Leaf fossils show a 40-million-year history for the Australian tropical rainforest genus Megahertzia (Proteaceae)
Raymond J. Carpenter A * and Andrew C. Rozefelds
A * and Andrew C. Rozefelds  B C
B C
A School of Biological Sciences, University of Adelaide, Adelaide, SA 5005, Australia.
B Collection and Research Centre, Queensland Museum, Hendra, Qld 4011, Australia.
C School of Engineering and Technology, Central Queensland University, Rockhampton, Qld 4702, Australia.
Australian Systematic Botany 36(4) 312-321 https://doi.org/10.1071/SB23005
Submitted: 28 February 2023 Accepted: 21 July 2023 Published: 18 August 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Well-preserved leaf fossils from the Middle Eocene Anglesea site in Victoria are assigned to a new species of Megahertzia (M. paleoamplexicaulis R.J.Carp. & Rozefelds), a genus of Proteaceae now represented by a single species, M. amplexicaulis A.S.George & B.Hyland, in the Wet Tropics rainforests of Queensland. Megahertzia-like cuticular remains also occur in the Eocene Mount Hotham assemblage of Victoria, and pollen closely conforming to Megahertzia (i.e. Proteacidites latrobensis W.K.Harris) occurs widely in Cenozoic sediments of Australia and in New Zealand. All these records add to other fossil evidence that many species of Australian rainforest Proteaceae are the last vestiges of formerly much more widespread lineages. The fossil leaves are near-identical in architecture and cuticular features to lobed leaves of M. amplexicaulis, including that they have small teeth, stomata in well-defined areoles, and fine cuticular striations. Moreover, where preserved, the leaf fossils show amplexicaul bases, a unique (apomorphic) trait of the extant species. The apparent absence at Anglesea of simple (unlobed) leaves in Megahertzia and two other taxa of fossil Proteaceae is discussed; this leaf type could have evolved convergently in response to forest canopy heat increase as Australia drifted towards the Equator.
Keywords: Australian flora, climate change, continental drift, Eocene, fossil pollen, leaf cuticle, lobed leaf, relictual lineage, Wet Tropics, World Heritage Area.
Introduction
A remarkable feature of the Australian rainforest flora is its high generic diversity of Proteaceae. This diversity is even higher than that of the much more species-rich scleromorphic Mediterranean-climate heathland flora, which is characterised by generally more familiar genera, including Persoonia Sm., Banksia L., Isopogon R.Br., Hakea Schrad. & J.C.Wendl. and Grevillea R.Br. ex Knight. Indeed, the rainforests of the Queensland Wet Tropics (i.e. Cooktown to Townsville region) host 26 currently recognised genera (not including Banksia), 15 of which that are endemic to the region (data from Weston and Barker 2006; Mast et al. 2008). By contrast, although still rich, the famous Mediterranean-climate south-western Australian biodiversity hotspot has 16 genera of Proteaceae, only four of which are endemic. In terms of phylogenetic diversity, almost all the Wet Tropics genera belong to subfamily Grevilleoideae, with each of the four clades recognised at tribal level by Weston and Barker (2006) being represented, as well as the endemic genera Carnarvonia F.Muell. and Sphalmium B.G.Briggs, B.Hyland & L.A.S.Johnson, whose tribal affinities remain unresolved. The only non-grevilleoids are single species each of Placospermum C.T.White & W.D.Francis (subfamily Persoonioideae) and Eidothea A.W.Douglas & B.Hyland (Proteoideae), but these genera belong to outlying lineages within their respective subfamilies (Sauquet et al. 2009).
A fossil taxon from the Victorian Middle Eocene Anglesea site was likened by Christophel et al. (1987) to leaves of a then newly discovered plant from the Daintree region of the Wet Tropics that was provisionally included in Orites R.Br. (as ‘Orites code #752’; Hyland 1982), but subsequently recognised as the only species in the new genus Megahertzia A.S.George & B.Hyland (George and Hyland 1995). Megahertzia was tentatively proposed to be closest to Orites and Neorites L.S.Sm.; however, molecular evidence now places it apart from these genera. Thus, in all recently published phylogenies a clade comprising only Orites, Neorites and Roupala Aubl. is strongly supported as subtribe Roupalinae of tribe Roupaleae, but the positions of Megahertzia and some other genera in Roupaleae (Knightia R.Br., Triunia L.A.S.Johnson & B.G.Briggs and Eucarpha (R.Br.) Spach) have yet to be resolved with confidence (Weston and Barker 2006; Weston 2014). Sauquet et al. (2009) placed Megahertzia as sister to Heliciinae (Helicia Lour. + Hollandaea F.Muell.) in Roupaleae, a topology supported with a posterior probability of 0.99, but parsimony bootstrap support of only 59%, whereas Onstein et al. (2016) placed Megahertzia as sister to Knightia + Heliciinae. Morphological support for a close relationship among Megahertzia, Helicia and Hollandaea is indicated by the fact that these genera share similar leaf cuticular morphologies (Carpenter 1994, 2012).
In this paper, we formally describe the Anglesea leaf taxon as a new species of Megahertzia, and further discuss (see Christophel and Greenwood 1988; Greenwood and Christophel 2005) the likely history of the genus within mesic rainforests of Australia.
Materials and methods
Fossil site and age of the sediments
As discussed extensively by Christophel et al. (1987) and Holdgate et al. (2001), abundant plant fossils were found in blocks of coastal plain fluvio-lacustrine (Salt Creek Formation–Eastern View Group–Torquay Basin) clastic sediments at the Anglesea open-cut coalmine of Victoria. Christophel et al. (1987) used spore–pollen ranges from the nearby Otway and Gippsland basins to conclude that the Anglesea clay lenses broadly correspond in age with the boundary of the Gippsland Basin Lower and Middle Nothofagidites asperus zones (see Partridge 2006), which is within the Bartonian stage (40.4–37.2 million years ago) of the Middle Eocene.
