Complete mitochondrial genome of the endangered Mary River turtle (Elusor macrurus) and low mtDNA variation across the species’ range
Daniel J. Schmidt A D , Brittany Brockett A , Thomas Espinoza B , Marilyn Connell C and Jane M. Hughes AA Australian Rivers Institute, Griffith University, Nathan, Qld 4111, Australia.
B Queensland Department of Natural Resources and Mines, Bundaberg, Qld 4670, Australia.
C Tiaro and District Landcare Group, Tiaro, Qld 4650, Australia.
D Corresponding author. Email: d.schmidt@griffith.edu.au
Australian Journal of Zoology 64(2) 117-121 https://doi.org/10.1071/ZO16013
Submitted: 26 February 2016 Accepted: 4 July 2016 Published: 18 July 2016
Abstract
Elusor macrurus is an endangered short-necked turtle restricted to the Mary River catchment in south-eastern Queensland. Shotgun sequencing of genomic DNA was used to generate a complete mitochondrial genome sequence for E. macrurus using the Illumina MiSeq platform. The mitogenome is 16 499 base pairs (bp) long with 37 genes arranged in the typical vertebrate order and a relatively short 918-bp control region, which does not feature extensive tandem repeats as observed in some turtles. Primers were designed to amplify a 1270-bp region that includes 81% of the typically hypervariable control region. Two haplotypes were detected in a sample of 22 wild-caught individuals from eight sites across its natural range. The Mary River turtle is a species with low mtDNA nucleotide variability relative to other Chelidae. The combination of a very restricted distribution and dramatic reduction in population size due to exploitation for the pet trade are the conditions likely to have led to very low mtDNA variability in this endangered species.
Additional keywords: control region, D loop, freshwater turtle, MiSeq, next generation sequencing.
Introduction
The Mary River turtle (Elusor macrurus) represents an ancient lineage of short-necked turtles restricted to a single coastal drainage of eastern Australia (Georges and Thomson 2006). Deep phylogenetic relationships among Australo-Papuan short-neck chelids, including placement of the monotypic genus Elusor, are not yet resolved (cf. Georges et al. 1999; Le et al. 2013; Spinks et al. 2015). A recent treatment shows Elusor as sister lineage to either Myuchelys purvisi or Rheodytes leukops, depending on whether mitochondrial or nuclear DNA datasets are analysed (Spinks et al. 2015). The narrow distribution of E. macrurus, which is limited to the Mary River in south-eastern Queensland, combined with a history of exploitation for the pet trade and recruitment failure due to nest predation, have conspired to make it one of the most threatened species of freshwater turtle in Queensland (Limpus 2012; Micheli-Campbell et al. 2013). The species is listed as ‘Endangered’ at state level under the Nature Conservation Act 1992 and at national level under the EPBC Act 1999, and recognised as endangered globally in the IUCN Red List of Threatened Species 2015-4. Complete mitochondrial genomes (mitogenomes) have proven utility in molecular systematics at both deep and shallow taxonomic scales (e.g. Duchene et al. 2012). Few mitogenomes are available for Australo-Papuan chelid turtles and here we document the first complete mitochondrial genome sequence of E. macrurus and also conduct an assessment of mtDNA variation within the restricted natural range of this highly threatened taxon.
