Lowering the water solubility of phosphorus fertilisers impacts leaching, plant growth and residual soil phosphorus
Robert Summers A * and David Weaver
A * and David Weaver  B
B
A Department of Primary Industries and Regional Development, 45 Mandurah Terrace, Mandurah, WA 6210, Australia.
B Department of Primary Industries and Regional Development, 444 Albany Highway, Albany, WA 6330, Australia.
Soil Research 61(1) 20-36 https://doi.org/10.1071/SR22037
Submitted: 7 February 2022 Accepted: 5 July 2022 Published: 1 August 2022
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Leaching of phosphorus (P) from water soluble agricultural fertilisers applied to sandy soil can adversely impact downstream water quality. Less soluble fertilisers may reduce P leaching and increase production. However, repeated application of low water soluble P (LWSP) fertiliser with high citrate soluble P (CSP) has potential to increase P leaching and P accumulation.
Methods: We examined the effect of LWSP fertilisers (single superphosphate, SSP; fertilisers low in water soluble P, WSP) on leaching losses in soil with low P retention/low P status; with/without bauxite residue amendment and with/without plants.
Key results: On low P retentive soils with a low P status, P leaching was reduced by reducing the WSP of fertiliser. Plants and soil amendment reduced P leaching further, but P loss remained proportional to WSP applied. Under field conditions, a subset of LWSP fertilisers greatly increased pasture dry matter production and increased soil test P values. Soil test P increases were positively correlated to fertiliser citrate soluble P content. Soils already containing at least 80% of the required plant available P did not require a WSP fertiliser to provide early season P.
Conclusions: LWSP fertilisers and soil amendment have potential to reduce P leaching in low P retentive soils. Fertiliser formulations targeting reduced WSP and high CSP require attention.
Implications: LWSP fertilisers may increase soil P residues reducing future P needs; however, ongoing soil testing is required for assessment of soil P accumulation, requirements and leaching potential.
Keywords: citrate soluble, eutrophication, fertiliser, leaching, pasture, phosphorus, retention, sand, water soluble.
Introduction
A Mediterranean climate with 600–1000 mm of rain over a short cool winter encourages phosphorus (P) leaching through sandy soils of coastal catchments draining to estuaries in south-west Western Australia (SWWA). Phosphorus application has been required to increase soil P fertility on the naturally infertile soils to support pastures grazed by cattle and sheep on these poorly P retentive sandy soils. Many of the soils are now P saturated (Weaver and Summers 2021) and represent a high risk of P loss from legacy P stocks (McDowell et al. 2020; von Arb et al. 2021) with little opportunity for P attenuation as they are situated close to affected water bodies (Weaver and Reed 1998; Weaver and Summers 2021). The loss of P, particularly from farmland, contributes to water quality problems because P ingress removes limitations to algal growth. Algal blooms are subsequently consumed by bacteria along with depletion of oxygen in the water column, resulting in fish kills (Environmental Protection Authority 1988).
Reducing the solubility of P fertiliser has the potential to reduce P leaching, thereby increasing P retained in the soil. Here, we are referring to low water soluble P (LWSP) fertilisers as those which have been manipulated to reduce the soluble P proportion by lime reversion, partial acidulation or combination of a soluble fertiliser with a less water soluble fertiliser. The effectiveness of LWSP fertilisers to manage leaching on sandy soil has been studied in the laboratory and in the field (Deeley et al. 1987; Weaver et al. 1988a, 1988b; Summers et al. 2000). Similar or increased yields were found when LWSP fertilisers were compared to highly soluble fertiliser such as single superphosphate (SSP) (Yeates et al. 1984; Bolland et al. 1995a, 1995b; Summers et al. 2000). Initial P leaching was about 50% lower with LWSP fertilisers, and this resulted in higher residues of P in the soil.
While most of the soils in the region have sufficient or excess P to maintain pasture dry matter yields (Weaver and Summers 2021), some have P deficiency and there is some requirement for early season P (Grant et al. 2001). LWSP fertilisers may not be soluble enough to provide adequate early season P in the rare occurrence of severe P limitation in SWWA.
Reducing the water soluble P fraction of fertilisers can reduce incidental P losses and increase the residual value of fertiliser for use in future years. This is particularly applicable to sandy soils on the Swan coastal plain in SWWA where high winter rainfall encourages leaching, and surface runoff when water tables rise to the surface. Short term laboratory leaching studies suggests a 40–50% reduction in P loss on sandy soils for single applications (Yeates et al. 1984; DCE 1985; Summers et al. 2000). However, repeated annual applications without soil testing can lead to increased systemic losses (Weaver et al. 1988a).
Of the limited runoff studies using LWSP fertilisers on leaching sands, a small catchment study (5 ha) on sandy soil by Schofield et al. (1985) showed that the use of LWSP called Coastal Super (the first commercially available lime reverted superphosphate for SWWA) reduced P losses by 40% relative to SSP, without any reduction in yield (3.64–3.97 t ha−1, respectively) or any reduction in plant P content (0.30–0.29% P, respectively). This small catchment study was only over a single year and reduction in P loss may diminish because a high proportion of annual losses can be derived from legacy soil P stores rather than fertiliser applications in the current year. The long-term re-application of LWSP fertilisers may result in increased soil P stores and contribute to an increase in P loss from the soil that would partially negate the effect of losing less P from currently applied P (Schofield et al. 1985; Weaver et al. 1988a, 1988b). This situation was encountered in a trial using 5 ha runoff plots on duplex soils where applications of New Coastal Super (the second commercially available product, a LWSP made from partially acidulated rock phosphate) were compared with applications of SSP at similar P rates. The LWSP fertiliser showed a slight increase in P loss over 4 years (Silberstein and Schofield 1990). Re-examination of the soil test results using contemporary nutrient response functions (Gourley et al. 2019) reveal that both soils in this trial contained P in excess of agronomic requirements prior to commencement of the trial, and therefore did not require further P applications for maximum pasture production. As a result, it is highly likely that there would be significant P loss from legacy soil P stores, reducing the likelihood that solubility effects of different fertiliser treatments could be detected.
Despite previous research and attempts to commercialise LWSP fertilisers in WA, these initial products were withdrawn from sale due to poor acceptance. A new lime reverted SSP fertiliser was subsequently released in WA in 2013 called Super SR at a price competitive to traditional SSP, again providing a commercially available LWSP fertiliser.
This paper is a meta-analysis of experiments conducted in SWWA comparing P leaching losses from fertilisers with differing water soluble P (WSP) and citrate soluble P (CSP) contents. The experiments include multiple fertiliser applications in the presence and absence of plants. Also included were experiments that used a P retentive soil amendment, bauxite residue, for comparison with previous studies of amended leaching sands at glasshouse and field scale (Summers et al. 1993, 1996b, 2020), and studies on the impact of LWSP on pasture production (Summers et al. 1996a, 2001).
The hypotheses tested in this meta-analysis are as follows:
-
On low P retention soils with a low P status, P leaching losses in the absence of plants will be positively correlated with the amount of water soluble P applied.
-
On low P retention soils with a low P status, P leaching losses in the presence of plants will be less than when plants are absent but will be positively correlated with the amount of water soluble P applied.
-
On low P retention soils with a low P status, P leaching losses in the presence of P retentive soil amendment will be reduced, but positively correlated with the amount of water soluble P applied.
-
LWSP fertilisers will be less effective for plant growth on low P retentive soils with no plant available P residues compared to soils with plant available soil P derived from a history of P application.
Materials and methods
Soils and environment
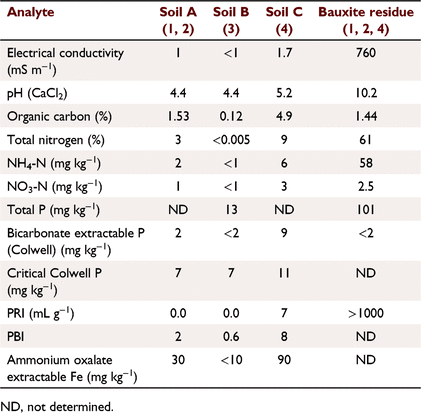
The soils used in the experiments were chosen as representative of widespread, problematic, leaching soils from SWWA; for example, coastal sandplain derived from broad dunal systems with deep grey imperfectly drained siliceous sands described as the Bassendean series [212 Bs_B6; van Gool (1990)]. The soils in the area are a combination of soils such as Bleached-Orthic Tenosols, Grey Sodosols and Redoxic Hydrosols. Arenic Bleached-Orthic Tenosol, Mesotropic Mottled-Mesonatric Grey Sodosol and Parapanic Humosesquic Semiaquic Podosol in this area commonly produce topsoils similar to those used here (Isbell and National Committee on Soil and Terrain 2021).
