Morphological and environmental variation within Hibiscus krichauffianus (Malvaceae), and the recognition of two new species, H. verecundus and H. calcareus
Todd G. B. McLay A B C * , Robyn M. Barker D and David E. Albrecht A E
A B C * , Robyn M. Barker D and David E. Albrecht A E
A
B
C
D
E
Abstract
The velvet-leaf hibiscus, Hibiscus krichauffianus F.Muell. (Malvaceae subfamily Malvoideae), is predominantly associated with sand dunes in the Australian arid zone but the name is presently also applied to plants occurring in different habitats on the southern edge of the range, and in disjunct locations in Western Australia and eastern Queensland. We investigated the morphological and environmental variation within H. krichauffianus throughout the geographic range. As a result, we have identified two new species, H. verecundus McLay & Albr. and H. calcareus McLay & Albr.; coined a phrase name, H. sp. Belele (D.W.Goodall 3417) for a putative new taxon; and recircumscribed H. krichauffianus. We also assessed material collected when the protologue of H. krichauffianus was published in the late 1850s and selected a lectotype from the original material identified.
Keywords: Hibiscus calcareus, Hibiscus krichauffianus, Hibiscus verecundus, lectotypification, Malvaceae, plant morphology, taxonomy, velvet-leaf hibiscus.
Introduction
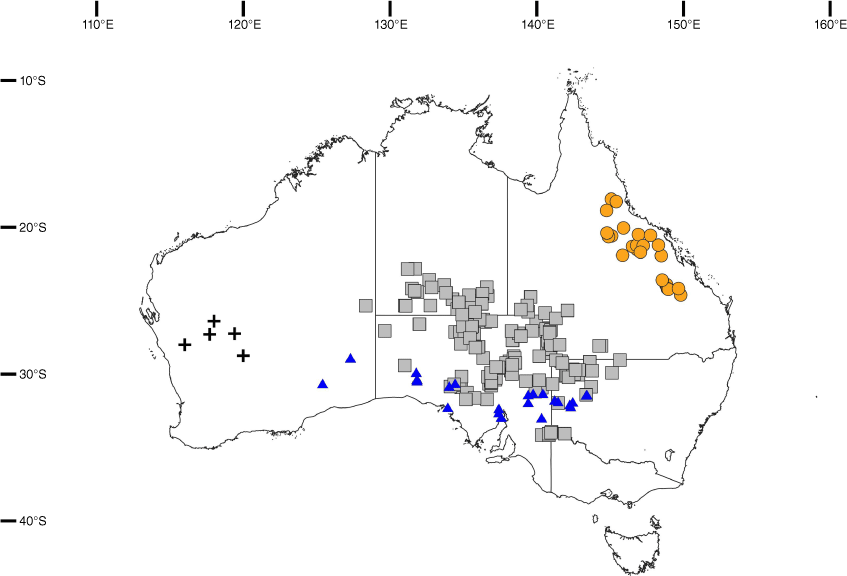
Hibiscus krichauffianus F.Muell. (velvet-leaf hibiscus) was first described by Ferdinand Mueller in a report on the plants collected on the Babbage Expedition of 1858 (Mueller 1859b). Two collection locations were cited in the protologue but a lectotype has not been designated to fix the application of the name. Based on the taxonomic concept of H. krichauffianus adopted by Mitchell (1981) that is generally followed by Australian herbaria, the species is a sand-loving arid zone specialist, occurring in South Australia (SA), southern Northern Territory (NT), western Queensland (Qld), western New South Wales (NSW) and far north-western Victoria (Vic.) (https://avh.ala.org.au/occurrences/search?q=taxa%3A%22hibiscus+krichauffianus%22#tab_mapView, accessed October 2022, but see Fig. 1 for distribution based on material confirmed by authors with misidentifications removed). Several collections from beyond this core distribution have also been included within H. krichauffianus but these are morphologically and environmentally atypical and have potentially been misidentified. These include populations from eastern Queensland that would likely experience vastly different seasonal rainfall from those in the core area; populations from the Nullarbor Plain and sites to the east at a similar latitude; and a few collections from the Murchison region of Western Australia (WA) that are separated by over 1000 km from the closest collections of H. krichauffianus to the east.
Distribution map for the four morphotypes of H. krichauffianus sens. lat. in Australia. Distributions are based only on material viewed by the authors. Morphotype A: Hibiscus krichauffianus sens. strict., grey squares; Morphotype B: Hibiscus verecundus, orange circles; Morphotype C: Hibiscus calcareus, blue triangles; Morphotype D: Hibiscus sp. Belele (voucher: D.W.Goodall 3417), black crosses.
Hibiscus L. diversity in Australia largely occurs in three main sections: H. sect. Furcaria DC., H. sect. Panduriformes Ulbr. and H. sect. Bombicella DC., with the former two having been studied by Lyn Craven and colleagues (see Craven et al. 2003; Juswara and Craven 2005, and the recently added Flora of Australia online profiles, https://profiles.ala.org.au/opus/foa/profile/Hibiscus, accessed 21 August 2023). Hibiscus sect. Bombicella was first proposed by de Candolle (1824) and Bentham (1863), and included four Australian species, with Mueller (1859c, 1868) assigning a further two species to the section in the Fragmenta Phytographiae Australiae series. Apart from the global treatment of Hibiscus by Hochreutiner (1900) in which H. sect. Bombicella was treated as ‘Bombycella’ and the Fryxell (1980) account of the American species of H. sect. Bombicella, this group has not been revised. Hibiscus sect. Bombicella occurs in Australia, Africa and North America, and the section is largely united on the basis of the long, silky seed hairs and many species occur in arid environments. Recent phylogenetic analyses of Hibisceae (Pfeil and Crisp 2005; Hanes et al., in press) indicate that the section is polyphyletic, with the Australian members likely requiring a new sectional classification.
As part of a larger revisionary work on the Australian members of Hibiscus section Bombicella, Clade /Euhibiscus (Pfeil and Crisp 2005) we assess the morphological and environmental variability within H. krichauffianus sens. lat. and recognise four taxa, including two segregate species and a phrase-named entity. We also investigate possible syntypes of H. krichauffianus and revisit the status of H. krichauffianus var. chippendalei Fryxell, currently a name of uncertain application in the Australian Plant Census (APC, https://biodiversity.org.au/nsl/services/search/taxonomy, accessed 16 May 2023).
Materials and methods
Assessment of morphological variation within H. krichauffianus sens. lat.
Specimens were loaned from all major mainland Australian herbaria and available for study at CANB, and more than 230 specimens of H. krichauffianus were reviewed. This included material from throughout the geographic range of H. krichauffianus as currently circumscribed (sens. lat.) (Fig. 1). Several specimens previously identified as H. krichauffianus, including some from far north-west WA and eastern Qld, were excluded as these were deemed to be part of the H. sturtii Hook. complex, with epicalyx lobes fused for more than 4 mm at the base.
Specimens were sorted into groups based on a range of characters including habitat preference, habit, branchlet indumentum, stipule, leaf and flower (including epicalyx) characters, and fruit and seed characters (see Table 1 for full list). Details of how various characters were specifically assessed are provided in Table 1.
| Character | Morphotype A – Hibiscus krichauffianus | Morphotype B – Hibiscus verecundus | Morphotype C – Hibiscus calcareus | Morphotype D – Hibiscus sp. Belele (D.W.Goodall 3417) | |
|---|---|---|---|---|---|
| Substrate preference | Deep sand | Typically shallow soils over sandstone, laterite or basalt | Lime-rich soils | Insufficient data | |
| Plant habit | Subshrub or shrub to ~1 m high, usually erect or ascending | Low spreading subshrub or shrub to ~0.5 m high, usually decumbent | Low subshrub to ~0.5 m high, spreading, dome-shaped or rounded | Low or dwarf subshrub or shrub to ~0.3 m high | |
| Branchlet indumentum colour | Silvery-white, white, sometimes fading to yellowish-white | Yellowish-brown, sometimes fading to white | White, or silvery-white | White on younger branchlets, older branchlets becoming ferruginous (ferruginous rays and white rays sometimes on the same hair) | |
| Stipule width (mm) | 0.16–0.4 | 0.1–0.17 | 0.2–0.5 | 0.2–0.35 | |
| Leaf lamina colour (adaxial) | Whitish-silver to grey, becoming greyish-green with age | Green to dark green | Grey to silvery-white | Silvery–ferruginous | |
| Leaf lamina shape | Mostly ovate to lanceolate or oblong, occasionally broadly ovate | Ovate to broadly ovate, rarely elliptic–ovate | Ovate, elliptic–ovate or oblong–ovate | Ovate to elliptic–ovate | |
| Leaf lamina in transverse | Flat to weakly concave or weakly folded | Flat to weakly concave or weakly folded | Mostly strongly concave to v-shaped (and appearing folded on many herbarium specimens) | Flat to weakly folded | |
| Leaf lamina length and width (mm) | 10–55 × 5–35 | 9–52 × 8–33 | 6–28 × 4–16 | 17–43 × 6–22 | |
| Leaf lamina base | Obtuse, truncate or very broadly cuneate | Broadly cuneate, obtuse or truncate | Broadly cuneate or truncate | Truncate, very slightly cordate or broadly obtuse | |
| Leaf lamina margin | Crenate to dentate | Serrate to dentate, rarely crenate | Serrate to dentate or crenate, undulate, sinus between teeth up to halfway to midvein | Dentate to crenate | |
| Abcision line (at peduncle–pedicel junction), whether visible and position | Not obvious, obscured by hairs, usually 1–2 mm from the base, rarely up to one-half length from the base | Sometimes obvious, one-third to one-half length from the base | ±Obvious, sometimes obscured by hairs, usually in upper half, 2–17 (–24) mm from the base | ±Obvious, sometimes obscured by hairs, approximately one-third length from the base | |
| Number of epicalyx lobes | 5–8 (rarely 10, rarely bifurcating) | 5–7 | 7–8 | 5–6 | |
| Epicalyx length (Including fused portion, excluding receptacle) (mm) | 7–16 | 6–10 | 4–14 | 6–12 | |
| Fusion of epicalyx lobes at base | Fused for 1–4 mm | Free or fused to 0.5 mm | Fused for 1–3.5 mm | Free or fused for up to 1 mm | |
| Epicalyx lobes degree of curvature | Straight in flower, becoming recurved or rarely incurved in fruit | Straight | Straight in flower, becoming recurved in fruit | Straight in flower, becoming recurved in fruit | |
| Corolla colour | Pale pink or mauve (rarely white) | Pale pink or white (sometimes drying pale yellow) | Pale pink to mauve, sometimes almost white | Purple | |
| Petal length (mm) | 17–35 | 15–28 | 20–44 | 39–42 | |
| Seed indumentum | Patchy indumentum of wispy spreading, white to off-white hairs | Short patchy indumentum of appressed white to yellowish-brown hairs | Short patchy indumentum of appressed white hairs | Short patchy indumentum of appressed white to yellowish hairs | |
| Seed length (mm, greatest dimension) | 2–3 | 1.7–2.3 | 2.5–3 | ~3 | |
| Seed characteristic features or notes | Reniform (rarely subangular–reniform), funicular remnants brown, membranous and wing-like, on either side of the hilum | Two straighter sides almost forming a right angle at intersection, funicular remnants brown, membranous and wing-like, one centrally placed and one on either side of the hilum | Two straighter sides almost forming a right angle at intersection, funicular remnants brown, membranous and wing-like, on either side of the hilum | Two straighter sides almost forming a right angle at intersection, funicular remnants brown, membranous and wing-like, on either side of the hilum | |
| Flower cleistogamy | Observed in some specimens | No evidence | Observed in a single specimen | Not observed in material examined |
Stipule width was measured at the widest point of a mature stipule. All leaf lamina characters were assessed on mature, fully expanded leaves. In practicality, the largest leaves away from terminal growth were used. The density of stellate hairs on the leaf lamina was assessed between veins and based on the following classes used by Bean (2004): very dense – stellate hairs overlapping, multi-layered, the subtending surface not visible with a hand lens; dense – stellate hairs overlapping, 0.1–0.5 mm in diameter apart (centre to centre), only one or two layers, the subtending surface visible with hand lens; moderate – stellate hairs slightly overlapping, 0.5–1 mm in diameter apart (centre to centre); sparse – stellate hairs not overlapping, 1–2 mm in diameter apart (centre to centre); and very sparse – stellate hairs not overlapping, >2 mm in diameter apart (centre to centre). Epicalyx and calyx lengths were measured at anthesis where material was available, as both of these can expand when in fruit. Epicalyx lobe width was measured at the base or where epicalyx lobe fusion ended. Petal length was measured externally from the base of the corolla to the tip of the longest lobe and therefore included the lower portion that is fused to the staminal column. Staminal column length was measured from the lower portion that is fused to the corolla to the apex of the longest apical lobe. Style exsertion length was measured from the apex of the longest apical staminal column lobe to the tip of the longest stigmatic lobe (excluding stigmatic hairs, noting that this character potentially varies with time). Flowering material was either limited or difficult to manipulate without destroying the specimen, therefore for some characters we use approximate the measurements (~) to encompass a broader range of dimensions than those measured (e.g. measured length may have been 6.8–7.2 mm but we used ~7 mm to avoid fixing this narrow range for the species). Cleistogamous flowers were identified as having a highly reduced corolla (resembling a shuttlecock) with the petals overlapping tightly, forming a cap that fully encloses the staminal column and style. Seed length was measured across the largest dimension.