Materials and methods
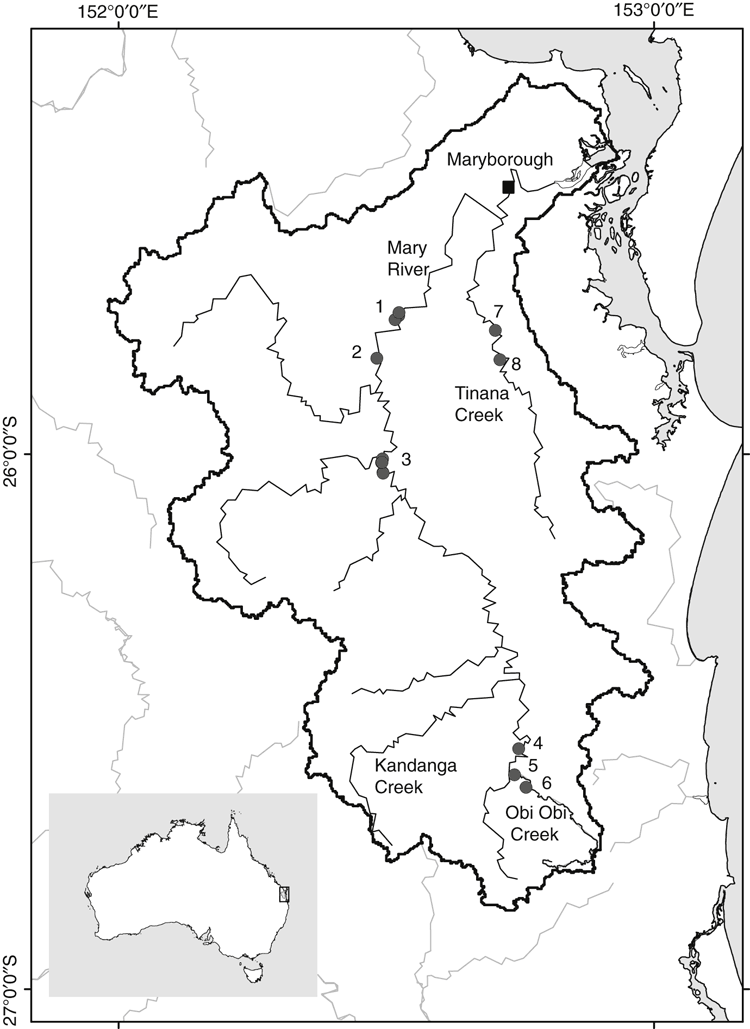
A total of 22 E. macrurus individuals were sampled from eight sites along the main trunk of the Mary River and a tributary separated by estuary, Tinana Creek (Fig. 1; Supplementary Material Table S1). All samples were collected using double-winged fyke nets set overnight in both upstream and downstream directions. A small section of skin (5 mm2) was taken from along the webbing of the hind foot, and preserved immediately in 100% ethanol. Tissue from voucher MRT4 was used for extraction of whole genomic DNA using the DNeasy blood and tissue kit (Qiagen). DNA was sheared to an approximate mean length of 400 bp using the M220 Focused-ultrasonicator (Covaris) and an Illumina MiSeq-compatible sequencing library was prepared. A double-index two-step library preparation was used, with all steps performed in the presence of solid-phase reversible immobilisation (SPRI) beads (iTru protocol: Travis Glenn, pers. comm.). The final full-length library construct was compatible with an Illumina TruSeq library except for unique 8-bp indexes within read 1 and read 2 adapters (Faircloth and Glenn 2012). Sequencing was performed on the Illumina MiSeq platform at Griffith University DNA Sequencing Facility using a 600-cycle MiSeq reagent kit v3, running 2 × 300-bp paired-end reads. The E. macrurus library was sequenced in parallel with four other libraries.
A total of 1.38 × 107 paired-end reads were generated from the E. macrurus shotgun library. Mitogenome assembly was performed using Geneious v8.1.7 (Kearse et al. 2012). Overlapping paired reads were first merged using the FLASH v1.2.9 plugin (minimum overlap 20, maximum overlap 200) (Magoc and Salzberg 2011). Then 5 × 105 merged reads in size range 301–550 bp were sampled. De novo and reference-guided assembly approaches were used for comparison (Schmidt 2015). De novo assembly was implemented in Geneious using default medium-sensitivity settings and allowing contigs with matching ends to circularise. A putative mitogenome was identified from the resulting contigs as the longest contig with circular topology. The putative mitogenome assembly was visually checked for errors derived from heteroplasmy or paralogues using the Geneious genome browser. A low-coverage region occurred towards the start of the putative control region from position 15 553 to 15 688 (mean coverage 3.2×; minimum 1; maximum 5) (Fig. 2). Subsequent mapping of an extra 680 000 merged reads (size range 200–300 bp) to the draft de novo–assembled mitogenome improved coverage in this region to a mean of 10×. A set of primers was designed to amplify across this low-coverage region in two overlapping fragments, spanning a total of 1270 bp from position 15 129 to 16 398 (Fig. 2). Primer pair MRT15129F (5ʹ-TTCGCCTATGCCATCCTACG) and MRT15705R (5ʹ-TGCTAGAGGTAAATAAATTTATGCACG) amplify a 603-bp fragment including partial cyt b, complete trnThr, trnPro and partial control region. Primer pair MRT15599F (5ʹ-CAACCACACCCTATCCGACA) and MRT16379R (5ʹ-TCGACACTGCACTTGGTGTA) amplify a 800-bp fragment of the control region. Overlap between these two fragments was 133 bp (Fig. 2). Standard PCR conditions of 35 cycles with annealing temperature of 58°C were used. Sequencing of PCR amplicons was performed by Macrogen Inc. (Seoul, Korea). Mitogenome annotation was performed with the Mitos webserver (Bernt et al. 2013) followed by adjustment of gene boundaries in Geneious v8.1.7. Folding of a putative light strand origin of replication (OL) was examined with the Vienna RNAfold webserver (Gruber et al. 2008), and a search for tandem repeats in the control region was performed with Tandem Repeat Finder (Benson 1999).
