The interaction of seasonal rainfall and nitrogen fertiliser rate on soil N2O emission, total N loss and crop yield of dryland sorghum and sunflower grown on sub-tropical Vertosols
G. D. Schwenke A B and B. M. Haigh AA NSW Department of Primary Industries, Tamworth Agricultural Institute, Tamworth, NSW 2340, Australia.
B Corresponding author. Email: graeme.schwenke@dpi.nsw.gov.au
Soil Research 54(5) 604-618 https://doi.org/10.1071/SR15286
Submitted: 6 October 2015 Accepted: 14 March 2016 Published: 27 June 2016
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Abstract
Summer crop production on slow-draining Vertosols in a sub-tropical climate has the potential for large emissions of soil nitrous oxide (N2O) from denitrification of applied nitrogen (N) fertiliser. While it is well established that applying N fertiliser will increase N2O emissions above background levels, previous research in temperate climates has shown that increasing N fertiliser rates can increase N2O emissions linearly, exponentially or not at all. Little such data exists for summer cropping in sub-tropical regions. In four field experiments at two locations across two summers, we assessed the impact of increasing N fertiliser rate on both soil N2O emissions and crop yield of grain sorghum (Sorghum bicolor L.) or sunflower (Helianthus annuus L.) in Vertosols of sub-tropical Australia. Rates of N fertiliser, applied as urea at sowing, included a nil application, an optimum N rate and a double-optimum rate.
Daily N2O fluxes ranged from –3.8 to 2734 g N2O-N ha–1 day–1 and cumulative N2O emissions ranged from 96 to 6659 g N2O-N ha–1 during crop growth. Emissions of N2O increased with increased N fertiliser rates at all experimental sites, but the rate of N loss was five times greater in wetter-than-average seasons than in drier conditions. For two of the four experiments, periods of intense rainfall resulted in N2O emission factors (EF, percent of applied N emitted) in the range of 1.2–3.2%. In contrast, the EFs for the two drier experiments were 0.41–0.56% with no effect of N fertiliser rate.
Additional 15N mini-plots aimed to determine whether N fertiliser rate affected total N lost from the soil–plant system between sowing and harvest. Total 15N unaccounted was in the range of 28–45% of applied N and was presumed to be emitted as N2O + N2. At the drier site, the ratio of N2 (estimated by difference) to N2O (measured) lost was a constant 43%, whereas the ratio declined from 29% to 12% with increased N fertiliser rate for the wetter experiment.
Choosing an N fertiliser rate aimed at optimum crop production mitigates potentially high environmental (N2O) and agronomic (N2 + N2O) gaseous N losses from over-application, particularly in seasons with high intensity rainfall occurring soon after fertiliser application.
Additional keywords: denitrification, N2O emission factor, N2O emission intensity.
Introduction
Grain sorghum (Sorghum bicolor L.) is the dominant summer cereal crop grown in the northern Australian grain cropping region (Carrigan et al. 2014) with production averaging 2.1 Mt year–1 (2005–14 range: 1.1–3.8 Mt year–1). Sunflower (Helianthus annuus L.) is a less commonly grown summer crop (Serafin et al. 2014) with production averaging 50 kt year–1 (2005–14 range: 18–98 kt year–1) (ABARES 2015). Sufficient nitrogen (N) supply for the optimum production of both crops is typically determined using a budgeting approach whereby growers select the N fertiliser rate based on the previous crop, the length of the fallow period and the expected yield (Herridge 2011). Rates of N fertiliser commonly applied in northern NSW are in the range of 0–140 kg N ha–1 for dryland sorghum and 0–100 kg N ha–1 for dryland sunflowers (Carrigan et al. 2014; Serafin et al. 2014).
Crop production using N fertilisers causes an increase in soil nitrous oxide (N2O) emitted from the biological processes of nitrification and denitrification (Bremner 1997) and therefore contributes to the increasing concentration of N2O in the atmosphere; a cause of global warming and stratospheric ozone depletion (Myhre et al. 2013). Previous research into N2O emissions from dryland annual crops grown in the sub-tropical Australian northern grains region has mainly focussed on winter crops such as canola, chickpea and wheat with emission factors (EF) often well below the IPCC default EF of 1.0% (Wang et al. 2011; Schwenke et al. 2015). The potential for N2O emissions during a summer crop should be greater owing to warmer temperatures and higher rainfall intensity (Dalal et al. 2003).
Soil properties influence the potential for direct and indirect losses of N2O through denitrification and leaching respectively. For example, De Antoni Migliorati et al. (2014) found that the moderate permeability of an Oxisol prevented prolonged waterlogging but its high clay content prevented nitrate leaching under sorghum and maize. Cropping in the northern Australian grains region is dominated by grey, brown and black Vertosols (Webb et al. 1997; Isbell 2002) of medium–heavy shrink/swell clay content and characteristically low permeability. Flood irrigation or high intensity rainfall on these soils can lead to temporary waterlogging and oxygen depletion causing denitrification and substantial total N loss (Strong et al. 1992; Armstrong et al. 1996; Rochester and Constable 2000). These soils are often quite wet throughout the profile at sowing as regional agronomic guidelines recommend that farmers only plant a crop when the soil has >1 m depth of plant available soil moisture (Carrigan et al. 2014). Actual emissions of N2O from Vertosols under waterlogging conditions may also be influenced by the ratio of N2 : N2O released during denitrification, which in turn may be influenced by depth of denitrification (Gilliam et al. 1978), soil pH (Rochester and Constable 2000), aeration, soluble carbon concentration (Weier et al. 1993) and nitrate concentration (Firestone et al. 1979). Ramu et al. (2012) reported greater N2O emissions from a well-drained Alfisol than from an adjacent poorly-drained Vertisol, demonstrating that factors other than drainage can affect cumulative N2O emitted. Soil N2O emissions from N-fertilised sorghum in tropical and sub-tropical regions have been reported from Oxisols (Mosier et al. 1998; De Antoni Migliorati et al. 2015) an Inceptisol (Storlien et al. 2014), an Entisol (Welzmiller et al. 2008) and an Alfisol (Ramu et al. 2012), but only Ramu et al. (2012) has documented N2O emissions from a Vertisol in central India. The potential for denitrification losses of N as N2O and N2 is high, particularly if high intensity rainfall occurs early in the growing season (Schwenke et al. 2015).
It is well established that adding N fertiliser raises soil N2O emissions above background levels (Snyder et al. 2009). However, the effects of ever-increasing N fertiliser rates on N2O emissions are less certain, with previous studies recording either a linear increase, an exponential increase, a hyperbola response, no change or even a decrease in the amount of N2O emitted as N fertiliser rate increased (Kim et al. 2013; Shcherbak et al. 2014). Kim et al. (2013) argued that most of these responses were merely different phases within an essentially common response function with distinct linear, non-linear or steady-state phases delineated by optimal N uptake thresholds by vegetation and soil microbes. These uptake thresholds vary depending on site vegetation, climate and soil properties. A global meta-analysis of published data of N2O response to increasing N fertiliser rate (Shcherbak et al. 2014) concluded that an exponential response was generally more applicable when soil inorganic N exceeded crop N demand. Of the 78 published studies reviewed, only one was conducted in Australia – N2O emissions in a high-rainfall sub-tropical sugarcane study on a coastal Hydrosol (Allen et al. 2010).
Choice of N fertiliser rate can be used as a N2O mitigation strategy by limiting the accumulation of surplus nitrate in soil from N applied in excess of crop demand (Snyder et al. 2009). Since crop N use is a key component of soil N dynamics, the use of a simple linear relationship (i.e. fixed EF) to describe N2O response to N rate in crop lands is likely to underestimate N2O emissions at N rates in excess of the crop uptake capacity. It is in this excess application zone that the largest mitigation gains are to be made by reducing N rate (Shcherbak et al. 2014). For N rate-based N2O mitigation strategies to be accepted and adopted by farmers, the proposed N rates must be both agronomically efficient and economically profitable (Van Groenigen et al. 2010), so yield-scaled emission relationships with N rate are needed. Where sufficient data on impacts of N rates on N2O emissions exist, IPCC Tier 2 EFs can be used in the place of the fixed-EF IPCC Tier 1 methodology for national greenhouse gas inventories and industry-based incentive schemes for mitigation (Millar et al. 2010). While there is a considerable global dataset of soil N2O emission responses to fertiliser N rate, most data are from temperate regions. There are little data available from sub-tropical summer cropping environments and none from the Australian northern grains region (Shcherbak et al. 2014).
This paper reports the effects of N fertiliser rate treatments on N2O emissions, crop yield and total N loss from three field-plot and two 15N mini-plot N fertiliser rate experiments with summer sorghum, and one field-plot N rate experiment with sunflower. Each experiment featured a nil-N control and at least two rates of N as urea applied as a side-band during sowing. The aim of these experiments was to determine how N fertiliser rate affected the N2O emission rate and therefore whether optimising the N rate can minimise direct N2O emissions. Such research has not been previously reported for summer cropping in this region, and rarely for this climate, soil and cropping system globally. The results of this work should inform greenhouse gas inventory calculations for dryland summer sorghum and sunflower and be used to validate and further develop models of soil N2O emissions during and after annual summer crops.
