from pressed material, but the difference in length of bracts was confirmed by our data.
The cypselae of Euchiton lateralis and E. traversii have clavate twin hairs present in varying densities. The glabrous nature of some E. lateralis material recorded in Webb and Simpson (2001) could be due to the frequent confusion between this species and A. mackayi in which the cypselae are indeed mostly glabrous. Allan (1961) included G. traversii var. mackayi (= A. mackayi) under G. traversii (= E. traversii), which explains why his description of the cypselae in E. traversii included a gradation from sparsely puberulous to glabrous.
There are no clear characters in cypsela ornamentation that separate E. lateralis and E. traversii, although there is a slight trend towards fewer hairs in E. lateralis.
The apparently clear geographic split between Tasmanian and mainland Australian material determined as E. traversii was unexpected. Although being a very clear split in our morphometric analysis, comparison with the rest of the relevant specimens on loan showed that most but not all Tasmanian material referred to E. traversii should be regarded as E. lateralis. Four specimens from Tasmania with high numbers of female florets, and so referrable to E. traversii rather than E. lateralis, were seen in the loans examined; however, they were not included in this analysis. These were all from Cradle Mountain or the Great Lake region. In terms of distribution, this makes all of the alpine, silver, stoloniferous material with a solitary capitulum on the Australian mainland E. traversii and most in Tasmania E. lateralis. This means E. traversii is uncommon in Tasmania, a similar pattern of distribution and relative abundance to that of A. poliochlorum and A. fordianum which are both listed as rare in Tasmania (Department of Primary Industry, Water and Environment, Tasmania 2005; Department of Sustainability and Environment, Victoria 2005) but are relatively common in the alpine regions of mainland Australia (Everett 1992; Walsh 1999a).
Euchiton argentifolius–E. traversii
One of the original questions, ‘Are E. argentifolius and E. traversii the same species?’, was clearly answered by this analysis. They are not the same species and, in fact, specimens previously regarded as E. argentifolius form a continuum with A. mackayi when analysed morphometrically. This means that the question that ends up being asked is whether A. mackayi (including E. argentifolius) is different from E. traversii sens. str. (excluding those specimens now referred to E. lateralis). The answer to this question is clearly ‘Yes’. In line with the generic difference supported by the overall pattern of the analysis, these two species fall into different groups in all analyses and are separated by many characters. The same characters that are important in distinguishing Argyrotegium from Euchiton are therefore relevant to the comparison of these species. The most obvious difference between A. mackayi and E. traversii appears to be habit related. E. traversii has a definite rosette and stolons connecting plantlets, as opposed to a highly developed branching structure at or near ground level, but with no stolons, a habit which is exhibited by A. mackayi (and A. poliochlorum and A. fordianum). The inflorescence is terminal in A. mackayi but lateral in E. traversii. Cauline leaves generally appear much more reduced on E. traversii specimens, which also have a generally higher number of female florets.
Material lacking clear information regarding stolons could easily have contributed to the previous taxonomic confusion, as identification of stolons and habit can be affected by the quality and presentation of the specimens. When redetermining the loans in the light of this analysis, several collections from Tasmania previously identified as E. traversii were transferred to A. mackayi.
On referring to the relevant literature it is apparent that much of this information has been known, but not synthesised into a comprehensible solution. Webb (1988a) noted that A. mackayi was treated as a variety of E. traversii at one point, and listed its mat-forming habit, terminal capitula and glabrous achenes as distinguishing characters. Walsh (1999a) also noted the distinctiveness of E. argentifolius and E. traversii in that the latter had larger, solitary capitula and larger, distinctly rosetted leaves. These are the same differences as those between A. mackayi and E. traversii. In the original description of E. argentifolius, Wakefield (1957) noted a superficial similarity between his new species and E. traversii but listed the definite rosettes of basal leaves, solitary, pedunculate flower heads and pubescent cypselae of E. traversii as proof of their difference. In conclusion, the differences between E. argentifolius and E. traversii found here are the same as those noted in the original description of E. argentifolius.
Scanning electron microscope evidence showed that the cypselae of A. mackayi are usually glabrous, with at most a sparse scattering of twin hairs (Fig. 6b, e, h, k, n), whereas the cypselae of E. traversii consistently have hairs present in a higher density (Fig. 8h, k). The presence of paired papillae on E. traversii (Fig. 8i, l), compared with the imbricate papillae of A. mackayi, is a character that clearly distinguishes the two species, even with a few reduced second papillae present on some material of A. mackayi (Fig. 6c, f, i, l, o).
Euchiton lateralis–A. mackayi
There is also an association between the specimens of A. mackayi and E. lateralis. These two species can easily be confused when examining herbarium specimens if material containing stolons is unavailable and capitulum attachment is unclear. It is interesting that they appear superficially similar but have been placed in different genera as Argyrotegium mackayi and Euchiton lateralis. Webb (1988a) separated these two taxa very early on in the key to Gnaphalium (including Euchiton), by using the characters ‘cypselae glabrous, flowering stems terminal and plants mat-forming’ to designate G. mackayi (= A. mackayi), and ‘cypselae hairy or sparsely papillate, flowering stems 1–3, lateral, plants growing singly or in loose patches’ for G. laterale (= E. lateralis). Because of the similarities between E. lateralis and E. traversii, these are the same characters that separate the latter from A. mackayi, as noted above. The scanning electron microscopy characters, as discussed above, also separate A. mackayi and E. lateralis in that the cypselae of the former are usually glabrous with imbricate papillae while the latter has twin hairs present and paired papillae (Figs 6, 8).
Conclusions
Historically, the species of the E. traversii complex have caused confusion in their circumscription and identification. A. mackayi was once treated as a subspecies of E. traversii. Some specimens referred to A. mackayi are now known to be E. lateralis. Similarities exist between E. lateralis and E. traversii. There has been confusion between E. traversii and E. argentifolius.
In summary, E. argentifolius is not the same species as E. traversii. It is, however, conspecific with A. mackayi. Inclusion of material identified as E. argentifolius in the circumscription of A. mackayi adds more evidence for variation in papillae already noted (Ward et al. 2003). The distribution of A. mackayi with this altered circumscription covers alpine regions of mainland Australia, Tasmania and New Zealand.
Euchiton lateralis is shown to be present in Tasmania as well as in New Zealand. Australian E. traversii material is confirmed as conspecific with its New Zealand counterpart and in Australia E. traversii is found in mainland alpine regions, with a few records from Cradle Mountain and the Great Lake region in Tasmania.
Segregation of the genus Argyrotegium from Euchiton was supported by the present study and the evidence, including scanning electron microscope imaging, confirmed the inclusion of A. fordianum and A. poliochlorum in Argyrotegium. These two species were confirmed to be distinct despite both previously being included in a more broadly circumscribed E. argentifolius.
Further issues regarding nomenclatural changes will be published in the future.
Acknowledgements
We are very grateful to Paul Wilson (PERTH) for access to his unpublished work on the group. We thank Dr Nicholas Hind (K) for help with type material; Dr Simon Crawford and Dr Ann Bohte for help with scanning electron microscopic work; Katy Sommerville, Catherine Gallagher, Helen Barnes, Helen Rommelaar, Wayne Gebert (MEL), Mary Korver and Ines Schönberger (CHR) for help with loans; all other herbaria mentioned for loans and electronic data; Steve Sinclair, Claire Marks, Katie Throssell, Charlotte Yeabsley and Ruth Harland for assistance with fieldwork; Steve Sinclair, Elizabeth Flann, Bob Flann and Rosanne Bersten for feedback on an early draft; two reviewers for improvements to the manuscript; and Kerry Ford and Adele Gibbs for help with plant specimens.
Anderberg AA
(1991) Taxonomy and phylogeny of the tribe Gnaphalieae (Asteraceae). Opera Botanica 104, 1–195.