Results
De novo assembly of 5 × 105 merged raw reads produced a 16 499-bp circular contig from 1053 reads with 23× mean coverage (s.d. 5.4; minimum 1; maximum 41) (Fig. 2). Mapping to two chelid reference mitogenomes (Elseya branderhorsti GenBank accession: NC_026047; Emydura subglobosa GenBank accession: NC_026048) produced the same 16 499-bp contig from either 1057 reads (NC_026047), or 1044 reads (NC_026048), both spanning the entire reference at 23× mean coverage (s.d. 5.4; minimum 1; maximum 41). The mean quality score for 1057 mapped reads was Q36, with 93% at Q30 or higher. The three independent assemblies (one de novo and two map-to- reference) produced contigs 100% identical in length and sequence identity. The top 10 blastn matches for this sequence from the NCBI nucleotide database were turtle complete mitochondrial genomes, with pairwise identity to the query sequence ranging from 78.1% to 88.9%. The mitogenome of Elseya branderhorsti used for reference-guided assembly had 88.4% sequence identity with the new E. macrurus mitogenome. Validation of the E. macrurus mitogenome was achieved by sequencing two overlapping PCR amplicons using primers MRT15129F/MRT15705R and MRT15599F/MRT16379R, which produced an edited 1129-bp contig that aligned with 100% identity to position 15 196–16 324 of the mitogenome consensus sequence.
The complete mitogenome of E. macrurus is available from GenBank under accession number KU736930. It was 16 499 bp in length, with 13 protein-coding genes, 22 tRNAs, 2 rRNAs and a 918-bp control region (Fig. 2; Supplementary Material Fig. S1A). All 37 genes and the control region were arranged in the order observed in other Chelidae (Wang et al. 2012; Zhang and Georges 2014). Base composition was A (34.9%), C (26.6%), G (12.7%), T (25.8%). A portion of tRNAAsn and tRNACys along with an 18-bp intergenic region between these tRNAs folded into a stem and loop secondary structure (Supplementary Material Fig. S1B). This structure may be homologous with the putative origin of light strand replication (OL) proposed for Chelodina longicollis by Zhang and Georges (2014). There was no evidence for extensive tandem repeats in the control region as observed in mitogenomes of some turtle taxa (e.g. Wang et al. 2012). The longest tandem repeat detected was (AT)5. The new mitogenome was compared with publicly available sequences of mitochondrial origin matching E. macrurus. Pairwise identity >99% was found for partial fragments of 12S rRNA (GenBank accession: U40639), 16S rRNA (AF113622), COI (GenBank HQ329617, AF113646, KP876791; KP876792), ND4 (GenBank KC755124; Dryad doi:10.5061/dryad.tf8q1), and cyt b (Dryad doi:10.5061/dryad.tf8q1). All differences between the mitogenome consensus sequence and GenBank accessions were due to the presence of ambiguous base calls in GenBank sequences (16S rRNA from Georges et al. 1999), or to base differences within the first 22 bp at the 5ʹ-end of GenBank sequences (12S rRNA, COI from Georges et al. 1999; Seddon et al. 1997). Sequencing of PCR amplicons for 21 additional E. macrurus samples, using primer sets MRT15129F/MRT15705R and MRT15599F/MRT16379R yielded a full-length edited fragment of 1127–1129 bp, including 246-bp cyt b, complete trnThr and trnPro, and 743 bp of the control region. Two haplotypes, designated A and B, were identified. Haplotype A (1129 bp; GenBank Accession: KX369542) was found in 18 individuals and is identical to the mitogenome reference sequence KU736930. Haplotype B (1127 bp; GenBank accession: KX369543) was found in 4 individuals and differed by one substitution A > G at Position 16 059 as well as by contraction of the (AT)5 tandem repeat unit to (AT)4 at Position 16284–16293. Both of these substitutions were located in the control region. Haplotype A was distributed throughout the study area and Haplotype B was located at Sites 2 and 3 in Fig. 1 (see Supplementary Material Table S1).
