Changes in soil C, N and δ15N along three forest–pasture chronosequences in New Zealand
P. L. Mudge A B C E , L. A. Schipper A , W. T. Baisden D , A. Ghani B and R. W. Lewis AA Department of Earth and Ocean Sciences, University of Waikato, Private Bag 3105, Hamilton 3240, New Zealand.
B AgResearch, Ruakura Research Centre, Private Bag 3115, Hamilton 3240, New Zealand.
C Landcare Research, Private Bag 3217, Hamilton, New Zealand.
D National Isotope Centre, GNS Science, Lower Hutt, New Zealand.
E Corresponding author. Email: mudgep@landcareresearch.co.nz
Soil Research 52(1) 27-37 https://doi.org/10.1071/SR13183
Submitted: 20 June 2013 Accepted: 23 September 2013 Published: 5 February 2014
Abstract
Changes in total soil carbon (C), nitrogen (N) and natural-abundance N isotopes (δ15N) were measured along three forest-to-pasture chronosequences on pumice soils in the Central North Island of New Zealand. On each of the three chronosequences, exotic pine forests had been converted to intensive dairy pastures 2–11 years before sampling and samples were also taken from remaining pine forests and long-term pastures (40–80 years old). The primary objective of the study was to test the hypothesis that surface-soil δ15N would increase over time following conversion of forest to pasture, due to greater N inputs and isotope-fractionating N losses (e.g. ammonia volatilisation) in pasture systems. Results supported our hypothesis, with linear regression revealing a significant (P < 0.001) positive correlation between log-transformed pasture age (log10[pasture age + 1]) and surface-soil δ15N. There was also a positive correlation (P < 0.001) between pasture age and total soil C and N, and a negative correlation of pasture age with C : N ratio. Surface-soil δ15N was also positively correlated (P < 0.001) with total soil N, and negatively correlated with C : N ratio when C : N was <13.6. These results suggested that as soils became more N-‘saturated’, isotope-fractionating N loss processes increased. Surface-soil δ15N in the pine forests was significantly less than subsoil δ15N, but there was no significant difference between the surface and subsoil in the long-term pastures, due to 15N enrichment of the surface soil. The difference in δ15N between the surface soil and subsoil may be a useful indicator of past land management, in addition to absolute δ15N values of surface soils.
Additional keywords: deforestation, land-use change, natural abundance, nitrogen isotope, pine forest.
Introduction
The understanding of soil properties and ecosystem development has been advanced by studying chronosequences, which are defined as ‘a set of sites formed from the same parent material or substrate that differ in the time since they were formed’ (Walker et al. 2010). Chronosequence studies are also referred to as space-for-time substitutions, and allow insight into how ecosystem properties change over time periods that are longer than would be possible via direct measurement (i.e. millennia, Vitousek et al. 1989) or studies over shorter time periods (i.e. decades) where samples have not been taken through time (Lemenih et al. 2005). Many decadal-scale chronosequence studies have investigated the effect that changes in land use, such as conversion of forest to agricultural land (Piccolo et al. 1996; Awiti et al. 2008) or reversion of agricultural land to natural ecosystems (Compton et al. 2007; Wang et al. 2007), have on soil properties. Soil carbon (C) and nitrogen (N) are commonly measured in chronosequence studies, due to the fundamental importance of soil organic matter in ecosystem processes such as nutrient cycling. The natural abundance of C and N isotopes in soil and vegetation has also been shown to be a useful tool in chronosequence studies, with nitrogen isotopes (δ15N), in particular, providing useful information on N cycling and loss processes (Piccolo et al. 1996; Martinelli et al. 1999; Brenner et al. 2001; Lemenih et al. 2005; Compton et al. 2007).
Before European settlement, New Zealand was predominantly covered by native forest or scrub, with forest clearance for pastoral agriculture commencing in earnest around 1850 and continuing until around 1980, although much of the currently productive land was cleared by 1920 (MacLeod and Moller 2006). Currently, 39% of New Zealand’s land area is in pastoral agriculture, 9% in exotic forest–scrub and 50% under native land cover, primarily forest in upland areas (MFE 2009). Despite the large scale and rapid conversion of forest to pasture in New Zealand, relatively few studies have measured how soil properties changed during the conversion process. Jackman (1964a, 1964b) and Walker et al. (1959) conducted chronosequence studies spanning 19–66 years and found that soil C and N generally increased when native scrub or poor-producing pasture was converted to high-producing pasture. Increases in C and N were rapid during the first few years following conversion, and then slowed as a new equilibrium was approached. The C : N ratio tended to decrease with time under pasture, indicating that the soils were becoming saturated with N, therefore increasing the likelihood of N losses via leaching and gaseous pathways (Walker et al. 1959; Schipper et al. 2004; Schipper and Sparling 2011).
New Zealand’s history of land-use change has been associated with various transitions between native and exotic forest and pasture. The clearing of native vegetation for pasture slowed or stopped by ~1980, but economic reforms and spikes in log prices led to conversions of large areas of pasture to exotic forest (mainly Pinus radiata) during the 1990s. A recent downturn in returns from plantation forestry, and good returns from dairy farming, led to conversion of large areas of exotic forest (mainly Pinus radiata) to dairy pastures, during the decade leading up to 2008 when article 3.3 of the Kyoto Protocol took effect. This provided an opportunity to further improve our understanding of how soil C and N changed during the first few years following forest clearance. There was particular interest in soil C dynamics because of the implications of forest conversion on New Zealand’s greenhouse gas obligations under the Kyoto Protocol, and replacement of forests with pasture was expected to significantly increase N leaching losses, with implications for water quality (Hamilton 2005). With a focus mainly on estimating soil C changes associated with afforestation of pasture, several studies have compared soil properties under pasture and plantation forest sites in New Zealand (Giddens et al. 1997; Davis and Condron 2002; Sparling and Schipper 2004; Tate et al. 2005; Baisden et al. 2006), but we are aware of only one published chronosequence study where plantation forest had been converted to pasture (Hedley et al. 2009).
Globally, few studies have looked at N isotopes along forest–pasture chronosequences, and no such studies have been carried out in New Zealand. Stevenson et al. (2010) measured natural-abundance N isotopes in surface soils from throughout New Zealand, and found a clear separation between land uses, with lower δ15N under forests (native and exotic) than under pastoral agriculture or cropping land. Lower δ15N in soils under forests than under pasture was largely attributed to differences in land-use intensity, with higher N inputs, cycling and isotope fractionating N losses (e.g. ammonia volatilisation) from the pasture sites. However, Stevenson et al. (2010) also pointed out that land use can be biased towards soil type or landscape position, and these factors may have also influenced soil δ15N values.