Experiments
Four studies were combined to investigate experimental and commercial fertilisers with a range of water soluble P (WSP) contents. Fertilisers were compared to SSP or triple superphosphate (TSP) for leaching of P, plant growth and effects on soil residues under high leaching conditions on soil with a low P retention capacity. Experiments were conducted with fertiliser alone, with fertiliser and pasture plants, and with fertiliser and soil amendments that increase the capacity of the soil to retain P.
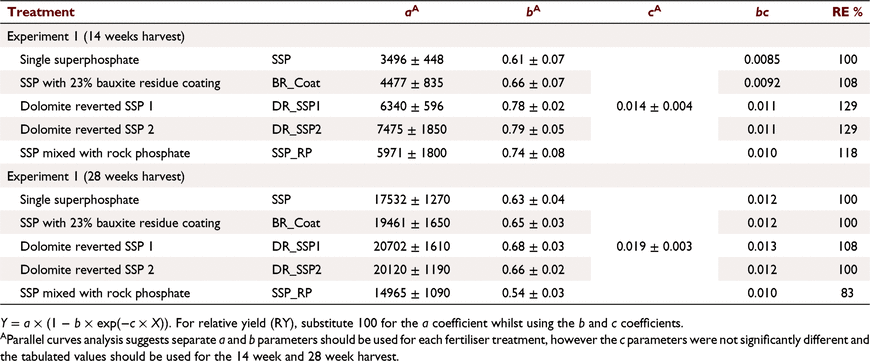
Experiment 1
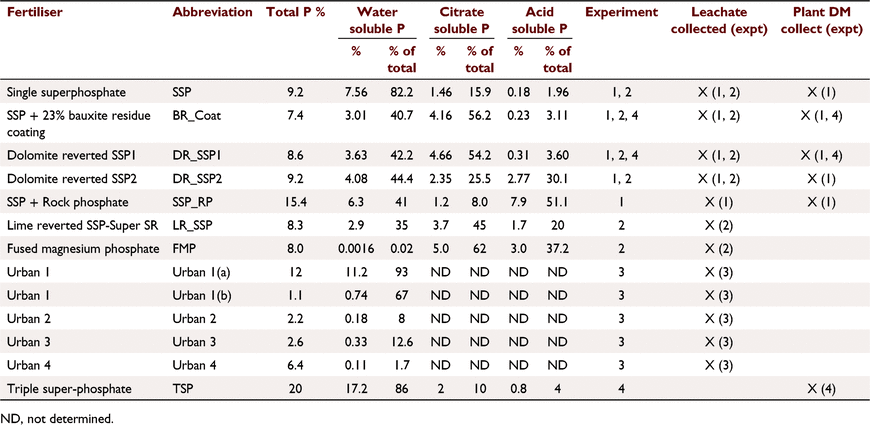
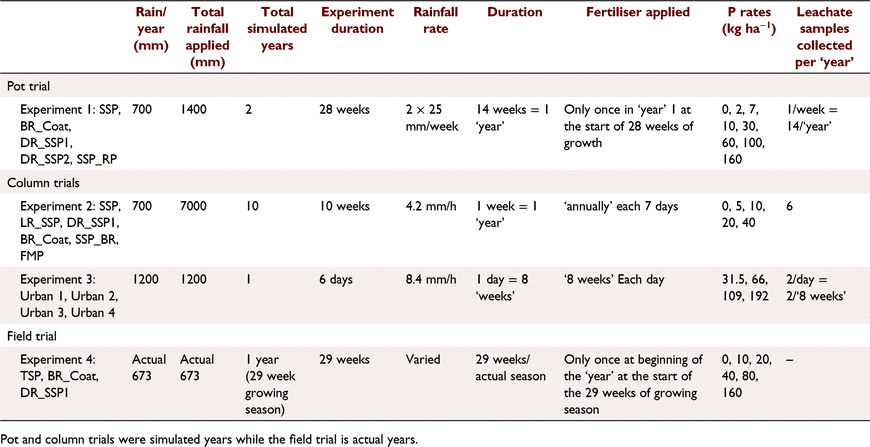
Soil A (Table 1) was treated with five fertilisers with varied soluble P contents (Tables 2, 3): SSP, single superphosphate; BR_Coat, SSP + 23% bauxite residue coating; DR_SSP1, dolomite reverted SSP; DR_SSP2, dolomite reverted SSP; and SSP_RP, SSP and rock phosphate. Each fertiliser was applied at nine different P rates (0, 2, 5, 7, 10, 30, 60, 100, 160 kg P ha−1) in a glasshouse pot experiment (note that all application rates referred to throughout were converted to kg P ha−1 equivalents). The trial used one replicate, consistent with the idea that the effects of rate on yield derived from curve fits was more important than other factors (Barrow 2021). Additional replicates of 0 kg P ha−1 were included, resulting in 46 pots, each 160 mm in diameter and containing about 5.0 kg of soil.
|
|
The soil was very low in P (Table 1) and P retention, and prior to the application of fertilisers and planting, the pots were pre-leached with the equivalent of 150 mm of rainfall using deionised (DI) water over 3 weeks. The fertiliser treatments were applied to the soil surface and incorporated into the top 2 cm of soil. Fertiliser was applied in granular form and smaller granules were selected to achieve the lowest application rates. The SSP_RP granules were selected so that half the application came from SSP and half from rock phosphate. Basal nutrients other than P were applied at planting following pre-leaching and again at week 12. The basal nutrients applied were (mg pot−1): NaMoO4·2H2O, 4.8; H3BO4, 0.24; ZnSO4·7H2O, 8.4; CuSO4·5H2O, 9.6; MnSO4·4H2O, 34.2; MgSO4·7H2O, 360; CoSO4·7H2O, 0.6; K2SO4, 194.4; CaCl2·2H2O, 150; NH4NO3, 180. An additional 324 mg pot−1 of K2SO4 was applied at week 20.
A total of 25 subterranean clover seeds were planted per pot (thinned to 16 plants pot−1 after 4 weeks), and the pots were lightly misted with DI for 6 weeks to avoid soil disturbance and floating seeds. Pots were then watered twice a week with 940 mL of DI water, equivalent to a total 50 mm of rainfall each week (Table 2). Each pot was raised above the base of a bucket to allow free draining of soil, and to facilitate leachate collection. Once each week, prior to watering, the volume of leachate was measured, and 50 mL subsamples were collected and frozen for P analysis.
After 14 weeks of growth, a total of 700 mm equivalent rainfall had been applied. Aboveground shoots were harvested for estimation of dry matter (DM) and P content. The P content of clover was combined with DM production to determine plant P uptake. To represent seasons and to assess the residues of fertilisers, the clover was allowed to re-grow for another 14 weeks without additional fertiliser or further sampling of leachate, and with the application of an additional 700 mm of equivalent rainfall. At the end of this additional 14 weeks of growth, aboveground shoots were harvested for DM and P content, and soil from each pot was collected and analysed for Colwell P and total P (TP).
Experiment 2
Soil A (Table 1) was treated with six fertilisers (Table 3) plus a control of 0 kg P ha−1. The fertilisers were: SSP, superphosphate; BR_Coat, SSP with a 23% bauxite residue coating; BR_SSP, SSP applied to 20 t ha−1 bauxite residue (Table 1); DR_SSP1, dolomite reverted SSP; LR_SSP, lime reverted SSP; and FMP, fused magnesium phosphate also known as calcium magnesium phosphate. Bauxite residue was supplied by Alcoa from the Kwinana alumina refinery.
