1H NMR-based metabolomic observation of a two-phased toxic mode of action in Eisenia fetida after sub-lethal phenanthrene exposure
Brian P. Lankadurai A , David M. Wolfe A , André J. Simpson A and Myrna J. Simpson A BA Department of Chemistry, University of Toronto, 1265 Military Trail, Toronto, ON, M1C 1A4, Canada.
B Corresponding author. Email: myrna.simpson@utoronto.ca
Environmental Chemistry 8(2) 105-114 https://doi.org/10.1071/EN10094
Submitted: 24 August 2010 Accepted: 2 December 2010 Published: 2 May 2011
Journal Compilation © CSIRO Publishing 2011 Open Access CC BY-NC-ND
Environmental context. Phenanthrene is a persistent soil contaminant, whose toxic mode of action in earthworms has not been fully examined. We adopt a metabolomics approach, using 1H nuclear magnetic resonance (NMR) spectroscopy, to measure the response of earthworms to sub-lethal phenanthrene exposure. The results indicate that NMR-based metabolomics may be used to monitor responses to sub-lethal levels of contaminants and to delineate their toxic mode of action.
Abstract. 1H NMR-based metabolomics was used to examine the response of the earthworm Eisenia fetida to sub-lethal phenanthrene exposure. E. fetida were exposed via contact tests to six sub-lethal (below the measured LC50 of 1.6 mg cm–2) concentrations of phenanthrene (0.8–0.025 mg cm–2) for 48 h. Multivariate statistical analysis of the 1H NMR spectra of earthworm tissue extracts revealed a two-phased mode of action (MOA). At exposures below 1/16th of the LC50, the MOA was characterised by a linear correlation between the metabolic response and exposure concentration. At exposures ≥1/16th of the LC50, the metabolic response to phenanthrene appeared to plateau, indicating a distinct change in the MOA. Further data analysis suggested that alanine, lysine, arginine, isoleucine, maltose, ATP and betaine may be potential indicators for sub-lethal phenanthrene exposure. Metabolite variation was also found to be proportional to the exposure concentration suggesting that NMR-based earthworm metabolomics is capable of elucidating concentration-dependent relationships in addition to elucidating the MOA of sub-lethal contaminant-exposure.
Additional keywords: concentration-dependence, contact tests, LC50, metabonomics.
Introduction
Earthworms have been used in ecotoxicological studies as indicators of soil toxicity[1–3] and are considered to be excellent model organisms, because they are exposed to soil contaminants by both ingestion and via passive absorption.[1,4,5] There are many studies that have examined the exposure of earthworms to lethal contaminant concentrations that result in mortality to 50% of the population; lethal dose (LD50) or lethal concentration (LC50).[1,4,5] However, these studies may not provide sufficient information concerning the toxic mode of action (MOA) of a given contaminant. Currently studies that explore changes in cellular metabolism, such as fluctuations in synthesis and breakdown of simple metabolites like amino acids and sugars due to exposure to very low or sub-lethal concentrations of contaminants, are lacking.[2,6,7] Sub-lethal contaminant exposure also results in adverse changes to the physiology of organisms.[8,9] Monitoring fluctuations in metabolite levels in response to sub-lethal contaminant exposure can potentially lead to an elucidation of the contaminant’s MOA.[10,11]
Nuclear magnetic resonance (NMR)-based metabolomics offers a reliable, reproducible and high-throughput platform that is currently being utilised to study earthworm responses to sub-lethal exposure of contaminants in both contact and soil exposure tests.[1–3,12–15] Recent metabolomic studies with polycyclic aromatic hydrocarbons (PAHs) have suggested that the earthworm responses are concentration-dependent, but these studies did not assess whether or not the toxic MOA could be ascertained over a wide range of ultra-low sub-lethal concentrations.[12,16,17] For example, Brown et al.[12] suggested that Eisenia fetida earthworm metabolic responses may be correlated with contaminant concentration but their study only included three different exposure concentrations and did not clearly identify the MOA. Soil exposure studies have shown that there is a positive linear correlation between earthworm metabolomic responses and PAH concentration.[16,17] These pioneering studies demonstrate the promise of NMR-based metabolomics as a novel soil testing tool; however, further studies are needed to test the ability of earthworm NMR-based metabolomic methods as a routine tool in the ecotoxicological assessment of PAHs in the environment. PAHs are prolific and persistent in soils,[18–20] thus developing better tools to monitor their risk to soil organisms is needed.
In the present study, the metabolic response of the earthworm Eisenia fetida to ultra-low sub-lethal phenanthrene exposure in contact tests was examined using 1H NMR analysis of earthworm tissue extracts in combination with multivariate statistical methods. We specifically test if 1H-NMR metabolomics can be used over a wide range of sub-lethal concentrations (i.e. fractions of the LC50), which is important before metabolomics can be used as a widespread tool for elucidating sub-lethal toxic responses in soil environments.[21,22] Furthermore, by using six, sub-lethal concentrations, we hope to build upon previous studies[12,17] and determine if NMR-based metabolomics can be used to ascertain the MOA over a wide range of sub-lethal phenanthrene concentrations. We report on the metabolomic responses of Eisenia fetida (the recommended earthworm species for use in toxicity tests by the Organization for Economic Cooperation and Development (OECD))[3,12,23] after exposure to six sub-lethal concentrations of a model PAH,[24,25] phenanthrene, ranging from 0.8 to 0.025 mg cm–2, which are 1/2 to 1/64th of the LC50 respectively. Metabolites that have the potential to be reliable indicators of phenanthrene exposure over a wide range of sub-lethal concentrations were also monitored to further build upon previous results[12,17] with the main goal of further determining the potential of NMR-based metabolomics as an ecotoxicity tool for soil contaminants.
Experimental methods
Determination of phenanthrene LC50
The 48-h LC50 of phenanthrene, using a concentration range from 0.016 to 10.4 mg cm–2, was determined using the contact filter paper test as described by the OECD Guidelines.[23] The LC50 for the OECD recommended reference substance, chloroacetamide, was also measured to assess the accuracy of the method. Standard 30-mL glass vials with Polytetrafluoroethylene (PTFE)-lined caps (Kimble Glass Inc., Fisher Scientific), which are recommended by the US Environmental Protection Agency,[26] were lined with Whatman no. 1 filter papers. Dichloromethane (DCM) was used as the carrier solvent. For all compounds, 1 mL of the carrier solution was added into each vial and evaporated under a slow stream of filtered, compressed nitrogen gas. In all cases, 1 mL of DCM was added to control vials (ten replicates) and vented. All vials were then vented for 4 h.
