A revision of Derris and Brachypterum (Leguminosae subfamily Papilionoideae) in Australia
Wendy E. Cooper A * , Frank A. Zich
A * , Frank A. Zich  A B , Lars Nauheimer
A B , Lars Nauheimer  A , Melissa J. Harrison A and Darren M. Crayn
A , Melissa J. Harrison A and Darren M. Crayn  A
A
A
B
Abstract
Derris Lour. and Brachypterum (Wight & Arn.) Benth. (Leguminosae subfamily Papilionoideae) in Australia are revised on the basis of examination of fresh, dried and spirit-preserved specimens and phylogenetic analysis of plastid matK and nuclear internal transcribed spacer (ITS) sequence data. Three species of Derris and four species of Brachypterum are recognised as occurring in Australia. Two new Brachypterum species, namely Brachypterum nitidum W.E.Cooper and Brachypterum opacum W.E.Cooper, are described and illustrated. Notes on habitat, distribution and an identification key are presented for all species of both genera that occur in Australia.
Keywords: Australian flora, Brachypterum, Derris, Fabaceae, identification key, Leguminosae, new species, Queensland flora, rainforest, Solori, taxonomy.
Introduction
Derris Lour. and Brachypterum (Wight & Arn.) Benth. belong to the tribe Millettieae within the Millettioid clade (Choi et al. 2022) within the subfamily Papilionoideae. Derris consists of ~50 species occurring in eastern Africa, Madagascar, India, Sri Lanka, South-East Asia, China, Malesia and Australia (Plants of the World Online, Royal Botanic Gardens, Kew, UK, see http://www.plantsoftheworldonline.org/, accessed August 2023). The following three species of Derris occur in Australia (Australian Plant Census IBIS database, Centre for Australian National Biodiversity Research, Council of Heads of Australasian Herbaria, see https://biodiversity.org.au/nsl/services/search/taxonomy, accessed August 2023): D. elliptica (Wall.) Benth., D. rubrocalyx Verdc. and D. trifoliata Lour. The status of D. elliptica in Australia is uncertain; it has been introduced and cultivated on islands of the Torres Strait for the rotenone contained in its roots (which has insecticidal, pesticidal and piscicidal properties; Lazo et al. 2014, pp. 74–75), but it is considered possibly native to Christmas Island (Alisdair Grigg, pers. comm., 2022).
Within Millettieae, Derris and Brachypterum belong to a group of six to nine genera referred to as the ‘Palaeotropic Derris-like taxa’ by Sirichamorn et al. (2012a). The two genera share many morphological features, including vine habit, leaves imparipinnate, leaflet numbers; inflorescence racemose or paniculate, branched or with wart-like or club-shaped brachyblasts; pods one or two-winged, but all these features are found in at least one other genus in the group. Sirichamorn et al. (2014) reported that the number of flowers per brachyblast (or lateral branches) in Derris is usually less than five and in Brachypterum mostly more than five. However, Australian material differs in that Derris specimens have one to seven flowers per brachyblast and Brachypterum specimens have 2–40. They can be clearly distinguished in fruit by the presence of seed chambers in Brachypterum (absent in Derris), and, within Australia, on ovule numbers (Brachypterum 8–10 v. Derris 2–5). Sirichamorn et al. (2012a, 2014) stated that the discs are annular, indistinct or absent in Derris, whereas they are tubular, cylindrical or 10-lobed in Brachypterum (Solori). However, Australian specimens of Derris trifoliata and Derris rubrocalyx seen by the first author have distinctly 10-lobed discs and therefore are not considered diagnostic.
Derris was reviewed by Adema (2003) who recognised ~50 species from Africa, India, South-East Asia, Australia and the Pacific. The relationships of Derris and Derris-like species were investigated by Sirichamorn et al. (2012a) by using molecular and morphological data to test the validity of the genera Aganope Miq., Deguelia Aublet, Leptoderris Dunn, Lonchocarpus Kunth, Philenoptera Hochst. ex A.Rich., Solori Adans. and to distinguish them from Derris and Paraderris (Miq.) Geesink. On the basis of the results, Sirichamorn et al. (2014) synonymised Paraderris with Derris to render the latter monophyletic and made the necessary nomenclatural changes, but did not attempt a full revision of Derris nor provide a list of recognised taxa.
Sirichamorn et al. (2012b, 2014) undertook parsimony and Bayesian analyses of sequence data from three plastid markers (trnL-F intergenic spacer, IGS; psbA–trnH IGS; and trnK–matK) and nuclear internal transcribed spacer (ITS) to test relationships and generic delimitation among Palaeotropical Derris-like taxa. Their analyses resolved a strongly supported clade of species (referred to as the ‘Brachypterum’ clade in Sirichamorn et al. 2012a, and the Solori clade in Sirichamorn et al. 2014) for which they resurrected the genus Solori (Sirichamorn et al. 2014). Later, Brachypterum Wight & Arn. was conserved against Solori (Sirichamorn et al. 2013; Applequist 2017; Adema and Sirichamorn 2019; Sirichamorn and Adema 2020). The genus Brachypterum currently consists of 12 species from India to Myanmar, China, Thailand, Laos, South-East Asia to Papua New Guinea and Australia (Plants of the World Online, see http://www.plantsoftheworldonline.org/). In Australia, two species are recognised, namely, B. involutum (Sprague) Adema & Sirich. and B. koolgibberah (F.M.Bailey) Adema & Sirich. The Australian Plant Census (see https://biodiversity.org.au/nsl/services/search/taxonomy) currently recognises these species under Solori as S. involuta (Sprague) Sirich. & Adema, and S. koolgibberah (F.M.Bailey) Sirich. & Adema respectively.
Sterile Derris and Brachypterum specimens are sometimes confused with Austrosteenisia Geesink. However, Austrosteenisia differs in having peltate stipules (v. never peltate in Derris and Brachypterum); stipels separated from the rachis and angled at ~45° somewhat midway between the rachis and petiolule axis (v. laying along the rachis); leaflet apex usually with a small mucro (v. mucro absent); and leaflet primary vein sunken (v. slightly raised).
Australian material of Brachypterum and Derris that cannot be accommodated within existing species has been accumulating in herbaria since 1937 (Derris sp. Daintree Sparvell s.n. CNS 4567A). In this study, we revised these two genera in Australia, including a test of generic limits using phylogenetic analysis of plastid matK and nuclear ITS sequence data. On the basis of the results, we recognise three species of Derris and four species of Brachypterum in Australia, including two new species of Brachypterum described herein, namely, Brachypterum nitidum W.E.Cooper and Brachypterum opacum W.E.Cooper. We speculate that more species remain to be discovered, opening the possibility of new sources of useful compounds as recently published by Boonprajan et al. (2024).
Materials and methods
Morphological study
This study is based on the examination of material from CNS as well as selected specimens from BRI, CANB, CFSHB, K, L, NE and PERTH, and images online. All specimens cited have been seen by the first author.
Measurements of the floral parts and fruits are based on dried or fresh material as well as material preserved in 70% ethanol.
Molecular phylogenetic analysis
To confirm the genus relationships of the study taxa and to test generic limits of Brachypterum and Derris, we constructed a dataset of sequences of the nuclear ribosomal ITS region (ITS1, 5.8S, ITS2) and plastid matK locus that included a broad sampling of representatives of Derris sensu lato (including Brachypterum) and their closest relatives in tribe Millettieae (Fabaceae) on the basis of the phylogenetic results of Sirichamorn et al. (2012a). We sourced ITS data of 36 taxa and matK data of 34 taxa from GenBank and generated new ITS data for 34 samples and matK data for 32 samples, together representing 18 taxa. Multiple samples of the putative new species Brachypterum nitidum and B. opacum, and of Derris rubrocalyx and D. trifoliata, were included to assess the validity and limits of those taxa. In total, 10 species of Brachypterum (excluding the 2 newly described species) and 25 species of Derris were included, representing respectively 83 and 38% of currently recognised species (Plants of the World Online, see http://www.plantsoftheworldonline.org/). Details of all samples used in this study are provided in Table 1.
Specimens for this study were collected under Queensland Government Permits WITK11258712, WISP11258812, PTU18-001474 and PTC18-001475.
| Sample | Laboratory DNA number | Herbarium voucher | GenBank Accession number matK | GenBank Accession number ITS | |
|---|---|---|---|---|---|
| Brachypterum cumingii (Benth.) Adema & Sirich. | JX506618 | JX506447 | |||
| Brachypterum eriocarpum (F.C.How) Adema & Sirich. | JX506625 | JX506454 | |||
| Brachypterum involutum (Sprague) Adema & Sirich. 1 | G05662 | CANB 591415 | OR531451 | OR531482 | |
| Brachypterum involutum (Sprague) Adema & Sirich. 2 | MN076309 | JX506451 | |||
| Brachypterum koolgibberah (F.M.Bailey) Adema & Sirich. | JX506624 | JX506453 | |||
| Brachypterum microphyllum Miq. | JX506619 | JX506448 | |||
| Brachypterum nitidum sp. nov. 1 | G08017 | Cooper, W. 2528 (CNS) | OR531452 | OR531483 | |
| Brachypterum nitidum sp. nov. 2 | G08018 | Cooper, W. 2529 (CNS) | OR531453 | OR531484 | |
| Brachypterum nitidum sp. nov. 3 | G08019 | Cooper, W. 2522 (CNS) | OR531454 | OR531485 | |
| Brachypterum nitidum sp. nov. 4 | G08806 | Cooper, W. 2546 (CNS) | OR531455 | OR531486 | |
| Brachypterum nitidum sp. nov. 5 | G08807 | Cooper, W. 2617 (CNS) | OR531456 | OR531487 | |
| Brachypterum nitidum sp. nov. 6 | G08811 | Cooper, W. 2576 (CNS) | OR531457 | OR531488 | |
| Brachypterum opacum sp. nov. 1 | G08801 | Cooper, W. 2533 (CNS) | OR531458 | OR531489 | |
| Brachypterum opacum sp. nov. 2 | G08802 | Cooper, W. 2534 (CNS) | OR531459 | OR531490 | |
| Brachypterum opacum sp. nov. 3 | G08803 | Cooper, W. 2535 (CNS) | OR531460 | OR531491 | |
| Brachypterum opacum sp. nov. 4 | G08812 | Cooper, W. 2578 (CNS) | OR531461 | OR531492 | |
| Brachypterum philippinense (Merr.) Adema & Sirich. | JX506627 | JX506455 | |||
| Brachypterum pseudoinvolutum (Verdc.) Adema & Sirich. | JX506623 | JX506452 | |||
| Brachypterum robustum Dalzell & A.Gibson | JX506617 | JX506446 | |||
| Brachypterum scandens Benth. | LC435419 | LC435418 | |||
| Brachypterum thorelii (Gagnep.) Adema & Sirich. | JX506620 | JX506449 | |||
| Canavalia rosea (Sw.) DC. | G05661 | CANB 686257 | OR531448 | OR531479 | |
| Dahlstedtia pentaphylla (Taub.) Burkart | G06453 | CANB 503755 | OR531462 | OR531493 | |
| Derris alborubra Hemsl. | JX506638 | JX506466 | |||
| Derris amoena Benth. | JX506628 | JX506456 | |||
| Derris caudatilimba F.C.How | AF467045 | ||||
| Derris cuneifolia Benth. | JX506649 | JX506478 | |||
| Derris elegans var. elegans Benth. | JX506641 | JX506469 | |||
| Derris elliptica (Wall.) Benth. | JX506648 | JX506477 | |||
| Derris ferruginea (Wall. ex Voigt) Benth. | JX506633 | JX506461 | |||
| Derris glabra Sirich. | JX506635 | JX506463 | |||
| Derris laotica Gagnep. | JX506645 | JX506473 | |||
| Derris laxiflora Benth. | AF142715 | AF467046 | |||
| Derris lianoides Elmer | JX506653 | JX506482 | |||
| Derris luzoniensis (Adema) Sirich. & Adema | JX506654 | JX506483 | |||
| Derris marginata (Roxb.) Benth. | JX506643 | JX506471 | |||
| Derris montana Benth. | JX506650 | JX506479 | |||
| Derris monticola (Kurz) Prain | JX506637 | JX506465 | |||
| Derris oblongifolia Merr. | JX506652 | JX506481 | |||
| Derris ovalifolia (Wight & Arn.) Benth. | JX856445 | ||||
| Derris piscatoria (Blanco) Sirich. & Adema | JX506651 | JX506480 | |||
| Derris pseudomarginata Sirich. | JX506639 | JX506467 | |||
| Derris pubipetala Miq. | JX506634 | JX506462 | |||
| Derris reticulata Craib | AB504375 | JX506460 | |||
| Derris rubrocalyx Verdc. 1 | G08804 | Cooper, W. 2543 (CNS) | OR531463 | OR531494 | |
| Derris rubrocalyx Verdc. 2 | G08805 | Cooper, W. 2544 (CNS) | OR531464 | OR531495 | |
| Derris rubrocalyx Verdc. 3 | G08814 | Cooper, W. 2545 (CNS) | OR531465 | OR531496 | |
| Derris rubrocalyx Verdc. 4 | G08816 | Cooper, W. 2401 (CNS) | OR531497 | ||
| Derris rubrocalyx Verdc. 5 | JX506644 | JX506472 | |||
| Derris sp. Maxwell 50–75 | JX506640 | JX506468 | |||
| Derris spanogheana Blume ex. Miq. | JX506464 | ||||
| Derris tonkinensis Gagnep. | JX506631 | JX506459 | |||
| Derris trifoliata Lour. 1 | G05663 | CANB 338067 | OR531466 | OR531499 | |
| Derris trifoliata Lour. 2 | G08808 | Cooper, W. 2573 (CNS) | OR531467 | ||
| Derris trifoliata Lour. 3 | G08809 | Cooper, W. 2574 (CNS) | OR531468 | OR531500 | |
| Derris trifoliata Lour. 4 | G08810 | Cooper, W. 2575 (CNS) | OR531469 | ||
| Derris trifoliata Lour. 5 | G08813 | Cooper, W. 2266 (CNS) | OR531501 | ||
| Derris trifoliata Lour. 6 | G08815 | Harrington 528 (CNS) | OR531470 | OR531502 | |
| Derris trifoliata Lour. 7 | G05913 | CANB 345454 | OR531471 | OR531503 | |
| Derris trifoliata Lour. 8 | HM049528 | GU217633 | |||
| Fordia splendidissima (Blume ex Miq.) J.R.M.Buijsen | G06465 | CANB 530177 | OR531472 | OR531504 | |
| Gen. sp. Takeuchi 4349 | G08817 | akeuchi, W. 4349 (CANB) | OR531498 | ||
| Gen. sp. Takeuchi 4349 | Takeuchi, W. 4349 (L) | JX506626 | |||
| Ibatiria furfuracea W.E.Cooper | G06447 | W. Cooper 1901 (CNS) | OR531473 | OR531505 | |
| Lonchocarpus capassa Rolfe | G05900 | CBG 9002245 | OR531449 | OR531480 | |
| Muellera campestris (Mart. ex Benth.) M.J.Silva & A.M.G.Azevedo | G06462 | CANB 484528 | OR531450 | OR531481 | |
| Millettia brandisiana Kurz | G05664 | CBG 7804175 | OR531474 | OR531506 | |
| Mundulea sericea (Wild) A.Chev. | G05668 | CBG 9210832 | OR531475 | OR531507 | |
| Pongamia pinnata var. minor (Benth.) Domin | G08000 | W. Cooper 2414 (CNS) | OR538399 | OR538883 | |
| Pongamia pinnata var. pinnata (L.) Merr. | G07995 | W. Cooper 2409 (CNS) | OR538400 | OR538884 | |
| Pongamiopsis amygdalina (Baill) R.Vig. | G05665 | CANB 598569 | OR531476 | OR531508 | |
| Tephrosia astragaloides Benth. | G05670 | CANB 688004 | OR531477 | OR531509 | |
| Tephrosia benthamii Pedley | G05671 | CANB 707912 | OR531478 | OR531510 |
Herbarium codes follow Index Herbariorum (New York Botanical Garden’s Virtual Herbarium, see http://sweetgum.nybg.org/ih/).
