Comparison of the penetration of primary and lateral roots of pea and different tree seedlings growing in hard soils
Gausul Azam A C , Cameron D. Grant A , Robert S. Murray A , Ian K. Nuberg A and Rabindra K. Misra BA Waite Research Institute, School of Agriculture, Food and Wine, University of Adelaide, PMB 1 Glen Osmond, SA 5064, Australia.
B Faculty of Engineering and Surveying, University of Southern Queensland, Toowoomba, Qld 4350, Australia.
C Corresponding author. Email: gausu.azam@uwa.edu.au
Soil Research 52(1) 87-96 https://doi.org/10.1071/SR13201
Submitted: 9 July 2013 Accepted: 20 September 2013 Published: 5 February 2014
Abstract
Establishment and survival of tree seedlings in hard soils depends on production of deep root systems. This study evaluated the primary and lateral roots of an annual crop and several tree species growing in soils of varying strength. We grew peas and acacias by direct seeding, plus three eucalypts by direct seeding and transplanting, and measured various root characteristics. At all levels of soil compaction, the primary roots of acacia were thicker and they elongated faster than did those of the eucalypts. However, lateral roots of transplanted eucalypts elongated faster than their primary roots, and the rate of root elongation was negatively correlated with soil penetration resistance, especially for Eucalyptus camaldulensis. The primary root diameter of all plants increased with increasing penetration resistance, but acacia roots continued to elongate faster than pea roots. Pea plants produced most of their roots in the top 5 cm, whereas tree roots were more uniformly distributed with depth. Although not statistically significant at P = 0.05, the relative rate of root elongation in very hard soil correlated modestly (P = 0.11) with the maximum root growth pressure of four tree species. These variations in root growth behaviour can be related to the intrinsic variability of root characteristics for each plant species and the natural abundance of each species in different environments.
Additional keywords: Acacia, compaction, direct seeding, Eucalyptus, Pisum, root penetration, transplanting.
Introduction
Revegetation of agricultural landscapes with trees has attracted much attention over the last three decades (e.g. Simpfendorfer 1975; Hillis and Brown 1984; Stirzaker et al. 2002; Donohue et al. 2009). Plant establishment, however, remains highly variable, especially in lower rainfall regions where seasonally dry environments often coincide with hard soils (Azam et al. 2012). High soil strength (i.e. >2 MPa penetration resistance) is one of the most limiting factors for tree establishment (Kozlowski 1999). In these situations, plant roots (especially the primary axes) must be able to grow rapidly and take advantage of water and nutrients before the topsoil becomes dry and impenetrable (Lloret et al. 1999). Therefore, trees that can ramify and explore the subsoil quickly during the first growing season may have an ecological advantage over those that grow more slowly (Grime 1977).
The ability of roots to penetrate hard soils varies among tree species (Kozlowski 1999; Alameda and Villar 2009). Azam et al. (2012, 2013a) argued that the natural spatial distribution of trees growing in low-rainfall zones could be related to genetic variations in the important root characteristics shown in Table 1. Plants that can exert large axial root growth pressures, for example, can elongate their roots faster without significant root thickening (Bengough 2012).

|
The question arises as to whether variations in root growth characteristics can be used to identify trees that are more robust for planting on hard soils in seasonally dry regions. Other factors, of course, also need to be taken into account in any such analysis. For example, seed size is known to correlate positively with root growth rate (Lloret et al. 1999), but it is still a matter of supposition that larger seeds and growth rates might coincide with greater penetration of hard soils. Planting method is also important here in controlling which roots (primary or lateral) are exposed to the soil first. When trees are direct-seeded, for example, the primary roots must do the initial soil exploration, whereas transplanted seedlings use their lateral roots for exploration. Although we know that both primary and lateral roots respond to soil compaction (Misra and Gibbons1996; Zou et al. 2001), we do not understand the extent to which the predominance of one or other type of root might confer an advantage in hard soils. A better understanding of the effects of seed size, plant species, and planting method on early plant establishment would increase the probability of successful tree establishment on hard soils exposed to seasonally dry conditions.
The present paper follows work conducted by Azam et al. (2013a), which found significant variation in root growth rates and maximum axial root growth pressures (σmax) exerted by young roots of several different tree species. The extent to which this variation correlates with the performance of these species in terms of root growth in hard soils is the subject of the current paper. We compacted soil to varying extents and grew four tree species (one Acacia and three Eucalyptus species) and an annual reference crop (Pisum sativum) in experiments designed to account for the effects of variation in seed size, root growth pressure, and planting method on root diameter, total root length, root elongation rate, and root distribution with depth.
Materials and methods
Soil characterisation
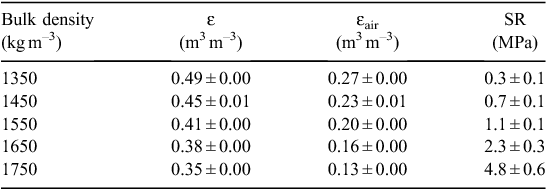
A non-saline (electrical conductivity (EC) of saturated paste extract, 0.49 ± 0.05 dS m–1), slightly alkaline (pH of saturated paste extract, 7.6 ± 0.2), loamy sand (87% 200–20 μm, 7% 20–2 μm, 6% <2μm) was collected from Monarto, South Australia (35°5′S, 139°4′E), air-dried, and sieved through a 2-mm mesh. It was a topsoil (5–20 cm depth) and contained 1.30% organic carbon, 53 mg kg–1 of total nitrogen (N), and 230 mg kg–1 of phosphorus (P). A simple compaction test (Proctor 1933) was conducted to determine the optimum gravimetric water content (θ) for efficient compaction (found to be 0.14 kg kg–1). To ensure that the volumetric air content of the most densely packed soil (i.e. 1750 kg m–3) did not impose poor soil aeration (Wesseling and van Wijk 1957), the soil was wetted at a slightly lower water content of θ = 0.12 kg kg–1 before packing; this ensured that the volumetric air content (εair) of the soil was always >0.10 m3 m–3 (Table 2).
