A revision of Chara sect. Protochara, comb. et stat. nov. (Characeae: Charophyceae)
Michelle T. Casanova A B D and Kenneth G. Karol CA Royal Botanic Gardens, Melbourne, Birdwood Avenue, South Yarra, Vic. 3141, Australia.
B Centre for Environmental Management, University of Ballarat, Mt Helen, Vic. 3350, Australia.
C New York Botanical Garden, 2900 Southern Boulevard, Bronx, NY 10458-5126, USA.
D Corresponding author. Present address: 273 Casanova Road, Westmere, Vic. 3351, Australia. Email: amcnova@netconnect.com.au
Australian Systematic Botany 27(1) 23-27 https://doi.org/10.1071/SB13016
Submitted: 2 April 2013 Accepted: 15 April 2014 Published: 30 June 2014
Abstract
A revision of a group of ecorticate species of Chara is presented, on the basis of fresh, pressed and spirit-preserved material. The following seven species are recognised, characterised by a very simple morphology, with few or inconspicuous accessory cells (cortication, stipulodes, bract cells, bracteoles) and large gametangia: Chara australis R.Br., C. lucida (A.Braun) Casanova & Karol comb et. stat. nov., C. porteri Casanova, sp. nov., C. protocharoides Casanova & Karol, nom. nov. (=Protochara australis Womersley & Ophel) and C. stuartiana (Kütz.) Casanova & Karol comb. et. stat. nov. from Australia, and C. corallina Klein ex Willd. and C. wallichii A.Braun from Asia. A new section, Chara subg. Charopsis sect. Protochara (Womersley & Ophel) Casanova & Karol, comb. et stat. nov., is erected to accommodate these taxa, formerly placed in sect. Charopsis.
Additional keywords: Chara australis, Chara stuartiana, Chara lucida, Chara protocharoides, Chara porteri, Protochara, systematics, Tolypellopsis.
Introduction
Charophytes (members of the extant family Characeae, Charophyceae) are submerged water plants that grow in fresh, brackish and saline water throughout the world. They are distinguished from other aquatic macrophytes on the basis of their overall simple morphology (consisting of essentially uniseriate, branched filaments of cells), their unique reproductive organs (antheridia producing motile sperm cells, and oogonia or oosporangia containing a non-motile egg cell), and distinctive oospores patterned with helical sutures. The genus Chara L. is characterised by a five-celled coronula on the oogonium (oosporangium), placement of the oogonium above the antheridium where they occur together, whorls of accessory cells below the branchlet whorls (stipulodes) and essentially monopodial branchlet structure. The family Characeae was revised for Australia by Wood (1971); however, Wood’s revision resulted in an unusable taxonomy (Casanova 2005, 2009) and recent studies have shown that the lumping of Australian species with those in the northern hemisphere, and the delineation of numerous subtaxa (varieties, forms) do not represent reality, or enhance utility. There are many more species in Australia than were recognised by Wood (1971) (Casanova 2005, 2009, 2013; García and Chivas 2006) and current advances in charophyte taxonomy, including investigation of oospore morphology with scanning electron microscopy, have allowed a new approach to the systematics of this group.
Historical review
Traditionally (in what has been termed the Braun–Wood taxonomy, Proctor 1980), the genus Chara has been divided into two groups, defined on the basis of the number of stipulode whorls. These groups have been variously treated as subgenera or sections by different authors (see Kützing 1843; Nordstedt 1883; Zaneveld 1940; Wood 1962; Wood and Imahori 1965; Proctor 1980). Current taxonomy recognises two subgenera, namely, subg. Chara and subg. Charopsis (Kütz.) Leonh. Within subg. Charopsis, the following two sections are currently recognised, following Wood and Imahori (1965): sect. Charopsis (totally ecorticate) and sect. Agardhia R.D.Wood (corticated axes, ecorticate branchlets), with Chara braunii C.C.Gmel. being the type of subg. and sect. Charopsis. However, phylogenetic analysis places C. braunii close to C. muelleri A.Braun, which is in sect. Agardhia (Meiers et al. 1999; McCourt et al. 2000). The remaining totally ecorticate Chara species are morphologically and phylogenetically (McCourt et al. 2000) more similar to each other than to C. braunii; however, a group comprising these ecorticate species, without C. braunii, has no name under the rules of the ICN (McNeill et al. 2012). The members of this group have ecorticate axes and branchlets, and have a relatively simple morphology (compared with corticated species in subgenus Chara). Some of these species have, in the past, been allocated to different genera (e.g. Charopsis Kütz., Nitella C.Agardh, Nitellopsis Hy, Tolypellopsis (Leonh.) Mig. and Protochara Womersley & Ophel). The first member of the group to be described was Chara corallina Klein ex Willd., a monoecious species first collected in India (Willdenow 1805). Soon after, a dioecious species was described from Australia, C. australis R.Br. (Brown 1810). Kützing (1857) placed Brown’s C. australis in the genus Nitella as N. australis (R.Br.) Kütz., described N. stuartiana Kütz. on the basis of more robust material collected from Tasmania, and illustrated both species. In a summary of all the taxa in the subgenus, presented by Nordstedt (1883), Brown’s C. australis was retained without further description, and N. stuartiana was placed in synonymy of that species. From a relatively small number of specimens, an additional six taxa at different ranks were also recognised, including C. australis var. lucida A.Braun, C. australis var. nobilis A.Braun, C. australis subsp. plebeja (R.Br. ex A.Braun) A.Braun, C. australis var. vieillardii A.Braun, C. corallina and C. wallichii A.Braun. The other ecorticate Chara species included in this group by Nordstedt (1883), C. braunii, will not be dealt with in the present study.
Zaneveld (1940) treated C. australis and C. australis var. nobilis as synonymous, but distinguished northern Australian and New Caledonian specimens as different forms of C. australis var. lucida: i.e. f. typica Zaneveld, nom. inval., f. tenerior Zaneveld and f. vieillardii (A.Braun) Zaneveld. Womersley and Ophel (1947) erected the genus Protochara to accommodate a totally ecorticate taxon from Western Australia, Protochara australis Womersley & Ophel, and transferred Nitellopsis inflata Fil. & G.O.Allen ex Fil. to that genus as Protochara inflata (Fil. & G.O.Allen ex Fil.) Womersley & Ophel. Filarszky (1934) also described a species with morphological similarities to C. australis, from Indonesia, as Tolypellopsis simplicissima Fil.
In the first experimental breeding study in the Characeae, Macdonald and Hotchkiss (1956) found that female Chara australis and a specimen from New South Wales identified by H. B. S. Womersley as male Protochara australis were inter-fertile to the point of oospore production, and so amalgamated C. australis and P. australis, separating material that had been called P. australis as a new subspecies, C. australis subsp. estipulodica M.B.Macdon. & Hotchk. In amalgamating Protochara australis with Chara, they also included P. inflata in Chara, as C. inflata (Fil. et G.O.Allen ex Fil.) M.B.Macdon. & Hotchk. Subsequently, on the basis of reproductive morphology and nucleotide sequences, García and Karol (2004) recognised Protochara inflata as a species of Lamprothamnium J.Groves, L. inflatum (Fil. & G.O.Allen ex Fil.) Adr.García & Karol. Wood (1962) amalgamated most of the members of subg. Charopsis into a single species, C. corallina, and delineated several infraspecific taxa, some of which were not validly published (cf. Art. 41.5 of the ICN; McNeill et al. 2012). These were subsequently treated as both forms and varieties of C. corallina, as well as being treated at ‘microspecies’ rank (equivalent in rank to Wood’s forms as well as his ‘monotypic’ varieties and species, i.e. those lacking subordinate taxa) by Wood and Imahori (1965). Infraspecific taxa are treated in the main body of the monograph, with alternative ‘microspecies’ status presented in the Microspecies Appendix at the end of the work. These names are here treated as invalid, alternative names under Art. 36.2 of the ICN (McNeill et al. 2012). Wood (1971) later revised his taxonomy for Australian material, delineating C. corallina var. corallina as those specimens with cylindrical branchlet segments, and C. corallina var. nobilis for those specimens with inflated branchlet segments. Within C. corallina var. nobilis, he tentatively recognised the following four forms: nobilis, inflata, simplicissima and stuartiana. These formae names are here treated as invalid provisional names under Art. 36.1 of the ICN (McNeill et al. 2012).
In the present study, the type material of all the Australian species and their close relatives, as well as more than 200 specimens from field collections and herbaria, were examined to determine the status and morphological limits of the species previously included in Chara subg. Charopsis sect. Charopsis (excluding C. braunii).