For chloroacetamide, five concentrations between 0.05 and 31.25 μg cm–2 were evaluated. A preliminary range-finding with five logarithmic concentrations of phenanthrene (between 0.001 and 10.0 mg cm–2) was first conducted. This range was narrowed down to 0.016 to 10.4 mg cm–2 (six concentrations), with ten worms per exposure concentration. Earthworms were depurated for 3 h to void the intestinal tracts. A single, mature earthworm, weighing between 300 and 600 mg was added to each vial and 1 mL of deionised water was added to moisten the filter paper. All vials were stored on their side in the dark at ambient room temperature. After 48 h, mortality was determined. LC50 values were calculated using the Trimmed Spearman–Karber Program Version 1.5 with automatic trim selected.[26,27] The LC50 value for chloroacetamide was 3.9 μg cm–2 (95% confidence interval of 2.6 to 5.8 μg cm–2). This is in good agreement with the reported value of 2.7 μg cm–2 for chloroacetamide.[28]
Earthworm contact tests and tissue extraction
Mature earthworms (Accessory publication, ‘Earthworm maintenance prior to contact tests’ section) were depurated in the dark for 96 h to empty their intestinal tracts before exposure tests.[3] Earthworms were exposed to six concentrations of phenanthrene: 0.8, 0.4, 0.2, 0.1, 0.05 and 0.025 mg cm–2 (corresponding to: 1/2, 1/4th, 1/8th, 1/16th, 1/32nd and 1/64th of the measured 48-h LC50 respectively). Filter papers were placed in amber glass jars before the addition of phenanthrene solutions (1 mL in chloroform).[12] For comparison to non-exposed earthworms (control set), only chloroform was added to filter papers (without phenanthrene).[12] The chloroform in all jars (exposed and controls) was allowed to evaporate and then 1 mL of distilled water was added before addition of earthworms. Twelve earthworms per exposure concentration were used and 12 earthworms comprised the control set (without phenanthrene added to filter papers). The glass jars were kept in the dark for 48 h.[23] Earthworms were then flash-frozen in liquid nitrogen, lyophilised and stored frozen until extraction.[3,15]
Lyophilised earthworms were homogenised in a 1.5-mL centrifuge tube using a 5 mm-wide stainless steel spatula.[15] The homogenised earthworm tissue was then extracted using 1.20 mL of a 0.2-M monobasic sodium phosphate buffer solution (NaH2PO4·2H2O, 99.3%, Fisher Chemicals) containing 0.1% (w/v) sodium azide (99.5% purity, Sigma Aldrich) as a preservative.[3] Buffer solution was made with D2O (99.9% purity, Cambridge Isotope Laboratories) and adjusted to a pD of 7.4 using NaOD (30% w/w in 99.5% D2O, Cambridge Isotope Laboratories Inc.). The buffer solution also contained 10 mg L–1 of 2,2-dimethyl-2-silapentane-5-sulfonate sodium salt (DSS, 97%, Sigma Aldrich) as an internal standard.[3,12] Samples were vortexed for 30 s and then sonicated for 15 min to aid with the extraction. Samples were then centrifuged at 14 000 rpm (~15 000g) for 20 min at room temperature (24°C) and the supernatant was transferred into a new 1.5-mL centrifuge tube. The centrifugation procedure was repeated twice more to remove any additional particulates. Samples were then transferred into 5-mm High Throughputplus NMR tubes (Norell Inc., NJ, USA) for 1H NMR analysis.
1H NMR spectroscopy and data analysis
1H NMR spectra of the earthworm extracts were acquired with a Bruker Avance 500 MHz spectrometer using a 1H–19F–15N–13C 5-mm Quadruple Resonance Inverse (QXI) probe fitted with an actively shielded Z gradient. 1H NMR experiments were performed using Presaturation Using Relaxation Gradients and Echoes (PURGE) water suppression, 128 scans, a recycle delay of 3 s, and 16 K time domain points.[29] Spectra were apodised through multiplication with an exponential decay corresponding to 0.3-Hz line broadening in the transformed spectrum, and a zero filling factor of 2. All spectra were manually phased and calibrated to the DSS internal reference methyl singlet, set to a chemical shift (δ) of 0.00 ppm.
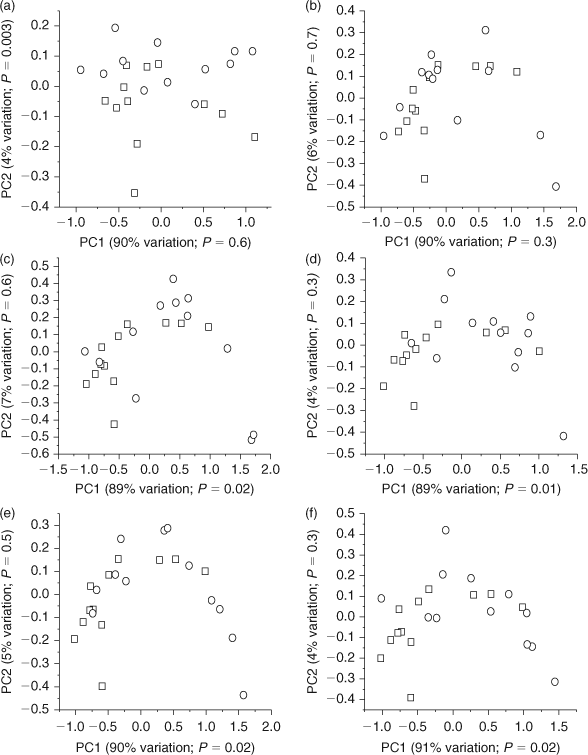
Multivariate statistical analyses were performed on processed 1H NMR data using the AMIX 3.8.4 (Bruker BioSpin, Rheinstetten, Germany) statistics tool to identify differences in the metabolic profiles of E. fetida following phenanthrene exposure. The 1H NMR spectra were analysed between δ of 0.5 and 10 ppm and divided into 0.02 ppm-wide buckets, for a total of 475 buckets.[30] The area between δ = 4.70 and 4.85 ppm was excluded to eliminate the small residual H2O/HOD signals. The integration mode was set to the sum of intensities and the spectra were scaled to total intensity.[12,15] This created a matrix in which each row represents an earthworm sample and each column contains the integrated area of the original spectral intensities contained within each bucket region. Individual principal component analysis (PCA) and partial least-squares discriminant analysis (PLS-DA) scores plots were calculated to compare the metabolic response of the control and exposed worms for each exposure level. PCA is an unsupervised statistical method that identifies the maximum variation in data.[31] However, PLS-DA is a supervised statistical method that identifies variation between groups (for example, between controls and phenanthrene exposed worms).[31,32] The R2X and R2Y values, which denote the explained variance of X and Y respectively were obtained for PLS-DA.[33,34] The PLS-DA models were also cross-validated (internal cross-validation) using leave-one-out cross-validation (LOOCV) and the cross-validated R2Y value (reported as Q2Y) was also obtained to determine the robustness of the model (Accessory publication, ‘Calculation of Q2Y of partial least-squares regression models’ section).[12,16,34,35] Levene’s test was used to test for variance homogeneity among the PCA and PLS-DA scores, which were found to have equal variances at α = 0.05.[36] A t-test (two-tailed, equal variances) was also performed on the first and second component PCA and PLS-DA scores to determine if there was a significant difference between the scores of the controls and the exposed worms at α = 0.05.[37] Corresponding PCA loadings plots, which show the relative weight for each bucket, were also acquired for each of the PCA scores plots to identify the metabolites that were contributing to the separation between the scores of the control and exposed earthworms. Both PCA and PLS-DA showed similar trends in discrimination (Fig. 1 and Accessory publication, Fig. A1). PLS-DA cross validation was also used to test the robustness of the separation and the resulting Q2Y values for all exposure concentrations were greater than 0.5, suggesting that the models are robust (Accessory publication, Fig. A1).[16] As all of the replicates were used to make the PLS-DA models, external validation was not performed with an independent test set to determine the predictive ability of the model.[32] Thus, the resulting PLS-DA models provided no additional discrimination to that obtained with PCA; therefore further discussions will focus solely on the PCA models for brevity.