|
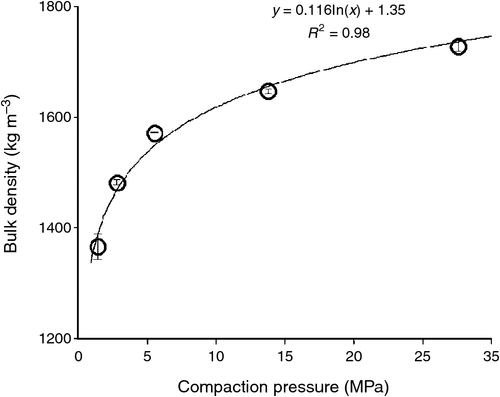
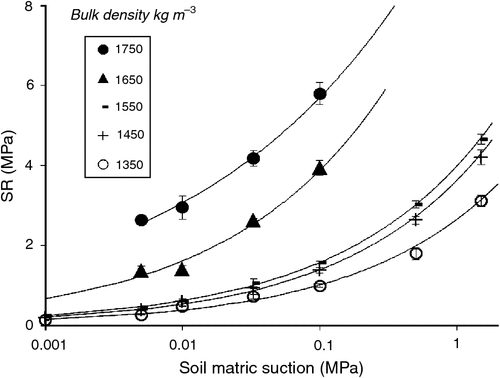
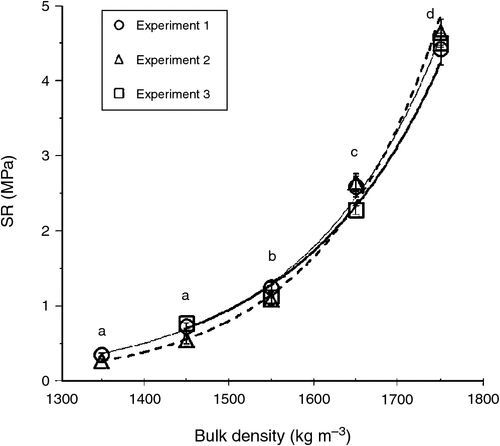
Soil volumetric water retention curves were determined at five different bulk densities (1350, 1450, 1550, 1650, and 1750 kg m–3) using three replicated soil samples. Soil was moistened and packed incrementally in stainless steel cylindrical rings (70 mm internal diameter, 50 mm height) to the required bulk densities using five hydraulically controlled piston pressures (Fig. 1). The packed soil samples were then saturated and placed on porous ceramic plates connected to hanging columns of water (0.001, 0.0033, and 0.01 MPa) for 48 h, then sealed in pressure chambers (models 1500F1 and 1600; Soil Moisture Equipment Co., Santa Barbara, CA, USA) set at 0.033 MPa for 2 weeks then 0.1 and 0.5 MPa for 3 weeks, and finally 1.5 MPa for 5 weeks. When samples came to equilibrium, which was checked by repeated weighing until constant weight at the given pressure, the final weight was recorded. The resistance to a penetrometer (SR) was then measured using an automated cone penetrometer having a recessed shaft and cone-base diameter of 2.38 mm with tip semi-angle 30° (model Lloyd LFPlus 1 kN; CSC Force Measurement Inc., Agawam, MA, USA); the penetrometer advanced at a constant speed of 3 mm min−1. The force required to push the cone into the hardest soils exceeded the capacity of the Lloyd instrument (5.5 MPa), so it was not possible to measure SR for the two driest conditions and greatest bulk densities. For the SR profile in each soil sample, the penetration force was averaged over 25–45 mm depth then divided by the cross-sectional area of the penetrometer cone to obtain a mean SR (MPa) at each matric suction and bulk density (Fig. 2). The five curves in Fig. 2 were used to select appropriate packing densities and watering regimes for the plant growth experiments. The mean saturated hydraulic conductivity (Ks) was measured on the soil samples and found to range from 2 mm h–1 (most compacted soil) up to 53 mm h–1 (least compacted soil), which indicated that hydraulic restrictions were not a problem even in the most compacted soil.
|
|
Preparation of PVC containers for plants
Moist soil (θ = 0.12 kg kg–1) was packed into rigid polyvinyl chloride (PVC) pots (220 mm height, 70 mm diameter) with transparent plexiglass bases, which enabled us to observe the average time taken for roots to approach the bottom of each pot (Fig. 3a, b). Soil was packed to the five bulk densities above by adding small increments of moist soil under the appropriate hydraulic compaction pressure. Soil was added until its height was 20 mm from the top of the pots (i.e. soil sample height, 200 mm) as shown in Fig. 3a, b. All pots were fertilised with 5 mL of a nutrient solution containing 5.0 mm N, 0.29 mm P, and 1.2 mm, potassium (K) plus essential micronutrients, according to Ingestad and Lund (1986). Additional water was added to each pot so that its volumetric water content was never greater than 0.22 m3 m–3, which ensured sufficient aeration at all times (Table 2). The pots were then sealed and stored for 1 week in a dark, constant-temperature chamber to allow hydraulic equilibrium and age-hardening to occur (Utomo and Dexter 1981).