In the current study, we sampled soils from three chronosequences where plantation pine forests had been converted to intensive dairy pastures. At each chronosequence, pine and pasture sites were on the same soils, on the same topography and under the same climatic regime, and therefore we could be more certain that any differences in δ15N were due to land use. Based on the work of Stevenson et al. (2010), we hypothesised that surface-soil δ15N values would increase over time following conversion of plantation pine forest to pasture, due to greater N inputs and isotope fractionating N losses in the new pasture systems (Fig. 1). We tested the hypothesis that surface-soil δ15N values would increase in two ways: (i) an increase in absolute δ15N values of surface soils, and (ii) a relative increase in surface-soil δ15N values compared with a baseline estimated using subsoil δ15N values.

|
Methods
Site descriptions and soil sampling
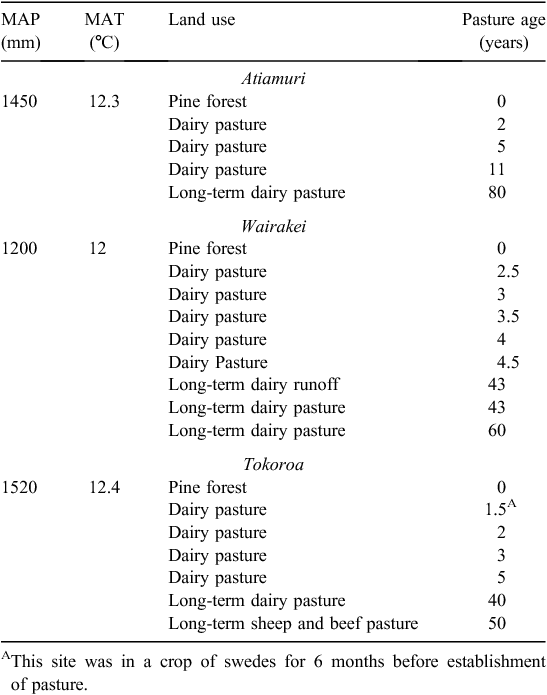
The three chronosequences were located in the Central North Island, near the settlements of Tokoroa, Atiamuri and Wairakei. All three chronosequences were on the Taupo Pumice soil (Immature Orthic Pumice Soil; Hewitt 1998), formed on the non-welded Taupo ignimbrite (of rhyolitic composition) deposited as a pyroclastic flow around AD 232 (Hogg et al. 2012). This soil is classified as a Typic Udivitrand according to USDA Soil Taxonomy (Soil Survey Staff 2010). The Atiamuri and Wairakei study areas were flat or very gently sloping, whereas at Tokoroa the topography was more varied, but all samples were taken from a remnant terrace landform that was virtually flat. The Tokoroa and Wairakei chronosequences were 60 km apart, with the Atiamuri chronosequence in-between. At Atiamuri all sampling sites were within 2 km of each other, at Tokoroa sites were within 3.5 km of each other, and at Wairakei sites were within ~10 km of each other (although most sites were within 5 km). Annual rainfall and temperature was similar at the three sites (Table 1).
|
At all three chronosequences, second- or third-rotation pine forest (Pinus radiata) had been converted to intensive pastoral dairy farms 2–11 years before sampling, which occurred in March–July 2010. Before land preparation for pasture, mature forests were harvested, while immature trees were simply pulled or pushed out with diggers or bulldozers. Pine-tree debris was deposited in windrows or slash heaps, most of which were still present at the time of sampling, although at Atiamuri and Wairakei, some had been removed (for fuel), burned or buried. Where windrows and slash heaps were no longer present, their location was determined from old aerial photographs and from talking to the respective farmers. These disturbed locations were also identifiable on the ground due to the abundance of woody debris and/or charcoal, and were avoided during sampling. Following removal of the pine trees at Tokoroa and Atiamuri, the soil was disc-cultivated, harrowed, rolled and then seeded. At Wairakei, heavy-duty mulchers were first used to break up remaining wood on or in the surface soil (excluding that deposited in windrows and slash heaps), and the land was then harrowed, rolled and seeded. At each of the three chronosequences, samples were also taken from remaining mature pine forests and long-term dairy pastures (40–80 years under pasture). A long-term sheep and beef pasture was also sampled at Tokoroa, and at Wairakei a dairy runoff (used to make silage, and to graze dairy cows during winter) was sampled (Table 1).
Management on all dairy farms was similar, with stocking rates of 2.5–2.9 cows ha–1 and milk-solids (milk fat + milk protein) production of 800–1100 kg ha–1 year–1. On the recently converted farms, N fertiliser inputs were ~200–300 kg ha–1 year–1 for the first 2 years, after which rates were 150–200 kg ha–1 year–1, which was similar to rates on the long-term dairy pastures. The long-term sheep and beef pasture at Tokoroa received ~40 kg N ha–1 year–1 and the dairy runoff at Wairakei 120 kg N ha–1 year–1. The recently converted sites received large inputs of lime (2–3.5 t ha–1), and superphosphate (~2 t ha–1) or diammonium phosphate (800 kg ha–1) during the first 2 years following pasture establishment, to raise soil pH and Olsen-P. Subsequently, all pastures received ~500 kg superphosphate (or other fertiliser with equivalent P and S) to maintain soil P and S, and other nutrients and trace elements (e.g. K, Co, Se) were applied as required. Pastures at all sampling sites were predominantly ryegrass (Lolium perenne) and white clover (Trifolium repens). Clover content in the recently converted pastures appeared to be higher than in the long-term pastures (although this was not specifically quantified).
At each of the sites identified in Table 1, three 50-m transects were laid out. For the Wairakei and Atiamuri pasture sites, the three transects were generally in separate paddocks, whereas at Tokoroa there was a limited number of suitable paddocks and therefore all three transects were usually within the same paddock. The three transects in the pine forest were distributed over a similar area to transects at the pasture sites (i.e. at all sites the three transects were within 200–300 m of each other). For each transect, 20 surface-soil cores (0–75 mm depth by 22 mm diameter) were taken at predetermined, random intervals and bulked into one sample. In addition, at seven of the points along each transect 25-mm-diameter cores were taken to a depth of 600 mm with the aid of a wooden maul. Each individual 600-mm core was split by horizon (with horizon depth recorded) and the seven cores were bulked by horizon to give one sample per horizon per transect. Random sampling intervals along the transects were used to avoid any potential for set sample spacing to coincide with pine tree rows. Pits were dug at 25 m along each transect, and three bulk-density samples were taken from each horizon (in each pit). Bulk-density cores were 60 mm in diameter and 50 mm deep.