|
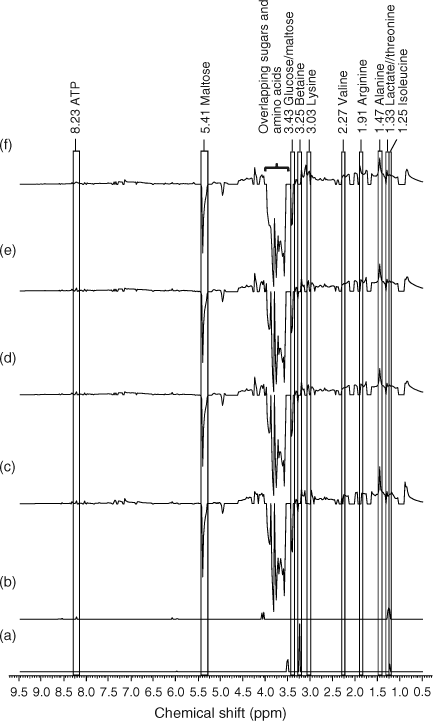
Difference class 1H NMR spectra were constructed to identify metabolites that had significantly increased or decreased relative to the control.[10,38] The buckets generated by AMIX 3.8.4 statistics tool, which represents the binned 1H NMR spectra of E. fetida extracts were then imported into Microsoft Excel (version 12.0.6504, Microsoft Corporation, Redmond, WA). A Levene’s test performed on the buckets revealed that there was equal variance at α = 0.05.[36] A t-test (two-tailed, equal variances) was then performed comparing the buckets of the controls with that of the exposure class to identify the buckets that were statistically different at α = 0.05. Average class 1H NMR spectra were obtained by averaging the buckets of each exposure class. Difference class 1H NMR spectra were then obtained by subtracting the buckets of the average controls from that of each average exposure class. The buckets representing metabolites peaks that were not statistically significant from the controls were then replaced with a zero resulting in a t-test filtered 1H NMR difference spectrum.[10,38] The buckets were then imported into ACD/1D NMR manager (Advanced Chemistry Development, version 12.0, Toronto, Canada) to acquire the difference spectra. The percentage changes in the intensity of metabolite peaks of exposed worms relative to the controls were obtained by first subtracting the buckets that pertain to the metabolites in the control earthworms from the exposed earthworms and then dividing again by the buckets in the control earthworms. The metabolite peaks were identified using a database of the 1H NMR spectra of a series of standard metabolites that were previously identified in E. fetida.[3,15]
A partial least-squares (PLS) scores plot was also calculated using the AMIX 3.8.4 statistics tool, having the exposure concentration as the Y-variable, to compare the control and exposed earthworms at all concentrations to deduce concentration-dependent relationships in the metabolic profile. A five-component model was found to be ideal using LOOCV. The scores from the PLS plot were then imported into Microsoft Excel and were averaged per class (concentration of phenanthrene exposure) and re-plotted with their associated standard errors.
Results and discussion
PCA scores plots of 1H NMR spectra of E. fetida tissue extracts identified some statistically significant (P < 0.05) changes in the metabolic profiles of the phenanthrene exposed earthworms relative to the unexposed (control) earthworms (Fig. 1). Brown et al.[12] also showed that exposure of E. fetida to sub-lethal PAH concentrations via contact tests elicits changes in the metabolic profile. The t-test performed on the first and second component PCA scores showed that the higher exposure concentrations (0.80. 0.40, 0.20 and 0.10 mg cm–2) resulted in a significant separation (P < 0.05) between the controls and the exposed worms along the x-axis (PC1; explains ~90% of the metabolic variation; Fig. 1c–f). However, the lower concentrations (0.025 and 0.05 mg cm–2) did not show significant separation (P > 0.05) from the controls along PC1 (Fig. 1a,b). The 0.025 mg cm–2 phenanthrene exposure had statistically significant separation (P = 0.003) from the controls along the y-axis (PC2; explains 4% of the metabolic variation); however, this accounts for very little of the variation in the metabolic profile (Fig. 1a). Therefore, higher phenanthrene exposure results in greater metabolic responses leading to greater differences in the overall metabolic profile. Brown et al.[17] also showed that high phenanthrene exposures in soil resulted in improved separation from the controls along PC1 (explains 74% of the metabolic variation), with lower concentrations separating from the controls only along PC4, which only explained ~2% of the metabolic variation.
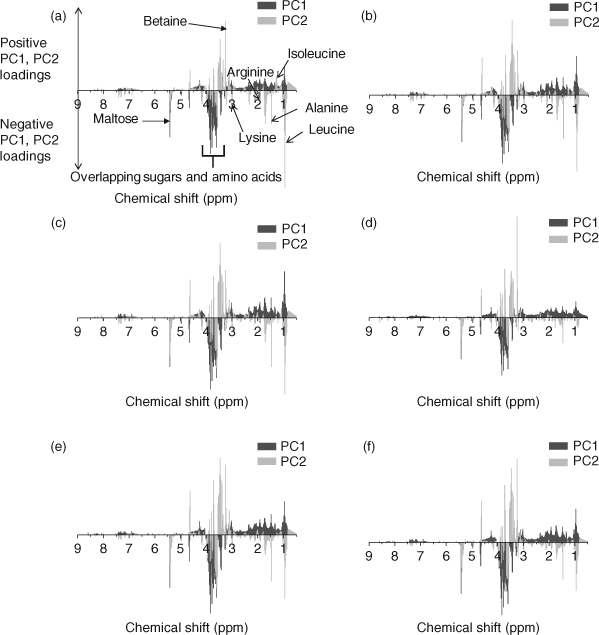
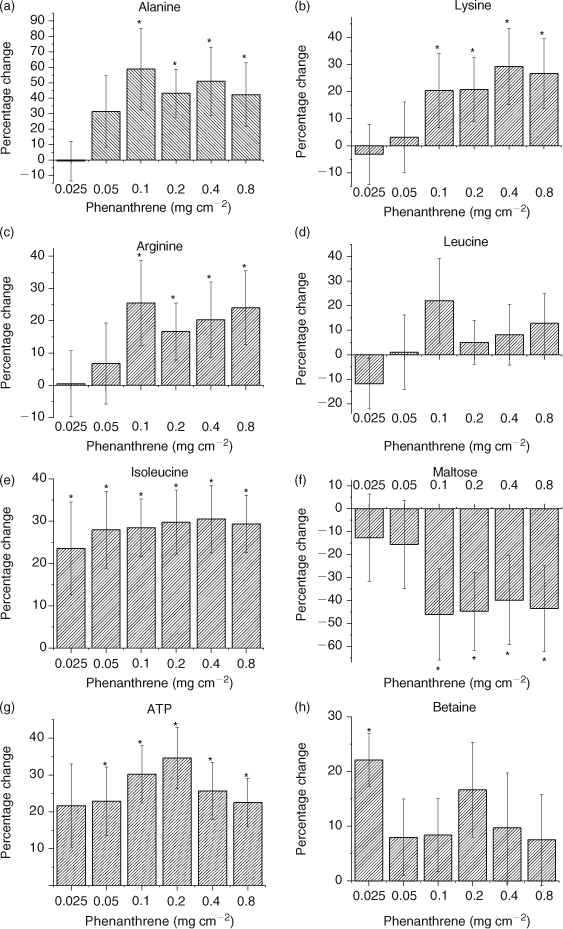
PCA (PC1 and PC2) loadings plots were used to identify metabolites contributing to the separation (Fig. 2).[1,12,17,39] Leucine (δ 0.95 ppm), isoleucine (δ 1.25 ppm), alanine (δ 1.47 ppm), arginine (δ 1.91 ppm), lysine (δ 3.03 ppm), betaine (δ 3.25 ppm) and maltose (δ 5.41 ppm) were identified as the major metabolites contributing to the PCA separation (Fig. 2). The t-test filtered difference 1H NMR spectra (Fig. 3) highlight these metabolites and the extent that the metabolic response varies with exposure to increasing phenanthrene concentration.[10,38] The percentage change of the identified metabolites relative to the control (Fig. 4) suggests that metabolic changes are also related to exposure concentration over the range of concentrations studied. This confirms previous studies that suggest that NMR-based metabolomics is capable of detecting concentration-dependent responses in soil and contact exposure tests.[12,16,17] Our study, which included a wider range of sub-lethal exposure concentrations, shows that significant (at α = 0.05) metabolic responses can be detected consistently at exposure concentrations as low as 0.1 mg cm–2 (1/16th of the LC50) for select metabolites. For example, significant (at α = 0.05) increases in alanine, lysine and arginine, along with a significant decrease in maltose relative to the control were observed at exposures ≥0.1 mg cm–2 (Fig. 4a–c,f). The percentage increase in isoleucine levels relative to the control was significant (α = 0.05) at all exposure levels (Fig. 4e). The magnitude of the percentage changes in alanine, lysine, arginine, maltose and the energy molecule adenosine triphosphate (ATP), all increase with exposure, indicating concentration-dependent responses to phenanthrene exposure (Fig. 4).[12,17] However, the levels of the amino acids and maltose began to plateau at exposures ≥0.1 mg cm–2. Brown et al.[17] also observed significant increases in amino acid levels and significant decreases in maltose relative to the controls at high phenanthrene exposures in soil. The consistency in the response of E. fetida to phenanthrene exposure in soil and in contact tests shows that contact tests are useful for fast contaminant-exposure studies that may be representative of exposure responses observed in soil.[17]