|
Planting and maintenance of seedlings
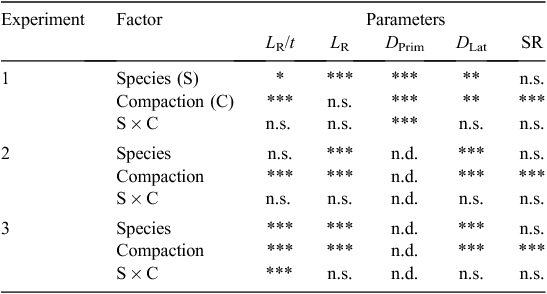
The experiments were conducted in a controlled temperature glasshouse having day and night temperatures of 25 ± 2°C and 18 ± 2°C, respectively. Five plant species—Pisum sativum L., Acacia salicina Lindl., Eucalyptus camaldulensis Dehnh., E. leucoxylon F.Muell., and E. kochii Maiden & Blakely—were selected from Azam et al. (2013a) for use in three different experiments (Table 3). Direct-seeding experiments, i.e. Expts 1 and 2, had five compaction treatments. The transplanting experiment, i.e. Expt 3, had four compaction treatments (there was no significant difference between soil penetration resistance of the two lowest compaction treatments, so the lowest compaction treatment was eliminated). The reason for growing Eucalyptus species using two planting methods was that the fine roots of Eucalyptus species were too small to get reliable measurements of root growth pressure (Azam et al. 2013), so the lateral (larger) roots of transplanted seedlings had to be used instead.
In Expt 1, the two large-seeded species, i.e. P. sativum and A. salicina, were planted from pre-germinated seeds (direct-seeded) and observations were collected on all (i.e. primary and lateral) roots (Table 3). Similarly, in Expt 2, three small-seeded Eucalyptus species (E. camaldulensis, E. leucoxylon, and E. kochii) were planted from pre-germinated seeds (direct-seeded), and observations were collected on their primary and lateral roots (Table 3). In Expt 3, the same three small-seeded Eucalyptus species were transplanted using 90-day-old seedlings grown from the same seed lot, and data were collected on their newly regenerated lateral roots only (i.e. roots from the early-growth root plug were excluded; Table 3).
For the direct-seeded experiments, three pre-germinated seeds were planted in a circle in each of the three replicated pots. For the transplanting experiment, only one seedling was transplanted into the centre of each pot (three replicates) by packing identical soil around the root plug (50 mm long) at the appropriate bulk density (Fig. 3b). After the seeds or seedlings were planted, 20 mm of polyethylene beads were poured onto the top of each pot (Fig. 3a, b), which reduced evaporation by 82 ± 2%. Plants were watered to weight daily at sunset, and if hot weather was forecast, an additional watering was made at 09 : 00 that day. The large-seeded, direct-seeded species and the transplanted species were harvested 14 days after planting or transplanting, whereas the small-seeded, direct-seeded species grew much more slowly and had to be harvested later, 35 days after planting.
Measurement of penetration resistance and root growth
At multiple intervals each day, the transparent base of each pot was observed to determine whether the roots had reached the bottom of the pot. When roots appeared at the bottom, the time was noted and the root elongation rate was estimated from the length of the soil sample (200 mm) divided by the total time taken for the first roots to appear at the bottom of the pot, LR/t. The length of the soil sample used in the transplanting experiments was only 150 mm because the newly grown lateral roots were regenerated from the bottom of the (50-mm-long) root plug (see Fig. 3b). In the two treatments that were most compacted, no roots reached the bottom of the pots; for these treatments, roots were simply washed from the soil using a 0.5-mm sieve and the length of their main axes was measured directly with a ruler (±0.5 mm accuracy). The root elongation rate, LR/t, for these pots was calculated by dividing the length of the main root axis by the duration of experiment.
At the nominated harvest time, shoots were clipped at their bases and then soil penetration resistance was measured to a depth of 50 mm on each of the three replicated, undisturbed pots. For the direct-seeding experiments, the soil sample was divided into four equal horizontal layers (5 cm thick) to quantify the vertical distribution of roots, LRDepth. Roots were separated from the soil in each of the three experiments using a gentle jet of water over a 0.5-mm sieve. For Expt 1, the primary root (of the large-seeded species) was scanned separately from its lateral roots to digitally measure its mean diameter, DR, and total length, LR, using a high-resolution scanner (600 dpi) combined with WinRhizo image analysis software (v. 2005c; Régent Instruments Inc., Quebec, Canada). For Expt 2, the primary and lateral roots were sufficiently similar in size that they could not be separated from each other, so they were scanned together to measure the mean diameter and length of all roots. For Expt 3, all root measurements were taken from the whole, non-subdivided soil sample.
Statistical analyses
Factorial designs were used for all experiments (see Table 3). Data were first subjected to a normality test of the variables to verify the requirement for analyses of variance (ANOVA). Data on SR, DR, LR, and LR/t, were all found to be normally distributed and were thus subjected to a two-way ANOVA with the factors plant species and compaction levels and their interactions at a significance level of P = 0.05 using Genstat for Windows (Edn 14, VSN International, Hemel Hempstead, UK). The distribution of root lengths in the four layers for Expts 1 and 2 was skewed towards much greater root lengths in the top layer, so the data for each layer were log-transformed using ln(1 + LRDepth) to achieve a normal distribution. This enabled the data to be subjected to a three-way ANOVA with the factors plant species, soil compaction, and soil depth, and their interactions, at a significance level of P = 0.05 using Genstat. Mean LRDepth values for each depth were back-transformed using procedures outlined in Misra et al. (1998).
Results
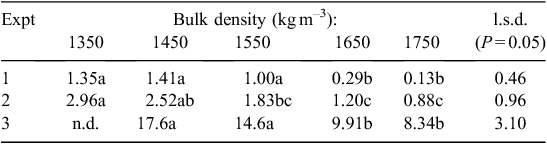
Table 4 summarises the effects of plant species, soil compaction, and species × soil compaction interaction on various root growth parameters in the three experiments. Obviously, soil resistance increased significantly (P = 0.05) with increasing bulk density (Fig. 4), although there was no significant difference in SR for the two lowest bulk densities; therefore, the lowest density (1350 kg m–3) was eliminated in Expt 3.
