Phylogeny and classification of Eucalyptus subgenus Eudesmia (Myrtaceae) based on nuclear ribosomal DNA, chloroplast DNA and morphology
Adele K. Gibbs A , Frank Udovicic B , Andrew N. Drinnan A and Pauline Y. Ladiges A CA School of Botany, The University of Melbourne, Vic. 3010, Australia.
B Royal Botanic Gardens Melbourne, Birdwood Avenue, South Yarra, Vic. 3141, Australia.
C Corresponding author. Email: p.ladiges@unimelb.edu.au
Australian Systematic Botany 22(3) 158-179 https://doi.org/10.1071/SB08043
Submitted: 11 September 2008 Accepted: 24 February 2009 Published: 10 June 2009
Abstract
Phylogenetic analysis of Eucalyptus subgenus Eudesmia is presented on the basis of the following three datasets: sequences of the internal transcribed spacer (ITS) and the external transcribed spacer (ETS) regions from nuclear rDNA, sequences of the psbA–trnH intergenic spacer region from chloroplast DNA, and morphological characters, including stamen bundling, operculum development, seeds and trichomes. Studies of floral development were essential for understanding the morphology of mature flowers and interpretation of synapomorphy and homoplasy. A summary phylogeny was constructed from a maximum parsimony analysis of those nodes coded as characters that had support in the molecular trees together with morphological characters. A revised infra-subgeneric classification is presented on the basis of the summary phylogeny, and compared with classifications of Hill and Johnson (1998) and Brooker (2000). Differences relate to relationships between clades and taxonomic rank (sections, series and subseries) and valid names of Brooker (2000) are conserved where possible. One main clade of 14 species (section Limbatae), many of mallee growth form, was found in all analyses; this clade is distributed in the South-West of Western Australia and adjacent Interzone and desert areas. A second main clade (section Complanatae) occurs in the northern and eastern tropical and subtropical regions of Australia, including Kimberley, Arnhem, Queensland and New South Wales. This section includes E. tetrodonta, previously treated as an isolated taxon in a monotypic section; however, this species is related to E. baileyana, E. similis, E. lirata and series Miniatae. The hypothesised phylogeny provides a framework for further analyses of biogeography and ecology, including functional traits.
Introduction
Classification of the eucalypts (Myrtaceae) has had a long history from the early writings of R. Brown, G. Bentham and F. von Mueller in the 19th century to the subsequent works of J. H. Maiden, W. T. Blakely, L. D. Pryor, L. A. S. Johnson and M. I. H. Brooker (see summary in Brooker 2000). In recent years, molecular phylogenetic studies have identified relationships and monophyletic groups, in particular confirming the monophyly of Eucalyptus sensu stricto (e.g. Ladiges et al. 1995; Udovicic et al. 1995; Sale et al. 1996; Steane et al. 1999; Udovicic and Ladiges 2000). Despite differences of opinion of generic limits, both Hill and Johnson (1998) and Brooker (2000) recognised similar groups, including Eucalyptus subgenus Eudesmia (R.Br.) L.A.S.Johnson & K.D.Hill.
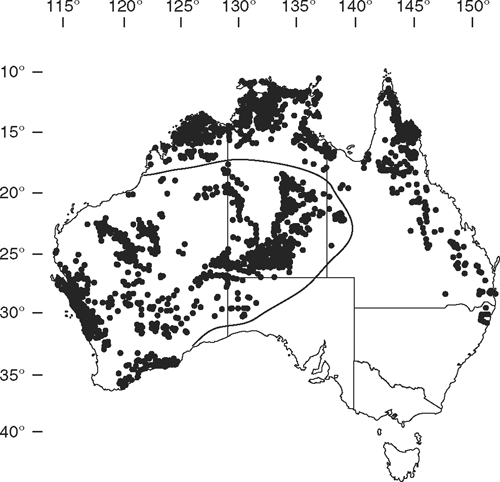
Eudesmia currently includes 26 species and subspecies (Hill and Johnson 1998; Table 1) distributed across tropical and temperate regions of Western Australia, Northern Territory, Queensland and central arid deserts of Western Australia and South Australia (Fig. 1). Eudesmia is a heterogeneous group of trees and mallees, with a range of morphological variation, including bark, flowers, fruit, seed, seedlings and adult foliage (some are neotenous). Seedlings have distinctive trichomes, hairs that radiate from raised oil glands (termed rE trichomes), a character considered a synapomorphy for the subgenus (Ladiges 1984), although E. gamophylla and E. odontocarpa lack trichomes, which has been interpreted as a secondary loss (Hill and Johnson 1998).