|
|
|
The observed amino acid increases may be attributed to an onset of protein catabolism that could have been triggered to meet the increased energy requirement in an attempt to counteract phenanthrene toxicity or a complete halt in amino acid breakdown.[40] Brown et al.[12] showed that maltose concentrations increased (but not statistically significantly) in earthworms exposed to phenanthrene concentrations of 0.05 and 0.1 mg cm–2 in contact tests. However, the variation in maltose concentration was high (i.e. large standard deviations). The large variation in maltose was likely due to inherent natural variation in the earthworms and potential errors from using DSS as an internal standard.[12] In our study, the intensity of the maltose peak decreased significantly (at α = 0.05) for phenanthrene exposures ≥0.1 mg cm–2 (Fig. 4f), which may be due to an increase in glycolysis to fulfil the energy needs of the cells.[17,40] An increase in glycolysis also results in an increase in ATP production (Fig. 4g). However, a significant accumulation of ATP at exposures ≥0.05 mg cm–2 also suggests that even though ATP is produced to meet the higher energy requirements, the mode that utilises ATP may be compromised due to phenanthrene exposure (Fig. 4g). The lack of efficiently utilising ATP contributes to the plateau observed in the response, because energy is no longer available.
Chemical toxicity may either depress or stimulate metabolic activity.[40,41] Depression of metabolic activity has been correlated with a decrease in alanine, leucine and isoleucine levels in the marine mussel Mytilus edulis, which was exposed to lindane.[40,41] In our study, E. fetida exposure to phenanthrene showed a general increase in amino acids. Betaine, an osmolyte that is produced when organisms are exposed to conditions of drought, high salinity or temperature stress, also decreases as metabolism slows down.[10,40,41] Betaine stabilises cellular metabolic functions under varying conditions of stress by enhancing the water retention of cells and by replacing inorganic salts.[37,42,43] Betaine significantly (at α = 0.05) increased at the lowest exposure of 0.025 mg cm–2 (Figs 3a, 4h). However, the increase in betaine at exposures >0.025 mg cm–2 was not significant (Fig. 4h). Therefore, the increases in amino acids and betaine levels suggests that there is stimulation in the metabolic activity of E. fetida on account of increasing phenanthrene exposure; however, this plateaus as the exposure increases above 0.1 mg cm–2 or 1/16th of the LC50.
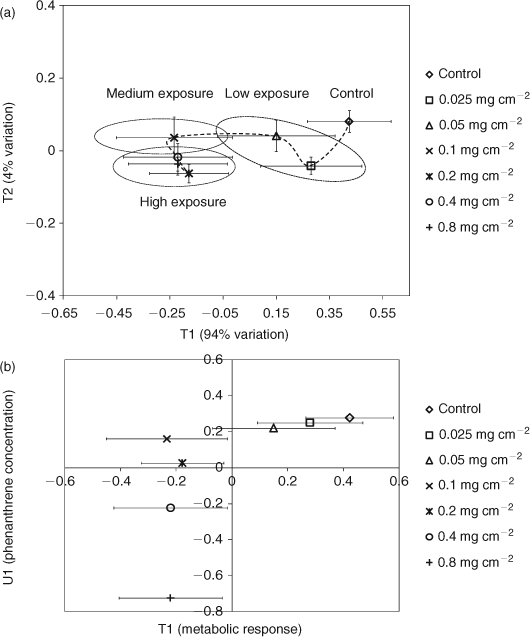
PLS regression analysis was also used to further ascertain the relationships between metabolic responses and phenanthrene exposure (Fig. 5a).[31,44–48] The PLS scores plot shows distinct regions for high (0.8, 0.4 and 0.2 mg cm–2), medium (0.1 mg cm–2), and low (0.05 and 0.025 mg cm–2) exposure levels (Fig. 5a). The low phenanthrene exposure level clusters near the controls whereas the higher exposure levels are further away, also suggesting a concentration-dependent response. A trajectory of responses to exposure of a chemical can signify its MOA, as exposure to chemicals with varying toxicities leads to trajectories that differ in their geometries or the overall shape of the curve.[10,11,38,49,50] The trajectory of the metabolic profile of E. fetida is shown in Fig. 5a. The metabolic response to phenanthrene exposure increases profoundly from 0.1 to 0.05 mg cm–2 (large shift along T1 which explains 94% of the metabolic variation). However, increasing the exposure concentration beyond 0.1 mg cm–2 alters the position only along T2 (explains only 4% of the metabolic variation). This suggests that at exposures ≥0.1 mg cm–2, there is little change in the intensity of the metabolic response. The PLS analysis also suggests a two-phased MOA for phenanthrene in E. fetida centred on the exposure of 0.1 mg cm–2. This agrees with the results from the difference spectra and the percentage change in metabolites, which also show that the metabolic response plateaus at exposures ≥0.1 mg cm–2 (Figs 3, 4). The PLS T1/U1 scores plot, which is used to delineate any correlations between the metabolic response and exposure concentration,[31,44,51] shows that there is a positive linear correlation (R2 = 0.99) between exposure level and the metabolic profile that spans from the control to an exposure concentration of 0.1 mg cm–2 (Fig. 5b). However, including exposure concentrations >0.1 mg cm–2 results in an overall non-linear correlation (R2 = 0.41) between exposure level and the metabolic profile. Therefore, the difference 1H NMR spectra, percentage changes in metabolites and PLS analysis indicate that phenanthrene exposure of 0.1 mg cm–2 is a critical concentration for E. fetida in contact tests. The MOA at the lower concentrations, which were 1/64th and 1/32nd fraction of the LC50, may change to a more potent phase at exposures higher than the critical concentration of 0.1 mg cm–2 and remains in that state as the exposure concentration increases towards the LC50. Similar concentration-dependent behaviour was also observed in E. fetida and other earthworm species exposed to PAHs where the concentration of cytochrome (Cyt) P450 was being monitored.