|
|
Rate of root elongation
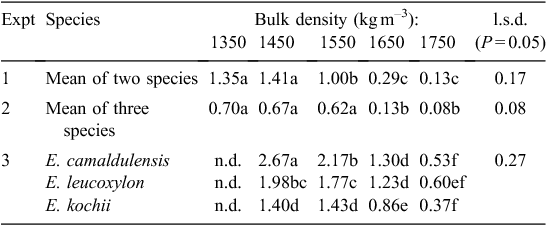
For the two large-seeded species (Expt 1), the rate of root elongation, LR/t, depended significantly on plant species at all levels of compaction (Table 4). The mean values of LR/t for the two large-seeded species decreased significantly as bulk density increased (P = 0.05; Table 5). Overall, roots of A salicina elongated significantly faster (0.89 ± 0.11 cm day–1) than those of P. sativum (0.78 ± 0.11 cm day–1). Among the direct-seeded Eucalyptus species (Expt 2), the mean elongation rates of primary roots were similar for all species (Table 4) and declined significantly with increasing compaction (P = 0.05; Table 5). Among the transplanted Eucalyptus species (Expt 3), the mean elongation rate of lateral roots also declined significantly with increasing compaction (P = 0.05), but the effects were more pronounced in some species (as indicated by a significant species × soil compaction interaction; Table 4). For example, LR/t declined significantly with each incremental increase in bulk density for the species in the order E. camaldulensis > E. leucoxylon > E. kochii. At the highest bulk density of ≥1650 kg m–3, there was no significant difference in LR/t between these species (P = 0.05; Table 5).
|
|
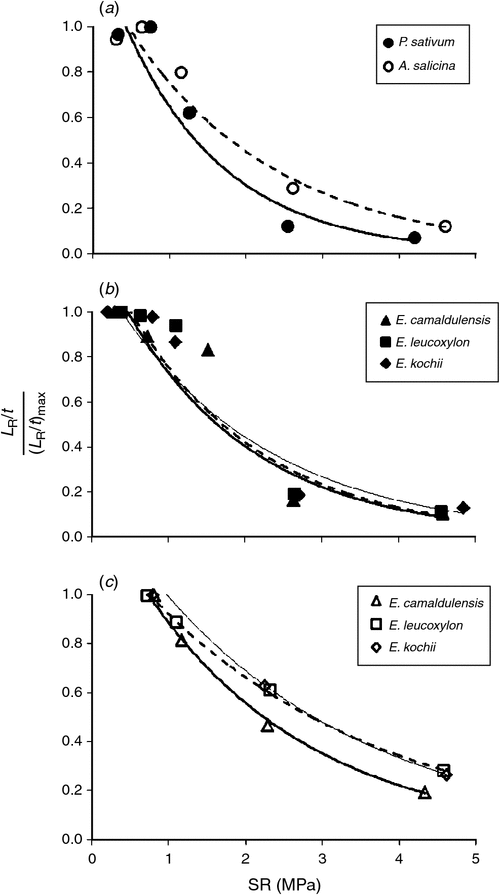
In all three experiments, the rate of root elongation relative to its maximum rate, (LR/t)/(LR/t)max (called R/Rmax by Dexter 1987), declined exponentially with increasing soil penetration resistance (SR). The effect of compaction on the different species is shown in Fig. 5a–c and can be quantified using the relation of Misra and Gibbons (1996):

where α is a freely adjustable fitting parameter (MPa–1). Equation 1 can be equated to the relation of Dexter (1987) to describe the effects of compaction on root growth relative to a critical point, SR0.5:

where SR0.5 is the soil penetration resistance at which the relative root elongation rate is halved, (LR/t)/(LR/t)max = 0.5, and where –0.6931 = ln(0.5).
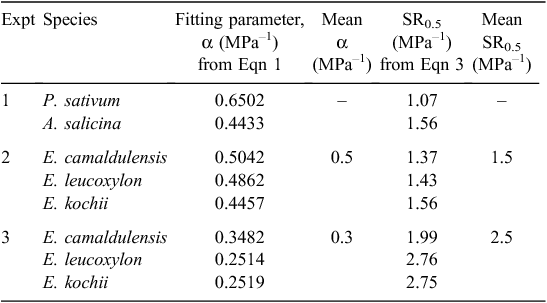
By equating and rearranging Eqns 1 and 2, we can obtain values of SR0.5 for each species, and these are shown in Table 6:
|

Total length of roots
In Expt 1, the total length of roots, LR, was significantly affected by plant species, and LR depended on neither soil compaction nor the interaction between plant species and soil compaction (Table 4). At all levels of compaction, LR was significantly greater for P. sativum (1.28 ± 0.15 m) than for A. salicina (0.33 ± 0.06 m).
For the direct-seeded and transplanted Eucalyptus seedlings (i.e. Expts 2 and 3), there were significant compaction and plant species effects but no interactions at P = 0.05 (Table 4). For example, in Expt 2, E. camaldulensis seedlings produced significantly (P = 0.05) greater mean total root length (3.04 ± 0.47 m) than did E. leucoxylon (1.44 ± 0.23 m) and E. kochii (1.15 ± 0.18 m). Similarly, in Expt 3, E. camaldulensis seedlings produced significantly (P = 0.05) greater mean LR (16.42 ± 2.01 m) than seedlings of E. leucoxylon (10.56 ± 0.87 m) and E. kochii (10.83 ± 0.74 m).
Mean LR decreased significantly for all species with increasing compaction (P = 0.05) (Table 7), but the level of compaction at which LR declined varied among species. For example, significant reductions in LR occurred for E. camaldulensis when bulk density increased to 1550 kg m–3, whereas significant reductions (P = 0.05) occurred for E. leucoxylon and E. kochii only when bulk density exceeded 1650 kg m–3. In Expt 3, differences among species were not statistically significant once bulk density exceeded 1550 kg m–3.
|
Root diameter
In Expt 1 (involving Pisum and Acacia), the diameter of the primary roots, DPrim, increased significantly (P = 0.05) with compaction, and differed according to plant species; the significant interaction term, species × compaction, in Table 4 suggests that the magnitude of the compaction effect depended on plant species. Pisum sativum produced significantly thicker (P = 0.05) primary roots (1.72 ± 0.11 mm) than A. salicina (1.09 ± 0.06 mm).
