Taxonomic revision of Riccia (Ricciaceae, Marchantiophyta) in the monsoon tropics of the Northern Territory, Australia
D. Christine Cargill A B H , Karen Beckmann C F and Rod Seppelt D E G
A B H , Karen Beckmann C F and Rod Seppelt D E G
A Australian National Botanic Gardens, GPO Box 1777, Canberra, ACT 2601, Australia.
B Australian National Herbarium, Centre for Australian National Biodiversity Research, (a joint venture between the Director of National Parks and CSIRO), GPO Box 1700, Canberra, ACT 2601, Australia.
C Royal Botanic Gardens, Birdwood Avenue, Melbourne, Vic., Australia.
D Tasmanian Herbarium, College Road, Sandy Bay, Hobart, Tas., Australia.
E Biological Sciences, Macquarie University, Balaclava Road, North Ryde. NSW 2109, Australia.
F Present address: PO Box 353, Monbulk, Vic. 3793, Australia.
G Present address: 1 Lothian Avenue, Bundall, Qld 4217, Australia.
H Corresponding author. Email: chris.cargill@environment.gov.au
Australian Systematic Botany 34(4) 336-430 https://doi.org/10.1071/SB20030
Submitted: 12 November 2020 Accepted: 31 March 2021 Published: 25 June 2021
Journal Compilation © CSIRO 2021 Open Access CC BY-NC
Abstract
The genus Riccia L. in the monsoon tropics of the Northern Territory north of the 18°S latitude is revised. Sixteen species are described in detail, including four new species (R. abdita Cargill, R. chrysocrinita Cargill, R. obchantiana Cargill and R. verrucosa Cargill), with accompanying images and line drawings. A key to the species and distribution maps are provided.
Keywords: liverworts, northern Australia, Riccia abdita, R. chrysocrinita, R. obchantiana, R. verrucosa.
Introduction
Riccia L. is the largest and most diverse genus within the complex thalloid liverworts (Class Marchantiopsida; Jovet-Ast 2000; Cargill et al. 2016), with over 200 species. Along with the monotypic genus Ricciocarpos Corda, it is one of two genera in the family Ricciaceae. The Ricciaceae are uniquely characterised by the capsule borne within thallus tissue and lacking a seta or foot and elaters. Riccia species are further characterised by a strap-shaped thallus that may be unbranched but is typically bifurcating in a Y-pattern either symmetrically or asymmetrically (for a more detailed description, see Cargill et al. 2016). The genus is most diverse in Mediterranean-type environments, particularly in southern Africa (~50 species, Perold 1999), Australia (~50 species, https://biodiversity.org.au/nsl/services/APNI, NSL, 11 March 2018), North America (43 species, A. Hagborg and L. Söderström, pers. comm., 2015), South America (~25 species, Hässel de Menéndez and Rubies 2009) and India (36 species, Singh 2014; see Cargill et al. 2016).
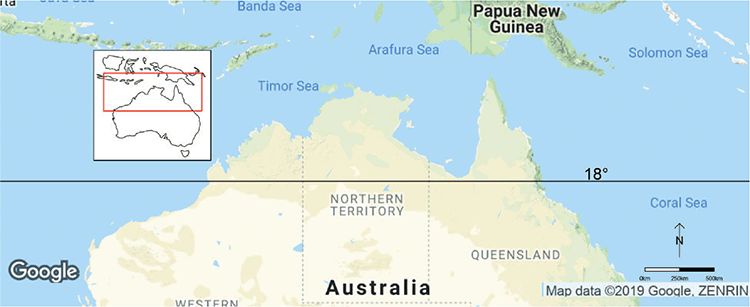
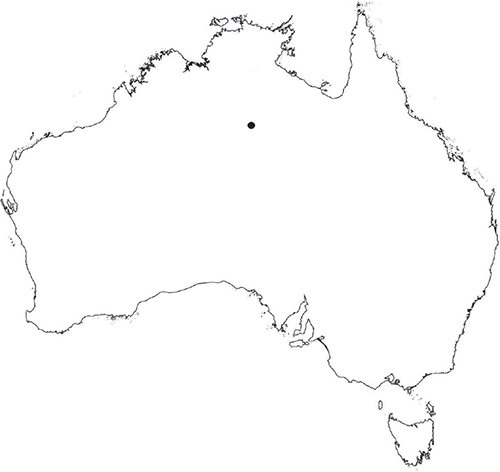
The Northern Territory is one of the least explored regions for bryophytes in Australia (Cargill et al. 2016). The northern part of the Northern Territory (north of 18°S latitude; Fig. 1) is characterised by a monsoonal climate with distinct wet and dry seasons. South of this latitude, the climate is semi-arid to arid, with variable rainfall and temperatures ranging from a minimum −7°C in winter to over 40°C in summer.
|
Of the almost 33 000 specimens of Australian liverworts (Marchantiophyta) currently held and databased in Australasian herbaria (The Australasian Virtual Herbarium 2021), only 748 collections are from the Northern Territory, and almost half of these (320 collections) are Riccia. Of these collections, 91 are from the northern part of the Northern Territory. The small number of collections may reflect unfavourable environmental conditions for Riccia, or the lack of intensive collecting of bryophytes in the area, or both. Collections have been made in the Northern Territory by botanists outside of Australia and duplicates have been deposited in Australian herbaria; however, the number of specimens that may have been deposited only in non-Australian herbaria is unknown. In the 2002 Catalogue of Australian Mosses, 113 species of mosses were listed for the Northern Territory (Streimann and Klazenga 2002), compared to 1074 Australia wide (10.5% of total). Only 38 species of liverworts and hornworts were listed for the Northern Territory by McCarthy (2003), compared to 869 Australia wide (4.4% of total). Table 1 lists all known species of Riccia recorded for the Northern Territory before this revision. During the wet season, the amount of water present in the landscape may be such that it does not allow for bryophytes to remain attached to their substrates. Areas around more permanent water bodies, such as gorges with waterfalls, do not have the otherwise typical bryophyte communities at the edges of splash zones, as is commonly found in southern Australia and other parts of northern Australia, such as northern Queensland. Instead, the bryophyte flora may be restricted to small patches of Riccia occurring only on ledges under rock overhangs on small areas of moist soil or as large patches along the edges of shallow rocky outcrops or in shallow depressions of rock slabs along some of the escarpments. During the dry season, high temperatures and lack of water appear to be prohibitive, and, hence, many bryophytes, including Riccia, are generally confined to creek banks, shaded vine thickets, monsoon forests, near waterfalls or on the margins of swamps and buffalo wallows.
|
This paper builds on the findings of Cargill et al. (2016), who sequenced 7 of the final 16 species covered in this revision. That paper identified the polyphyly of the second-largest subgenus within the group (subgenus Ricciella) as well as that of several common species. Of the Northern Territory species identified and sampled, several morphological species were confirmed by the molecular data, but others were found to be new species. This paper documents all known Riccia taxa occurring in the northern part of the Northern Territory, north of 18°S. Several collections used in this study were made during a Bush Blitz biodiversity survey expedition undertaken by the first author in 2012.
Materials and methods
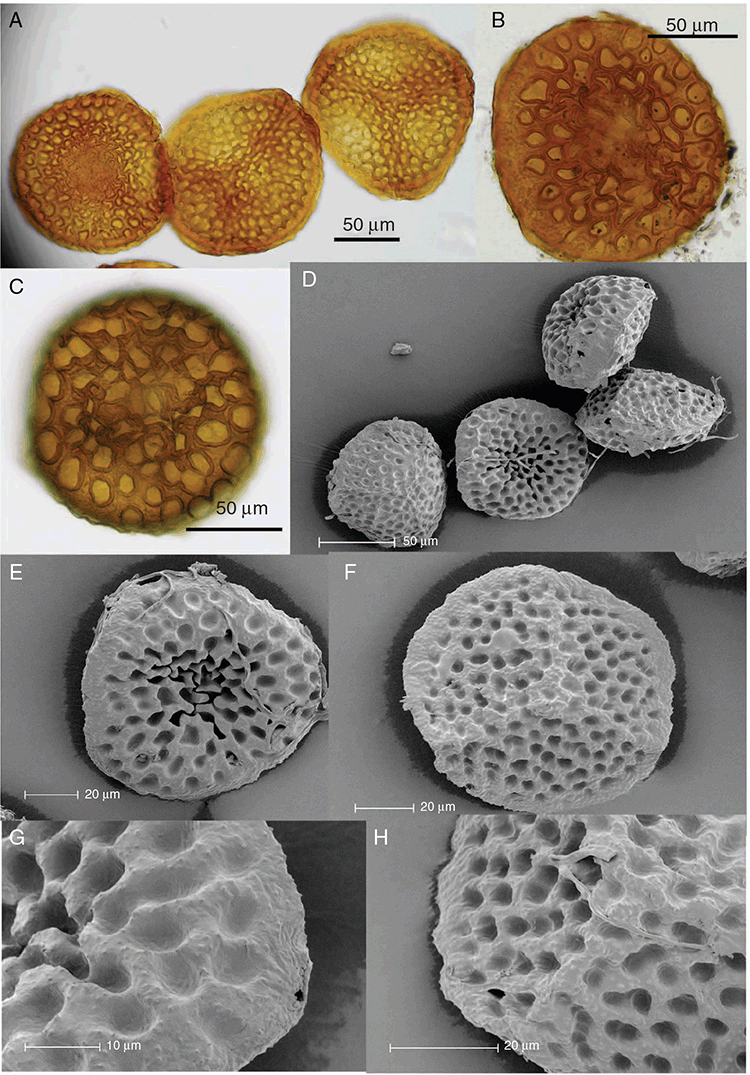