[52–54] Exposure to xenobiotics results in an increased expression of Cyt P450 enzymes, allowing the monitoring of total Cyt P450 content to be a biomarker for exposure of xenobiotics to organisms.[22,53,55] PAHs have generally been shown to induce the Cyt P4501a isoenzyme in many species.[22,55–57] Zhang et al.[53] investigated the concentration-dependent behaviour of E. fetida to pyrene (Py) and benzo[a]pyrene (BaP) by monitoring the total Cyt P450 content. Total Cyt P450 content did not display any consistent correlation with PAH exposure and it was concluded that this observation was made because at certain concentrations, PAHs act as inducers of specific Cyt P450 isoenzymes while being an inhibitor for others. This may explain the observation of a MOA with two distinct phases in E. fetida to phenanthrene exposure. At concentrations ≥0.1 mg cm–2 phenanthrene may act as an inhibitor for specific Cyt P450 isoenzymes. This would compromise the earthworm’s ability to combat the toxic MOA of phenanthrene and the chances for mortality are increased. This trend culminates with almost 50% mortality at the LC50. The inconsistency in leucine levels, which contributed to the separation observed in the PCA scores plots, but did not increase significantly (at α = 0.05) may also be partially attributed to the initiation in Cyt P450 expression (Figs 3, 4d). Leucine, which comprises close to 15% of Cyt P450s amino acid composition, is the major amino acid in its makeup.[58] Therefore, initiation in Cyt P450 production in exposed worms may have resulted in the observed fluctuations of free leucine levels in E. fetida after phenanthrene exposure. However, as we did not measure Cyt P450 levels directly, future studies are needed to confirm the observed MOA within the context of Cyt P450 activity.
|
Conclusion
Our study further indicates that 1H NMR-based metabolomics is able to detect earthworm responses to sub-lethal concentrations of phenanthrene and also suggests that earthworm responses are correlated with exposure concentration.[12,17] Furthermore, in addition to being able to detect earthworm responses over a wide range of ultra-low sub-lethal phenanthrene concentrations, metabolomics also has the potential to delineate the MOA of the contaminant in earthworms. Two phases of the toxic MOA were observed for E. fetida in response to phenanthrene exposure. At exposures below 1/16th of the LC50 phase I of the toxic MOA is in action, highlighted by a linear correlation between the metabolic response and the exposure concentration. At exposures ≥1/16th of the LC50, the metabolic response to phenanthrene appeared to plateau, indicating the operation of a distinct phase II in the MOA. As in previous studies,[12,17] amino acids such as alanine, arginine, isoleucine and lysine and the sugar maltose emerged as potential response indicators of phenanthrene exposure. The consistency between the response of E. fetida to phenanthrene exposure in both contact and soil exposure studies suggests that even though contact tests may not fully represent the soil environment, they provide a rapid and reliable screening method for potentially measuring soil exposure responses. Therefore, the results of our study shows that NMR-based earthworm metabolomics holds great potential for development as a routine tool in the ecotoxicological assessment of low and sub-lethal levels of contaminants in the environment.
Accessory publication
Detailed information on the protocols followed for earthworm maintenance, the procedure followed by AMIX to calculate Q2Y, and PLS-DA scores plots of the 1H NMR spectra of E. fetida control and phenanthrene exposed earthworms.
Acknowledgements
Funding was provided by the Natural Sciences and Engineering Research Council (NSERC) Strategic Grants Program. Brian Lankadurai would also like to thank NSERC for a Post Graduate Scholarship (PGS M). We would like to extend thanks to Dr Jennifer McKelvie, Dr Melissa Whitfield Ã…´slund and Jimmy Yuk for technical assistance and valuable discussions.
References
[1] J. G. Bundy, E. M. Lenz, N. J. Bailey, C. L. Gavaghan, C. Svendsen, D. Spurgeon, P. K. Hankard, D. Osborn, J. A. Weeks, S. A. Trauger, Metabonomic assessment of toxicity of 4-fluoroaniline, 3,5-difluoroaniline and 2-fluoro-4-methylaniline to the earthworm Eisenia veneta (Rosa): identification of new endogenous biomarkers. Environ. Toxicol. Chem. 2002, 21, 1966..| 12206438PubMed |
[2] M. J. Simpson, J. R. McKelvie, Environmental metabolomics: new insights into earthworm ecotoxicity and contaminant bioavailability in soil. Anal. Bioanal. Chem. 2009, 394, 137.
| Environmental metabolomics: new insights into earthworm ecotoxicity and contaminant bioavailability in soil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFWisb0%3D&md5=b57bc5aa80a551588927c245e903630bCAS | 19194697PubMed |
[3] S. A. E. Brown, A. J. Simpson, M. J. Simpson, Evaluation of sample preparation methods for nuclear magnetic resonance metabolic profiling studies with Eisenia fetida. Environ. Toxicol. Chem. 2008, 27, 828.
| Evaluation of sample preparation methods for nuclear magnetic resonance metabolic profiling studies with Eisenia fetida.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXktFGntLg%3D&md5=f8467651471cd45df8ab5ab4160d6142CAS | 18333692PubMed |
[4] C. A. Edwards, P. J. Bohlen, The effects of toxic-chemicals on earthworms. Rev. Environ. Contam. Toxicol. 1992, 125, 23..