Total genomic DNA was extracted from ~10 to 20 mg of field-collected silica-dried, or herbarium leaf material. Extractions were conducted with commercial extraction kits (Qiagen DNeasy plant kit, Venlo, Netherlands; ChargeSwitch gDNA plant kit, Invitrogen, Carlsbad, CA, USA) following the manufacturer’s protocols.
All PCR amplification reactions were performed in 20-µL volumes. PCR amplifications of ITS were initially performed with primers 17SE and 26SE (Table 2); however, in cases where amplification failed or resulted in poor-quality sequence reads, amplifications were repeated using the ITS4 and ITS5 primers. For ITS 17SE and 26SE, reactions contained 4 µL of 5× iProof High-Fidelity Buffer, 0.8 µL of each primer (10 µM), 0.4 µL of dNTP mix (10 mM), 0.6 µL of dimethylsulfoxide (DMSO), 0.25 µL of iProof High-Fidelity DNA polymerase (5 U µL–1) (Bio-Rad Laboratories Inc., Hercules, CA, USA), 11.15 µL of H2O, and 2 µL of gDNA (~5 ng µL–1). Cycling conditions consisted of an initial denaturation step of 98°C for 45 s followed by 35 cycles of 98°C for 10 s, 63°C for 30 s, 72°C for 45 s, with a final extension at 72°C for 10 min.
| Name | Sequence 5′–3′ | Source | |
|---|---|---|---|
| ITS4 | TCCTCCGCTTATTGATATGC | White et al. (1990) | |
| ITS5 | GGAAGTAAAAGTCGTAACAAGG | White et al. (1990) | |
| 17SE | ACGAATTCATGGTCCGGTGAAGTGTTCG | Sun et al. (1994) | |
| 26SE | TAGAATTCCCCGGTTCGCTCGCCGTTAC | Sun et al. (1994) | |
| 3F_KIM | CGTACAGTACTTTTGTGTTTACGAG | Ki-Joong Kim, pers. comm. | |
| 1R_KIM | ACCCAGTCCATCTGGAAATCTTGGTTC | Ki-Joong Kim, pers. comm. |
For ITS4 and ITS5, reactions contained 2.5 µL of PCR buffer (200 mM of Tris-HCl pH 8.4, 500 mM of KCl), 2.5 µL of MgCl (25 mM), 0.6 µL of each primer (10 µM), 0.5 µL of dNTP mix (10 mM), 0.8 µL of DMSO, 0.25 µL of bovine serum albumin (BSA, 4 mg mL–1), 0.2 µL of KAPA Taq-DNA polymerase (5 U µL–1) (Kapa Biosystems, Wilmington, DE, USA), 10.05 µL of H2O and 2 µL of genomic DNA (~5 ng µL–1). Cycling conditions consisted of an initial denaturation step of 95°C for 2 min, followed by 30 cycles of 95°C for 30 s, 50°C for 30 s, 72°C for 1 min, with a final extension at 72°C for 5 min.
PCR amplifications of matK were performed with the primers 3F_KIM and 1R_KIM (Table 1). Reactions contained 4 µL of 5× iProof High-Fidelity Buffer, 0.6 µL of each primer (10 µM), 0.4 µL of dNTP mix (10 mM), 0.6 µL of DMSO, 0.25 µL of iProof High-Fidelity DNA polymerase (5 U µL–1; Bio-Rad Laboratories Inc.), 11.55 µL of H2O and 2 µL of genomic DNA (~5 ng µL–1). Cycling conditions consisted of an initial denaturation step of 98°C for 45 s followed by 35 cycles of 98°C for 10 s, 56°C for 30 s, 72°C for 45 s, with a final extension at 72°C for 10 min.
Sequencing reactions were performed using the amplification primers and sequencing was conducted on an AB3730xl 96-capillary sequencer (Australian Genome Research Facility, Brisbane, Qld, Australia).
Chromatograms for matK and ITS were assembled into sequences by using the inbuilt assembler in Geneious Prime software (ver. 2022.2.2, see https://help.geneious.com/hc/en‐us/articles/360044627352‐How‐do‐I‐cite‐Geneious‐or‐Geneious‐Prime‐in‐a‐paper) and visually checked for errors and misinterpretations.
The resulting consensus sequences (32 for matK, 34 for ITS) were combined with sequences available on GenBank (34 for matK, 36 for ITS) and aligned for each marker separately in MAFFT (ver. 7.487, see https://mafft.cbrc.jp/alignment/software/; Katoh and Standley 2013) by using the default setting. The generated alignments were subsequently checked and corrected manually.
Phylogenetic reconstructions were performed using maximum likelihood in IQ-TREE 2 (ver. 2.2.0, see http://www.iqtree.org; Minh et al. 2020). The best-matching substitution model for each marker (matK, GTR + F + R2; ITS, TN + F + I + I + R3) was determined using ModelFinder (ver. 2.2.0, see http://www.iqtree.org/ModelFinder/; Kalyaanamoorthy et al. 2017) on the basis of the Bayesian information criterion. Clade support was estimated using ultra-fast bootstrap (BS) analysis (Hoang et al. 2018) with 1000 replicates. Clades were regarded as strongly supported with a BS of 95 or more. Phylogenetic trees were rooted using Canavalia rosea (Sw.) DC., on the basis of Hu et al. (2000, 2002) and Sirichamorn et al. (2012a).
Results
The resulting matK and ITS alignments comprised 2818 base pairs (bp) and 941 bp with the newly generated sequences spanning 915 and 713 bp, containing 8.0 and 10.4% gaps, and 223 and 247 parsimony-informative sites respectively.
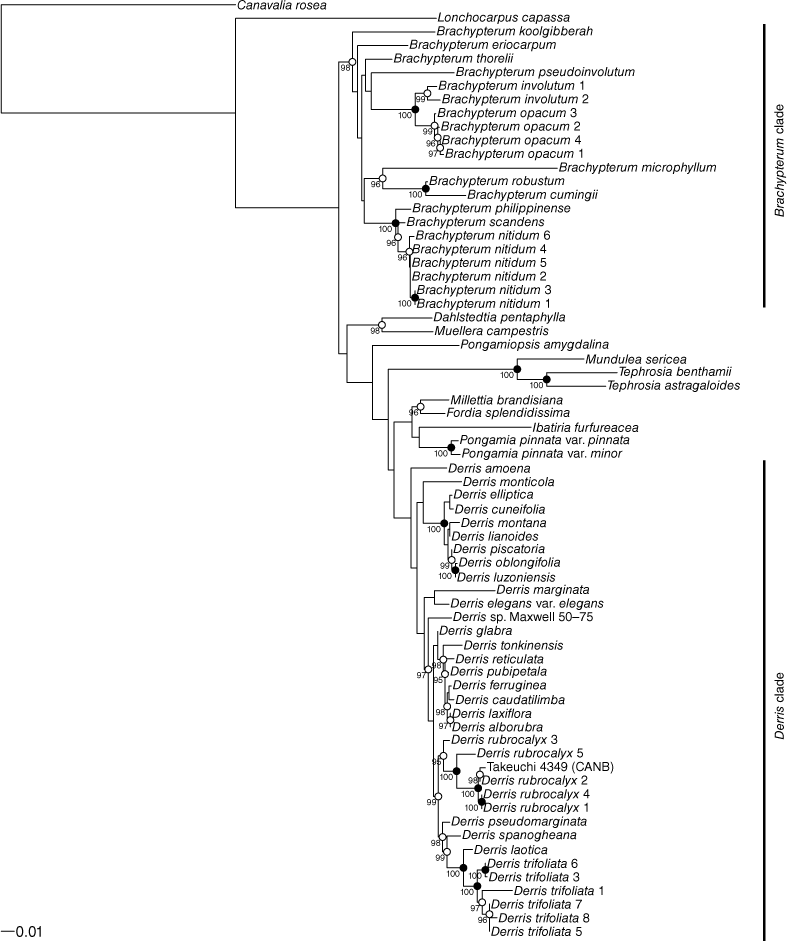
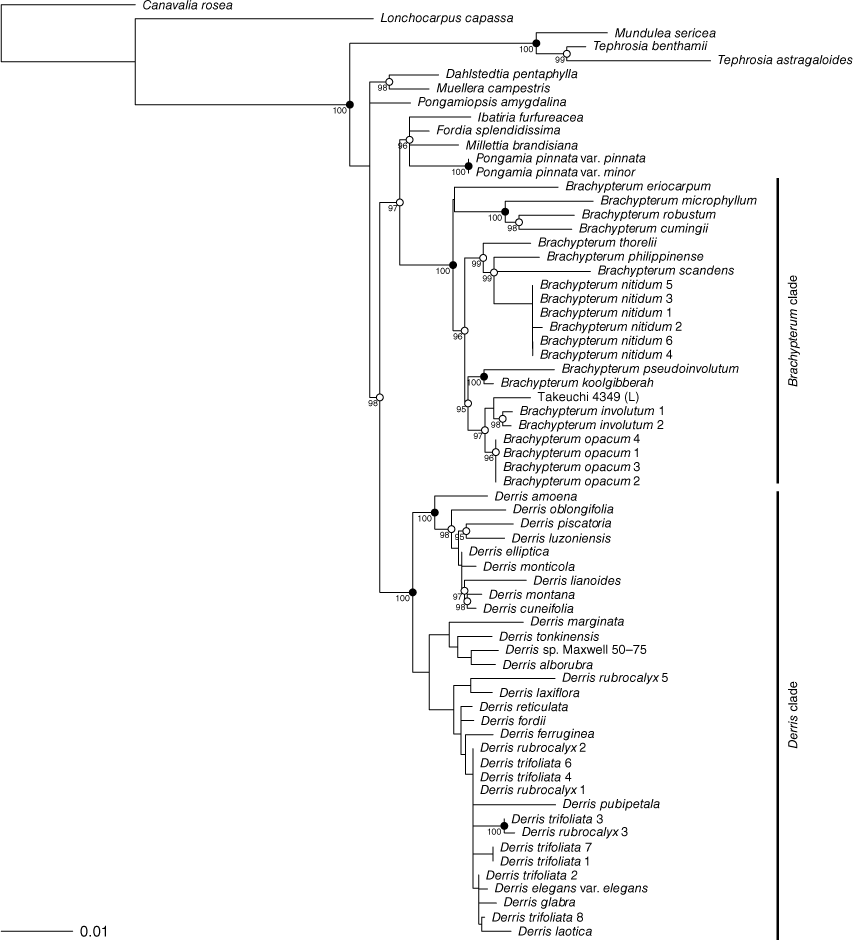
The trees resulting from the maximum-likelihood (ML) analysis of the ITS and matK data are shown in Fig. 1 and 2 respectively. In both the ITS and matK trees Brachypterum and Derris are resolved as monophyletic with robust bootstrap support (Brachypterum 99 and 100, Derris 96 and 100 for ITS and matK respectively).
Within these two groups, relationships on ITS data are generally well supported, but very few of the branches resolved in the ITS tree receive strong support, particularly within the Derris clade. Conversely, relationships between the main clades on the trees are better supported by matK than by ITS data. Although the ML trees from the two loci show conflict in the topology at some nodes, none of the conflicts is strongly supported.
The six included samples of B. nitidum and the four of B. opacum each form robust clades within Brachypterum. In the ITS analysis, they are sisters, whereas in the matK analysis, Brachypterum involutum (represented by two samples) forms a clade with B. submontanum (Verdc.) Adema & Sirich., and that clade is sister to B. opacum. However, the taxonomic identity of the sample of B. submontanum (Takeuchi 4349) is unresolved, which is discussed below.
Within B. opacum, ITS data resolve relationships among the four samples with strong support, whereas there is no support in the matK data for any internal structure in this clade.
The sister to Brachypterum nitidum is resolved by ITS data as B. scandens (Roxb.) Miq., but relationships in this part of the matK tree are not supported. Except for one pair of samples grouped on the ITS tree, there is no supported structure within B. nitidum.
Discussion
Brachypterum
The analyses of plastid (trnL-F IGS, psbA–trnH IGS and trnK–matK) and nuclear (ITS) sequence data by Sirichamorn et al. (2012a, 2014) retrieved a strongly supported clade (the ‘Brachypterum’ clade in Sirichamorn et al. 2012a, and the Solori clade in Sirichamorn et al. 2014), which is also retrieved in the present analyses (Fig. 1, 2). Regarding relationships within this clade, the ITS trees of Sirichamorn et al. (2012a, 2014) and the present study both retrieve the following relationships with strong support: B. robustum + B. cumingii, and the sister relationship of B. microphyllum to this clade; and B. philippinense + B. scandens, which also includes B. nitidum sp. nov. in our tree (taxon not included in Sirichamorn et al. 2012a). In the matK tree (Fig. 2), the congruent strongly supported clades are B. robustum + B. cumingii + B. microphyllum; B. pseudoinvolutum + B. koolgibberah; B. involutum + B. submontana; and B. philippinense + B. scandens + B. thorelii + B. nitidum sp. nov. in our tree.
The reciprocal monophyly of the samples of B. nitidum and B. opacum, and their non-sister relationship on matK data, strongly support the recognition of two novel species on the basis of the genealogical species concept (Baum 1992). Interestingly, samples of B. nitidum from Restoration Island (W. Cooper 2546, G08806; 2576, G08811; and 2617, G08807) exhibit nucleotide polymorphisms at several positions in the ITS sequences (data not shown), which, taken together with observations of morphological differences compared with other B. nitidum (discussed in Notes under Brachypterum nitidum in the Taxonomy section), suggest that hybridisation may have played a role in the history of this population. Plants collected from the Wenlock River area representing a form that was previously considered distinct (e.g. Derris sp. Wenlock River (B.Hyland 21082V)) are robustly grouped within the B. nitidum clade.
Derris
The Derris clade resolved by our ITS and matK analyses (albeit unsupported in the ITS analyses; Fig. 1, 2) is consistent with the ‘Derris clade’ resolved by Sirichamorn et al. (2012a, 2014). Within that clade, there are no strongly supported conflicts between the Sirichamorn analyses and those presented here, except (1) a clade comprising Derris piscatoria + D. oblongifolia + D. luzonensis (Sirichamorn et al. 2012a, 2014) is contradicted by our matK results (Fig. 2), but supported by our ITS results (Fig. 1), and (2) the species D. tonkinensis, D. pseudomarginata, D. alborubra, and D. marginata form a clade in Sirichamorn et al. (2012a, 2014), whereas these species are dispersed throughout the Derris clade on our ITS analysis (Fig. 1; albeit being grouped without strong support on our matK tree, Fig. 2).
In our ITS tree, the samples of Derris rubrocalyx and D. trifoliata each form robust clades (Fig. 1). However, these clades are not resolved in the matK tree (Fig. 2). Instead, these samples are part of a large clade of Derris samples, among which relationships are not strongly supported.