The fertilisers were applied to the soil surface at rates to supply 0, 5, 10, 20 and 40 kg P ha−1 with one replicate to columns 93 mm internal diameter and 100 mm deep, filled with a poorly P retentive soil containing very little plant available P (Table 1). Deionised water was applied by peristaltic pump to each column equivalent to 700 mm rain in 7 days to simulate a year of leaching (Table 2). Samples of leachate were collected each day (six samples per week) and analysed for P (Murphy and Riley 1962). Fertilisers were applied at the beginning of each 7 day period for a total of 10 weeks, resulting in the equivalent of 10 years of rainfall and fertiliser applied with a total of approximately 7000 mm equivalent rainfall applied.
Experiment 3
This experiment used soil B (Table 1) and four different fertiliser treatments applied according to package recommendations (Table 3) plus a control of 0 kg P ha−1. The fertilisers were commercially available products designed for the urban environment. The fertiliser treatments were applied according to package labelling. Urban 1 was a granulated inorganic lawn fertiliser treatment that recommended a different formulation for the first application, and subsequent applications, whilst Urban 2 and 4 were granulated inorganic fertilisers, and Urban 3 was pelletised chicken manure. Package recommended annual P application rates were 31.5, 66, 109, 192 kg P ha−1 for Urban 1, Urban 2, Urban 3, and Urban 4 fertilisers respectively. The application of P was uniformly split over 6 days (Table 2) to achieve the recommended annual package P rates. The fertilisers were ground with a mortar and pestle and applied to the surface of soil B (Table 2) with poor P retention capacity and low Colwell P. Leaching columns were the same as Experiment 1 with 1200 mm equivalent rainfall applied by peristaltic pump over 6 days. The leachate was sampled at 08:00 hours and 16:00 hours each day (12 times in all). At the end of the trial, the soil was sampled and analysed for Colwell P and TP.
Experiment 4
This experiment was a field trial and soil testing identified the soil (Soil C, Table 1) contained plant available P residues from historical fertilisation to permanent pasture. The experiment included three P fertiliser treatments and three replicates (Table 2). The fertilisers were: TSP, superphosphate; BR_Coat, SSP with a 23% bauxite residue coating; and DR_SSP1, dolomite reverted SSP (Table 3). Each fertiliser was applied to supply 0, 10, 20, 40, 80, 160 kg P ha−1 to a field experiment on a sandy Bassendean soil in Coolup, WA, and a clover ryegrass sward was established. Basal fertilisers were applied 3 weeks after pasture emergence to supply 5 kg ha−1 manganese (Mn) as manganese sulfate (32% Mn), 3 kg ha−1 copper (Cu) as copper sulfate (25% Cu), 2 kg ha−1 zinc (Zn) as zinc sulfate (22.4% Zn), and 80 g ha−1 molybdenum (Mo) as sodium molybdate (39% Mo). Basal fertilisers containing potassium (K) and sulfur (S) were applied in autumn and spring of each year, as potassium chloride or gypsum, with 75 kg K ha−1 and 10 kg S ha−1 being applied at each application, and a total of 150 kg K ha−1 and 20 kg S ha−1 applied in the growing season. The total rainfall for the year was 673 mm.
Analyses
Soil samples were analysed for pH (CaCl2) (Rayment and Lyons 2011), Colwell P and K (Colwell 1965; Rayment and Lyons 2011), ammonium oxalate extractable iron and aluminium (Ammox Fe; Ammox Al) (Tamm 1922), P retention index (PRI) (Allen and Jeffery 1990), P buffering index (PBI) (Burkitt et al. 2002) and TP (Rayment and Lyons 2011).
All fertiliser samples were analysed for WSP and TP. All fertilisers except the urban fertilisers were analysed for citrate soluble P in neutral ammonium citrate (CSP), acid soluble P (ASP) (AOAC 1990).
Data analysis
Data was analysed using linear and non-linear regression and Analysis of Covariance using DataDesk RP ver. 8.2.0 Data Description Inc, Igor Pro ver. 9 from WaveMetrics, Inc., and Genstat ver. 20.1 from VSN International Ltd. Parallel curves analysis using accumulated analysis of variance was used to determine whether equation parameters for different fertiliser treatments and nutrient rates was justified (Powers 2021).
Where plants were grown in an experiment, the relationship between yield and the amount of P applied was described by the Mitscherlich equation:
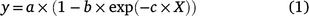
where y is the yield of dried pasture (kg ha−1), X is the amount of P applied (kg P ha−1), the coefficient a provides an estimate of maximum yield (kg ha−1), the coefficient b quantifies the yield response (maximum yield minus the mean yield for the nil fertiliser application, as estimated by the fitted equation), and coefficient c describes the curvature or shape of the response curve such that as c increases, the response curve moves to the left, becomes steeper and less P is required to produce the same relative yield. Hence smaller values of c are regarded as less responsive than larger values of c.
The effectiveness of P treatments was calculated relative to the effectiveness of freshly applied SSP (RE %) using the initial slope (bc) of the fitted Mitscherlich equation. It is not valid to use the curvature coefficient c, where there are different yield plateaux (Barrow and Campbell 1972; Bolland et al. 1995a).
Relationships were explored for data grouped by the presence of fertiliser only, with fertiliser and plants, and with fertiliser and soil amendments. In addition, the data for the first harvest from Experiment 1 was used to account for P applied as WSP, P uptake in aboveground dry matter and P lost in leachate. By difference, the remaining WSP was assumed to be retained in plant roots and in soil solution. The maximum amounts of P in above ground dry matter from Mitscherlich equations were averaged across fertiliser treatments, which along with estimates of P in plant roots were then used to estimate rates of P leached when WSP application exceeded that to achieve maximum yield.
Phosphorus concentrations of leachates collected in Experiment 1 were compared to soil solution P concentrations (rhizosphere.com) collected from a similar sandy soil to which different rates of a P binding clay had been added (Summers et al. 2020). This comparison was included to assist with interpretation of observed plant growth responses.
Results
Experiment 1
Plant growth
Three to five times more biomass was measured at 28 weeks than 14 weeks (Table 4). The c coefficients for response to the different fertilisers (sum of the fractions WSP + CSP + ASP) for the 14 week harvest (Fig. 1e) were similar and not significantly different (P > 0.05). Similarly the c coefficients for the 28 week harvest (Fig. 1j, Table 4) were not significantly different (P > 0.05). Plants grown with fertilisers containing less ASP (more WSP and CSP) showed greater DM response than fertilisers containing more ASP (Fig. 1a, f). Parallel curves analysis indicated that separate a, b and c coefficients were justified (P < 0.05) when DM yield was expressed in response to ASP for harvests at 14 and 28 weeks (Fig. 1a, f), and CSP when harvested at 28 weeks (Fig. 1g), accounting for 86.7%, 93.8% and 93.8% variance, respectively. The response curve coefficients (a, b, c in Eqn 1) were not significantly different (P > 0.05) for each of the fertiliser treatments when the dry matter yield was expressed in response to WSP + CSP at 28 weeks (Fig. 1i), and the model with a single a, b and c coefficient for all fertiliser treatments accounted for 93.4% of the variance. Analysis for all other response curves (CSP harvested at 14 weeks, WSP harvested at 14 weeks, WSP + CSP harvested at 14 weeks, WSP + CSP + ASP harvested at 14 weeks, WSP harvested at 28 weeks, WSP + CSP + ASP harvested at 28 weeks) suggested there was justification (P < 0.05) to vary the a and b coefficients but not the c coefficient (Table 4, Fig. 1b–e, h, j). These models accounted for 86.5%, 87.3%, 87.4%, 87.0%, 94.2% and 93.8% of the variance, respectively.