Materials and methods
Field experimental design and treatments
We conducted four separate field-plot experiments (Expts 1–4) and two 15N mini-plot experiments (Expts 5 and 6) (Table 1). Each experiment had a randomised complete block design. All field-plot experiments were sown with a four-row minimum-disturbance precision disc planter at 0.75-m row spacing in plots that were 12 m long, and so individual plot area was 36 m2. Grain sorghum (S. bicolor (L.) Moench) was sown at 75 000 plants ha–1 and sunflower (H. annuus L.) at 36 000 plants ha–1.

|
The 15N experiments (Expts 5 and 6) were conducted within additional field plots of Expts 3 and 4, respectively. Each mini-plot covered one plant row and one fertiliser band. The mini-plots in Expt 5 were 0.75 m × 1.33 m = 1 m2, while those in Expt 6 were 0.75 m × 1.0 m = 0.75 m2. All mini-plots were bounded by steel frames that were hammered 0.1 m into the soil leaving 0.05 m protruding above the surface to prevent surface runoff.
The N rates for each experiment are listed in Table 1. All N was applied as urea side-banded 0.1 m from the seed row and 0.05 m deep in the soil at sowing. The N used in the mini-plot experiments was applied in the same side-band location as in the larger field plots, but was applied as a solution of 10% atom-enriched 15N urea (Sigma-Aldrich Chemicals, St Louis, Missourri, USA).
Sites, locations and soils
The Expts 1, 3 and 5 were located at the Tamworth Agricultural Institute (31.152°S, 150.982°E) near Tamworth NSW, while Expts 2 (31.490°S, 150.485°E), 4 and 6 (31.515°S, 150.599°E) were located on the Liverpool Plains. All experiments were on alkaline (pH1 : 5 water in the range of 8.2–8.5 in 0–0.3 m) Black Vertosols (Isbell 2002) of medium-heavy clay content (54% clay at Tamworth and 81% clay on Liverpool Plains). Soil organic carbon in 0–0.1 m was 1.0% at the Liverpool Plains sites and 1.2% at the Tamworth site. Soil drained upper limit (DUL) and crop lower limit (CLL) for sorghum was determined at the Tamworth and the Quirindi sites, as described by Dalgliesh and Cawthray (1998). The total plant available water capacity (PAWC) was 299 and 277 mm for the Tamworth and Liverpool Plains soils respectively. Actual PAW measured at sowing of each experiment was 256, 145, 253 and 89 mm for Expts 1–4 respectively.
Soil and plant sampling
All field-plot sites were soil cored (nine cores per site) to either 1.2 or 1.5 m for gravimetric moisture content before sowing. The Expts 1 and 2 were sampled for soil moisture (0–0.1 m) at each gas sampling time using a 0.05-m diameter corer. Mineral N was measured in the pre-sowing and post-harvest cores from Expts 3 and 4, as well as to 0.20 m depth approximately monthly for a year post-fertiliser application. Sampling for soil mineral N (ammonium and nitrate) in Expts 3 and 4 was done by combining 10 × 0.02-m diameter cores per plot for surface (0–0.1 m) and sub-surface (0.1–0.2 m) samples. Two cores out of each 10 were located on the fertiliser band, with the remainder located across the inter-band area. The deeper pre-sow and post-harvest sampling consisted of two 0.05-m diameter cores per plot (one core on the fertiliser band and the other between bands) divided into 0.2–0.3, 0.3–0.6, 0.6–0.9, 0.9–1.2 and 1.2–1.5 m. Soil bulk density for 0–0.1 and 0.1–0.2 m depths was determined by the small core volumetric ring method (Method 502.03) (Cresswell and Hamilton 2002) and, for deeper samples, from the oven-dry weight of the two core samples per plot. Soil water content was determined by gravimetric difference before and after drying for 48 h at 105°C. Water filled pore space (WFPS) was calculated based on soil bulk density, gravimetric water content and particle density (Linn and Doran 1984). Ammonium and nitrate N in filtered (Whatman 42) soil extracts (2 M potassium chloride), prepared on the day of sampling, were determined by standard colourimetric analyses using a flow injection analyser (Lachat Instruments, Colorado, USA). Soil moisture at 0.05 m depth was also monitored continuously (15-min logging) in Expt 3 using site-calibrated ThetaProbe ML2x Soil Moisture Sensors (Delta-T Devices) inserted into the soil.
Aboveground whole plant samples (two 0.75-m rows per plot) were collected by hand cutting at flowering (Expts 3 and 4) and at harvest (Expts 1–4). Samples were dried at 70°C for 48 h, weighed, sub-sampled and finely ground to <0.125 mm, then those from Expts 3 and 4 were analysed for total N concentration (%N) by combustion analysis (EA1112, Thermo Finnigan). Samples of harvested grain were analysed for moisture content by oven drying, and N concentration by combustion analysis as above. Grain protein was calculated by multiplying grain %N by a factor of 5.7. All grain yield and protein results were standardised to a moisture content of 12% before treatments were compared. In Expt 3, all post-harvest plant residue material from a plot was distributed evenly across that same plot on the day after harvesting.
Weather conditions measured at all experimental sites included ambient air temperature, relative humidity and daily rainfall. Soil temperature was also measured at 15-min intervals at depths of 0.05 and 0.15 m in Expt 3.
Greenhouse gas measurements – automatic chambers (Expt 3)
Greenhouse gas emissions were measured using chambers (0.5 m × 0.5 m × 0.15 m height) secured to bases pushed 0.1 m into the soil. Each base covered one crop row and one N fertiliser band. During crop growth, chambers were moved weekly between three bases within each plot to minimise the impact of the chamber on plant growth. Chamber height was increased using extensions so that the chamber covered the crops as they grew to include N2O emitted through plant transpiration (Chang et al. 1998). At maturity, plants growing within the chamber bases were hand harvested, the grain was removed and the plant residues returned to the same base.
We used a 12-chamber automatic gas measuring system (Scheer et al. 2011). Four automatically operated chambers (first replicate) were closed for 60 min, during which time four separate samples of air were collected at 15-min intervals and analysed immediately using a gas chromatograph (8610C, SRI Instruments, CA, USA) fitted with an electron capture detector for N2O measurement. After the closure period, the chambers in the first replicate were opened and those located in the second replicate were closed for 60 min, followed by the chambers in the third replicate 60 min later. After 180 min the cycle restarted, giving a total of eight measurements per chamber per day.
The N2O concentrations in the four air samples from each chamber during each closure period were regressed against closure time. We used a routine developed by Pedersen et al. (2010) that selects the most appropriate model for regression based on the data from each measurement period. The routine first fitted a non-linear model to the data. Where this fit was not statistically significant, a linear model was then fitted. If neither model was statistically significant, a slope of zero was assigned. The calculated slope of the selected regression was integrated back to the time of chamber closure, then used in the calculation of N2O flux using a formula derived from that given by Rochette and Hutchinson (2005) as follows:
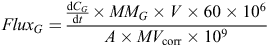
where FluxG is the hourly flux of the measured gas ‘G’ (µg m–2 h–1), 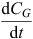 is extrapolated from the slope of the regression between gas concentration (CG, parts per billion = ppb mol mol–1) and time (t, min) integrated back to the time of chamber closure, MMG is the molar mass of the element of interest in gas ‘G’ (28 g mol–1 for N in N2O), V is the volume of the measurement chamber (m3), A is the surface area of the measuring chamber (m2), 60 converts from min to h, 106 converts g to µg, 109 converts ppb mol mol–1 to mol mol–1, and MVcorr is the molar volume of air in the sampling chamber (m3 mol–1) as follows:
is extrapolated from the slope of the regression between gas concentration (CG, parts per billion = ppb mol mol–1) and time (t, min) integrated back to the time of chamber closure, MMG is the molar mass of the element of interest in gas ‘G’ (28 g mol–1 for N in N2O), V is the volume of the measurement chamber (m3), A is the surface area of the measuring chamber (m2), 60 converts from min to h, 106 converts g to µg, 109 converts ppb mol mol–1 to mol mol–1, and MVcorr is the molar volume of air in the sampling chamber (m3 mol–1) as follows:
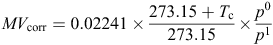
where 0.02241 m3 mol–1 is the molar volume of an ideal gas at 0°C and 101.325 kPa, Tc is the chamber temperature (°C), and p0 and p1 are the barometric pressures (kPa) at sea level and at the trial site respectively. Chamber temperature was measured using thermocouple probes mounted within one chamber in each replicate group of four. Bi-daily (9 a.m. and 3 p.m.) barometric pressure data for Tamworth (Station 055325: 430 m above sea level) were obtained from the Australian Bureau of Meteorology (http://www.bom.gov.au/climate/data/).
Since the chambers were 0.5 m in width and the crop row width was 0.75 m, we calculated a weighted N2O flux for each N-treated plot as the sum of the treated (× 0.67) and untreated (× 0.33) chamber results. The average N2O flux of the non-N-fertilised plots was used as the untreated result for these calculations. This weighting method was justified by separate on-fertiliser-row and off-fertiliser-row measurements in Expt 1, which showed no significant difference between N2O fluxes from untreated plots and those from between-band chambers in treated plots. Cumulative N2O emitted during the experiment was calculated by linear interpolation between measurement times.