The Anglesea flora could comprise over 100 woody taxa represented by macrofossils (Christophel 1994) and is indicative of warm, mesic rainforest vegetation, being so far known to include the fern Lygodium Sw. (Schizaeaceae: Rozefelds et al. 1992, 2017), cycads including Bowenia Hook.f. (Hill 1978, 1980; Hill et al. 2019), several Podocarpaceae (Greenwood 1987; Hill and Carpenter 1991; Hill and Pole 1992; Hill and Scriven 1998), Austrodiospyros Basinger & Christophel (Ebenaceae: Basinger and Christophel 1985), Elaeocarpaceae (O’Dowd et al. 1991), Gymnostoma L.A.S.Johnson (Casuarinaceae: Christophel 1980), Syzygium-like Myrtaceae (Christophel and Lys 1986), monocots (Conran et al. 1994; Conran and Christophel 1999; Greenwood and Conran 2000) including a palm (Greenwood and Christophel 2005), numerous Lauraceae (Christophel et al. 1987; O’Dowd et al. 1991; Christophel 1994), and flowers (Christophel 1984) and leaves (Hill and Christophel 1988; Carpenter et al. 2016) of rainforest Banksieae (Proteaceae).
Fossil collections, preparation and photography
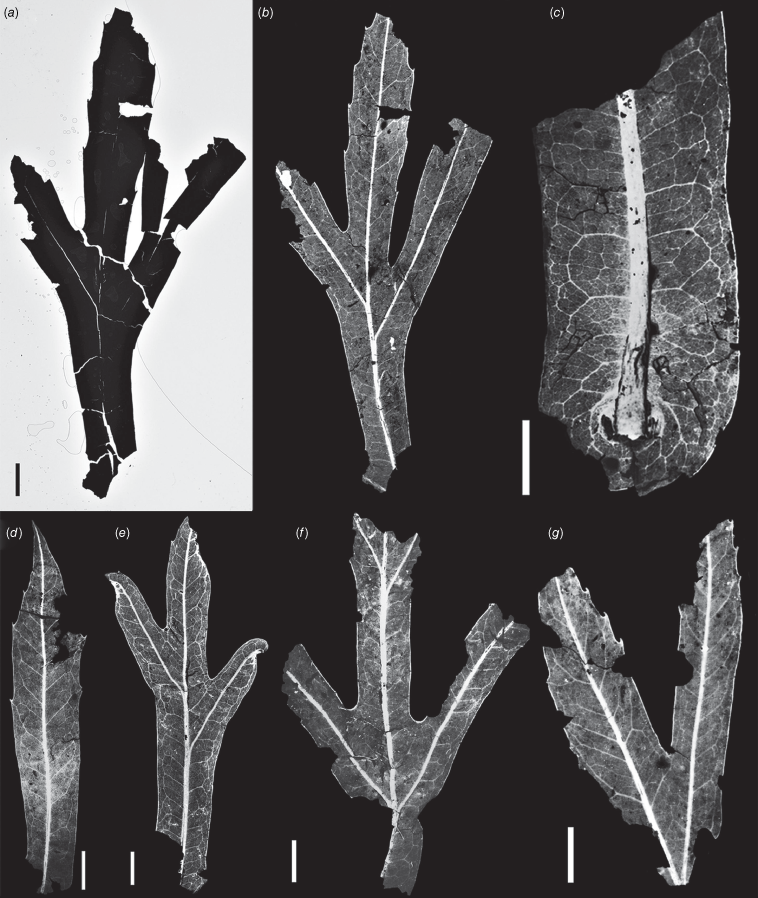
Christophel et al. (1987) detailed the methods used to recover fossils from the Anglesea sediments and prepare them for analysis. Most of the leaves that are the subject of the present study exist as mummifications that were recovered from disaggregated clay matrix and mounted in 80% Karo corn syrup solution between glass slides, after first cutting out a small fragment of leaf to obtain a cuticle sample for microscopy. These specimens, originally given Anglesea (AN) numbers, are now registered (with P numbers) in the Palaeobotany Collection of Museum Victoria (MV), where they were examined by the first author. One leaf specimen, P257421 (AN2342) (Fig. 1), was illustrated by Christophel et al. (1987, their fig. 7C) as ‘lobed Proteaceae’ and more recently by Greenwood and Christophel (2005, their fig. 6b) as ‘aff. Megahertzia’. As explained by Christophel et al. (1987), although this leaf is one of many from Anglesea that are opaque, its venation fluoresces by using a yellow filter and appropriate lighting, and thus could be imaged.
Megahertzia paleoamplexicaulis leaf specimens. All except (a) are scans by R. S. Hill, University of Adelaide, of D. C. Christophel photographs that were obtained using yellow filtering and fluorescence to show venation; all such images are held in the D.T. Blackburn collection at the University of Adelaide. Compare features shown in Fig. 3. (a, b) P257421 (holotype); (a) recent image, (b) image first shown as fig. 7C in Christophel et al. (1987). (c) AN1806. Note the amplexicaul leaf base. (d) P257291. Note the spinose, acute apex. (e) P257303. (f) P257294. This specimen is likely to have five lobes (including the terminal lobe). (g) AN1807. Scale bar: 1 cm.
The cuticular morphology of the lobed taxon has previously been described only in brief; Rowett and Christophel (1990) provided an illustration of a stomatal field (their fig. 6D, as ‘aff. Orites’), with a caption that noted the presence of prominent cuticular striations and large multicellular hair bases. Relevant cuticle slides from MV were further examined and photographed using a Nikon E200 microscope with Tucsen 5.0-MP digital camera. Pieces of cuticle from one slide (P231732: leaf AN1807) as well as from material (from AN1806, P257295 and an uncatalogued specimen) provided by D. C. Christophel to the first author were placed on aluminium stubs and carbon coated for scanning electron microscopy (SEM) using a Philips XL 30 FEGSEM operated at 10 kV at the University of Adelaide, South Australia. The leaf specimen designated here as the holotype was photographed at the Queensland Museum by using a Nikon SMZ25 stereomicroscope fitted with a Nikon Digital Sight DS-Ri1 camera linked to NIS-Elements imaging software.
Also available for study in the D. T. Blackburn Palaeobotany Collection, University of Adelaide, is a large collection of Christophel’s photographs (and negatives), as well as SEM stubs with cuticle of both extant and fossil species. Among these leaf images is the one published previously by Christophel et al. (1987) and Greenwood and Christophel (2005).