Comparing environmental variables
For specimens examined by the authors, data for the associated collection and nine environmental variables were downloaded from the Atlas of Living Australia (Atlas of Living Australia website at http://www.ala.org.au, accessed July 2019). The nine environmental variables selected included the following: Temperature – annual mean (Bio01), Temperature – seasonality (Bio04), Precipitation – seasonality (Bio15), Precipitation – driest quarter (Bio17), Radiation – seasonality (Bio23), Radiation – warmest quarter (Bio26), Moisture Index – highest quarter mean (Bio32), Moisture Index – annual mean (Bio28) and Moisture Index – seasonality (Bio31). Analyses of the environmental variables were performed using R (ver. 3.5.3, R Foundation for Statistical Computing, Vienna, Austria, see http://www.R-project.org/). The environmental variables are likely to be autocorrelated and therefore violate the assumption of independence between data for Principal Components Analysis (PCA; Vanhatalo and Kulahci 2016). To test for autocorrelation, pairwise comparisons of all environmental variables were performed using the command pairs. Variables were removed leaving only those with correlations of less than 0.60 R2 to be included. The variables included for PCA included the following: Temperature – seasonality (Bio04), Precipitation – driest quarter (Bio17), Radiation – seasonality (Bio23) and Moisture Index – seasonality (Bio31). These remaining variables were subsequently transformed using dudi.pca in the package ade4 (ver. 1.7-22, S. Dray, A.-B. Dufour and J. Thioulouse, see https://cran.r-project.org/package=ade4; Dray and Dufour 2007) and visualised using the package factoextra (ver. 1.0.7, A. Kassambara and F. Mundt, see https://cran.r-project.org/package=factoextra).
Results and discussion
Morphological variation within H. krichauffianus sens. lat.
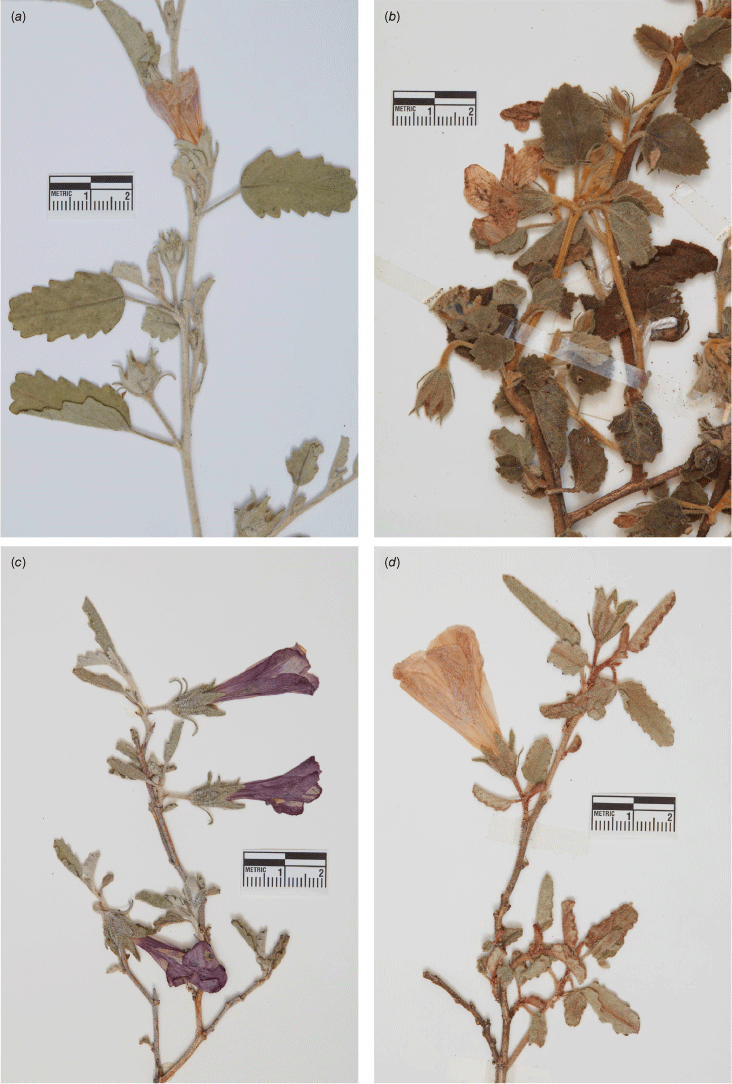
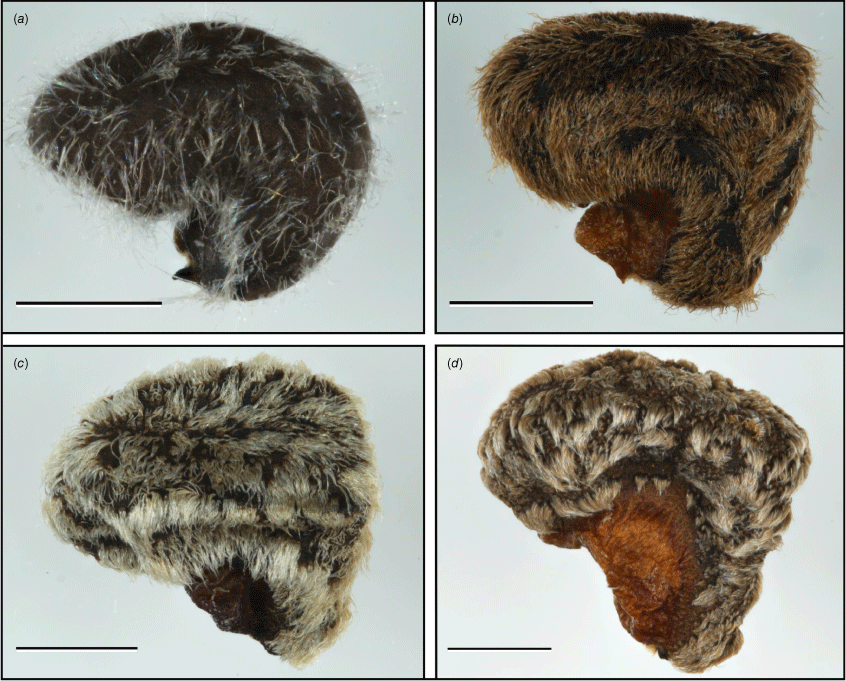
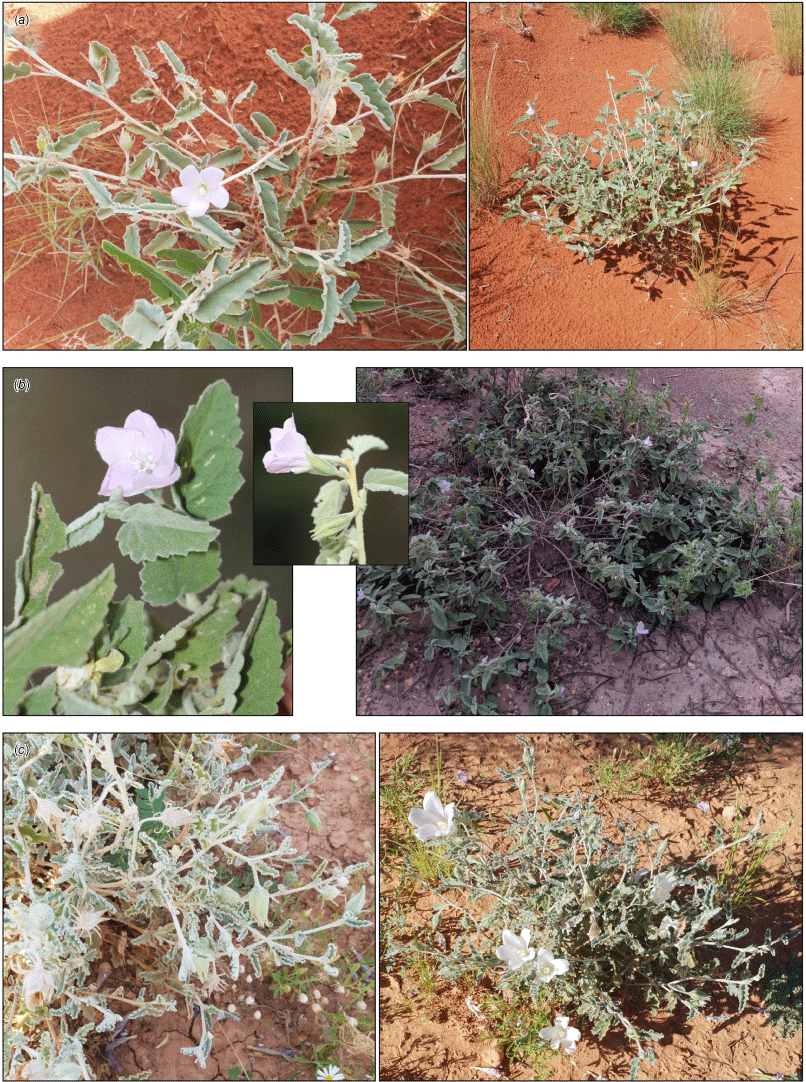
Specimens of Hibiscus krichauffianus were sorted into four morphotypes. A representative specimen of each is shown in Fig. 2, seeds in Fig. 3 and typical habitats of morphotypes A–C in Fig. 4. The morphotype encompassing the largest number of specimens, including those from NT and Vic., and many from SA, Qld and NSW was assigned morphotype A (Fig. 2a, 3a, 4a). The morphotype encompassing the second largest number of specimens, all from eastern Qld, was assigned morphotype B (Fig. 2b, 3b, 4b). Specimens from the Nullarbor region of WA and SA, along with those collected as far east as near Wilcannia in western NSW were assigned morphotype C (Fig. 2c, 3c, 4c). Specimens from the Murchison region in WA, of which only four were available for study (there were five specimens in total in herbaria), were assigned morphotype D (Fig. 2d, 3d). Features distinguishing the morphotypes are summarised in Table 1 and the distribution of each morphotype is shown in Fig. 1.
Example herbarium specimens of the four morphotypes of H. krichauffianus sens. lat. (a) Morphotype A Hibiscus krichauffianus sens. strict. (voucher: C.J.Brodie & P.J.Lang 3402, AD 249644); (b) morphotype B Hibiscus verecundus (voucher: R.J.Fensham 2991, BRI AQ653057); (c) morphotype C Hibiscus calcareus (voucher: P.Hudson s.n., AD 98321044); and (d) morphotype D Hibiscus sp. Belele (voucher: D.W.Goodall 3417, PERTH 3427285). Scale bar: 2 cm.
