Modelling nitrous oxide emissions: comparing algorithms in six widely used agro-ecological models
Hongtao Xing A B , Chris. J. Smith A * , Enli Wang A , Ben Macdonald
A * , Enli Wang A , Ben Macdonald  A and David Wårlind A C
A and David Wårlind A C
A CSIRO Agriculture, GPO BOX 1666, Canberra, ACT 2601, Australia.
B Present address: Department of Environment and Science, Queensland Government, Brisbane, Qld 4001, Australia.
C Institute for Physical Geography and Ecosystem Science, Lund University, Lund, Sweden.
Soil Research 61(6) 523-541 https://doi.org/10.1071/SR22009
Submitted: 12 January 2022 Accepted: 14 February 2023 Published: 6 March 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Agricultural soils are the most important anthropogenic source of nitrous oxide (N2O) emissions. This occurs via two main pathways: (1) from microbial-mediated oxidation of ammonium to nitrite and nitrate; and (2) denitrification. Most agro-ecological models explicitly deal with these two pathways albeit with different degrees of process understanding and empiricism. Models that integrate the impact of multiple environmental factors on N2O emissions can provide estimates of N2O fluxes from complex agricultural systems. However, uncertainties in model predictions arise from differences in the algorithms, imperfect quantification of the nitrification and denitrification response to edaphic conditions, and the spatial and temporal variability of N2O fluxes resulting from variable soil conditions. This study compared N2O responses to environmental factors in six agro-ecological models. The comparisons showed that environmental factors impact nitrification and denitrification differently in each model. Reasons include the inability to apportion the total N2O flux to the specific N transformation rates used to validate and calibrate the simplifications represented in the model algorithms, and incomplete understanding of the multiple interactions between processes and modifying factors as these are generally not quantified in field experiments. Rather, N2O flux data is reported as total or net N2O emissions without attributing emissions to gross and/or net rates for specific N processes, or considering changes that occur between production and emissions. Additional measurements that quantify all processes understand the multiple interactions that affect N2O emissions are needed to improve model algorithms and reduce the error associated with predicted emissions.
Keywords: agriculture, agro-ecological models, algorithm comparison, denitrification, N2O emissions, nitrification, soil, soil-atmosphere flux.
Introduction
Nitrous oxide is a long-lived greenhouse gas and the major source of stratospheric nitric oxides (NOx; Cicerone 1989; Williams et al. 1992), contributing approximately 6% to global warming (Dalal et al. 2003; Smith et al. 2008). Atmospheric N2O concentration has risen from the pre-industrial value (1850–1990) of 270 to 332 ppb (v/v) in 2019, with 70% of the increase occurring since 1970 (IPCC 2014, 2021). Soils are estimated to contribute between 15 and 30% of the anthropogenic N2O at the global scale (Bouwman 1990; Tubiello et al. 2013), and agriculture is cited as being the single largest contributor (Mosier et al. 1996, 1998; IPCC 2014). Since 1980, global agricultural expansion and intensification (increased nitrogen fertiliser and manure use) has accounted for 30% of the total increased N2O concentration (Tian et al. 2020; IPCC 2021). Globally, about 3–5% of the synthetic N fertiliser used in agriculture is estimated to be returned to the atmosphere as N2O (Crutzen et al. 2016). The predicted warmer and wetter conditions in response to climate change are expected to further increase N2O emissions (Griffis et al. 2017). The IPCC synthesis reports (IPCC 2014, 2021) and other studies (Bouwman 1989; Mosier et al. 1998; FAO/IFA 2001) suggest that more than 60% of the annual global N2O emissions from soils are from arable land.
Although N2O production relates to three or four microbial processes (Baggs 2011), about 70% of global N2O emissions are estimated to originate from microbial nitrification and denitrification (Braker and Conrad 2011; Syakila and Kroeze 2011). However, most models only simulate emissions from nitrification and denitrification, which are regulated by the oxygen (O2) status in soil profiles, mainly controlled by soil moisture (SW), temperature (Ts), carbon (C) and nitrogen (N) substrates, soil pH and the microbial community (Nömmik 1956; Focht 1974; Weier et al. 1993; Smith et al. 1998; Weier 1999; Saggar et al. 2013). The complex microbial production and consumption processes, and the links to biotic (species competition, plant–microbe interactions) and abiotic (climate, soil chemistry and physics) factors are not fully understood and quantified (Baggs 2011; Butterbach-Bahl et al. 2013). Emissions are highly variable and difficult to predict because soil N2O production and consumption are controlled by multiple biotic and abiotic factors within soil micro-sites (Butterbach-Bahl et al. 2013; Griffis et al. 2017). Recently, mechanistic approaches have been proposed to represent microsites in soil aggregates in models (Ebrahimi and Or 2018; Sihi et al. 2020). An ‘anaerobic balloon’, as used in the model DNDC, can be considered an early representation of non-uniformity in soil aeration status within any soil layer. Given that none of the models reviewed include microsites within soil aggregates, this was not considered in this review.
N2O emissions estimates from agricultural soils range from a simple emission factor (Bouwman 1996; IPCC 2014; Del Grosso et al. 2020 and reference within) to more complex process-based models such as APSIM (Keating et al. 2003; Holzworth et al. 2014), DayCent (Parton et al. 1996, 2001; Del Grosso et al. 2000), WNMM (Li et al. 2007) or DNDC (Li et al. 1992, 2000; Li 2000). Although emission factors are generally used for regional-scale inventories, process-based models capable of predicting the effect of environmental conditions and management practices play an important role in modifying emission factors (Xing et al. 2013; Shen et al. 2018), and may possibly replace them. Bouwman (1996) highlighted the inadequacy of emission factors for estimating emissions at the local scale. The recent trend is to use process-based models, such as DayCent and DNDC, to calculate field-scale N2O emissions, or to develop a series of emission factors for different land uses and management to estimate the larger scale fluxes (Li 2000; Del Grosso et al. 2005, 2006, 2020; Giltrap et al. 2010, 2011; Leip et al. 2011). Irrespective of the process-based model used, it is important to understand how processes are represented, the environmental rate modifiers and subsequent impact on model predictions.
Models play an essential role in estimating soil N2O emissions, understanding management impacts on N2O fluxes, simulating emissions over variable time scales, and extrapolating results to regional or national scales. Despite increasing applications, questions remain as to whether models can accurately predict the fluxes from soils in response to climate variability and change and agronomic management modifications (including fertiliser use). Giltrap et al. (2020) discussed how inaccuracies with model parameters can propagate through the model leading to uncertainty in predictions. When comparing a number of simplified denitrification process models, Heinen (2006) found that changes in SW were critical to estimating N2O emissions and must be accurately determined. Pan et al. (2022), using a global data set, found that denitrification rate was positively related to soil water-filled pore space (WFPS), NO3− concentration and soil temperature (P < 0.01), whereas the rate decreased with higher soil oxygen content (P < 0.01).
The most commonly used process-based models for predicting N2O production from soils include APSIM (Thorburn et al. 2010; Xing et al. 2011), DayCent (Del Grosso et al. 2000; Parton et al. 2001), DNDC (Li 2000; Li et al. 2000), FASSET (Chatskikh et al. 2005), NOE (Hénault et al. 2005) and WNMM (Li et al. 2007). Chen et al. (2008) reviewed and summarised the strengths, limitations and applications of commonly used field scale N2O emissions models (i.e. DayCent, DNDC, NLOSS, ecosyss, Expert-N, FASSET, WNMM, and CERES-NOE). They concluded that significant gaps existed in the accurate partitioning of denitrification products and the diffusion and assimilation of N2O from depth. More recently, Giltrap et al. (2020) reviewed the concepts of APSIM, DayCent and DNDC. Other studies have compared the performance of process-based models at predicting N2O emissions (e. g., Li et al. 2007; Xing et al. 2011; Ehrhardt et al. 2018). There are two ways to compare model performance: (1) extracting the specific algorithms from the different models and recoding them in the same platform (model), then comparing N2O emissions prediction performance after calibrating and validating against measurements (e.g. Li et al. 2007; Xing et al. 2011; Inatomi et al. 2019); and (2) comparing the predicted productivity and N2O emissions by applying default parameters (benchmark simulations) to multiple process-based models using experimental data from multiple sites (e.g. Ehrhardt et al. 2018). Interestingly in the latter study, the prediction errors for N2O emissions only marginally improved after model calibration. Evaluating model structure and performance helps to explain model differences. The consensus from these studies is that the processes controlling N2O emissions involve multiple complex interactions.
Uncertainty still exists regarding the spatial and temporal variability of N2O emission as a function of soil properties and nitrogen fertiliser applications. Furthermore, not all the relevant environmental and biogeochemical drivers and processes are currently represented in the most widely used models. Del Grosso et al. (2020) suggested that a systematic evaluation of N2O and N2 production from nitrification and denitrification in relation to key drivers and comparison to measurements is needed to highlight the algorithms and parameter values most responsible for inaccuracies in predicted N2O emissions.
As models become more mechanistic, they should, in principle, be better suited to simulate the impacts of site-specific climate, soil drivers, and management on N2O emissions (Giltrap et al. 2020). However, increasing the complexity in a model is only useful if, for example, the measured total N2O emission can be attributed to specific N transformation rates, microbial, and physical processes (Zhang et al. 2015a). Most process-based models have been evaluated against a range of field experiments using net N2O emissions (see for example Frolking et al. 1998; Li et al. 2005; Vogeler et al. 2011). Li et al. (2005) compiled the algorithms for predicting N2O emissions from WNMM, DayCent and DNDC into the WNMM framework, and compared the performance of the different modules. Vogeler et al. (2011) compared APSIM and DNDC predictions for nitrification, denitrification and N2O emissions from pastoral systems. They found the SW threshold value, Ts, and potential denitrification rate were important for predicting N2O emissions from agricultural soils. Thorburn et al. (2010) and Xing et al. (2011) evalutated APSIM against field and laboratory data sets on net N2O emissions and found that APSIM underestimated N2O emissions from denitrification. Xing et al. (2011) highlighted the importance of soil depth when simulating emissions as the atmospheric emission includes variable contributions of N2O produced in deeper layers. Del Grosso et al. (2010) showed that in DayCent, spatial variblity was mainly a result of differences in fertiliser or organic N additions, whereas temporal variablity in the annual N2O emission was correlated with N mineralisation rates and climatic varialibity. Such comparisons indicate a need to better quantify the controlling variables on nitrification, denitrification and N2O emissions. Accurate simulation of SW dynamics and its linkage to denitrification and N2O flux is a key component of each model (Frolking et al. 1998). Predicting N2O emissions is further challenged by a range of measurement artefacts, primarily SW dynamics and Ts, caused by the use of field chambers and laboratory incubations in calibration and validation data sets (Davidson et al. 2002; Livingston et al. 2006; Debouk et al. 2018). The temporal frequency of these measurements is also critical thus ensuring relatively large episodic events are captured (Grace et al. 2020).
Model comparisons, across a range of scales, indicate that model structure and parameterisation lead to different spatial and temporal patterns of the simulated N2O emissions (Chen et al. 2008; Wu et al. 2015; Tian et al. 2018). At global and regional scales, uncertainty in agronomic management (cultivation, nitrogen fertiliser applications and species) results in significant variations in simulated N2O emissions (Wu et al. 2015; Tian et al. 2018). Simulations of annual cumulative N2O emissions by DNDC, LandscapeDNDC and IAP-N-GAS aligned well with annual observations, but there were discrepancies between simulated and observed daily fluxes related to the date, duration, and the magnitude of the peak fluxes (Zhang et al. 2015a). The error in daily predicted N2O fluxes was hypothesised to be due to inaccurate SW prediction.