Plants from the Claudie River area of Cape York Peninsula, first collected by Len Webb (1920–2008) and J. Geoff Tracey (1930–2004) in 1968, have previously been considered distinct and treated under the informal names Derris sp. (Claudie River), Derris sp. (Claudie River L.J.Webb + 8348), and Derris sp. Claudie River (L.J.Webb + 8348) Qld Herbarium. All samples of this variant included in this study clustered with Derris rubrocalyx samples. Therefore, we consider these informal names to be synonyms of Derris rubrocalyx Verdc.
Takeuchi 4349
Sirichamorn et al. (2012a) included sequences of the plastid markers trnL-F IGS, psbA–trnH IGS and trnK–matK for Derris rubrocalyx Verdc. (≡Brachypterum submontanun), which were vouchered by the specimen Takeuchi 4349 et al. (L). We sequenced ITS from a sample taken from the CANB sheet of Takeuchi 4349 (CANB 510221), with the intent to complement the existing plastid data. However, this ITS sequence clustered robustly with samples of Derris rubrocalyx Verdc. (Fig. 1), whereas the matK sequence used in Sirichamorn et al. (2012a) (GenBank Accession number JX506626) clustered robustly within a subclade of Brachypterum with B. involutum (Fig. 2), a placement also resolved by Sirichamorn et al. (2012a, 2014) on the basis of three plastid markers (ITS data of Takeuchi 4349 was not included in that study). The first author has viewed available high-quality images of Takeuchi (CANB 510221, L 1941488), but these are not sufficient to challenge the identification because they lack fruit, which are required to definitively distinguish Derris and Brachypterum. Further investigation of the incongruence between the ITS and matK results for Takeuchi 4349 will require either matK data from the CANB material or ITS data from the L material.
Taxonomy
Derris Lour., Fl. Cochinch. 2: 432 (1790), nom. cons.
Type: Derris trifoliata Lour.
In Australia: monoecious, slender twiner, which often becomes a liana or shrubby, evergreen, bark lenticellate. Stipules caducous or persistent. Leaves compound, alternate, imparipinnate; stipels rarely present; leaflets 3–15, glabrous or with indumentum; venation brochidromous or camptodromous. Inflorescence an axillary, terminal or ramiflorous pseudoraceme or rarely a pseudopanicle, bracts at base of branches or brachyblasts; 1–7-flowered; bracteoles near pedicel apex; standard with basal callosities; wings auricled; keel connate at apex, lateral pockets adherent to wings; stamens monadelphous, 10, basal fenestrae present; disc lobed; ovary fusiform, stipe absent or indistinct; ovary fusiform, ovules 2–5. Pod indehiscent, 1-winged or 2-winged, wing along upper margin 1–5 mm wide.
Derris elliptica (Wall.) Benth., J. Proc. Linn. Soc. 4 (Suppl.): 111–112 (1860)
Pongamia elliptica Wall., Pl. Asiat. Rar. 3: 30, t.237 (1832); Paraderris elliptica (Wall.) Adema, Thai. For. Bull., Bot. 28: 11 (2001). Type: Ambon, cult. Bot. Gard. Calcutta, N.Wallich Cat. 5881 (holo: K K000898268 [image!]; iso.: CAL (fragments), n.v. (fide Y. Sirichamorn et al., Taxon 63(3): 534 (2014).).
D. J. Du Puy (1993, p. 220). J. Claussen (2005, p. 83). Y. Sirichamorn et al. (2012b, p. 433).
Scandent shrub or liana up to 12 m long. Bark dull greenish to brown. Twig diameter 4–15 mm, hirsute becoming glabrescent, lenticellate. Stipules triangular or ovate, 3–3.5 mm long, ~3 mm wide, abaxially sericeous, adaxially glabrous. Leaves with 7–15 leaflets; rachis + petiole 10–350 mm long, terete, very shallowly adaxially, pilose, pulvinus 3–11 mm long. Stipels caducous, acicular, ~1.5 mm long; petiolules 4–9 mm long. Leaflets obovate, elliptical or narrowly elliptical, 40–250 mm long, 20–65 mm wide, chartaceous or sub-coriaceous, abaxially rusty sericeous especially on veins; adaxial sericeous to glabrous, discolourous, glaucous; base rounded, obtuse or cuneate; apex shortly acuminate or rounded; venation camptodromous, primary vein slightly raised; secondary veins 7–14, +flush on adaxial surface and raised abaxially, angle of divergence ~40°, tertiary venation indistinct, reticulate. Inflorescence an axillary (rarely terminal) pseudoraceme or pseudopanicle 25–300 mm long; lateral branches 5–33 mm long, mostly 3-flowered, rusty sericeous; bracts at base of pedicels triangular, ~1 mm long, 0.5 mm wide, sericeous; pedicels 10–13 mm long; bracteoles at pedicel apex triangular ~1 × 0.5 mm. Flower fragrance not recorded; calyx cup-shaped, usually with an incurved apex, 3–7 mm long, abaxially sericeous, rusty or reddish-brown; corolla pink and white, with green nectar guides; standard petal: claw 3–4 mm long; blade + orbicular, 12–17 mm long, 10–17 mm wide, abaxially sericeous, apex emarginate and incurved, basal callosities present; wing petals: claw ~2.5 mm long; blade oblong, straight or curved around keel, ~8 mm long, 3 mm wide, upper auricle triangular, ~1.5 mm long; lower auricle absent; apex rounded; keel petals: claw ~2.8 mm long; blades oblong or boat-shaped, connate at apex, ~8 mm long and 3 mm wide, lateral pocket ~3 mm long; stamens monadelphous, up to 13 mm long, basal fenestrae ~3 mm long; anthers ~0.7 mm long, sparse hairs abaxially; ovary fusiform, not stiped, 6–10 mm long, sericeous; ovules 3–5; style 8–14 mm long, sparse hairs near base; stigma capitate. Fruit pedicel ~8 mm long; pod indehiscent, flat, broadly elliptic, strap-shaped or oblong, 35–110 mm long, 10–25 mm wide, leathery, thinly sericeous, rusty brown or glaucous, winged along upper side, wing ~1.52 mm wide, wing along underside up to 1.5 mm wide; seeds 1–3, discoid-reniform, ~13 × 8–8.5 × 3 mm; hilum central, 2.5–3 mm long.
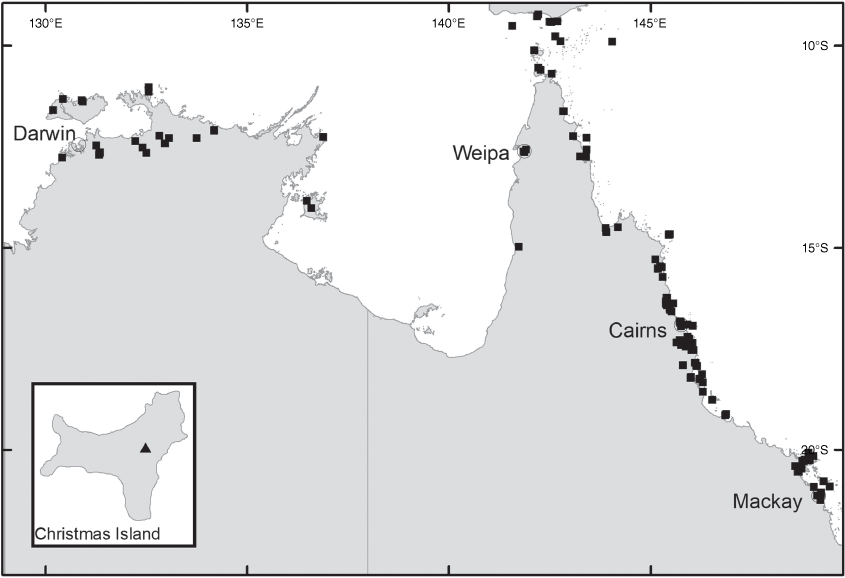
Derris elliptica occurs naturally in India, Myanmar, Thailand, Laos, Vietnam, Cambodia, Malay Peninsula, Indonesia, Philippines, Caroline Islands and on Christmas Island (Australia) (Fig. 3, inset).
Derris elliptica has become naturalised in many countries, including Brazil, China, Democratic Republic of the Congo, Hawaii and Australia. It is likely to be native on Christmas Island where it occurs on land that has been disturbed or cultivated. Its occurrence in the Torres Strait is presumably a deliberate introduction, being that it is cultivated by the local people for its rotenone content, which is used as a piscicide and insecticide (Lazo et al. 2014). The plant appears to be non-invasive in the Torres Strait.
Flowers have been collected in December. No fruiting collections have been made in Australia.
The epithet elliptica is from the Latin, ellipticus (elliptical) possibly referring to the fruit, which are mostly elliptical.
Given the paucity of herbarium vouchers from Christmas Island (one specimen at PERTH) in Australia, this description for Derris elliptica is an amalgamation of information gleaned from images at BRI, PERTH, L and K, as well as those on JSTOR Global Plants, in addition to written descriptions by Du Puy (1993) and Sirichamorn et al. (2012b).
Derris elliptica occurs in regrowth or disturbed areas on Christmas Island and could have been introduced by early settlers; however, the species is not common on the island, nor does it have a weedy habit and is a plausible native to Christmas Island, given the close proximity to Indonesia where it is native.
AUSTRALIA. TERRITORY OF CHRISTMAS ISLAND. Pipeline Track from Grants Well to Ross Hill Gardens, above cliffs, Christmas Island, Sep. 1996, Swarbrick 13230 (PERTH). QUEENSLAND. Property of Margaret Mareko, Badu Island, Torres Strait, May 2006, Waterhouse BMW7397 (BRI); South Johnstone Experimental Station, Dec. 1941, White s.n. (BRI). INDONESIA. JAVA. Natural Reserve Tjibodas, Oct. 1962, Hasan 120 (BRI). PAPUA NEW GUINEA. MOROBE. Markham Road near Lae, Oct. 1959, Henty NGF57114 (BRI); SANDAUN (WEST SEPIK). Amoi Village, Bewani, Aug. 2002, Waterhouse BMW6466 (BRI). THAILAND. Siam, Bangkok, Mar. 1920, Kerr 4094 (TCD). PHILIPPINES. Luzon: Laguna Province, Mt Markling, Apr. 1947, Sulit 5259-2 (BRI); Ilocos Norte Province, Mt Nagapatan, Aug. 1918, Ramos 2-107 (BRI).
Derris rubrocalyx Verdc., Kew Bull. 32(2): 466 (1978)
Type citation: ‘Papua New Guinea, Schodde 2759 (holotypus, K; isotypi, CANB, L, LAE)’. Type: Papua New Guinea, ~1 mile [~1.6 km] north of Rigo, Central District, Schodde 2759 (holo: K 00898353 [image!]; iso: A 00105820 [image!], BO, BRI AQ0052379 [image!], CANB 121656 & 121657 [image!], L 0476239 & 0476240 [image!], LAE, MEL 117597 [image!], PNH, US 00810602 [image!]).
Derris rubrocalyx subsp. acuminata Verdc., Kew Bull. 32(2): 468 (1978). Type: Papua New Guinea. Morobe District: Huon Peninsula, along Kua R., N. of Zalimpa, in low river bank forest, 420 m, Hoogland 9003 (holo: K 000898354 & 000898355 [images!]; iso: A 00105823 & 00105824 [image!], BRI AQ0052372 [image!], CANB 531346 [image!], L 0476241 & 0476242 [images!], LAE, US 00730764 & 00730765 [images!]), syn. nov.
Derris sp. (Claudie River L.J.Webb + 8348), Queensl. Vasc. Pl.: Names and Distribution 122 (1994); Queensl. Pl.: Names and Distribution 79 (1997); Names and Distribution Queensl. Fl. 78 (2002); Census Queensl. Fl. 75 (2007); Census Queensl. Fl. 71 (2010); Census Queensl. Fl. (2013); Census Queensl. Fl (2016).
Derris sp. (Kamerunga C.E.Wood AQ234578), Queensl. Vasc. Pl.: Names and Distribution 122 (1994).
Derris sp. Claudie River (L.J.Webb 8348), Austral. Tropical Rain Forest Pl. (2003).
Derris sp. (Claudie River), Fruits Austral. Trop. Rainforest 208 (2004).
Derris sp. Claudie River (L.J.Webb + 8348) Qld Herbarium, CHAH, Council of Heads of Australian Herbaria (2005), Australian Plant Census database (see https://biodiversity.org.au/nsl/services/rest/name/apni/218840, accessed 11 April 2022).
B. Verdcourt (1978), p. 467. W. Cooper and W. T. Cooper (2004, p. 208). F. A. Zich, B. P. M. Hyland, T. Whiffin & R. A. Kerrigan (Australian Tropical Rainforest Plants, edition 8, see https://apps.lucidcentral.org/rainforest/text/intro/index.html).