Sample preparation and analysis
Soil samples were air-dried and sieved through a 2-mm sieve, and any wood or pumice that would not pass through the sieve was discarded. Subsamples were obtained by passing each sample through a riffle, which split the sample in half until ~10 g was obtained. Each subsample was then fine-ground with an agate mortar and pestle and analysed for total C and N using a FP 2000 TruSpec Micro analyser (LECO Corporation, St. Joseph, MI, USAi). All 0–75-mm samples were analysed for δ15N using a 20–20 Stable Isotope Analyser (Europa Scientific, Cambridge, UK) at the University of Waikato Stable Isotope Unit, Hamilton, New Zealand. Profile samples from one forest and one long-term pasture site at each of the three chronosequences were analysed for δ15N using an Isoprime mass spectrometer (Elementar Analysensysteme GmbH, Hanau, Germany) coupled to an EA3000 Elemental Analyser (EuroVector, Milan, Italy) modified with large-capacity carousel and combustion furnace at GNS Science, Lower Hutt, New Zealand. Profile samples were run on the Isoprime at GNS Science because this instrument provided enhanced sensitivity on samples with lower N contents in the subsoil horizons. The estimated repeatability of δ15N measurement was 0.1‰ for surface soil samples, and was inversely related to %N for subsoil samples, reaching ~0.2‰ at 0.1% N and ~0.3‰ for samples measured with the lowest N content. Ten samples were run in both laboratories, and this confirmed that results were comparable within 0.1 ‰.
Removal of pumice and wood fragments from soils (during sieving) meant that only the fine earth fraction (FEF) of soil was analysed for C, N and δ15N. Therefore, the bulk density of the FEF was required to express C and N on an area basis. Bulk density of the FEF was determined by sieving the whole bulk-density cores (after drying and weighing) through a 2-mm sieve, then re-drying the FEF and dividing the weight of soil in the FEF by the total volume of the bulk-density core. Measurements of bulk density were not specifically made for the 0–75 mm depth, and therefore, soil %C and %N data for this depth increment were converted to an area basis using measurements of bulk density from the A horizon.
Statistical analyses
Before statistical analysis, data from the three replicate transects at each site (pasture age) were averaged. This conservative approach was taken because samples from the three transects at the same site were essentially pseudo-replicates (particularly where transects were located in the same paddock). Transects in separate paddocks could have been treated as separate replicate samples because management would have differed between paddocks. However, it was decided not to take this approach, and instead standard errors are presented for each point in Fig. 2 to show the variability between transects at each site. Data were plotted against the number of years the sites had been in pasture, but due to the large spread and uneven distribution of pasture ages, the time data was log-transformed. The transformation log10 + 1 was used to account for the pine forest sites where the pasture age was zero. Data were also analysed with the long-term pasture sites excluded to better understand changes soon after the conversion process. In this case, data were not log-transformed. For full profile samples, the significance of differences between depths within the forests or pastures, and between the forests and pastures for each depth, were determined using analysis of variance in Genstat 13 (VSN International, Hemel Hempstead, UK).

|
Results
Surface soil samples (0–75 mm depth)
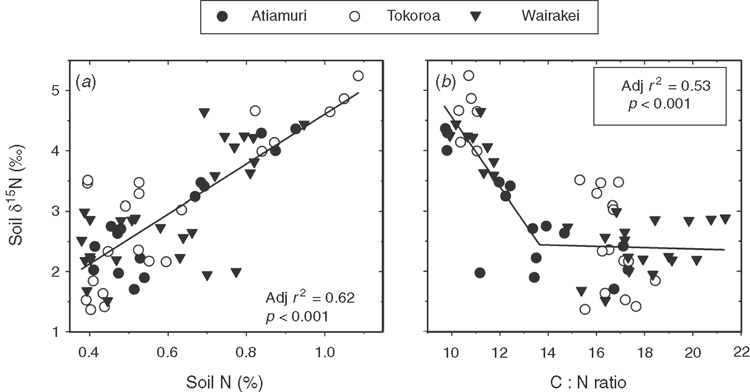
The clearest trend in the data was higher total N and δ15N, and lower C : N ratio in the surface soils at long-term pasture sites compared with pine forest or recently converted sites (Fig. 2). For example, average δ15N, %N and C : N ratio in the forest sites were 2‰, 0.47% and 17 respectively, compared with 4.3‰, 0.86% and 10.7 in the long-term pasture sites. For the three individual chronosequences, there was a significant positive correlation between log-transformed pasture age (log10[pasture age + 1]) and soil δ15N, total N and total C and a negative correlation with C : N ratio (Table 2). One exception was the Wairakei chronosequence, where there was no significant correlation between pasture age and soil C (Table 2). Values for soil C, N and δ15N were similar between the three chronosequences (Fig. 2), and regression analysis of all data combined showed a highly significant (P < 0.001) positive relationship between pasture age (log-transformed) and C, N and δ15N, and a negative relationship between pasture age and C : N (Table 2). The combined data were also analysed with the long-term pasture sites excluded, which revealed that there was still a significant positive correlation (P < 0.05) between pasture age (not log-transformed in this case) and C, N and δ15N (data not shown).
Based on the model fitted to data from the three chronosequences combined (Table 2), total N in the 0–75 mm depth increased by 165 kg ha–1 year–1 during the first 10 years following pasture establishment, with the rate slowing to 26 ha–1 year–1 between 10 and 50 years. Soil C showed a similar trend, increasing by 908 kg ha–1 year–1 during the first 10 years, with the rate slowing to 145 kg ha–1 year–1 between 10 and 50 years.
Plotting data from all individual transects revealed a significant (P < 0.001) positive correlation between soil δ15N values and soil %N (Fig. 3a). Soil δ15N and C : N ratio data could be fitted with the ‘broken stick’ model, with a significant negative correlation between δ15N and C : N when C : N was <13.6, but no significant relationship when C : N was >13.6 (Fig. 3b).
Full soil profile samples
For the full profiles in the pine forests, %N decreased and δ15N increased significantly from the A to the B horizon, but there were no significant changes between the B and C horizons (Fig. 4a, b). At the long-term pasture sites, both %N and δ15N were significantly higher in the A horizon than they were at the forest sites, and %N decreased with depth as at the forest sites. However, there was no significant change in δ15N with depth in the long-term pastures (Fig. 4a). At all three chronosequences, total mass of N to 600 mm depth was significantly higher in the long-term pastures, and average δ15N (weighted by mass of N in each horizon) was significantly higher in the pasture profiles than the forest profiles at two of three chronosequences. This suggests the higher total N and δ15N in the surface soils of the long-term pastures were not simply due to a difference in N or 15N distribution within the profile (i.e. Billings and Richter 2006; Högberg et al. 2011).