Morphology
Chara species grow as totally submerged water plants, rooted in the sediment by colourless rhizoids, with a photosynthetic axis in the water column. The axis consists of single-celled internodes and whorls of multicellular branchlets, on which the reproductive structures are borne. The axes look very similar to submerged flowering plants, and can be confused with species of Myriophyllum L. Species of Chara in this group are entirely ecorticate, i.e. with no cortex on the axis or branchlets, and, in general, have little in the way of ‘accessory cells’ (i.e. stipulodes, bract cells or bracteoles). Thus, there are few morphological characteristics on which to separate species, which has led to the current taxonomic confusion. They are, in general, somewhat large, with robust axes and spreading whorls of branchlets. The antheridia and oogonia are typical of the genus, but large (up to 1 mm long) in comparison with some other species, and visible to the naked eye. The antheridia are usually bright orange, and the oogonia have few circumvolutions of the enveloping spiral cells and very small, apiculate coronula cells. Their persistent reproductive units (oospores) are among the largest recorded for Australian charophytes, but with relatively few striae (spiral sutures), and some species accumulate starch in the lower and rhizoidal nodal cells (bulbils), which allows them to persist vegetatively. The major characteristics used to separate species in this group are sexuality (monoecious v. dioecious), development and abundance of accessory cells, axis and branchlet diameter and length, characteristics of the branchlet nodes and oospore morphology. They are distinguished from similar species of Lamprothamnium by the presence, in Lamprothamnium, of gyrogonites (calcified covering on the oospore), rounded coronula cells, downward pointing stipulodes and verticillate bract cells.
Materials and methods
Approximately 200 fresh, pressed and spirit-preserved specimens of charophytes in Australian and overseas herbaria and several private collections were examined for the present study, including 15 specimens designated as type material of species, subspecies, varieties or forms within the group. Each specimen was allocated a letter–number combination (p###, r###, t### or v###), so that all data obtained and stored for that specimen (i.e. measurements, photographs, chromosome counts, scanning electron micrographs) could be related back to the specimen. Initial examination involved measurements (size, number) directly from the specimen (e.g. number of branchlets, number of branchlet segments) and with the use of a microscope (e.g. axis and branchlet diameter, number and morphology of stipulodes and bract cells, arrangement of gametangia). Oospore features were examined and measured with the aid of light microscopy and scanning electron microscopy.
Fresh specimens were obtained in field surveys in all states of Australia (following the methods of Casanova 2004), and from culture of seed-bank material in a greenhouse (following the methods of Casanova 2004). In the greenhouse, plastic containers (185 mm × 125 mm × 50 mm) containing ~300 g of wetland soil were inundated to a depth of 10–14 cm in large tanks and the charophytes that germinated from them were examined. When oospores were present they were removed from the specimens for examination with a scanning electron microscope (SEM), then plants were gathered and pressed, or preserved in 70% alcohol. If oospores were not present, the plants were either returned to culture to mature, or kept in jars on a windowsill until they matured and oospores were freely released. If antheridia were present, chromosome counts were attempted, following the methods outlined in Casanova (1997).
Where possible and appropriate, oospores were removed from the herbarium specimens or obtained as above from live material. Approximately a quarter (50) of the specimens examined had oospores. They were prepared for SEM examination by cleaning (if required) with a detergent solution, using a modification of the methods of Crawford et al. (2001). Sometimes the enveloping cells were removed by hand, using fine needles. For type specimens, very old material, or specimens with a single oospore available for examination, the oospores were handled with great care, with minimal manipulation. Oospores were removed from the stubs after microscopy and are stored in alcohol and deposited with the specimen for possible future examination.
Results
Chara L., Sp. Pl. 2: 1156 (1753)
Type: Chara vulgaris L.
Chara subgen. Charopsis (Kütz.) Leonh., Lotos 13: 73 (1863)
Type: Chara braunii C.C.Gmel.
Monoecious or dioecious. Plant axis corticated or ecorticate, stipulodes in a single whorl (haplostephanous), bract cells unilateral or verticillate, branchlets ecorticate and terminated by a single cell, a cluster of bract cells or a group of three equal-sized cells (corona).
Chara subgen. Charopsis sect. Protochara (Womersley & Ophel) Casanova & Karol, comb. et stat. nov.
Type: Chara protocharoides Casanova & Karol.
Axes ecorticate. Stipulodes in a single tier when present, sometimes obscure, up to 2 per branchlet. Branchlets ecorticate, with a single-celled end segment, sometimes surrounded by smaller bract cells. Bract cells 2 or 3, often obscure. Bracteoles 2, often replaced by gametangia or obscure. Gametangia sessile; solitary, geminate or aggregate, on the branchlet nodes and at the base of the branchlets, conjoined (monoecious) or on separate plants (dioecious). Oogonia with few circumvolutions, coronula cells slightly apiculate. Oospores with fewer than 8 striae, without a gyrogonite. Annual or perennial.
Species in this section have been variously placed in Chara, Nitella, Nitellopsis, Protochara and Tolypellopsis by previous authors. All these names refer to currently recognised genera, except Protochara and Tolypellopsis (=Nitellopsis). Thus, the name Protochara appears to be an appropriate name for the new section.
Key to the species in Chara subgen. Charopsis sect. Protochara|
1. Plants dioecious |
|
2. Bract cells and stipulodes small or absent |
|
3. Axis diameter usually >1.1 mm, branchlets somewhat inflated, 6–13 mm long |
|
4. Branchlets 20–50 mm long, axes tangled and wiry …C. lucida |
|
5. Stipulodes, bract cells or bracteoles absent …C. protocharoides |
|
6. Branchlets and internodes 1.2–1.8 mm in diameter …C. australis |
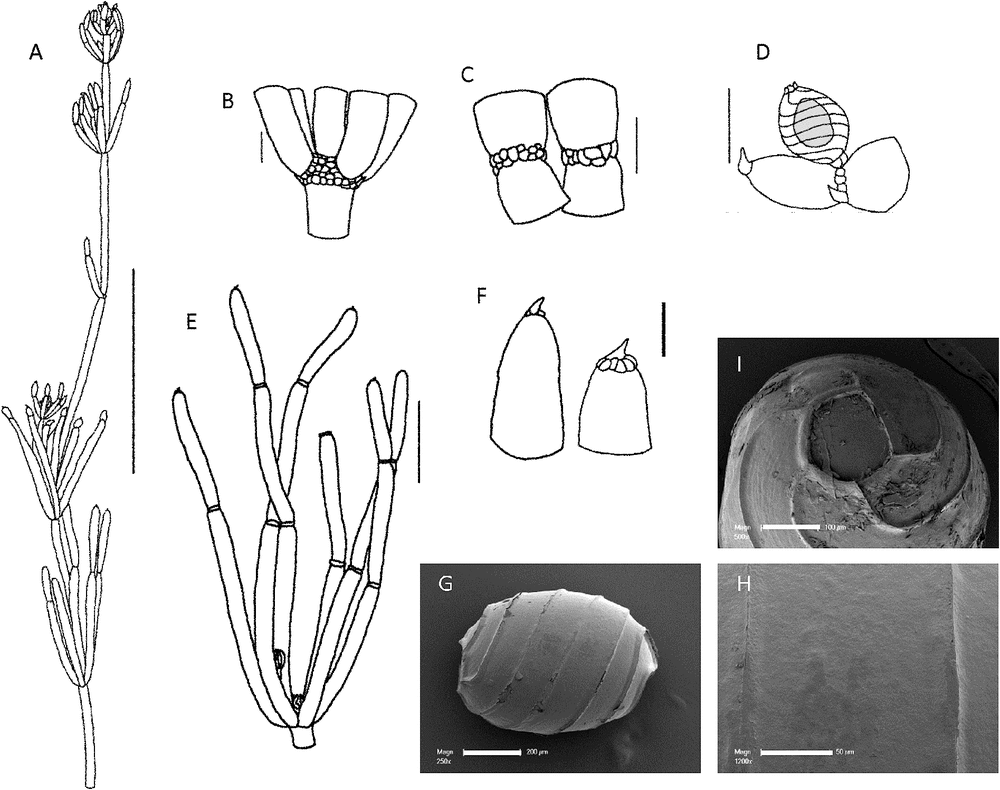
Chara australis R.Br., Prodr. 1: 346 (1810)
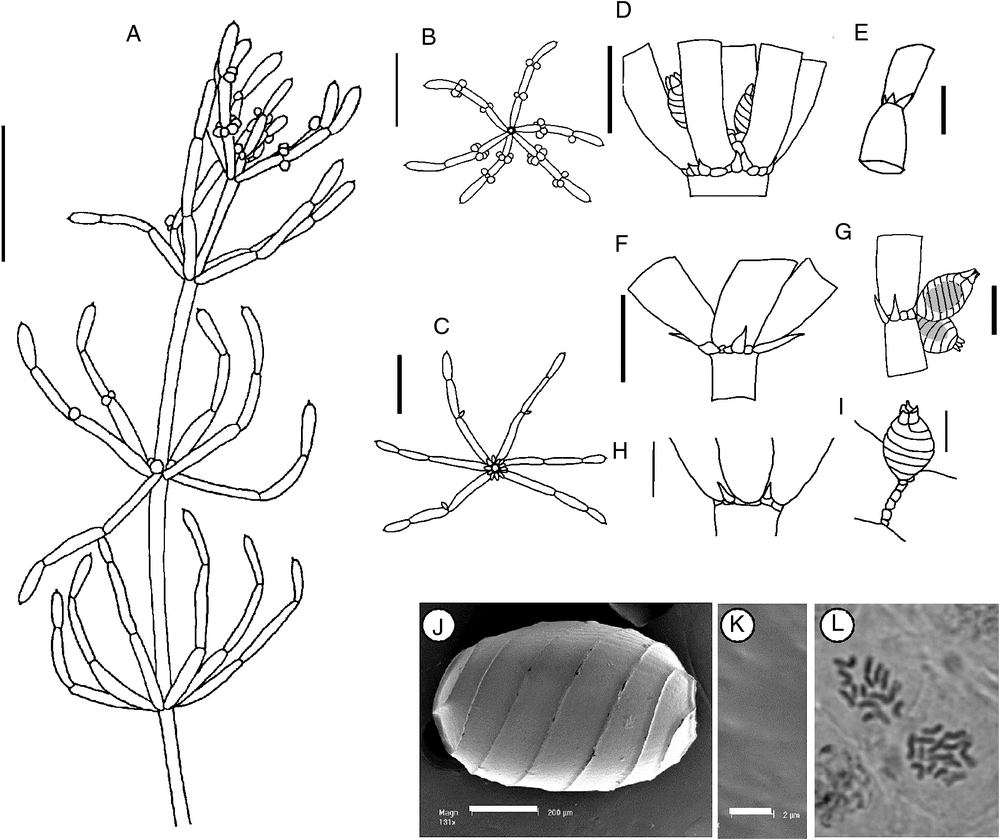
(Fig. 1)
Type: NEW SOUTH WALES: Port Jackson, 1804, R.Brown 277. Holo: BM; iso: LD.