Specific comparisons between model components to understand the effect of process modifiers on the simulated N2O fluxes are rarely done. This study evaluated the algorithms in six process-based models (APSIM, DayCent, DNDC, FASSET, NOE, and WNMM) for nitrification and denitrification, and N2O emissions from these processes, including the effect of environmental rate modifiers. We show the difference in the algorithms for modelling nitrification, denitrification and N2O fluxes and identify the measurements needed to improve model process-understanding and predictability for soil N2O emissions.
Major modelling processes
Conceptual approach to model nitrification and denitrification
The biochemical processes that lead to N2O production during nitrification and denitrification are well known (Sahrawat and Keeney 1986; Firestone and Davidson 1989; Davidson 1993; Braker and Conrad 2011; Syakila and Kroeze 2011). Most of the research related to N2O emissions explores emission response to environment, soil and land management in different agro-ecological zones (see for example, Li 2000; Del Grosso et al. 2010; Tian et al. 2018; Yu and Zhuang 2019).
All of the models reviewed in this study predict N2O emissions from nitrification and denitrification and except for DNDC, ignore chemical pathways of N2O production (Chalk and Smith 1983, 2020; Howden and O’Leary 1997). Additionally, N2O emission during the NO3− reducing processes of nitrate ammonification (dissimilatory NO3− reduction to NH4+), and co-denitrification are not represented in current models (Baggs 2011).
Nitrification is regarded as the main source of N2O emissions during the oxidation of NH4+ or heterotrophic nitrification of organic N to nitrate (NO3−) in aerobic soil (Bremner and Blackmer 1978; Butterbach-Bahl et al. 2013; Zhang et al. 2015b; Inatomi et al. 2019). In contrast, denitrification is the main source of N2O emissions under anaerobic conditions (Bremner and Shaw 1958; Focht 1974; Bremner and Blackmer 1980; Tiedje et al. 1983; Butterbach-Bahl et al. 2013) where NO3− is reduced to dinitrogen (N2) and oxygen (O2) and the NO3− is used as the terminal electron acceptor. Although denitrification is an anaerobic process, in soils it can occur at high O2 pressures because of anaerobic microsites within aggregates in otherwise aerobic soil (Khalil et al. 2004; Sihi et al. 2020).
The models reviewed in this study use two different approaches to model N2O production as a function of aeration and the availability of oxygen, which is commonly indexed using SW or more precisely the WFPS (the equations used to calculate soil moisture content (SW) and soil water-filled porosity (WFPS) are given in Cresswell and Hamilton (2002)). The first approach assumes that denitrification occurs only if WFPS exceeds a ‘threshold’ value that would indicate anaerobiosis. The WFPS threshold value for the various models range from 0.55 to 0.62. Nitrification is also a function of WFPS and nitrification and denitrification can occur simultaneously in the same soil layer. DayCent, WNMM, NOE, FASSET and APSIM adopt this approach. Field or laboratory measurements generally give total N2O emissions and cannot ascribe the fluxes to either nitrification or denitrification. The use of 15N isotopes, added to different mineral N pools, is required to attribute N2O emissions to their source processes (Zhang et al. 2015b; Denk et al. 2019).
The second approach assumes that the soil is partitioned into aerobic and anaerobic fractions within each soil layer. Nitrification occurs in the aerobic fraction while denitrification happens in the anaerobic fraction. The size of the aerobic or anaerobic fraction is calculated as a function of the ‘bulk’ soil redox potential (Eh). This approach is used by DNDC to simulate N2O emission from both nitrification and denitrification in the same soil layer at the same time (Li et al. 2000). The size of the anaerobic fraction is defined by the simulated O2 partial pressure calculated from O2 diffusion and consumption rates in the soil (Table 1, Li et al. 2000).
|
Nitrification and associated N2O production
Modelling N2O production from nitrification
Each of the reviewed models simulate net nitrification rates (see Supplementary Table S1). The algorithms therein do not distinguish the distinct phases of nitrification, namely NH4+ oxidation to NO2− and NO2− oxidation to NO3−. Nitrite is generally not considered because of its low concentration relative to NH4+ and/or NO3−. However, high concentrations of NO2− can occur following banded applications of urea or anhydrous ammonia (NH3) that uncouples the two phases of nitrification (Chalk et al. 1975; Venterea et al. 2020). In the models reviewed, NH4+ concentration, SW, Ts, and pH regulate nitrification, although not all factors are included in the different algorithms (Hanson et al. 2000; Li et al. 2000, 2007; Parton et al. 2001; Thorburn et al. 2010). Nitrifier biomass is included in DNDC (Li et al. 2000). Nitrous oxide is produced as an intermediate gaseous product during the microbial oxidation of NH4+ to NO3−. Since there is insufficient data to quantify the intermediary steps and regulatory mechanisms for N2O production during nitrification, the process is represented as a ‘hole-in-the-pipe’, or ‘leaky pipe’ first proposed by Firestone and Davidson (1989). In most models, a proportion of nitrified NH4+ is emitted as N2O (Fig. 1; Li et al. 2000, 2007; Parton et al. 2001; Khalil et al. 2004; Chatskikh et al. 2005). Recently, Zhang et al. (2015b) reported significant gross heterotrophic nitrification of organic N and N2O emissions from acidic soil with high C/N ratio. They proposed introducing N2O production via heterotrophic nitrification of organic N into the conceptual ‘hole-in-the-pipe’ model of N2O emission. Currently, the models reviewed in this study are calibrated to represent net N2O emissions without distinguishing between autotrophic and heterotrophic nitrification.
Effect of NH4+ concentration on nitrification
The models reviewed in this study simulate different nitrification rates under a given NH4+ concentration even when other soil conditions (temperature, water, and pH) are at the optimal level. Nitrification is therefore not limited by any factor except NH4+ concentration (Fig. 2a). The relationship between nitrification rates and NH4+ concentration is as a linear response in DNDC, FASSET and WNMM. Michaelis–Menten kinetics is used in APSIM, and a Langmuir isotherm equation in used NOE. The detailed equations for nitrification and the modifiers in the models are given in Table S1.
Potential nitrification in APSIM follows Michaelis–Menten kinetics (Meier et al. 2006) with a maximum reaction rate (Vmax) of 40 mg NH4+-N kg−1 soil day−1 and a NH4-N concentration of 90 mg NH4+-N kg−1 soil at Vmax/2 (Km) as default. Potential nitrification rate is also modified by SW, and Ts. Meier et al. (2006) have suggested that the values of Vmax and Km should be reduced to 12 mg NH4+-N kg−1 soil day−1 and 30 mg NH4+-N kg−1 soil, respectively. Similarly, Smith et al. (2020) suggested that the parameter values in APSIM need to be reduced and a minimum NH4+ concentration introduced into the model. Meier et al. (2006) cite Reuss and Innis (1977) as the source of APSIM’s default values. However, there is some confusion regarding the units as Reuss and Innis (1977) list the units for Vmax and Km as being g N m−3 day−1 and g m−3, respectively. They do have the same value when the soil bulk density is 1.
In DayCent, nitrification follows exponential decay (1 – EXP form) that asymptotes at the maximum rate of 1 (Fig. 2a; Table S1) with a base flux equivalent to 0.1 g N ha−1 day−1. For comparison purposes we convert all the model outputs to the dimension ha−1 day−1 and volumetric soil water content, assuming a bulk of 1480 kg m−3 in the surface 0.15 m. A minimum NH4+ concentration is set to allow nitrification to occur. The effect of NH4+ on nitrification is very similar in DayCent and APSIMmax (scaled using the maximum value), but if the APSIM rate is scaled using Vmax, the maximum value is 83% of what is given in DayCent.
Although the effect of NH4+ concentration in DayCent and APSIM appear to be similar (Fig. 2a), there is a maximum fraction of NH4+ nitrified in DayCent (Parton et al. 1996, 2001). The equations are (Parton et al. 1996):
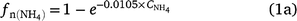
where 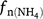 is the fraction of NH4+ nitrified and
is the fraction of NH4+ nitrified and 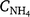 is the ammonium concentration in mg kg−1 soil. Eqn (1a) was revised to include net daily mineralisation (Eqn 1b; Parton et al. 2001).
is the ammonium concentration in mg kg−1 soil. Eqn (1a) was revised to include net daily mineralisation (Eqn 1b; Parton et al. 2001).
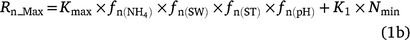
Rn_Max is the maximum fraction of NH4+ that goes to NO3−˙ (gN m−2 day−1), Nmin is the daily net N mineralisation from the soil organic matter decomposition, K1 is the fraction of Nmin that is assumed to be nitrified each day (K1 = 0.2), 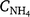 is the model-derived soil ammonium concentration (gN m−2), Kmax is the maximum fraction of NH4+ nitrified (Kmax = 0.10 day−1), fn(ST) is the effect of soil temperature on nitrification, fn(SW) is the effect of soil water content and soil texture, and fn(pH) is the effect of soil pH on nitrification. The absolute maximum rate (Rp_n = 0.4; it has a similar meaning to the potential nitrification rate used in other models) used for the comparison (Fig. 3), was taken from the nitrification code (SJ Del Grosso, pers. comm.).
is the model-derived soil ammonium concentration (gN m−2), Kmax is the maximum fraction of NH4+ nitrified (Kmax = 0.10 day−1), fn(ST) is the effect of soil temperature on nitrification, fn(SW) is the effect of soil water content and soil texture, and fn(pH) is the effect of soil pH on nitrification. The absolute maximum rate (Rp_n = 0.4; it has a similar meaning to the potential nitrification rate used in other models) used for the comparison (Fig. 3), was taken from the nitrification code (SJ Del Grosso, pers. comm.).
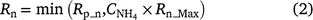
In NOE, the relationship between nitrification and NH4+ follows a Langmuir isotherm-type equation (Fig. 2a) where NH4+ only effects the nitrification rate at low concentrations and achieves 80% of the maximum rate at 10 mg NH4+-N kg−1 soil.
Nitrification rate response to soil moisture, temperature, and pH
Whilst all the models recognise O2 as a key controlling factor of nitrification, soil O2 dynamics are difficult to measure and predict. Soil moisture content is often used as a proxy for O2 status (Heinen 2006). DNDC simulates O2 partial pressure, which is calculated based on O2 diffusion and consumption rates in the soil and partitions the soil into aerobic and anaerobic fractions (Li et al. 1992, 2000; Li 2000). As stated previously, nitrification in DNDC is only allowed to occur in the aerobic fraction. APSIM and WNMM use volumetric water content, DayCent and NOE use WFPS, and FASSET uses soil water potential (ψ) to describe the impacts of O2 on nitrification. For a given soil type, volumetric water content and soil water potential are related to WFPS, which enables the response to nitrification rate to changes in soil water conditions to be compared. For the comparison in this review, SW was converted to ψ using the equation in van Genuchten (1980) and the soil water characteristic, [θ(ψ)], measured on surface layer of a Solodic soil (Smith et al. 2020). The comparison revealed significant differences between the models in depicting the effect of SW on nitrification (Fig. 2b). The differences are reflected not only in the threshold values (i.e. the range of WFPS within which nitrification occurs), but also in the type of response within the ranges (Fig. 2b). The response to SW was the same in WNMM and APSIM until about 0.65 WFPS. The modifier increases around 0.4 WFPS and decreases around 0.8 WFPS. The response in FASSET, which uses water potential, was different. Decreasing the ψ from −310 MPa to −31 MPa (Petersen et al. 2005a) means that the modifier remains at 1 over a larger range of WFPS before declining to 0.6 as ψ approaches 0. As outlined in Cresswell (2002), the shape of the water characteristic curves varies with soil texture. The use of ψ requires knowledge of the θ(ψ) characteristics of the soil layers.