Leaf-fossil identification and taxonomy
The fossils were compared with extant Megahertzia and other Proteaceae leaf specimens held at BRI (AQ numbers), and cuticle preparations from those and a sample from a plant collected by the first author (see Carpenter 1994). The cuticles were examined and photographed as for the fossils. Taxonomy follows Weston and Barker (2006). No macrofossils have previously been assigned to Megahertzia (Olde 2017).
Taxonomy
Megahertzia paleoamplexicaulis R.J.Carp. & Rozefelds, sp. nov.
Holotype. P257421 (Fig. 1). Middle Eocene Salt Creek Formation, Eastern View Group (Holdgate et al. 2001), Anglesea, Victoria. Museum Victoria Christophel collection, Melbourne, Victoria, Australia. A photograph of the holotype is also held in the D.T. Blackburn Palaeobotany Collection, University of Adelaide.
Fossil leaves conforming closely to M. amplexicaulis in gross morphology and cuticular features.
Images of Megahertzia paleoamplexicaulis cuticle under (a–d, i) light and (e–h, j) scanning electron microscopy. Compare features shown in Fig. 3. (a) Slide P231730, showing stomata on abaxial side within small areoles. (b) Slide P231734. Note trichome base at upper left and sinuous anticlinal walls. (c) Slide P231730. Note trichome base at upper left and relatively straight anticlinal walls. (d) Slide P231730. Note striations. (e) Outer abaxial surface from leaf AN1806, showing stomatal pores and fine striations. (f) Inner abaxial cuticle from slide P231732. Note slightly granular surface mostly associated with normal pavement cells, and evidence of subsidiary cell striations. (g) Inner abaxial cuticle from leaf P257295. Note granulations. (h) Inner abaxial cuticle from an uncatalogued leaf specimen. Note granulations especially associated with pavement cells. (i) P231735, showing trichome base on adaxial side associated with 11 cells. Note striations radiating from base. (j) Inner adaxial cuticle from slide P231732, showing the position of a trichome base associated with five cells at upper left. Scale bars: (a) 200 µm; (b, c) 100 µm; (e) 50 µm; (d, i) 25 µm; (f, g, j) 20 µm; (h) 10 µm.
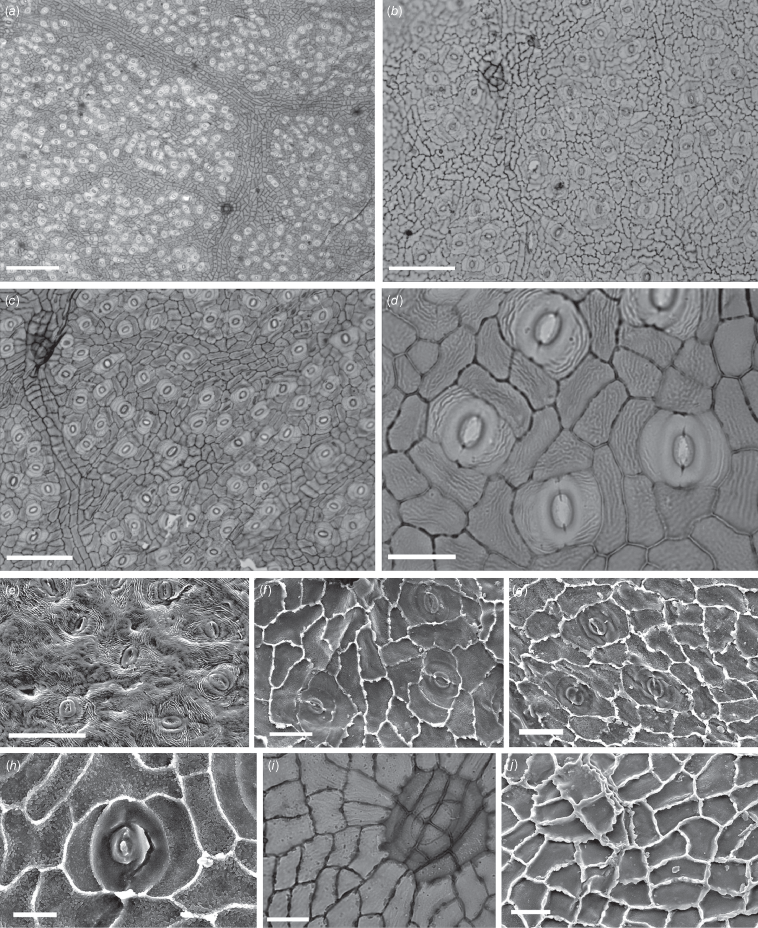
Leaves (Fig. 1) mostly incomplete; amplexicaul, typically 3-lobed, entire or sparsely toothed; simple (i.e. unlobed) leaves unknown. Leaf length up to ~16 cm; individual lobes ~10 cm long and ~1.2–2 cm wide (narrow oblong), apex acute and ±falcate, tip spinose. Teeth mostly in apical half of lobes, ~0.5–1mm long, apically directed, narrow and ±straight apically and basally (spinose) or convex to slightly concave apically and convex basally, sinus angular. Venation brochidodromous to semicraspedodromous where teeth present; ~10 alternately placed secondary veins per side in lobes, arising at ~45° from midvein, with higher angles near leaf bases. Cuticle of both outer leaf surfaces finely striated with smooth to variably granular inner (periclinal) surfaces; abaxial anticlinal cell wall cuticle more-or-less straight to sinuous and slightly buttressed. Stomatal complexes (i.e. guard cells + subsidiary cells) (Fig. 2) confined to abaxial side, randomly oriented within distinct small areoles; brachyparacytic, or very rarely, with 1 of the subsidiary cells divided into 2, guard cell length ~24 µm; inner surface cuticle associated with subsidiary cells occasionally with evidence of striations. Trichome bases (Fig. 2) present but infrequent on both adaxial and abaxial sides, large, associated with multiple underlying epidermal cells (6–30).