Type: NEW ZEALAND: Whangape [Whangapa?] Lake, T.K[irk] s.n., v.-1870, Lecto: NY (fide Wood and Imahori (1965: 275)); ?isolecto: WELT A9062 n.v., CHR n.v.
Dioecious. Plants robust, translucent and turgid, up to 40 cm high. Axes 0.9–1.75 mm wide, totally ecorticate, internodes 30–100 mm long. No spine cells. Stipulodes in a single row when present, 6–12 in a whorl, up to 0.38 mm long, but often absent or obscure. Branchlets almost uniformly 6 in a whorl, rarely 7, completely ecorticate, 3–5 cells long, cylindrical, 15–43 mm long, basal branchlet cell elongate, up to 15 mm long, branchlet end segments usually a single mucronate cell, sometimes with up to two subtending bract cells, bract cells 2–4 at a node, up to 0.2 mm long, frequently obscure or absent. Branchlets are often strongly incurved at the apices, especially in shallow water. Bracteoles obscure, possibly 2 per oogonium. Gametangia solitary, geminate or aggregate at the lowest 1 or 2 branchlet nodes and inside the base of the whorl, rarely antheridia sessile outside the base of the whorl. Oogonia orange, up to 1 mm long, 0.8 mm wide, coronula cells obtuse to slightly apiculate and usually very small on mature oogonia. Oospores black, cylindrical to ovate, 700–800 µm long, 450–600 µm wide, with 5 or 6 striae, appearing smooth or finely granulate, impression of the basal cell 180–200 µm in diameter. Antheridia up to 800 µm in diameter, orange and often very obvious. Vegetative reproduction by perennial basal internodes and the deposition of starch in the lower and rhizoid nodes. Chromosomes n = 14 (M.T.Casanova p426, p788).
Recognition
Chara australis is the most common totally ecorticate species of Chara in eastern mainland Australia. It can be distinguished as dioecious, robust, ecorticate plants with large gametangia and small, often inconspicuous, stipulodes, bract cells and bracteoles. The internodes are usually as long as or longer than the branchlets and the axes are usually greater than 1 mm in diameter.
Distribution
Occurring in cool, deep, still or slow-flowing water from east of Brisbane in Queensland, south through New South Wales (including the Australian Capital Territory) to Victoria, with an isolated collection from the Flinders Ranges in South Australia. Also occurs in New Zealand.
Etymology
From the Latin ‘australis’, referring to the southern distribution of the species.
Specimens examined
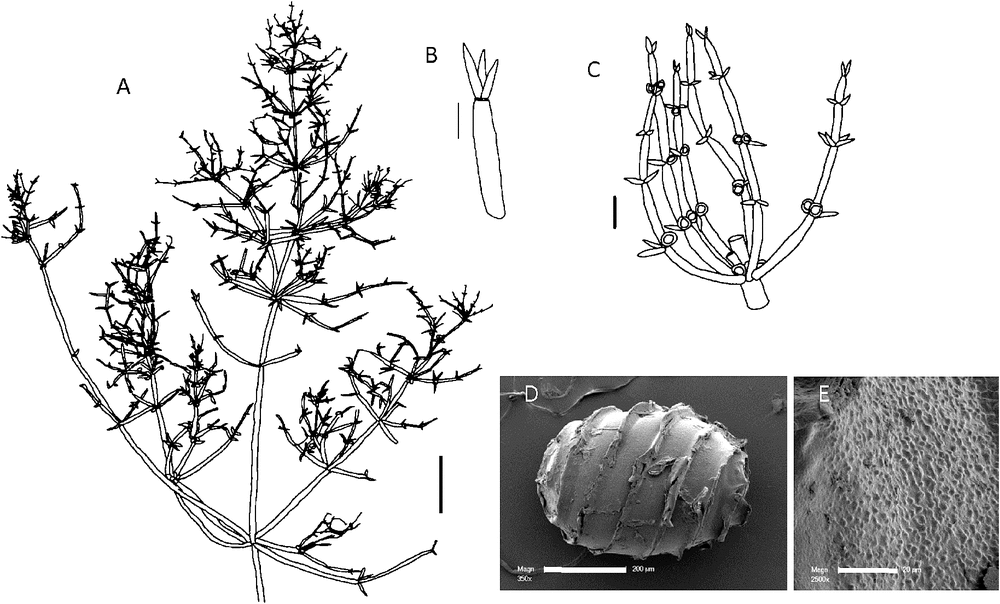
Chara corallina Klein ex Willd., Mém. Acad. Roy. Sci. Hist. (Berlin): 89 (1805)
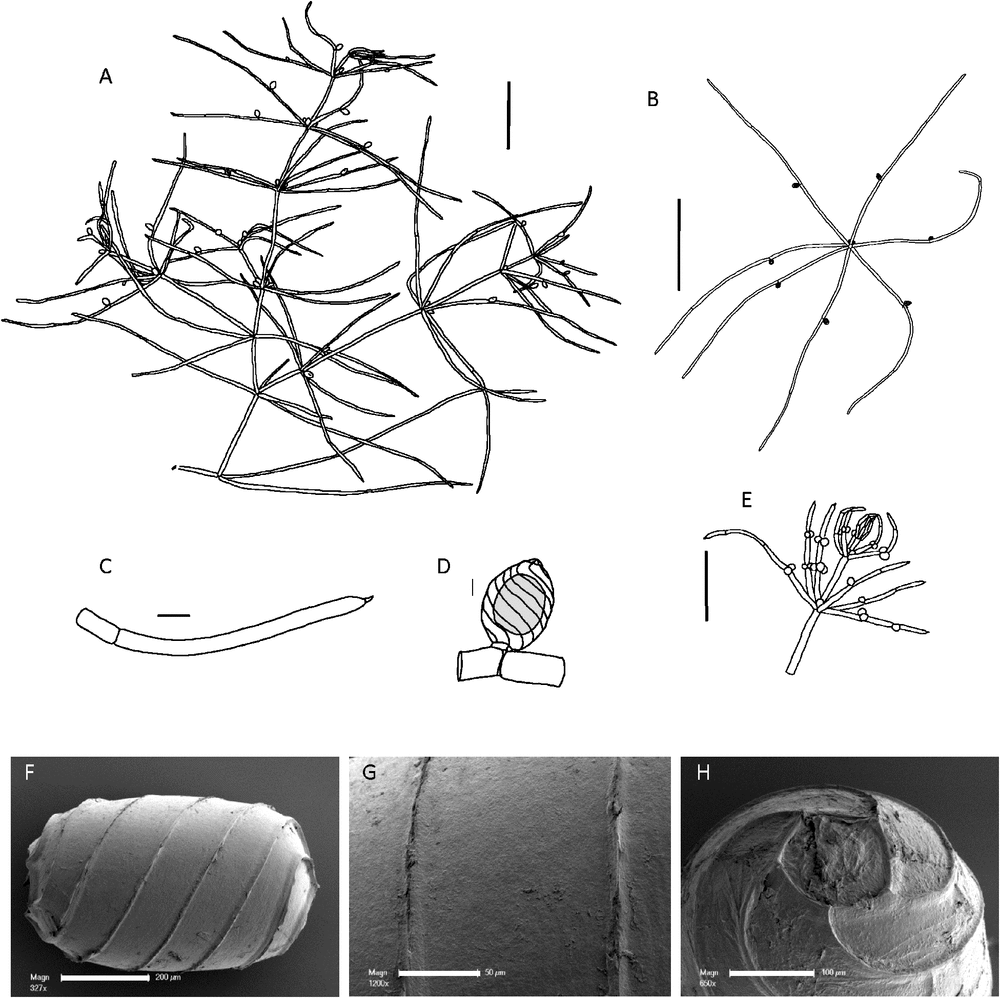
(Fig. 2)
|
Type: INDIA: Wöppanpasi, Tranquebar (Tharamgambadi), near Madras (Chennai), Tamil Nadu, 7 Jan. 1799, Klein 510. Holo: B-W 17105; iso: MEL 2315415 & 2367911.