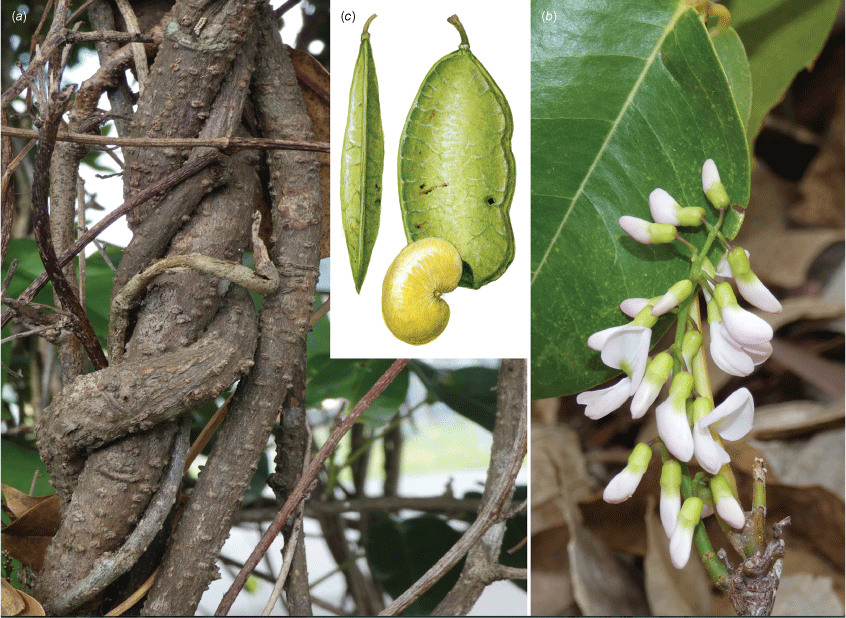
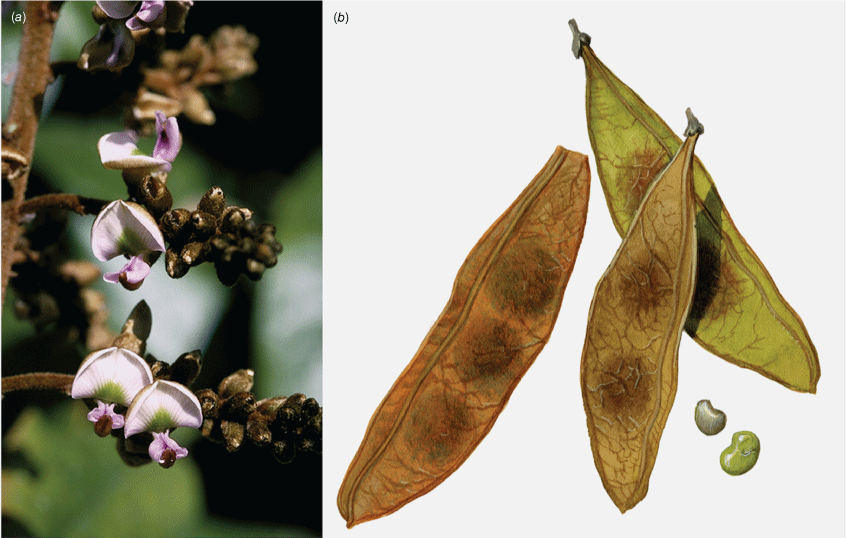
In Australia: Liana, twiner to forest canopy, stem diameter up to 18 cm; bark on older stems greyish to reddish-brown, somewhat flaky with some vertical fissures, numerous lenticels present, new stems green, becoming grey or cream with pale lenticels, corky tubercles up to 10 mm long are sometimes densely clustered on some young stems with a diameter up to 15 mm, a mix of stems with or without tubercles may be present on the same plant, tubercle presence on stems greater than 30 mm in diameter has not been observed. Stipules mostly caducous, ovate or triangular, ~2 mm long and 1.5–2 mm wide at base, puberulent to glabrous, margin ciliate. Leaves with new growth red; leaf rachis + petiole terete, very shallowly channelled on adaxial side, 40–210 mm long; indumentum puberulent (denser at nodes) to glabrous; pulvinus 7–10 mm long, 1.5–4 mm wide, puberulent to glabrous, rugose; stipels absent; lateral petiolules 4–8 mm long, sparsely clothed in minute hairs; terminal petiolules 15–33 mm long, a few minute hairs may be visible. Leaflets 3–5, subpeltate (often not distinct on dried specimens), ovate, elliptical, oblong–elliptical, obovate, orbicular, 30–170 mm long, 25–87 mm wide, leathery, emerging leaves sparsely hairy soon glabrous on both surfaces, discolourous, abaxially somewhat glaucous; base obtuse, rounded, cuneate or truncate; apex short or long acuminate (mostly abruptly tapering) and often ultimately minutely retuse; margin entire; venation brochidodromous throughout or at least in distal half; primary vein depressed or +flush; secondary veins 7–10 pairs, flush or slightly raised on both surfaces, angle of divergence from primary vein 20–50°, forming loops 2–3 mm from margin; tertiary venation reticulate. Inflorescence an axillary, ramiflorous (rarely terminal) pseudoraceme or pseudopanicle, 40–365 mm long; rachis 1.25–1.5 mm thick at base, puberulent, branches up to 140 mm long; bracts at base of branches triangular, ~1 mm long and 0.75 mm wide at base, puberulent with ciliate margin; branch-like brachyblasts 3–30 mm long, 1–7-flowered (mostly 3- or 4-flowered), puberulent; pedicels 2.5–5 mm long, puberulent; bracteoles clasping calyx base and persisting through to fruit, ovate, ~0.5 mm long, 0.3 mm wide at base, puberulent, margin ciliate. Flowers: no fragrance detected; calyx cup-shaped with incurved apex, ~3 mm long, 3.5 mm wide, purple or greenish-red, adaxially puberulent towards apex, abaxially puberulent and indumentum denser near base and apex; margin ciliate; standard orbicular, ~9 mm long (including claw ~1.5 mm long), ~8 mm wide, glabrous, pink with green nectar guides, base truncate, apex incurved and emarginate, basal callosities present; wing petals becoming strongly revolute, oblong, ~9 mm long (incl. claw ~1.75 mm long), 2 mm wide, glabrous, pink, lower base cuneate, upper base auricled, auricle triangular, 0.75 mm long, 0.75 mm wide; keel petals oblong, ~9 mm long (incl. claw ~2 mm long), ~2.5 mm wide, sparse short hairs near apex, pink, lateral pocket adherent to wing, ~2 mm long, lower margin attenuate, upper margin auricled; auricle triangular, ~0.75 mm long and wide; stamens monadelphous, up to 8 mm long, basal fenestrae ~1 mm long, glabrous; filaments glabrous; anthers ~0.6 mm long, 0.4 mm wide, glabrous; disc lobed and with a few hairs at centre; ovary fusiform, not stiped, ~7 mm long, 0.5 mm wide, thinly sericeous; style glabrous, stigma capitate, ovules 2–5. Fruit pedicel 4–5.5 mm long, thin, puberulent; pod indehiscent, flat and strap-shaped or oblong, 25–110 mm long, 10–25 mm wide, papery, glabrous or scattered minute appressed hairs mostly at base and along wings, straw-coloured, upper wing 2.25–5 mm wide, lower wing 1–2.5 mm wide, base cuneate, apex rounded and mucronate; seeds discoid-reniform, 1–4, 7–10 mm long, 11–14 mm wide, 1 mm thick, chestnut-brown; hilum ~1.75 mm long. Germination hypogeal (Fig. 4, 5).
Derris rubrocalyx is known from New Guinea (West Papua and Papua New Guinea) and south to the Wet Tropics in northern Queensland. In Queensland, it occurs from islands in the Torres Strait south to the Palmerston area of Wooroonooran National Park (Fig. 6).
Derris rubrocalyx in Queensland occurs in complex mesophyll vine forest, complex notophyll vine forest and semi-deciduous mesophyll forest from near sea level up to 780 m.
Flowers have been recorded from September to November; fruits have been recorded from December to February.
Derris rubrocalyx is a variable species throughout its range in Australia and New Guinea. Verdcourt (1978) separated Derris rubrocalyx into two subspecies, namely, D. rubrocalyx subsp. rubrocalyx and D. rubrocalyx subsp. acuminata, on the basis of leaf apices, inflorescences open or compressed, inflorescence branches or brachyblast length and corolla colour. Australian specimens have various features and combinations of both described subspecies. Whereas the Type specimen for D. rubrocalyx subsp. acuminata has a distinctly acuminate leaflet and a compressed inflorescence, most Australian specimens have similarly acuminate leaflets and open inflorescences, resembling D. rubrocalyx subsp. rubrocalyx. Other variances between material from Australia and New Guinea are new growth (glabrescent v. glabrous); inflorescence branches or brachyblasts (usually 3-flowered and rarely up to 7-flowered v. 5–15-flowered); inflorescence branch or brachyblast thickness (0.3 mm v. 0.75–1 mm); calyx (sparsely sericeous in upper third v. glabrous); standard (glabrous v. sericeous or at least some hairs abaxially); wings (1–2.5 mm wide v. 3.5–4.5 mm wide); disc (distinct v. annular, indistinct or absent); anthers (glabrous v. at least a few hairs; Adema 2003); pod (with a few sparse hairs v. sericeous at apex and base).
AUSTRALIA. QUEENSLAND. Murray Island, July 1974, Heatwole & Cameron 642 (CNS); Mer Island, Apr. 2021, Fell DGF-MER34 & Bon (CNS); Mer Island, Apr. 2021, Fell DGF-MER28, Bon & Tapau-Bon (CNS); Gebar Island, Oct. 2017, Fell DGF GB85 (CNS); 5 km N of old homestead, Lockerbie Scrub, Sep. 1985, Williams 85206 (BRI); roadside near West Claudie crossing, Oct. 2018, Cooper 2544 (CNS); Round Mountain, Embley Range, June 1992, Forster PIF 10483, Kenning, Sankowsky & Tucker (BRI); Goana Creek, E of McIlwraith Range, Nov. 1956, Webb 3135 (BRI); McIvor River, July 1972, Stocker 895 (BRI); Shiptons Flat, Oct. 1997, Cooper & Cooper 1160 (CNS); Barron River, below Lake Placid, Oct. 1994, Jago 3196 (BRI); SFR 607, Dinden, Bridle LA, Oct. 1991, Hyland 14264 (CNS); Danbulla Forest Drive, Oct. 2018, Cooper 2543 & Jensen (CNS); Danbulla Forest Drive, Oct. 2017, Cooper 2526 & Ford (CNS); Danbulla Forest Drive, Jan. 2017, Cooper 2401 & Ford (CNS); Lake Barrine, Oct. 1929, Kajewski 1298 (BRI); Tolga Scrub, Oct. 1978, Gray 1059 (CNS); Johnstone River, Sep. 1998, Elick 166 (CNS); K-Tree Road, Wooroonooran NP, Oct. 2017, Cooper 2517 & Ford (CNS); K-Tree Road, Wooroonooran NP, Oct. 2017, Cooper 2518 & Tuck (CNS). PAPUA NEW GUINEA. Kaiser-Wilhelmsland, July 1908, Schlecter 17927 (K); ~1 mile [~1.6 km] N of Rigo, Central District, Aug. 1962, Schodde 2759 (K); Along Kua River, N of Zalimpa, May 1964, Hoogland 9003 (BRI); Western Province, Baia River Survey tracks A and B near the Tikawe Creek junction, Feb. 2008, Takeuchi 22813, Gambia & Jisaka (L). INDONESIA. WEST PAPUA (IRIAN JAYA). Manokwari District. Manokwari subdistrict, Mupi Dessa, Apr. 1995, Davis 567 (L); Reremi, Manokwari, Dec. 1960, Moll BW9807 (WAG).
Derris trifoliata Lour., Fl. Cochin. Edn. 1, 2: 433 (1790)
Type citation: ‘Habitat in sylvis provinciae Cantoniensis Sinarum.’ Type: China. Guangdong, s. dat., Loureiro s.n. (holo: P00150849 [image!]).
Robinia uliginosa Roxb. ex Willd., Sp. Pl. 4th edn., 3(2): 1133–1134 (1802); Pongamia uliginosa (Roxb. ex Willd.) DC., Prodr. 2: 416 (1825); Galedupa uliginosa (Roxb. ex Willd.) Roxb., Fl. Ind. 2nd edn., 3: 243 (1832); Derris uliginosa (Roxb. ex Willd.) Benth. in F. A. W. Miquel (Ed.), Pl. Jungh. 2: 252 (1852). Type citation: ‘Habitat in India orientali.’ Type: India, s. dat., Roxburgh s.n. (holo: B W13666-010 [image!]; iso: BM 000958747 [image!]).
Dalbergia heterophylla Willd., Sp. Pl., 4th edn., 3(2): 901 (1803); Derris heterophylla (Willd.) Backer ex K.Heyne, Nutt. Pl. Ned.-Ind. 2nd ed., 2: 806 (1927). Type citation: ‘Habitat in India orientali.’ Type: India, s. dat., Roxburgh s.n. (lecto, designated by V. E. Rudd in M. D. Dassanayake and F. R. Fosberg (Eds), Revised handb. Fl. Ceylon 7: 232 (1991): fruiting specimen, B W13073-030 [image!]; syn.: flowering specimen B W13073-010 [image!].
Derris trifoliata var. macrocarpa Domin, Biblio. Bot. 22(89): 786 (1926). Type citation: ‘Queensland: in der Nähe der Russel-Mündung (DOMIN I. 1910)’. Type: Queensland: at the delta of the Russell River, Jan. 1910, Domin ‘4837’ (holo: PR 527544 [image!]), syn. nov.
For a full synonymy, see Sirichamorn et al. (2012b).
A. B. Cribb and J. W. Cribb (1985, p. 129). W. Cooper and W. T. Cooper (2004, p. 208). Y. Sirichamorn, F. Adema and P. C. van Welzen (2012a, p. 406). Y. Sirichamorn (2013, fig. 1-2C, 1-3A, 1-4C, 9C). Y. Sirichamorn, F. Adema, M. C. Roos and P. C. van Welzen (2014, p. 529). F. Zich, B. P. M. Hyland, T. Whiffin and R. A. Kerrigan (Australian Tropical Rainforest Plants, edition 8, see https://apps.lucidcentral.org/rainforest/text/intro/index.html).
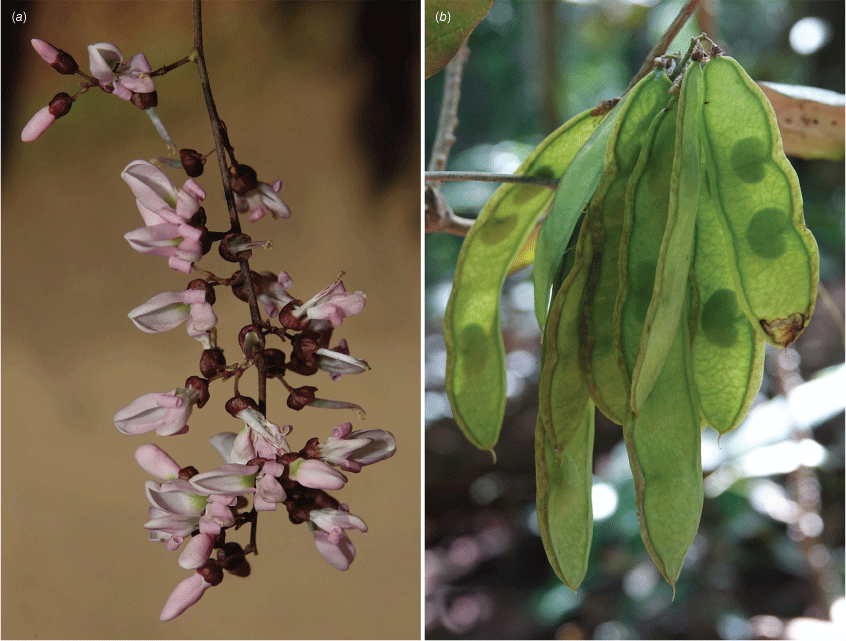
In Australia: slender twiner that can become a liana, stem diameter up to 50 mm, prostrate or climbing to subcanopy, evergreen; new stems green. Bark warty with numerous lenticels or corky pustules, which sometimes produces adventitious roots, grey. Twigs glabrous with numerous lenticels. Stipules mostly persistent, triangular, 0.5–1.5–2 mm long and 1–2 mm wide at base, glabrous. Leaves with new growth green; petiole 18–50 mm long; petiole + rachis 40–120 mm long; rachis terete, narrowly channelled along adaxial side, 30–160 mm long, sparse minute hairs at nodes becoming glabrescent; pulvinus 3–10 mm long, rugose, glabrous or glabrescent; stipels rarely present, caducous, narrowly triangular, 0.5–1.5 mm long, sparsely hairy or glabrous; lateral petiolules 3–10 mm, rarely with a few sparse hairs; terminal petiolules 15–35 mm long, rarely with a few sparse hairs; stipellae caducous or absent, triangular, ~0.75 mm long. Leaflets 3–7 (mostly 3 or 5), ovate, cordate or elliptic, 25–160 mm long, 10–90 mm wide, chartaceous or coriaceous, glabrous or with a few sparse hairs on new growth and soon glabrescent, adaxially glossy dark green, discolourous, base subpeltate, cordate or rarely obtuse (sometimes cuneate on terminal leaflet); lateral petiolules 3–7 mm long; terminal petiolules 7–25 mm long; apex long or shortly acuminate or almost caudate and ultimately retuse or narrowly rounded, entire; venation brochidodromous, primary vein slightly channelled adaxially; secondary veins 5–8 pairs, flush or slightly raised on both surfaces, angle of divergence to primary vein 10–30°, forming loops 1–3 mm from margin, tertiary venation reticulate. Inflorescence an axillary, terminal or ramiflorous, a pseudoraceme or rarely a pseudopanicle, 10–180 mm long, rachis with a few sparse minute hairs; bracts at base of brachyblasts triangular, ~0–65 mm long and wide, glabrous, margin ciliate; branching racemose, 3–6.5 mm long, 1–5-flowered (mostly 3-flowered), glabrous or with a few sparse minute hairs; pedicels 3–7 mm long, glabrous or with a few sparse minute hairs; bracteoles at or near pedicel apex, elliptical, cordate or orbicular, ~0.6 mm long and ~0.5 mm wide at base, glabrous or with a few sparse minute hairs, margin ciliate. Flowers fragrant; calyx cup-shaped with incurved apex, 2.5–3 mm long and wide, abaxially glabrescent, adaxially sparsely or densely sericeous towards apex, margin ciliate, green or reddish-green; corolla pale pink or mauve, aging to white, nectar guides green; standard orbicular, 7–11 mm long (including claw 2–3 mm long), 7.5–10 mm wide, glabrous, apex emarginate and incurved, basal callosities present; wing petals slightly curved to straight, oblong, ~11.5 mm long (including claw ~2.5 mm long) and 2–3 mm wide, apex rounded, glabrous, upper auricle triangular and ~0.5 mm long; keel petals boat-shaped, connate near apex, 11–12 mm long (including claw 2–3 mm long) and 3–4 mm wide, base rounded; apex rounded, glabrous, lateral pockets ~2 mm long and adherent to wing, upper auricle triangular and ~0.2 mm long; stamens monadelphous with basal fenestrae ~1.25 mm long, alternating long and short, up to 10 mm long, glabrous; anthers 0.6–0.8 mm long and 0.3–0.5 mm wide, glabrous; disc 10-lobed; ovary fusiform, ~4 mm long and 1 mm wide, sericeous, stipe indistinct; style 4–5 mm long, glabrous, stigma capitate, ovules 3–5. Fruit pedicel 3–8 mm long; pod elliptic, obovoid or discoid and flat, 30–55 mm long and 20–37 mm wide, apex often with persistent style, glabrous or sparse hairs at base and apex; wing along upper margin 1–3 mm wide, prominently ridged along lower margin; seeds discoid-reniform, 1–3, 13–23 mm long, 12–21 mm wide, 2–5 mm thick; hilum central, ~1 mm long. Derris, Threeleaf Derris (Fig. 7).
