Revision of the Pultenaea setulosa species complex (Fabaceae: Mirbelieae) including 14 new species
Russell L. Barrett A B * , James A. R. Clugston
A B * , James A. R. Clugston  A , David E. Albrecht C D , Lesley Elkan A , John R. Hosking E , Peter C. Jobson A , Seanna F. McCune A , Andrew E. Orme A , Ruth L. Palsson
A , David E. Albrecht C D , Lesley Elkan A , John R. Hosking E , Peter C. Jobson A , Seanna F. McCune A , Andrew E. Orme A , Ruth L. Palsson  E , Matthew A. M. Renner
E , Matthew A. M. Renner  A , Catherine Wardrop A and Peter H. Weston A
A , Catherine Wardrop A and Peter H. Weston A
A
B
C
D
E
Abstract
A taxonomic revision of the Pultenaea setulosa species complex (Fabaceae, tribe Mirbelieae) is presented. Prior to this study, P. setulosa Benth. was broadly circumscribed as a single, morphologically variable species. Here, we present evidence supporting the recognition of 18 species, 14 of which are new to science. Pultenaea setulosa is recircumscribed as a morphologically uniform taxon endemic to the Marlborough region in south-east Queensland. We reinstate Pultenaea boormanii H.B.Will., Pultenaea campbellii Maiden & Betche and Pultenaea lapidosa Corrick from synonymy of P. setulosa and describe an additional 14 new species: Pultenaea acanthocalyx R.L.Barrett & Clugston, Pultenaea corrickiae R.L.Barrett, Orme & Clugston, Pultenaea estelleae R.L.Barrett & Clugston, Pultenaea farmeriana R.L.Barrett, Orme & P.H.Weston, Pultenaea hoskingii R.L.Barrett & Clugston, Pultenaea imminuta R.L.Barrett & S.F.McCune, Pultenaea murrayi R.L.Barrett, Pultenaea palssoniae R.L.Barrett & Clugston, Pultenaea praetermissa R.L.Barrett & Albr., Pultenaea purdieae R.L.Barrett & Clugston, Pultenaea renneri R.L.Barrett & Clugston, Pultenaea venusta R.L.Barrett & Orme, Pultenaea westonii R.L.Barrett & Clugston and Pultenaea woolcockiorum R.L.Barrett & Clugston. Sixteen of these species are endemic to New South Wales, one to Queensland and one to Victoria. All taxa are described and illustrated, and habitats and conservation status are discussed. Two additional related species, Pultenaea procumbens A.Cunn. and P. setigera A.Cunn. ex Benth. are recircumscribed. Pultenaea setigera is reinstated here, known from the type collection made in 1822 and is possibly extinct. Lectotypes are selected for the names Pultenaea boormanii, P. campbellii, P. procumbens, P. setigera and P. setulosa.
Keywords: Australia, legumes, New South Wales, plant taxonomy, Queensland, rare species, short-range endemics, speciation, systematics, Victoria.
Introduction
Significant numbers of new plant species continue to be described in Australia every year (Barrett 2015; Preece et al. 2015; Taxonomy Decadal Plan Working Group 2018). New species are recognised from across the continent, from well populated regions to the remotest parts of Australia (Wilde and Barrett 2021; Collins et al. 2022; Curtis et al. 2022; Barrett et al. 2022a, 2022b; Barrett and Barrett 2023; O’Donnell et al. 2023; Wilson and Barrett 2023). The publication of taxonomic revisions and flora treatments can have both positive and negative effects on the likelihood of new species being discovered. There is a shortage of systematic botanists in Australia relative to the known number of undescribed species, therefore attention is most commonly focussed on groups that have not been revised in recent decades or for which no flora treatment exists. Conversely, publication of revisions can promote further collection effort in groups that previously lacked identification keys and such research may assist in the recognition of further new species, e.g. Calectasia R.Br. (Barrett and Dixon 2001; Barrett and Barrett 2015) and Poranthera Rudge (Halford and Henderson 2005; Barrett 2012; Barrett and Barrett 2015).
In the last 30 years, Pultenaea Sm. species in New South Wales have been treated in two editions of the Flora of New South Wales (Weston 1991; Weston and de Kok 2002) and a series of taxonomic revisions (de Kok and West 2002, 2003, 2004). An assessment that diversity within Pultenaea is well documented and most of the species diversity described would usually be considered fair, given the recency and comprehensiveness of the most recent revisions in the genus. However, new identification tools and reference texts can stimulate collecting effort that increases the specimen resource, thereby enabling concepts to be revisited and refined, based on more representative material and larger sample sizes. A case in point is presented by Pultenaea glabra Benth., long regarded as a morphologically variable species. Using combined morphological and molecular evidence from a considerable resource of specimens built since the early 2010s, Renner et al. (2022) comprehensively demonstrated that this complex actually represents eight discrete species, of which six were new. The revision of Pultenaea glabra refuted the hypothesis that intraspecific morphological variation explained the diversity of morphotypes attributed to that species (as proposed by de Kok and West 2002), and leaves the possibility open that the same hypothesis does not adequately explain morphological variation within other inferred variable Pultenaea species so defined by those authors. Additional morphologically distinct entities have also been overlooked (e.g. Telford et al. 2022).
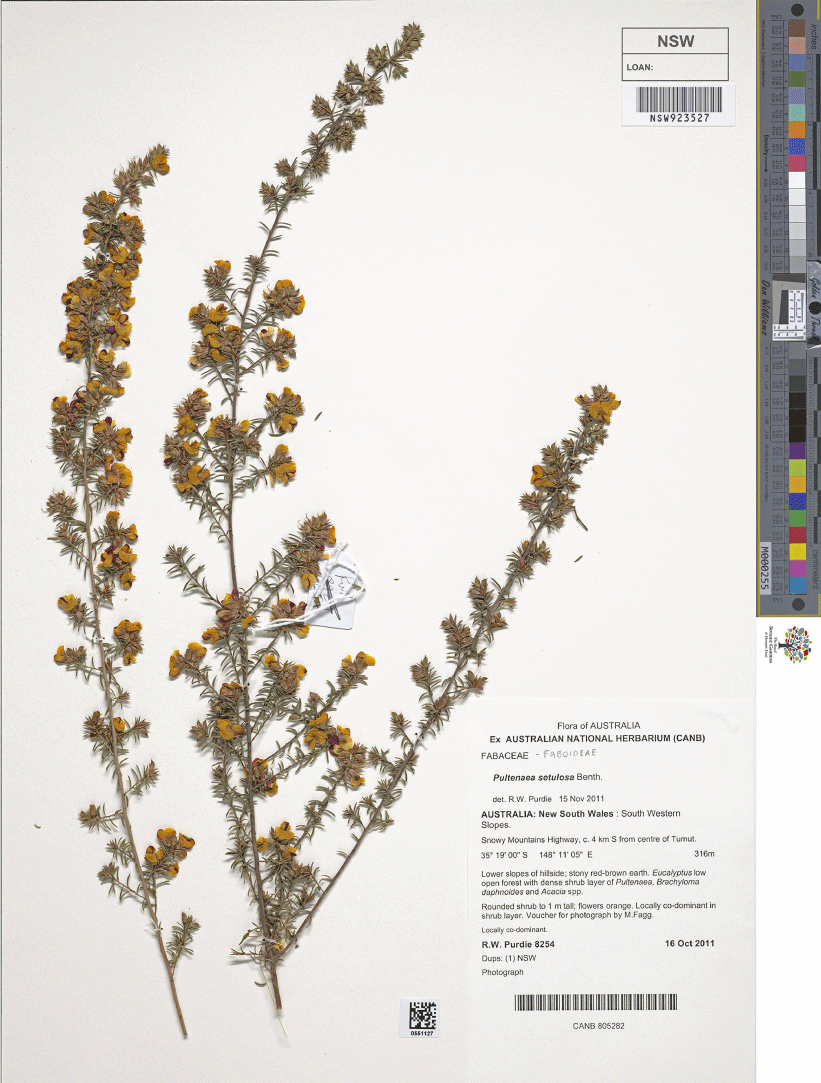
Pultenaea setulosa Benth. is one such morphologically variable species. This species was circumscribed by de Kok and West (2002) to include a suite of relatively isolated populations distributed from near Omeo in Victoria to Mount Kaputar and the Northern Tablelands of northern New South Wales, and subsequently disjunct to the Wide Bay district of south-east Queensland. De Kok and West (2002) circumscribed P. setulosa based on the following characters: (1) the presence of a tuft of hairs at the apex of the ovary; (2) margins of bracteolar stipules entire and dark red; (3) bracteoles with free stipules; (4) leaves with a long or short acuminate to aristate point; (5) leaves with one main vein, sometimes with a false marginal vein present but not palmate; and (6) leaves linear, on branchlets mostly >3.5 mm long. As we define the group, all species have narrow, mucronate leaves that are involute throughout length (though sometimes flat), with only a central vein and only P. imminuta has a style inserted centrally on the ovary; all other species have distinctly eccentric styles. We note that one entity included under P. setulosa by de Kok and West (2002), described here as P. renneri R.L.Barrett & Clugston, is morphologically anomalous in this group and clearly more closely related to P. glabra. The morphological affinity of some members of this group to P. procumbens A.Cunn. has not been fully appreciated and this is discussed below where appropriate.
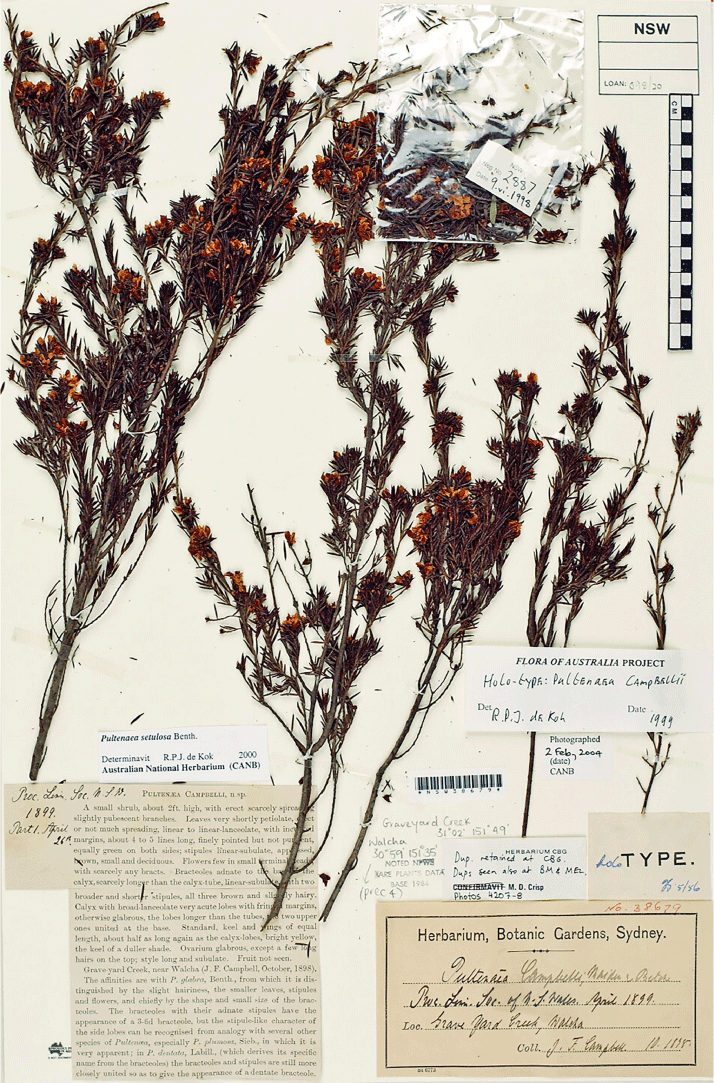
Pultenaea setulosa was described by Bentham (1864) based on specimens from the Broad Sound district in south-east Queensland. Three species (P. campbellii, P. boormanii and P. lapidosa) were included in synonymy of P. setulosa by de Kok and West (2002). Pultenaea campbellii Maiden & Betche was described by Maiden and Betche (1899) based on specimens collected from near Walcha, on the edge of the New England Tableland. Pultenaea boormanii H.B.Will. was described by Williamson (1922) from several collections made between Dubbo and Gilgandra. Corrick (1994a) described P. lapidosa Corrick based on wide-ranging collections from northern Victoria to central New South Wales, including specimens previously ascribed to P. sp. F of Weston (1991). Each of these species have generally been recognised as distinct in recent taxonomic reviews and floras (Thompson 1958, 1961; Stanley 1983; Hacker 1990; Weston 1991; Corrick 1996; Elliot and Jones 2002).
Recent field studies have suggested that several geographically discrete populations are morphologically uniform and exhibit fixed character states. We undertook a comprehensive morphological revision of the group, primarily based on herbarium material held by CANB, NE, NSW and SYD, and complemented by fieldwork. In contrast to the revision by de Kok and West (2003), we recognise P. boormanii, P. campbellii and P. lapidosa as species distinct from P. setulosa, along with 14 additional species.
To date, relationships within Pultenaea have only been assessed to a limited extent, with a molecular phylogeny generated by Orthia et al. (2005) establishing that P. setulosa sensu de Kok and West (2003) is a member of Pultenaea clade B described in the paper, with a close relationship with P. subspicata strongly supported based on plastid DNA data. The voucher for this sample is R.P. de Kok 716 (CANB); however, we consider this specimen to be morphologically closer to P. procumbens than other taxa included in this study, therefore the phylogenetic relationships of any of the 18 taxa recognised here were not tested by Orthia et al. (2005), or Barrett et al. (2021) in a review of available data. This situation has partially been remedied through a Genomics of Australian Plants phylogeny phase 2 project, with target capture data being newly generated for six representatives of this group (Clugston, Barrett, Murphy, Renner, Weston, Cook, Jobson, Lepschi and Crisp, unpubl. data). This phylogenomic data has demonstrated that the group of species treated here as the P. setulosa complex is not entirely a natural one, as might be expected from the highly variable morphology within the species complex. Although all the species in this complex are placed into the same broad phylogenetic clade, at least 10 additional species (including P. procumbens sens. lat.) are also placed in this same clade and some of these additional species are more closely related to individual species in this complex than members of the complex are to each other.
Generic circumscription
Orthia et al. (2005) suggested that generic circumscriptions in tribe Mirbelieae may need major revision. Barrett et al. (2021) analysed publicly available plastid data for the trnL-F marker and concluded that additional sequence data would likely resolve relationships sufficiently to allow the core of Pultenaea to be maintained, and these species belong within the core Pultenaea clade. We have demonstrated, by undertaking representative sampling with the MyBaits Angiosperms353 kit, that this approach is justified, resolving a very similar topology with strong support for critical nodes (Clugston et al., unpubl. data). A paper providing a recircumscription of Pultenaea sens. lat. has been submitted.
Materials and methods
The following descriptions are based on examination of herbarium specimens at CANB, NE, NSW and SYD using light microscopy, and field observations of approximately half the species recognised here by at least one of the authors. Type specimens of related species have been studied first-hand at CANB, MEL and NSW. Images of additional type specimens have been examined on JSTOR Plants (https://plants.jstor.org/, accessed November 2022), individual herbarium online databases when not present in JSTOR Plants or as digital images provided by individual herbaria (AD, BRI, K and MEL).
We note that stipules commonly take on different forms on the branchlets, within the inflorescence (subtending pedicels) and on the calyx, and are therefore described separately in the species descriptions for each position. Petiole length measurements include the often-wrinkly pulvinus-like structure at the distal end of the petiole, which is probably below the point of abscission.
Results and discussion
Morphological examination of herbarium specimens has resulted in the recognition of 18 species in the complex that were all included under a broad definition of Pultenaea setulosa by de Kok and West (2002). As some of these entities were found to share particular characteristics with the Pultenaea procumbens species complex, P. procumbens and P. setigera (here reinstated) are also each defined in a strict sense to enable direct comparison to all of the species recognised in this work. All the species keyed out here will usually lead to P. setulosa in the key of de Kok and West (2002), including a strict definition of P. procumbens, de Kok and West’s (2002) concept of the latter species referring to a broader species complex that requires recircumscription. However, two species recognised here (P. corrickiae and P. palssoniae), have ovaries that are hairy throughout (or almost so), contrasting with the definition of this group by de Kok and West (2002) and we presume that each case could represent a reversal to an ancestral character state.
Pultenaea procumbens and P. setigera – two allied species
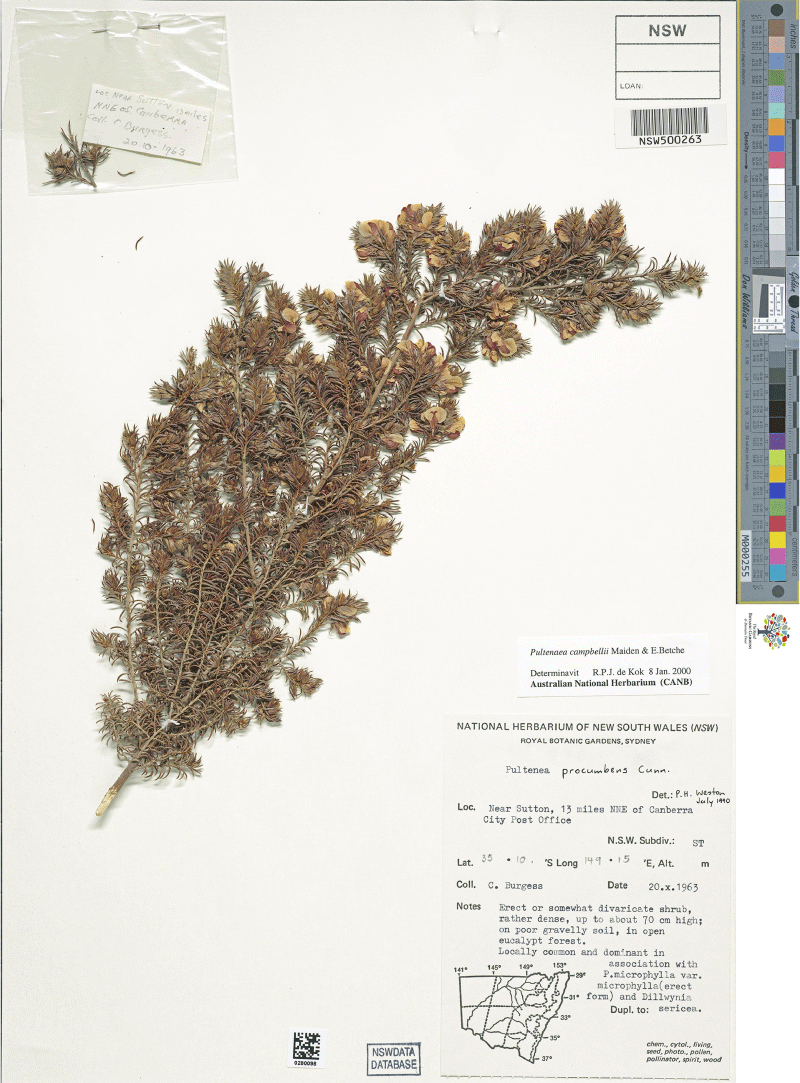
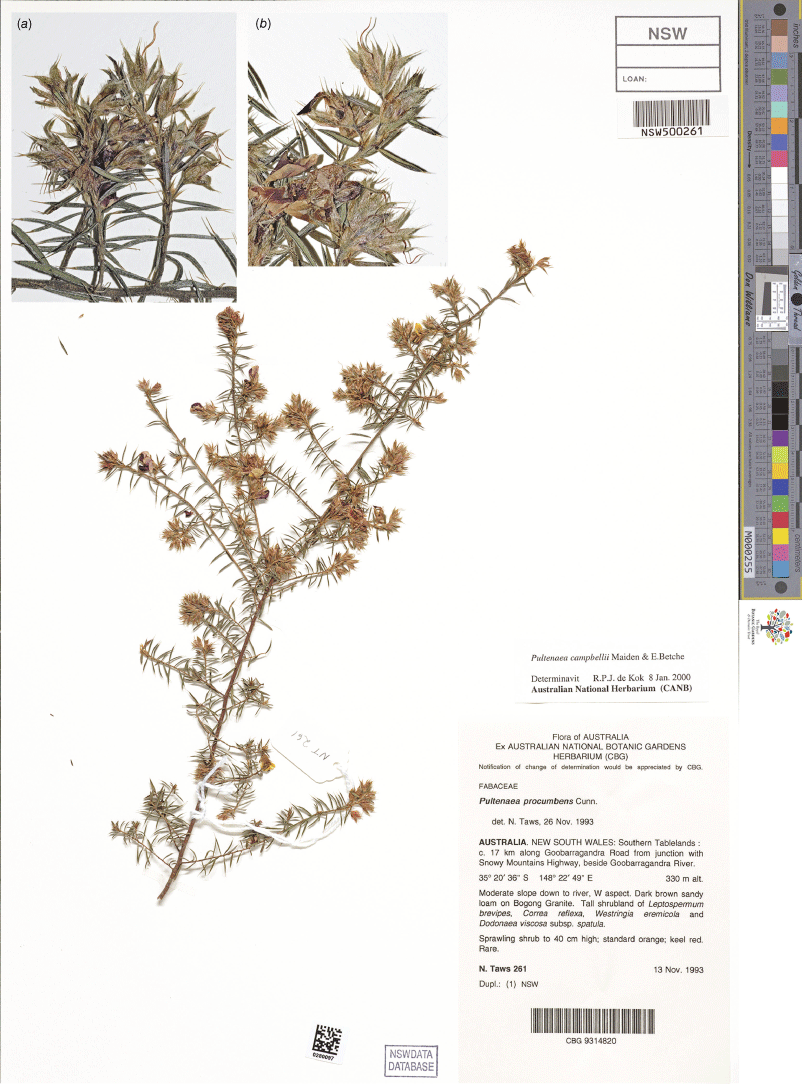
While not considered part of the Pultenaea setulosa complex sensu de Kok and West (2002), P. procumbens sens. lat. appears to be morphologically more similar to some of the entities included in the complex by de Kok and West (2002) than P. setulosa sens. str., therefore the circumscription must be addressed logically. However, based on our preliminary studies, Pultenaea procumbens sensu de Kok and West (2002) evidently also represents a species complex, perhaps including as many as 10 morphological entities that will require future revision. Molecular data place one of the entities in the P. procumbens complex as sister to P. corrickiae (Clugston et al., unpubl. data).
In considering the identity of Pultenaea procumbens, the fact that a type for the name had not been clearly identified in the literature became apparent, even though the relevant type specimens were examined by both M.D. Crisp and R.P.J de Kok at different times. Orchard and Orchard (2020) located type material for both P. procumbens and P. setigera from the Cox’s River and ENE of Bathurst respectively. While Bentham (1837) had validated the name P. setigera, Bentham (1864) treated the two names as synonyms, creating a broad concept for the name P. procumbens that has persisted to the present. However, the two type specimens are morphologically distinct and we choose to treat these as separate species. We restrict Pultenaea procumbens to the type collection made 200 years ago, a second collection made 82 years ago and two collections from 1963. Based on photographs provided to the authors by R. Medd, the species is apparently extant to the NW of Bathurst and likely under-collected. Pultenaea setigera is only known from the type collection made over 200 years ago and the species is therefore potentially extinct.
We formally designate lectotypes for both names. Novel descriptions are provided for P. setigera based on the isolectotype specimen held at NSW and P. procumbens based on a single collection from ‘Tarana’, the only collection held at NSW that has similar morphology to the lectotype. Two additional specimens matching the type are held at CANB. This represents a very different definition of P. procumbens relative to the circumscription accepted by de Kok and West (2002), and P. setigera is reinstated as a distinct species. As noted above, a detailed revision of the complex is required to determine the most appropriate circumscriptions of numerous additional entities previously included under this name, with most other entities in this complex currently being unnamed.
Our provision of descriptions of P. procumbens and P. setigera establishes a clear reference point for distinguishing all the entities recognised within the P. setulosa complex and a basis for future revision of the broader P. procumbens complex.
Geological affinities
Geological associations are described here based primarily on the Geological Survey of New South Wales Simplified Surface Geology Map (Table 1; https://datasets.seed.nsw.gov.au/dataset/nsw-1500k-simplified-surface-geology, accessed October 2022).
| Species | Distribution | Stratum | Characteristics | |
|---|---|---|---|---|
| P. acanthocalyx | Snowy Mountains, Southern Tablelands, NSW | Silurian (419–443 Ma) sedimentary rocks | Slate and quartzite | |
| P. boormanii | Dubbo to Pilliga, Central W and NW Slopes of NSW | Quaternary (0–2 Ma) alluvial and colluvial deposits | Sand and gravel | |
| P. campbellii | Northern Tablelands of NSW | Various | Granites, adamellites, sedimentary rock, acid volcanic soils and basalt | |
| P. corrickiae | Snowy Mountains to Wagga Wagga, SW Slopes of NSW | Ordovician (443–491 Ma) sedimentary rocks | Interbedded quartz-rich sandstone, siltstone and mudstone | |
| P. estelleae | N of Yass, S Tablelands of NSW | Ordovician (443–491 Ma) sedimentary rocks | Interbedded quartz-rich sandstone, siltstone and mudstone | |
| P. farmeriana | S of Orange, Central Tablelands of NSW | Ordovician (443–491 Ma) sedimentary rocks | Interbedded quartz-rich sandstone, siltstone and mudstone | |
| P. hoskingii | Between Woolomin and Nundle, NW Slopes and N Tablelands of NSW | Devonian (359–419 Ma) sedimentary rocks | Conglomerate, sandstone, siltstone and mudstone | |
| P. imminuta | Near Boggabri, NW Slopes of NSW | Permian (252–299 Ma) sedimentary rocks | Sandstone, siltstone and mudstone | |
| P. lapidosa | Northern central Victoria | Middle or Late Devonian (359–419 Ma) sedimentary rocks | Conglomerates and interbedded coarse sandstone | |
| P. murrayi | SE of Canberra, S Tablelands of NSW | Ordovician (443–491 Ma) sedimentary rocks | Interbedded quartz-rich sandstone, siltstone and mudstone | |
| P. palssoniae | Nandewar Range, N Tablelands of NSW | Cenozoic (0–66 Ma) mafic volcanics | Volcanic rocks | |
| P. praetermissa | Near Sutton, S Tablelands of NSW | Ordovician (443–491 Ma) sedimentary rocks | Interbedded quartz-rich sandstone, siltstone and mudstone | |
| P. purdieae | Snowy Mountains, S Tablelands of NSW | Devonian (359–419 Ma) sedimentary rocks; Silurian (419–443 Ma) sedimentary rocks | Conglomerate sandstone, siltstone and mudstone; slate and quartzite | |
| P. procumbens | Megalong Valley to Lithgow, Central Tablelands of NSW | Carboniferous I-type granites (300–359 Ma) igneous source rocks | Granite tors with quartz, felspar and biotite | |
| P. renneri | Bucketty area, Central Coast of NSW | Triassic (201–252 Ma) sedimentary rocks | Quartz-lithic to quartz-rich sandstone with conglomerate, mudstone and siltstone | |
| P. setigera | NW of Bathurst, Central Tablelands of NSW | Carboniferous I-type granites (300–359 Ma) igneous source rocks | Granite tors with quartz, felspar and biotite | |
| P. setulosa | Wide Bay area, SE Queensland | Late Devonian (359–384 Ma) metamorphic rocks | Serpentinite | |
| P. venusta | NW of Albury, SW Slopes of NSW | Ordovician (443–491 Ma) sedimentary rocks | Interbedded quartz-rich sandstone, siltstone and mudstone | |
| P. westonii | Orange to Bathurst, Central Tablelands of NSW | Cenozoic (0–66 Ma) mafic volcanics; Devonian (359–419 Ma) sedimentary and volcanic rocks | Basalt lava flows; associated with volcanic rocks | |
| P. woolcockiorum | W of Cowra, Central W Slopes of NSW | Late Devonian (359–384 Ma) sedimentary rocks | Quartz-rich sandstone, sandstone, siltstone, mudstone and pebbly conglomerate units |
Speciation patterns and processes
New South Wales presents a complex evolutionary landscape dominated by the Great Dividing Range, with complex topography, geology and microclimates. Each of these factors may be strong drivers of speciation, particularly during extended periods of climatic fluctuation (Hill 1998). The extent of these climatic fluctuations and the degree to which habitats remain isolated following climate shifts become the primary drivers of speciation at a landscape level (Truswell 1993; Garrick et al. 2004; Elliott et al. 2023). There is evidence that many Australian plant species were once widespread along the Great Dividing Range, this wide distribution likely enabled by a consistently high topography, fertile soils and consistent rainfall patterns (Hnatiuk and Maslin 1988; Crisp et al. 2001). However, Australia’s history includes many periods of aridification, sometimes associated with broader global glaciation patterns. These periods of fluctuation cause habitats to repeatedly expand and contract but actual extinction levels appear to be much lower than for northern hemisphere environments, especially those affected by glaciation, with a larger number of refugial environments persisting in SE Australia (Endo et al. 2015). Restricted moisture availability can cause relatively rapid restriction in the environmental envelope suitable for particular species. Such contractions can lead to rapid and localised speciation events (Byrne 2008; Williams et al. 2011).
Understanding broadscale patterns of evolution in complex landscapes requires detailed datasets, and larger genera such as Acacia Mill., Eucalyptus L’Hér., Banksia L.f., Hibbertia Andrews and Pultenaea offer unique opportunities for undertaking such studies (Crisp et al. 2004, 2005; Bui et al. 2017; Skeels and Cardillo 2017; Thornhill et al. 2019; Renner et al. 2021a, 2022; Barrett et al. 2021; Hua et al. 2022).
In complex landscapes such as the Great Dividing Range and associated geological formations, isolation patterns can be strong over short distances (Garrick et al. 2004; Pepper et al. 2014). Determining whether gene flow has indeed ceased between populations that may only be separated by a few kilometres can be challenging (Rutherford et al. 2016; Rutherford 2020). However, that such restricted gene flow at relatively small scales is more common than previously considered is becoming more apparent (Chase et al. 2023; O’Donnell et al. 2023; Orel et al. 2023a, 2023b; Wilson et al. 2023). Population-level molecular markers applied to the Pultenaea glabra complex demonstrated a clear demarcation of species within the greater Blue Mountains (Renner et al. 2022). Taxa within the P. glabra complex were recognised as distinct species as there are strong morphological correlations with this genetic isolation. This high degree of genetic isolation and structure is likely to be reflected in many other groups in the same region. Other plant groups may not show such strong morphological distinction but may yet be readily definable based on patterns of genetic distinction (Wilson et al. 2023).
Geological complexity may separate otherwise similar environments and be sufficient for speciation to occur. For example, species that require cooler climates and might persist at high altitudes at more inland locations, such as Mount Canobolas and Mount Kaputar but may well be genetically distinct from plants found at similar altitudes in neighbouring regions with different geological constituents (commonly basalt v. sandstone). Such a driver of speciation has been inferred in eucalypts (Bell and Nicolle 2020). Species occurring specifically on ranges and peaks often have narrow climatic niches (Murphy et al. 2019) and may also have specific edaphic requirements. Therefore, species that adapt to rocky habitats are often highly specialised and more subject to speciation over shorter distances, and this can also make these more vulnerable to climatic fluctuations (Shay et al. 2021).
Rocky substrates of differing geological components vary in water and nutrient holding capacities and therefore influence species distributions (Hahm et al. 2014, 2019a, 2019b). Sandstone is variable in porosity but known to hold large volumes of water and these environments are therefore able to provide a more stable climate, particularly in gorges and ravines or at the bases of cliffs, especially when south-facing, avoiding extremes of heat from direct sunshine (Pickett and Alder 1997; Keith 2004; Washington and Wray 2011). This is demonstrated effectively by the persistence of the Wollemi pine (Wollemia nobilis W.G.Jones, K.D.Hill & J.M.Allen) in deep canyons in the Blue Mountains (Mackenzie et al. 2022). The persistence of the Wollemi pine and other species occupying similar refugial microclimates through many millions of years of climate change can largely be attributed to the water holding capacity of sandstone, as water can filter through the sandstone into the lower parts of these canyons, maintaining relative stability in these locations regardless of aboveground rainfall patterns, provided that on average, there is sufficient rainfall to keep the internal aquifers charged (Mackenzie et al. 2022). This is naturally only a very small proportion of average annual rainfall in these rocky environments as most rain dissipates very quickly. These internal aquifers can therefore be maintained under much lower rainfall conditions (Manna et al. 2016).
Our understanding of speciation across the Blue Mountains is accumulating slowly (e.g. Rutherford et al. 2016; Rutherford 2020). Research to date points to a concentration of short-range endemic species being present in the Blue Mountains in particular (Baker and Corringham 2004; Bell 2008; Hager and Benson 2010; Martyn 2018; Renner et al. 2022; Wilson et al. 2023) and many more likely await discovery. The identification of a number of locally endemic species occurring in the Western Slopes of New South Wales is interesting in this study. Pultenaea, the Australian bush peas, represents an excellent group for inferring patterns of speciation along the Great Dividing Range in New South Wales and adjacent regions of Victoria and Queensland, as there is a large number of species endemic to this region (de Kok and West 2002, 2003, 2004). These shrubs have similar form and are therefore subject to similar selective pressures, sharing the same pollination systems, similar ecological requirements and similar responses to disturbance, therefore the evolutionary history likely reflects the underlying environmental conditions that have driven the speciation. This is reflected in the saying that ‘rare species are common where you find them’ and environmentally driven niche specialisation may also result in co-occurring rare species (Lesica et al. 2006).
There are likely to have been underlying factors simultaneously driving speciation in multiple plant groups, with primary factors being time and distance but ultimately, the degree to which genetic isolation is reflected in morphology varies greatly. Determining the significance of morphological variation in large, complex genera such as Pultenaea is therefore particularly challenging and requires careful assessment. As there are no set characters for defining species in general, each case must be considered individually and each species pair assessed against each other (De Queiroz 2007). Morphological variation therefore becomes only one point reflecting landscape-level diversity. We must increasingly look to genetic markers to understand plants within landscape and evolutionary contexts (e.g. Renner et al. 2022; Wilson et al. 2023).
Taxonomy
Two keys are presented below, one to sterile specimens and one to fertile specimens.
| 1 | Branchlets glabrous, long-ribbed below petioles, almost winged; leaf lamina strongly discolourous with a dark purplish median stripe abaxially ...P. renneri Branchlets pubescent, shortly ribbed below petioles, not at all winged; leaf lamina weakly discolourous, green abaxially ...2 |
| 2 | Branchlet stipules fused for 1/20–1/6 of length ...3 Branchlet stipules fused for (1/5–)1/3–3/4 of length ...10 |
| 3 | Leaf lamina ±linear, mucro 0.8–2.2 mm long (and leaves to 0.9 mm wide), hairs on leaf lamina with swollen bases ...P. acanthocalyx Leaf lamina usually narrow–lanceolate to narrowly ovate (sometimes ±linear), mucro 0.1–0.8(–1.6 but then leaves ≥1 mm wide) mm long, hairs on leaf lamina without swollen bases ...4 |
| 4 | Leaf lamina glabrous or sparsely pubescent when young; stipules each 0.2–0.3 mm wide at sinus ...5 Leaf lamina moderately to densely pubescent; stipules each 0.4–0.5 mm wide at sinus ...6 |
| 5 | Branchlets with straight, appressed, matted hairs, the longest to 0.4 mm long; leaves with petiole 0.4–0.8 mm long, lamina ±straight along length, concolourous, with scattered hairs when young, margins slightly incurved, mucro to 0.1 mm long ...P. campbellii Branchlets with crisped, curved, matted hairs, the longest to 0.2 mm long; leaves with petiole 0.2–0.3 mm long, lamina curved along length, discolourous, glabrous, margins strongly incurved, mucro 0.3–0.6 mm long ...P. purdieae |
| 6 | Low shrubs at maturity, usually 0.4–0.6 m tall; leaves with lamina narrowly oblong to narrowly ovate or somewhat obovate, margins strongly incurved but not hiding the adaxial surface ...7 Tall shrubs at maturity, usually 0.8–1.5 m tall; leaves with lamina linear or narrow–lanceolate, margins strongly incurved and usually hiding the adaxial surface ...9 |
| 7 | Leaves with petioles 0.3–0.7 mm long, lamina 1-nerved, strongly incurved along length ...P. venusta Leaves with petioles 0.1–0.3 mm long, lamina 3-nerved (at least at base), margins strongly incurved for much of length but ±flat at base ...8 |
| 8 | Procumbent subshrub; branchlets with wavy, matted to ±spreading hairs, the longest to 0.5(–0.8) mm long; leaves 4.3–6.8 mm long, 1.5–2.4 mm wide, with soft, usually spreading hairs to 1.5 mm long on the lamina ...P. procumbens Erect subshrub; branchlets with straight, ±erect hairs, the longest to 0.2 mm long; leaves 2.0–4.1 mm long, 1.0–1.6 mm wide, with ±mostly erect, straight or gently curved, ±stiff hairs to 0.3 mm long on the lamina ...P. setigera |
| 9 | Branchlet hairs, the longest to 0.5 mm long; petioles 0.2–0.4 mm long; leaf lamina narrow–lanceolate, 0.5–1.4 mm wide ...P. corrickiae Branchlet hairs, the longest to 0.1 mm long; petioles 0.4–0.8 mm long; leaf lamina ±linear, 0.4–0.7 mm wide ...P. praetermissa |
| 10 | Leaf mucro 0.1–0.4(–0.5) mm long ...11 Leaf mucro 0.4–1.7 mm long ...16 |
| 11 | Branchlet hairs, the longest commonly to 1 mm long; stipules narrow–lanceolate, fused for 1/5–1/4 of length, 3.2–4.6 mm long, ~0.5 mm wide ...P. setulosa Branchlet hairs, the longest 0.2–0.6 mm long; stipules lanceolate, fused for 1/3–1/2 of length, 1.2–3.1(–3.6 in P. boormanii) mm long, 0.3–0.4 mm wide ...12 |
| 12 | Branchlet stipules with glabrous margins, usually with at least a few small teeth; longest hairs on branchlets 0.5–0.6 mm long ...13 Branchlet stipules with hairy margins, at least in mid-third, usually without small teeth; longest hairs on branchlets 0.2–0.3 mm long ...14 |
| 13 | Leaf lamina ±lanceolate (to oblanceolate), 3.6–5.8 mm long; branchlet hairs straight to curved, erect; stipules brown (almost black when dried) ...P. estelleae Leaf lamina ±linear, 3.9–14 mm long; branchlet hairs slightly crisped or straight, appressed, matted; stipules brown to red–brown ...P. imminuta |
| 14 | Stipules 2.7–3.6 mm long; leaf lamina 0.3–0.6 mm wide ...P. boormanii Stipules 1.3–2.4 mm long; leaf lamina 0.6–1.5 mm wide ...15 |
| 15 | Leaf lamina ±linear, 0.6–1.5 mm wide; stipules 1.3–2.0 mm long, margins with hairs only in mid-third ...P. hoskingii Leaf lamina ±lanceolate, 0.6–1.1 mm wide; stipules 1.7–2.4 mm long, margins with hairs throughout ...P. palssoniae |
| 16 | Leaf lamina 0.4–0.8(–0.9) mm wide, margins strongly incurved, usually hiding the adaxial surface ...17 Leaf lamina 0.9–1.8 mm wide, margins incurved but not hiding the adaxial surface ...19 |
| 17 | Petioles 1.0–1.4 mm long; stipules dark brown to black, with long, appressed hairs ...P. murrayi Petioles 0.6–1.0 mm long; stipules brown to red–brown, glabrous ...18 |
| 18 | Branchlet hairs dense, crisped, persistent; stipules 2.0–3.6 mm long, ~0.4 mm wide at sinus; petiole 0.5–0.7 mm long; leaf lamina 4.7–7.3 mm long, glabrous ...P. farmeriana Branchlet hairs sparse, straight, soon glabrescent; stipules 3.4–4.5 mm long, ~0.6 mm wide at sinus; petiole 0.6–1.0 mm long; leaf lamina 7.5–11.9 mm long, with at least some tubercle-based hairs abaxially ...P. woolcockiorum |
| 19 | Branchlet hairs, the longest to 0.4 mm long; stipules lanceolate, fused for 2/3–3/4 of length, margins and keels with sparse, long hairs ...P. lapidosa Branchlet hairs, the longest to 0.2 mm long; stipules broadly lanceolate, fused for ~1/2 of length, margins glabrous, keels with sparse, long hairs ...P. westonii |
| 1 | Branchlets glabrous; bracteolar stipules absent; keel petal shorter than wing petals ...P. renneri Branchlets pubescent; bracteolar stipules present; keel petal longer than wing petals ...2 |
| 2 | Bracteoles fused to stipules for >1/2 of length ...3 Bracteoles free from stipules or fused only at the very base ...8 |
| 3 | Branchlet stipules 1.3–2.0 mm long; leaf mucro 0.1–0.4 mm long; pedicels 2.3–4.9 mm long, subtended by stipules 1.7–2.1 mm long, 0.4–0.5 mm wide; bracteolar stipules 1.3–1.4 mm long, ~0.3 mm wide at sinus; calyx tube 1.1–2.0 mm long, moderately densely pubescent ...P. hoskingii Branchlet stipules 2.0–5.3 mm long; leaf mucro 0.4–1.7 mm long; pedicels 0.1–1.8 mm long, subtended by stipules 3.9–7.0 mm long, 0.7–1.6 mm wide; bracteolar stipules 2.6–5.1 mm long, 0.6–0.9 mm wide at sinus; calyx tube 2.6–3.8 mm long, glabrous or hairy towards apex ...4 |
| 4 | Flowers ±sessile or on pedicels up to 0.2 mm long; stipules 4.6–5.1 mm long; ovary ~1.9 mm long; bracteoles inserted 0.3–0.5 mm above pedicel ...P. woolcockiorum Flowers on distinct pedicels 0.4–1.8 mm long; stipules 2.6–4.4 mm long; ovary 1.0–1.5 mm long; bracteoles inserted 0.1–0.6 mm below pedicel apex ...5 |
| 5 | Leaf lamina 0.4–0.8(–0.9) mm wide, margins strongly incurved, usually hiding the adaxial surface; pedicels 0.4–0.9 mm long ...6 Leaf lamina 0.9–1.8 mm wide, margins incurved, but not hiding the adaxial surface; pedicels 0.9–1.8 mm long ...7 |
| 6 | Leaf lamina glabrous, 4.7–7.3 mm long, mucro 0.5–0.7 mm long; petioles 0.5–0.7 mm long; branchlet stipules brown to red–brown, glabrous; pedicels subtended by stipules 3.9–4.3 mm long ...P. farmeriana Leaf lamina with at least some tubercle-based hairs abaxially, 6.4–10.7 mm long, mucro 0.6–1.1 mm long; petioles 1.0–1.4 mm long; branchlet stipules dark brown to black, with long, appressed hairs; pedicels subtended by stipules 4.7–5.6 mm long ...P. murrayi |
| 7 | Branchlet hairs, the longest to 0.4 mm long, straight; stipules on branchlets fused for 2/3–3/4 of length; leaf lamina with prominent tubercules at the base of hairs; calyx lobes 2.8–4.3 mm long, upper pair fused for almost 1/2 of length; standard 8.5–9.6 mm long, 5.8–7.2 mm wide ...P. lapidosa Branchlet hairs, the longest to 0.2 mm long, crisped; stipules on branchlets fused for ~1/2 of length; leaf lamina with obscure tubercules at the base of hairs; calyx lobes 4.0–5.5 mm long, upper pair fused for up to 1/8 of length; standard 11.7–13.1 mm long, 8.4–10.7 mm wide ...P. westonii |
| 8 | Branchlet stipules fused for 1/20–1/6 of length ...9 Branchlet stipules fused for 1/3–3/4 of length (1/5–1/4 in P. setulosa) ...16 |
| 9 | Bracteoles inserted 0.1–0.4 mm below pedicel apex, bract-like (scarious, brown), reaching ~1/3 length of calyx; pedicels subtended by stipules 0.8–0.9 mm long; leaf lamina margins only slightly incurved ...P. campbellii Bracteoles inserted 0.7–1.6 mm above pedicel apex, leaf-like (herbaceous, green), reaching 3/4 length of calyx; pedicels subtended by stipules 1.1–3.9 mm long; leaf lamina margins strongly incurved, at least near apex ...10 |
| 10 | Calyx lobes acuminate with a pungent mucro to 1.6 mm long; hairs on abaxial surface of leaves appressed, with small persistent tuberculate bases, leaf mucro 0.8–2.2 mm long ...P. acanthocalyx Calyx lobes acute to acuminate but not or only shortly and bluntly mucronate (mucro to 0.4 mm in P. corrickiae); leaves with hairs lacking tuberculate bases or leaves glabrous, mucro usually 0.2–0.8 mm long (to 1.2 mm long in P. procumbens; rarely to 1.6 mm in P. setigera) ...11 |
| 11 | Pedicels 1.9–3.5 mm long, subtended by stipules 3.2–3.9 mm long; leaf mucro, 0.2–0.3 mm long; staminal filaments 7.5–9.1 mm long; ovary hairy for most of length; style 7.5–8.6 mm long ...P. corrickiae Pedicels 0.5–2.1 mm long, subtended by stipules 1.1–2.3 mm long; leaf mucro 0.2–0.8(–1.6) mm long; staminal filaments 4.4–7.2 mm long; ovary hairy only near apex; style 4.4–6.6 mm long ...12 |
| 12 | Leaf lamina glabrous; pedicels subtended by stipules 1.1–1.4 mm long; bracteoles 1.9–2.8 mm long, glabrous; bracteolar stipules 0.8–1.3 mm long, ~0.4 mm wide at sinus ...P. purdieae Leaf lamina pubescent; pedicels subtended by stipules 1.7–2.3 mm long; bracteoles 2.7–4.1 mm long, pubescent; bracteolar stipules 1.3–2.4 mm long, ~0.2 or 0.7 mm wide at sinus ...13 |
| 13 | Leaf lamina 0.4–0.7 mm wide, linear, indumentum mostly appressed, margins strongly incurved and usually hiding the adaxial surface; calyx lobes 3.9–5.8 mm long, with long, silky, appressed hairs on lamina ...P. praetermissa Leaf lamina 0.7–2.2 mm wide, narrowly oblong to narrowly ovate or somewhat obovate, indumentum mostly spreading to erect, margins strongly incurved at least towards apex but not hiding the adaxial surface; calyx lobes 2.4–4.3 mm long, with long, erect or spreading hairs on lamina ...14 |
| 14 | Branchlet hairs, the longest to 0.6 mm long; petioles 0.3–0.7 mm long; leaf lamina 1-nerved, strongly incurved along length; pedicels 1.5–2.1 mm long; ovary 1.5–1.9 mm long ...P. venusta Branchlet hairs, the longest to 0.2 mm long; petioles 0.1–0.3 mm long; leaf lamina 3-nerved (at least at base), ±flat at base, strongly incurved above; pedicels 0.7–1.6 mm long; ovary 0.8–1.3 mm long ...15 |
| 15 | Procumbent shrub; branchlets with wavy, matted hairs, the longest to 0.5(–0.8) mm long; leaves 4.3–6.8 mm long, 1.5–2.4 mm wide, with soft spreading hairs on the lamina to 1.5 mm long; pedicels 0.9–1.6 mm long, subtended by stipules 2.3–2.6 mm long, 0.8–0.9 mm wide at sinus; upper calyx lobes fused for ~1/2 of length, 3.3–4.1 mm long; ovary 1.2–1.3 mm long ...P. procumbens Erect shrub; branchlets with straight, ±erect hairs, the longest to 0.2 mm long; leaves 2.0–4.1 mm long, 1.0–1.6 mm wide, with ±mostly erect, straight or gently curved, ±stiff hairs on the lamina to 0.3 mm long; pedicels 0.7–0.9 mm long, subtended by stipules 1.7–2.3 mm long, 0.6–0.7 mm wide at sinus; upper calyx lobes fused for ~1/4 of length, 2.4–3.4 mm long; ovary ~0.8 mm long ...P. setigera |
| 16 | Branchlet hairs, the longest to 1 mm long; branchlet stipules fused for 1/5–1/4 of length, 3.2–4.6 mm long, ~0.5 mm wide; pedicels subtended by stipules 2.4–2.8 mm long, ~0.3 mm wide at sinus; bracteoles inserted 1.0–1.4 mm above pedicel apex ...P. setulosa Branchlet hairs, the longest 0.2–0.6 mm long, branchlet stipules fused for 1/3–1/2 of length, 1.2–3.1 mm long (–3.6 mm in P. boormanii), ~0.3 mm wide; pedicels subtended by stipules 0.9–2.3 mm long (–2.8 mm in P. imminuta but then 0.5–0.6 mm wide), 0.4–0.6 mm wide at sinus; bracteoles inserted at or up to 0.6 mm above pedicel apex ...17 |
| 17 | Branchlet stipules with glabrous margins, usually with at least a few small teeth; longest hairs on branchlets 0.5–0.6 mm long ...18 Branchlet stipules with hairy margins, at least in mid-third, usually without small teeth; longest hairs on branchlets 0.2–0.3 mm long ...19 |
| 18 | Leaf lamina ±lanceolate, 3.6–5.8 mm long; branchlet hairs straight to curved, erect; stipules brown (almost black when dried); pedicels 1.0–1.5(–1.9) mm long; bracteoles 2.5–3.6 mm long; calyx tube 2.1–3.6 mm long; calyx lobes 2.7–3.1 mm long; style eccentric on ovary apex ...P. estelleae Leaf lamina ±linear, 3.9–14 mm long; branchlet hairs slightly crisped or straight, appressed, matted; stipules brown to red–brown; pedicels 1.6–2.3 mm long; bracteoles 3.8–6.4 mm long; calyx tube 1.2–1.5 mm long; calyx lobes 3.1–4.4 mm long; style ±central on ovary apex ...P. imminuta |
| 19 | Branchlet stipules 2.7–3.6 mm long; leaf lamina ±linear, 0.3–0.6 mm wide, margins strongly incurved, usually hiding the adaxial surface; standard yellow–orange; ovary hairy in apical 1/2; style 6.3–6.7 mm long ...P. boormanii Branchlet stipules 1.7–2.4 mm long; leaf lamina lanceolate to oblanceolate, 0.6–1.1 mm wide, margins strongly incurved but usually not completely hiding the adaxial surface; standard yellow; ovary hairy throughout; style 4.8–5.0 mm long ...P. palssoniae |
| 10 | Branchlets sparsely hairy; stipules ~3 mm long; leaves sparsely hairy adaxially, surface with a glaucous bloom; pedicels ~3 mm long, sparsely hairy; floral bracteoles ~2/5 length of calyx; calyx lobes with a line of hairs on the medial lamina; standard <3 mm long ...P. mutabilis var. angusta Branchlets glabrous; stipules 1.6–2.3 mm long; leaves glabrous adaxially, surface green; pedicels 1.2–2.0 mm long, glabrous; floral bracteoles ~2/3 length of calyx; calyx lobes glabrous except for short hairs on the margins; standard >5 mm long ...P. renneri |
In addition to the identifying characteristics included in the key above, diagnostic characters for all species are presented in Tables 2–6 to allow direct comparison. A full set of morphological characters comparable to the descriptions is available in a spreadsheet as Supplementary material to maximise reuse of the data.