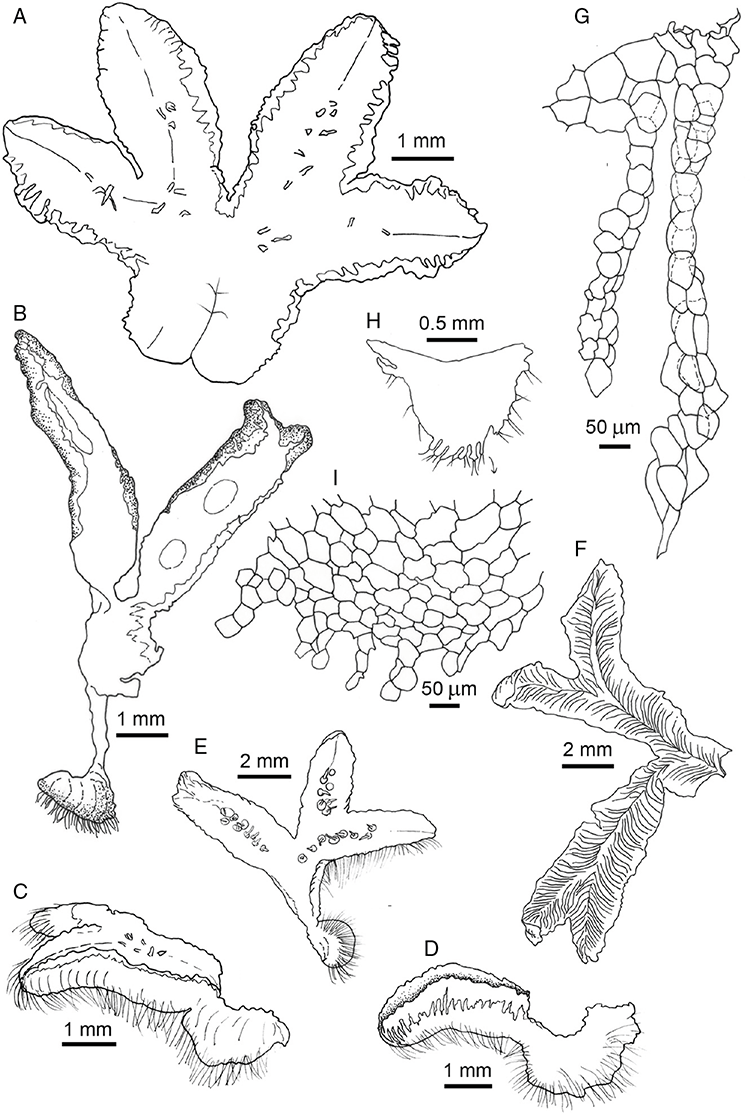
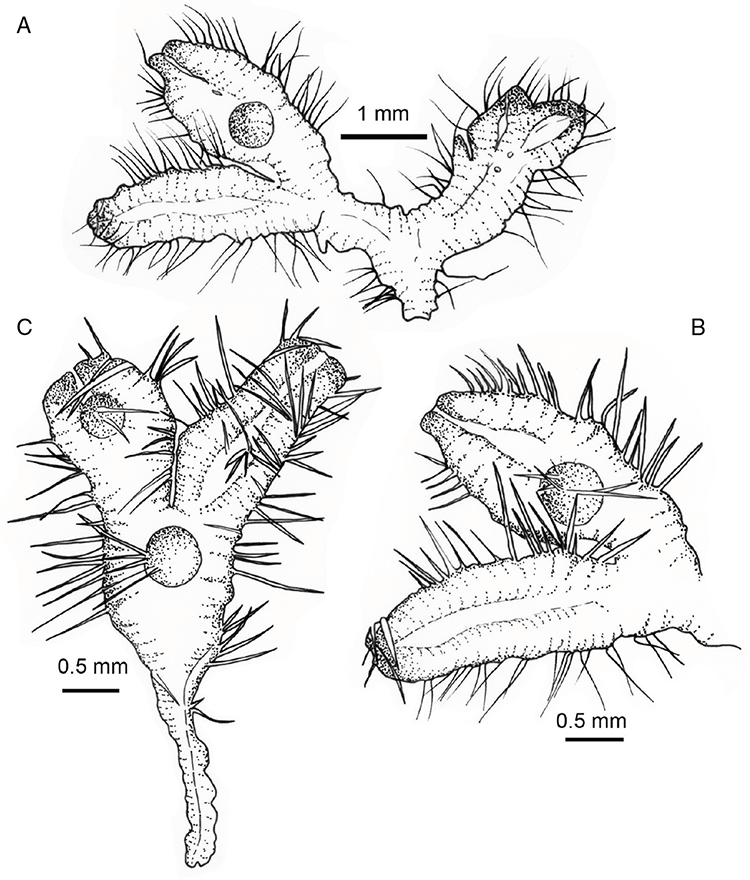
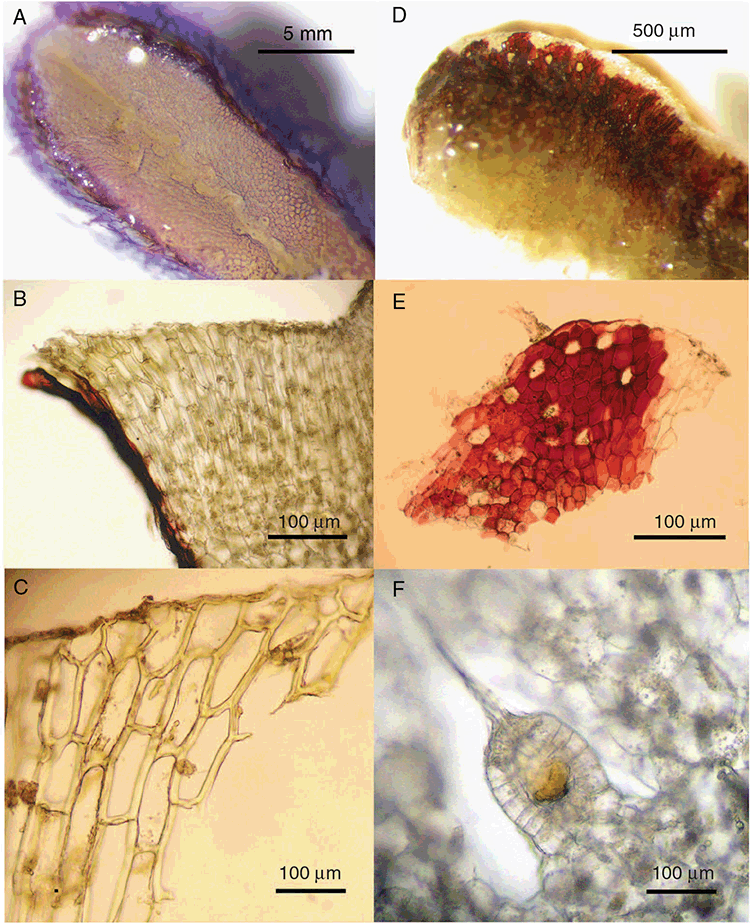
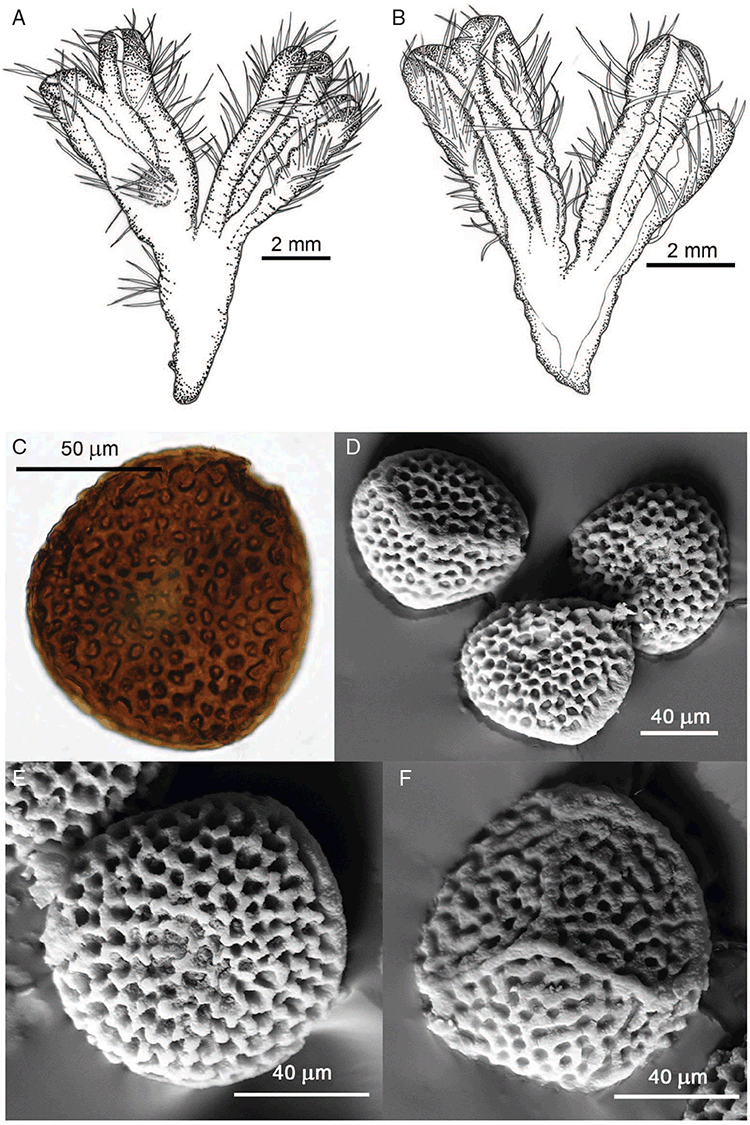
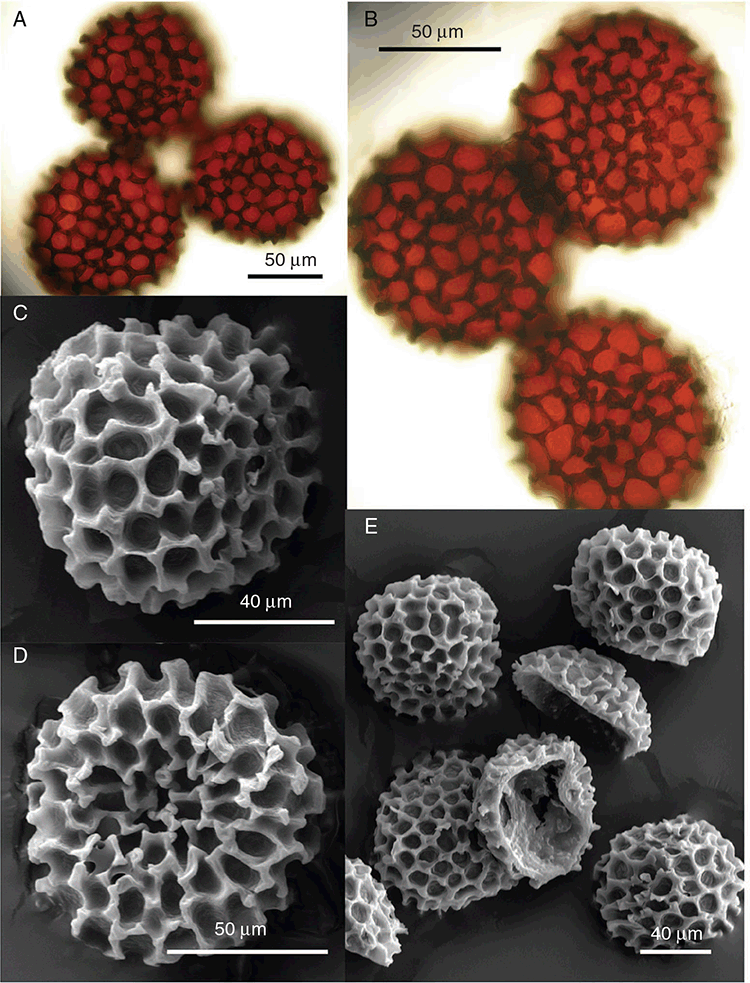
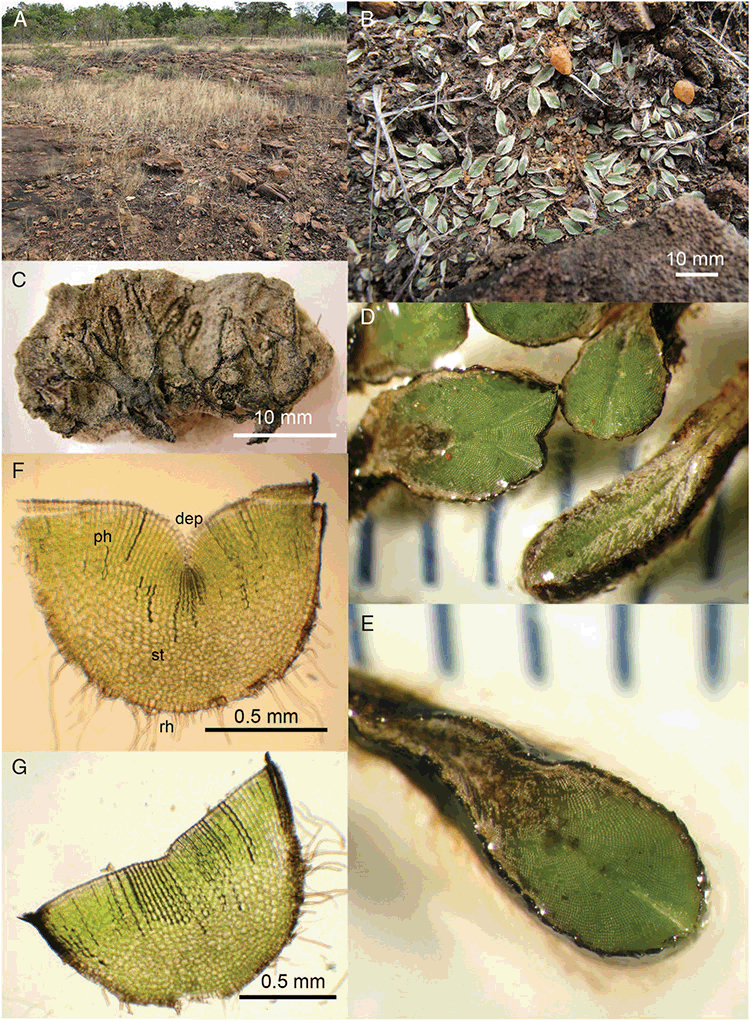
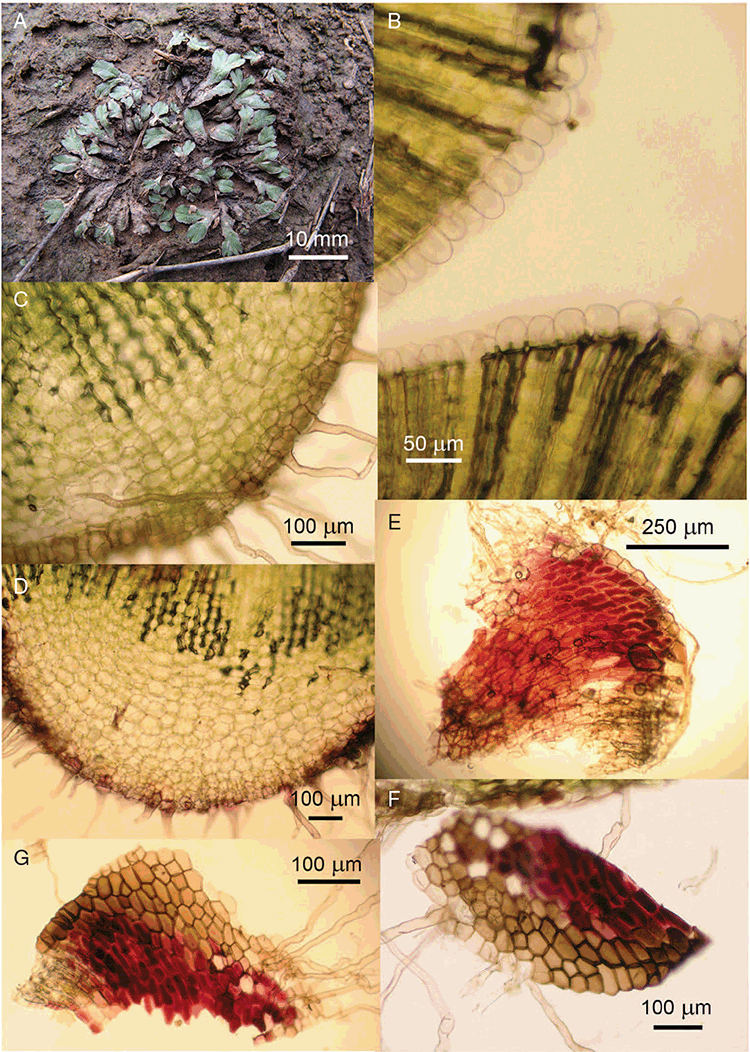
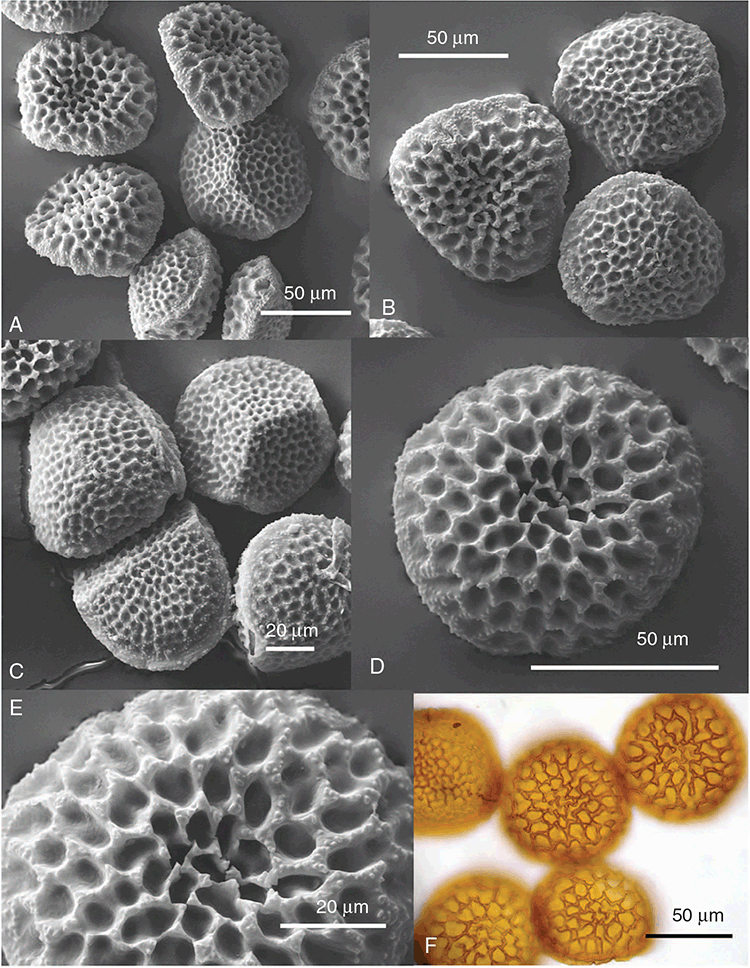
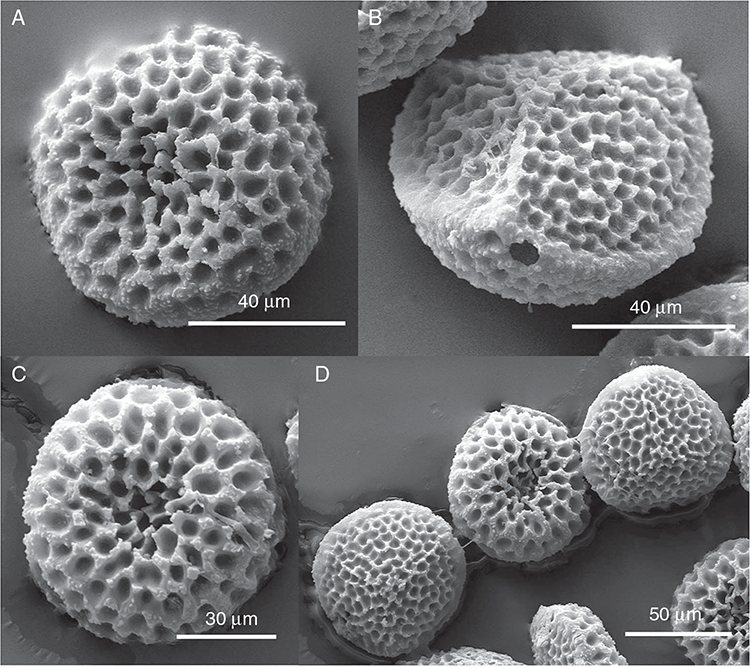
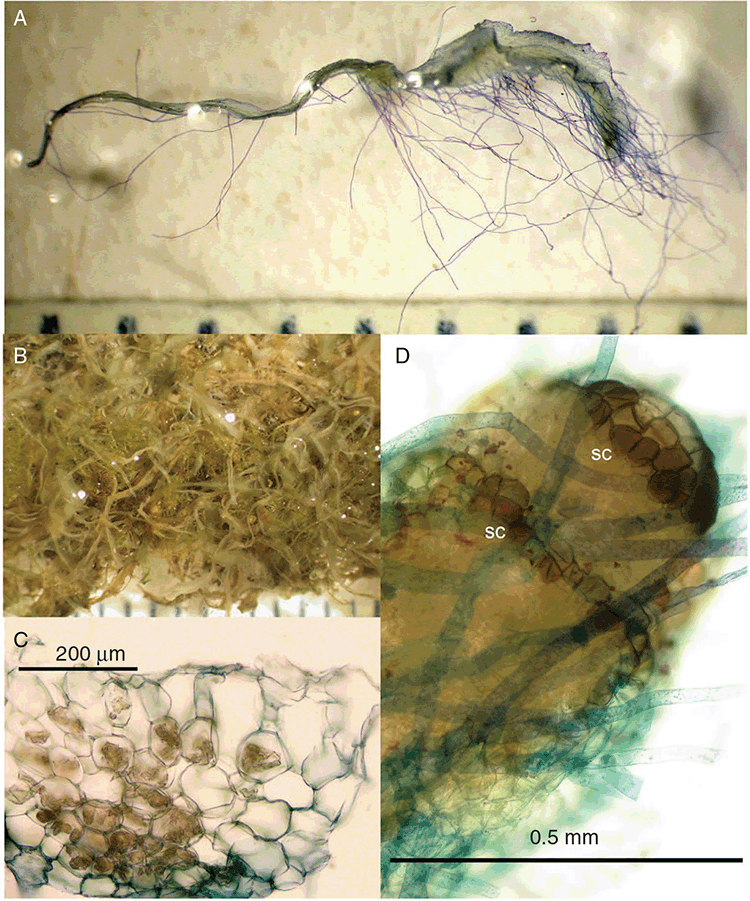
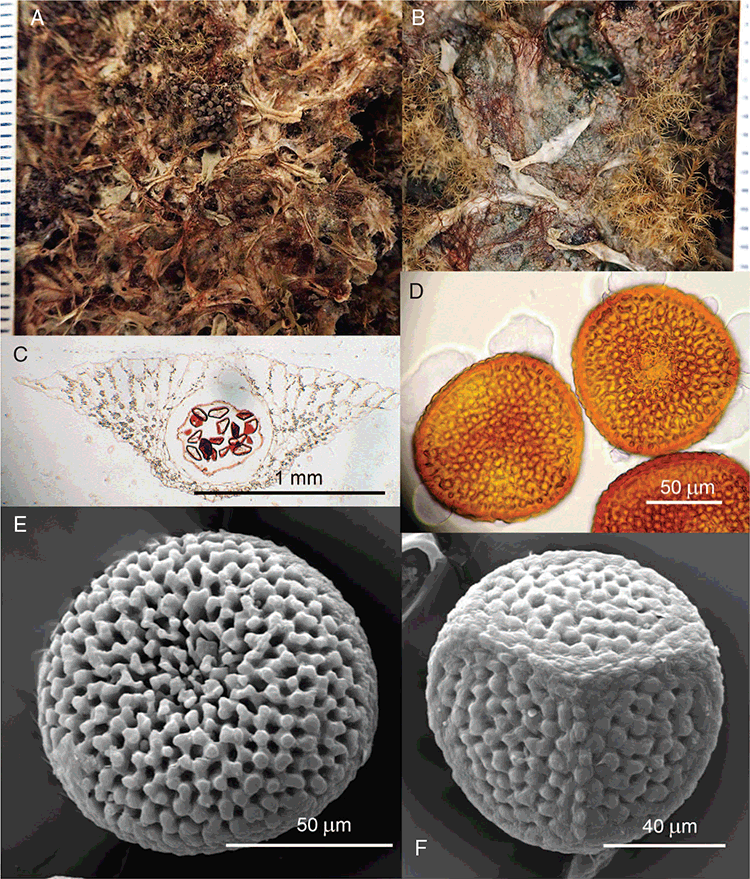
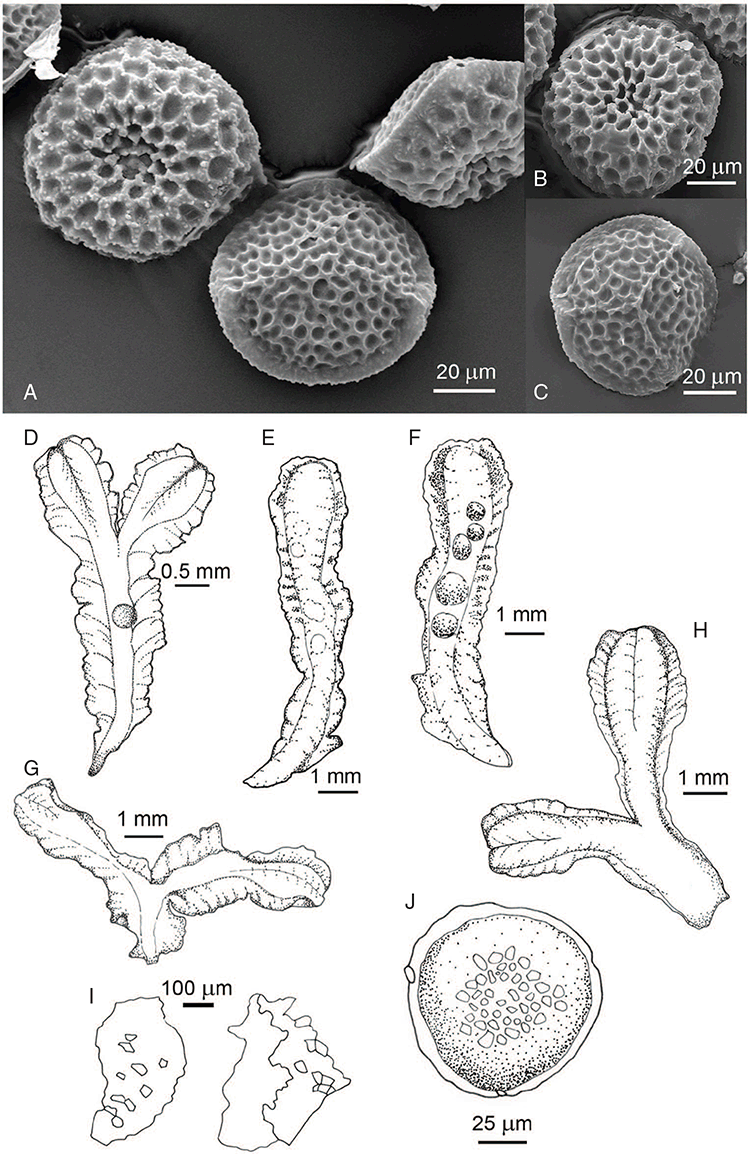
This study is based on examination of (rehydrated) herbarium specimens from CANB, DNA, NSW and SYD, with selected material examined from G and P, as well as living material. Rehydration was achieved by soaking plants attached to soil in tap water. All soil and other plant material was then carefully cleaned from both live and rehydrated plants with forceps and fine paintbrushes. Both living plants and rehydrated herbarium specimens were measured, and characters scored using Leitz compound and dissecting microscopes. At least one plant per accession was sampled and cleaned for observations, measurements and dissections. Where capsules were present, spores were mounted in water on microscope slides for light microscopy (LM) or mounted on adhesive carbon tabs on aluminium stubs, gold-coated using a Xenosput coater (Dynavac) and viewed using an FEI XL 30 FEG scanning electron microscope (SEM) or on double-sided sticky tape on aluminium stubs, gold-coated and viewed using a Zeiss EVO LS 15 Environmental SEM. Spore descriptions are a combination of observations from both LM and SEM. The Royal Horticultural Society Colour Chart (RHSCC; Royal Horticultural Society 1995) was utilised to describe colours of whole plants, spores and other associated structures. Light micrographs were obtained with a Nikon Coolpix 5000 digital camera, other images were taken with a Canon Powershot 12 digital camera. All line drawings were made using drawing tube attachments to both microscopes.
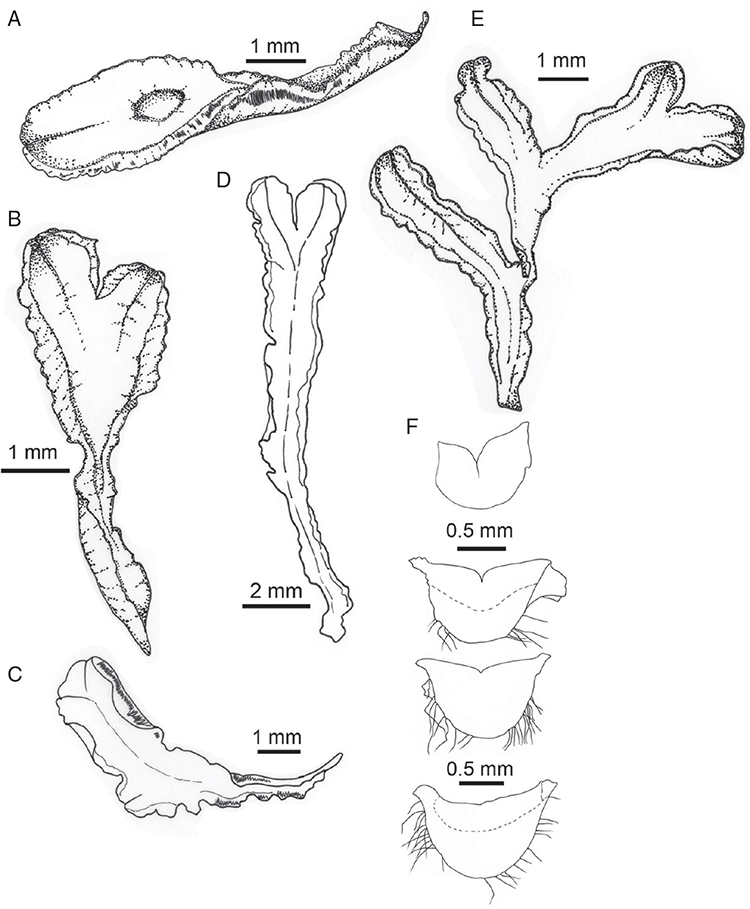
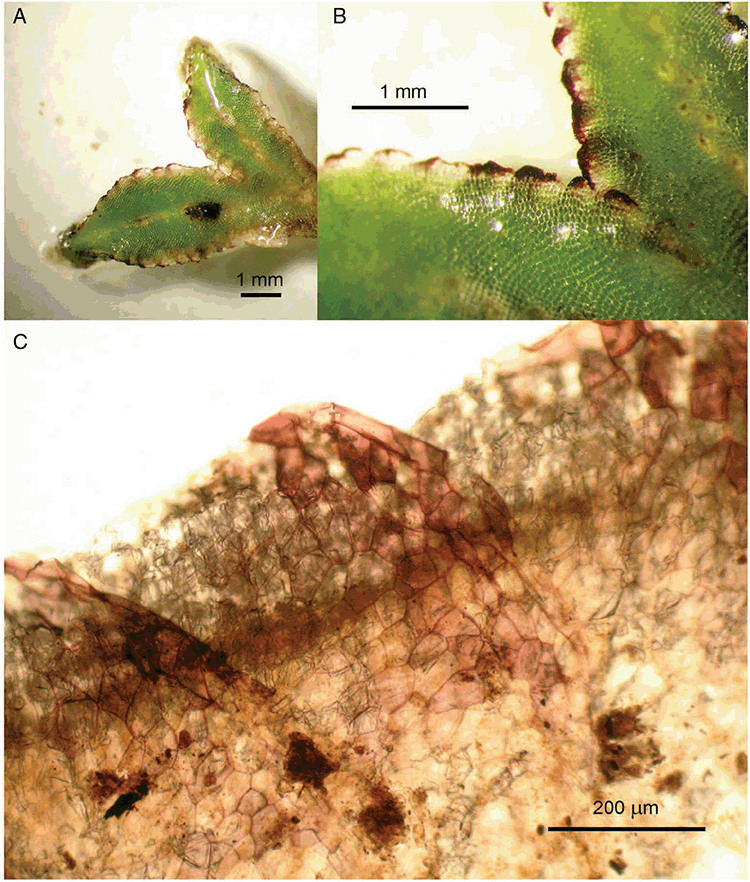
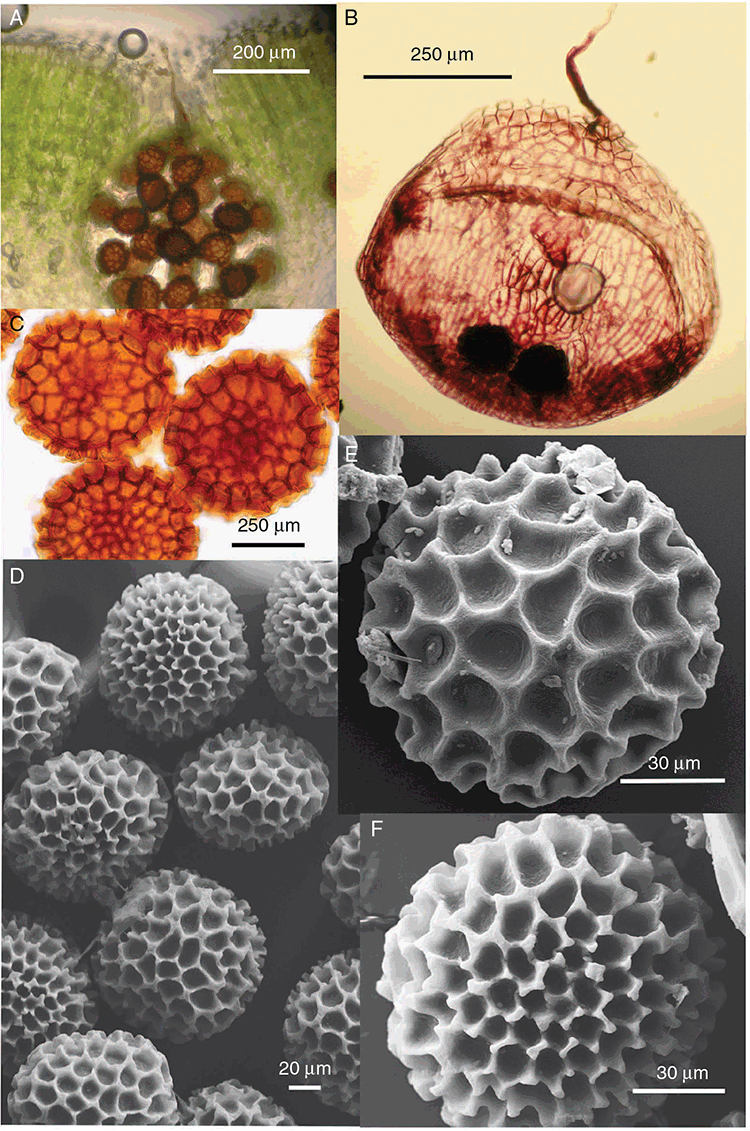
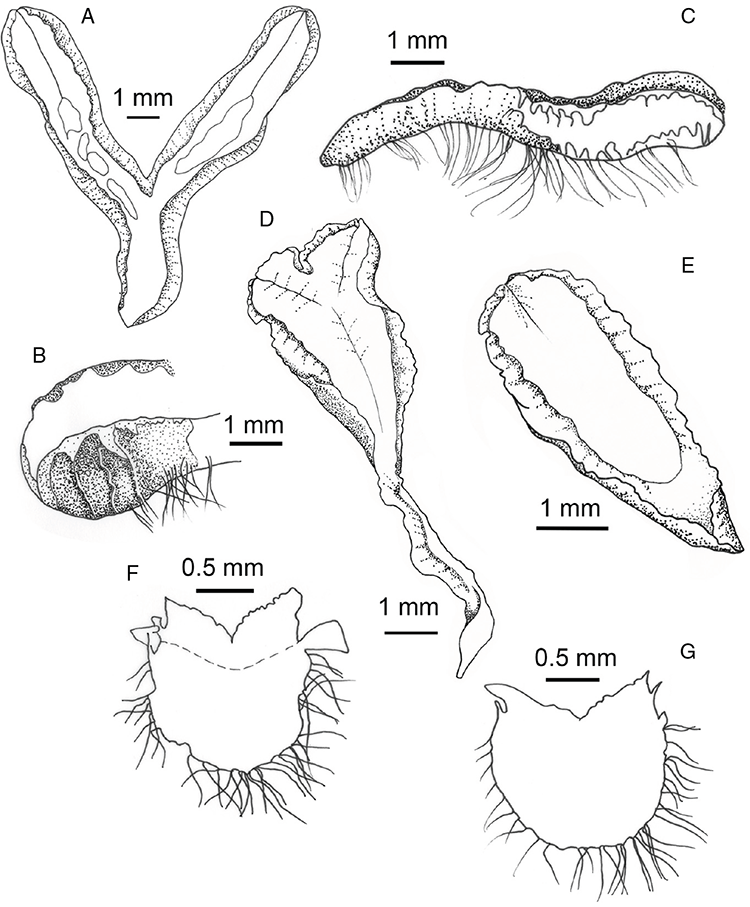
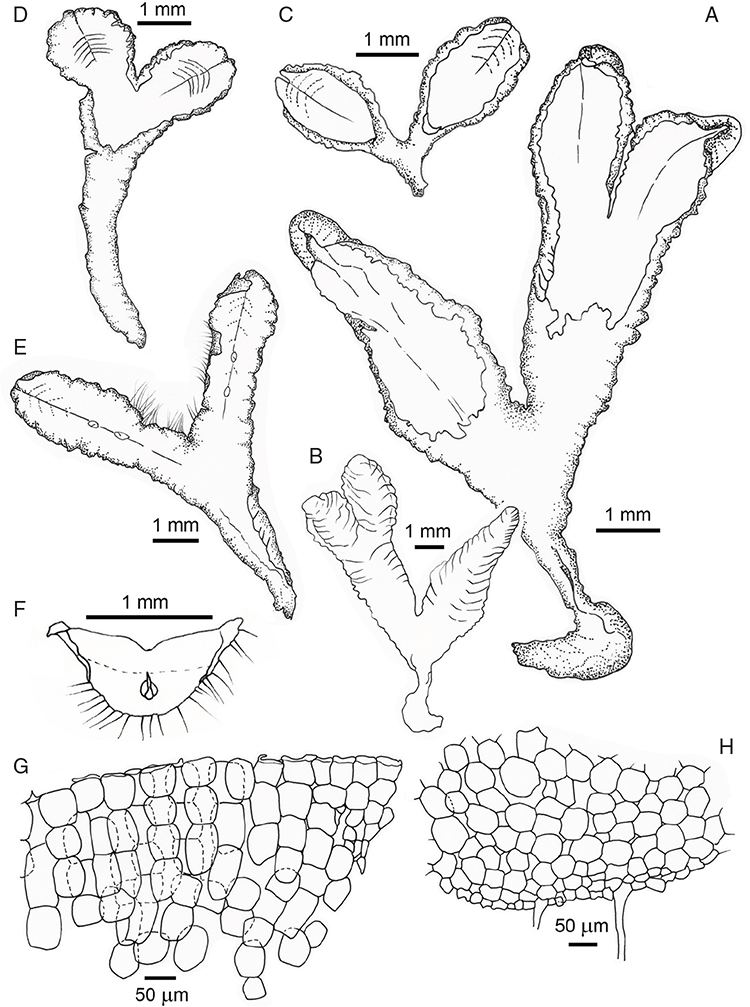
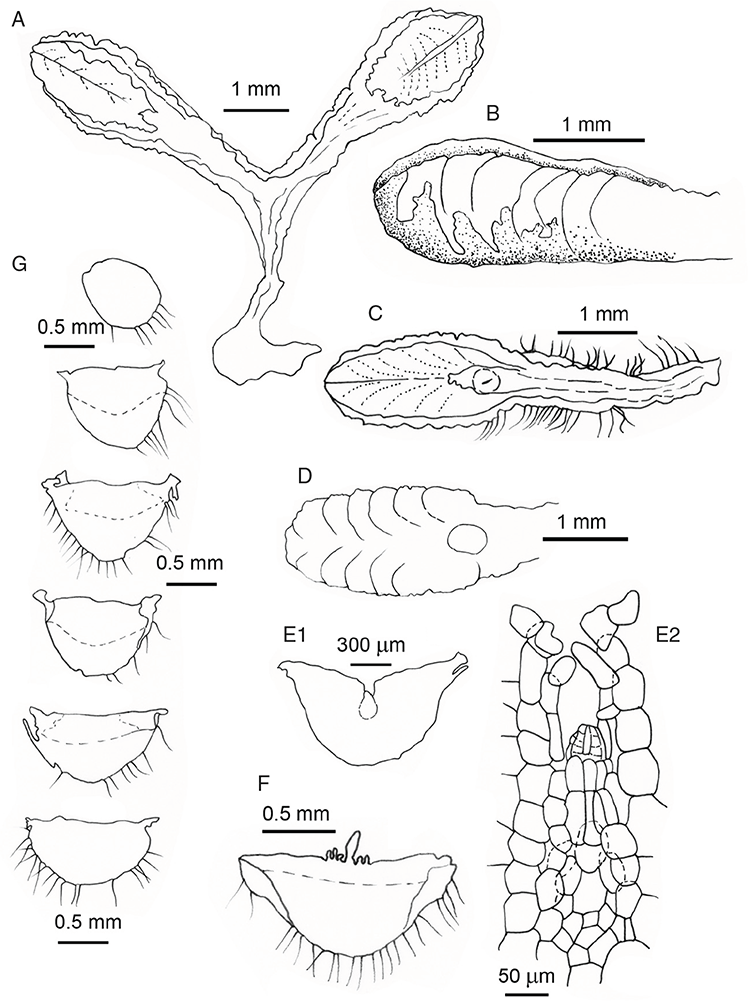
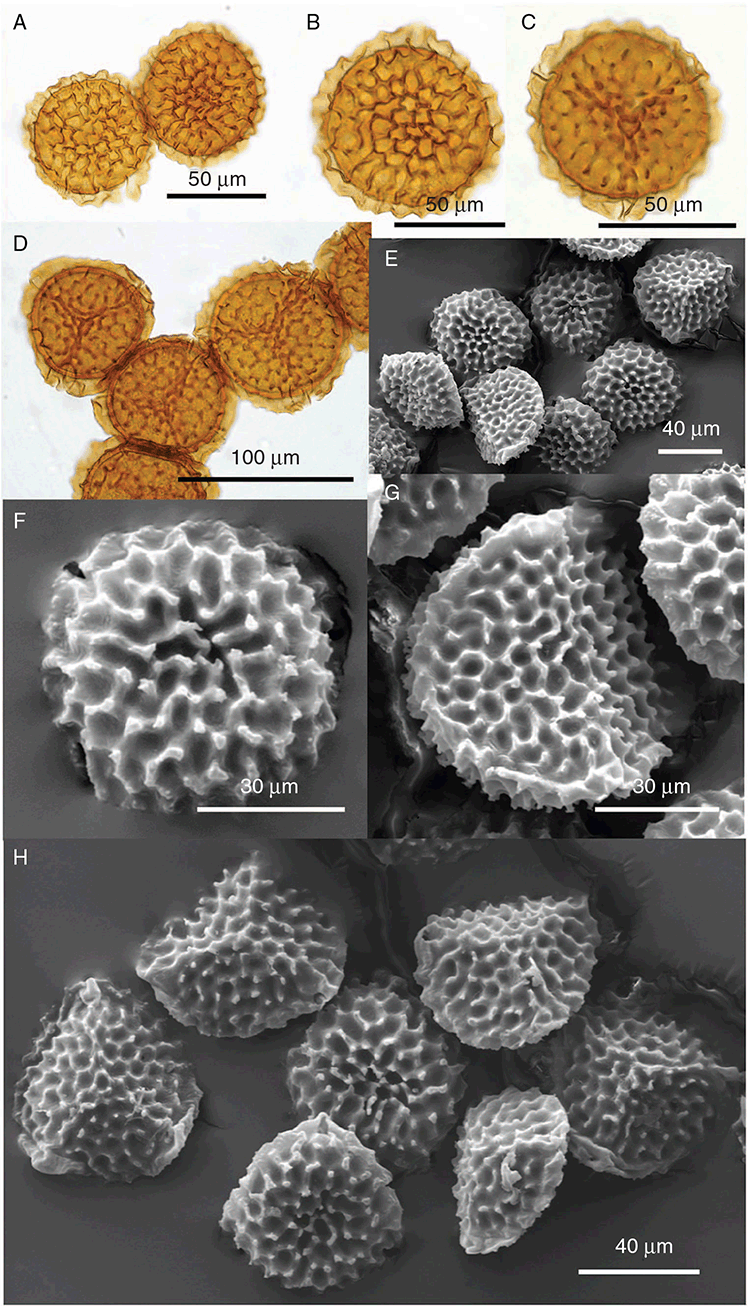
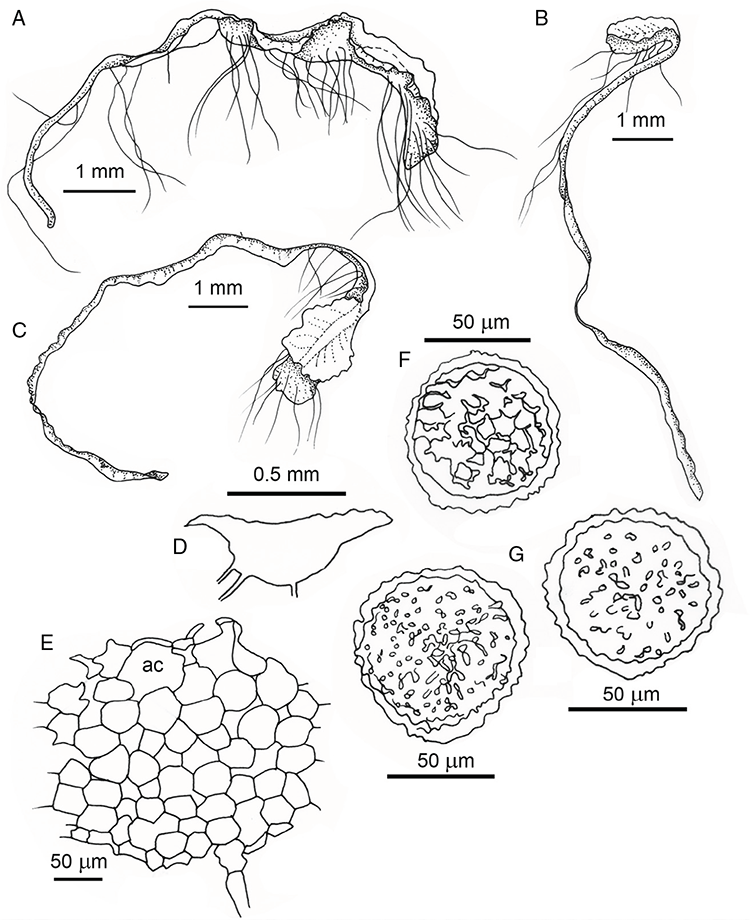
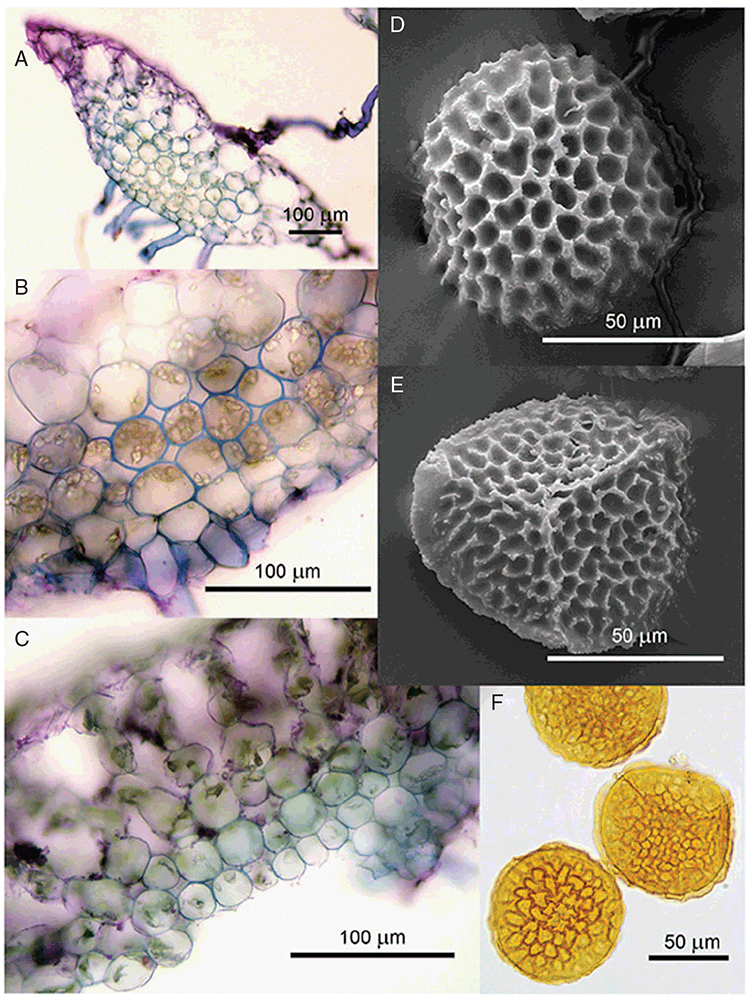
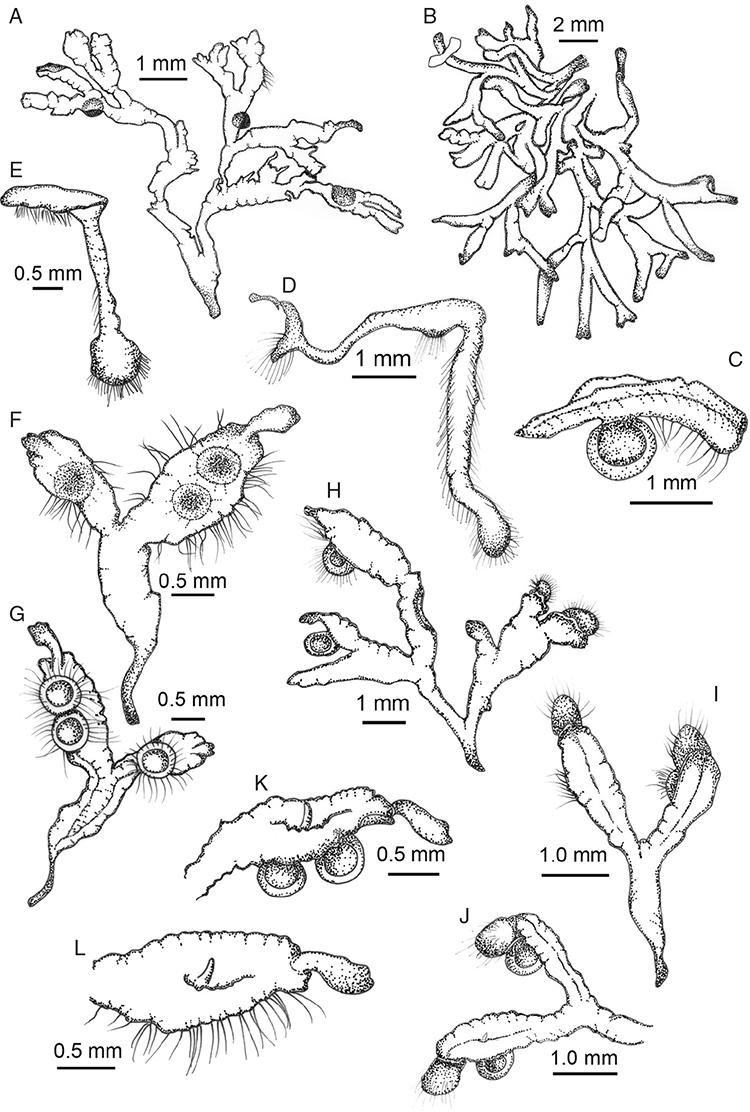
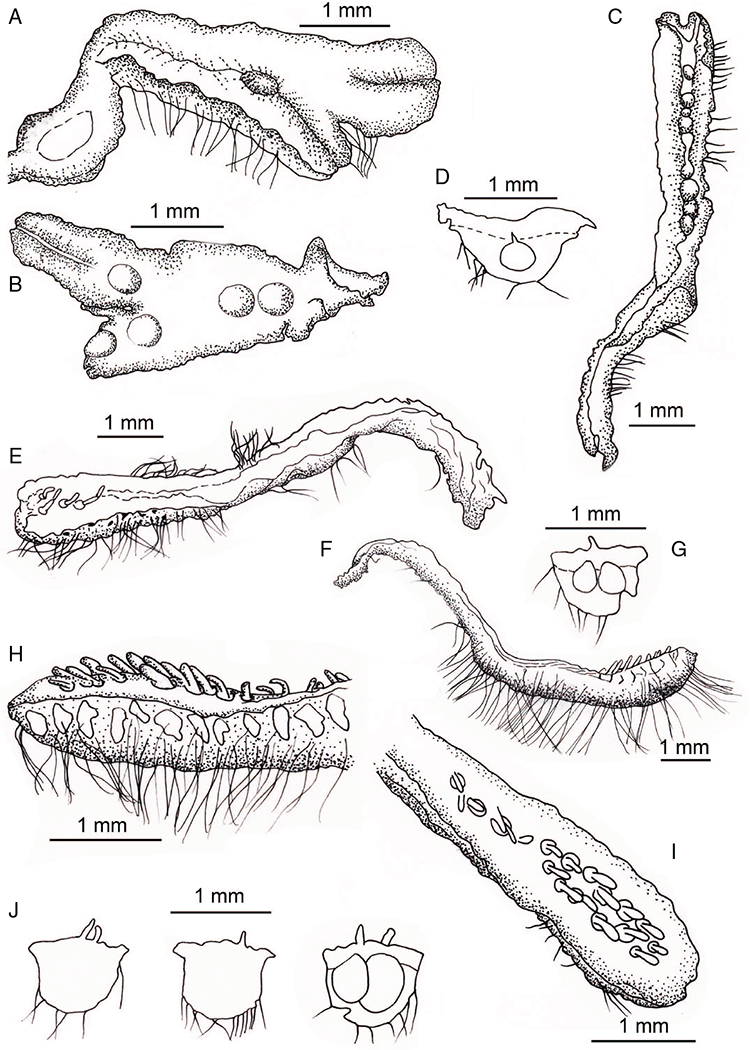
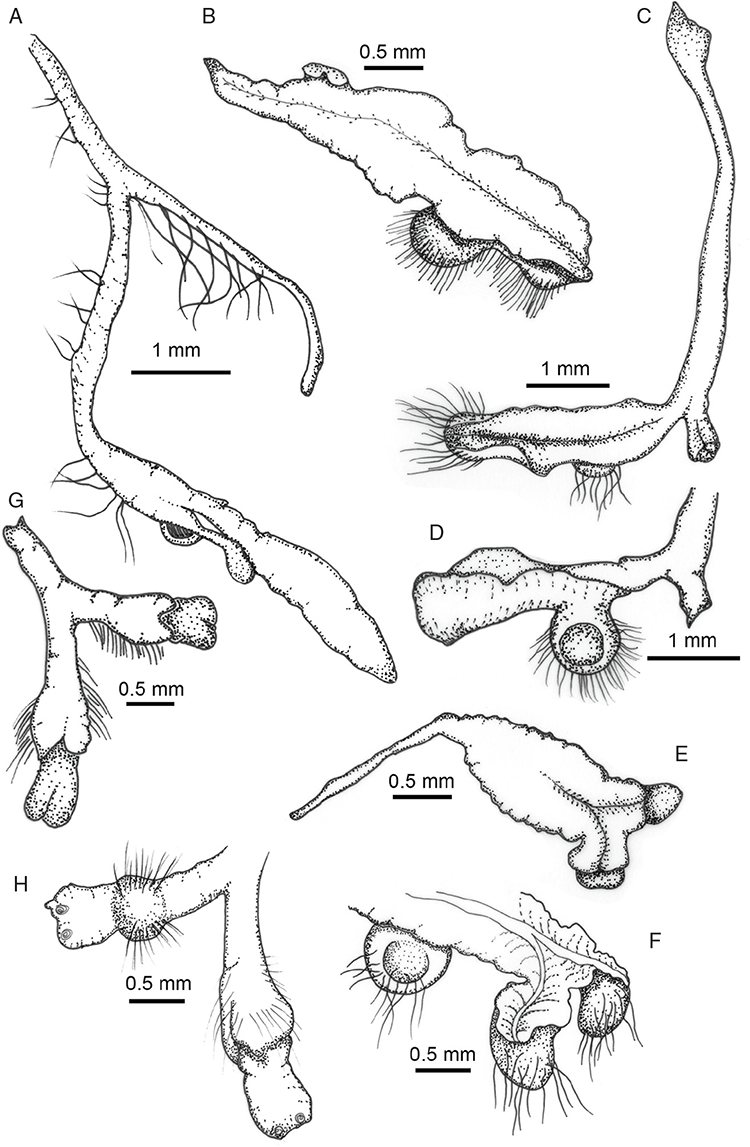
Specific characters for this treatment include the length and width of the whole plant (Fig. 2A); length and width of thallus branches (Fig. 2B); polygonal air chambers that connect to an air pore (Fig. 2C), or air spaces that do not (Malcolm and Malcolm 2006); dorsal groove of the thallus (Fig. 2D); spore diameter in polar view (Fig. 2E); height of the spore in equatorial view (Fig. 2G); and appearance and position of spore wing, mucilage pore, alveoli (Fig. 2E) and triradiate mark on the proximal spore face (Fig. 2F).

|
Maps were generated using the Atlas of Living Australia (ALA) spatial portal (https://spatial.ala.org.au/webportal//), using data only from specimens examined by the authors.
Taxonomic treatment
Riccia L. Sp. Pl. 2: 1138 (1753)
Type: Riccia glauca L., typ. cons. (fide Barrie 2006).
Genus description
Dioicous or monoicous. Thallus simple wedge- or Y-shaped or elongated straps; scattered or in gregarious patches or rosettes, on soil or rarely aquatic. Thallus unbranched (simple) or branched 1–3(–4) times furcate, symmetrical or asymmetrical; each branch, designated here as a segment, linear to obovate in shape. Apex truncate to rounded, emarginate to retuse, dorsal groove almost always present, deep and narrow at the apex, becoming shallow or becoming shallow and broader and often disappearing posteriorly. Margins acute to rounded. Ventral face rounded to V-shaped and forming a keel. Scales lateral or ventral, conspicuous to inconspicuous but rarely absent, hyaline or variously coloured purple, maroon, red, or black. Cilia present in some species at margins or on upper surface of thallus. Rhizoids numerous, dimorphic, smooth or pegged arising from the ventral surface.
Thallus differentiated into upper chlorophyllose photosynthetic and lower parenchymatous storage layers (in Riccia caroliniana and R. sayhadrica Manju & Cargill, the positions of these layers are reversed). Tubers frequent either as geotropic stalked bulbs or as swollen ventral tissue. Gemmae never present.
Gametangia embedded, only necks projecting, median along groove or scattered, in 1–2(–3) rows. Sporophyte without seta or foot, capsule globose, unistratose capsule wall resorbed during maturation. Venter wall and calyptra disintegrating on dorsal or ventral surface of thallus to passively release spores. Spores range in diameter from 40–150 μm and 35–102.5 μm high, triangular globose to subglobose to globose shape in polar view, tetrahedral to fusiform shape in equatorial view or are apolar. Ornamentation mostly reticulate, often species-specific. Triradiate mark on proximal face, distinct to indistinct, or absent; pores present or absent. Elaters absent. Chromosome number n = 8, 10, 16, 48.
Key to the species of Riccia in monsoon tropical Northern Territory|
1 Thallus without cilia |
|
2 Thallus with white cilia |
|
3 Thallus without ventral lamellae; internal photosynthetic layer located in dorsal half of thallus internally |
|
4 Thallus photosynthetic tissue consisting of internal polygonal-shaped air chambers scattered through photosynthetic layer |
|
5 Dorsal surface of thallus green or crimson |
|
6 Dorsal epidermal cells without calcite crystals; fertile spores orange–brown, with a reticulate pattern on both surfaces under light microscopy |
|
7 Plants always green dorsally, not tinged with fuchsia, ventral flanks always green; monoicous, male plant thallus segments as broad as female segments; antheridial necks short, not projecting conspicuously from dorsal surface |
|
8 Spores tetrahedral in shape; distal face reticulate, proximal face with much a smaller reticulate pattern; triradiate mark and pores present |
|