[5] L. C. Fitzpatrick, R. Sassani, B. J. Venables, A. J. Goven, Comparative toxicity of polychlorinated-biphenyls to earthworms Eisenia foetida and Lumbricus terrestris. Environ. Pollut. 1992, 77, 65.
| Comparative toxicity of polychlorinated-biphenyls to earthworms Eisenia foetida and Lumbricus terrestris.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XksVaitLo%3D&md5=d0b0ccd388e0635384a0a4855289f267CAS | 15091979PubMed |
[6] M. R. Viant, Recent developments in environmental metabolomics. Mol. Biosyst. 2008, 4, 980.
| Recent developments in environmental metabolomics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtFeitrfK&md5=c3993a0cee1e75c6a46b549a3f163f1bCAS | 19082136PubMed |
[7] M. Oldiges, S. Lutz, S. Pflug, K. Schroer, N. Stein, C. Wiendahl, Metabolomics: current state and evolving methodologies and tools. Appl. Microbiol. Biotechnol. 2007, 76, 495.
| Metabolomics: current state and evolving methodologies and tools.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXpt1ahsbs%3D&md5=df34d7a8117ff350a8b9f0d8abd438ddCAS | 17665194PubMed |
[8] Y. Y. Mosleh, S. Paris-Palacios, M. Couderchet, G. Vernet, Acute and sublethal effects of two insecticides on earthworms (Lumbricus terrestris L.) under laboratory conditions. Environ. Toxicol. 2003, 18, 1.
| Acute and sublethal effects of two insecticides on earthworms (Lumbricus terrestris L.) under laboratory conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXhtFKjsLc%3D&md5=67f840669273a3a24eb9a5b6c050c319CAS | 12539138PubMed |
[9] E. F. Neuhauser, C. A. Callahan, Growth and reproduction of the earthworm Eisenia fetida exposed to sublethal concentrations of organic-chemicals. Soil Biol. Biochem. 1990, 22, 175.
| Growth and reproduction of the earthworm Eisenia fetida exposed to sublethal concentrations of organic-chemicals.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXhvFegtL0%3D&md5=c9b3c7229eac85a21c88ff7d2931fc7fCAS |
[10] D. R. Ekman, Q. Teng, D. L. Villeneuve, M. D. Kahl, K. M. Jensen, E. J. Durhan, G. T. Ankley, T. W. Collette, Investigating compensation and recovery of fathead minnow (Pimephales promelas) exposed to 17 alpha-ethynylestradiol with metabolite profiling. Environ. Sci. Technol. 2008, 42, 4188.
| Investigating compensation and recovery of fathead minnow (Pimephales promelas) exposed to 17 alpha-ethynylestradiol with metabolite profiling.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXlt1Sks7o%3D&md5=ad652146af4f861f9db0b30febc09dfdCAS | 18589986PubMed |
[11] B. M. Beckwith-Hall, J. K. Nicholson, A. W. Nicholls, P. J. D. Foxall, J. C. Lindon, S. C. Connor, M. Abdi, J. Connelly, E. Holmes, Nuclear magnetic resonance spectroscopic and principal components analysis investigations into biochemical effects of three model hepatotoxins. Chem. Res. Toxicol. 1998, 11, 260.
| Nuclear magnetic resonance spectroscopic and principal components analysis investigations into biochemical effects of three model hepatotoxins.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXisVSiu7Y%3D&md5=5a11c1904758ede1c091bd76386f1b17CAS | 9548796PubMed |
[12] S. A. E. Brown, A. J. Simpson, M. J. Simpson, 1H NMR metabolomics of earthworm responses to sub-lethal PAH exposure. Environ. Chem. 2009, 6, 432.
| 1H NMR metabolomics of earthworm responses to sub-lethal PAH exposure.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFSkurnK&md5=ce1e72442ee2dcc90157930d537ca8a4CAS |
[13] J. G. Bundy, M. P. Davey, M. R. Viant, Environmental metabolomics: a critical review and future perspectives. Metabolomics 2009, 5, 3.
| Environmental metabolomics: a critical review and future perspectives.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXisFSls7k%3D&md5=f8528d88bc47af243aadb4abbb08cdbbCAS |
[14] J. G. Bundy, D. Osborn, J. M. Weeks, J. C. Lindon, J. K. Nicholson, An NMR-based metabonomic approach to the investigation of coelomic fluid biochemistry in earthworms under toxic stress. FEBS Lett. 2001, 500, 31.
| An NMR-based metabonomic approach to the investigation of coelomic fluid biochemistry in earthworms under toxic stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXkvVemu7w%3D&md5=d53370733042d10c6abdb9500697faebCAS | 11434921PubMed |
[15] J. R. McKelvie, J. Yuk, Y. P. Xu, A. J. Simpson, M. J. Simpson, 1H NMR and GC/MS metabolomics of earthworm responses to sub-lethal DDT and endosulfan exposure. Metabolomics 2009, 5, 84.
| 1H NMR and GC/MS metabolomics of earthworm responses to sub-lethal DDT and endosulfan exposure.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXisFSls7g%3D&md5=1688328b4ca9fcb0b33fd8ef8867b01bCAS |
[16] O. A. H. Jones, D. J. Spurgeon, C. Svendsen, J. L. Griffin, A metabolomics based approach to assessing the toxicity of the polyaromatic hydrocarbon pyrene to the earthworm Lumbricus rubellus. Chemosphere 2008, 71, 601.
| A metabolomics based approach to assessing the toxicity of the polyaromatic hydrocarbon pyrene to the earthworm Lumbricus rubellus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXisVWjsbY%3D&md5=7445330843c7108859a9b535664969dfCAS | 17928029PubMed |
[17] S. A. E. Brown, J. R. McKelvie, A. J. Simpson, M. J. Simpson, 1H NMR metabolomics of earthworm exposure to sub-lethal concentrations of phenanthrene in soil. Environ. Pollut. 2010, 158, 2117.
| 1H NMR metabolomics of earthworm exposure to sub-lethal concentrations of phenanthrene in soil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXlvVent7w%3D&md5=040d4c79d9b40051a63f9b35826e7b24CAS | 20338676PubMed |
[18] S. Lundstedt, P. Haglund, L. Oberg, Simultaneous extraction and fractionation of polycyclic aromatic hydrocarbons and their oxygenated derivatives in soil using selective pressurized liquid extraction. Anal. Chem. 2006, 78, 2993.
| Simultaneous extraction and fractionation of polycyclic aromatic hydrocarbons and their oxygenated derivatives in soil using selective pressurized liquid extraction.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XivVels7g%3D&md5=d649d73c1bff64f02607a6d45d347530CAS | 16642985PubMed |
[19] M. J. Smith, T. H. Flowers, H. J. Duncan, J. Alder, Effects of polycyclic aromatic hydrocarbons on germination and subsequent growth of grasses and legumes in freshly contaminated soil and soil with aged PAHs residues. Environ. Pollut. 2006, 141, 519.
| Effects of polycyclic aromatic hydrocarbons on germination and subsequent growth of grasses and legumes in freshly contaminated soil and soil with aged PAHs residues.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XjtFGkt74%3D&md5=8ebbe7373b6db1ff074696fac6984352CAS | 16246476PubMed |
[20] M. K. Chung, R. Hu, K. C. Cheung, M. H. Wong, Pollutants in Hong Kong soils: polycyclic aromatic hydrocarbons. Chemosphere 2007, 67, 464.
| Pollutants in Hong Kong soils: polycyclic aromatic hydrocarbons.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXnslKktg%3D%3D&md5=c7a557d7ebb4c074b53a470cffe29fa9CAS | 17109918PubMed |
[21] C. Svendsen, D. J. Spurgeon, P. K. Hankard, J. M. Weeks, A review of lysosomal membrane stability measured by neutral red retention: is it a workable earthworm biomarker? Ecotoxicol. Environ. Saf. 2004, 57, 20.
| A review of lysosomal membrane stability measured by neutral red retention: is it a workable earthworm biomarker?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXpsVSiu7g%3D&md5=b670defa639f48521c4261921ecf4ebeCAS | 14659363PubMed |
[22] R. van der Oost, J. Beyer, N. P. E. Vermeulen, Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ. Toxicol. Pharmacol. 2003, 13, 57.
| Fish bioaccumulation and biomarkers in environmental risk assessment: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XptFeqsro%3D&md5=e8f487a8d9b9c22d87eb26c4e9f1dfd8CAS |
[23] Earthworm acute toxicity tests, in OECD Guideline 207 1984 (Organisation for Economic Co-operation and Development: Paris).