Discussion
Based on Stevenson et al. (2010), we hypothesised that surface soil δ15N would increase with time following conversion of forest to pasture. Data from the three chronosequences supported this hypothesis, with a clear increase in soil δ15N between the pine forests and the long-term pastures (Fig. 2a). These results help to confirm that the differences observed by Stevenson et al. (2010) were indeed driven by differences in land use, since soil and climatic conditions were the same (or very similar) between forest and pasture sites in the current study.
Varied results have been reported for soil δ15N in other land use change, chronosequence studies. In two (of the few) forest–pasture chronosequence studies where N isotopes were measured, Piccolo et al. (1994a, 1996) found either no difference, or lower surface soil δ15N in pasture sites compared with forest sites, in the Brazilian Amazon. At the sites where pasture soils had lower δ15N than forest soils, δ15N tended to be lowest in older pastures, which was opposite to what was observed in the current study (Fig. 2a). The lower δ15N in some pasture sites reported by Piccolo et al. (1994a, 1996) was suggested to be due to increased N inputs from N2 fixation (by free-living bacteria, since legumes were not sown), and N derived via fixation typically has δ15N values of ~0‰, which was much lower than the surface soils (~7–12‰). In addition, no fertiliser was applied to the pastures, and both N mineralisation and nitrification rates were higher in the forest soils (Piccolo et al. 1994a, 1994b, 1996), which suggests N cycling was tighter in the pastures, and isotope-fractionating N losses (e.g. denitrification) may have also been lower. We did not measure N mineralisation in the current study, but Stevenson et al. (2010) found that N mineralisation rates were twice as high in long-term pastures compared with native or exotic forests in New Zealand.
The increases in soil δ15N along the chronosequences in this study were similar to those reported when forests have been converted to cropland (Lemenih et al. 2005; Lemma and Olsson 2006; Awiti et al. 2008; Llorente et al. 2010). In those studies the increase in δ15N was mirrored by a decrease in total N (and C), which suggests that conversion led to increased N losses, and the N lost was depleted in 15N. During the conversion of agricultural land to forest, Compton et al. (2007) and Billings and Richter (2006) also found that soil δ15N increased with time, although this was largely attributed to re-distribution of N (depleted in 15N) from the mineral soil to above-ground plant biomass, facilitated by mycorrhizal fungi, which can cause strong isotopic fractionation (Hobbie and Ouimette 2009). By contrast, Boutton and Liao (2010) and Wang et al. (2007) found that soil δ15N decreased in chronosequences where agricultural land had been allowed to revert to forest or scrub. Boutton and Liao (2010) attributed the decrease in soil δ15N to an increase in soil N that was largely derived via N2 fixation, and hence had a delta value of ~0‰.
The increase in both δ15N and total N with pasture age in the three chronosequences in the current study differed compared with most other land-use-change chronosequence studies, where soil N and δ15N often had opposite trends (see above). The main difference between the chronosequences in the current study, and those in the previously mentioned studies, was the high N-loading rate to the pastures. Total N input to the forest sites would have been <10 kg ha–1 year–1, mainly from atmospheric deposition (Parfitt et al. 2006; Stevenson et al. 2010), while inputs to the pasture soils would have probably been >200 kg ha–1 year–1, via inputs from N fertiliser and N2 fixation associated with clover. Total N in the 0–75 mm depth increased significantly with time under pasture (55 kg N ha–1 year–1 during the first 50 years), indicating that a portion of the added–fixed N was immobilised in the soil. Fixed N and fertiliser N generally have δ15N values of ~0‰ (Högberg 1997; Bateman and Kelly 2007), and therefore N immobilisation should have led to a decrease in soil δ15N (Piccolo et al. 1996; Boutton and Liao 2010; Gubsch et al. 2011). The increase in soil δ15N despite N immobilisation in the soil therefore indicates that isotope-fractionating N-loss processes (e.g. volatilisation and nitrification followed by nitrate leaching or denitrification) must have occurred, leading to a loss of 15N-depleted N. It is well established in New Zealand pastoral systems that isotope-fractionating N-loss processes such as volatilisation and nitrification followed by nitrate leaching or denitrification increase as N inputs increase (Ledgard et al. 1999; Monaghan et al. 2005), and that N losses are much higher under pasture than forest (Di and Cameron 2002; Menneer et al. 2004; Stevenson et al. 2010).
A simple mass-balance calculation, based on a total N input of 200 kg ha–1 year–1 (with a δ15N value of 0‰) and a net N immobilisation rate of 55 kg ha–1 year–1 during 50 years of pasture development (calculated from the regression equation from Fig. 2b, Table 2), revealed that the net δ15N of N immobilised in the surface soil must have been ~5.7‰, and net δ15N of N lost –2.1‰. These values were similar to those calculated for immobilised and lost N in two long-term (~50-year) superphosphate and irrigation trials at Winchmore in Canterbury, where archived soils taken through time were analysed for δ15N (Mudge et al. 2013). The average rate of change in surface-soil δ15N between the pine forests and 50-year-old pastures was 0.05‰ (calculated from the regression equation in Table 2), which was also of a similar magnitude to the average rate of change (~0.03‰) reported in the more intensive treatments of the two Winchmore trials (Mudge et al. 2013).
Average soil δ15N (0–75 mm depth) under the pine forests and long-term dairy pastures was 2‰ and 4.1‰, respectively. These values were lower than the average reported by Stevenson et al. (2010) for 30 plantation-forest sites (2.8‰) and 50 dairy-farm sites (5.4‰) throughout New Zealand. Lower δ15N values in the current study under both pine forest and long-term dairy pasture may have been due to the pumice soil being young (~1800 years) relative to many other soils in New Zealand. Soil δ15N tends to increase with soil age (Vitousek et al. 1989; Martinelli et al. 1999; Brenner et al. 2001), and therefore, there would have been less time for 15N enrichment to occur in the pumice soils. In addition, the long-term pasture sites in the current study had probably been in pasture for a shorter time than many of the sites reported by Stevenson et al. (2010), because land clearance in the Central North Island would have occurred, on average, later than in many other parts of New Zealand. Some of the pine forests reported by Stevenson et al. (2010) were also planted on sites that had previously been under pasture, and therefore δ15N may have become elevated before establishment of the trees. As far as we can determine, none of the pine forest sites in the current study were previously under pasture. The similarity of δ15N from the long-term dairy pastures and the long-term sheep and beef pasture at Tokoroa and the dairy runoff at Wairakei was not consistent with Stevenson et al. (2010), where average δ15N was higher in dairy soils. However, this was probably because both of the dry-stock pastures were intensively managed, similar to the dairy pastures.