Museum Victoria registered specimens, glass-mounted in Karo (registered cuticle slide numbers, if corresponding cuticle prepared and stored at MV, in parentheses): P237339 (P243232), P237493 (P243285), P237500 (P243116), P237527 (P231733), P239637 (P242550), P239643 (P243049), P239644 (P242546), P239645 (P242551), P239653 (P242512), P239654 (P242814), P239656 (P242548), P239666 (P242486), P239689 (P242994), P239691 (P242706), P240210 (P243371), P240221, P257290 (P242808), P257291 (Fig. 1) (P242809), P257292 (P242810), P257293, P257294 (Fig. 1), P257295 (Fig. 2), P257296, P257297, P257298 (P243325), P257299 (P243340), P257300 (P243341), P257301 (P243343), P257302 (P243344 and P231734, Fig. 2), P257303 (Fig. 1), P257858 (P243339). The following MV cuticle slides also show Megahertzia; the location(s) of corresponding leaf specimens are unknown, but photographs of several such leaves with Anglesea specimen numbers (prefix ‘AN’ shown in parentheses) are held at the University of Adelaide: P231730 (Fig. 2), P231732 (Fig. 2) (AN1807, Fig. 1), P231735 (Fig. 2), P242806 (AN1806, Fig. 1, 2), P243120. The location(s) of leaf specimens AN1549, AN1552, AN1769, AN1995 and AN2028 are similarly unknown, but all are accepted in the new species based on photographic evidence. Also stored at MV are a few incomplete leaf impression fossils, including P236490 and P236468a, b (part and counterpart).
First part of epithet from Greek palaio; named to indicate an ancient occurrence of leaves closely conforming to those of the extant M. amplexicaulis.
Christophel et al. (1987) confirmed their placement of the fossil leaves in Proteaceae by reporting the presence of paracytic stomata, an apparent synapomorphy for the family (Carpenter et al. 2005), and complex trichome bases.
Amplexicaul (i.e. stem-clasping) and lobed leaves are a highly distinctive feature of the single extant species of Megahertzia. To our knowledge, no other species of Proteaceae have such leaves; amplexicaul but highly dissimilar leaves occur only among a few species of non-rainforest habitats, perhaps most notably Hakea amplexicaulis R.Br. of Western Australia. This species and closely related taxa in the Prostrata group of Hakea (Barker et al. 1999), which is strongly supported by molecular analyses (Cardillo et al. 2017), all show amplexicaul or auriculate leaves (Barker et al. 1999), a state that is unusual in Hakea and which must be considered synapomorphic for the Prostrata group. Amplexicaul leaves are not known in the putative Hollandaea + Helicia sister group of Megahertzia, which has over 100 species, nor elsewhere in Roupaleae. We are therefore confident that amplexicaul leaves in M. amplexicaulis are autapomorphic among at least the extant members of Roupaleae. The presence of this state in the fossils not only allows close comparison with M. amplexicaulis, but also that this trait can be regarded as diagnostic at genus level, not just for M. amplexicaulis as treated by George and Hyland (1995).
All the fossil Megahertzia leaves are three (rarely five)-lobed, and entire or with sparse and minute teeth, exactly as for many juvenile leaves of M. amplexicaulis, although the modern foliage may be larger and may have more prominent teeth (Fig. 3). However, the smaller leaf size of the fossils may in part be due to collection biases; Rowett and Christophel (1990) noted that leaves were particularly brittle and difficult to recover as wholly or mostly intact specimens, but that some were observed to exceed 30 cm in length. This is approximately twice the length of specimens examined in the current study, and consistent in size with some extant specimens held at BRI.
Images of extant Megahertzia foliage (a, b), and cuticle, under (c–e, h) light and (f, g, i) scanning electron microscopy. Compare features shown in Fig. 1 and 2. (a) Note amplexicaul leaf bases (courtesy G. Sankowsky, Tolga, Qld). (b) AQ020534. Note lobes, spinose teeth and the acute apex of the lobe at upper right. (c) AQ645333. Note the stomata within obvious areoles. (d) AQ645333. Note cuticular striations; a trichome base is located near centre. (e) AQ645333. Note cuticular striations. (f) Outer abaxial surface, showing stomatal pores and striations. (g) Inner abaxial cuticle associated with a stomate. Note granulations. (h) AQ645333, showing obvious striations. (i) Outer adaxial cuticle showing a trichome base at lower left with radiating striations. Scale bars: (c) 200 µm; (d) 100 µm; (i) 50 µm; (e, h) 25 µm; (f, g) 20 µm.

The cuticle of M. amplexicaulis (Fig. 3) (as ‘Orites code #752’) was described by Carpenter (1994). It shows quite rare trichome bases that are associated with numerous (commonly eight) basal epidermal cells, sinuous to buttressed anticlinal cell-wall cuticle, prominent and finely striated outer cuticle surfaces and granular inner cuticle surfaces. As has been demonstrated (Fig. 2), the same features are found in the fossil cuticles, although there is typically less evidence of adaxial striations and inner granulations, and some specimens show many more cells associated with the trichome bases (compare these features in Fig. 2 and 3). There is also variation in epidermal pavement cell anticlinal wall outlines, with some specimens showing more-or-less straight walls, and others sinuous and slightly buttressed walls. This variation is likely to reflect litter input to depositional sites from different light levels within the local Anglesea vegetation, because it is well known that, in shade, these cells will have more undulated outlines than do those exposed to full sun (Watson 1942; Kürschner 1997; Dunn et al. 2015). In conclusion, we regard all the above differences as minor, and moreover note that among species of all Proteaceae genera studied by the first author, only M. amplexicaulis has a cuticular morphology that is consistent with that of the fossils. On this basis, referral of the fossil leaves to the extant species is possible, but this option was rejected in consideration of the massive time separation and absence of supporting evidence. Of possible future relevance here is that Christophel (1984, p. 184) mentioned that two proteaceous inflorescences of unknown affinities had been recovered from the Anglesea clays, as well as a Grevillea-like follicle. Unfortunately, the fate of these fossils is unknown, but it is noted that the fruits of M. amplexicaulis are readily comparable with those of at least the larger-fruited species of Grevillea (Makinson 2000), being 30–35 mm long dehiscent follicles (George and Hyland 1995).