In all three experiments, the diameter of the lateral roots, DLat, increased significantly (P = 0.05) with compaction, and this differed for each plant species, but there were no interaction effects (i.e. none of the species × compaction interaction terms for DLat in Table 4 was significant at P = 0.05). For example, P. sativum produced significantly thicker (P = 0.05) lateral roots (0.84 ± 0.03 mm) than A. salicina (0.72 ± 0.03 mm) in Expt 1. Direct-seeded Eucalyptus camaldulensis produced significantly thicker (P = 0.05) roots (0.50 ± 0.02 mm) than both E. leucoxylon (0.41 ± 0.02 mm) and E. kochii (0.45 ± 0.03 mm) in Expt 2. Finally, the transplanted E. camaldulensis (0.62 ± 0.02 mm) and E. leucoxylon (0.65 ± 0.02 mm) produced significantly thicker (P = 0.05) lateral roots than E. kochii (0.51 ± 0.02 mm) in Expt 3.
Distribution of roots with depth (direct seeded only)
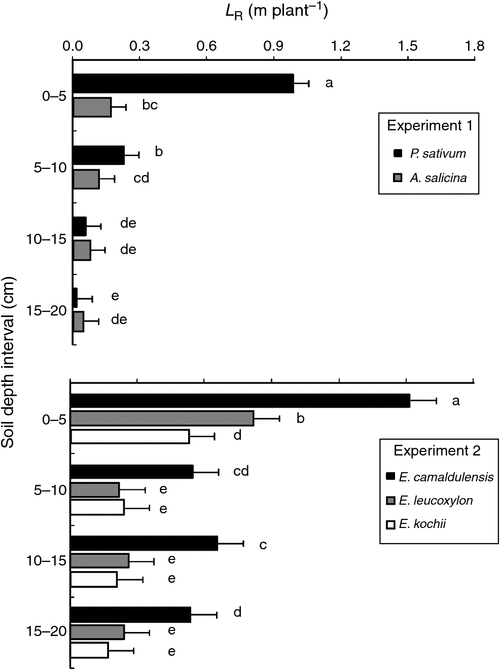
The statistical significance of the variables and their interactions on root distribution with depth is summarised in Table 8. In both Expts 1 and 2, there were significant interactions (P ≤ 0.05) of species × soil depth, which influenced the distribution of roots with depth. Figure 6 shows that most of the roots for all species grew in the top few centimetres of soil (0–5 cm layer); this was especially so for the more compacted soils. In particular, the 14-day-old P. sativum plants (Expt 1) and the 35-day-old seedlings of E. camaldulensis (Expt 2) had a greater proportion of their roots in the top 5 cm than did other species, whose roots were more uniformly distributed with depth (Fig. 6). In Expt 2, however, E. camaldulensis produced significantly greater LR than the other Eucalyptus species. At the relatively high bulk density of 1650 kg m–3, A. salicina roots grew into the 5–10 cm layer within only 2 weeks (Expt 1), whereas none of the Eucalyptus species used in Expt 2 produced any roots in that soil depth at the same level of compaction, even after 5 weeks following germination (data not presented). Furthermore, there were no significant differences in the distribution of roots among the three direct-seeded Eucalyptus species (Fig. 6).
|
|
Discussion
Root elongation rate
The magnitudes of α and SR0.5 shown in Table 6 suggest that the relative growth rate of P. sativum roots is more sensitive to compaction than is that of the trees, although there are insufficient data at present to assign statistical significance. Table 6 shows that (LR/t)/(LR/t)max was halved for P. sativum by soil compaction in the order of only 1 MPa (SR0.5 = 1.07 MPa), whereas it took >1.5 MPa to halve the relative growth rate of A. salicina roots (SR0.5 = 1.56 MPa) and 2–2.8 MPa to halve the relative rates for the lateral roots of the transplanted trees.
Among the three direct-seeded Eucalyptus species (Expt 2) there were no significant differences in (LR/t)/(LR/t)max (Fig. 5b); their α values averaged 0.5 MPa–1 and their SR0.5 values averaged 1.5 MPa, which was similar to A. salicina and greater than P. sativum (Table 6). For the transplanted Eucalyptus species (Expt 3), the values of (LR/t)/(LR/t)max responded similarly to those for direct-seeded Eucalyptus species except that the lateral roots involved here (being larger) showed slightly less sensitivity to compaction than the (primary) roots of the direct-seeded trees (Fig. 5c); their α values were slightly smaller (mean α = 0.3 MPa–1) and their SR0.5values were all greater (mean SR0.5 = 2.5 MPa) than those by direct-seeding (Table 6).
The greater elongation rates at low soil compaction for the fast-growing tree, E. camaldulensis, relative to those of E. leucoxylon and E kochii (Table 5) are consistent with the domination of E. camaldulensis in regions that have wetter (i.e. softer) soils (Marcar et al. 1995), and thus, it becomes established faster than other trees. By contrast, E. leucoxylon and E kochii have higher SR0.5 values than E. camaldulensis (Table 6), so they tend to thrive on harder, drier soils. For example, E. kochii is a small, slow-growing tree that occurs most commonly in the drier regions of Western Australia (Robinson et al. 2006). Similarly, in South Australian woodlands where E. leucoxylon occurs with E. camaldulensis, E. leucoxylon is found on shallower, high-altitude areas of the same soil catena (Boomsma and Lewis 1980).
Total root length
In Expt 1, LR was significantly greater for P. sativum than for A. salicina because P. sativum is highly sensitive to compaction (Hebblethwaite and McGowan 1980) and so produces lateral roots close to the soil surface as an early response (Goss 1977). By contrast, A. salicina does not generate laterals as quickly; rather, it directs resources into generating large axial root growth pressures over time (Azam et al. 2013) to maintain its root growth rate (Table 5) and continue pushing through the harder soil (Fig. 5a).