|
|
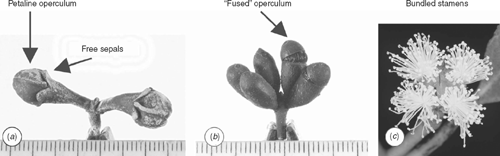
A combination of other morphological characters also supports the monophyly of subgenus Eudesmia. All eudesmids have grey-black seeds, with a ventral hilum and double seed coat (Gauba and Pryor 1959, 1961; Boland et al. 1980). All species have a corolline operculum (Drinnan and Ladiges 1989a, 1989b), with either free sepals on the rim of the hypanthium (Fig. 2a) or have small sepals carried to the top of the corolline operculum (Fig. 2b). In all species, stamens develop on the basal adaxial components (buttresses) of young corolline parts, the basal components becoming the staminophore of the mature flower. In some species, e.g. E. pleurocarpa (= E. tetragona in Drinnan and Ladiges 1989a) and E. erythrocorys, the corolline parts remain more or less free and the stamens develop as four distinct bundles on an undulating staminophore (bundles incorrectly termed fascicles by some authors, because the filaments are not fused). In other species, early corolline continuity leads to a more or less continuous ring of stamens, e.g. in E. jucunda, E. lirata and E. gamophylla. These differences seen in the mature flower, despite having an underlying similarity of development, have been emphasised in previous classifications of the subgenus.
|
Taxonomic history of eudesmid eucalypts
Robert Brown (1814) first described E. tetragona, placing it in the genus Eudesmia because he believed it was significantly different from other species of Eucalyptus. Bentham (1867) classified five species in Eucalyptus subseries Eudesmiae, and E. miniata in subseries Robustae (with E. tetraptera Turcz.). Mueller (1879–1884) included E. eudesmioides, E. tetragona, E. erythrocorys, E. miniata and E. phoenicea in section Parallelantherae, and E. baileyana in section Renantherae, having mixed material with the stringybark E. tindaliae Blakely (see discussion in Hill and Johnson 1998). Maiden (1903–1931) placed nine species in subseries Eudesmiae within series Non-corymbosae, and E. miniata and E. phoenicea in an unnamed subseries within series Corymbosae. Blakely (1934) elevated the two subseries of Maiden (1903–1931) to two series, namely Eudesmieae and Miniatae. Carr and Carr (1968) divided the series Eudesmieae into the following two groups: Group A, with free persistent sepals on fruit, and Group B, with sepals carried on the corolline operculum (Fig. 2). Pryor and Johnson’s informal taxonomic treatment (Pryor and Johnson (1971) classified the eudesmid eucalypts at the rank of subgenus Eudesmia with two sections, Quadraria and Apicaria. Chippendale (1988) followed Pryor and Johnson’s classification (Pryor and Johnson (1971), and placed the known species into seven series, although with no additional ranks or indication of relationships.
Hill and Johnson (1998) and Nicolle (2000) added nine new eudesmid taxa, most of which were subspecies of previously recognised species. Hill and Johnson’s extracodical classification (see Table 1) recognised several monophyletic groups, congruent with those of Pryor and Johnson (1971), and the names of Hill and Johnson (1998) are used throughout the present paper for convenience. Nicolle (2000) used the name series Heteroptera Maiden (1903–1931) for Hill and Johnson’s series Tetragonae and lumped some species; he regarded E. pallida as indistinct from E. eudesmioides, and E. tetragona as an intergrade between E. pleurocarpa and E. extrica.
The formal classification of subgenus Eudesmia by Brooker (2000) is the most recent and includes four sections, two of which are monotypic, four series and six subseries (Table 1), characterised in part by seed characters. The hierarchy of these 14 higher taxa suggests relationships although the classification is not based on any published phylogeny.
Phylogenetic analyses
Molecular phylogenetic analyses have focussed on the broad eucalypt group and have included different combinations of a few eudesmid species (Sale et al. 1993, 1996; Ladiges et al. 1995; Udovicic et al. 1995; Steane et al. 1999; Udovicic and Ladiges 2000). Although based on a small sample of eudesmid species, analyses of Sale et al. (1993, chloroplast DNA) and Udovicic and Ladiges (2000, chloroplast and nuclear DNA) provided further support for the monophyly of the subgenus, in addition to morphology discussed above. In a large sample of ITS nuclear rDNA sequences for species of Eucalyptus, including six eudesmids, Steane et al. (2002) also found subgenus Eudesmia to be monophyletic, and related to subgenus Eucalyptus (the ‘monocalypt’ clade) and E. tenuipes (Maiden & Blakely) Blakely & C.T.White, although nodes lacked bootstrap support.
The only analysis to focus on relationships within Eudesmia is that of Hill and Johnson (1998). These authors, however, analysed only a small morphological dataset of 14 characters, with their eudesmid sections and series as terminal taxa together with outgroups. They found a high level of homoplasy and their resulting cladogram was virtually unresolved except for two nodes.
The aim of the present paper is to determine the phylogenetic relationships of all taxa within subgenus Eudesmia, by using sequence data from both nuclear and chloroplast DNA, and morphology. A revised classification is presented on the basis of the phylogeny.
Materials and methods
Sampling
Location and accession details for the taxa investigated are listed in Appendix 1. Herbarium vouchers are housed at the seed supplier/institution or University of Melbourne Herbarium (MELU). Outgroup taxa included were E. curtisii Blakely & C.T.White (monotypic subgenus Acerosae), E. tenuipes (subgenus Cuboidea) and E. cloeziana F.Muell. (monotypic subgenus Idiogenes) based on previous eucalypt studies (Ladiges et al. 1995; Sale et al. 1996; Steane et al. 2002). As indicated by their treatment as subgenera by Brooker (2000), there is evidence from morphology and molecular data that these species are outside the main clades of Eucalyptus (subgenera Eudesmia, Eucalyptus and Symphyomyrtus) and therefore they are useful as outgroups. Previous studies indicate that E. curtisii is sister taxon to all other taxa within Eucalyptus sensu stricto (Ladiges et al. 1995; Steane et al. 2002); E. tenuipes may be related to either subgenus Eudesmia or subgenus Eucalyptus (Steane et al. 2002) and E. cloeziana to subgenus Eucalyptus.
DNA isolation and amplification
Leaf material was collected at Currency Creek Arboretum (South Australia), Kings Park (Western Australia) and Australian Botanic Gardens, or from glasshouse-grown seedlings (some seed from Top End seeds). Leaf tissue was manually disrupted in a mortar and pestle with liquid nitrogen. DNA was isolated from fresh leaf tissue with the DNeasy Plant Mini Kit (QIAGEN, Hilden, Germany), according to the manufacturer’s handbook and stored at −20°C.
Choice of DNA regions
The ITS and ETS regions of nuclear rDNA were chosen on the basis of previous studies of eucalypts (Steane et al. 1999, 2002; Udovicic and Ladiges 2000; Whittock et al. 2003; Parra-O. et al. 2006; Ochieng et al. 2007). The psbA–trnH intergenic spacer region of chloroplast DNA was selected on the basis of Udovicic and Ladiges (2000), where psbA–trnH was found to be more informative than the trnL intron and trnL–trnF spacer regions, and more conserved than ITS.
PCR with nested primers was initially used for the entire ITS region by using the protocol of Udovicic and Murphy (2002); ITS26 and ITS18 primers were used for the initial PCR, followed by a second PCR with S3 and S4 primers and the product from the initial PCR. Because of poor reproducibility and with only three taxa successfully sequenced, the internal spacers were amplified and sequenced separately. Primers S3 and S5 were used for the ITS1 region, and S6 and ITS26 were used for the ITS2 region (Käss and Wink 1997). The ETS primers used were ETS–18S (Wright et al. 2001) and ETSmyrtF (Lucas et al. 2007). The psbA–trnH intergenic primers were psbAF and trnHR (Sang et al. 1997).
The PCR reactions for ITS1 + 2 and psbA–trnH regions consisted of ×10 buffer (containing 15 mM MgCl2) (QIAGEN), 1.5 μL of 25 mM MgCl2 (QIAGEN), 2 μL of 2.5 mM dNTPs (Fisher Biotec, Perth, Australia), 0.5 μM of each primer, 2.5U HotStarTaq DNA polymerase (QIAGEN), 20–50 ng of Eucalyptus DNA, and ultra-pure water to make a total volume of 25 μL. A negative control for each batch of PCR reactions was always included to test whether the reagents were free of DNA contamination. PCR amplification reactions were conducted in a Mastercycler gradient thermal cycler (Eppendorf, Foster City, CA), beginning with incubation at 95°C for 15 min to activate the HotStarTaq DNA polymerase. PCR conditions for psbA–trnH and ITS1 + 2 were as follows: 30 cycles of 94°C for 30 s, 55°C for 30 s, 72°C for 10 s (psbA–trnH) or 20 s (ITS1 + 2), incubation at 72°C for 5 min, then held at 4°C (Käss and Wink 1997; Udovicic and Ladiges 2000). The PCR reagent concentrations and conditions for the ETS region are described in Parra-O. et al. (2006).