| Species | Branchlet indumentum | Branchlet stipules | Stipule size | Stipule margins | Petiole length | Inflorescence leaves | |
|---|---|---|---|---|---|---|---|
| P. acanthocalyx | Crisped, spreading, matted hairs to 0.2 mm long | Fused for ~1/8 length | 1.8–3.0 mm long, ~0.2 mm wide | Margins with short hairs throughout | 0.2–0.3 mm long | Not distinct | |
| P. boormanii | Crisped, appressed, matted hairs to 0.3 mm long | Fused for 1/3–1/2 length | 2.7–3.6 mm long, ~0.3 mm wide | Margins ±entire or an occasional tooth-like projection, with a small area of short, crisped hairs | 0.3–0.8 mm long | Not distinct | |
| P. campbellii | Straight, appressed, matted hairs to 0.4 mm long | Fused for 1/8–1/6 length | 1.7–2.3 mm long, ~0.2 mm wide | Margins entire or with a few tooth-like projections, otherwise glabrous | 0.4–0.8 mm long | Not distinct | |
| P. corrickiae | Crisped, spreading to erect, tangled hairs to 0.5 mm long | Fused for 1/10–1/8 length (almost appearing free) | 1.8–3.5 mm long, ~0.5 mm wide | Margins glabrous or with a few long hairs towards apex | 0.2–0.4 mm long | Not distinct | |
| P. estelleae | Straight to curved, spreading to erect hairs ~0.6 mm long | Fused for 1/3(–1/2) length | 1.4–2.3 mm long, ~0.3 mm wide | Margins glabrous, sometimes with a few small teeth | 0.4–0.6 mm long | With dense, long, erect, stiff hairs to 0.4 mm long on both surfaces, 2.8–4.4 mm long, 0.9–1.3 mm wide | |
| P. farmeriana | Crisped, appressed, matted hairs to 0.4 mm long | Fused for 1/2–3/4 length | 2.0–3.6 mm long, ~0.4 mm wide | Margins with scattered tooth-like projections, otherwise glabrous | 0.5–0.7 mm long | Not distinct | |
| P. hoskingii | Crisped, appressed, matted hairs to 0.3 mm long | Fused for ~1/2 length | 1.3–2.0 mm long, ~0.4 mm wide | Margins with short, crisped hairs in mid-third | 0.2–0.8 mm long | Not distinct | |
| P. imminuta | Slightly crisped or straight, appressed, matted hairs to 0.5 mm long | Fused for ~1/3 length | 1.2–3.1 mm long, ~0.3 mm wide | Margins with scattered tooth-like projections, glabrous or with a few hairs | 0.4–0.9 mm long | Not distinct | |
| P. lapidosa | Straight, appressed, pale hairs to 0.4 mm long, glabrescent | Fused for 2/3–3/4 length | 3.1–4.2 mm long, ~0.5 mm wide | Margins with scattered, short tooth-like projections, margins and keels with sparse, long hairs | 0.9–1.4 mm long | Not distinct | |
| P. murrayi | Crisped, matted or appressed, pale hairs to 0.3 mm long | Fused for 1/2–3/5 length | 3.1–4.3 mm long, ~0.5 mm wide | Margins with scattered, short tooth-like projections, margins, keels and lower lamina with long, appressed hairs | 1.0–1.4 mm long | Not distinct | |
| P. palssoniae | Crisped, appressed, matted hairs to 0.2 mm long | Fused for 1/3–1/2 length | 1.7–2.4 mm long, ~0.3 mm wide | Margins with short, crisped hairs | 0.2–0.5 mm long | Not distinct | |
| P. praetermissa | Crisped, appressed, matted hairs ~0.1 mm long | Fused for 1/8–1/6 length | 1.6–3.3 mm long, ~0.4 mm wide | Margins with a few scattered stiff hairs or glabrous | 0.4–0.8 mm long | Not distinct | |
| P. procumbens | Wavy, matted to ±spreading hairs to 0.5(–0.8) mm long | Fused for 1/20 length | 1.7–3.3 mm long, 0.4–0.5 mm wide | Margins with a few scattered stiff hairs in apical 2/3 | 0.2–0.3 mm long | Not distinct | |
| P. purdieae | Crisped, curved and matted hairs to 0.2 mm long | Fused for ~1/8 length | 1.8–2.4(–3.0) mm long, ~0.3 mm wide | Glabrous | 0.2–0.3 mm long | Not distinct | |
| P. renneri | Glabrous | Fused for 1/5–1/4 length | 1.6–2.3 mm long, ~0.2 mm wide | Margins ±entire or an occasional tooth-like projection | 0.4–0.6 mm long | Not distinct | |
| P. setigera | Straight, ±erect hairs to 0.2 mm long | Fused for 1/20 length | 1.5–2.5 mm long, ~0.4 mm wide | Margins with scattered stiff hairs in apical 1/3 | 0.1–0.3 mm long | Not distinct | |
| P. setulosa | Straight or curved, appressed to spreading hairs to 1 mm long | Fused for 1/5–1/4 length | 3.2–4.6 mm long, ~0.5 mm wide | Margins ±entire, with a few fine hairs | 0.3–0.7 mm long | Not distinct | |
| P. venusta | Straight or curved, ±erect hairs to 0.6 mm long | Fused for 1/10–1/8 length | 2.3–3.9 mm long, ~0.4 mm wide | Margins with scattered stiff hairs | 0.3–0.7 mm long | Not distinct | |
| P. westonii | Crisped, appressed to spreading, pale hairs to 0.2 mm long, glabrescent | Fused for ~1/2 length | 2.8–5.3 mm long, ~0.4 mm wide | Margins with scattered, short tooth-like projections, margins glabrous, keels with sparse, long hairs | 0.6–1.3 mm long | Not distinct | |
| P. woolcockiorum | Straight, appressed hairs to 0.4 mm long | Fused for 1/2–3/5 length | 3.4–4.5 mm long, ~0.6 mm wide | Margins with scattered tooth-like projections, otherwise glabrous | 0.6–1.0 mm long | Not distinct |
| Species | Leaf lamina | Leaf lamina width | Leaf lamina margins | Mucro length | Pedicel length | Pedicels | |
|---|---|---|---|---|---|---|---|
| P. acanthocalyx | ±Linear, with appressed hairs to 0.7 mm long abaxially, with small persistent tuberculate hair-bases | 0.4–0.9 mm wide | Margins strongly incurved, commonly hiding adaxial surface at maturity | 0.8–2.2 mm long | 1.7–3.1 mm long | Subtended by stipules 1.9–2.7 mm long, ~0.5 mm wide, partly hiding pedicels; pedicels densely hairy | |
| P. boormanii | ±Linear, with appressed, straight hairs to 0.4 mm long abaxially | 0.3–0.6 mm wide | Margins strongly incurved usually hiding adaxial surface | 0.1–0.2(–0.5) mm long | 1.5–2.5 mm long | Subtended by stipules 1.9–2.3 mm long, 0.6–0.7 mm wide, partly hiding pedicels; pedicels densely hairy | |
| P. campbellii | Lanceolate, with long, straight hairs to 0.4 mm long abaxially | 0.6–0.9(–1.1) mm wide | Margins slightly incurved, not hiding adaxial surface | To 0.1 mm long | 1.0–1.6 mm long | Subtended by stipules 0.8–0.9 mm long, 0.4–0.5 mm wide, not or partly hiding pedicels; pedicels densely hairy | |
| P. corrickiae | Narrow–lanceolate, with somewhat spreading hairs to 0.5 mm long abaxially | 0.5–1.4 mm wide | Margins strongly incurved, partly or completely hiding adaxial surface | 0.2–0.3 mm long | 1.9–3.5 mm long | Subtended by stipules 3.2–3.9 mm long, ~0.6 mm wide, not or partly hiding pedicels; pedicels densely hairy | |
| P. estelleae | ±Lanceolate, with long, appressed hairs on abaxial surface | 0.6–1.1 mm wide | Margins strongly incurved, usually mostly hiding adaxial surface | 0.1–0.2 mm long | 1.0–1.5(–1.9) mm long | Subtended by stipules 1.7–2.2 mm long, ~0.6 mm wide, partly hiding pedicels; pedicels densely hairy | |
| P. farmeriana | ±Linear, glabrous | 0.4–0.7(–0.9) mm wide | Margins strongly incurved, usually hiding adaxial surface | 0.5–0.7 mm long | 0.5–0.9 mm long | Subtended by stipules 3.9–4.3 mm long, 0.7–1.1 mm wide, hiding pedicels; pedicels glabrous | |
| P. hoskingii | ±Linear, with appressed, straight hairs to 0.4 mm long on abaxial surface | 0.6–1.5 mm wide | Margins strongly incurved but not hiding adaxial surface | 0.1–0.4 mm long | 2.3–4.9 mm long | Subtended by stipules 1.7–2.1 mm long, 0.4–0.5 mm wide, partly hiding pedicels; pedicels densely hairy | |
| P. imminuta | Linear, with scattered, appressed, straight hairs to 0.7 mm long abaxially (adaxially only on newest growth), eventually glabrescent | 0.5–0.9(–1.4) mm wide | Strongly incurved, mostly or completely hiding adaxial surface | (0.1–)0.2–0.4 mm long | 1.6–2.3 mm long | Subtended by enlarged stipules 1.6–2.8 mm long, each 0.5–0.6 mm wide at sinus, with short, stiff, with erect hairs on margins, partly hiding pedicels; pedicels densely hairy with hairs appressed to spreading, straight, white | |
| P. lapidosa | ±Linear to narrowly elliptic, long, tubercle-based hairs on abaxial surface | 0.9–1.3 mm wide | Margins incurved but not hiding adaxial surface | 0.8–1.7 mm long | 1.0–1.2 mm long | Subtended by stipules 4.9–6.7 mm long, 1.2–1.3 mm wide, hiding pedicels; pedicels glabrous except at apex | |
| P. murrayi | ±Linear, with very sparse, long, tubercle-based hairs abaxially | 0.5–0.8 mm wide | Margins incurved, ±completely hiding adaxial surface | 0.6–1.1 mm long | 0.4–0.7 mm long | Subtended by stipules 4.7–5.6 mm long, fused for most of length, each ~0.7 mm long at sinus, but enlarged to 1.6 mm wide at broadest point, hiding pedicels | |
| P. palssoniae | Lanceolate to oblanceolate, appressed, hispid hairs to 0.5 mm long on abaxial surface | 0.6–1.1 mm wide | Margins strongly incurved but usually not completely hiding adaxial surface | 0.2–0.4 mm long | 1.7–2.6 mm long | Subtended by stipules 0.9–1.5 mm long, 0.4–0.5 mm wide, not hiding pedicels; pedicels densely hairy | |
| P. praetermissa | ±Linear, often curved along length, with appressed to somewhat spreading long, sometimes curved, stiff hairs to 0.5 mm long abaxially | 0.4–0.7 mm wide | Margins strongly incurved, usually hiding adaxial surface | 0.2–0.6 mm long | 0.5–1.4 mm long | Subtended by stipules 1.8–2.0 mm long, ~0.4 mm wide, partly hiding pedicels; pedicels densely hairy | |
| P. procumbens | Narrowly ovate to somewhat obovate, 3-nerved, ±straight or gently recurved along length, with dense, appressed to spreading or almost erect, gently curved, ±soft hairs to 1.5 mm long | 1.5–2.4 mm wide | Margins ±flat near base, moderately to strongly incurved towards apex but not or only somewhat hiding adaxial surface | 0.3–1.2 mm long | 0.9–1.6 mm long | Subtended by stipules 2.3–2.6 mm long, each 0.8–0.9 mm wide at sinus, hiding pedicels; pedicels moderately hairy with hairs spreading | |
| P. purdieae | ±Lanceolate, glabrous, recurved along length | 0.5–0.7(–1.3) mm wide | Margins usually strongly incurved, commonly hiding adaxial surface | 0.3–0.6 mm long | 1.0–1.9 mm long | Subtended by stipules 1.1–1.4 mm long, ~0.5 mm wide, partly hiding pedicels; pedicels densely hairy | |
| P. renneri | ±Linear, glabrous, with a purplish abaxial stripe | 0.5–0.8 mm wide | Margins moderately to strongly incurved but not hiding adaxial surface | 0.2–0.4 mm long | 1.2–2.0 mm long | Subtended by stipules 1.1–1.3 mm long, ~0.6 mm wide, not hiding pedicels; pedicels glabrous | |
| P. setigera | Narrowly ovate to somewhat obovate, 3-nerved, ±straight or gently curved along length, with dense, ±mostly erect or some hairs spreading, straight or gently curved, ±stiff hairs to 0.3 mm long | 1.0–1.6 mm wide | Margins ±flat near base, moderately to strongly incurved towards apex but not or only somewhat hiding adaxial surface | 0.3–0.6(–1.6) mm long | 0.7–0.9 mm long | Subtended by stipules 1.7–2.3 mm long, 0.6–0.7 mm wide, hiding pedicels; pedicels densely hairy | |
| P. setulosa | ±Linear, appressed to spreading, straight or curved hairs to 0.8 mm long on abaxial surface | 0.4–0.6(–0.8) mm wide | Margins strongly incurved, usually hiding adaxial surface | 0.1–0.3 mm long | 1.1–1.7 mm long | Subtended by stipules 2.4–2.8 mm long, ~0.3 mm wide, not hiding pedicels; pedicels densely hairy | |
| P. venusta | Narrowly oblong to narrowly ovate, curved along length, with dense, spreading to somewhat erect (some almost appressed), sometimes curved, stiff hairs to 0.4 mm long | 0.7–1.2(–1.9 when flattened) mm wide | Margins strongly incurved but not hiding adaxial surface | 0.4–0.8 mm long | 1.5–2.1 mm long | Subtended by stipules 1.9–2.3 mm long, each 0.6–0.7 mm wide, partly hiding pedicels; pedicels densely hairy with hairs erect | |
| P. westonii | Narrowly elliptic, glabrous or hairy near apex on abaxial surface | 0.9–1.8 mm wide | Margins incurved but not hiding adaxial surface | 0.6–1.5 mm long | 0.9–1.8 mm long | Subtended by stipules 5.7–7.0 mm long, 1.3–1.6 mm wide, not hiding pedicels; pedicels glabrous except at apex | |
| P. woolcockiorum | ±Linear, with appressed hairs to 0.4 mm long abaxially | 0.5–0.8 mm wide | Margins strongly incurved, usually mostly hiding adaxial surface | 0.4–0.9 mm long | ±Sessile or on pedicels up to 0.2 mm long | Subtended by stipules 5.6–6.6 mm long, 1.3–1.6 mm wide, hiding pedicels; pedicels glabrous |
| Species | Bracteoles | Bracteole length | Bracteolar Stipules | Calyx tube | Calyx lobes | |
|---|---|---|---|---|---|---|
| P. acanthocalyx | Inserted 0.9–1.2 mm above pedicel, linear, leaf-like, ~3/4 length of calyx, long–mucronate, glabrous or with a few hairs | 2.4–3.7 mm long | 1.4–1.9 mm long, ~0.3 mm wide, with hairs on margins | 1.1–1.7 mm long, sparsely hairy near bracteoles with hairs erect | ±Equal in width, upper pair fused for ~1/4 length; 2.6–4.9 mm long, with pungent mucro to 1.6 mm long, with many long, spreading to erect hairs, densest near margins, with fine, short hairs inside near margins | |
| P. boormanii | Inserted ~0.4 mm above pedicel, linear, leaf-like, to 2/3 length of calyx, mucronate, pubescent | 3.2–5.1 mm long | 1.7–2.1 mm long, ~0.4 mm wide | 1.3–2.0 mm long, moderately to densely hairy with hairs erect | Upper pair broader, fused for ~1/2 length; 3.2–5.7 mm long, with long appressed to ascending hairs except towards apex | |
| P. campbellii | Inserted 0.1–0.4 mm below pedicel apex, linear, bract-like, ~1/3 length of calyx, shortly mucronate, glabrous | 1.7–2.1 mm long | 0.8–1.1 mm long, ~0.2 mm wide | 1.5–2.0 mm long, glabrous | ±Equal in width, upper pair fused for ~1/10 length; 3.0–3.6 mm long, with dense, short, crisped hairs on margins | |
| P. corrickiae | Inserted 1.0–1.4 mm above pedicel with a rib extending down to pedicel apex, linear, leaf-like, 4/5–9/10 lengh of calyx, long–mucronate, hairy throughout | 3.6–4.8 mm long | 1.9–2.2 mm long, ~0.3 mm wide | 2.2–2.8 mm long, densely hairy with hairs erect | Upper pair broader, fused for ~1/3 length; 3.5–4.3 mm long, with many long, spreading to erect hairs throughout | |
| P. estelleae | Inserted immediately at apex of pedicel, linear, leaf-like, ~3/5 length of calyx, weakly mucronate, densely pubescent with hairs erect | 2.5–3.6 mm long | 1.5–1.8 mm long, ~0.3 mm wide | 2.1–3.6 mm long, moderately long hairs erect | Upper pair broader, fused for almost 1/2 length, 2.7–3.1 mm long, with long, straight erect hairs on lamina and dense, short, crisped hairs on margins inside | |
| P. farmeriana | Inserted ~0.3 mm below pedicel apex, linear, bract-like, ~1/2 length of calyx, not mucronate, pubescent in mid-1/3 | 4.1–4.7 mm long, free portion 1.5–2.0 mm long | 3.2–3.5 mm long, ~0.8 mm wide | 2.7–3.3 mm long, glabrous | ±Equal in width, upper pair fused for ~1/3 length, 3.9–5.3 mm long, with long spreading hairs mostly on margins | |
| P. hoskingii | Inserted exactly at pedicel apex, linear, bract-like, 1/2–2/5 length of calyx, mucronate, pubescent except at apex | 2.3–2.7 mm long, free portion 0.8–1.0 mm long | 1.3–1.4 mm long, ~0.3 mm wide | 1.1–2.0 mm long, moderately densely pubescent | Upper pair broader, fused for ~2/3 length, 1.7–3.5 mm long, with long spreading hairs except towards apex and margins with dense, short, crisped hairs | |
| P. imminuta | Inserted at apex or up to 0.6 mm above pedicel apex, appressed at base, linear, leaf-like, reaching length of calyx, mucronate, pubescent | 3.8–6.4 mm long | 1.4–1.8 mm long, each ~0.2 mm wide | 1.2–1.5 mm long, green, moderately densely pubescent | Upper pair broader, fused for almost 1/3 length, 3.1–4.4 mm long, acuminate or narrowly triangular, with long spreading hairs except towards apex, margins with dense, short, crisped hairs inside | |
| P. lapidosa | Inserted ~0.3 mm below pedicel apex, linear, leaf-like, ~2/3 lengh of calyx, long–mucronate, densely long, erect pubescent throughout | 3.5–4.9 mm long, free portion 1.6–2.4 mm long | 3.1–3.2 mm long, ~0.6 mm wide | 2.6–3.3 mm long, glabrous | ±Equal in width, upper pair fused for almost 1/2 length; 2.8–4.3 mm long, acuminate, with many long, spreading to erect hairs | |
| P. murrayi | Inserted at pedicel apex, appressed at base, linear, bract-like, reaching 2/3–1/2 length ofcalyx, not or weakly mucronate, densely pubescent throughout with hairs long, erect | 4.3–5.7 mm long, free portion 1.4–2.4 mm long | 3.8–4.6 mm long, each ~0.9 mm wide at sinus, to 1.5 mm wide below | 2.6–3.8 mm long, glabrous or with short, appressed hairs on tube and long erect or spreading hairs towards apex | ±Equal in width, upper pair fused for up to 3/10 length, 3.5–4.8 mm long, with long, spreading to erect hairs | |
| P. palssoniae | Inserted 0.5–0.6 mm above pedicel apex, linear, leaf-like, almost reaching length of calyx, shortly mucronate, pubescent | 3.3–4.0 mm long | 0.8–1.1 mm long, ~0.2 mm wide | 1.1–1.7 mm long, with dense appressed hairs | ±Equal in width, upper pair fused for ~1/4 length, 2.6–3.6 mm long, with long appressed hairs on lamina and short, crisped hairs on margins | |
| P. praetermissa | Inserted 0.7–1.2 mm above pedicel apex, linear, reduced leaf-like, equal in length to calyx, mucronate, pubescent | 3.4–4.1 mm long | 1.6–2.1 mm long, ~0.2 mm wide | 1.4–1.8 mm long, densely silky, hairs appressed | ±Equal in width, upper pair fused for ~1/4 length, 3.9–5.8 mm long, with long, silky, appressed hairs on lamina and dense, short, crisped hairs on margins inside | |
| P. procumbens | Inserted 1.0–1.2 mm above pedicel but with prominent rib below extending to apex of pedicel, appressed at base, lanceolate (not or scarcely fused to subtending stipules), leaf-like, to ~7/8 length of calyx, mucronate, spreading–pubescent | 3.1–3.5 mm long | 2.3–2.7 mm long, ~0.8 mm wide | 1.5–1.7 mm long, green, almost glabrous or sparsely hairy with hairs erect to spreading | Upper pair ~2× as broad, fused for ~1/2 length, narrowly triangular, 3.3–4.1 mm long, acuminate, with long erect hairs on lamina at base of lower lobes and dense, short, curved hairs on margins inside | |
| P. purdieae | Inserted 0.7–1.1 mm above pedicel apex, linear, leaf-like, ~4/5 length of calyx, short–mucronate, glabrous | 1.9–2.8 mm long | 0.8–1.3 mm long, ~0.4 mm wide | 1.2–1.6 mm long, a few hairs near base | ±Equal in width, upper pair fused for ~1/6 length; 2.5–3.6 mm long, with long, spreading to erect hairs along margins and fine, short hairs inside near margins | |
| P. renneri | Inserted 0.5–0.8 mm above pedicel apex but with a rib extending down to pedicel apex, narrowly triangular, bract-like, 2/3 length of calyx, acuminate, glabrous | 1.6–1.7 mm long | Stipules absent | 2.2–2.4 mm long, glabrous | ±Equal in width, upper pair fused for ~1/5 length; 1.5–1.9 mm long, glabrous except for short, crisped hairs on margins | |
| P. setigera | Inserted 0.9–1.1 mm above pedicel but with prominent rib below extending full length of pedicel, linear, reduced leaf-like, to ~7/8 length of calyx, mucronate, pubescent with hairs erect | 3.3–3.7 mm long | 2.1–2.4 mm long, ~0.7 mm wide | 1.5–1.9 mm long, moderately hairy with hairs erect to spreading | Upper pair ~2× as broad, fused for ~1/4 length, 2.4–3.4 mm long, acuminate, with long erect hairs on lamina and dense, short, curved hairs on margins inside | |
| P. setulosa | Inserted 1.0–1.4 mm above pedicel apex, linear, leaf-like, to 4/5 length of calyx, mucronate, pubescent | 4.2–5.3 mm long | 1.9–2.3 mm long, 0.3–0.4 mm wide | 1.4–2.7 mm long, sparsely pubescent | Upper pair broader, fused for ~1/2 length, 3.3–4.8 mm long, with scattered long spreading hairs on lamina and fine, short, crisped hairs on outer margin | |
| P. venusta | Inserted 1.0–1.6 mm above pedicel but with prominent rib below extending full length of pedicel, linear, reduced leaf-like, ~7/8 length of calyx, mucronate, pubescent with hairs erect | 2.7–3.9 mm long, | 1.3–2.4 mm long, ~0.2 mm wide | 1.4–2.9 mm long, green, densely hairy with hairs erect | Upper pair fused for 1/4–1/2 length, ~2× as broad as lower; 3.4–4.3 mm long, acuminate, with long erect hairs on lamina and dense, short, crisped hairs on margins inside | |
| P. westonii | Inserted ~0.6 mm below pedicel apex, linear, leaf-like, ~2/3 length of calyx, short-mucronate, densely pubescent throughout, hairs long, erect | 5.0–6.3 mm long, free portion 2.0–4.8 mm long | 2.6–4.4 mm long, each ~0.8 mm wide | 2.7–3.4 mm long, glabrous except at apex | Upper pair fused for up to 1/8 length, ~2× as broad as lower; 4.0–5.5 mm long, long–acuminate, with many, long, spreading to erect hairs on lamina | |
| P. woolcockiorum | Inserted 0.3–0.5 mm above pedicel, linear, bract-like, ~3/4 length of calyx, not or weakly mucronate, hairy in lower 2/3 | 3.9–4.5 mm long, free portion 1.4–1.9 mm long | 4.6–5.1 mm long, ~0.9 mm wide | 3.4–3.8 mm long, glabrous except near lobes | ±Equal in width, upper pair fused for ~1/3 length, 4.4–5.1 mm long, with long spreading hairs on lamina and margins |
| Species | Standard length & width | Standard colour | Wing petals | Keel | Staminal filaments | |
|---|---|---|---|---|---|---|
| P. acanthocalyx | 5.8–6.8 mm long, 5.8–7.4 mm wide | Yellow–orange | 5.4–6.6 mm long, 0.9–1.3 mm wide, mostly yellow | 5.9–6.3 mm long, 2.4–2.6 mm wide, dark brick red | 4.8–5.7 mm long | |
| P. boormanii | 6.3–6.9 mm long, 7.1–8.0 mm wide | Yellow–orange | 5.9–6.9 mm long, 1.1–1.4 mm wide, mostly yellow–orange | 7.4–7.9 mm long, 2.3–2.8 mm wide, brownish-red, paler at base | 5.1–5.6 mm long | |
| P. campbellii | 5.9–6.8 mm long, 5.6–6.5 mm wide | Yellow–orange | 6.2–6.9 mm long, 1.5–2.1 mm wide, mostly yellow–orange | 7.2–8.2 mm long, 2.6–2.8 mm wide, mostly yellow, grading to red at base | 5.7–6.2 mm long | |
| P. corrickiae | 7.8–8.7 mm long, 7.6–8.4 mm wide | Orange–yellow | 7.1–8.5 mm long, 2.2–2.4 mm wide, orange | 8.2–8.7 mm long, 2.9–3.5 mm wide, dark brick red to burgundy | 7.5–9.1 mm long | |
| P. estelleae | 7.8–8.9 mm long, 7.0–8.1 mm wide | Orange–golden | 6.3–8.0 mm long, 1.7–2.1 mm wide, mostly yellow–orange | 6.2–7.9 mm long, 2.0–2.3 mm wide, deep red, grading to orange at base | 5.5–7.6 mm long | |
| P. farmeriana | 7.2–9.1 mm long, 6.4–8.0 mm wide | Orange–yellow | 6.5–7.2 mm long, 2.0–2.2 mm wide, mostly yellow–orange | 6.8–8.3 mm long, 2.6–2.9 mm wide, deep red, grading to pink at base | 5.3–7.5 mm long | |
| P. hoskingii | 5.8–7.1 mm long, 6.2–7.6 mm wide | Yellow–orange | 7.7–8.2 mm long, 2.0–2.6 mm wide, mostly yellow–orange | 7.0–8.5 mm long, 2.9–3.3 mm wide, brownish-red, paler at base | 4.5–5.8 mm long | |
| P. imminuta | 5.5–7.7 mm long, 4.0–5.8 mm wide | Yellow–orange | 4.7–6.8 mm long, 1.2–1.7 mm wide, yellow | 5.1–7.0 mm long, 1.6–3.3 mm wide, slightly exceeding wings, yellow–orange | 3.7–4.9 mm long | |
| P. lapidosa | 8.5–9.6 mm long, 5.8–7.2 mm wide | Deep orange | 6.2–7.2 mm long, 1.1–1.4 mm wide, mostly deep orange | 7.1–8.5 mm long, 2.1–2.6 mm wide, dark brick red, grading to red at base | 4.8–6.7 mm long | |
| P. murrayi | 8.2–12.1 mm long, 6.7–9.2 mm wide | Yellow–orange | 8.6–9.8 mm long, 1.6–2.1 mm wide, yellow–orange | 9.3–9.7 mm long, 2.4–3.2 mm wide, dark red | 5.8–8.5 mm long | |
| P. palssoniae | 5.2–6.5 mm long, 7.0–7.5 mm wide, | Yellow | 5.6–7.0 mm long, 1.9–2.1 mm wide, mostly yellow | 6.1–7.1 mm long, 2.3–2.9 mm wide, mostly yellow | 4.5–4.9 mm long | |
| P. praetermissa | 7.8–9.6 mm long, 6.6–7.3 mm wide | Yellow–orange | 6.8–7.6 mm long, 1.6–1.8 mm wide, mostly yellow–orange | 7.4–8.4 mm long, 2.8–3.2 mm wide, deep red, grading to red at base | 5.3–6.0 mm long | |
| P. procumbens | 5.1–6.1 mm long, 4.9–5.4 mm wide | Yellow–orange | 4.7–5.8 mm long, 1.5–1.7 mm wide, yellow–orange | 5.9–6.9 mm long, 1.9–2.5 mm wide (folded), ±equal to wings, deep red to red | 4.0–5.6 mm long | |
| P. purdieae | 4.8–6.4 mm long, 5.1–6.0 mm wide | Orange–yellow | 5.8–6.5 mm long, 1.2–1.6 mm wide, mostly yellow–orange | 5.5–7.0 mm long, 2.1–2.5 mm wide, dark brick red | 4.7–6.1 mm long | |
| P. renneri | 5.4–7.1 mm long, 7.4–8.0 mm wide | Yellow–orange | 6.2–7.4 mm long, 1.6–1.9 mm wide, mostly yellow | 7.1–7.7 mm long, 2.4–2.8 mm wide, slightly shorter than wings, brownish-red | 5.1–6.7 mm long | |
| P. setigera | ~5 mm long, ~4.2 mm wide | Yellow–orange | ~5 mm long, ~1.2 mm wide, yellow–orange | 5.0–5.7 mm long, 1.3–1.6 mm wide, ±equal to wings, deep red to red | 4.4–5.8 mm long | |
| P. setulosa | 7.1–8.1 mm long, 5.8–7.6 mm wide | Bright yellow | 6.9–8.0 mm long, 1.2–1.9 mm wide, mostly yellow | 8.0–8.9 mm long, 2.6–3.4 mm wide, yellow–orange, paler at base | 4.8–6.0 mm long | |
| P. venusta | 6.4–7.9 mm long, 6.8–8.2 mm wide | Orange–yellow | 6.4–7.1 mm long, 1.3–1.6 mm wide, mostly dark orange | 6.9–8.1 mm long, 1.7–2.6 mm wide, slightly exceeding wings, deep red to red | 4.7–7.2 mm long | |
| P. westonii | 11.7–13.1 mm long, 8.4–10.7 mm wide | Deep orange | 8.3–9.1 mm long, 1.3–1.5 mm wide, mostly deep orange | 9.6–10.5 mm long, 3.2–3.6 mm wide, dark reddish-brown or maroon, grading to red at base | 7.4–9.8 mm long | |
| P. woolcockiorum | 8.3–11.4 mm long, 7.8–8.6 mm wide | Yellow–orange | 7.7–9.0 mm long, 1.8–2.1 mm wide, yellow–orange grading to purple–red | 8.4–9.7 mm long, 2.7–2.9 mm wide, dark maroon at apex, paler at base | 6.5–8.3 mm long |
| Species | Ovary | Style | Fruit | Seeds | |
|---|---|---|---|---|---|
| P. acanthocalyx | ~1.4 mm long, hairy in apical 1/5–1/6 | 4.6–5.8 mm long | ~4.9 mm long, ~2.2 mm in diameter, sparsely hairy in apical 1/5 | [not seen] | |
| P. boormanii | 1.1–1.2 mm long, hairy in apical 1/2 | 6.3–6.7 mm long | 4.7–5.9 mm long, 3.3–3.9 mm in diameter, sparsely hairy in apical 1/2 | ~1.5 mm long, ~1.2 mm wide, aril curved through 90°, ~1 mm long | |
| P. campbellii | 1.3–1.6 mm long, glabrous | 4.3–5.8 mm long | 6.2–6.8 mm long, ~3.4 mm in diameter, glabrous | [not seen] | |
| P. corrickiae | ~1.2 mm long, hairy for most of length | 7.5–8.6 mm long | 3.8–4.2 mm long, 2.5–2.9 mm in diameter, hairy at least in apical 1/2 | [imm.] > 1.9 mm long, >1.0 mm wide, aril probably entire, straight, >0.5 mm long | |
| P. estelleae | 1.5–1.6 mm long, hairy in apical 1/8 | 3.6–5.7 mm long | [not seen] | [not seen] | |
| P. farmeriana | 1.0–1.2 mm long, hairy in apical 1/8 | 7.6–8.1 mm long | [not seen] | [not seen] | |
| P. hoskingii | 2.2–3.7 mm long, hairy in apical 3/4 | 6.0–6.7 mm long | 5.5–6.1 mm long, 2.4–2.6 mm in diameter, hairy at least in apical 3/4 | 2.2–2.4 mm long, 1.5–1.9 mm wide, with dark brown mottling or flecks, aril ±straight, apex divided into course, hair-like lobes, 1.9–2.2 mm long | |
| P. imminuta | 1.3–2.1 mm long, hairy in apical 5/6–1/2 | Broad at base, ±central on ovary apex, 4.1–6.5 mm long | [not seen] | [not seen] | |
| P. lapidosa | ~1.5 mm long, hairy in apical 1/3 | 6.5–8.1 mm long | 5.4–6.9 mm long, 2.6–3.1 mm in diameter, hairy in apical 1/3 | 2.1–2.8 mm long, 1.2–1.8 mm wide, aril highly divided into course, hair-like lobes, 0.5–0.7 mm long | |
| P. murrayi | ~1.4 mm long, hairs to 1.1 mm long in apical 1/5 on abaxial side of style | 6.8–8.7 mm long | 4.9–6.2 mm long, 2.8–3.6 mm in diameter, sparsely hairy near apex | 2.5–2.8 mm long, 1.5–1,8 mm wide, smooth, dark brown, aril white, curved, ornate (coralline), with many short projections, 1.3–1.4 mm long | |
| P. palssoniae | 1.3–1.5 mm long, hairy throughout | 4.8–5.0 mm long | 4.6–5.8 mm long, 2.4–2.8 mm in diameter, hairy for most of length or at least in apical 1/2 | ~3.0 mm long, ~2.2 mm wide, aril irregular with many thick projections, ~1.3 mm long | |
| P. praetermissa | ~1.7 mm long, hairy in apical 1/4 | 6.2–6.6 mm long | 4.2–5.4 mm long, 2.3–2.8 mm in diameter, hairy in apical 1/4 | [not seen] | |
| P. procumbens | ovary 1.2–1.3 mm long, hairy in apical 1/6 | ~5.6 mm long | [not seen] | [not seen] | |
| P. purdieae | ~1.3 mm long, hairy in apical 1/3 | 4.4–5.8 mm long | [not seen] | [not seen] | |
| P. renneri | 1.1–1.2 mm long, hairy in apical 1/8 | 4.3–5.7 mm long | [not seen] | [not seen] | |
| P. setigera | ~0.8 mm long, hairy in apical 1/8 | ~5.1 mm long | [not seen] | [not seen] | |
| P. setulosa | 1.7–1.9 mm long, hairy in apical 1/3 | 6.2–7.5 mm long | 4.3–5.4 mm long, 2.3–3.5 mm in diameter, hairy in apical 1/3 | 1.9–2.2 mm long, 1.2–1.4 mm wide, aril ±straight, entire, ~0.8 mm long | |
| P. venusta | 1.5–1.9 mm long, hairy in apical 1/5 | 5.5–6.2 mm long | 4.5–4.9 mm long, 2.9–3.2 mm in diameter, sparsely hairy in apical 1/4 | 2.0–2.2 mm long, 1.5–1.6 mm wide; aril irregular bowl-shaped with scattered papillae outside, ~1.1 mm long | |
| P. westonii | ~1.4 mm long, hairy in apical 1/8 | 8.3–9.1 mm long | 4.2–5.4 mm long, 2.1–2.8 mm in diameter, glabrous or sparsely hairy near apex on sutures | [imm.] ~1.2 mm long, 0.7 mm wide, aril straight or curved, entire, ~0.3 mm long | |
| P. woolcockiorum | ~1.9 mm long, hairy in apical 1/8 | 6.6–7.1 mm long | [not seen] | [not seen] |
Species descriptions
Species are presented in alphabetical order as the phylogenetic relationships remain mostly speculative, although these are known to not form a monophyletic clade. Pultenaea procumbens and P. setigera are treated after the other 18 species as Pultenaea procumbens sens. lat. was excluded from this group by de Kok and West (2002).
Pultenaea acanthocalyx R.L.Barrett & Clugston, sp. nov.
Type: New South Wales: Southern Tablelands: ~17 km along Goobarragandra Road from junction with Snowy Mountains Highway, beside Goobarragandra River, 330 m, 13 Nov. 1993, N.Taws 261 (holo: NSW 500261; iso: CBG 9314820).
Pultenaea sp. Snowy Mountains (N.Taws 261) NSW Herbarium, PlantNet [https://plantnet.rbgsyd.nsw.gov.au/, accessed 25 July 2022].
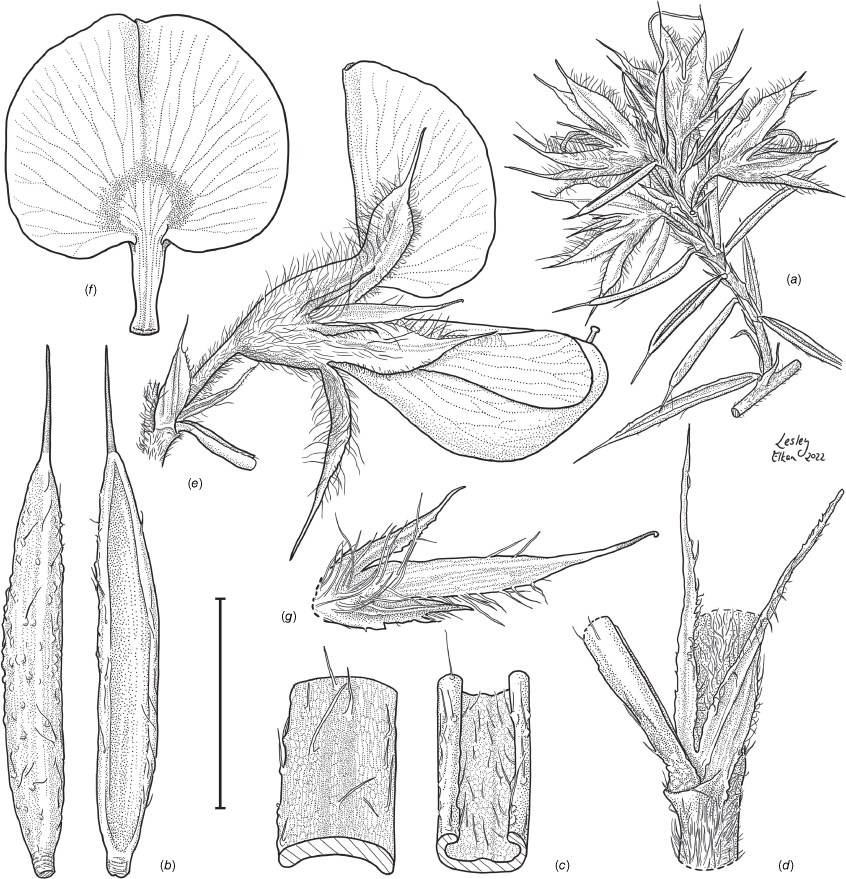
Sprawling shrub 0.4–0.6 m high, multi-stemmed from the base, width of spread not known (>40 cm). Branchlets erect to spreading, stiff, shortly ribbed below petioles, moderately pubescent with short, crisped, spreading, matted hairs to 0.2 mm long. Stipules lanceolate, fused for ~1/8 of length, 1.8–3.0 mm long, each ~0.2 mm wide at sinus, weakly keeled, brown to red–brown with broad pale margins; apex spreading, long–acuminate; margins with short hairs throughout. Leaves alternate: petiole 0.2–0.3 mm long; lamina ±linear, moderately pubescent with appressed hairs to 0.7 mm long abaxially, eventually glabrescent but with small tuberculate hair-bases remaining, moderately pubescent adaxially when young, weakly discolourous, slightly paler above but adaxial surface partly or mostly hidden by incurved margins, 5.3–10.9 mm long, 0.4–0.9 mm wide, with scattered, irregular papillose cells adaxially, surface smooth with distinctly oblong papillose cells abaxially; apex straight to slightly oblique, acute, rigidly and abruptly mucronate, mucro pungent, 0.8–2.2 mm long; margins usually strongly incurved, commonly hiding the adaxial surface at maturity. Inflorescence of 5–10 solitary flowers borne in upper axils, appearing loosely clustered. Flowers: pedicels subtended by enlarged stipules 1.9–2.7 mm long, each ~0.5 mm wide at sinus, with short hairs on margins, partly hiding the pedicels; pedicels 1.7–3.1 mm long, densely hairy; floral bracteoles inserted on calyx tube, 0.9–1.2 mm above pedicel, appressed at base, linear (not or scarcely fused to subtending stipules), leaf-like, herbaceous, green, 2.4–3.7 mm long, reaching ~3/4 length of calyx, long–mucronate, glabrous or with a few long, erect hairs; bracteolar stipules narrow, 1.4–1.9 mm long, each ~0.3 mm wide at sinus, with scattered hairs on margins; calyx tube broadly obconic, 1.1–1.7 mm long, greenish, with sparse, erect hairs outside near bracteoles; calyx lobes 5, papery, narrowly triangular, 2.6–4.9 mm long, long–acuminate with a pungent mucro to 1.6 mm long, with many long, spreading to erect hairs, densest near margins, with fine, short hairs inside near the margins, subequal, the upper pair broader, fused for ~1/4 of length; standard petal ±circular in appearance, obcordate when flattened and claw is visible, emarginate, 5.8–6.8 mm long including 2.0–2.5 mm claw, 5.8–7.4 mm wide, yellow–orange with red marks around a dull orange ‘eye’, reverse side dark yellow–orange; wing petals narrow–oblong, 5.4–6.6 mm long including 2.1–2.4 mm claw, 0.9–1.3 mm wide, yellow, orange towards the base; keel petals naviculate, ±narrow–oblong to obovate in outline, 5.9–6.3 mm long including claw 1.1–1.7 mm long, 2.4–2.6 mm wide (when folded), slightly exceeding wings, dark brick red at the apex grading to dark red at the base; stamens free to the base, subequal in length; filaments 4.8–5.7 mm long; anthers ~0.4 mm long; ovary sessile, 2-ovulate, ~1.4 mm long, with many long, appressed hairs in apical 1/5–1/6; style 4.6–5.8 mm long, gently curving, glabrous; stigma inconspicuous, very slightly capitate. Fruit ovoid, pale brown, compressed, asymmetrically beaked, ~4.9 mm long including stylar remnant, ~2.2 mm in diameter, sparsely hairy in apical 1/5. Seeds not seen. (Fig. 1, 2.)
Holotype of Pultenaea acanthocalyx R.L.Barrett & Clugston (N.Taws 261; NSW) with inset photos (a, b) showing details of leaves and inflorescence.
Line drawing of Pultenaea acanthocalyx R.L.Barrett & Clugston. (a) Flowering branchlet. (b) Leaves: abaxial surface (L), adaxial surface (R). (c) Leaf lamina surface: abaxial surface (L), adaxial surface (R). (d) Stipules and petiole. (e) Lateral view of flower showing pair of stipules subtending pedicel. (f) Standard. (g) Bracteole and stipules. Voucher: N.Taws 261 (NSW). Scale bars: a, 10 mm; b, 4 mm; c, d, 2 mm; e, f, 5 mm; g, 2.5 mm. Illustration by L. Elkan.
The very long leaf mucro (0.8–2.2 mm long), enlarged upper calyx lobes and papery calyx with prominently mucronate calyx lobes suggest that this species might be allied to P. procumbens. However, P. acanthocalyx is readily distinguished by having narrower and longer leaves 5.3–10.9 mm long and 0.4–0.9 mm wide, with only a central vein and strongly incurved margins. In contrast, P. procumbens has narrowly ovate to somewhat obovate leaves 1.5–2.4 mm wide that are 3-nerved and with margins only strongly incurved towards the apex. Pultenaea praetermissa and P. purdieae, that both occur in relative geographic proximity to P. acanthocalyx, differ in the calyx lobes being acute to acuminate but not or only shortly mucronate and the leaves being glabrous or if hairy, then hairs without tuberculate bases, and shorter leaf mucro (0.2–0.8 mm long).
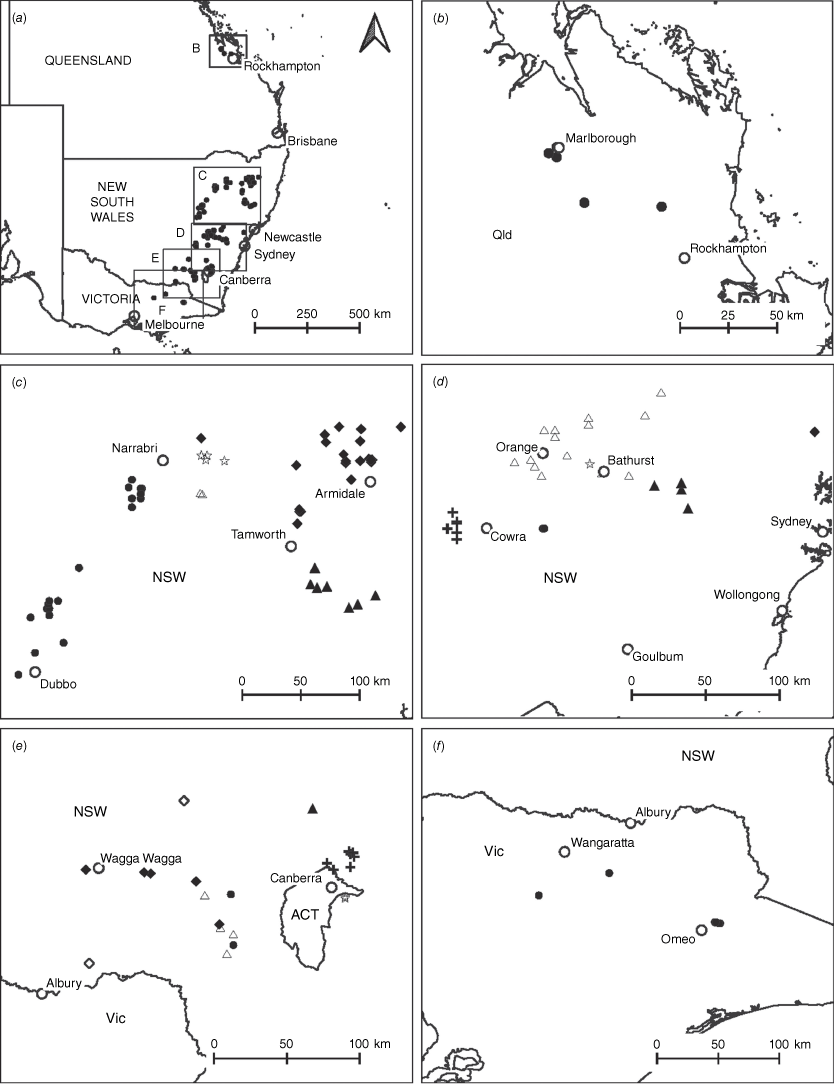
Only known from two locations at the northern end of the Snowy Mountains, one east of Tumut, at 330 m and one S of Yarrangobilly at 750 m in the Southern Tablelands of New South Wales (Fig. 3e).