Seeds of the four morphotypes. (a) Morphotype A Hibiscus krichauffianus sens. strict. (voucher: C.J.Brodie & P.J.Lang 3402, AD 249644); (b) morphotype B Hibiscus verecundus (voucher: R.J.Fensham 2991, BRI AQ653057); (c) morphotype C Hibiscus calcareus (voucher: P.Hudson s.n., AD 98321044); and (d) morphotype D Hibiscus sp. Belele (voucher: D.W.Goodall 3417, PERTH 3427285). Scale bar: 1 mm.
Habit of morphotypes A–C. (a) Morphotype A Hibiscus krichauffianus sens. strict. (voucher: D.E.Albrecht 16356, CANB; images: Dave Albrecht); (b) morphotype B Hibiscus verecundus (voucher: T.G.B.McLay TM333, CANB; images: Mike Bayly); and (c) morphotype C Hibiscus calcareus (voucher: D.E.Albrecht 16345, CANB [holotype]; images: Dave Albrecht).
Environmental variation within H. krichauffianus sens. lat.
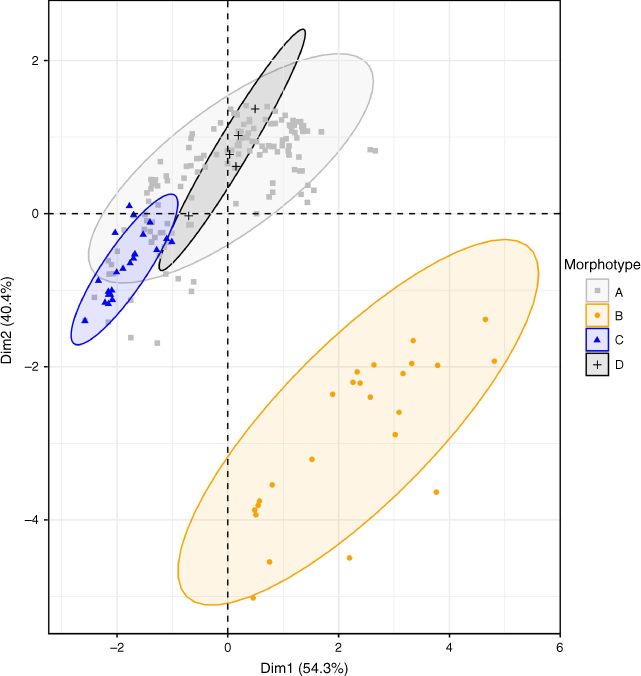
PCA (Fig. 5) shows a clear segregation of morphotype B samples (orange triangles) from morphotype A (grey squares), C (blue circles) and D (black crosses) samples. Morphotypes A, C and D largely occupy the same space within the PCA and the environmental variables used in the final dataset do not differentiate the climate envelopes of those morphotypes. Given the association of morphotype C with calcrete or limestone soils, edaphic variables may be more suitable for discriminating this species but were not included due to the lack of a comprehensive soil dataset for Australia (though see https://portal.tern.org.au/metadata/22160, accessed July 2023).
Taxonomic implications
We have shown that there is considerable morphological variation within H. krichauffianus and that four morphotypes can be distinguished.
After reviewing the putative type material (including the collection localities of specimens), we determined that morphotype A correlates with H. krichauffianus sens. strict. An amended description of H. krichauffianus, designation of a lectotype and identification of possible syntypes are presented below. The status of the name H. krichauffianus var. chippendalei Fryxell was investigated and is discussed below under excluded taxa.
Morphotype B can be distinguished from the other three morphotypes on the basis of several morphological characters and has a distinct environmental envelope relative to the other three morphotypes (Fig. 5). This morphotype is described below as the new species H. verecundus McLay & Albr.
Morphotype C is similar to morphotype A (H. krichauffianus sens. strict.), differing in the shorter stature and growth form, smaller and often more deeply toothed leaves and seeds with an indumentum of short appressed hairs (Fig. 2–4). The consistent morphological differences between morphotype C and H. krichauffianus sens. strict., and the association of this morphotype with finer-textured calcareous soils (e.g. Milthorpe & Cunningham 1928, NSW 624403; Symon NYPE-1300, AD 98805401), indicates that this entity is worthy of formal recognition and is described below as H. calcareus McLay & Albr. The environmental variables we used did not capture the association of this taxon with calcareous soils (Fig. 5).
Morphotype D is tentatively recognised as distinct on the basis of the ferruginous indumentum, petals longer than 39 mm long and geographic separation from other H. krichauffianus sens. lat. taxa. We have given morphotype D the informal phrase name H. sp. Belele (D.W.Goodall 3417). Further collections are required to confirm the morphological and environmental differences identified in this study that were based on limited material (five sheets in total).
Informal conservation assessments were made using International Union for Conservation of Nature (2012) criteria, though this should be more rigorously considered, and assessments also made against the threat classification system in use for each Australian state in which the taxa occur.
Taxonomy
| 1. | Epicalyx lobes fused at the base for more than 4 mm or if fused for less than 4 mm then free part never narrowly linear nor with a length to width (l:w) ratio higher than 4:1...H. sturtii complex Epicalyx free or fused at base for less than 4 mm, free part narrowly linear with a l:w ratio higher than 4:1...2 H. krichauffianus and allies |
| 2. | Young branchlet indumentum yellowish-brown; adaxial leaf surface green to dark green; stipules 0.1–0.17 mm wide; epicalyx lobes free or fused at base for up to 0.5 mm, free part straight in flower and fruit; seeds less than 2.5 mm long, with a patchy covering of appressed hairs...H. verecundus Young branchlet indumentum white, silvery-white, grey or yellowish-white; adaxial leaf surface whitish, greyish, silvery or ferruginous; stipules 0.16–0.5 mm wide; epicalyx lobes free or fused at base for up to 3.5(–4) mm, free part straight in flower and becoming recurved in fruit; seeds 2–3 mm long but if less than 2.5 mm long then with a sparse to moderate covering of non-appressed hairs...3 |
| 3. | Branchlet indumentum becoming ferruginous on older branchlets; adaxial leaf surface silvery–ferruginous; epicalyx lobes free or fused at base for up to 1 mm; petals 39–42 mm long, apparently purple; seeds with a patchy covering of appressed hairs ...H. sp. Belele (D.W.Goodall 3417) Branchlet indumentum not becoming ferruginous with age; adaxial leaf surfaces not ferruginous; epicalyx lobes free or fused at base for up to 3.5(−4) mm; petals 17–44 mm long, pale pink or mauve; seeds with covering of appressed or non-appressed hairs...4 |
| 4. | Erect or ascending shrub or subshrub to ~1 m high, growing in deep sands; leaf lamina 10–55 mm long and 5–35 mm wide, flat to weakly concave or weakly folded in transverse section, the sinus between marginal dentations never reaching halfway to the midvein, pedicel abscission point usually 1–2 mm from the base; seeds rounded in profile with an indumentum of wispy hairs that are not regularly appressed (see Fig. 3a)...H. krichauffianus Low, spreading dome-shaped or rounded shrub to ~0.5 m high, growing on plains with lime-rich soils; leaf lamina 6–28 mm long and 4–16 mm wide, mostly strongly concave to folded or conduplicate in transverse section, the sinus between at least some marginal dentations reaching halfway to the midvein; pedicel abscission point usually in the upper half of the flowering stalk, 2–17(–24) mm from the base; seeds angular in profile with an indumentum of short, rigid appressed hairs (see Fig. 3c)...H. calcareus |
Hibiscus krichauffianus F.Muell., Rep. pl. Babbage’s Exped. 7–8 (Oct. 1859)
Type citation: ‘Lake Gregory, Darling River’. Type: So. [South] Australia, s. dat., Mr Babbage 1, Com. R.Schomburgk 9/71 (lecto, here designated: K000659853!, Supplementary Fig. S1, bottom right corner). Residual syntypes: Minindee Creek, River Darling [=Menindee Creek, Darling River], T.H.Goodwin s.n (MEL 0068091A!, excluding upper left packet and lower two fragments, Supplementary Fig. S2); possible syntypes: mixed collections from Herb. Mueller mounted on same sheet as the lectotype, Darling river, Near Spencer’s Gulf etc. (K s.n., p.p.) [excluding the left-most specimen, as this is H. calcareus; see notes below under Typification].
Hibiscus krichauffianus F.Muell. var. krichauffianus [Autonym established by P.A.Fryxell, Proc. Linn. Soc. New South Wales 92(3): 263 (1968)].
Shrub or subshrub to 0.4–1 m tall, typically erect and ascending. Branchlets very densely covered with sessile to shortly stalked stellate hairs 0.3–0.8 mm in diameter, indumentum white, silvery-white, grey or yellowish-white. Stipules persistent or abscising with age, filiform, filiform–linear or filiform–subulate, 2.5–8 mm long, 0.16–0.4 mm wide. Mature leaves simple and unlobed, petiolate; petiole 5–25 mm long, moderately to very densely covered with sessile or shortly stalked stellate hairs; lamina mostly ovate to lanceolate or oblong, occasionally broadly ovate, flat to weakly concave or sometimes weakly folded or conduplicate, 10–55 mm long, 5–35 mm wide; base obtuse, truncate or very broadly cuneate; margins dentate to crenate; apex obtuse to broadly acute; adaxial surface whitish-silver to grey, becoming greyish-green with age, abaxial surface paler (except in young leaves); abaxial main and lateral veins raised and obvious; stellate hairs on adaxial surface dense to very dense (rarely moderate), 0.2–0.8 mm in diameter, sessile to shortly stalked, multiradiate with 7–20(–30) rays; stellate hairs on abaxial surface dense to very dense, 0.25–0.75 mm in diameter, sessile to shortly stalked, multiradiate with 10–25 rays. Flowers solitary in leaf axils, occasionally cleistogamous; combined peduncle and pedicel 5–26 mm long, usually elongating in fruit, abscission line not obvious, usually 1–2(–13) mm from base, indumentum as for young stems and petioles, broadening distally and sometimes becoming obviously flattened, occasionally recurving in fruit. Epicalyx lobes 5–8 (rarely 10), narrowly linear or subulate, 7–16 mm long, to ~1 mm wide, one-half to the same length as the calyx at anthesis, fused basally for 1–4 mm (fused part of epicalyx sometimes difficult to distinguish from the pedicel), straight in flower and becoming recurved (rarely incurved) in fruit, usually entire at the apex but rarely bifurcating, with moderate to dense stellate hairs abaxially on the lobes and usually very dense hairs on the fused portion. Calyx 9–18 mm long at anthesis, enlarging to 20 mm long in fruit; lobes narrowly triangular to triangular, 5–9 mm long at anthesis, enlarging to 12 mm long in fruit, abaxial indumentum of moderate to very dense stellate hairs, adaxial indumentum of appressed or ascending 1- or 2-armed hairs intermixed with stellate hairs particularly distally, obscurely 1-nerved under hairs. Petals 17–35 mm long, adnate to staminal column at base but otherwise free, pale pink or mauve (rarely white), lacking basal spot, glabrous adaxially, with sparse to moderate stellate hairs abaxially towards the margin and apex on one side, sometimes extending towards the petal base. Staminal column 10–18 mm long, apex irregularly 5-lobed, with the stamens usually distributed singly along the distal ~7 mm of the column but sometimes distributed along almost the full length of the column; staminal filaments 1–5 mm long; anthers yellow. Style 5-branched, with branches 1.75–4 mm long, exserted 2–9 mm beyond the apex of the staminal column. Stigmas capitate, 0.5–0.6 mm wide, distinctly hairy, hairs 0.25–0.6 mm long. Ovary 5-locular, with hairs 0.5–1 mm long. Cleistogamous flowers with a cap 3–4 mm long, with simple and stellate hairs on the apical ~1.5-mm segment, the hairs very dense. Capsule ovoid to globose, 5–12 mm long, usually beaked, beak 1–2 mm long, densely covered with simple shiny appressed hairs, the apical hairs erect, 0.5–1 mm long and extending beyond apex of capsule. Seeds reniform (rarely subangular reniform), 2–3 mm long, dark brown, with a sparse to moderate indumentum of simple, white, wispy spreading hairs 0.2–0.4 mm long; funicular remnants brown, membranous and wing-like, on either side of the himum. (Fig. 2a, 3a, 4a.)