The QIAquick PCR Purification Kit (QIAGEN) was used to purify the PCR products, according to the manufacturer’s handbook. The concentration of the purified DNA was determined by electrophoresis. Direct sequencing of the purified PCR products was conducted with the ABI Prism Big Dye Terminator Cycle Sequencing Ready Reaction Kits (Applied Biosystems, Foster City, CA), version 3.1. Sequencing reactions were analysed at the Australian Genome Research Facility in Brisbane. Contiguous sequences were edited with Sequencher version 3.0 (Gene Codes Corporation, Ann Arbor, MI), and deposited in GenBank (Accession Numbers FJ654340–FJ654428). Owing to sections of highly conserved sequence across all taxa, edited sequences were aligned manually with BioEdit version 7.0.1 (Hall 1999).
Analysis of datasets
Both maximum parsimony and Bayesian inference methods of phylogenetic reconstruction were used. Molecular datasets were analysed separately and combined. Parsimony analyses were conducted with PAUP* version 4.0b10 (Swofford 2002), with individual bases coded as unordered multi-state characters and gaps treated as missing data. All heuristic searches were conducted with 1000 random addition sequences by using tree bisection and reconnection (TBR) branch swapping, and trees were rooted by using outgroups. Multiple most parsimonious trees were summarised as a strict consensus tree. Branch lengths were calculated for one of the equally most parsimonious trees by using DELTRAN character-state optimisation. Node support was tested with bootstrap analyses using 1000 heuristic replicates. Nodes were considered supported with bootstrap values (bs) of ≥50%.
For Bayesian inference, Modelltest 3.7 (Posada and Crandall 1998) was used to select the model of nucleotide substitution that best fitted the data, from the 56 models available. The two model-selection tests used by Modelltest are the hierarchical likelihood ratio test (hLRT), and the Akaike information criterion (AIC). The two tests suggested different models for each dataset. The hLRT was rejected because this test has been suggested to be dependent on the starting model and significance level, and may not select the best model (Posada and Buckley 2004).
The AIC test selected the TrN + I model as the best fit for the ITS2 region data. The estimated base frequencies (A = 0.2078, C = 0.3233, G = 0.2803, T = 0.1886), the substitution-rate matrix (A–C = 1.0000, A–G = 2.2544, A–T = 1.0000, C-G = 1.0000, C–T = 4.0677, G–T = 1.0000), the gamma–distribution shape (equal), and the proportion of invariable sites (0.6575) were defined. The AIC test selected the K81uf + I + Γ model as the best fit for the ETS region data. The estimated base frequencies (A = 0.2722, C = 0.2647, G = 0.2763, T = 0.1867), the substitution-rate matrix (A–C = 1.0000, A–G = 5.7694, A–T = 0.3646, C–G = 0.3646, C–T = 5.7694, G–T = 1.0000), the gamma-distribution shape (0.9177), and the proportion of invariable sites (0.6639) were defined. The AIC test selected the K81uf + Γ model as the best fit for the psbA region data. The estimated base frequencies (A = 0.3111, C = 0.1233, G = 0.1575, T = 0.4081), the substitution-rate matrix (A–C = 1.0000, A–G = 1.1036, A–T = 0.6585, C–G = 0.6585, C–T = 1.1036, G–T = 1.0000) and the gamma-distribution shape (0.2720) were defined.
Bayesian inference analyses were conducted with MrBayes 3.1.2 (Huelsenbeck and Ronquist 2001). Indels from the datasets were partitioned from the nucleotides to allow the Jukes Cantor plus gamma (JC + Γ) model to be applied (Ronquist et al. 2005). The model and parameters estimated in Modelltest were used. To increase the mixing of chains and congruence, two replicate runs of 10 chains each were run for 2 000 000 generations with tree sampling every 100th generation (Martins and Hellwig 2005; Brown et al. 2006). To ensure that an analysis had run to completion, two runs needed to converge to a stationary distribution and the standard derivative of split frequencies approached zero (Ronquist et al. 2005). Trees sampled at the beginning of the run were discarded when log-likelihood values were increasing rapidly during the burn-in period, and remaining trees summarised as a 50% majority rule tree. Posterior probabilities (pp) were used as the measure of support (Larget and Simon 1999; Huelsenbeck et al. 2002); nodes were considered supported with pp ≥ 95% (Wilcox et al. 2002). Each Bayesian analysis was repeated five times to ensure convergence to the same tree topology (Huelsenbeck et al. 2002).
Morphological characters
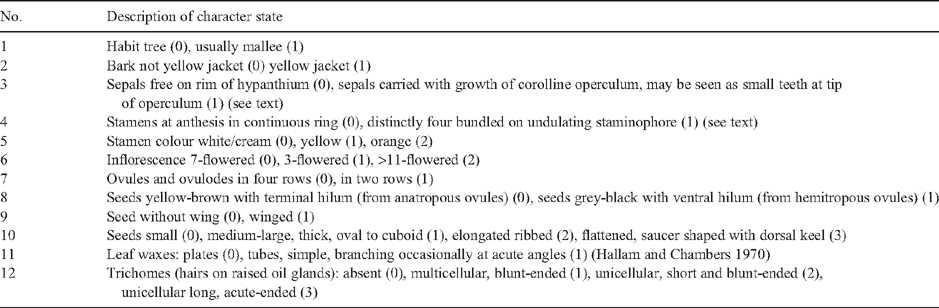
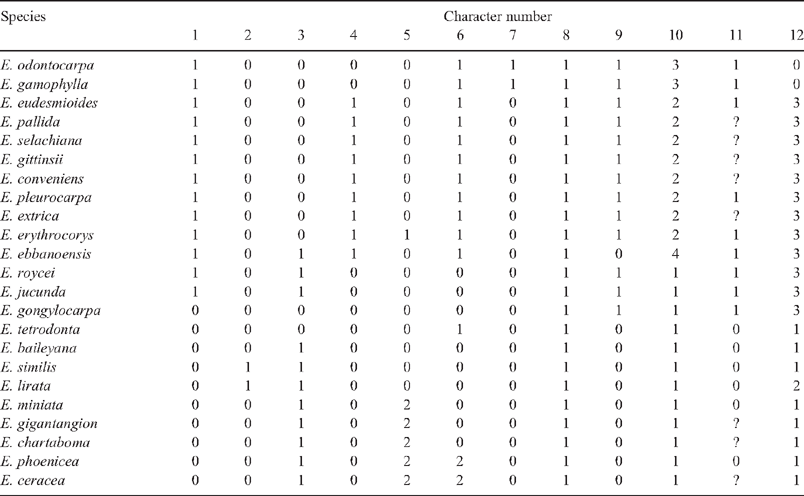
The morphology of taxa was reviewed on the basis of herbarium specimens (on average, minimum of five samples per taxon) from AD, BRI, CANB, DNA, MEL, NSW, PERTH (see Appendix 1), field collections and literature. Light and scanning electron microscopy were used to investigate trichomes (including from glasshouse-grown seedlings) and seeds. Characters relating to size and shape of fruits, flower buds and leaves or bark are useful for identifying subspecies and species or small clades of sister taxa; however, they were difficult to score as non-overlapping discrete states for analysis across the whole subgenus. For cladistic analysis, 12 characters were scored across all taxa that were potentially informative of relationships within subgenus Eudesmia and that have been emphasised in previous infrageneric classifications. Nodes that had support in each of the nrDNA and cpDNA trees were coded as characters and added to the matrix of morphological characters. The matrix was analysed in PAUP using maximum parsimony, with multistate characters treated as unordered and an all-zero outgroup included.
Results
ITS1 region
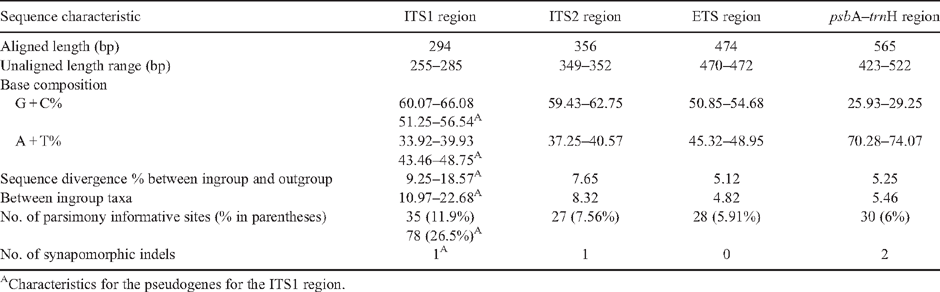
The ITS1 region included the ITS1 intergenic spacer and 34 bp of the 5.8S rRNA gene. The region was more difficult to amplify, sequence and align than the ITS2 region. In part, this was due to five sequences identified as probable pseudogenes (E. gamophylla, E. odontocarpa, E. pleurocarpa, E. extrica and E. lirata) on the basis of an inferred increase in methylation-induced substitutions. The methylation-induced substitutions occur mainly at CpG and CpNpG sites, where N can be any nucleotide, followed by deamination (Gardiner-Garden et al. 1992). Deamination corresponds with a rise in A + T content, with the presumed pseudogenes in eudesmid eucalypts increasing up to 43.46–48.23%, compared with 33.93–39.92% in the ‘typical’ orthologous copies (Table 2). This corresponds with G + C content in the pseudogenes decreasing down to 51.77–56.54%, compared with 60.07–66.08% in the ‘typical’ orthologous copies. The details of the pseudogene sequences for E. gamophylla, E. odontocarpa and E. lirata have been reported by Bayly et al. (2008); none of the sequences in Steane et al. (2002) appears to be pseudogenes (M. Bayly, pers. comm.).
|
Pseudogenes are a potential problem because of comparison of non-orthologous copies in datasets (Bayly and Ladiges 2007); thus, they were excluded from the alignment. After their removal, ITS1 provided little resolution of phylogenetic relationships. For this reason, and because of the potential for other undetected paralogous ITS1 spacer copies within this dataset, the ITS1 region was not used in further analyses of the nrDNA dataset.
ITS2 region
The ITS2 region refers to the remaining 126 bp of the 5.8S rRNA gene, the ITS2 spacer and 26S rRNA gene. In all, 27 accessions representing 23 species of subgenus Eudesmia and three outgroup species were successfully aligned for the ITS2 region, including three sequences obtained from the GenBank (E. ceracea, E. eudesmioides and E. curtisii; Appendix 1). However, DNA for E. gittinsii subsp. gittinsii and E. roycei was unable to be sequenced successfully, despite repeated attempts, and could not be included in the analyses. The total alignment length of the ITS2 region was 356 bp (Table 2). In total, 28 characters, including one indel of 2 bp (Table 3), were found to be parsimony informative (7.65%).