Distributions of species in the Pultenaea setulosa complex, P. procumbens and P. setigera (closed circle). (a) All species and location of map insets. (b) P. setulosa (closed circle). (c) P. boormanii (closed circle), P. campbellii (closed diamond), P. hoskingii (closed triangle), P. imminuta (open triangle), P. palssoniae (open star). (d) P. farmeriana (closed cicle), P. procumbens (closed triangle), P. renneri (closed diamond), P. setigera (open star), P. westonii (open triangle), P. woolcockiorum (cross). (e) P. acanthocalyx (closed circle), P. corrickiae (closed diamond), P. estelleae (closed triangle), P. murrayi (open star), P. praetermissa (cross), P. purdieae (open triangle), P. venusta (open diamond). (f) P. lapidosa. Maps by R. L. Palsson based on data from the Australasian Virtual Herbarium.
Recorded from moderate rocky slopes in tall shrubland on dark brown, sandy loam on Bogong Granite. Recorded in association with Correa reflexa, Crowea exaltata subsp. exaltata, Dodonaea viscosa subsp. spatulata, Eucalyptus dives, E. macrorhyncha, Grevillea wilkinsonii, Leptospermum brevipes, Pomaderris cotoneaster and Westringia eremicola.
The epithet is derived from the Greek acantho- (spine) and calyx, in reference to the prominently mucronate calyx lobes (and bracteoles).
Pultenaea acanthocalyx is currently known from two locations ~50 km apart. Area of extent is difficult to determine but possibly ~450 km2. Based on current data, this species would warrant an IUCN Category of Endangered (B1a, B2a); however, given the poor collection record and largely intact habitat, we recommend listing the species as Data Deficient until further survey work can be undertaken.
First identified as P. campbellii by de Kok on specimens at NSW and subsequently included under P. setulosa by de Kok and West (2002). Superficially similar in appearance to the P. juniperina Labill. complex, where one collection had been placed but the ovary is only hairy at the apex and the calyx lobes are much longer than the calyx tube, both in contrast to P. juniperina and allied species. The species is likely more closely related to P. procumbens.
There are several specimens that likely represent a related but unnamed taxon that differ in having leaves that are broad at the base and also strongly recurved slightly above the base. These are listed here due to morphological similarities but will be assessed further as part of a planned revision of the P. procumbens group:
Victoria: Eastern Highlands, Jemba Reference Area, Burrowa National Park, 25 Oct. 1987, A.C.Beauglehole 89303 & L.W.Huebner (CBG, MEL 2019891, NSW 368456); Eastern Highlands, Burrowa National Park, 22 Oct. 1987, A.C.Beauglehole 89046 (CBG, MEL 2019930, NSW 369671).
New South Wales: Burrin Juck [Burrinjuck], Jan. 1912, E.Cheel s.n. (NSW 38701); Tumut–Tumbarumba–Holbrook district, Dec. 1947, P.J.Norland 85 (NSW 38704).
Pultenaea boormanii H.B.Will., Proc. Roy. Soc. Victoria ser. 2, 35: 103, t. VII (1922)
Type citation: ‘It was originally collected at Minore, N.S.W., by Mr J.L. Boorman, in Feb., 1899 … In 1904, some additional specimens were collected at Bidden Road, 7 miles [~11.3 km] from Gilgandra, North of Dubbo, by Mr R.H. Cambage, who sent them in under No. 1110, Oct. 15, ‘04 … Duplicates of the same plant from the same locality were sent in later by Mr Cambage … In August, 1908, Mr Boorman collected a small specimen at Goonoo, near Mudgee, and again in June, 1909, from the same locality…’. Type: New South Wales: Bidden Rd, 7 miles [~11.3 km] from Gilgandra, [north of Dubbo], 15 Oct. 1904, R.H.Cambage 1097, east of Bidden Rd, Gilgandra, 15 Oct. 1904, R.H.Cambage 1110 (lecto, here designated: NSW 38731, left hand specimen) [the two labels cannot be specifically matched to the two specimens with any confidence].
Residual syn: New South Wales: Minore, NSW, Feb. 1899, J.L.Boorman s.n. (NSW 38728); Bidden Rd, 7 miles [~11.3 km] from Gilgandra, [north of Dubbo], 15 Oct. 1904, R.H.Cambage 1097, east of Bidden Rd, Gilgandra, 15 Oct. 1904, R.H.Cambage 1110 (MEL 524047, NSW 38731 right hand specimen); Gilgandra, Oct. 1904, R.H.Cambage s.n. (MEL 2057258, p.p.); Goonoo, Aug. 1908, J.L.Boorman s.n. (NSW 38727); Goonoo to Mudgee, Oct. 1908, J.L.Boorman s.n. (NSW 38729, MEL 2057258, p.p.).
Illustration: O’Halloran (2018, pl. p. 47, as P. setulosa).
Erect or spreading shrub 0.4–1 m high, multi-stemmed from the base, spreading 0.6–1.5 m wide. Branchlets erect to spreading, stiff, shortly ribbed below petioles, moderately pubescent with short, crisped, appressed, matted hairs to 0.3 mm long. Stipules lanceolate, fused for 1/3–1/2 of length, 2.7–3.6 mm long, ~0.3 mm wide at sinus, moderately keeled, brown to red–brown with paler margins; apex spreading, long–acuminate; margins ±entire or with occasional tooth-like projections, with a small area of short, crisped hairs. Leaves alternate: petiole 0.3–0.8 mm long; lamina ±linear, with scattered, appressed, straight hairs to 0.4 mm long, eventually glabrescent, weakly discolourous but adaxial surface usually hidden by incurved margins, 5.7–10.5 mm long, 0.3–0.6 mm wide, with dense, irregularly rounded papillose cells adaxially, surface smooth with rounded to irregularly oblong papillose cells abaxially, apex typically distinctly oblique, sometimes straight, acute, rigidly and abruptly mucronate, mucro pungent, 0.1–0.2(–0.5) mm long; margins strongly incurved usually hiding the adaxial surface. Inflorescence of 4–8 solitary flowers borne in upper axils, appearing loosely to tightly clustered. Flowers: pedicels subtended by enlarged stipules 1.9–2.3 mm long, each 0.6–0.7 mm wide at sinus, with short hairs on margins, partly hiding the pedicels; pedicels 1.5–2.5 mm long, with dense, appressed to spreading straight white hairs to 0.1 mm long; floral bracteoles inserted ~0.4 mm above pedicel apex, appressed at base, linear (free from subtending stipules), leaf-like, herbaceous, green, 3.2–5.1 mm long, reaching 2/3 to full length calyx, mucronate, pubescent; stipules narrowly triangular, 1.7–2.1 mm long, each ~0.4 mm wide at sinus; calyx tube broadly obconic, 1.3–2.0 mm long, green, moderately densely pubescent outside; calyx lobes 5, narrowly triangular, 3.2–5.7 mm long, acuminate or narrowly triangular, with long appressed to ascending hairs except towards the apex, the upper pair broader, fused for ~1/2 of ther length; standard petal ±circular in appearance or obcordate when flattened and claw is visible, emarginate, 6.3–6.9 mm long including 2.3–2.6 mm claw, 7.1–8.0 mm wide, yellow–orange with red crescent (broader on the sides) around a small yellow ‘eye’; wing petals narrow–oblong, 5.9–6.9 mm long including 1.8–3.5 mm claw, 1.1–1.4 mm wide, mostly yellow–orange, red towards the base; keel petals naviculate, ±narrow–oblong in outline, 7.4–7.9 mm long including claw 2.0–2.3 mm long, 2.3–2.8 mm wide (when folded), slightly exceeding wings, brownish-red, paler at the base; stamens free, subequal in length; filaments 5.1–5.6 mm long; anthers ~0.6 mm long; ovary sessile, 2-ovulate, 1.1–1.2 mm long, with a dense tuft of appressed hairs to 2.1 mm long in apical 1/2; style 6.3–6.7 mm long, curving ~45° ~1/3 from base, with long, semi-appressed hairs only in lower 1/8–1/6; stigma inconspicuous, very slightly capitate. Fruit ovoid, brown, compressed, asymmetrically beaked, 4.7–5.9 mm long including stylar remnant, 3.3–3.9 mm in diameter, sparsely hairy at least in apical half. Seeds compressed irregular–ovoid, ~1.5 mm long, ~1.2 mm wide, smooth, dark brown; aril white, curved through 90°, ~1 mm long. (Fig. 4–6.)
Lectotype and syntype of Pultenaea boormanii H.B.Will. (R.H.Cambage 1097, R.H.Cambage 1110; NSW 38731 (lecto: left hand specimen; syn: right hand specimen)).
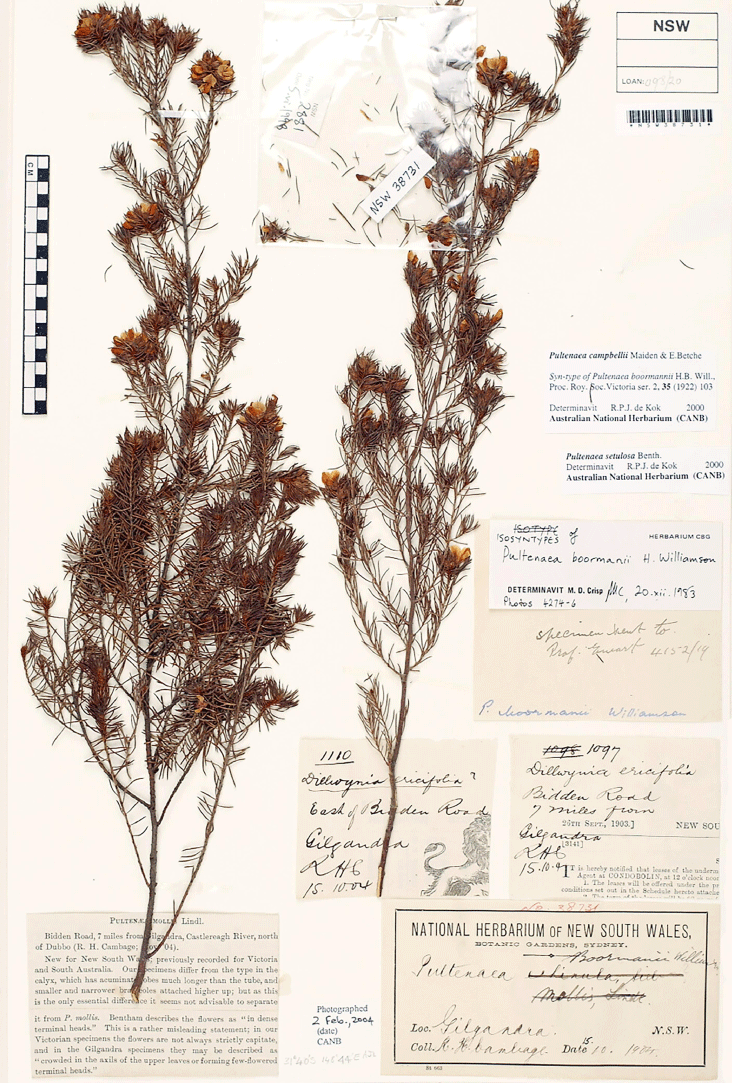
Field photographs of Pultenaea boormanii H.B.Will. Photos: (a, c, d) by P. Woodall; (b) by R. W. Medd. Photos (a, c, d) also posted on iNaturalist.

Line drawing of Pultenaea boormanii H.B.Will. (a) Flowering branchlet. (b) Leaves: abaxial surface (R), adaxial surface (L). (c) Leaf lamina surface: abaxial surface (centre), adaxial surface (L), leaf apex (R). (d) Stipules and petiole. (e) Lateral view of flower. (f) Standard. (g) Bracteole and stipules. Voucher: R.Coveny 2351 (NSW). Scale bars: a, 10 mm; b, 4 mm; c, d, 2 mm; e, f, 5 mm; g, 2.5 mm. Illustration by L. Elkan.
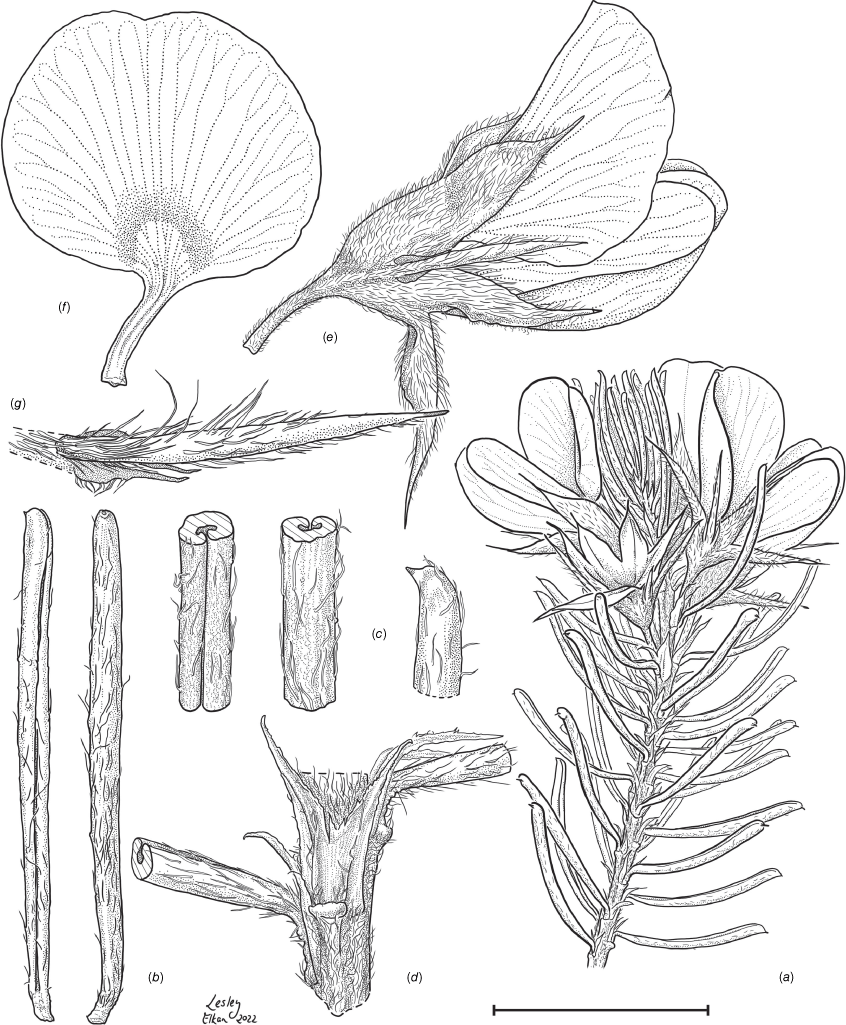
Similar in appearance to P. campbellii, P. farmeriana, P. hoskingii, P. praetermissa and P. setulosa but distinguished from these species by the following combination of characters: branchlet stipules fused for 1/3–1/2 of length, 2.7–3.6 mm long; leaf lamina ±linear, 0.3–0.6 mm wide, margins strongly incurved usually hiding the adaxial surface; pedicels subtended by stipules 1.9–2.3 mm long, 0.6–0.7 mm wide; floral bracteoles inserted ~0.4 mm above pedicel, linear, leaf-like, to 2/3 length of calyx, mucronate, pubescent, 3.2–5.1 mm long; calyx lobes with upper pair broader, fused for ~1/2 length; 3.2–5.7 mm long, with long spreading hairs; ovary 1.1–1.2 mm long, hairy in apical 1/2; and style 6.3–6.7 mm long.
Occurs in two disjunct areas, bounded by Dubbo, the Warrumbungle Range and the Pilliga, in the Central Western Slopes and North Western Slopes of New South Wales at altitudes of 200–500 m (Fig. 3c). Habitats between these areas were presumably cleared without any collections having being made.
Grows in open woodland or heath on yellow or red sandy flats, sometimes over sandstone or near creeklines. Recorded in association with Acacia caroleae, A. cheelii, A. cultriformis, A. gladiiformis, A. granitica, A. triptera, Allocasuarina gymnanthera, Brachyloma daphnoides, Brunonia australis, Callitris glaucophylla, Calytrix tetragona, Dampiera lanceolata, D. maideniana, Dillwynia sericea, Dodonaea peduncularis, D. truncatiales, D. viscosa subsp. mucronata, Eucalyptus crebra, E. dwyeri, E. elegans, E. fibrosa subsp. nubia, E. sideroxylon, Goodenia sp., Grevillea arenaria subsp. canescens, G. obtusiflora, Hibbertia obtusifolia, Isopogon petiolaris, Melaleuca uncinata, Microtis parviflora, Myoporum platycarpum subsp. platycarpum, Olax stricta, Ozothamnus diosmifolius, Phebalium nottii, P. squamulosum subsp. coriaceum, P. stenophyllum, Platysace ericoides, Pterostylis boormanii, Symphyomyrtus crebra, Westringia cheelii and Xanthorrhoea glauca subsp. angustifolia.
Flowering recorded mainly for September–December (but also recorded for August and March). Fruiting recorded for November–December.
The epithet honours botanist John Luke Boorman (1864–1938), who worked at the Sydney Botanic Gardens from January 1887 to c. 1930 (George 2009).
Pultenaea boormanii is found in somewhat disjunct regions, between Dubbo and Gilgandra, and in the Pilliga, south of Narrabri to the Warrumbungle Range. Habitats between these regions may have been cleared prior to any collections being made or the species may have always been restricted to an association with relict, windblown sands from the west that are largely absent between these locations. Notably only two collections have been made since 1984, though this may represent a general decline in collection effort rather than the species. Area of extent: 5500 km2. IUCN Category: Least Concern; but may warrant listing as Vulnerable if ongoing climate change affects populations through long-term drought.
Selection of a lectotype is required as several collections were mentioned by Williamson (1922), but these are mentioned under correspondence from Edwin Cheel, therefore discerning which material was seen by Williamson is difficult. Many of the collections cited are sterile, therefore the best flowering sheet is the clear choice. However, the best sheet, held at NSW (NSW 38731) and recorded as being sent on loan to MEL, comprises two pieces of material that correspond to two collections, R.H.Cambage 1097 and R.H.Cambage 1110, both made near Bidden Road, NE of Gilgandra, on 15 Oct. 1904. There is no indication as to which collection number corresponds with which piece, therefore the largest (on the left) is designated as the lectotype to unequivocally select a single gathering as the lectotype. A sheet at MEL has two pieces of material formed from the NSW sheet, likely corresponding to both collections. The sheet also has both Cambage collection numbers indicated but these are regarded as syntypes as once again these cannot be matched to the individual pieces on the NSW sheet with any confidence, though one of the pieces is most likely a duplicate of the lectotype.
Cheel included a note ‘Identical plants found at Nundle, and at Warialda.’ However, these likely represent Pultenaea campbellii or the Nundle plants possibly P. hoskingii, though specimens from these locations have not been matched (the original observations being attributable to Boorman).
A pure yellow-flowered form has been observed in the Gilgandra region but appears to only be a colour morph, growing in proximity to normally coloured flowers (G. Williams, pers. comm.).
Specimens of P. bracteaminor de Kok, a species endemic to south-east Queensland, were previously included under P. boormanii (see Stanley 1983; Hacker 1990; de Kok and West 2004).
Between Dubbo and Mendooran, Mar 1946, G.Althofer 31 (NSW 38722): possibly a hybrid between P. boormanii and P. foliolosa A.Cunn. ex Benth. This specimen differs in the much smaller leaves, 3–4.5 mm long, that are somewhat recurved and smaller flowers relative to P. boormanii, and these features are large relative to those in P. foliolosa.
NEW SOUTH WALES: between Dubbo and Mendooran, Mar. 1946, G.W.Althofer 18 (NSW 38724); Mendooran–Ballimore road, 1947, G.W.Althofer s.n. (NSW 38725); Ganoo [Goonoo] Forest, near Dubbo, Nov. 1947, G.W.Althofer s.n. (QRS 32103, n.v.); 4 miles [~6.4 km] E of Mogriguy, 19 Sep. 1957, E.F.Biddiscombe 37 (CANB 24923); Bearbong, Nov. 1958, A.J.Buckley s.n. (NSW 53016); Goonoo Goonoo State Forest, 18 Oct. 1979, R.C.Carolin 13161 (SYD); Goonoo State Forest, Dubbo to Mendooran, 23 Sep. 1951, G.Chippendale & E.Constable s.n. (L 2034127, NSW 17492); Newell Hwy, 37 km (~23 miles) SW of Narrabri towards Coonabarabran, 9 Oct. 1969, R.G.Coveny 2318 (L 2034532*, MEL 0546850); Gilgandra Flora Reserve, ~13 km (~8 miles) NE of Gilgandra towards Bearbong, 10 Oct. 1969, R.Coveny 2351 (AD 97610229Z, CANB 321191, MEL 2093518, NSW 467643); Goonoo State Forest, 5.5. km E of Mogriguy on Mendooran road, 18 Oct. 1978, R.Coveny 10313 & J.Armstrong (L 2034535*); Gilgandra Flora Reserve, 28 July 1996, S.Donaldson 939 (CBG 9612911); Warrumbungle area, Aug. 1959, A.Floyd 2 (NSW 53017); Bearbung [Bearbong] road, 6 miles [~9.7 km, NW] of Gilgandra, 22 Oct. 1962, A.G.Graham s.n. (NSW 467644); Bidden [Biddon] State Forest, ~10 miles [~16 km] N of Gilgandra, 30 Aug. 1959, B.McReaddie s.n. (NSW 53018); Bidden [Biddon] State Forest, 11 Sep. 1959, B.McReaddie s.n. (CANB 135887); Yalcogrin State Forest, [~10 km NE of Gilgandra], 16 Sep. 1959, B.McReaddie s.n. (CANB 135884, NSW 47635); Coolbaggie Nature Reserve, Near Eumungerie, 23 Nov. 1976, A.K.Morris C 74 (NSW 420483); 35 km from Narrabri on Coonabarabran Road, 27 Aug. 1973, B.Muffet 173/163 (CBG 53096); Goonoo Forest (Dubbo–Mendooran), Sep. 1974, R.T.Perry s.n. (NSW 467642); 3.5 miles [~5.6 km] along road to Bearbong from Newell Highway (Gilgandra district), 14 Sep. 1972, M.E.Phillips 143 (CBG 50376, L 2034536*; NSW 500269); Kenebri Road, Pilliga Scrub, 17 km SW of Newell Highway, 240 m, Dec. 1984, A.N.Rodd 4252 (NSW 500268, PERTH 817058, n.v.); Goonoo State Forest, 1 Oct. 1950, H.M.R.Rupp s.n. (NSW 38726); Pilliga scrub, 10 Aug. 1962, V.E.Sands 628.9.4 (SYD); Pilliga scrub, 2 Oct. 1964, D.Shoobridge s.n. (CBG 14924, K, n.v., L 2034540*); 35 km from Narrabri on Coonabarabran Road, 8 Dec. 1973, H.Streimann HS 663 (CBG 53759); 13 km NE of Gilgandra, 300 m, 13 Dec. 1973, H.Streimann HS 771 (A, n.v., CBG 53564, K, n.v., L 2034537*); Pilliga State Forest, 9 Sep. 1962, L.K.Wescombe 89 (CBG 11362); near Dubbo, Dec. 2001, G.Williams s.n. (NSW 496643); 22 miles [~37 km] S of Narrabri on Newell Highway 29 Aug. 1973, J.B.Williams s.n. (BRI, n.v., MEL, n.v., NE 94036).
Pultenaea campbellii Maiden & Betche, Proc. Linn. Soc. New South Wales 24: 144–145 (1899) (as ‘Campbelli’)
Type: New South Wales: Grave-yard Creek, near Walcha, Oct. 1898, J.F.Campbell s.n. (lecto, here designated: NSW 38679; isolecto: AD, n.v., BM 000544759*, BRI AQ0025147, CBG 8313097, K 000119010*, M 0219168*, MEL 516220, MEL 516221, MEL 516223, MEL 2055265, MPU 021265*, NSW 371377, US 1092080*).
Erect to spreading shrub 0.4–0.9(–1.4) m high, multi-stemmed from the base, spreading 0.5–1.2(–2) m wide. Branchlets erect to spreading, stiff, shortly ribbed below petioles, densely pubescent with short, straight, appressed, matted hairs to 0.4 mm long. Stipules lanceolate to falcate, fused for 1/8–1/6 of length, 1.7–2.3 mm long, ~0.2 mm wide at sinus, weakly keeled, dark red–brown with paler margins; apex spreading to out-curved, acuminate; margins entire or with a few tooth-like projections, otherwise glabrous. Leaves alternate: petiole 0.4–0.8 mm long; lamina lanceolate, not discolourous, mostly glabrous but with a few scattered, long, straight hairs to 0.4 mm long on both surfaces of young leaves, 3.4–9.2(–11.5) mm long, 0.6–0.9(–1.1) mm wide, adaxial surface smooth with scattered, irregular, flat papillose cells; abaxial surface with distinctly oblong papillose cells; apex almost straight when young, becoming strongly recurved, acute, rigidly and sharply mucronate, mucro pungent, to 0.1 mm long; margins slightly incurved (strongly so when young). Inflorescence of 3–8 solitary flowers borne in upper axils, appearing tightly clustered. Flowers: pedicels subtended by slightly enlarged stipules 0.8–0.9 mm long, each 0.4–0.5 mm wide at sinus, free almost to the base, glabrous but with toothed margins, not or partly hiding the pedicels; pedicels 1.0–1.6 mm long, with dense, appressed, straight or crisped hairs to 0.2 mm long; floral bracteoles inserted 0.1–0.4 mm below pedicel apex, appressed at base, fused to stipules near the base, linear, bract-like, scarious, brownish, 1.7–2.1 mm long, reaching ~1/3 length of calyx, shortly mucronate, glabrous; stipules narrow, 0.8–1.1 mm long, each ~0.2 mm wide at sinus, fused for most of length; calyx tube broadly obconic, 1.5–2.0 mm long, glabrous outside; calyx lobes 5, narrowly triangular, 3.0–3.6 mm long, acuminate, lamina glabrous but with dense, short, crisped hairs on margin, ±equal in width, the upper pair fused for ~1/10 of length; standard petal ±circular in appearance or obcordate when flattened and claw is visible, emarginate, 5.9–6.8 mm long including 1.6–1.9 mm claw, 5.6–6.5 mm wide, yellow–orange with a crescent of red flecks around a small yellow ‘eye’; wing petals narrow–oblong, 6.2–6.9 mm long including 1.6–1.8 mm claw, 1.5–2.1 mm wide, mostly yellow–orange, red towards the base; keel petals naviculate, ±narrow–oblong in outline, 7.2–8.2 mm long including claw 2.0–2.2 mm long, 2.6–2.8 mm wide (when folded), slightly exceeding wings, mostly yellow, grading to red at the base; stamens free, ±equal in length; filaments 5.7–6.2 mm long; anthers 0.4–0.5 mm long; ovary sessile, 2-ovulate, 1.3–1.6 mm long, glabrous; style 4.3–5.8 mm long, curving 70–90° towards apex, glabrous; stigma inconspicuous, very slightly capitate. Fruit ovoid, brown, compressed, asymmetrically beaked, 6.2–6.8 mm long including stylar remnant, ~3.4 mm in diameter, glabrous. Seeds not seen. (Fig. 7–9.)
Field photographs of Pultenaea campbellii Maiden & E.Betche. Vouchers: (a) Not vouchered; (b–d) J.R.Hosking 3642 (NE); (e, f) J.R.Hosking 3656 (NE). Photos: (a) by G. Derrin; (b–f) by J. R. Hosking. Photo (a) also posted on iNaturalist.
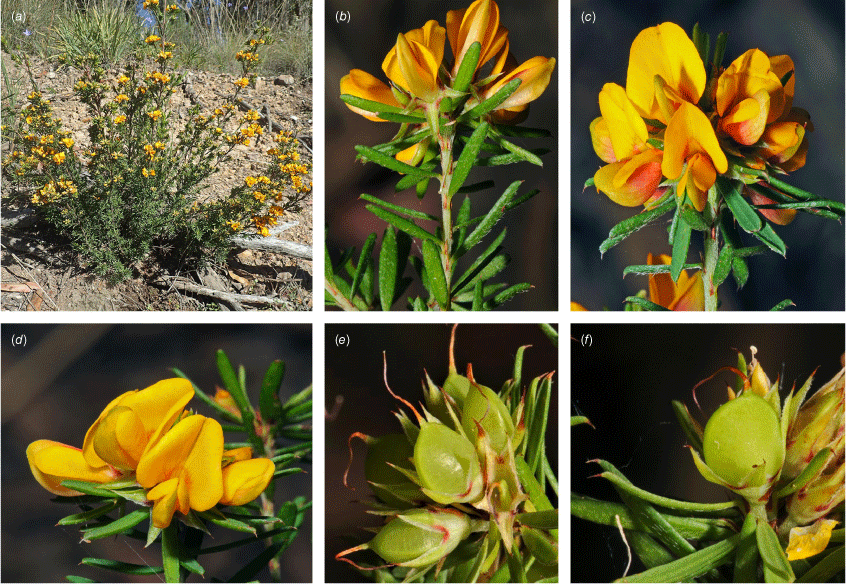
Line drawing of Pultenaea campbellii Maiden & E.Betche. (a) Flowering branchlet. (b) Leaves: abaxial surface (R), adaxial surface (L). (c) Leaf lamina surface: abaxial surface (below), adaxial surface (above). (d) Leaf cross-section. (e) Stipules. (f) Lateral view of flower. (g) Standard. (h) Bracteole and stipules. Vouchers: (a) L.M.Copeland 1915 & N.E.Noble (NSW); (e) J.R Hosking 2237 & R.J.Sippel (NSW); (b–d, f–h) L.M.Copeland 3246 (NSW). Scale bars: a, 10 mm; b, 4 mm; c–e, 2 mm; g, 5 mm; h, 1.6 mm. Illustration by C. Wardrop.
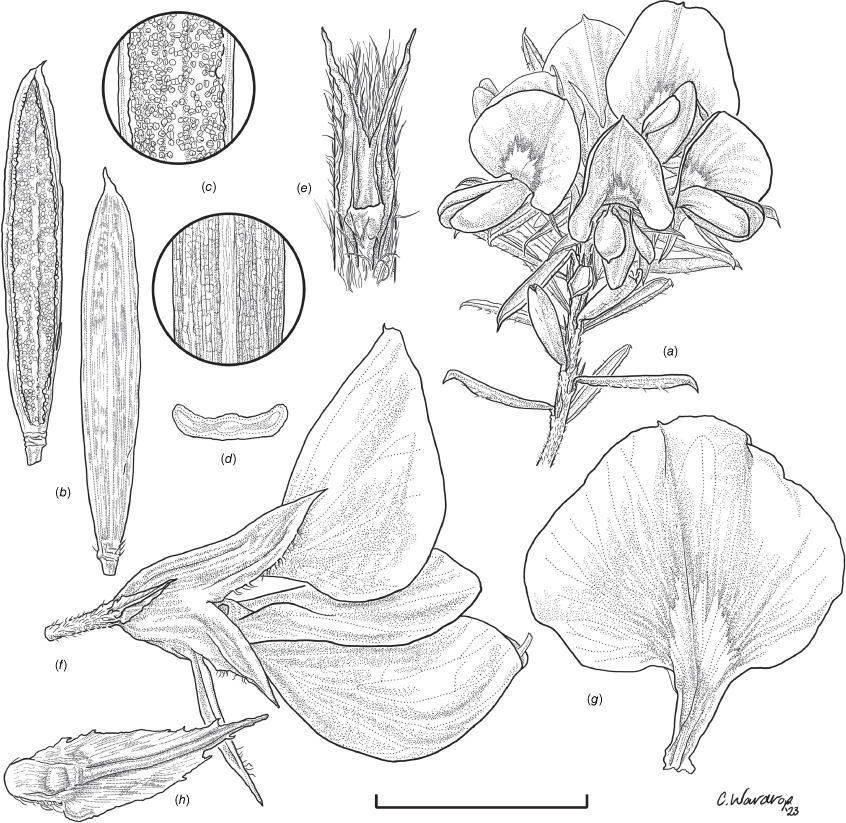
Most similar in appearance to P. palssoniae and P. hoskingii but distinguished from these species by straight hairs on the branchlets; branchlet stipules fused for 1/8–1/6 of length; short pedicels 1.0–1.6 mm long, subtended by stipules 0.8–0.9 mm long; floral bracteoles bract-like, glabrous; glabrous calyx tube and calyx lobes with hairs only on the margins, the upper lobes only fused for ~1/10 of length; glabrous ovary and fruit.
Restricted to the Northern Tablelands of New South Wales between Tingha, Nundle and Walcha, and possibly extending W to Rocky Creek, SW of Bingara (Fig. 3c).
Recorded from open forest to woodland on a variety of substrates at altitudes of 890–1250 m, including granites (adamellites), sedimentary rock, and basalt on flats and gentle slopes, in yellow–brown, grey or grey–brown sand, clay, loam and acid volcanic soils. Recorded in association with Acacia dealbata, A. decora, Brachyloma daphnoides, Brachyscome procumbens, Bursaria spinosa subsp. spinosa, Caleana major, Callitris endlicheri, Calochilus gracillimus, Calotis dentex, Caustis flexuosa, Daviesia genistifolia, Dianella sp., Dichelachne micrantha, Eucalyptus andrewsii, E. bridgesiana, E. caliginosa, E. crebra, E. dalrympleana, E. laevopinea, E. nova-anglica, E. prava, E. youmanii, Festuca asperula, Goodenia paradoxa, Hardenbergia violacea, Imperata cylindrica, Luzula flaccida, Lyperanthus suaveolens, Melichrus urceolatus, Persoonia fastigiata, Poa sieberiana var. sieberiana, Pultenaea cuneata, Pteridium esculentum, Rytidosperma pallidum, Themeda triandra, Thysanotus tuberosus subsp. tuberosus and Triodia scariosa.
The epithet honours the collector of the type specimen, John Fauna Campbell (1853–1938), a surveyor for the New South Wales Department of Lands who was based at Walcha when the type collection was made (George 2009).
Pultenaea campbellii is known from numerous populations, including in Single National Park, Indwarra Nature Reserve, Stony Batter Creek Nature Reserve, The Basin Nature Reserve and Watsons Creek Nature Reserve. Area of extent: 16 000 km2. IUCN Category: Least Concern.
As the type gathering comprises several sheets in different herbaria, we designate a lectotype for P. campbellii, selecting the largest specimen held at NSW. Some of the labels have only ‘near Walcha’, however the material appears to be consistent and we accept the other sheets cited as isolectotypes.
A set of collections from Rocky Creek, Killarney Gap Road, NE of Narrabri (e.g. R.L.Palsson RLP 472) is anomalous in having ovaries that are hairy in the apical half. This population should be studied further to determine whether any taxonomic distinction may be warranted. Rocky Creek, Killarney Gap Road, 45 km from intersection with Newell Highway north of Narrabri, 495 m, 4 Sep. 2021, R.L.Palsson RLP 472 (NE 111974, NSW); and Killarney Gap Road, 45 km from intersection with Newell Highway north of Narrabri, 495 m, 12 Oct. 2021, R.L.Palsson RLP 481 (NE 106074, NSW). A specimen from near New England Gully Road has hairs inside the calyx lobes and the upper calyx lobes are fused for ~1/3 of length (J.R.Hosking 2237 & R.J.Sippel).
Insect galling of inflorescences can result in the production of a terminal cluster of leaves that develop long, erect hairs but non-galled growth tips on the same specimen are glabrous as is usual for this species (e.g. L.M.Copeland 3246).
NEW SOUTH WALES: Northern Tablelands: Wandsworth Road, 7 km W Ben Lomond, on roadside, 6 Dec. 1992, J.S.Benson 2015 (NSW 269193); ‘Caer Tiuvil’, 300 m W of New England Highway, N of Armidale, 20 Jan. 1994, J.S.Benson s.n. & R.J.Denham (NSW 408157); The Parlor, Guyra District, 28 Oct. 1929, W.F.Blakely s.n., E.N.McKie & T.Yowman (NSW 38674); Top of range near Backwater, 30 Oct. 1029, W.F.Blakely s.n., E.N. McKie & T.Yowman (NSW 38680); along New England Hwy near Devil’s Pinch, N of Armidale, 3 Apr. 1997, L.M.Copeland 97-963 (NSW 438220); NE section, Watsons Creek Nature Reserve, 1 May 1998, L.M.Copeland 98-1004 (CANB 544868, NE 67052, NSW 502421); New Valley Fire Trail near Single National Park S boundary, 27 Oct. 1999, L.M.Copeland 1915 & N.E.Noble (NE 70423, NSW 599832); along 4WD track just within E boundary, ~20 km NE of Bundurra, Indwarra Nature Reserve, 1 Nov. 2001, L.M.Copeland 3246 (CANB 550141, NE 76919, NSW 599834); 2.8 km S of ‘Lutana’, ~20 km E of Bundarra, The Basin Nature Reserve, 14 Nov. 2001, L.M.Copeland 3261 & P.Croft (NE 79010, NSW 541989); ~60 km WNW of Armidale, along creekline in NW section of reserve, Stony Batter Creek Nature Reserve, 980 m, 21 Nov. 2001, L.M.Copeland 3271 (CANB 559987, NE 79091, NSW 599833); Booroolong to Longford, 4 Nov. 1953, M.Gray 2854 (CANB 670757); ‘Minbalup’, property of J. Hystrek, end of New England Gully Road via Moonbi, 21 Oct. 2002, J.R.Hosking 2237 & R.J.Sippel (CANB 572262, MEL, NE 84623, NSW 631391); Watsons Creek Nature Reserve, 15 Oct. 2012, J.R.Hosking 3642 (CANB 817603, MEL, NE 99138, NSW 2 sheets); Watsons Creek State Conservation Area west of old tin mining dam, 4 Nov. 2012, J.R.Hosking 3656 (CANB 817597, MEL, NE 99155, NSW 2 sheets); Langford area, 4 Nov. 1953, R.W.Jessup & M.Gray 122 (CANB 93133); Baldersleigh area, 25 Nov. 1953, R.W.Jessup & M.Gray 263 (CANB 93134); Guyra-Inverell Road, 8 Nov. 1990, S.McIntyre 30-2 (NSW 415173); Guyra, Nov. 1927, E.N.McKie s.n. (NSW 38675); Guyra, Feb. 1928, E.N.McKie FF (NSW 38676); Guyra, Apr. 1928, E.N.McKie 104 (NSW 38678); Guyra District, Sep. 1928, E.N.McKie s.n. (SYD); Baldersleigh, SW of Guyra, (0.25 mile [~0.4 km] S of Baldersleigh on Longford road), Nov. 1965, J.B.Williams s.n. (NE 52271, NSW 371376); Devils Pinch, on New England Highway between Armidale and Black Mountain, Oct. 1959, J.B.Williams 705 (NE 10608, NE 11314, NSW 371375); ~3 km from Hanging Rock, Nundle Road, 22 Oct. 1977, J.B.Williams s.n. (NE 35628, NSW 930354).
Pultenaea corrickiae R.L.Barrett, Orme & Clugston, sp. nov.
Type: New South Wales: South Western Slopes: 3.2 km NE on Mundarlo Road (Old Hume Hwy) from the Sturt Hwy, 27 Sep. 2022, A.E.Orme 1770 & E.C.Orme (holo: NSW 1129631; iso: CANB, MEL).
Pultenaea sp. Wagga Wagga (G.Burrows 13) NSW Herbarium, PlantNet [https://plantnet.rbgsyd.nsw.gov.au/, accessed 25 July 2022].
Erect to spreading shrub 0.6–1.6 m high, multi-stemmed from the base, spreading 0.6–1.2 m wide. Branchlets erect to spreading, stiff, shortly ribbed below petioles, densely pubescent with short, crisped, spreading to erect, tangled hairs to 0.5 mm long. Stipules lanceolate, fused for 1/10–1/8 of length (almost appearing free), 1.8–3.5 mm long, ~0.5 mm wide at sinus, weakly to moderately keeled, brown to red–brown with broad pale margins; apex spreading, long–acuminate; margins glabrous or with a few long hairs towards the apex. Leaves alternate: petiole 0.2–0.4 mm long; lamina narrow–lanceolate, moderately pubescent with somewhat spreading hairs to 0.5 mm long abaxially, almost glabrous adaxially, weakly discolourous but adaxial surface commonly hidden by incurved margins, 4.3–8.5 mm long, 0.5–1.4 mm wide, with scattered, irregular papillose cells adaxially, surface smooth with distinctly oblong papillose cells abaxially; apex straight to slightly oblique, acute, rigidly and abruptly mucronate, mucro pungent, 0.2–0.3 mm long; margins strongly incurved, partly or completely hiding the adaxial surface at maturity. Inflorescence of 4–12 solitary flowers borne distinctly below the branchlet apex, appearing well-separated to loosely clustered. Flowers: pedicels subtended by slightly enlarged stipules 3.2–3.9 mm long, each ~0.6 mm wide at sinus, not or partly hiding the pedicels; pedicels 1.9–3.5 mm long, densely hairy; floral bracteoles inserted 1.0–1.4 mm above the pedicel with a rib extending down to the pedicel apex, appressed at base, linear (not or scarcely fused to subtending stipules), leaf-like, herbaceous, green, 3.6–4.8 mm long, reaching 4/5–9/10 length of calyx, long–mucronate, moderately long, erect pubescent throughout; stipules narrow, 1.9–2.2 mm long, each ~0.3 mm wide at sinus, with scattered hairs in margins; calyx tube broadly obconic, 2.2–2.8 mm long, greenish, with dense, erect hairs outside; calyx lobes 5, triangular, 3.5–4.3 mm long, acuminate (or with a short mucro to 0.4 mm long), with many, long, spreading to erect hairs right to the apex, upper pair broader, fused for ~1/3 of length; standard petal ±circular in appearance or obcordate when flattened and claw is visible, emarginate, 7.8–8.7 mm long including 3.2–3.4 mm claw, 7.6–8.4 mm wide, orange–yellow with a small red irregular band grading to orange upwards, with red lines along veins, around a dull orange–yellow ‘eye’, reverse side orange–yellow to red at the base; wing petals narrow–oblong, 7.1–8.5 mm long including 2.0–2.7 mm claw, 2.2–2.4 mm wide, orange, darker towards the base; keel petals naviculate, ±narrow–oblong to obovate in outline, 8.2–8.7 mm long including claw 2.3–2.6 mm long, 2.9–3.5 mm wide (when folded), slightly exceeding wings, dark brick red to burgundy at the apex, grading to dark red at the base; stamens free to the base, subequal in length; filaments 7.5–9.1 mm long; anthers ~0.4 mm long; ovary sessile, 2-ovulate, ~1.2 mm long, with a tuft of many long hairs along most of length; style 7.5–8.6 mm long, gently curving, glabrous; stigma inconspicuous, very slightly capitate. Fruit ovoid, pale brown, compressed, asymmetrically beaked, 3.8–4.2 mm long including stylar remnant, 2.5–2.9 mm in diameter, moderately hairy in at least apical 1/2. Seeds (only seen immature) compressed, obliquely ovoid, >1.9 mm long, >1.0 mm wide, smooth; aril white, likely entire, straight, >0.5 mm long. n = 7, 2n = 14; based on V.E.Sands 639.3.1. (Fig. 10–12.)
Holotype of Pultenaea corrickiae R.L.Barrett, Orme & Clugston (A.E.Orme 1770 & E.C. Orme; NSW).

Field photographs of Pultenaea corrickiae R.L.Barrett, Orme & Clugston (a–d). Voucher: A.E.Orme AO1770 (NSW). Photos by A. E. Orme.
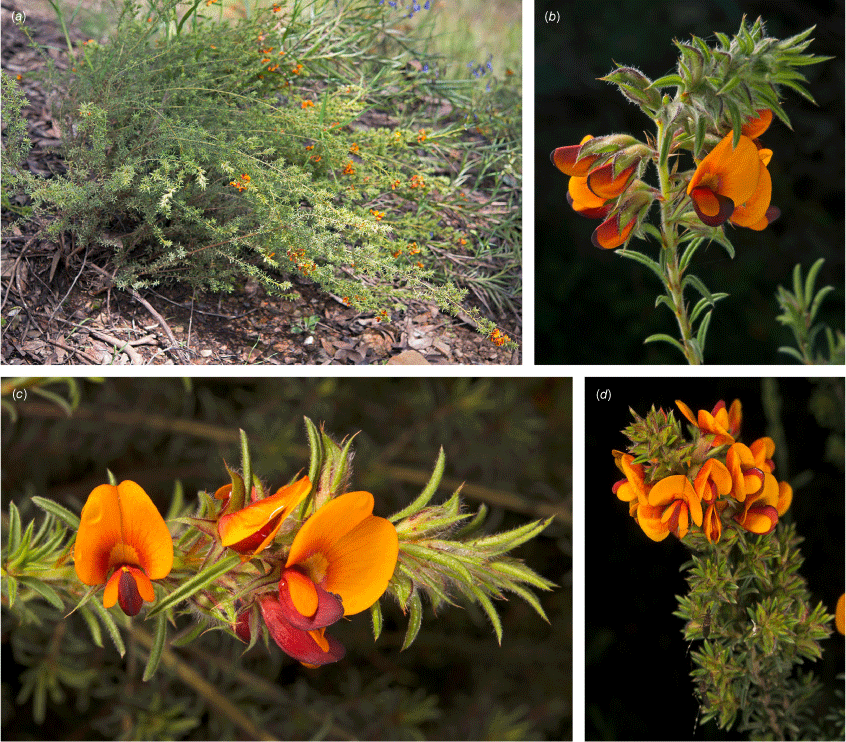
Line drawing of Pultenaea corrickiae R.L.Barrett, Orme & Clugston. (a) Flowering branchlet. (b) Leaves: abaxial surface (R), adaxial surface (L). (c) Leaf lamina surface: abaxial surface (R), adaxial surface (L). (d) Stipules and petiole. (e) Lateral view of flower. (f) Standard. (g) Bracteole and stipules. Voucher: G.Burrows 13 (NSW). Scale bars: a, 10 mm; b, 4 mm; c, d, 2 mm; e, f, 5 mm; g, 2.5 mm. Illustration by L. Elkan.
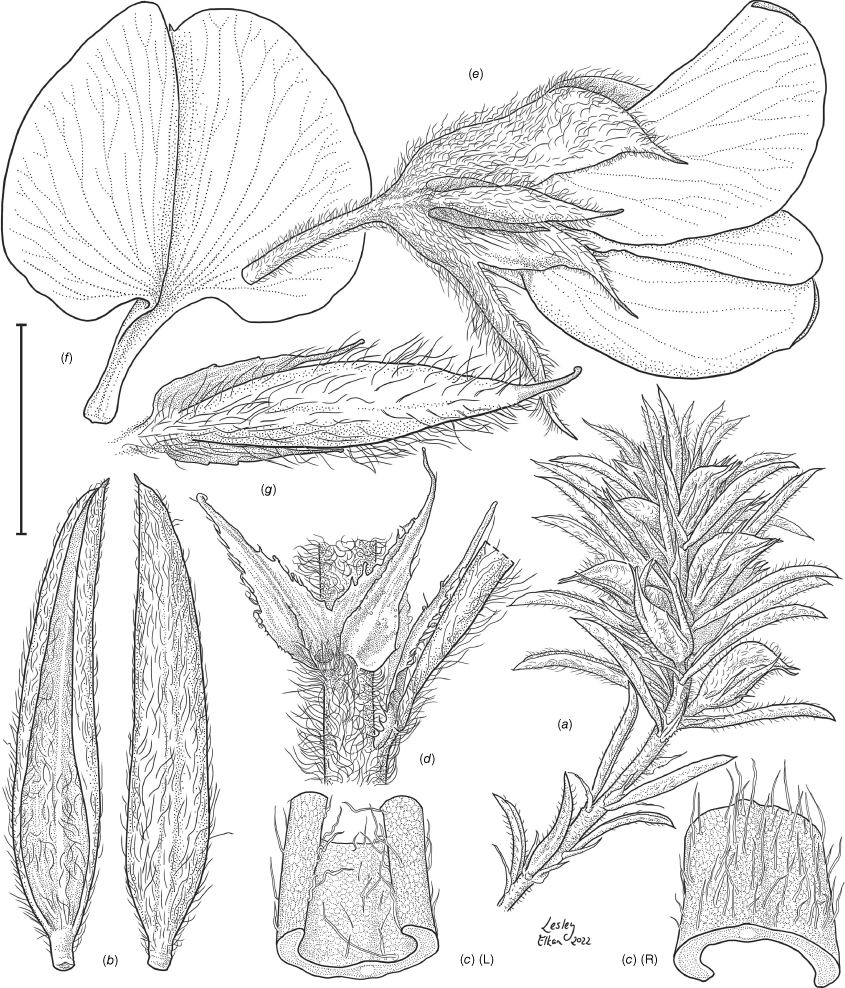
Somewhat similar in appearance to P. lapidosa and P. woolcockiorum but lacking the long leaf mucro and enlarged bracteolar stipules found in these species, and distinguished from these species by the following combination of characters: branchlet hairs crisped; branchlet stipules fused for 1/10–1/8 of length (almost appearing free); petioles 0.2–0.4 mm long; leaf mucro short, 0.2–0.3 mm long; pedicels 1.9–3.5 mm long, densely hairy; floral bracteoles 3.6–4.8 mm long; calyx tube 2.2–2.8 mm long, with dense, erect hairs; ovary ~1.2 mm long, hairy for most of length; style 7.5–8.6 mm long. Differs most distinctly from P. purdieae, with which collections suggest the species co-occurs near Talbingo, in the moderately pubescent leaves with somewhat spreading hairs to 0.5 mm long on the abaxial surface.