Hibiscus krichauffianus is widely distributed in arid Australia, predominantly occurring in southern NT, SA, south-western Qld and western NSW, with more restricted occurrences in WA near the NT border and in north-western Victoria (Fig. 1, grey squares). The species occurs on deep sandy substrates (yellow, brown or red) sometimes overlying limestone on sand dunes, sandy rises or sand plains. Most occurrences are in open shrublands and hummock grasslands.
Associated overstorey species include Acacia species (e.g. A. aneura F.Muell. ex Benth., A. ligulata A.Cunn. ex Benth., A. brachystachya Benth. and A. melleodora Pedley), Atalaya hemiglauca (F.Muell.) F.Muell. ex Benth., Dodonaea viscosa Jacq., Eucalyptus concinna Maiden & Blakely, Eucalyptus socialis F.Muell. ex Miq. and Grevillea stenobotrya F.Muell.
Associated shrubs and herbs include Abutilon otocarpum F.Muell., Aluta maisonneuvei (F.Muell.) Rye & Trudgen, Aristida holathera Domin, Chrysocephalum eremaeum (Haegi) Anderb., Crotalaria cunninghamii R.Br., Crotalaria eremaea F.Muell., Eremophila macdonnellii F.Muell., Eremophila willsii F.Muell., Gyrostemon ramulosus Desf., Lechenaultia divaricata F.Muell., Phyllanthus lacunellus Airy Shaw, Polycalymma stuartii F.Muell. & Sond. ex Sond., Portulaca oleracea L., Salsola australis R.Br., Senna artemisioides (Gaudich. ex DC.) Randell, Sida ammophila F.Muell. ex J.H.Willis, Solanum coactiliferum J.M.Black, Tribulus hystrix R.Br., Triodia basedowii E.Pritz. and Zygochloa paradoxa (R.Br.) S.T.Blake.
Buds, flowers or fruit were recorded in all months of the year and flowering likely occurred in response to rainfall.
Given the wide distribution and common occurrence of this species on sand substrates, the conservation status is of Least Concern in Australia (International Union for Conservation of Nature 2012). In Victoria, this species only occurs in Ned’s Corner in the far north-western part of the state and is therefore considered Critically Endangered in the state (Flora and Fauna Guarantee Act Threatened list issued October 2021, https://www.environment.vic.gov.au/conserving-threatened-species/threatened-list, accessed August 2023). The addition of Hibiscus calcareus and H. sp. Belele Station (D.W.Goodall 3417) to the WA flora will require reassessment of H. krichauffianus in that state, as the species may only occur on the WA–NT border.
Named for Friedrich Eduard Heinrich Wulf Krichauff (1824–1904), a South Australian parliamentarian and friend of Ferdinand Mueller (O’Neill 1974). Although originally published as krichauffianus, in later references and on some specimen labels Mueller changed the spelling of the epithet to either ‘krichauffi’ or ‘krichauffii’ for unknown reasons (e.g. Mueller 1868).
Hibiscus krichauffianus appears to be most closely allied to H. calcareus but is also similar to H. verecundus and H. sp. Belele (D.W.Goodall 3417). Features distinguishing these species from H. krichauffianus can be found in the ‘Affinities’ sections for the species, Table 1 and the key.
Populations of the highly variable species Hibiscus sturtii and H. leptocladus Benth. occur within the distributional range of H. krichauffianus and could possibly be confused with this species. Both H. sturtii and H. leptocladus represent species complexes that are currently undergoing taxonomic revision. Members of the H. sturtii complex have epicalyx lobes that are distinctly fused basally for >4 mm (or if rarely less then the epicalyx lobes are never narrowly linear) and seed hairs that are appressed (or if rarely non-appressed then hairs dense and not wispy), whereas in H. krichauffianus the epicalyx lobes are only fused for 1–4 mm, and the seed hairs are wispy and not appressed (Fig. 3). Members of the H. leptocladus complex differ from H. krichauffianus in having petals with a dark basal spot, capsules never having a dense covering of appressed hairs throughout, seeds with denser hairs and leaves that are green rather than whitish-silver to greyish-green.
We observed examples of specimens with both cleistogamous and chasmogamous flowers, indicating that the two reproductive modes can overlap on the same plant or potentially occur simultaneously. Our finding of cleistogamy being present in H. krichauffianus sens. strict. but absent in H. verecundus and only present on one specimen of H. calcareus, suggests that there may be a connection between this reproductive mode and an arid environment (Culley and Klooster 2007).
WESTERN AUSTRALIA. Rawlinson Range, beside Gun Barrel Highway, 30–80 km north-west of Giles, May 1980, J.M.Bechervaise & J.Kelso 149 (PERTH). NORTHERN TERRITORY. 20 km SW Adelaide t/o, Sth Stuart Highway, 1 Mar. 1994, D.E.Albrecht 5774 (DNA, MEL, NT); Mt Wedge Station, 19 Jan. 1972, C.Dunlop 2450 (CANB, DNA, PERTH); 15 miles [~24 km] North of Andado Homestead, s. dat., J.R.Maconochie 487 (NT); W side of Stuart Hwy, 34.0 km by road S of the intersection of the road from Alice Springs township to the Alice Springs Airport., 12 Mar. 2021, D.E.Albrecht 16356 (CANB). SOUTH AUSTRALIA. On N side of plant fence, ~30 m away Merrimelia Oil, 22 Nov. 2011, C.J.Brodie & P.J.Lang 3402 (AD); Lake Littra, 16 Mar. 1997, R.Bates 46564 (AD); 6.1 km W from Mt Hoare, 22 Sep. 2001, P.D.Canty BS23-38895 (AD 121328, DNA D0182575, NT D0182575); Officer Creek, 26 Apr. 1961, R.Bates 58204 (AD); Dulkaninna Station, 5 Apr. 1997, H.Smyth 38 (AD); Mount Gason, 11 Apr. 1997, D.E.Symon 15642 (AD); ~9 km east from base camp. Base camp ~61 km east of Dalhousie Springs. Dalhousie Springs, ~115 km north of Oodnadatta, 11 Aug. 1963, T.R.N.Lothian 1779 (AD); 7 km south of Roxby Downs township, Purple Downs Stations, 21 Feb. 1990, F.J.Badman 4149 (AD); Wilgena, Carnding block, 13 Dec. 1962, leg. ign. s.n. (AD 97629404); 1.9 km NE from Candradecka Dam, 11 Nov. 1996, R.Brandle & S.E.Ruff BS69-29551 (AD). QUEENSLAND. 28.8 km south of Thylungra on Windorah–Quilpie Road, 12 Sep. 1990, M.E.Ballingall 2629 (AD, BRI); ~58 km NW of Birdsville ~10 km S of Halffinish via road to Birdsville, Adria Downs Stn, 22 Feb. 1997, W.E.Edmunds AD179 (BRI). NEW SOUTH WALES. Eastern edge of Lake Victoria, ~50 km WNW from Wentworth, I.Sluiter s.n. (CANB); Sturt National Park, 1 Oct. 1976, W.E.Mulham W915 (NSW); 5.4 km WSW of Bottom Bore on the Fort Grey road, 8 May 1977, R.Chinnock 3545 (AD, MEL); Wanaaring–Milparinka Road, 25 km E of Clifton Bore, 7 Nov. 1971, A.N.Rodd 1935 (AD, BRI, NSW); Mt Jack cattle paddock, east of Whitecliffs, 9 Mar. 1979, J.W.Lawrie 1646 (NSW). VICTORIA. Freehold land of Rod Stoeckel in far NW corner of state (N of Lindsay Point [Homestead]), 15 Mar. 2007, I.R.K.Sluiter 07/05 (MEL).
The name Hibiscus krichauffianus was first published by Mueller in 1859 in a ‘Report on the plants collected during Mr Babbage’s expedition into the north-western interior of South Australia in 1858’ but there were two reports with the same title (Mueller 1859a, 1859b). The first (Mueller 1859a) was published in April in the Quarterly Journal & Transactions of the Pharmaceutical Society of Victoria. This article was a short, two-page summary of the Babbage expedition. Although several plant species are mentioned in the text, Hibiscus krichauffianus was not listed and this publication was not the original source of the name.
The second and more comprehensive report (Mueller 1859b) was tabled in the Victorian Parliament in October 1859 (Churchill et al. 1978). This report began with the same two pages that appeared in Mueller (1859a) and was followed by two pages that described the localities from which collections were obtained. An enumeration of the plants collected followed on page 6. A description of H. krichauffianus was provided on pages seven and eight of the report. Mueller’s (1859b) description of H. krichauffianus was detailed, and the specimen examined clearly possessed flowers but no fruit and was only a small fragment:
Everywhere covered with an ashy velvet-like indument; upper leaves ovate or nearly orbicular, blunt, crenate-toothed; stipuls [sic.] almost setaceous; peduncles axillary and terminal, solitary, one flowered, as long as the petiole; involucre deeply divided into seven linear segments, which are of equal length with the nerveless calyx; petals three times longer than the calyx, blunt, oblong-cuneate, notched, slightly ciliate otherwise glabrous; styles and stamens glabrous; free parts of filaments arising singly from the columna [Latin: ‘column’], several times longer than the anthers.
Lake Gregory, Darling River.
The branches of the only small specimen which the collection contains are terete, slightly angular towards the summit. Leaves longer than the petiole, measuring about one inch. Calyx about five lines long [i.e. 11–12 mm long; see Stearn 1973, p. 113], with semilanceolate teeth. Petals probably pink, in the dried state pale, without spots, scarcely longer than one inch, blunt, not like in Hibiscus multifidus produced into an acute angle. Stamens half as long as the corolla [likely the staminal column], pale yellow. Pollen golden-yellow. Styles five, their free parts 1–1 ½ line long, yellow. Stigmas depressed, bearded [likely meaning hairy]. Capsules unknown.
It differs from Hibiscus Sturtii in longer peduncles, larger flowers, deeply cleft involucre, longer stipules, and a more ashy and velvety covering. [emphasis ours]
Prior to the publication of these two reports, Mueller discussed some of the plants from Babbage’s expedition in a letter to the director of Kew Gardens, William Hooker, dated 5 Feb. 1859 (Home et al. 2022, letter 59.02.05, accessed July 2023), including a new species of Hibiscus and a new Eremophila speciesintended to be named after the South Australian governor of the time, Sir Richard Graves MacDonnell (1814–1881). Mueller also described the state of the collection (https://vmcp.rbg.vic.gov.au/text/letters/1850-9/1859/59-02-05-final/, accessed August 2023):
I have since commencing to write to you finished the examination of Mr Babbages plants… The collection contains 8 new sp of genera know [sic.], vize Hibiscus, Helichrysum, Trichinium, & Mr Dallachy has also returned and found like Mr Babbage a magnificent Eremophila… But the great mass of his collection has not yet arrived & may contain some novelty also… Mr Babbage has only 1 specimen of each plant glued up, so that I do not possess them myself, but I will print a full enumeration of them. I have broken a piece of[f] from his specimen of Babbagia. [emphasis ours].