Discussion
We examined a large portion (81%) of the mitochondrial control region, which is the most hypervariable mtDNA region in the Chelidae (Zhang and Georges 2014). Our sample included most of the known geographic range of E. macrurus, including Tinana Creek, which represents a distinct genetic subpopulation in both the Australian lungfish and Mary River cod (Huey et al. 2013; Hughes et al. 2015). Samples from Tinana Creek shared the same control region haplotype (Haplotype A) found throughout the main stem of the Mary River. Limited mtDNA variation means that further data such as polymorphic microsatellites will be required to assess fine-scale genetic subdivision within the Mary River catchment. Overall, control region variation detected across the range of E. macrurus was low relative to most chelid species examined at the within-drainage scale (Souza et al. 2003; Todd et al. 2013, 2014a, 2014b; Georges et al. 2014; Hodges et al. 2014, 2015). One exception is the Bellinger River turtle (Myuchelys georgesi), a critically endangered species with a single control region haplotype known from a sample consisting of 428 bp sequenced in 16 individuals (Georges et al. 2011). Like that of E. macrurus, the range of M. georgesi is restricted to a single small drainage.
Comparison of the new mitogenome sequence with publicly available partial mtDNA sequences of E. macrurus did not yield convincing evidence for additional mtDNA nucleotide variation in this species. All differences detected were ambiguous base calls or variation clustered at the beginning of old sequence accessions, which may be due to editing issues rather than real variation. The distribution of E. macrurus is restricted to a single coastal drainage so we should expect natural levels of mtDNA variability to be limited, as observed in other chelids at the within-drainage scale (e.g. Georges et al. 2011; Todd et al. 2014a). In addition to this, E. macrurus has undergone a dramatic population decline over the last 50 years, attributed to egg harvesting for the pet trade compounded by habitat modification and increased predation (Limpus 2012; Micheli-Campbell et al. 2013). Extreme population bottlenecks of this kind can remove most or all mtDNA variation within a population (Wilson et al. 1985).
Previous studies suggested that absence of an origin of light strand replication located between tRNAAsn and tRNACys may be characteristic of suborder Pleurodira (Wang et al. 2012). However, evidence for folding of this intergenic region in the new mitogenome of E. macrurus, along with similar evidence for Chelodina longicollis, suggests that this is not the case (Zhang and Georges 2014). Deep phylogenetic relationships among Australo-Papuan chelid genera have proved challenging to resolve (Georges et al. 1999; Georges and Thomson 2006; Le et al. 2013; Spinks et al. 2015). Uncorrected pairwise distance between complete mitochondrial genomes of E. macrurus and those of other Chelidae currently available on the NCBI nucleotide database was >10%, so the new E. macrurus mitogenome will be a useful resource, particularly for resolving the deep mtDNA relationships among Australo-Papuan short-necked Chelidae.
Acknowledgements
We thank Tiaro and District Landcare members for project support, Kathryn Real (Griffith University), Sharon Marshall (Queensland Department of Natural Resources and Mines), Travis Glenn (University of Georgia) for advice on the iTru library protocol, and Griffith University DNA sequencing facility for performing the MiSeq run and providing access to the ultrasonicator. This work was funded by the Queensland State Government ‘Everyones Environment Grant’ scheme and Tiaro and District Landcare Group under project code EER1400016. The project complies with Australian animal ethics permit no. ENV/08/15/AEC and DAFF Animal Experimentation Ethics Committee permit no. SA 2012/11/394, 395, 398, 399, 400 and General Fisheries Permit 139122.