9 Subepidermal cells thin-walled; fertile spore surface patterns various but not covered with rounded protuberances |
|
10 Pigmentation of scales along the ventral flanks variable, either mottled or entirely maroon or extending onto the ventral surface |
|
11 Ventral flanks, ventral surface and scales deep purple to purplish-red to black; dorsal epidermal layer always unistratose; rhizoids usually hyaline, rarely pale brown |
|
12 Ventral flanks mottled hyaline and purplish-red; remaining green on ventral surface; tubers absent |
|
13 Plants with spathulate to ligulate, broader segments (0.45–2.55 mm wide) growing gregariously, or in thick mats or in rosettes |
|
14 Internal cell walls of cells surrounding air chambers unlike other cells, i.e. thick walled |
|
15 Ventral scales not triangular or bilobed or hemispherical, instead reduced to 1 or 2 rows of cells in 3 or 4 bands across width of thallus; dioicous |
|
16 Plants with segments up to 2.55 mm broad, spores almost round, distal face with a reticulate pattern, 13–19 alveoli across the diameter, alveoli 2.5–10 μm in diameter, with thick, low borders around the alveoli |
Riccia subgenus Riccia L., H.G.L.Reichenbach, Deutsch. Bot. Herb.-Buch. 23: 213 (1841)
Thallus typically differentiated into upper chlorophyllose and lower parenchymatous layers. Chlorophyllose layer either composed of compact pillars capped by a hyaline epidermal cell with narrow, vertical air spaces, or polyhedral air chambers bounded by unistratose layer of cells, air chambers opening to the surface through a well-defined pore or air spaces that do not. Or chlorophyllose tissue ventral and parenchymatous tissue dorsal; with ventral lamellae occurring in a repeated V-shape arrangement along the entire length of the thallus, each lamella is two cell layers thick projecting from ventral surface with rhizoids along their ventral margins. Only known for the species R. caroliniana and R. sayhadrica.
Na-Thalang (1980) placed Riccia caroliniana in a separate section, section Viridisquamata, subsequently regarded to warrant subgeneric rank by Jovet-Ast (1984), although Jovet-Ast failed to validly publish this combination as she did not provide a full and direct reference to the basionym and its author and its place of publication as required by ICN Art. 41.5 (Shenzhen Code, Turland et al. 2018). However, recent research by Rabeau (2019) has indicated that R. caroliniana is embedded within subgenus Riccia; hence, our synonymising of the subgenus.
Of the remaining five subgenera in the genus, only the monotypic subgenus Triseriata occurs in Australia. The species Riccia singularis Jovet-Ast is currently confirmed to occur only in Western Australia.
1. Riccia abdita Cargill, sp. nov.
Type: AUSTRALIA. Northern Territory. Fish River Station, under rock ledge on damp algal mat over soil, surrounded by spinifex, 29 Apr. 2012, D.C.Cargill 1298 (holo: CANB 811430!; iso: DNA!).
Description
Plants observed both in nature and from rehydrated herbarium collections.
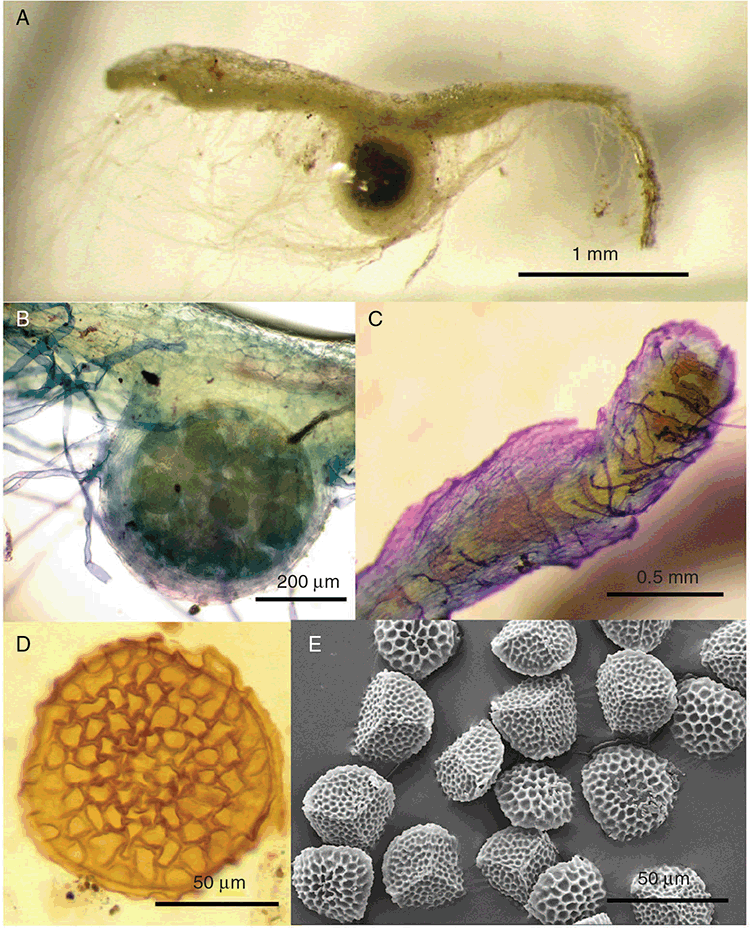
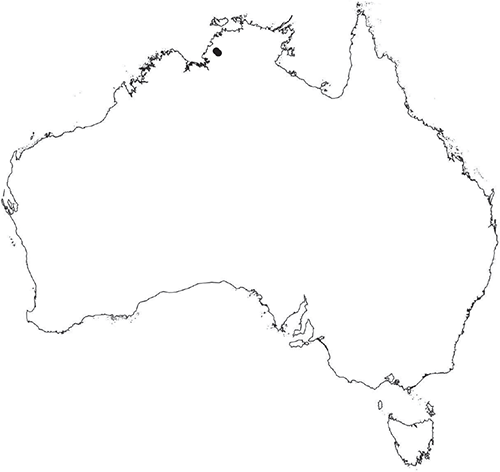
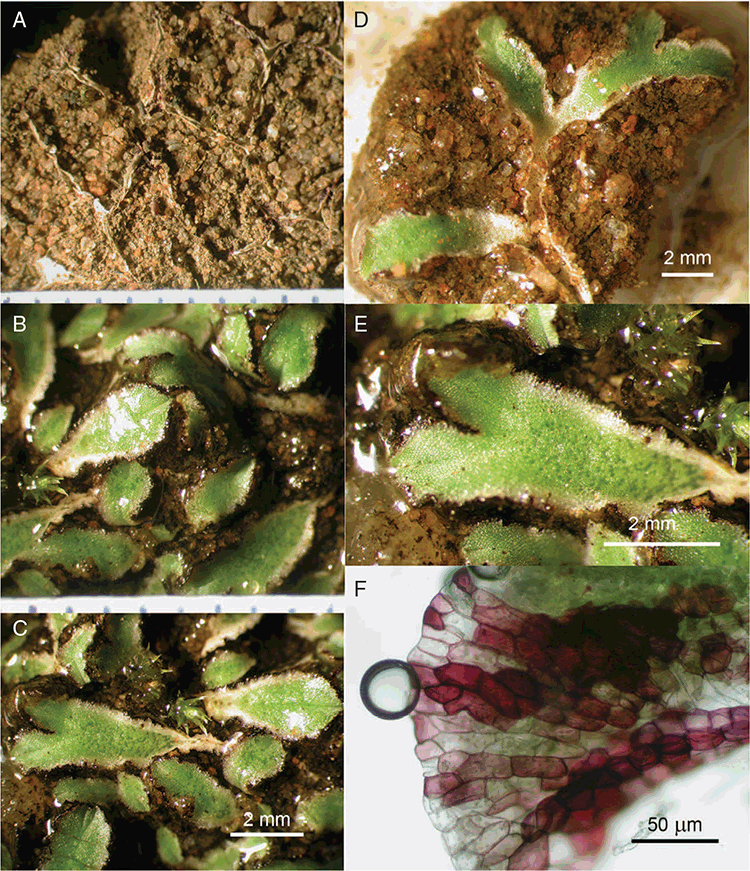
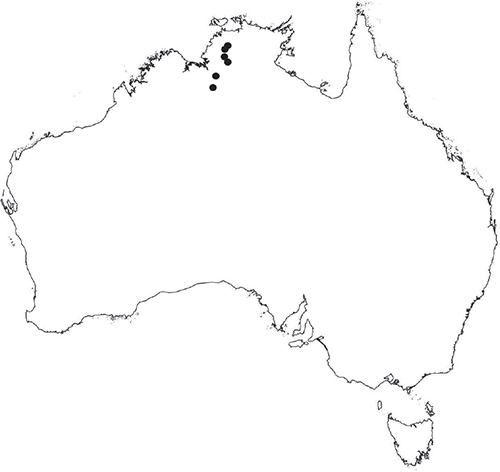
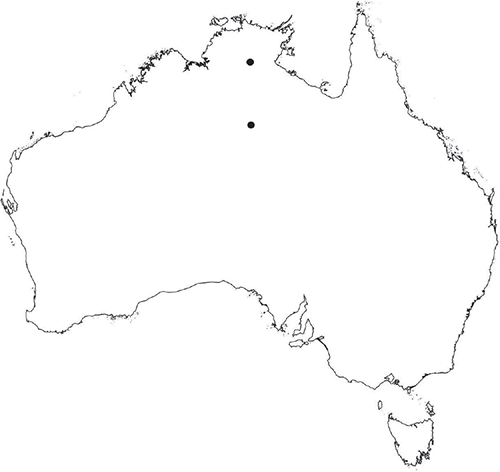
Plants prostrate, scattered, in rosettes or in mats, rosettes loose and up to 16.5 mm in diameter. Thallus, when moist or rehydrated, bright green (RHS 143C, 144A,B, 145A,B 146D, yellow–green group); when dry, plants shrunken, margins curling up into a vertical position not completely covering dorsal surface, giving a partial view of dorsal surface, almost folded over at the apex; pale green, or white, sometimes with dark margins (RHS 142B, 145C, yellow–green group). Whole plants 1.5–13.8 mm long and 0.8–8.8 mm wide. Segments 0.3–12.0 mm long, × 0.7–1.7 mm wide, ligulate to lingulate to rectangular. Dorsal surface smooth, with a finely reticulate pattern due to dorsal epidermal cells. Ventral flanks mottled hyaline, purplish-red (RHS 59A, red–purple group, or 187A, greyed-purple group) due to scales and green due to photosynthetic tissue. Branching simple or 1–3 times furcate, asymmetrical, branches moderately to widely divergent, diverging 45–90°. Apex rounded to squarish; emarginate to retuse or not dissected. Dorsal groove present, deep and narrow anteriorly, disappearing shortly afterwards or mid-thallus or shallow posteriorly. Margin of thallus wing-like; undulating to wavy, acute to obtuse in cross-section. Scales present, 300–720 × 120–240 μm, narrowly triangular to crescent-shaped, sometimes undulate along margin of scale or dentate; a very slim lunar shape to hemispherical; spread well apart, or overlapping or only at the apex, closely appressed to the ventral flank; scales extend to edge of margin of flank or just below; mottled hyaline and maroon (RHS 187A, greyed-purple). Cilia absent. Dorsal epidermal cells unistratose, hyaline, globose to rectangular at the apex and within the dorsal groove, becoming deflated, disintegrating with age. Thallus in transverse section biconvex–convex to campanulate; 500–1100 μm thick and 900–2400 μm wide. Photosynthetic tissue in vertical columns occupying 1/3–1/2 the thallus thickness, 4–8 cells long per column. Rhizoids covering the entire ventral surface of the thallus up to the base of scales, hyaline, smooth only. Tubers absent. ?Monoicous. Archegonia seen only along dorsal groove or either side of dorsal groove. Antheridia not seen. Capsules 3 per plant (only 1 fertile plant seen), situated mid-thallus, embedded, seen from both the dorsal and ventral surface; hyaline to pale brown. Spores 85–97.5 μm in diameter, globose in polar view, tetrahedral in equatorial view, 60–70 μm high, golden-brown (RHS 166A, greyed-orange group); wing present, 5–10 μm wide, protuberances around the circumference may be truncated; spore margins entire. Distal surface ornamentation finely reticulate, alveoli borders variable in height; alveoli shallow, 9–13 across the diameter, 5–15 μm in size; proximal surface ornamentation reticulate, but reduced in area to the centre of the triradiate faces, 5 or 6 alveoli across each triradiate facet. Triradiate mark present, pores present. Chromosome number unknown. (Fig. 3 (map), 4–7.)