|
ETS sequences
The ETS region from 20 accessions, representing 17 species of subgenus Eudesmia and three outgroup species, was aligned successfully. The ETS region for E. roycei was unable to be sequenced. M. Bayly (University of Melbourne) provided the ETS sequences for E. similis and E. tenuipes. ETS sequences for E. pleurocarpa, E. curtisii and E. cloeziana were from GenBank. The total alignment length of the ETS region was 474 bp and no pseudogenes were identified (Table 2). In total, 28 characters were found to be informative (5.91%) and no indels were coded for this dataset. The ETS region sequenced was longer than the ITS2 region, although it had the same number of informative characters.
Combined nrDNA analyses
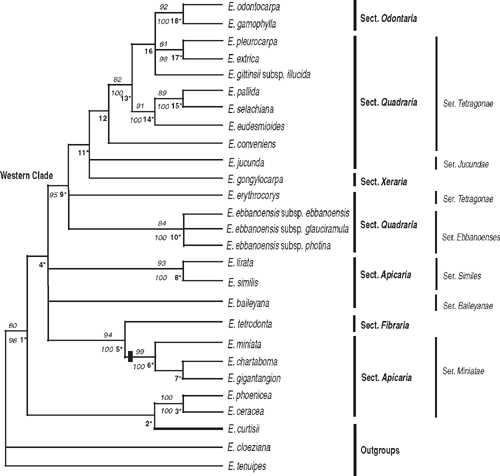
The ITS2 and ETS regions were combined into a single matrix since analyses (not shown) of them separately were not in conflict. An heuristic search in PAUP* identified 72 most parsimonious trees, each with a tree length of 161, CI = 0.77, RI = 0.76. The parsimony strict consensus tree had 16 nodes, 11 resolved with bs ≥ 50%. The Bayesian tree had 18 nodes, 12 with pp ≥ 95%. This tree is illustrated in Fig. 3, with the sections and series of Hill and Johnson (1998, referred to as H&J in the text that follows) shown and nodes identified by parsimony analysis marked with an asterisk. The parsimony and Bayesian analyses produced largely congruent trees, except that Nodes 12 and 16 collapsed in the parsimony strict consensus tree.
|
Eucalyptus phoenicea and E. ceracea (series Miniatae subseries Phoeniceosae H&J) grouped with one of the outgroup species, E. curtisii (although Node 2 lacks support), rather than with members of series Miniatae (H&J). The phylogram of one of the most parsimonious trees (Fig. 4) illustrates relatively long branches leading to these two eudesmid taxa. Other relatively long branches lead to E. gongylocarpa (monotypic section Xeraria H&J), and the well supported clade of E. tetrodonta (section Fibraria H&J) and E. miniata, E. chartaboma and E. gigantangion (series Miniatae). Series Miniatae is strongly supported at node 6 (bs 99% and pp 100%), including one indel.
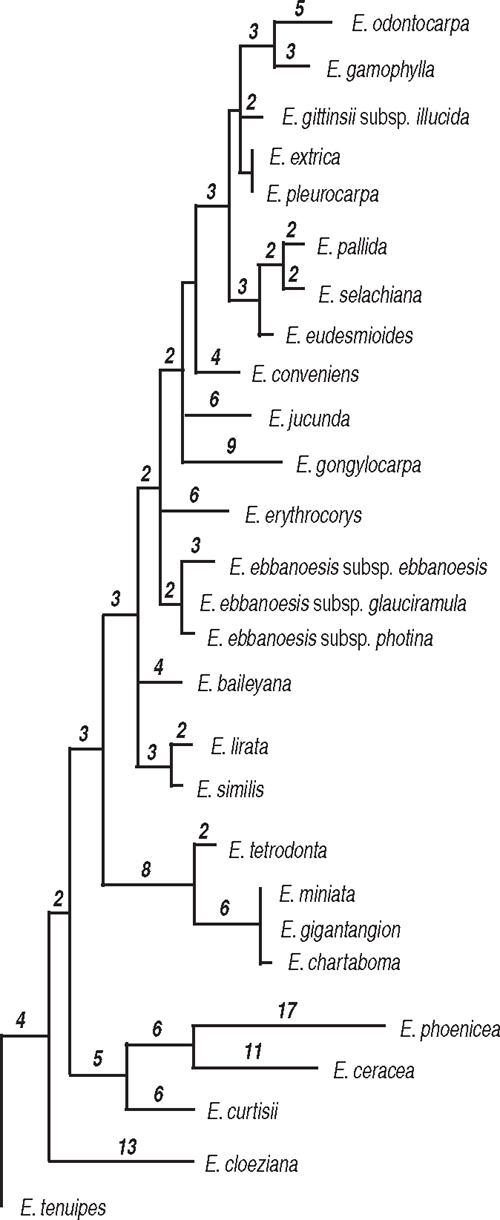
|
Other well supported relationships are as follows: sister taxa E. lirata and E. similis (series Similes H&J) at Node 8 (bs 93% and pp 100%); the three subspecies of E. ebbanoensis at Node 10, (bs 84%, pp 100%); and the clade at Node 13 (bs 82%, pp 100%) that includes six species of series Tetragonae H&J (with subgroups well supported at Nodes 14 and 15), together with E. odontocarpa and E. gamophylla of series Odontaria H&J, which are shown as sister species at node 18 (bs 92%, pp 100%). Although Node 9 lacks bootstrap support (pp 95%), it suggests that all taxa from Western Australia and adjacent desert regions form a clade (here termed the Western clade). E. erythrocorys (part of the polytomy at Node 9) is outside the clade that includes the other members of series Tetragonae and its relationship is unresolved by this dataset.
psbA–trnH intergenic spacer region
Sequences from the psbA–trnH intergenic spacer region from 26 accessions, representing the 23 species of subgenus Eudesmia, were aligned successfully. These included the sequence for E. erythrocorys obtained from the GenBank, and the three outgroup species (Appendix 1). The total alignment length of this spacer region was 565 bp (Table 2). Thirty characters, including two indels (Table 3), were found to be informative (6%), excluding regions of ambiguity. Regions of ambiguity (11.3%) included three different autapomorphic, direct repeat sequence regions, one poly A repeat and one poly T repeat.
An heuristic search in PAUP* identified 396 most parsimonious trees, with a minimum length of 112, CI = 0.86, RI = 0.88. The parsimony strict consensus tree had 13 nodes, 11 with bs ≥ 50%. The Bayesian tree had 14 nodes, 8 with pp ≥ 95% (Fig. 5). The parsimony and Bayesian analyses produced largely congruent trees, except that Nodes 2 and 4 (Fig. 5) in the Bayesian tree collapsed in the parsimony strict consensus tree, and Node 6 (bs 57%) in the parsimony tree was not identified by Bayesian inference. The phylogram of one of the most parsimonious trees showed overall short branches of five or fewer changes resolving clades and up to 11 changes for branches leading to terminal taxa.
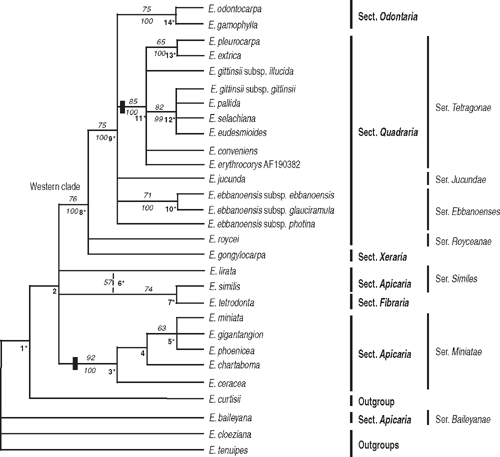
|
On the basis of the psbA–trnH intergenic spacer dataset, the relationship of E. baileyana (series Baileyanae H&J) is unresolved at the base of the cladogram with one of the outgroups, E. curtisii, at a higher node. Basal Nodes 1 and 2 lack support, and monophyly of Eudesmia is not rejected.
Of clades with support, series Miniatae (node 3, bs 92%, pp 100%) is characterised by a large indel (Table 3). Node 8 (bs 76%, pp 100%) confirms the Western clade. E. roycei (series Royceanae H&J) and E. gongylocarpa (section Xeraria) at Node 8 are related to a supported clade of 15 other taxa. Again E. odontocarpa and E. gamophylla (section Odontaria) are sister taxa (Node 14, bs 75%, pp 100%) and, in contrast with the nuclear DNA data, series Tetragonae is monophyletic, supported by an indel character (Node 11, bs 85%, pp 100%).
One surprising result is the relationship of E. tetrodonta with E. similis (Node 7). Examination of individual trees from the parsimony analysis shows for some a branch length of zero leading to Node 7, and sometimes these two species group with E. lirata, which has the longest terminal branch (11 steps) of all accessions.
Combined nrDNA and cpDNA analyses
The ITS2 + ETS and the psbA–trnH datasets were combined. An heuristic search in PAUP* identified 1080 most parsimonious trees, with a minimum length of 289, CI = 0.76, RI = 0.77. The parsimony strict consensus tree had 14 nodes, 13 of which had bs ≥ 50%. The Bayesian tree had more resolved nodes, totalling 24, 16 with pp ≥ 95% (Fig. 6).
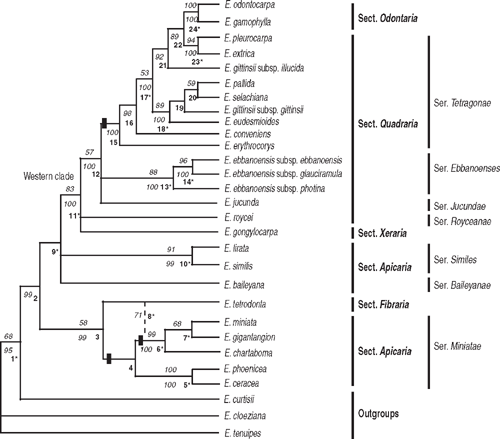
|
Of the clades with support, series Miniatae (Node 4) is characterised by the large indel found in the psbA–trnH region (Table 3), and within that clade both subseries Miniatosae H&J (E. miniata, E. gigantangion and E. chartaboma) and Phoeniceosae (E. phoenicea and E. ceracea) are highly supported as monophyletic groups (Nodes 5 and 6, bs 99–100%, pp 100%). E. tetrodonta (monotypic section Fibraria) is the sister taxon to series Miniatae, although it nests within that group in the parsimony strict consensus tree.
The clade at Node 9 includes three lineages, including E. baileyana (series Baileyanae), sister species E. similis and E. lirata (series Similes) and the Western clade including sections Xeraria, Odontaria and Quadraria (series Royceanae, Ebbanoenses, Jucundae and Tetragonae). Section Odontaria (E. odontocarpa and E. gamophylla) appears to be nested within Tetragonae, although Nodes 15–22 have low or no bootstrap support; the indel identified in the chloroplast data supports Tetragonae as monophyletic and it may not be necessary to invoke a secondary loss of this character in Odontaria, which may be better placed at a lower node as in Fig. 4.
Nodes 2, 3, 4, 12, 15, 16, 19, 20, 21 and 22 (Fig. 6) in the Bayesian tree were collapsed in the parsimony strict consensus tree, and one node (8) in the parsimony tree was not identified by Bayesian inference. The collapse of more nodes in the parsimony tree suggests that the parsimony method more correctly reflected character conflict, and that some nodes resolved in the Bayesian analysis, although with low pp values, are artefactual. The incongruence length difference (ILD) test (Farris et al. 1994, the partition homogeneity test in PAUP) was applied to test for incongruence between the datasets. Heterogeneity was detected, suggesting conservatively that combining the dataset in this way into a single matrix may not be appropriate.
Combined morphology and molecules
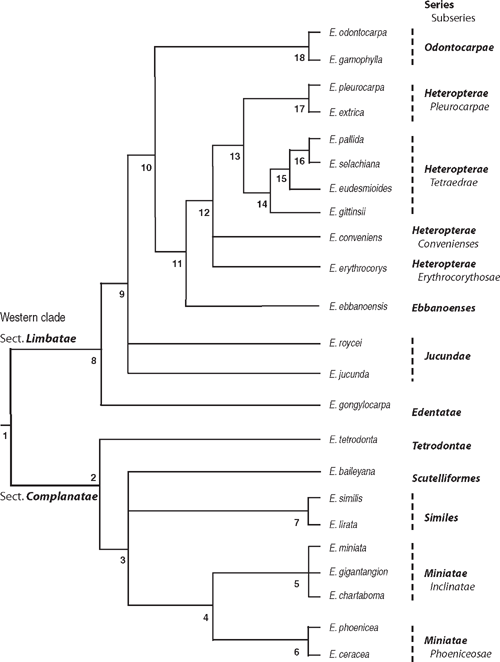
An alternative way of analysing data in combination was to code as characters the nodes of each of the molecular trees (Figs 3, 5) that had bootstrap support ≥50% and posterior probability support ≥95%. In all, 19 characters were scored, and to these were added 12 morphological characters (Tables 4, 5). Character 8, grey-back seeds with ventral hilum, supported the monophyly of the subgenus (Node 1).
|
|
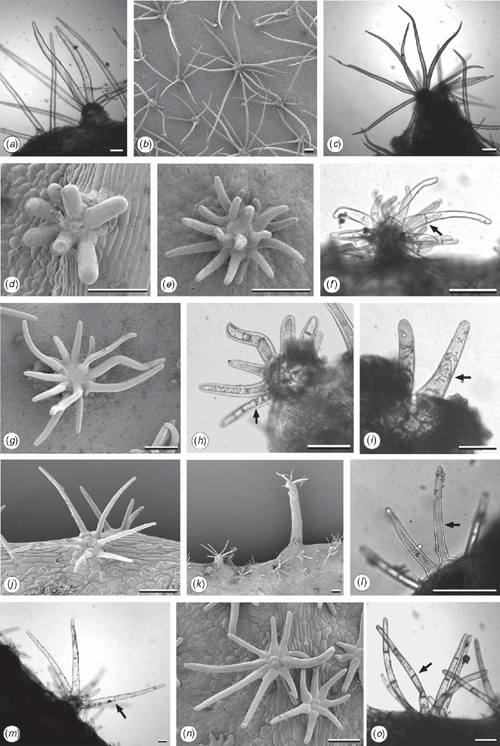
Parsimony analysis resulted in two trees, each of length 45, CI = 0.82, RI = 0.94 (Fig. 7). The two trees differed only with respect to the relationship of E. tetrodonta, which was either unresolved at the base of the tree (Node 1) or resolved at Node 2 as sister to the other northern species on the basis of the presence of multicellular, blunt-ended hairs radiating from raised oil glands (Character 12). Radiating hairs of eudesmids (rE) have previously been described as one type (Ladiges 1984), but further investigation here identified the following three character states (Fig. 8): hairs may be unicelluar blunt-ended (unique to E. lirata), multicellular blunt-ended (E. tetrodonta, E. baileyana, E. similis and series Miniatae) or unicellular elongated-acute (Western clade except series Odontaria, see below).
|
|
The clade at Node 3 relates E. baileyana, sister species E. similis and E. lirata (bark termed ‘yellow jacket’, Character 2) and the five species in series Miniatae. Series Miniatae (at Node 4) is supported by the synapomorphy of orange stamens (Character 5), and the two subclades (Nodes 5 and 6) each have distinctive urceolate, ribbed fruits (illustrated in Fig. 9s–w). Series Miniatae is characterised also by woollybutt bark, large clavate buds, long peduncles, large seeds and large, fleshy cotyledons (not scored in matrix), in addition to the indel found in the psbA–trnH spacer region.