Only known from four locations, near Wagga Wagga, south of Mundarlo, north of Tumut, and near Talbingo (Fig. 3e). However, there are also reports of this species occurring between Mangoplah and Cookardinia, and west of Adelong (https://waggaflora.com/shrubs-trees/stony-bush-pea/), though there is possibly confusion with a form of P. procumbens.
Grows in Eucalyptus woodland on gentle slopes on clay loam. Recorded in association with Acacia decora, A. pycnantha, A. verniciflua, Allocasuarina verticillata, Austrostipa densiflora, A. scabra subsp. falcata, Brachyloma daphnoides, Callitris glaucophylla, Cheilanthes austrotenuifolia, Chrysocephalum apiculatum, C. semipapposum, Dillwynia sericea, Eucalyptus albens, E. blakelyi, E. polyanthemos subsp. vestita, E. sideroxylon, Glycine clandestina, Lomandra filiformis subsp. coriacea, Plantago varia, Poa sieberiana, Rytidosperma erianthum, Stypandra glauca, Themeda triandra and Xerochrysum viscosum.
Flowering recorded for September–November, with one outlying record from March. Fruiting recorded for October–November.
The epithet honours Victorian botanist Margaret G. Corrick (née Vaughan) (1922–2020), who had a special interest in Pultenaea, describing several new species and revising the concepts of Victorian taxa (Corrick 1976a, 1976b, 1977a, 1977b, 1977c, 1977d, 1977e, 1977f, 1978a, 1978b, 1978c, 1978d, 1980a, 1980b, 1980c, 1981, 1983a, 1983b, 1984a, 1984b, 1984c, 1987a, 1987b, 1988a, 1988b, 1993a, 1993b, 1994a, 1994b, 1996; Corrick and Walsh 2009). Corrick was able to draw on the significant earlier work by Williamson (1920, 1921, 1922, 1925, 1928) and associated specimens subsequently donated to MEL. Corrick also co-authored two popular books, Wildflowers of Southern Western Australia (Corrick and Fuhrer 1996, 2002, 2009) and Wildflowers of Victoria (Corrick and Fuhrer 2000).
Pultenaea corrickiae is currently known from a relatively restricted area but is recorded as locally common at known sites. The conservation status requires further assessment but the habitat is highly cleared and fragmented by agriculture. Area of extent: 2500 km2. IUCN Category: Vulnerable (B1a, B1bii,iii).
A superficially similar specimen was collected less than 200 m from the type location in a wetter habitat beside a small creek but the stipules are narrower, the leaves and mucro are shorter, the mucro is more recurved and the hairs on the leaf lamina are more appressed. This specimen is considered to be part of the P. procumbens species complex as the leaves are 3-veined: 3.3 km NE on Mundarlo Road (Old Hume Hwy) from the Sturt Hwy, 27 Sep. 2022, A.E.Orme 1775 (BRI, CANB, K, MEL, NSW).
NEW SOUTH WALES: 10 [~4.3] km NNW of the intersection of the Sturt and Hume Highways on Mundarlo Road, Mar. 1993, G.Burrows 10 (NSW 277011); Pomingalarna Reserve, Wagga [Wagga], 5 Sep. 1990, G.Burrows 13 (NSW 279035); Minjary National Park, 2.25 km S of Minjary Mountain, and 10.75 km NW of Tumut, 6 Nov. 2001, I.Crawford 6688 (CANB 733597, NSW 830216); Ellerslie National Park: headwaters of unnamed tributary of Yaven Yaven Creek, 4.8 km SE of Mount Yaven, and 12 km ENE of Tarcutta, 475 m, 4 Nov. 2003, I.Crawford 7807 (CANB 778099); opposite the hotel, Talbingo, Sep. 1950, J.Niewand 277 (NSW 38707); 1.5 miles [~2.4 km] S of Tumblong on Hume Highway, 18 Oct. 1965, M.E.Phillips s.n. (CBG 25145); 11 miles [~17.7 km, W of the] E end of Sturt Highway, 12 Sep. 1963, V.E.Sands 639.3.1 (SYD); South Gundagai Cemetery, ~3.5 km SW of Gundagai on road to Tumut, 240 m, 14 May 1999, M.N.Taws 1087 (CANB 617011); Pomingalarna Reserve, Wagga Wagga, 23 Oct. 1988, E.Whiting s.n. (NSW 278937).
Pultenaea estelleae R.L.Barrett & Clugston, sp. nov.
Type: New South Wales: [on Blakney Creek North Road] 0.3 km from Yass–Dalton [Rye Creek] Road, towards Blakney Creek hamlet, 560 m, 16 Nov. 1992, E.M.Canning 6809 (holo: CBG 9216404.1; iso: CBG 9216404.2, MEL 234368, n.v., NSW 500262).
Pultenaea sp. Blakney Creek (E.M.Canning 6809) NSW Herbarium, PlantNet [https://plantnet.rbgsyd.nsw.gov.au/, accessed 25 July 2022].
Erect to spreading shrub ~0.3 m high, multi-stemmed from the base, spreading ~0.8 m wide. Branchlets erect to spreading, stiff, shortly ribbed below petioles, densely pubescent with long, straight to curved, spreading to erect hairs ~0.6 mm long. Stipules lanceolate, fused for 1/3(–1/2) of length, 1.4–2.3 mm long, ~0.3 mm wide at sinus, moderately keeled, brown (almost black when dried) with narrow pale margins; apex spreading, long–acuminate; margins glabrous, sometimes with a few small teeth. Leaves alternate: petiole 0.4–0.6 mm long; lamina ±lanceolate; distinct inflorescence leaf-type with dense, erect, long, stiff hairs to 0.4 mm long on both surfaces, lamina 2.8–4.4 mm long, 0.9–1.3 mm wide; branchlet leaves sometimes with long appressed hairs on abaxial surface but quickly glabrescent, weakly discolourous, paler above but adaxial surface commonly hidden by incurved margins, lamina 3.6–5.8 mm long, 0.6–1.1 mm wide, with very dense, irregular papillose cells adaxially, surface smooth with distinctly oblong papillose cells abaxially; apex straight to slightly oblique, acute, rigidly and abruptly mucronate, mucro ±pungent, 0.1–0.2 mm long; margins strongly incurved usually mostly hiding the adaxial surface. Inflorescence of 5–12 solitary flowers borne in upper axils of short lateral branches, appearing tightly clustered. Flowers: pedicels subtended by enlarged stipules 1.7–2.2 mm long, each ~0.6 mm wide at sinus, partly hiding the pedicels; pedicels 1.0–1.5(–1.9) mm long, densely hairy; floral bracteoles inserted immediately at pedicel apex, appressed at base, linear (fused to subtending stipules in lower 1/4–1/3), leaf-like, herbaceous, green, 2.5–3.6 mm long, reaching ~3/5 length of calyx, weakly mucronate, densely pubescent with hairs erect; stipules narrow, 1.5–1.8 mm long, each ~0.3 mm wide at sinus; calyx tube broadly obconic, 2.1–3.6 mm long, green, moderately long, hairy outside with hairs erect; calyx lobes 5, narrowly triangular, 2.7–3.1 mm long, acuminate, with long, straight erect hairs on the lamina and dense, short, crisped hairs on the margins inside, upper pair broader, fused for almost 1/2 of length; standard petal ±circular in appearance or obcordate when flattened and claw is visible, emarginate, 7.8–8.9 mm long including 2.4–3.1 mm claw, 7.0–8.1 mm wide, orange–golden with red marks around a dull orange ‘eye’; wing petals narrow–oblong, 6.3–8.0 mm long including 2.0–2.5 mm claw, 1.7–2.1 mm wide, mostly yellow–orange, red towards the base; keel petals naviculate, ±narrow–oblong in outline, 6.2–7.9 mm long including claw 1.9–2.5 mm long, 2.0–2.3 mm wide (when folded), slightly exceeding wings, deep red at the apex, grading to orange at the base; stamens free to the base, subequal in length, 5.5–7.6 mm long; anthers ~0.5 mm long; ovary sessile, 2-ovulate, 1.5–1.6 mm long, with appressed hairs to 0.7 mm long in apical 1/8; style 3.6–5.7 mm long, gently curving through 90° in apical 1/3, with long, semi-appressed hairs in lower 1/8; stigma inconspicuous, very slightly capitate. Fruit not seen. (Fig. 13, 14.)
Isotype of Pultenaea estelleae R.L.Barrett & Clugston with inset photo showing details of leaves (a) and inflorescence (b) (E.M.Canning 6809; NSW 500262).
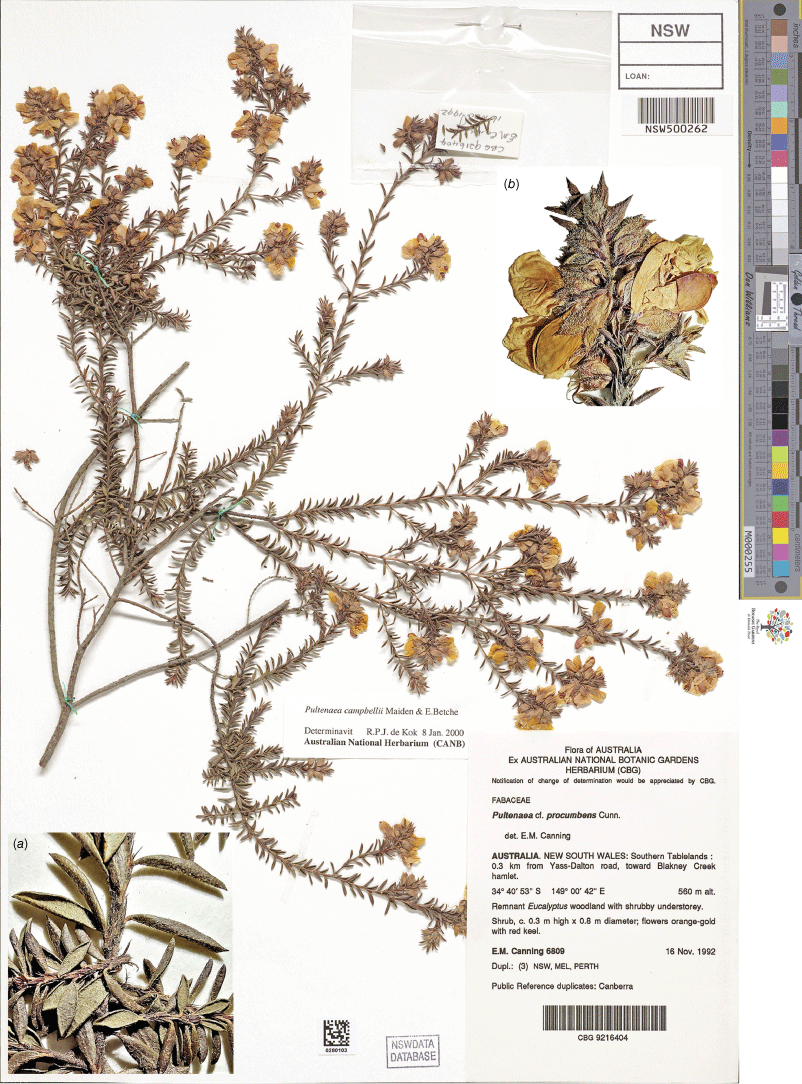
Line drawing of Pultenaea estelleae R.L.Barrett & Clugston. (a) Flowering branchlet. (b) Leaves: abaxial surface (R), adaxial surface (L). (c) Leaf lamina surface: abaxial surface (above), adaxial surface (below). (d) Leaf cross-section. (e) Stipules and petiole. (f) Lateral view of flower. (g) Standard. (h) Bracteole and stipules. Voucher: E.M.Canning 6809 (NSW). Scale bars: a, 10 mm; b, 4 mm; c–e, 2 mm; f, g, 5 mm; h, 2.5 mm. Illustration by C. Wardrop.
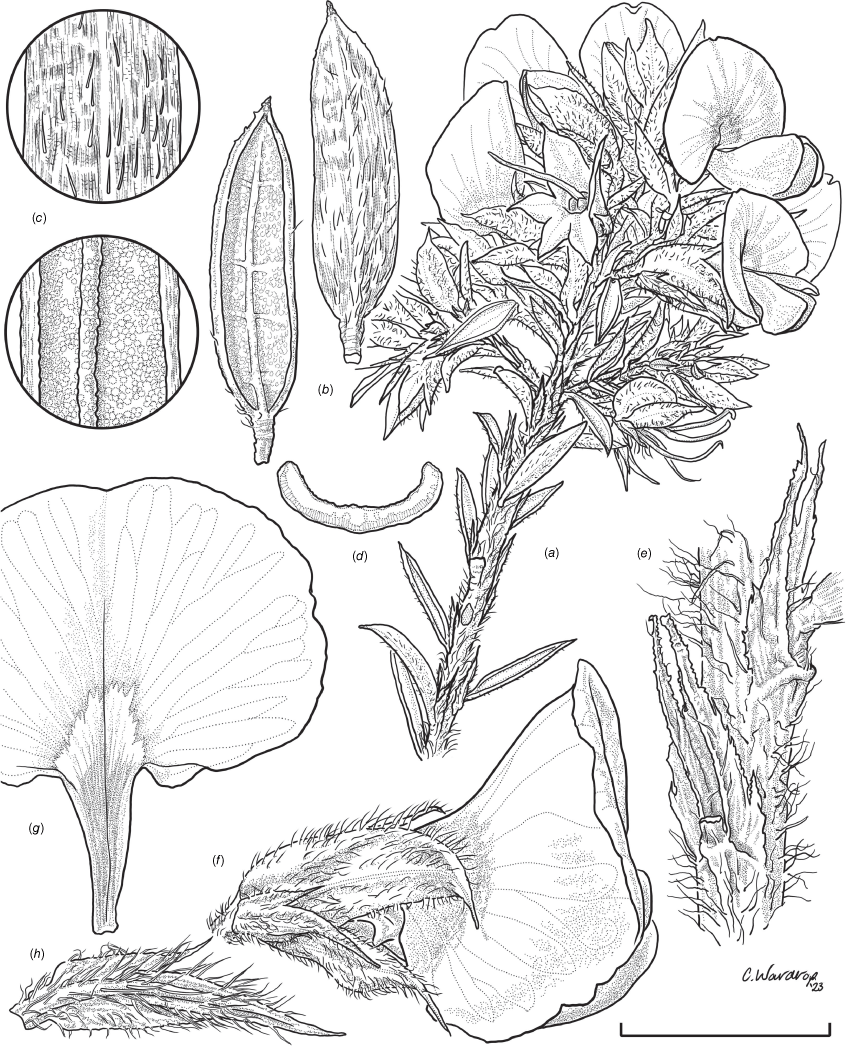
Morphologically most similar to P. corrickiae, P. purdieae and P. praetermissa but distinguished from these species by the following combination of characters: branchlets with straight to curved hairs; branchlet stipules fused for 1/3(–1/2) of length; petioles 0.4–0.6 mm long; leaves within inflorescence morphologically distinct from branchlet leaves, 2.8–4.4 mm long, 0.9–1.3 mm wide, with dense, erect, long, stiff hairs to 0.4 mm long on both surfaces; branchlet leaves with appressed hairs on abaxial surface; pedicels subtended by stipules 1.7–2.2 mm long; floral bracteoles inserted at apex of pedicel; calyx lobes with upper pair broader, fused for almost 1/2 length, 2.7–3.1 mm long, with long, straight erect hairs on lamina and dense, short, crisped hairs on margins inside; ovary 1.5–1.6 mm long, hairy in apical 1/8.
Only known from a single location near Blakney Creek, north of Yass in the Southern Tablelands of New South Wales (Fig. 3e).
Recorded from remnant Eucalyptus woodland with a shrubby understorey. Species recorded nearby include Acacia dealbata subsp. dealbata, A. deanei subsp. paucijuga, A. genistifolia, Brunonia australis, Cassinia sp., Daviesia genistifolia, Dillwynia sp., Eucalyptus spp., Pimelea curviflora var. sericea, Pultenaea polifolia, P. spinosa, P. subspicata, Ranunculus lappaceus, Rytidosperma pilosum, Senecio quadridentatus, Stypandra glauca and Themeda triandra.
The epithet honours Estelle Margaret Canning (1936–2021) who made the only known collection of the species near Dalton, where Canning lived after a long career at the Australian National Botanic Gardens, Canberra. In conjunction with many co-workers, Estelle Canning made over 10 500 plant collections across southern Australia (CHAH 2022; https://www.anbg.gov.au/biography/canning-estelle-margaret.html).
Pultenaea estelleae is currently known from a single location in a landscape with a high degree of clearing for agriculture. Targeted surveys are required to assess the conservation status but the species may well qualify for listing as Endangered. Two searches of the type locality in August and November 2022 failed to relocate the species, however sufficient native vegetation with similar habitat remains within a few kilometres of the site, in which the species is likely still present and further regional surveys should be prioritised. IUCN Category: Endangered (B1a, B2a).
Duplicates of this collection have variously been identified as Pultenaea campbellii, P. procumbens or P. setulosa. Two additional collections are superficially similar in appearance but differ in indumentum and calyx features, and are considered more appropriately treated under the Pultenaea procumbens complex: Top Creek Property, ~27.5 km NNW of Boorowa, ~2.5 km along entrance road to the property from the Cowra–Boorowa Road, 580 m, 30 Oct. 1985, P.Beesley 520, I.Gleadhill & B.Rimes (MEL, n.v., NSW 474270); Old Frogmore cemetery (near school), 21 Dec. 1999, W.Semple OR 1807 (NSW 539156).
Pultenaea subspicata Benth. has been recorded at the type location of P. estelleae but differs in having obtuse rather than mucronate leaf apices, broader leaves 1–2 mm wide and longer stipules 2–4 mm long.
Pultenaea farmeriana R.L.Barrett, Orme & P.H.Weston, sp. nov.
Type: New South Wales: Central Tablelands: Pound Creek Station, ~0.15 km SE of farmhouse, 7 Nov. 2009, P.H.Weston 3370 & P.Bernhardt (holo: NSW 798476; iso: BRI AQ0881077, CANB 723251, LP, MEL 2344631, NE 99432).
Pultenaea sp. Pound Creek Station (P.H.Weston 3370 & P.Bernhardt) NSW Herbarium, PlantNet [https://plantnet.rbgsyd.nsw.gov.au/, accessed 25 July 2022].
Erect, spreading or decumbent shrub 0.25–0.5 m high, multi-stemmed from the base, spreading 0.4–1.2 m wide. Branchlets erect to spreading, shortly ribbed below petioles, stiff, moderately pubescent with short, crisped, appressed, matted hairs to 0.4 mm long. Stipules lanceolate, fused for 1/2–3/4 of length, 2.0–3.6 mm long, ~0.4 mm wide at sinus, prominently keeled, brown to red–brown with paler margins; apex spreading, long–acuminate; margins with scattered tooth-like projections, otherwise glabrous. Leaves alternate: petiole 0.5–0.7 mm long; lamina ±linear, glabrous, weakly discolourous, 4.7–7.3 mm long, 0.4–0.7(–0.9) mm wide, with dense, irregular papillose cells adaxially, surface smooth with distinctly oblong papillose cells abaxially; apex straight to slightly oblique, acute, rigidly and abruptly mucronate, mucro pungent, 0.5–0.7 mm long; margins strongly incurved usually hiding the adaxial surface. Inflorescence of 8–12 solitary flowers borne in upper axils, appearing tightly clustered. Flowers: pedicels subtended by enlarged stipules 3.9–4.3 mm long, each 0.7–1.1 mm wide at sinus, hiding the pedicels; pedicels 0.5–0.9 mm long, glabrous; floral bracteoles inserted ~0.3 mm below pedicel apex, not appressed at base, linear (fused to subtending stipules for > 1/2 of length), bract-like, scarious, brown, 4.1–4.7 mm long, free portion 1.5–2.0 mm long, reaching ~1/2 length of calyx, not mucronate, pubescent in mid-1/3; stipules broad, 3.2–3.5 mm long, each ~0.8 mm wide at sinus, calyx tube broadly obconic, 2.7–3.3 mm long, green, glabrous outside; calyx lobes 5, narrowly triangular, 3.9–5.3 mm long, acuminate, with long spreading hairs mostly restricted to margins, upper pair slightly broader, fused for ~1/3 of length; standard petal ±circular in appearance or obcordate when flattened and claw is visible, emarginate, 7.2–9.1 mm long including 2.4–3.2 mm claw, 6.4–8.0 mm wide, orange–yellow with a broad red crescent around a dull orange ‘eye’, reverse side dark red; wing petals narrow–oblong, 6.5–7.2 mm long including 2.7–3.2 mm claw, 2.0–2.2 mm wide, mostly yellow–orange, red towards the base; keel petals naviculate, ±narrow–oblong in outline, 6.8–8.3 mm long including claw 2.2–3.1 mm long, 2.6–2.9 mm wide (when folded), slightly exceeding wings, deep red at the apex, grading to pink at the base; stamens free to the base, subequal in length, 5.3–7.5 mm long; anthers ~0.4 mm long; ovary sessile, 2-ovulate, 1.0–1.2 mm long, with long, appressed hairs in apical 1/8; style 7.6–8.1 mm long, curving almost 90° towards apex, with long, semi-appressed hairs only in lower 1/6–1/4; stigma inconspicuous, very slightly capitate. Fruit not seen. (Fig. 15–17.)
Holotype of Pultenaea farmeriana R.L.Barrett, Orme & P.H.Weston (P.H.Weston 3370 & P.Bernhardt; NSW 798476).
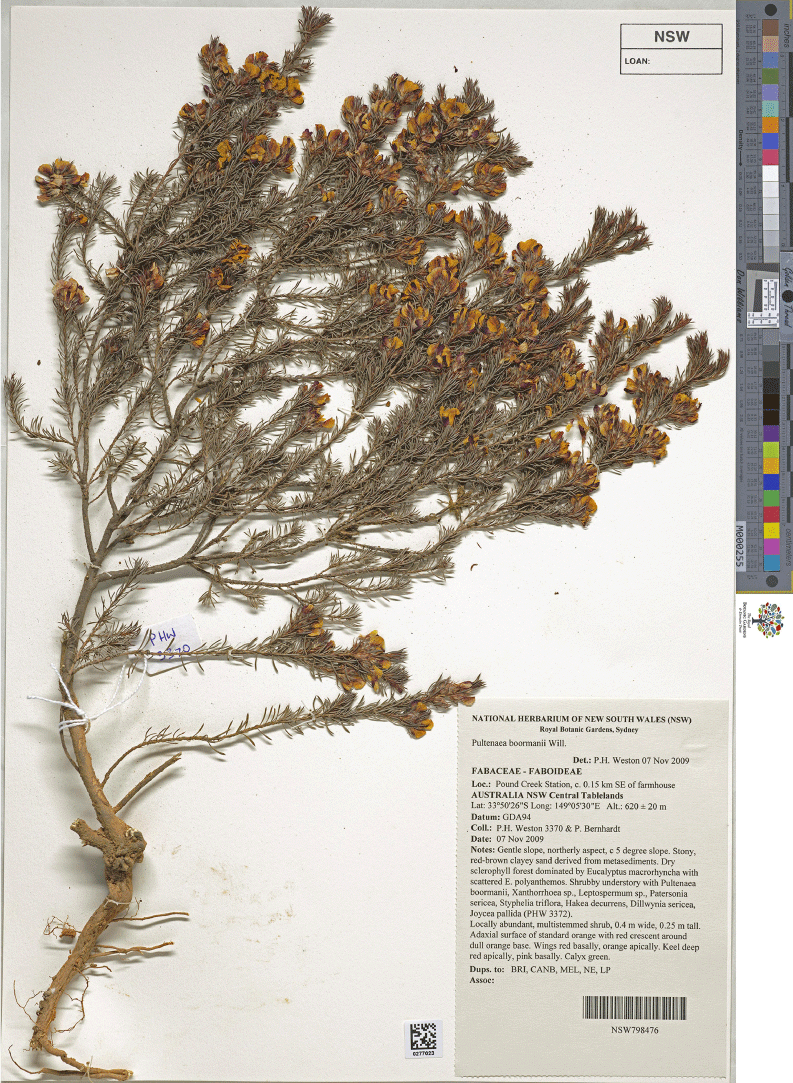
Field photographs of Pultenaea farmeriana R.L.Barrett, Orme & P.H.Weston (a–d). (e, f) Flowers being visited by a native bee (Leioproctus wilsonii). Vouchers: (a–c) A.E.Orme AO1617 (NSW); (d–f) P.H.Weston 3370 & P.Bernhardt (NSW). Photos: (a–c) by A. E. Orme; (d–f) by P. H. Weston.
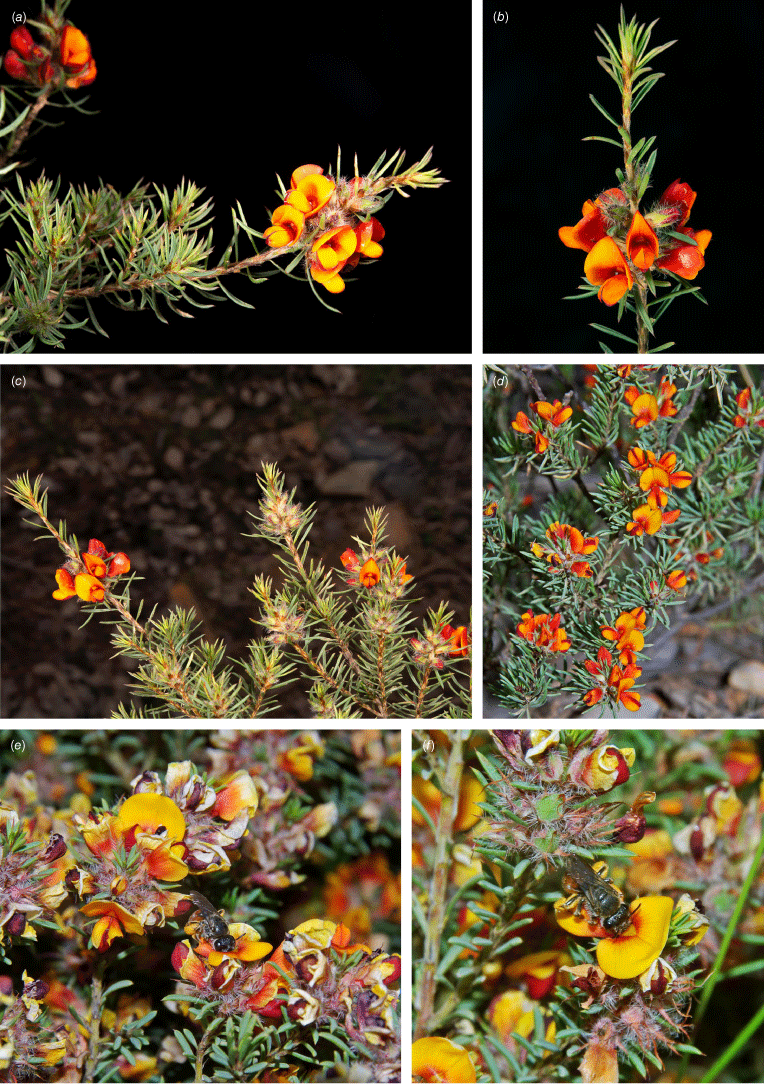
Line drawing of Pultenaea farmeriana R.L.Barrett, Orme & P.H.Weston. (a) Flowering branchlet. (b) Leaves: abaxial surface (L), adaxial surface (R). (c) Leaf lamina surface: abaxial surface (R), adaxial surface (L). (d) Leaf cross-section. (e) Stipules and petiole. (f) Lateral view of flower. (g) Standard. (h) Bracteole and stipules. Voucher: P.H.Weston 3370 & P.Bernhardt (NSW). Scale bars: a, 10 mm; b, 10 mm; c–e, 2 mm; f, g, 5 mm; h, 2.5 mm. Illustration by C. Wardrop.
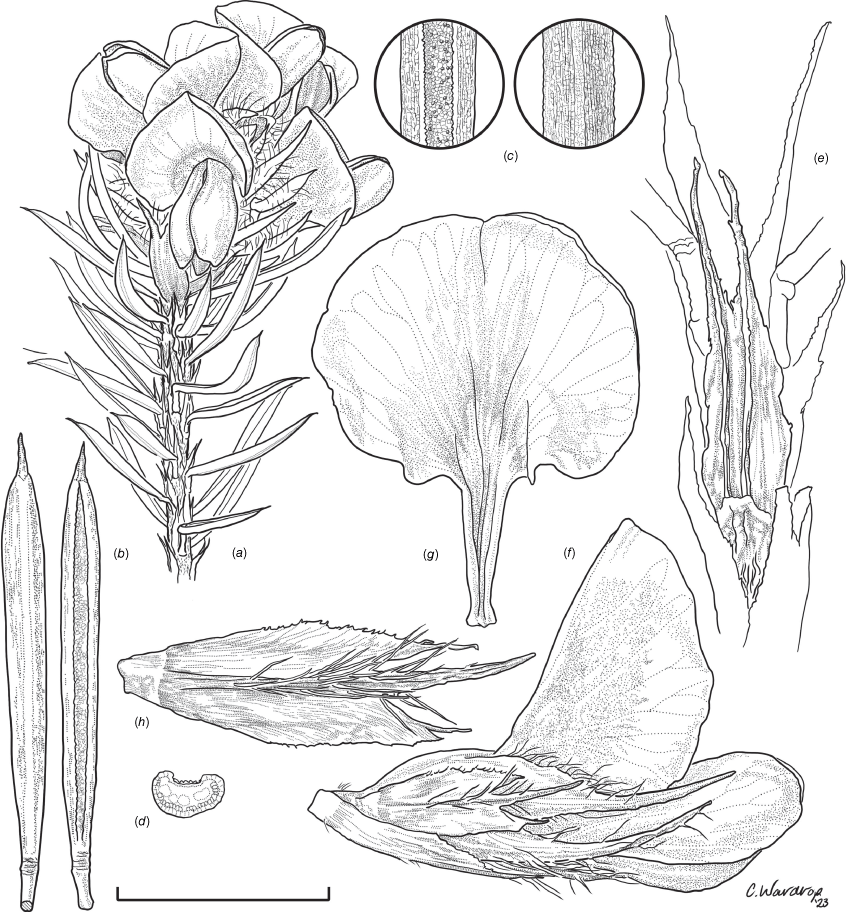
Similar in appearance to P. boormanii, P. westonii and P. woolcockiorum but distinguished from these species by the following combination of characters: branchlet hairs crisped; leaves ±linear, glabrous, 0.4–0.7(–0.9) mm wide, mucro 0.5–0.7 mm long; pedicels 0.5–0.9 mm long, glabrous, subtended by stipules 3.9–4.3 mm long, 0.7–1.1 mm wide, hiding the pedicels; floral bracteoles inserted ~0.3 mm below pedicel apex, linear, bract-like, ~1/2 length of calyx, not mucronate, pubescent in mid-1/3 of length; calyx tube 2.7–3.3 mm long, glabrous, calyx lobes with upper pair slightly broader, fused for ~1/3 length, 3.9–5.3 mm long, with long spreading hairs mostly on margins; style 7.6–8.1 mm long.
Only known from a single location on Pound Creek Station, at an altitude of ~600 m, south of Orange, in the Central Tablelands of New South Wales (Fig. 3d).
Recorded from a gentle slope with a northerly aspect, on stony, red–brown clay and sand derived from metasediments in dry sclerophyll forest dominated by Eucalyptus macrorhyncha with scattered E. melliodora and E. polyanthemos, and a shrubby understorey. Associated species include Acacia genistifolia, A. vestita, Brachyloma daphnoides, Bursaria spinosa, Caladenia atrovespa, Callitris endlicheri, Calytrix tetragona, Centrolepis strigosa, Dillwynia sericea, Diuris praecox, Eucalyptus blakelyi, E. goniocalyx, E. rossii, Hakea decurrens, Hibbertia aff. calycina, H. obtusifolia, H. sp. Pound Creek (P.H.Weston 3367 & P.Bernhardt), Hydrocotyle sp., Laxmannia gracilis, Lepidosperma lineare, Leptospermum multicaule, Lomandra longifolia, Microseris walteri, Patersonia sericea, Persoonia rigida, Plantago sp., Pomaderris prunifolius, Pultenaea procumbens, Ranunculus sp., Rytidosperma pallidum, Schoenus apogon, Stuartina muelleri, Stylidium despectum, Styphelia triflora, Thelymitra ixioides, Thysanotus patersonii, Viola betonicifolia, Wurmbea sp. and Xanthorrhoea sp.
In recognition of the support of Antionette Farrow and John Palmer, owners of Pound Creek Station, in documenting the native flora on the rural property on which this species was discovered. The epithet is formed as a contraction and combination of the surnames Farrow and Palmer (far- and -mer) with the termination -iana, and thus combined also refers to the English ‘farmer’ as a rural occupation.
Pultenaea farmeriana is currently known from a single, large population. The species has been recorded as locally abundant at the known locality on private property. At this location, there is grazing pressure from goats and deer, and disturbance by pigs. Further survey work in the surrounding region is required to determine the distribution and conservation status. Area of extent: 1.5 km2. IUCN Category: Endangered (B1a, B2a).
The species has been recognised at NE and NSW under the informal phrase name Pultenaea sp. Pound Creek Station (P.H.Weston 3370 & P.Bernhardt). The collections cited here were previously assigned to P. boormanii and P. campbellii.
Observed to be pollinated by the native bee Leioproctus wilsoni (Rayment).
NEW SOUTH WALES: Central Western Slopes: Pound Creek Station, N of farmhouse, 7 Oct. 2020, A.E.Orme AO1617 & S.F.McCune (NSW 1119349); Pound Creek Station, 600 m, 9 July 2002, P.H.Weston 2557 (NSW 493982); Pound Creek Station, ~0.1 km SSE of farmhouse, 580 m, 4 Oct. 2003, P.H.Weston 2629 (NSW 610364); Pound Creek Station, ~0.15 km SE of farmhouse, 16 July 2022, P.H.Weston 3745 (NSW 1125072); Pound Creek Station, ~0.15 km SE of farmhouse, 16 July 2022, P.H.Weston 3746 (NSW 1125073).
Pultenaea hoskingii R.L.Barrett & Clugston, sp. nov.
Type: New South Wales: North Western Slopes: alongside Woolomin tip, 27 Sep. 2001, J.R.Hosking 2072, A.J.Lawler, R.J.Sippel & P.J.Christian (holo: NSW 522117; iso: CANB, MEL 2198337, NE 79380).
Pultenaea sp. Woolomin (J.R.Hosking 2072 et al.) NSW Herbarium, PlantNet [https://plantnet.rbgsyd.nsw.gov.au/, accessed 25 July 2022].
Erect or sprawling shrub 0.4–1.5 m high, bark grey, multi-stemmed from the base, spreading 0.5–0.7 m wide. Branchlets erect to spreading, stiff, shortly ribbed below petioles, moderately pubescent with short, crisped, appressed, matted hairs to 0.3 mm long. Stipules lanceolate, fused for ~(1/4–)1/2 of length, 1.3–2.0 mm long, ~0.4 mm wide at sinus, weakly to moderately keeled, brown to red–brown with paler margins; apex spreading, long–acuminate; margins with short, crisped hairs in mid-third of length. Leaves alternate: petiole 0.2–0.8 mm long; lamina ±linear, with sparse, appressed, straight hairs to 0.4 mm long (adaxially only on the midrib), eventually glabrescent, weakly discolourous, 2.3–8.6(–12.1) mm long, 0.6–1.5 mm wide, with moderately dense, irregularly rounded cells adaxially (cells not papillose), surface smooth or finely striate (when dry) with linear to narrow–oblong cells abaxially (not papillose); apex straight to slightly oblique, acute, rigidly and abruptly mucronate, mucro pungent, 0.1–0.4 mm long; margins strongly incurved but not hiding the adaxial surface. Inflorescence of 6–15 solitary flowers borne in upper axils, appearing tightly clustered. Flowers: pedicels subtended by slightly enlarged stipules 1.7–2.1 mm long, each 0.4–0.5 mm wide at sinus, partly hiding the pedicels; pedicels 2.3–4.9 mm long, with dense, appressed to spreading straight white hairs to 0.2 mm long; floral bracteoles inserted exactly at pedicel apex, appressed at base, linear (fused to subtending stipules in lower 1/2–2/3), bract-like, scarious, brown, 2.3–2.7 mm long, free portion 0.8–1.0 mm long, reaching 1/2–3/5 length of calyx, mucronate, pubescent except at apex; stipules narrowly triangular, 1.3–1.4 mm long, each ~0.3 mm wide at sinus; calyx tube broadly obconic, 1.1–2.0 mm long, green, moderately densely pubescent outside; calyx lobes 5, the upper pair usually broader, fused for (1/2–) ~2/3 of length, broadly triangular, 1.7–2.3 mm long, acuminate, with long spreading hairs except towards the apex and margins with dense, short, crisped hairs, the lower lobes narrowly triangular, 2.7–3.5 mm long, acuminate, with long spreading hairs except towards the apex, margins with dense, short, crisped hairs; standard petal ±circular in appearance or obcordate when flattened and claw is visible, emarginate, 5.8–7.1 mm long including 2.7–2.9 mm claw, 6.2–7.6 mm wide, yellow–orange with an irregular red rim around a dull orange ‘eye’; wing petals narrow–oblong, 7.7–8.2 mm long including 0.7–0.9 mm claw, 2.0–2.6 mm wide, mostly yellow–orange, reddish towards the base; keel petals naviculate, ±narrow–oblong in outline, 7.0–8.5 mm long including claw 2.0–2.2 mm long, 2.9–3.3 mm wide (when folded), slightly exceeding wings, brownish-red, paler at the base; stamens free, unequal in length; filaments 4.5–5.8 mm long; anthers ~0.5 mm long; ovary sessile, 2-ovulate, 2.2–3.7 mm long, with appressed hairs to 1.1 mm long in apical 3/4; style 6.0–6.7 mm long, curving ~90° ~2/3 from base, glabrous; stigma inconspicuous, very slightly capitate. Fruit ovoid, pale brown, compressed, asymmetrically beaked, 5.5–6.1 mm long including stylar remnant, 2.4–2.6 mm in diameter, sparsely hairy at least in apical 3/4, the hairs spreading, to 1.7 mm long. Seeds compressed, irregular–ovoid, 2.2–2.4 mm long, 1.5–1.9 mm wide, smooth, olive–green with dark brown mottling or flecks; aril white, ±straight, the apex highly divided into course, hair-like lobes, 1.9–2.2 mm long. (Fig. 18–20.)
Holotype of Pultenaea hoskingii R.L.Barrett & Clugston (J.R.Hosking 2072 et al.; NSW 522117).

Field photographs of Pultenaea hoskingii R.L.Barrett & Clugston. Vouchers: (a, b, d, h) J.R.Hosking 4167 (NE); (c, e) J.R.Hosking 3651 (NE); (f, g, i) J.R.Hosking 3820 (NE). Photos by J. R. Hosking.
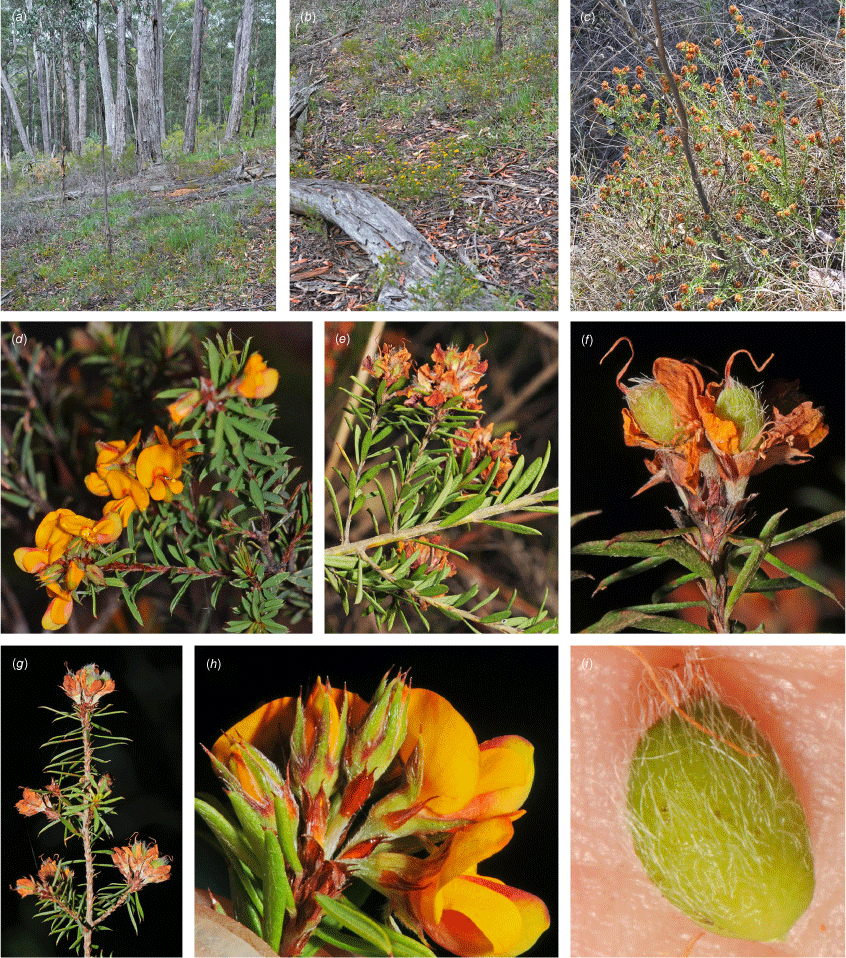
Line drawing of Pultenaea hoskingii R.L.Barrett & Clugston. (a) Flowering branchlet. (b) Leaves: adaxial surface (L), abaxial surface (R). (c) Leaf lamina surface: adaxial surface (L), abaxial surface (R). (d) Leaf cross-section. (e) Stipules. (f) Lateral view of flower. (g) Standard. (h) Bracteole and stipules. Voucher: J.R.Hosking 2072 et al. (NSW). Scale bars: a, 10 mm; b, 4 mm; c–e, 2 mm; f, g, 5 mm; h, 2.5 mm. Illustration by C. Wardrop.
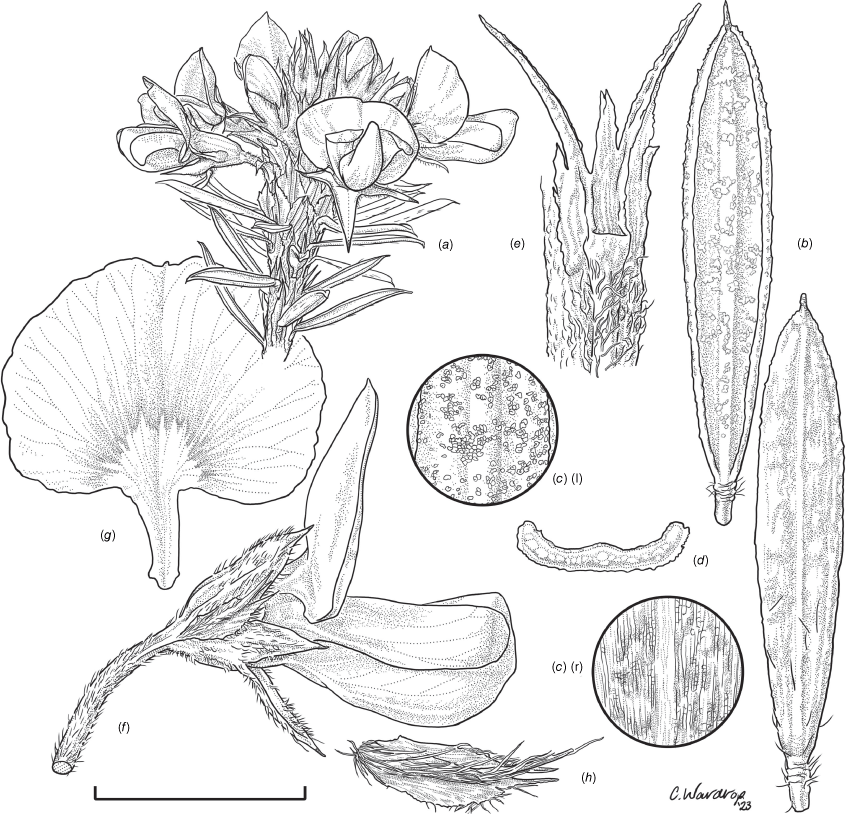
Similar in appearance to P. campbellii and P. palssoniae but distinguished from these species by the following combination of characters: crisped branchlet hairs; branchlet stipules fused for ~1/2 of length; leaves ±linear, pedicels 2.3–4.9 mm long, subtended by stipules 1.7–2.1 mm long, 0.4–0.5 mm wide; floral bracteoles 1.8–2.5 mm long, inserted exactly at pedicel apex, linear, bract-like, 1/3–2/5 length of calyx, mucronate, pubescent except at apex; bracteolar stipules 1.3–1.4 mm long; calyx tube densely pubescent, calyx lobes with upper pair broader, fused for ~2/3 of length, 1.7–3.5 mm long, with long spreading hairs except towards the apex; ovary 2.2–3.7 mm long, hairy in apical 3/4; style 6.0–6.7 mm long. Pultenaea campbellii has glabrous pods while P. hoskingii and P. palssoniae have hairy pods.
Only known from SE of Tamworth in the North Western Slopes and Northern Tablelands of New South Wales between Woolomin, Hanging Rock, Nundle, Bralga Tops and Nowendoc (Fig. 3c).
Recorded from basalt, serpentinite, metasediment, jasper and quartz soils in low heath, woodland or tall open forest communities with numerous shrubs, grasses and herbs, on gentle slopes from 500 to 1100 m. Recorded in association with Acacia falciformis, A. filicifolia, Billardiera scandens, Brachyscome sieberi, Callitris glaucophylla, Cassinia laevis, C. leptocephala, Cheilanthes sieberi subsp. sieberi, Cryptandra amara, Dichelachne micrantha, Dillwynia juniperina, Dodonaea viscosa subsp. angustifolia, Eucalyptus albens, E. cypellocarpa, E. elliptica, E. laevopinea, E. macrorhyncha, E. moluccana, E. pauciflora, E. radiata subsp. sejuncta, E. viminalis, Festuca asperula, Hakea microcarpa, Hovea similis, Indigofera adesmiifolia, Leptospermum brevipes, Leucopogon affinis, Lomandra sp., Macrozamia concinna, Olearia fulgens, Podolobium ilicifolium, Pomaderris betulina subsp. betulina, Pultenaea campbellii, P. microphylla, Senecio quadridentatus, Tasmannia stipitata and Themeda australis.
The epithet recognises the extensive work of botanist John R. Hosking in documenting the Australian flora while based at the Department of Agriculture in Tamworth and more recently as a volunteer at NE, including collection of this species.
Pultenaea hoskingii is locally restricted and extant populations are fragmented by clearing for agriculture and pine plantations. The species is conserved within Curracabundi National Park and is considered locally common here (>1000 plants in collection area, but only at one known site, ~100 × 30 m), and in Back River Nature Reserve (>30 plants in collection area). Area of extent: 500 km2.
John Hosking drew the attention of Rogier de Kok to this taxon in 2002 (correspondence on specimen sheets at NE and NSW); however, at that time the taxon was determined to be part of the broader P. setulosa complex. Pultenaea hoskingii is closely related to P. campbellii and the two species notably occur in proximity between Nundle and Hanging Rock based on specimen records, though mixed populations are yet to be observed. The upper calyx lobes on J.R.Hosking 4188 are only fused for ~1/2 length.
NEW SOUTH WALES: Nowendoc State Forest, 1200 m, Mar. 1995, W.Chapman s.n. (NSW 414066); at Bralga Tops – Glenrock, 14 Nov. 1985, H.Davie s.n. (NSW 371378); Ferman Plot – Hanging Rock State Forest No. 671, 3500 ft [~1.07 km], 11 Nov. 1969, A.G.Floyd H20 (NSW 371374); alongside Woolomin tip, 500 m, 6 Dec. 2001, J.R.Hosking 2160 (BRI AQ0980109, CANB, MEL 2198336, NE 79399, NSW 522128); Woolomin Gap Road near Woolomin, 24 Oct. 2012, J.R.Hosking 3651 (CANB 817600, MEL 2382113, NE 99150, NSW 825845); Back River Nature Reserve, 1100 m, 10 Dec. 2014, J.R.Hosking 3820 (CANB 825114, MEL 2395189, NE 101593, NSW 930742); western section of Curracabundi National Park, 990 m, 12 Oct. 2020, J.R.Hosking 4167 (BRI, CANB, MEL, NE 110907, NSW); western section of Curracabundi National Park, 990 m, 3 Nov. 2020, J.R.Hosking 4188 (BRI, CANB, MEL, NE 110964, NSW); near Hanging Rock [near Nundle], S.E. of Tamworth, 3800–4000 [~3640] ft [1.16–1.22, ~1.11 km], Nov. 1976, J.Turner s.n. (NSW 467165); 2–3 km from Nundle on the road to Hanging Rock, 1 Nov. 1982, C.E.Woolcock s.n. (NSW 500270).
Pultenaea imminuta R.L.Barrett & S.F.McCune, sp. nov.
Type: New South Wales: [precise locality withheld] Leard State Forest, [NE of] Boggabri, 395 m, 24 Sep. 2009, D.Landenberger s.n. (holo: NSW 865925).