References
Benson, G. (1999). Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Research 27, 573–580.| Tandem repeats finder: a program to analyze DNA sequences.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXhtVKmtrg%3D&md5=3c96e7cbaf41aab6caf3698f64e3c3ebCAS | 9862982PubMed |
Bernt, M., Donath, A., Juhling, F., Externbrink, F., Florentz, C., Fritzsch, G., Putz, J., Middendorf, M., and Stadler, P. F. (2013). MITOS: improved de novo metazoan mitochondrial genome annotation. Molecular Phylogenetics and Evolution 69, 313–319.
| MITOS: improved de novo metazoan mitochondrial genome annotation.Crossref | GoogleScholarGoogle Scholar | 22982435PubMed |
Duchene, S., Frey, A., Alfaro-Nunez, A., Dutton, P. H., Gilbert, M. T. P., and Morin, P. A. (2012). Marine turtle mitogenome phylogenetics and evolution. Molecular Phylogenetics and Evolution 65, 241–250.
| Marine turtle mitogenome phylogenetics and evolution.Crossref | GoogleScholarGoogle Scholar | 22750111PubMed |
Faircloth, B. C., and Glenn, T. C. (2012). Not all sequence tags are created equal: designing and validating sequence identification tags robust to indels. PLoS One 7, e42543.
| Not all sequence tags are created equal: designing and validating sequence identification tags robust to indels.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xht1Olsb7K&md5=6b87ef2ee0e80dddd7918606189f2cd8CAS | 22900027PubMed |
Georges, A., and Thomson, S. (2006). Evolution and zoogeography of Australian freshwater turtles. In ‘Evolution and Zoogeography of Australasian Vertebrates’. (Eds J. R. Merrick, M. Archer, G. M. Hickey and M. S. Y. Lee.) pp. 291–308. (Auscipub Pty Ltd: Sydney.)
Georges, A., Birrell, J., Saint, K. M., McCord, W., and Donnellan, S. C. (1999). A phylogeny for side-necked turtles (Chelonia: Pleurodira) based on mitochondrial and nuclear gene sequence variation. Biological Journal of the Linnean Society 67, 213–246.
| A phylogeny for side-necked turtles (Chelonia: Pleurodira) based on mitochondrial and nuclear gene sequence variation.Crossref | GoogleScholarGoogle Scholar |
Georges, A., Spencer, R.-J., Welsh, M., Shaffer, H. B., Walsh, R., and Zhang, X. (2011). Application of the precautionary principle to taxa of uncertain status: the case of the Bellinger River turtle. Endangered Species Research 14, 127–134.
| Application of the precautionary principle to taxa of uncertain status: the case of the Bellinger River turtle.Crossref | GoogleScholarGoogle Scholar |
Georges, A., Zhang, X. W., Unmack, P., Reid, B. N., Le, M., and McCord, W. P. (2014). Contemporary genetic structure of an endemic freshwater turtle reflects Miocene orogenesis of New Guinea. Biological Journal of the Linnean Society 111, 192–208.
| Contemporary genetic structure of an endemic freshwater turtle reflects Miocene orogenesis of New Guinea.Crossref | GoogleScholarGoogle Scholar |
Gruber, A. R., Lorenz, R., Bernhart, S. H., Neuboock, R., and Hofacker, I. L. (2008). The Vienna RNA Websuite. Nucleic Acids Research 36, W70–W74.
| The Vienna RNA Websuite.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXptlKkurg%3D&md5=f5f69cc0a0e6fd6dc570a7c9802a6673CAS | 18424795PubMed |
Hodges, K., Donnellan, S., and Georges, A. (2014). Phylogeography of the Australian freshwater turtle Chelodina expansa reveals complex relationships among inland and coastal bioregions. Biological Journal of the Linnean Society 111, 789–805.