Greenhouse gas measurements – manual chambers (Expts 1, 2 and 4)
Manual chambers of 0.25-m diameter cylindrical PVC were pushed into the soil to 0.1 m in depth, leaving 0.2 m above the soil surface. The chambers remained in place for the duration of the experiment. In Expts 1 and 2, there were two chambers within each measured plot; one directly over the fertiliser band, and one directly in the plant row (between the fertiliser bands). In Expt 4 there was just one chamber per plot located directly over the fertiliser band. Gas emissions measurements were conducted on an irregular basis, with several daily measurements following significant rainfall events (>10 mm). The Expts 1, 2 and 4 were sampled 18, 9 and 29 times during the growing season respectively. Most measurements began between 10 a.m. and 12 p.m. in the day to approximate an average daily flux. At the time of sampling, a lid fitted with a rubber O-ring was put onto the top of the chamber. Several chambers were immediately sampled after fitting the lid to give an average ambient gas concentration which was then used for all calculations. We collected 20 mL of chamber air using a hypodermic needle inserted through a rubber septum in the chamber lid. The sample was then injected into a pre-evacuated 12-mL glass Exetainer (Labco, Lampeter, UK) vial. Samples were collected 0.5 and 1 h after lid closure. All samples were analysed for N2O concentration using a Varian 450-GC gas chromatograph fitted with an electron capture detector. Fluxes were determined using the same formula as used for the automated chamber measurements. Since the chambers were 0.25 m in diameter and the row width was 0.75 m, we calculated a weighted N2O flux for each plot as the sum of the on-band (× 0.33) and the off-band (× 0.67) chamber results from that plot (Expts 1 and 2). In Expt 4, an averaged flux from the non-treated plots was used in place of off-band chamber results to calculate the weighted plot flux. Cumulative N2O emitted during the experiment was calculated by multiplying the average daily flux rate of consecutive samples by the number of days between when those samples were collected.
15N mini-plot measurements (Expts 5 and 6)
At the time of harvest, all plants from the central 0.75 m of plant row within the mini-plots were cut at ground level, dried at 70°C, divided into grain and biomass, then representatively sub-sampled and finely ground (<0.125 mm) before total N and 15N analysis by combustion and mass spectroscopy (Sercon Instruments, UK). Plants from 0.75 m of the two adjacent plant rows outside each mini-plot were also sampled and analysed in the same manner to assess scavenging of the 15N from outside plants. Following plant removal, a central block of soil measuring 0.4 m along the row × 0.75 m across the row × 0.1 m depth was excavated from the mini-plot, weighed, dried at 40°C, representatively sub-sampled, ground to <0.125 mm and then analysed for total N and 15N as for the plant samples. All distinct plant root material was removed from the soil sample and analysed separately after any adhering soil was removed. The root material was dried at 70°C, and then treated as per plant samples. Another 0.1-m deep block of soil was removed below the first block (0.1–0.2 m depth) and treated as above. Below this, five soil cores of 0.05 m in diameter were collected using a hydraulic soil corer. These cores were segmented by depth into 0.2–0.3, 0.3–0.6, 0.6–0.9, 0.9–1.2 and 1.2–1.5 m depths, and then treated as per the soil block samples. One of the five soil cores was located directly under the fertiliser band and the others were across the inter-band space. Soil cores were also collected from adjacent unfertilised plots for use as background 15N measurements. All results were calculated according to formulae given by Armstrong et al. (1996).
Statistical analysis
Statistical comparisons of treatment results were made using analysis of variance (Genstat v 14), with individual means tested for difference using the least significant difference test at a probability level of 5%. Smoothing splines (Verbyla et al. 1999) were used to model daily N2O emission fluxes in Expt 3. Histograms of daily N2O fluxes showed the data to be highly skewed, and so the data were first log-transformed to reduce skewness. Values below zero (6% of all data, average negative result = –0.4 g N2O-N ha–1 day–1) were set to zero and 1 was added to all the data before applying the log-transformation. Weekly N2O readings were used instead of daily readings in order to reduce the high variation in the data. Plots of predicted means ± 1 standard error were constructed for each of the four treatments to compare treatment effects.
Results
Environmental conditions
Growing season rainfall varied considerably between the four field-crop sites, with monthly rainfall totals often well below, but sometimes above, long-term monthly medians (Fig. 1). Total December rainfall for Expt 1 was 166 mm, which was just below the 95th percentile for the site, although only 17 mm of this fell after the trial was sown. Rainfall in January 2011 was average, but in February was below the 25th percentile. In contrast, Expts 4 and 6 had rainfall in the 90th and 93rd percentile of the district long-term figures during January and March but only in the 9th and 30th percentile after this. Total in-crop (sowing–harvest) rainfall was 212, 79, 322 and 407 mm for Expts 1–4 respectively. The site at Expt 3 had another 211 mm during the post-crop fallow gas measurement period. When considered in conjunction with the starting soil moisture conditions, the experiments reported here constitute a range of crop growth conditions including a wet soil with normal in-crop rain (Expt 1), a drier soil with low in-crop rain (Expt 2) and a very dry soil with high in-crop rainfall (Expt 4).
Soil mineral N
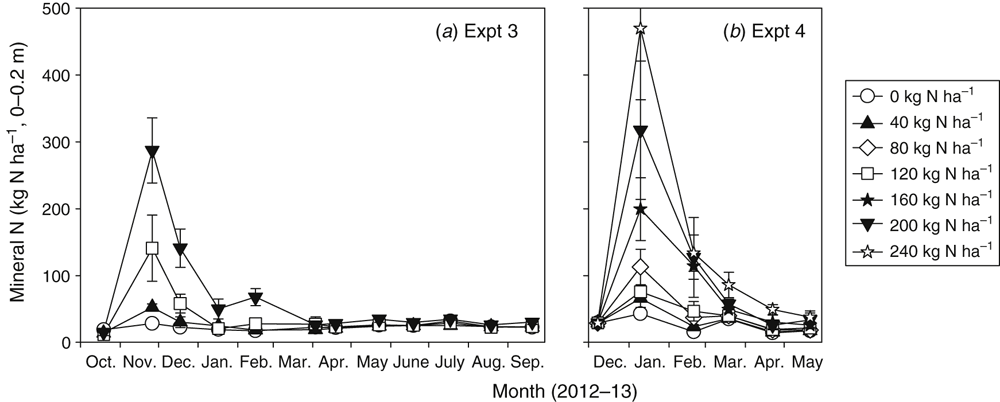
Soil mineral N sampled to 0.2 m depth in the gas-measured treatments of Expts 3 and 4 showed a distinct peak in total mineral N in the first sampling after fertiliser application (Fig. 2). The size of these peaks typically exceeded the amounts of N applied as fertiliser and the experimental error associated with these measurements was very large, particularly for the higher N rate treatments. Accurately measuring average soil mineral N across a 0.75-m row spacing unit after fertiliser was applied in a narrow band was problematic, despite multiple cores taken across a row spacing unit. However, the trends shown in Fig. 2 are indicative of the large quantity of mineral N concentrated in the fertiliser band soon after fertilising, and the rapid depletion of this mineral N by plant uptake and N loss mechanisms over the succeeding months. By the time of harvest, there was no discernible difference in soil mineral N between any of the N rate treatments.
|
N2O emissions
Mean daily N2O emissions measured in Expts 1–4 are shown in Figs 3 and 4, along with the soil WFPS (0–0.1 m), daily rainfall and average daily temperature at each site. Median daily N2O flux was 3.9 g N2O-N ha–1 day–1 in Expt 1 (range: –0.1 to 291 g N2O-N ha–1 day–1), 3.0 in Expt 2 (range: 0.3 to 26), 0.4 in Expt 3 (range: –3.8 to 111) and 5.7 in Expt 4 (range: –0.9 to 2734). In general, N2O emissions occurred in response to high intensity rainfall conditions, especially where these occurred on already-wet soils in the early period of crop growth in early–mid-summer (Expts 1 and 4). The Expt 1 was sown into a soil profile that had received 147 mm of rainfall in the preceding 3 weeks, and so was at the soil’s DUL. The site then received another 107 mm of rainfall during the first 30 days after sowing. In contrast, Expt 2 was sown at a similar date into a profile approaching DUL, but received only 9 mm of rainfall during the first month post-sowing. Maximum N2O fluxes in Expt 2 were approximately one-tenth of those recorded for Expt 1.
Extremely high N2O flux results were recorded in Expt 4 (Fig. 3i–l) which was sown on a soil profile that was initially dry in the surface, but quite moist lower in the profile. Two weeks after sowing, emissions of N2O increased by up to two orders of magnitude when 61 mm of rain fell over three days, then increased by another order of magnitude a month later when 146 mm of rain fell over four days, followed by another 37 mm four days later. These high fluxes were repeated a month later with another 109 mm of rain over a two-day period. Daily temperatures during these rainfall events averaged 25°C. The Expt 3, run in the same season as Expt 4 but at a drier location, showed a much lower range of N2O fluxes, although emission rates did briefly exceed 100 g N2O-N ha–1 day–1 (Fig. 4a–d). The rainfall events for Expt 3 were less intense and less in total than for Expt 4, with the most intense event (48 mm) occurring in early March 2013 (Fig. 4e). This late-in-the-season rainfall did not lead to a spike in N2O flux, presumably because there was little soil mineral N remaining by this late maturity growth stage (Fig. 2a).