In seeking candidate specimens for lectotypification we considered any material that could be linked to either Babbage or Herrgott1 that could be reasonably matched to Mueller’s (1859b) description, and included either of the localities mentioned in the protologue, i.e. Lake Gregory and Darling River. Although Mueller’s description in the protologue clearly indicated that only a single specimen was observed, this was at odds with the citation of two localities. Only Lake Gregory can relate to Babbage (or Herrgott) collections because the 1858 expedition did not approach the Darling River2. Based on the protologue we identified several herbarium specimens as including possible syntypes of H. krichauffianus and evaluated these as being original material based on appearance and collection details (summarised in Table 2). These collections are housed in K, MEL and AD; there are no Babbage Expedition specimens of H. krichauffianus in MEL.
| Accession | Pro | Con | Decision | |
|---|---|---|---|---|
| K000659853 | Attributed to Babbage, a small fragment. Sent to Kew by Schomburgk in Sep.1871. | Includes fruit (label in Schomburgk’s hand). | Likely not seen by Mueller as this lacks an identification but part of original material. | |
| K000659852 | Annotated by Mueller. | A single fruit with seeds annotated as H. krichauffii, not krichauffianus. Dated 1885 by Mueller therefore too late for consideration. | Not a syntype. | |
| AD 97611531 | H. krichauffianus, in the Schomburgk herbarium, collected in South Australia. No further collecting information. Buds and flower, no fruit. | A large branched specimen with minimal flowering material. Connection to Babbage tenuous and looks different from the material at K. | Probably not a syntype. | |
| MEL 0068091A | Locality of Darling River, annotated by Mueller as H. krichauffianus, collected by Goodwin. Mentioned in protologue. | A collection of six small fragments, one of which has a young capsule. Description in protologue does not (appear to) encompass these. | Syntype, as part of the type gathering but not material used in describing the species. | |
| Herb. F. Mueller: five branches from different collectors mounted on same K sheet as those above. Without barcode. | Herbarium of the author of the species. Includes a specimen locality of Darling River. All but the one on the right have flowers and no fruit. These specimens are all from the Mueller Herb. Bentham cites a Babbage collection in Flora australiensis supporting the concept that one of these five is the original Babbage collection used by Mueller. | As discussed in text, specimens 1, 2, and 5 can be excluded. Either or both specimens 3 and 4 represent original material used by Mueller to describe the species and could be chosen. | Specimens 3 and 4 are possible syntypes, possibly matching either the Babbage or Goodwin material. |
All potential type specimens of H. krichauffianus at Kew are mounted on a single sheet (though with two accession numbers, K00659852 and K000659853, and another one required; see Supplementary Fig. S1). On the bottom right-hand side of the sheet is a specimen that is attributed to Babbage (K000659853). The material largely matches the protologue description well with an ashy indumentum, a deeply divided epicalyx and petals three times longer than the calyx, ~1 inch (~2.5 cm) long. However, there is a fruit on the specimen and no species name, locality information or date of collection. Notes on this specimen indicate that this was sent to Kew by Richard Schomburgk, director of the Adelaide Botanic Garden, in September 1871. Schomburgk sent several other specimens from Babbage’s expedition, including material of Eremophila macdonnellii3 F.Muell. (K000961426) and Datura leichhardtii F.Muell. ex Benth. (K000759495) at the same time. Although specimen K000659853 matches the overall description and is attributed to Babbage, the presence of the young fruit, the lack of locality information (aside from ‘So. [South] Australia’) and most significantly the lack of any scientific name on the label, means this is likely a duplicate of the specimen Mueller used in describing the species but was likely not observed.
The label on specimen K000659852 (bottom left-hand side) was written by Mueller. However, this cannot be part of the type gathering, consisting of a single fruit with seeds, that the protologue explicitly stated were ‘unknown’ at the time of writing (Mueller 1859b, p. 8). This was also annotated as H. krichauffii, an orthographic variant introduced by Mueller after 1859, and was dated 1885, the year of collection and receipt by Kew. The unnumbered suite of specimens from Herb Mueller on the upper half of the same Kew sheet are from various localities and collectors, and discussed later.
The source of the ‘Darling River’ locality cited in the protologue requires clarification. Specimens of Hibiscus krichauffianus bearing this locality must have come from another of Mueller’s collectors since Babbage did not visit the locality. Although the collections from the Babbage expedition are the major subject of the report by Mueller (1859b), material gathered by other collectors from arid areas and some northern Australian collections are also mentioned. The most likely source of Darling River collections was from the work of John Dallachy, a curator of the Melbourne Botanic Gardens, who, in 1858, ‘collected drought-resistant species along the River Murray near Wentworth and the Darling River as far north as Mount Murchison near Wilcannia’ (Gross 1972). Dallachy was assisted in this task by the Rev. Thomas Hill Goodwin, a resident of the area, and the collections feature throughout Mueller’s report. Eremophila goodwinii F.Muell. (Mueller 1859b, p. 18) is named for Goodwin (also see the letter to Hooker, above, that mentions the collections made by Dallachy).
Specimen MEL 0068091A (Supplementary Fig. S2) bears a white label attributing collection to Goodwin, with the locality ‘on sandhills near Minindee Creek, River Darling’, collection date February 1859, and an annotation by Mueller on a blue label as H. krichauffianus. Information on the white label matches the information on the envelope in the upper right packet, linking that material with the four uppermost sprigs on the sheet based on morphology. The packet contains two fruit and seeds and is written in Mueller’s hand. The packet and both labels are annotated with a ‘B’, indicating that Bentham saw this material. However, this material cannot have been used in producing the original description as fruit are included and the vegetative material is not a good overall match for the description (consisting of many fragments, a poor match for the Babbage specimen in Kew). Although the material was almost certainly not used in drawing up the description in the protologue, this was likely the source of the Darling River locality cited in the protologue and nonetheless a syntype.
Further adding to the complexity is that this sheet comprises two different entities based on our recircumscription of the species: Hibiscus krichauffianus sens. strict. and H. calcareus. The four uppermost mounted sprigs and fragments within the packet (comprising fruit, seeds and a leaf) fixed in the top right-hand corner of the sheet in Supplementary Fig. S2 match Hibiscus krichauffianus sens. strict. (morphotype A). Details on the white label notably match the information written on the packet mounted at the top right-hand side of the sheet containing material of morphotype A. The two lowermost mounted sprigs and fragments within the packet fixed in the top left-hand corner of the sheet in Supplementary Fig. S2 match H. calcareus (morphotype C), including the overall colour, leaf dentation, leaf folding and epicalyx characters. These specimens are also presumably from the Darling River where the two species overlap in distribution. In recircumscribing H. krichauffianus, we have excluded these specimens from our concept of H. krichauffianus sens. strict. and consider these specimens of H. calcareus.
Other MEL collections of Hibiscus krichauffianus from the Darling River were collected after 1860 and these were too late to be syntypes for the name (e.g. Beckler, Victorian Expedition (MEL 0068092A); H.Forde s.n. (MEL 2223351A) (likely Helena Forde (née Scott), who collected for Mueller in the Darling River area in the late 1860s and 1870s; see Maroske and Vaughan 2014, p. 132).
A specimen housed at the State Herbarium of South Australia in Adelaide (South Australia, s. dat., Anon. s.n. (AD 97611531)), part of Schomburgk’s herbarium, has minimal information but may have come from Babbage as Schomburgk presented Babbage material to Kew in 1871. However, this is not a ‘small specimen’ as described in the protologue of the original material, the specimen does not match the Babbage specimen in K and the lack of any information means that other candidates could be the collector. We have discounted this specimen as a potential type.
On the Kew sheet that includes the specimens K000659853 and K000659852 are five additional sprigs that have no Kew catalogue number associated with them. They are all from Herb. Mueller and reference multiple locations (‘Darling River, near Spencer’s Gulf, etc.’). Bentham labelled this collection as H. krichauffii and the collections likely relate to the treatment in Flora australiensis (Bentham 1863). In the treatment the Victorian Expedition (that departed from Melbourne in 1860 after publication of the protologue of H. krichauffianus) was listed as the source of specimens from the Darling River and Cooper’s Creek, the Babbage Expedition of specimens from Lake Gregory and Major Peter Warburton (1813–1889) collected specimens from Spencer’s Gulf4. However, the initial ‘B’ on the Goodwin collection (MEL 0068091A) indicates that Bentham also saw this collection for Flora australiensis (Bentham 1863), therefore Goodwin could also have been cited for the Darling River locality. Bentham notably cited the Babbage Expedition as the source of material from Lake Gregory, therefore one of the fragments from Mueller’s herbarium was almost certainly original material. The Babbage specimen (K000659853) was sent to Kew in 1871 and could therefore not be the material Bentham referred to in Flora australiensis.
As one or more of these five unnumbered specimens possibly relate to the original Babbage material (K000659853) or the Goodwin material (MEL 0068091A), these could be considered original material or syntypes. The leftmost fragment on the sheet at K appears to be different from the other specimens as this is a paler grey with smaller, more dentate leaves and appears to be similar to morphotype C, the newly described H. calcareus, as mentioned above. This fragment matches a MEL specimen (MEL 2223362) collected by Warburton ‘towards Spencers Gulf’. This specimen lacks a date but is not a syntype of H. krichauffianus as no Warburton collections were mentioned in the protologue (although Warburton had been in the Gawler Range area in 1857 and replaced Babbage on the expedition to Lake Torrens in 1858; Orchard 1999, p. 75). Moving left to right, the second fragment of the five can be ruled out as original material as this is small and lacks any reproductive material. The fifth fragment can also be ruled out as this includes a fruit. However, fragments three and four could be original material related to the Babbage collection (K000659853).
To typify H. krichauffianus, a type must be selected from K000659853, MEL 0068091A or the unlabelled Kew specimens from Mueller’s herbarium, though each has drawbacks. K000659853 is attributed to Babbage but includes a fruit and therefore does not perfectly match the protologue. Specimens mounted as MEL 0068091A are likely part of the type gathering, representing the Darling River locality in the protologue but do not match the protologue description well. The material from Mueller’s herbarium mounted on the same sheet as K000659853 potentially includes material from both the Babbage and Goodwin collections, though we cannot be certain as to whether specimens three or four on that sheet represent the original material. We therefore decided to lectotypify the name Hibiscus krichauffianus on K000659853, as this is likely a duplicate of the material Mueller used to prepare the protologue. MEL 0068091A therefore becomes a residual syntype and the unlabelled Kew specimens (the third and fourth fragments) are possible residual syntypes.
Hibiscus krichauffianus var. chippendalei Fryxell, Proc. Linn. Soc. New South Wales 92(3): 263 (1968).
=Hibiscus leptocladus Benth., in part (taxonomic synonym, includes holotype).
=Melhania oblongifolia F.Muell., in part (misapplication with respect to the Nicholls’ collections).
Specimens cited by Fryxell (1968, p. 263): ‘Northern Territory: Rare in grey sandy soil in woodland, 52.2 miles [~84 km] N.W. of Newcastle Waters, G. Chippendale s.n., 18 April 1959, (NT 5826, Holotype): ¼ mile [~400 m] N. of Alice Springs trucking yards, A. Nicholls 519 (NT) 524 (NT) 525 (NT, TAES), 11 May 1967’.
Hibiscus krichauffianus var. chippendalei was described by Fryxell (1968, p. 263) based on material collected from the Northern Territory and is currently cited as a name of uncertain application in the Australian Plant Census (APC, https://biodiversity.org.au/nsl/services/rest/treeElement/51731610/51229594; accessed August 2023). However, this has previously been cited as a synonym of Melhania oblongifolia (Jacobs and Pickard 1981) and Hibiscus leptocladus (Mitchell 1981).
Fryxell designated a specimen from NW of Newcastle Waters (G.Chippendale s.n., NT5826; DNA A0005826) as the holotype of the new variety name and cited four additional specimens collected from near Alice Springs (viz. Nicholls 519 (NT), 524 (NT) and 525 (NT, TAES)). Andrew Mitchell (NT) examined the holotype on 9 April 1975 and later listed H. krichauffianus var. chippendalei in synonymy under H. leptocladus in Mitchell (1979) and in the Flora of Central Australia treatment of Hibiscus (Mitchell 1981). We have examined the holotype of H. krichauffianus var. chippendalei and agree with Mitchell’s assessment that this is a specimen of H. leptocladus.5
The other specimens of Hibiscus krichauffianus var. chippendalei cited by Fryxell (Nicholls 519 (NT), 524 (NT) and 525 (NT, TAES)) were redetermined by Ian Cowie (DNA) as Melhania oblongifolia while preparing the Melhania treatment for the Flora of the Darwin Region (Ian Cowie, pers. comm., November 2018). Jacobs and Pickard (1981) also listed H. krichauffianus var. chippendalei as a synonym of Melhania oblongifolia in the census of the plants of New South Wales. Fryxell’s concept is therefore a partial misapplication to Melhania oblongifolia and should therefore be listed as such under the synonymy of that taxon.
Hibiscus verecundus McLay & Albr., sp. nov.
Type: Queensland, Poison Valley Track, White Mountains National Park, 13 Apr. 2000, K.R.McDonald KRM463 (holo: BRI AQ 496863! [Supplementary Fig. S3]; iso: DNA D0156915!).