Whilst DNDC uses a simple equation to describe the effect of moisture when WFPS > 0.05, (and 0 when WFPS is <0.05), the water factor modifies the relative growth rate of the nitrifiers (Li 2000). In turn, the nitrification rate is a function of the net increase in nitrifier biomass. DNDC also allocates NH4+ substrate between the aerobic and anaerobic soil fractions and only the substrate in the aerobic fraction is available for nitrification. The detailed equations for DNDC are given in the Appendix of Li (2000).
APSIM and WNMM have a narrow range for the effect of SW (and WPFS) on nitrification (Fig. 2b). Pan et al. (2021) reported that APSIM and WNMM estimated no nitrification when the WFPS was <0.3. However, their global analysis (Pan et al. 2022) indicates nitrification can occur when WFPS is <0.3. Based on studies in sandy soils of Western Australia, Asseng et al. (1998) reduced the lower limit for nitrification in APSIM to 5% of the potential rate at the lower limit of plant available water (LL). The default potential mineralisation rate and the production of NH4+ substrate for nitrification in APSIM was developed for medium- and fine-textured soils and higher mineralisation rates are more appropriate for coarser-textured soils (Asseng et al. 1998; Mielenz et al. 2016a).
Large variations exist in the models in the values used to represent the dimensionless modifier of nitrification to Ts (Fig. 2c). There are contrasting temperature thresholds (minimum, optimum and maximum temperatures), and different response curves employed between models. For example, DayCent uses an optimal temperature for nitrification that is a function of the average maximum monthly air temperature for the warmest month of the year (Parton et al. 2001).
Both DNDC and DayCent assume nitrification occurs in pH range 0–14. DNDC increases the nitrification rate linearly with soil pH, while DayCent uses a logistic curve and simulates the maximum nitrification rate at pH 9.0 (Fig. 2d). Nitrification is assumed to only occur between pH values of 4–9 in APSIM, and 4.5 to 10 in WNMM, with both models using a trapezoid response curve (Fig. 2d). FASSET and NOE do not consider the effect of pH on nitrification. However, there is compelling evidence that under alkaline conditions, NO2− accumulates due to the inhibition of NO2− oxidising organisms and is a precursor to biogenic and abiotic formation of N2O (Chalk and Smith 1983, 2020).
Of all the models reviewed, only DNDC considers the impact of soluble organic carbon on nitrification. It assumes that the population of nitrifiers (Ug) increases with dissolved organic carbon (CDOC; kg C ha−1) according to the equation:
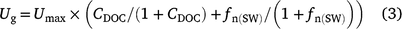
where Umax is the maximum growth rate for nitrifiers (4.87 day−1) and fn(SW) is a moisture factor (Li et al. 2000). The relative growth rate is calculated with double-Monod kinetics, which has the same form as the Michaelis–Menten equation (Li et al. 1992, 2000).
Combined effect of NH4+ concentration and soil moisture on the absolute nitrification rate
Ammonium concentration and SW content are the two most important factors that influence the rate of nitrification. 3D response surfaces (Fig. 3) demonstrate the combined effects of these two factors on the daily nitrification rate in the different models with the WFPS scaled between the lower limit (LL; volumetric water content) and drained upper limit (DUL). The other rate modifiers (soil pH and Ts) were set to 1, equivalent to the most favourable conditions for nitrification.
Changes in NH4+ concentrations and SW (and the derived WFPS) produce distinctly different daily nitrification rates for each of the models (Fig. 3). WNMM produces the highest nitrification rate, although the effect of SW on nitrification rate is similar to that observed in APSIM (Fig. 2b). At SW below LL, the water factor for nitrification is zero, and this increases linearly to 1 at a soil water content of LL + 0.25 × (DUL-LL). The lowest daily nitrification rates were predicted by the NOE and DayCent models. The nitrification rate in DayCent reached a maximum value at a NH4+ concentration of 12 mg N kg−1 soil but increased in response to increases in WFPS. In the NOE model, there is a large initial increase in the daily nitrification rate with increasing NH4+-N concentration.
N2O production from nitrification
The regulating mechanisms for quantifying the fraction of N2O 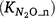 produced in the nitrification process are important but poorly understood (Inatomi et al. 2019). Only a few studies have investigated the responses of
produced in the nitrification process are important but poorly understood (Inatomi et al. 2019). Only a few studies have investigated the responses of 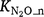 to Ts and moisture content (Stark and Firestone 1996; Bateman and Baggs 2005). Farquharson (2016) found no strong relationship between the fraction of N2O emitted from nitrification and environmental factors that included soil moisture. Between 0.03% and 1% of N was released as N2O accompanying nitrification in soils. Inatomi et al. (2019) also found no consistent relationship between
to Ts and moisture content (Stark and Firestone 1996; Bateman and Baggs 2005). Farquharson (2016) found no strong relationship between the fraction of N2O emitted from nitrification and environmental factors that included soil moisture. Between 0.03% and 1% of N was released as N2O accompanying nitrification in soils. Inatomi et al. (2019) also found no consistent relationship between 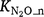 and environmental factors. When summarised, the results from these studies indicate
and environmental factors. When summarised, the results from these studies indicate 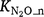 ranged from 0.006 to 29.4% with a median value of 0.19%. After removing outliers from the data, the mean was 0.43% and the median was 0.14%.
ranged from 0.006 to 29.4% with a median value of 0.19%. After removing outliers from the data, the mean was 0.43% and the median was 0.14%.
The generalised equations used to predict N2O production from nitrification (N2On) are given in Eqns 5–7. The equations used in each model are given in Table S1.
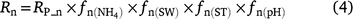
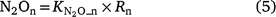
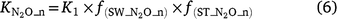
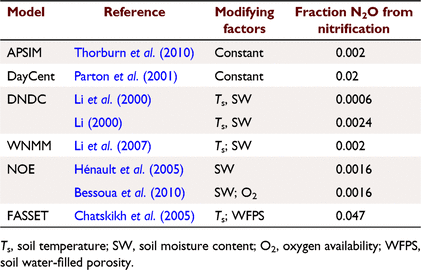
where Rn and RP_n are the actual and potential nitrification rates (kg N ha−1 day−1), respectively; and 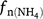 , fn(SW), fn(ST) and fn(pH) are the rate modifiers for the effects of NH4+ concentration, soil moisture, Ts and pH conditions on nitrification, respectively. N2On is the N2O flux produced from nitrification; K1, is the potential fraction of nitrified N lost as N2O flux, and ranges from 0.002 to 0.047 in different models (Table 1);
, fn(SW), fn(ST) and fn(pH) are the rate modifiers for the effects of NH4+ concentration, soil moisture, Ts and pH conditions on nitrification, respectively. N2On is the N2O flux produced from nitrification; K1, is the potential fraction of nitrified N lost as N2O flux, and ranges from 0.002 to 0.047 in different models (Table 1); 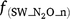 and
and 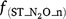 are the rate modifiers for the effects of soil moisture and Ts on K1, respectively. Additionally, soil moisture and Ts influence N2O production and the diffusion of N2O to the atmosphere (Davidson and Swank 1986; Clough et al. 2005). In APSIM and DayCent, once the N2O is produced, it is directly released to the atmosphere.
are the rate modifiers for the effects of soil moisture and Ts on K1, respectively. Additionally, soil moisture and Ts influence N2O production and the diffusion of N2O to the atmosphere (Davidson and Swank 1986; Clough et al. 2005). In APSIM and DayCent, once the N2O is produced, it is directly released to the atmosphere.
Although DNDC uses the same ‘leaky pipe’ concept, it is represented as (Li et al. 2000):
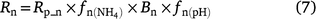
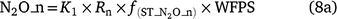
The equation to predict N2O from nitrification reported in Li (2000) is:

where K1 is 0.0006 (or 0.0024 in Eqn 8b), Rn is the nitrification rate, Rp_n is maximum nitrification rate (1/h), which is similar to the definition of potential nitrification rate used in other models, 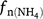 is the rate modifiers for the effects of NH4+ concentration (kg N ha−1) on nitrification and Bn is the biomass of nitrifiers (kg C ha−1),
is the rate modifiers for the effects of NH4+ concentration (kg N ha−1) on nitrification and Bn is the biomass of nitrifiers (kg C ha−1), 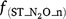 is temperature factor and WFPS is water-filled porosity. The main difference between Eqn 7 and Eqn 4 is that the biomass of nitrifiers (Bn) is included in Eqn 7, which is driven by dissolved organic carbon, SW and Ts (Table S1). In summary, the proportion of K1 is a constant in APSIM, and DayCent (Parton et al. 2001; Thorburn et al. 2010), whereas it is modified by changes in soil moisture content in NOE (Khalil et al. 2004), and changed with Ts and SW in DNDC, FASSET and WNMM (Li et al. 2000, 2007; Chatskikh et al. 2005).
is temperature factor and WFPS is water-filled porosity. The main difference between Eqn 7 and Eqn 4 is that the biomass of nitrifiers (Bn) is included in Eqn 7, which is driven by dissolved organic carbon, SW and Ts (Table S1). In summary, the proportion of K1 is a constant in APSIM, and DayCent (Parton et al. 2001; Thorburn et al. 2010), whereas it is modified by changes in soil moisture content in NOE (Khalil et al. 2004), and changed with Ts and SW in DNDC, FASSET and WNMM (Li et al. 2000, 2007; Chatskikh et al. 2005).
The fraction of N2O produced by nitrification increases linearly with WFPS in FASSET to a maximum of 1% (Fig. 4a). In WNMM, no N2O is produced when WFPS is <0.25, a linear increase to 1% at a WFPS of 0.4, plateauing between 0.4 and 0.8 WFPS and then a linear decline to 0% at SW saturation. In NOE, the WFPS modifier increases slowly from 0.06 to 0.12 within the range of WFPS that nitrification is considered to occur (Fig. 4a).
FASSET and WNMM both assume that there is an optimum Ts (35°C) for the fraction of N2O emitted during nitrification (Fig. 4b). This fraction decreases when Ts is higher than the optimum temperature in FASSET, whereas in WNMM Ts above the optimum temperature has limited effect.
Denitrification and associated N2O production
Concept in modelling denitrification and N2O production
Except for DNDC, the models reviewed use the ‘leaky pipe’ concept to simulate denitrification and associated N2O production (Fig. 1). The general equations for calculating daily denitrification rate and N2O emission from denitrification are given in Eqns 9–11. The equations used in each model are given in Tables S2 and S3. Denitrification is calculated as a function of NO3−, SW, Ts, pH, and soil organic carbon (SOC) (Eqn 9). The amount of N2O produced from denitrification is a function of the total denitrification (N2O plus N2). The fraction of N2O to total denitrification (Eqn 11) is positively related to SOC, SW, and Ts, and inversely related to NO3− concentration (Parton et al. 2001; Khalil et al. 2004; Chatskikh et al. 2005; Li et al. 2007). The algorithms to calculate N2O from denitrification in APSIM came from DayCent (Thorburn et al. 2010). The models reviewed use different OC components to modify the denitrification rate and associated N2O emissions. To develop a generic set of equations, we used OC as a surrogate for the different C pools in Eqn 9–11. The specific component of OC used is given in Tables S2 and S3.