|
All of the taxa at Node 3 have the sepals carried up on the corolline operculum (Character 3), as do E. jucunda and E. roycei (Node 9) and E. ebbanoensis (Node 11). Carr and Carr (1968) emphasised this character to group these taxa as Eudesmieae B; however, the character is homoplasious (Fig. 7). If petals develop quickly after sepal initiation, petal growth can carry the sepals upwards, whereas if sepals grow to sufficient size before petal initiation, they remain on the rim of the hypanthium (Drinnan and Ladiges 1989c). Thus, homoplasy (either parallelism or reversal) may be simply the result of different growth rates. Those taxa with free and persistent sepals on the fruit are members of the clades at Nodes 13 and 18, plus E. gongylocarpa (Node 8) and E. tetrodonta (Node 2; Fig. 7), which Carr and Carr (1968) grouped in Eudesmieae A. E. tetrodonta may have a unique (apomorphic) pattern of development because the persistent sepals are located below the rim of the mature fruit, which extends upwards forming a narrow neck (Fig. 9o).
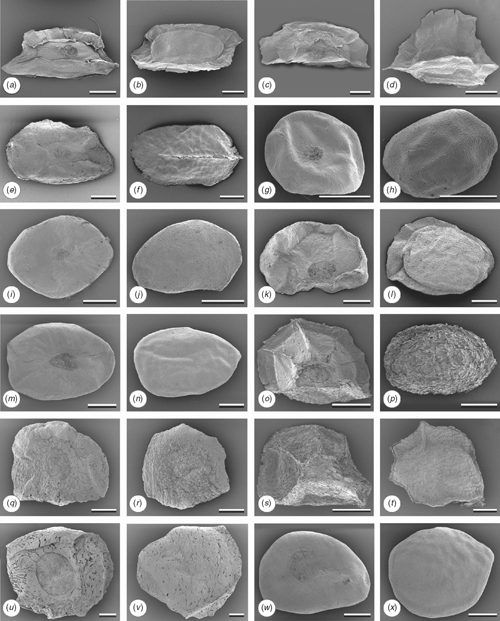
The Western clade (Node 8, Fig. 7), which has strong support from both the nrDNA and cpDNA analyses, is characterised by leaf waxes that are formed as tubes (Character 11; Hallam and Chambers 1970), winged seeds (Character 9, Fig. 10) and, as mentioned above, unicellular, long acute hairs (Character 12, State 3). Two of these characters are homoplasious. Seeds of E. ebbanoensis (Fig. 10g, h) lack a wing and are interpreted as autapomorphic (also described as uniquely ‘obese’ by Brooker 2000). Lack of hairs in E. odontocarpa and E. gamophylla is interpreted as a loss. These two species are sister taxa on the basis of several characters, including two rows of ovules (Character 7; Bohte and Drinnan 2005).
|
Within the Western clade, taxa at Node 9 are all mallees (Character 1), and those at Node 10 all have flowers in clusters of three (Character 6, paralleled in E. tetrodonta). The clade (Node 11), consisting of taxa in series Ebbanoensis + Tetragonae, have stamens at anthesis in four distinct bundles on an undulating or discontinuous staminophore (Character 4). Bundling of stamens arises from four separate basal buttresses of the corolline whorl. All eudesmid species examined (Drinnan and Ladiges 1989a) develop these buttresses; a degree of stamen bundling is evident in flowers of E. baileyana and E. tetrodonta (Maiden 1922: vol. 5, p. 136) as well as E. gongylocarpa; however, at anthesis this is indistinct, the stamens forming a continuous ring owing to early meristematic fusion of the basal epipetalous buttresses (Drinnan and Ladiges 1989c). Various conditions in this character may be nothing more than the result of minor differences in timing of meristematic fusion.
Discussion
Phylogenetic analyses and utility of the datasets
The parsimony and Bayesian methods were mostly congruent, with all strongly supported nodes identified in the parsimony analysis also found by Bayesian inference, although the latter consistently resolved more nodes.
All datasets have their advantages and limitations. The DNA regions sequenced here provided informative characters, including three indels. For the eudesmids, the ITS2 region had greater sequence conservation than the ITS1 region, as reported for other taxa (Hershkovitz and Lewis 1996). The ETS region was easily aligned with no regions of ambiguity, and had a similar number of informative characters as did the ITS2 region. The combined dataset of the ITS and ETS regions produced a more resolved phylogeny than each dataset separately, a finding similar to what has been reported in other studies (e.g. Baldwin and Markos 1998; Parra-O. et al. 2006).
The psbA–trnH intergenic spacer region provided a number of informative characters similar to each of the nrDNA regions and yielded two informative indels, near the 5′ end of the trnH gene, and several poly A/T repeats (Shaw et al. 2005). Direct repeats of sequence blocks of 6–12 nucleotides in the alignment were autapomorphic indels and thus not informative.
Limitations include the problems of pseudogenes (ITS), possible hybridisation with chloroplast capture confounding phylogenetic analyses and homoplasy in morphology that requires understanding of developmental processes. The identification of pseudogenes in the ITS-1 region meant that concerted evolution of this region cannot be assumed; thus, care needs to be taken when analysing ITS sequences. On the basis of analysis of chloroplast DNA, McKinnon et al. (1999) detected hybridisation between closely related eucalypt species sampled from the same geographic area, and concluded that cpDNA may provide information about geographic patterns rather than taxon relationships. This does not appear to be a general problem in Eudesmia although there may be exceptions. For example, the relationship of E. tetrodonta with E. similis and E. lirata according to the maternally inherited cpDNA was in conflict with the biparentally inherited nrDNA, where E. tetrodonta grouped closer to series Miniatae. E. tetrodonta overlaps the distribution range of these taxa in northern Queeensland, and DNA may have been derived from plants with a history of hybridisation or introgression events. This widespread species needs further study across its broad distribution range to test for population variation and the possibility of hybridisation; Bean (2006) recently recognised a new species E. megasepala, reportedly related to E. tetrodonta, which we have not sequenced.
Addition of morphological characters to supported nodes scored as characters from the nuclear trees, improved resolution of relationships. Morphology provided additional synapomorphies, particularly for the main clades (basal nodes). At the same time, evidence of relationships based on DNA sequencing helped identify homoplasy among morphological characters, and thus contributed to an understanding of characters that have in the past appeared problematic.
Phylogeny of Eucalyptus subgenus Eudesmia and revised classification
The summary phylogenetic tree (Fig. 7) is the basis of the revised classification in Table 6. Taxa are monophyletic and arranged in phyletic sequence, such that the phylogenetic tree can be constructed from the hierarchy of names. In phyletic sequencing (Wiley 1981), the first taxon in the list is the sister group to those below it of the same rank. For polytomous nodes, each lineage is given an equal rank. In our revised treatment, the valid names in the classification of Brooker (2000) are conserved where appropriate because the classification of Hill and Johnson (1998) was extracodical.