[24] M. L. Hannam, S. D. Bamber, T. S. Galloway, A. J. Moody, M. B. Jones, Effects of the model PAH phenanthrene on immune function and oxidative stress in the haemolymph of the temperate scallop Pecten maximus. Chemosphere 2010, 78, 779.
| Effects of the model PAH phenanthrene on immune function and oxidative stress in the haemolymph of the temperate scallop Pecten maximus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtValtL0%3D&md5=3b014970e052beedfca553d5e77eb8cbCAS | 20074773PubMed |
[25] C. M. Hurdzan, N. T. Basta, P. G. Hatcher, O. H. Tuovinen, Phenanthrene release from natural organic matter surrogates under simulated human gastrointestinal conditions. Ecotoxicol. Environ. Saf. 2008, 69, 525.
| Phenanthrene release from natural organic matter surrogates under simulated human gastrointestinal conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXivVOitbs%3D&md5=b0500da9fb316623219bfb1ab7fd6470CAS | 17433439PubMed |
[26] Trimmed Spearman–Karber (TSK) Program, Version 1.5 1991 (Ecological Monitoring Research Division, Environmental Monitoring Systems Laboratory, US Environmental Protection Agency: Cincinnati, OH).
[27] M. A. Hamilton, R. C. Russo, R. V. Thurston, Trimmed Spearman–Karber method for estimating median lethal concentrations in toxicity bioassays. Environ. Sci. Technol. 1977, 11, 714.
| Trimmed Spearman–Karber method for estimating median lethal concentrations in toxicity bioassays.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE2sXksl2it7s%3D&md5=290149ce379b8a45c8a01abd3db87d9fCAS |
[28] C. A. Edwards, J. E. Bater, The use of earthworms in environmental-management, in The 4th International Symposium on Earthworm Ecology 1990, pp. 1683–1689 (Pergamon-Elsevier Science Ltd: Avignon, France).
[29] A. J. Simpson, S. A. Brown, N. M. R. Purge, Effective and easy solvent suppression. J. Magn. Reson. 2005, 175, 340.
| Effective and easy solvent suppression.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXls1ygsrY%3D&md5=67f12730c50ea4ae54303eca7e7a36cfCAS | 15964227PubMed |
[30] J. G. Bundy, D. J. Spurgeon, C. Svendsen, P. K. Hankard, J. M. Weeks, D. Osborn, J. C. Lindon, J. K. Nicholson, Environmental metabonomics: Applying combination biomarker analysis in earthworms at a metal contaminated site. Ecotoxicology 2004, 13, 797.
| Environmental metabonomics: Applying combination biomarker analysis in earthworms at a metal contaminated site.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhslyrs7o%3D&md5=95873eae73546df65733e9f724c0319dCAS | 15736850PubMed |
[31] L. Eriksson, E. Johansson, N. Kettaneh-Wold, J. Trygg, C. Wikstrom, S. Wold, Multi- and Megavariate Data Analysis Part 1: Basic Principles and Applications 2006 (Umetrics AB: Umea, Sweden).
[32] D. I. Broadhurst, D. B. Kell, Statistical strategies for avoiding false discoveries in metabolomics and related experiments. Metabolomics 2006, 2, 171.
| Statistical strategies for avoiding false discoveries in metabolomics and related experiments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhsVWktL4%3D&md5=4228914b3a43ed4f6839bdf27ee981d6CAS |
[33] S. Wold, M. Sjostrom, L. Eriksson, PLS-regression: a basic tool of chemometrics. Chemometr. Intell. Lab. 2001, 58, 109.
| PLS-regression: a basic tool of chemometrics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXotF2mtLw%3D&md5=3c29bb47eff6ccd96a20f683964223b9CAS |
[34] S. Wold, J. Trygg, A. Berglund, H. Antti, Some recent developments in PLS model building. Chemometr. Intell. Lab. 2001, 58, 131.
| Some recent developments in PLS model building.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXotF2mtLo%3D&md5=abfe021b5246cd72dcd79278fd9fe9e8CAS |
[35] S. Wold, L. Eriksson, Statistical validation of QSAR results, in Chemometrics Methods in Molecular Design (Ed. H. van de Waterbeemd) 1995, pp. 309–318 (VCH: Weinheim, Germany).
[36] M. B. Brown, A. B. Forsythe, Robust Tests for Equality of Variances. J. Am. Stat. Assoc. 1974, 69, 364.
| Robust Tests for Equality of Variances.Crossref | GoogleScholarGoogle Scholar |
[37] A. F. B. Boroujerdi, M. I. Vizcaino, A. Meyers, E. C. Pollock, S. L. Huynh, T. B. Schock, P. J. Morris, D. W. Bearden, NMR-based microbial metabolomics and the temperature-dependent coral pathogen Vibrio coralliilyticus. Environ. Sci. Technol. 2009, 43, 7658.
| NMR-based microbial metabolomics and the temperature-dependent coral pathogen Vibrio coralliilyticus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFCqtb7J&md5=aa9d7b13c4af1b842e9a80df0c7a98e6CAS | 19921875PubMed |
[38] D. R. Ekman, Q. N. Teng, D. L. Villeneuve, M. D. Kahl, K. M. Jensen, E. J. Durhan, G. T. Ankley, T. W. Collette, Profiling lipid metabolites yields unique information on sex- and time-dependent responses of fathead minnows (Pimephales promelas) exposed to 17 alpha-ethynylestradiol. Metabolomics 2009, 5, 22.
| Profiling lipid metabolites yields unique information on sex- and time-dependent responses of fathead minnows (Pimephales promelas) exposed to 17 alpha-ethynylestradiol.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXisFSls7Y%3D&md5=c8efcf49af259f8012808abba5505f65CAS |
[39] J. G. Bundy, H. C. Keun, J. K. Sidhu, D. J. Spurgeon, C. Svendsen, P. Kille, A. J. Morgan, Metabolic profile biomarkers of metal contamination in a sentinel terrestrial species are applicable across multiple sites. Environ. Sci. Technol. 2007, 41, 4458.
| Metabolic profile biomarkers of metal contamination in a sentinel terrestrial species are applicable across multiple sites.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXltVKqs70%3D&md5=676a6197a402726b6f710004b7bef7f8CAS | 17626452PubMed |
[40] W. Tuffnail, G. A. Mills, P. Cary, R. Greenwood, An environmental H-1 NMR metabolomic study of the exposure of the marine mussel Mytilus edulis to atrazine, lindane, hypoxia and starvation. Metabolomics 2009, 5, 33.
| An environmental H-1 NMR metabolomic study of the exposure of the marine mussel Mytilus edulis to atrazine, lindane, hypoxia and starvation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXisFSls7w%3D&md5=a82832ab67dc19ff63d6cf47a06a2146CAS |
[41] N. S. El-Shenawy, R. Greenwood, I. M. Ab-Del-Nabi, Z. I. Nabil, Effect of atrazine and lindane on the scope for growth of marine mussels Mytilus edulis. Acta Zool. Sin. 2006, 52, 712..