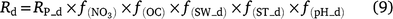
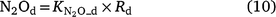
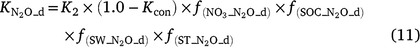
where Rd and RP_d are the actual and potential rates of denitrification (kg N ha−1 day−1); 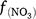 , f(OC), f(SW_d), f(ST_d) and f(pH_d) are dimensionless rate modifiers for the effects of NO3−, OC, SW, Ts and pH on denitrification, respectively. N2Od is the N2O flux produced from denitrification (kg N ha−1 day−1); K2 is the potential fraction of N2O production to the total denitrification gases (NO, N2O and N2). Kcon is the N2O consumption rate during diffusion in the soil and is estimated from clay content and the depth at which the N2O was produced in the soil profile. The terms
, f(OC), f(SW_d), f(ST_d) and f(pH_d) are dimensionless rate modifiers for the effects of NO3−, OC, SW, Ts and pH on denitrification, respectively. N2Od is the N2O flux produced from denitrification (kg N ha−1 day−1); K2 is the potential fraction of N2O production to the total denitrification gases (NO, N2O and N2). Kcon is the N2O consumption rate during diffusion in the soil and is estimated from clay content and the depth at which the N2O was produced in the soil profile. The terms 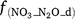 ,
, 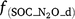 ,
, 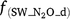 and
and 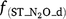 are rate modifiers for the effects of NO3− concentration, OC, SW and Ts on K2.
are rate modifiers for the effects of NO3− concentration, OC, SW and Ts on K2.
In DNDC (Li 2000; Li et al. 2000), denitrification is simulated using a stepwise approach according to the biochemical process. The relative intensity of each step is related to the activity of the generic denitrifying microorganisms and is controlled by Eh, SW, Ts, pH, concentration of nitrogen oxides (NO3−, NO2−, NO and N2O) and dissolved organic carbon (DOC). The general equation for denitrification (Eqn 9) can be modified to Eqn 12 to estimate the activity of all denitrifier groups. The stepwise equations are listed in Table S3. Denitrifier activity for each step is a function of the activity of all the denitrifying populations and is modified by soil moisture (f(SW_d)), temperature (f(ST_d)), pH (f(pH_d)) and the substrates (DOC and NO3−, or NO2−, or NO, or N2O; see Eqn 13). Net N2O production (N2Od_E) is estimated as the difference in denitrifier activity between N2O production (NO2− → N2O and NO → N2O) and N2O reduction (N2O → N2), and N2O consumption (reduction of N2O to N2) as the gas diffuses through the soil to the atmosphere (Eqn 14).
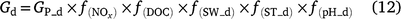
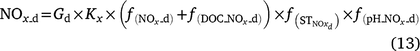
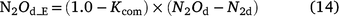
where Gd is the activity of all denitrifying microorganisms; GP_d is the potential denitrifier activity; 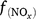 , f(DOC), f(SW_d), f(ST_d), and f(pH_d) are the dimensionless rate modifiers for the effects of nitrogen oxides (that includes NO3−, NO2−, NO, N2O) concentrations, DOC, SW, Ts and pH on denitrifier activity, respectively; NOx_d is the production of NOx (NO2−, NO, N2O and N2); Kx is the potential fraction of NOx (NO2−, NO, N2O) production; The terms
, f(DOC), f(SW_d), f(ST_d), and f(pH_d) are the dimensionless rate modifiers for the effects of nitrogen oxides (that includes NO3−, NO2−, NO, N2O) concentrations, DOC, SW, Ts and pH on denitrifier activity, respectively; NOx_d is the production of NOx (NO2−, NO, N2O and N2); Kx is the potential fraction of NOx (NO2−, NO, N2O) production; The terms 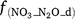 ,
, 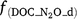 ,
, 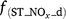 and
and 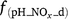 are the rate modifiers based on the concentrations of the substrates (NO3−, NO2−, NO, N2O and DOC), soil temperature and pH on Gd; Kcom is the N2O consumption rate during diffusion in the soil.
are the rate modifiers based on the concentrations of the substrates (NO3−, NO2−, NO, N2O and DOC), soil temperature and pH on Gd; Kcom is the N2O consumption rate during diffusion in the soil.
Denitrification in response to NO3− and soil organic carbon
If other factors are non-limiting, the effect of NO3− concentration on denitrification, or denitrifier activity is handled differently in the various models. APSIM and WNMM assume that the denitrification rate increases linearly with NO3− concentration. In contrast, DayCent adopts a power function to describe the impact of NO3−, and NOE and FASSET use a Langmuir-type equation (Fig. 5a; Tables S2 and S3).
Assuming other factors are non-limiting, the effect of SOC, or its surrogate (e.g. soil heterotrophic CO2 respiration as used in DayCent) has either no impact (NOE) or a significant impact (APSIM, DayCent, DNDC, FASSET and WNMM) on denitrification. DNDC assumes that denitrifier activity continues to increase in response to increasing DOC. Denitrification potential (g N m−2 day−1) in FASSET is assumed to be proportional to the SOC mineralisation rate (g C m−2 day−1) and to clay content (Chatskikh et al. 2005). DayCent and WNMM assume that denitrification rate asymptotes to the maximum with increasing soil heterotrophic CO2 respiration (DayCent) or organic carbon content (Fig. 5b). DayCent and FASSET assume a combined effect of NO3− and SOC on denitrification. Liebig’s Law of the Minimum is used in DayCent to describe the combined effect, thus, at any given time either NO3− or CO2 limits the denitrification rate to a value permitted by the most limiting substrate (Parton et al. 1996; Del Grosso et al. 2000). APSIM uses active carbon defined by the organic C concentration in soil humus (HUM) and fresh organic (FOM) carbon pools (Thorburn et al. 2010).
Response to soil physical factors
Apart from DNDC, the models assume that there is a lower limit to WFPS at which denitrification occurs. The dimensionless SW modifier (f(SW_d)) increases from 0 to 1 as SW or WFPS increase from the lower limit to the upper limit, following different curves (Fig. 5c). The lower and upper limits of WFPS vary among the models, reflecting slightly different representations of the same generalised equation. In DNDC, SW content determines the size of the anaerobic zone that controls the size of the denitrifier population, and this will modify the activity and capacity of each stage during the transformation of NO3− to N2 (Li 2000). Denitrification was found to be very sensitive to WFPS or porosity at which denitrification commences in APSIM (dnitlim; Mielenz et al. 2016a, 2016b). Mielenz et al. (2016a) hypothesised that the relationship between dnitlim and WFPSDUL is a soil specific variable that may be predicted from DUL.
Denitrification rate is modified by Ts in all the models except DayCent. Although DayCent assumes Ts has no direct effect on denitrification, Ts it does modify nitrification and NO3− production (Fig. 5d). The other models assume that denitrifier activity increases in response to Ts between a lower and upper limit. The lower limit, optimum Ts and the response function vary in each of the models.
Except for DNDC, all the models assume that the pH has no effect on denitrification. In DNDC, denitrification does not occur if the soil pH is <4.2 or >9.5 and occurs at the maximum rate when the soil pH is between 6.0 and 7.0 (Fig. 5e).
Soil texture (clay content) is directly considered in FASSET (Fig. 5f) whereas, APSIM, DayCent, DNDC and WNMM consider it to have an indirect effect. APSIM and DNDC consider soil texture effects denitrification through soil water retention curves. For example, WFPS at DUL is the threshold for denitrification in APSIM, and DUL varies with soil texture and depth. Mielenz et al. (2016a) reported variability in WFPS or porosity at which denitrification commences for different soils. Consistent with the suggestion of Mielenz et al. (2016a), DayCent and WNMM assume that denitrification response curves to SW change with texture. Three soil textural classes (coarse, medium, and fine) are used in DayCent to predict denitrification response to WFPS, whereas six soil textural classes are considered in WNMM.
Soil water content or WFPS may not adequately represent the O2 partial pressure that regulates nitrifier and denitrifier activity in different soils or within soil aggregates (Li et al. 2000). Del Grosso et al. (2000) points out that WFPS interacts with soil physical properties and O2 demand, to determine the O2 status of the soil. Modellers have addressed this by using ‘soil texture’ to characterise SW or WFPS effect on denitrification. WNMM (Li et al. 2005) adopts six response curves, related to soil texture, to describe the effect of WFPS on denitrification rate. However, the ‘soil texture’ classes are not directly related to measurable soil texture. Experience is needed to judge the selection of the arbitrary ‘soil textural class’, which introduces uncertainty into N2O emission predictions. Using measured soil particle size data would be an improvement on using the arbitrary textural classes but will require modellers to develop equations that use measured soil particle-size distribution to modify the denitrification–WFPS function.
Comparison of actual denitrification rate
The combined effect of WFPS and NO3− concentration on denitrification rate for the different models is shown in Fig. 6. The WFPS scale is limited to the range of values where denitrification occurs. The other rate modifiers (soil pH, and Ts) were set to 1, equivalent to the most favourable condition for denitrification. A potential denitrification rate (Rp_d, gN m−2 day−1) was required in order to make comparisons between FASSET and the other models (Hansen et al. 1991; Chatskikh et al. 2005; Petersen et al. 2005a, 2005b). This was estimated using the algorithm in FASSAT, which is proportional to mineralisation of organic C and depends on clay content (Eqn 15).
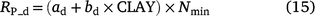
where ad and bd (ad = 0.151 gN gC−1, bd = 0.015 gN gC−1) are constants and CLAY is clay content (%) and Nmin (g C m−2 day−1) is the soil organic matter mineralisation rate (Chatskikh et al. 2005). For the comparison in this study, the mineralisation rate was set to 30 kg C ha−1 day−1 (equivalent to 3 g C m−2 day−1), which is equivalent to the non-limiting soil respiration used in DayCent (Fig. 3, Parton et al. 1996). The actual denitrification rate (Rd, g N m−2 day−1) is the product of Rp_d, which is modified by the Ts function (f(ST_d)), water filled porosity (FQ(Qwfp)) and NO3− concentration (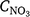 , mg N kg−1). The equation (Chatskikh et al. 2005) is:
, mg N kg−1). The equation (Chatskikh et al. 2005) is:
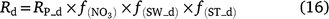
There was more than a 1000-fold difference in total denitrification rates between the models using the default parameters set for each model (Fig. 6). The values shown in Fig. 6 have been converted to the same soil depth (0.2 m) because the DayCent, NOE and WNMM models only calculate denitrification from this soil layer. The lowest rate was observed with DayCent, whilst the highest rate was with FASSET. The denitrification rate plateaued when the NO3− concentration was >200 mg N kg−1 soil in FASSET and NOE (not shown). By contrast the denitrification rate continued to increase with NO3− in APSIM and DayCent (Figs 5, 6).
N2O production from denitrification
APSIM, DayCent, FASSET and WNMM assume the fraction of N2O to the total denitrification fluxes (NO, N2O and N2) declines as WFPS increases (Fig. 7a). Values for the threshold and the optimal WPFS, similar to the denitrification rate modifier, vary amongst the models. The other two major differences with respect to WFPS are the minimum and maximum values of the dimensionless WFPS modifier, and the response functions (Fig. 7a).
The effect of soil temperature on the proportion of N2O to the total denitrification fluxes (NO, N2O and N2) is only considered in DNDC and FASSET (Fig. 7b). DNDC assumes that microbe activity for NO2 → N2O, NO → N2O and N2O → N2 increases with soil temperature when it is below the optimal value (22.5°C). The temperature factor is a standard exponential function equal to 1.0 at 22.5°C (Q10 = 2; Li et al. 1996, 2000). The same temperature factor is applied to the activities of NO3−, NO2− and N2O denitrifiers. In addition, DOC and N oxides are the main substrates controlling the growth of denitrifier growth (Li et al. 2000). The fraction of N2O produced during denitrification decreases with increasing temperature in FASSET (Fig. 7b; Fang et al. 2015).