| Phylogeography of the Australian freshwater turtle Chelodina expansa reveals complex relationships among inland and coastal bioregions.Crossref | GoogleScholarGoogle Scholar |
Hodges, K., Donnellan, S., and Georges, A. (2015). Significant genetic structure despite high vagility revealed through mitochondrial phylogeography of an Australian freshwater turtle (Chelodina longicollis). Marine and Freshwater Research 66, 1045–1056.
| Significant genetic structure despite high vagility revealed through mitochondrial phylogeography of an Australian freshwater turtle (Chelodina longicollis).Crossref | GoogleScholarGoogle Scholar |
Huey, J. A., Espinoza, T., and Hughes, J. M. (2013). Natural and anthropogenic drivers of genetic structure and low genetic variation in the endangered freshwater cod, Maccullochella mariensis. Conservation Genetics 14, 997–1008.
| Natural and anthropogenic drivers of genetic structure and low genetic variation in the endangered freshwater cod, Maccullochella mariensis.Crossref | GoogleScholarGoogle Scholar |
Hughes, J. M., Schmidt, D. J., Huey, J. A., Real, K. M., Espinoza, T., McDougall, A., Kind, P. K., Brooks, S., and Roberts, D. T. (2015). Extremely low microsatellite diversity but distinct population structure in a long-lived threatened species, the Australian lungfish Neoceratodus forsteri (Dipnoi). PLoS One 10, e0121858.
| Extremely low microsatellite diversity but distinct population structure in a long-lived threatened species, the Australian lungfish Neoceratodus forsteri (Dipnoi).Crossref | GoogleScholarGoogle Scholar | 25853492PubMed |
Kearse, M., Moir, R., Wilson, A., Stones-Havas, S., Cheung, M., Sturrock, S., Buxton, S., Cooper, A., Markowitz, S., Duran, C., Thierer, T., Ashton, B., Meintjes, P., and Drummond, A. (2012). Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28, 1647–1649.
| Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data.Crossref | GoogleScholarGoogle Scholar | 22543367PubMed |
Le, M., Reid, B. N., McCord, W. P., Naro-Maciel, E., Raxworthy, C. J., Amato, G., and Georges, A. (2013). Resolving the phylogenetic history of the short-necked turtles, genera Elseya and Myuchelys (Testudines: Chelidae) from Australia and New Guinea. Molecular Phylogenetics and Evolution 68, 251–258.
| Resolving the phylogenetic history of the short-necked turtles, genera Elseya and Myuchelys (Testudines: Chelidae) from Australia and New Guinea.Crossref | GoogleScholarGoogle Scholar | 23563271PubMed |
Limpus, C. (2012). Freshwater turtles in the Mary River: review of biological data for turtles in the Mary River, with emphasis on Elusor macrurus and Elseya albagula. Queensland Government, Brisbane.
Magoc, T., and Salzberg, S. L. (2011). FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27, 2957–2963.
| FLASH: fast length adjustment of short reads to improve genome assemblies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtlGkur7M&md5=2c0d3d2c2c23dbf05f2c0bd505a3757cCAS | 21903629PubMed |
Micheli-Campbell, M. A., Campbell, H. A., Connell, M., Dwyer, R. G., and Franklin, C. E. (2013). Integrating telemetry with a predictive model to assess habitat preferences and juvenile survival in an endangered freshwater turtle. Freshwater Biology 58, 2253–2263.
| Integrating telemetry with a predictive model to assess habitat preferences and juvenile survival in an endangered freshwater turtle.Crossref | GoogleScholarGoogle Scholar |
Schmidt, D. J. (2015). The complete mitogenome of an Australian carp gudgeon, hybridogenetic biotype HAHB (Hypseleotris: Eleotridae). Mitochondrial DNA , .
| The complete mitogenome of an Australian carp gudgeon, hybridogenetic biotype HAHB (Hypseleotris: Eleotridae).Crossref | GoogleScholarGoogle Scholar | 26643618PubMed |
Seddon, J. M., Georges, A., Baverstock, P. R., and McCord, W. (1997). Phylogenetic relationships of chelid turtles (Pleurodira: Chelidae) based on mitochondrial 12S rRNA gene sequence variation. Molecular Phylogenetics and Evolution 7, 55–61.