Monoecious. Plants usually elongate with stout branchlets, up to 40 cm tall, sometimes lightly encrusted with calcium carbonate. Axes up to 1.5 mm in diameter (~2 mm in flattened, preserved specimens), ecorticate, internodes up to 30 mm long, as long as or longer than the branchlets. Stipulodes crowded out by gametangia, 1 per branchlet, up to 380 µm long, often difficult to discern on pressed specimens. Branchlets 6 or 7 in a whorl, 20–60 mm long, somewhat pinched-in at the nodes, and appearing quite wide on pressed material, ecorticate, 4 or 5 cells long, branchlet end segment pointed, conical and acute, basal branchlet cell long, in diameter similar to the other branchlet cells. Bract cells small, 2–4 per branchlet node, 200–600 µm long, bracteoles difficult to distinguish, 2, similar to bract cells. Gametangia conjoined, clustered inside and below the branchlet whorls, and solitary or geminate on the lowest two branchlet nodes. Oogonia 0.8–1.0 mm long, 0.6–0.8 mm wide, coronula cells appressed, slightly apiculate. Oospores black, cylindrical, 600–830 µm long (778 ± 5 µm: Mandal et al. 2008) and 450–600 µm wide, 6 or 7 striae of low ridges, sometimes slightly flanged, the impression of the basal cell 165–180 µm in diameter. In a population from China (Lu & Soulié-Märshe, 1996), a proportion of the oospores had a 6-sided basal-cell impression, rather than a pentagonal basal-cell impression. Antheridia below or adjacent to the oogonia where they are at the same node, 500–750 µm in diameter. The antheridia appear tetrascutate. Chromosomes n = 42 (Khatun et al. 2009).
Nomenclatural notes
Living material of Chara corallina was not available for examination and the above description is based on the type material, the protologue, Nordstedt (1883), Imahori (1954) and the description of Indian material by Wood and Imahori (1964, icon 111). The status of C. corallina var. basilaris A.Braun, with gametangia absent from the branchlets and restricted to the base of the whorls, and C. corallina var. kyusyensis Imahori characterised by an almost complete absence of bract cells and stipulodes, cannot be commented on without reference to fresh specimens. The holotype material of C. corallina is lodged in the Willdenow collection in B, but oospores were not available for examination, apparently having been removed (R. Jahn, pers. comm.). Examination of material in the Sonder Herbarium at MEL revealed two specimens with morphology identical to the type material in B-W. One appears to have been used as the model for Kützing’s (1857) tabulae 80. These have been identified as isotypes of C. corallina, and the material is in good condition compared with the material in B-W (which appears to have been moistened, dissected and re-pressed).
Recognition
Chara corallina is the only monoecious member of Chara sect. Protochara.
Distribution
Chara corallina is apparently widespread in Asia, occurring in India, Bangladesh, Malaysia, Japan and China, at least as far north as Nanjing.
Etymology
The term ‘corallina’ is derived from Latin corallinus meaning ‘coral-red’, referring to the bright colour of the gametangia.
Specimens examined
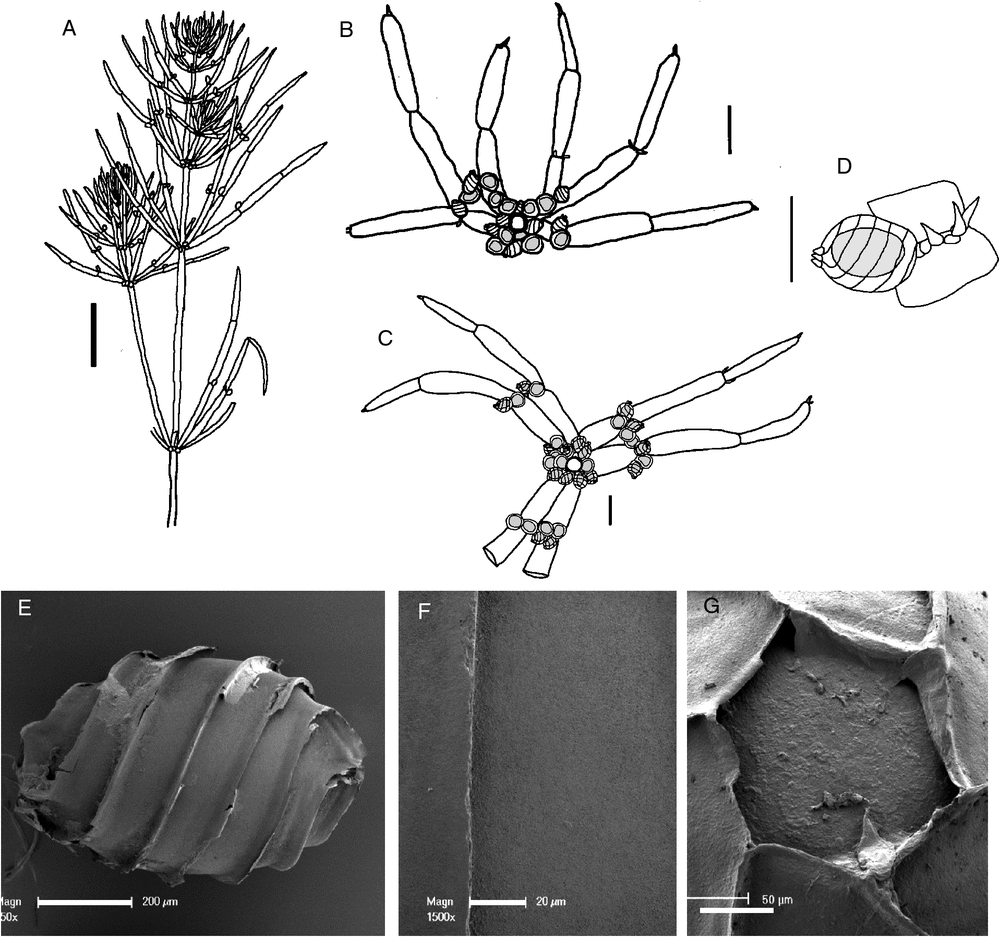
Chara lucida (A.Braun) Casanova & Karol, comb. et stat. nov.