In Proteaceae, the foliage of many taxa changes throughout development, including transitions from lobed juvenile and new shoot forms to simple adult leaves (Johnson and Briggs 1975). However, beyond the seedling stage, only a few extant species, in subfamily Grevilleoideae, are known to produce types of pinnately lobed leaves with occasional teeth similar to those in Megahertzia, notably Orites excelsus R.Br. (tribe Roupaleae) and Athertonia diversifolia (C.T.White) L.A.S.Johnson & B.G.Briggs (Johnson and Briggs 1975) of tribe Macadamieae subtribe Virotiinae. None of these species has remotely similar leaf cuticular morphology to that of Megahertzia, with for example O. excelsus and A. diversifolia having obviously waxy surfaces (Carpenter 1994). The fossil-leaf species Maslinia grevilleoides D.T.Blackburn from the Eocene Maslin Bay site in South Australia is of a size similar to Megahertzia leaves and is also lobed with sparse, small teeth (Blackburn 1981). Unfortunately, the single specimen was described as lacking a base, and its cuticular features were not clearly illustrated. However, Blackburn made no mention of large trichome bases, and instead described (Blackburn 1981, p. 19) ‘indistinct’ trichome bases ‘with one or two hair base support cells’ that are ‘frequently associated with stomates’. Such trichome bases do not occur in Megahertzia. Another similar lobed and toothed taxon from the Early to Middle Eocene of New Zealand is ‘cf. Orites excelsa’ (Pole 1994). These specimens cannot be closely compared with Megahertzia because they lack cuticle, but they appear to be distinct in having acute leaf bases. The Early Eocene Patagonian Lomatia occidentalis (Berry) Frenguelli also lacks cuticular preservation, but is anyway distinct in having imparipinnate or pinnatisect, clearly serrate leaves with lateral leaflets or lobes incised nearly to the midvein (Frenguelli 1943; Gonzalez et al. 2007).
Discussion
The past distribution of Megahertzia
As well as at Anglesea, Megahertzia-type cuticle (‘Proteaceae sp. 7’) occurs in the mid-Eocene Hotham Heights foliar assemblage of south-eastern Victoria (Carpenter et al. 2004, their fig. 56). This assemblage represents a rainforest-derived flora very similar to that which was present at Anglesea, sharing Athertonia- and Musgravea-like cuticles, Ebenaceae, Gymnostoma, and abundant evidence of Lauraceae, Cunoniaceae and Elaeocarpaceae. An even greater past distribution of Megahertzia is indicated by fossil occurrence records of the Megahertzia-like (Macphail 1999) pollen morphospecies Proteacidites latrobensis W.K.Harris. This species was not included in the phylogenetic study of Proteaceae pollen by Sauquet et al. (2009), but its distinctive small sexine spines (Harris 1965, 1966; Stover and Partridge 1973) could be homologous with the supratectal spines recorded by Sauquet et al. (2009) for M. amplexicaulis. Proteacidites latrobensis was described by Harris (1965, 1966) from the Princetown Member (Dilywn Formation – Otway Basin) of western Victoria (Early Eocene: e.g. McGowran et al. 2004) and has since been widely recorded in Cenozoic sediments from across Australia (e.g. Stover and Partridge 1973, 1982; Nott and Owen 1992; Alley et al. 1996; Itzstein-Davey 2004), including Tasmania (Hill and Macphail 1983), the Northern Territory (Truswell and Marchant 1986) and the Pilbara of north-western Australia (Macphail and Stone 2004). Records of P. latrobensis from Queensland include from the Eocene of the Yaamba Basin (Foster 1982), the Early–Late Miocene inland of Mackay (Beeston 1994) and the Early Miocene sequence of Sandy Cape, K’gari (Fraser Island) (Wood 1986). Adding to our inference that Megahertzia had an extensive past distribution, P. latrobensis is also found within the Neogene of New Zealand (Raine et al. 2011).
Megahertzia paleoamplexicaulis adds to the list of proteaceous taxa that belong to lineages that were widespread in the past, but are now confined to the northern Queensland Wet Tropics and Gondwana Rainforests of Australia World Heritage Area of north-eastern New South Wales and south-eastern Queensland. These taxa include Athertonia (Rozefelds 1992, 1995), Eidothea (Douglas and Hyland 1995; Rozefelds et al. 2005), Musgraveinae (Christophel 1984; Carpenter et al. 2016) and Orites excelsus (Carpenter and Jordan 1997).
Christophel et al. (1987) found Megahertzia leaves in two of the six lenses they studied, the ‘Mesophyll Lens’, and ‘Site II Lens A’. Only a few of their highly diverse leaf taxa have been identified, but both lenses are known to also host the extinct cycad Pterostoma R.S.Hill as well as Ebenaceae, Cunoniaceae and Elaeocarpaceae, Musgraveinae and abundant and diverse Lauraceae (Christophel et al. 1987; R. J. Carpenter, pers. obs.). As well as these taxa (except Pterostoma), the greater Anglesea flora reportedly has numerous other taxa with extant relatives in tropical and subtropical rainforests of eastern Australia, including Bowenia, large-leaved Podocarpus Labill., Gymnostoma, Brachychiton Schott & Endl. and Myrtaceae (Christophel et al. 1987; Christophel and Greenwood 1988; Christophel 1994). Undoubtedly, the disappearance from much of Australia of mesic rainforest elements, including Megahertzia and the other Proteaceae discussed above, was a consequence of a general decline over millions of years in the availability of equably wet and warm conditions, especially since the final separation of Australia from Antarctica (reviewed by Byrne et al. 2011).