Pultenaea sp. Maules Creek (M.V.Robinson s.n., NSW 1115296).
Shrub ~0.6 m high, width of spread not recorded. Branchlets erect to spreading, stiff, shortly ribbed below petioles, moderately to densely pubescent with short, slightly crisped or straight, appressed, matted hairs to 0.5 mm long. Stipules lanceolate, fused for ~1/3 of length, 1.2–3.1 mm long, ~0.3 mm wide at sinus, moderately keeled, brown to red–brown with paler margins; apex spreading, long–acuminate; margins with scattered tooth-like projections, glabrous. Leaves alternate: petiole 0.4–0.9 mm long; lamina ±linear, with scattered, appressed, straight hairs to 0.7 mm long abaxially (adaxially only on newest growth), eventually glabrescent, weakly discolourous but adaxial surface mostly or completely hidden by incurved margins, 3.9–14.0 mm long, 0.5–0.9(–1.4) mm wide, with dense, irregularly rounded papillose cells adaxially, surface smooth with irregularly oblong papillose cells abaxially; apex slightly to strongly oblique, acute, rigidly mucronate, mucro pungent, (0.1–)0.2–0.4 mm long; margins strongly incurved, mostly or completely hiding the adaxial surface. Inflorescence of 6–12 solitary flowers borne in upper axils, appearing very loosely to tightly clustered. Flowers: pedicels subtended by enlarged stipules 1.6–2.8 mm long, each 0.5–0.6 mm wide at sinus, with short, stiff, erect hairs on margins, partly hiding the pedicels; pedicels 1.6–2.3 mm long, with dense, appressed to spreading, straight, white hairs to 0.1 mm long; floral bracteoles inserted at the apex or up to 0.6 mm above pedicel apex, appressed at base, linear (free from subtending stipules), leaf-like, herbaceous, green, 3.8–6.4 mm long, reaching ±full length of calyx, mucronate, pubescent; stipules narrowly triangular, 1.4–1.8 mm long, each ~0.2 mm wide at sinus; calyx tube broadly obconic, 1.2–1.5 mm long, green, moderately densely pubescent outside; calyx lobes 5, narrowly triangular, 3.1–4.4 mm long, acuminate or acute, with long spreading hairs except towards the apex, margins with dense, short, crisped hairs inside, upper pair broader, fused for almost 1/3 of length; standard petal ±circular in appearance or obcordate when flattened and claw is visible, emarginate, 5.5–7.7 mm long including 2.1–2.6 mm claw, 4.0–5.8 mm wide, yellow–orange with minute red marks around a small yellow ‘eye’; wing petals narrow–oblong, 4.7–6.8 mm long including 0.9–1.4 mm claw, 1.2–1.7 mm wide, yellow throughout; keel petals naviculate, ±narrow–oblong in outline, 5.1–7.0 mm long including claw 1.1–1.3 mm long, 1.6–3.3 mm wide (when folded), slightly exceeding wings, yellow–orange, paler at the base; stamens free, subequal in length; filaments 3.7–4.9 mm long; anthers ~0.4 mm long; ovary sessile, 2-ovulate, 1.3–2.1 mm long, with dense, appressed hairs to 0.6 mm long in apical 5/6–1/2; style broad at base, ±central on ovary apex, 4.1–6.5 mm long, curving ~90° ~5/6 from base, glabrous; stigma inconspicuous, very slightly capitate. Fruit not seen. (Fig. 21, 22.)
Holotype of Pultenaea imminuta R.L.Barrett & S.F.McCune with inset photo showing details of leaves and inflorescence (D.Landenberger s.n.; NSW 865925).
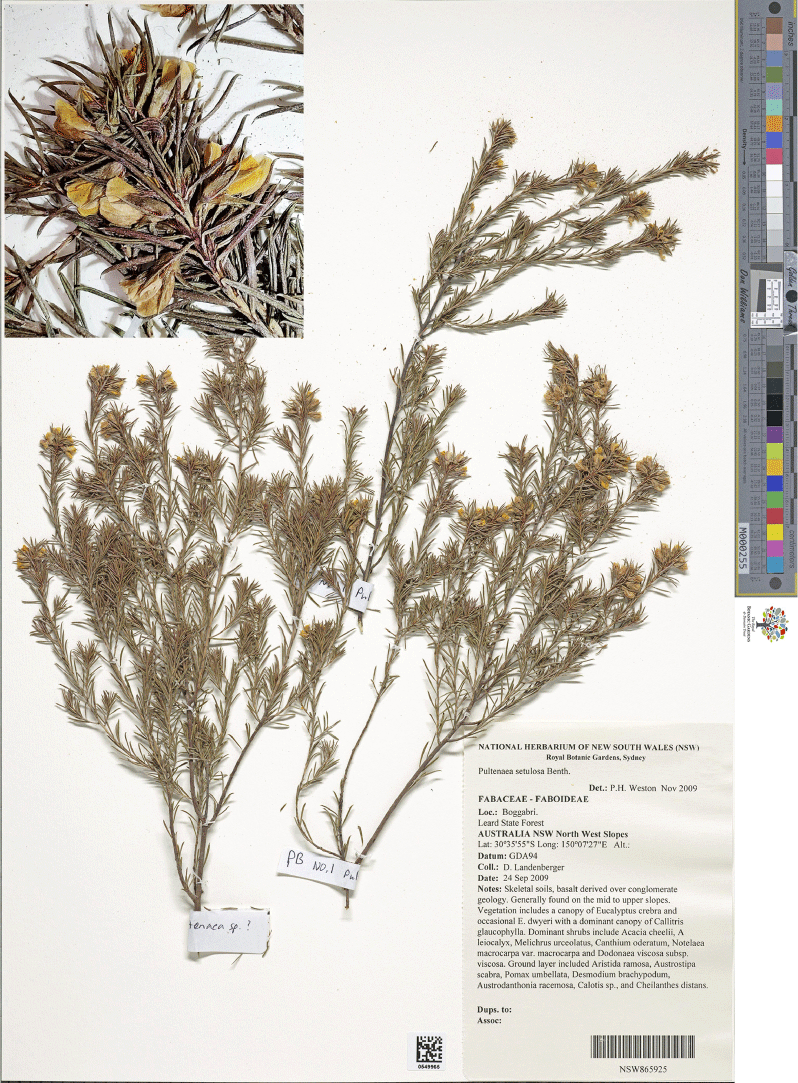
Line drawing of Pultenaea imminuta R.L.Barrett & S.F.McCune. (a) Flowering branchlet. (b) Leaves: adaxial surface (L), abaxial surface (centre), leaf apex (R). (c) Leaf lamina surface: abaxial surface (R), adaxial surface (L). (d) Stipules and petiole. (e) Lateral view of flower. (f) Standard. (g) Bracteole and stipules. Voucher: D.Landenberger s.n. (NSW 865925). Scale bars: a, 10 mm; b, 4 mm; c, d, 2 mm; e, f, 5 mm; g, 2.5 mm. Illustration by L. Elkan.
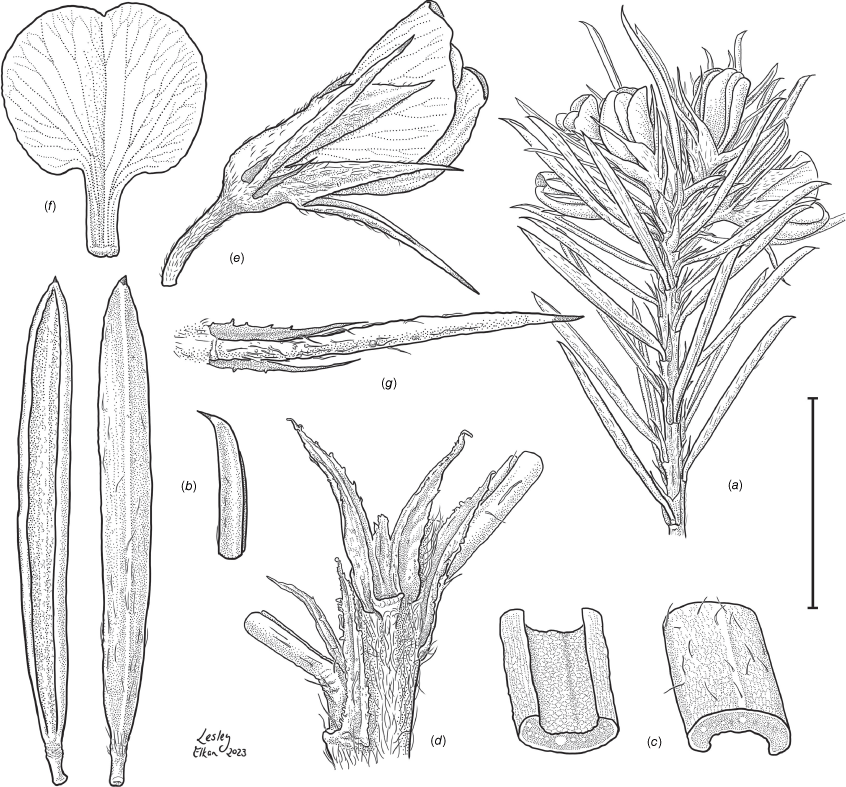
Similar in appearance to P. boormanii but distinguished by the following characters: branchlet stipules 1.2–3.1 (v. 2.7–3.6) mm long, margins with scattered (v. few) tooth-like projections, glabrous (v. with a patch of crisped hairs); leaves held at 30–45° (v. 45–90°) from branchlets; leaf lamina 0.5–0.9(–1.4) (v. 0.3–0.6) mm wide, drying smooth (v. with a rough, sculpted texture); inflorescence of 6–12 (v. 4–8) solitary flowers; calyx sparsely to moderately hairy (v. densely hairy), hairs ±appressed (v. somewhat spreading to erect); standard 4.0–5.8 (v. 7.1–8.0) mm wide; keel yellow–orange (v. usually brick red); staminal filaments 3.7–4.9 (v. 5.1–5.6) mm long; ovary 1.3–2.1 (v. 1.1–1.2) mm long, with dense, appressed hairs to 0.6 (v. to 2.1) mm long; style inserted centrally (v. distinctly eccentric) on ovary apex.
Only known from the Leard State Forest, north-east of Boggabri, south-east of Narrabri, in the North Western Slopes of New South Wales at an altitude of ~400 m, very close (<1 km) to both Boggabri Coal Mine and Maules Creek Coal Mine (Fig. 3c).
Grows in eucalypt and cypress pine woodland on skeletal soils derived from basalt over conglomerate on mid to upper slopes of hills. Recorded in association with Acacia cheelii, A. leiocalyx, Aristida ramosa, Austrostipa scabra, Callitris glaucophylla, Calotis sp., Cheilanthes distans, Dodonaea stenophylla, D. truncatiales, D. viscosa subsp. angustifolia, D. viscosa subsp. mucronata, D. viscosa subsp. viscosa, Eucalyptus crebra, E. dwyeri, Geijera parviflora, Gonocarpus elatus, Melichrus urceolatus, Notelaea microcarpa var. microcarpa, Oxytes brachypoda, Pimelea neo-anglica, Pomax umbellata, Psydrax odorata, Rytidosperma fulvum, Rytidosperma racemosum and Senna aciphylla.
The epithet is from the Latin imminuo (to encroach upon, lessen or diminish), in reference to the extant habitat of this species that has historically been significantly encroached upon by agriculture and logging, and is currently being encroached upon by open-pit coal mining.
Pultenaea imminuta is known from two vouchered collections made ~1.5 km apart, with a third unvouchered sighting by Tanya Bangel on 3 Oct. 2018 (BioNet Atlas Record No. LJJSI0149462; as Pultenaea setulosa) ~1.5 km away and all from active mining tenements within Leard State Forest, south-east of Narrabri. The three locations are all situated between the Boggabri Coal Mine and the Maules Creek Coal Mine. Collection notes from one of the known sites lists fire, past logging, grazing, slashing, soil and water alteration, weeds and roads as disturbance factors. The Area of Extent is likely to be less than 20 km2 and the known Area of Occurrence is currently less than 1.5 km2, with known, ongoing, imminent threats to all known populations. No estimates of population size are currently available as searches in the vicinity of the three known locations in October 2022 failed to relocate this species. IUCN Category: Critically Endangered (A2, B1a, B1b(i–v), C2a(i)).
This species is morphologically similar in many respects to P. boormanii that occurs in the Pilliga, ~65 km west of P. imminuta. However, molecular data demonstrate that these two species are related but not sister taxa, justifying distinction at specific rank (Clugston et al., in prep.). The style positioned centrally on the ovary is an unusual feature in the P. setulosa group. The only other Pultenaea species recorded from Leard State Forest is P. cuneata Benth. that is easily distinguished by the broad, cuneate leaves. Pultenaea cinerascens Maiden & Betche has also been reported from nearby but is readily distinguished by the clustered leaves.
Pultenaea lapidosa Corrick, Muelleria 8(2): 119, fig. 1 (1994)
Type: Victoria: Eastern Highlands, 16 km ENE of Omeo township on Old [Mill] Track, NE of its junction with Scrubby Creek Track, 23 Nov. 1986, M.G.Corrick 10029 (holo: MEL 0686134, MEL 2103285 [spirit], n.v.; iso: BRI-AQ0520789*, CBG 9400485, HO 307676*, K, NSW 500260, PERTH 9315209).
Illustration: Corrick (1994, p. 119, fig. 1).
Erect to spreading or decumbent shrub 0.4–0.6 m high, multi-stemmed from the base, spreading 0.5–0.7 m wide. Branchlets erect to spreading, stiff, shortly ribbed below petioles, young growth sparsely pubescent with short, straight, appressed, pale hairs to 0.4 mm long, glabrescent. Stipules lanceolate, fused for 2/3–3/4 of length, 3.1–4.2 mm long, ~0.5 mm wide at sinus, prominently keeled, dark brown to almost black; apex spreading, long–acuminate; margins with scattered, short tooth-like projections; margins and keels with sparse, long hairs. Leaves alternate: petiole 0.9–1.4 mm long; lamina ±linear to narrowly elliptic, discolourous, (5.5–)7.2–9.6 mm long, 0.9–1.3 mm wide, with dense, irregular papillose cells adaxially, otherwise glabrous; sparse, long, tubercle-based hairs abaxially and midrib slightly raised, surface otherwise smooth with rounded to shortly oblong cells; apex acute, rigidly and abruptly mucronate, mucro straight to recurved, pungent, 0.8–1.7 mm long; margins incurved but not hiding the adaxial surface (except on new growth). Inflorescence of 10–25 solitary flowers borne in upper axils, appearing tightly clustered, each flower subtended by a slightly reduced leaf with enlarged stipules. Flowers: pedicels subtended by enlarged stipules 4.9–6.7 mm long, each 1.2–1.3 mm wide at sinus, hiding the pedicels; pedicels 1.0–1.2 mm long, glabrous except at apex; floral bracteoles inserted ~0.3 mm below pedicel apex, appressed at base, linear (fused to subtending stipules for almost 1/2–2/3 of length), leaf-like, herbaceous, green, 3.5–4.9 mm long, free portion 1.6–2.4 mm long, reaching ~2/3 length of calyx, long–mucronate, densely long, erect pubescent throughout; stipules narrow, 3.1–3.2 mm long, each ~0.6 mm wide at sinus, glabrous; calyx tube broadly obconic, 2.6–3.3 mm long, greenish, glabrous outside; calyx lobes 5, triangular, 2.8–4.3 mm long, acuminate, with many, long, spreading to erect hairs, subequal, the upper pair fused for almost 1/2 of length; standard petal ±circular in appearance or obcordate when flattened and claw is visible, emarginate, 8.5–9.6 mm long including 3.1–4.1 mm claw, 5.8–7.2 mm wide, deep orange with a small crescent of red lines around a dull orange ‘eye’, reverse side dark orange–red; wing petals narrow–oblong, 6.2–7.2 mm long including 2.5–3.2 mm claw, 1.1–1.4 mm wide, deep orange, red towards the base; keel petals naviculate, ±narrow–oblong to obovate in outline, 7.1–8.5 mm long including claw 2.8–3.1 mm long, 2.1–2.6 mm wide (when folded), slightly exceeding wings, dark brick red at the apex, grading to red at the base; stamens free to the base, subequal in length; filaments 4.8–6.7 mm long; anthers ~0.4 mm long; ovary sessile, 2-ovulate, ~1.5 mm long, with long, appressed hairs in apical 1/3; style 6.5–8.1 mm long, gently curving, with long, semi-appressed hairs only in lower 1/8–1/6; stigma inconspicuous, very slightly capitate. Fruit ovoid, pale brown, compressed, asymmetrically beaked, 5.4–6.9 mm long including stylar remnant, 2.6–3.1 mm in diameter, sparsely hairy in apical 1/3. Seeds compressed, obliquely ovoid, 2.1–2.8 mm long, 1.2–1.8 mm wide, smooth, dark brown; aril white, ornate (coralline), highly divided into coarse, hair-like lobes, 0.5–0.7 mm long. (Fig. 23–25.)
Field photographs of Pultenaea lapidosa Corrick from the Myrtleford population (a–e). Photos (a–c) by M. Jusaitis; (d, e) by M. Cincotta. All previously posted on iNaturalist. Photo (f) From Hinnomunjie population, by J. Turner.
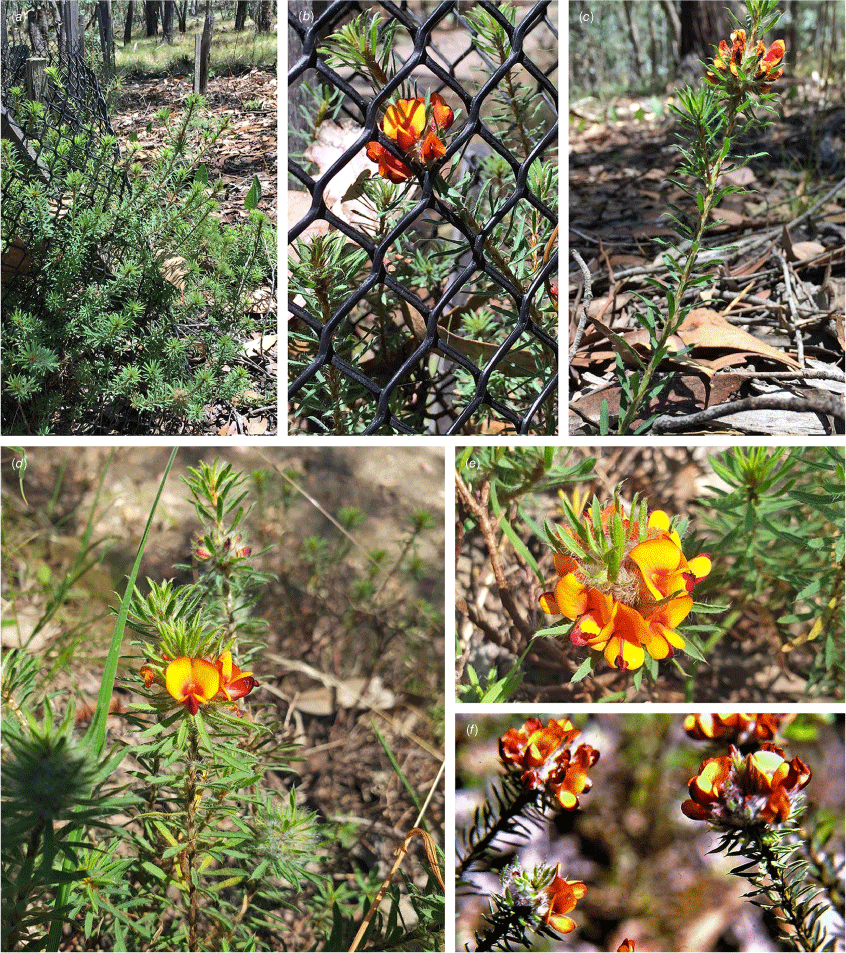
Line drawing of Pultenaea lapidosa Corrick. (a) Flowering branchlet. (b) Leaves: adaxial surface (R), abaxial surface (L). (c) Leaf lamina surface: abaxial surface (below), adaxial surface (above). (d) Stipules. (e) Lateral view of flower. (f) Standard. (g) Bracteole and stipules. Voucher: G.W.Carr 10268 (NSW). Scale bars: a, 10 mm; b, 4 mm; c, d, 2 mm; e, f, 5 mm; g, 2.5 mm. Illustration by C. Wardrop.
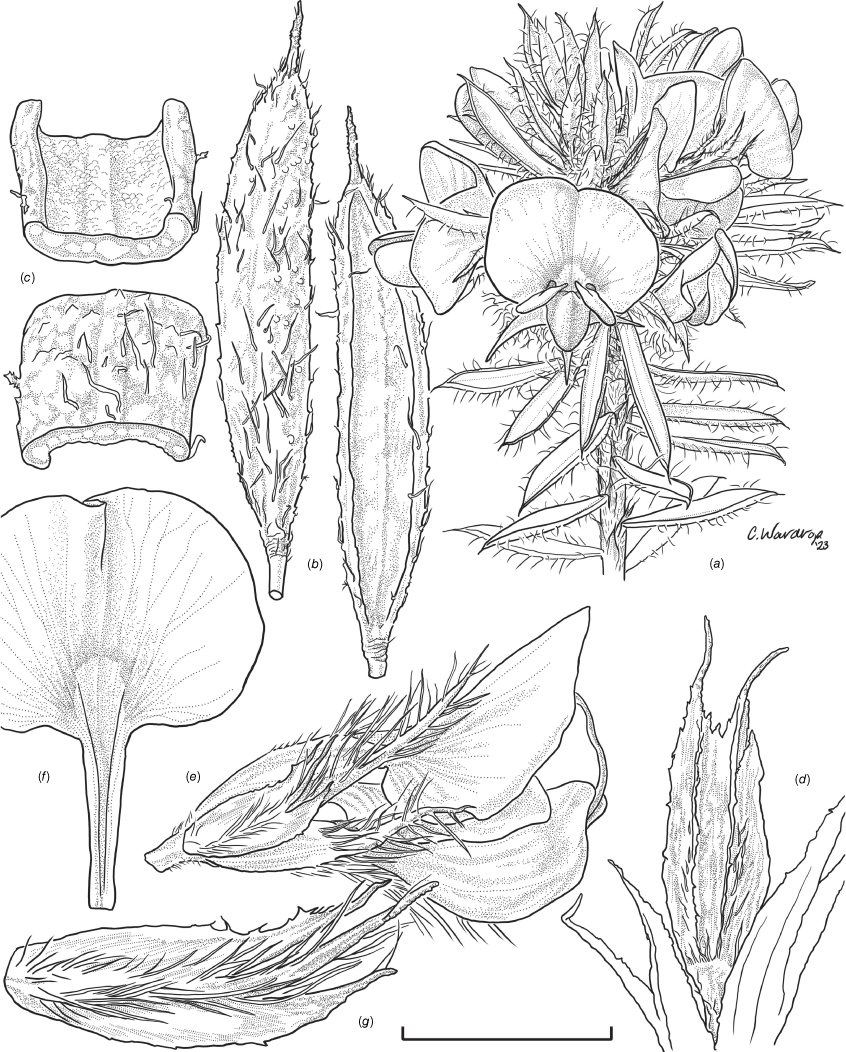
Similar in appearance to P. corrickiae, P. farmeriana, P. praetermissa, P. westonii and P. woolcockiorum but distinguished from these species by the following combination of characters: straight hairs on the branchlets; branchlet bracteoles fused for 2/3–3/4 of length; petioles 0.9–1.4 mm long; leaf lamina ±linear to narrowly elliptic, 0.9–1.3 mm wide, with long, tubercle-based hairs on abaxial surface, margins incurved but not hiding the adaxial surface, mucro 0.8–1.7 mm long; pedicels 1.0–1.2 mm long, glabrous except at apex; subtended by stipules 4.9–6.7 mm long, 1.2–1.3 mm wide, hiding the pedicels; floral bracteoles 2.0–3.1 mm long, inserted ~0.3 mm below pedicel apex, linear, leaf-like, ~2/3 length of calyx, long–mucronate; bracteolar stipules 3.1–3.2 mm long, ~0.6 mm wide; calyx tube 2.6–3.3 mm long, glabrous, calyx lobes with upper pair broader, fused for almost 1/2 length; 2.8–4.3 mm long; standard 8.5–9.6 mm long, 5.8–7.2 mm wide, deep orange; ovary ~1.5 mm long, hairy in apical 1/3; aril highly divided into coarse, hair-like lobes.
Only known from three locations in north-eastern Victoria near Mount Samaria, Myrtleford and Omeo at altitudes of 600–700 m (Fig. 3f).
Recorded from open box/stringybark woodland and forest over sparse shrubs and grasses, on rocky hillsides over pale brown sandy loam with gravel. Recorded in association with Acacia dealbata, A. implexa, Bossiaea sericea, B. distichoclada, Cassinia aculeata, Correa lawrenceana var. latrobeana, Dichelachne hirtella, Dillwynia phylicoides, D. sericea, Eucalyptus camphora subsp. humeana, E. goniocalyx, E. largiflorens, E. macrorhyncha, E. mannifera, E. polyanthemos, Grevillea lanigera, Hydrocotyle hirta, Kunzea ericoides, Leptospermum brevipes, Persoonia confertifolia, Platylobium montanum, Pimelea sp., Poa morrisii, P. sieberiana, Pultenaea spinosa, Rytidosperma pallidum, Schoenus apogon, Tetratheca bauerifolia and Xanthorrhoea glauca subsp. angustifolia.
Flowering recorded for November–December(–February). Fruiting recorded for December–January.
The epithet is from the Latin lapidus [lapideus] (of stone) in reference to the favoured habitat on rocky slopes.
Pultenaea lapidosa is listed as Vulnerable in Victoria. The species is currently known from three localities in north-eastern Victoria near Mount Samaria, Myrtleford and Omeo. Collections record this species as locally common, but highly restricted in extent. The Reform Hill population near Myrtleford consists of over 1500 plants in an area of ~0.5 ha. The Hinnomunjie population near Omeo was recorded as consisting of ~200 plants in an area of ~0.5 ha (J.A.Jeanes 2582 & A.J.Brown). Area of Extent: 3500 km2. Area of Occupancy: <5 km2. IUCN Category: Vulnerable (B2a, C1, D2).
The original concept of this species included NSW populations that are significantly disjunct (Corrick 1994). Further investigation of the NSW populations included by Corrick (1994) identified that these represent three morphologically distinct taxa we recognised at the rank of species, P. corrickiae, P. westonii and P. woolcockiorum, rendering P. lapidosa endemic to Victoria. Pultenaea murrayi also belongs to this group but no specimens were available to Corrick and this species has never been formally included within P. lapidosa.
VICTORIA: ~16 km ENE of Omeo township on Old Mill Track, 2.4 km NE of its junction with Scrubby Creek Track, ~5.3 km SW of Mt Tambo, ~600 m, 11 Dec. 1984, G.W.Carr 10268 (CBG 8502067, MEL 0671346, NSW 500259 (2 sheets)); Mount Samaria (~35 km S of Benalla), 16 Dec. 1974, M.G.Corrick 4880 (MEL 1575701); 16 km ENE Omeo township on Old Mill Track 2.3 km NE of its junction with Scrubby Creek Track ~5.3 km SW of Mt Tambo, 23 Nov. 1986, M.G.Corrick 10025 (MEL 0685981); Myrtleford – beside Halls Road, below the lookout, ~3 km east of the town; on public land next to pine plantation, 20 Feb. 1990, M.G.Corrick 10680 (MEL 2094102A); Hinnomunjie, ~700 m along Sisters Track from McKenzie Road, 15 Feb. 2011, J.A.Jeanes 2582 & A.J.Brown (CANB 728724, MEL 2338212, S A1244-2, n.v.); Myrtleford Lookout Reserve, NE Study Area, Sector I, Sub-block 55c, 14 Nov. 1989, J.Strudwick 677 (MEL 1579940); Myrtleford lookout, NE Study area, Sector I, Sub-block 55a, 29 Nov. 1989, J.Strudwick 744 (MEL 1579916); Myrtleford lookout, 28 Dec. 1989, J.Strudwick 776 (MEL 1579915A); by rough track south of Benambra, 22 Nov. 1995, J.Turner 1070 & P.Tratt (MEL 2041758); Myrtleford lookout, 29 Nov. 1989, W.S.Wilson s.n. (MEL 1579965).
Pultenaea murrayi R.L.Barrett, sp. nov.
Type: New South Wales: W of Old Sydney Road on Queanbeyan Fault Escarpment, ~2 km E of Queanbeyan River, 29 Oct. 1995, E.Gilbert 950001, N.Williams & W.Gray (holo: CBG 9517770).
Erect to spreading shrub 0.4–0.5 m high, multi-stemmed from the base, spreading 0.6–1.0 m wide. Branchlets erect to spreading, stiff, very shortly ribbed below petioles, young growth densely pubescent with short, crisped, matted or appressed, pale hairs to 0.3 mm long. Stipules broadly lanceolate, fused for 1/2–3/5 of length, 3.1–4.3 mm long, ~0.5 mm wide at sinus, much broader below, prominently keeled, dark brown to black; apex spreading, long–acuminate; margins with scattered, short tooth-like projections; margins, keels and lower lamina with scattered to moderately dense, long, appressed hairs. Leaves alternate: petiole 1.0–1.4 mm long, glabrous; lamina ±linear, discolourous, paler adaxially, 6.4–10.7 mm long, 0.5–0.8 mm wide, with dense, irregular granular cells adaxially, otherwise glabrous; very sparse, long, tubercle-based hairs abaxially, midrib indistinct, surface otherwise smooth with oblong cells; apex acute, rigidly and abruptly mucronate, mucro straight to slightly oblique, pungent, 0.6–1.1 mm long; margins incurved, ±completely hiding the adaxial surface (except on new growth). Inflorescence of 3–24 solitary flowers borne in upper axils, appearing very tightly clustered or occasionally spike-like. Flowers: pedicels subtended by enlarged stipules 4.7–5.6 mm long, fused for most of length, each ~0.7 mm long at sinus but enlarged to 1.6 mm wide at broadest point, hiding the pedicels; pedicels 0.4–0.7 mm long, glabrous except at apex; floral bracteoles inserted ±at pedicel apex, appressed at base, linear (fused to subtending stipules for 3/5–2/3 of length), bract-like, scarious, brown, 4.3–5.7 mm long, free portion 1.4–2.4 mm long, reaching 2/3–1/2 length of calyx, not or weakly mucronate, densely long, pubescent throughout with hairs erect; stipules broad, 3.8–4.6 mm long, each ~0.9 mm wide at sinus, to 1.5 mm wide below, glabrous except where fused to bracteole; calyx tube broadly obconic, 2.6–3.8 mm long, pale brown to greenish, glabrous or with short, appressed hairs on tube, with long erect or spreading hairs to 3.3 mm long towards apex; calyx lobes 5, very narrowly triangular, 3.5–4.8 mm long, long–acuminate, with many long, spreading to erect hairs, upper pair fused for up to 3/10 length, ~2× as broad as lower lobes; standard petal ±circular in appearance or obcordate when flattened and claw is visible, slightly emarginate, 8.2–12.1 mm long including 4.3–5.7 mm claw, 6.7–9.2 mm wide, yellow–orange with a broad red crescent around an orange ‘eye’, reverse side orange; wing petals narrow–oblong, 8.6–9.8 mm long including 3.1–3.8 mm claw, 1.6–2.1 mm wide, yellow–orange; keel petals naviculate, ±narrow–oblong to obovate in outline, 9.3–9.7 mm long including claw 2.8–3.8 mm long, 2.4–3.2 mm wide (when folded), slightly exceeding wings, dark red; stamens free to the base, subequal in length; filaments 5.8–8.5 mm long; anthers ~0.5 mm long; ovary sessile, 2-ovulate, ~1.4 mm long, with long, appressed hairs to 1.1 mm long in apical 1/5 on the abaxial side of the style; style 6.8–8.7 mm long, curving through 45° in apical 1/3, with a few long, semi-appressed hairs only at very base; stigma inconspicuous, very slightly capitate. Fruit ovoid, pale brown, slightly wrinkled at maturity, compressed, asymmetrically beaked, 4.9–6.2 mm long including stylar remnant, 2.8–3.6 mm in diameter, sparsely hairy near apex. Seeds compressed, obliquely ovoid, 2.5–2.8 mm long, 1.5–1.8 mm wide, smooth, dark brown; aril white, curved, ornate (coralline), with many short projections, 1.3–1.4 mm long. (Fig. 26–28.)
Holotype of Pultenaea murrayi R.L.Barrett with insets (a, b) showing detail of leaves and inflorescence (E.Gilbert 950001; CBG 9517770).

Field photographs of Pultenaea murrayi R.L.Barrett (a–d). Voucher: R.W.Purdie 13371 (CANB). Photos by R. W. Purdie.
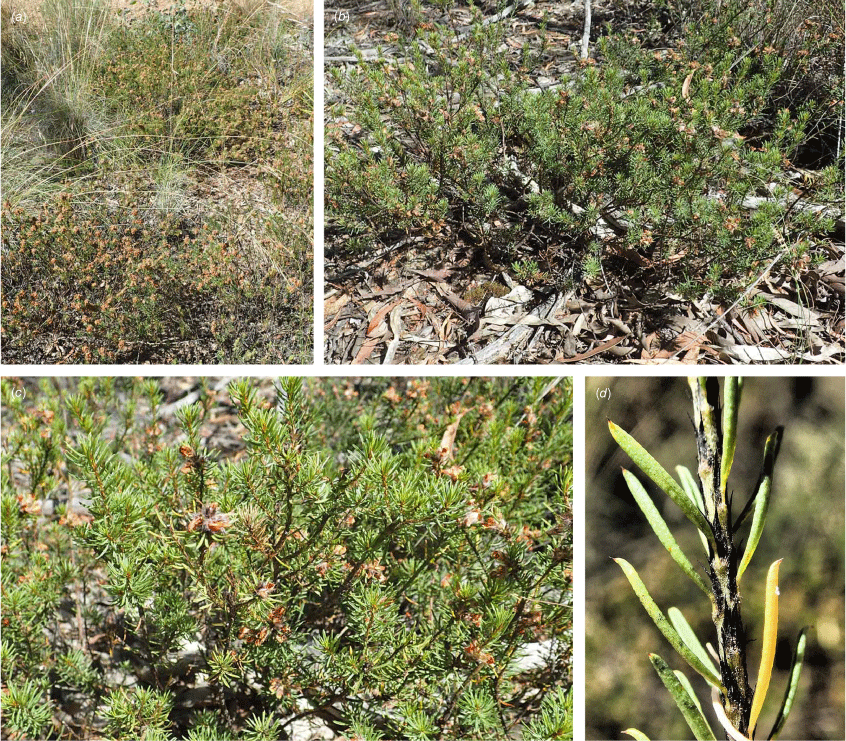
Line drawing of Pultenaea murrayi R.L.Barrett. (a) Flowering branchlet. (b) Leaves: abaxial surface (R), adaxial surface (L). (c) Leaf lamina surface: abaxial surface (below), adaxial surface (above). (d) Leaf cross-section. (e) Stipules. (f) Lateral view of flower. (g) Standard. (h) Bracteole and stipules. Voucher: R.W.Purdie 13371 (NSW). Scale bars: a, 10 mm; b, 4 mm; c–e, 2 mm; f, g, 5 mm; h, 2.5 mm. Illustration by C. Wardrop.
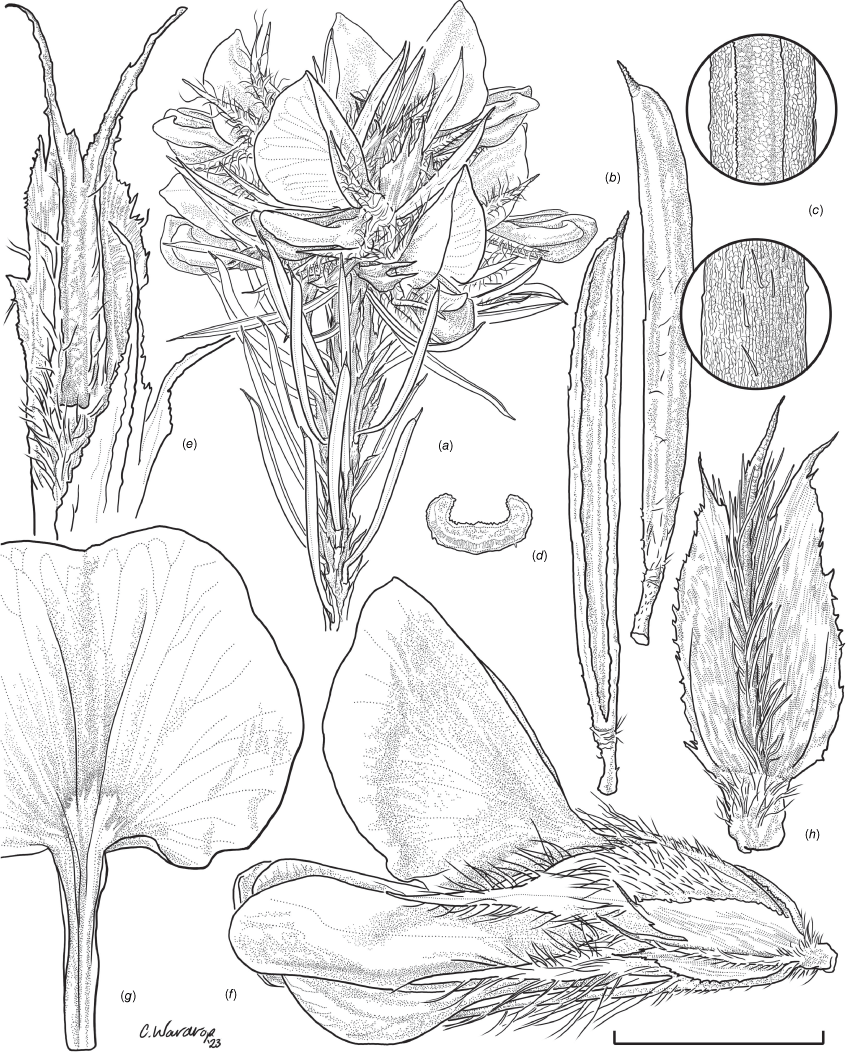
Similar in appearance to P. farmeriana, P. lapidosa, P. westonii and P. woolcockiorum but distinguished from these species by the following combination of characters: crisped branchlet hairs; leaf lamina 0.5–0.8 mm wide, ±linear, sparsely hairy on abaxial surface, bases of these hairs with obscure tubercles, margins incurved and ±hiding the adaxial surface; pedicels 0.6–1.1 mm long, straight, subtended by stipules 4.7–5.6 mm long, ~0.7 mm wide at sinus but enlarged to 1.6 mm wide at broadest point, hiding the pedicels; stipules caducous as fruit mature, exposing the prominently hairy calyx; bracteoles 4.3–5.7 mm long, inserted ±at pedicel apex, linear, bract-like, 1/2–2/3 length of calyx, not or weakly mucronate; subtending stipules 3.8–4.6 mm long; calyx tube glabrous or with short, appressed hairs and long, erect or spreading hairs to 3.3 mm long towards apex, calyx lobes with upper pair fused for up to 3/10 length, ~2× as broad as lower lobes; standard 8.2–12.1 mm long, 6.7–9.2 mm wide; ovary ~1.4 mm long, hairs in apical 1/5 in a tuft on the abaxial side of the style; style 6.8–8.7 mm long; aril ornate (coralline), with many short projections, 1.3–1.4 mm long.
Only known from three collections ~3 km apart, both on the escarpment within Cuumbeun Nature Reserve, near the suburb of Greenleigh, Queanbeyan, in the Southern Tablelands of New South Wales at an altitude of ~800 m (Fig. 3e).
Recorded from gentle to steep hill slopes with stony brown soil over sandstone, in low open forest or woodland. Recorded in association with Acacia dawsonii, A. parramattensis, Dillwynia sieberi, Eucalyptus macrorhyncha, E. mannifera, E. polyanthemos, E. rossii, Leucopogon sp. and Rytidosperma pallidum.
The epithet recognises the extensive work of Murray Allan Fagg in photographing the Australian flora and communicating the beauty of Australian natives to a broad audience through a series of popular books (e.g. Wrigley and Fagg 1989, 1990, 1993, 2010, 2013; Lockwood et al. 2001; https://www.anbg.gov.au/people/fagg.murray.html). This species had been recognised as a potentially new species known from a single collection and attempts by R. Purdie and M. Fagg to relocate the original population in Cuumbeun Nature Reserve had been unsuccessful. The species was serendipitously relocated by R. Purdie and M. Fagg while picnicking at a nearby location on New Year’s Day, 2023.
Pultenaea murrayi is known from the Cuumbeun Nature Reserve and state forest near Queanbeyan in the southern tablelands of NSW. The single population located in 2023 was divided by a fire trail and consisted of 15–20 plants. Further surveys are required in the region to determine the extent of this poorly known species. Area of extent: 5 km2. IUCN Category: Vulnerable (B1a, B1b(iii)).
Pultenaea palssoniae R.L.Barrett & Clugston, sp. nov.
Type: New South Wales: Northern Tablelands: near Jokers Spring, 36 km E of Narrabri, Mount Kaputar National Park, 1040 m, 19 Nov. 1976, R.G.Coveny 8811 & S.K.Roy (holo: NSW 228400; iso: BRI-AQ510737*, CBG 9100369, MEL 1583602).
Pultenaea sp. I, P.H.Weston in G.J.Harden, (ed.), Fl. New South Wales 2: 495 (1991).
Pultenaea sp. Narrabri (R.G.Coveny 8811 & S.K.Roy) NSW Herbarium, PlantNet [https://plantnet.rbgsyd.nsw.gov.au/, accessed 25 July 2022].
Erect or sprawling shrub 0.4–0.8(–1) m high, single- or multi-stemmed from the base, spreading 0.4–1 m wide. Branchlets erect to spreading, stiff, shortly ribbed below petioles, densely pubescent with short, crisped, appressed, matted hairs to 0.2 mm long. Stipules lanceolate, fused for 1/3–1/2 of length, 1.7–2.4 mm long, ~0.3 mm wide at sinus, moderately keeled, margins entire, dark red–brown with paler margins; apex spreading, acuminate; margins with short, crisped hairs. Leaves alternate: petiole 0.2–0.5 mm long; lamina lanceolate to oblanceolate, not or weakly discolourous, 3.4–10.0 mm long, 0.6–1.1 mm wide, with dense, irregularly rounded papillose cells adaxially and scattered, appressed, hispid hairs to 0.5 mm long (glabrescent with age), surface otherwise smooth with distinctly oblong papillose cells abaxially; apex straight to recurved, acute, rigidly and sharply mucronate, mucro pungent, 0.2–0.4 mm long; margins strongly incurved, usually completely hiding the abaxial surface. Inflorescence of 6–12 solitary flowers borne in upper axils, appearing loosely to tightly clustered. Flowers: pedicels subtended by slightly enlarged stipules 0.9–1.5 mm long, each 0.4–0.5 mm wide at sinus, not hiding the pedicels; pedicels 1.7–2.6 mm long, with dense, appressed, straight or crisped hairs to 0.3 mm long; bracteoles inserted 0.5–0.6 mm above pedicel apex, appressed at base (only slightly fused to stipules), linear, leaf-like, herbaceous, greenish-brown, 3.3–4.0 mm long, almost reaching length of calyx, shortly mucronate, pubescent; stipules narrow, 0.8–1.1 mm long, each ~0.2 mm wide at sinus, glabrous; calyx tube broadly obconic, 1.1–1.7 mm long, with dense, short to long, crisped or straight, appressed hairs outside; calyx lobes 5, narrowly or broadly triangular, 2.6–3.6 mm long, acuminate, with long, appressed hairs on lamina and short, crisped hairs on margin, ±equal in width, the upper pair fused for ~1/4 of length; standard petal ±circular in appearance or obcordate when flattened and claw is visible, emarginate, 5.2–6.5 mm long including 1.2–1.4 mm claw, 7.0–7.5 mm wide, yellow with a partial red crescent (fading to yellow at the apex) around a small yellow ‘eye’, reverse side yellow; wing petals narrow–oblong, 5.6–7.0 mm long including 1.7–1.9 mm claw, 1.9–2.1 mm wide, mostly yellow, red towards the adaxial margin; keel petals naviculate, ±narrow–oblong in outline, 6.1–7.1 mm long including claw 2.0–2.2 mm long, 2.3–2.9 mm wide (when folded), slightly exceeding wings, mostly yellow; stamens free, ±equal in length; filaments 4.5–4.9 mm long; anthers 0.5–0.6 mm long; ovary sessile, 2-ovulate, 1.3–1.5 mm long, with long, appressed hairs throughout; style 4.8–5.0 mm long, curving almost 90° towards apex, with long, semi-appressed hairs only in lower 1/6; stigma inconspicuous, very slightly capitate. Fruit ovoid, brown, compressed, asymmetrically beaked, 4.6–5.8 mm long including stylar remnant, 2.4–2.8 mm in diameter, sparsely hairy for most of length or at least in apical 1/2. Seeds compressed ovoid, ~3.0 mm long, ~2.2 mm wide, smooth, dark brown; aril white, irregular with many thick projections, ~1.3 mm long. (Fig. 29–31.)
Holotype of Pultenaea palssoniae R.L.Barrett & Clugston (R.G.Coveny 8811 & S.K.Roy; NSW 228400).
Field photographs of Pultenaea palssoniae R.L.Barrett & Clugston. Vouchers: (a, c, d) R.L.Barrett et al. RLB 9432 (NSW); (b, e) R.L.Palsson RLP 487 & K.D.Durham (NE); (f, g) R.P.O’Donnell (CANB); (h) R.L.Palsson RLP 494 & K.D.Durham (NE). Photos: (a, c, d) by R. L. Barrett; (b, e, h) by R. L. Palsson; (f, g) by R. P. O’Donnell.
Line drawing of Pultenaea palssoniae R.L.Barrett & Clugston. (a) Flowering branchlet. (b) Leaves: abaxial surface (R), adaxial surface (L). (c) Leaf lamina surface: abaxial surface (R), adaxial surface (L). (d) Stipules and petiole. (e) Lateral view of flower. (f) Standard. (g) Bracteole and stipules. Voucher: R.G.Coveny 8811 & S.K.Roy (NSW). Scale bars: a, 10 mm; b, 4 mm; c, d, 2 mm; e, f, 5 mm; g, 2.5 mm. Illustration by L. Elkan.
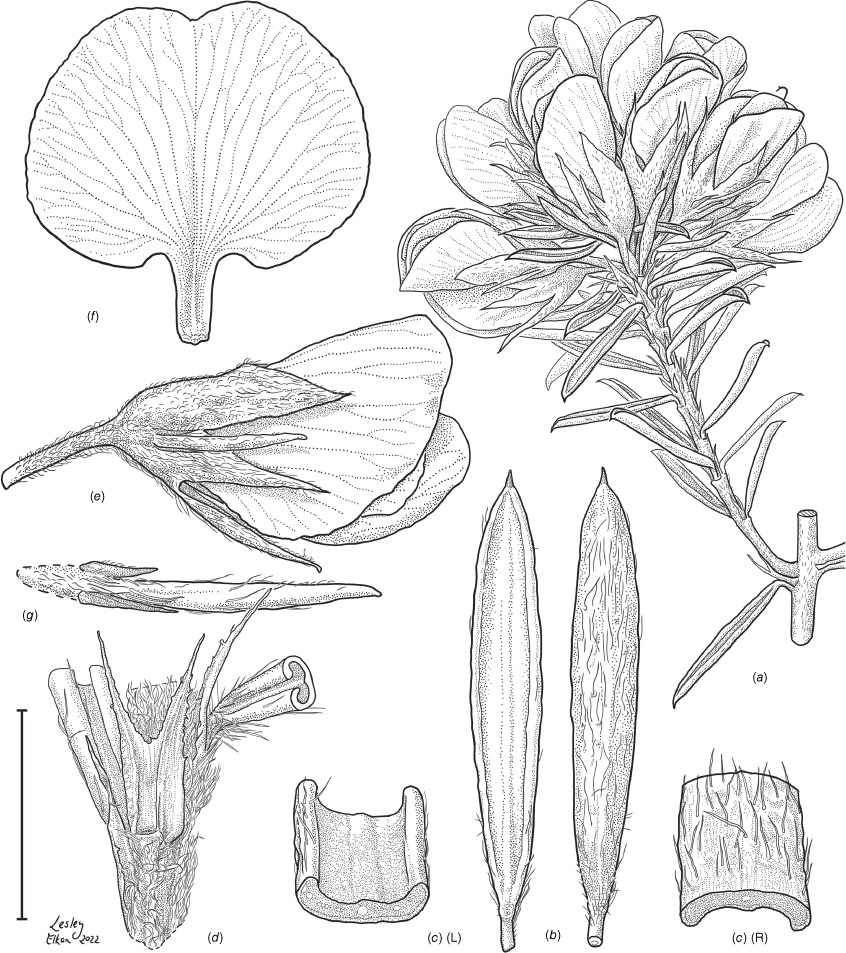
Similar in appearance to Pultenaea campbellii and P. hoskingii but distinguished from these species by the following combination of characters: crisped hairs on the branchlets; branchlet stipules fused for 1/3–1/2 of length; pedicels 1.7–2.6 mm long, subtended by stipules 0.9–1.5 mm long, 0.8–1.0 mm wide; floral bracteoles 3.3–4.0 mm long, inserted 0.5–0.6 mm above pedicel apex, linear, leaf-like, reaching almost full length of calyx, calyx tube with dense, appressed hairs, calyx lobes with long, appressed hairs on lamina, ±equal in width, upper pair fused for ~1/4 length; standard yellow; ovary 1.3–1.5 mm long, hairy throughout. Pultenaea campbellii has glabrous pods while P. palssoniae and P. hoskingii have hairy pods.