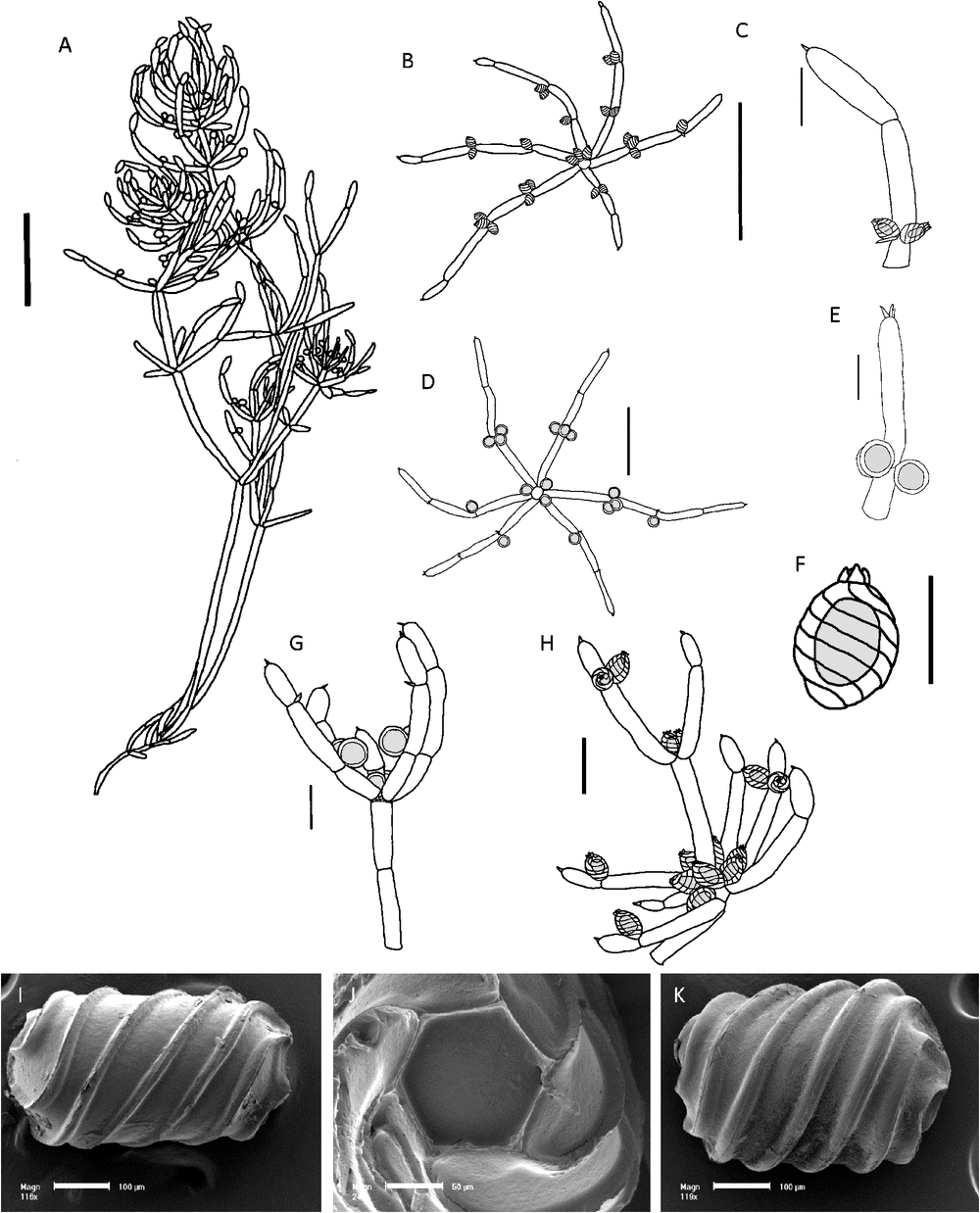
(Fig. 3)
Type: Northern Territory: Victoria River, s. dat., F.Mueller 5. Lecto (fide Zaneveld 1940): LD; isolecto: L. Syn: Northern Territory: Baines Creek, 1856, F.Mueller s.n.: LD.
Type: Gulf of Carpentaria, 1856, F.Mueller s.n. Holo: B (destroyed; fide Wood and Imahori (1965: 276)); iso: LD, L.
Dioecious. Plants flexible, transparent, often in tangled clumps, up to 200 mm tall. Axes 0.35–0.7 mm wide (when flat and dried, narrower in fresh material), ecorticate, internodes 8–20 mm long, generally much shorter than the adjacent branchlets. Stipulodes in a single row, 6–12 in total, usually fewer apparent, up to 0.5 mm long. Branchlets 6 or 7 in a whorl, ecorticate, 11–50 mm long, 0.3–0.6 mm wide, 4 or 5 cells long, basal branchlet cell up to 10 mm long, branchlet end segments small, conical and acute, sometimes subtended by 1 or 2 bract cells, bract cells obscure or short. Bracteoles obscure or short, occasionally up to 0.5 mm long. Fertile parts sometimes somewhat contracted with shorter branchlets and internodes. Specimens with only fertile branchlets were distinguished as form tenerior but recent collections (e.g. M.T.Casanova r758) have both long sterile and short fertile branchlets on the same plant. Gametangia sessile inside the base of the whorl and solitary, geminate or clustered at the lowest 1 or 2 branchlet nodes. Oogonia 0.9 mm long, 0.5 mm wide, coronula cells very short, obtuse. Oospores black, cylindrical, 600–670 µm long, 380–400 µm wide, 7 or 8 striae of low ridges, ornamentation smooth to minutely granulate, basal-cell impression 120–150 µm in diameter at the widest part, edges 95–100 µm long. Antheridia from 400–850 µm in diameter. Vegetative reproduction not known. Chromosomes not known.
Nomenclatural notes
Wood’s (1962) combination Chara corallina f. lucida is here treated as invalid, under Art. 41.5 of the ICN (McNeill et al. 2012). Wood and Imahori (1965) subsequently simultaneously published C. corallina f. lucida and C. lucida, the latter at the rank of ‘microspecies’, equivalent in rank to Wood’s forms as well as his ‘monotypic’ varieties and species (i.e. those lacking subordinate taxa). Both these names are here treated as invalid alternative names, under Art. 36.2 of the ICN (McNeill et al. 2012).
Taxonomic notes
Zaneveld (1940) distinguished the following two formae in his concept of C. australis var. lucida, on the basis of material from New Guinea and northern Australia: f. typica, with somewhat stout (~600 µm wide), long branchlets, and f. tenerior, with shorter, narrower (~350 µm wide) branchlets. New collections from the Northern Territory (M.T.Casanova r784, r758) represent material with both long and short branchlets on apical parts of the same plant, and branchlet width varies in relation to branchlet length. Additionally, specimens from the Northern Territory and the Gulf of Carpentaria have identical oospores, and accordingly, no infraspecific taxa are here recognised in this species.
Recognition
The species can be distinguished as a totally ecorticate Chara species with narrow axes, distinguished from narrower morphs of C. australis by the short internodes in comparison with the branchlets. C. lucida forms tangled masses in warm waters, compared to the upright, turgid stems of C. australis in colder, deeper waters.
Distribution
Tropical and subtropical Queensland, Western Australia and the Northern Territory in dams, ponds, still rivers and lagoons. Also occurs in Papua New Guinea.
Etymology
From the Latin ‘lucidus’, meaning shining, clear, transparent. Zaneveld (1940) and Wood (1971) thought this referred to a shiny surface on the pressed specimens, but that feature is neither obvious nor consistent. More likely, it refers to the clear and transparent, almost colourless appearance (fide Nordstedt 1883) of the pressed type material.
Specimens examined
Chara porteri Casanova, sp. nov.
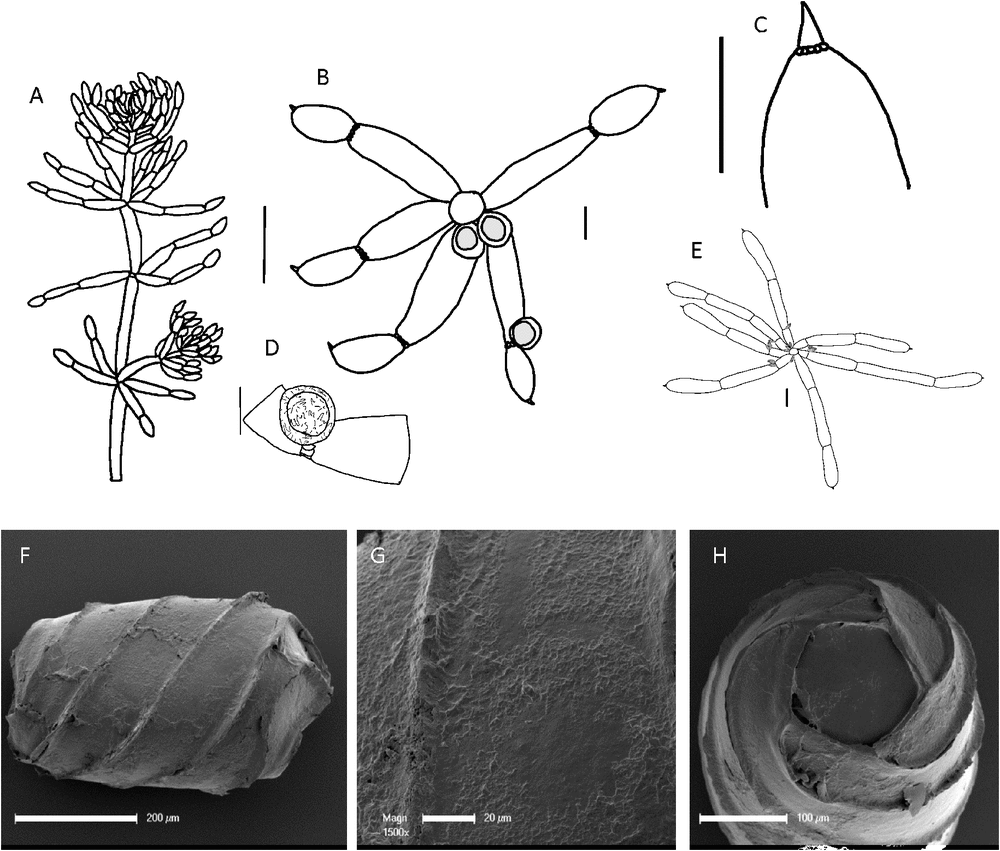
(Fig. 4)
Type: Western Australia: Ethel Creek Claypan, Pilbara Biological Survey site 041, T2, 1 June 2004, M.N.Lyons & D.A.Mickle 3077. Holo: PERTH; iso: MEL.