Lobed-only leaves of Anglesea Proteaceae
Only two Proteaceae leaf types appear to be represented in the Anglesea Karo-mounted glass slide collection at MV, namely, M. paleoamplexicaulis and Banksieaefolia cuneata (R.S.Hill & Christophel) R.J.Carp., G.J.Jord. & R.S.Hill, and both these leaf species are only known from lobed leaves. In their nearest extant relatives (i.e. M. amplexicaulis and Musgraveinae), lobed leaves mostly occur in juvenile and understorey foliage, whereas adult and canopy leaves are simple (Johnson and Briggs 1975; George and Hyland 1995; Hyland 1999a, 1999b; R. J. Carpenter, pers. obs.). Of further interest is that a third Anglesea lobed leaf-type was reported by Rowett and Christophel (1990), although without illustrations or other description, and not being identified. It is probable that this leaf type was Athertonia-like, as fossil cuticles extracted from Anglesea dispersed cuticle preparations are very similar to Athertonia diversifolia cuticle (R. J. Carpenter, unpubl. data). As for the other two taxa, adult foliage in Athertonia is simple and juvenile foliage is lobed (Johnson and Briggs 1975; Weston 1995).
It is possible that lobed-only leaves among these three taxa of Anglesea Proteaceae are an artefact of collection bias; Christophel (1994) remarked that most of the leaves recovered from Anglesea are architecturally indistinct, elliptical to ovate notophylls, and, therefore, adult leaves belonging to these proteaceous taxa may be present in the collections, but not recognised as such. However, arguing against this is the possibility that Christophel specifically searched for these leaves, because he would have been aware of the simple adult types of the nearest extant relatives, including Musgraveinae, inflorescence fossils of which also occur in the Anglesea sediments (Christophel 1984). Also, because the routine procedure for curating Anglesea leaf fossils involved cuticle preparations (Christophel et al. 1987), any simple leaves with proteaceous cuticular features should have been identified.
If correct, our inference of lobed-only leaves from three distinct tribes of subfamily Grevilleoideae is of ecological and evolutionary interest. Large, lobed leaves are a distinctive feature of numerous other tropical and subtropical Proteaceae, including Darlingia F.Muell., Placospermum and Heliciopsis Sleumer (Johnson and Briggs 1975), but this leaf state is very unusual among co-occurring rainforest angiosperms. At the outer canopy level, unlobed and much smaller leaf forms predominate, likely serving the function of reducing heat loads (Leigh et al. 2017). We propose that simple leaves evolved convergently in rainforest Proteaceae as post-Eocene environments became hotter and less equable with the northward drift of the Australian continent towards the Equator. In the much higher-latitude Eocene (and probably earlier) forests exclusively lobed-leaves sufficed whatever the size of the trees, and conferred a range of advantages, including efficient light capture (e.g. Givnish 1979).
Conclusions
The fossils described here showed that the sole living species of Megahertzia in the Wet Tropics is the last vestige of a lineage that formerly occurred in southern Australia 40 million years ago, and was likely to be widespread in rainforest vegetation across Australia and New Zealand. This clear fossil evidence highlights the globally significant importance of our remaining rainforests, and the imperative to conserve them in the face of increasing threats.
Dedication
This paper is dedicated to our teachers David Christophel (1947–2018) and Bob Hill, who provided opportunities for us to undertake studies in paleobotany.
Acknowledgements
We are very grateful to Museum Victoria staff, especially Tim Ziegler and Rolf Schmidt, for facilitating R. J. Carpenter’s visit to MV, and Bob Hill for providing the original Anglesea leaf-fossil images held in the D.T. Blackburn Palaeobotany Collection, University of Adelaide. Access to extant specimens and laboratory space for R. J. Carpenter were provided by BRI. Garry Sankowsky kindly provided a photo of Megahertzia foliage. The reviewers and editorial board members (especially Brendan Lepschi) are thanked for providing useful suggestions for improving the manuscript.
References
Alley NF, Krieg GW, Callen RA (1996) Early Tertiary Eyre Formation, lower Nelly Creek, southern Lake Eyre Basin, Australia: palynological dating of macrofloras and silcrete, and palaeoclimatic interpretations. Australian Journal of Earth Sciences 43, 71-84.
| Crossref | Google Scholar |
Barker RM, Haegi L, Barker WR (1999) Hakea. Flora of Australia 17B, 31-170.
| Google Scholar |
Basinger JF, Christophel DC (1985) Fossil flowers and leaves of the Ebenaceae from the Eocene of southern Australia. Canadian Journal of Botany 63, 1825-1843.
| Crossref | Google Scholar |
Beeston JW (1994) Tertiary palynology in the Mount Coolon and Riverside areas. Queensland Geology 6, 127-179.
| Google Scholar |
Blackburn DT (1981) Tertiary megafossil flora of Maslin Bay, South Australia: numerical taxonomic study of selected leaves. Alcheringa: An Australasian Journal of Palaeontology 5, 9-28.
| Crossref | Google Scholar |
Byrne M, Steane DA, Joseph L, Yeates DK, Jordan GJ, Crayn D, Aplin K, Cantrill DJ, Cook LG, Crisp MD, Keogh JS, Melville J, Moritz C, Porch N, Sniderman JMK, Sunnucks P, Weston PH (2011) Decline of a biome: evolution, contraction, fragmentation, extinction and invasion of the Australian mesic zone biota. Journal of Biogeography 38, 1635-1656.
| Crossref | Google Scholar |
Cardillo M, Weston PH, Reynolds ZKM, Olde PM, Mast AR, Lemmon EM, Lemmon AR, Bromham L (2017) The phylogeny and biogeography of Hakea (Proteaceae) reveals the role of biome shifts in a continental plant radiation. Evolution 71, 1928-1943.
| Crossref | Google Scholar |
Carpenter RJ (1994) Cuticular morphology and aspects of the ecology and fossil history of North Queensland rainforest Proteaceae. Botanical Journal of the Linnean Society 116, 249-303.
| Crossref | Google Scholar |
Carpenter RJ (2012) Proteaceae leaf fossils: phylogeny, diversity, ecology and austral distributions. The Botanical Review 78, 261-287.
| Crossref | Google Scholar |
Carpenter RJ, Jordan GJ (1997) Early Tertiary macrofossils of Proteaceae from Tasmania. Australian Systematic Botany 10, 533-563.
| Crossref | Google Scholar |
Carpenter RJ, Hill RS, Greenwood DR, Partridge AD, Banks MA (2004) No snow in the mountains: Early Eocene plant fossils from Hotham Heights, Victoria, Australia. Australian Journal of Botany 52, 685-718.