Among the three Eucalyptus species, E. camaldulensis had significantly greater total root length at all levels of soil compaction compared with all other species, regardless of planting method (Table 7). This, presumably, is why E. camaldulensis trees are so much larger than either E. leucoxylon or E. kochii when they have access to plenty of water and nutrients (Marcar et al. 1995).
Although the root lengths of the transplanted E. leucoxylon and E kochii were slightly less than those of E. camaldulensis, they appear to be less sensitive to compaction because their root lengths declined by only 53% (from 14.4 to 6.8 m plant–1) and 33% (from 12.5 to 8.4 m plant–1), respectively, from the least to the most compacted soil, whereas root lengths for E. camaldulensis declined by 62% (from 25.8 to 9.8 m plant–1) under the same conditions. This is perhaps not surprising in view of the fact that E. leucoxylon and E kochii both can exert significantly greater axial root growth pressures than E. camaldulensis (Azam et al. 2013) and they naturally thrive on shallower, drier (i.e. harder) soils than E. camaldulensis (Boomsma and Lewis 1980; Robinson et al. 2006). The significantly greater total root lengths for the three transplanted Eucalyptus species supports the practical finding in the field that transplanted, small-seeded species tend to establish more successfully than direct-seeded trees of the same species with adequate watering and weed control (Clemens 1980; Young and Evans 2000).
Root thickening
Table 8 suggests that the investment in primary root thickening as a response to compaction was greater for P. sativum (DPrim increased from 1.34 to 2.23 mm) than for the tree species, A. salicina (DPrim increased from 0.83 to 1.37 mm). Materechera et al. (1991) suggested that root thickening comes at a cost in terms of soil exploration. On the one hand, thickening may help weaken the soil by opening cracks for further root development, but it may also reduce root elongation rates, which can be critical to maintain for survival. In our Expt 1, the difference in root thickening corresponded with a 93% reduction in root growth rate for P. sativum (LR/t dropped from 1.35 to 0.09 cm day–1) v. an 87% reduction in root growth rate for A. salicina (LR/t dropped from 1.34 to 0.17 cm day–1) when exposed to bulk densities ranging from 1350 to 1750 kg m–3. This is because tree species such as A. salicina develop several layers of suberised cells behind the root tip (Steudle 2000), which makes them less susceptible to cell deformation with increasing soil strength, and this allows them to maintain a greater relative root elongation rate (Lipiec et al. 2012).The greater thickening of P. sativum roots, by contrast, caused them to invest more energy in thickening (especially at the two higher compaction levels), which left them with less stored energy to maintain elongation rates (Thaler and Pagès 1996).
Root distribution
The heavy concentration of P. sativum roots in the top 5 cm at all levels of compaction suggests that peas are more sensitive to compaction (Goss 1977; Hebblethwaite and McGowan 1980) than most of the tree species (Fig. 6). This was especially so in our experiments at the lower levels of compaction; >60% of the pea roots were restricted to the top 5 cm, whereas at the same levels of compaction the tree root systems were more uniformly distributed with depth.
Among the tree species, at comparatively high levels of compaction (e.g. bulk density 1650 kg m–3), A. salicina roots grew down into the 5–10 cm layer within only 14 days, whereas none of the Eucalyptus species produced any roots in that layer at the same level of compaction, even after 35 days. This suggests that if direct seeding is the only option in the field, large-seeded Acacias are better able to penetrate hard soils than are small-seeded Eucalyptus trees. This also supports the observation that when species of Acacia and Eucalyptus are mixed-seeded, the Acacias establish themselves as pioneer species during the first 2–3 years, thereby creating a favourable microclimate for the subsequent establishment of Eucalyptus species (Richardson et al. 2011). The lack of any significant difference in root distribution of three direct-seeded Eucalyptus species implies no superiority of one Eucalyptus species over another, especially immediately after germination.
Relation between root growth pressure and root growth rate
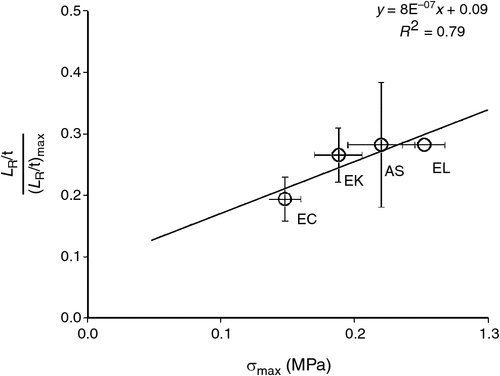
Figure 5a–c and Table 5 suggest there is species variation in the way root elongation rates decline in response to soil compaction. It has been postulated that sustained, high axial root growth pressures may play a crucial role in maintaining root elongation rates of plants growing in hard soils (Bengough 2012; Azam et al. 2013a). This suggestion can be at least partly evaluated by plotting the relative root growth rates (three replicates) found in the most compacted soils (bulk density 1750 kg m–3) against the maximum axial root growth pressures (at least nine replicates) reported for the same species by Azam et al. (2013). Only four species are available for this analysis (Fig. 7), but the linear dependency was fair (R2 = 0.79) and the relationship was modest at P = 0.11. While this level of significance is not strong, it is still possible to speculate that in very hard soils (particularly in the absence of biopores and cracks), species that can exert greater axial root growth pressures may be able to maintain somewhat greater root elongation rates (Fig. 7). Given that only four points (i.e. four species) were used in this analysis, the veracity of this idea requires further testing using a wider range of plant species.
|
Conclusions
We conclude that root growth rates of tree seedlings (e.g. A. salicina) are considerably less sensitive to soil compaction than are those of the annual species, P. sativum. Furthermore, tree roots are able to penetrate hard soils and distribute their roots more uniformly with depth than the annual, P. sativum, which expends more resources on root thickening. This confers an obvious advantage in terms of survival in seasonally dry environments; roots growing to greater depths can sustain plant life longer by accessing a greater volume of soil before the surface soil dries out.