Changes in soil C and N, and correlations with δ15N
The increase in total C and N from the pine forests to the long-term pastures for the 0–75 mm samples (Fig. 2b, c) was consistent with results from the full profiles (Lewis et al. 2014). Average accumulation rates for C and N during the first 10 years following conversion were 908 kg C and 165 kg N ha–1 year–1, which were within the range of values reported from other similar studies in New Zealand (Walker et al. 1959; Jackman 1964a, 1964b; Hedley et al. 2009; Schipper and Sparling 2011). The decline in C : N ratio from forests or recently converted sites to long-term pastures was also consistent with previous studies (Sparling and Schipper 2004; Hedley et al. 2009). Relatively high rates of N accumulation were probably due to high N inputs to the pasture systems from N fixation and N fertiliser, which, coupled with the initially high C : N ratios of the forest soils, would have provided the ideal environment for net N immobilisation. However, caution is required when drawing conclusions about changes in total C and N stocks when only surface soils are sampled, because changes often occur throughout the profile (Schipper and Sparling 2011).
The positive correlation between δ15N and total N and the negative correlation with C : N (Fig. 3a, b) suggested that, as soils became more ‘saturated’ with N, isotope-fractionating N-loss processes increased. The lack of any clear relationship between δ15N and the C : N ratio when C : N was >13.6 (Fig. 3b) was probably because, at higher C : N ratios, isotope-fractionating N-loss processes would have been lower, due to high rates of N immobilisation into soil organic matter, and since fertiliser and fixed N generally have δ15N values of ~0‰, immobilisation would tend to balance out 15N enrichment due to isotope-fractionating N losses. In a broader survey of New Zealand soils, Stevenson et al. (2010) also found a significant negative correlation between δ15N and C : N, which suggests that the combination of total N, C : N ratio and δ15N could provide more information on the ‘N status’ or history of a system than if only one of these variables were measured. For example, if a soil had high total N, high δ15N and low C : N ratio, it would probably have been subject to high N inputs and losses for several years (i.e. long-term pastures in the current study). A soil with low total N and low C : N ratio, but with high δ15N, has likely lost organic N (i.e. soil from a long-term cultivated site; Stevenson et al. 2010). By contrast, if a soil had low N, low δ15N and high C : N ratio, it is likely from a natural ecosystem or an extensively managed pasture, with low N inputs and losses. These hypotheses need to be further explored.
Full soil profile samples
The significant difference in A-horizon δ15N between the forest and pasture sites, but no difference for the two subsoil horizons (Fig. 4a), was consistent with other studies (Piccolo et al. 1994a; Lemma and Olsson 2006; Llorente et al. 2010), and was presumably because processes affecting δ15N (e.g. volatilisation and nitrification) were higher in the surface horizon (Piccolo et al. 1994a).
Previous studies have shown that, in general, %N decreases and δ15N increases with soil depth in both forest and pasture ecosystems (Steele and Wilson 1981; Ledgard et al. 1984; Piccolo et al. 1996; Hobbie and Ouimette 2009). The increase in δ15N with depth is thought to occur because organic N at depth is older and more processed and, therefore, has had more opportunities for isotope-fractionating N losses, and also because plants (particularly those associated with ectomycorrhizal fungi) tend to take up N that is depleted in 15N relative to bulk soil N, and thus litter incorporated into the surface soil has low δ15N (Hobbie and Ouimette 2009). The trend of decreasing %N and increasing δ15N with depth in the soil profiles under the pine forests (Fig. 4a, b) was consistent with most other studies, but the lack of change in δ15N with depth in the long-term pastures was not. However, a few other studies have also found little difference between topsoil and subsoil δ15N in modified ecosystems, compared with more ‘natural’ systems where there was the typical increase in δ15N with depth. For example, Eshetu and Högberg (2000), and Lemma and Olsson (2006) found a greater increase in soil δ15N with depth under forests than under pastures or cropped land; in a forest ecosystem, Högberg et al. (1996) found that δ15N in plots fertilised with high rates of N decreased with depth (from the top of the litter layer to 50 mm depth in the mineral soil), whereas in control plots that did not receive N there was a typical increase in δ15N with depth (Table 3). By contrast, Piccolo et al. (1994a) found the opposite trend, with the surface soil of pastures being more depleted in 15N relative to subsoils than in forests (Table 3). This was probably because of immobilisation of fixed N (with low δ15N) in the surface soils of pastures.

|
These results suggest that differences between topsoil and subsoil δ15N may be a useful indicator of past land management. Undisturbed or extensively managed sites with low N inputs and losses will likely show the typical increase in δ15N with depth, whereas at more intensively managed sites, there may be no change with depth, or even decreases in δ15N. One advantage of using the difference between topsoil and subsoil δ15N is that it may be more applicable for comparing across soils that have different ‘baseline’ δ15N values. Subsoil δ15N in general appears less affected by land use than does surface-soil δ15N (except see Billings and Richter 2006), and therefore the difference between the topsoil and subsoil could reflect the impact of management irrespective of the soils initial δ15N value. An example of this can be seen in Table 3, where differences in mineral soil δ15N between studies varied by up to 9‰, but differences between surface soil and subsoil were relatively consistent, ranging from 0 to 2.5‰, depending on land use. Further research on multiple soil types under similar management will be required to test this hypothesis.
The much higher δ15N values (~7–13‰) reported by Eshetu and Högberg (2000), Lemma and Olsson (2006) and Piccolo et al. (1994a) than in the current study (~2–5‰) (Table 3) were presumably because the soils were older (thus had more time to become enriched) and were also from the tropics where N cycling tends to be more open, thus providing more opportunity for isotope-fractionating N losses (Martinelli et al. 1999).
Conclusion
The key hypothesis tested in this study was that soil δ15N would increase with time following clearance of forest or scrub and conversion to pasture. Results provided strong support for this hypothesis, with a clear increase in δ15N in surface (0–75 mm) soils between pine forests (2‰) and long-term pastures (4.1‰). There was also a significant increase in total C and N, and a decrease in the C : N ratio, in the surface soils. Analysis of full soil profiles under long-term pasture and remaining forest revealed a significant increase in δ15N between the A horizon and subsoils of the pine forests, but no significant differences between depth increments in the long-term pastures (presumably due to 15N enrichment of the A horizon). These profile results suggest that differences in δ15N between the surface soil and subsoil may be a useful indicator of past land management, with the added advantage of potentially being more applicable for comparing across soil types with different ‘baseline’ δ15N values. This hypothesis needs to be tested further.