Dioecious. Plants somewhat inflated and turgid, sometimes calcified, up to 150 mm tall, somewhat densely branched, with in-curved branchlets in shallow water. Axes 0.7 mm wide (up to 1 mm on pressed specimens), ecorticate, internodes 8–25 mm long, shorter and somewhat contracted at the apices. Rarely internodes appear 2 cells long. Stipulodes in a single row when present, frequently obscure or absent. Branchlets 6 in a whorl, sometimes made up of a few elongate branchlets and some dwarf branchlets, particularly at the apices, ecorticate, 8–15 mm long, 0.4–0.6 mm wide, 3 or 4 cells long, basal branchlet cell up to 6 mm long, usually the longest cell in the branchlet, branchlet end segments small, conical and acute, occasionally subtended by bract cells, bract cells usually obscure, up to 0.2 mm long, or absent. Bracteoles obscure, up to 0.2 mm long, or absent. Gametangia on separate plants, sessile inside the base of the whorl, and solitary, geminate or clustered at the lowest 1 or 2 branchlet nodes. Oogonia 0.6–0.75 mm long, 0.5 mm wide, coronula cells short, somewhat spreading, slightly apiculate. Oospores black, cylindrical, 470–525 µm long, 250–350 µm wide, 5 or 6 striae of wide ridges (pachygyra), ornamentation smooth to minutely granulate, basal-cell impression 100–120 µm in diameter at the widest part, edges 70–76 µm long, rarely 6-sided. Antheridia octoscutate up to 850 µm in diameter. Vegetative reproduction not known. Chromosomes n = 14 (M.T.Casanova r416).
Recognition
Specimens are distinctly turgid and brightly festooned with gametangia, with incurved branchlets. The oospores have markedly enlarged striae (pachygyra).
Distribution
Occurs in temporary wetlands (claypans and riparian overflows) in the Paroo region of New South Wales, the Pilbara region of Western Australia and in south-western Queensland.
Etymology
Named in honour of John L. Porter, who collected the first herbarium material of this species, from the Paroo region in New South Wales.
Specimens examined
Chara protocharoides Casanova & Karol, nom. nov.
(Fig. 5)
Type: Western Australia: In shallow swamp on peneplain of breakaway country between Mingenew and Irwin River coal seam, south-west of Geraldton, 28 Aug. 1947, H.B.S.Womersley s.n. Holo: AD A5917a; Iso: AD A5917c, NY 01089127.
Dioecious. Plants up to 40 cm high, somewhat branched, variably inflated. Axes ecorticate, internodes 10–100 mm long, 0.9–1.5 mm in diameter in life (up to 2 mm when flattened in pressing). Stipulodes completely absent. Branchlets 6 or 7 in a whorl, entirely ecorticate, segments swollen or inflated in shallow water, elongate and cylindrical in deep-water populations, pinched in at the nodes, similar in diameter to the axes, 14–30 mm long, 3 or 4 cells long including end segment, basal branchlet cell variable in size, ~300 µm long in upper, fertile branchlets, similar to second branchlet segments on sterile branchlets, branchlet end segments unicellular, very small and mucronate, up to 200 µm long, with nodal cells at the base, bract cells and bracteoles completely absent from all branchlet nodes. Upper axes somewhat contracted. Gametangia on separate plants, singly and geminate on first and second branchlet nodes, occasionally oogonia inside the base of the branchlet whorl. Oogonia 670–900 µm long × 600–780 µm wide with 6 or 7 convolutions, coronula cells connivent and blunt, 80 µm high. Oospores black, 490–560 µm long and 310–390 µm wide, almost rectangular in side view, with 4 or 5 striae, sometimes flanged, and impression of the basal cell 80 µm in diameter. Antheridia 800–1150 µm in diameter, octoscutate. Vegetative reproduction not known; appears to be an annual in temporary water bodies. Chromosomes not known.
Taxonomic notes
Originally placed in the segregate genus Protochara on the basis of very simple, inflated axes and the lack of accessory cells (bract cells, stipulodes or bracteoles) (Womersley and Ophel 1947). Apart from the absence of any accessory cells, type material of Protochara australis is otherwise consistent with the morphology of the genus Chara. The epithet australis is preoccupied in Chara (C. australis R.Br., 1810), and a new name is required for this taxon.
Recognition
The complete absence of stipulodes, bract cells and bracteoles are the main characteristics that allow this species to be distinguished.
Distribution
Grows in permanent, seasonal and temporary water bodies in Western Australia.
Etymology
From the Latin ‘protocharoides’ in reference to the genus Protochara for which this species remains the type.
Specimens examined
Chara stuartiana (F.Muell. ex Kütz.) Casanova & Karol, comb. et stat. nov.
(Fig. 6)
Type: Tasmania: South Esk River, R.C.Gunn 1565, 1852. Holo: L; iso: PC.
Dioecious. Plants large and inflated, transparent and turgid, up to 27 cm high, but fragile, fragmenting easily when handled. Axes 1.5–2.8 mm wide, ecorticate, internodes 30–120 mm long. Stipulodes in a single row, tiny, up to 12 per whorl, up to 400 µm long, alternate to the branchlets and pointing upwards. Branchlets 6 in a whorl, 20–30 mm long, 1.6–2.8 mm wide, 3 or 4 cells long, all cells cylindrical, end segment a single cell, or subtended by small bract cells, basal branchlet cell elongate and cylindrical, the second branchlet cell is usually shorter than the basal branchlet cell, abbreviated and sometimes almost spherical, particularly on the upper parts of the axis; bract cells 2 or 3 at branchlet nodes, up to 400 µm long. Bracteoles 2, up to 200 µm long. Gametangia on separate plants, solitary or geminate at all branchlet nodes. Oogonia at the lowest branchlet node, up to 1 mm long and 700 µm wide, with 8 convolutions, coronula very small and minutely apiculate. Oospores black, cylindrical to ovate, 615–700 µm long, 450–550 µm wide, with 6 or 7 striae of low ridges, basal-cell impression up to 140 µm in diameter at widest point, edges 85–115 µm long. Antheridia up to 500 µm in diameter, solitary, geminate or aggregate at the lowest branchlet node and inside the base of the whorl. Vegetative reproduction by starch accumulations in the lower and rhizoidal nodes. Chromosomes not known.
Nomenclatural notes
Wood’s (1962) combination Chara corallina f. stuartiana is here treated as invalid, under Art. 41.5 of the ICN (McNeill et al. 2012). Wood and Imahori (1965) subsequently simultaneously published C. corallina f. stuartiana and C. stuartiana, the latter at the rank of ‘microspecies’, equivalent in rank to Wood’s forms as well as his ‘monotypic’ varieties and species (i.e. those lacking subordinate taxa). Both these names are here treated as invalid alternative names, under Art. 36.2 of the ICN (McNeill et al. 2012).