The effect of soil pH on denitrification and N2O production is only considered in DNDC through pH factor (FPH2; Li et al. 2000), which modifies the relative growth rate of NO2− and NO denitrifiers, and FPH3, which is a pH factor for N2O denitrifiers (see table 4 in Li et al. 2000). The production and reduction of N2O both increase with increasing soil pH (Fig. 8).
The direct impact of NO3− on the fraction of N2O produced is considered in APSIM and DayCent (Fig. 7d). DNDC is not included in Fig. 7d because NO3− modifies the relative growth of denitrifiers and, through a series of interactions, modifies the N2O fraction produced (see Appendix: equation and parameters; Li 2000). APSIM and DayCent assume that the proportion of N2O increases as NO3− concentration increases from 0 to optimal and does not increase above the optimal NO3− concentration at a given soil heterotrophic respiration level (Del Grosso et al. 2000). In DNDC, N2O and N2 production increases in response to increases in substrate concentration following Monod type kinetics (Li et al. 1992) with the same value for the half-saturated nitrogen oxides (Fig. 8). Net N2O production is estimated by the difference between N2O produced from NO and NO2−, and reduction of N2O to N2.
APSIM, DayCent and DNDC consider the effect of SOC on the relative N2O production from denitrification (Fig. 7d). APSIM uses ‘active’ carbon which is a function of SOC (defined as the carbon concentration in the HUM and FOM soil C pools; Thorburn et al. 2010), DayCent and APSIM assume that the proportion of N2O declines to a minimum as soil heterotrophic CO2 respiration (which is a surrogate for labile carbon) increases from 0 to the optimal level for any given NO3− concentration. In DNDC, denitrifier activity for N2O production and reduction is directly linked with DOC and follows double-Monod kinetics (nutrient-dependent Michaelis–Menten-type growth; Li et al. 1996). The proportion of N2O emitted relative to total denitrification declined with increasing DOC, which is comparable to the assumption used in DayCent and APSIM. Increasing DOC allows the denitrifiers to complete the denitrification process to N2 (Li et al. 1996).
N2O consumption during diffusion
Nitrous oxide consumption is only considered in DNDC, FASSET, WNMM and in the APSIM version modified by Xing et al. (2011) (Fig. 9). The NOE and DayCent models assume that there is no N2O consumption, and once N2O is produced it is released to the atmosphere, irrespective of depth in the soil profile. The N2O consumption rate increases with clay content in DNDC and FASSET. The N2O consumption algorithm in the modified APSIM (Xing et al. 2011) was adopted from FASSET. This algorithm assumes that N2O consumption increases with soil depth and all N2O produced below 0.55 m is reduced to N2. The WNMM model has a similar assumption, but the depth limitation does not exist (Fig. 9b). DNDC assumes that N2O produced in deeper soil layers diffuses into the aerobic and anaerobic zones in the above layer (Li et al. 2000). The fraction that diffused into the aerobic zone passes directly into the next aerobic or anaerobic zones or is emitted from the soil surface. The portion that diffuses into the anaerobic zone in the upper layers is reduced to N2, depending on the substrate (DOC) and electron acceptors (NO, N2O) that control denitrifier growth (Li et al. 2000). The proportion of N2O produced that diffuses into the aerobic and anaerobic zones in the upper soil layers depends on the fraction of aerobic and anaerobic zones in the soil layer, which is controlled by the soil O2 condition.
|
|
Although N2O diffusion and consumption are both known to be influenced by soil variables (Li et al. 2000; Warneke et al. 2015), the two major reasons limiting the incorporation of the N2O consumption and diffusion processes into models are: (1) the lack of data on N2O movement through soil profiles; and (2) the calibration and validation of these processes are limited, because only net fluxes to the air are reported from the soil surface. Moldrup et al. (2000) established a predictive relationship between gaseous diffusion coefficient and air-filled porosity which is an improvement on the widely used soil type-independent gas diffusivity models. Whilst consumption terms can be approximated, accurate measurements are needed to improve the modelling of N2O consumption, net N2O emission and N balance in the soil profiles.
Summary and future research needs
While there are differences in the algorithms used to predict N2O emissions from nitrification and denitrification in the models, conceptually the models use a similar approach. For example, different kinetics are used to represent the biogeochemical processes. These differences arise from the availability of data on gaseous (NOx, N2O, N2) production and emissions from the nitrification and denitrification pathways and the simplified representations of complex mechanisms needed to describe the impact of soil type, climate, land use, and vegetation. Although N2 is a major product of denitrification, modelling studies rarely evaluate simulated N2 emissions because few observations are available (see Del Grosso et al. 2020). Measuring N2, the final product of denitrification, remains an analytical challenge and as is the partitioning of N2O and N2 ratio. Progress in this area will be most effective if experimental scientists and modellers work together to design and implement the appropriate experiments to cover the diverse range of soil and climatic conditions. There are a larger number of historical laboratory and field experiments that use inhibitor (e.g. acetylene), which have proven to be unreliable.
The use of 15N isotopes and a stable isotope model (e.g. SIMONE), are promising tools to obtain information on rates of nitrification and denitrification and address weaknesses in N cycling of ecosystem models (Denk et al. 2019). The differences in the modelling approaches demonstrate: (1) the importance of calibrating and validating models before they are used to predict N2O emissions and analyse responses to environment and management at any scale; and (2) the need for detailed data sets, including diffusion and consumption that can be used to calibrate and validate the different processes to reduce uncertainty. The scarcity of accessible measurements as compared with the extent of simulated processes is a general problem for the calibration and validation of complex biogeochemical models (Del Grosso et al. 2020). There is renewed interest in using helium/O2 chambers to measure N2, in addition to N2O. Although the technique has the potential to improve the quantitative understanding of denitrification, the methodology needs to ensure: (1) stringent quality control to ensure there are no air leaks; (2) an N2-free soil atmosphere is established; and (3) flushing with helium/O2 does not modify the anaerobic zones or micropores within the soil column, before reliable denitrification data sets can be obtained (Friedl et al. 2020).
A potential improvement is to quantify nitrification and denitrification responses to O2 pressure in the soil atmosphere, analogous to that used in DNDC (Li et al. 1992, 2000). Adopting this approach will require prediction capability for soil O2 concentration to be incorporated into the models (e.g. Cook 1995) and measurement of O2 concentrations in the soil profile using the techniques given by Hanslin et al. (2005). This will increase the model complexity and require more parameters, but these are measurable and physically based.
Previous laboratory and field studies focused on one or a limited number of variables that influence N2O production and ignore the other controlling factors. For example, soil texture is not always reported, or disturbed soil samples are used in most experiments making it difficult to relate the finding to field conditions. This leads to difficulties in quantifying the reasons why response curves are different when the same treatments are used in the different experiments. Several processes that control N2O emissions are missing from the models (e.g. chemodenitrification, the two steps in nitrification, and heterotrophic nitrification of organic N), although the literature shows that they are important and contribute to total N2O emissions. These processes and their response to environmental factors need to be quantified and understood before it is possible to incorporate them into models. Measurements that apportion N2O to the different biogeochemical process are also needed, rather than measurements of net emissions at the soil-air surface.
Finally, future model development needs to balance complexity and precision. Models may potentially increase in response to process understanding but the number of parameters and how they are estimated must be rigorously evaluated to reduce model uncertainty.
Supplementary material
Supplementary material is available online.
Data availability
Data sharing is not applicable as no new data generated or analysed during this study.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This study was supported in part by funding from the Department of Agriculture and the Grains Research and Development Corporation, Australia.
Acknowledgements
We acknowledge Drs Cresswell and Cook for providing the soil physics supports, Tania Moore from NSW DPI for editing, the anonymous referees and the Associate Editor for their constructive comments and suggestions to improve the manuscript.
References
Asseng S, Fillery IRP, Anderson GC, Dolling PJ, Dunin FX, Keating BA (1998) Use of APSIM wheat model to predict yield, drainage, and NO3- leaching for a deep sand. Australian Journal of Agricultural Research 49, 363–377.| Use of APSIM wheat model to predict yield, drainage, and NO3- leaching for a deep sand.Crossref | GoogleScholarGoogle Scholar |
Baggs EM (2011) Soil microbial sources of nitrous oxide: recent advances in knowledge, emerging challenges and future direction. Current Opinion in Environmental Sustainability 3, 321–327.
| Soil microbial sources of nitrous oxide: recent advances in knowledge, emerging challenges and future direction.Crossref | GoogleScholarGoogle Scholar |
Bateman EJ, Baggs EM (2005) Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space. Biology and Fertility of Soils 41, 379–388.
| Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space.Crossref | GoogleScholarGoogle Scholar |
Bessoua C, Mary B, Léonard J, Roussel M, Gréhan E, Gabriell B (2010) Modelling soil compactions impacts on nitrous oxide emissions in arable fields. European Journal of Soil Science 61, 348–363.
| Modelling soil compactions impacts on nitrous oxide emissions in arable fields.Crossref | GoogleScholarGoogle Scholar |
Bouwman AF (1989) The role of soils and land use in the greenhouse effect. Netherlands Journal of Agricultural Science 37, 13–19.
| The role of soils and land use in the greenhouse effect.Crossref | GoogleScholarGoogle Scholar |
Bouwman AF (1990) Exchange of greenhouse gases between terrestrial ecosystems and the atmosphere. In ‘Soils and the greenhouse effect’. (Ed AF Bouwman) pp. 61–127. (J. Wiley & Sons Ltd.: Chichester)
Bouwman AF (1996) Direct emission of nitrous oxide from agricultural soils. Nutrient Cycling in Agroecosystems 46, 53–70.
| Direct emission of nitrous oxide from agricultural soils.Crossref | GoogleScholarGoogle Scholar |
Braker G, Conrad R (2011) Diversity, structure, and size of N2O-producing microbial communities in soils-what matters for their functioning? In ‘Advances in applied microbiology’. (Eds AI Laskin, S Sariaslani, GM Gadd) pp 33–70. (Elsevier Academic Press Inc, San Diego)
Bremner JM, Blackmer AM (1978) Nitrous oxide: emission from soils during nitrification of fertilizer nitrogen. Science 199, 295–296.
| Nitrous oxide: emission from soils during nitrification of fertilizer nitrogen.Crossref | GoogleScholarGoogle Scholar |
Bremner JM, Blackmer AM (1980) Mechanisms of nitrous oxide production in soils. In ‘Biogeochemistry of ancient and modern environments’. (Eds PA Trudinger, MR Walter, BJ Ralph) pp. 279–291. (Springer Berlin Heidelberg)
Bremner JM, Shaw K (1958) Denitrification in soil. II. Factors affecting denitrification. Journal of Agricultural Science 51, 22–39.
| Denitrification in soil. II. Factors affecting denitrification.Crossref | GoogleScholarGoogle Scholar |
Butterbach-Bahl K, Baggs EM, Dannenmann M, Kiese R, Zechmeister-Boltenstern S (2013) Nitrous oxide emissions from soils: how well do we understand the processes and their controls? Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences 368, 20130122
| Nitrous oxide emissions from soils: how well do we understand the processes and their controls?Crossref | GoogleScholarGoogle Scholar |
Chalk PM, Smith CJ (1983) Chemodenitrification. In ‘Gaseous loss of nitrogen from plant-soil systems’. (Eds JR Freney, JR Simpson) pp. 65–89. (Martinus Nijhoff/Dr. W. Junk: The Hague)
Chalk PM, Smith CJ (2020) The role of agroecosystems in chemical pathways of N2O production. Agriculture, Ecosystems and Environment 290, 106783
| The role of agroecosystems in chemical pathways of N2O production.Crossref | GoogleScholarGoogle Scholar |
Chalk PM, Keeney DR, Walsh LM (1975) Crop recovery and nitrification of fall and spring applied anhydrous ammonia 1. Agronomy Journal 67, 33–37.