Results from this study show that soil δ15N can provide useful information about soil N dynamics in addition to that derived from total N or the C : N ratio. For example, the increase in δ15N along the three chronosequences indicated that a large amount of N must have been lost via isotope-fractionating pathways (e.g. ammonia volatilisation), otherwise the net immobilisation of fixed or fertiliser N (with δ15N values of ~0‰) in the soil would have resulted in a decrease in soil δ15N.
Acknowledgements
We thank the many farm owners and managers for allowing access for sampling, and for providing information on farm history and management. Dr Megan Balks had input to study design, and assisted with soil classification and sampling. Djuro Paripovic also assisted with sampling. Operational funding; and stipend funding for P. Mudge was from the Foundation for Research Science and Technology via a subcontract with GNS Science (C05X0803). P. Mudge was also grateful to receive funding from the Wilf and Ruth Malcolm Postgraduate Scholarship, administered by the University of Waikato.
References
Awiti AO, Walsh MG, Kinyamario J (2008) Dynamics of topsoil carbon and nitrogen along a tropical forest-cropland chronosequence: Evidence from stable isotope analysis and spectroscopy. Agriculture, Ecosystems & Environment 127, 265–272.| Dynamics of topsoil carbon and nitrogen along a tropical forest-cropland chronosequence: Evidence from stable isotope analysis and spectroscopy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXns1SmsL8%3D&md5=7255b8492c2be0d07ecd05c805ec7e15CAS |
Baisden WT, Beets P, Davis M, Wilde H, Arnold G, Trotter C (2006) ‘Changes in New Zealand’s soil carbon stocks following afforestation of pastures.’ (Ministry for the Environment: Wellington, New Zealand)
Bateman AS, Kelly SD (2007) Fertilizer nitrogen isotope signatures. Isotopes in Environmental and Health Studies 43, 237–247.
| Fertilizer nitrogen isotope signatures.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXpvVeltrw%3D&md5=aef966a039026d0654db32a8338300c0CAS | 17786669PubMed |
Billings SA, Richter DD (2006) Changes in stable isotopic signatures of soil nitrogen and carbon during 40 years of forest development. Oecologia 148, 325–333.
| Changes in stable isotopic signatures of soil nitrogen and carbon during 40 years of forest development.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD28znvVGgtA%3D%3D&md5=a4154a66eba25678b081df50369806bfCAS | 16465541PubMed |
Boutton TW, Liao JD (2010) Changes in soil nitrogen storage and δ15N with woody plant encroachment in a subtropical savanna parkland landscape. Journal of Geophysical Research-Biogeosciences 115, G03019
| Changes in soil nitrogen storage and δ15N with woody plant encroachment in a subtropical savanna parkland landscape.Crossref | GoogleScholarGoogle Scholar |
Brenner DL, Amundson R, Baisden WT, Kendall C, Harden J (2001) Soil N and 15N variation with time in a California annual grassland ecosystem. Geochimica et Cosmochimica Acta 65, 4171–4186.
| Soil N and 15N variation with time in a California annual grassland ecosystem.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXot1Kitbg%3D&md5=4c6487cc55f984ba2bb0aceaa7b46172CAS |
Compton JE, Hooker TD, Perakis SS (2007) Ecosystem N distribution and δ15N during a century of forest regrowth after agricultural abandonment. Ecosystems 10, 1197–1208.
| Ecosystem N distribution and δ15N during a century of forest regrowth after agricultural abandonment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtlKrsrrP&md5=b351672437ef68010bf2025ed95b8fb2CAS |
Davis MR, Condron LM (2002) Impact of grassland afforestation on soil carbon in New Zealand: a review of paired-site studies. Australian Journal of Soil Research 40, 675–690.
| Impact of grassland afforestation on soil carbon in New Zealand: a review of paired-site studies.Crossref | GoogleScholarGoogle Scholar |
Di HJ, Cameron KC (2002) Nitrate leaching in temperate agroecosystems: sources, factors and mitigating strategies. Nutrient Cycling in Agroecosystems 64, 237–256.
| Nitrate leaching in temperate agroecosystems: sources, factors and mitigating strategies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xptl2quro%3D&md5=442b68cf1d52b7592f0ff1f7e96cb66eCAS |
Eshetu Z, Högberg P (2000) Effects of land use on 15N natural abundance of soils in Ethiopian highlands. Plant and Soil 222, 109–117.
| Effects of land use on 15N natural abundance of soils in Ethiopian highlands.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXmtleit74%3D&md5=b7e2f2200a9b14fdd6381957a0d0edf0CAS |
Frank DA, Evans RD, Tracy BF (2004) The role of ammonia volatilization in controlling the natural 15N abundance of a grazed grassland. Biogeochemistry 68, 169–178.
| The role of ammonia volatilization in controlling the natural 15N abundance of a grazed grassland.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjsV2nt7s%3D&md5=fd59b1588647b3d943288ade6a48f94eCAS |
Giddens KM, Parfitt RL, Percival HJ (1997) Comparison of some soil properties under Pinus radiata and improved pasture. New Zealand Journal of Agricultural Research 40, 409–416.
| Comparison of some soil properties under Pinus radiata and improved pasture.Crossref | GoogleScholarGoogle Scholar |
Gubsch M, Roscher C, Gleixner G, Habekost M, Lipowsky A, Schmid B, Schulze ED, Steinbeiss S, Buchmann N (2011) Foliar and soil δ15N values reveal increased nitrogen partitioning among species in diverse grassland communities. Plant, Cell & Environment 34, 895–908.
| Foliar and soil δ15N values reveal increased nitrogen partitioning among species in diverse grassland communities.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXos1SgtL0%3D&md5=070943678049e96f085c7ffd7eb3b46eCAS |
Hamilton D (2005) Land use impacts on nutrient export in the Central Volcanic Plateau, North Island. New Zealand Journal of Forestry 49, 27–31.