Taxonomic notes
Braun (1852) included a specimen collected by C. Stuart in Tasmania (Stuart 1565) in Chara australis. Kützing (1857) named the species Nitella stuartiana based on Stuart 1565 and provided an illustration (t. 28). Braun (1860) listed a collection from Tasmania, Gunn 1565, in his concept of C. australis. This is likely to refer to the same specimen as cited by Braun (1852) and Kützing (1857), and was collected by Gunn rather than Stuart, because the specimens attributed to Gunn have collection numbers for charophytes in the vicinity of Launceston (and the South Esk River) in the 1560s, and Stuart’s collection numbers for the South Esk River are either very low (3, 4, 5), in the 210s or in the 1020s. Only two specimens in sect. Protochara collected from Tasmania in the 1800s are known, including material used by Kützing for his illustration, almost certainly obtained from Sonder’s herbarium (L), and a specimen labelled ‘Nitella australis R.Br. Donné par Sir William Hooker, 1863; Ex Herb. Hook. Chara australis Br, Tasmania, Coll. R.C.Gunn’ (PC). It is possible that these are parts of the same gathering, and therefore the PC specimen would constitute isotype material. Braun, in Nordstedt (1883), retained N. stuartiana in synonymy of C. australis, but cited another specimen from Tasmania (Oldfield 283 n.v.) as C. australis var. nobilis. Zaneveld (1940) treated N. stuartiana as a form of C. australis var. nobilis. Proctor (1999) found that specimens identified as C. australis and C. stuartiana could be crossed to produce oospores, and explained C. stuartiana as a ‘genetically fixed morphotype of C. australis’. Examination of the type material and more recent collections indicated that most of specimens from Tasmania in sect. Protochara can be clearly separated from C. australis on the basis of more robust axes and branchlets, complex axial and branchlet nodes, overall fragility and different oospores. C. stuartiana is rarely collected as a whole plant, because the heavy, turgid axes are fragile and tend to break as they are removed from the water. The species still occurs in what is presumed to be the type locality, namely, the first basin of Cataract Gorge in Launceston. Specimens collected from Kimberley Warm Springs (T.Dugdale & S.Talman 20, HO, MEL, NY; H. & A.Wapstra HAW002, MEL, HO) appear to be C. australis rather than C. stuartiana, but if so, that is the only occurrence of C. australis known from Tasmania.
Recognition
Chara stuartiana has large, fragile, often inflated, ecorticate axes and branchlets, with orange antheridia and oogonia. It can be distinguished from C. australis by its wide axes and branchlets, and by the abbreviated penultimate cell on the upper branchlet whorls.
Distribution
Grows in Tasmania in rivers and streams, at depth.
Etymology
Named for Charles Stuart (1802–1877) who collected charophytes in Tasmania from 1842 to 1852, initially for R.C.Gunn, later for F. von Mueller (Orchard 1999).
Specimens examined
Chara wallichii A.Braun in C.F.O. Abh. Königl. Akad. Wiss. Berlin 1882: 107 (1883)
(Fig. 7)
Type: INDIA: Gangetic Plain, leg. ign., 9 Jan. 1809. Holo: LINN, n.v.
Dioecious. Plants up to 25 cm high, prickly in appearance. Axes ecorticate, 875–1100 µm wide, internodes somewhat shorter than the branchlets. Stipulodes well developed and somewhat inflated where present, up to 450 µm long. Branchlets 6 in a whorl, ecorticate, up to 30 mm long, 4–6 cells long, basal branchlet cell longest, the end segment short, conical and acute. Bract cells well developed, 1–4 at a node, up to 2 mm long. Gametangia on separate plants, clustered at the base of the whorl and singly or geminate at all branchlet nodes. Oogonia up to 745 µm long, 700 µm wide, with 7 or 8 convolutions. Oospores 500–610 µm long, 330–440 µm wide with 6 or 7 flanged ridges. Antheridia 750–950 µm in diameter, tetrascutate (Wood and Imahori 1965). Chromosomes not known.
Nomenclatural notes
Wood’s (1962) combination Chara corallina f. wallichii is here treated as invalid, under Art. 41.5 of the ICN (McNeill et al. 2012). Wood and Imahori (1965) subsequently simultaneously published C. corallina var. wallichii and C. wallichii, the latter at the rank of ‘microspecies’, equivalent in rank to Wood’s forms as well as his ‘monotypic’ varieties and species (i.e. those lacking subordinate taxa)). C. corallina var. wallichii is here treated as invalid, under Art. 36.2 of the ICN (McNeill et al. 2012).
Recognition
Chara wallichii is a dioecious species that, in contrast to most other species in the group, is distinguished by well developed, somewhat inflated, stipulodes and bract cells. Material seen in BM and BP is relatively uniform.
Distribution
India and Bangladesh.
Etymology
Named in honour of Nathaniel Wallich (1786–1854), a Danish botanist involved in the development of the Calcutta Botanic Garden.
Specimens examined
Additional taxa of uncertain status
Tolypellopsis simplicissima Fil., Arch. Hydrobiol., Suppl. 12, Tropische Binnengewässer 3: 716 (1934)
Known only from the type gathering from Lake Toba, a deep, oligotrophic caldera in Sumatra, in 1929. The morphology of this species is similar to that of C. australis; however, the oogonia are much smaller and more spherical than those of any other species in sect. Protochara. Because of its overwhelming morphological similarity to other species in sect. Protochara, it is likely to be a species of Chara. It does not appear to have many accessory cells (stipulodes, bract cells); however, further collections of mature female material is required for confirmation of the identity and placement of this taxon.
Chara plebeja R.Br. ex A.Braun, Linnaea 17: 118 (1843)
Known only from the type gathering from ‘Carpentaria Point’, possibly the ‘Carpentaria Main’ of Brown’s and Flinders’ diaries, now known as Cape Shield (Groves and Moore 1989), in Arnhem Land near Groote Eylandt in the Gulf of Carpentaria. Satellite images of the locality of Cape Shield revealed a hind-dune wetland area that could be where Brown collected this species. Treated as a subspecies of C. australis by Braun in Nordstedt (1883) and as a variety of C. australis by Zaneveld (1940), and distinguished from C. australis sens. str. on the basis of obtuse end cells on the branchlets. Detailed examination of the type material revealed a few stipulodes and bract cells, and what appear to be obtuse end cells are probably penultimate cells, with the end cells dehiscent. Given the possible habitat and evidence from the morphology (7 or 8 branchlets rather than 6, stipulodes opposite the branchlet and somewhat decumbent), it seems likely that C. plebeja is a species of Lamprothamnium. If so, it would be the only known coastal Lamprothamnium from the tropics. Further collections of fertile female material are required to confirm this.
Chara sp. Woorndoo (M.T.Casanova p246)
This specimen is likely to represent a new species. Oospores are similar in appearance to those of C. porteri, but are over 700 μm long (c.f. C. porteri with oospores 470–525 µm long), and its distribution is disjunct from C. porteri. However, there are insufficient gatherings to confirm its status at the present time.
Several other species have been recorded from the Indian subcontinent (Chara fulgens Fil., C. pashanii S.C.Dixit and C. nuda Pal) and oceanic islands of the southern hemisphere (Chara socotrensis Nordst. from Socotra, C. corallina f. vieillardii (A.Braun) R.D.Wood from New Caledonia, C. corallina f. vitiensis Nordst. from Fiji and C. corallina f. mascarensis J.Groves & Stephens, nom. inval., nom. prov. from Mauritius); however, because specimens have not been examined, no comment on their status can be made in the present study.
Discussion
Species in sect. Protochara have few characters to aid discrimination, a fact that has contributed to the confused history of generic and species names in this group. Axis and branchlet diameter and the expression of accessory cells (stipulodes, bract cells) can allow discrimination in well grown plants; however, the occurrence and expression of basal gametangia and abundance of branchlet gametangia probably vary in relation to plant nutrition and age and there are examples of specimens that fail to conform to the ‘average’ morphology (e.g. from cultured material, young plants growing in shallow water, or etiolated plants from deep water). Reference to the oospore morphology is necessary to distinguish such doubtful specimens.
Separation of Chara sect. Protochara and the genus Lamprothamnium (also ecorticate, sometimes inflated, sometimes with few accessories) on the basis of vegetative morphology will always be something of a challenge, particularly with material lacking diagnostic characters. These two taxa appear to be closely related on the basis of nucleotide-sequencing studies (Meiers et al. 1999; McCourt et al. 2000). The main characters to aid identification are that Lamprothamnium species usually have more than six branchlets in a whorl, their stipulodes are opposite and decumbent when present, and the oospores possess gyrogonites (a calcified covering). Additionally, most members of sect. Protochara occur in freshwater, whereas Lamprothamnium species occur in brackish to saline water.
Chara braunii (sect. Charopsis) can be separated from members of sect. Protochara phylogenetically (Meiers et al. 1999; McCourt et al. 2000) and morphologically. C. braunii has well developed stipulodes and bract cells, and the branchlets are terminated by a ‘corona’ of approximately three equal-sized cells, rather than a single cell, as in sect. Protochara. C. braunii is a relatively ephemeral charophyte, and is rarely found in Australia, with most specimens referred to C. braunii in Australian herbaria being referable to C. muelleri.