| Crop recovery and nitrification of fall and spring applied anhydrous ammonia 1.Crossref | GoogleScholarGoogle Scholar |
Chatskikh D, Olesen JE, Berntsen J, Regina K, Yamulki S (2005) Simulation of effects of soils, climate and management on N2O emission from grasslands. Biogeochemistry 76, 395–419.
| Simulation of effects of soils, climate and management on N2O emission from grasslands.Crossref | GoogleScholarGoogle Scholar |
Chen D, Li Y, Grace P, Mosier AR (2008) N2O emissions from agricultural lands: a synthesis of simulation approaches. Plant and Soil 309, 169–189.
| N2O emissions from agricultural lands: a synthesis of simulation approaches.Crossref | GoogleScholarGoogle Scholar |
Cicerone RJ (1989) Analysis of sources and sinks of atmospheric nitrous oxide (N2O). Journal of Geophysical Research Atmospheres 94, 18265–18271.
| Analysis of sources and sinks of atmospheric nitrous oxide (N2O).Crossref | GoogleScholarGoogle Scholar |
Clough TJ, Sherlock RR, Rolston DE (2005) A review of the movement and fate of N2O in the subsoil. Nutrient Cycling in Agroecosystems 72, 3–11.
| A review of the movement and fate of N2O in the subsoil.Crossref | GoogleScholarGoogle Scholar |
Cook FJ (1995) One-dimensional oxygen diffusion into soil with exponential respiration: analytical and numerical solutions. Ecological Modelling 78, 277–283.
| One-dimensional oxygen diffusion into soil with exponential respiration: analytical and numerical solutions.Crossref | GoogleScholarGoogle Scholar |
Cresswell HP (2002) The soil water characteristic. In ‘Soil physical measurements and interpretation for land evaluation’. (Eds N McKenzie, K Coughlan, H Cresswell) pp. 59–84. (CSIRO Publishing: Collingwood, Vic., Australia)
Cresswell HP, Hamilton GJ (2002) Bulk density and pore space relations. In ‘Soil physical measurements and interpretation for land evaluation’. (Eds N McKenzie, K Coughlan, H Cresswell) pp. 35–58. (CSIRO Publishing: Collingwood, Vic., Australia)
Crutzen PJ, Mosier AR, Smith KA, Winiwarter W (2016) N2O release from agro-biofuel production negates global warming reduction by replacing fossil fuels. In ‘Paul J. Crutzen: a pioneer on atmospheric chemistry and climate change in the anthropocene’, vol. 50. (Eds P Crutzen, HG Brauch). SpringerBriefs on Pioneers in Science and Practice. (Springer: Cham). https://doi.org/10.1007/978-3-319-27460-7_12
Dalal RC, Wang WJ, Robertson GP, Parton WJ (2003) Nitrous oxide emission from Australian agricultural lands and mitigation options: a review. Australian Journal of Soil Research 41, 165–195.
| Nitrous oxide emission from Australian agricultural lands and mitigation options: a review.Crossref | GoogleScholarGoogle Scholar |
Davidson EA (1993) Soil-water content and the ratio of nitrous-oxide to nitric-oxide emitted from soil. In ‘Biogeochemistry of global change’. (Ed. RS Oremland) pp. 369–386. (Springer US: Boston, MA) https://doi.org/10.1007/978-1-4615-2812-8_20
Davidson EA, Swank WT (1986) Environmental parameters regulating gaseous nitrogen losses from two forested ecosystems via nitrification and denitrification. Applied and Environmental Microbiology 52, 1287–1292.
| Environmental parameters regulating gaseous nitrogen losses from two forested ecosystems via nitrification and denitrification.Crossref | GoogleScholarGoogle Scholar |
Davidson EA, Savage K, Verchot LV, Navarro R (2002) Minimizing artifacts and biases in chamber-based measurements of soil respiration. Agricultural and Forest Meteorology 113, 21–37.
| Minimizing artifacts and biases in chamber-based measurements of soil respiration.Crossref | GoogleScholarGoogle Scholar |
Debouk H, Altimir N, Sebastia M-T (2018) Maximizing the information obtained from chamber-based greenhouse gas exchange measurements in remote areas. MethodsX 5, 973–983.
| Maximizing the information obtained from chamber-based greenhouse gas exchange measurements in remote areas.Crossref | GoogleScholarGoogle Scholar |
Del Grosso SJ, Parton WJ, Mosier AR, Ojima DS, Kulmala AE, Phongpan S (2000) General model for N2O and N2 gas emissions from soils due to denitrification. Global Biogeochemical Cycles 14, 1045–1060.
| General model for N2O and N2 gas emissions from soils due to denitrification.Crossref | GoogleScholarGoogle Scholar |
Del Grosso SJ, Mosier AR, Parton WJ, Ojima DS (2005) DAYCENT model analysis of past and contemporary soil N2O and net greenhouse gas flux for major crops in the USA. Soil and Tillage Research 83, 9–24.
| DAYCENT model analysis of past and contemporary soil N2O and net greenhouse gas flux for major crops in the USA.Crossref | GoogleScholarGoogle Scholar |
Del Grosso SJ, Parton WJ, Mosier AR, Walsh MK, Ojima DS, Thornton PE (2006) DAYCENT national-scale simulations of nitrous oxide emissions from cropped soils in the United States. Journal of Environmental Quality 35, 1451–1460.
| DAYCENT national-scale simulations of nitrous oxide emissions from cropped soils in the United States.Crossref | GoogleScholarGoogle Scholar |
Del Grosso SJ, Ogle SM, Parton WJ, Breidt FJ (2010) Estimating uncertainty in N2O emissions from U.S. cropland soil. Global Biogeochemical Cycles 24, GB1009
| Estimating uncertainty in N2O emissions from U.S. cropland soil.Crossref | GoogleScholarGoogle Scholar |
Del Grosso SJ, Smith W, Kraus D, Massad RS, Vogeler I, Fuchs K (2020) Approaches and concepts of modelling denitrification: increased process understanding using observational data can reduce uncertainties. Current Opinions in Environmental Sustainability 47, 37–45.
| Approaches and concepts of modelling denitrification: increased process understanding using observational data can reduce uncertainties.Crossref | GoogleScholarGoogle Scholar |
Denk TRA, Kraus D, Kiese R, Butterbach-Bahl K, Wolf B (2019) Constraining N cycling in the ecosystem model LandscapeDNDC with the stable isotope model SIMONE. Ecology 100, e02675
| Constraining N cycling in the ecosystem model LandscapeDNDC with the stable isotope model SIMONE.Crossref | GoogleScholarGoogle Scholar |
Ebrahimi A, Or D (2018) On upscaling of soil microbial processes and biogeochemical fluxes from aggregates to landscapes. Journal of Geophysical Research: Biogeosciences 123, 1526–1547.
| On upscaling of soil microbial processes and biogeochemical fluxes from aggregates to landscapes.Crossref | GoogleScholarGoogle Scholar |
Ehrhardt F, Soussana JF, Bellocchi G, Grace P, McAuliffe R, Recous S, Sándor R, Smith P, Snow V, de Antoni Migliorati M, Basso B, Bhatia A, Brilli L, Doltra J, Dorich CD, Doro L, Fitton N, Giacomini SJ, Grant B, Harrison MT, Jones SK, Kirschbaum MUF, Klumpp K, Laville P, Léonard J, Liebig M, Lieffering M, Martin R, Massad RS, Meier E, Merbold L, Moore AD, Myrgiotis V, Newton P, Pattey E, Rolinski S, Sharp J, Smith WN, Wu L, Zhang Q (2018) Assessing uncertainties in crop and pasture ensemble model simulations of productivity and N2O emissions. Global Change Biology 24, e603–e616.
| Assessing uncertainties in crop and pasture ensemble model simulations of productivity and N2O emissions.Crossref | GoogleScholarGoogle Scholar |
Fang QX, Ma L, Halvorson AD, Malone RW, Ahuja LR, Del Grosso SJ, Hatfield JL (2015) Evaluating four nitrous oxide emission algorithms in response to N rate on an irrigated corn field. Environmental Modelling & Software 72, 56–70.
| Evaluating four nitrous oxide emission algorithms in response to N rate on an irrigated corn field.Crossref | GoogleScholarGoogle Scholar |
FAO/IFA (2001) Global estimates of gaseous emissions of NH3, NO and N2O from agricultural land. Food and Agriculture Organization of the United Nations (FAO)/International Fertilizer Industry Association (IFA), Rome, p. 106. Available at https://www.fao.org/3/y2780e/y2780e01b.htm
Farquharson R (2016) Nitrification rates and associated nitrous oxide emissions from agricultural soils – a synopsis. Soil Research 54, 469–480.
Firestone MK, Davidson EA (1989) Microbiological basis of NO and N2O production and consumption in soil. In ‘Exchange of trace gases between terrestrial ecosystems and the atmosphere. Report of the Dahlem workshop on exchange of trace gases between terrestrial ecosystems and the atmosphere’. (Eds MO Andreae, DS Schimel) pp. 7–21. (John Wiley & Sons)
Focht DD (1974) The effect of temperature, pH and aeration on the production of nitrous oxide and gaseous nitrogen: a zero-order kinetic model. Soil Science 118, 173–179.
| The effect of temperature, pH and aeration on the production of nitrous oxide and gaseous nitrogen: a zero-order kinetic model.Crossref | GoogleScholarGoogle Scholar |
Friedl J, Cardenas LM, Clough TJ, Dannenmann M, Hu C, Scheer C (2020) Measuring denitrification and the N2O: (N2O+N2) emission ratio from terrestrial soils. Current Opinion in Environmental Sustainability 47, 61–71.
| Measuring denitrification and the N2O: (N2O+N2) emission ratio from terrestrial soils.Crossref | GoogleScholarGoogle Scholar |
Frolking SE, Mosier AR, Ojima DS, Li C, Parton WJ, Potter CS, Priesack E, Stenger R, Haberbosch C, Dörsch P, Flessa H, Smith KA (1998) Comparison of N2O emissions from soils at three temperate agricultural sites: simulations of year-round measurements by four models. Nutrient Cycling Agroecosystems 52, 77–105.
| Comparison of N2O emissions from soils at three temperate agricultural sites: simulations of year-round measurements by four models.Crossref | GoogleScholarGoogle Scholar |
Giltrap DL, Li CS, Saggar S (2010) DNDC: a process-based model of greenhouse gas fluxes from agricultural soils. Agriculture Ecosystems and Environment 136, 292–300.
| DNDC: a process-based model of greenhouse gas fluxes from agricultural soils.Crossref | GoogleScholarGoogle Scholar |
Giltrap DL, Thakur KP, Ausseil AG (2011) Sensitivity analysis of emission factors for regional-scale nitrous oxide emissions estimates using NZ-DNDC. In ‘19th International Congress on Modelling and Simulation, 12–16 December 2011, Perth, Australia’. pp. 2606–2612. Available at http://mssanz.org.au/modsim2011
Giltrap D, Yeluripati J, Smith P, Fitton N, Smith W, Grant B, Dorich CD, Deng J, Topp CFE, Abdalla M, Liáng LL, Snow V (2020) Global research alliance N2O chamber methodology guidelines: summary of modeling approaches. Journal of Environmental Quality 49, 1168–1185.