The other noteworthy trend in this N2O flux data is that fluxes tended to be greater in treatments with more fertiliser N applied, with the nil-N treatment having the lowest rate of N2O emission on most days of measurement. This trend is best seen in the splines (± standard error) for each N rate treatment of Expt 3 (Fig. 5). The hatched zone (± standard error) about the splined N2O fluxes for each N treatment were clearly separate for the first 100 days after N application, but gradually overlapped later in the growing season, indicating less probability of significant treatment differences in N2O fluxes once the crop had taken up most of the available N from the soil. Both Figs 3 and 4 also demonstrate that the nil-N treatment (background) fluxes also increased in conjunction with high WFPS conditions after intense rainfall. In Expt 4 (Fig. 3i), daily N2O fluxes from the nil-N plots exceeded 10 g N2O-N ha–1 day–1 for a short period in January 2011.
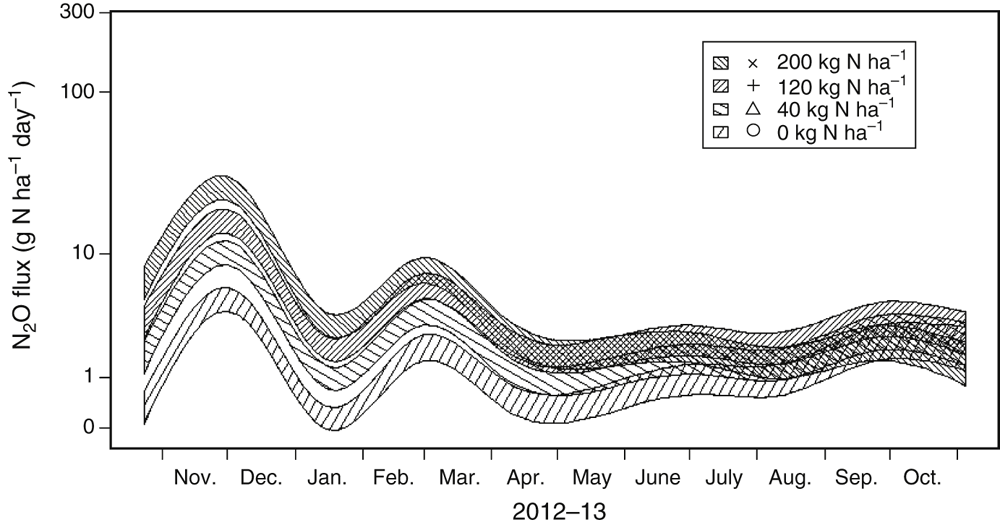
|
Cumulative N2O emitted over the growth season (Expts 1, 2 and 4) or whole year from N application (Expt 3) is shown in Fig. 6. Total emissions were in the ranges of 570–4369, 96–753, 130–1050 and 101–6659 g N2O-N ha–1 for Expts 1–4 respectively. The lowest total emission in each experiment was from the nil-N treatment. Cumulative N2O losses in each experiment tended to be in order of N addition rate, although in some cases the high variation about the mean losses meant that some N rate treatments were statistically similar in total N2O-N lost over the study period. In Expt 4, the lower N rate treatments (40–160 kg N ha–1) showed much less response to the late-February high WFPS conditions caused by intense rainfall than the 200–240 kg N ha–1 treatments, which showed large losses of N2O-N at this time. All experiments showed a plateau in cumulative N2O loss in the latter half of the summer cropping season, coinciding with drier soil conditions and depleted soil mineral N. Of the whole year’s net N2O emissions (background corrected) in Expt 3, 80–94% occurred between sowing and harvest (Fig. 6d).
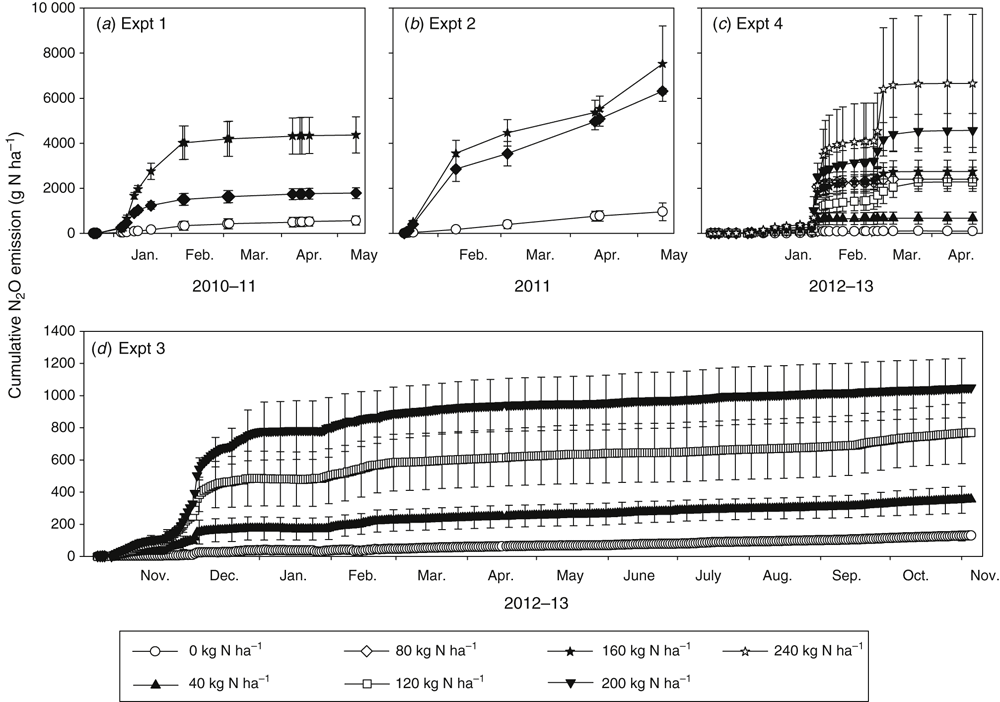
|
Increasing the N fertiliser rate increased N2O emissions linearly at all experimental sites (Fig. 7a). However, the slope of the linear regression between N fertiliser rate and cumulative N2O emitted differed markedly between the experimental sites. For Expts 2 and 3, the slopes were 4.6 and 4.1 g N2O-N kg–1 fertiliser N respectively; while at the wetter sites (Expts 1 and 4), the slopes were 23.7 and 24.8 g N2O-N kg–1 fertiliser N respectively. An exponential regression was also fitted to the data from Expt 4 but the r2 for this was 0.81 compared with 0.91 for the linear fit.
When the cumulative N2O-N lost was corrected for background emissions and compared against the amount of N applied in fertiliser, we found two trends in the data from the four experiments (Fig. 7b). For Expts 1 and 4, EFs were high (>1.2%) and tended to increase with increasing N fertiliser rate, with a maximum EF of 3.2% at the 240 kg N ha–1 rate. In contrast, Expts 2 and 3 had EFs in the range of 0.41–0.56%, and showed only a slight decreasing trend with increasing N fertiliser rate.
Similarly, the intensity of cumulative N2O emissions per tonne of grain (or oilseed in Expt 2) also showed two contrasting trends with increasing N fertiliser rate (Fig. 7c). Emissions intensity results for the nil-N treatments in all experiments were quite similar, in the range of just 30–85 g N2O-N t–1 grain. In Expts 2 and 3, the intensity increased with N rate, but only up to 80 and 120 kg N ha–1 respectively. The intensity did not increase further at higher N rates. In contrast, the emissions intensity in Expts 1 and 4 continued to increase as N fertiliser rate increased with intensities of 615–624 g N2O-N t–1 grain at 160 kg N ha–1 (Expts 1 and 4), and 1578 g N2O-N t–1 grain at the highest N rate in Expt 4.
The yield-scaled N2O emissions from Expts 3 and 4 versus the N surplus for each treatment in these experiments are shown in Fig. 8. The yield in this case refers to the sum of grain-N and crop residue-N contents at harvest (i.e. the aboveground crop N uptake), while the N surplus is the difference between this ‘yield-N’ and the amount of N applied in fertiliser (Van Groenigen et al. 2010).