Derris trifoliata occurs from eastern Africa (South Africa, Mozambique, Tanzania, Kenya and Somalia) to Madagascar, Mascarene Is., India, China, Japan, South-East Asia, Guam, East Timor, New Guinea, Australia and the Pacific including New Caledonia, Micronesia, Fiji and Samoa. In Australia, it is found on the mainland as well as offshore islands in the Northern Territory, and in Queensland from the Torres Strait south to the Mackay area (Fig. 3).
Within Australia, Derris trifoliata is a plant of areas with marine influences, including beaches, strands, rocky headlands, littoral rainforest, coastal floodplain, mangroves and back mangal areas at altitudes near sea level.
Flowers have been recorded from September to March and May; fruit has been recorded from October to February.
The epithet trifoliatus is from the Latin tri- (three) and foliatus (leaved); referring to the compound leaves that often possess three leaflets.
Sirichamorn et al. (2014, p. 528, fig. 3b) stated that the disc in Derris trifoliata is annular, indistinct or absent. However, in Australian specimens, the disc is distinct and prominently lobed. Furthermore, South-East Asian plants are reportedly glabrous (Adema 2003, p. 406), whereas some Australian specimens have sparse hairs on young leaves, inflorescence rachis, pedicels and calyces.
AUSTRALIA. NORTHERN TERRITORY. East Alligator River, 19.5 km NW of Cannon Hill Ranger Station, Mar. 1981, Craven 6560 (CANB). QUEENSLAND. Cook: Dauan Island, July 1975, Cameron 2386 (CNS); Horn Island, July 1975, Cameron 2179 (CNS); Mer Island, Aug. 2021, Fell DGF-Mer35 & Bon, Tapau-Bon & Noah (CNS); Jardine River, Oct. 2014, Cooper 2266, Jensen, Kemp & Zdenek (CNS); adjacent to N side of Captain Billy Creek, Jan. 1992, Jones 8873 & Broers (CBG); Forbes Island, May 1994, Hyland 15107 (CNS); Cape Weymouth, Nov. 2018, Cooper 2575 & Pritchard (CNS); Wangi Point, Oct. 2016, Cooper 2344, Venables & Pannell (CNS); Lake McLeod, Oct. 1999, Hyland 16290 (CNS); ‘Greenhills’, Horseplanter Creek, W of Annan River Bridge Site 76, Nov. 2002, Ford 3714 & Holmes (CNS); Finch Bay Road, Cooktown, Dec. 2022, Cooper 2947 & Jensen (CNS); Bloomfield River, W. Poland s.n., Nov. 1902 (K 000898169 [image!]; K 000898170, fruit only [image!]; K 000898171, fruit only [image!]; Daintree River ferry crossing, Oct. 1999, Gray 20341V (CNS); Barron River boatramp, Stratford, Oct 2009, Harrington 528 (CNS); Rolling Bay, Yarrabah Road, Mar. 1999, Gray 7561 (CNS); Bramston Beach, Sep. 2011, Holland 3011 (BRI); bank of Ninds Creek, S of Innisfail, Mar. 1994, Waterhouse 3107 (CNS); Mulgrave River, Deeral Landing, Feb. 2002, Jago 1439 (CNS); Garners Beach, Nov. 2018, Cooper 2573 (CNS). NPR 771, Edmund Kennedy, N of Cardwell, Feb. 1996, Forster 18352 (CNS); NPR 95, Hinchinbrook Island, Sep. 1980, Hyland 10617 (CNS); South Kennedy: Lamberts Beach Lookout, Slade Point, Mackay, Nov. 2008, Champion 2071 (BRI) and East Point, Mackay, Feb. 2004, Kemp TH7375 & Paterson (BRI). CHINA. Cantoniensis Sinarum, undated, Loureiro 526-2 (P). INDIA. Bengala, s. dat., Rottler 888 (M); Peninsula India, s. dat., Wight 935 (K). INDONESIA. Java, s. dat., Junghuhn 47 (K); JAPAN. Loo Choo Islands, 1853, Wright 67 (US); MADAGASCAR. s. loc., s. dat., Auguste s.n. (K). MOZAMBIQUE: Maputo, Incomati River mouth, Burrows & Burrows 8128 (BNRH).
Brachypterum (Wight & Arn.) Benth., Commentat. Legum. Gen.: 37 (1837), nom. cons.
Dalbergia subg. Brachypterum Wight & Arn., Prodr. Fl. Ind. Orient. 264 (1834); Derris sect. Brachypterum (Wight & Arn.) Benth., J. Proc. Linn. Soc., Bot. IV, Suppl.: 101–102 (1860); Deguelia sect. Brachypterum (Wight & Arn.) Taub., in H. G. A. Engler and K. A. E. Prantl (eds), Nat. Pflanzenfam. 3, 3: 345 (1894).
Type: Brachypterum scandens (Roxb.) Miq. (≡Dalbergia scandens Roxb.; ≡Derris scandens (Roxb.) Benth.; ≡Solori scandens (Roxb.) Sirich. & Adema).
Solori Adans., Fam. Pl. 3.: 327 (1763), nom. rej.
Type (designated by Y. Sirichamorn et al., Taxon 63(3): 532 (2014)): Solori scandens (Roxb.) Sirich. & Adema (≡Dalbergia scandens Roxb.; ≡Derris scandens (Roxb.) Benth.; ≡ Brachypterum scandens (Roxb.) Miq.
In Australia: monoecious evergreen slender vines, often becoming lianas. Bark mostly with a cross-hatched pattern or tessellated and lenticellate. Stipules caducous or persistent. Leaves compound, alternate, imparipinnate; stipels persistent, caducous or absent; leaflets 3–13; venation camptodromous or brochidromous throughout or camptodromous proximally and brochidodromous distally. Inflorescence an axillary or terminal pseudoraceme or pseudopanicle, 2–40-flowered; bracts and bracteoles persistent or caducous; standard with basal callosities present or absent; keel connate, lateral pockets adherent to wings; stamens 10, monadelphous with basal fenestrae, alternating long and short; disc tubular or lobed; ovary fusiform with 8–11 ovules. Fruit an indehiscent pod, papery, winged along upper margin 1–4 mm wide; seeds 1–4.
Brachypterum involutum (Sprague) Adema & Sirich., Thai Forest Bull., Bot. 48(1): 58 (2020)
Wisteria involuta Sprague, Gard. Chron. Ser. 3, 36: 141 (1904), as ‘Wistaria’; Derris involuta (Sprague) Sprague, Gard. Chron. Ser. 3, 38: 3 (1905); Solori involuta (Sprague) Sirich. & Adema, Taxon 63(3): 532 (2014).
Type citation: ‘has been lately in flower in the Temperate-house at Kew, … It comes from the Richmond River district of New South Wales’. Type: ‘United Kingdom. Royal Botanic Gardens, Kew, Temperate House (cultivated from material collected in Australia, New South Wales, Richmond River), 15 July 1904, Sprague s.n. (holotype: K 000898356 [image!])’, fide Y. Sirichamorn et al., Taxon 63(3): 532 (2014).
Harden et al. (2014, pp. 5, 6, 8, 9, 13, 14, 16, 17, 23). Y. Sirichamorn et al. (2014, p. 529).
Liana, twiner to rainforest canopy, diameter up to ~150 mm; bark grey with scattered lenticels and a cross-hatched pattern; twigs green to brown and with pale round lenticels; new growth rusty hairy. Stipules persistent, ovate or triangular 1–3 mm long, 0.7–1 mm wide, sericeous becoming glabrescent. Leaves with 9–13 leaflets; leaf rachis + petiole shallowly channelled on adaxial side, 60–150 mm long, pubescent; pulvinus 5–6 mm long, 1.6–1.8 mm wide, pubescent; stipels caducous, narrowly triangular up to 0.5 mm long, clothed in appressed hairs; lateral petiolules 2–3 mm long, pubescent; terminal petiolules 8–14 mm long, pubescent. Leaflets ovate, elliptic, obovate, oblong or rarely almost orbicular, 18–55 mm long, 13–34 mm wide, coriaceous, adaxially clothed in prostrate whitish or colourless hairs becoming sparse, although some erect dense hairs remain along midrib, glossy dark green; abaxially glaucous and mostly with prostrate rusty or yellowish hairs; base truncate, rounded, cuneate or oblique; apex mostly broadly or narrowly obtuse, may be retuse, margins entire; venation camptodromous proximally and brochidodromous distally; primary vein slightly raised adaxially and raised abaxially, very small papule usually present at apex on abaxial surface; secondary veins 4–8 pairs, slightly raised or flush adaxially, raised abaxially, angle of divergence from primary vein 40–50°, forming loops 1–2 mm from margin; tertiary venation reticulate. Inflorescence an axillary or terminal pseudoraceme or pseudopanicle, 35–210 mm long; rachis 1.2–1.6 mm thick at base, puberulous; branches to 120 mm long, puberulous; bracts at base absent; tufts of rusty hairs at base; brachyblasts wart-like, 1–2 mm long and 0.75 mm thick, 3–5-flowered, pubescent; bracts at base of pedicels ovate, ~1.25 mm long, 0.5 mm wide, sericeous, entire; pedicels 4.5–5.5 mm long, pubescent; bracteoles paired at pedicel apex, narrowly triangular, ~1 mm long, 0.5 mm wide, pubescent. Flowers fragrant; calyx bell-shaped, ~3 mm long, sericeous abaxially, glabrous adaxially, green; standard: claw ~2 mm long; blades oblong–orbicular, 8–10 mm long, glabrous, pink, mauve or purple with green nectar guides; apex incurved, emarginate or obtuse; base attenuate, basal callosities usually present; wing petals: claw ~2.25 mm long; blades curved around keel or reflexed, oblong, ~8.5 mm long, glabrous, pink, mauve or purple, auricles absent, apex acute; keel petals: claw ~2.5 mm long; blades oblong–obovate, connate towards apex, ~9 mm long, sericeous towards apex abaxially and with sparse hairs elsewhere, pink, mauve or purple, lower auricle absent, upper auricle truncate, apex rounded; lateral pockets 1.75–2 mm long, adherent to wings; stamens monadelphous, 8–9 mm long, with basal fenestrae, free section ~1.25 mm long, filaments with a few sparse hairs or glabrous; disc tubular, lobed; ovary fusiform, ~6 mm long, 1.25 mm wide, sericeous; style ~3 mm long; stigma captitate; ovules ~8. Fruit pedicel 5–7 mm long, puberulous; pod indehiscent, flat and strap-shaped, 45–95 mm long, 11–15 mm wide, papery, sericeous, hairs rusty or becoming colourless, wing on upper margin 1.2–2.5 mm wide at widest section, margin on lower side ribbed, straw-coloured, base cuneate, apex mostly rounded with the remnant style usually persistent as a beak; seeds 1–3, discoid-reniform, 4–5 mm long, 5–7 mm wide; hilum central, ~0.75 mm long. Native Derris, Native Derris (Fig. 8).
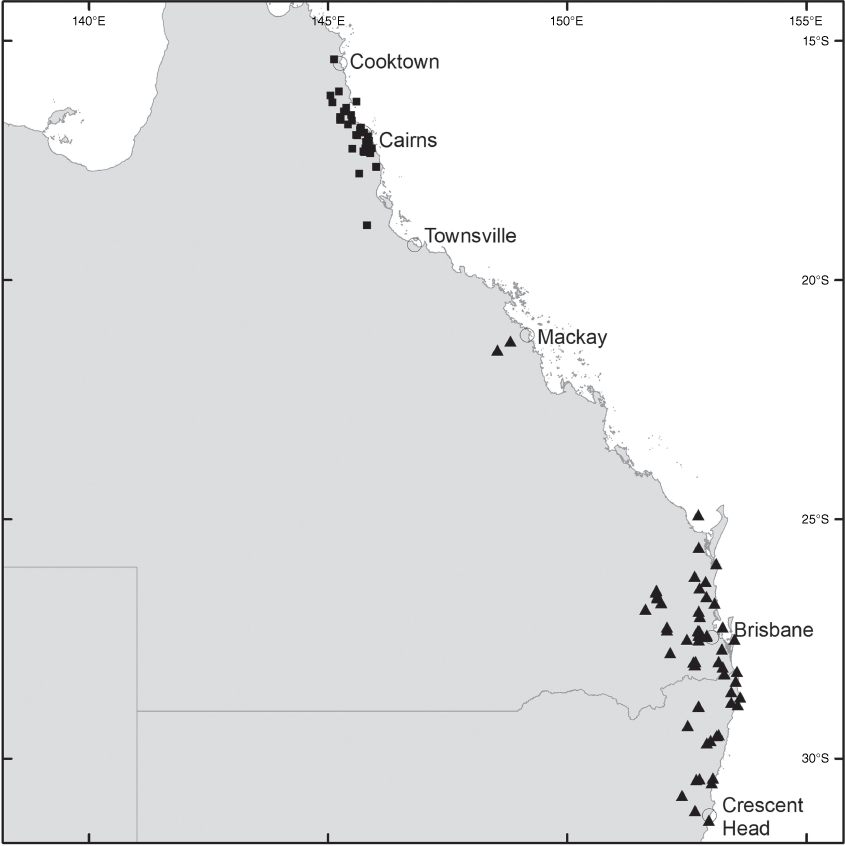
Brachypterum involutum is endemic to Australia, where it occurs from Homevale Resources Reserve (south-west of Mackay, Qld) to Crescent Head (NSW) (Fig. 9).