|
|
|
P content
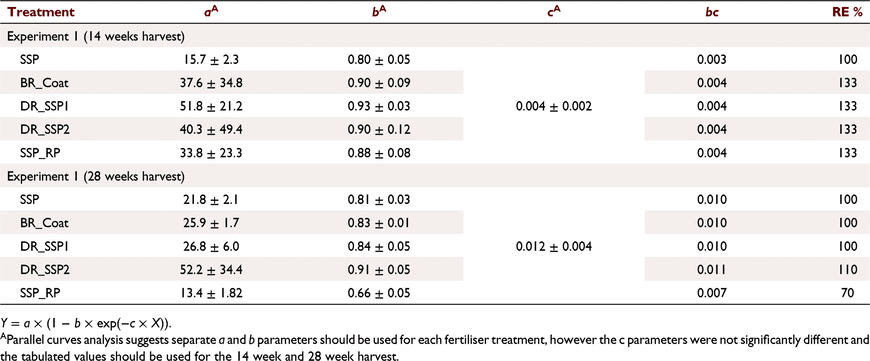
The P content of clover at 14 weeks was about double that measured at 28 weeks. By 28 weeks, the response of P content to applied P was small (Fig. 2a, c; Table 5). The rate of increase in P content to applied P varied for the different fertilisers at 14 weeks as indicated by a significant difference (P < 0.05) identified in c coefficients (Table 5), and 88.6% of the variance was accounted for. SSP and BR_Coat treatments had the highest P contents at the maximum rate of P applied. The rate of increase of P content in clover to applied P for the 28 week harvest was similar for all of the fertilisers with as indicated by the parallel curves analysis and similar c coefficient (P > 0.05) (Table 5, Fig. 2). In this model 72.8% of the variance was accounted for.
|
|

|
P uptake
Parallel curves analysis indicated that plant P uptake was greater at 14 weeks (38.7 kg ha−1) than at 28 weeks (22.7 kg ha−1) (Fig. 2b, c; Table 6) when the model coefficients were not separated for the different fertilisers, and these models accounted for 87.5% and 84.0% of the variance, respectively. The c coefficients and hence rates of P uptake were similar (P > 0.05) for all fertilisers (Fig. 2; Table 6) however there were some differences in a and b coefficients (P < 0.05), increasing the variance accounted for to 90.6% and 90.9% for the 14 week and 28 week harvest respectively when these differences were included.
|
Soil P
TP in the soil at the conclusion of Experiment 1 was generally less than 30 mg kg−1 (Fig. 3a–d), and not much higher than the TP measured in virgin soil (13 mg kg−1) at the start of the experiments (Table 1). A positive relationship between TP and P applied in various forms from the different fertilisers was seen in most cases (Fig. 3). Analysis of covariance indicated a significant interaction between amount of WSP applied and fertiliser type (P < 0.05), but no significant difference in intercept (P > 0.05) (Fig. 3a). Slopes of the lines decreased in the following order (SSP_PR; 0.29, DR_SSP2; 0.29, BR_Coat; 0.17, SSP; 0.02, DR_SSP1; −0.03) with a common intercept of 13.5.
|
|
For the relationship between TP and CSP applied, analysis of covariance indicated a significant interaction (P < 0.05) between fertiliser type and P applied, but no significant difference in the intercepts of the linear fits (P > 0.05). Slopes of the lines decreased in the following order (SSP_PR; 1.5, DR_SSP2; 0.51, SSP; 0.13, BR_Coat; 0.12, DR_SSP1; −0.02) with a common intercept of 13.5. For the relationship between TP and CSP + ASP applied, there was no significant interaction (P > 0.05), nor a significant effect of fertiliser type (P > 0.05). Hence the relationship of TP with CSP + ASP applied could be described by a single equation (TP = 13.5 + 0.126 × (CSP + ASP) applied; r2 = 0.17). Similarly, the relationship between TP and WSP + CSP + ASP applied showed no significant interaction (P > 0.05), nor a significant effect of fertiliser type (P > 0.05). Hence, the relationship of TP with WSP + CSP + ASP applied could be described by a single equation (TP = 13.5 + 0.065 × (CSP + ASP) applied; r2 = 0.15).
Colwell P (not shown) was generally lower than the limits of reporting (<2 mg kg−1) except for DR_SSP2, which was 7 mg kg−1 only at the highest rate of P applied.
P balance
The mean value of maximum P uptake by DM after 14 weeks growth was 10.5 kg P ha−1. The mean value of maximum P stored either in roots or soil solution was estimated to be 28.7 kg P ha−1. Across the fertiliser treatments, the average percentage of applied WSP measured in the above ground plant biomass ranged from over 200% at the lowest rate of P applied (2 kg P ha−1), to 14% at the highest rate of P applied (160 kg P ha−1).
Experiments 1, 2, and 3
Leaching
In Experiment 2, SSP leached more than twice as much P as the other fertilisers. The pattern of leaching followed a stepwise pattern, with a rapid leaching of P after each application, followed by a slower leaching rate after the initial pulse of P (Fig. 4). The stepwise pattern of P leaching was most pronounced for fertilisers with high WSP contents, with a rapid pulse of P leaching immediately after application decreasing as fertiliser WSP content decreased. The concentration of P in solution was initially high for the fertilisers with highest WSP especially SSP (Fig. 5a) followed by a rapid fall. Fig. 5b compares measured leachate P concentrations in these experiments with soil solution P concentrations collected using rhizons (rhizosphere.com) in Bassendean sand with SSP applied (Summers et al. 2020).
|
|
|
|
The amount of P leached in the absence of plants was strongly related to the amount of WSP applied (Fig. 6). The inclusion of plants reduced P leaching by 41%, whilst applying P to a soil amended with bauxite residue without plants resulted in a 53% reduction in P leaching. Analysis of covariance identified that slopes of the relationships between P leached and WSP applied (Fig. 6) were significantly different (P < 0.05) between fertiliser, fertiliser plus plants, and soil amendment. Slopes and 95% confidence intervals were 0.996 ± 0.047, 0.581 ± 0.070 and 0.479 ± 0.135 for fertiliser, fertiliser with plants and fertiliser with soil amendment, respectively. The rate (slope) of P leaching once WSP application exceeded 100 kg WSP ha−1 and plant growth was maximised (Fig. 1) was estimated to be the same as when plants were not included (Fig. 6). Whilst the rate of P leaching was the same, the amount lost was reduced by the amount of P retained by above and below ground plant biomass.
Experiment 4
Yield and soil P
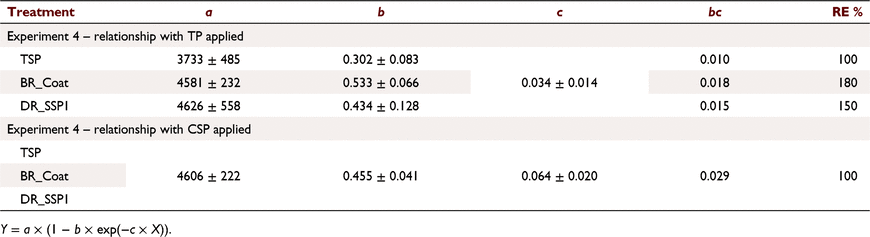
Both DR_SSP1 and BR_Coat increased dry matter more than TSP for the same amount of P applied (Fig. 7a; Table 7). The dry matter response to P applied as CSP was similar for the three fertilisers (Fig. 7g), much more so than when the independent variable was WSP + CSP + ASP, CSP + WSP and WSP (Fig. 7a, c, e). Parallel curves analysis indicated that there was no justification (P > 0.05) to use different a, b or c coefficients for the different fertilisers to describe the relationship between DM and P applied as CSP, and a single relationship could be used to describe the response (Table 7). The same analysis when WSP (Fig. 7e), WSP + CSP (Fig. 7c) or TP (Fig. 7a) were used as the dependent variable indicated that there was justification to vary some of the a and b coefficients (P < 0.05), but not the c coefficient (P > 0.05) (Table 7).
|
|
|
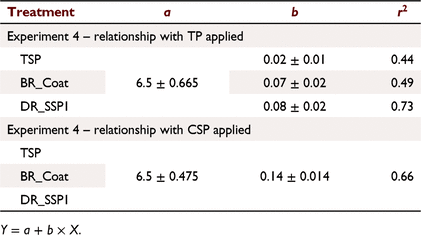
For relationships between Colwell P and CSP + WSP + ASP applied (Fig. 7b), analysis of covariance indicated a significant interaction (P < 0.05) between fertiliser type and P rate, but the intercept was not significantly different (P > 0.05). The resulting model accounted for 66.7% of the variance with a common intercept of 6.5, and slopes of 0.02, 0.07 and 0.08 for TSP, BR_Coat and DR_SSP1, respectively. Both the DR_SSP1 and BR_Coat had more than twice the soil P residues than TSP when measured in the following year based on TP applied (Fig. 7b; Table 8). Results for relationships between Colwell P and WSP applied (f) were similar to those for CSP + WSP + ASP applied. That is, a common intercept (6.5) and slopes of 0.02, 0.17 and 0.20 for TSP, BR_Coat and DR_SSP1, respectively, with 66.7% of the variance accounted for.
|
Colwell P was related to the CSP applied and the relationships of the three fertilisers (Fig. 7h) were not significantly different (P > 0.05) compared to combinations of the other fertiliser fractions (Fig. 7b, c, f, Table 8). This relationship could be described with an intercept of 6.5, a slope of 0.14 and accounted for 66% of the variance. The relationships between Colwell P and P applied as CSP + WSP (Fig. 7d) showed a common intercept and different slopes from analysis of covariance. The common intercept was 6.5 and slopes of 0.02, 0.07 and 0.08 for TSP, BR_Coat and DR_SSP1, respectively, with 66% of the variance accounted for.