Distribution
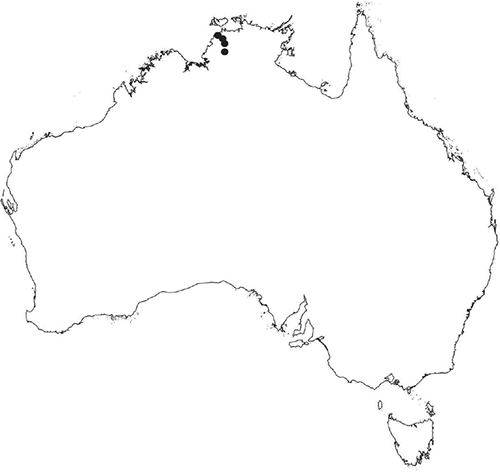
Known from Fish River Station reserve near Daly River in the northern part of the Northern Territory.
Habitat and ecology
All populations were found growing on bare soil or over damp algal mats over soil under rock ledges.
Etymology
From the Latin ‘abdita’ (hidden, concealed, secret), in reference to the known microhabitat of this taxon (under rock ledges), which allows soil to remain shaded and damp for longer periods, therefore providing suitable habitat for this species.
Notes
Superficially similar to Riccia macrospora and R. verrucosa, with which it shares reddish-maroon scales along the ventral flanks of the thallus; molecular data (Cargill et al. 2016) indicate that it is genetically distinct from these taxa. Riccia abdita has scales mottled hyaline, green and maroon colour, and they do not extend beyond the margins of the segments v. deep reddish scales that extend beyond the segment margins in R. macrospora and scales that vary in colour from hyaline to deep purple to almost black in R. verrucosa. Other differences include a flaccid, wing-like extension of the thallus margin, which is generally hyaline in R. abdita and R. verrucosa, and absent in R. macrospora. The presence or absence of tubers is also an important vegetative difference among the three species, with Riccia abdita and R. macrospora lacking tubers and Riccia verrucosa bearing tubers in the form of swollen apices.
Spore morphology of R. abdita is similar to that of R. verrucosa, although limited fertile material is available for R. abdita (Cargill 1310), and these spores appear to be not quite fully mature. Both species bear a reticulate pattern on the distal and proximal faces, with 7–11 punctae on the proximal face for R. verrucosav. 5 or 6 in R. abdita. The ridges surrounding the punctae are smooth in all species except R. verrucosa, in which they are papillate (although some populations can be smooth). In comparison, the spores of R. macrospora have 8–10 punctae. However, it is the distal face that distinguishes the three species from one another. Both R. abdita and R. verrucosa possess spores with a regular reticulate pattern on the distal face, which radiate more or less in rows out from the centre of the face. The alveoli are surrounded by low ridges in these two species, but, as noted above, they are papillate in R. verrucosa and smooth in R. abdita. The spores of R. macrospora have an irregular reticulate pattern in an almost ‘swirl’ pattern, with high ridges that are smooth and laciniate along their margins.
Specimens examined
NORTHERN TERRITORY. Fish River Station, at a rock seepage next to a pool under a rock ledge, D.C.Cargill 1310 (CANB); Fish River Station, under rock ledge of a gorge, D.C.Cargill 1311 (CANB, DNA).
|

|
|
2. Riccia billardierei Mont. & Nees in C.M.Gottsche, J.B.W.Lindenberg & C.G.D.Nees von Esenbeck, Syn. hepat. 4: 602 (1846)
Type citation: ‘In Java insula ad terram (La Billardière Hb. M.et N)’. Type: INDONESIA. Java insula, s. dat., J.J.H. de Labillardière 237 (lecto: STR [image!]; isolecto: Java, s. dat., J.J.H. deLabillardière, ex herb. Labillardièrii, Com. D. Webb (PC 0049036!)).
Description
Plants observed both in nature and from rehydrated herbarium collections.
Plants prostrate, growing variously as scattered individuals, in scattered or loose patches or gregarious in thick overlapping mats, in rosettes or semi-rosettes up to 20 mm in diameter. Thallus, when moist, bright green to pale green to yellowish-green (RHS 144A, B, C; 145A, B; 146A, D; 151B, D) becoming creamy yellow to mustard with age, (RHS 153B, 166C); when dry, plant margins fully inflexed over dorsal surface or margin only curled into a perpendicular position; black or white and maroon or pale green (RHS 145D) or entirely white in colour. Whole plants 1.3–14.7 mm long and 0.75–11.0 mm wide. Segments 0.9–12.1 mm long and 0.6–3.0 mm wide; varying in shape from ovoid to obovoid to ligulate or lingulate, fusiform to cordate to ellipsoid, canaliculated anteriorly, becoming flattened posteriorly. Dorsal surface smooth with a reticulated pattern formed by epidermal cells. Ventral flanks striped maroon or mottled maroon and hyaline because of pigmentation of the scales, becoming green below scales. Branching simple or 2–3 times furcate, asymmetrical; branches almost parallel to widely divergent, diverging from 10 to 100°. Apex variable; rounded to squarish, truncate, lanceolate, obtuse, canaliculated, emarginate, occasionally becoming tuberous. Dorsal groove present, anteriorly narrow and deep, becoming broader and shallower posteriorly, particularly over developing capsules but generally present along the entire length of segment. Margin crenate because of scales extending beyond the margin; becoming attenuate in cross-section. Scales present, large, 350–1008 × 144–1050 μm, extending shortly beyond thallus margins, imbricate and reflexed at their apex, mottled hyaline and dark maroon (RHS 187A, greyed-purple group), frequently maroon colouring only along the distal margin of scale emphasising the crescentic shape of the scales, but becoming a solid maroon colour with maturity. Cilia absent. Dorsal epidermal cells unistratose; globose to pyriform to mammillate in shape at the dorsal groove near the apex, deflating or disintegrating with age; hyaline, up to 47.5 μm wide, up to 40 μm high, air pores absent. Thallus in transverse section almost terete apically to biconvex-convex to campanulate to broadly concave-convex mid-thallus to posteriorly, half as deep as wide; 250–1000 μm deep and 600–2920 μm wide. Photosynthetic tissue in vertical columns occupying 1/3–2/3 of the thallus thickness, 3–7 cells long per column. Rhizoids densely covering the ventral surface of the thallus up to the base of scales, hyaline to distinctly pale orange–brown, dimorphic, both pegged and smooth, sometimes mainly smooth, sometimes mainly pegged; infrequently all smooth, pegs small and sparse, sometimes dome-shaped or very short. Ventral epidermal cells hyaline. Tubers present, frequently apices becoming swollen and geotropic or segments forming perennating stolons. Odour when crushed faintly of coumarins (cut grass) or rarely of burnt sugar.
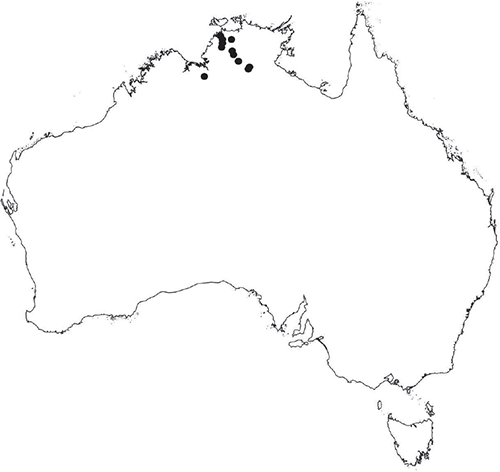
Monoicous. Gametangia in 1 or 2 rows, along or on either side of dorsal groove; separated both spatially and temporally; antheridia occurring apically, archegonia clustered mid-thallus. Antheridia ovoid to pyriform to spherical, 150–225 × 135–275 μm; antheridial stalk 150–250 μm long. Capsules 1–6 to many per plant, along the length of segment or clustered proximally, embedded, visible from both dorsal and ventral surface, often bulging from ventral surface, rupturing on dorsal surface, capsule tissue varying in colour from hyaline to bright maroon, to pale orange to pale reddish-purple brown (RHS 59A, red–purple group). Spores 82.5–137.5 μm in diameter, globose to subglobose in polar view, baculate to fusiform to hemispherical in equatorial view; 57.5–97.5 μm high, varying from golden-brown to pinkish-brown or red–brown when developing, becoming deep maroon to almost black at maturity (RHS 187A, greyed-purple group and 166A, greyed-orange group); wing present, 2.5–12.0 μm wide, as an incomplete webbing between protuberances around the circumference of the spores, spore margin crenate to crenulate owing to protuberances, which may be truncated, T-shaped or rounded at tips, 5.0–12.5 μm long. Distal and proximal surface ornamentation regularly reticulate on both faces, alveoli more or less angular, 4–8(–11) alveoli across diameter, 2.5–27.5 μm in diameter, alveoli walls mainly thin but occasionally thick, projections at corners of alveoli; proximal alveoli becoming smaller and appearing ‘pinched in’ at its centre. Triradiate mark and pores absent. Chromosome number n = 8, 16 (Na-Thalang 1980). (Fig. 8 (map), 9–13.)
Illustrations
Meijer (1958, p. 116, fig. 8); Na-Thalang (1980, p. 67, pl.1J., 89, fig. 8); Jovet-Ast (2000, p. 311, fig. 2, pl. 18.).
Distribution
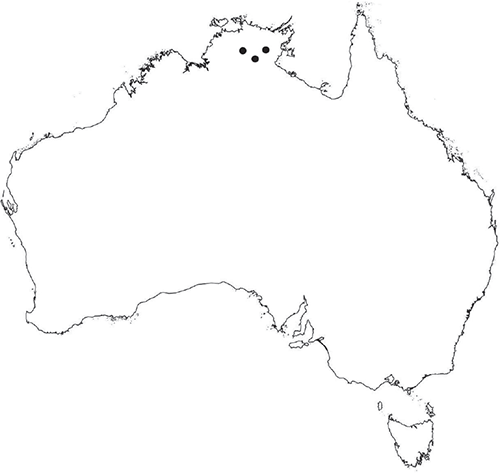
Occurs in the western part of the Northern Territory, from the Darwin region south to the Kata Tjuta (The Olgas) of the central Australian region. Also occurs in northern Queensland, from Townsville to Quilpie in central Queensland, northern Western Australia and northern South Australia. Also recorded from New South Wales (AVH), but the identity of these collections has yet to be confirmed. Also occurs in Indonesia, India, Sri Lanka, Vietnam and the Philippines.
Habitat and ecology
Growing on calcareous, sandy and laterite soils; in bare sites, on creek banks or in protected sites under rock ledges or under trees, in vine thicket forest or grasslands.
Etymology
Named for the French explorer and botanist J. J. H. de Labillardière (1775–1834), collector of the type material.
Notes
A variable species, Riccia billardierei, in its current circumscription may prove to be polyphyletic, but further research throughout its range is required. Riccia billardierei is morphologically similar to several widespread, mostly tropical species, including R. congoana Steph., R. discolor, R. gangetica, R. himalayensis, R. macrospora (Pandé and Udar 1957; Udar 1957; Na-Thalang 1980; Perold 1986) and R. sp. Darwin (O.Na-Thalang 281). Of all these species, a recent unpublished molecular analysis has shown that R. congoana is closely related to or conspecific with R. billardierei (Rabeau 2019), whereas all other species are not closely related at all (Rabeau 2019; Cargill et al. 2016). Within Australia, R. billardierei may be distinguished from R. sp. Darwin (O.Na-Thalang 281) by sexual condition, colouration of the thallus and scales and spore morphology. Riccia sp. Darwin (O.Na-Thalang 281) is dioicous and the thallus deep fuchsia pink or purplish-pink, with scales similar in colour and fused to the ventral flank. Riccia billardierei is monoicous, the thallus is always bright green except for the scales, which are pigmented dark maroon along the distal margin, giving them a scalloped appearance (Fig. 9D, E, 10A–C). Spores of R. billardierei bear a wing around the circumference (Fig. 11C), but this is usually absent or incomplete in R. sp. Darwin (O.Na-Thalang 281). The number of alveoli across the diameter of the distal face can vary but appears to be more variable in R. billardierei (4–8(–11)) v. 7–10 in R. sp. Darwin (O.Na-Thalang 281). Riccia billardierei may also be distinguished from R. gangetica by spore characters and scale pigmentation (see under R. gangetica for discussion of scale characters). Spores in R. billardierei are dark maroon to almost black, apolar, coarsely reticulate over the distal face, and have smaller reticulations on the proximal faces with curved, high borders around each of the alveoli (Fig. 11C, D), v. dark reddish-brown to dark brown in R. gangetica, large (100–142.5 μm), globose and apolar, finely reticulate with sinuous borders around each of the alveoli.
Meijer (1958) cited the type of the name Riccia billardierei as ‘Java: without exact locality, La Billardière (Herb. Nees: Strassbourg, type)’ (p. 117). This is here treated as effective lectotypification by Meijer in accordance with ICN Art. 7.11 (Shenzhen Code, Turland et al. 2018). Original material of this name at PC, treated by Jovet-Ast (2000) as the ‘type’, is here regarded as an isolectotype.
Specimens examined
NORTHERN TERRITORY. Tipperary, 24 km NE of Daly River Crossing, J.Brock 554 (CANB); Fish River Station, 200 km S of Darwin, D.C.Cargill 1275 (CANB, DNA), Fish River Station, 200 km S of Darwin, I.D.Cowie 13387 (CANB); Newcastle Range, head of Little Horse Creek, 7 km SSW of Timber Creek, J.A.Curnow 2824 (CANB); Gregory NP, tributary of Upper East Baines River, 50 km SW from Bullita Outstation G.J.Jones 98 (MEL); Fish River Station, ~60 km S of Douglas Research Farm. Nearest place: Hungry Knob, D.Lewis 2685 (CANB); Darwin River, O.Na-Thalang 278 p.p. (SYD); Daly River Rd, near Adelaide River, O.Na-Thalang 290 p.p. (SYD); Fish River Station, 200 km S of Darwin, B.Wirf 795A (CANB, DNA).
|
3. Riccia caroliniana Na-Thalang, Brunonia 3: 72 (1980)
Type citation: Holotype: Robin Water Fall, N.T., Na-Thalang 288, 28.i.1969 (NSW); (isotype (SYD [see ‘Comments’, below]).’ Type: CULTIVATED. Australia. New South Wales, University of Sydney, 19 Sep. 1969 (grown from live material collected from Robin Falls, Northern Territory), O.Na-Thalang 288 (neo, here designated: SYD (no accession number)!; isoneo: CANB 914649!).
Description
Plants not observed in nature, all observations from rehydrated herbarium collections.
Plants prostrate, growing in scattered patches or gregarious in mats with other bryophytes. Thallus, when moist, varying in colour from bright to pale green (RHS 145C, 152D, 153D, yellowish-green group) with maroon margins (RHS 187A, greyed-purple group) or pinkish-red (RHS 59, red–purple group; 186B, greyed-purple group) or losing colour with age to become straw coloured, creamish (RHS 159C, orange–white group; 160B, 161C, 162C, greyed-yellow group; 163C,D; 164 B, C, D, greyed-orange group) or eventually becoming whitish with age; when dry, becoming shrunken, dorsal groove deepening, dorsal surface remaining exposed but margins inflexed inward to a perpendicular position; pale green to rose pink to straw yellow to creamish to whitish. Whole plants 2.5–12.5 mm long and 1.7–13.8 mm wide. Segments 0.6–12.5 mm long and 0.7–3.1 mm wide, ligulate to lingulate, fusiform to lanceolate, ellipsoid, frequently canaliculate. Dorsal surface smooth except for depressions for antheridial necks or cavities owing to erosion of epidermal layer with age. Ventral flanks with a row of scales often a mottled maroon, narrowly triangular in shape at the top of the flank, with multiple rows of thin bands of lamellae along the bottom of the flank. Branching simple or 2 or 3 times furcate; branches moderately to widely divergent, diverging from 40 to 130°. Apex mainly lanceolate, sometimes truncate or rounded, infrequently emarginate, sometimes becoming tuberous. Dorsal groove present, anteriorly deep and narrow giving thallus a V-shape in cross-section, becoming broader and shallower posteriorly. Margin of thallus entire to irregularly crenulated owing to projecting scales or rarely because of inflated pigmented cells, attenuate or acute in cross-section. Scales present, 500–1100 × 100–370 μm along top of ventral flank, green or mottled maroon (RHS 187A, greyed-purple group) or darker, appressed to the ventral flanks, imbricate; crescent to triangular-shaped, gill-like. Cilia absent. Dorsal epidermal cells unistratose, globose to u-shaped to predominantly flat, parallel to the surface (i.e. wider than deep in cross-section), or disintegrating with age, hyaline, variable in size up to 27 μm wide, up to 48 μm high, air pores absent. Thallus in transverse section generally U- or V-shaped to plano-convex or concave-convex to campanulate, rarely T-shaped, 300–1720 μm thick and 530–3300 μm wide. Photosynthetic tissue occupying the ventral regions of the thallus in a u-shape, also occurring in the ventral lamellae of the ventral flanks and ventral surface of thallus, composed of smaller, tightly packed cells containing oil droplets, not starch. Storage tissue of the upper portion of the thallus hyaline, up to 700 μm thick, composed of a sub-epidermal layer of large, thick-walled cells, with all other cells below that thin walled, irregularly arranged and lacking airspaces or air chambers. Rhizoids dimorphic, mainly smooth, very few pegged, arising from the tips of the ventral lamellae, hyaline. Tubers present infrequently as geotropic apical tips or tuberous swollen apical tips or new plants growing from swollen triangular tubers.
Dioicous. Male plants generally similar in size to female plants, but occasionally dimorphic, with males more narrow, dorsal groove more pronounced. Archegonia in 1 row along dorsal groove or in 2 rows either side of the dorsal groove. Antheridia in 2 or 3 rows either side of the dorsal groove; variable in shape from ovoid to pyriform to spherical to baculate, (75–)100–450 × 75–360 μm; antheridial necks extending 200–560 μm above the dorsal surface. Capsules 1–5 per plant, along the entire length of the segment, pinkish-brown to pale brown, embedded in thallus, sometimes protruding slightly from the ventral surface. Spores 62.5–140 μm in diameter, globose to triangular globose in polar view, hemispherical to tetrahedral in equatorial view, 55–87.5 μm high, golden-brown, (RHS 164A, 165A, greyed-orange group); wing present, narrow, entire to slightly undulate, 2.5–15 μm wide. Distal surface ornamentation reticulate, with an irregular patterning of alveoli, varying in shape from ovoid to spherical, becoming more compressed in the centre of the face, 7–16 alveoli across diameter, 5–15 μm in diameter, alveoli with smooth, even, low borders, proximal surface ornamentation also reticulate with smaller, more numerous alveoli, varying in shape from spherical to ovoid to dumb-bell shaped, with low borders, 6–11 alveoli across diameter of each facet, 2.5–15(–17.5) μm in diameter. Both surfaces finely papillate at the micro level. Triradiate mark present, ±distinct, pores present, small but not distinct under light microscopy. Chromosome number n = 8, 10 (Na-Thalang 1980; Jovet-Ast 1984). (Fig. 14 (map), 15–21.)
Illustrations
Na-Thalang (1980, p. 73, fig. 5); Jovet-Ast (1984, pp. 390–392, pl. I, II, fig. 1, 394, pl. III, 396, pl. IV, 398, pl. V, 400, pl. VI; 2000, p. 298, fig. 1).
Distribution
Previously considered endemic to the Northern Territory (Na-Thalang 1980; Jovet-Ast 1984); however, more recently collected from the Gregory Ranges near Croydon in Queensland. May also occur in similar habitat in northern Western Australia, although no specimens have been seen from that state.
Habitat and ecology
Riccia caroliniana grows on creek banks, among grass, on acid soil in shady habitats, in open grassland and low woodlands with other monsoonal species such as R. sp. Darwin (O.Na-Thalang 281), R. billardierei and R. gangetica.
Etymology
Named for Dr Roger Carolin (1929–), Na-Thalang’s PhD advisor at the time, and now a retired University of Sydney lecturer.
Comments
Na-Thalang originally collected material of Riccia caroliniana from a site near Robin Falls (as ‘Robin Water Fall’) in the Northern Territory on 28 January 1969, with the collection number Na-Thalang 288. Part of this material was subsequently cultivated at the University of Sydney and a gathering was made from cultivation on 19 September 1969. On the packet for this cultivated material, Na-Thalang repeated the wild collection information, including the collection number, but added an annotation ‘H.B. 19.9.69’ (Fig. 15A). When publishing the name Riccia caroliniana Na-Thalang (1980, p. 74) cited the wild collection information and stated that the ‘holotype’ was lodged at NSW and an ‘isotype’ at SYD. However, the holotype is now missing (Katherine Downs, cryptogam curator, NSW, pers. comm., 12 October 2018) and the ‘isotype’ at SYD cited by Na-Thalang is, in fact, cultivated material gathered on 19 September 1969. Because no original material appears to be extant, a neotype is here designated in accordance with ICN Art. 9.13 (Turland et al. 2018). The specimen Na-Thalang 288 (SYD) has been chosen as the neotype because it is fertile and derived from material of the original wild collection. Jovet-Ast (1984) did not cite either the holotype or isotype of Riccia caroliniana, although she did complete a thorough investigation of 10 other Riccia specimens held at SYD.
Notes
Chromosome numbers for this species had been reported as n = 10 by Na-Thalang (1980); however, several years later, Jovet-Ast was able to make numerous preparations from freshly collected material and recorded the karyotype as n = 8 (Jovet-Ast 1984). Jovet-Ast also redefined organs occurring on the ventral surface of the thallus, referred to as ‘scales’ by Na-Thalang. These organs were treated by Jovet-Ast (1984) as lamellae, on the basis that lamellae are chlorophyllous and bistratose, whereas scales are echlorophyllose, unistratose and lacking rhizoids. Scales are distributed on the ventral flanks of the thallus, whereas lamellae are situated on the underside of the thallus. Jovet-Ast elevated Riccia section Viridisquamata to subgeneric status on the basis of these unique morphological characters. Molecular data have now shown that subgenus Viridisquamata is nested within subgenus Riccia and, therefore, has now been synonymised under subgenus Riccia (see above).
Specimens examined
NORTHERN TERRITORY. Southern end of Pinkerton Ranges, ~74 miles [~119 km] W of Timber Creek, R.Carolin 6678, 6680 (SYD); Coomalie Creek, O.Na-Thalang 258, (SYD); Home [Holmes] Jungle, 8 miles [~12.9 km] from Darwin, O.Na-Thalang 260 (SYD); Elizabeth River, O.Na-Thalang 266 (SYD); on the way to Mt Bundy [Bundey], O.Na-Thalang 270 (SYD); Casuarina Beach, E Darwin, O.Na-Thalang 275 (SYD); Daly River Rd, near exp [experimental] farm O.Na-Thalang 286 p.p. (mixed collection with R. sp. Darwin (O.Na-Thalang 281) (SYD); ~5 miles [~8 km] S of Pine Creek, O.Na-Thalang 296 (SYD); Maluka Cemetery, O.Na-Thalang 310 (SYD); ~100 yards from Mataranka Cr, O.Na-Thalang 313 (SYD); Au N de Mataranka su la Stuart Hwy, A.See s.n. (PC 0049028).
|

|
4. Riccia chrysocrinita Cargill, sp. nov.
Type: CULTIVATED. Australia. New South Wales, University of Sydney, 14 June 1969 (grown from live material collected from c. 325+ miles Stuart Highway, Northern Territory), O.Na-Thalang 312 (holo: SYD (no accession number)!, iso: CANB 914650!).
Description
Plants observed from rehydrated herbarium collections.