Soil compaction causes an increase in root diameter as well as a decrease in elongation rate and total length of roots. When compaction is very severe, some tree species such as A. salicina, E. leucoxylon, and E. kochii are able to maintain root growth more successfully than an annual or another tree (e.g. E. camaldulensis). Given that significant variation in root responses to compaction have been demonstrated here among only a few tree species and an annual, it is reasonable to expect considerably more variation among other species, both annual and perennial.
The lateral roots of transplanted Eucalyptus are able to elongate faster than the primary roots of direct-seeded Eucalyptus and A. salicina at all levels of soil compaction. This has implications for species selection and tree planting methods in revegetation programs. If a dry season is anticipated, for example, it may be better to invest in transplanting to get faster establishment, rather than risk losing direct-seeded plants even though they may appear to be more economical.
A modest relationship was observed between the relative root elongation rate in very hard soils and the maximum root growth pressure that four different tree species can exert. However, because of the limited number of species examined, we consider this relationship speculative until additional work on other species is available.
Finally, the ability of established trees to maintain high root growth rates, or to distribute roots uniformly in a larger volume of soil, or to increase the diameter of roots may be important for sustaining mature trees whose roots grow in urban soils that become compacted (e.g. parkland and street trees).
Acknowledgements
The senior author acknowledges the support from the University of Adelaide in the form of an International Postgraduate Student Scholarship and the Cooperative Research Centre for Future Farm Industries for a supplementary scholarship and research support.
References
Alameda D, Villar R (2009) Moderate soil compaction: Implications on growth and architecture in seedlings of 17 woody plant species. Soil & Tillage Research 103, 325–331.| Moderate soil compaction: Implications on growth and architecture in seedlings of 17 woody plant species.Crossref | GoogleScholarGoogle Scholar |
Azam G, Grant CD, Nuberg IK, Murray RS, Misra RK (2012) Establishing woody perennials on hostile soils in arid and semi-arid regions – A review. Plant and Soil 360, 55–76.
| Establishing woody perennials on hostile soils in arid and semi-arid regions – A review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsFWisLfE&md5=68ee8d00b42f5ebc4b781ab4c27f932cCAS |
Azam G, Grant CD, Misra RK, Murray RS, Nuberg IK (2013) Growth of tree roots in hostile soil: A comparison of root growth pressures of tree seedlings with peas. Plant and Soil 368, 569–580.
| Growth of tree roots in hostile soil: A comparison of root growth pressures of tree seedlings with peas.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXps1Gmtbo%3D&md5=a380395f3b48706cff161d8ae9a290acCAS |
Barley KP (1962) The effects of mechanical stress on the growth of roots. Journal of Experimental Botany 13, 95–110.
| The effects of mechanical stress on the growth of roots.Crossref | GoogleScholarGoogle Scholar |
Barlow PW (1987) Cellular packets, cell division and morphogenesis in the primary root meristem of Zea mays L. New Phytologist 105, 27–56.
| Cellular packets, cell division and morphogenesis in the primary root meristem of Zea mays L.Crossref | GoogleScholarGoogle Scholar |
Barlow PW, Parker JS, Brain P (1994) Oscillations of axial plant organs. Advances in Space Research 14, 149–158.
| Oscillations of axial plant organs.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3Mnls1CmtQ%3D%3D&md5=1956de246ec8e4455cfd76f4858426a5CAS | 11537913PubMed |
Bengough AG (2012) Root elongation is restricted by axial but not by radial pressures: so what happens in field soil? Plant and Soil 360, 15–18.
| Root elongation is restricted by axial but not by radial pressures: so what happens in field soil?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsFWisbzJ&md5=ffee6b135781fef3f34df6dc0391d1edCAS |
Bengough AG, Mullins CE (1991) Penetrometer resistance, root penetration resistance and root elongation rate in two sandy loam soils. Plant and Soil 131, 59–66.
Boomsma CD, Lewis NB (1980) The native forest and woodland vegetation of South Australia. Bulletin of the South Australian Woodlands and Forestry Department, No. 25, Adelaide, S. Aust.
Clark LJ, Whalley WR, Barraclough PB (2003) How do roots penetrate strong soil? Plant and Soil 255, 93–104.
| How do roots penetrate strong soil?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXotV2js7g%3D&md5=6253947202f9962b5767c5d899bbe8e1CAS |
Clemens J (1980) Direct seeding of native woody plants. Landscape Australia 80, 280–284.
Cockroft B, Barley KP, Greacen EL (1969) The penetration of clays by fine probes and root tips. Australian Journal of Soil Research 7, 333–348.
| The penetration of clays by fine probes and root tips.Crossref | GoogleScholarGoogle Scholar |
Dexter AR (1987) Mechanics of root growth. Plant and Soil 98, 303–312.
| Mechanics of root growth.Crossref | GoogleScholarGoogle Scholar |
Donohue RJ, McVicar TR, Roderick ML (2009) Climate-related trends in Australian vegetation cover as inferred from satellite observations, 1981–2006. Global Change Biology 15, 1025–1039.
| Climate-related trends in Australian vegetation cover as inferred from satellite observations, 1981–2006.Crossref | GoogleScholarGoogle Scholar |
Ehlers W, Köpke U, Hesse F, Böhm W (1983) Penetration resistance and root growth of oats in tilled and untilled loess soil. Soil & Tillage Research 3, 261–275.
| Penetration resistance and root growth of oats in tilled and untilled loess soil.Crossref | GoogleScholarGoogle Scholar |
Goss MJ (1977) Effects of mechanical impedance on root growth in barley (Hordeum vulgare L.): I. Effects of the elongation and branching of seminal root axes. Journal of Experimental Botany 28, 96–111.