Pultenaea palssoniae is endemic to elevated areas of the Nandewar Range, between altitudes of 800 and 1500 m, east of Narrabri and west of Barraba (Fig. 3c).
The species grows in Eucalyptus shrubby woodland on rocky sites, in reddish-brown clay loam on basalt soils. Other associated species recorded include Acacia dealbata, A. falcata, A. gunnii, Acrothamnus hookeri, Anogramma leptophylla, Arthropodium milleflorum, Asperula conferta, Asterolasia rupestris subsp. rupestris, Bertya mollissima, Billardiera scandens, Brachyscome multifida, B. kaputarensis, Bulbine bulbosa, Callitris endlicheri, Carex appressa, C. fascicularis, C. gaudichaudiana, Chiloglottis palachila, Chrysocephalum apiculatum, C. semipapposum, Coprosma hirtella, C. quadrifida, Coronidium scorpioides, Cyperus secubans, Dampiera purpurea, Daviesia ulicifolia, Dianella revoluta, Diplazium australe, Discaria pubescens, Dodonaea viscosa subsp. angustifolia, Eucalyptus dalrympleana, E. elliptica, E. laevopinea, E. nandewarica, E. nobilis, E. pauciflora, E. viminalis, E. volcanica, Geranium solanderi, Glycine clandestina, Hakea microcarpa, Hardenbergia violacea, Hibbertia pedunculata, H. vestita, Hovea lanceolata, Hydrocotyle tripartita, Indigofera adesmiifolia, Isolepis subtilissima, Juncus filicaulis, J. laeviusculus, J. vaginatus, Kunzea occidentalis, K. opposita, Lagenophora stipitata, Leionema viridiflorum, Leptospermum brevipes, L. gregarium, L. polygalifolium subsp. montanum, Leucopogon attenuatus, L. ericoides, Lilaeopsis polyantha, Lomandra longifolia, L. multiflora, Lomatia arborescens, Melichrus urceolatus, Monotoca scoparia, Muehlenbeckia sp. Mount Norman (J.T.Hunter 3847), Myoporum montanum, Oxylobium oxylobioides, Poa labillardierei, Poa sieberiana, Poranthera microphylla, Prostanthera aff. lasianthos, P. nivea, Pterostylis longicurva, Ranunculus lappaceus, Scaevola albida, Schoenus apogon, Senecio microbasis, Stylidium graminifolium, Swainsona galegifolia, Trachymene incisa, Veronica derwentiana, V. velutina, Viola betonicifolia and Xanthorrhoea glauca subsp. angustifolia.
The epithet acknowledges the work of Ruth L. Palsson in documenting the flora of the Nandewar Range. Ruth Palsson and Kay Durham are attempting to voucher unvouchered species recorded in Mount Kaputar National Park.
Pultenaea palssoniae is currently known from seven broadly defined populations. The subpopulation immediately to the south of Mount Kaputar numbers several hundred plants over a distance of ~500 m. This population is primarily represented by plants regenerated from seed following the 2019–2020 fires; however, some parts of this population were not burnt, especially those growing on shallow soils around sloping wetlands. The size of other populations is not known but the species is noted as locally common in collection notes. The species must currently be regarded as Data Deficient under IUCN Standards and Petitions Subcommittee (2014) categories but listing as Vulnerable may be appropriate given the limited area of extent (230 km2) and threats of climate change shifting species composition in these montane communities. The species is conserved in Mount Kaputar National Park and Horton Falls National Park.
The species has been recognised at NE and NSW under the informal phrase name Pultenaea sp. Narrabri (R.G.Coveny 8811 & S.K.Roy) and was previously confused with either P. boormanii or P. campbellii before being included under P. setulosa by de Kok and West (2001).
Most likely closest to P. campbellii that generally occurs further to the east in the Armidale region, although a variant of that species is also found in Killarney Gap (NW of Mount Kaputar) but clearly differing in the calyx indumentum (glabrous except for the lobe margins in P. campbellii) and insertion point of the bracteole (inserted at or slightly below the base of the floral tube in P. campbellii). The anomalous populations of P. campbellii are similar to P. palssoniae in having a hairy apex to the ovary and fruit.
Pultenaea palssoniae and P. hoskingii appear to be closely related but are readily distinguished by branchlet and bracteolar stipules. In P. palssoniae, the bracteolar stipules are short (0.8–1.1 mm long) and slender, not at all winged (to 0.2 mm wide) and less than 1/3 length of the bracteole (that is 3.3–4.0 mm long). In P. hoskingii, the bracteolar stipules are long (1.3–1.4 mm long), broadly winged at the base (each 0.3 mm wide) and ~2/3 to almost full length of the bracteole (that is 2.3–2.7 mm long).
NEW SOUTH WALES: Northern Tablelands: Mount Kaputar National Park, north side of the junction of the Barraba Track and Kaputar Road, 1416 m, 1 Apr. 2022, R.L.Barrett, Z.E.Davies, X.V.Davies & F.F.Barrett RLB 9432 (CANB, NE, NSW); Mount Kaputar National Park, Dawsons Spring Nature Trail, N of campground, ~1400 m, 1 Apr. 2022, R.L.Barrett, Z.E.Davies, X.V.Davies & F.F.Barrett RLB 9433 (NE, NSW); Mount Kaputar National Park, Dawsons Spring Nature Trail, NW of campground, ~1400 m, 1 Apr. 2022, R.L.Barrett, Z.E.Davies, X.V.Davies & F.F.Barrett RLB 9434 (MEL, NSW); Mount Kaputar Plateau, ~4500 ft [~1.37 km], Dec. 1965, B.V.Booth s.n. (NSW 421387); Coryah Gap, Nandewar Range, 3500 ft [~1.07 km], 2 Apr. 1961, B.G.Briggs s.n. (NSW 421386); Nandewar Range, Horton Falls, ~30 km WNW of Barraba, 809 m, 15 Nov. 2000, J.J.Bruhl 1957, I.R.Telford & Odyssey volunteers (CANB 559952, NE 73430, NSW 622077, TENN, WAIK); Mount Lindsay, Nandewars, 4500–4700 ft [~1.37–1.43 km], 2 Nov. 1909, R.H.Cambage 2408 (NSW 38723, SYD); upper part of Barraba Track, Mount Kaputar National Park, 4 Oct. 1975, G.J.Harden s.n. (NE 38841); Barraba Track, 3/4 of the way down, Mount Kaputar National Park, 26 Oct. 1976, G.J.Harden s.n. (NE 38840); Mount Kaputar (Nandewars), 6 Sep. 1949, K.Ingram s.n. (NSW 38730); Barraba District [presumably Nandewar Range], 15 July 1962, J.A.Loveridge s.n. (NSW 421389); Mount Kaputar, Nandewar Range, 31 Oct. 1961, per J.A.O’Reilly s.n. (NSW 421385); Mount Kaputar National Park, north side of the junction of the Barraba Track and Kaputar Road, 1416 m, 1 Nov. 2021, R.L.Palsson RLP 487 & K.D.Durham (CANB, NE 112322, NSW); Mount Kaputar National Park, north side of the junction of the Barraba Track and Kaputar Road, 1416 m, 16 Jan. 2022, R.L.Palsson RLP 494 & K.D.Durham (CANB, MEL, NE 112371, NSW); 36 miles [~58 km] from Bingara on road to Narrabri, 11 Aug. 1958, B.R.Paterson s.n. (NE 52229); Mount Kaputar area, 1230 m, 11 Mar. 1967, J.B.Williams s.n. (NE 39232).
Pultenaea praetermissa R.L.Barrett & Albr., sp. nov.
Type: New South Wales: near Sutton, 13 miles [~20.9 km] NNE of Canberra City Post Office, 20 Oct. 1963, C.Burgess s.n. [Sands 6310.6.1] (holo: NSW 500263; iso: AD 96642134*, L 2034539*, L 2034541*, NSW 500266, PERTH 624152, n.v., SYD).
Pultenaea sp. Sutton (C.Burgess s.n., 20 Oct. 1963), NSW Herbarium, PlantNet [https://plantnet.rbgsyd.nsw.gov.au/, accessed 25 July 2022].
Erect or spreading shrub 0.4–1.8 m high, single or multi-stemmed from the base, spreading 0.5–1.2 m wide. Branchlets erect to spreading, stiff, shortly ribbed below petioles, moderately pubescent with short, crisped, appressed, matted hairs ~0.1 mm long. Stipules lanceolate, fused for 1/8–1/6 of length, 1.6–3.3 mm long, ~0.4 mm wide at sinus, weakly keeled, brown to red–brown with broad pale margins; apex spreading, long–acuminate; margins with a few scattered stiff hairs or glabrous. Leaves alternate: petiole 0.4–0.8 mm long; lamina ±linear, often curved along length, with dense, appressed to somewhat spreading, sometimes curved, stiff hairs to 0.5 mm long, weakly discolourous but adaxial surface usually hidden by incurved margins, 5.9–7.7 mm long, 0.4–0.7 mm wide, with very dense, irregular papillose cells adaxially, surface smooth with distinctly oblong papillose cells abaxially; apex straight to slightly oblique, acute, rigidly and abruptly mucronate, mucro pungent, 0.2–0.6 mm long; margins strongly incurved usually hiding the adaxial surface. Inflorescence of 4–12 solitary flowers borne in upper axils, appearing tightly clustered. Flowers: pedicels subtended by slightly enlarged stipules 1.8–2.0 mm long, each ~0.4 mm wide at sinus, partly hiding the pedicels; pedicels 0.5–1.4 mm long, densely hairy; floral bracteoles inserted 0.7–1.2 mm above pedicel, appressed at base, linear (not or scarcely fused to subtending stipules), reduced leaf-like, herbaceous, green, 3.4–4.1 mm long, reaching ±full length of calyx, mucronate, pubescent; stipules narrow, 1.6–2.1 mm long, each ~0.2 mm wide at sinus; calyx tube broadly obconic, 1.4–1.8 mm long, green, densely, silky, with appressed hairs outside; calyx lobes 5, narrowly triangular, 3.9–5.8 mm long, acuminate, with long, silky, appressed hairs on the lamina and dense, short, crisped hairs on the margins inside, ±equal in width, the upper pair fused for ~1/4 of length; standard petal ±circular in appearance or obcordate when flattened and claw is visible, emarginate, 7.8–9.6 mm long including 2.8–3.3 mm claw, 6.6–7.3 mm wide, yellow–orange with a small broad red crescent around a dull orange ‘eye’, reverse side yellow–orange; wing petals narrow–oblong, 6.8–7.6 mm long including 2.3–2.8 mm claw, 1.6–1.8 mm wide, mostly yellow–orange, red towards the base; keel petals naviculate, ±narrow–oblong in outline, 7.4–8.4 mm long including claw 2.1–2.4 mm long, 2.8–3.2 mm wide (when folded), slightly exceeding wings, deep red at the apex, grading to red at the base; stamens free to the base, subequal in length, 5.3–6.0 mm long; anthers ~0.5 mm long; ovary sessile, 2-ovulate, ~1.7 mm long, with long, appressed hairs in apical 1/4; style 6.2–6.6 mm long, gently curving through 90° in apical 1/3, with long, semi-appressed hairs only at very base; stigma inconspicuous, very slightly capitate. Fruit ovoid, brown, compressed, asymmetrically beaked, 4.2–5.4 mm long including stylar remnant, 2.3–2.8 mm in diameter, sparsely hairy in apical 1/4. Seeds not seen. N = 7; based on V.E.Sands 6310.6.1. (Fig. 32–34.)
Field photographs of Pultenaea praetermissa R.L.Barrett & Albr. Vouchers: (a, c–f) R.W.Purdie 13303 (CANB); (b) R.W.Purdie 13302 (CANB). Photos by M. Fagg.
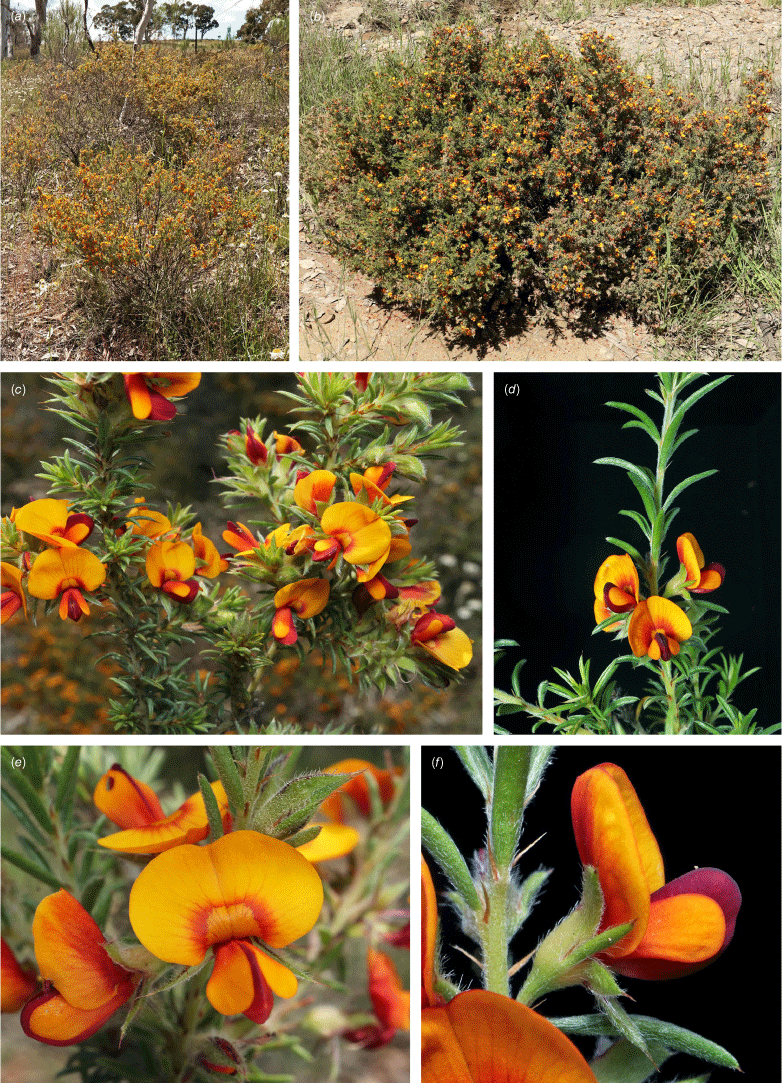
Line drawing of Pultenaea praetermissa R.L.Barrett & Albr. (a) Flowering branchlet. (b) Leaves: abaxial surface (R), adaxial surface (L). (c) Leaf lamina surface: abaxial surface (R), adaxial surface (L). (d) Stipules and petiole. (e) Lateral view of flower. (f) Standard. (g) Bracteole and stipules. Voucher: C.Burgess s.n. (NSW 500263). Scale bars: a, 10 mm; b, 4 mm; c, d, 2 mm; e, f, 5 mm; g, 2.5 mm. Illustration by L. Elkan.
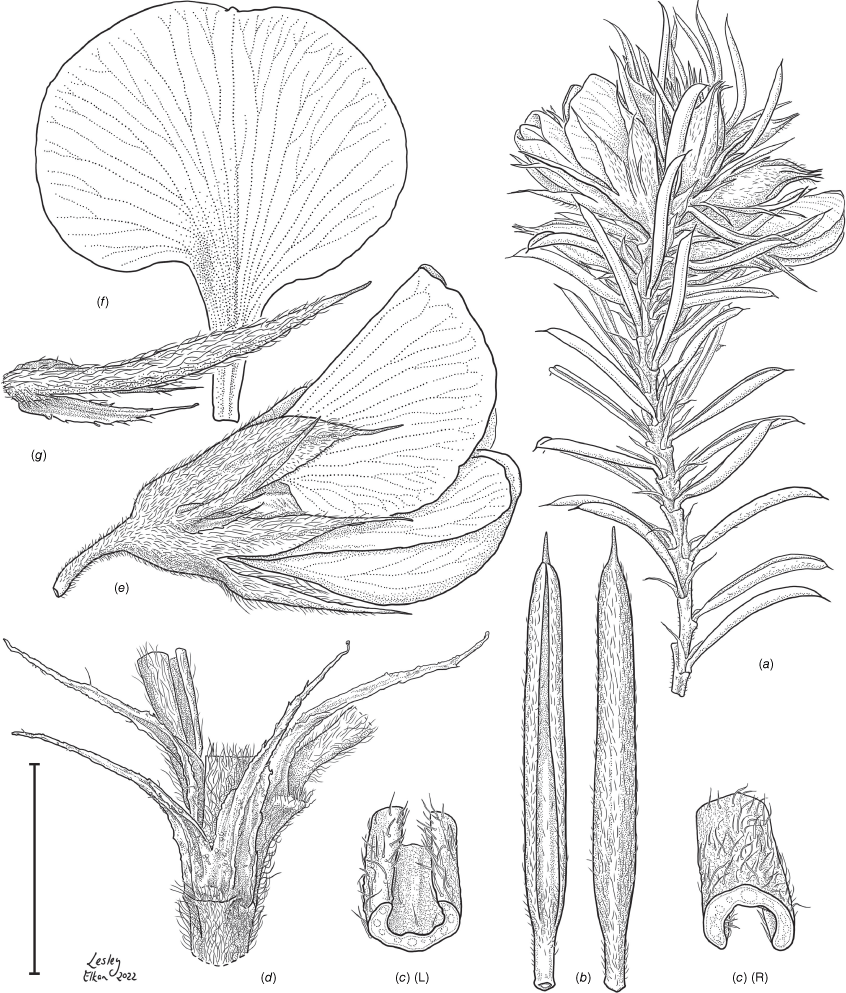
Similar in appearance to P. boormanii, P. corrickiae and P. farmeriana but distinguished from these species by the following combination of characters: branchlet stipules fused for 1/8–1/6 of length; leaf lamina ±linear, with appressed to somewhat spreading hairs abaxially; pedicels 0.5–1.4 mm long, densely hairy, subtended by stipules 1.8–2.0 mm long, ~0.4 mm wide, partly hiding the pedicels; floral bracteoles 3.4–4.1 mm long, inserted 0.7–1.2 mm above pedicel apex, reduced leaf-like, ±full length of calyx, mucronate; calyx tube 1.4–1.8 mm long, densely hairy with hairs silky, appressed, calyx lobes with long, silky, appressed hairs on lamina and dense, short, crisped hairs on margins inside; ovary ~1.7 mm long, hairy in apical 1/4.
Only known from the Southern Tablelands of New South Wales between Gundaroo, Sutton and near Gininderra. A record from ‘Yass’ is probably from the hills to the east of Yass (Fig. 3e).
Recorded from poor gravelly and alluvial soils, pale stony clay, red sandy clay, brown clay loam or on rocky shales, in open eucalypt woodland and forest or heath on gentle to steep slopes. Recorded in association with Acacia baileyana, A. parramattensis, Brachyloma daphnoides, Cassinia sifton, Cheiranthera linearis, Daviesia leptophylla, D. virgata, Dillwynia juniperina, D. sericea, Eucalyptus blakelyi, E. bridgesiana, E. macrorhyncha, E. mannifera, E. melliodora, E. polyanthemos, E. rossii, Goodenia hederacea, Leucochrysum albicans var. tricolor, Melichrus urceolatus, Pimelea curviflora, Poa sieberiana, Pultenaea microphylla, Rytidosperma pallidum, R. pilosum, R. tenuius and Themeda triandra.
The epithet is from the Latin praetermissus (to overlook) in reference to this species not having been previously recognised, despite the geographic proximity to the Australian National Herbarium (CANB).
Pultenaea praetermissa is currently known from four broad population areas that are highly fragmented due to historical clearing for agriculture. The species is relatively common on roadsides for several kilometres between Gundaroo and Gunning (R. Purdie and M. Fagg, pers. comm.). Area of extent: 180 km2. Current populations appear to be stable, with at least some being relatively extensive but limited information on the number of individuals is available. Roadside populations and some on private property are likely to be at long-term risk of weed invasion and habitat decline. Not known from any conservation reserves but may occur in Mcleods Creek Nature Reserve. IUCN Category: Vulnerable (B1a, B1b(iii)).
A chromosome count was obtained for this species and reported under P. boormanii by Sands (1975).
Most similar in appearance to P. corrickiae that occurs on the western footslopes of the Snowy Mountains. Sometimes also confused with P. laxiflora, a species with velutinous ovaries and long–pedunculate flowers, due to both species having narrow leaves. Occasionally identified as P. boormanii that has similar leaves but differs in having longer pedicels 1.5–2.5 (v. 0.5–1.4) mm long, bracteoles inserted ~0.4 (v. 0.7–1.2) mm above pedicel and the ovary 1.1–1.2 (v. ~1.7) mm long, hairy in the apical 1/2 (v. 1/4).
A single collection from near Binalong (7 km SE of Binalong Post Office, 25 Oct. 1992, B.J.Lepschi 854, CANB 437438, NSW 474051) is similar to P. praetermissa but has weakly 3-veined leaves, placing this in the P. procumbens species complex, a more sparsely hairy calyx and shorter calyx lobes.
NEW SOUTH WALES: Southern Tablelands: 5 miles [~8 km] W of Lake George, 3 miles [~4.8 km] NE of Sutton, Gunning Shire, 27 May 1971, L.G.Adams 2597 (CANB 213310, NSW 500267); Gininderra – Sutton road (near A.C.T.), 5 Nov. 1964, R.W.Boden s.n. (CBG 39976); Private property near eastern end of Dairy Creek Road, E of Gundaroo, 4 Nov. 2022, H.Brewer s.n. (CANB); Private property near eastern end of Dairy Creek Road, E of Gundaroo, 11 Nov. 2022, H.Brewer s.n. (CANB, MEL, NSW); 3.5 km from Gundaroo on road towards Collector, 17 Dec. 1990, G.Butler 1597 (CBG 9012295); Yass, 12 May 1962, C.Burgess s.n. (CBG 9618); near Sutton, 10 Oct. 1963, C.Burgess s.n. (CBG 13263, NSW 445494); near Gundaroo, 18 Dec. 1961, C.Burgess s.n. (NSW 500264, NSW 500265); near Sutton, on the road to Gininderra, 20 Oct. 1963, C.Burgess s.n. (CBG 4161); Gininderra road on the border of ACT and NSW, 7 Oct. 1973, C.Burgess B 291 (L 2034533*, P 02929143*); Gundaroo; Dairy Creek Road, 1.6 km past the rubbish tip; road verge, 2 Dec. 2010, J.D.Marges 106 (CANB 797702); Dairy Creek Road, ~3.2 km from Gundaroo Post Office, 626 m, 12 Nov. 2022, R.W.Purdie 13302 (CANB, NSW); along Marked Tree Road, ~1.6 km E of junction with Dairy Creek Road, NE of Gundaroo, 630 m, 12 Nov. 2022, R.W.Purdie 13303 (CANB, NSW); 3.8 km E from Gundaroo Post Office, along Dairy Creek Road, 5 Sep. 2000, D.A.Taylor 70 (CANB 617625); Collector–Gunning road, N of Canberra, 800 m, 10 Nov. 1974, P. van Royen 10600 (BISH, n.v., L 2034194*).
Pultenaea purdieae R.L.Barrett & Clugston, sp. nov.
Type: New South Wales: Snowy Mountains Highway, ~4 km S from centre of Tumut, 316 m, 16 Oct. 2011, R.W.Purdie 8254 (holo: CANB 805282; iso: NSW 923527).
Pultenaea sp. Lobs Hole (M.E.Phillips 176) NSW Herbarium, PlantNet [https://plantnet.rbgsyd.nsw.gov.au/, accessed 25 July 2022].
Erect to sprawling shrub 0.4–0.7 m high, multi-stemmed from the base, spreading 0.5–1.2 m wide. Branchlets erect to spreading, stiff, shortly ribbed below petioles, densely pubescent with short, crisped, curved and matted hairs to 0.2 mm long. Stipules lanceolate, fused for ~1/8 of length, 1.8–2.4(–3.0) mm long, ~0.3 mm wide at sinus, strongly keeled, brown to red–brown with broad pale margins; apex spreading, long–acuminate; margins glabrous. Leaves alternate: those subtending the inflorescence distinctly smaller, petiole 0.2–0.3 mm long; lamina ±lanceolate, distinctly recurved, glabrous, discolourous, paler above, 2.3–6.7 mm long, 0.5–0.7(–1.3) mm wide, with dense, irregular papillose cells adaxially, surface smooth with distinctly oblong papillose cells abaxially; apex straight to slightly oblique, acute, rigidly and abruptly mucronate, mucro pungent, 0.3–0.6 mm long; margins usually strongly incurved, commonly hiding the adaxial surface at maturity. Inflorescence of 3–12 solitary flowers borne in upper axils, appearing loosely clustered. Flowers: pedicels subtended by enlarged stipules 1.1–1.4 mm long, each ~0.5 mm wide at sinus, partly hiding the pedicels; pedicels 1.0–1.9 mm long, densely hairy; floral bracteoles inserted on calyx tube, 0.7–1.1 mm above pedicel, appressed at base, linear (not or scarcely fused to subtending stipules), leaf-like, herbaceous, green, 1.9–2.8 mm long, reaching ~4/5 length of calyx, short–mucronate, glabrous; bracteolar stipules narrow, 0.8–1.3 mm long, each ~0.4 mm wide at sinus, with scattered hairs on margins; calyx tube broadly obconic, 1.2–1.6 mm long, greenish, a few scattered hairs near the base; calyx lobes 5, narrowly triangular, 2.5–3.6 mm long, long–acuminate, with long, spreading to erect hairs along the margins, with fine, short hairs inside near the margins, ±equal in width, the upper pair fused for ~1/6 of length; standard petal ±circular in appearance or obcordate when flattened and claw is visible, emarginate, 4.8–6.4 mm long including 1.6–1.9 mm claw, 5.1–6.0 mm wide, orange–yellow with a narrow red crescent around an orange–yellow ‘eye’, reverse side dark yellow–orange; wing petals narrow–oblong, 5.8–6.5 mm long including 1.4–1.6 mm claw, 1.2–1.6 mm wide, yellow–orange, darker orange towards the base; keel petals naviculate, ±narrow–oblong to obovate in outline, 5.5–7.0 mm long including claw 1.3–1.7 mm long, 2.1–2.5 mm wide (when folded), slightly exceeding wings, dark brick red at the apex, grading to dark red at the base; stamens free to the base, subequal in length; filaments 4.7–6.1 mm long; anthers ~0.6 mm long; ovary sessile, 2-ovulate, ~1.3 mm long, with many long hairs in apical 1/3; style 4.4–5.8 mm long, gently curving through 90° in apical 1/3, glabrous; stigma inconspicuous, very slightly capitate. Fruit not seen. (Fig. 35–37.)
Field photographs of Pultenaea purdieae R.L.Barrett & Clugston (a–e). Voucher: R.W.Purdie 8254 (CANB). Photos from the Australian Plant Image Index (https://www.anbg.gov.au/photo/apii/), © M. Fagg, 2011.

Line drawing of Pultenaea purdieae R.L.Barrett & Clugston. (a) Flowering branchlet. (b1, b2) Leaves: abaxial surface (L), adaxial surface (R). (c) Leaf lamina surface: adaxial surface (L), abaxial surface (R). (d) Stipules and petiole. (e) Lateral view of flower. (f) Standard. (g) Bracteole and stipules. Voucher: R.W.Purdie 8254 (NSW). Scale bars: a, 10 mm; b, 4 mm; c, 2 mm; d, e, 5 mm; f, 2.5 mm. Illustration by L. Elkan.
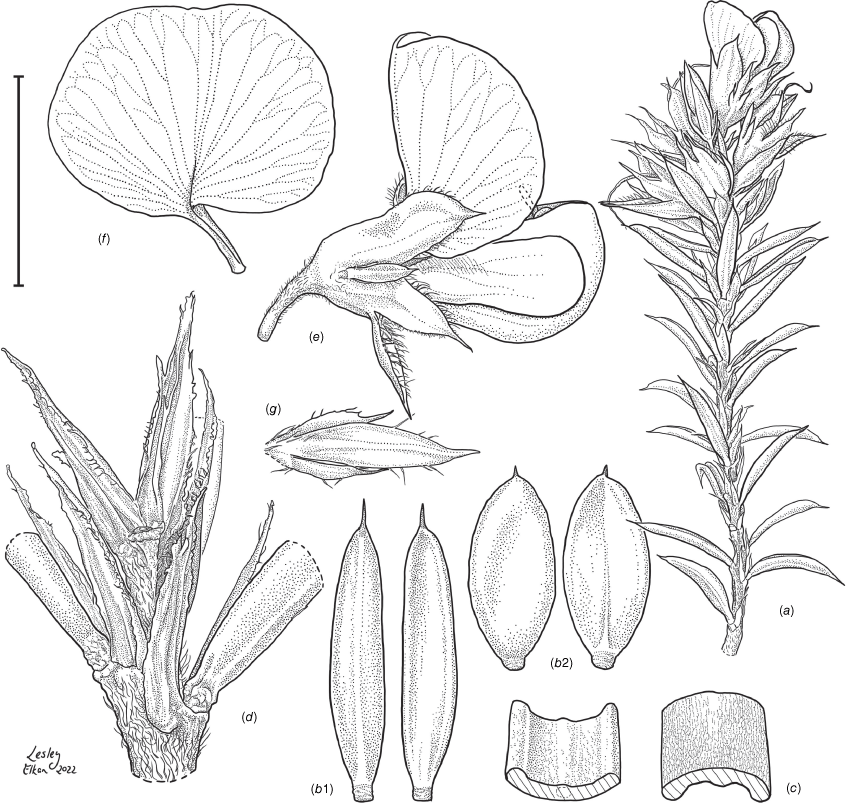
A relatively distinctive species, most similar to P. corrickiae and P. estelleae but distinguished from these species by the following combination of characters: crisped branchlet hairs; branchlet bracteoles fused for ~1/8 of length; leaves within inflorescence similar to branchlet leaves; leaf lamina ±lanceolate, glabrous, recurved along length, mucro 0.3–0.6 mm long; pedicels 1.0–1.9 mm long, subtended by stipules 1.1–1.4 mm long; floral bracteoles inserted 0.7–1.1 mm above pedicel apex, glabrous; bracteolar stipules 0.8–1.3 mm long; calyx tube 1.2–1.6 mm long, a few hairs near base, calyx lobes ±equal in width, with upper pair fused for ~1/6 length; 2.5–3.6 mm long; standard 4.8–6.4 mm long, 5.1–6.0 mm wide; ovary sessile, 2-ovulate, ~1.3 mm long, hairy in apical 1/3. Possibly also related to P. procumbens s.l., differing in the calyx lobes ±equal in width (upper pair ~2× as broad as lower lobes in the P. procumbens species complex).
Only known from a small area at the northern end of the Snowy Mountains between Tumut and Talbingo, from 330 to 550 m, in the Southern Tablelands of New South Wales (Fig. 3e).
Recorded from moderate to steep rocky slopes in eucalypt woodland or open forest on dark brown to red–brown, rocky loam. Recorded in association with Acacia melanoxylon, Banksia canei, Boronia nana var. hyssopifolia, Cassinia aculeata, Cryptandra amara, Dodonaea viscosa subsp. spatulata, Eucalyptus elaeophora, E. macrorhyncha, Galium gaudichaudii, Grevillea rosmarinifolia, Hibbertia riparia, Juncus subsecundus, Leptospermum brevipes, L. trinervium, Leucopogon affinis, Olearia stricta subsp. parvilobata, Poa petrophila, Prostanthera rotundifolia, Pultenaea juniperina, Rytidosperma pilosum, Senecio phelleus and Wahlenbergia stricta.
The epithet honours botanist Rosemary Winifred Purdie, an avid collector of Australian plants, vouchering most of the species photographed by Purdie’s partner Murray Fagg for the Australian Plant Image Index and leading expeditions of discovery, often in remote parts of Australia (https://www.anbg.gov.au/biography/purdie-rosemary.html). Purdie has authored publications on ecology (Purdie 1977a, 1977b) and plant taxonomy (Purdie et al. 1982).
Pultenaea purdieae is currently known from a relatively restricted area, with most collections made within a few kilometres of Talbingo and one from near Tumut. Likely more widespread as clearing of hills in the region is minimal but the preferred habitat appears to follow the highly cleared valleys. This species may also have occurred in some locations that are currently inundated by the original Snowy Hydro scheme. Area of extent: 150 km2. IUCN Category: Vulnerable (B2a, B2b(iii)).
When infected by an insect gall, the leaves can form dense apical tufts and sometimes but not consistently, develop dense, long, erect hairs on the abaxial surface (see J.C.Newman s.n.; NSW 38708).
NEW SOUTH WALES: Southern Tablelands: Talbingo, 15 Feb. 1974, G.Althofer 97 (NSW 500088); Jouanama [Jounama] Ck, Upper Tumut R area, 2 July 1952, J.C.Newman s.n. (NSW 38708); Lobs Hole, Snowy Mountains, 9 Nov. 1961, M.E.Phillips 176 (CBG 48404, NSW 500087); Snowy Mountains Highway, 1/2 mile [~0.8 km] W of Talbingo turnoff, 28 Sep. 1972, D.J.Wimbush KOS693 (CANB 320151).
Pultenaea renneri R.L.Barrett & Clugston, sp. nov.
Type: New South Wales: Central Coast: WSW [of] Bucketty, 28 Sep. 1963, C.E.B.H.Burgess s.n. (holo: NSW 2124458; iso: AD 97026113*, CBG 009693, NSW 469941, NSW 2124457, SYD).
Pultenaea sp. Bucketty (C.Burgess s.n., 28 Sept. 1963) NSW Herbarium, PlantNet [https://plantnet.rbgsyd.nsw.gov.au/, accessed 25 July 2022].
Erect shrub 1–2 m high, single-stemmed from the base, width of spread not known. Branchlets erect to spreading, stiff, glabrous; long-ribbed or almost winged below petioles. Stipules lanceolate, fused for 1/5–1/4 of length, 1.6–2.3 mm long, ~0.2 mm wide at sinus, moderately keeled, brown to red–brown; apex spreading, long–acuminate; margins ±entire or an occasional tooth-like projection, glabrous. Leaves alternate: petiole 0.4–0.6 mm long; lamina ±linear, glabrous, strongly discolourous with a dark purplish median stripe abaxially, (3.3–)4.8–12.2 mm long, 0.5–0.8 mm wide, with dense, irregularly rounded papillose cells adaxially, surface smooth with rounded to irregularly oblong papillose cells abaxially; apex straight to slightly oblique, acute, rigidly and abruptly mucronate, mucro pungent, 0.2–0.4 mm long margins moderately to strongly incurved but not hiding the adaxial surface. Inflorescence of 4–10 solitary flowers borne in upper axils, appearing loosely clustered. Flowers: pedicels subtended by enlarged stipules 1.1–1.3 mm long, each ~0.6 mm wide at sinus, not hiding the pedicels; pedicels 1.2–2.0 mm long, glabrous; floral bracteoles inserted 0.5–0.8 mm above pedicel apex but with a rib extending down to pedicel apex, appressed at base, narrowly triangular, bract-like, 1.6–1.7 mm long, reaching 2/3 length of calyx, scarious, acuminate, glabrous, green; stipules absent; calyx tube broadly obconic, 2.2–2.4 mm long, green, glabrous; calyx lobes 5, broadly triangular, 1.5–1.9 mm long, acuminate, glabrous except for short, crisped hairs on the margins, ±equal in width, the upper pair fused for ~1/5 of length; standard petal ±circular in appearance or obcordate when flattened and claw is visible, emarginate, 5.4–7.1 mm long including 2.3–2.5 mm claw, 7.4–8.0 mm wide, yellow–orange with a radiating red crescent at the base around a paler ‘eye’; wing petals narrow–oblong, 6.2–7.4 mm long including 1.7–2.0 mm claw, 1.6–1.9 mm wide, mostly yellow, orange towards the base; keel petals naviculate, ±narrow–oblong in outline, 7.1–7.7 mm long including claw 2.1–2.3 mm long, 2.4–2.8 mm wide (when folded), slightly shorter than wings, brownish-red; stamens free, subequal in length; filaments 5.1–6.7 mm long; anthers 0.5–0.6 mm long; ovary sessile, 2-ovulate, 1.1–1.2 mm long, with a small tuft of appressed hairs to 2.1 mm long in apical 1/8 on one side; style 4.3–5.7 mm long, curving through ~90° ~1/3 from base, glabrous; stigma inconspicuous, very slightly capitate. Fruit not seen. n = 7, 2n = 14; based on V.E.Sands 648.1.3, from NSW 469940. (Fig. 38–40.)
Line drawing of Pultenaea renneri R.L.Barrett & Clugston. (a) Flowering branchlet. (b) Leaves: abaxial surface (L), adaxial surface (R). (c) Leaf lamina surface: abaxial surface (above), adaxial surface (below). (d) Stipules and petiole. (e) Lateral view of flower. (f) Standard. (g) Bracteole. Voucher: C.Burgess s.n. (NSW 2124458). Scale bars: a, 10 mm; b, 4 mm; c, d, 2 mm; e, f, 5 mm; g, 2.5 mm. Illustration by L. Elkan.
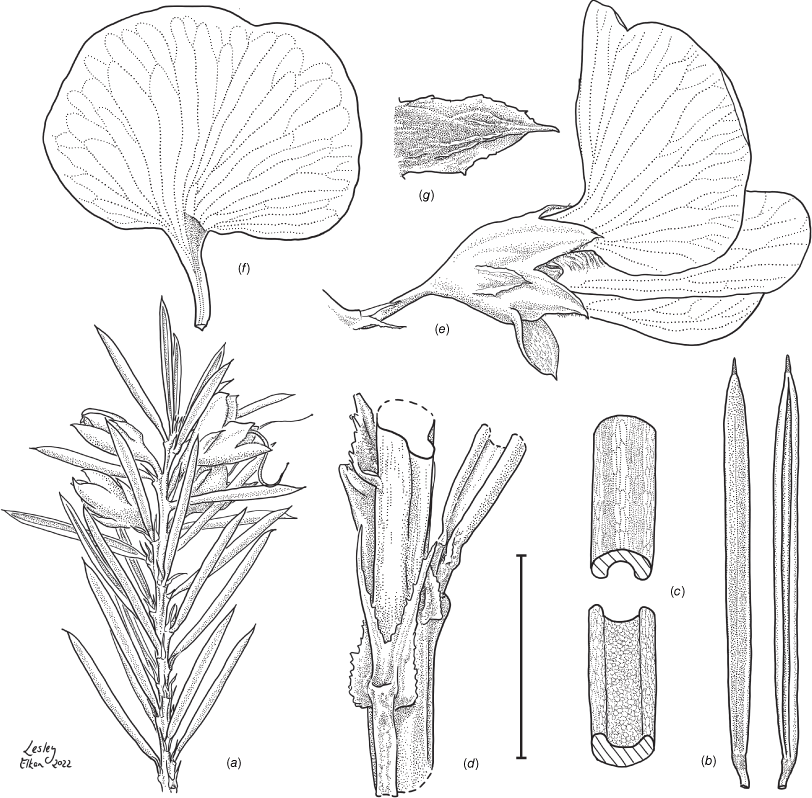
Field photographs of Pultenaea renneri R.L.Barrett & Clugston (a, b). Voucher: C.E. Woolcock 1445 (AD). Photos from the Australian Plant Image Index (https://www.anbg.gov.au/photo/apii/), © C. E. Woolcock, 1983.

Similar to P. mutabilis Renner & P.H.Weston and specifically P. mutabilis var. angusta M.A.M.Renner, P.H.Weston & S.Clarke but distinguished by branchlets glabrous (v. sparsely hairy); stipules 1.6–2.3 (v. ~3) mm long; leaves glabrous adaxially (v. sparsely hairy), surface green with a dark purplish median stripe (v. green with a glaucous bloom); pedicels 1.2–2.0 (v. ~3) mm long, glabrous (v. sparsely hairy); floral bracteoles ~2/3 (v. ~2/5) length of calyx; calyx lobes glabrous except for short hairs on the margins (v. with a line of hairs on the medial lamina); corolla standard >5 (v.<3) mm long.
While some specimens of this species were included under P. setulosa by de Kok and West (2002) and hence we treat this here, the species differs from all members of the P. setulosa group in the glabrous branchlets, leaves with a dark purplish median stripe abaxially, lack of bracteolar stipules and keel petal slightly shorter than the wing petals. Based on these characters, this species can be confidently assigned to the P. glabra group (see Renner et al. 2022).
Only known from the vicinity of Bucketty south to Mount Manning, adjacent to Yengo National Park, inland from Newcastle, New South Wales (Fig. 3d).
Grows in ‘rather sheltered, damp sites in dry sclerophyll forest’. No other habitat details are known. The single locality that can be identified with confidence, ‘Mount Manning’ is a sandstone hill with some outcropping rock and ledges with thin soil, similar to habitats occupied by other members of the Pultenaea glabra complex. However the locality could equally apply to one of the deep adjacent valleys as there were few geographic points of reference at the time of collection. While ‘Bucketty’ is a region, the location referred to on collection labels is most likely in proximity to the intersection of Great North Road and George Downes Drive, where vegetation is tall woodland over a variously dense understorey depending on proximity to drainage lines. Also, as searching sandstone platforms and slopes has proven unsuccessful, vegetation adjacent to drainage lines may be the most likely habitat for P. renneri.
The epithet recognises the contributions of Matthew A.M. Renner to plant systematics in Australia, New Zealand and the Pacific, including in the legume genera Acacia and Pultenaea (Barrett et al. 2021; Renner et al. 2021a, 2022). Renner has made particular contributions to bryophyte systematics (e.g. Renner and de Lange 2020; Feldberg et al. 2021; Pócs and Renner 2021; Renner 2020, 2021; Renner et al. 2021b; Patiño et al. 2022), always demonstrating tenacity in the face of challenges.
Pultenaea renneri is currently only known from around Bucketty, adjacent to Yengo National Park, over a distance of less than 10 km. The species was first collected in 1907, followed by four collections in the 1960s, and one in 1983, but has not been recollected for over 40 years. Two targeted surveys, in September 2022 and September 2023, failed to locate any populations and the species remains poorly known. Based on a known area of extent of less than 20 km2, the species should be assessed for listing as endangered. IUCN Category: Data Deficient.
The affinity of this taxon has been subject to considerable historical uncertainty, with specimens variously placed under P. campbellii, P. flexilis, P. glabra and P. setulosa s.l. or remaining unidentified to species. During a revision of the P. glabra Benth. complex (Renner et al. 2022), M.A.M. Renner and R.L. Barrett suggested a possible relationship between a few specimens now included in P. renneri and P. mutabilis Renner & P.H.Weston, however fertile material was not seen at the time of that revision. Fertile specimens were subsequently located, filed under P. setulosa at NSW and variously filed at SYD, and clarity emerged that P. renneri was indeed a distinctive entity with a sound number of distinguishing characters. Based on morphology, P. renneri is most likely related to P. mutabilis rather than any members of the P. setulosa complex, therefore the description here complements that revision.
A chromosome count was obtained for this species and reported under P. campbellii by Sands (1975).
NEW SOUTH WALES: 4 miles [~6.4 km] N of Bucketty (Calga–Wollombi), 30 Aug. 1960, R.Carolin 1999 (SYD); Mount Manning, 14 Sep. 1907, A.Chapman s.n. (SYD, 2 sheets); Bucketty (S of the range), 4 Aug. 1964, L.H.Williams s.n. (AD 97646649, n.v.; NSW 469940, SYD); between Bucketty and Laguna on Wollombi road, by Cruiets’ drinking trough, Sept. 1963, L.H.Williams s.n. (NSW 469939, SYD); Bucketty, 1 Sep. 1983, C.E.Woolcock 1445 (AD 98423080).
Pultenaea setulosa Benth., Fl. Austral. 2: 132 (1864)
Type: Queensland: Broad Sound [probably Marlborough], [1862], E.M.Bowman s.n. (lecto, here designated: K 000118900*; isolecto: BRI-AQ0540172, MEL 516219, NSW 38978).
Queensland: Marlborough, [1862], E.M.Bowman 82 (residual syn or possible isolecto: MEL 0035339).
Erect or spreading shrub 0.6–2.5 m high, single-stemmed from the base, spreading 0.6–1.2 m wide. Branchlets erect to spreading, stiff, shortly ribbed below petioles, moderately to densely pubescent with short, straight or curved, appressed to spreading hairs to 1 mm long. Stipules narrow–lanceolate, fused for 1/5–1/4 of length, 3.2–4.6 mm long, ~0.5 mm wide at sinus, weakly keeled, brown to red–brown with paler margins; apex spreading, long–acuminate; margins ±entire, with a few fine hairs. Leaves alternate: petiole 0.3–0.7 mm long; lamina ±linear, with scattered, appressed to spreading, straight or curved hairs to 0.8 mm long, rarely glabrescent, weakly discolourous, slightly darker abaxially, 2.8–7.1 mm long, 0.4–0.6(–0.8) mm wide, with scattered, irregular, flat cells and scattered hairs adaxially; surface smooth with rounded to irregularly oblong papillose cells and scattered hairs abaxially, apex straight to slightly oblique, acute, rigidly and abruptly mucronate, mucro pungent, 0.1–0.3 mm long; margins strongly incurved usually hiding the adaxial surface. Inflorescence of 5–12 solitary flowers borne in upper axils, appearing loosely to tightly clustered. Flowers: pedicels subtended by slightly enlarged stipules 2.4–2.8 mm long, each ~0.3 mm wide at sinus, not hiding the pedicels; pedicels 1.1–1.7 mm long, with dense, appressed to spreading, straight, white hairs to 0.5 mm long; floral bracteoles inserted 1.0–1.4 mm above pedicel apex, appressed at base, linear (free from subtending stipules), leaf-like, herbaceous, green, 4.2–5.3 mm long, reaching 4/5 to full length of calyx, mucronate, pubescent; stipules narrowly triangular, 1.9–2.3 mm long, each 0.3–0.4 mm wide at sinus, fused for ~1/4 of length; calyx tube broadly obconic, 1.4–2.7 mm long, green, sparsely pubescent outside; calyx lobes 5, ±oblong, 3.7–4.3 mm long, acuminate, with scattered long spreading hairs on the lamina and fine, short, crisped hairs on the outer margin, the lower lobes narrowly triangular, 3.3–4.8 mm long, long–acuminate, with sparse long spreading hairs, the upper pair much broader, fused for ~1/2 of length; standard petal ±circular in appearance, or obcordate when flattened and claw is visible, emarginate, 7.1–8.1 mm long including 2.1–2.9 mm claw, 5.8–7.6 mm wide, bright yellow with a small crescent of red lines around a dull orange ‘eye’; wing petals narrow–oblong, 6.9–8.0 mm long including 2.1–2.3 mm claw, 1.2–1.9 mm wide, yellow, orange towards the base; keel petals naviculate, ±narrow–oblong in outline, 8.0–8.9 mm long including claw 2.1–2.7 mm long, 2.6–3.4 mm wide (when folded), slightly exceeding wings, yellow–orange, paler at the base; stamens free, subequal in length; filaments 4.8–6.0 mm long; anthers ~0.4 mm long; ovary sessile, 2-ovulate, 1.7–1.9 mm long, with a tuft of sparse, appressed hairs to 1.7 mm long in apical 1/3; style 6.2–7.5 mm long, curving ~30° ~3/5 from base, glabrous or with a few long, semi-appressed hairs only in lower 1/8; stigma inconspicuous, very slightly capitate. Fruit ovoid, brown, compressed, asymmetrically beaked, 4.3–5.4 mm long including stylar remnant, 2.3–3.5 mm in diameter, sparsely hairy in apical 1/3. Seeds compressed, irregular-ovoid, 1.9–2.2 mm long, 1.2–1.4 mm wide, smooth, dark brown; aril white, ±straight, entire, ~0.8 mm long. (Fig. 41, 42.)
Isolectotype of Pultenaea setulosa Benth. (E.M.Bowman s.n.; NSW 38978) and inset photo from G.N.Batianoff 91081 & P.Robins (NSW).

Line drawing of Pultenaea setulosa Benth. (a) Flowering branchlet. (b) Leaves: abaxial surface (R), adaxial surface (L). (c) Leaf lamina surface: abaxial surface (R), adaxial surface (L). (d) Stipules and petiole. (e) Lateral view of flower. (f) Standard. (g) Bracteole and stipules. Voucher: G.N.Batianoff 91081 & P.Robins (NSW). Scale bars: a, 10 mm; b, 4 mm; c, d, 2 mm; e, f, 5 mm; g, 2.5 mm. Illustration by L. Elkan.