[Hibiscus krichauffianus auct. non F.Muell.: F.J.H. von Mueller, Fragm. 6(46): 169 (1868), as ‘H. krichauffi’; E.J.Thompson et al., White Mountains Scientific Study Report, Geogr. Monogr. Ser. 9: 106 (2003); F.A.Zich et al., Australian Tropical Rainforest Plants Ed. 7 (2018); G.P.Guymer, Malvaceae in G.K.Brown & P.D.Bostock, Census Queensland Fl. 2020 (2021), http://www.data.qld.gov.au/dataset/census-of-the-queensland-flora-2020, accessed Aug. 2023].
Low, spreading, usually decumbent subshrub to 0.5 m tall. Branchlets very densely covered with sessile to shortly stalked stellate hairs 0.5–1 mm in diameter, indumentum yellowish-brown on younger branchlets, sometimes fading to white with age. Stipules persistent or abscising with age, filiform to filiform–subulate, 1.5–6 mm long, 0.1–0.17 mm wide. Mature leaves simple and unlobed, petiolate; petiole 5–15 mm long, densely to very densely covered with sessile or shortly stalked stellate hairs; lamina ovate to broadly ovate, rarely elliptic–ovate, flat to weakly concave or weakly folded, 9–52 mm long, 8–33 mm wide; base broadly cuneate, obtuse or truncate; margins serrate to dentate, rarely crenate; apex obtuse or occasionally acute; adaxial surface green to dark green, abaxial surface paler (except in young leaves); abaxial main and lateral veins raised and obvious or obscured by hairs; stellate hairs on adaxial surface moderate to dense, 0.4–0.8(–1) mm in diameter, sessile to shortly stalked, multiradiate with 5–18 rays; stellate hairs on abaxial surface moderate to very dense, 0.3–1 mm in diameter, sessile to shortly stalked, multiradiate with 7–22 rays. Flowers solitary in leaf axils; combined peduncle and pedicel 3–22 mm long, usually elongating in fruit, abscission line sometimes obvious, one-third to one-half the length from the base, indumentum as for young stems and petioles, pedicel broadening distally. Epicalyx lobes 5–7, narrowly linear or subulate, 6–10 mm long, to ~1 mm wide, one-half to three-quarters the length of the calyx at anthesis, free to base or fused basally for up to ~0.5 mm, straight, not recurved apically, with moderate to dense stellate hairs abaxially. Calyx 9–15 mm long at anthesis, enlarging to 20 mm long in fruit; lobes narrowly triangular to triangular, 4.5–7 mm long at anthesis, enlarging to 12 mm long in fruit, abaxial indumentum of dense to very dense stellate hairs, adaxial indumentum of appressed or ascending 1- or 2-armed hairs intermixed with stellate hairs particularly distally. Petals 15–28 mm long, adnate to staminal column at base but otherwise free, light pink or white, sometimes drying pale yellow, lacking basal spot, glabrous adaxially, with sparse to moderate stellate hairs abaxially toward the margin on one side. Staminal column ~15.5 mm long, apex irregularly 5-lobed, with the stamens distributed singly along the distal 4–7 mm of the column; staminal filaments 2.5–5 mm long; anthers yellow. Style 5-branched, with branches 2.5–4 mm long, exserted 4–6 mm beyond the apex of the staminal column. Stigmas capitate, ~0.5 mm wide, distinctly hairy, hairs ~0.25 mm long. Ovary 5-locular, with hairs 0.4–0.9 mm long. Capsule ovoid to ellipsoid, 6–10 mm long, usually very shortly beaked, densely covered with stout shiny simple appressed hairs, the apical hairs erect, 1–1.5 mm long and extending beyond apex of capsule. Seeds angular–reniform with the two abaxial sides flat to convex, 1.7–2.3 mm long and almost forming a right angle at the junction, dark brown, with a short patchy indumentum of simple appressed white to yellowish-brown hairs with a minute persistent tubercle base, 0.25–0.4 mm long; funicular remnants brown, membranous and wing-like, one centrally placed and one on either side of the hilum, the central one occasionally detaching. (Fig. 2b, 3b, 4b.)
Hibiscus verecundus is endemic to eastern central Queensland. Scattered populations occur within an area bound by Emerald in the south, Mt Garnet in the north, Duaringa in the east and Muttaburra in the west. Most occurrences are within the Leichhardt, Kennedy South and Kennedy North botanical regions, with fewer records from adjoining parts of the Cook, Burke and Mitchell botanical regions (Fig. 1, orange circles).
The species occurs in shrublands, woodlands and forests featuring an overstorey of Acacia (especially A. shirleyi Maiden, and also A. rhodoxylon Maiden and A. catenulata C.T.White), or Eucalyptus (e.g. E. persistens L.A.S.Johnson & K.D.Hill, E. melanophloia F.Muell., E. thozetiana (F.Muell. ex Maiden) R.T.Baker, E. drepanophylla F.Muell. ex Benth. and E. similis, Maiden) or Corymbia (e.g. C. citriodora (Hook.) K.D.Hill & L.A.S.Johnson, C. trachyphloia (F.Muell.) K.D.Hill & L.A.S.Johnson and C. clarksoniana (D.J.Carr & S.G.M.Carr) K.D.Hill & L.A.S.Johnson). One specimen was collected in the remnants of semi-deciduous vine thicket. The species occurs over a range of topographical positions including on ridges, plains, hillslopes, rocky outcrops, escarpments and gullies, and is typically associated with shallow soils overlying sandstone, laterite or basalt.
Flowering specimens have been collected in January, March, April, May, June and November. Flowering is likely dependent on rainfall that predominantly occurs in summer within the geographic range of Hibiscus verecundus, but a reasonable amount of rainfall can occur in any month in the region.
Following International Union for Conservation of Nature (2012) threat assessment definitions, Hibiscus verecundus has an approximate Extent of Occurrence (EOO) of 15 400 km2 and occurs in several nature reserves (Epping Forest National Park, White Mountains National Park and Blackwood National Park). The species has a much smaller Area of Occupancy (AOO) of 180 km2 based on ~25 collections and populations seen by the authors are generally small (<10 plants) (but due to the small stature of the species individual plants could easily be overlooked). The conservation status is possibly of Least Concern but could be Vulnerable. Until further surveying is undertaken to obtain a more accurate estimation of population number and size, and population decline, the species should be considered Data Deficient.
The species epithet is the Latin adjective verecundus, meaning modest or shy (Lewis and Short 1879), referring both to the fact that this entity was overlooked despite being very distinct from Hibiscus krichauffianus and to the difficulty in finding this species in the field.
Specimens of Hibiscus verecundus have previously been included within H. krichauffianus, though whether the two species are particularly closely related is uncertain. Hibiscus verecundus is readily distinguished from H. krichauffianus by the branchlet indumentum colour (yellowish-brown, sometimes fading to white, cf. white or silvery sometimes fading to yellowish-white in H. krichauffianus), narrower stipules (0.1–0.17 mm wide, cf. >0.16 mm in H. krichauffianus), adaxial leaf lamina colour (green to dark green, cf. typically silvery-white in H. krichauffianus), free or slightly basally fused epicalyx lobes (fused for up to 0.5 mm, cf. fused for 1–4 mm in H. krichauffianus) that remain straight in fruiting specimens (cf. recurved or incurved in H. krichauffianus) and seeds that are angular in profile (cf. rounded in profile in H. krichauffianus), with an indumentum of shorter, more rigid appressed hairs (cf. longer wispy hairs that are not regularly appressed in H. krichauffianus), and with a central and two marginal flaps of tissue associated with the hilum (cf. with only two marginal flaps in H. krichauffianus). Hibiscus verecundus has a low usually decumbent habit, whereas in H. krichauffianus the stems are mostly erect or ascending. The habitat of H. verecundus is also completely different from that of H. krichauffianus, the former occurring in woodlands, forests and shrublands (rarely vine-thickets), whereas the latter occurs in more arid environments, typically on sand dunes.
Populations of the highly variable species Hibiscus sturtii and H. leptocladus occur within the distributional range of H. verecundus and could possibly be confused with this. Both H. sturtii and H. leptocladus represent species complexes that are currently undergoing taxonomic revision. Members of the H. sturtii complex have epicalyx lobes that are distinctly fused basally for >4 mm (or if rarely less then the epicalyx lobes are never narrowly linear), whereas in H. verecundus the epicalyx lobes are free at the base or virtually so. The leaves of H. sturtii var. campylochlamys F.Muell. ex Benth. are similar to those of H. verecundus and the two taxa could be confused when flowers are lacking. Members of the H. leptocladus complex differ from H. verecundus in the erect or ascending habit, petals with a dark basal spot, capsules that never have a dense covering of appressed hairs throughout and the non-appressed seed hairs.
Due to the risk of damaging the few open flowers present on herbarium specimens (three specimens), some measurements, particularly of the androecium and gynoecium, are based on a small sample and may not truly reflect the range of variation in this species. Only chasmogamous flowers were seen in this species though an extensive field-based investigation has not been undertaken. This contrasts with Hibiscus krichauffianus that can have cleistogamous flowers.
QUEENSLAND. White Mountain National Park – NW of Warang, 3 Apr. 2000, B.S.Wannan 1736 & T.Daniel (BRI, CANB); Red soil tableland south of Jervoise homestead, 9 June 1999, E.Addicott 248 (BRI, MBA); On Myola Road, 48 km from Powlathanga homestead, 13 Mar. 1988, P.I.Forster 3665 & M.P.Bolton (BRI); ~40 miles [~64 km] S of Mt Garnet, 24 June 1967, S.A.Morain 6 (BRI); ~45 miles [~72 km] SE of Mt Garnet, 23 Jan. 1967, S.A.Morain 283 (BRI); 15 km NE of Mt Cooper homestead in St Pauls Scrub, 15 June 1992, E.J.Thompson CHA145 & P.R.Sharpe (BRI); Collinsville Coal Pty. Ltd. Mine lease area, near Collinsville and Scottville, 11 Nov. 1984, H.S. Thompson s.n. (CANB); 3.5 km SW of Scott homestead, 3 Apr. 1992, E.J.Thompson BUC841A & B.K.Simon (BRI); 31.7 km from Gregory Development Rd into eastern Disney, 14 June 1997, S.Thompson 373 (BRI); 48 km N of Belyando Crossing, 11 Dec. 1999, K.R.McDonald 190 (BRI); SSW of junction of Belyando & Suttor Rivers, 24 May 1945, S.T.Blake 15714 & L.J.Webb (BRI); 10 km W of St Anns homestead, 11 June 1992, E.J.Thompson BUC626 & P.R.Sharpe (BRI, K); Vine Creek Holding, NW of Mt Coolon, 12 June 1997, R.J.Cumming 16035 (BRI); Cometville (=Comet), 1879, P’A.O’Shanesy s.n. (MEL); Dawson Range, 1.6 km S of Baralaba–Woorabinda Road, 26 Mar. 2005, A.R.Bean 23521 (BRI); Dawson Highway, ~10 km W of Dawson River crossing, 2009, G.Harvey s.n. (CANB); Humboldt, S of Blackwater, 7 Jan. 1997, R.J.Fensham 2991 (BRI); Humboldt, S of Blackwater, 8 Jan. 1997, R.J.Fensham 2990 (BRI); Terang Holdings, 4 km NE of South Blackwater Mine administration, 24 Nov. 1998, T.S.Ryan 1264 (BRI); South Blackwater Mine, Laleham, 3 Jan. 1986, E.J.Thompson s.n. (BRI); ~10 km due NNW of ‘Exevale’ along the Pipeline–Eungella Dam Road, Nebo Shire, 18 Mar. 2003, A.B.Pollock 1564 & I.G.Champion (BRI); Harrybrandt Station, 7 km W of Dingo/Mt Flora, Beef Road, 24 Jan. 1998, S.Thompson 566 & I.Fox (BRI); Blackdown Tableland, 9 Apr. 1982, S.Pearson 471 (BRI). Bullock Creek, NNW of Torrens Creek township, 27 Apr. 1990, R.J.Cumming 9553 (BRI).