Discussion
Experiment 1
A requirement for early season P for optimal plant growth on this low P retention low P status soil may explain why the highly water soluble SSP showed a greater initial yield response than the LWSP fertilisers (Grant et al. 2001). The soil used was depleted in P, then leached prior to planting, and application of SSP with a high WSP content would rapidly increase soil solution P and yield despite P leaching. The initial yield response to applied P was shown by the steepness in the fitted Mitscherlich curve. This is particularly noticeable when the responses have the less soluble ASP or CSP fractions as the dependent variables (Fig. 1a, b, f, g), and where the ASP and CSP content of the fertiliser is low, or conversely the WSP content of the fertiliser is high. It is speculated that transient initial P toxicity at the highest application rates of SSP (100 and 160 kg P ha−1) may have limited the early growth in such a poorly P buffered soil. This is evident to some degree in the DM response (a coefficient) for SSP (Table 4, Fig. 1c, h) being significantly less than the other fertilisers for the harvest at 14 weeks, and less than all but SSP_RP at 28 weeks. This can only be speculated because the tissue P concentration was not measured until the end of the experiment.
There are several studies that support this speculation and indicate a need for further research. Rossiter (1952) found yield reductions in clover and oats on Bassendean sand at 25–50 kg P ha−1 applied as SSP. He later showed toxicity from 37 kg P ha−1 applied as SSP to clover in a pot trial to the same Bassendean sand used here. Further, no toxicity was found using soil with higher P retention capacity soil, nor at a lower application rate of 12 kg P ha−1 on Bassendean sand (Rossiter 1955). These trials were not leaching trials and the rapid leaching of P found here would explain why P concentrations in the plant by the end our experiment was not at the toxic levels that Rossiter (1952) observed. In a field experiment, Summers et al. (2001) found similar yield suppression above 40 kg P ha−1 applied as SSP on Bassendean sand and this effect was not seen when the soil was modified to increase P retention capacity. Asher and Loneragan (1967) demonstrated that clover in solution culture, showed reductions in final yield at some point below 0.75 mg P L−1 depending on the species. Additionally, Summers et al. (2020) found soil solution concentration collected by rhizons (rhizosphere.com) in a pot trial using Bassendean sand with application of equivalent of 31.7 kg P ha−1 resulted in soil solution P concentrations up to 30 mg L−1. These high soil solution P concentrations were greatly reduced to less than 4.4 mg L−1 by the application of 2.6% clay to the sand when 39.2 kg P ha−1 was applied as SSP (Fig. 5b). Although toxicity in early growth was not explored, the concentration of P in the unamended soil was sustained at very high levels for the duration of the experiment. These observations and the potential to use LWSP fertilisers to reduce the excessive soil solution P concentration requires further investigation to determine whether P toxicity from high WSP fertilisers has a transient negative effect early in the season on low P retention sandy soils. The cases mentioned above were in soils low in P and if such an effect exists, it is likely to be further amplified in situations where sandy soils already have high P fertility levels, as is the case for many sandy soils in SWWA that have a long fertiliser history (Rogers et al. 2021; Weaver and Summers 2021). Alternatively, application of LWSP fertilisers may still result in application rates of WSP and CSP sufficient to support plant growth, either directly or through residues over a plant growth season. This is evident to some degree in Fig. 1i, where dry matter at 28 weeks was well described by the amount of WSP + CSP applied, independent of fertiliser type. By the second assessment after the application of another 700 mm of rainfall, the response to applied P was similar for most of the fertilisers. It may be that some of P uptake from SSP during the first growth period was carried over in plant roots into the second growth period. This is clearly evident by the estimated average retention of 28.7 kg P ha−1 in plant roots and soil solution at the end of the first 14 weeks of plant growth for the highest rates of P applied. In contrast, the CSP and ASP fractions from the LWSP fertilisers continued to contribute P in the second growth period, even after 700 mm of leaching. Late in the growing season, ‘the growth rate of the plants with adequate or inadequate P converges, the limiting factor to plant growth is not nutrient supply’ (Grant et al. 2001). The high proportion of WSP in SSP initially increased the plant tissue P content which would have been available as reserves stored in remaining plant tissue for the second assessment. The higher CSP of some of the LWSP fertilisers were less soluble and were likely available longer for plant uptake.
At the end of the experiment, Colwell P (plant available soil test P) was completely exhausted presumably because of 1400 mm of leaching combined with plant uptake and harvest. Only the LWSP treatments had P residues (TP) while no treatments had any plant available P remaining.
The cumulative P leached from SSP was almost double that of BR_Coat, DR_SSP1, and DR_SSP (Fig. 4), consistent with the almost double WSP content that SSP has compared to these LWSP fertilisers (Fig. 6).
Experiment 2
Reducing the solubility of SSP with a coating of bauxite residue decreased the proportion of WSP and increased CSP, resulting in a measured reduction in P leaching, which was similar to earlier reports (Summers et al. 2000). In the current experiments, we found a slight reduction in yield relative to SSP while earlier studies (Summers et al. 2000) found substantial increases in production from both glasshouse and field studies over 3 years on the same soil. This may be due to rainfall simulation in earlier glasshouse studies more consistent with rainfall and leaching in the field.
The bauxite residue treatment was included to compare the effect of soil amendment with a high P retention material and fertilisers with reduced water solubility. Leaching of P from SSP was 53% lower following addition of 20 t ha−1 of bauxite residue. This reduction is similar to that found in previous glasshouse (Summers et al. 1996b) and large scale field studies over 20 years (Summers et al. 2020). A substantial reduction in P concentration occurred when increasing the native clay content from 2.9 to 5.5% by adding subsurface clay to the topsoil (data from Summers et al. (2020), Fig. 5b). The soil solution P concentration was much lower with clay amendment to the sand despite more P being applied. The clay amendment used by Summers et al. (2020) is comparable to the SSP_BR treatment used here. All concentrations measured in Fig. 5a, b exceeded the optimum soil solution P concentration of 0.15–0.3 mg L−1 (Asher and Loneragan 1967; Russell and Russell 1973). Use of bauxite residue at this application rate also increases pasture production (Summers et al. 1996a, 2001). The similarity in leaching between these two studies provides further confidence that soil amendments can be applied at scale to reduce P losses from WSP fertilisers whilst maintaining or increasing production.
Experiment 3
Even under intense simulated rainfall, P leaching from FMP was less than 10% of that from SSP reflecting the relative amounts of WSP in these fertilisers. The high proportion of CSP and ASP in FMP, a water insoluble P source appeared resistant to leaching.
FMP has been found to be more effective for plant growth than SSP in coarse, acidic, highly P fixing soils when finely ground and surface applied, followed by surface watering (Sinclair 1975a, 1975b). The FMP they used was also water-insoluble with 60% CSP which only reacts through intimate contact with the soil. Intimate contact with the soil was proposed to have occurred through lessivage or percolation of the dispersed fine particles into the coarse textured soil carried in by the water. Further studies found similar improvement in yield from FMP relative to SSP where the fertilisers were mixed into the soil (Otabbong and Persson 1992, 1994). The application of water in our study was through a constant and slow flow rate via peristaltic pump, and this may not have resulted in lessivage and the same intimate contact observed by Sinclair (1975a) and Sinclair (1975b). In addition, the FMP we used was pelletised and whilst the soils were coarse textured and acidic, they had very poor P retention (Table 1) and pelletisation reduces the release of nutrients of sparingly water soluble fertilisers (Degryse et al. 2017). Despite this, there is potential for use of pelletised FMP on acidic sands because as Sinclair (1975b) noted, the granulated form slowly improved response to P over the growing season likely due to the gradual break down and mixing of fertiliser pellet. This is worthy of field exploration on very acidic sands with low P retention such as in WA (Weaver and Summers 2021) where re-application over several seasons may combine to provide continuity of P supply from finer FMP particles in close contact with the soil.