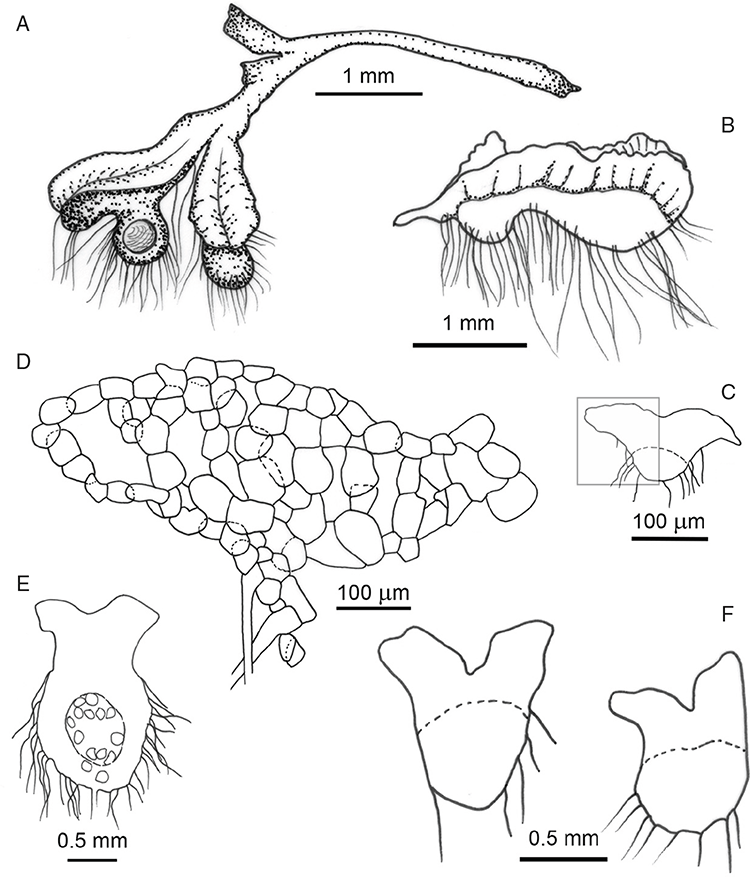
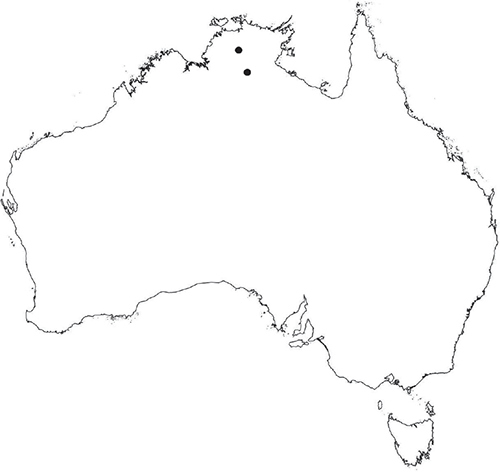
Plants prostrate, gregarious in loose mats or overlapping, forming complete or incomplete rosettes up to 35 mm in diameter. Thallus, when moist, olive green (RHS 152A, yellow–green group); when dry, plants shrunken flat, dorsal surface remaining exposed (cultured plants) or margins inflexed, cilia covering dorsal surface (wild plants); pale green (RHS 191D, greyed-green group; cultured plants) or white and black owing to cilia and inflexed ventral surface (wild plants). Whole plants 2.0–7 mm long, 2.55–5.75 mm wide. Segments 0.3–4.75 mm long and 0.45–1.5 mm wide, fusiform, ribbon-like or lingulate. Ventral flanks darkly pigmented or green. Plants simple, 1–3 times furcate, branches narrow to moderately to widely divergent, diverging from 40 to 110°. Apex rounded to truncate to cordate, shortly emarginate. Dorsal groove present, anteriorly narrow and shallow, becoming broader and shallower to flat posteriorly. Margin of thallus tumid near apex becoming rounded, obtuse or flat posteriorly. Scales not observed. Cilia 120–1120 μm long × 16–90 μm wide at base, conical, numerous, scattered along the ventral flanks and margins in up to 5 obscure rows, often crowded at apex, golden, smooth, density and length variable, stiff and erect, slightly flexuose, often with a loose, more or less spiral twist, from a bulbous base, gradually tapering to the tip, hollow, split on one side. Dorsal epidermal cells unistratose, globose to mammillate, hyaline, disintegrating except along thallus dorsal groove, up to 28 μm high and up to 35 μm wide. Photosynthetic tissue in tightly packed vertical columns, 4–9 cells long. Rhizoids dimorphic, mainly smooth, very few pegged, hyaline to very pale brown. Tubers absent. ?Monoicous No gametangia observed. Capsules 1–several per plant, dark brown, embedded in thallus, very prominent and rupturing on the dorsal surface, frequently with 5–7 cilia protruding from apex of capsule. Spores 92.5–137.5 μm in diameter, irregularly globose to triangular-globose in polar view, 75–95 μm high, dark brown to blackish-brown (no match on RHS chart); wingless; margins entire to crenulate. Distal surface ornamentation irregularly reticulate, alveoli with thick, rounded vermiculate-like borders, variable in shape, from 7 to 11 alveoli across diameter, 5–25 μm in diameter; proximal surface also reticulate, alveoli variable in shape from circular to dumb-bell shaped, with broad low borders, 5–19 alveoli across diameter of each facet, 2.5–32.5 μm in diameter, entire spore is finely papillate over both distal and proximal surfaces. Triradiate mark distinct, pores present. Chromosome number unknown. (Fig. 22 (map), 23–24.)
Distribution
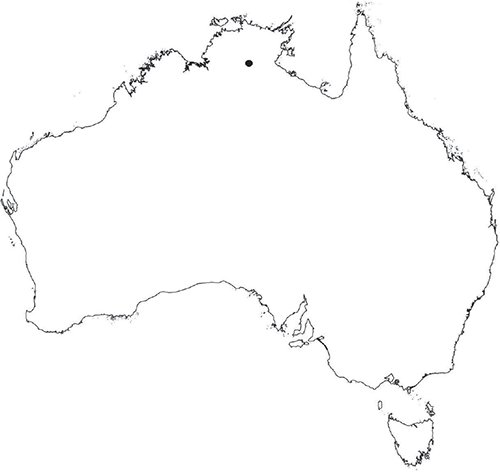
An apparently rare species, endemic to the Northern Territory where it occurs near Katherine.
Habitat and ecology
Grows on bare soil in disturbed sites.
Etymology
Named for its close resemblance to the common southern species Riccia crinita, and from the Greek ‘chryso’, meaning ‘golden’, in reference to the presence of distinctive, golden-coloured cilia along the thallus margins.
Comments
Na-Thalang originally collected material of Riccia chrysocrinita from ‘ca. 325+ miles, Stuart H’way (s)’ in the Northern Territory on 3 February 1969, under the name Riccia crinita and with a collection number Na-Thalang 312 (Fig. 15C). Part of this collection was subsequently cultivated in a laboratory at the University of Sydney and a further specimen was made from cultivated plants on 14 June 1969. Na-Thalang repeated the wild collection information, adding ‘under tree shade’, and re-used the original collection number on the packet containing this cultivated material. However, she added an annotation ‘Herb. 14.6.69’ (Fig. 15D). This cultivated specimen has been chosen as the holotype because it is fertile and appears to contain purely material of R. chrysocrinita, whereas the wild gathering represents a mixture of several species of Riccia. Despite Na-Thalang’s use of the same collection number for both the wild and cultivated material, only the gathering from cultivation constitutes type material of R. chrysocrinita.
Notes
This species is vegetatively very similar to R. crinita but differs from that taxon in the presence of golden coloured cilia on the thallus (v. cilia that are white when dry, hyaline when wet in R. crinita) and a coarsely vermiculate spore surface ornamentation (v. a regularly reticulated pattern in R. crinita). Spore ornamentation is unique in this species and has not been seen in any other Australian Riccia species. It should be noted that the cultivated specimens are morphologically different from the single wild collection. The gametophytes are larger, and the plants have, for the most part, remained open and flat following drying, in contrast to the wild plants that have inflexed margins, thus covering the dorsal surface with their cilia.
Specimens examined
CULTIVATED. New South Wales. Cultivated at University of Sydney, 14 June 1969 (grown from live material collected from Maluka cemetery, Stuart Hwy, 3 Feb. 1969), O.Na-Thalang 308 (SYD); cultivated at University of Sydney, 14 June 1969 (grown from live material collected from Maluka cemetery, Stuart Hwy, 3 Feb. 1969), O.Na-Thalang 308–310 (SYD).
|
5. Riccia crassivenia Jovet-Ast, Cryptog. Bryol. 21(4): 312–313 (2000)
Type: AUSTRALIA. Northern Territory. Katherine, Caravan Park, 19 Apr. 1979, O.H. Volk 111 (holo: PC 0049023!, iso: CANB 871892!)
Description
Plants observed from rehydrated herbarium collections.
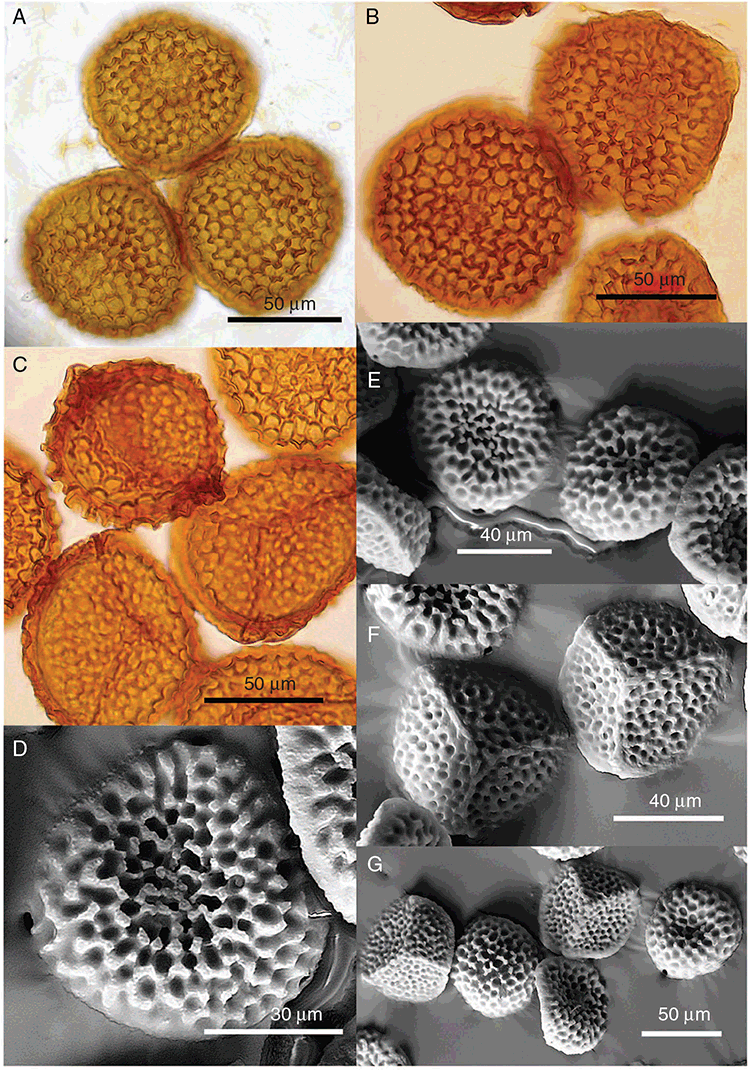
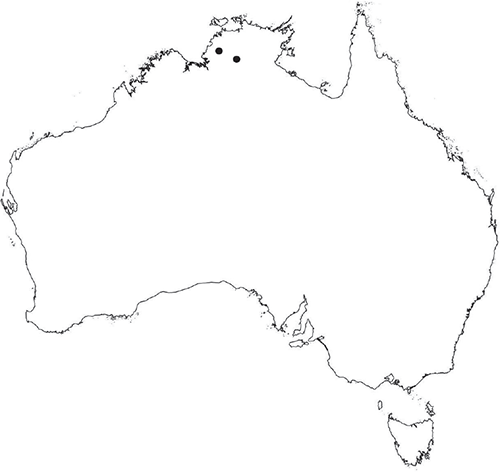
Plants prostrate, gregarious not in rosettes. Thallus, when moist, pale green to yellowish (RHS 18B, yellow–orange group) to cream coloured; when dry, thallus margins curling into a perpendicular position, partially covering dorsal surface, cream or white in colour. Female plants 1.85–11.9 mm long and 0.55–9.25 mm wide. Segments 1.0–7.7 mm long and 0.9–1.8 mm wide; varying in shape from ligulate to tear-drop shaped contracting to a shrunken ‘tail’ posteriorly. Dorsal surface smooth with a reticulate pattern due to epidermal cells and air spaces in between. Ventral flanks mainly a maroon colour but also dark purple, mottled maroon and cream. Branching simple or 1–3 times furcate, branches moderately to widely divergent, diverging up to 60°. Apex rounded or obtuse to lanceolate ±dissected. Dorsal groove present, anteriorly narrow and deep, becoming broader and shallower posteriorly or narrow throughout or only at the anterior end, ranging from 0.4 to 1.4 mm long, Margin of thallus hyaline to white in colour, thin, delicate and undulate, crenate, attenuated to rounded in transverse section. Scales present, 430–1100 × 216–800 μm, imbricate, mainly bright crimson (RHS 187A) dotted with hyaline cells, the crimson pigmentation is such that the scales do appear pointed. Cilia absent. Dorsal epidermal cells unistratose, globose, mainly deflated or disintegrating with age and distance from the segment apex, hyaline, up to 45 μm high. Thallus in transverse section V-shaped, margins reflexed; 600–1240 μm deep and 500–2000 μm wide. Photosynthetic tissue in vertical columns, occupying 1/3–1/2 thallus thickness; columns 3–8 cells long, upper 3 or 4 subepidermal layers are distinctly thick-walled, elongate cells up to 220 μm long and up to 70 μm wide; cells below thin-walled, containing non-starch storage substances, and variable-sized air spaces. Rhizoids as a fine mat covering the ventral surface, dimorphic, a mixture of pegged and smooth. Tubers absent. Dioicous. Gametangia either side of dorsal groove in 2 or 3 rows or along dorsal groove. Male plants similar in size to female plants, 5.0–8.0 mm long and segments 1.0–1.5 mm wide. Antheridia with hyaline necks protruding 200–215 μm from dorsal surface. Capsules 4–6 per plant, occurring from mid-segment to the posterior, embedded, seen from both the dorsal and ventral surface, rupturing on dorsal surface. Spores 80–122.5 μm in diameter, triangular-globose to globose in polar view, fusiform in equatorial view, 70–102.5 μm high, dark reddish-brown to burnt sienna (no colour match on RHS chart); wing may or may not be present, even from spores in same capsule, 5.0–12.5 μm wide. Distal surface ornamentation reticulate, made up of numerous alveoli, borders incomplete, sinuous and vermiculate with protuberances at their corners; 10–13 alveoli across spore diameter, 2.5–15 μm in diameter; proximal surface similar but with smaller alveoli, 6–12 across each facet, 2.5–12.5 μm in diameter. Triradiate mark present but can be prominent in some spores and indistinct in others; pores present. Tubers absent. Chromosome number unknown. (Fig. 25 (map), 26–29.)
Illustrations
Jovet-Ast (2000, fig. 5–8, pl. 11, 12, 19.)
Distribution
This species is so far known only from Fish River Station and near Katherine, in the northern part of the Northern Territory.
Habitat and ecology
Recorded growing on a bare sandy footpath in a caravan park, also found growing in less disturbed areas or on bare or exposed soil at edge of rock slabs in undisturbed habitat.
Etymology
From the Latin ‘crassus’ (thick) and ‘venis’ (veins), in reference to the thick-walled cells of the veins in the dorsal thallus tissue (Jovet-Ast 2000).
Notes
Spore ornamentation in R. crassivenia is quite distinct with a complex, thick, vermiculate pattern on both distal and proximal faces (Fig. 28A–E). However, the gametophyte of this species is superficially similar to several other Riccia species, including R. abdita, Riccia billardierei, R. gangetica and, R. verrucosa. However, in the absence of spores R. crassivenia can be distinguished from similar species by its elongate, thick-walled cells of the subepidermal layer (Fig. 27B, C).
Specimens examined
NORTHERN TERRITORY. Fish River Station, ~200 km S of Darwin, D.C.Cargill 1267 (CANB, DNA).
|
6. Riccia crinita Taylor, London J. Bot. 5: 415. (1846)
Type citation: ‘On clay; n. 42. Swan River. Mr. James Drummond. Hook. Herb.’ Type: AUSTRALIA. Western Australia. Swan River, 1843, J.Drummond 42 (lecto: designated by W.Rauh & G.Buchloh Revue Bryologique et Lichénologique, 30: 261 (1961).: BM 000824078!; isolecto: MEL 19788!, NY 01076780 [image!], NY 01076785 [image!], NY 01076786 [image!], NY 01076787 [image!],).
Description
Plants observed from rehydrated herbarium collections.
Plants prostrate, gregarious in small scattered complete or incomplete rosettes 10.5–13 mm in diameter. Thallus, when moist, dark olive green (RHS 198A, greyed-green group); when dry, plants with margins inflexed, with cilia covering entire dorsal surface; white and black due to cilia and inflexed ventral surface. Whole plants 4.3–5.1 mm long, 3.6–4.55 mm wide. Segments 2.75–4.3 mm long and 0.5–1.3 mm wide, rectangular to obcuneate-oblong or lingulate. Ventral flanks very darkly pigmented due to scales. Plants bifurcate, branches narrow to moderately divergent, diverging from 50 to 60°. Apex truncate to squarish, emarginate. Dorsal groove present, anteriorly narrow and deep, becoming broader and shallower from mid-thallus to posterior. Margin of thallus tumid, obtuse in cross section. Scales present, dark purple (RHS 79A, purple group) appressed against the ventral flank and tightly attached. Cilia 295–1230 μm long × 10–50 μm wide at base, conical, numerous, scattered along the ventral flanks and margins in up to 5 obscure rows, hyaline or white, smooth, density and length variable, stiff and erect, hollow, split on one side. Dorsal epidermal cells unistratose, globose to mammillate, pointed at the tips, hyaline, up to 67.5 μm high and up to 60 μm wide. Photosynthetic tissue in tightly packed vertical columns, occupying 1/3–1/2 of upper thallus, 4–7 cells long. Rhizoids dimorphic, both smooth and pegged, mainly smooth, hyaline to very pale brown. Tubers absent.
Monoicious. Gametangia in two rows either side of dorsal groove. Antheridia pyriform to spherical, 87.5–125 × 75–87.5 μm; antheridial stalk 185 μm long. Capsules 1–several per plant, brown, embedded in thallus, prominent and rupturing on the dorsal surface, with cilia protruding from apex of capsule. Spores 82.5–105 μm in diameter, irregularly globose in polar view, dark brown (no match on RHS chart); wingless; margins entire to shallowly crenate. Distal surface ornamentation irregularly reticulate, alveoli with thick, rounded vermiculate-like borders, variable in shape, from 7 to 12 alveoli across diameter, 2.5–12.5 μm in diameter; proximal surface also reticulate, alveoli variable in shape from circular to dumb-bell shaped, with broad low borders, 6–8 (–10) alveoli across diameter of each facet. Triradiate mark indistinct, pores absent. Chromosome number n = 8 (Na-Thalang 1980). (Fig. 30 (map), 31.)
Distribution
A cosmopolitan species, occurring in all Australian States and Territories, except Tasmania. Known in the Northern Territory from a single collection at Tennant Creek, at the southern margin of the geographical area covered in this paper. Also occurs in the Mediterranean, Macaronesia, southern Africa, North and South America.
Etymology
Named from the Latin ‘crinitus’ (having tufts of long weak hairs), in reference to the hair-like cilia present on the margins of the thallus and often over the surface of the developing capsules.
Notes
Riccia crinita is a common southern Australian species with a complex taxonomic history, which will be discussed in later publications. Riccia crinita is easily distinguished from the only other ciliate species known from the northern part of the Northern Territory by the colour of its cilia and spore ornamentation (see R. chrysocrinita, above). Rauh and Buchloh (1961) stated ‘Durch gütige Vermittlung von Herrn Dr. G. TAYLOR (Kew) könnten wir das Original der R. crinita aus dem Herbarium HOOKER’S (Nr. 42, Swan River, leg. Drummond) untersuchen.’ (p. 261) and also refer to the ‘der Typ-Pflanzen von Riccia crinita Taylor aus dem Herbarium Hooker’s (Australien, Swan River, leg. Drummond)’ in the abstract to their paper. This is here treated as effective lectotypification by Rauh and Buchloh in accordance with ICN Art. 7.11 (Shenzhen Code, Turland et al. 2018). At the time of Rauh and Buchloh’s publication, type material of R. crinita was held at K; however, since that time it has been transferred to BM.
Specimens examined
NORTHERN TERRITORY. Bank of Tennant Creek at Stuart Hwy crossing, A.E.Orchard 922 (AD).
|
7. Riccia crustata Trabut ex Grolle, Lindbergia 3: 54 (1975)
Type citation: ‘Algerien, Tiaret, leg. TRABUT; Holotypus: MPU (fide JOVET-AST in lit.).’ Type: n.v.
Description
Plants observed from rehydrated herbarium collections.
Plants prostrate, growing in scattered or loose patches, gregarious, not growing in rosettes or semi-rosettes. Thallus when moist, bright green to pale green (RHS 144A, 145B, C; 146C, yellow-green group); when dry, plants white, owing to calcite crystals in dorsal epidermal cells. Whole plants 1.9–5.0 mm long and 0.65–3.1 mm wide. Segments 0.5–4.0 mm long × 0.4–3.0 mm wide; varying in shape from elliptical to lingulate to ellipsoid-fusiform to lanceolate. Dorsal surface appears smooth macroscopically but cracked and chalky microscopically because of calcite crystals in surface cells. Ventral flanks tumid; mainly green with infrequent blotches of maroon pigmentation. Branching usually simple or bifurcate, rarely 2 times furcate, branching asymmetrical, branches moderately to widely divergent, diverging from 30 to 90°. Apex variable, lanceolate or blunt, rounded-truncate or squarish. Dorsal groove present, anteriorly narrow and deep, more prominent when dry, becoming broader and shallower posteriorly, particularly over developing capsules. Margin tumid; very finely crenulate because of bulging dorsal epidermal cells, obtuse in cross-section. Scales present, hyaline, confined to ventral surface beneath apex, consisting of a few cells appressed to ventral surface, extending across ventral keel in 1 or 2 bands. Cilia absent. Dorsal epidermal cells unistratose, possibly also bistratose, globose only in the apical region of the dorsal groove, deflating with maturity over the dorsal surface, hyaline but containing calcite crystals, 25–45 μm × 22–62.5 μm. Thallus in transverse section campanulate with reflexed marginal wings, or an inverted hat-shape, plano-convex to concave-convex; half as deep as wide; 235–800 μm × 610–1525 μm. Photosynthetic tissue in vertical columns occupying the upper 1/5–2/3 of thallus depth, 4–9 cells high per column. Rhizoids covering entire ventral keel surface of segments, hyaline, smooth only. Tubers absent, but thallus swollen ventrally along length of segment.
Dioicous. Male plants similar in size to female plants. Gametangia in 1 row along dorsal groove or 2 rows either side of dorsal groove. Antheridia pyriform to spherical, 140–300 × 180–360 μm; antheridial stalk up to 125 μm long. Capsules 1 per plant in centre of segment, embedded, visible from both the dorsal and ventral surface, sometimes bulging from the ventral surface, capsule tissue varying in colour from green when immature to golden-brown to dark brown when mature. Spores 80–100 μm in diameter, globose to rounded-hemispherical in polar and equatorial view; 60–100 μm high, dark red to very dark brown (darker than RHS 200A) or very dark purple-black; wing absent. Distal and proximal surface ornamentation generally absent, surface smooth. Triradiate mark present in some spores but thin or completely absent; pores absent. Chromosome number unknown. (Fig. 32 (map), 33–35.)
Illustrations
Jovet-Ast (1956, fig. II); Jovet-Ast (1973, pl. 1, 2); Hugonnot et al. (2014, fig. 1).
Distribution
Known from several collections at Roper River in northern part of the Northern Territory. Also occurs in South Australia, on Flinders Island, Tasmania, Victoria and Western Australia. Also occurs in North America, northern Africa (Algeria, Egypt, Libya, Morocco, Tunisia), the Middle East (Israel, Lebanon, Jordan), Europe (Cyprus, France, Spain and the Balearic Islands) and Eurasia (Bulgaria and Kazakhstan; Hugonnot et al. 2014).
Habitat and ecology
Recorded as growing on bare soil, sometimes in crevices in limestone, on creek or river banks.
Etymology
Named from the Latin ‘crusta’, crust-like or forming a crust, in reference to the crust-like top layer of the thallus, formed by the presence of calcite crystals embedded in the epidermal layer.
Notes
Riccia crustata is similar to R. albida, and these species have variously been treated as either distinct (Jovet-Ast 1973) or possibly conspecific (Schuster 1992; Seppelt 1999; Jovet-Ast 2000), but there are morphological differences between the two species (as noted by Jovet-Ast 2000), including the presence of air spaces in R. albida (v. absent in R. crustata) and the shape of the dorsal epidermal cells (globose in R. crustatav. conical in R. albida). Geographical separation is also evident, with plants corresponding to R. crustata occurring in northern Western Australia and the Northern Territory, and R. albida in Victoria and South Australia. Specimens examined as part of this study are from the same region as those seen by Jovet-Ast (2000) and exhibit the globose dorsal epithelial cells she described for R. crustata. Grolle (1975), in his Latin description to validate the name Riccia crustata, described the spores of that species as dark red, seen in one collection (Curnow 2799), which may be due to variation within the species or to the maturity of the spores. Spore colour in other collections of R. crustata examined in this study otherwise range from very dark brown to very dark purplish-black. The trnL-F tree of Cargill et al. (2016) indicated that northern Western Australian accessions identified in that study as R. albida and accessions from North America, also identified as R. albida, are closely related but not conspecific, supporting the hypothesis that northern Australian populations are likely to represent R. crustata rather than R. albida. Sampling and sequencing of additional populations of R. crustata and R. albida in Australia are required to confirm the identity of the taxa occurring in Australia. However, on the basis of current molecular and morphological data, we consider plants occurring in the northern part of the Northern Territory to represent R. crustata.
Specimens examined
NORTHERN TERRITORY. Roper River, 13 km E of Mataranka, J.A.Curnow 2788; J.A.Curnow 2792; J.A.Curnow 2799 (all CANB).
|
8. Riccia sp. Darwin (O.Na-Thalang 281) Cargill
Description
Plants observed from rehydrated herbarium collections.