Acknowledgements
Thanks go to Professor Jim Ross, who, early in the project, said ‘if you don’t do it, who will?’. For this study, access to type material in various European (B, BM, BP, GFW, GOET, HBG, L, LD, PC and W) and Australian (AD, BRI, CANB, DNA, HO, MEL, NSW, PERTH) herbaria was essential; I thank the directors and staff of these institutions for their kind assistance and permission to remove oospores for examination. Many people have assisted with providing specimens, fresh and preserved, since 2000, some of their names are noted in the specimen lists. This study would have been impossible without their efforts. Mary Beth Williams provided notes, translations and literature in an early part of this study. Professor W. Prud’homme van Reine offered valuable references from Joop van Raam’s literature collection. Funds for this work were provided by the Australian Biological Resources Study Grant number 209-49, Bush Blitz and Charophyte Services, and by the US National Science Foundation under Grant numbers DEB-1020660 and DEB-1036466. We thank Joan Powling for her meticulous proofreading and an anonymous referee for suggesting improvements. The assistance of Brendan Lepschi, Peter Wilson and Nick Turland with difficult nomenclatural issues is greatly appreciated.
References
Braun A (1852) Characeae. F. L. von Schlechtendal plantae Mullerianae. Linnaea 25, 705–709.Braun A (1860) Characeae. In ‘The Botany of the Antarctic Voyage III. Flora Tasmaniae. Vol. 2’. (Ed. JD Hooker) pp. 159–160. (Lovell Reeve: London)
Brown R (1810) ‘Prodromus florae novae hollandiae et insulare van diemen. Vol. 1.’ (Richard Taylor and Sons: London)
Casanova MT (1997) Oospore variation in three species of Chara (Charales, Chlorophyta). Phycologia 36, 274–280.
| Oospore variation in three species of Chara (Charales, Chlorophyta).Crossref | GoogleScholarGoogle Scholar |
Casanova MT (2004) A census of submerged plants of the Angas River and Tookayerta Creek catchments. Consultants report to the River Murray Catchment Management Board. (Charophyte Services: Lake Bolac, Vic.)
Casanova MT (2005) An overview of Chara L. in Australia (Characeae, Charophyta). Australian Systematic Botany 18, 25–39.
| An overview of Chara L. in Australia (Characeae, Charophyta).Crossref | GoogleScholarGoogle Scholar |
Casanova MT (2009) An overview of Nitella in Australia (Characeae, Charophyta). Australian Systematic Botany 22, 193–218.
| An overview of Nitella in Australia (Characeae, Charophyta).Crossref | GoogleScholarGoogle Scholar |
Casanova MT (2013) Lamprothamnium in Australia (Characeae, Charophyta). Australian Systematic Botany 26, 268–290.
| Lamprothamnium in Australia (Characeae, Charophyta).Crossref | GoogleScholarGoogle Scholar |
Crawford SA, Higgins SJ, Mulvaney P, Wetherbee R (2001) Nanostructure of the diatom frustule as revealed by atomic force and scanning electron microscopy. Journal of Phycology 37, 543–554.
| Nanostructure of the diatom frustule as revealed by atomic force and scanning electron microscopy.Crossref | GoogleScholarGoogle Scholar |
Filarszky F (1934) Die Characeen der Deutschen Limnologischen Sunda-Expedition. Archiv fuer Hydrobiologie 4, 705–726.
García A, Chivas AR (2006) Diversity and ecology of extant and Quaternary Australian charophytes (Charales). Cryptogamie. Algologie 27, 323–340.
García A, Karol KG (2004) A paradigm in the taxonomy of charophytes: the oospore and gyrogonite of Nitellopsis inflata (Fil. & G.O.Allen ex Fil.) = Lamprothamnium inflatum comb. nov. In ‘Proceedings of the Fourth International Congress on Extant and Fossil Charophytes’, October 2000, Robertson, NSW, Australia. (Eds A García, A Chivas) p. 42. (University of Wollongong: Wollongong, NSW)
Groves EW, Moore DT (1989) A list of the cryptogams and gymnospermous plant specimens in the British Museum (Natural History) gathered by Robert Brown in Australia 1801–5. Proceedings of the Linnean Society of New South Wales 111, 65–102.
Imahori K (1954) ‘Ecology, Phytogeography and Taxonomy of the Japanese Charophyta.’ (Kanazawa University: Kanazawa, Japan)
Khatun W, Ud-Deen MM, Kabir G (2009) Karyotype of some species of Chara and Nitella from Bangladesh. Bangladesh Journal of Botany 38, 205–207.
Kützing FT (1843) ‘Phycologia generalis, oder Anatomie, Physiologie und Systemkunde der Tange. Vol. 2’. (F.A. Brockhaus: Leipzig)
Kützing FT (1857) ‘Tabulae Phycologiae, oder Abbildungen der Tange. Vol. 7’, pp. 10–32. (Nordhausen)
Macdonald MB, Hotchkiss AT (1956) An estipulodic form of Chara australis R.Br. (= Protochara australis Woms. and Ophel). Proceedings of the Linnean Society of New South Wales 80, 274–284.
Mandal DK, Ojha SN, Ray S (2008) Species-specificity of oospore dimensions in three ecorticate species of Chara (Charales, Charophyceae). Aquatic Botany 88, 181–183.
| Species-specificity of oospore dimensions in three ecorticate species of Chara (Charales, Charophyceae).Crossref | GoogleScholarGoogle Scholar |
McCourt RM, Karol KG, Proctor V, Feist M, Delwiche CF (2000) Molecular phylogeny of tribe Charae (Characeae) based on rbcL sequences, with comments on classification of Chara sensu Wood. In ‘The Third International Symposium on Extant and Fossil Charophytes’, October 2000, Nanjing, Peoples Republic of China. (Eds W Qifei, L Hui-nan, I Soulié-Märche) p. 29. (Nanjing Institute of Geology and Palaeontology, Academia Sinica: Nanjin, China)
McNeill J, Barrie FR, Buck WR, Demoulin V, Greuter W, Hawksworth D, Herendeen PS, Knapp S, Marhold K, Prado J, Prud’homme van Reine WF, Smith GF, Wiersema JH, Turland NJ (Eds) (2012) International Code of Nomenclature for algae, fungi and plants (Melbourne Code), adopted by the Eighteenth International Botanical Congress Melbourne, Australia, July 2011. (International Association for Plant Taxonomy: Bratislava, Slovakia) Available at http://www.iatp-taxon.org/nomen/main.php [Verified September 2013]
Meiers ST, Proctor VW, Chapman RL (1999) Phylogeny and biogeography of Chara (Charophyta) inferred from 18S rDNA sequences. Australian Journal of Botany 47, 347–360.
| Phylogeny and biogeography of Chara (Charophyta) inferred from 18S rDNA sequences.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXlsFynsLk%3D&md5=695c15837dc05154a475d40a0a3bb580CAS |
Nordstedt CFO (1883) Fragmente einer Monographie der Characeen. Abhandlungen der Königlichen Akademie der Wissenschaften in Berlin 1882, 1–211.
Orchard AE (1999) A history of systematic botany in Australia. In ‘Flora of Australia. Vol. 1’. 2nd edn. (Ed. AE Orchard) pp. 11–103. (ABRS: Canberra)
Proctor VW (1980) Historical biogeography of Chara (Charophyta): an appraisal of the Braun–Wood classification plus a falsifiable alternative for future consideration. Journal of Phycology 16, 218–233.
| Historical biogeography of Chara (Charophyta): an appraisal of the Braun–Wood classification plus a falsifiable alternative for future consideration.Crossref | GoogleScholarGoogle Scholar |
Proctor VW (1999) Charophytivory, Playas y Papalotes, a local paradigm of global relevance. Australian Journal of Botany 47, 399–406.
| Charophytivory, Playas y Papalotes, a local paradigm of global relevance.Crossref | GoogleScholarGoogle Scholar |
Willdenow CL (1805) Du genere nommé Chara. Mémoires de l’Académie Royale des Sciences 1803, 79–90.
Womersley HBS, Ophel IL (1947) Protochara, a new genus of Characeae from Western Australia. Transactions of the Royal Society of South Australia 71, 311–317.
Wood RD (1962) New combinations and taxa in the revision of the Characeae. Taxon 11, 7–25.
| New combinations and taxa in the revision of the Characeae.Crossref | GoogleScholarGoogle Scholar |
Wood RD (1971) Characeae of Australia. Nova Hedwigia 22, 1–120.
Wood RD, Imahori KI (1964) Iconograph of the Characeae. In ‘A Revision of the Characeae. Vol. 2’. (Eds RD Wood, KI Imahori) pp. 1–395. (Cramer: Weinheim, Germany)
Wood RD, Imahori KI (1965) Monograph of the Characeae. In ‘A Revision of the Characeae. Vol. 1’. (Eds RD Wood, KI Imahori) pp. 1–904. (Cramer: Weinheim, Germany)
Zaneveld JS (1940) The Charophyta of Malaysia and adjacent countries. Blumea 4, 1–223.