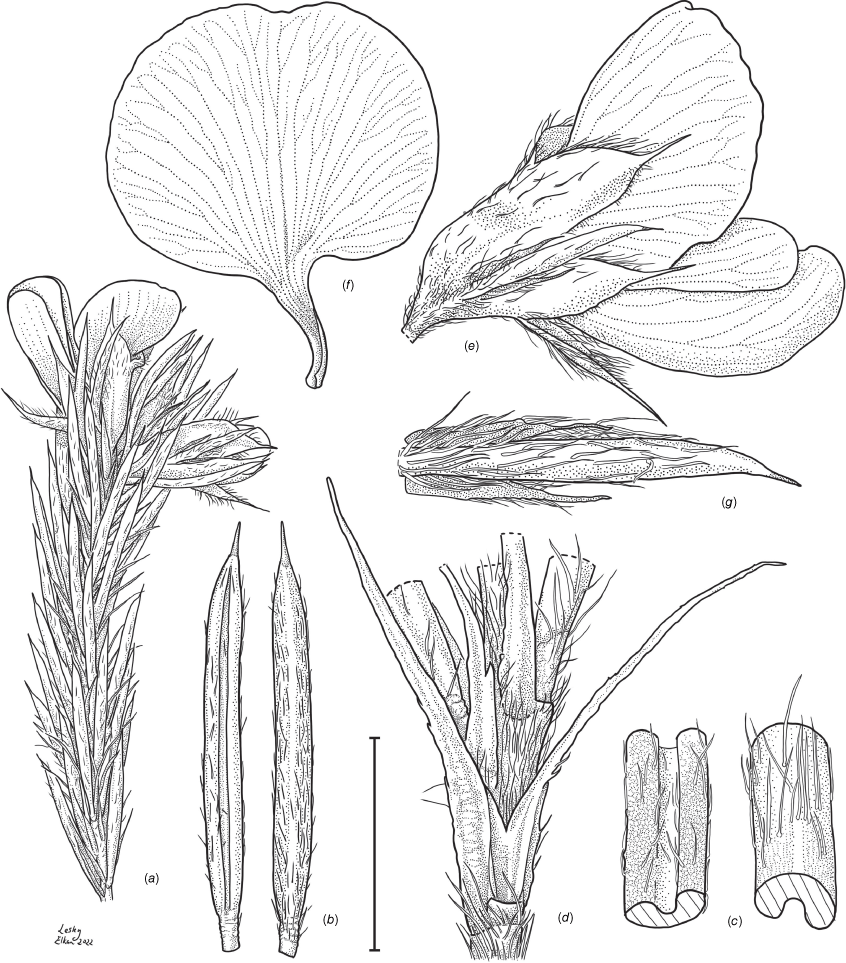
Probably most similar to P. boormanii but distinguished by the following combination of characters: branchlet hairs straight or curved, to 1 mm long (v. crisped, to 0.3 mm long); branchlet stipules fused for 1/5–1/4 (v. 1/3–1/2) of length; pedicels 1.1–1.7 (v. 1.5–2.5) mm long, subtended by stipules 2.4–2.8 (v. 1.9–2.3) mm long, ~0.3 (v. 0.6–0.7) mm wide; ovary 1.7–1.9 (v. 1.1–1.2) mm long, hairy in apical 1/3 (v. 1/2); seeds 1.9–2.2 (v. ~1.5) mm long; aril ±straight (v. curved through 90°).
Only known from south of Broad Sound, between Marlborough and The Caves in central eastern Queensland (Fig. 3b).
Recorded from serpentine ridges, hills and slopes, in woodlands or open forests with a heathy or grassy understorey on serpentinite geology, dominated by Eucalyptus fibrosa or Corymbia xanthope. Recorded in association with Acacia leptostachya, A. sp. Canoona (S.T.Blake 15321), Amorphospermum antilogum, Bursaria reevesii, Cajanus confertus, Callistemon viminalis, Capparis canescens, C. thozetiana, Cymbopogon queenslandicus, C. refractus, Dodonaea triquetra, Euphorbia laciniloba, E. ophiolitica, Hakea trineura, Leucopogon cuspidatus, Macrozamia serpentina, Melaleuca styphelioides, Neoroepera buxifolia, Olearia orientalis, Panicum effusum, Pimelea leptospermoides, Psychotria daphnoides, Stackhousia tryonii, Triodia mitchellii and Xanthorrhoea johnsonii (see Batianoff et al. 2000; Batianoff and Singh 2001).
Pultenaea setulosa is listed as Vulnerable under the Queensland Nature Conservation Act 1992 and Vulnerable under the Commonwealth Environment Protection and Biodiversity Conservation Act 1999. The species is currently known from ~15 populations. Known threats include habitat destruction through mineral extraction activities and grazing by feral animals (Batianoff et al. 2000). Area of extent: 700 km2. IUCN Category: Vulnerable (B2b(ii, iii)).
While de Kok and West (2002) included some material of P. setulosa under P. glabra, no morphological distinction is identifiable in the material examined and all specimens from this region are referrable to P. setulosa.
While the lectotype is labelled ‘Broad Sound’, a syntype at MEL is specifically labelled ‘Marlborough’ and all the Bowman material is most likely from this location, and may even constitute duplicates of a single collection, in which case the residual syntype listed above would be an isolectotype.
QUEENSLAND: Port Curtis District: The Caves Fault/Yaamba, 4 km NE of Yaamba town, 30 Aug. 1991, G.N.Batianoff 91081 & P.Robins (BRI-AQ633395*, MEL 725878, NSW 503411); Mount Redcliffe, 6 km SW of Marlborough town, 24 Oct. 1991, G.N.Batianoff 911011 & A.Franks (AD, BRI-AQ633390*, CANB 490822, DNA, L 0216991*, MEL 725875, NSW 503410); Mount Redcliffe, near Marlborough, 9 Aug. 1992, G.N.Batianoff 920801 (BRI-AQ565401*, K, L, MEL, MEXU, MO, NSW 503409, PR, PRE, QRS); Mount Fairview, W of Ridgelands State Forest 114, 12 May 1998, G.N.Batianoff 980529W & T.Ryan (BRI-AQ671590*, NSW 461947); Site 1+, State Forest 114, Rockhampton, 8 Dec. 1998, G.N.Batianoff 9812156, V.J.Nelder & S.Bidwell (BRI-AQ676689*, NSW 674665); Mount Slopeaway, near Marlborough, 30 Aug. 1963, N.H.Speck 1753 (BRI-AQ0237114*, CANB 138046, NSW 507569).
Pultenaea venusta R.L.Barrett & Orme, sp. nov.
Type: New South Wales: ~4 km E on Tunnel Road, ESE of Woomargama, 27 Sep. 2022, A.E.Orme 1776 & E.C.Orme (holo: NSW 1130081; iso: AD, CANB, K, MEL).
Pultenaea sp. Woomargama (E.J.McBarron 2493), NSW Herbarium, PlantNet [https://plantnet.rbgsyd.nsw.gov.au/, accessed 24 November 2022].
Spreading or scrambling shrub 0.4–1.0 m high, multi-stemmed from the base, spreading 0.7–1.2 m wide. Branchlets erect to spreading, stiff, shortly ribbed below petioles, moderately to densely pubescent with short, straight or curved, ±erect hairs to 0.6 mm long. Stipules narrowly lanceolate, fused for 1/10–1/8 of length, 2.3–3.9 mm long, 0.3–0.4 mm wide at sinus, weakly keeled, pale brown with pale narrow margins; apex spreading, long–acuminate; margins with scattered stiff hairs. Leaves alternate: petiole 0.3–0.7 mm long; lamina narrowly oblong to narrowly ovate, curved along length, with dense, spreading to somewhat erect (some almost appressed), sometimes curved, stiff hairs to 0.4 mm long, weakly discolourous, 3.3–5.1(–6.9) mm long, 0.7–1.2(–1.9 when flattened) mm wide, with scattered, irregular papillose cells adaxially, surface smooth with distinctly oblong cells abaxially; apex straight to slightly oblique, acute, rigidly and abruptly mucronate, mucro pungent, 0.4–0.8 mm long; margins strongly incurved but not hiding the adaxial surface. Inflorescence of 5–35 solitary flowers borne in upper axils, appearing tightly clustered. Flowers: pedicels subtended by slightly enlarged but shorter stipules 1.9–2.3 mm long, each 0.6–0.7 mm wide at sinus, partly hiding the pedicels; pedicels 1.5–2.1 mm long, densely hairy with hairs erect; floral bracteoles inserted 1.0–1.6 mm above pedicel but with prominent rib below extending full length of the pedicel (more obviously a rib when dried, fresh pedicels look compressed in section), appressed at base, linear (not or scarcely fused to subtending stipules), reduced leaf-like, herbaceous, green, 2.7–3.9 mm long, reaching ~7/8 length of calyx, mucronate, erect pubescent; stipules narrow, 1.3–2.4 mm long, each ~0.2 mm wide at sinus; calyx tube broadly obconic, 1.4–2.9 mm long, green, with dense, erect hairs outside; calyx lobes 5, narrowly or oblong–triangular, 3.4–4.3 mm long, acuminate, with long erect hairs on the lamina and dense, short, crisped hairs on the margins inside, the upper pair broader, fused for 1/4–1/2 of length; standard petal ±circular in appearance or obcordate when flattened and claw is visible, emarginate, 6.4–7.9 mm long including 2.4–2.6 mm claw, 6.8–8.2 mm wide, orange–yellow with a broad red crescent around a dull orange ‘eye’, reverse side orange–yellow grading to red; wing petals narrow–oblong, 6.4–7.1 mm long including 1.3–1.7 mm claw, 1.3–1.6 mm wide, mostly dark orange, red towards the base; keel petals naviculate, ±narrow–oblong in outline, 6.9–8.1 mm long including claw 1.4–2.2 mm long, 1.7–2.6 mm wide (when folded), slightly exceeding wings, deep red at the apex, grading to red at the base; stamens free to the base, subequal in length, 4.7–7.2 mm long; anthers ~0.5 mm long; ovary sessile, 2-ovulate, 1.5–1.9 mm long, with long, appressed hairs to 1.7 mm long in apical 1/5; style 5.5–6.2 mm long, gently curving through 45° near mid-point, glabrous or with a few with long, semi-appressed hairs only at very base; stigma inconspicuous, very slightly capitate. Fruit ovoid, brown, with pale marginal rib, faces with slightly raised venation, compressed, asymmetrically beaked, 4.5–4.9 mm long including stylar remnant, 2.9–3.2 mm in diameter, sparsely hairy in apical 1/4. Seeds compressed ovoid, 2.0–2.2 mm long, 1.5–1.6 mm wide, smooth or very shallowly reticulate, dark brown; aril white, irregular bowl-shaped with scattered papillae outside, ~1.1 mm long. (Fig. 43–45.)
Field photographs of Pultenaea venusta R.L.Barrett & Orme (a–f). Voucher: A.E.Orme AO1776 (NSW). Photos by A. E. Orme.
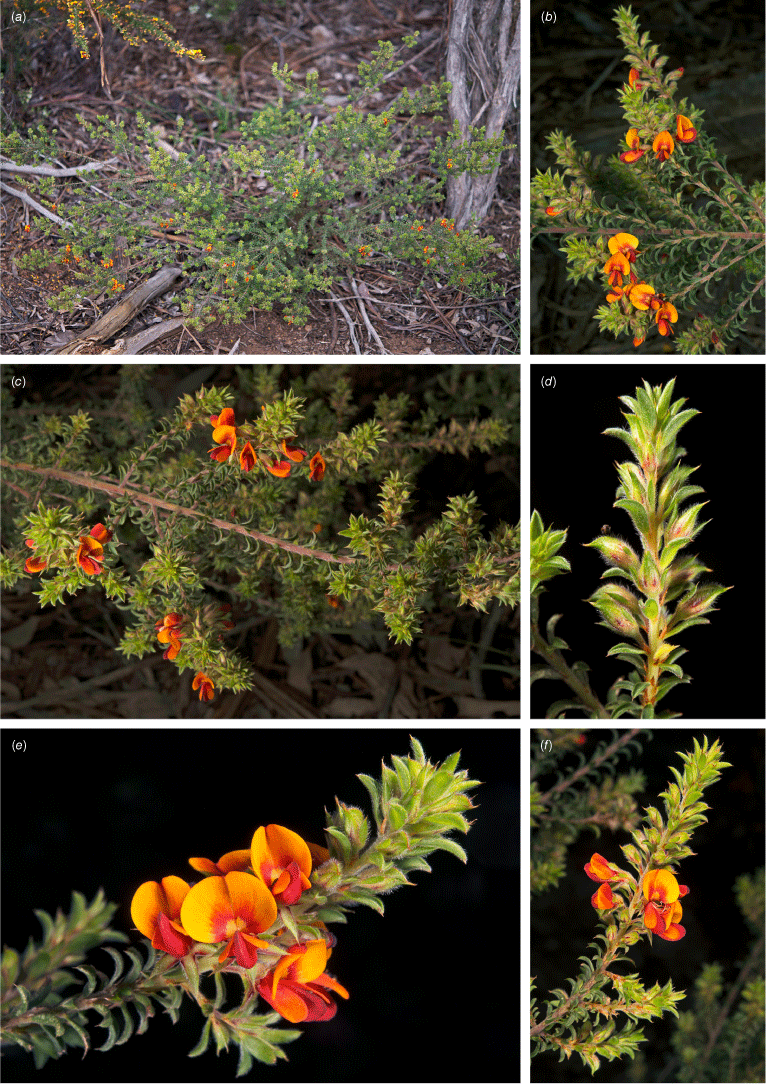
Line drawing of Pultenaea venusta R.L.Barrett & Orme. (a) Flowering branchlet. (b) Leaves: abaxial surface (R), adaxial surface (L), side view (bottom). (c) Leaf lamina surface: abaxial surface (below), adaxial surface (above). (d) Stipules and petiole. (e) Lateral view of flower. (f) Standard. (g) Bracteole and stipules. Voucher: E.J.McBarron 2493 (NSW). Scale bars: a, 10 mm; b, 4 mm; c, d, 2 mm; e, f, 5 mm; g, 2.5 mm. Illustration by L. Elkan.
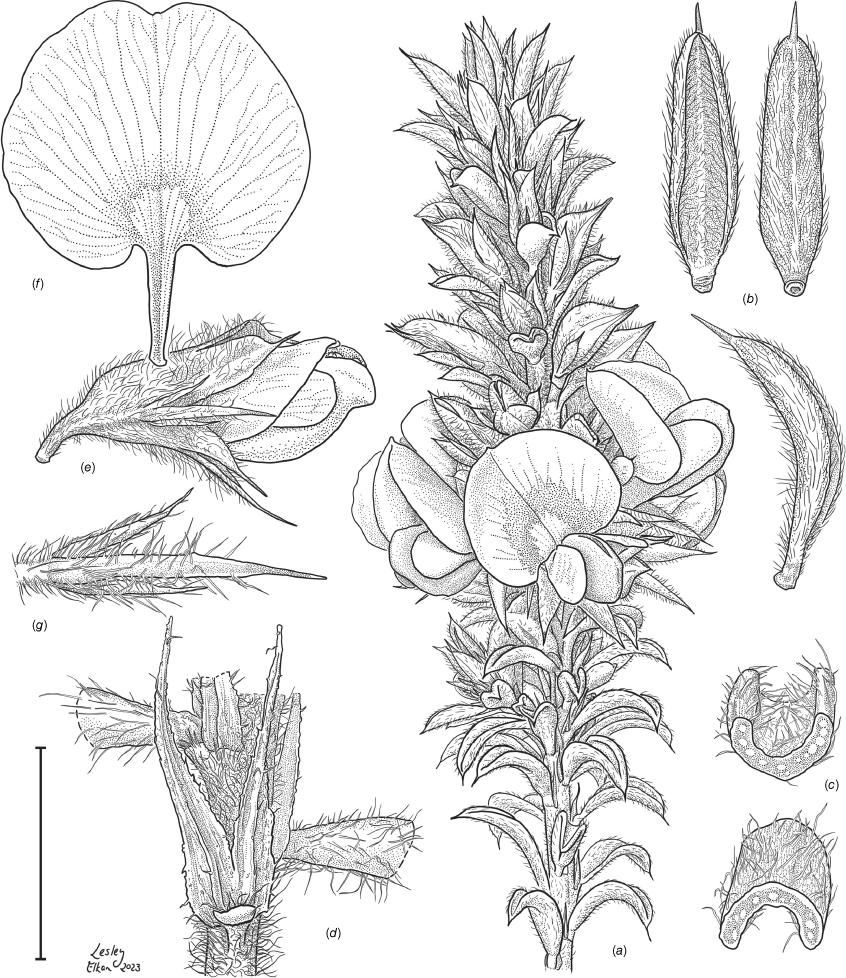
Distinguished from all other members of the P. setulosa group by the rib extending below the bracteoles to the base of the pedicel. Differs from P. praetermissa in the dense erect hairs on the calyx and leaves. Differs from P. corrickiae in shorter pedicels 1.5–2.1 (v. 1.9–3.5) mm long, subtended by shorter stipules 1.9–2.3 (v. 3.2–3.9) mm long and leaf mucro 0.4–0.8 (v. 0.2–0.3) mm long. Differs from other members of the P. setulosa group in the enlarged upper calyx lobes, ~2× as broad as the lower lobes. Differs from members of the P. procumbens species complex in the single-veined, narrow leaves, 0.7–1.0(–1.6 when flattened) mm wide.
Only known from the South Western Slopes of New South Wales, near Woomargama, ENE of Albury and possibly also near Cootamundra. ‘Woomargama’ presumably refers to the nature reserve of the same name, as that is where the species was relocated (Fig. 3e).
The epithet is from the Latin venustus (attractive) in reference to the spreading habit and many-flowered inflorescences that give this species an elegant appearance.
Pultenaea venusta is currently known from one or possibly two populations. Fieldwork in September 2022 relocated the Woomargama population that had not been recollected for 75 years, demonstrating that the species is extant but appears to be highly localised. A specimen collected from ‘Cootamundra’ 50 years ago that appears to be the same taxon suggests the present restricted distribution is likely attributable to land clearing for agriculture. Given the current limited distribution, the species is vulnerable to stochastic events. Area of extent and occupancy: likely ~2 km2. IUCN Category: Endangered (B2a, B2b(iii)).
This species has morphological affinity to Pultenaea praetermissa and P. corrickiae, and also some members of the P. procumbens species complex. Molecular data ally P. venusta to P. setulosa (Clugston et al., in prep.) but P. praetermissa and P. corrickiae are yet to be included in a phylogeny.
NEW SOUTH WALES: ?Cootamundra, 9 Nov. 1972, B.A.Andrews s.n. (NSW 444471); Woomargama, 5 Dec. 1947, E.J.McBarron 1262 (NSW 38710); Tunnel Road, Woomargama, 6 Nov. 1948, E.J.McBarron 2493 (NSW 38711, SYD); ~4 km E on Tunnel Road, ESE of Woomargama, 1 Jan. 2023, A.E.Orme AO1868 (CANB, NSW 1129587).
Pultenaea westonii R.L.Barrett & Clugston, sp. nov.
Type: New South Wales: Central Tablelands: Mount Canobolas, 10 miles [~16.1 km] SW of Orange, 8 Nov. 1960, E.F.Constable s.n. (holo: NSW 52794; iso: AD 98716054, BRI AQ0427018, CANB 362404, CBG 8504147, HO 93219, MEL 0677227, PERTH, n.v.).
Pultenaea sp. F, P.H.Weston in G.J.Harden (ed.), Fl. New South Wales 2: 494 (1991).
Pultenaea sp. Mount Canobolas (R.Coveny 4191b) NSW Herbarium, PlantNet [https://plantnet.rbgsyd.nsw.gov.au/, accessed 25 July 2022].
[Pultenaea lapidosa auct non Corrick: p.p., as to some NSW populations].
Erect to spreading, decumbent or prostrate shrub 0.25–1.3(–2) m high, multi-stemmed from the base, spreading 0.5–1.2 m wide. Branchlets erect to spreading, stiff, shortly ribbed below petioles, young growth sparsely pubescent with short, crisped, appressed to spreading, pale hairs to 0.2 mm long, glabrescent. Stipules broadly lanceolate, fused for ~1/2 of length, 2.8–5.3 mm long, ~0.4 mm wide at sinus, prominently keeled, dark brown to almost black; apex spreading, long–acuminate; margins with scattered, short tooth-like projections, glabrous; keels with sparse, long hairs. Leaves alternate: petiole 0.6–1.3 mm long, glabrous, or hairy near the apex; lamina narrowly elliptic, discolourous, paler adaxially, 5.6–10.5 mm long, 0.9–1.8 mm wide, with dense, irregular papillose cells adaxially, otherwise glabrous; sparse, long, tubercle-based hairs abaxially and midrib slightly raised, surface otherwise smooth with rounded to shortly oblong cells; apex acute, rigidly and abruptly mucronate, mucro straight to oblique, pungent, 0.6–1.5 mm long; margins incurved but not hiding the adaxial surface (except on new growth). Inflorescence of 8–15 solitary flowers borne in upper axils, appearing tightly clustered, each flower subtended by a slightly reduced leaf with enlarged stipules. Flowers: pedicels subtended by enlarged stipules 5.7–7.0 mm long, each 1.3–1.6 mm wide at sinus, not hiding the pedicels; pedicels 0.9–1.8 mm long, glabrous except at apex; floral bracteoles inserted ~0.6 mm below pedicel apex, appressed at base, linear (fused to subtending stipules for 2/5–1/2 of length), leaf-like, herbaceous, green, 5.0–6.3 mm long, free portion 2.0–4.8 mm long, reaching 2/3–3/4 length of calyx, short-mucronate, densely long, erect pubescent throughout; stipules broad, 2.6–4.4 mm long, each ~0.8 mm wide at sinus, glabrous except where fused to bracteole; calyx tube broadly obconic, 2.7–3.4 mm long, greenish, glabrous outside except towards apex; calyx lobes 5, narrowly triangular, 4.0–5.5 mm long, long–acuminate, with many, long, spreading to erect hairs, the upper pair fused for up to 1/8 length, ~2× as broad as lower lobes; standard petal ±circular in appearance, or obcordate when flattened and claw is visible, slightly emarginate, 11.7–13.1 mm long including 4.3–5.1 mm claw, 8.4–10.7 mm wide, deep orange with a small red crescent around an orange ‘eye’, reverse side dark orange–red; wing petals narrow–oblong, 8.3–9.1 mm long including 3.0–4.3 mm claw, 1.3–1.5 mm wide, deep orange, maroon towards the base; keel petals naviculate, ±narrow–oblong to obovate in outline, 9.6–10.5 mm long including claw 3.8–4.3 mm long, 3.2–3.6 mm wide (when folded), slightly exceeding wings, dark reddish-brown or maroon at the apex, grading to red at the base; stamens free to the base, subequal in length; filaments 7.4–9.8 mm long; anthers ~0.4 mm long; ovary sessile, 2-ovulate, ~1.4 mm long, with long, appressed hairs in apical 1/8; style 8.3–9.1 mm long, curving through 90° in apical 1/6, with a few long, semi-appressed hairs only at very base; stigma inconspicuous, very slightly capitate. Fruit ovoid, pale brown, slightly wrinkled at maturity, compressed, asymmetrically beaked, 4.2–5.4 mm long including stylar remnant, 2.1–2.8 mm in diameter, glabrous or sparsely hairy near apex on sutures. Seeds only seen immature, compressed, obliquely ovoid, ~1.2 mm long, 0.7 mm wide, smooth, dark brown; aril white, straight or curved, appearing entire, ~0.3 mm long. (Fig. 46–48.)
Field photographs of Pultenaea westonii R.L.Barrett & Clugston. Photos: (a) J. Fry; (b–e) R. W. Medd; (f, g) A. E. Orme. Photo (a) also posted on iNaturalist.
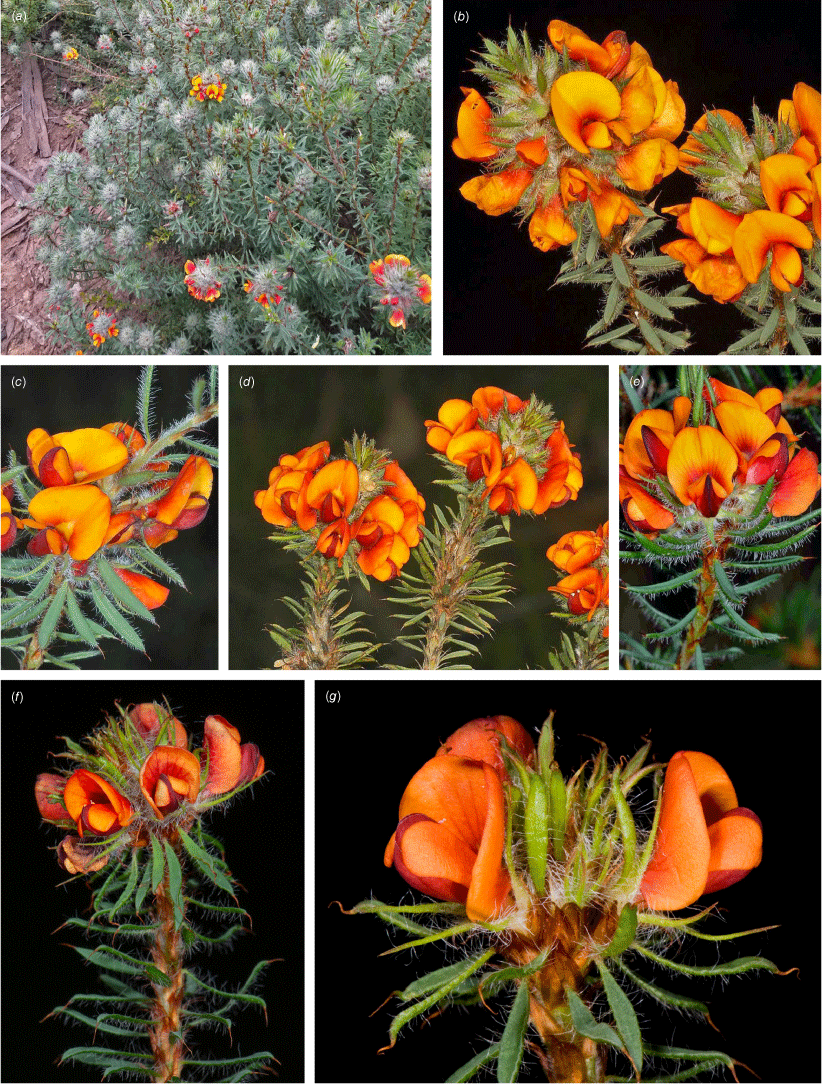
Line drawing of Pultenaea westonii R.L.Barrett & Clugston. (a) Flowering branchlet. (b) Leaves: adaxial surface (L), abaxial surface (R). (c) Leaf lamina surface: abaxial surface (above), adaxial surface (below). (d) Stipules. (e) Lateral view of flower. (f) Standard. (g) Bracteole and stipules. Voucher: U.Johnson s.n. (NSW). Scale bars: a, 16 mm; b, 4 mm; c, d, 2 mm; e, f, 5 mm; g, 2.5 mm. Illustration by C. Wardrop.
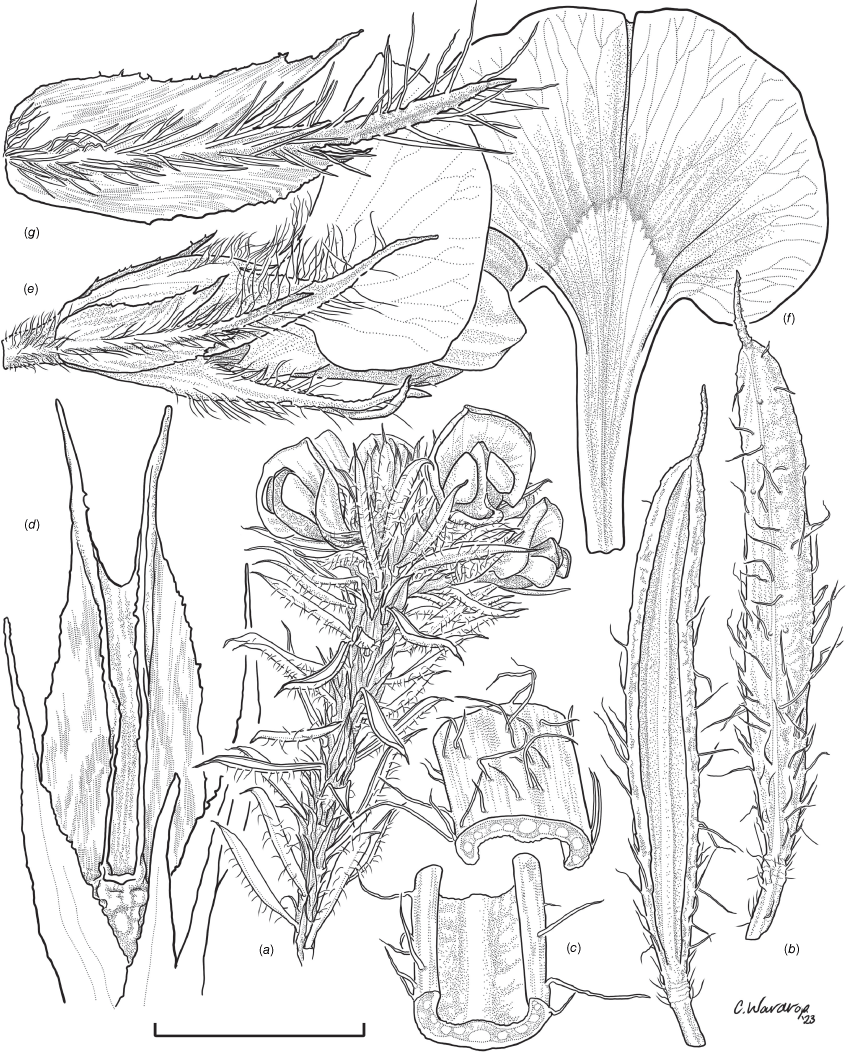
Similar in appearance to P. farmeriana, P. lapidosa and P. woolcockiorum but distinguished from these species by the following combination of characters: crisped branchlet hairs; leaf lamina 0.9–1.8 mm wide, narrowly elliptic, glabrous or hairy near the apex on abaxial surface, bases of these hairs with obscure tubercles, margins incurved but not hiding the adaxial surface; pedicels 0.9–1.8 mm long, subtended by stipules 5.7–7.0 mm long, each ~0.8 mm wide, not hiding the pedicels; floral bracteoles 5.0–6.3 mm long, inserted ~0.6 mm below pedicel apex, linear, leaf-like, 2/3–3/4 length of calyx, short-mucronate; bracteolar stipules 2.6–4.4 mm long; calyx lobes with upper pair fused for up to 1/8 length, ~2× as broad as lower lobes; standard 11.7–13.1 mm long, 8.4–10.7 mm wide; ovary ~1.4 mm long, hairy in apical 1/8; style 8.3–9.1 mm long.
Restricted to an area in the Central Tablelands of New South Wales bounded by Orange (including The Pinnacle and Mount Canobolas), The Mullions Range, Rylstone and Bathurst, with a southern outlier at Caloola (SE of Blayney), at altitudes of 800–1400 m (Fig. 3d).
Recorded from sandy loam and gravelly soils, usually on rocky hills and escarpments, sometimes on disturbed roadsides, in open forest, woodland or montane forest on basalt. Recorded in association with Acacia lanigera, A. penninervis, Brachyloma daphnoides, Daviesia latifolia, Dillwynia phylicoides, Eucalyptus macrorhyncha, E. mannifera, E. rossii, E. rubida, Grevillea polybracta, G. ramosissima, Pultenaea subternata, Sannantha cunninghamii and Stypandra glauca.
iNaturalist records: https://inaturalist.ala.org.au/observations/99932373.
The epithet recognises the extensive work of Peter H. Weston on Australian peas, particularly the tribe Mirbelieae and New South Wales Pultenaea (e.g. Crisp and Weston 1987, 1991, 1995; Weston 1991; Jobson and Weston 1998, 1999, 2001; Crisp et al. 1999; Weston and de Kok 2002, 2022; Barrett et al. 2021; Renner et al. 2022; https://www.anbg.gov.au/biography/weston-peter-henry.html). Weston was the first to recognise this as a distinct species (Weston 1991; as Pultenaea sp. F).
Pultenaea westonii is known from ~11 broad population areas, however most of these are at relatively high elevation on isolated peaks and the species may be under threat from climate change due to the relatively narrow ecological niche. Area of extent: 5500 km2. IUCN Category: Vulnerable (B1a, B1b(iii)).
Previously confused with P. lapidosa from Victoria, P. westonii can be readily distinguished by branchlet hairs ~0.2 (v. 0.4) mm long, crisped (v. straight); stipules on branchlets fused for ~1/2 (v. 2/3–3/4) of length; leaf lamina with obscure (v. prominent) tubercules at the base of hairs; calyx lobes with the upper pair fused for up to 1/8 (v. almost 1/2) of length; 4.0–5.5 (v. 2.8–4.3) mm long; standard 11.7–13.1 (v. 8.5–9.6) mm long, 8.4–10.7 (v. 5.8–7.2) mm wide. Eastern populations of P. westonii tend to have narrower, straighter leaves and regional variation in this taxon should be investigated further in the field.
Historically, this species has also been confused with P. aristata Sieber ex DC., P. canescens A.Cunn. and P. subspicata Benth. A number of records from sandstone substrates between Clarence and Bell previously included under Pultenaea sp. F (Weston 1991), P. lapidosa (Corrick 1994) or P. setulosa (Lollback et al. 2014) are here included under P. canescens. These populations can readily be separated from P. westonii by the leaves being rapidly glabrescent (only those near the apex of branchlets being hairy), longer leaf mucros (to 2.3 mm long), larger flowers with wing petals to 10.6 mm long and keel petals to 11.6 mm long, and larger ovaries, to 1.9 mm long. The plants are smaller, clonal shrubs that grow in open heath on sand, usually in swamps or sometimes in woodland. The populations with rapidly glabrescent leaves may deserve taxonomic recognition as distinct from P. canescens in the strict sense.
NEW SOUTH WALES: Mullions Range, 26 Sep. 1962, Y.Bennett s.n. (NSW 57956); 10 miles [~16.1 km] E of Bathurst, 7 Oct. 1961, C.Burgess s.n. (BRI, MEL 0677228, NSW 56419); 10 miles [~16.1 km] E of Bathurst, 8 Oct. 1961, C.Burgess s.n. (CANB 362404, CBG 8504149, NSW 63226); near the Great Western Highway, 10 miles [~16.1 km] E of Bathurst, 9 Oct. 1961, C.Burgess s.n. (CBG 366); S side of Bald Hill, Hill End, 31 July 1911, R.H.Cambage 2761 (BRI AQ0848381, CBG 8504148, NSW 38026); The Pinnacle, Orange, 3300 ft [~1.01 km], 5 Oct. 1957, E.F.Constable s.n. (NSW 17573, SYD); The Mullions Range, 6.6 miles [~10.6 km] NE of Mullion Creek (~14 miles [~22.5 km] NNE of Orange), 15 Apr. 1972, R.G.Coveny 4178 (CANB 590151, NSW 257618); slope of Mount Canobolas, 8 miles (~12.9 km) SW of Orange, 16 Apr. 1972, R.Coveny 4191b (NSW 467086); 4 km from Mount Canobolas, 1400 m, 04 July 1986, A.Curry 8648 (NSW 439512); Mount Canobolas, 5 Dec. 1964, P.Easton s.n. (NSW 257615); Springside via Orange, Nov. 1949, W.E.Giles s.n. (NSW 38028); 4th Crossing, Summer Hill Creek, 23 Sep. 1962, C.K.Ingram 16035 (NSW 2053798); Mudgee Road, southern outskirts of Ilford, 14 Oct. 1978, U.Johnson s.n. (NSW 2576170); Mullions Range State Forest, 19 Oct. 1959, B.Lane s.n. (NSW 47742); cultivated at ANBG, Canberra, (CBG 731956), originally from Bathurst (CBG 702343), 4 Oct. 1980, L.Lockwood ST 52 (CBG 8007373); corner of Ullamulla Road and Hill End Road, ~5 km north of Hill End, 28 Oct. 2018, K.McDougall 1494 (CANB 914467); ‘Kooroo’, Casula Hills, ~24 km N of Orange, 24 Oct. 1993, R.Medd 211409 & C.Bower (NSW 395742); 5.6 km along Lookout Road (Mullion Creek Ophir Road) from Long Point Road, ~7 km ENE of Mullion Creek, 20 Dec. 2006, A.E.Orme 632 & R.L.Johnstone (NSW 746860); cultivated at Mt Annan Botanic Garden [from A.E.Orme AO632], 13 Aug. 2008, A.E.Orme AO663 (NSW 773559); Barton Nature Reserve, near Orange, 1974, C.H.Pratten 16 (NSW 257616, PERTH); Post Office Lane, Lewis Ponds, ~25 km E of Orange, 16 Nov. 1989, W.Semple OR203 (NSW 439512); 1.5 km S of Ilford, 14 Nov. 1993, T.Tame s.n. (NSW 281584); 10 miles [~16.1 km] east of Bathurst, Oct. 1963, M.Tindale s.n. (AD 98716085*, BRI, n.v., CANB 366211, CBG 8600275, HO 98584, n.v., MEL 0677492, NSW 178865, PERTH, n.v.); Rylestone [Rylstone], Sep. 1932, A.H.Winter s.n. (NSW 38027).
Pultenaea woolcockiorum R.L.Barrett & Clugston, sp. nov.
Type: New South Wales: E side of Warrumba Range, NE of Grenfell, 12 Oct. 1973, R.Coveny 5245 (holo: NSW 257614; iso (all n.v. except L): ISC, K, L 2034534*, MO, RSA).
Pultenaea sp. Conimbla (D.E.Albrecht 15178) NSW Herbarium, PlantNet [https://plantnet.rbgsyd.nsw.gov.au/, accessed 25 July 2022].
Illustration: Woolcock and Woolcock (1989c, p. 132, fig.), as Pultenaea sp. (unnamed species).
Erect shrub 0.3–1.5 m high, single- or multi-stemmed from the base, spreading 0.3–0.6 m wide. Branchlets erect to spreading, stiff, shortly ribbed below petioles, moderately pubescent with long, straight, appressed hairs to 0.4 mm long. Stipules lanceolate, fused for 1/2–3/5 of length, 3.4–4.5 mm long, ~0.6 mm wide at sinus, prominently keeled, brown to red–brown with paler margins; apex spreading, long–acuminate; margins with scattered tooth-like projections, otherwise glabrous. Leaves alternate: petiole 0.6–1.0 mm long; lamina ±linear, with sparse, appressed hairs to 0.4 mm long, with at least some tubercle-based hairs abaxially, eventually glabrescent, weakly discolourous, paler above, 7.5–11.9 mm long, 0.5–0.8 mm wide, with dense, irregular papillose cells adaxially, surface smooth with distinctly oblong papillose cells abaxially; apex straight to slightly oblique, acute, rigidly and abruptly mucronate, mucro pungent, 0.4–0.9 mm long; margins strongly incurved usually mostly hiding the adaxial surface. Inflorescence of 5–9 solitary flowers borne in upper axils, appearing tightly clustered. Flowers: pedicels subtended by greatly enlarged stipules 5.6–6.6 mm long, each 1.3–1.6 mm wide at sinus, fused for 7/8 of length, hairy on lamina and margins, margins also toothed, hiding the pedicels; pedicels from ±sessile up to 0.2 mm long, glabrous; floral bracteoles inserted 0.3–0.5 mm above pedicel apex, appressed at base, linear (fused to subtending stipules for >4/5 of length), bract-like, scarious, brown, 3.9–4.5 mm long, free portion 1.4–1.9 mm long, reaching ~3/4 length of calyx, not or weakly mucronate, pubescent in lower 2/3; stipules broad, 4.6–5.1 mm long, each ~0.9 mm wide at sinus; calyx tube broadly obconic, 3.4–3.8 mm long, green, glabrous outside except near base of lobes; calyx lobes 5, subequal, the upper pair fused for ~1/3 of length, narrowly triangular, 4.4–5.1 mm long, acuminate, with long spreading hairs on lamina and margins; standard petal ±circular in appearance or obcordate when flattened and claw is visible, emarginate, 8.3–11.4 mm long including 2.9–3.4 mm claw, 7.8–8.6 mm wide, yellow–orange with a small red crescent around an orange to pale yellow ‘eye’; wing petals narrow–oblong, 7.7–9.0 mm long including 2.7–3.0 mm claw, 1.8–2.1 mm wide, yellow–orange at the apex, purple-red in the middle and towards the base; keel petals naviculate, ±narrow–oblong in outline, 8.4–9.7 mm long including claw 2.8–3.2 mm long, 2.7–2.9 mm wide (when folded), slightly exceeding wings, dark maroon or dark red at the apex, grading paler towards the base; stamens free to the base, subequal in length, 6.5–8.3 mm long; anthers ~0.5 mm long; ovary sessile, 2-ovulate, ~1.9 mm long, with long, appressed hairs in apical 1/8; style 6.6–7.1 mm long, curving almost 90° towards apex, with long, semi-appressed hairs only in lower 1/8; stigma inconspicuous, very slightly capitate. Fruit not seen. (Fig. 49–51.)
Field photographs of Pultenaea woolcockiorum R.L.Barrett & Clugston. Vouchers: (a, b, e) Conimbla, not vouchered; (c, d) R.W.Purdie 5596 (CANB). Photos: (a, b, e) R. W. Medd; (c, d) M. Fagg.

Line drawing of Pultenaea woolcockiorum R.L.Barrett & Clugston. (a) Flowering branchlet. (b) Leaves: abaxial surface (R), adaxial surface (L). (c) Leaf lamina surface: abaxial surface (L), adaxial surface (R). (d) Stipules and petiole. (e) Lateral view of flower showing pair of stipules subtending pedicel. (f) Standard. (g) Bracteole and stipules. Voucher: R.Coveny 5245 (NSW). Scale bars: a, 10 mm; b, 4 mm; c, d, 2 mm; e, f, 5 mm; g, 2.5 mm. Illustration by L. Elkan.
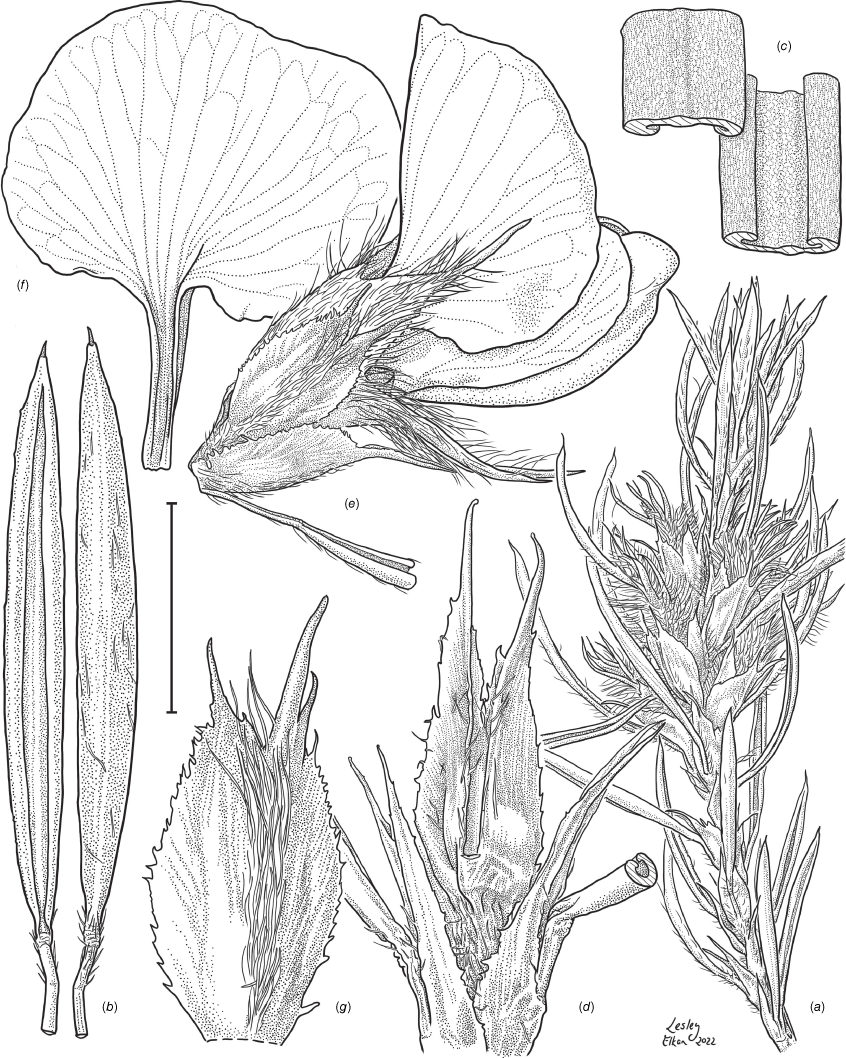
Similar in appearance to P. farmeriana, P. lapidosa and P. westonii but distinguished from these species by the following combination of characters: straight branchlet hairs; leaf lamina 0.5–0.8 mm wide, ±linear, with appressed hairs on abaxial surface, margins strongly incurved, usually hiding the adaxial surface; flowers ±sessile or on pedicels up to 0.2 mm long, subtended by stipules 5.6–6.6 mm long, each ~0.9 mm wide at sinus, hiding the pedicels; floral bracteoles mostly fused to broad subtending stipules, free portion of floral bracteoles 1.4–1.9 mm long, inserted 0.3–0.5 mm above pedicel apex (or base of calyx when sessile), linear, bract-like, ~3/4 length of calyx, not or weakly mucronate, scarious, brownish; bracteolar stipules 4.6–5.1 mm long; calyx lobes ±equal in width, with upper pair fused for ~1/3 length; standard 8.3–11.4 mm long, 7.8–8.6 mm wide; ovary ~1.9 mm long, hairy in apical 1/8; style 6.6–7.1 mm long.
Only known from the Warrumba Range, Kangarooby Range, Bumbaldry and Conimbla National Park in the Central Western Slopes of New South Wales at altitudes of 500–600 m (Fig. 3d).
Recorded from open eucalypt forest, woodland and shrubland on slopes on pale red sandy soils. Recorded in association with Acianthus collina, Brachyloma daphnoides, Daviesia latifolia, Dillwynia sericea, Gompholobium huegelii, Grevillea floribunda, G. polybractea, G. ramosissima, Hibbertia calycina, Leptospermum multicaule, Ozothamnus diosmifolius, Pterostylis parviflora, P. rubescens, P. stenosepala, Sannantha cunninghamii and Stypandra glauca.
The epithet recognises the work of Dorothy Theresa Woolcock (1914–2001) and Colin Elwyn Woolcock (1914–1990) who first collected this species while collecting, illustrating and photographing peas for the book A fieldguide to native peaflowers of Victoria and Southeastern Australia (D. T. Woolcock and C. E. Woolcock 1989). Woolcock and Woolcock also wrote numerous articles on the genus Pultenaea for Australian Plants (D. T. Woolcock and C. E. Woolcock 1983; C. E. Woolcock and D. T. Woolcock 1984, 1985, 1986a, 1986b, 1987a, 1987b, 1988a, 1988b, 1989a, 1989b, 1989c, 1989d). (https://www.anbg.gov.au/biography/woolcock-collin.html).
Pultenaea woolcockiorum is currently known from six collections, mostly within a 10-km radius. Recorded as occasional or locally common at collection sites. Area of extent: <200 km2. IUCN Category: Vulnerable (B2a, B2b(iii).
Previously confused with P. lapidosa from Victoria due to the long, spreading hairs on the calyx lobes but with narrower leaves 0.5–0.8 (v. 0.9–1.3) mm wide that are more strongly incurved, hiding the adaxial surface, with a shorter mucro 0.4–0.9 (v. 0.8–1.7) mm long. Pultenaea woolcockiorum was first recognised as an unnamed species by Woolcock and Woolcock (1989c), who published a short description and detailed line drawing that is clearly ascribable to this species, with a voucher specimen held at MEL, prior to the description of P. lapidosa Corrick. This was subsequently overlooked as part of the variation present in P. lapidosa sensu Corrick (1994). Also confused with P. boormanii that has stipules free from the bracteole.
NEW SOUTH WALES: Conimbla National Park, Fire trail, ~830 m due S of the Ironbark picnic area, 497 m, 1 Oct. 2017, D.E.Albrecht 15178 (CANB 902429); Kangarooby Road, 4 km N of corner with Mid Western Highway (W of Cowra), 17 Oct. 2014, I.Denton 468 (NSW 907211); Kangarooby Range, 18 July 1964, C.K.Ingram 18504 (NSW 2053972); Major West Road, near junction with Codogong turnoff (between east and west sections of Conimbla National Park), 6 Oct. 2002, R.W.Purdie 5596 (CANB 641387); Bumbaldry, 25 Oct. 1980, C.E.Woolcock s.n. (MEL 0674740).
Pultenaea procumbens A.Cunn. in B.Field (ed.), Geogr. Mem. New South Wales 347 (1825)
Type citation: ‘…frequent in the western interior, on exposed hills.’
Type: New South Wales: [stony hill beside] Coxs River [~9 km due S of the junction with the River Lett], 8 Oct. 1822, A.Cunningham 67 (lecto, here designated: K 000270273; isolecto: BM, n.v., K 000270272, K 000270374).