Hedley CB, Kusumo BH, Hedley MJ, Tuohy MP, Hawke M (2009) Soil C and N sequestration and fertility development under land recently converted from plantation forest to pastoral farming. New Zealand Journal of Agricultural Research 52, 443–453.
| Soil C and N sequestration and fertility development under land recently converted from plantation forest to pastoral farming.Crossref | GoogleScholarGoogle Scholar |
Hewitt AE (1998) ‘New Zealand Soil Classification.’ 2nd edn (Manaaki Whenua Press: Lincoln, New Zealand)
Hobbie EA, Ouimette AP (2009) Controls of nitrogen isotope patterns in soil profiles. Biogeochemistry 95, 355–371.
| Controls of nitrogen isotope patterns in soil profiles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtV2hs7%2FO&md5=dffc1997267768acfd142dffcec9d4e0CAS |
Högberg P (1997) Tansley review No. 95 – 15N natural abundance in soil–plant systems. New Phytologist 137, 179–203.
| Tansley review No. 95 – 15N natural abundance in soil–plant systems.Crossref | GoogleScholarGoogle Scholar |
Högberg P, Högbom L, Schinkel H, Högberg M, Johannisson C, Wallmark H (1996) 15N abundance of surface soils, roots and mycorrhizas in profiles of European forest soils. Oecologia 108, 207–214.
Högberg P, Johannisson C, Yarwood S, Callesen I, Nasholm T, Myrold DD, Högberg MN (2011) Recovery of ectomycorrhiza after ‘nitrogen saturation’ of a conifer forest. New Phytologist 189, 515–525.
| Recovery of ectomycorrhiza after ‘nitrogen saturation’ of a conifer forest.Crossref | GoogleScholarGoogle Scholar | 20880225PubMed |
Hogg A, Lowe DJ, Palmer J, Boswijk G, Ramsey CB (2012) Revised calendar date for the Taupo eruption derived by 14C wiggle-matching using a New Zealand kauri 14C calibration data set. The Holocene 22, 439–449.
| Revised calendar date for the Taupo eruption derived by 14C wiggle-matching using a New Zealand kauri 14C calibration data set.Crossref | GoogleScholarGoogle Scholar |
Houlton BZ, Bai E (2009) Imprint of denitrifying bacteria on the global terrestrial biosphere. Proceedings of the National Academy of Sciences of the United States of America 106, 21713–21716.
| Imprint of denitrifying bacteria on the global terrestrial biosphere.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXltlKitw%3D%3D&md5=b8c7c0b91af89827dee2f9a854566e8aCAS | 19995974PubMed |
Jackman RH (1964a) Accumulation of organic matter in some New Zealand soils under permanent pasture. I. Patterns of change of organic carbon, nitrogen, sulphur and phosphorus. New Zealand Journal of Agricultural Research 7, 445–471.
| Accumulation of organic matter in some New Zealand soils under permanent pasture. I. Patterns of change of organic carbon, nitrogen, sulphur and phosphorus.Crossref | GoogleScholarGoogle Scholar |
Jackman RH (1964b) Accumulation of organic matter in some New Zealand soils under permanent pasture. II. Rates of mineralisation of organic matter and the supply of available nutrients. New Zealand Journal of Agricultural Research 7, 472–479.
| Accumulation of organic matter in some New Zealand soils under permanent pasture. II. Rates of mineralisation of organic matter and the supply of available nutrients.Crossref | GoogleScholarGoogle Scholar |
Ledgard SF, Freney JR, Simpson JR (1984) Variations in natural enrichment of 15N in the profiles of some Australian pasture soils. Australian Journal of Soil Research 22, 155–164.
| Variations in natural enrichment of 15N in the profiles of some Australian pasture soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2cXksFeqt7k%3D&md5=097834e9947336d66d4e33b7c3e4309eCAS |
Ledgard SF, Penno JW, Sprosen MS (1999) Nitrogen inputs and losses from clover/grass pastures grazed by dairy cows, as affected by nitrogen fertilizer application. The Journal of Agricultural Science 132, 215–225.
| Nitrogen inputs and losses from clover/grass pastures grazed by dairy cows, as affected by nitrogen fertilizer application.Crossref | GoogleScholarGoogle Scholar |
Lemenih M, Karltun E, Olsson M (2005) Soil organic matter dynamics after deforestation along a farm field chronosequence in southern highlands of Ethiopia. Agriculture, Ecosystems & Environment 109, 9–19.
| Soil organic matter dynamics after deforestation along a farm field chronosequence in southern highlands of Ethiopia.Crossref | GoogleScholarGoogle Scholar |
Lemma B, Olsson M (2006) Soil δ15N and nutrients under exotic tree plantations in the southwestern Ethiopian highlands. Forest Ecology and Management 237, 127–134.
| Soil δ15N and nutrients under exotic tree plantations in the southwestern Ethiopian highlands.Crossref | GoogleScholarGoogle Scholar |
Lewis RW, Mudge PL, Schipper LA, Sparling GP, Balks M (2014) Changes in soil organic C and N contents at three study areas after conversion from plantation pine forest to dairy pasture on a New Zealand Pumice soil. Soil Research 52,
Llorente M, Glaser B, Turrion MB (2010) Anthropogenic disturbance of natural forest vegetation on calcareous soils alters soil organic matter composition and natural abundance of 13C and 15N in density fractions. European Journal of Forest Research 129, 1143–1153.
| Anthropogenic disturbance of natural forest vegetation on calcareous soils alters soil organic matter composition and natural abundance of 13C and 15N in density fractions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtlSjt7%2FK&md5=cda4e429acfaca634844ff5e6a770dafCAS |
MacLeod CJ, Moller H (2006) Intensification and diversification of New Zealand agriculture since 1960: An evaluation of current indicators of land use change. Agriculture, Ecosystems & Environment 115, 201–218.
| Intensification and diversification of New Zealand agriculture since 1960: An evaluation of current indicators of land use change.Crossref | GoogleScholarGoogle Scholar |
Martinelli LA, Piccolo MC, Townsend AR, Vitousek PM, Cuevas E, McDowell W, Robertson GP, Santos OC, Treseder K (1999) Nitrogen stable isotopic composition of leaves and soil: Tropical versus temperate forests. Biogeochemistry 46, 45–65.
| Nitrogen stable isotopic composition of leaves and soil: Tropical versus temperate forests.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXlslSmu7k%3D&md5=f8acc82e5af5fd933031681e42f68b5bCAS |
Menneer JC, Ledgard SF, Gillingham AG (2004) Land use impacts on nitrogen and phosphorus loss and management options for intervention. Client Report, Prepared for Environment Bay of Plenty Regional Council, Whakatane, New Zealand. Available at: www.boprc.govt.nz/media/34344/TechReports-040601-LandUseImpactsNandPloss.pdf
MFE (2009) The New Zealand Land Cover Database. Ministry for the Environment, Wellington, New Zealand. Available at: www.mfe.govt.nz/issues/land/land-cover-dbase/
Monaghan RM, Paton RJ, Smith LC, Drewry JJ, Littlejohn RP (2005) The impacts of nitrogen fertilisation and increased stocking rate on pasture yield, soil physical condition and nutrient losses in drainage from a cattle-grazed pasture. New Zealand Journal of Agricultural Research 48, 227–240.