Brachypterum involutum occurs in Araucarian microphyll vineforest, notophyll vineforest, remnant rainforest, semi-evergreen vine thicket, wet sclerophyll forest, eucalypt woodland and open forest.
Flowers have been recorded in November and December and fruit has been recorded in March, May, July and August.
The epithet involutum is from the Latin, involutus (rolled inwards), referring to the involute calyx margin.
The names Derris scandens (Roxb.) Benth. and Brachypterum scandens have mistakenly applied to plants in Australia. Bentham (1860, pp. 7, 102) suggested that Derris scandens occurred in tropical Australia, which, without a list of specimens cited, is presumably a reference to Brachypterum involutum. Adema (2003) described Derris involuta (Sprague) Sprague (now Brachypterum involutum (Sprague) Adema & Sirich.) as endemic to northern Australia. However, specimens of Brachypterum involutum are not known from north of the Mackay area of central-eastern Queensland. Sirichamorn et al. (2012b) described Derris scandens (now Brachypterum scandens) as occurring in Australia from a specimen at Leiden (Hubbard 5355 (L 1941538)) collected at Boonah (cited as ‘Boonal’) in south-eastern Queensland. This specimen has been incorrectly determined and belongs to Brachypterum involutum.
The map in Sirichamorn and Adema (2020, p. 59) indicates the distribution of Brachypterum involutum to be much more widespread than is realistic. Their map includes west and north Qld and southern NSW but we herein confirm B. involutum to be restricted to an area from Mackay in central eastern Qld to Crescent Head in north east NSW.
AUSTRALIA. QUEENSLAND. Leichhardt: Homevale Station Resources Reserve, July 1995, Champion 1220, Korn & McDougall (BRI). Wide Bay: S of Kin Kin, Nov. 1977, Williams 77283 (BRI); Happy Jack Creek, SE of Kadanga, Nov. 1993, Bean 7045 (BRI). Burnett: Hoggs Road, Tingoora, N of Kingaroy, Nov. 2001, Bean 18104 (BRI); Tessman’s Road, 2 km NE of Kingaroy, May 2007, Forster PIF32500 & Fechner (BRI); corner of West Coolabunia Road and Royles Road, 3 km SW of Coolabunia, Dec. 2002, Forster PIF29156, Smyrnell & Haskard (BRI); Franklins Road, Coolabunia, 8 km SE of Kingaroy, May 2007, Forster PIF 32510 & Fechner (BRI); Semgreen Road, SSE of Kingaroy, Mar. 2001, Bean 17431 (BRI). Moreton: near Boonah, Nov. 1930, Hubbard 5355 (L); Treviot Brook, Main Range, Aug. 1995, Forster PIF17416, Figg & Leiper (CNS); Maidenwell Road, E slopes of Bunya Mountains, Dec. 1994, Grimshaw PG1040 & Turpin (BRI). NEW SOUTH WALES. Cultivated from Richmond River at Temperate House Kew, NSW, Jul. 1904, leg. ign. s.n. (K000898356); 0.4 miles [~0.6 km] NNE of Dunoon, Sep. 1972, Coveny 4439 & Rodd (L1941275); Cudgera Creek, NSW, Dec. 1977, Coveny 9923 & Haegi (L1941281); Flinton Hill, World’s End Pocket, Nov. 1990, Forster PIF7620 (L 1941280); Richmond Range, 0.8 km along Bulmer Road from Bruxner Highway, 2.7 km E of Mallanganee, Telford 12739 & Bruhl (NE); Lyons Road, Sawtell, Dec. 1999, Kemp BK652 & England (CFSHB); 7 miles [~11.3 km] S of Crescent Head, Dec. 1973, Williams s.n. (NE 044784A)
Brachypterum koolgibberah (F.M.Bailey) Adema & Sirich., Blumea 64(1): 278 (2019)
Derris koolgibberah F.M.Bailey, in A. Meston, Votes Proc. Legislative Assembly during the Session of 1889: 16 (1889); Deguelia koolgibberah (F.M.Bailey) S.F.Blake, J. Wash. Acad. Sci. 19(21): 475 (1929); Solori koolgibberah (F.M.Bailey) Adema & Sirich., Taxon 63(3): 532 (2014).
Type citation: ‘Border of the scrubs along the Mulgrave River’ [Queensland]. Type: AUSTRALIA. Queensland, along the Mulgrave River, F.M. Bailey s.n. (lecto, designated by F. Adema and Y. Sirichamorn, Blumea 64(1): 278 (2019)): BRI AQ0022850 [image!]; isolecto: K 000898358 [image!], MEL 282865 [image!].
W. Cooper and W. T. Cooper (1994, pp. 90–91; 2004, p. 207). F. Adema (2003, p. 394). F. A. Zich, B. P. M. Hyland, T. Whiffin and R. A. Kerrigan (Australian Tropical Rainforest Plants, edition 8, see https://apps.lucidcentral.org/rainforest/text/intro/index.html).
Liana, twiner to forest canopy, stem diameter up to 50 mm; bark with scattered lenticels or corky pustules, grey; twigs terete with scattered lenticels, thinly sericeous, grey. Stipules persistent or caducous, ovate, 2–5 mm long and 1–2 mm wide, clothed in prostrate hairs. New growth densely rusty hairy. Leaves with 5–9 leaflets; leaf rachis + petiole terete, channelled adaxially, 110–260 mm long and ~2.5 mm wide at base, all parts rusty pubescent; pulvinus hardly distinct, 8–10 mm long; stipels absent or not obvious among a densely rusty pubescent rachis; lateral petiolules 3–7 mm long; terminal petiolules 20–38 mm long. Leaflets 5–9, oblong, broadly elliptic, oblong–elliptic, obovate, rarely almost orbicular, 60–138 mm long, 34–138 mm wide, leathery; both sides velvety pubescent, indumentum denser on primary vein and along secondary veins; discolourous; base cuneate, truncate or rounded; apex rounded, acute or obtuse; margin entire; venation camptodromous throughout or camptodromous proximally and brochidodromous distally, primary vein depressed on adaxial side, raised abaxially; secondary veins 6–8 pairs, depressed or flush adaxially and raised abaxially, angle of divergence from primary vein 40–50°, veins looping 2–5 mm from margin; tertiary venation reticulate. Inflorescence axillary or terminal, a panicle or pseudoraceme with lateral branches 30–70 mm long, 1.8 mm wide, rachis up to 6.5 mm thick at base, densely rusty pubescent, up to 40-flowered; bracts at pedicel bases ovate, 2–2.5 mm long, ~1.5 mm wide, densely rusty pubescent; pedicels 1.3–2 mm long, densely rusty pubescent; bracteoles not obvious. Flower fragrance not noted; calyx cup-shaped, ~5 mm long and wide, rusty sericeous abaxially, glabrous adaxially; corolla mauve, pink or aging to whitish, nectar guides green; standard: claw ~1 mm long; blades orbicular, 6–8 mm long, ~8.5 mm wide, densely clothed in rusty sericeous hairs abaxially; base cuneate, apex incurved and emarginate; adaxially glabrous except for a few hairs near base, basal callosities present; wing petals: claw ~5.5 mm long; blades curved around keel, oblong, ~4.5 mm long, glabrous, auricles absent; keel petals: claw ~2.5 mm long; blades obovate or boat-shaped, connate at apex, 5.5–6 mm long, densely clothed in rusty sericeous hairs, auricles absent, apex rounded, lateral pockets ~1.5 mm long and adherent to wings; stamens 10, monadelphous, ~6.5 mm long, free section at base ~1.5 mm long, sparsely pubescent in distal half; disc tubular, lobed; ovary fusiform, shortly stiped, ~6 mm long, densely clothed in pale brown sericeous hairs; style ~3 mm long, glabrous; stigma capitate, some hairs at base; ovules 9–11. Fruit pedicel ~3 mm long; pod indehiscent, flat and oblong or strap-shaped, 50–110 long, 16–25 mm wide, densely rusty velvet pubescent, upper wing 2–4 mm wide, lower margin a thickened ridge; seeds 1–3, discoid-reniform, 4–5 mm long, 6–7 mm wide; hilum 1 mm long. Hairy Derris (Fig. 10).
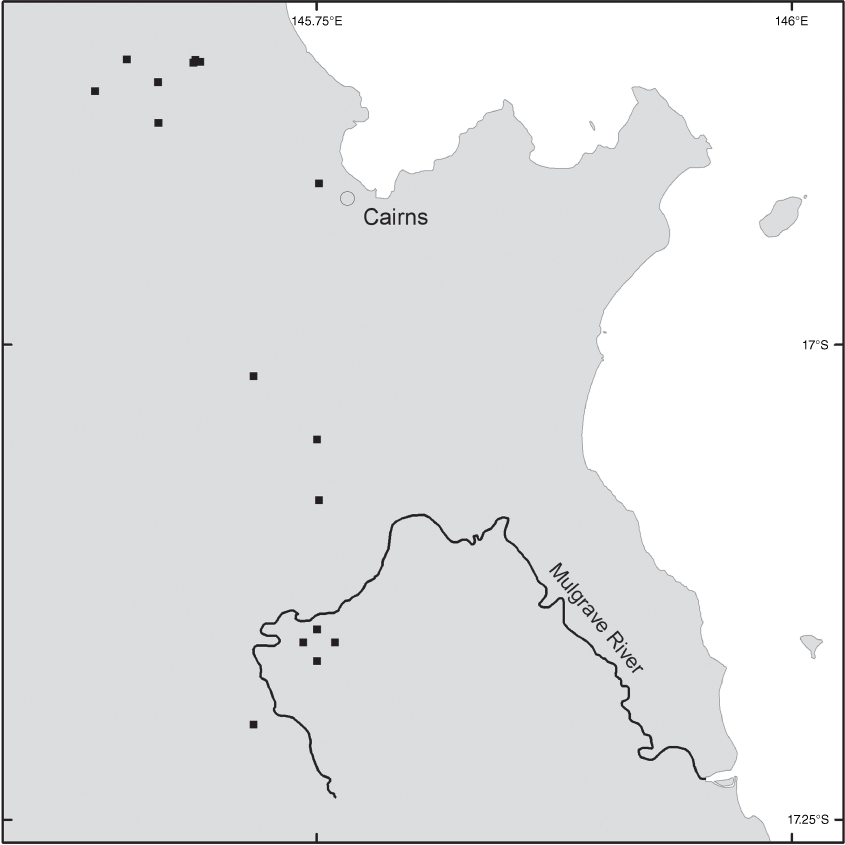
Brachypterum koolgibberah occurs in Australia and Papua New Guinea. In Australia, it occurs in a small area around Cairns between the Mulgrave River and the Macalister Range (Fig. 11).
Brachypterum koolgibberah in Australia grows in lowland mesophyll vine forest (often on the disturbed margins) and in monsoon forest up to 120-m altitude.
Flowers have been recorded in July and September and fruit has been recorded in October and December.
The epithet koolgibberah is from the Aboriginal name for the Mulgrave River, where the type specimen was collected.
AUSTRALIA. QUEENSLAND. Cook. Macalister Range, hillslope beside Kennedy Highway, Dec. 1999, Wannan 1000 & Jago (BRI); Barron River at Smithfield, Oct. 1995, Ford 1633 (CNS); Barron Gorge, Oct. 1995, Gray 20290V (CNS); Barron Gorge Road, ~5 m east of stairs to river, July 2011, Dowe 1090 (CNS); Lower Stoney Creek, July 1946, Flecker s.n. (CNS 10323); Cairns, s. dat., Cowley 56B (BRI); Wright Creek, Mt Peter, Aug. 1941, Stephens s.n. (CNS); White’s Farm Plot, Goldsborough Road, Dec. 1980, Gray 1980 (CNS); White’s Farm Plot, Goldsborough Road, July 1980, Hyland 10542 (CNS); White’s Farm Plot, Goldsborough Road, Aug. 1969, Miller s.n. (CNS); Mulgrave River, 1889, Bailey s.n. (BRI).
Brachypterum nitidum W.E.Cooper, sp. nov.
Type: AUSTRALIA. QUEENSLAND. Cook District: Stones Crossing, Wenlock River, 12 Dec. 2017, W.Cooper 2529 & R.Jensen (holo: CNS [CNS152497]; iso: BRI, CANB, K, L, LAE, MEL, MO, NSW, SING, US distribuendi).
Derris sp. (Wenlock River BH 21082V), Fruits of the Rainforest, 305 (1994).
Derris sp. Wenlock River (B.Hyland 21082V), Australian Tropical Rainforest Plants [online only] (2010).
F. A. Zich, B. P. M. Hyland, T. Whiffin and R. A. Kerrigan (Australian Tropical Rainforest Plants, edition 8, see https://apps.lucidcentral.org/rainforest/text/intro/index.html).
Brachypterum nitidum is similar to Brachypterum scandens (Roxb.) Sirich. & Adema, but differs in stipule width 0.5 mm (v. 0.9–1.3 mm); leaflet number 3–9, rarely 11 (v. 7–19); standard claw length 2–3 mm (v. 1 mm); standard petal abaxially sericeous (v. slightly sericeous at apex to glabrous); wing claw 1.5 mm long (v. 2–2.5 mm long); wing petals reflexed widely and becoming slightly revolute (v. slightly curved to reflexed); wings abaxially sparsely sericeous with ciliate auricles (v. slightly sericeous and auricles densely strigose); wing auricles flabellate (v. obtuse); stamen length ~7 mm (v. 9–12 mm); staminal fenestrae free section 0.6 mm long (v. 1.5–3.5 mm long; style ~1.5 mm long (v. 4–5.5 mm long); seeds 4.5–6 mm long, 6–6.5 wide × 2 mm thick (v. 7 mm long, 5 mm wide, 1 mm thick).