| Crossref | Google Scholar |
Carpenter RJ, Hill RS, Jordan GJ (2005) Leaf cuticular morphology links Platanaceae and Proteaceae. International Journal of Plant Sciences 166, 843-855.
| Crossref | Google Scholar |
Carpenter RJ, Jordan GJ, Hill RS (2016) Fossil leaves of Banksia, Banksieae and pretenders: resolving the fossil genus Banksieaephyllum. Australian Systematic Botany 29, 126-141.
| Crossref | Google Scholar |
Christophel DC (1980) Occurrence of Casuarina megafossils in the Tertiary of south-eastern Australia. Australian Journal of Botany 28, 249-259.
| Crossref | Google Scholar |
Christophel DC (1984) Early Tertiary Proteaceae: the first floral evidence for the Musgraveinae. Australian Journal of Botany 32, 177-186.
| Crossref | Google Scholar |
Christophel DC, Lys SD (1986) Mummified leaves of two new species of Myrtaceae from the Eocene of Victoria, Australia. Australian Journal of Botany 34, 649-662.
| Crossref | Google Scholar |
Christophel DC, Harris WK, Syber AK (1987) The Eocene flora of the Anglesea locality, Victoria. Alcheringa: An Australasian Journal of Palaeontology 11, 303-323.
| Crossref | Google Scholar |
Conran JG, Christophel DC (1999) A redescription of the Australian Eocene fossil monocotyledon Petermanniopsis (Lilianae: aff. Petermanniaceae). Transactions of the Royal Society of South Australia 123, 61-67.
| Google Scholar |
Conran JG, Christophel DC, Scriven L (1994) Petermanniopsis angleseaënsis Gen. & Sp. Nov.: an Australian fossil net-veined monocotyledon from Eocene Victoria. International Journal of Plant Sciences 155, 816-827.
| Crossref | Google Scholar |
Douglas AW, Hyland BPM (1995) Eidothea. Flora of Australia 16, 127-129.
| Google Scholar |
Dunn RE, Strömberg CAE, Madden RH, Kohn MJ, Carlini AA (2015) Linked canopy, climate, and faunal change in the Cenozoic of Patagonia. Science 347, 258-261.
| Crossref | Google Scholar |
Foster CB (1982) Illustrations of early Tertiary (Eocene) plant microfossils from the Yaamba Basin, Queensland. Geological Survey of Queensland 381, 1-33.
| Google Scholar |
Frenguelli J (1943) Proteáceas del Cenozoico de Patagonia. [Proteaceae from the Cenozoic of Patagonia.]. Notas del Museo de La Plata 8, 201-213 [In Spanish].
| Google Scholar |
George AS, Hyland BPM (1995) Megahertzia. Flora of Australia 16, 355.
| Google Scholar |
Gonzalez CC, Gandolfo MA, Zamaloa MC, Cúneo NR, Wilf P, Johnson KR (2007) Revision of the Proteaceae macrofossil record from Patagonia, Argentina. The Botanical Review 73, 235-266.
| Crossref | Google Scholar |
Greenwood DR (1987) Early Tertiary Podocarpaceae: megafossils from the Eocene Anglesea locality, Victoria, Australia. Australian Journal of Botany 35, 111-133.
| Crossref | Google Scholar |
Harris WK (1965) Basal Tertiary microfloras from the Princetown area, Victoria, Australia. Palaeontographica B 115, 75-106.
| Google Scholar |
Harris WK (1966) News and notes. Taxon 15, 332-336.
| Crossref | Google Scholar |
Hill RS (1978) Two new species of Bowenia Hook. ex Hook. f. from the Eocene of eastern Australia. Australian Journal of Botany 26, 837-846.
| Crossref | Google Scholar |
Hill RS (1980) Three new Eocene cycads from eastern Australia. Australian Journal of Botany 28, 105-122.
| Crossref | Google Scholar |
Hill RS, Carpenter RJ (1991) Evolution of Acmopyle and Dacrycarpus (Podocarpaceae) foliage as inferred from macrofossils in south-eastern Australia. Australian Systematic Botany 4, 449-479.
| Crossref | Google Scholar |
Hill RS, Christophel DC (1988) Tertiary leaves of the tribe Banksieae (Proteaceae) from south-eastern Australia. Botanical Journal of the Linnean Society 97, 205-227.
| Crossref | Google Scholar |
Hill RS, Macphail MK (1983) Reconstruction of the Oligocene vegetation at Pioneer, northeast Tasmania. Alcheringa: An Australasian Journal of Palaeontology 7(4), 281-299.
| Crossref | Google Scholar |
Hill RS, Pole MS (1992) Leaf and shoot morphology of extant Afrocarpus, Nageia and Retrophyllum (Podocarpaceae) species, and species with similar leaf arrangement from Tertiary sediments in Australasia. Australian Systematic Botany 5, 337-358.
| Crossref | Google Scholar |
Hill RS, Scriven LJ (1998) Falcatifolium (Podocarpaceae) macrofossils from Paleogene sediments in south-eastern Australia: a reassessment. Australian Systematic Botany 12, 711-720.
| Crossref | Google Scholar |
Hill RS, Hill KE, Carpenter RJ, Jordan GJ (2019) New macrofossils of the Australian cycad Bowenia and their significance in reconstructing the past morphological range of the genus. International Journal of Plant Sciences 180, 128-140.
| Crossref | Google Scholar |
Holdgate GR, Smith TAG, Gallagher SJ, Wallace MW (2001) Geology of coal-bearing Palaeogene sediments, onshore Torquay Basin, Victoria. Australian Journal of Earth Sciences 48, 657-679.
| Crossref | Google Scholar |
Hyland BPM (1999a) Musgravea. Flora of Australia 17b, 170-172.
| Google Scholar |
Hyland BPM (1999b) Austromuellera. Flora of Australia 17b, 173-175.
| Google Scholar |
Itzstein-Davey F (2004) A spatial and temporal Eocene palaeoenvironmental study, focusing on the Proteaceae family, from Kambalda, Western Australia. Review of Palaeobotany and Palynology 131, 159-180.