Leaching in experiments 1, 2, and 3
The leaching of P from fertilisers without plants was directly related to the amount of WSP applied and this continued even with multiple applications of fertiliser (Fig. 6). A slope very close to one for the relationship between P leached and WSP applied indicates that for these soils with low P retention, P losses are correlated directly with the amount of WSP applied, and none of the applied WSP is retained in the soil. This supports the first hypothesis that P losses would be positively correlated to WSP content of the fertiliser in a low P retentive soil.
Phosphorus leaching was reduced by about 40% by including plants as indicated by the slope of around 0.58 between P leached and WSP applied (Fig. 6). This supports the second hypothesis that P leaching is reduced by the presence of plants; however, leaching is still related to WSP applied. This phenomenon of a lower slope has upper limits since higher rates of WSP application will at some point exceed the capacity of plant P uptake or will simply leach faster than the plants can take up, leading to rates of P leaching similar to P application of fertiliser alone, albeit offset by the aboveground and belowground plant tissue P reservoir (Fig. 6). In addition, the low P fertility and P retention of the soil used in Experiment 1 where plants were present, as well as the range of WSP application rates used likely contribute to the linear nature of the response shown in Fig. 6. At low WSP application rates, both P leaching and plant growth are limited, whilst at high WSP application rates, both P leaching and plant P uptake are increased proportionally. Only at the highest rates of WSP applied from SSP does the amount of P leached start to increase above the fitted line, most likely because plant growth has been maximised, P uptake by plants is therefore capped, and any further application of WSP will simply contribute to P leaching losses, and possibly to plant toxicity. In grazing situations, the aboveground biomass is consumed by animals when not cut for hay, and around 80–90% of the consumed P (Hutton et al. 1967) is transferred back to the soil mostly in dung in a form more leachable than growing pasture. This means that for these sandy soils, the rate and amount of P leaching when plants and animals are present will be almost identical to the amount of WSP applied. This supports the notion that when soil P fertility increases to the point where additional P application results in no additional plant growth, then the amount of P applied should be equivalent to the amount of P removed in products (Helyar and Godden 1977; Barrow 2015), predominantly in the form of livestock or livestock products. For typical sheep, beef and dairy grazing enterprises in SWWA, this equates to average values of 1, 2 and 7 kg P ha−1, respectively (Weaver and Wong 2011).
Phosphorus leaching was reduced by around 52% by the addition of the bauxite residue soil amendment (SSP_BR) even with multiple SSP applications. This was indicated by the lower slope of 0.48 between P leached and WSP applied (Fig. 6). This supports the third hypothesis that a nutrient retentive soil amendment (bauxite residue) will reduce P leaching; however, it is still related to WSP applied. This linear response will be limited by the capacity of the soil amendment to retain P, and the amount of soil amendment applied. In the same way that plants offer an upper limit of P uptake, soil amendment will offer an upper limit of P retention. This is no different than soils having limited P retention capacity. Once this is exceeded, the amount of P leached will be likely be correlated to the amount of WSP applied. In some cases, the amount of P leached can exceed the amount of WSP applied if the soil has retained sufficient P residues from previous fertiliser application and plant residues to contribute to P leaching losses over and above applied WSP (Bromfield and Jones 1972; Weaver et al. 1988a; Noack et al. 2012).
Experiment 4
The field experiment had half the rainfall (673 mm of rainfall over 29 weeks) of the glasshouse experiment (1400 mm over 28 weeks). this rainfall difference might explain why the LWSP fertiliser treatments (BR_Coat, DR_SSP1) from the field experiment had elevated Colwell P levels relative to TSP, in contrast with the glasshouse trials having leached all the plant available P from all treatments. The differences between field results and controlled leaching experiments may also be due to the higher initial P status of the soil in the field. The field soil contained more than 80% of the P required to reach 95% of maximum production (Gourley et al. 2019), which would provide the required early season P, whilst the soil for the pot trial contained less than 30% of the P required to reach 95% of maximum production. Comparing the pot with field trial results supports the fourth hypothesis that LWSP fertilisers are less effective than WSP fertilisers on soil with limited plant available P. In the case of the field trial, addition of WSP did little to increase pasture production above the control, suggesting that when 80% of the required available P is already present in the soil, addition of soluble early season P was not necessary. In contrast, fertilisers containing less WSP and largely CSP showed larger production increases, most likely because these fertilisers were able to dissolve more slowly over the growing season, providing pastures with a more continuous P source. The differences in rates of dissolution and therefore, plant availability for fertilisers with differing WSP contents is seen clearly in Fig. 4. Highly water soluble fertilisers release P in a rapid pulse, whilst those containing less WSP and a large proportion of CSP show a more constant P release.
Agricultural soils in SWWA are high in P with the majority having more P than required for 90% of maximum production (Weaver and Wong 2011; Weaver and Summers 2021). A very limited proportion of agricultural soils in SWWA are as exhausted of P as that used in the glasshouse trials. Existing soil P reserves would remove the potential for limitations from early season P on most leaching sands. However, it would be prudent to soil test to assess if the rare situation of insufficient soil P exists before applying a LWSP fertiliser.
Care should be taken to avoid excessive build-up of plant available P that may become a future source of P loss to the drainage system (Sharpley et al. 2013; Kleinman 2017; Kusmer et al. 2019; von Arb et al. 2021). In addition, increases in organic P from higher production may also contribute to legacy P stocks, also contributing to P losses. The increase in plant residues is particularly important to recognise and manage on soils with low P retention capacity, where the P equilibrium strongly favours the soil solution, increasing the risk of P loss. The release of P from soils with little or no P retention capacity is governed by dissolution of residual P in the granules of applied fertiliser, adsorption onto organic matter, direct release from plant residues or from the release from organic residues via mineralisation. Soil amendment with high P retention materials such as clays can increase the available P retention on these leaching sands, supplementing the reserves in organic matter and also increasing the soil organic matter content through organic matter interaction with clay (Harper et al. 2012; Churchman et al. 2014). Soil testing offers potential to reduce the likelihood of unnecessary build-up of legacy P, signalling the application of P only when soil test P levels shows it is required.
Conclusion
The amount of P leached from LWSP fertilisers was directly related to the amount of WSP applied in a low P status, low P retentive soil. Plants reduced P leaching, but P leaching was still positively correlated with the amount of WSP applied. P leaching was further reduced by the application of a P retentive soil amendment but was still positively correlated with WSP application in a low P soil. CSP content was a good indicator of the effectiveness of the fertiliser to increase dry matter.
Under the leaching conditions studied here, P loss was reduced by reducing the WSP applied, similar to other field studies and over the long-term. Low initial soil P used in the glasshouse limited the P for early season growth from LWSP fertilisers relative to SSP that provided more P in these early stages of growth despite rapid leaching. The opposite was found in the field where plant available P reserves provided access for early season P highlighting the need for soil testing to decide the amount and form of P fertiliser required.
There is potential for further increases in growth and reduction in incidental P losses directly from fertilisers through further reduction in the WSP while maintaining high CSP components of P fertilisers. Further assessment of suitable candidates on acidic leaching soils is warranted using the findings from this study.
Data availability
Data is available on request.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
Acknowledgements
Assistance was provided by current and past staff of the Department of Primary Industries and Regional Development: John Grant, Simon Clarendon, Garry Dean, Christine Smart, the late Dr. Mike Bolland who initiated the field trial, and Martin Clarke. The field trial was carried out on the farm of Damien Curtis of Coolup. The authors are grateful for comments and suggestions made by the reviewers which have greatly improved the manuscript.