|
At the base of Tree 1 (Fig. 7), there are three possible relationships for E. tetrodonta, including sister taxon to all eudesmids, sister taxon to the Western clade or sister taxon to the other northern taxa. The evidence presented as Tree 2 (Fig. 7) points to its relationship with the northern group and, consequently, we have classified them together. This relationship is supported by multicellular hairs and nrDNA, and these species also have rough persistent bark. The two main clades in Tree 2 (Fig. 7) are classified as two sections, Complanatae and Limbatae, conserving two sectional names of Brooker (Table 6). These two sections equate to informal sections Quadraria and Apicaria of Pryor and Johnson (1971), except for their placement of E. tetrodonta in the former.
Section Complanatae is the clade of nine species from northern and eastern Australia, grouped in four series. In sequence, these are series Tetrodontae (E. tetrodonta), series Scutelliformes (E. baileyana), series Similes (E. similis and E. lirata) and series Miniatae. Within series Miniatae, the clades E. phoenicea + E. ceracea and E. miniata + E. gigantangion + E. chartaboma, which are sister groups, are treated as two subseries Phoeniceosae and Inclinatae, respectively, similar to Hill and Johnson (1998).
Section Limbatae is the clade of 14 species from Western Australia. Within this section, we recognise six series and four subseries whereas Brooker’s treatment included only two series, each with two subseries (Table 1). In comparison Hill and Johnson (1998) placed these species in three sections, one of which included four series and three subseries (Table 1). Some of these differences are a matter of rank, whereas others imply monophyletic groups not supported by our analysis. One significant change in our classification is the rank of the group of sister species E. odontocarpa and E. gamophylla. We treat them as series Odontocarpae, whereas Hill and Johnson (1998) elevated them to sectional level, implying a more basal phylogenetic position. Brooker (2000) treated them as a subseries related to subseries Tetraedrae, a relationship supported by our findings. Brooker’s treatment of E. ebbanoensis as a monotypic section isolated from its relatives identified here as the Western clade, is not supported.
Nicolle (2000) suggested that E. pallida was not significantly distinct from E. eudesmioides; however, on the basis of molecular sequences and morphology it is concluded to be a separate species from E. eudesmioides and most closely related to E. selachiana. Our results support the view of Nicolle (2000) that E. extrica is the closest relative of E. pleurocarpa, which we have classified as subseries Pleurocarpae.
Conclusions
Phylogenetic analysis revealed several clades congruent across molecular datasets of ITS2, ETS and psbA–trnH spacer regions; however, no molecular dataset was sufficiently informative to resolve all sister group relationships. Limitations of datasets include pseudogenes in ITS, and the possibility that the cpDNA dataset is influenced by historic interspecific hybridisation and chloroplast-capture events. Morphological characters that have been emphasised in various classification schemes of eudesmids require interpretation through developmental studies, and provided further resolution of relationships. Our results provide a basis for a revised phylogenetic classification, recognising a level of congruence with previous formal and informal treatments (including those of Blakely, Chippendale, Hill and Johnson, and Brooker).
Acknowledgements
Dean Nicolle, Marjorie King, Royal Botanic Gardens Sydney Seedbank, and Kings Park and Botanic Gardens provided plant material and seeds. Wayne Gebert and Neville Walsh provided Latin diagnoses. This work was funded by ARC Linkage Grant LP0455375, with support from Royal Botanic Gardens Melbourne, and Maud Gibson Trust. AG was supported by the Hansjörg Eichler Scientific Research Award (Australian Systematic Botany Society), a Melbourne Research Scholarship, the Ethel McLennan Award (The Botany Foundation) and an Albert Shimmin Award, Faculty of Science, University of Melbourne.
Baldwin BG, Markos S
(1998) Phylogenetic utility of the external transcribed spacer (ETS) of 18S–26S rDNA: congruence of ETS and ITS trees of Calycadenia (Compositae). Molecular Phylogenetics and Evolution 10, 449–463.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Bayly MJ, Ladiges PY
(2007) Divergent paralogues of ribosomal DNA in eucalypts (Myrtaceae). Molecular Phylogenetics and Evolution 44, 346–356.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Bayly MJ,
Udovicic F,
Gibbs AK,
Parra-O. C, Ladiges PY
(2008) Ribosomal DNA pseudogenes are widespread in eucalypts (Myrtaceae): implications for phylogenetic analysis. Cladistics 24, 131–146.
| Crossref | GoogleScholarGoogle Scholar |