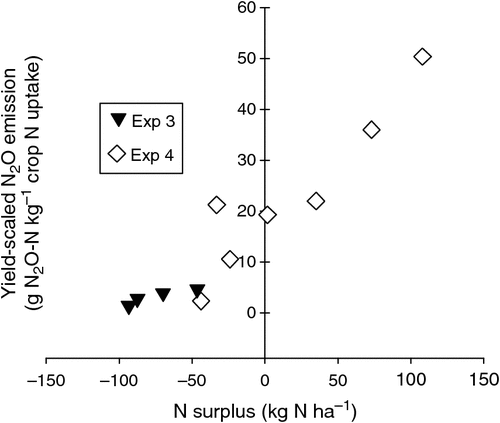
|
15N labelling experiments
Table 2 details the results from the two concurrent 15N labelling studies (Expts 5 and 6) run within the trial areas of Expts 3 and 4 respectively. Total recovery of applied 15N averaged 80.3% for Expt 5 (no N rate effect), while for Expt 6 the recovery was in the range of 55.0–71.9% with the greater recovery for the 200 kg N ha–1 treatment. Plants in adjoining rows scavenged 2–10% of the applied 15N, which is not unusual in a cropping region where N is often mid-row banded and rows may be spaced at >1 m. Assuming no lateral movement of the applied 15N in the soil below the steel barrier, this means that unrecovered 15N, presumed lost from the soil–plant system as gases, averaged 19.7% for Expt 5, which equates to N losses from the applied urea of 8, 24 and 40 kg N ha–1 from the 40, 120 and 200 kg N ha–1 treatments respectively. For Expt 6, the unrecovered N equated to N losses of 17, 54 and 56 kg N ha–1 from the 40, 120 and 200 kg N ha–1 treatments respectively.
Nitrogen fertiliser use efficiency (NUE), the proportion of applied N recovered in the harvested grain, was not affected by N rate in Expt 5 where average NUE was 28.6%. However, for Expt 6, the 40 and 120 kg N ha–1 rates had an NUE of 21.7%, whereas NUE in the 200 kg N ha–1 treatment was significantly higher at 38.3%. Recovery of applied 15N in the soil was significantly greater at the lowest N rate in Expt 5 than at the higher N rates, with the majority of 15N found in the surface soil (0–0.1 m). In contrast, there was no significant N rate effect on 15N recovery in soil for Expt 6.
Plant biomass and grain yields
In Expt 1, there was no significant effect of N fertiliser rate on either crop residue biomass (site mean of 8.3 t ha–1) or grain yield (site mean of 6.75 t ha–1) (Fig. 9a, b). Similarly, Expt 2 also showed no significant effect of N fertiliser rate on sunflower seed yield (site mean of 3.2 t ha–1). However, N fertiliser rate had a strong effect on crop biomass, grain yield and grain protein in Expts 3 and 4, with maximum biomass and grain production achieved at N rates around 120 kg N ha–1 (Fig. 9a, b). Higher N rates did not further increase biomass or grain yield in Expt 3, while in Expt 4 the highest N rate led to an unexpected increase in grain yield above that recorded for the four lower N rates. Grain protein showed similar trends in Expts 3 and 4, with a consistent increase in protein with N rate from a low at 40 kg N ha–1 (Fig. 9c). This was a characteristic response to N applied at a severely N-deficient site as the small amount of N applied at 40 kg N ha–1 went into boosting leaf and stem production but was not enough to boost grain protein.
Discussion
Soil N2O emissions from sorghum and sunflower
This study reports four site-seasons of N2O emissions data during the growth of dryland grain sorghum or sunflower on sub-tropical Vertosols. Emissions during the post-harvest fallow were included in Expt 3, which covered a full 12 months from the fertiliser N application at sowing. Daily N2O fluxes varied enormously in scale (Figs 3 and 4) across the four experiments, and occurred in response to high moisture conditions, particularly during the first 1–2 months after N fertiliser application. De Antoni Migliorati et al. (2015) recently reported N2O emissions under grain sorghum grown on an Australian Oxisol in the 2012–13 summer under high intensity rainfall conditions similar to those experienced in Expt 4 in this study. Cumulative N2O losses from the Vertosol in Expt 4 were 1.6 times that for a similar rate of N fertiliser applied to the Oxisol, perhaps reflecting the difference in permeability between the heavy clay Vertosol (81% clay) and the medium clay Oxisol (55% clay). In addition, the N2O emissions intensities in Expt 4 (Fig. 7c) were nearly twice those reported by De Antoni Migliorati et al. (2015) at a similar N application rate.
Ramu et al. (2012) found N2O emissions peaking at 377 g N2O-N ha–1 day–1 on a Vertosol used to grow sorghum in India, which is of similar magnitude to maximum daily fluxes in Expts 1–3 in our study (26–291 g N2O-N ha–1 day–1), but well below the maximum in Expt 4 of 2734 g N2O-N ha–1 day–1. Cumulative N2O lost from the Indian Vertosol study (700 g N2O-N ha–1) (Ramu et al. 2012) was similar to the total N2O emitted (631 and 531 g N2O-N ha–1) from comparable N rates at the two drier sites (Expts 2 and 3 respectively), but much less than that from the two wetter sites in the present study. The total N2O emissions at the two wetter sites (Expts 1 and 4) were more in the range typical for dryland summer cropping in temperate areas of the USA, where annual N2O emissions of up to 15 400 g N2O-N ha–1 have been reported, even in normal-dry seasons (Parkin and Kaspar 2006; Storlien et al. 2014). Previous research on N2O emissions from sunflower crops is sparse, with only Flessa et al. (1995) reporting annual N2O fluxes of 9.4 and 12.9 kg N2O-N ha–1 year–1 for a sandy and a clay soil respectively in Germany, which had been fertilised with 12 t ha–1 of farmyard manure. The cumulative N2O emissions from Expts 1, 2 and 4 were calculated from manual chamber measurements, which at infrequent and irregular sampling frequencies of days to weeks could vary significantly from actual total N2O losses (Barton et al. 2015). However, our sampling was tailored using prior knowledge of the temporal variability in this environment (Schwenke et al. 2015), with sampling triggered by rainfall events which cause temporary bursts of N2O emissions between otherwise dry conditions with nil-emissions.
The Australian grain sorghum industry typically produces 2.1 Mt grain year–1. For an average N application rate of 100 kg N ha–1 (Carrigan et al. 2014), we found an average N2O emissions intensity of 292 g N2O-N t–1 grain from the three trial-years of data in this study, a figure similar to that of De Antoni Migliorati et al. (2015). This amounts to an annual Australian sorghum industry loss of 613 200 kg N2O-N year–1 or 287 150 t CO2e (carbon dioxide equivalent) year–1 using a global warming potential for N2O of 298 times that of CO2 (Myhre et al. 2013). For sunflower, an average N2O emissions intensity from Expt 2 of 201 g N2O-N t–1 seed at a fertiliser rate of 80 kg N ha–1 (Serafin et al. 2014), when applied across an Australian sunflower industry producing an average of 50 kt year–1, gives an annual industry-wide N2O emission of 10 050 kg N2O-N year–1 or 4700 t CO2e year–1.
Response of soil N2O emission to N rate
Increasing N fertiliser rate increased N2O emissions linearly at all experimental sites (r2 = 0.88–0.99) (Fig. 7a). While there was also a significant exponential relationship between cumulative N2O emissions and N rate in Expt 4, the goodness-of-fit was less (r2 = 0.81) than for the linear fit (r2 = 0.91). The slopes of the linear regression between N fertiliser rate and cumulative N2O emitted differed markedly between the experimental sites. At Expts 2 and 3, the slopes were 4.6 and 4.1 g N2O-N kg–1 fertiliser N respectively, while at the wetter sites (Expts 1 and 4), the slopes were 23.7 and 24.8 g N2O-N kg–1 fertiliser N respectively. Clearly, the N2O emission response to N rate interacted with seasonal rainfall intensity in the sub-tropical Vertosols of this study. The linear responses in this study contrast with the majority of non-linear (exponential) relationships found between cumulative N2O emitted and N fertiliser rate in North American corn grown in temperate climates (McSwiney and Robertson 2005; Ma et al. 2010; Hoben et al. 2011), although a global review into N rate responses did also find a significant number of studies showing a linear N response (Kim et al. 2013; Shcherbak et al. 2014). Kim et al. (2013) hypothesised that linear responses may indicate that the N rates used were below those likely to accumulate excessive soil nitrate N.
Linear increases imply that for each experiment a fixed EF would be appropriate to describe the impact of N rate on N2O emissions. However, while this is likely for Expts 2 and 3, it appears less likely for Expts 1 and 4 (Fig. 7b), although Expt 1 had only two EFs, while the EFs in Expt 4 were too variable to be significantly regressed against N rate. Production-linked indicators of N2O emissions, such as emissions intensity (Scheer et al. 2012) or yield-scaled emissions (Van Groenigen et al. 2010) may be more informative in developing N rate strategies for both optimum crop productivity and minimal environmental impact. While the drier sites in this study showed a stable (Expt 2) or slightly linearly increasing (Expt 3) N2O emission intensity with increasing N rate, the wetter sites showed significant exponential increases in N2O emission intensity (Fig. 7c). An optimal N rate could be chosen from the inflection point in these exponential curves, at around 110–120 kg N ha–1.