References
Allen DG, Jeffery RC (1990) Methods for analysis of phosphorus in Western Australian soils. Report of investigation No: 37 March 1990. Agricultural Chemistry Laboratory, Chemistry Centre of Western Australia.AOAC (1990) Official Methods of Analysis of the Association of Analytical Chemists, 15th edn. (Association of Official Analytical Chemists: Arlington, Virginia, USA)
Asher CJ, Loneragan JF (1967) Response of plants to phosphate concentration in solution culture I. Growth and phosphorus content. Soil Science 103, 225–233.
| Response of plants to phosphate concentration in solution culture I. Growth and phosphorus content.Crossref | GoogleScholarGoogle Scholar |
Barrow NJ (2015) Soil phosphate chemistry and the P-sparing effect of previous phosphate applications. Plant and Soil 397, 401–409.
| Soil phosphate chemistry and the P-sparing effect of previous phosphate applications.Crossref | GoogleScholarGoogle Scholar |
Barrow NJ (2021) Presenting data and distinguishing response curves. Plant and Soil 462, 1–5.
| Presenting data and distinguishing response curves.Crossref | GoogleScholarGoogle Scholar |
Barrow NJ, Campbell NA (1972) Methods of measuring residual value of fertilizers. Australian Journal of Experimental Agriculture 12, 502–510.
| Methods of measuring residual value of fertilizers.Crossref | GoogleScholarGoogle Scholar |
Bolland MDA, Clarke MF, Boetel FC (1995a) Comparison of single and coastal superphosphate for subterranean clover on phosphorus leaching soils. Fertilizer Research 40, 49–61.
| Comparison of single and coastal superphosphate for subterranean clover on phosphorus leaching soils.Crossref | GoogleScholarGoogle Scholar |
Bolland MDA, Clarke MF, Yeates JS (1995b) Effectiveness of rock phosphate, coastal superphosphate and single superphosphate for pasture on deep sandy soils. Fertilizer Research 41, 129–143.
| Effectiveness of rock phosphate, coastal superphosphate and single superphosphate for pasture on deep sandy soils.Crossref | GoogleScholarGoogle Scholar |
Bromfield SM, Jones OL (1972) The initial leaching of hayed-off pasture plants in relation to the recycling of phosphorus. Australian Journal of Agricultural Research 23, 811–824.
| The initial leaching of hayed-off pasture plants in relation to the recycling of phosphorus.Crossref | GoogleScholarGoogle Scholar |
Burkitt LL, Moody PW, Gourley CJP, Hannah MC (2002) A simple phosphorus buffering index for Australian soils. Australian Journal of Soil Research 40, 497–513.
| A simple phosphorus buffering index for Australian soils.Crossref | GoogleScholarGoogle Scholar |
Churchman GJ, Noble A, Bailey G, Chittleborough D, Harper R (2014) Clay addition and redistribution to enhance carbon sequestration in soils. In ‘Soil carbon’. (Eds A Hartemink, K McSweeney) pp. 327–335. (Springer: Cham, Switzerland)
| Crossref |
Colwell J (1965) An automatic procedure for the determination of phosphorus in sodium hydrogen carbonate extracts of soils. Chemical Industry 22, 893–895.
DCE (1985) The Peel-Harvey Estuarine System - proposals for management. Report 14. Department of Conervation and Environment, Perth, WA. Available at https://library.dbca.wa.gov.au/#record/151608
Deeley DM, Yeates JS, Gilkes RJ (1987) Chemical testing procedures for poorly soluble P fertilizers used on acid leaching sands. Fertilizer Research 14, 101–111.
| Chemical testing procedures for poorly soluble P fertilizers used on acid leaching sands.Crossref | GoogleScholarGoogle Scholar |
Degryse F, Baird R, da Silva RC, McLaughlin MJ (2017) Dissolution rate and agronomic effectiveness of struvite fertilizers – effect of soil pH, granulation and base excess. Plant and Soil 410, 139–152.
| Dissolution rate and agronomic effectiveness of struvite fertilizers – effect of soil pH, granulation and base excess.Crossref | GoogleScholarGoogle Scholar |
Environmental Protection Authority (1988) Peel inlet and harvey estuary management strategy - environmental review and management programme - Stage 2 (SUMMARY). Summary report prepared for Department of Agriculture and Department of Marine and Harbours by Kinhill Engineers: Victoria Park, WA. Available at https://catalogue.nla.gov.au/Record/394761
Gourley CJP, Weaver DM, Simpson RJ, Aarons SR, Hannah MM, Peverill KI (2019) The development and application of functions describing pasture yield responses to phosphorus, potassium and sulfur in Australia using meta-data analysis and derived soil-test calibration relationships. Crop and Pasture Science 70, 1065–1079.
| The development and application of functions describing pasture yield responses to phosphorus, potassium and sulfur in Australia using meta-data analysis and derived soil-test calibration relationships.Crossref | GoogleScholarGoogle Scholar |
Grant CA, Flaten DN, Tomasiewicz DJ, Sheppard SC (2001) The importance of early season phosphorus nutrition. Canadian Journal of Plant Science 81, 211–224.
| The importance of early season phosphorus nutrition.Crossref | GoogleScholarGoogle Scholar |
Harper R, Sochacki S, Bell R, Chittleborough D, Summers R, Tibbett M (2012) Increasing soil carbon storage in sandy soils with clay amendments. In ‘Climate change strategy for the primary industries conference, Melbourne, Vic.’ Available at https://researchrepository.murdoch.edu.au/id/eprint/19728/1/increasing_soil_carbon_storage.pdf
Helyar KR, Godden DP (1977) The biology and modelling of fertilizer response. Journal of the Australian Institute of Agricultural Science 43, 22–30.
Hutton JB, Jury KE, Davies EB (1967) Studies of the nutritive value of New Zealand dairy pastures. New Zealand Journal of Agricultural Research 10, 367–388.
| Studies of the nutritive value of New Zealand dairy pastures.Crossref | GoogleScholarGoogle Scholar |
Isbell RF, National Committee on Soil and Terrain (2021) ‘The Australian soil classification.’ 3rd edn. (CSIRO Publishing: Melbourne, Vic.)
Kleinman PJA (2017) The persistent environmental relevance of soil phosphorus sorption saturation. Current Pollution Reports 3, 141–150.
| The persistent environmental relevance of soil phosphorus sorption saturation.Crossref | GoogleScholarGoogle Scholar |
Kusmer AS, Goyette J-O, MacDonald GK, Bennett EM, Maranger R, Withers PJA (2019) Watershed buffering of legacy phosphorus pressure at a regional scale: a comparison across space and time. Ecosystems 22, 91–109.
| Watershed buffering of legacy phosphorus pressure at a regional scale: a comparison across space and time.Crossref | GoogleScholarGoogle Scholar |
McDowell R, Dodd R, Pletnyakov P, Noble A (2020) The ability to reduce soil legacy phosphorus at a country scale. Frontiers in Environmental Science 8, 6
| The ability to reduce soil legacy phosphorus at a country scale.Crossref | GoogleScholarGoogle Scholar |
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta 27, 31–36.
| A modified single solution method for the determination of phosphate in natural waters.Crossref | GoogleScholarGoogle Scholar |
Noack SR, McLaughlin MJ, Smernik RJ, McBeath TM, Armstrong RD (2012) Crop residue phosphorus: speciation and potential bio-availability. Plant and Soil 359, 375–385.
| Crop residue phosphorus: speciation and potential bio-availability.Crossref | GoogleScholarGoogle Scholar |
Otabbong E, Persson J (1992) Relative agronomic merit of fused calcium phosphate II. Dry matter production and P yields of ryegrass (Lolium perenne L.) and barley (Hordeum vulgare L.) in pot experiments. Fertilizer Research 32, 269–277.
| Relative agronomic merit of fused calcium phosphate II. Dry matter production and P yields of ryegrass (Lolium perenne L.) and barley (Hordeum vulgare L.) in pot experiments.Crossref | GoogleScholarGoogle Scholar |
Otabbong E, Persson J (1994) Relative agronomic merit of fused calcium phosphate: III. Forms of phosphorus in soils repeatedly cropped in pot experiments. Acta Agriculturae Scandinavica, Section B — Soil & Plant Science 44, 2–11.
| Relative agronomic merit of fused calcium phosphate: III. Forms of phosphorus in soils repeatedly cropped in pot experiments.Crossref | GoogleScholarGoogle Scholar |
Powers SJ (2021) Regression analysis in the context of designed experiments: neglect not thy opportunity to test for position and parallelism. Annals of Applied Biology 179, 4–11.