| Crossref | Google Scholar |
Johnson LAS, Briggs BG (1975) On the Proteaceae: the evolution and classification of a southern family. Botanical Journal of the Linnean Society 70, 83-182.
| Crossref | Google Scholar |
Kürschner WM (1997) The anatomical diversity of recent and fossil leaves of the durmast oak (Quercus petraea Lieblein/Q. pseudocastanea Goeppert): implications for their use as biosensors of palaeoatmospheric CO2 levels. Review of Palaeobotany and Palynology 96, 1-30.
| Crossref | Google Scholar |
Leigh A, Sevanto S, Close JD, Nicotra AB (2017) The influence of leaf size and shape on leaf thermal dynamics: does theory hold up under natural conditions? Plant, Cell & Environment 40, 237-248.
| Crossref | Google Scholar |
Macphail MK (1999) Palynostratigraphy of the Murray Basin, inland southeastern Australia. Palynology 23, 197-240.
| Crossref | Google Scholar |
Macphail MK, Stone MS (2004) Age and palaeoenvironmental constraints on the genesis of the Yandi channel iron deposits, Marillana Formation, Pilbara, northwestern Australia. Australian Journal of Earth Sciences 51, 497-520.
| Crossref | Google Scholar |
Makinson RO (2000) Grevillea. Flora of Australia 17A, 1-460.
| Google Scholar |
Mast AR, Willis CL, Jones EH, Downs KM, Weston PH (2008) A smaller Macadamia from a more vagile tribe: inference of phylogenetic relationships, divergence times, and diaspore evolution in Macadamia and relatives (tribe Macadamieae; Proteaceae). American Journal of Botany 95, 843-870.
| Crossref | Google Scholar |
McGowran B, Holdgate GR, Li Q, Gallagher SJ (2004) Cenozoic stratigraphic succession in southeastern Australia. Australian Journal of Earth Sciences 51, 459-496.
| Crossref | Google Scholar |
Nott JF, Owen JAK (1992) An Oligocene palynoflora from the middle Shoalhaven catchment N.S.W. and the Tertiary evolution of flora and climate in the southeast Australian highlands. Palaeogeography, Palaeoclimatology, Palaeoecology 95, 135-151.
| Crossref | Google Scholar |
O’Dowd DJ, Brew CR, Christophel DC, Norton RA (1991) Mite-plant associations from the Eocene of southern Australia. Science 252, 99-101.
| Crossref | Google Scholar |
Olde PM (2017) A checklist of fossil names in extant genera of the Proteaceae. Telopea 20, 289-324.
| Crossref | Google Scholar |
Onstein RE, Jordan GJ, Sauquet H, Weston PH, Bouchenak-Khelladi Y, Carpenter RJ, Linder HP (2016) Evolutionary radiations of Proteaceae are triggered by the interaction between traits and climates in open habitats. Global Ecology and Biogeography 25, 1239-1251.
| Crossref | Google Scholar |
Pole M (1994) An Eocene macroflora from the Taratu Formation at Livingstone, North Otago, New Zealand. Australian Journal of Botany 42(3), 341-367.
| Crossref | Google Scholar |
Raine JI, Mildenhall DC, Kennedy EM (2011) New Zealand fossil spores and pollen: an illustrated catalogue, 4th edn. Miscellaneous series number 4. (GNS Science). Available at http://pal.gns.cri.nz/catalog [Verified January 2023]
Rozefelds AC (1992) The subtribe Hicksbeachiinae (Proteaceae) in the Australian Tertiary. Memoirs of the Queensland Museum 32, 195-202.
| Google Scholar |
Rozefelds AC (1995) Miocene Wilkinsonia fruits (Hicksbeachiinae, Proteaceae) from the base of the Yallourn Formation, Latrobe Valley, Victoria. Papers and Proceedings of the Royal Society of Tasmania 129, 59-62.
| Crossref | Google Scholar |
Rozefelds AC, Christophel DC, Alley NF (1992) Tertiary occurrence of the fern Lygodium (Schizaeaceae) in Australia and New Zealand. Memoirs of the Queensland Museum 32, 303-322.
| Google Scholar |
Rozefelds AC, Dettmann ME, Clifford HT (2005) Xylocaryon lockii F.Muell. (Proteaceae) fruits from the Cenozoic of south eastern Australia. Kanunnah 1, 91-102.
| Google Scholar |
Rozefelds AC, Dettmann ME, Clifford HT, Carpenter RJ (2017) Lygodium (Schizaeaceae) in southern high latitudes during the Cenozoic. A new species and new insights into character evolution in the genus. Review of Palaeobotany and Palynology 247, 40-52.
| Crossref | Google Scholar |
Sauquet H, Weston PH, Anderson CL, Barker NP, Cantrill DJ, Mast AR, Savolainen V (2009) Contrasted patterns of hyperdiversification in Mediterranean hotspots. Proceedings of the National Academy of Sciences 106, 221-225.
| Crossref | Google Scholar |
Stover LE, Partridge AD (1973) Tertiary and Late Cretaceous spores and pollen from the Gippsland Basin, southeastern Australia. Proceedings of the Royal Society of Victoria 85, 237-286.
| Google Scholar |
Stover LE, Partridge AD (1982) Eocene spore-pollen from the Werillup Formation, Western Australia. Palynology 6, 69-96.
| Crossref | Google Scholar |
Truswell EM, Marchant NG (1986) Early Tertiary pollen of probable Droseracean affinity from Central Australia. Special Papers in Palaeontology 35, 163-178.
| Google Scholar |
Watson RW (1942) The effect of cuticular hardening on the form of epidermal cells. New Phytologist 41, 223-229.
| Crossref | Google Scholar |
Weston PH (1995) Athertonia. Flora of Australia 16, 413-415.
| Google Scholar |
Weston P, Barker N (2006) A new suprageneric classification of the Proteaceae, with an annotated checklist of genera. Telopea 11, 314-344.
| Crossref | Google Scholar |
Wood GR (1986) Late Oligocene to early Miocene palynomorphs from GSQ Sandy Cape 1-3R. Geological Survey of Queensland Publication 387, 1-28.
| Google Scholar |