When N2O emissions were yield-scaled in terms of crop N uptake (N content of grain plus aboveground residue) and compared against N surplus (N applied minus crop N uptake), we found no N surplus in Expt 3 and hence yield-scaled N2O emissions remained low (Fig. 8). However, in Expt 4, yield-scaled emissions remained around 20 g N2O kg–1 crop N uptake for N rates of 80–160 kg N ha–1, but increased dramatically as N surplus successively increased with 34, 73 and 108 kg N ha–1 for N rates of 160, 200 and 240 kg N ha–1 (Fig. 8). It was in this upper range of applied N treatments where N2O emissions were stimulated by intense late-season rainfall events, while lower N rate treatments recorded no N2O emissions (Fig. 6c). The trend in Fig. 8 is similar to the meta-analysis of previous research by Van Groenigen et al. (2010), although the y-intercept in their relationship (~12 g N2O-N kg–1) was half that in our data. In Expt 4, much of the ‘surplus’ N was actually lost from the soil during waterlogging, rather than being surplus to crop requirements, whereas Expt 3 had no ‘surplus N’ at any N rate because N losses were water limited but crop production was not. Therefore, the concept of using yield-scaling versus N surplus is less useful in determining optimal N rates in highly variable rainfall environments with poorly draining clay soils.
Total gaseous N loss in response to N rate (15N studies)
In our studies, the total recovery of applied 15N was affected by N rate for Expt 6, but not Expt 5 (Table 2). For Expt 5, 15N recovery in soil + plant components totalled 80%, which is very similar to recoveries of 78% (Strong et al. 1992) and 76% (Armstrong et al. 1996) measured after sorghum crops grown on Vertosols in the northern Australian grains region. However, it is less than the 97–99% recovery reported by De Antoni Migliorati et al. (2014) for sorghum grown on a more permeable Oxisol with a split-N application. Strong et al. (1992) reported much lower recoveries (46%) when fertiliser N was applied >6 months before sowing, which was considered to be the result of waterlogging. While all our fertiliser was applied at the time of sowing, we recovered much less of the applied 15N where prolonged waterlogging would have promoted denitrification (Expt 6). Only 55–57% of the applied N was recovered from the 40 and 120 kg N ha–1 treatments, although 72% was recovered in the 200 kg N ha–1 treatment. Despite these percentage differences between treatments, the total amount of N lost was similar (54–56 kg N ha–1) between the 120 and 200 kg N ha–1 treatments. This may indicate that an optimal N uptake threshold by microbes had been reached, due to an available carbon limitation (Kim et al. 2013), beyond which further denitrification of nitrate N could not proceed. The major difference between these treatments was in the significantly greater recovery of 15N in the grain (Table 2), which suggests that the plants in the 200 kg N ha–1 treatment had late-season access to soil mineral N to partition into grain protein that the lower rate treatments lacked, possibly due to downward movement of soil water carrying un-denitrified nitrate. At harvest, there was no treatment difference in 15N recovered in the soil between N rates for Expt 6, but in Expt 5 a greater proportion of the applied N was found under the 40 kg N ha–1 treatment.
Recovery of 15N by the crop
Armstrong et al. (1996) found that recovery of applied 15N to Vertosols growing sorghum did not vary with the N response by the crop but instead with the rainfall pattern in each season and its likely influence on denitrification. Similarly, in Expt 5 of this study, we found no effect of N rate on plant N uptake, with uptake in the treated plots averaging 46% of the applied N, which compared closely to the 36–50% recoveries reported previously (Strong et al. 1992; Armstrong et al. 1996). However, the highest N rate at Expt 6 did increase the plant N recovery from 35% to 52%, with the difference occurring in the grain N uptake (Table 2). We suggest that this was because denitrification was limited at the highest N rate, perhaps by availability of labile carbon, so that more of the N applied at the high rate remained for plant uptake than at the lower N rates. Downward movement of the un-denitrified nitrate N meant that more was taken up into the plant during grain-filling.
Since the applied 15N urea was immediately covered by 0.05 m of soil to prevent volatilisation, and no 15N leached below 0.60 m, it is likely that all of the unaccounted N was lost as N2 or N2O. We used the mean cumulative N2O emission results from the larger plot experiments at harvest (corrected for background emissions) to calculate the likely ratio of N2 : N2O in the emitted gases. For Expts 3 and 5, the N2 : N2O ratio was not affected by N application rate, with an average ratio of 43.4 across the three N rate treatments. These ratios were almost identical to the mean ratio of 42 estimated for alkaline grey clay soils from a review of field and laboratory N2O emissions studies by Rochester (2003). In contrast, for Expts 4 and 6 the N2 : N2O ratio decreased with increasing N rate, with ratios of 29, 24 and 12 for the 40, 120 and 200 kg N ha–1 treatments respectively. The decreasing ratio with N rate at this site may be related to increasing concentrations of soil nitrate and nitrite that can inhibit the biochemical reduction of N2O to N2 during denitrification (Firestone et al. 1979; Smith and Tiedje 1979).
Crop responses to N rate
In this study, Expts 1 and 2 showed no yield or seed protein response to applied fertiliser N, presumably because the soil in both cases already had sufficient N for the crop’s demand. While we did not measure soil mineral N at the commencement of these experiments, it is reasonable to assume that both soils would have been well stocked with mineral N. The Nbudget model (Herridge 2011) predicts that these medium fertility soils would have mineralised at least 98 kg N ha–1 during the preceding long fallow period. It is also likely that in Expt 1 the waterlogged soil conditions led to a significant denitrification loss of the applied fertiliser N early in the growing season before the crop could benefit from the additional N applied. In contrast, the soils used for Expts 3 and 4 were sufficiently low in mineral N at sowing (Fig. 2) such that they were N deficient and showed a classic yield and protein response to increasing N rate (Fig. 9a, b). Armstrong et al. (1996) found that the response of dryland sorghum to applied N depended heavily on soil water content, with the response to added N dependent on the timing of rainfall, as demonstrated by Holland and Herridge (1992) for sorghum grown previously at the same location as Expt 3.
Many farmers do not carry out soil testing on their paddocks before planting a crop to determine the available mineral N content, instead preferring to use a standard N fertiliser rate. Knowing the soil’s stock of available mineral N when going into a crop can assist in better agronomy through ensuring they are either applying adequate N for optimum yield or not applying excessive N that will not further increase yield above the optimum. The economics of optimal fertiliser application are also an important factor in knowing the soil mineral N stock, as applying N above the productive optimum (120 kg N ha–1 for Expt 3 and 80 kg N ha–1 for Expt 4) decreased gross margins (data not shown). An alternative to soil testing can be the use of a regionally calibrated model or decision support tool to estimate the likely soil mineral N based on the recent crop history, soil type, age of cultivation and weather conditions (Herridge 2011). This approach is less useful in instances where denitrification is likely to have occurred and a potentially large amount of the stored soil mineral N may be lost. Biophysical models capable of simulating denitrification losses are generally not accessible to farmers. Chemically or physically delaying the availability of nitrate N in the soil until the period of rapid crop N uptake are alternative options for reducing the risk of denitrification losses of N and N2O emissions, and are the subject of subsequent research in this region.
Conclusions
We confirmed that applying N fertiliser at the time of sowing a summer crop generated N2O emissions and that the amount of N2O lost depended on the N rate and the seasonal rainfall. High intensity rainfall led to waterlogging and denitrification, which increased the rate of N2O loss with increasing N fertiliser up to five-fold compared with drier sites. Emissions of N2O from Vertosols growing sorghum or sunflower crops in seasons without prolonged waterlogging can be reasonably well predicted using an EF of 0.53, whereas the use of a standard EF, currently 0.3 for dryland cropping in Australia, in calculating N2O losses in very wet conditions is much less advisable. Linking crop productivity, N rate and N2O emissions is needed to construct guidelines for optimal production with minimal environmental and economic impact.
Total gaseous N losses also increased with increasing N fertiliser rate, but under waterlogged conditions produced an increasing proportion of N2O : N2 in the overall gases emitted, exacerbating the environmental impacts of waterlogging events on recently-fertilised Vertosols.
As with our previous research with winter crops grown on Vertosols in this region (Schwenke et al. 2015, 2016), we confirmed that the critical period for N2O emission losses from applied N fertiliser for summer crops is during the initial weeks after fertilising, before plant uptake can reduce the large pool of nitrate N available for denitrification should waterlogging occur. Further research is needed to devise N fertiliser application strategies that mitigate the large potential for N2O emissions from Vertosols used for summer cropping without compromising optimum crop production.
Acknowledgements
This research was part of the National Agricultural Nitrous Oxide Research Program (NANORP) funded by the Australian Government Department of Agriculture, with the support of NSW Department of Primary Industries and the Grains Research and Development Corporation. Many people assisted with various aspects of field operations, sample processing and sample analyses including Matthew Gardner, Peter Formann, Mustafa Kamal Hossain, William Keene, Kelly Leedham, Annabelle Mcpherson, Adam Perfrement, Peter Sanson, Zara Temple-Smith, Steve Harden, Guy McMullen and Loretta Serafin (NSW DPI, Tamworth). Thanks to Leanne Lisle at the University of New England for greenhouse gas analyses of samples from Expts 1 and 2. Thanks also to Brad Keen, Stephen Kimber and co-workers (NSW DPI, Wollongbar) for analysis of manual chamber samples from Expt 4. We also gratefully thank Ian Carter for the use of ‘Connemara’ (Expt 2) and ‘Romney Vale’ (Expt 4).