| Phylogenetic relationships of chelid turtles (Pleurodira: Chelidae) based on mitochondrial 12S rRNA gene sequence variation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXhtFGhu7g%3D&md5=90918230bbf3932f5c9898bfdaa65a21CAS | 9007020PubMed |
Souza, F. L., Cunha, A. F., Oliveira, M. A., Pereira, G. A. G., and Dos Reis, S. F. (2003). Preliminary phylogeographic analysis of the Neotropical freshwater turtle Hydromedusa maximiliani (Chelidae). Journal of Herpetology 37, 427–433.
| Preliminary phylogeographic analysis of the Neotropical freshwater turtle Hydromedusa maximiliani (Chelidae).Crossref | GoogleScholarGoogle Scholar |
Spinks, P. Q., Georges, A., and Shaffer, H. B. (2015). Phylogenetic uncertainty and taxonomic re-revisions: an example from the Australian short-necked turtles (Testudines: Chelidae). Copeia 103, 536–540.
| Phylogenetic uncertainty and taxonomic re-revisions: an example from the Australian short-necked turtles (Testudines: Chelidae).Crossref | GoogleScholarGoogle Scholar |
Todd, E. V., Blair, D., Farley, S., Farrington, L., Fitzsimmons, N. N., Georges, A., Limpus, C. J., and Jerry, D. R. (2013). Contemporary genetic structure reflects historical drainage isolation in an Australian snapping turtle, Elseya albagula. Zoological Journal of the Linnean Society 169, 200–214.
| Contemporary genetic structure reflects historical drainage isolation in an Australian snapping turtle, Elseya albagula.Crossref | GoogleScholarGoogle Scholar |
Todd, E. V., Blair, D., Georges, A., Lukoschek, V., and Jerry, D. R. (2014a). A biogeographical history and timeline for the evolution of Australian snapping turtles (Elseya: Chelidae) in Australia and New Guinea. Journal of Biogeography 41, 905–918.
| A biogeographical history and timeline for the evolution of Australian snapping turtles (Elseya: Chelidae) in Australia and New Guinea.Crossref | GoogleScholarGoogle Scholar |
Todd, E. V., Blair, D., and Jerry, D. R. (2014b). Influence of drainage divides versus arid corridors on genetic structure and demography of a widespread freshwater turtle, Emydura macquarii krefftii, from Australia. Ecology and Evolution 4, 606–622.
| Influence of drainage divides versus arid corridors on genetic structure and demography of a widespread freshwater turtle, Emydura macquarii krefftii, from Australia.Crossref | GoogleScholarGoogle Scholar | 25035802PubMed |
Wang, L., Zhou, X. M., Nie, L. W., Xia, X. Q., Liu, L., Jiang, Y., Huang, Z. F., and Jing, W. X. (2012). The complete mitochondrial genome sequences of Chelodina rugosa and Chelus fimbriata (Pleurodira: Chelidae): implications of a common absence of initiation sites (O–L) in pleurodiran turtles. Molecular Biology Reports 39, 2097–2107.
| The complete mitochondrial genome sequences of Chelodina rugosa and Chelus fimbriata (Pleurodira: Chelidae): implications of a common absence of initiation sites (O–L) in pleurodiran turtles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvVWmsLc%3D&md5=3272a76750b1063fcc7e496650b7eb75CAS | 21655955PubMed |
Wilson, A. C., Cann, R. L., Carr, S. M., George, M., Gyllensten, U. B., Helmbychowski, K. M., Higuchi, R. G., Palumbi, S. R., Prager, E. M., Sage, R. D., and Stoneking, M. (1985). Mitochondrial DNA and two perspectives on evolutionary genetics. Biological Journal of the Linnean Society 26, 375–400.
| Mitochondrial DNA and two perspectives on evolutionary genetics.Crossref | GoogleScholarGoogle Scholar |
Zhang, X. W., and Georges, A. (2014). A complete mitochondrial genome sequence for the Australian turtle, Chelodina longicollis, obtained using 454-pyrosequencing. Conservation Genetics Resources 6, 555–557.
| A complete mitochondrial genome sequence for the Australian turtle, Chelodina longicollis, obtained using 454-pyrosequencing.Crossref | GoogleScholarGoogle Scholar |