Breitwieser I, Ward JM
(2003) Phylogenetic relationships and character evolution in New Zealand and selected Australian Gnaphalieae (Compositae) inferred from morphological and anatomical data. Botanical Journal of the Linnean Society 141, 183–203.
| Crossref | GoogleScholarGoogle Scholar |

Buchanan J
(1882) On the alpine flora of New Zealand. Transactions of the New Zealand Institute 14, 342–356.

Crisp MD, Weston PH
(1993) Geographic and ontogenetic variation in morphology of Australian waratahs (Telopea: Proteaceae). Systematic Biology 42, 49–76.
| Crossref | GoogleScholarGoogle Scholar |

http://www.dpiw.tas.gov.au/inter.nsf/Attachments/SROS-6VH52J/$FILE/Argyrotegium%20fordianum.pdf
Drury DG
(1970) A fresh approach to the classification of the genus Gnaphalium with particular reference to the species present in New Zealand (Inuleae–Composite). New Zealand Journal of Botany 8, 228–248.

Drury DG
(1971) The American spicate cudweeds adventive to New Zealand: (Gnaphalium Section Gamochaeta – Compositae). New Zealand Journal of Botany 9, 157–185.

Drury DG
(1972) The cluster and solitary-headed cudweeds native to New Zealand (Gnaphalium section Euchiton – Compositae). New Zealand Journal of Botany 10, 112–179.

Faith DP,
Minchin PR, Belbin L
(1987) Compositional dissimilarity as a robust measure of ecological distance. Vegetatio 69, 57–68.
| Crossref | GoogleScholarGoogle Scholar |

Gower JC
(1971) A general coefficient of similarity and some of its properties. Biometrics 27, 857–874.
| Crossref | GoogleScholarGoogle Scholar |

Gray M
(1976) Miscellaneous notes on Australian plants. 3. Craspedia, Gnaphalium, Epacris, Tasmannia, Colobanthus and Deyeuxia. Contributions from Herbarium Australiense 26, 1–11.

Rozefelds AC
(2001) Cypsela morphology and a reassessment of the record of Omalotheca supina (Asteraceae) from Tasmania. Muelleria 15, 65–68.

Short P
(1987) A note on Gnaphalium L. in Australia. Australian Systematic Botany Society Newsletter 52, 7–11.

Wakefield NA
(1957) Flora of Victoria: new species and other additions—12. Victorian Naturalist 73, 186–188.

Walsh NG
(1999b) New species in Asteraceae from the subalps of southeastern Australia. Muelleria 12, 223–228.

Ward JM
(1993) Systematics of New Zealand Inuleae (Compositae–Asteraceae)—2. A numerical phenetic study of Raoulia in relation to allied genera. New Zealand Journal of Botany 31, 29–42.

Ward JM,
Breitwieser I, Flann C
(2003) Argyrotegium, a new genus of Gnaphalieae (Compositae). New Zealand Journal of Botany 41, 603–611.

Webb CJ
(1988b) Gnaphalium laterale, a new species for New Zealand. New Zealand Journal of Botany 26, 485–488.



 ). Group 2: E. argentifolius (▲). Group 3: A. fordianum (■). Group 4: A. poliochlorum (□). Group 5: E. lateralis and Tasmanian E. traversii (●). Group 6: E. traversii (○).
). Group 2: E. argentifolius (▲). Group 3: A. fordianum (■). Group 4: A. poliochlorum (□). Group 5: E. lateralis and Tasmanian E. traversii (●). Group 6: E. traversii (○).