References
ABARES (2015) Agricultural commodity statistics 2015. Australian Bureau of Agricultural and Resource Economics and Sciences. Available at www.agriculture.gov.au/abares/publications [accessed 27 May 2016]Allen DE, Kingston G, Rennenberg H, Dalal RC, Schmidt S (2010) Effect of nitrogen fertilizer management and waterlogging on nitrous oxide emission from subtropical sugarcane soils. Agriculture, Ecosystems & Environment 136, 209–217.
| Effect of nitrogen fertilizer management and waterlogging on nitrous oxide emission from subtropical sugarcane soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXisFentb8%3D&md5=3d8ca5935f97bbe807b7a0c7de966c8dCAS |
Armstrong R, Halpin N, McCosker K, Standley J, Lisle A (1996) Applying nitrogen to grain sorghum in central Queensland: residual value and effect of fallowing and tillage practice. Australian Journal of Agricultural Research 47, 81–95.
| Applying nitrogen to grain sorghum in central Queensland: residual value and effect of fallowing and tillage practice.Crossref | GoogleScholarGoogle Scholar |
Barton L, Wolf B, Rowlings D, Scheer C, Kiese R, Grace P, Stefanova K, Butterbach-Bahl K (2015) Sampling frequency affects estimates of annual nitrous oxide fluxes. Scientific Reports 5, 15912
| Sampling frequency affects estimates of annual nitrous oxide fluxes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhslKgsb%2FE&md5=9e294a6c60f85b158120f4387322e158CAS | 26522228PubMed |
Bremner JM (1997) Sources of nitrous oxide in soils. Nutrient Cycling in Agroecosystems 49, 7–16.
| Sources of nitrous oxide in soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXmslCqs7Y%3D&md5=765c0e54cc956c83b7125ab88da26131CAS |
Carrigan N, Serafin L, McMullen G (2014) Grain sorghum. In ‘Summer crop production guide’. (Eds N Moore, L Serafin, L Jenkins) pp. 5–16. (NSW Department of Primary Industries: Orange, NSW)
Chang C, Janzen HH, Cho CM, Nakonechny EM (1998) Nitrous oxide emission through plants. Soil Science Society of America Journal 62, 35–38.
| Nitrous oxide emission through plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXhtlakurk%3D&md5=f854e43f831032703c97aaaff12303e9CAS |
Cresswell HP, Hamilton GJ (2002) Bulk density and pore space relations. In ‘Soil physical measurement and interpretation for land evaluation’. (Eds N McKenzie, K Coughlan, H Cresswell) pp. 35–58. (CSIRO Publishing: Melbourne)
Dalal RC, Wang WJ, Robertson GP, Parton WJ (2003) Nitrous oxide emission from Australian agricultural lands and mitigation options: a review. Australian Journal of Soil Research 41, 165–195.
| Nitrous oxide emission from Australian agricultural lands and mitigation options: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXktFKisr8%3D&md5=82cb3d74aaae67b11972b27b0ea300f6CAS |
Dalgliesh NP, Cawthray S (1998) Determining plant available water capacity. In ‘Soil matters: monitoring soil water and nutrients in dryland farming’. (Eds NP Dalgliesh, M Foale) pp. 71–92. (Agricultural Production Systems Research Unit, CSIRO: Toowoomba, Qld)
De Antoni Migliorati M, Bell M, Grace P, Rowlings D, Scheer C, Strazzabosco A (2014) Assessing agronomic and environmental implications of different N fertilisation strategies in subtropical grain cropping systems on Oxisols. Nutrient Cycling in Agroecosystems 100, 369–382.
| Assessing agronomic and environmental implications of different N fertilisation strategies in subtropical grain cropping systems on Oxisols.Crossref | GoogleScholarGoogle Scholar |
De Antoni Migliorati M, Bell M, Grace PR, Scheer C, Rowlings DW, Liu S (2015) Legume pastures can reduce N2O emissions intensity in subtropical cereal cropping systems. Agriculture, Ecosystems & Environment 204, 27–39.
| Legume pastures can reduce N2O emissions intensity in subtropical cereal cropping systems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXjtFegsL4%3D&md5=1e18334b81692abfbeea6425732375d5CAS |
Firestone MK, Smith MS, Firestone RB, Tiedje JM (1979) The influence of nitrate, nitrite, and oxygen on the composition of the gaseous products of denitrification in soil. Soil Science Society of America Journal 43, 1140–1144.
| The influence of nitrate, nitrite, and oxygen on the composition of the gaseous products of denitrification in soil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3cXht1Olsro%3D&md5=1ca22a3462a988fcbbedacca544e25cdCAS |
Flessa H, Dorsch P, Beese F (1995) Seasonal variation of N2O and CH4 fluxes in differently managed arable soils in southern Germany. Journal of Geophysical Research, D, Atmospheres 100, 23115–23124.
| Seasonal variation of N2O and CH4 fluxes in differently managed arable soils in southern Germany.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XltlGmsQ%3D%3D&md5=cb9920ca8fec4670425b933c77e61cd9CAS |
Gilliam JW, Dasberg S, Lund LJ, Focht DD (1978) Denitrification in four California soils: effect of soil profile characteristics. Soil Science Society of America Journal 42, 61–66.
| Denitrification in four California soils: effect of soil profile characteristics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1cXhvVyqsL0%3D&md5=c2bac36414751e136ffd496554685a73CAS |
Herridge DF (2011) ‘Managing legume and fertiliser N for northern grains cropping.’ (Grains Research and Development Corporation: Kingston, ACT, Australia)
Hoben JP, Gehl RJ, Millar N, Grace PR, Robertson GP (2011) Nonlinear nitrous oxide (N2O) response to nitrogen fertilizer in on-farm corn crops of the US Midwest. Global Change Biology 17, 1140–1152.
| Nonlinear nitrous oxide (N2O) response to nitrogen fertilizer in on-farm corn crops of the US Midwest.Crossref | GoogleScholarGoogle Scholar |
Holland J, Herridge D (1992) Production of summer crops in northern New South Wales. II. Effects of tillage and crop rotation on yields of sorghum. Australian Journal of Agricultural Research 43, 123–134.
| Production of summer crops in northern New South Wales. II. Effects of tillage and crop rotation on yields of sorghum.Crossref | GoogleScholarGoogle Scholar |
Isbell RF (2002) ‘The Australian soil classification.’ Revised edn (CSIRO Publishing: Melbourne)
Kim D-G, Hernandez-Ramirez G, Giltrap D (2013) Linear and nonlinear dependency of direct nitrous oxide emissions on fertilizer nitrogen input: A meta-analysis. Agriculture, Ecosystems & Environment 168, 53–65.
| Linear and nonlinear dependency of direct nitrous oxide emissions on fertilizer nitrogen input: A meta-analysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXltVyhs7k%3D&md5=3f8a4f707d6fb031dbf8da0b9d13c498CAS |
Linn DM, Doran JW (1984) Effect of water-filled pore space on carbon dioxide and nitrous oxide production in tilled and nontilled soils. Soil Science Society of America Journal 48, 1267–1272.
| Effect of water-filled pore space on carbon dioxide and nitrous oxide production in tilled and nontilled soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXhtFaitL4%3D&md5=0b26b0412d09d2e99729b94c3b26fe9aCAS |
Ma BL, Wu TY, Tremblay N, Deen W, Morrison MJ, McLaughlin NB, Gregorich EG, Stewart G (2010) Nitrous oxide fluxes from corn fields: on-farm assessment of the amount and timing of nitrogen fertilizer. Global Change Biology 16, 156–170.
| Nitrous oxide fluxes from corn fields: on-farm assessment of the amount and timing of nitrogen fertilizer.Crossref | GoogleScholarGoogle Scholar |
McSwiney CP, Robertson GP (2005) Nonlinear response of N2O flux to incremental fertilizer addition in a continuous maize (Zea mays L.) cropping system. Global Change Biology 11, 1712–1719.
| Nonlinear response of N2O flux to incremental fertilizer addition in a continuous maize (Zea mays L.) cropping system.Crossref | GoogleScholarGoogle Scholar |
Millar N, Robertson GP, Grace PR, Gehl RJ, Hoben JP (2010) Nitrogen fertilizer management for nitrous oxide (N2O) mitigation in intensive corn (maize) production: an emissions reduction protocol for US Midwest agriculture. Mitigation and Adaptation Strategies for Global Change 15, 185–204.
| Nitrogen fertilizer management for nitrous oxide (N2O) mitigation in intensive corn (maize) production: an emissions reduction protocol for US Midwest agriculture.Crossref | GoogleScholarGoogle Scholar |
Mosier AR, Delgado JA, Keller M (1998) Methane and nitrous oxide fluxes in an acid Oxisol in western Puerto Rico: effects of tillage, liming and fertilization. Soil Biology & Biochemistry 30, 2087–2098.
| Methane and nitrous oxide fluxes in an acid Oxisol in western Puerto Rico: effects of tillage, liming and fertilization.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXmslegsbY%3D&md5=7d7b68e1fa8581bb685ce834ff6bff2fCAS |
Myhre G, Shindell D, Bréon F-M, Collins W, Fuglestvedt J, Huang J, Koch D, Lamarque J, Lee D, Mendoza B, Nakajima T, Robock A, Stephens G, Takemura THZ (2013) Anthropogenic and natural radiative forcing. In ‘Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment. Report of the Intergovernmental Panel on Climate Change’. (Eds TF Stocker, D Qin, GK Plattner, M Tignor, SK Allen, J Boschung, A Nauels, Y Xia, V Bex, PM Midgley). (Cambridge University Press: Cambridge, United Kingdom and New York, NY, USA)
Parkin TB, Kaspar TC (2006) Nitrous oxide emissions from corn–soybean systems in the Midwest. Journal of Environmental Quality 35, 1496–1506.