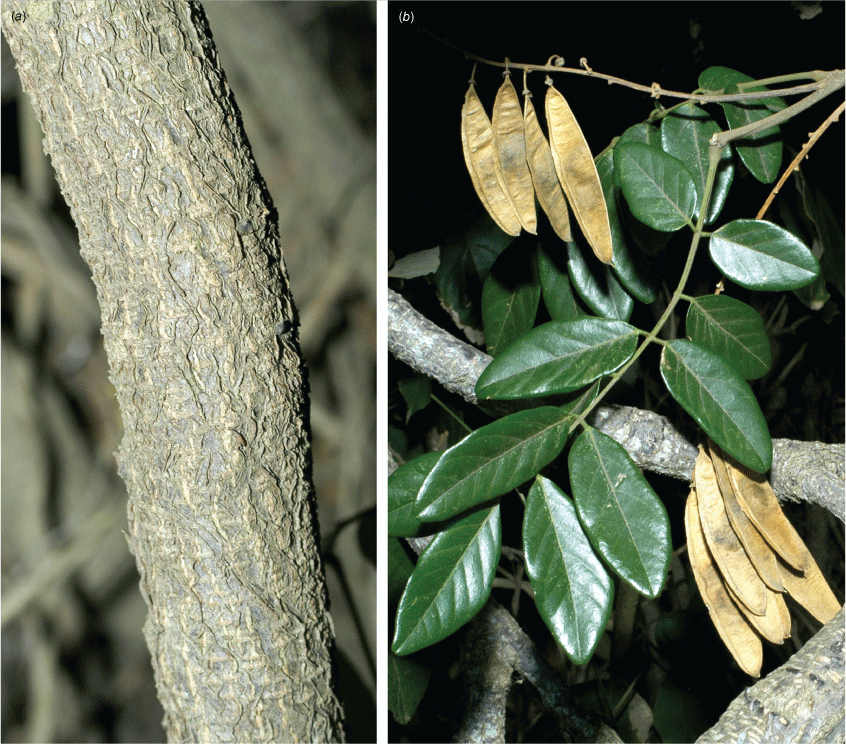
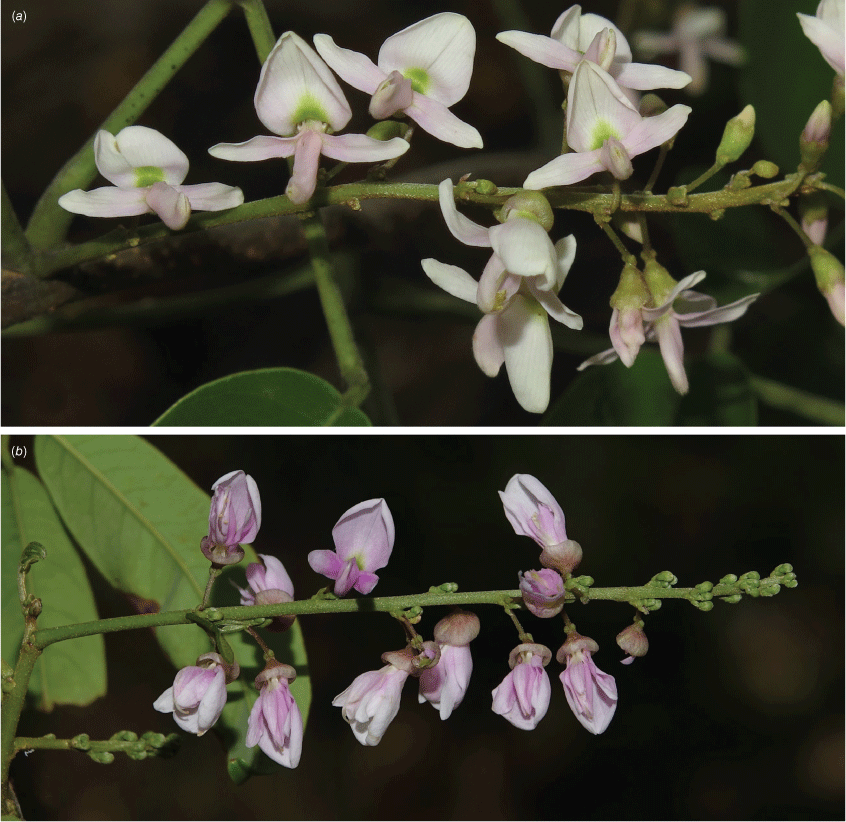
Liana to forest canopy or a sprawling slender vine; stem diameter up to 120 mm; bark on medium-sized stems tessellated or with a flaky-edged herringbone pattern, cream, grey or light brown, with rusty-coloured round and horizontal lenticels, bark on older stems becoming less patterned, brown, cream or grey with vertical fissures and thinly flaky; twigs green with numerous pale lenticels and scattered minute hairs, or glabrous. Stipules persistent, triangular, 0.6–1.5 mm long and 0.5–1 mm wide at base, sericeous; new growth green. Leaves with 3–9 (rarely 11) leaflets; rachis + petiole terete, channelled adaxially, 45–105 mm long, thinly and minutely sericeous; pulvinus 3.5–6 mm long, 1.5–2 mm wide, puberulent; stipels mostly persistent, narrowly triangular, 0.20–1 mm long, sparsely sericeous and often with a denser tuft of hairs at apex; lateral petiolules 2–5 mm long, puberulent; terminal petiolules 3–29 mm long, puberulent. Leaflets elliptical, narrowly elliptical, narrowly ovate, ovate, orbicular or obovate, 15–120 mm long, 10–65 mm wide, coriaceous, adaxially surface shiny and thinly sericeous along midrib, with sparse scattered hairs elsewhere or glabrous, abaxially thinly sericeous to glabrous, glaucous, discolourous; base subpeltate, obtuse, rounded, cordate, cuneate or truncate; apex long or shortly acuminate and ultimately retuse; margin entire and scarious; venation brochidodromous, primary vein depressed adaxially, raised abaxially and with a small papule at apex; secondary veins 10–13 pairs, flush or slightly raised on both sides, angle of divergence from primary vein 20–50°, forming loops 1–2 mm from margin; tertiary venation reticulate. Inflorescence axillary or mostly terminal or near terminal, a pseudoraceme or rarely a branched panicle, 45–200 mm long; rachis 0.8–1.8 mm thick at base, sericeous; bracts at base of brachyblasts solitary, persistent, ovate or triangular, 0.6–1 mm long and 0.5 mm wide at base, sericeous; pedicels 2.5–4 mm long, sericeous; brachyblasts thickened stems or wart-like, 1.5–3.5 mm long, 3–15-flowered, puberulent; bracts at base of pedicels caducous, ovate or triangular, 0.6–0.9 mm long, 0.5–0.7 mm wide at base, puberulent; pedicels 2.5–7 mm long, sparsely puberulent; bracteoles paired near pedicel apex, triangular, 0.7–1 mm long, ~0.5 mm wide at base, puberulent. Flowers either faintly and pleasantly fragrant or unpleasantly scented and ammonia-like; calyx vase-shaped or campanulate with incurved apex, 2–3 mm long and wide, thinly sericeous abaxially, glabrous adaxially, purplish, green or green blotched with crimson; corolla pink to palest pink with green nectar guides; standard claw 0.5–1 mm long; blades oblong–orbicular, 6.5–8 mm long, 6.5–7.5 mm wide, abaxially glabrous or thinly sericeous, adaxially glabrous, base attenuate or cuneate, basal callosities absent; apex incurved and emarginate; wing petals claw 1.5–2 mm long; blades spread at right angles becoming slightly revolute, oblong or oblong–obovate, ~6–7.5 mm long and 1.5–2.5 mm wide, adaxially glabrous or thinly sericeous mostly along main vein, abaxially glabrous except for hairs near margin in basal half; upper margin auricled, auricle triangular or flabellate and folded, lower margin attenuate or cuneate; apex rounded or obtuse; keel petals claw 1.5–2.5 mm long; blades obovate, connate, 5–6.5 mm long, 2.5–3 mm wide, abaxially thinly puberulent, adaxially glabrous, auricles absent; apex rounded; lateral pocket 1–2.5 mm long, adherent to wing near base; stamens monadelphous, 7–9 mm long, basal fenestrae 0.5–0.6 mm long, glabrous; anthers ~0.35 mm long, 0.3 mm wide, glabrous; disc tubular, lobed; ovary fusiform, not stiped, 5–5.5 mm long, 0.5 mm wide, sericeous; style 1.5–2 mm long, thinly sericeous in lower section; ovules 8–10; stigma capitate. Fruit pedicel 6–7 mm long; pod indehiscent, flat and oblong or strap-shaped, thin and chartaceous, 25–60 mm long, 11–13 mm wide, straw-coloured, clothed in minute appressed hairs, wing along upper suture 1–2 mm wide, base attenuate, apex acuminate, seed chambers distinct and enclosing each seed, funicle ~1.75 mm long; seeds 1–4, discoid, flat, 4.5–6 mm long, 6–6.5 mm wide, 2 mm thick; hilum central, 1–1.2 mm long (Fig. 12, 13).
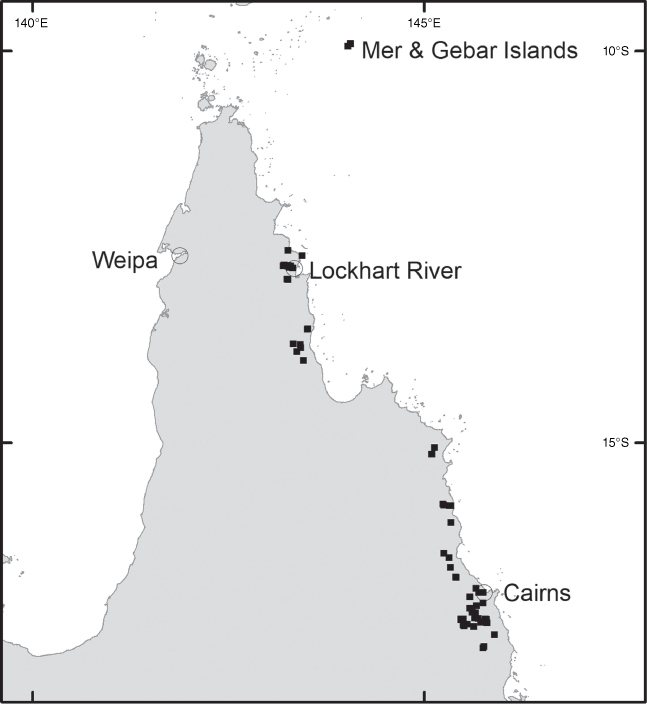
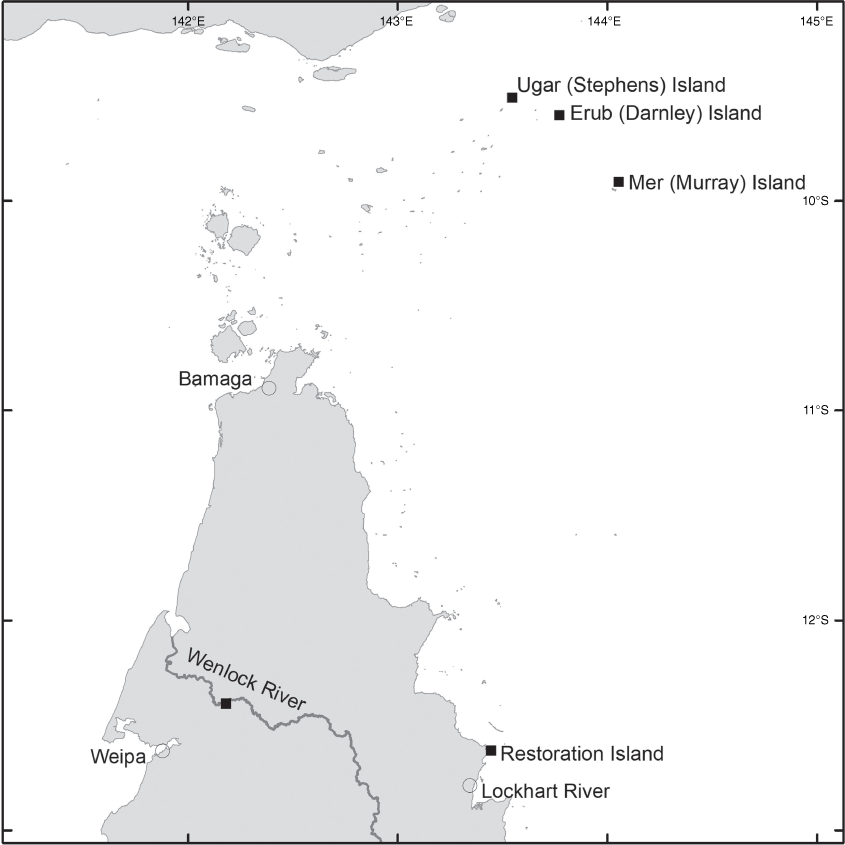
Brachypterum nitidum is endemic to far-northern Queensland from Stones Crossing on the Wenlock River north of Weipa, Restoration Island adjacent to Cape Weymouth as well as Ugar (Stephens) Island, Mer (Murray) Island and Erub (Darnley) Island (all in the Torres Strait), up to ~20 m above sea level (Fig. 14).
Brachypterum nitidum mostly grows as a slender vine or liana to the forest canopy in complex mesophyll vineforest and semi-deciduous notophyll vineforest. On Erub (Darnley) Island, it also grows as a sprawling shrubby vine, resprouting from rootstock after regular burns. In this habitat, it co-occurs as the dominant species with the grass Imperata cylindrica.
The epithet nitida is from the Latin nitidus (shining), referring to the adaxial surface of the leaflet.
Nucleotide polymorphisms at several positions in the ITS sequences (data not shown) were observed in all samples from Restoration Island. Restoration Island plants also differ somewhat morphologically from the rest of the population in the following attributes: bark pale creamy-grey with vertical fissures and round or horizontal pale lenticels, becoming tessellated on old stems (v. medium-sized stems having a flaky-edged herringbone pattern with rusty-coloured round and horizontal lenticels, becoming almost patternless and flaky on larger stems); pedicel length 2.5–4 (v. 4–7 mm); flower fragrance pleasantly floral (v. unpleasant and ammonia-like); petal colour rose pink (v. very pale pink); wing petal indumentum adaxially glabrous (v. adaxially thinly sericeous); wing auricle shape triangular (v. flabellate); and wing/keel lateral pocket ~2.5 mm long (v. ~1 mm long) (Fig. 13). Taken together, these observations suggest possible hybridisation in the history of this population. Detailed population genetic analysis of B. nitidum and related species would be required to confirm this.
AUSTRALIA. QUEENSLAND. Ugar (Stephens) Island, eastern Torres Strait, Apr. 2021, Fell DGF UG30, Stephen, Stephen & Savage (CNS); Mer (Murray) Island, Torres Strait, Apr. 2021, Fell DGF-MER26, Bon, Tapau-Bon & Noah (CNS); Wenlock River, Stones Crossing, Oct. 1980, Hyland 21082V (CNS); Stones Crossing, Wenlock River, Oct. 1999, Hyland 21377V (CNS); Stones Crossing, Wenlock River, Oct. 2017, Cooper 2522, Stubbs & Burch (CNS); Stones Crossing, Wenlock River, Dec. 2017, Cooper 2528 & Jensen (CNS); Restoration Island (off Cape Weymouth), Nov. 2001, Waterhouse BMW 6331 (BRI); Restoration Island, Oct. 2018, Cooper 2546 & Pritchard (CNS); Restoration Island, Dec. 2019, Cooper 2617, Jensen & Pritchard (CNS).
Brachypterum opacum W.E.Cooper, sp. nov.
Type: AUSTRALIA. QUEENSLAND. Cook District, Windsor Tableland Road, Daintree National Park, 28 Jan. 2016, W.Cooper 2315, A.Ford & R.Jensen (holo: CNS [CNS152498]; iso: BRI, CANB, K, L, MEL, MO, NSW distribuendi).
Derris sp. (Daintree D.E.Boyland + 469), Queensland Plants: Names and Distribution 79 (1997).
Derris sp. (Daintree), Fruits Austral. Trop. Rainforest 208 (2004).
Derris sp. Daintree (D.E.Boyland + 469), Australian Plant Census database (see https://biodiversity.org.au/nsl/services/rest/name/apni/219345, accessed 11 April 2022).
W. Cooper and W. T Cooper (2004, p. 208). F. A. Zich, B. P. M. Hyland, T. Whiffin and R. A. Kerrigan (Australian Tropical Rainforest Plants, edition 8, see https://apps.lucidcentral.org/rainforest/text/intro/index.html).
Brachypterum opacum is similar to Brachypterum involutum (Sprague) Adema & Sirich, but differs in being a short slender climber up to 3 m (v. woody climber to forest canopy); leaflet numbers 5–9 (v. 9–13); leaflet texture thinly leathery (v. coriaceous); primary vein on the adaxial surface of the leaflet depressed (v. slightly raised); secondary veins + flush (v. raised on abaxial surface); pedicels 1.5–4 mm long (v. 4.5–5.5 mm long); flower petals palest green or lemon to cream or white (v. pink or purple).