Bean AR
(2006)
Eucalyptus megasepala A.R.Bean (Myrtaceae), a new species from Queensland allied to E. tetrodonta F.Muell. Austrobaileya 7, 305–310.

Bohte A, Drinnan AN
(2005) Ontogeny, anatomy and systematic significance of ovular structures in the ‘eucalypt group’ (Eucalyptae, Myrtaceae). Plant Systematics and Evolution 255, 17–39.
| Crossref | GoogleScholarGoogle Scholar |

Brooker MIH
(2000) A new classification of the genus Eucalyptus L’Hér. (Myrtaceae). Australian Systematic Botany 13, 79–148.
| Crossref | GoogleScholarGoogle Scholar |

Brown GK,
Craven LA,
Udovicic F, Ladiges PY
(2006) Phylogeny of Rhododendron section Vireya (Ericaceae) based on two non-coding regions of cpDNA. Plant Systematics and Evolution 257, 57–93.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Carr SGM, Carr DJ
(1968) Operculum development and the taxonomy of eucalypts. Nature 219, 513–515.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Drinnan AN, Ladiges PY
(1989a) Corolla and androecium development in some Eudesmia eucalypts (Myrtaceae). Plant Systematics and Evolution 165, 239–254.
| Crossref | GoogleScholarGoogle Scholar |

Drinnan AN, Ladiges PY
(1989b) Operculum development in Eucalyptus cloeziana and Eucalyptus informal subg. Monocalyptus (Myrtaceae). Plant Systematics and Evolution 166, 183–196.
| Crossref | GoogleScholarGoogle Scholar |

Drinnan AN, Ladiges PY
(1989c) Operculum development in the Eudesmieae B eucalypts and Eucalyptus caesia (Myrtaceae). Plant Systematics and Evolution 165, 227–237.
| Crossref | GoogleScholarGoogle Scholar |

Farris JS,
Källersjö M,
Kluge AG, Bult C
(1994) Testing significance of congruence. Cladistics 10, 315–319.
| Crossref | GoogleScholarGoogle Scholar |

Gardiner-Garden M,
Sved JA, Frommer M
(1992) Methylation sites in angiosperm genes. Journal of Molecular Evolution 34, 219–230.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Gauba E, Pryor LD
(1959) Seed coat anatomy and taxonomy in Eucalyptus 2. Proceedings of the Linnean Society of New South Wales 84, 278–291.