| Nitrous oxide emissions from corn–soybean systems in the Midwest.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xns1Ckuro%3D&md5=6faed321832ffe93bdde349411bb624cCAS | 16825470PubMed |
Pedersen AR, Petersen SO, Schelde K (2010) A comprehensive approach to soil-atmosphere trace-gas flux estimation with static chambers. European Journal of Soil Science 61, 888–902.
| A comprehensive approach to soil-atmosphere trace-gas flux estimation with static chambers.Crossref | GoogleScholarGoogle Scholar |
Ramu K, Watanabe T, Uchino H, Sahrawat KL, Wani SP, Ito O (2012) Fertilizer induced nitrous oxide emissions from Vertisols and Alfisols during sweet sorghum cultivation in the Indian semi-arid tropics. The Science of the Total Environment 438, 9–14.
| Fertilizer induced nitrous oxide emissions from Vertisols and Alfisols during sweet sorghum cultivation in the Indian semi-arid tropics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsFyitbnL&md5=743ac5802e3cd65f273e6736612384ebCAS | 22967492PubMed |
Rochester IJ (2003) Estimating nitrous oxide emissions from flood-irrigated alkaline grey clays. Australian Journal of Soil Research 41, 197–206.
| Estimating nitrous oxide emissions from flood-irrigated alkaline grey clays.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXktFKisrw%3D&md5=3a475561eea619e333bdd85ec4b7f899CAS |
Rochester IJ, Constable GA (2000) Denitrification and immobilisation in flood-irrigated alkaline grey clays as affected by nitrification inhibitors, wheat straw, and soil texture. Soil Research 38, 633–642.
| Denitrification and immobilisation in flood-irrigated alkaline grey clays as affected by nitrification inhibitors, wheat straw, and soil texture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXktlGisrs%3D&md5=9aabd92a8d371868055804cd723bd739CAS |
Rochette P, Hutchinson GL (2005) Measurement of soil respiration in situ: chamber techniques. In ‘Micrometeorology in agricultural systems’. (Eds JL Hatfield, JM Baker, MK Viney) pp. 247–286. (American Society of Agronomy, Inc., Crop Science Society of America, Inc., Soil Science Society of America, Inc.: Madison, WI, USA)
Scheer C, Grace PR, Rowlings DW, Kimber S, Van Zwieten L (2011) Effect of biochar amendment on the soil-atmosphere exchange of greenhouse gases from an intensive subtropical pasture in northern New South Wales, Australia. Plant and Soil 345, 47–58.
| Effect of biochar amendment on the soil-atmosphere exchange of greenhouse gases from an intensive subtropical pasture in northern New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXptFymt7o%3D&md5=035eaf908fa5f45f19e185ef59a541bbCAS |
Scheer C, Grace P, Rowlings D, Payero J (2012) Nitrous oxide emissions from irrigated wheat in Australia: impact of irrigation management. Plant and Soil 359, 351–362.
| Nitrous oxide emissions from irrigated wheat in Australia: impact of irrigation management.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtlGmsrrJ&md5=0a0042c45a025374b5e699c821e096f1CAS |
Schwenke GD, Herridge DF, Scheer C, Rowlings DW, Haigh BM, McMullen KG (2015) Soil N2O emissions under N2-fixing legumes and N-fertilised canola: a reappraisal of emissions factor calculations. Agriculture, Ecosystems & Environment 202, 232–242.
| Soil N2O emissions under N2-fixing legumes and N-fertilised canola: a reappraisal of emissions factor calculations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhtVOgsL4%3D&md5=5e74db16ebc6b862a9ac74b9a0396a34CAS |
Schwenke GD, Herridge DF, Scheer C, Rowlings DW, Haigh BM, McMullen KG (2016) Greenhouse gas (N2O and CH4) fluxes under nitrogen-fertilised dryland wheat and barley on sub-tropical Vertosols: risk, rainfall and alternatives. Soil Research, 54, 634–650.
| Greenhouse gas (N2O and CH4) fluxes under nitrogen-fertilised dryland wheat and barley on sub-tropical Vertosols: risk, rainfall and alternatives.Crossref | GoogleScholarGoogle Scholar |
Serafin L, McCaffery D, Thompson S (2014) Sunflower. In ‘Summer crop production guide’. (Eds N Moore, L Serafin, L Jenkins) pp. 80–92. (NSW Department of Primary Industries: Orange, NSW)
Shcherbak I, Millar N, Robertson GP (2014) Global metaanalysis of the nonlinear response of soil nitrous oxide (N2O) emissions to fertilizer nitrogen. Proceedings of the National Academy of Sciences of the United States of America 111, 9199–9204.
| Global metaanalysis of the nonlinear response of soil nitrous oxide (N2O) emissions to fertilizer nitrogen.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXpsVamurY%3D&md5=8382fab4f0e01c7b581e0fdf91fd058aCAS | 24927583PubMed |
Smith MS, Tiedje JM (1979) The effect of roots on soil denitrification. Soil Science Society of America Journal 43, 951–955.
| The effect of roots on soil denitrification.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3cXitFemtQ%3D%3D&md5=6ca665c0dd1169c6f70bca1283e8cf23CAS |
Snyder CS, Bruulsema TW, Jensen TL, Fixen PE (2009) Review of greenhouse gas emissions from crop production systems and fertilizer management effects. Agriculture, Ecosystems & Environment 133, 247–266.
| Review of greenhouse gas emissions from crop production systems and fertilizer management effects.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXptl2ht7w%3D&md5=bbb71b3e2c594debba00dc99e62f971cCAS |
Storlien JO, Hons FM, Wight JP, Heilman JL (2014) Carbon dioxide and nitrous oxide emissions impacted by bioenergy sorghum management. Soil Science Society of America Journal 78, 1694–1706.
| Carbon dioxide and nitrous oxide emissions impacted by bioenergy sorghum management.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhslaqurzE&md5=ea4e9c1e7e1bf907142772196c70b973CAS |
Strong W, Saffigna P, Cooper J, Cogle A (1992) Application of anhydrous ammonia or urea during the fallow period for winter cereals on the Darling Downs, Queensland. II. The recovery of 15N by wheat and sorghum in soil and plant at harvest. Soil Research 30, 711–721.
| Application of anhydrous ammonia or urea during the fallow period for winter cereals on the Darling Downs, Queensland. II. The recovery of 15N by wheat and sorghum in soil and plant at harvest.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXlvVOrug%3D%3D&md5=e68a0d6bd36d046dd83985e0a45253d0CAS |
Van Groenigen JW, Velthof GL, Oenema O, Van Groenigen KJ, Van Kessel C (2010) Towards an agronomic assessment of N2O emissions: a case study for arable crops. European Journal of Soil Science 61, 903–913.
| Towards an agronomic assessment of N2O emissions: a case study for arable crops.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXks1Gr&md5=eeb4cb3f453a9f9bab1a9285109f287cCAS |
Verbyla AP, Cullis BR, Kenward MG, Welham SJ (1999) The analysis of designed experiments and longitudinal data by using smoothing splines. Journal of the Royal Statistical Society. Series C, Applied Statistics 48, 269–311.
| The analysis of designed experiments and longitudinal data by using smoothing splines.Crossref | GoogleScholarGoogle Scholar |
Wang W, Dalal RC, Reeves SH, Butterbach-Bahl K, Kiese R (2011) Greenhouse gas fluxes from an Australian subtropical cropland under long-term contrasting management regimes. Global Change Biology 17, 3089–3101.
| Greenhouse gas fluxes from an Australian subtropical cropland under long-term contrasting management regimes.Crossref | GoogleScholarGoogle Scholar |
Webb AA, Grundy MJ, Powell B, Littleboy M (1997) The Australian sub-tropical cereal belt: soils, climate and agriculture. In ‘Sustainable crop production in the sub-tropics: an Australian perspective’. (Eds AL Clarke, PB Wylie) pp. 8–26. (Queensland Department of Primary Industries: Brisbane)
Weier KL, Doran JW, Power JF, Walters DT (1993) Denitrification and the dinitrogen/nitrous oxide ratio as affected by soil water, available carbon, and nitrate. Soil Science Society of America Journal 57, 66–72.
| Denitrification and the dinitrogen/nitrous oxide ratio as affected by soil water, available carbon, and nitrate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXisFeqtLk%3D&md5=fd56bf11bee9b78d91071f533d1e8f08CAS |
Welzmiller JT, Matthias AD, White S, Thompson TL (2008) Elevated carbon dioxide and irrigation effects on soil nitrogen gas exchange in irrigated sorghum. Soil Science Society of America Journal 72, 393–401.
| Elevated carbon dioxide and irrigation effects on soil nitrogen gas exchange in irrigated sorghum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXjsFynsLc%3D&md5=627c4cf174ac011d9d7f227b12ab34b6CAS |