| Global research alliance N2O chamber methodology guidelines: summary of modeling approaches.Crossref | GoogleScholarGoogle Scholar |
Grace PR, van der Weerden TJ, Rowlings DW, Scheer C, Brunk C, Kiese R, Butterbach-Bahl K, Rees RM, Robertson GP, Skiba UM (2020) Global research alliance N2O chamber methodology guidelines: considerations for automated flux measurement. Journal of Environmental Quality 49, 1126–1140.
| Global research alliance N2O chamber methodology guidelines: considerations for automated flux measurement.Crossref | GoogleScholarGoogle Scholar |
Griffis TJ, Chen Z, Baker JM, Wood JD, Millet DB, Lee X, Venterea RT, Turner PA (2017) Nitrous oxide emissions are enhanced in a warmer and wetter world. Proceedings of the National Academy of Sciences of the United States of America 114, 12081–12085.
| Nitrous oxide emissions are enhanced in a warmer and wetter world.Crossref | GoogleScholarGoogle Scholar |
Hansen S, Jensen HE, Nielsen NE, Svendsen H (1991) Simulation of nitrogen dynamics and biomass production in winter wheat using the Danish simulation model DAISY. Fertilizer Research 27, 245–259.
| Simulation of nitrogen dynamics and biomass production in winter wheat using the Danish simulation model DAISY.Crossref | GoogleScholarGoogle Scholar |
Hanslin HM, Sæbø A, Bergersen O (2005) Estimation of oxygen concentration in the soil gas phase beneath compost mulch by means of a simple method. Urban Forestry & Urban Greening 4, 37–40.
| Estimation of oxygen concentration in the soil gas phase beneath compost mulch by means of a simple method.Crossref | GoogleScholarGoogle Scholar |
Hanson PJ, Edwards NT, Garten CT, Andrews JA (2000) Separating root and soil microbial contributions to soil respiration: a review of methods and observations. Biogeochemistry 48, 115–146.
| Separating root and soil microbial contributions to soil respiration: a review of methods and observations.Crossref | GoogleScholarGoogle Scholar |
Heinen M (2006) Simplified denitrification models: overview and properties. Geoderma 133, 444–463.
| Simplified denitrification models: overview and properties.Crossref | GoogleScholarGoogle Scholar |
Holzworth DP, Huth NI, deVoil PG, Zurcher EJ, Herrmann NI, McLean G, Chenu K, van Oosterom EJ, Snow V, Murphy C, Moore AD, Brown H, Whish JPM, Verrall S, Fainges J, Bell LW, Peake AS, Poulton PL, Hochman Z, Thorburn PJ, Gaydon DS, Dalgliesh NP, Rodriguez D, Cox H, Chapman S, Doherty A, Teixeira E, Sharp J, Cichota R, Vogeler I, Li FY, Wang E, Hammer GL, Robertson MJ, Dimes JP, Whitbread AM, Hunt J, van Rees H, McClelland T, Carberry PS, Hargreaves JNG, MacLeod N, McDonald C, Harsdorf J, Wedgwood S, Keating BA (2014) APSIM – Evolution towards a new generation of agricultural systems simulation. Environmental Modelling & Software 62, 327–350.
| APSIM – Evolution towards a new generation of agricultural systems simulation.Crossref | GoogleScholarGoogle Scholar |
Howden SM, O’Leary GJ (1997) Evaluating options to reduce greenhouse gas emissions from an Australian temperate wheat cropping system. Environmental Modelling & Software 12, 169–176.
| Evaluating options to reduce greenhouse gas emissions from an Australian temperate wheat cropping system.Crossref | GoogleScholarGoogle Scholar |
Hénault C, Bizouard F, Laville P, Gabrielle B, Nicoullaud B, Germon JC, Cellier P (2005) Predicting in situ soil N2O emission using NOE algorithm and soil database. Global Change Biology 11, 115–127.
| Predicting in situ soil N2O emission using NOE algorithm and soil database.Crossref | GoogleScholarGoogle Scholar |
Inatomi M, Hajima T, Ito A (2019) Fraction of nitrous oxide production in nitrification and its effect on total soil emission: a meta-analysis and global-scale sensitivity analysis using a process-based model. PLoS ONE 14, e0219159
| Fraction of nitrous oxide production in nitrification and its effect on total soil emission: a meta-analysis and global-scale sensitivity analysis using a process-based model.Crossref | GoogleScholarGoogle Scholar |
IPCC (2014) Climate Change 2014: Synthesis Report. pp. 1–32. (IPCC)
Keating BA, Carberry PS, Hammer GL, Probert ME, Robertson MJ, Holzworth D, Huth NI, Hargreaves JNG, Meinke H, Hochman Z, McLean G, Verburg K, Snow V, Dimes JP, Silburn M, Wang E, Brown S, Bristow KL, Asseng S, Chapman S, McCown RL, Freebairn DM, Smith CJ (2003) An overview of APSIM, a model designed for farming systems simulation. European Journal of Agronomy 18, 267–288.
| An overview of APSIM, a model designed for farming systems simulation.Crossref | GoogleScholarGoogle Scholar |
Khalil K, Mary B, Renault P (2004) Nitrous oxide production by nitrification and denitrification in soil aggregates as affected by O2 concentration. Soil Biology & Biochemistry 36, 687–699.
| Nitrous oxide production by nitrification and denitrification in soil aggregates as affected by O2 concentration.Crossref | GoogleScholarGoogle Scholar |
Leip A, Busto M, Winiwarter W (2011) Developing spatially stratified N2O emission factors for Europe. Environmental Pollution 159, 3223–3232.
| Developing spatially stratified N2O emission factors for Europe.Crossref | GoogleScholarGoogle Scholar |
Li CS (2000) Modeling trace gas emissions from agricultural ecosystems. Nutrient Cycling in Agroecosystems 58, 259–276.
| Modeling trace gas emissions from agricultural ecosystems.Crossref | GoogleScholarGoogle Scholar |
Li C, Frolking S, Frolking TA (1992) A model of nitrous oxide evolution from soil driven by rainfall events. 2. Model applications. Journal of Geophysical Research Atmospheres 97, 9777–9783.
| A model of nitrous oxide evolution from soil driven by rainfall events. 2. Model applications.Crossref | GoogleScholarGoogle Scholar |
Li C, Narayanan V, Harriss RC (1996) Model estimates of nitrous oxide emissions from agricultural lands in the United States. Global Biogeochemical Cycles 10, 297–306.
| Model estimates of nitrous oxide emissions from agricultural lands in the United States.Crossref | GoogleScholarGoogle Scholar |
Li C, Aber J, Stange F, Butterbach-Bahl K, Papen H (2000) A process-oriented model of N2O and NO emissions from forest soils: 1. Model development. Journal of Geophysical Research: Atmospheres 105, 4369–4384.
| A process-oriented model of N2O and NO emissions from forest soils: 1. Model development.Crossref | GoogleScholarGoogle Scholar |
Li Y, Chen DL, Zhang Y, Edis R, Ding H (2005) Comparison of three modeling approaches for simulating denitrification and nitrous oxide emissions from loam-textured arable soils. Global Biogeochemical Cycles 19, GB3002
| Comparison of three modeling approaches for simulating denitrification and nitrous oxide emissions from loam-textured arable soils.Crossref | GoogleScholarGoogle Scholar |
Li Y, White R, Chen D, Zhang J, Li B, Zhang Y, Huang Y, Edis R (2007) A spatially referenced water and nitrogen management model (WNMM) for (irrigated) intensive cropping systems in the North China Plain. Ecological Modelling 203, 395–423.
| A spatially referenced water and nitrogen management model (WNMM) for (irrigated) intensive cropping systems in the North China Plain.Crossref | GoogleScholarGoogle Scholar |
Livingston GP, Hutchinson GL, Spartalian K (2006) Trace gas emission in chambers: a non-steady state diffusion model. Soil Science Society America Journal 70, 1459–1469.
| Trace gas emission in chambers: a non-steady state diffusion model.Crossref | GoogleScholarGoogle Scholar |
IPCC (2021) Summary for policymakers. In ‘Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change’ (Eds V Masson-Delmotte, P Zhai, A Pirani, SL Connors, C Péan, S Berger, N Caud, Y Chen, L Goldfarb, MI Gomis, M Huang, K Leitzell, E Lonnoy, JBR Matthews, TK Maycock, T Waterfield, O Yelekçi, R Yu, B Zhou) pp. 3–32. (Cambridge University Press. United Kingdom and New York, NY, USA) https://doi.org/10.1017/9781009157896.001
Meier EA, Thorburn PJ, Probert ME (2006) Occurrence and simulation of nitrification in two contrasting sugarcane soils from the Australian wet tropics. Australian Journal of Soil Research 44, 1–9.
| Occurrence and simulation of nitrification in two contrasting sugarcane soils from the Australian wet tropics.Crossref | GoogleScholarGoogle Scholar |
Mielenz H, Thorburn PJ, Harris RH, Officer SJ, Li G, Schwenke GD, Grace P (2016a) Nitrous oxide emissions from grain production systems across a wide range of environmental conditions in eastern Australia. Soil Research 54, 659–674.
| Nitrous oxide emissions from grain production systems across a wide range of environmental conditions in eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Mielenz H, Thorburn PJ, Scheer C, De Antoni Migliorati M, Grace PR, Bell MJ (2016b) Opportunities for mitigating nitrous oxide emissions in subtropical cereal and fiber cropping systems: a simulation study. Agriculture, Ecosystems and Environment 218, 11–27.
| Opportunities for mitigating nitrous oxide emissions in subtropical cereal and fiber cropping systems: a simulation study.Crossref | GoogleScholarGoogle Scholar |
Moldrup P, Olesen T, Gamst J, Schjønning P, Yamaguchi T, Rolston DE (2000) Predicting the gas diffusion coefficient in repacked soil water-induced linear reduction model. Soil Science Society of America Journal 64, 1588–1594.
| Predicting the gas diffusion coefficient in repacked soil water-induced linear reduction model.Crossref | GoogleScholarGoogle Scholar |
Mosier AR, Duxbury JM, Freney JR, Heinemeyer O, Minami K (1996) Nitrous oxide emissions from agricultural fields: assessment, measurement and mitigation. Plant and Soil 181, 95–108.
| Nitrous oxide emissions from agricultural fields: assessment, measurement and mitigation.Crossref | GoogleScholarGoogle Scholar |
Mosier A, Kroeze C, Nevison C, Oenema O, Seitzinger S, van Cleemput O (1998) Closing the global N2O budget: nitrous oxide emissions through the agricultural nitrogen cycle: OECD/IPCC/IEA phase II development of IPCC guidelines for national greenhouse gas inventory methodology. Nutrient Cycling in Agroecosystems 52, 225–248.
| Closing the global N2O budget: nitrous oxide emissions through the agricultural nitrogen cycle: OECD/IPCC/IEA phase II development of IPCC guidelines for national greenhouse gas inventory methodology.Crossref | GoogleScholarGoogle Scholar |
Nömmik H (1956) Investigations on denitrification in soil. Acta Agriculturae Scandinavica 6, 195–228.