Hibiscus calcareus McLay & Albr., sp. nov.
Type: South Australia, Curnamona Station, W side of the Yunta–Arkaroola Road, ~590 m due W of Curnamona Homestead, 2 Mar. 2021, D.E.Albrecht 16345 (holo: CANB 914032! [Supplementary Fig. S4]; iso: AD!, NSW!).
[Hibiscus krichauffianus auct. non F.Muell.: F.J.H. von Mueller, Rep. pl. Babbage’s Exped. 7 (1859), p.p. [only as to some Goodwin specimens from Darling River labelled ‘B’ on MEL 0068091A (Supplementary Fig. S2)]; G.Bentham, Fl. austral. 1: 216 (1863), p.p. [only as to Warburton specimen from Spencer’s Gulf]; F.J.H. von Mueller, Fragm. 9(78): 129 (1875), p.p. [as to Giles specimen from N of Fowler’s Bay (MEL2223365A)], as H. krichauffii; J.M.Black, Fl. S. Australia 3: 381 (1926), p.p.; J.M.Black, Fl. S. Australia 2nd edn, 3: 566 (1952), p.p.; Hj.Eichler, Suppl. Black’s Fl. S. Australia 223 (1965), p.p.; Western Australian Herbarium (1998), Florabase, Department of Biodiversity, Conservation and Attractions. https://florabase.dpaw.wa.gov.au/browse/profile/4932c, p.p. [only as to collections from south-eastern Western Australia]; PlantNET (The NSW Plant Information Network System). Royal Botanic Gardens and Domain Trust, Sydney. https://plantnet.rbgsyd.nsw.gov.au/cgi-bin/NSWfl.pl?page=nswfl&lvl=sp&name=Hibiscus~krichauffianus, p.p. [only as to specimens from the Broken Hill–Wilcannia area].
Low, spreading, many-branched, dome-shaped or rounded subshrub to 0.5 m tall but often less than 0.4 m tall. Branchlets very densely covered with sessile to shortly stalked stellate hairs 0.25–0.9 mm in diameter, indumentum white to silvery white. Stipules persistent or abscising with age, filiform–subulate, linear or very narrowly triangular, 2–6 mm long, 0.2–0.5 mm wide. Mature leaves simple and unlobed, petiolate; petiole 2–10 mm long, densely to very densely covered with sessile or shortly stalked stellate hairs; lamina narrowly to broadly ovate (the lowermost leaves tend to be broader), elliptic–ovate or oblong–ovate, mostly strongly concave to folded or conduplicate (distal leaves particularly strongly V-shaped in cross-section), 6–28 mm long, 4–16 mm wide; base broadly cuneate or truncate; margins serrate, dentate or crenate, undulate, the sinus between teeth to halfway to midvein, one or more proximal teeth sometimes larger than the other teeth; apex obtuse or broadly acute; adaxial surface grey to whitish-silver, abaxial surface similar or occasionally paler; midvein and primary veins equally covered by hairs on both surfaces, the lateral veins weakly or moderately raised and obscured by hairs; stellate hairs on adaxial surface moderate to very dense, occasionally sparse in oldest leaves, 0.4–0.6 mm in diameter, sessile to shortly stalked, multiradiate with 10–20 rays; stellate hairs on abaxial surface dense to very dense, 0.35–0.7 mm in diameter, sessile to shortly stalked, multiradiate with 8–25 rays. Flowers solitary in leaf axils; combined peduncle and pedicel 3–25 mm long, usually elongating in fruit, abscission line not always distinct, usually in distal half, 2–17(–24) mm from the base, indumentum as for young stems and petioles, broadening and flattening distally, often bent or recurved in fruit. Epicalyx lobes 7–8, narrowly linear or subulate and rarely linear–oblanceolate, 4–14 mm long, to ~1 mm wide, one-half to three-quarters the length of the calyx at anthesis, fused basally for 1–3.5 mm, becoming recurved in fruit, with sparse to dense stellate hairs abaxially. Calyx 10–19 mm long at anthesis; lobes narrowly triangular to triangular, 5–12 mm long at anthesis, abaxial indumentum of moderate to very dense stellate hairs, adaxial indumentum of dense appressed or ascending 1- or 2-armed hairs intermixed with stellate hairs particularly distally. Petals 20–44 mm long, adnate to staminal column at base but otherwise free, pale pink to pale lilac–purple, sometimes almost white, pigment often more intense after flowers senesced, lacking basal spot, glabrous adaxially, with sparse to dense stellate hairs abaxially towards the apex and near the margin on one side. Staminal column 12–18 mm long, apex irregularly 5-lobed, with the stamens distributed singly along the distal 6.5–8 mm of the column; staminal filaments 1.5–3 mm long; anthers yellow. Style 5-branched, with branches 2–3 mm long, exserted 3–4 mm beyond the apex of the staminal column. Stigmas capitate, 0.4–0.7 mm wide, distinctly hairy, hairs 0.2–0.3 mm long. Ovary 5-locular, with hairs 0.4–1 mm long. Capsule ovoid to globose, 8–12 mm long, usually very shortly beaked, beak to ~1 mm long, densely covered with simple shiny appressed hairs, the apical hairs erect, 0.4–1.3 mm long and extending beyond apex of capsule. Seeds angular–reniform with the two abaxial sides flat to convex, 2.5–3 mm long and almost forming a right angle at the junction, dark brown, with a short patchy indumentum of appressed white to yellow–brown simple hairs with a minute persistent tubercle base; funicular remnants brown, membranous and wing-like, on either side of the hilum. (Fig. 2c, 3c, 4c.)
Hibiscus calcareus occurs from eastern Western Australia near the Great Victoria Desert, through South Australia and into western New South Wales near Barringun, between latitudes 29 and 35°S (Fig. 1, blue triangles). The distribution of this species is likely strongly associated with landform and soil type, as many collection records report plants growing on plains with lime-rich soils (e.g. Milthorpe & Cunningham 1928, NSW 624403; Symon NYPE-1300, AD 98805401; Milthorpe 680, NSW 684440, as ‘solonised brown soil’; and Bates 14221, AD 98821007). The substrate at the sites supporting this species typically has a low rate of percolation of water. Some sites have surface stones or even a thin surface veneer of sandy loam. The record Constable s.n. (NSW 684435) from ‘high rocky ground’ is anomalous and requires further investigation. The limited notes on the specimens seen suggest this species grows in grasslands, open woodlands and shrublands, featuring chenopodiaceous shrubs and Acacia (including A. papyrocarpa Benth.). At the type locality the species occurs in run-on areas on a calcareous plain. Very sparse shrubs of Acacia victoriae Benth., Eremophila duttonii F.Muell. and Atalaya hemiglauca occur at this site. The lower shrub stratum is dominated by Maireana astrotricha (L.A.S.Johnson) Paul G.Wilson, with associated species including Cullen cinereum (Lindl.) J.W.Grimes, Erodiophyllum elderi F.Muell., Sida fibulifera Lindl., Minuria leptophylla DC., Rhodanthe floribunda (A.Cunn. ex DC.) Paul G.Wilson, Enneapogon cylindricus N.T.Burb., Sclerolaena patenticuspis (R.H.Anderson) Ulbr. and Swainsona viridis J.M.Black.
Specimens with flowers (17 in total) were collected from January to October, with 11 flowering collections made between March and May.
The status of this species would be considered Data Deficient (International Union for Conservation of Nature 2012). Owing to the small number of specimens seen, the relatively restrictive habitat type in calcareous soils and that attempts to relocate the species at some existing collection sites in NSW were unsuccessful, a Vulnerable or Endangered status could be required.
The epithet calcareus is a Latin adjective meaning 'of or pertaining to lime' and refers to the species' association with soils overlying limestone or calcrete.
Hibiscus calcareus is similar to H. krichauffianus and these two are likely closely related or sister taxa. Hibiscus calcareus is distinguished from H. krichauffianus in habit (low, spreading dome-shaped or rounded, cf. upright, erect or ascending stems in H. krichauffianus) and habitat (occurring on plains with slow-draining lime-rich soils, cf. deep sands in H. krichauffianus), lamina size (although this character can overlap, H. calcareus usually has smaller leaves 6–28 mm long and 4–16 mm wide, cf. 10–55 mm long and 5–35 mm wide in H. krichauffianus), lamina shape in transverse section (mostly strongly concave to folded or conduplicate in H. calcareus, cf. flat to weakly concave or weakly folded in H. krichauffianus), leaf margins (undulate, serrate to dentate or crenate, often deeply so with the sinus between dentations extending up to half-way to the midvein, cf. crenate or dentate in H. krichauffianus), with pedicel abscission point usually in the upper half of the flowering stalk, 2–17 mm from the base (cf. only 1–2 mm from the base in H. krichauffianus) and seeds that are angular in profile (cf. rounded in profile in H. krichauffianus), with an indumentum of shorter, more rigid appressed hairs (cf. longer wispy hairs that are not regularly appressed in H. krichauffianus). Sometimes depauperate plants of H. krichauffianus growing in marginal habitats can approach H. calcareus in appearance, typically by being smaller in stature and having smaller leaves. Examples of these specimens of H. krichauffianus include Mills & Cox 238 (NSW 624404), Percival 73 (CANB 866287), Clarke 2999 (MEL 2073274A) and Lawrie 1646 (NSW 624396). However, these can be distinguished from H. calcareus by leaf dentation and seed characters. These differences are stable under common growth conditions and not strictly controlled by growth on limestone or calcrete (Supplementary Fig. S5).
The available specimens limited our certainty about some characters, especially flower measurements.
WESTERN AUSTRALIA. 78 km S by track of Ilkurlka roadhouse, Great Victoria Desert, Laverton, Oct. 2010, R.Davis & J.Jackson 11710 (PERTH). SOUTH AUSTRALIA. 10 km east-south-east of Benagarie, 10 Apr. 1976, L.D.Williams 7927 (AD); Erudina Station, 25 Apr. 1988, R.Bates 14221 (AD); Nillinghoo Mine, N.E of Koonamore Station, 10 Oct. 1987, A.G.Spooner 10905 (AD); Sandy flat S of Frome Downs, 15 May 1980, K.Paijmans 3483 (CANB); Black Oak Plains, Quandong Homestead (Quandong Homestead is ~140 km east of Peterborough), 31 Jan. 1968, S.Barker 180 (AD); Towards Spencers Gulf, s. dat., P.E.Warburton s.n. (MEL 2223362); Vulcan Paddock, Roopena Station, via Whyalla, s. dat., R.T.Lange s.n. (AD 99616857); Whyalla, 14 Apr. 1983, P.Hudson s.n. (AD); ~13 km WSW of Tarcoola township, 23 Apr. 1992, P.Hornsby s.n. (AD); Mt Finke Site, 2.0 km east Mt Finke trig, Quadrat 2, 8 Oct. 1987, D.E.Symon for N.P.W.S 1300 (AD); Ooldea near siding sign, 22 May 2014, D.E.Murfet 7738 & D.J. Duval (AD); 29 km west north-west of Oak Valley, Great Victoria Desert, (TERN site code: SAAGVD0002) 9 Mar. 2014, E.Leitch SAA003896 (CANB). NEW SOUTH WALES. 1 mile [~1.6 km] north Wilcannia, 30 Mar. 1974, P.L.Milthorpe & G.M.Cunningham 1928 (NSW); Byrnedale Station via Broken Hill, 1 Apr. 1949, R.L.Caskey (NSW); Broken Hill, 1 March 1920, A.Morris 162 (NSW); Broken Hill, 29 Apr. 1956, F.Apperkuch McB. No. 6492 (NSW); Silverton, 25 Nov. 1947, E.J.Constable s.n. (NSW).
Hibiscus sp., Belele (D.W.Goodall 3417) McLay & Albr
Note: the description below is tentative, and presented to facilitate recognition and the collection of additional specimens and associated data. This is based on very limited material and should only be considered a draft description requiring modification as more specimens become available.