Plants prostrate, in tangled mats and loose patches. Thallus, when rehydrated, dorsal surface a pale yellow–green (RHS 160D, greyed-yellow group) or dull mustard (RHS 161A, C, D, greyed-yellow group) or sandy yellow (RHS 164D, greyed-orange group) or a deep fuchsia over the entire dorsal surface (RHS 59A, red–purple group) with margins a deep maroon (RHS 187A, greyed-purple group); when dry, plants shrunken, frequently ventral scales closed over the dorsal surface or less frequently with the margins inflexed only to a perpendicular position exposing the dorsal surface, pale cream to crimson purple (RHS 187B, greyed-purple group). Whole plants 3.0–11.2 mm long, 1–11.2 mm broad. Segments variable in shape, elongate-cordate, lingulate, narrowly spathulate, elongated obovoid, fusiform or ellipsoid, 0.55–11.6 mm long and 0.25–2.2 mm wide. Branching simple to 1–4 times furcate, branches almost parallel to moderately to widely divergent, diverging from 20 to 130°. Apex rounded to lanceolate, often with a developing tuber at the tip. Dorsal groove anteriorly deep and narrow, becoming broader and shallower or disappearing or becoming an elongated cavity posteriorly. Margin crenulate owing to scales, to wavy and becoming ragged as it degrades; acute in transverse section. Ventral flanks crimson–purple. Scales present, fused to flanks except along their distal edge, imbricate, deep pink or purplish-pink, extending beyond thallus margins. Cilia absent. Dorsal epidermal cells unistratose globose–pyriform, hyaline, disintegrating except along thallus dorsal groove, vertical side walls persisting. Thallus in transverse section almost terete at apex when tuber developing but otherwise biconvex-convex to concave-convex in female plants, 290–670 μm thick and 700–1700 μm wide; male plants variable from U-shaped to V-shaped to plano-convex to campanulate, 250–900 μm thick and 800–2010 μm wide. Photosynthetic tissue in vertical columns, occupying upper half of thallus, 3–6 cells long. Rhizoids dimorphic, mostly smooth but some also pegged. Tubers present, plants frequently growing out from a distinctly triangular-shaped tuber, geotropic tubers also developing at apices of segments.
Dioicous. Male plants and female plants similar in size, males slightly narrower. Antheridia mainly in 1 row, infrequently in 2 rows along dorsal groove with long, conspicuous hyaline or purplish-pink necks, spherical to pyriform, occupying most of the depth of the segment tissue 82.5–700 × 100–550 μm, antheridial necks 185–565 μm long; fuchsia–purple papillae, associated with the necks run along the dorsal groove, 1 or 2 cells long. Archegonia in 1 row, without prominent necks, papillae absent. Capsules 1 per plant at base of segment, embedded in thallus, visible from dorsal surface; maroon to reddish-purple brown. Spores 102.5–127.5 μm diameter, globose to subglobose in polar view, 80–97.5 μm high, hemispherical in equatorial view; dark reddish-purple–brown becoming opaque at maturity; wing generally absent or if present, incomplete, 5–10 μm wide, margin crenulate or with truncate protuberances. Distal surface ornamentation similar on both surfaces, reticulate over entire spore, 7–10 alveoli across diameter; 5–25 μm in diameter, alveoli very regular in shape, borders low and even in width; proximal surface ornamentation reticulate, pinched in at the centre. Triradiate mark and pores absent. Chromosome number n = 8 (Na-Thalang 1980). (Fig. 36 (map), 37–43.)
Distribution
Recorded from the Darwin area in Northern Territory.
Habitat and ecology
Recorded growing on bare soil among grasses or along man-made tracks in the Northern Territory.
Notes
The identity of plants referred to this taxon is uncertain, but they may represent the widespread and variable Riccia discolor Lehm. & Lindenb., which occurs in Nepal, India, Pakistan, Sri Lanka, Bangladesh, Myanmar, Bali, Indonesia and Africa (Benin, Democratic Republic of Congo, Ivory Coast, Kenya, Malawi, Nigeria, Niger, Príncipé, Tanzania, Uganda and Zambia; Wigginton 2018), or a related taxon. Australian material matches descriptions of R. discolor in Stephani (1900) and Pandé and Udar (1957). However, syntype material of R. discolor held at W (W 2010– 02662!), examined by Cargill in 2018, does not match Australian plants. Plants comprising the W syntype gathering are more robust, with larger scales that partially curl over onto the dorsal surface of the thallus. Plants also do not show the strong pinkish pigmentation seen in Australian material. The complex taxonomic history of R. discolor is discussed by Srivastava (1964), who adopts a broad concept of this taxon, including some taxa more recently treated as distinct by subsequent authors (e.g. R. himalayensis Steph. ex Kashyap; Mondal 2007). Taxonomic resolution of R. discolor within its extra-Australian range would assist in determining the identity of Australian plants currently referred to R. sp. Darwin (O.Na-Thalang 281). See also notes under R. billardierei for further discussion of R. discolor.
Specimens examined
NORTHERN TERRITORY. Berry Spring, south of Darwin, O.Na-Thalang 262 (SYD); Darwin River, O.Na-Thalang 278 (SYD); South Port, Darwin, O.Na-Thalang 281 (SYD); Daly River Rd, near exp [experimental] farm, O.Na-Thalang 286 p.p. (SYD). CULTIVATED. New South Wales. Cultivated at University of Sydney, 8 May 1969 (grown from live material collected from Coomalie Creek, Darwin, 19 Jan. 1969), O.Na-Thalang 259 (SYD).
|

|
9. Riccia duplex var. megaspora Na-Thalang, Brunonia 3: 128–129 (1980)
Type: AUSTRALIA. Northern Territory. East Alligator River, South Cahill’s Crossing, ~7 miles, 16 May 1968 [given as 15 May in protologue], R.Carolin 6880 (holo: NSW).
Description
Plants observed both in nature and from rehydrated herbarium collections.
Plants prostrate, growing variously in loose patches or tangled mats, not forming rosettes. Thallus when moist bright green to pale green or yellow–green and pale yellow, (RHS 145C; 150D, 151D yellow–green group); when dry, plants shrunken, often remaining open or margins partially inflexed over dorsal surface; remaining green or pale green (RHS 144A; 145C). Whole plants 1.35–10.0 mm long and 0.4–2.35 mm wide. Segments 0.4–10.0 mm long, and 0.1–0.95 mm wide; varying in shape from ellipsoid to lingulate, canaliculate, mainly ligulate. Dorsal surface smooth, air chambers visible below. Ventral flanks green or mottled with few purple cells (RHS 79A). Branching simple or bifurcate, asymmetrical; branches growing parallel or widely diverging at 90°. Apex rounded or truncate, emarginate, often becoming tuberous. Dorsal groove present, anteriorly narrow and deep, becoming broader and shallower posteriorly, more distinct over developing capsules or when dry. Margin entire or crenulate or undulate; becoming attenuate to rounded in cross-section. Scales present, often only under apex but also in bands along ventral surface; variable in shape from hemispherical or triangular or bifid; 36–216 × 72–288 μm. Cilia absent. Dorsal epidermal cells unistratose; globose; hyaline. Thallus in transverse section variable from terete to cordate to broadly campanulate to fusiform; 130–820 μm deep and 370–1150 μm wide. Photosynthetic tissue with one layer of air chambers or air spaces occupying from 1/5 to 2/3 of the thallus thickness. Rhizoids over ventral surface, hyaline to very pale brown, dimorphic, variable: mainly pegged or both pegged and smooth. Tubers present as swollen geotropic apices. Monoicous. Archegonia along midline of dorsal groove. Antheridia not seen. Capsules 1–4 per plant, produced anywhere along the length of segment, embedded, bulging as a spherical ball from the ventral surface, capsule tissue pale brown or hyaline. Spores 75–92.5 μm in diameter, globose to triangular-globose in polar view, tetrahedral in equatorial view; 47.5–65 μm high, golden-brown (RHS 164A, greyed-orange group); wing present, crenulate and finely papillate, wing 5–10 μm wide. Distal surface ornamentation regularly reticulate, alveoli more or less angular, (6–)7–12 alveoli across diameter, alveoli 2.5–22.5 μm in diameter, alveolar walls evenly thin, projections at corners; proximal surface reticulate, alveoli smaller than dorsal alveoli, walls thinner with projections at corners; 6–10 alveoli across each facet, 2.5–15 μm in diameter. Triradiate mark distinct and pores present. Chromosome number n = 16 (Na-Thalang 1980). (Fig. 15B, 44 (map), 45–48.)
Illustrations
Na-Thalang (1980, 69, fig. 3K); Jovet-Ast (2000, pl. 15).
Distribution
Widespread across the northern part of the Northern Territory and also occurs in Queensland (Jovet-Ast 2000).
Habitat and ecology
In the Northern Territory, recorded growing on bare soil under shade on creek banks or on muddy banks of billabongs.
Etymology
From the Latin ‘duplex’ (meaning ‘double’), in reference to the diploid chromosome number (Müller 1954) and the Greek ‘mega’ (large) and the Latin ‘spora’ (spore), in reference to the size of the spores, which are quite large in comparison to R. duplex var. duplex (85–106 μm v. 70–85 μm; see Na-Thalang 1980).
Notes
This species is very similar to Riccia multifida, with narrow, strap-like thallus segments, and frequently growing in similar habitats near permanent water sources such as creeks. Riccia duplex differs from R. multifida by the absence of thick-walled cells surrounding the air chambers. Na-Thalang (1980) recognised two varieties of Riccia duplex, distinguished from each other by the size of their spores. R. duplex var. duplex has spores 70–85 μm in diameter, whereas those of var. megaspora are larger at 85–106 μm in diameter. The number of alveoli across the distal face ranges from 6 or 7 at 10–20 μm wide in var. duplexv. 9 or 10 at 6–9 μm wide in var. megaspora. Specimens examined in this study bear spores that fall within the size range for var. duplex, but also in the lower range for var. megaspora. They also have 9–12 alveoli across the distal face, which is within the range described for var. megaspora. Riccia duplex may be better treated as a single, variable species without infraspecific taxa, but additional collections are required for morphological and sequencing studies. In the absence of additional collections, we retain Na-Thalang’s circumscription here and treat material from the Northern Territory as var. megaspora, pending further evaluation.
As with other Na-Thalang specimens, plants were frequently grown on in the laboratory following collection from the wild. In the case of R. duplex var. megaspora, the holotype comprises wild-collected material gathered by Roger Carolin (Carolin 6880), lodged at NSW. Additional material of this taxon, labelled as ‘R.C.Carolin 6880’ is also held at SYD (Fig. 15B). However, this material does not represent duplicate material of Carolin 6880, rather it comprises cultivated material from this population, harvested at a later date. The annotation ‘Herb. 30.5.1969’ is pencilled underneath the collection date for the original, wild gathering, indicating the date the material was collected from cultivation. Na-Thalang also added yet another collection to this packet, wrapped in paper towel, from glasshouse-grown material, collected at a later date (24 September1969). These subsequent collections are separate collecting events from the original wild collection, and therefore cannot be considered part of the original material for this name (ICN Art 8.2, Turland et al. 2018).
Neither the holotype (NSW) nor the isotype (SYD) of Riccia multifida var. divaricata has been located (Katherine Downs, cryptogam curator, NSW, pers. comm., 12 October 2018; and Murray Henwood, Curator SYD, pers. comm., 22 October 2018), despite extensive searches at both NSW and SYD. Only one other collection of Riccia multifida var. divaricata (Na-Thalang 324) was cited by Na-Thalang (1980) in the protologue, and this collection is here designated as the neotype for this name. However, Na-Thalang 324 is a monoicous plant with thin-walled cells surrounding air chambers in the thallus, characters typical of Riccia duplex var. megaspora, and Riccia multifida var. divaricata is accordingly placed in synonymy of that species.
Specimens examined
NORTHERN TERRITORY. Wongalara Homestead, near Mainoru boundary, N of Wongalara Homestead, S.Hirst 278 (CANB, DNA); Katherine Gorge NP, plateau above lily pond, L.A.Craven 6716 (CANB); near Mt Sir James, 62 km E of Mataranka, J.A.Curnow 2782 p.p. (CANB). CULTIVATED. Sydney University laboratory, 30 May 1969, O.Na-Thalang s.n. (SYD).
|
|
10. Riccia eburnea Jovet-Ast, Cryptog. Bryol. 21(4): 300 (2000)
Type: AUSTRALIA. Northern Territory. Mt Olga, ‘Valley of the Winds’, 24 July 1983, A.Schäfer-Verwimp 4628a (holo: PC 0049024!).
Description
Plants observed from rehydrated herbarium collections.
Plants prostrate, gregarious, in loose rosettes or half rosettes up to 24 mm in diameter, 1 population found growing mixed with Riccia multifida in entangled mats. Thallus, when moist after rehydration, pale green (RHS 145B, C, yellow–green group) to a straw colour owing to age of collection; when dry, plants shrunken and sunken into substrate, becoming flattened and thin, remaining reflexed, dorsal groove broadening; becoming pale green to pale brown or whitish. Whole plants from 5.4–12.5 mm long, and 2.0–8.0 mm wide. Segments 0.65–8.2 mm long, and 0.65–2.5 mm wide, lingulate, flat and ribbon-like elongate cordate, rectangular in shape. Dorsal surface quilt-like owing to air chambers below epidermal layer, thallus tumid either side of dorsal groove. Ventral flanks green. Branching 1 or 2 times furcate, asymmetrical, branches almost parallel to moderately divergent, diverging 45–80°. Apex emarginate to retuse, truncate or rounded. Dorsal groove present, deep and narrow at the apex, becoming broad and shallow shortly behind apex, running the length of the segment. Margin of thallus entire and wavy to finely crenate; acute in cross-section. Scales present, small, 150 × 950 μm, appressed to the ventral keel in bands along the length of the segment, not reaching thallus margins. Cilia absent. Dorsal epidermal cells unistratose, dimorphic, smaller cells surrounding air pores, with larger cells surrounding these, up to 20 μm high, globose, becoming deflated with maturity, hyaline. Thallus in transverse section concave-convex to plano-convex, margins reflexed, 380–925 μm deep and 850–2700 μm wide. Photosynthetic tissue in broad, vertical columns, occupying 1/2–2/3 of the thallus thickness, 3–9 cells long. Rhizoids thickly covering the ventral surface keel, hyaline, dimorphic, sometimes mainly smooth with few pegged rhizoids or mainly pegged with few smooth rhizoids. Tubers absent.
Monoicous. Gametangia in rows along either side of dorsal groove. Antheridia ovoid, 70–110 × 62.5–145 μm; antheridial necks not seen. Capsules 1–12 per plant, anywhere along the length of the segment, embedded, visible from dorsal and ventral surface; ornamentation absent; pale brown to hyaline. Spores 60–77.5 μm in diameter, globose to triangular globose in polar view, 35–50 μm high, orange brown (RHS 165A, greyed-orange group) becoming opaque; wing present, 1.25–7.5 μm wide, margins irregularly crenulate, sometimes papillose at edges. Distal face reticulate, with incomplete borders with protuberances at corners; 8–12 alveoli across diameter; proximal face reticulate, with low, evenly thick, incomplete borders, 8–11 alveoli across each facet, 2.5–7.5 μm in diameter. Chromosome number n = 8 (Jovet-Ast 2000). (Fig. 49 (map), 50–52.)
Illustrations
Jovet-Ast (2000, pl. 1, 2, 3, 14).
Distribution
Known from two widely separated localities in the northern part of the Northern Territory, at Tennant Creek and Mataranka. Widespread in the southern part of the Northern Territory and also occurs in the Meekatharra–Gascoyne River region of Western Australia.
Habitat and ecology
Recorded growing on soil in disturbed sites in the northern part of the Northern Territory.
Etymology
From the Latin ‘eburneus’ (ivory white), referring to the colouring of the thallus.
Notes
Superficially similar to R. albida or R. crustata, which are also often distinctly whitish. However, plants of R. eburnea do not have the calcite crystals embedded in their epidermal layer, which is typical of the former two species. The assimilative tissues of R. eburnea also contain large, vertical, polygonal air chambers and air spaces, rather than the very narrow, vertical columns typically found in R. albida and R. crustata. The spores of both R. albida and R. crustata and are virtually smooth, unlike the reticulate patterned spores of R. eburnea.
Specimens examined
NORTHERN TERRITORY. Barkly Tableland. Bank of Tennant Creek at Stuart Hwy Crossing, A.E.Orchard 921 (AD); near Mt Sir James, 62 km E of Mataranka, J.A.Curnow 2782 p.p. (CANB).
|

|
11. Riccia gangetica Ahmad ex L.Söderstr., A.Hagborg & von Konrat, Phytotaxa 65: 57 (2012)
Type: INDIA. Uttar Pradesh. Lucknow, Unao et Aligardh, Aug. 1940, S. Ahmad s.n. (LWU, n.v.).
Description
Plants observed both in nature and from rehydrated herbarium collections.
Plants prostrate, growing in scattered or loose patches or in rosettes, up to 31.6 mm in diameter. Thallus, when moist, variable in colour from bright green to pale green to yellowish-green to brownish, (RHS 137C; 138C; 139B, green group; 144A, B, C; 146A, B, yellow–green group; 199B, greyed-brown group); when dry, plant margins fully inflexed over dorsal surface or curled into a perpendicular position and only partially covering the dorsal surface; black, owing to scales closed over dorsal surface or, when partially closed, pale green in colour. Plants 2.2–13.1 mm long and 0.7–9.8 mm wide. Segments 1.0–8.35 mm long, and 0.8–3.0 mm wide; varying in shape from ovate to ellipsoid to lanceolate to lingulate anteriorly, becoming shrunken into a ‘tail’ posteriorly. Dorsal surface smooth with a reticulated pattern formed by epidermal cells and air spaces. Ventral flanks dark purple to dark brown (RHS 200B, brown group), owing to scales, becoming mottled green and black or yellowish-brown below scales. Branching simple or 1 or 2 times furcate, asymmetrical, branches almost parallel to moderately or widely divergent, diverging 30–180°. Apex pointed or lanceolate, sometimes dissected. Dorsal groove present, anteriorly narrow and deep, becoming shallow or disappearing posteriorly. Margin wavy, crenate, owing to scales extending beyond the margin; becoming attenuate or obtuse in cross-section. Scales present, 500–900 × 350–735 μm, extending shortly beyond thallus margins, imbricate closely appressed to ventral flanks, bicoloured, both brown (RHS 200B, brown group) and purplish-maroon (RHS 187A, greyed-purple group), crescent to triangular in shape. Cilia absent. Dorsal epidermal cells uni- or bistratose; globose to pyriform to quadrate to bilobed to u-shaped to deflated or disintegrating with age; hyaline. Thallus in transverse section biconvex-convex, with margins reflexed to campanulate to bowl-shaped, half as deep as wide; 500–1080 μm deep and 850–2500 μm wide. Photosynthetic tissue in vertical columns occupying 1/3–2/3 of the thallus thickness, 4–14 cells long per column. Rhizoids densely covering the ventral surface of the thallus up to the base of scales, pale brown to pale purplish-brown to brownish-purple; dimorphic, both pegged and smooth, occasionally mainly smooth or all smooth, pegs small and dense, sometimes branched. Tubers absent.
Monoicous. Archegonia along dorsal groove or either side of groove. Antheridia not seen. Capsules 2–9 per plant, produced from mid-segment to posterior, embedded, situated in the top half of thallus, visible from the dorsal surface; dark yellowish to yellowish-brown to reddish-yellow–brown to orange–yellow to pale brown to hyaline. Spores 100–142.5 μm in diameter, globose in polar view, hemispherical in equatorial view, 90–102.5 μm high, darkly coloured from dark red–brown (RHS 187A, greyed-purple group) to dark brown; wing absent; spore margin shallowly to coarsely crenulate. Distal and proximal surface ornamentation regularly and finely reticulate on both faces, alveoli small, borders ±angular or sinuous, 9–15 alveoli across diameter, alveoli 5.0–17.5 μm in diameter, alveolar walls mainly evenly wide and high; proximal alveoli same as distal alveoli. Triradiate mark and pores absent. Chromosome number n = 8, 16, 24, 48 (Udar and Chopra 1957; Na-Thalang 1980; Jovet-Ast 2000). (Fig. 53 (map), 54–57.)
Illustrations
Ahmad (1942, fig, 1–3); Pandé and Udar (1957, fig. 33–44); Na-Thalang (1980, pl. 1I); Jovet-Ast (2000, fig. 4, pl. 16. 6–9).
Distribution
In Australia, R. gangetica is widespread throughout the Northern Territory, and is also recorded from the Pilbara region in Western Australia. Also occurs in Indonesia and northern and southern India.
Habitat and ecology
Recorded from a variety of habitats, including bare, exposed soil, over humus or algal mats on sandy soils at the base of flat sandstone rock slabs or in the shade of shrubs in disturbed areas.
Etymology
The epithet ‘gangetica’ is derived from the Ganges River in India from where the species was originally described.
Notes
Riccia gangetica was originally published as a nom. inval. by Ahmad (1942; and, subsequently, by several other authors, before finally being validated by Söderström et al. 2012). Similarities to other northern Australian species were noted by both Na-Thalang (similar to R. macrospora) and Jovet-Ast (similar to R. billardierei), but both can be distinguished from R. gangetica by spore morphology, the spores of R. gangetica being apolar with no triradiate mark or pores, highly reticulate on both distal and proximal sides with alveoli surrounded by a network of sinuous borders (Fig. 56C–D) v. the tetrahedral spores of R. macrospora with a distinct triradiate mark, three pores and pattern of high ridges surrounding irregular shaped alveoli (see Cargill and Beckmann 2020 for images) v. the apolar spores of R. billardierei with larger, fewer alveoli surrounded by high ridges with projections at the corners of the alveoli (Fig. 11D–E). Gametophyte characters also distinguish R. gangetica from the two other species, including the presence of brown-pigmented rhizoids and maroon–brown scales (Fig. 55E–G). Riccia macrospora has large, dark reddish-purple scales, whereas R. billardierei has scales with crimson pigmentation confined only to 1 or 2 cell layers of the distal margins, emphasising their scalloped appearance (Fig. 10B-C).
Specimens examined
NORTHERN TERRITORY. Fish River Station, ~200 km S of Darwin, D.C.Cargill 1276 (CANB, DNA); Fish River Station, on top of escarpment, D.C.Cargill 1318 p.p. (CANB); Fish River Station, down slope from the top of the escarpment, D.C.Cargill 1319 (CANB); near Mataranka, J.A.Curnow 2780 p.p. (CANB).
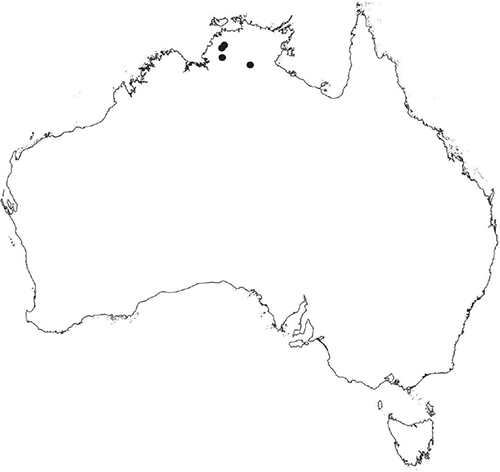
|
12. Riccia junghuhniana Nees & Lindenb. ex Gottsche, Lindenb. & Nees, in C.M.Gottsche, J.B.W.Lindenberg & C.G.D.Nees von Esenbeck, Syn. hepat. 4: 609 (1846)
Type citation: In Java Insula legit cl. Junghuhn (Hb. N. ab E.). Type: INDONESIA. Java, s. dat., F.Junghuhn s.n. (STR [image! (provided by STR)]).
Description
Plants observed both in nature and from rehydrated herbarium collections.
Plants prostrate, scattered in half-rosettes (insitu) up to 8.65 mm in diameter or in thick mats (when grown on) or scattered and mixed among mats of Riccia multifida. Thallus when moist light green (RHS 145B, 147C, 150D, yellow–green group) becoming yellowish after drying and rehydrating (RHS 8D, yellow group; 18C and D, 19C, yellow–orange group; 158A, yellow–white group); when dry, margins inflexed or remaining flat but not covering dorsal surface; remaining green (RHS 145B, C and D, 147C, yellow–green group; 195A greyed-green) or becoming yellow (RHS 18D, 20C, yellow–orange group). Whole plants 1.6–11.7 mm long and 0.5–11.7 mm wide. Segments 0.6–11.7 mm long, and 0.5–2.2 mm wide; varying in shape from lingulate to canaliculate-lingulate to elongated ribbons to spathulate to obtriangular to elongate ovate. Dorsal surface smooth, except for surface cavities in the older parts of the thallus. Ventral flanks mottled hyaline and purplish-maroon (RHS 79A, purple group; 187A, greyed-purple). Branching simple or 1–3 times furcate, asymmetrical, almost parallel to moderately to widely divergent, diverging 60–110°. Apex lanceolate, rounded, truncated or; shallowly retuse. Dorsal groove present, deep and narrow anteriorly becoming shallower and broader posteriorly but persisting the entire length of segments, particularly obvious when plants dry. Margin may be narrowly to broadly wing-like; irregularly crenulate to entire, sometimes undulating, sometimes reflexed, acute in transverse section. Scales present, 160–400 × 120–200 μm, not readily seen, appressed at base of ventral flanks and across ventral surface as bands with irregular distal margins, cell walls straight, mottled hyaline and maroon (RHS 187A, greyed-purple). Cilia absent. Dorsal epidermal cells unistratose, hyaline, dimorphic, smaller cells surrounding air spaces, globose to pyriform, wider than high. Thallus in transverse section biconvex-convex with margins reflexed to concave-convex to campanulate to plano-convex; 170–1000 μm thick and 650–2250 μm wide. Photosynthetic tissue with air chambers occupying from 1/5 to 1/2 of the thallus thickness, smaller air spaces also present throughout storage tissue. Rhizoids covering the ventral surface of the thallus, dimorphic, both smooth and pegged, hyaline. Tubers absent, sometimes segments with swollen apices.
Monoicous. Gametangia in 1–3 rows either side of dorsal groove. Antheridial necks only seen. Capsules 2–19 per plant, along entire length of segments or only from mid-segment to posterior, embedded, seen from both the dorsal and ventral surface, bulging from the ventral surface when mature, varying from pale brown to pale orange–brown to hyaline. Spores 65–95 μm in diameter, globose to triangular-globose to ovoid in polar view, tetrahedral to hemispherical in equatorial view, 47.5–72.5 μm high, light brown to golden-brown (RHS 165A, greyed-orange group); wing present, 2.5–10 μm wide, incomplete, spore margin, irregularly crenulate and finely papillate. Distal surface ornamentation reticulate with elevated borders around the alveoli, varying in height, 6–13 alveoli across diameter, each 2–25 μm in diameter. Proximal surface ornamentation similar to distal face, irregularly reticulate, alveoli frequently incomplete, 7–15 alveoli across each facet, alveoli 2.5–25 μm in diameter, alveoli borders low and thin and ±vermicular. Triradiate mark indistinct, pores present. Chromosome number n = 8 (Na-Thalang 1980). (Fig. 58 (map), 59–64.)
Illustrations
Na-Thalang (1980, p. 120, fig. 13).
Distribution
Known from the northern part of the Northern Territory and from Eungella in Queensland. Also known from Java, Indonesia.
Habitat and ecology
Recorded growing on damp soil in shade of trees or under rock overhangs near water holes or creeks.