| Effects of mechanical impedance on root growth in barley (Hordeum vulgare L.): I. Effects of the elongation and branching of seminal root axes.Crossref | GoogleScholarGoogle Scholar |
Grime JP (1977) Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. American Naturalist 111, 1169–1194.
| Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory.Crossref | GoogleScholarGoogle Scholar |
Hebblethwaite PD, McGowan M (1980) The effects of soil compaction on the emergence, growth and yield of sugar beet and peas. Journal of the Science of Food and Agriculture 31, 1131–1142.
| The effects of soil compaction on the emergence, growth and yield of sugar beet and peas.Crossref | GoogleScholarGoogle Scholar |
Hillis WE, Brown AG (1984) ‘Eucalypts for wood production.’ (Academic Press: Sydney)
Ingestad T, Lund AB (1986) Theory and techniques for steady state mineral nutrition and growth of plants. Scandinavian Journal of Forest Research 1, 439–453.
| Theory and techniques for steady state mineral nutrition and growth of plants.Crossref | GoogleScholarGoogle Scholar |
Kozlowski TT (1999) Soil compaction and growth of woody plants. Scandinavian Journal of Forest Research 14, 596–619.
Lipiec J, Horn R, Pietrusiewicz J, Siczek A (2012) Effects of soil compaction on root elongation and anatomy of different cereal plant species. Soil & Tillage Research 121, 74–81.
| Effects of soil compaction on root elongation and anatomy of different cereal plant species.Crossref | GoogleScholarGoogle Scholar |
Lloret F, Casanovas C, Peñuelas J (1999) Seedling survival of Mediterranean shrubland species in relation to root : shoot ratio, seed size and water and nitrogen use. Functional Ecology 13, 210–216.
| Seedling survival of Mediterranean shrubland species in relation to root : shoot ratio, seed size and water and nitrogen use.Crossref | GoogleScholarGoogle Scholar |
Marcar N, Crawford D, Leppert P, Jovanovic T, Floyd R, Farrow R (1995) ‘Trees for saltland: A guide to selecting native species for Australia.’ (CSIRO Publishing: Melbourne)
Materechera SA, Dexter AR, Alston AM (1991) Penetration of very strong soils by seedling roots of different plant species. Plant and Soil 135, 31–41.
| Penetration of very strong soils by seedling roots of different plant species.Crossref | GoogleScholarGoogle Scholar |
Misra RK, Gibbons AK (1996) Growth and morphology of eucalypt seedling-roots, in relation to soil strength arising from compaction. Plant and Soil 182, 1–11.
| Growth and morphology of eucalypt seedling-roots, in relation to soil strength arising from compaction.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XmtVGrt7o%3D&md5=38aa1074390f3ce39e593a8b28d31574CAS |
Misra RK, Turnbull CRA, Cromer RN, Gibbons AK, LaSala AV (1998) Below- and above-ground growth of Eucalyptus nitens in a young plantation. I. Biomass. Forest Ecology and Management 106, 283–293.
| Below- and above-ground growth of Eucalyptus nitens in a young plantation. I. Biomass.Crossref | GoogleScholarGoogle Scholar |
Proctor RR (1933) Fundamental principles of soil compaction. Engineering News Record 111, 245–248.
Richardson DM, Carruthers J, Hui C, Impson FAC, Miller JT, Robertson MP, Rouget M, Le Roux JJ, Wilson JRU (2011) Human-mediated introductions of Australian acacias—a global experiment in biogeography. Diversity & Distributions 17, 771–787.
| Human-mediated introductions of Australian acacias—a global experiment in biogeography.Crossref | GoogleScholarGoogle Scholar |
Robinson N, Harper R, Smettem KRJ (2006) Soil water depletion by Eucalyptus spp. integrated into dryland agricultural systems. Plant and Soil 286, 141–151.
| Soil water depletion by Eucalyptus spp. integrated into dryland agricultural systems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xps1Wju74%3D&md5=489b2f2cbc65f22c96e03470782faffbCAS |
Simpfendorfer K (1975) ‘An introduction to trees for south eastern Australia.’(Inkata Press: Melbourne)
Steudle E (2000) Water uptake by plant roots: an integration of views. Plant and Soil 226, 45–56.
| Water uptake by plant roots: an integration of views.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXotVGrtw%3D%3D&md5=53a38ba273f3c7417b64c3b74192e435CAS |
Stirzaker R, Vertessy R, Sarre A (2002) ‘Trees, water and salt. An Australian guide to using trees for healthy catchments and productive farms.’ (Commonwealth of Australia: Canberra)
Thaler P, Pagès L (1996) Root apical diameter and root elongation rate of rubber seedlings (Hevea brasiliensis) show parallel response to photoassimilate availability. Physiologia Plantarum 97, 365–371.
| Root apical diameter and root elongation rate of rubber seedlings (Hevea brasiliensis) show parallel response to photoassimilate availability.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28Xjslyht7k%3D&md5=5a323809dac8441368b5099826204893CAS |
Utomo WH, Dexter AR (1981) Age hardening of agricultural soils. Journal of Soil Science 32, 335–350.
| Age hardening of agricultural soils.Crossref | GoogleScholarGoogle Scholar |
Wesseling J, van Wijk WR (1957) Soil physical conditions in relation to drain depth. In ‘Drainage of agricultural lands’. (Ed. JN Luthin) pp. 461–504. (American Society of Agronomy: Madison, WI)
Young TP, Evans RY (2000) Container stock versus direct seeding for woody species in restoration sites. Combined Proceedings of the International Plant Propagators’ Society 50, 577–582.
Zou C, Penfold C, Sands R, Misra RK, Hudson I (2001) Effects of soil air-filled porosity, soil matric potential and soil strength on primary root growth of radiata pine seedlings. Plant and Soil 236, 105–115.
| Effects of soil air-filled porosity, soil matric potential and soil strength on primary root growth of radiata pine seedlings.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXotlajtr8%3D&md5=024d01bbb78c9d2fead4ea6344173a04CAS |