| The impacts of nitrogen fertilisation and increased stocking rate on pasture yield, soil physical condition and nutrient losses in drainage from a cattle-grazed pasture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXosVerur4%3D&md5=f9ddec2243906f97d4b9928271b1175aCAS |
Mudge PL, Schipper LA, Ghani A, Upsdell M, Baisden WT (2013) Changes in natural 15N abundance in pastoral soils receiving differing amounts of superphosphate fertilizer and irrigation for 50 years. Soil Science Society of America Journal 77, 830–841.
| Changes in natural 15N abundance in pastoral soils receiving differing amounts of superphosphate fertilizer and irrigation for 50 years.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXnsl2htbs%3D&md5=73ae30808210eb9c72455bb5326cfd19CAS |
Parfitt RL, Schipper LA, Baisden WT, Elliott AH (2006) Nitrogen inputs and outputs for New Zealand in 2001 at national and regional scales. Biogeochemistry 80, 71–88.
| Nitrogen inputs and outputs for New Zealand in 2001 at national and regional scales.Crossref | GoogleScholarGoogle Scholar |
Piccolo MC, Neill C, Cerri CC (1994a) Natural-abundance of 15N in soils along forest-to-pasture chronosequences in the Western Brazilian Amazon Basin. Oecologia 99, 112–117.
| Natural-abundance of 15N in soils along forest-to-pasture chronosequences in the Western Brazilian Amazon Basin.Crossref | GoogleScholarGoogle Scholar |
Piccolo MC, Neill C, Cerri CC (1994b) Net nitrogen mineralization and net nitrification along a tropical forest-to-pasture chronosequence. Plant and Soil 162, 61–70.
| Net nitrogen mineralization and net nitrification along a tropical forest-to-pasture chronosequence.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXlslylsr8%3D&md5=2ea2cccb69299f5d3d0ff9bfc9d20d04CAS |
Piccolo MC, Neill C, Melillo JM, Cerri CC, Steudler PA (1996) 15N natural abundance in forest and pasture soils of the Brazilian Amazon Basin. Plant and Soil 182, 249–258.
Schipper LA, Sparling GP (2011) Accumulation of soil organic C and change in C:N ratio after establishment of pastures on reverted scrubland in New Zealand. Biogeochemistry 104, 49–58.
| Accumulation of soil organic C and change in C:N ratio after establishment of pastures on reverted scrubland in New Zealand.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXmslehsro%3D&md5=c71fa3766b1220777a99e24ff1ac9fa4CAS |
Schipper LA, Percival HJ, Sparling GP (2004) An approach for estimating when soils will reach maximum nitrogen storage. Soil Use and Management 20, 281–286.
| An approach for estimating when soils will reach maximum nitrogen storage.Crossref | GoogleScholarGoogle Scholar |
Soil Survey Staff (2010) ‘Keys to Soil Taxonomy.’ 11th edn (USDA-Natural Resources Conservation Service: Washington, DC)
Sparling G, Schipper L (2004) Soil quality monitoring in New Zealand: trends and issues arising from a broad-scale survey. Agriculture, Ecosystems & Environment 104, 545–552.
| Soil quality monitoring in New Zealand: trends and issues arising from a broad-scale survey.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhtVakt7rM&md5=ffbd1a4f56906ae9fbf56854ebbc80e4CAS |
Steele KW, Wilson AT (1981) Nitrogen isotope ratios in surface and sub-surface horizons of New Zealand improved grassland soils. New Zealand Journal of Agricultural Research 24, 167–170.
| Nitrogen isotope ratios in surface and sub-surface horizons of New Zealand improved grassland soils.Crossref | GoogleScholarGoogle Scholar |
Stevenson BA, Parfitt RL, Schipper LA, Baisden WT, Mudge P (2010) Relationship between soil δ15N, C/N and N losses across land uses in New Zealand. Agriculture, Ecosystems & Environment 139, 736–741.
| Relationship between soil δ15N, C/N and N losses across land uses in New Zealand.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjs1ehsg%3D%3D&md5=e4cec1fac4813dd37119f20cda450d66CAS |
Tait A, Henderson R, Turner R, Zheng XG (2006) Thin plate smoothing spline interpolation of daily rainfall for New Zealand using a climatological rainfall surface. International Journal of Climatology 26, 2097–2115.
| Thin plate smoothing spline interpolation of daily rainfall for New Zealand using a climatological rainfall surface.Crossref | GoogleScholarGoogle Scholar |
Tate K, Wilde RH, Giltrap DJ, Baisden WT, Saggar S, Trustrum NA, Scott NA, Barton JP (2005) Soil organic carbon stocks and flows in New Zealand: System development, measurement and modelling. Canadian Journal of Soil Science 85, 481–489.
| Soil organic carbon stocks and flows in New Zealand: System development, measurement and modelling.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xjs1GgsQ%3D%3D&md5=93925b0ff2ecd5c7487473fbbea6e74fCAS |
Vitousek PM, Shearer G, Kohl DH (1989) Foliar 15N natural abundance in Hawaiian rainforest: patterns and possible mechanisms. Oecologia 78, 383–388.
| Foliar 15N natural abundance in Hawaiian rainforest: patterns and possible mechanisms.Crossref | GoogleScholarGoogle Scholar |
Walker TW, Thapa BK, Adams AFR (1959) Studies on soil organic matter: 3. Accumulation of carbon, nitrogen, sulphur, organic and total phosphorus in improved grassland soils. Soil Science 87, 135–140.
| Studies on soil organic matter: 3. Accumulation of carbon, nitrogen, sulphur, organic and total phosphorus in improved grassland soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF3MXktlGj&md5=a0ed9cd36424fdcb8f7d3130d21e236cCAS |
Walker LR, Wardle DA, Bardgett RD, Clarkson BD (2010) The use of chronosequences in studies of ecological succession and soil development. Journal of Ecology 98, 725–736.
| The use of chronosequences in studies of ecological succession and soil development.Crossref | GoogleScholarGoogle Scholar |
Wang LX, Shaner PJL, Macko S (2007) Foliar δ15N patterns along successional gradients at plant community and species levels. Geophysical Research Letters 34, L16403
| Foliar δ15N patterns along successional gradients at plant community and species levels.Crossref | GoogleScholarGoogle Scholar |