Pan B, Lam SK, Wang E, Mosier A, Chen D (2021) New approach for predicting nitrification and its fraction of N2O emissions in global terrestrial ecosystems. Environmental Research Letters 16, 034053
| New approach for predicting nitrification and its fraction of N2O emissions in global terrestrial ecosystems.Crossref | GoogleScholarGoogle Scholar |
Pan B, Xia L, Lam SK, Wang E, Zhang Y, Mosier A, Chen D (2022) A global synthesis of soil denitrification: driving factors and mitigation strategies. Agriculture, Ecosystems and Environment 327, 107850
| A global synthesis of soil denitrification: driving factors and mitigation strategies.Crossref | GoogleScholarGoogle Scholar |
Parton WJ, Mosier AR, Ojima DS, Valentine DW, Schimel DS, Weier K, Kulmala AE (1996) Generalized model for N2 and N2O production from nitrification and denitrification. Global Biogeochemical Cycles 10, 401–412.
| Generalized model for N2 and N2O production from nitrification and denitrification.Crossref | GoogleScholarGoogle Scholar |
Parton WJ, Holland EA, Del Grosso SJ, Hartman MD, Martin RE, Mosier AR, Ojima DS, Schimel DS (2001) Generalized model for NOx and N2O emissions from soils. Journal of Geophysical Research: Atmospheres 106, 17403–17419.
| Generalized model for NOx and N2O emissions from soils.Crossref | GoogleScholarGoogle Scholar |
Petersen BM, Berntsen J, Hansen S, Jensen LS (2005a) CN-SIM—a model for the turnover of soil organic matter. I. Long-term carbon and radiocarbon development. Soil Biology and Biochemistry 37, 359–374.
| CN-SIM—a model for the turnover of soil organic matter. I. Long-term carbon and radiocarbon development.Crossref | GoogleScholarGoogle Scholar |
Petersen BM, Jensen LS, Hansen S, Pedersen A, Henriksen TM, Sørensen P, Trinsoutrot-Gattin I, Berntsen J (2005b) CN-SIM: a model for the turnover of soil organic matter. II. Short-term carbon and nitrogen development. Soil Biology Biochemistry 37, 375–393.
| CN-SIM: a model for the turnover of soil organic matter. II. Short-term carbon and nitrogen development.Crossref | GoogleScholarGoogle Scholar |
Reuss JO, Innis GS (1977) A grassland nitrogen flow simulation model. Ecology 58, 379–388.
| A grassland nitrogen flow simulation model.Crossref | GoogleScholarGoogle Scholar |
Saggar S, Jha N, Deslippe J, Bolan NS, Luo J, Giltrap DL, Kim DG, Zaman M, Tillman RW (2013) Denitrification and N2O:N2 production in temperate grasslands: Processes, measurements, modelling and mitigating negative impacts. Science of the Total Environment 465, 173–195.
| Denitrification and N2O:N2 production in temperate grasslands: Processes, measurements, modelling and mitigating negative impacts.Crossref | GoogleScholarGoogle Scholar |
Sahrawat KL, Keeney DR (1986) Nitrous oxide emission from soils. Advances in Soil Science 4, 103–148.
Shen J, Treu R, Wang J, Nicholson F, Bhogal A, Thorman R (2018) Modeling nitrous oxide emissions from digestate and slurry applied to three agricultural soils in the United Kingdom: fluxes and emission factors. Environmental Pollution 243, 1952–1965.
| Modeling nitrous oxide emissions from digestate and slurry applied to three agricultural soils in the United Kingdom: fluxes and emission factors.Crossref | GoogleScholarGoogle Scholar |
Sihi D, Davidson EA, Savage KE, Liang D (2020) Simultaneous numerical representation of soil microsite production and consumption of carbon dioxide, methane, and nitrous oxide using probability distribution functions. Global Change Biology 26, 200–218.
| Simultaneous numerical representation of soil microsite production and consumption of carbon dioxide, methane, and nitrous oxide using probability distribution functions.Crossref | GoogleScholarGoogle Scholar |
Smith KA, Thomson PE, Clayton H, McTaggart IP, Conen F (1998) Effects of temperature, water content and nitrogen fertilisation on emissions of nitrous oxide by soils. Atmospheric Environment 32, 3301–3309.
| Effects of temperature, water content and nitrogen fertilisation on emissions of nitrous oxide by soils.Crossref | GoogleScholarGoogle Scholar |
Smith P, Martino D, Cai Z, Gwary D, Janzen H, Kumar P, McCarl B, Ogle S, O’Mara F, Rice C, Scholes B, Sirotenko O, Howden M, McAllister T, Pan G, Romanenkov V, Schneider U, Towprayoon S, Wattenbach M, Smith J (2008) Greenhouse gas mitigation in agriculture. Philosophical Transactions of the Royal Society B: Biological Sciences 363, 789–813.
| Greenhouse gas mitigation in agriculture.Crossref | GoogleScholarGoogle Scholar |
Smith CJ, Macdonald BCT, Xing H, Denmead OT, Wang E, McLachlan G, Tuomi S, Turner D, Chen D (2020) Measurements and APSIM modelling of soil C and N dynamics. Soil Research 58, 41–61.
| Measurements and APSIM modelling of soil C and N dynamics.Crossref | GoogleScholarGoogle Scholar |
Stark JM, Firestone MK (1996) Kinetic characteristics of ammonium-oxidizer communities in a California oak woodland-annual grassland. Soil Biology and Biochemistry 28, 1307–1317.
| Kinetic characteristics of ammonium-oxidizer communities in a California oak woodland-annual grassland.Crossref | GoogleScholarGoogle Scholar |
Syakila A, Kroeze C (2011) The global nitrous oxide budget revisited. Greenhouse Gas Measurement and Management 1, 17–26.
| The global nitrous oxide budget revisited.Crossref | GoogleScholarGoogle Scholar |
Thorburn PJ, Biggs JS, Collins K, Probert ME (2010) Using the APSIM model to estimate nitrous oxide emissions from diverse Australian sugarcane production systems. Agriculture Ecosystems & Environment 136, 343–350.
| Using the APSIM model to estimate nitrous oxide emissions from diverse Australian sugarcane production systems.Crossref | GoogleScholarGoogle Scholar |
Tian H, Yang J, Lu C, Xu R, Canadell JG, Jackson RB, et al. (2018) The global N2O model intercomparison project. Bulletin of the American Meteorological Society 99, 1231–1251.
| The global N2O model intercomparison project.Crossref | GoogleScholarGoogle Scholar |
Tian H, Xu R, Canadell JG, et al. (2020) A comprehensive quantification of global nitrous oxide sources and sinks. Nature 586, 248–256.
| A comprehensive quantification of global nitrous oxide sources and sinks.Crossref | GoogleScholarGoogle Scholar |
Tiedje JM, Sexstone AJ, Myrold DD, Robinson JA (1983) Denitrification: ecological niches, competition and survival. Antonie van Leeuwenhoek 48, 569–583.
| Denitrification: ecological niches, competition and survival.Crossref | GoogleScholarGoogle Scholar |
Tubiello FN, Salvatore M, Rossi S, Ferrara A, Fitton N, Smith P (2013) The FAOSTAT database of greenhouse gas emissions from agriculture. Environmental Research Letters 8, 015009
| The FAOSTAT database of greenhouse gas emissions from agriculture.Crossref | GoogleScholarGoogle Scholar |
van Genuchten MT (1980) A closed form equation for predicting the hydraulic conductivity of unsaturated soils. Soil Science Society of America Journal 44, 892–898.
| A closed form equation for predicting the hydraulic conductivity of unsaturated soils.Crossref | GoogleScholarGoogle Scholar |
Venterea RT, Coulter JA, Clough TJ (2020) Nitrite accumulation and nitrogen gas production increase with decreasing temperature in urea-amended soil: experiments and modelling. Soil Biology and Biochemistry 142, 107727
| Nitrite accumulation and nitrogen gas production increase with decreasing temperature in urea-amended soil: experiments and modelling.Crossref | GoogleScholarGoogle Scholar |
Vogeler I, Giltrap D, Li F, Snow V (2011) Comparison of models for predicting nitrification and denitrification in pastoral systems. In ‘19th International Congress on Modelling and Simulation, 12–16 December 2011, Perth, Australia’. pp. 884–890. Available at http://mssanz.org.au/modsim2011
Warneke S, Macdonald BCT, Macdonald LM, Sanderman J, Farrell M (2015) Abiotic dissolution and biological uptake of nitrous oxide in Mediterranean woodland and pasture soil. Soil Biology and Biochemistry 82, 62–64.
| Abiotic dissolution and biological uptake of nitrous oxide in Mediterranean woodland and pasture soil.Crossref | GoogleScholarGoogle Scholar |
Weier KL (1999) N2O and CH4 emission and CH4 consumption in a sugarcane soil after variation in nitrogen and water application. Soil Biology and Biochemistry 31, 1931–1941.
| N2O and CH4 emission and CH4 consumption in a sugarcane soil after variation in nitrogen and water application.Crossref | GoogleScholarGoogle Scholar |
Weier KL, Doran JW, Power JF, Walters DT (1993) Denitrification and the dinitrogen/nitrous oxide ratio as affected by soil-water, available carbon, and nitrate. Soil Science Society of America Journal 57, 66–72.
| Denitrification and the dinitrogen/nitrous oxide ratio as affected by soil-water, available carbon, and nitrate.Crossref | GoogleScholarGoogle Scholar |
Williams EJ, Hutchinson GL, Fehsenfeld FC (1992) NOx and N2O emissions from soil. Global Biogeochemical Cycles 6, 351–388.
| NOx and N2O emissions from soil.Crossref | GoogleScholarGoogle Scholar |
Wu L, Rees RM, Tarsitano D, Zhang X, Jones SK, Whitmore AP (2015) Simulation of nitrous oxide emissions at field scale using the SPACSYS model. Science of the Total Environment 530–531, 76–86.
| Simulation of nitrous oxide emissions at field scale using the SPACSYS model.Crossref | GoogleScholarGoogle Scholar |
Xing H, Wang E, Smith CJ, Rolston D, Yu Q (2011) Modelling nitrous oxide and carbon dioxide emission from soil in an incubation experiment. Geoderma 167–168, 328–339.
| Modelling nitrous oxide and carbon dioxide emission from soil in an incubation experiment.Crossref | GoogleScholarGoogle Scholar |
Xing HT, Liu DL, Wang E, Smith CJ, Anwar MR, Yu Q (2013) Modelling the response of N2O emission factor to nitrogen application rates and inter-annual climate variability. In ‘20th International Congress on Modelling and Simulation, Adelaide, Australia’.
Yu T, Zhuang Q (2019) Quantifying global N2O emissions from natural ecosystem soils using trait-based biogeochemistry models. Biogeosciences 16, 207–222.
| Quantifying global N2O emissions from natural ecosystem soils using trait-based biogeochemistry models.Crossref | GoogleScholarGoogle Scholar |
Zhang W, Liu C, Zheng X, Zhou Z, Cui F, Zhu B, Haas E, Klatt S, Butterbach-Bahl K, Kiese R (2015a) Comparison of the DNDC, LandscapeDNDC and IAP-N-GAS models for simulating nitrous oxide and nitric oxide emissions from the winter wheat-summer maize rotation system. Agriculture Systems 140, 1–10.
| Comparison of the DNDC, LandscapeDNDC and IAP-N-GAS models for simulating nitrous oxide and nitric oxide emissions from the winter wheat-summer maize rotation system.Crossref | GoogleScholarGoogle Scholar |
Zhang J, Muller C, Cai Z (2015b) Heterotrophic nitrification of organic N and its contribution to nitrous oxide emissions in soils. Soil Biology and Biochemistry 84, 199–209.
| Heterotrophic nitrification of organic N and its contribution to nitrous oxide emissions in soils.Crossref | GoogleScholarGoogle Scholar |