Etymology
Named for the collector of the type specimen, German botanist and traveller (Friedrich) Franz Wilhelm Junghuhn (1809–1864).
Notes
Described by Nees and Lindenberg in 1846 (Gottsche et al. 1846) from plants from Java, Indonesia, and formerly only known from this region (Stephani 1900; Schiffner 1900; Meijer 1958) until the work of Na-Thalang (1980). She noted that this species had been considered intermediate between subgenus Riccia and subgenus Ricciella because of the small size of its air chambers. Two Riccia junghuhniana specimens (V.Schiffner s.n., both CANB specimens: CBG 8205605, CBG 8205604) from West Java, were also available for comparison with Australian specimens. The Javanese plants are recorded as growing in rosettes or half rosettes, sometimes crowded, but not in mats. Australian plants were recorded as growing in half rosettes in the wild (Cargill 1317, Cowie 13386), whereas cultivated material (Na-Thalang 267A) appears as thick mats of somewhat etiolated plants (Fig. 59A). The common element among all the specimens is that thallus segments always have membranous, wing-like margins (Fig. 59, 60, 63, 64), whether the segments are broad and spathulate (Fig. 59B) or narrower and strap-like (Fig. 60A). Spores have a finely papillate, regularly reticulate distal face, with the number of alveoli across the diameter of the distal face ranging from 8 to 13 (see Fig. 61, 62).
Comparison of Australian and Indonesian populations at the molecular level would confirm conspecificity.
Specimens examined
NORTHERN TERRITORY. Fish River Station, ~200 km S of Darwin, swampy site, D.C.Cargill 1317 (CANB, DNA); same location, I.D.Cowie 13386 (DNA); Melville Island, Conder Point, J.A.Curnow 3126 (CANB); Mary River, Darwin, O.Na-Thalang 273A & B (SYD). CULTIVATED. University of Sydney (from material collected from Mt Bundy [Bundey], 500 yds [yards] from Iron Ore mining area, 22 Jan.1969), 1 May 1969), O.Na-Thalang 267A (SYD); University of Sydney (from material collected from the base of Mt Bundy [Bundey], along the track among grasses, 22 Jan. 1969), O.Na-Thalang 271 (SYD).
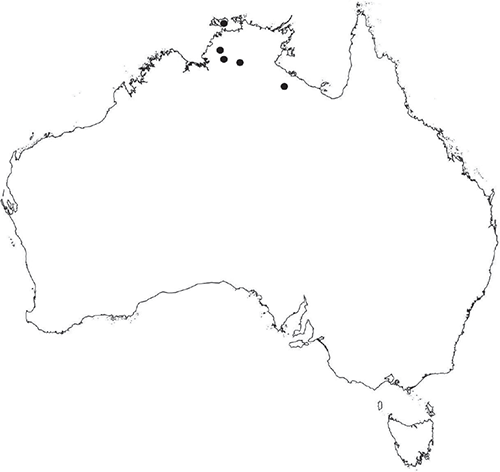
|

|
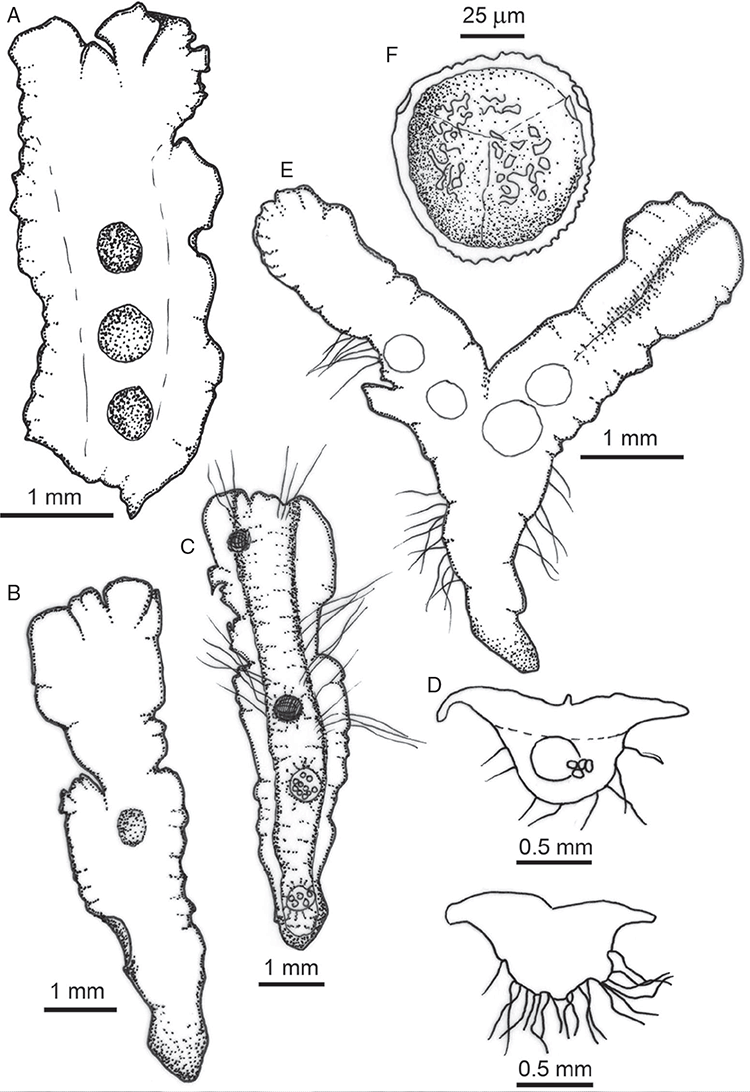
|
13. Riccia luticola Na-Thalang, Brunonia. 3: 123 (1980)
Type: AUSTRALIA. Northern Territory. ca. 5 miles, S. Katherine, at edge of swamp, among grass, 5 Feb. 1969, O.Na-Thalang 321 (holo: NSW 251927!; iso: SYD (no accession number)! CANB 914652!).
Description
Plants observed from rehydrated herbarium collections.
Plants prostrate, in thick tangled mats. Thallus when moist, pale green at tips, (RHS 145D, yellow-green group) becoming yellowish with age, thin bands of maroon-coloured scales across ventral surface; when dry, plants shrunken but remain ribbon-like and flat, remaining pale green at tips and yellowish elsewhere. Whole plants 8.0–10.5 mm long and 0.6–1.11 mm wide. Segments 8.0–10.5 mm long, 0.6–1.1 mm wide; ribbon-like, spathulate with a very short growing tip and a long, shrunken tail-like posterior. Dorsal surface smooth, air pores and air chambers only visible when stained. Ventral flanks without scales, same colour as dorsal surface of segment. Branching simple or bifurcate, branches moderate to widely divergent, up to 90°. Apex rounded or truncate, narrow or broad, shallowly dissected, sometimes with tubers developing at the tip. Dorsal groove present, deep and narrow 1/3 of the length of segment, becoming shallow and broad and disappearing posteriorly. Margin of thallus with undulating wing, entire, ±crenulate, eroding with age; acute to obtuse in transverse section. Scales present, 260–350 × 50–100 μm, confined and appressed to the ventral surface, hemispherical at the apex, becoming 3 or 4 bands of 1 or 2 rows of cells across ventral keel, hyaline or purplish-maroon (RHS 79A, purple group). Cilia absent. Dorsal epidermal cells unistratose, hyaline, globose, small, wider than high. Thallus in transverse section terete apically but becoming biconvex-convex to concave-convex mid-segment; 240–500 μm thick and 330–1150 μm wide. Photosynthetic tissue with air chambers confined to lateral wings, occupying 1/2 of the thallus thickness. Rhizoids only over ventral keel; hyaline, dimorphic, both smooth and pegged. Tubers sometimes present at apex, tips becoming geotropic.
Dioicous. Antheridia not seen. Archegonia in 1 row along dorsal groove. Capsules 1 per plant, mid-segment, embedded, bulging from the ventral surface when mature, hyaline. Spores 72.5–92.5 μm in diameter, globose in polar view, fusiform in equatorial view, (45–)50–65.5 μm high, golden-brown (RHS 164A, greyed-orange group); wing present, 2.5–10(–12.5) μm wide spore margins deeply crenulate and finely papillate along edge. Distal surface ornamentation reticulate, alveoli borders thin with clavate-shaped protuberances at the corners, 7–10 alveoli across diameter, alveoli 7.5–12.5(–17.5) μm in diameter. Proximal surface ornamentation with fine, thin irregular lamellae and papillae. Triradiate mark present ±distinct; pores present. Chromosome number n = 8 (Na-Thalang 1980). (Fig. 65 (map), 66–69.)
Illustrations
Na-Thalang (1980, p. 120, fig. 13).
Distribution
Known only from the type locality near Katherine in the Northern Territory.
Habitat and ecology
Recorded growing at the edge of a swamp among grasses, which is a habitat typical of many of the semi-aquatic Riccia species occurring in the Northern Territory.
Etymology
From the Latin ‘lutensis’, meaning living in or on mud, and cola meaning dweller, referring to this species preference for growing on damp, muddy substrates.
Notes
Riccia luticola differs from other semi-aquatic Riccia species in Australia by sexual condition and size. Riccia luticola is dioicous, whereas all other species are monoicous. The strap-like segments in R. luticola are between 1 and 2 mm broad, whereas in all other semi-aquatic species the segments are up to 1 mm broad.
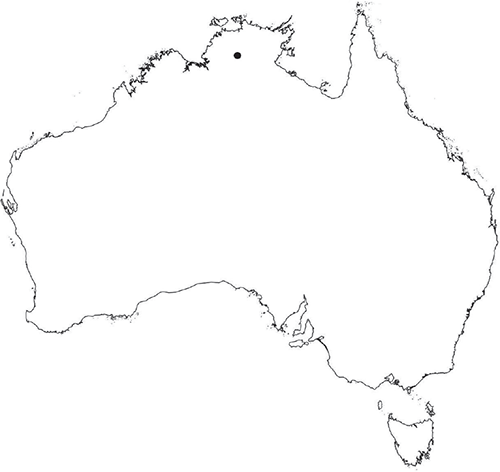
|
14. Riccia multifida (Steph.) Steph., Hedwigia, 28: 273 (1898)
Notes
Na-Thalang (1980) recognised four varieties in R. multifida: R. multifida var. divaricata Na-Thalang, R. multifida var. filiformis Na-Thalang, R. multifida var. multifida and R. multifida var. torticolla Na-Thalang, based on thallus morphology and anatomy, spore and gametophyte characters and geography. Of these taxa, only R. multifida var. divaricata and R. multifida var. multifida have been recorded from the Northern Territory. The taxonomic status of these varieties is uncertain (cf. Jovet-Ast 2000), but a full revision of R. multifida is beyond the scope of this paper, and Na-Thalang’s taxonomy is retained for this study, with the exception of Riccia multifida var. divaricata (here treated as a synonym of R. duplex var. megaspora).
14a. Riccia multifida (Steph.) Steph. var. multifida, Sp. hepat. 1: 40(1900)
Description
Plants observed both in nature and from rehydrated herbarium collections.
Plants prostrate, may or may not be in rosettes, commonly found growing gregariously in thick, tangled, or loose overlapping mats. Thallus, when moist, varies from bright green to olive green to pale green or yellowish, becoming transparent with age following drying and storing (RHS 144C, 145B, D, 146D, 152B, D, 152B, D 153A, D, yellow–green group; and 160D, greyed-yellow group); when dry, plants shrunken often sinking back into the soil, margins sometimes inflexed perpendicular to surface, usually remaining flat, dorsal groove becoming more prominent; pale green to pale cream (RHS 144B, 145A, B, C, D, yellow–green group). Whole plant 1.0–15.2 mm long and 0.25–13.85 mm wide. Segments 0.5–15.2 mm long, 0.15–1.6 mm wide; with a distinct ventral keel and wings either side of keel, wings undulating; thallus typically ribbon-like straps, varying in shape from fusiform, to lanceolate, spathulate, sagittate, sometimes becoming canaliculate. Dorsal surface smooth, striated due to air chambers below the surface with air pores punctuating the surface, only visible when stained. Ventral flanks variable in colour from green to pale green or pale yellowish-green or hyaline, sometimes with purple or violet flecks. Branching simple or 1–5 times furcate, asymmetrical, frequently with ventral lateral branches; branches almost parallel to widely divergent, diverging from 10 to 90°. Apex rounded, lanceolate to truncate to pointed; emarginate to deeply dissected, frequently bulbous because of the development of apical geotropic tubers. Dorsal groove present but confined to apical region, not present when rehydrated, but more obvious when dry. Margin variable; margins wing-like, undulating, entire, sometimes becoming canaliculate at the apex, attenuate in cross-section. Scales present, 72–285 × 110–400 μm, confined to the ventral surface under the apex in 3 or 4 bands across the ventral keel, hyaline, sometimes pigmented purple, frequently only visible when stained, variable in shape from a line of cells to triangular to crescent-shape, often erose along the distal edge. Cilia absent. Dorsal epidermal cells present, unistratose, hyaline, dimorphic, smaller cells surrounding air pores with larger cells surrounding these. Thallus in transverse section fusiform to plano-convex to concave-convex in shape; 80–350 μm thick and 107–115 μm wide. Photosynthetic tissue occupying almost all internal tissue except for 2 or 3 ventral cell layers, air chambers present in 1 row, up to 2 cells thick; internal cells surrounding cavities characterised by thickened cell walls. Rhizoids over the ventral keel and along the stem of the tubers, hyaline, dimorphic, sometimes a mixture of smooth and pegged, sometimes all smooth, pegs sparse to numerous, short to long, straight to sinuous. Tubers present as stalked, geotropic, apical bulbs, non-chlorophyllous, up to 2.25 mm long.
Monoicous. Only archegonia seen along the dorsal groove with hyaline necks protruding out from the dorsal surface, only visible with staining. Antheridia not seen. Capsules 1–11 per plant, present at any position along the length of segment, all capsules bulging from the ventral surface when mature, capsule tissue hyaline or pale brown. Spores 65–102.5 μm in diameter, globose to triangular-globose to ovate in polar view, tetrahedral in equatorial view, 42–80 μm high, golden-brown (RHS 165A; 166A, greyed-orange group) to brown (RHS 200B, brown group); wing present, sometimes incomplete, 2.5–12.5 μm wide, spore margins irregularly crenulate and ornamented with intermittent patches of tiny papillae along the wing edge. Distal surface ornamentation reticulate, with elevated thin borders, alveoli complete or incomplete, with protuberances at corners; 5–10 (–11) alveoli across diameter, large, 2.5–20 (–22.0) μm in diameter. Proximal surface ornamentation similar to distal face, irregularly reticulate, alveoli smaller, frequently incomplete or appearing as just sinuous lamellae, 5–10 alveoli across each facet, alveoli 2.5–15.0 μm in diameter, borders around alveoli low and thin. Triradiate mark ±distinct, pores present. Chromosome number n = 8 (Na-Thalang 1980). (Fig. 70 (map), 71–75.)
Illustrations
Na-Thalang (1980, p. 130, fig. 14).
Distribution
Occurs across the northern part of the Northern Territory, the Kimberley, Pilbara and south-west regions of Western Australia, and on the eastern seaboard from far-northern Queensland, through central New south Wales, central Victoria to the south-eastern corner of South Australia. Also occurs in northern Tasmania and King and Flinders Islands in Bass Strait.
Habitat and ecology
Recorded growing on bare, damp soil at the edge of watercourses, lagoons and swampy areas or in deep shade on bare soil or mud or over algal mats.
Etymology
From the Latin ‘multi’, meaning many, and ‘fidus’ meaning divided, in reference to the multi-branched thallus of this species.
Specimens examined
NORTHERN TERRITORY. Fish River Station, D.C.Cargill & B.Wirf 1278 (CANB, DNA, MEL); D.C.Cargill & I.D.Cowie 1284 (CANB, DNA); D.C.Cargill & I.D.Cowie 1285 (CANB, DNA, MEL); D.C.Cargill & D.Lewis 1291 (CANB, DNA); D.C.Cargill & C.Symonds 1316 (CANB, DNA); B.Wirf 831 (CANB, DNA); Katherine Gorge, A.C.Beauglehole 13773 (MEL); near Deaf Adder Creek, 24 km NNE of Jim Jim Falls, L.A.Craven 6119A (CANB); S of Darwin, Barry Springs, along track near creek bank, O.Na-Thalang 263 (SYD); on the way to Mt Bundy [Bundey], at edge of swamp, O.Na-Thalang 269 (SYD); Darwin, Woolner Rd, at edge of swamp, O.Na-Thalang 273B (SYD).
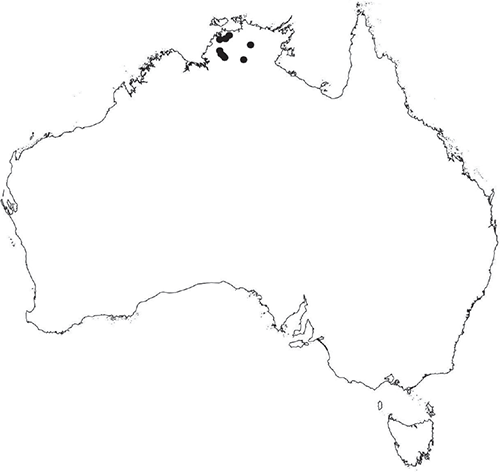
|
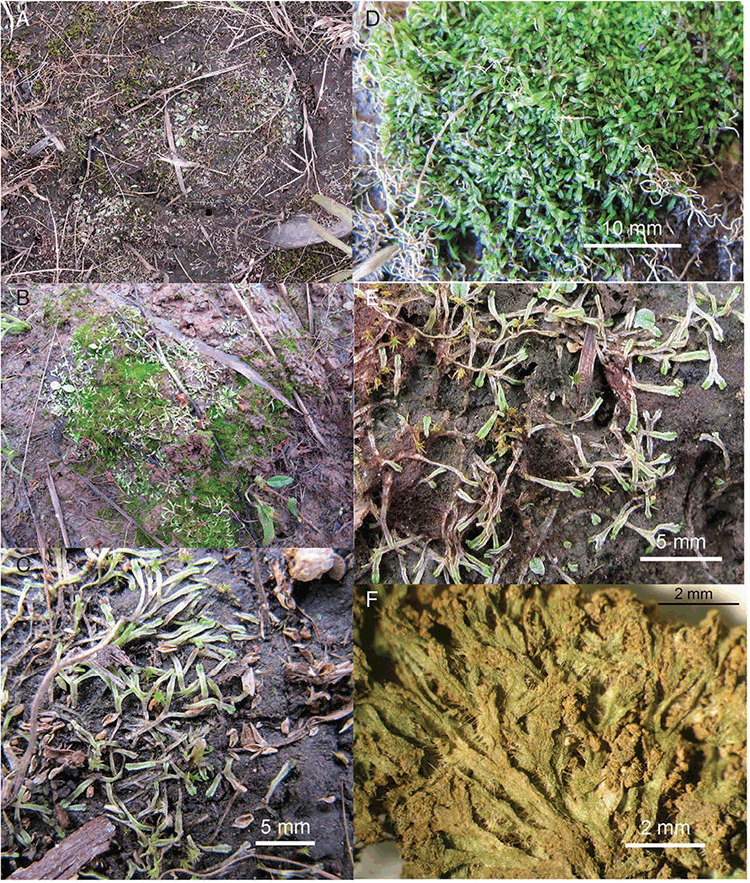
|
15. Riccia obchantiana Cargill, sp. nov.
Type: CULTIVATED. Australia. New South Wales, University of Sydney, 23 July 1969 (grown from live material collected from ca. 380 mile(s) Stuart H’way(s), on dry creek, under tree shade, 4 Feb. 1969), O.Na-Thalang 315 (holo: SYD (no accession number)!; iso: CANB 914651!).
Description
Plants observed from rehydrated herbarium collections.
Plants prostrate, growing in gregarious tangled mats. Thallus when moist, varying in colour from pale green to pale yellowish-green to yellowish (RHS 11B, yellow group; 19B, yellow–orange group; 145C, yellow–green group) with maroon margins (RHS 187A, greyed-purple group) pale green (RHS 145D, yellow–green group) to pale orange (RHS 19B, yellow–orange group) when dry; becoming shrunken; antheridial necks of males conspicuous. Plants 4.5–11.75 mm long and 0.75–6.9 mm wide. Segments 0.5–11.75 mm long and 0.5–1.7 mm wide, ligulate to lingulate in females, elongate triangular to long thin lingulate to spathulate with a long thin tail or narrowly pyriform in males. Dorsal surface smooth except for depressions for antheridial necks or cavities due to erosion with age of epidermal layer. Ventral flanks similar in female and male plants, yellowish-green with flecks of maroon owing to scales. Branching simple or 1 or 2 times furcate; branches moderately to widely divergent, diverging from 40 to 90°. Apex variable, emarginate to truncate to broadly lanceolate, rounded. Dorsal groove present or sometimes absent in males, narrow, shallow and short in length. Margin of thallus irregularly crenulate, undulate or wavy due a broad wing in females; acute in cross-section. Scales present, 350–600 × 50–275 μm, along ventral flank, hyaline or mottled maroon (RHS 187A, greyed-purple group), appressed to the ventral flanks, separate, crescent-shaped. Cilia absent. Dorsal epidermal cells unistratose, globose, hyaline, only visible at apex, up to 35 μm wide, up to 50 μm high, air pores absent. Thallus in transverse section generally plano-convex or concave-convex to biconvex-convex with a deep ventral keel in males, 140–950 μm thick and 770–2000 μm wide. Photosynthetic tissue in vertical columns occupying 1/4–1/2 of the thallus thickness, columns 4–8 cells long. Rhizoids dimorphic, smooth and pegged, across ventral surface; hyaline. Tubers absent.
Dioicous. Dimorphic, female plants broader and flatter than the longer, narrower and thicker males. Archegonia in 1 row along the mid-line, well spread out. Antheridia in 2 rows along mid-line; pyriform, 210–515 × 160–480 μm; antheridial necks 165–415 μm long. Capsules 3–6 per plant, along the entire length of the segment, pale brown, embedded in thallus, but visible from both dorsal and ventral surfaces of thallus. Spores 72.0–92.5 μm in diameter, globose to irregularly globose to triangular globose in polar view, hemispherical to tetrahedral in equatorial view, 55–70 μm high, golden-brown, (RHS 165A, greyed-orange group); wing present, not unlike a cog-wheel in outline, 2.5–5(–7.5) μm wide. Distal surface ornamentation reticulate, 7–11 areolae across diameter, 2.5–17 μm in diameter, with narrow borders surrounding areolae, projections at corners, proximal surface ornamentation also reticulate with smaller more numerous alveoli, borders around areolae forming more of a zig-zag pattern, low, up to 10 incomplete areolae across diameter of each facet, from 2.5–5 μm in diameter. Triradiate mark present, not distinct, pores present, not distinct under light microscopy. Chromosome number unknown. (Fig. 76 (map), 77–79.)
Distribution
Known only from the type collection, from the area around Birdum between the National Carpentaria and Buchanan Highways in the northern part of the Northern Territory.
Habitat and ecology
Recorded growing on a dry creek bank, under the shade of a tree.
Etymology
Named for Professor Obchant Thaithong (neé Na-Thalang) (1936–), Chulalongkorn University, Bangkok, Thailand. Na-Thalang published a landmark revision of the genus Riccia for Australia in 1980, based on her PhD thesis undertaken at the University of Sydney in the 1960s.
Notes
Type material of this species was originally collected and identified by Na-Thalang as Riccia collata Na-Thalang, a species she described from a collection made at Tummallallee [Timmallallie] on the North Western slopes of New South Wales. Na-Thalang placed R. collata within the subgenus Ricciella because of internal polygonal air chambers and air spaces. However, R. obchantiana has a solid thallus without air chambers, as well as more areolae on the distal spore faces than does R. collata. Both species occupy completely different environments, with R. obchantiana occurring in the northern monsoon tropics of the Northern Territory, whereas R. collata is found in the dry, warm bioregion of the North Western Slopes of New South Wales with rainfall falling generally in summer.
It is also similar morphologically to R. sp. Darwin (O.Na-Thalang 281). Both are dioicous species, with a male plant that is usually narrower than the female plant. However, the males of R. obchantiana have numerous antheridial necks in two rows or more along the dorsal surface. They are far fewer in R. sp. Darwin. However, the main difference is in the spore. Riccia obchantiana has tetrahedral spores with a distinct wing, three pores and a reticulate distal pattern (Fig. 78). Riccia sp. Darwin (O.Na-Thalang 281) has apolar spores with no wing or pores, and large, regular areolae over the whole spore (Fig. 40).
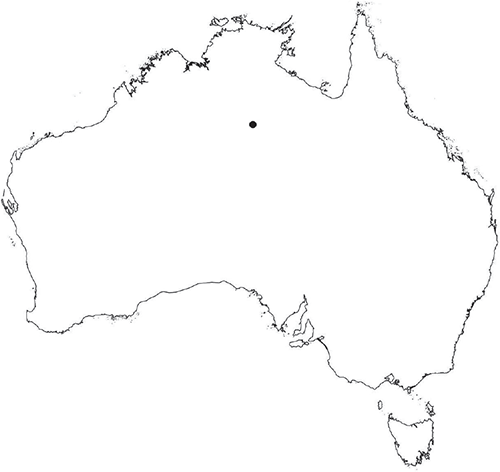
|
16. Riccia papulosa var. variabilis Na-Thalang, Brunonia 3: 112 (1980)
Type: Northern Territory, south of Darwin c. 150 miles on Stuart Highway, O.Na-Thalang 300 29.i.1969 (holo: SYD (specimen no longer extant except for 3 microscope slides of spores)).
Description
Plants observed from rehydrated herbarium collections.
Plants prostrate in loose gregarious mats, not in rosettes. Thallus when rehydrated, a pale olive green (RHS 145C, 146D, yellow–green group; 160A, C and D, 162C, greyed-yellow group); when dry, plants become shrunken, remaining flat or margins folded up into a perpendicular position to the surface forming an open tube, pale green to cream (RHS 145D, yellow-green group; 159A, orange-white group). Plants 5.0–27.5 mm long and 1.2–15.4 mm wide. Segments 0.6–27.5 mm long and 0.45–2.55 mm wide; varying in shape from elongate spathulate to ovate to ligulate to fusiform, all with a narrow ‘wing’ either side of a thickened central segment. Dorsal surface smooth with a reticulate pattern owing to the arrangement of air pores. Ventral flanks hyaline with patches of maroon. Branching simple or 2 × or 3 × furcate, asymmetrical, branches moderately to widely divergent, diverging from 50 to 90°. Apex pointed or rounded, sometimes swollen; retuse or not dissected. Dorsal groove present, shallow and broad or deep and narrow at the apex disappearing posteriorly. Margin wing-like, undulating to crispate, irregularly crenate; attenuate in cross-section. Scales present, 300–1200 × 100–580 μm, appressed against flank, with distal margin free; hyaline with patches of maroon (RHS 83A, violet group; or 187A, greyed-purple group). Cilia absent. Dorsal epidermal cells unistratose, hyaline, globose at the dorsal groove at the apex, becoming deflated and disintegrating with age. Thallus in transverse section sometimes almost terete at the apex, V- or U-shaped, concave-convex, with marginal wing reflexed; thallus 200–1070 μm thick and 700–2310 μm wide. Photosynthetic tissue with air chambers occupying 1/3–2/3 the thallus thickness, 3–9 cells thick. Rhizoids sparsely to thickly covering ventral surface of thallus, hyaline, dimorphic, mainly smooth with pegged rhizoids also. Tubers present infrequently as swollen apices.