| Regression analysis in the context of designed experiments: neglect not thy opportunity to test for position and parallelism.Crossref | GoogleScholarGoogle Scholar |
Rayment G, Lyons D (2011) ‘Soil chemical methods - Australasia.’ (CSIRO Publishing: Collingwood, Vic.). Available at https://www.publish.csiro.au/book/6418/
Rogers D, Weaver D, Summers R, et al. (2021) Critical phosphorus values from the Better Fertiliser Decisions for Pastures project: early insights from validation trials. Crop & Pasture Science 72, 731–741.
| Critical phosphorus values from the Better Fertiliser Decisions for Pastures project: early insights from validation trials.Crossref | GoogleScholarGoogle Scholar |
Rossiter RC (1952) Phosphorus toxicity in Subterranean clover and oats grown on Muchea sand, and the modifying effects of lime and nitrate-nitrogen. Australian Journal of Agricultural Research 3, 227–243.
| Phosphorus toxicity in Subterranean clover and oats grown on Muchea sand, and the modifying effects of lime and nitrate-nitrogen.Crossref | GoogleScholarGoogle Scholar |
Rossiter RC (1955) The influence of soil type on phosphorus toxicity in subterranean clover (Trifolium subterraneum L.). Australian Journal of Agricultural Research 6, 1–8.
| The influence of soil type on phosphorus toxicity in subterranean clover (Trifolium subterraneum L.).Crossref | GoogleScholarGoogle Scholar |
Russell EJ, Russell EW (1973) ‘Soil conditions and plant growth.’ (Longmans, Green: London)
Schofield N, Bettenay E, McAlpine K, Height M, Hurle D, Ritchie G, Birch P (1985) Water and phosphorus transport processes in permeable grey sands at Talbot’s site near Harvey, Western Australia. Available at https://www.academia.edu/29315248/Water_and_Phosphorus_Transport_Processes_in_Permeable_Grey_Sands_at_Talbots_site_near_Harvey_Western_Australia_6l
Sharpley A, Jarvie HP, Buda A, May L, Spears B, Kleinman P (2013) Phosphorus legacy: overcoming the effects of past management practices to mitigate future water quality impairment. Journal of Environmental Quality 42, 1308–1326.
| Phosphorus legacy: overcoming the effects of past management practices to mitigate future water quality impairment.Crossref | GoogleScholarGoogle Scholar |
Silberstein R, Schofield N (1990) Phosphorus export and runoff from Coolup (duplex) soils in the Peel-Harvey Catchment, Western Australia The Stacey Experiment. Report No. WS 66, June 1990. Water Authority of Western Australia. Available at https://dwer.wa.gov.au/
Sinclair AG (1975a) Reactions of fused calcium-magnesium phosphate and superphosphate on a highly phosphate-fixing soil. I. Particle size effects. New Zealand Journal of Experimental Agriculture 3, 105–110.
| Reactions of fused calcium-magnesium phosphate and superphosphate on a highly phosphate-fixing soil. I. Particle size effects.Crossref | GoogleScholarGoogle Scholar |
Sinclair AG (1975b) Reactions of fused calcium-magnesium phosphate and superphosphate on a highly phosphate-fixing soil. II. Placement effects. New Zealand Journal of Experimental Agriculture 3, 111–116.
| Reactions of fused calcium-magnesium phosphate and superphosphate on a highly phosphate-fixing soil. II. Placement effects.Crossref | GoogleScholarGoogle Scholar |
Summers RN, Guise NR, Smirk DD (1993) Bauxite residue (red mud) increases phosphorus retention in sandy soil catchments in Western Australia. Fertilizer Research 34, 85–94.
| Bauxite residue (red mud) increases phosphorus retention in sandy soil catchments in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Summers RN, Guise NR, Smirk DD, Summers KJ (1996a) Bauxite residue (red mud) improves pasture growth on sandy soils in Western Australia. Australian Journal of Soil Research 34, 569–581.
| Bauxite residue (red mud) improves pasture growth on sandy soils in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Summers RN, Smirk DD, Karafilis D (1996b) Phosphorus retention and leachates from sandy soil amended with bauxite residue (red mud). Australian Journal of Soil Research 34, 555–567.
| Phosphorus retention and leachates from sandy soil amended with bauxite residue (red mud).Crossref | GoogleScholarGoogle Scholar |
Summers R, Clarke M, Pope T, O’Dea T (2000) Comparison of single superphosphate and superphosphate coated with bauxite residue for subterranean clover production on phosphorus-leaching soils. Australian Journal of Soil Research 38, 735–744.
| Comparison of single superphosphate and superphosphate coated with bauxite residue for subterranean clover production on phosphorus-leaching soils.Crossref | GoogleScholarGoogle Scholar |
Summers RN, Bolland MDA, Clarke MF (2001) Effect of application of bauxite residue (red mud) to very sandy soils on subterranean clover yield and P response. Australian Journal of Soil Research 39, 979–990.
| Effect of application of bauxite residue (red mud) to very sandy soils on subterranean clover yield and P response.Crossref | GoogleScholarGoogle Scholar |
Summers R, Richards P, Weaver D, Rowe D (2020) Soil amendment and soil testing as nutrient reduction strategies for the Peel Integrated Water Initiative. Resource Management Technical Report 416. Department of Primary Industries and Regional Development, Perth. Available at https://researchlibrary.agric.wa.gov.au/rmtr/401/
Tamm O (1922) Eine Methode zur Bestimmung de der anorganischen Komponente des Bodens. Meddelanden fran Statens skogsforsoksanstalt Stockholm 19, 387–404. https://pub.epsilon.slu.se/10087/1/medd_statens_skogsforskningsanst_019_04.pdf
van Gool D (1990) Land resources in the northern section of the Peel-Harvey catchment, Swan Coastal Plain, Western Australia Map. Department of Primary Industries and Regional Development, Western Australia, Perth. Available at https://researchlibrary.agric.wa.gov.au/land_res/28/
von Arb C, Stoll S, Frossard E, Stamm C, Prasuhn V (2021) The time it takes to reduce soil legacy phosphorus to a tolerable level for surface waters: what we learn from a case study in the catchment of Lake Baldegg, Switzerland. Geoderma 403, 115257
| The time it takes to reduce soil legacy phosphorus to a tolerable level for surface waters: what we learn from a case study in the catchment of Lake Baldegg, Switzerland.Crossref | GoogleScholarGoogle Scholar |
Weaver DM, Reed AEG (1998) Patterns of nutrient status and fertiliser practice on soils of the south coast of Western Australia. Agriculture, Ecosystems & Environment 67, 37–53.
| Patterns of nutrient status and fertiliser practice on soils of the south coast of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Weaver D, Summers R (2021) Phosphorus status and saturation in soils that drain into the Peel Inlet and Harvey Estuary of Western Australia. Soil Research 59, 699–714.
| Phosphorus status and saturation in soils that drain into the Peel Inlet and Harvey Estuary of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Weaver DM, Wong MTF (2011) Scope to improve phosphorus (P) management and balance efficiency of crop and pasture soils with contrasting P status and buffering indices. Plant and Soil 349, 37–54.
| Scope to improve phosphorus (P) management and balance efficiency of crop and pasture soils with contrasting P status and buffering indices.Crossref | GoogleScholarGoogle Scholar |
Weaver DM, Ritchie GSP, Anderson GC (1988a) Phosphorus leaching in sandy soils. II. Laboratory studies of the long-term effects of the phosphorus source. Australian Journal of Soil Research 26, 191–200.
| Phosphorus leaching in sandy soils. II. Laboratory studies of the long-term effects of the phosphorus source.Crossref | GoogleScholarGoogle Scholar |
Weaver DM, Ritchie GSP, Anderson GC, Deeley DM (1988b) Phosphorus leaching in sandy soils. I. Short-term effects of fertilizer applications and environmental conditions. Australian Journal of Soil Research 26, 177–190.
| Phosphorus leaching in sandy soils. I. Short-term effects of fertilizer applications and environmental conditions.Crossref | GoogleScholarGoogle Scholar |
Yeates JS, Deeley DM, Clarke MF, Allen D (1984) Modifying fertiliser practices. Journal of the Department of Agriculture, Western Australia, Series 4 25, 87–91. https://researchlibrary.agric.wa.gov.au/cgi/viewcontent.cgi?article=3017&context=journal_agriculture4