Slender twiner usually to up to 3 m, occasionally up to ~7 m, stem diameter up to ~7 mm, evergreen; stems and twigs green to brown with round or slightly elongated vertical or horizontal pale or orange–brown lenticels. Stipules persistent or caducous, triangular, 1–2 mm long, 1–1.25 mm wide at base, clothed in minute appressed coppery hairs; new growth green. Leaves with 5–9 leaflets; rachis + petiole narrowly channelled adaxially, 60–200 mm long, sparsely clothed in minute coppery appressed hairs, denser at nodes; pulvinus 3.5–11 mm long, 1–3.5 mm wide, clothed in coppery appressed hairs; stipels persistent or caducous, narrowly triangular, 1.3–2 mm long, sparsely sericeous; lateral petiolules 2.5–5.5 mm long, puberulent; terminal petiolules 6–24 mm long, puberulent. Leaflets ovate, elliptical or obovate, 13–135 mm long, 9–44 mm wide, thinly leathery, adaxially dull, glabrous or a few sparse minute hairs on midrib, abaxially glaucous and clothed in minute fawn or coppery sericeous hairs, discolourous; base cuneate or rounded (rarely truncate or cordate); apex long acuminate (sometimes ultimately obtuse or retuse); margin entire; venation brochidodromous, primary vein depressed adaxially and raised abaxially with a small papule at apex; secondary veins 5–10 pairs, virtually flush or slightly raised on both surfaces, angle of divergence from primary vein 40°–60°, forming loops ~1 mm from margin, tertiary venation reticulate. Inflorescence an axillary, ramiflorous or rarely a terminal pseudoraceme, 20–190 mm long; rachis clothed in coppery sericeous hairs; brachyblasts thickened and wart-like, 1.25–2.5 mm long, ~1 mm wide, 2–5-flowered; bracts at base of pedicels caducous, triangular, ~0.75 mm long, 0.5 mm wide at base, clothed in coppery sericeous hairs; pedicels 1.5–4 mm long, clothed in minute coppery sericeous hairs; bracteoles persistent at calyx base, ovate, ~0.7 mm long, 0.5 mm wide at base, clothed in minute coppery sericeous hairs. Flowers: fragrance not detected; calyx campanulate with an incurved apex, 1.5–3.5 mm long, 2.5–4 mm wide, clothed in minute coppery hairs abaxially, glabrous adaxially, green or coppery green; corolla palest green, lemon, cream or whitish with green nectar guides; standard petal ~2 mm long; blade orbicular, 6–8 mm long, ~8 mm wide, glabrous, base truncate, basal callosities present, apex emarginate; wing petals: claw ~2 mm long; blades slightly curved around keel, oblong, ~8 mm long, ~3.3 mm wide, glabrous, upper margin narrowly auricled at base, auricle rounded, ~0.25 mm long, 0.3 mm wide; apex obtuse; keel petals: claw ~2.5 mm long; blades oblong, ~7.5 mm long, ~3 mm wide, glabrous or minute hairs at apex and near lower margin; upper margin attenuate at base, lower margin truncate or slightly rounded ~0.5 mm long, wide; apex rounded; lateral pockets ~2 mm long, adherent to wings; stamens monadelphous, up to 10 mm long, basal fenestrae ~1.25 mm long, glabrous; anthers ~0.5 mm long, glabrous; disc tubular, lobed; ovary fusiform, ~11 mm long and 3 mm wide, sericeous; style ~3 mm long, sparsely hairy; ovules ~8; stigma capitate, clothed in erect hairs. Fruit: pedicel 3.5–4.5 mm long, slender; pod indehiscent, flat and oblong–elliptical or strap-shaped, 70–100 mm long, 18–27 mm wide, papery, base attenuate, apex beaked, winged along upperside only, wings 2–4 mm wide in mid-section, straw-coloured, clothed in minute appressed coppery hairs, glandular-dots or blisters may be evident and numerous, seed chambers distinct and enclosing each seed; seeds solitary (rarely 2), reniform, 5.5–6.25 mm long, 9–9.75 mm wide, 2.8 mm thick, beige; hilum 0.75 mm long. Native Derris (Fig. 15).
Brachypterum opacum is endemic to Australia and mostly restricted to the Wet Tropics bioregion, occurring from Mount Fox near Paluma to Mount Fantastic near Cooktown (which is slightly outside of the northern boundary of the bioregion) (Fig. 9).
Brachypterum opacum occurs in a wide range of wet to drier forest types, including complex notophyll vineforest, semi-deciduous complex notophyll vineforest, complex mesophyll vineforest, gallery forest, littoral rainforest, rainforest margins or regrowth, back mangal, wet sclerophyll forest, Eucalyptus woodland and open forest, at altitudes from near sea level to 670 m.
Flowers have been recorded in October, November, January and February and ripe fruits have been recorded in August and September.
The epithet opacum is from the Latin opacus (dull or not shining); referring to the leaflets, which are distinctly dull on both the abaxial and adaxial surfaces.
AUSTRALIA. QUEENSLAND. Cook: Mt Fantastic, Hann State Forest ~12 km W of Cooktown, Dec. 2009, Roberts s.n. & Covacevich (BRI); 15.3 km SE of Annan River mouth, Caloola Holding, South Endeavour Trust, Fell DGF CAL3a/4, Geyle & Kulka, Apr. 2022 (CNS); ~15 miles [~24 km] NNW of Daintree, Nov. 1967, Boyland 469 & Gillieatt (BRI); Whyanbeel Creek, near the inlet between Dayman Point and Newell Beach, Nov. 1978, Moriarty 2521 (CNS); Whyanbeel Valley Road, Dec. 2018, Cooper 2578 & Hawkes (CNS); High Falls Creek near Whyanbeel, Daintree National Park, July 2021, Cooper 2778 & Hawkes (CNS); Mowbray, Nov. 1937, Sparvell (CNS); ~11.2 km NW of Mt Molloy, Brooklyn Wildlife Sanctuary, Jensen 3535 & Stanton (BRI); 4 km N of Mt Molloy, Peninsula Developmental Road, Jan. 1993, Gray 5604 (CNS); Hunter Creek, Brooklyn (Australian Wildlife Conservancy), Feb. 2020, Cooper 2634 & Jensen (CNS); Clohesy River, Sep. 2018, Cooper 2534 & Ford (CNS); Bridle Creek, Sep. 2018, Cooper 2533 & Ford (CNS); Bridle Creek, Sep. 2018, Cooper 2535 & Ford (CNS); Dinden Forest Reserve, 4.5 km along Bridle Creek Road from Davies Creek Road, E of Mareeba, Aug. 2004, Ford 4416 & Hewitt (CNS); James Cook Uni Cairns Campus, Nov. 1995, Jago 3679 (BRI); SFR 933, Nov. 1989, Irvine 2345 (CNS); Kamerunga, Barron River, undated, Cowley 11D (BRI); Walsh’s Pyramid, Apr. 1996, Lyons 170 (CNS); Double Barrel Creek, Boulders Road, Babinda, Oct. 1996, Jago 550 (CNS); Russell River S of Mt Bartle Frere, Jago 441 (BRI); Bosbaa Creek, Basilisk Range, 2 km SW of Moresby, Oct 1997, Forster PIF21794, Booth & Jensen (BRI); Garners Beach, Nov. 2016, Cooper 2392 (CNS); Upper Tully Valley, near Kareeya, Jan. 2006, McDonald KRM4761 (BRI); Mt Fox, Dec. 1949, Clemens s.n. (BRI).
| 1. | Stipels absent or caducous; standard with basal callosities; ovary with 2–5 ovules; seeds not enclosed in individual segments of the pod but within a single open chamber...2 Stipels mostly persistent or not obvious among densely rusty hairs on the rachis; standard with or without basal callosities; ovary with 8–11 ovules; each seed enclosed within thickened individual chambers within pod...4 |
| 2. | Leaflets subpeltate...3 Leaflets neither peltate nor subpeltate...D. elliptica |
| 3. | New growth green; lateral leaf bases cordate or rarely obtuse and sometimes cuneate on terminal leaflet; wing petals slightly curved around keel, becoming widely reflexed but not recurved or revolute; pods 1-winged, leathery; occurs only in habitats with marine influences...D. trifoliata New growth red; lateral and terminal leaflet bases obtuse, rounded, cuneate or truncate; wing petals reflexing widely and becoming revolute; pods 2-winged, papery; occurs in various forms of lowland to upland rainforest...D. rubrocalyx |
| 4. | Leaf rachis conspicuously rusty pubescent...5 Leaf rachis sparsely clothed in minute rusty or colourless hairs...6 |
| 5. | Leaves with 5–9 leaflets; leaflets 60–138 × 34–138 mm; inflorescence is a pseudoraceme or panicle with slender branches; occurs in the Cairns area, northern Qld...B. koolgibberah Leaves with 9–13 leaflets; leaflets 18–55 × 13–34 mm; inflorescences are wart-like brachyblasts; occurs from Mount Britton (south-west of Mackay), Qld to Crescent Head, NSW...B. involutum |
| 6. | Leaflet matt on adaxial surface; flowers white or cream-coloured, standard with basal callosities; stigma hairy; occurs south from the Cooktown area to Mount Fox near Paluma and mostly within the Wet Tropics bioregion, QLD...B. opacum Leaflet shiny on abaxial surface; flowers pink or palest pink, standard without basal callosities, stigma glabrous; occurs on Cape York Peninsula and off-shore islands, Qld...B. nitidum |
Data availability
The data that support this study are available in the article and in online DNA sequence repositories under the accession numbers listed in Table 1.
Conflicts of interest
Darren Crayn is Editor-in-Chief for Australian Systematic Botany but did not at any stage have editor-level access to this manuscript while in peer review, as is the standard practice when handling manuscripts submitted by an editor to this journal. Australian Systematic Botany encourages its editors to publish in the journal and they are kept totally separate from the decision-making processes for their manuscripts. The authors have no further conflicts of interest to declare.
Acknowledgements
The first author is grateful to Rigel Jensen, Andrew Ford and John Pritchard for their assistance during several field trips hunting Derris and Brachypterum. I also thank Peter Bostock (BRI), David Fell, Andrew Franks (BRI), Alisdair Grigg (Christmas Island), Tim Hawkes, David Mackay, Mike Mathieson (BRI), Steve Murphy, Hugh Nicholson, Nan Nicholson, Trixie Tuck and Brian Venables for their various forms of assistance. Charles Budby (past Director, Mokwiri Aboriginal Corporation), Phillip Mango (Napranum Senior Ranger) and Geoff Wharton are thanked for their encouragement and interest in B. nitidum. We are especially thankful for Matt Barrett’s comments on a draft of the manuscript.
References
Adema F (2003) Notes on Malesian Fabaceae (Leguminosae-Papilionoideae). 11. The genus Derris. Blumea – Biodiversity, Evolution and Biogeography of Plants 48, 393-419.
| Crossref | Google Scholar |
Adema F, Sirichamorn Y (2019) Notes on Malesian Fabaceae (Leguminosae–Papilionoideae 20. The genus Brachypterum. Blumea - Biodiversity, Evolution and Biogeography of Plants 64, 278-279.
| Crossref | Google Scholar |
Applequist WL (2017) Report of the Nomenclature Committee for Vascular Plants: 69. Taxon 66(2), 500-513.
| Crossref | Google Scholar |
Baum D (1992) Phylogenetic species concepts. Trends in Ecology & Evolution 7, 1-2.
| Crossref | Google Scholar | PubMed |
Bentham G (1860) Synopsis of Dalbergieæ, a Tribe of Leguminosæ. Journal of the Proceedings of the Linnean Society of London, Botany 4, 1-128.
| Crossref | Google Scholar |
Boonprajan P, Leeratiwong C, Sirichamorn Y (2024) From morphology to molecules: a comprehensive study of a novel Derris species (Fabaceae) with a rare flowering habit and reddish leaflet midribs, discovered in Peninsular Thailand. PhytoKeys 237, 51-77.
| Crossref | Google Scholar | PubMed |
Choi I-S, Cardoso D, de Queiroz LP, de Lima HC, Lee C, Ruhlman TA, Jansen RK, Wojciechowski MF (2022) Highly resolved papilionoid legume phylogeny based on plastid phylogenomics. Frontiers in Plant Science 13, 823190.
| Crossref | Google Scholar | PubMed |
Hoang DT, Chernomor O, von Haeseler A, Minh BQ, Vinh LS (2017) UFBoot2: improving the ultrafast bootstrap approximation. Molecular Biology and Evolution 35(2), 518-522.
| Crossref | Google Scholar |
Hu J, Lavin M, Wojciechowski MF, Sanderson MJ (2000) Phylogenetic systematics of the tribe Millettieae (Leguminosae) based on chloroplast trnK/matK sequences and its implications for evolutionary patterns in Papilionoideae. American Journal of Botany 87(3), 418-430.
| Crossref | Google Scholar |
Hu J, Lavin M, Wojciechowski M, Sanderson M (2002) Phylogenetic analysis of nuclear ribosomal ITS/5.8S sequences in the tribe Millettieae (Fabaceae): Poecilanthe–Cyclolobium, the core Millettieae, and the Callerya group. Systematic Botany 27(4), 722-733.
| Crossref | Google Scholar |
Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS (2017) ModelFinder: fast model selection for accurate phylogenetic estimates. Nature Methods 14, 587-589.
| Crossref | Google Scholar | PubMed |
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30, 772-780.
| Crossref | Google Scholar | PubMed |
Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, von Haeseler A, Lanfear R (2020) IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Molecular Biology and Evolution 37, 1530-1534.
| Crossref | Google Scholar | PubMed |
Sirichamorn Y, Adema F (2020) Four new combinations in the legume genus Brachypterum. Thai Forest Bulletin (Botany) 48(1), 57-60.
| Crossref | Google Scholar |
Sirichamorn Y, Adema F, Gravendeel B, van Welzen PC (2012a) Phylogeny of paleotropic Derris-like taxa (Fabaceae) on chloroplast and nuclear sequences shows reorganization of (infra)generic classifications is needed. American Journal of Botany 99(11), 1793-1808.
| Crossref | Google Scholar |
Sirichamorn Y, Adema F, van Welzen PC (2012b) The genera Aganope, Derris, and Paraderris (Fabaceae, Millettieae) in Thailand. Systematic Botany 37(2), 404-436.
| Crossref | Google Scholar |
Sirichamorn Y, Adema FACB, van Welzen PC (2013) (2121) Proposal to conserve the name Brachypterum against Solori (Fabaceae). Taxon 62(1), 179-180.
| Crossref | Google Scholar |
Sirichamorn Y, Adema FACB, Roos MC, van Welzen PC (2014) Molecular and morphological phylogenetic reconstruction reveals a new generic delimitation of Asian Derris (Fabaceae): reinstatement of Solori and synonymisation of Paraderris with Derris. Taxon 63(3), 522-538.
| Crossref | Google Scholar |
Sun Y, Skinner DZ, Liang GH, Hulbert SH (1994) Phylogenetic analysis of Sorghum and related taxa using internal transcribed spacers of nuclear ribosomal DNA. Theoretical and Applied Genetics 89, 26-32.
| Crossref | Google Scholar | PubMed |
Verdcourt B (1978) New taxa of Leguminosae from Papua New Guinea. Kew Bulletin 32(2), 455-473 ( ).
| Crossref | Google Scholar |