Monoicous. Gametangia along midline of segments in 2 rows. Antheridia 175 × 200 μm, tear-drop shaped. Capsules 2–4 per plant, present all along the length of segments, embedded, seen from both dorsal and ventral surface, opening on dorsal surface when mature, capsule tissue varying from hyaline to pale brown. Spores 97.5–117.5 μm in diameter, globose to triangular-globose in polar view, fusiform or hemispherical in equatorial view, 55–85 μm high, golden brown (RHS 166A, greyed-orange group); wing absent, spore margin crenulate. Distal surface ornamentation finely reticulate pattern, alveoli ±ovoid or dumbbell-shape, 13–19 alveoli across diameter, alveoli 2.5–10 μm in diameter, alveoli borders low and thick; proximal surface ornamentation punctate, indentations 9–14 across diameter of each facet, surrounded by slightly raised vermiculate borders. Triradiate mark present from distinct to indistinct; pores absent. Chromosome number n = 8 (Na-Thalang 1980). (Fig. 80 (map), 81–82.)
Illustrations
Na-Thalang (1980, fig. 11).
Distribution
Occurs in the Darwin area and immediately to the south of Darwin. Also recorded from the Pinkerton Ranges west of Timber Creek, in the Northern Territory.
Habitat and ecology
Recorded growing in grassland alongside roads, rivers and hills.
Etymology
From the Latin ‘papulosus’ (meaning pustular), in reference perhaps to the air-pores on the dorsal surface, which are surrounded by a ring of cells that may protrude from the dorsal surface, therefore giving the surface an almost pustulate appearance, and ‘variabilis’ (meaning variable), in reference to the variability of the thallus under different environmental conditions.
Notes
Riccia papulosa var. variabilis was described by Na-Thalang (1980) as a variety of the southern Australian species, Riccia papulosa. The type material of Riccia papulosa is sterile, as noted by Stephani (1889) in the protologue. The type comprises a few, bleached plants which bear a reticulate pattern on the dorsal surface of the thallus, as a result of erosion of cells around the air pores, which dot the dorsal surface of the thallus. Owing to the sterility of the type, correct application of the name R. papulosa is therefore problematic; however, we have adopted Na-Thalang’s (1980) concept of this species for this paper, pending further research on the correct application of this name.
Vegetative morphology of R. papulosa var. variabilis is similar to that of several large Riccia species, which also share internal polyhedral air chambers and air spaces and dorsal air pores.
The vegetative morphology of R. papulosa var. variabilis is similar to that of R. spongiosula Na-Thalang, widespread in southern Australia, and R. vesiculosa known from eastern Australia. Riccia papulosa var. variabilis may be separated from R. spongiosula on spore morphology (fewer areolae across the diameter, 12–15 v. 6–8, without pores v. pores present respectively), number of cells between air pores (2–5 cells v. 6–10 cells respectively) and chromosome number (16 v. 8 respectively). Riccia papulosa var. variabilis may be separated from R. vesiculosa on spore morphology (12–15 areolae across the diameter v. 7–9 respectively), distance between air pores (2–5 cells v. 4–6 respectively) and possibly plant size, although this may be influenced by environmental factors (8–15 mm long and 2.5–5 mm wide v. 5–7 mm long and 2.5–3.5 mm wide respectively). See Na-Thalang (1980) for further information.
With regards to differences between the two varieties of R. papulosa, Na-Thalang (1980) noted that vegetative characters of R. papulosa var. variabilis were ‘…very varied under different environmental conditions’ (p. 113) qualifying it by describing the thallus under different moisture regimes. Spore ornamentation also differs between the varieties. Spores in var. papulosa (according to Na-Thalang 1980) bear a broad wing, whereas the wing in var. variabilis is very narrow or absent altogether.
Riccia papulosa var. variabilis is also vegetatively similar to Riccia junghuhniana. Both are confined to northern Australia and both have internal air chambers and grow gregariously on damp soil in grassland habitats. Riccia papulosa var. variabilis generally has larger thallus segments; however, the two taxa may reliably be distinguished by spore ornamentation. The spores of R. junghuhniana are smaller (65–95 μm v. 97.5–117.5 μm in diameter) and are tetrahedral, v. almost rounded in R. papulosa var. variabilis. The distal face pattern is reticulate in both species, but in R. junghuhniana the areolae are generally large (2–25 μm in diameter) and fewer in number (7–15 across the face) v. 2.5–10 μm in diameter and 13–19 across the face). The borders surrounding the areolae are thinner and more prominent in R. junghuhniana (Fig. 61F) than the thicker and less prominent areolae borders seen in R. papulosa var. variabilis (Fig. 81D).
Specimens examined
NORTHERN TERRITORY. Woolner Rd, Darwin. O.Na-Thalang 274 (SYD); Darwin River, O.Na-Thalang 279 (SYD); ~5 miles [~8 km] W of El Sharana, O.Na-Thalang 294 (SYD); southern end of Pinkerton Ranges, ~74 m [miles, ~119 km] W of Timber Creek, R.C.Carolin 6678 (SYD).
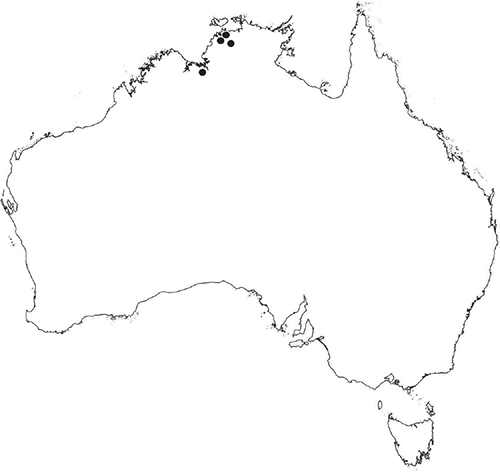
|
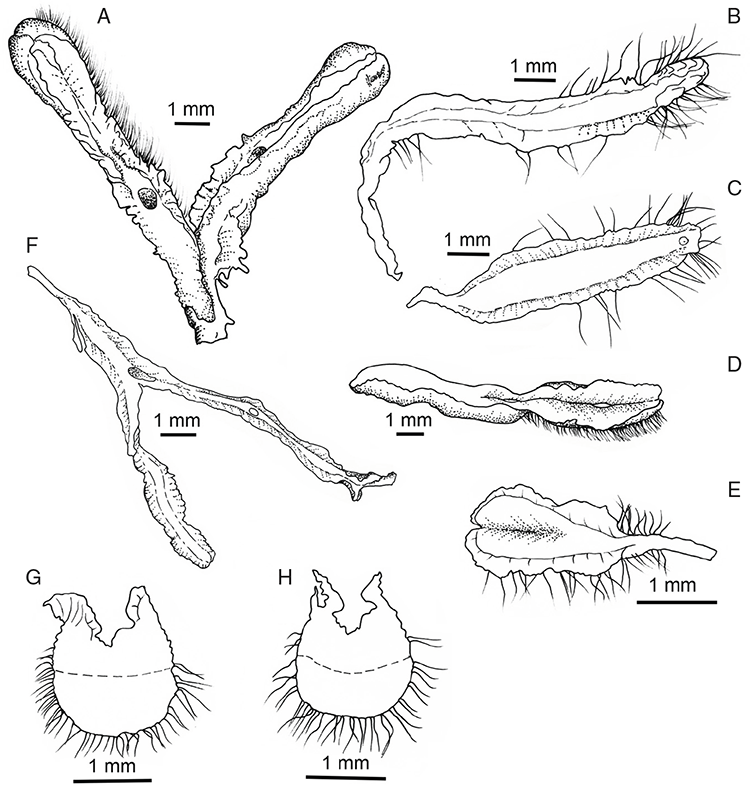
|
17. Riccia verrucosa Cargill, sp. nov.
Type: AUSTRALIA. Northern Territory. Fish River Station, under shrub on sticky algal mat over silty/sandy soil, 25 Apr. 2012, D.C.Cargill 1277p.p. (holo: CANB 811408!; iso: DNA!).
Description
Plants observed both in nature and from rehydrated herbarium collections.
Plants prostrate in scattered or loose patches or gregarious in thick overlapping mats with a second Riccia species, also in half or full rosettes. Thallus, when live or rehydrated, bright green (RHS145A, 145B, 153A, yellow–green group) to light green (RHS 144A and B, yellow–green group) at the tips, becoming a bright lime green colour (RHS 149B, yellow–green group) at mid-segment; when dry, plants become shrunken, margins partially closed over the dorsal surface at the anterior end of segments or folded up into a perpendicular position to the surface, whereas the posterior remains open and flat; when dry, pale green (RHS 139D; green group) or bleached white. Plants 2.7–14.55 mm long and 1.2–11.7 mm wide. Segments 1.0–11.6 mm long and 1.0–2.45 mm wide; varying in shape from obovate or lingulate to lanceolate to cylindrical because of swelling of the segments, all with a narrow ‘wing’ either side of a thickened central segment. Dorsal surface smooth or sometimes with a blister-like, reticulate pattern owing to the arrangement of swollen epidermal cells and air pores. Ventral flanks quite variable in colour from hyaline to deep purple, mottled or often two distinct colours from dark purple on the flanks to almost black on the ventral surface, or maroon along flanks and dark purple on ventral surface (margin is RHS 59A, red–purple group; and flank is RHS 187A, greyed-purple group), hyaline and with some mottling of purple cells or mottled maroon and green. Branching simple or bifurcate, asymmetrical, branches moderately to widely divergent, diverging from 45 to 130°. Apex lanceolate to truncate; retuse or not dissected. Dorsal groove present, deep and narrow at the apex disappearing shortly beyond apex or visible up to mid-segment before disappearing. Margin wing-like, undulating to crispate, irregularly crenate; attenuate in cross-section. Scales present, 200–1200 × 230–800 μm, not distinct, flaccid, appressed against flank, occurring separated or imbricate and up to the edge of the margin, not beyond; hyaline or green at the apex, becoming mottled hyaline and dark maroon with maturity. Cilia absent. Dorsal epidermal cells unistratose, hyaline, globose to quadrate to rectangular at the dorsal groove at the apex, becoming deflated and disintegrating with age. Thallus in transverse section biconvex-convex to campanulate to concave-convex, with marginal wing reflexed; thallus 450–1300 μm thick and 170–2260 μm wide. Photosynthetic tissue in vertical columns occupying 1/4–1/2 the thallus thickness, 3–8 cells long per column. Rhizoids thickly covering ventral surface of thallus up to base of scales, hyaline to pale brown, dimorphic, mainly smooth with few pegged rhizoids. Tubers present frequently as swollen apices.
Monoicous. Gametangia along the segments in 1 or 2 rows either side of the dorsal groove. Antheridia 125–200 × 125–200 μm, tear-drop shaped; antheridial necks typically not seen, but up to 350 μm long. Capsules 1–12 per plant, present all along the length of segments, embedded, seen from both dorsal and ventral surface, bulging from the ventral surface when mature, capsule tissue hyaline to pale brown. Spores 80–115 μm in diameter, globose to triangular-globose to quadrate in polar view, fusiform, ellipsoid or tetrahedral in equatorial view, 50–97.5 μm high, brown to yellowish-brown to orange–brown (RHS 165A, greyed-orange group); wing may be present, 5–12.5 μm wide, spore margin entire, slightly undulating or crenulate or finely papillate; projections, in equatorial view, truncate. Distal surface ornamentation a regular reticulate pattern, alveoli ±ovoid or spherical in shape, 9–14 alveoli across diameter, alveoli 2.5–20 μm in diameter, alveoli borders low, thick and covered in papillae; proximal surface ornamentation also reticulate, alveoli slightly smaller, (6–)7–11 across diameter of each facet, alveoli borders lower and even in thickness. Triradiate mark variable from distinct to faint; pores present. Chromosome number unknown. (Fig. 83 (map), 84–86.)
Distribution
Known from Fish River Station reserve near Daly River in the northern part of the Northern Territory.
Habitat and ecology
Recorded growing on bare soil in the shade of trees or shrubs, on silty sandy soils, or over algal mats on sticky mud.
Etymology
From the Latin ‘verrucosus’ (warty), in reference to the warty-like papillate patterning over the surface of the spores.
Notes
Originally identified as Riccia macrospora on the basis of the maroon scales along the ventral flanks; subsequent examination of type material R. macrospora from G (Cargill and Beckmann 2020) made it clear that plants from Fish River Station represented another, undescribed taxon. Riccia macrospora possesses large dark scales that extend beyond the margins of the segments. They are also pigmented a rich crimson colour and reflex over the dorsal surface of the thallus on drying. Riccia verrucosa has mottled scales, which do not extend beyond the margins of the segments and the thallus has a more flaccid wing-like extension of the margin, which is generally hyaline. The spores of the lectotype of R. macrospora are quite dissimilar. The spores of R. verrucosa are regularly reticulate, with the alveoli radiating in almost regular rows out from the centre of the distal face. The ridges surrounding the alveoli bear papillae that continue over the ridges around the alveoli on the proximal face as well, but they can be smooth in some populations (Fig. 85D–F, 86A–C). The spores of R. macrospora are also reticulate but these are arranged in a more ‘swirl’ pattern over the distal face, with a network of irregular shaped alveoli. The ridges surrounding the alveoli in R. macrospora are high, smooth and laciniate at their margins. The reticulate pattern of the proximal face is more regular and the surrounding ridges are smooth. (Cargill and Beckmann 2020).
Specimens examined
NORTHERN TERRITORY. Fish River Station, D.C.Cargill 1260 (CANB); Fish River Station, D.C.Cargill 1279 (CANB).
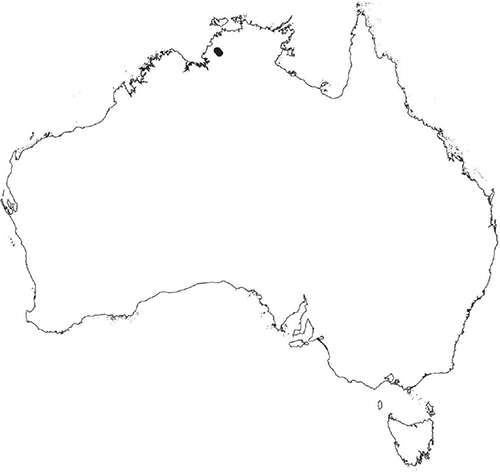
|
Taxa of uncertain status
Riccia vesiculosa (Carrington & Pearson) Steph., Bull. Herb. Boissier 6: 375 (1898).
Riccia bullosa var. vesiculosa Carrington & Pearson, Proc. Linn. Soc. New South Wales, ser. 2, 2: 1058 (1887). Type citation: ‘Hab. On earth, Parramatta, August, 1885.’ Type: AUSTRALIA. New South Wales. Parramatta, Aug 1885, T.Whitelegge 21 (lecto, designated by O.Na-Thalang, Brunonia 3: 116 (1980): NSW, n.v.; isolecto: G 00264386 [image seen!], SYD (no accession number)
The identity of this species is uncertain as the lectotype at NSW (Na-Thalang 1980) and both isolectotypes at G and SYD are sterile (D. C. Cargill, pers. obs.). Na-Thalang (1980) cited the type of the name Riccia bullosa var. vesiculosa as ‘Holotype: New South Wales, Parramatta, Whitelegge 8, 1885 (NSW H87; isotype (SYD).’ This is here treated as effective lectotypification by Na-Thalang. As Na-Thalang’s citation meets the relevant requirements of ICN Art. 7.11, her use of the terms ‘holotype’ and ‘isotype’ are correctable under ICN Art. 9.10.
Conservation status
No bryophytes have been listed as threatened for the Northern Territory (https://nt.gov.au/environment/native-plants/threatened-plants, accessed 15 October 2020). It is difficult for the authors to assess the conservation status of the genus Riccia for the Northern Territory because we have not had the opportunity to assess firsthand populations of the taxa treated in this paper. However, several species treated in this paper are either known only from the type locality, very few locations or have not been collected for many years. These taxa, in particular, warrant proper assessment of their conservation status, but this would be best achieved by botanists based in the NT who have expertise in this group of plants.
Conflicts of interest
The authors declare they have no conflicts of interest.
Declaration of funding
Dr D. Christine Cargill acknowledges the support of the Bush Blitz Program to attend the Fish River Station fieldtrip in 2012 and also for a Bush Blitz Tactical Taxonomy grant (TTC212-02) awarded in 2013, which partially supported the results of this publication. Dr Karen Beckmann acknowledges the support of ABRS for an Australian Biological Resources Study Participatory Grants Programme grant (number 204-55) awarded in 2004 until 2007 for the project ‘A Flora Treatment of the Family Ricciaceae (Marchantiales) in Australia’.
Acknowledgements
The authors thank the curators of the following herbaria for the loan of specimens for this study or for images if loans were not possible: AD, BM, DNA, G, MEL, NSW, P and in particular Dr Murray Henwood at SYD for the loan of Obchant Na-Thalang’s specimens, which are a particularly important collection. In particular, we acknowledge the professionalism of Ms Isabella Valette at G and Dr GisèArchipoff and Ms Marion Martinez-Martin at STR for sending us quality images of types at short notice; these were much appreciated. Also, thanks go to the curation support staff who have helped in the curation, databasing and maintenance of the specimens used in this study. Dr Karen Beckmann thanks the Biosciences Microscopy Unit, School of Biosciences, The University of Melbourne for access to the FEG SEM and the Xenosput gold coater. Dr Cargill thanks the CSIRO Black Mountain Imaging Centre for the use of their SEM facilities. We thank Dr Anna Monro for discussions on and the correct interpretations of the typification of several specimens. Dr Cargill particularly thanks Dr Peter Wilson of NSW herbarium for suggesting the epithet for Riccia abdita. A huge thank you goes to Brendan Lepschi (Centre for Australian National Biodiversity Research) for his careful and meticulous reading and formatting of the manuscript and bringing to the attention of the authors several taxonomic and nomenclatural discrepancies and irregularities. This has improved the quality of the paper immensely. And finally, we also thank Dr Matt Renner and Dr Matt von Konrat for their constructive review of the manuscript.
References
Ahmad S (1942) Three new species of Riccia from India. Current Science 11, 433–434.Barrie FR (2006) Report of the general committee: 9. Taxon 55, 795–800.
| Report of the general committee: 9.Crossref | GoogleScholarGoogle Scholar |
Cargill DC, Beckmann K (2020) Typification and identity of Riccia macrospora Stephani (Ricciaceae) Swainsona 33, 113–124.
Cargill DC, Neal WC, Sharma I, Gueidan C (2016) A preliminary molecular phylogeny of the genus Riccia L. (Ricciaceae) in Australia. Australian Systematic Botany 29, 197–217.
| A preliminary molecular phylogeny of the genus Riccia L. (Ricciaceae) in Australia.Crossref | GoogleScholarGoogle Scholar |
Gottsche CM, Lindenberg JBG, Nees von Esenbeck CG (1846) ‘Synopsis hepaticarum.’ (Meissner: Hamburg)
Grolle R (1975) Miscellanea hepaticologica 151–160. Lindbergia 3, 47–56.
Hässel de Menéndez GG, Rubies MF (2009) Catalogue of Marchantiophyta and Anthocerotophyta of southern South America. Nova Hedwigia 134, 1–672.
Hugonnot V, Pépin F, Servière L (2014) Temperate extension of the European range of Riccia crustata Trab. Nova Hedwigia 98, 473–480.
| Temperate extension of the European range of Riccia crustata Trab.Crossref | GoogleScholarGoogle Scholar |
Jovet-Ast S (1956) Trois Hépatiques marocaines. Revue Bryologique et Lichénologique 25, 128–133.
Jovet-Ast S (1973) Complément à l’étude du Riccia crustata Trabut. Présence en Australie. Spores et paroi sporale. Revue Bryologique et Lichénologique 39, 167–174.
Jovet-Ast S (1984) Riccia (Subg. Viridisquamata) caroliniana Na-Thalang, espèce endémique rélictuelle d’Australie. Cryptogamie. Bryologie, Lichenologie 5, 389–402.
Jovet-Ast S (2000) Documents pour la connaissance des Riccia Australiens (Hépatiques, Marchantiales): nouvelles récoltes. Taxons nouveaux. Commentaires morphologiques et écologiques. Cryptogamie. Bryologie 21, 289–343.
| Documents pour la connaissance des Riccia Australiens (Hépatiques, Marchantiales): nouvelles récoltes. Taxons nouveaux. Commentaires morphologiques et écologiques.Crossref | GoogleScholarGoogle Scholar |
Malcolm B, Malcolm N (2006) ‘Mosses and Other Bryophytes, an Illustrated Glossary’, 2nd edn. (Micro-Optics Press: Nelson, New Zealand)
McCarthy PM (2003) ‘Catalogue of Australian Liverworts and Hornworts. Flora of Australia Supplementary Series Number 21.’ (ABRS Publishing: Canberra, ACT, Australia)
Meijer W (1958) Notes on species of Riccia from the Malaysian region. The Journal of the Hattori Botanical Laboratory 20, 107–118.
Mondal AK (2007) A reinvestigation into the taxonomic status of Riccia himalayensis and Riccia discolor based on scanning electron microscopic and chemotaxonomical studies. Nordic Journal of Botany 25, 190–193.
| A reinvestigation into the taxonomic status of Riccia himalayensis and Riccia discolor based on scanning electron microscopic and chemotaxonomical studies.Crossref | GoogleScholarGoogle Scholar |
Müller K (1954) Die Lebermoose Europas. In ‘Dr. L. Rabenhorst’s Kryptogamen: Flora von Deutschland, Österreich und der Schweiz’, Vol. 6, 3rd edn. (Akademische Verlagsgesellschaft: Leipzig, Germany)
Na-Thalang O (1980) A revision of the genus Riccia (Hepaticae) in Australia. Brunonia 3, 61–140.
| A revision of the genus Riccia (Hepaticae) in Australia.Crossref | GoogleScholarGoogle Scholar |
Pandé SK, Udar R (1957) The genus Riccia in India. I. A reinvestigation of the taxonomic status of the Indian species of Riccia. Journal of the Indian Botanical Society 36, 564–579.
Perold S (1986) Studies in the genus Riccia (Marchantiales) from southern Africa. 7. R. congoana and its synonyms. Bothalia 16, 193–201.
| Studies in the genus Riccia (Marchantiales) from southern Africa. 7. R. congoana and its synonyms.Crossref | GoogleScholarGoogle Scholar |
Perold S (1999) Hepatophyta. Part 1: Marchantiopsida. Fascicle 1: Marchantiidae. In ‘Flora of Southern Africa’. (Ed. OA Leistner) pp. 111–240. (National Botanical Institute: Pretoria, South Africa)
Rabeau L (2019) Diversity and evolution in [the] liverwort genus Riccia (Ricciaceae, Marchantiidae): integrative and phylogenetic approaches with a focus on southern Africa and western Indian Ocean. PhD thesis, Sorbonne Université, Paris, France.
Rauh W, Buchloh G (1961) Riccia crinita Taylor und Riccia atromarginata Levier. Revue Bryologique et Lichénologique 30, 260–262.
Royal Horticultural Society (1995) ‘RHS Colour Chart.’ (The Royal Horticultural Society: London, UK)
Schiffner V (1900) ‘Die Hepaticae der Flora von Buitzenzorg.’ (Brill Publishers: Leiden, Netherlands)
Schuster RM (1992) ‘The Hepaticae and Anthocerotae of North America, East of the Hundredth Meridian. Vol 6.’ (Field Museum of Natural History: Chicago, IL, USA)
Seppelt RD (1999) Riccia albida Sull. (Marchantiales; Ricciaceae) in Australia. Haussknechtia Beiheft (Riclef-Grolle-Festschrift) 9, 339–342.
Singh SK (2014) An appraisal of genus Riccia in India with a note on diversity and distribution of species. International Journal of Sustainable Water and Environmental Systems 6, 35–43.
Söderström L, Hagborg A, von Konrat M (2012) Notes on early land plants today. 9. Validation of Riccia gangetica Ahmad. Phytotaxa 65, 57
| Notes on early land plants today. 9. Validation of Riccia gangetica Ahmad.Crossref | GoogleScholarGoogle Scholar |
Srivastava KP (1964) Bryophytes of India: I. Ricciaceae. Bulletin of the National Botanic Gardens 104, 1–103.
Stephani F (1889) Hepaticae australiae III. Hedwigia 28, 257–278.
Stephani F (1898) Species Hepaticarum. Bulletin de l’Herbier Boissier 6, 309–343.
Stephani F (1900) ‘Species hepaticarum Vol.1. Anacrogynae.’ (Georg & Cie: Geneva, Switzerland)
Streimann H, Klazenga N (2002) ‘Catalogue of Australian Mosses. Flora of Australia Supplementary Series Number 17.’ (ABRS publishers: Canberra, ACT, Australia)
The Australasian Virtual Herbarium (2021) Occurrence records: PHYLUM: Marchantiophyta; Country: “Australia”. Available at https://avh.ala.org.au/occurrences/search?q=lsid%3Ahttps%3A%2F%2Fid.biodiversity.org.au%2Ftaxon%2Fausmoss%2F51259285&qc=data_hub_uid%3Adh9&fq=country%3A%22Australia%22 [Verified 23 April 2021]
Turland NJ, Wiersema JH, Barrie FR, Greuter W, Hawksworth DL, Herendeen PS, Knapp S, Kusber WH, Li DZ, Marhold K, May TW, McNeill J, Monro AM, Prado J, Price MJ, Smith GF (Eds) (2018) ‘International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code) adopted by the Nineteenth International Botanical Congress’, Shenzhen, PR China, July 2017. Regnum Vegetabile 159. (Koeltz Botanical Books: Glashütten, Germany).
Udar R (1957) On the synonymy of some Indian species of Riccia Current Science 26, 20–22.
Udar R, Chopra N (1957) Cytotaxonomic studies in the genus Riccia (Mich.) L.I., R. billardieri Mont. et. Nees and R. gangetica Ahmad Journal of the Indian Botanical Society 36, 46–50.
Wigginton MJ (2018) Checklist and distribution of the liverworts and hornworts of sub-Saharan Africa, including the East African Islands. Tropical Bryology Research Report 9. (British Bryological Society) Available at https://www.britishbryologicalsociety.org.uk/wp-content/uploads/2021/01/Wigginton-M.-2018.-Checklist-of-the-liverworts-and-hornworts-of-sub-Saharan-Africa-edn.-4.pdf [Verified 23 April 2021]