Procumbent shrub 10–15 cm high, sprawling 30–60 cm wide. Branchlets erect to spreading, stiff, shortly ribbed below petioles, densely pubescent with long, wavy, matted to ±spreading hairs to 0.5(–0.8) mm long. Stipules narrowly lanceolate, fused for 1/20 of length, 1.7–3.3 mm long, 0.4–0.5 mm wide at sinus, weakly keeled, apex somewhat spreading, long–acuminate, pale brown with narrow pale margins (broad near the base), margins with a few scattered stiff hairs in the apical 2/3. Leaves alternate: petiole 0.2–0.3 mm long; lamina narrowly ovate to somewhat obovate, 3-nerved, at least at the base, ±straight or gently recurved along length, with dense, appressed to spreading, or almost erect, gently curved, ±soft hairs to 1.5 mm long, weakly discolourous, paler above, adaxial surface not or only partly hidden by incurved margins towards the apex, 4.3–6.8 mm long, 1.5–2.4 mm wide, with scattered, irregular papillose cells adaxially, surface smooth with distinctly oblong cells abaxially; apex straight, acute, rigidly and abruptly mucronate, mucro pungent, 0.3–1.2 mm long; margins ±flat near the base, moderately to strongly incurved towards the apex but not or only somewhat hiding the adaxial surface. Inflorescence of 3–5 solitary flowers borne in upper axils, appearing tightly clustered. Flowers: pedicels subtended by greatly enlarged stipules 2.3–2.6 mm long, each 0.8–0.9 mm wide at sinus, hiding the pedicels; pedicels 0.9–1.6 mm long, moderately hairy with hairs spreading; floral bracteoles inserted 1.0–1.2 mm above pedicel but with prominent rib below extending to the apex of the pedicel, appressed at base, lanceolate (not or scarcely fused to subtending stipules), leaf-like, herbaceous, green, 3.1–3.5 mm long, ~0.6 mm wide, reaching ~7/8 to length of calyx, mucronate, spreading-pubescent; stipules broad, 2.3–2.7 mm long, each ~0.8 mm wide at sinus; calyx tube broadly obconic, 1.5–1.7 mm long, green, almost glabrous or sparsely hairy outside with hairs erect to spreading; calyx lobes 5, narrowly triangular, 3.3–4.1 mm long, acuminate, with long erect hairs on the lamina at the base of the lower lobes and dense, short, curved hairs on the margins inside, the upper pair ~2× as broad as lower lobes, fused for ~1/2 of length; standard petal ±circular in appearance or obcordate when flattened and claw is visible, emarginate, 5.1–6.1 mm long including 1.6–2.0 mm claw, 4.9–5.4 mm wide, yellow–orange, reverse side yellow–orange; wing petals narrow–oblong, 4.7–5.8 mm long including 1.4–1.6 mm claw, 1.5–1.7 mm wide, yellow–orange; keel petals naviculate, ±narrow–oblong in outline, 5.9–6.9 mm long including claw 1.8–2.2 mm long, 1.9–2.5 mm wide (when folded), ±equal to wings, deep red at the apex, grading to red at the base; stamens free to the base, subequal in length, 4.0–5.6 mm long; anthers ~0.4 mm long; ovary sessile, 2-ovulate, 1.2–1.3 mm long, with long, appressed hairs to 1.4 mm long in apical 1/6; style ~5.6 mm long, curving through 90° near mid-point, with a few with long, semi-appressed hairs only at very base; stigma inconspicuous, very slightly capitate. Fruit not seen. (Fig. 52, 53.)
Lectotype of Pultenaea procumbens A.Cunn. (A.Cunningham 67; K 000270273), with inset photo showing details of leaves and inflorescence, and isolectotype (K 000270274). http://specimens.kew.org/herbarium/K000270273, © Board of Trustees of the Royal Botanic Gardens, Kew.

Field photographs of Pultenaea procumbens A.Cunn. (a, b). Cultivated at Blue Wren Nursery from stock collected near Walang. Photos: R. W. Medd.

Distinguished from P. setigera by the low, procumbent (v. presumably erect) habit; branchlets with wavy, matted to ±spreading (v. straight, ±erect) hairs to 0.5(–0.8) (v. to 0.2) mm long; leaves 4.3–6.2 (v. 2.0–4.1) mm long, 1.5–2.2 (v. 1.0–1.6) mm wide, with soft spreading (v. stiffly erect) hairs on the lamina to 1.5 (v. to 0.3) mm long; pedicels 0.9–1.6 (v. 0.7–0.9) mm long, subtended by stipules 2.3–2.6 (v. 1.7–2.3) mm long, 0.8–0.9 (v. 0.6–0.7) mm wide; calyx tube almost glabrous or sparsely hairy with hairs erect to spreading (v. moderately hairy); calyx lobes with upper pair fused for ~1/2 (v. 1/4) of length, 3.3–4.1 (v. 2.4–3.4) mm long, with long erect hairs on the lamina only at the base of the lower lobes (v. mostly hairy); ovary 1.2–1.3 (v. ~0.8) mm long. Distinguished from all members of the P. setulosa group by the distinctly 3-nerved leaves 1.5–2.2 mm wide.
Occurs in the Central Western Slopes of New South Wales, ‘in the western interior’ (Fig. 3d). The original collection location has been identified through examination of Cunningham’s diary as a hill adjacent the Coxs River, ~9 km due south of the junction with the River Lett (in Cunningham’s time known as ‘The Rivulet’), south of Lithgow and WSW of Blackheath. There is a single collection from ‘Tarana’, two collections from near Lithgow and photographs have been seen of cultivated plants apparently originating from Walang.
Based on Cunningham’s diary, Pultenaea procumbens was collected from a brushy, exposed rocky hill. The underlying geological component of the area is granite. Cunningham records a number of species co-occurring on the hill with P. procumbens (also the type locality for species marked with #): #Acacia verniciflua [as A. vernix], A. sp. [as straminea], #Bossiaea buxifolia, Dillwynia retorta [as tortilis], Eucalyptus pulverulenta [#Eucalyptus pulvigera], Hibbertia hermanniifolia, #Leucopogon attenuatus, L. virgatus, Mirbelia grandiflora [origin of seeds for #], [?#]M. pungens, Patersonia sericea, Philotheca myoporoides subsp. myoporoides [#Eriostemon cuspidatus], #Pomaderris ledifolia, Pultenaea dentata [#P. argentea], Stylidium graminifolium [as staticaefolium] and Stypandra glauca.
The epithet is from the Latin procumbere (prostrate) in reference to the low, spreading habit of the species.
The typical form of Pultenaea procumbens remains poorly collected with only a single specimen at NSW. Many vegetated hills remain in the general area in which the species was originally collected and these have been poorly surveyed. Photographs have been taken of plants apparently cultivated from Walang, an area from which there are no known vouchers for this taxon. Area of extent 320 km2. IUCN Category: Data Deficient but may well be Vulnerable due to the degree of land clearing in the vicinity of known collection locations.
The sheet selected here as the lectotype, K 000270273, is in the best condition with the largest number of flowers. While not all of the sheets bear Cunningham’s number 67, we have no reason to believe that more than one collection is involved as only a single collection is noted in Cunningham’s diary from the time, therefore the remaining sheets are all considered to be isolectotypes.
Probably related to P. setigera that has distinctive, stiffly erect hairs to 0.2 mm long on the leaf lamina and within the P. setulosa complex, perhaps most similar to P. venusta, differing in having distinctly 3-veined leaves.
NEW SOUTH WALES: Tarana, 29 Oct. 1940, W.F.Blakely & F.J.Ludowici s.n. (NSW 38698); Bowens Creek (Coxs River), 2 Nov. 1963, C.Burgess s.n. (CBG 12971); South Bowenfels, 4 Nov. 1963, C.Burgess s.n. (CBG 12871); Coxs River, 3 Oct. 1904, R.H.Cambage & J.H.Maiden 1168 (SYD); above Coxs R[iver], Up[per] Blue M[oun]t[ain]s, 27 Oct. 1963, V.E.Sands 6310.8.12 (AD 96642295, n.v., SYD).
Pultenaea setigera A.Cunn. ex Benth., Comm. Legum. Gen. 18 (1837)
Type citation: ‘Prope Bathurst, versus septentrionem, in Nova Cambria australi. A. Cunningham.’
Type: New South Wales: brushy hills north of Bathurst [‘Pine Range’ (now Pine Ridge), between Rock Forest and the Macquarie River, ~16 km along the Macquarie River from Bathurst], Nov. [24 Oct.] 1822, A.Cunningham s.n. [124] (lecto, here designated: K 000270271*; isolecto: BM 000544583*, BM 000544584*; BRI AQ0025097*, CANB 259092*, MEL 525036 (fragm.), NSW 469942, W, n.v. [de Kok and West 2002 listed this as ‘holo’]).
Possibly erect shrub. Branchlets erect to spreading, stiff, shortly ribbed below petioles densely pubescent with short, straight, ±erect hairs to 0.2 mm long. Stipules narrowly lanceolate, fused for 1/20 of length, 1.5–2.5 mm long, ~0.4 mm wide at sinus, weakly keeled, pale brown with narrow pale margins; apex spreading, long–acuminate; margins with scattered stiff hairs in the apical 1/3. Leaves alternate: petiole 0.1–0.3 mm long; lamina narrowly ovate to somewhat obovate, 3-nerved, at least at the base, ±straight or gently curved along length, with dense, ±mostly erect or some hairs spreading, straight or gently curved, ±stiff hairs to 0.3 mm long, weakly discolourous, 2.0–4.1 mm long, 1.0–1.6 mm wide, adaxial surface with scattered, irregular papillose cells adaxially, surface smooth with distinctly oblong cells abaxially; apex straight to slightly oblique, acute, rigidly and abruptly mucronate, mucro pungent, 0.3–0.6(–1.6) mm long; margins ±flat near the base, moderately to strongly incurved towards the apex but not or only somewhat hiding the adaxial surface towards the apex. Inflorescence of 3–8 solitary flowers borne in upper axils, appearing tightly clustered. Flowers: pedicels subtended by greatly enlarged stipules 1.7–2.3 mm long, each 0.6–0.7 mm wide at sinus, hiding the pedicels; pedicels 0.7–0.9 mm long, with dense, erect hairs; floral bracteoles inserted 0.9–1.1 mm above pedicel but with prominent rib below extending full length of the pedicel, appressed at base, linear (not or scarcely fused to subtending stipules), reduced leaf-like, herbaceous, green, 3.3–3.7 mm long, ~0.6 mm wide, reaching ~7/8 to length of calyx, mucronate, erect-pubescent; stipules broad, 2.1–2.4 mm long, each ~0.7 mm wide at sinus; calyx tube broadly obconic, 1.5–1.9 mm long, green, moderately hairy with hairs erect to spreading outside; calyx lobes 5, narrowly or oblong–triangular, 2.4–3.4 mm long, acuminate, with long erect hairs on the lamina and dense, short, curved hairs on the margins inside, the upper pair ~2× as broad as lower lobes, fused for ~1/4 of length; standard petal ±circular in appearance or obcordate when flattened and claw is visible, emarginate, ~5 mm long including ~1.1 mm claw, ~4.2 mm wide, yellow–orange, reverse side yellow–orange; wing petals narrow–oblong, ~5 mm long including ~1.2 mm claw, ~1.2 mm wide, yellow–orange; keel petals naviculate, ±narrow–oblong in outline, 5.0–5.7 mm long including claw 1.6–1.9 mm long, 1.3–1.6 mm wide (when folded), ±equal to wings, deep red at the apex, grading to red at the base; stamens free to the base, subequal in length, 4.4–5.8 mm long; anthers ~0.5 mm long; ovary sessile, 2-ovulate, ~0.8 mm long, with long, appressed hairs to 1.2 mm long in apical 1/8; style ~5.1 mm long, gently curving through 45° near mid-point, glabrous or with a few long, semi-appressed hairs only at very base; stigma inconspicuous, very slightly capitate. Fruit not seen. (Fig. 54).
Isolectotype of Pultenaea setigera A.Cunn. ex Benth. with inset photos (a, b) showing details of leaves and inflorescence (A.Cunningham 124; NSW 469942).
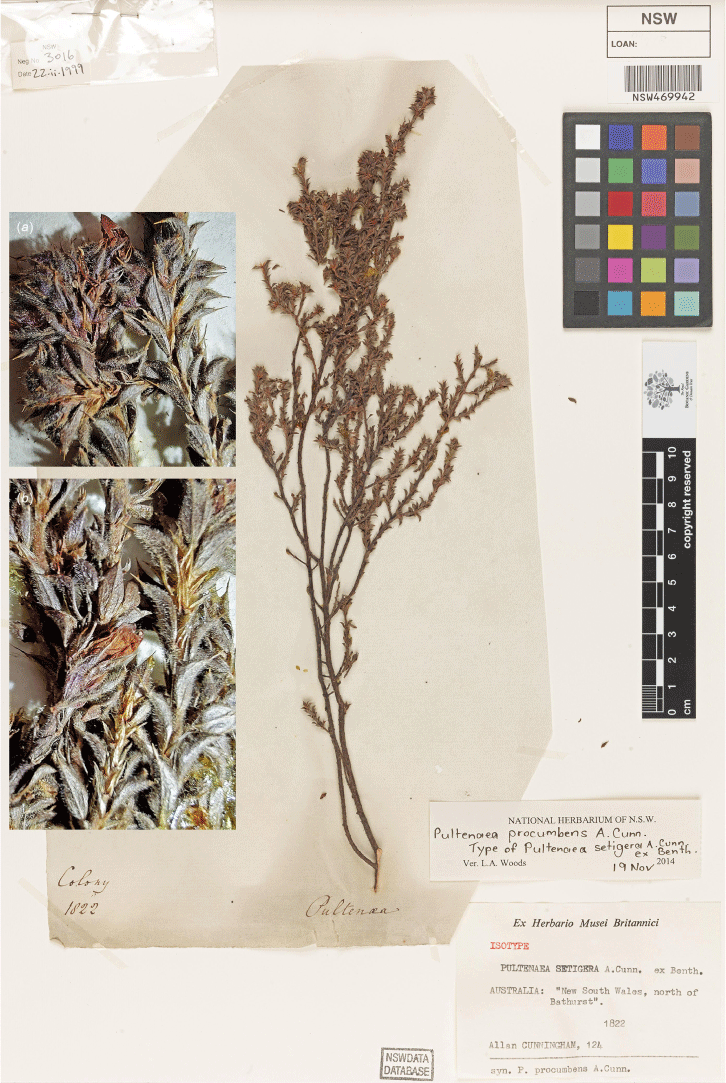
Distinguished from most members of the P. setulosa group by the following combination of characters: branchlet hairs to 0.2 mm long; petioles 0.1–0.3 mm long; leaf lamina 3-nerved, ±flat at base, margins strongly incurved above; pedicels 0.7–0.9 mm long; the rib extending below the bracteoles to the base of the pedicel; calyx lobes 2.4–3.4 mm long, with upper pair ~2× as broad as lower lobes; ovary ~0.8 mm long. Pultenaea venusta is somewhat similar in appearance and was previously confused with P. setigera but P. venusta has branchlet hairs to 0.6 mm long; petioles 0.3–0.7 mm long; and the leaf lamina strongly incurved along length. The type form of P. procumbens is morphologically similar but P. setigera can be distinguished by the presumably erect (v. low, procumbent) habit; branchlets with straight, ±erect (v. wavy, matted to ±spreading) hairs to 0.2 (v. to 0.5(–0.8)) mm long; leaves 2.0–4.1 (v. 4.3–6.8) mm long, 1.0–1.6 (v. 1.5–2.4) mm wide, with stiffly erect (v. soft spreading) hairs on the lamina to 0.3 (v. to 1.5) mm long; pedicels 0.7–0.9 (v. 0.9–1.6) mm long, subtended by stipules 1.7–2.3 (v. 2.3–2.6) mm long, 0.6–0.7 (v. 0.8–0.9) mm wide; calyx tube moderately hairy (v. almost glabrous or with sparse, erect to spreading hairs); calyx lobes with upper pair fused for ~1/4 (v. 1/2) of length, 2.4–3.4 (v. 3.3–4.1) mm long, with long erect hairs on the lamina only at the base of the lower lobes (v. mostly hairy); ovary ~0.8 (v. 1.2–1.3) mm long.
Only known from the Central Tablelands of New South Wales, ‘north’ of Bathurst. The original collection location has been identified through examination of Cunningham’s unpublished diary as the Pine Ridge, ~15 km WNW of Bathurst near the modern locality of Rock Forest (A. & T. Orchard, pers. comm.) (Fig. 3d).
Based on Cunningham’s diary, the species was collected from a white to pink, red-speckled granite boulder range in ‘country moderately wooded with a small timber’ growing on granite with grey–brown sandy loam. The following species are recorded for the area: Acacia buxifolia, Acacia doratoxylon, Acacia lanigera, A. vestita, Actinotus helianthi, Arthropodium sp., Caladenia dilatata, C. filamentosa, C. gracilis, C. testacea, Callitris endlicheri, Calytrix tetragona, Cheilanthes sp., Correa reflexa var. reflexa, Cryptandra amara var. floribunda, Cyphanthera albicans, Dampiera purpurea subsp. albicans, Daucus glochidiatus, Diuris sulphurea, Dodonaea sp., Drosera sp., Eucalyptus blakelyi, E. dwyeri, E. goniocalyx, E. macrorhyncha, Exocarpos strictus, Gonocarpus sp., Goodenia decurrens, G. hederacea, Grevillea arenaria, G. mucronulata, Hibbertia cf. calycina, Hydrocotyle laxiflora, Indigofera adesmiifolia, Isotoma axillaris, Kunzea sp., Lepidosperma sp., Microtis unifolia, Olearia ramosissima, Parietaria debilis, Platysace sp., Pomaderris betulina subsp. betulina, Prostanthera crocodyloides, Pterostylis cycnocephala, P. revoluta, Senecio phelleus, Solanum sp., Stellaria pungens, Stypandra glauca, Styphelia sp., Thelymitra paniculata, Tricoryne sp., Wahlenbergia gracilenta, W. stricta, Westringia eremicola and Zieria obcordata.
The epithet is from the Latin seta (bristle) and gerere (to bear) in reference to the distinctive erect bristle-like hairs on the leaves.
Pultenaea setigera is currently known only from the type and is possibly extinct. Several vegetated hills remain in the general area in which the species was originally collected, therefore the species is possibly extant and targeted surveys should be undertaken to determine whether the species can be relocated. IUCN Category: Data Deficient, but if extant, likely Critically Endangered.
The sheet at K selected here as the lectotype is in the best condition with the largest number of flowers and is also the only sheet with the full location description ‘brushy hills north of Bathurst’. While the sheets are variable in the information provided with each (e.g. s.n. or 124, and varying location and date precision), we have no reason to believe that more than one collection is involved as only a single collection is noted in Cunningham’s diary from the time, therefore the remaining sheets are all considered to be isolectotypes.
Data availability
The data that support this study are available in the article and accompanying online supplementary material.
Conflicts of interest
Dr Russell Barrett is an editor of Australian Systematic Botany but did not at any stage have editor-level access to this manuscript while in peer review, as is the standard practice when handling manuscripts submitted by an editor to this journal. Australian Systematic Botany encourages editors to publish in the journal who are kept totally separate from the decision-making processes for personal manuscripts. The authors have no further conflicts of interest to declare.
Declaration of funding
This study was funded by a Postdoctoral Fellowship Grant from the Australian Biological Resources Study (ABRS) National Taxonomy Research Grant Program (NTRGP 4-EHP5TK3) to James Clugston and collaborators at the Royal Botanic Gardens and Domain Trust, Sydney. The Genomics for Australian Plants Framework Initiative (who generated the data used in this publication) is supported by funding from Bioplatforms Australia (enabled by NCRIS), the Ian Potter Foundation, Royal Botanic Gardens Foundation (Victoria), Royal Botanic Gardens Victoria, the Royal Botanic Gardens and Domain Trust, the Council of Heads of Australasian Herbaria, CSIRO, Centre for Australian National Biodiversity Research and the Department of Biodiversity, Conservation and Attractions, Western Australia.
Author contributions
R. L. Barrett and J. A. R. Clugston conceived the study, prepared taxon treatments and wrote the first draft of the manuscript. R. L. Barrett, J. A. R. Clugston, D. E. Albrecht, J. R. Hosking, P. C. Jobson, A. E. Orme, R. L. Palsson and P. H. Weston conducted fieldwork. All authors interpreted results and revised the paper. L. Elkan and C. Wardrop created the line drawings. R. L. Barrett, J. R. Hosking, A. E. Orme, R. L. Palsson and P. H. Weston took photographs. R. L. Palsson prepared the maps.
Acknowledgements
We are grateful to Brendan Lepschi, Hannah McPherson, Val Stajsic, Lisa Woods, Kristina McColl, Trevor Wilson, Zoe Davies, Xanthe Davies, Felix Barrett, Rosemary Purdie, Murray Fagg, Antionette Farrow, John Palmer, Kerry Gibbons, Melissa Wong, Ryan O’Donnell and Helen Brewer for assistance in the field, providing photos and observations on particular species. Photographs included in this paper have been generously provided by Michael Cincotta, Geoff Derrin, Murray Fagg, Jack Fry, Manfred Jusaitis, Richard (Dick) Medd, Ryan O’Donnell, Rosemary Purdie, James Turner and Pete Woodall. Geoff Williams, from Diversity Native Seeds, is thanked for providing observations and photographs of P. boormanii and Helen Brewer for collecting and photographing P. praetermissa. Michael Batley identified the bee pollinating flowers of Pultenaea farmeriana for Peter Weston. Specimen images were kindly provided by Nigel Fechner (BRI), Jurgen Kellerman (AD), Nimal Karunajeewa (MEL) and Maggie Jones (K). Glenda Wardle, Murray Henwood and Bobby Tamayo kindly facilitated a visit to SYD to examine specimens. Tony and Tessa Orchard provided valuable information relating to two species collected and named by Allan Cunningham through detailed investigations of Cunningham’s diaries and plant specimens. Rosemary Purdie and Murray Fagg are particularly thanked for undertaking targeted searches for P. estelleae, P. murrayi and P. praetermissa. We acknowledge the contribution of the Genomics for Australian Plants Framework Initiative consortium (https://www.genomicsforaustralianplants.com/consortium/) in the generation of data used in this publication. We are grateful to Kevin Thiele and an anonymous reviewer for very constructive comments on the manuscript.
References
Barrett RL (2012) Poranthera moorokatta (Phyllanthaceae), a rare new species from Perth, Western Australia. Nuytsia 22, 399-407.
| Crossref | Google Scholar |
Barrett RL (2015) Bush Blitz and biodiversity discovery in Australia. Australian Systematic Botany 27, i.
| Crossref | Google Scholar |
Barrett RL, Barrett MD (2015) Twenty-seven new species of vascular plants from Western Australia. Nuytsia 26, 21-87.
| Crossref | Google Scholar |
Barrett RL, Barrett MD (2023) Taxonomic revision of Australian Erythrophleum (Fabaceae: Caesalpinioideae) including description of two new species. Australian Systematic Botany 36, 401-426.
| Crossref | Google Scholar |
Barrett RL, Dixon KW (2001) A revision of the genus Calectasia (Calectasiaceae) with eight new species described from south-west Western Australia. Nuytsia 13, 411-448.
| Crossref | Google Scholar |
Barrett RL, Clugston JAR, Cook LG, Crisp MD, Jobson PC, Lepschi BJ, Renner MAM, Weston PH (2021) Understanding diversity and systematics in Australian Fabaceae tribe Mirbelieae. Diversity 13, 391.
| Crossref | Google Scholar |
Barrett RL, Barrett MD, Clements MA (2022a) A revision of Orchidaceae from the Kimberley region of Western Australia with new species of tropical Calochilus and Dipodium. Telopea 25, 203-270.
| Crossref | Google Scholar |
Barrett RL, Plunkett GT, Bruhl JJ, Wilson KL (2022b) Lepidosperma prospectum (Cyperaceae), a new species from Sydney coastal heath and notes on usage of sword sedges. Telopea 25, 81-92.
| Crossref | Google Scholar |
Batianoff GN, Singh S (2001) Central Queensland serpentine landforms, plant ecology and endemism. South African Journal of Science 97, 495-500 Available at https://hdl.handle.net/10520/EJC97260.
| Google Scholar |
Batianoff GN, Neldner VJ, Singh S (2000) Vascular plant census and floristic analysis of serpentine landscapes in central Queensland. Proceedings of the Royal Society of Queensland 109, 1-30.
| Google Scholar |
Bell SAJ (2008) Rare or threatened plant species of Wollemi National Park, central eastern New South Wales. Cunninghamia 10, 331-371.
| Google Scholar |
Bell S, Nicolle D (2020) Glen Gallic Mallee (Eucalyptus dealbata subsp. aperticola, Myrtaceae), a new taxon from the sandstone escarpment of the Hunter Valley, New South Wales. Telopea 23, 141-150.
| Crossref | Google Scholar |
Bui EN, Thornhill AH, González-Orozco CE, Knerr N, Miller JT (2017) Climate and geochemistry as drivers of eucalypt diversification in Australia. Geobiology 15, 427-440.
| Crossref | Google Scholar | PubMed |
Byrne M (2008) Evidence for multiple refugia at different time scales during Pleistocene climatic oscillations in southern Australia inferred from phylogeography. Quaternary Science Reviews 27, 2576-2585.
| Crossref | Google Scholar |
Chase MW, Christenhusz MJM, Cauz-Santos LA, Nollet F, Bruhl JJ, Andrew DD, Palsson R, Jobson RW, Taseski GM, Samuel R (2023) Nine new species of Australian Nicotiana (Solanaceae). Australian Systematic Botany 36, 167-205.
| Crossref | Google Scholar |
Collins TL, Schmidt-Lebuhn AN, Andrew RL, Telford IRH, Bruhl JJ (2022) There’s gold in them thar hills! Morphology and molecules delimit species in Xerochrysum (Asteraceae; Gnaphalieae) and reveal many new taxa. Australian Systematic Botany 35, 120-185.
| Crossref | Google Scholar |
Corrick MG (1976a) Bush-peas of Victoria – genus Pultenaea no.1. The Victorian Naturalist 93, 176-179.
| Google Scholar |
Corrick MG (1976b) Bush-peas of Victoria – genus Pultenaea no.2. The Victorian Naturalist 93, 250-252.
| Google Scholar |
Corrick MG (1977a) Pultenaea paludosa J. Thompson – first record for Victoria. The Victorian Naturalist 94, 151.
| Google Scholar |
Corrick MG (1977b) Bush-peas of Victoria – genus Pultenaea no.3. The Victorian Naturalist 94, 26-28.
| Google Scholar |
Corrick MG (1977c) Bush-peas of Victoria – genus Pultenaea no.4. The Victorian Naturalist 94, 68-70.
| Google Scholar |
Corrick MG (1977d) Bush-peas of Victoria – genus Pultenaea no. 5. The Victorian Naturalist 94, 112-114.
| Google Scholar |
Corrick MG (1977e) Bush-peas of Victoria – genus Pultenaea No. 6. The Victorian Naturalist 94, 148-151.
| Google Scholar |
Corrick MG (1977f) Bush-peas of Victoria – genus Pultenaea No. 7. The Victorian Naturalist 94, 198-200.
| Google Scholar |
Corrick MG (1978a) Bush-peas of Victoria – genus Pultenaea – 8. The Victorian Naturalist 95, 26-30.
| Google Scholar |
Corrick MG (1978b) Bush-peas of Victoria – genus Pultenaea – 9. The Victorian Naturalist 95, 56-60.
| Google Scholar |
Corrick MG (1978c) Bush-peas of Victoria – genus Pultenaea – 10. The Victorian Naturalist 95, 92-94.
| Google Scholar |
Corrick MG (1978d) Bush-peas of Victoria – genus Pultenaea – 11. The Victorian Naturalist 95, 188-190.
| Google Scholar |
Corrick MG (1980a) Bush-peas of Victoria – genus Pultenaea – 12. The Victorian Naturalist 97, 19-22.
| Google Scholar |
Corrick MG (1980b) Bush-peas of Victoria – genus Pultenaea – 13. The Victorian Naturalist 97, 151-156.
| Google Scholar |
Corrick MG (1980c) Bush-peas of Victoria – genus Pultenaea – 14. The Victorian Naturalist 97, 217-221.
| Google Scholar |
Corrick MG (1981) Bush-peas of Victoria – genus Pultenaea – 15. The Victorian Naturalist 98, 42-44.
| Google Scholar |
Corrick MG (1983a) Bush-peas of Victoria – genus Pultenaea – 16. The Victorian Naturalist 100, 55-58.
| Google Scholar |
Corrick MG (1983b) Bush-peas of Victoria – genus Pultenaea – 17. The Victorian Naturalist 100, 207-210.
| Google Scholar |
Corrick MG (1984a) Bush-peas of Victoria – genus Pultenaea [– 18]. The Victorian Naturalist 101, 119-122.
| Google Scholar |
Corrick MG (1984b) Bush-peas of Victoria – genus Pultenaea – 19. The Victorian Naturalist 101, 166-167.
| Google Scholar |
Corrick MG (1984c) Bush-peas of Victoria – genus Pultenaea – 20. The Victorian Naturalist 101, 200-203.
| Google Scholar |
Corrick MG (1987a) Bush-peas of Victoria – genus Pultenaea Sm. (Fabaceae) – 21. The Victorian Naturalist 104, 64-68.
| Google Scholar |
Corrick MG (1987b) Bush-peas of Victoria – genus Pultenaea Sm. (Fabaceae) – 22. The Victorian Naturalist 104, 141-144.
| Google Scholar |
Corrick MG (1988a) Bush-peas of Victoria – genus Pultenaea Sm. (Fabaceae) – 23. The Victorian Naturalist 105, 36-40.
| Google Scholar |
Corrick MG (1988b) Bush-peas of Victoria – genus Pultenaea Sm. (Fabaceae) no. 24. A key to Pultenaea species in Victoria and an index to previous articles. The Victorian Naturalist 107, 99-104.
| Google Scholar |
Corrick MG (1993a) A new species of Pultenaea (Fabaceae) in Victoria. Muelleria 8, 51-53.
| Google Scholar |
Corrick MG (1993b) Notes on Pultenaea gunnii Benth. (Fabaceae) in Australia and description of a new subspecies from Victoria. Muelleria 8, 55-56.
| Google Scholar |
Corrick MG (1994a) A new species of Pultenaea (Fabaceae) from south-east Australia. Muelleria 8, 119-122.
| Google Scholar |
Corrick MG (1994b) Notes on Pultenaea Sm. (Fabaceae) in Victoria. Muelleria 8, 391-394.
| Google Scholar |
Corrick MG, Walsh NG (2009) A new species of Pultenaea (Fabaceae) from central Victoria. Muelleria 27, 179-182.
| Crossref | Google Scholar |
Crisp MD, Weston PH (1991) Almaleea, a new genus of Fabaceae from south-eastern Australia. Telopea 4, 307-311.
| Crossref | Google Scholar |
Crisp MD, Gilmore SR, Weston PH (1999) Phylogenetic relationships of two anomalous species of Pultenaea (Fabaceae: Mirbelieae), and description of a new genus. Taxon 48, 701-714.
| Crossref | Google Scholar |
Crisp MD, Laffan S, Linder HP, Monro A (2001) Endemism in the Australian flora. Journal of Biogeography 28, 183-198.
| Crossref | Google Scholar |
Crisp M, Cook L, Steane D (2004) Radiation of the Australian flora: what can comparisons of molecular phylogenies across multiple taxa tell us about the evolution of diversity in present-day communities? Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences 359, 1551-1571.
| Crossref | Google Scholar | PubMed |
Curtis AL, Grierson PF, Batley J, Naaykens J, Fowler RM, Severn-Ellis A, Thiele KR (2022) Resolution of the Eremophila tietkensii (Scrophulariaceae) species complex based on congruence between morphological and molecular pattern analyses. Australian Systematic Botany 35, 1-18.
| Crossref | Google Scholar |
de Kok RPJ, West JG (2002) A revision of Pultenaea (Fabaceae) 1. Species with ovaries glabrous and/or with tufted hairs. Australian Systematic Botany 15, 81-113.
| Crossref | Google Scholar |
de Kok RPJ, West JG (2003) A revision of the genus Pultenaea (Fabaceae) 2. Eastern Australian species with velutinous ovaries and incurved leaves. Australian Systematic Botany 16, 229-273.
| Crossref | Google Scholar |
de Kok RPJ, West JG (2004) A revision of the genus Pultenaea (Fabaceae). 3. The eastern species with recurved leaves. Australian Systematic Botany 17, 273-326.
| Crossref | Google Scholar |
De Queiroz K (2007) Species concepts and species delimitation. Systematic Biology 56, 879-886.
| Crossref | Google Scholar | PubMed |
Elliott TL, Spalink D, Larridon I, Zuntini AR, Escudero M, Hackel J, Barrett RL, Martín-Bravo S, Márquez-Corro JI, Granados Mendoza C, Mashau AC, Romero-Soler KJ, Zhigila DA, Gehrke B, Andrino CO, Crayn DM, Vorontsova MS, Forest F, Baker WJ, Wilson KL, Simpson DA, Muasya AM (2023) Global analysis of Poales diversification – parallel evolution in space and time into open and closed habitats. New Phytologist [Published online 27 November 2023].
| Crossref | Google Scholar | PubMed |
Endo Y, Nash M, Hoffmann AA, Slatyer R, Miller AD (2015) Comparative phylogeography of alpine invertebrates indicates deep lineage diversification and historical refugia in the Australian Alps. Journal of Biogeography 42, 89-102.
| Crossref | Google Scholar |
Feldberg K, Gradstein SR, Gröhn C, Heinrichs J, von Konrat M, Mamontov YS, Renner MAM, Roth M, Schäfer-Verwimp A, Sukkharak P, Schmidt AR (2021) Checklist of fossil liverworts suitable for calibrating phylogenetic reconstructions. Bryophyte Diversity and Evolution 43, 14-71.
| Crossref | Google Scholar |
Garrick RC, Sands CJ, Rowell DM, Tait NN, Greenslade P, Sunnucks P (2004) Phylogeography recapitulates topography: very fine-scale local endemism of a saproxylic ‘giant’ springtail at Tallaganda in the Great Dividing Range of south–east Australia. Molecular Ecology 13, 3329-3344.
| Crossref | Google Scholar | PubMed |
Hager T, Benson D (2010) The eucalypts of the Greater Blue Mountains World Heritage Area: distribution, classification and habitats of the species of Eucalyptus, Angophora and Corymbia (family Myrtaceae) recorded in its eight conservation reserves. Cunninghamia 10, 425-444.
| Google Scholar |
Hahm WJ, Riebe CS, Lukens CE, Araki S (2014) Bedrock composition regulates mountain ecosystems and landscape evolution. Proceedings of the National Academy of Sciences of the United States of America 111, 3338-3343.
| Crossref | Google Scholar | PubMed |
Hahm WJ, Dralle DN, Rempe DM, Bryk AB, Thompson SE, Dawson TE, Dietrich WE (2019a) Low subsurface water storage capacity relative to annual rainfall decouples Mediterranean plant productivity and water use from rainfall variability. Geophysical Research Letters 46, 6544-6553.
| Crossref | Google Scholar |
Hahm WJ, Rempe DM, Dralle DN, Dawson TE, Lovill SM, Bryk AB, Bish DL, Schieber J, Dietrich WE (2019b) Lithologically controlled subsurface critical zone thickness and water storage capacity determine regional plant community composition. Water Resources Research 55, 3028-3055.
| Crossref | Google Scholar |
Halford DA, Henderson RJF (2005) Studies in Euphorbiaceae s.lat. 6. A revision of the genus Poranthera Rudge (Antidesmeae, Porantherinae) in Australia. Austrobaileya 7, 1-27.
| Crossref | Google Scholar |
Hill RS (1998) Poor soils and a dry climate: the evolution of the Australian scleromorphic and xeromorphic vegetation. Australian Biologist 11, 26-29.
| Crossref | Google Scholar |
Hnatiuk RJ, Maslin BR (1988) Phytogeography of Acacia in Australia in relation to climate and species-richness. Australian Journal of Botany 36, 361-383.
| Crossref | Google Scholar |
Hua X, Cardillo M, Bromham L (2022) Adapting to extremes: Reconstructing evolution in response to changing climate over time and space in the diverse Australian plant genus Acacia. Journal of Biogeography 49, 727-738.
| Crossref | Google Scholar |
IUCN Standards and Petitions Subcommittee (2014) ‘Guidelines for Using the IUCN Red List Categories and Criteria. Version 11.’ (International Union for Conservation of Nature and Natural Resources) Available at http://www.iucnredlist.org/technical-documents/categories-and-criteria
Jobson PC, Weston PH (1998) Dillwynia glaucula (Fabaceae: Mirbelieae), a new species from the Southern Tablelands, New South Wales. Telopea 8, 1-5.
| Crossref | Google Scholar |
Jobson PC, Weston PH (1999) Two new species of Dillwynia (Fabaceae: Mirbelieae) from the Southern Tablelands of New South Wales. Telopea 8, 363-369.
| Crossref | Google Scholar |
Jobson PC, Weston PH (2001) Dillwynia rupestris (Fabaceae: Mirbelieae), a new species from the New England Tableland of New South Wales. Telopea 9, 323-327.
| Crossref | Google Scholar |
Lesica P, Yurkewycz R, Crone EE (2006) Rare plants are common where you find them. American Journal of Botany 93, 454-459.
| Crossref | Google Scholar | PubMed |
Mackenzie BDE, Clarke SW, Zimmer HC, Liew ECY, Phelan MT, Offord CA, Menke LK, Crust DW, Bragg J, McPherson H, Rossetto M, Coote DM, Yap J-YS, Auld TD (2022) Ecology and conservation of a living fossil: Australia’s Wollemi Pine (Wollemia nobilis). In ‘Imperilled: the encyclopedia of conservation’. (Eds DellaSala DA, Goldstein MI) pp. 884–894. (Elsevier: Amsterdam, Netherlands) 10.1016/B978-0-12-821139-7.00188-4
Maiden JH, Betche E (1899) Notes from the Botanic Gardens, Sydney. No. 4. Proceedings of the Linnean Society of New South Wales 24, 143-153.
| Crossref | Google Scholar |
Manna F, Cherry JA, McWhorter DB, Parker BL (2016) Groundwater recharge assessment in an upland sandstone aquifer of southern California. Journal of Hydrology 541, 787-799.
| Crossref | Google Scholar |
Murphy MJ, Murphy JK, Faris CJ, Mulholland MJ (2019) Marooned on an extinct volcano: the conservation status of four endemic land snails (Gastropoda: Pulmonata) at Mount Kaputar, New South Wales. Proceedings of the Linnean Society of New South Wales 141, S33-S44.
| Google Scholar |
O’Donnell RP, Bruhl JJ, Telford IRH, Wilson TC, Zimmer HC, Taseski GM, Andrew RL (2023) Molecular and morphological analyses support recognition of Prostanthera volucris (Lamiaceae), a new species from the Central Tablelands of New South Wales. Australian Systematic Botany 36, 1-20.
| Crossref | Google Scholar |
Orel HK, Briggs JD, Duretto MF (2023a) Zieria nubicola (Rutaceae), a highly restricted and newly described species from New South Wales, Australia. Telopea 26, 169-174.
| Crossref | Google Scholar |
Orel HK, McLay TGB, Guja LK, Duretto MF, Bayly MJ (2023b) Genomic data inform taxonomy and conservation of critically endangered shrubs: a case study of Zieria (Rutaceae) species from eastern Australia. Botanical Journal of the Linnean Society boad069.
| Crossref | Google Scholar |
Orthia LA, Crisp MD, Cook LG, de Kok RPJ (2005) Bush peas: a rapid radiation with no support for monophyly of Pultenaea (Fabaceae: Mirbelieae). Australian Systematic Botany 18, 133-147.
| Crossref | Google Scholar |
Patiño J, Bisang I, Goffinet B, Hedenäs L, McDaniel S, Pressel S, Stech M, Ah-Peng C, Bergamini A, Caners RT, Christine Cargill D, Cronberg N, Duckett J, Eppley S, Fenton NJ, Fisher K, González-Mancebo J, Hasebe M, Heinrichs J, Hylander K, Ignatov MS, Martínez-Abaigar J, Medina NG, Medina R, Quandt D, Rensing SA, Renzaglia K, Renner M, Ros RM, Schäfer-Verwimp A, Villarreal JC, Vanderpoorten A (2022) Unveiling the nature of a miniature world: a horizon scan of fundamental questions in bryology. Journal of Bryology 44, 1-34.
| Crossref | Google Scholar |
Pepper M, Barquero MD, Whiting MJ, Keogh JS (2014) A multi-locus molecular phylogeny for Australia’s iconic Jacky Dragon (Agamidae: Amphibolurus muricatus): Phylogeographic structure along the Great Dividing Range of south-eastern Australia. Molecular Phylogenetics and Evolution 71, 149-156.
| Crossref | Google Scholar | PubMed |
Pócs T, Renner MAM (2021) Contribution to the bryoflora of Australia, V. Radula tonitrua sp. nov. from Queensland. Telopea 24, 189-201.
| Crossref | Google Scholar |
Preece M, Harding J, West JG (2015) Bush Blitz: journeys of discovery in the Australian outback. Australian Systematic Botany 27, 325-332.
| Crossref | Google Scholar |
Purdie RW (1977a) Early stages of regeneration after burning in dry sclerophyll vegetation. I. Regeneration of the understorey by vegetative means. Australian Journal of Botany 25, 21-34.
| Crossref | Google Scholar |
Purdie RW (1977b) Early stages of regeneration after burning in dry sclerophyll vegetation. II. Regeneration by seed germination. Australian Journal of Botany 25, 35-46.
| Crossref | Google Scholar |
Renner MAM (2020) Opportunities and challenges presented by cryptic bryophyte species. Telopea 23, 41-60.
| Crossref | Google Scholar |
Renner MAM (2021) The typification of Australasian Plagiochila species (Plagiochilaceae: Jungermanniidae): a review with recommendations. New Zealand Journal of Botany 59, 323-375.
| Crossref | Google Scholar |
Renner MAM, de Lange PJ (2020) A revised circumscription for Siphonolejeunea and a new species from New Zealand. Australian Systematic Botany 33, 311-326.
| Crossref | Google Scholar |
Renner MAM, Foster CSP, Miller JT, Murphy DJ (2021a) Phyllodes and bipinnate leaves of Acacia exhibit contemporary continental-scale environmental correlation and evolutionary transition-rate heterogeneity. Australian Systematic Botany 34, 595-608.
| Crossref | Google Scholar |
Renner MAM, de Lange PJ, Glenny DS (2021b) A synopsis of Aotearoa/New Zealand Lejeunea (Lejeuneaceae: Jungermanniopsida) and new species in the Lejeunea epiphylla Colenso complex. Arctoa: Journal of Bryology 30, 187-212.
| Crossref | Google Scholar |
Renner MAM, Barrett RL, Clarke S, Clugston JAR, Wilson TC, Weston PH (2022) Morphological and molecular evidence refute a broad circumscription for Pultenaea glabra (Fabaceae: Mirbelieae), with implications for taxonomy, biogeography, and conservation. Australian Systematic Botany 35, 225-277.
| Crossref | Google Scholar |
Rutherford S (2020) Insights into speciation and species delimitation of closely related eucalypts using an interdisciplinary approach. Australian Systematic Botany 33, 110-127.
| Crossref | Google Scholar |
Rutherford S, Wilson PG, Rossetto M, Bonser SP (2016) Phylogenomics of the green ash eucalypts (Myrtaceae): a tale of reticulate evolution and misidentification. Australian Systematic Botany 28, 326-354.
| Crossref | Google Scholar |
Sands VE (1975) The cytoevolution of the Australian Papilionaceae. Proceedings of the Linnean Society of New South Wales 100, 118-155 Available at https://www.biodiversitylibrary.org/part/46884.
| Google Scholar |
Shay JE, Pennington LK, Mandussi Montiel-Molina JA, Toews DJ, Hendrickson BT, Sexton JP (2021) Rules of plant species ranges: applications for conservation strategies. Frontiers in Ecology and Evolution 9, 700962.
| Crossref | Google Scholar |
Skeels A, Cardillo M (2017) Environmental niche conservatism explains the accumulation of species richness in Mediterranean-hotspot plant genera. Evolution 71, 582-594.
| Crossref | Google Scholar | PubMed |
Telford IIRH, Clugston JAR, Barrett RL (2022) Pultenaea williamsii (Fabaceae: Mirbelieae), a new species endemic to the New England Tableland Bioregion of New South Wales. Telopea 25, 165-171.
| Crossref | Google Scholar |
Thompson J (1958) Systematic notes on some Eastern Australian members of the Papilionaceae. Proceedings of the Linnean Society of New South Wales Series 2 83, 187-189 Available at https://www.biodiversitylibrary.org/item/108609#page/199/mode/1up.
| Google Scholar |
Thompson J (1961) Papilionaceae. Flora of New South Wales 101, 46-79.
| Google Scholar |
Thornhill AH, Crisp MD, Külheim C, Lam KE, Nelson LA, Yeates DK, Miller JT (2019) A dated molecular perspective of eucalypt taxonomy, evolution and diversification. Australian Systematic Botany 32, 29-48.
| Crossref | Google Scholar |
Truswell E (1993) Vegetation changes in the Australian Tertiary in response to climatic and phytogeographic forcing factors. Australian Systematic Botany 6, 533-558.
| Crossref | Google Scholar |
Washington H, Wray RAL (2011) The geoheritage and geomorphology of the sandstone pagodas of the north-western Blue Mountains Region (NSW). Proceedings of the Linnean Society of New South Wales 132, 1-143.
| Google Scholar |
Weston PH, de Kok RPJ (2022) Pultenaea. In ‘New South Wales Flora Online’. Available at https://plantnet.rbgsyd.nsw.gov.au/cgi-bin/NSWfl.pl?page=nswfl&lvl=gn&name=Pultenaea [Verified 7 June 2022]
Wilde BC, Barrett RL (2021) Hidden in plain sight: Ficus desertorum (Moraceae), a new species of rock fig from Central Australia. Telopea 24, 283-301.
| Crossref | Google Scholar |
Williams KJ, Ford A, Rosauer DF, De Silva N, Mittermeier R, Bruce C, Larsen FW, Margules C (2011) Forests of east Australia: the 35th biodiversity hotspot. In ‘Biodiversity hotspots’. (Eds F Zachos, J Habel) pp. 295–310. (Springer) 10.1007/978-3-642-20992-5_16
Williamson HB (1920) A revision of the genus Pultenaea, Part 1. Proceedings of the Royal Society of Victoria 32, 210-224.
| Google Scholar |
Williamson HB (1921) A revision of the genus Pultenaea, Part II. Proceedings of the Royal Society of Victoria 33, 133-148.
| Google Scholar |
Williamson HB (1922) A revision of the genus Pultenaea, Part III. Proceedings of the Royal Society of Victoria 35, 97-107.
| Google Scholar |
Williamson HB (1925) A revision of the genus Pultenaea, Part IV. Proceedings of the Royal Society of Victoria 37, 125-129.
| Google Scholar |
Williamson HB (1928) A revision of the genus Pultenaea, Part V. Proceedings of the Royal Society of Victoria 40, 57-61.
| Google Scholar |
Wilson KL, Barrett RL (2023) Revision of the tropical genus Diplacrum (Cyperaceae: Bisboeckelereae) in Australia. Australian Systematic Botany 36, 143-156.
| Crossref | Google Scholar |
Wilson TC, Rutherford S, Yap J-YS, Douglas SM, Lee E, Rossetto M (2023) Eucalyptus cryptica (Myrtaceae): a critically endangered new species. Australian Systematic Botany 36, 386-400.
| Crossref | Google Scholar |
Woolcock CE, Woolcock DT (1984) Bush Peas. The genus Pultenaea – Part 2. Australian Plants 12(99 June), 304-309.
| Google Scholar |
Woolcock CE, Woolcock DT (1985) Bush Peas. The genus Pultenaea – Part 3. Australian Plants 13(102 March), 71-84.
| Google Scholar |
Woolcock CE, Woolcock DT (1986a) Bush Peas. The genus Pultenaea – Part 4. Australian Plants 13(106 March), 249-257.
| Google Scholar |
Woolcock CE, Woolcock DT (1986b) Bush Peas. The genus Pultenaea – Part 5. Australian Plants 13(107 June), 321-329.
| Google Scholar |
Woolcock CE, Woolcock DT (1987a) Bush Peas. The genus Pultenaea – Part 6. Australian Plants 14(111 June), 129-137.
| Google Scholar |
Woolcock CE, Woolcock DT (1987b) Bush Peas. The genus Pultenaea – Part 7. Australian Plants 14(112 September), 179-185.
| Google Scholar |
Woolcock CE, Woolcock DT (1988a) Bush Peas. The genus Pultenaea – Part 8. Australian Plants 13(115 June), 310-313.
| Google Scholar |
Woolcock CE, Woolcock DT (1988b) Bush Peas. The genus Pultenaea – Part 9. Australian Plants 14(116 September), 374-377.
| Google Scholar |
Woolcock CE, Woolcock DT (1989a) Fabaceae – the pea-flowered wildflower family. Australian Plants 15(119 June), 122-125.
| Google Scholar |
Woolcock CE, Woolcock DT (1989b) The pea-flowers called Pultenaea. Australian Plants 15(119 June), 126.
| Google Scholar |
Woolcock CE, Woolcock DT (1989c) Bush Peas - The genus Pultenaea Part 10. Australian Plants 15(119 June), 126-136.
| Google Scholar |
Woolcock CE, Woolcock DT (1989d) Bush Peas. The genus Pultenaea – Part 11. Australian Plants 15(120 September), 177-185.
| Google Scholar |
Woolcock DT, Woolcock CE (1983) Pultenaea – 1 with reference to related genera of the pea-lowered family. Australian Plants 12(95 June), 95-103, 116–121.
| Google Scholar |