Subshrub to ~0.3 m tall. Branchlets very densely covered with sessile to shortly stalked stellate hairs, indumentum white on young branchlets, becoming ferruginous. Stipules persistent or abscising with age, filiform to filiform–subulate, 1.5–3.5 mm long, 0.2–0.32 mm wide. Mature leaves simple and unlobed, petiolate; petiole 6–16 mm long, moderately to very densely covered with sessile or shortly stalked stellate hairs; lamina ovate to elliptic ovate, flat to weakly folded, 17–43 mm long, 6–22 mm wide; base truncate, very slightly cordate, or broadly obtuse; margins dentate to crenate; apex obtuse to broadly acute; adaxial surface silvery–ferruginous, abaxial surface usually paler (except in young leaves); abaxial main and lateral veins raised and very obvious; stellate hairs on adaxial surface dense to very dense (rarely moderate); stellate hairs on abaxial surface dense to very dense, ferruginous hairs more prominent on veins. Flowers solitary in leaf axils; combined peduncle and pedicel 8–19 mm long, broadening distally, abscission line not always distinct, approximately one-third of the length from the base, indumentum as on young stems and petioles. Epicalyx lobes 5–6, linear, 6–12 mm long, one-half to three-quarters the length of the calyx at anthesis, free or very slightly fused basally for ~1 mm, recurved in fruit, with dense stellate hairs abaxially on the lobes. Calyx 10.5–17 mm long at anthesis; lobes narrowly triangular to triangular, 7–10 mm long in fruit, abaxial indumentum of moderate to very dense stellate hairs, adaxial indumentum of ascending 1-few armed hairs intermixed with stellate hairs particularly distally. Petals 39–42 mm long, adnate to staminal column at base but otherwise free, apparently purple, glabrous adaxially, with sparse to moderate stellate hairs abaxially towards the margin and apex on one side. Staminal column ~20 mm long, apex irregularly 5-lobed, with the stamens distributed singly along the distal ~5 mm of the column; staminal filaments ~4 mm long; anthers yellow. Style 5-branched, with branches ~3 mm long, exserted ~5 mm beyond the apex of the staminal column. Stigmas capitate, ~0.5 mm wide, distinctly hairy, hairs ~0.4 mm long. Ovary 5-locular, with hairs. Capsule ovoid, 13–17 mm long, usually beaked, beak ~1 mm long, densely covered with simple shiny appressed hairs, the apical hairs erect, 0.8–1 mm long and extending beyond apex of capsule. Seeds angular–reniform with the two abaxial sides flat to convex, ~3 mm long and almost forming a right angle at the junction, dark brown, short patchy indumentum of appressed white–yellowish simple hairs, ~0.25 mm long; funicular remnants brown, membranous and wing-like, on either side of the hilum. (Fig. 2d, 3d.)
Western Australia, IBRA Bioregions of Murchison and Yalgoo (Fig. 1, black crosses).
The limited herbarium material available indicates that the entity flowers in April, producing fruit in July and October.
Hibiscus sp. Belele (D.W.Goodall 3417) is similar to H. krichauffianus and H. calcareus and these are likely closely related or sister taxa. Based on the limited material available, H. sp. Belele (D.W.Goodall 3417) appears to be distinct in having ferruginous hairs on older branchlets and leaves (cf. predominantly silvery-white or white in H. krichauffianus and H. calcareus), and apparently deeper coloured petals (recorded as purple) that are large (petals 39–42 mm long, cf. 17–35 mm in H. krichauffianus and 20–44 mm in H. calcareus). The leaf margins resemble those of plants of H. krichauffianus, being less deeply incised than those in H. calcareus; however, the seeds are angular and have appressed hairs, resembling H. calcareus rather than H. krichauffianus. The five specimens of H. sp. Belele (D.W.Goodall 3417) are also significantly geographically isolated from the current known ranges of H. krichauffianus or H. calcareus, with over 1000 km between collections (Fig. 1).
WESTERN AUSTRALIA: Cliffs Natural Resources Mount Richardson, ~138 km NW of township of Ularring, 7 Sep. 2012, D.Coultas Opp-48 (PERTH); Woolorong Station, 7 Apr. 1999, M.Officer 214 (PERTH); Gidgee Station (I447), 20 July 1993, H.Pringle 3683 (PERTH); Belele Station, WNW of Meekatharra, 26 & 30 Oct. 1965, D.W.Goodall 3417 (PERTH); Yuin, 1888, S.J.B.Hymus s.n. (MEL 2223356A).
Data availability
The data that support this study are available in the article and accompanying online supplementary material.
Declaration of funding
This research was funded through the Australian Government’s Australian Biological Resources Study National Taxonomy Research Grant Programme for the project ‘An eFlora treatment for Australian Hibiscus and novel genomic markers for addressing taxonomic challenges in Malvaceae sensu lato’. T. G. B. McLay was supported by an ABRS National Taxonomy Research Grant awarded to Sarah Mathews.
Acknowledgements
We thank the staff and directors at the Western Australian Herbarium (PERTH), the Northern Territory Herbarium (NT), the State Herbarium of South Australia (AD), the Queensland Herbarium (BRI), the National Herbarium of New South Wales (NSW), the Australian National Herbarium (CANB) and the National Herbarium of Victoria (MEL). Ian Cowie (DNA) confirmed the identity of specimens annotated as H. krichauffianus var. chippendalei for us. Dan Duval shared seed images and grew seedlings of H. krichauffianus and H. calcareus for us to observe any morphological differences side-by-side. Brendan Lepschi (CANB) assisted a great deal with advice about lectotypification. Neville Walsh (RBGV) provided help with Latin for the specific epithets. The South Australian Department of Environment and Water granted a permit to collect specimens. We thank Jeff Pumpa for hospitality at Curnamona Station while DEA was collecting H. calcareus. The image of the type of H. krichauffianus is reproduced with permission from RBG Kew. The image of the syntype specimen of H. krichauffianus is reproduced with permission from the Royal Botanic Gardens Victoria (MEL, RBGV) and the type image of H. verecundus is reproduced with permission from the Queensland Herbarium. Sarah Maroske (RBGV) and John Dowe (JCU) are thanked for helping identify relevant letters Mueller sent regarding the collection of H. krichauffianus. Lydia Guja (ANBG) kindly allowed access to the seed imaging camera available at the Australian National Botanic Gardens. Andre Messina (RBGV) helped with International Union for Conservation of Nature classification. Peter de Lange, Russell Barrett, Anna Monro and one anonymous reviewer are thanked for their contributions in improving the manuscript.
References
Bean AR (2004) The taxonomy and ecology of Solanum subg. Leptostemonum (Dunal) Bitter (Solanaceae) in Queensland and far north-eastern New South Wales, Australia. Austrobaileya 6, 639-816.
| Crossref | Google Scholar |
Bentham G (1863) Flora australiensis: a description of the plants of the Australian territory. Vol. 1. (L. Reeve and Co: London, UK) 10.5962/bhl.title.141
Churchill DM, Muir TB, Sinkora DM (1978) The published works of Ferdinand J.H. Mueller (1825–1896). Muelleria 4(1), 1-120.
| Crossref | Google Scholar |
Craven LA, Wilson FD, Fryxell PA (2003) A taxonomic review of Hibiscus sect. Furcaria (Malvaceae) in Western Australia and the Northern Territory. Australian Systematic Botany 16, 185-218.
| Crossref | Google Scholar |
Culley TM, Klooster MR (2007) The cleistogamous breeding system: a review of its frequency, evolution, and ecology in angiosperms. Botanical Review 73, 1-30.
| Crossref | Google Scholar |
Dray S, Dufour A-B (2007) The ade4 package: implementing the duality diagram for ecologists. Journal of Statistical Software 22(4), 1-20.
| Crossref | Google Scholar |
Fryxell PA (1968) Taxonomic notes on Australian Malvaceae. Proceedings of the Linnean Society of New South Wales 92(3), 262-265.
| Google Scholar |
Fryxell PA (1980) A revision of the American species of Hibiscus Section Bombicella (Malvaceae). Technical Bulletin 1624. (US Department of Agriculture) Available at https://naldc.nal.usda.gov/catalog/CAT89930168
Gross A (1972) Dallachy, John (1808–1871). In ‘Australian Dictionary of Biography’. (National Centre of Biography, Australian National University) Available at https://adb.anu.edu.au/biography/dallachy-john-3355/text5055 [Verified 25 July 2023]
Hochreutiner BPG (1900) Revision du genre Hibiscus. Annuaire du Conservatoire et du jardin botaniques de Genève 4, 23-191 [as Sect. Bombycella] Available at https://www.biodiversitylibrary.org/item/111659#page/35/mode/1up [In French].
| Google Scholar |
Home RW, Darragh TA, Lucas AM, Maroske S, Sinkora DM, Voigt JH, Wells M (Eds) (2022) Correspondence of Ferdinand von Mueller. (Royal Botanic Gardens Victoria) Available at https://vmcp.rbg.vic.gov.au/id/59-02-05 [Verified 25 July 2023]
Juswara LS, Craven LA (2005) The Hibiscus panduriformis complex (Malvaceae) in Australia. Blumea – Biodiversity, Evolution and Biogeography of Plants 50, 389-405.
| Crossref | Google Scholar |
Maroske S, Vaughan A (2014) Ferdinand Mueller’s female plant collectors: a biographical register. Muelleria 32, 92-172.
| Crossref | Google Scholar |
Mitchell AS (1979) A new Hibiscus (Malvaceae) from central Australia. Nuytsia 2(6), 336-340.
| Crossref | Google Scholar |
Mueller FJH (1859a) [April] Report on the plants collected during Mr Babbage’s expedition into the north-western interior of South Australia in 1858. Quarterly Journal & Transactions of the Pharmaceutical Society of Victoria 2(5), 44-46.
| Google Scholar |
Mueller FJH (1859b) Report on the plants collected during Mr Babbage’s expedition into the north-western interior of South Australia in 1858. In ‘Victoria – Parliamentary Papers – Votes and Proceedings of the Legislative Assembly 1859-60 3(1): 1-21.’ (Government Printer: Melbourne, Vic.) [tabled 18 October 1859] Available at https://www.biodiversitylibrary.org/item/217546
O’Neill, S (1974) Krichauff, Friedrich Eduard (1824–1904). In ‘Australian Dictionary of Biography’. (National Centre of Biography, Australian National University) Available at https://adb.anu.edu.au/biography/krichauff-friedrich-eduard-3973/text6273 [published first in hardcopy 1974, accessed August 2023]
Pfeil BE, Crisp MD (2005) What to do with Hibiscus? A proposed nomenclatural resolution for a large and well-known genus of Malvaceae and comments on paraphyly. Australian Systematic Botany 18, 49-60.
| Crossref | Google Scholar |
Vanhatalo E, Kulahci M (2016) Impact of autocorrelation on principal components and their use in statistical process control. Quality and Reliability Engineering International 32, 1483-1500.
| Crossref | Google Scholar |
Footnotes
1 Joseph Albert Franz David Herrgott (1823–1861), the botanist on the Babbage expedition, referred to by Mueller (1859b, p. 4) as David Hergolt. The surname is also sometimes given as Hergott (see records in the Australasian Virtual Herbarium https://avh.chah.org.au/).
2 See list of locations in Mueller (1859b, pp. 5–6).
3 Eremophila macdonnellii was lectotypified by Chinnock (2007, p. 406) on MEL 83174, a specimen from Lake Gregory that was attributed to Herrgott even though Herrgott’s name is not present and there is a blue label with the initial ‘B’, presumably indicating Babbage. The history surrounding the type specimens of E. macdonnellii likely mirrors that of H. krichauffianus but there is no material of the latter species at MEL bearing Herrgott’s name.
4 Distribution statement (Bentham 1863, p. 216): ‘N.S.Wales. Darling river, Victorian Expedition. S. Australia. Lake Gregory, Babbage’s Expedition; Cooper’s Creek, Victorian Expedition; towards Spencer’s Gulf, Warburton.’
5 Note this specimen is currently identified as Melhania oblongifolia on the Australasian Virtual Herbarium (AVH) site, but this is incorrect (https://avh.ala.org.au/occurrences/fc878fa7-9670-4272-a8d1-c6932c6a4757, viewed 15 November 2022).