Gauba E, Pryor LD
(1961) Seed coat anatomy and taxonomy in Eucalyptus 3. Proceedings of the Linnean Society of New South Wales 86, 96–111.

Hall TA
(1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41, 95–98.
|
CAS |

Hallam ND, Chambers TC
(1970) The leaf waxes of the genus Eucalyptus L’Heritier. Australian Journal of Botany 18, 335–386.
| Crossref | GoogleScholarGoogle Scholar |

Hershkovitz MA, Lewis LA
(1996) Deep-level diagnostic value of the rDNA-ITS region. Molecular Biology and Evolution 13, 1276–1295.
|
CAS |
PubMed |

Hill KD, Johnson LAS
(1998) Systematic studies in the eucalypts. 8. A review of the eudesmioid eucalypts, Eucalyptus subgenus Eudesmia (Myrtaceae). Telopea 7, 375–414.

Huelsenbeck JP, Ronquist F
(2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17, 754–755.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Huelsenbeck JP,
Larget B,
Miller RE, Ronquist F
(2002) Potential applications and pitfalls of Bayesian inference of phylogeny. Systematic Biology 51, 673–688.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Käss E, Wink M
(1997) Molecular phylogeny and phylogeography of Lupinus (Leguminosae) inferred from nucleotide sequences of the rbcL gene and ITS 1 + 2 regions of rDNA. Plant Systematics and Evolution 208, 139–167.
| Crossref | GoogleScholarGoogle Scholar |

Ladiges PY
(1984) A comparative study of trichomes in Angophora Cav. and Eucalyptus L’Hérít. – a question of homology. Australian Journal of Botany 32, 561–574.
| Crossref | GoogleScholarGoogle Scholar |

Ladiges PY,
Udovicic F, Drinnan AN
(1995) Eucalypt phylogeny – molecules and morphology. Australian Systematic Botany 8, 483–497.
| Crossref | GoogleScholarGoogle Scholar |

Larget B, Simon DL
(1999) Markov chain Monte Carlo algorithms for the Bayesian analysis of phylogenetic trees. Molecular Biology and Evolution 16, 750–759.
|
CAS |

Lucas E,
Harris SA,
Mazine FF,
Belsham SR,
Lughadha N,
Eimear M,
Telford A,
Gasson PE, Chase MW
(2007) Suprageneric phylogenetics of Myrteae, the generically richest tribe in Myrtaceae (Myrtales). Taxon 56, 1105–1128.

Martins L, Hellwig FH
(2005) Systematic position of the genera Serratula and Klasea within Centaureinae (Cardueae, Asteraceae) inferred from ETS and ITS sequence data and new combinations in Klasea. Taxon 54, 632–638.

McKinnon GE,
Steane DA,
Potts BM, Vaillancourt RE
(1999) Incongruence between chloroplast species phylogenies in Eucalyptus subgenus Monocalyptus (Myrtaceae). American Journal of Botany 86, 1038–1046.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Nicolle D
(2000) Three new taxa of Eucalyptus subgenus Eudesmia (Myrtaceae) from Queensland and Western Australia. Nuytsia 13, 317–329.

Ochieng JW,
Henry RJ,
Baverstock PR,
Steane DA, Shepherd M
(2007) Nuclear ribosomal pseudogenes resolve a corroborated monophyly of the eucalypt genus Corymbia despite misleading hypotheses at functional ITS paralogues. Molecular Phylogenetics and Evolution 44, 752–764.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Parra-O. C,
Bayly M,
Udovicic F, Ladiges P
(2006) ETS sequences support the monophyly of the eucalypt genus Corymbia (Myrtaceae). Taxon 55, 653–663.

Posada D, Buckley TR
(2004) Model selection and model averaging in phylogenetics: advantages of Akaike information criterion and Bayesian approaches over likelihood ratio tests. Systematic Biology 53, 793–808.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

Posada D, Crandall KA
(1998) MODELTEST: testing the model of DNA substitution. Bioinformatics 14, 817–818.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Sale MM,
Potts BM,
West AK, Reid JB
(1993) Relationships within Eucalyptus using chloroplast DNA. Australian Systematic Botany 6, 127–138.
| Crossref | GoogleScholarGoogle Scholar |

Sale MM,
Potts BM,
West AK, Reid JB
(1996) Relationships within Eucalyptus (Myrtaceae) using PCR amplification and southern hybridisation of chloroplast DNA. Australian Systematic Botany 9, 273–282.
| Crossref | GoogleScholarGoogle Scholar |

Sang T,
Crawford DJ, Stuessy TF
(1997) Chloroplast DNA phylogeny, reticulate evolution, and biogeography of Paeonia (Paeoniaceae). American Journal of Botany 84, 1120–1136.
|
CAS |
Crossref |

Shaw J,
Lickey EB,
Beck JT,
Farmer SB,
Liu W,
Miller J,
Siripun KC,
Winder CT,
Schilling EE, Small RL
(2005) The tortoise and the hare II: relative utility of 21 noncoding chloroplast DNA sequences for phylogenetic analysis. American Journal of Botany 92, 142–166.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Steane DA,
McKinnon GE,
Vaillancourt RE, Potts BM
(1999) ITS sequence data resolve higher level relationships among the eucalypts. Molecular Phylogenetics and Evolution 12, 215–223.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Steane DA,
Nicolle D,
McKinnon GE,
Vaillancourt RE, Potts BM
(2002) Higher-level relationships among the eucalypts are resolved by ITS-sequence data. Australian Systematic Botany 15, 49–62.
| Crossref | GoogleScholarGoogle Scholar |

Udovicic F, Ladiges PY
(2000) Informativeness of nuclear and chloroplast DNA regions and the phylogeny of the eucalypts and related genera (Myrtaceae). Kew Bulletin 55, 633–645.
| Crossref | GoogleScholarGoogle Scholar |

Udovicic F, Murphy DJ
(2002) Successful DNA amplification from Acacia (Leguminosae) and other refractory Australian plants and fungi using a nested/semi-nested PCR protocol. Muelleria 16, 47–53.

Udovicic F,
McFadden GI, Ladiges PY
(1995) Phylogeny of Eucalyptus and Angophora based on 5S rDNA spacer sequence DNA. Molecular Phylogenetics and Evolution 4, 247–256.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Whittock S,
Steane DA,
Vaillancourt RE, Potts BM
(2003) Molecular evidence shows that the tropical boxes (Eucalyptus subgenus Minutifructus) are over-ranked. Transactions of the Royal Society of South Australia 127, 27–32.

Wilcox TP,
Zwickl DJ,
Heath TA, Hillis DM
(2002) Phylogenetic relationships of the dwarf boas and a comparison of Bayesian and bootstrap measures of phylogenetic support. Molecular Phylogenetics and Evolution 25, 361–371.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Wright SD,
Yong CG,
Wichman SR,
Dawson JW, Gardner RC
(2001) Stepping stones to Hawaii: a trans-equatorial dispersal pathway for Metrosideros (Myrtaceae) inferred from nrDNA (ITS + ETS). Journal of Biogeography 28, 769–774.
| Crossref | GoogleScholarGoogle Scholar |