[42] P. H. Yancey, M. E. Clark, S. C. Hand, R. D. Bowlus, G. N. Somero, Living with water-stress – evolution of osmolyte systems. Science 1982, 217, 1214.
| Living with water-stress – evolution of osmolyte systems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL38XlsFyisbw%3D&md5=442ff2b660e5ba77fdc5051b8f40c7faCAS | 7112124PubMed |
[43] S. A. S. Craig, Betaine in human nutrition. Am. J. Clin. Nutr. 2004, 80, 539..
| 15321791PubMed |
[44] L. Eriksson, P. L. Andersson, E. Johansson, M. Tysklind, Megavariate analysis of environmental QSAR data. Part I – A basic framework founded on principal component analysis (PCA), partial least squares (PLS), and statistical molecular design (SMD). Mol. Divers. 2006, 10, 169.
| Megavariate analysis of environmental QSAR data. Part I – A basic framework founded on principal component analysis (PCA), partial least squares (PLS), and statistical molecular design (SMD).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XntFykt7o%3D&md5=13de0d6f1ca1b0ad92dd479293cfa9e8CAS | 16770514PubMed |
[45] L. Eriksson, P. L. Andersson, E. Johansson, M. Tysklind, Megavariate analysis of environmental QSAR data. Part II – Investigating very complex problem formulations using hierarchical, non-linear and batch-wise extensions of PCA and PLS. Mol. Divers. 2006, 10, 187.
| Megavariate analysis of environmental QSAR data. Part II – Investigating very complex problem formulations using hierarchical, non-linear and batch-wise extensions of PCA and PLS.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XntFyrs7Y%3D&md5=8ff0ac9b445aa216af2fa5e68c1f8feeCAS | 16802062PubMed |
[46] M. Henningsson, E. Sundbom, B. A. Armelius, P. Erdberg, PLS model building: a multivariate approach to personality test data. Scand. J. Psychol. 2001, 42, 399.
| PLS model building: a multivariate approach to personality test data.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD38%2FksVCksg%3D%3D&md5=8cf9a75aba98270fd214f4f001fc704eCAS | 11771809PubMed |
[47] S. Carraro, S. Rezzi, F. Reniero, K. Heberger, G. Giordano, S. Zanconato, C. Guillou, E. Baraldi, Metabolomics applied to exhaled breath condensate in childhood asthma. Am. J. Resp. Crit. Care 2007, 175, 986.
| Metabolomics applied to exhaled breath condensate in childhood asthma.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXmsFyqsLw%3D&md5=5241dd5d76d2702d4f4dead3810fc350CAS |
[48] P. Geladi, B. R. Kowalski, Partial least-squares regression – a tutorial. Anal. Chim. Acta 1986, 185, 1.
| Partial least-squares regression – a tutorial.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL28XmtVahs7c%3D&md5=ecdde10a303a213efaba96f43297bd65CAS |
[49] M. R. Viant, J. G. Bundy, C. A. Pincetich, J. S. de Ropp, R. S. Tjeerdema, NMR-derived developmental metabolic trajectories: an approach for visualizing the toxic actions of trichloroethylene during embryogenesis. Metabolomics 2005, 1, 149.
| NMR-derived developmental metabolic trajectories: an approach for visualizing the toxic actions of trichloroethylene during embryogenesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1SmsrjE&md5=1a48125c3ed554b0fc8b1e243c54947dCAS |
[50] O. Beckonert, H. C. Keun, T. M. D. Ebbels, J. G. Bundy, E. Holmes, J. C. Lindon, J. K. Nicholson, Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat. Protoc. 2007, 2, 2692.
| Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtlSlsr3E&md5=204325afd210fb611b89aad5cd844184CAS | 18007604PubMed |
[51] G. Balcerowska, R. Siuda, H. Czarnik-Matusewicz, Orthogonal signal correction to PLS modelling in application to spectral data. Acta Agrophysica 2005, 6, 7..
[52] M. Saint-Denis, J. F. Narbonne, C. Arnaud, E. Thybaud, D. Ribera, Biochemical responses of the earthworm Eisenia fetida andrei exposed to contaminated artificial soil: effects of benzo(a)pyrene. Soil Biol. Biochem. 1999, 31, 1837.
| Biochemical responses of the earthworm Eisenia fetida andrei exposed to contaminated artificial soil: effects of benzo(a)pyrene.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXmslGjtLs%3D&md5=b3fde7c598787852b3aab0d06ca1471bCAS |
[53] W. W. Zhang, Y. F. Y. F. Song, P. P. Gong, T. H. T. H. Sun, Q. X. Zhou, M. M. Liu, Earthworm cytochrome P450 determination and application as a biomarker for diagnosing PAH exposure. J. Environ. Monit. 2006, 8, 963.
| Earthworm cytochrome P450 determination and application as a biomarker for diagnosing PAH exposure.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XovFKlt7Y%3D&md5=b573a277fda5fa52daf2a21687de8587CAS | 16951757PubMed |
[54] R. K. Achazi, C. Flenner, D. R. Livingstone, L. D. Peters, K. Schaub, E. Scheiwe, Cytochrome P450 and dependent activities in unexposed and PAH-exposed terrestrial annelids. Comp. Biochem. Phys. C 1998, 121, 339..
[55] P. J. Brown, S. M. Long, D. J. Spurgeon, C. Svendsen, P. K. Hankard, Toxicological and biochemical responses of the earthworm Lumbricus rubellus to pyrene, a non-carcinogenic polycyclic aromatic hydrocarbon. Chemosphere 2004, 57, 1675.
| Toxicological and biochemical responses of the earthworm Lumbricus rubellus to pyrene, a non-carcinogenic polycyclic aromatic hydrocarbon.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXpt1arsrg%3D&md5=d5fa0315324825ee1766746b27f1161dCAS | 15519413PubMed |
[56] J. J. Whyte, R. E. Jung, C. J. Schmitt, D. E. Tillitt, Ethoxyresorufin-O-deethylase (EROD) activity in fish as a biomarker of chemical exposure. Crit. Rev. Toxicol. 2000, 30, 347.
| Ethoxyresorufin-O-deethylase (EROD) activity in fish as a biomarker of chemical exposure.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXmsVWjtrg%3D&md5=bdb7e906e747234f311a27928449aefcCAS | 10955715PubMed |
[57] R. F. Lee, Annelid cytochrome P-450. Comp. Biochem. Phys. C 1998, 121, 173..
[58] E. Gasteiger, C. Hoogland, A. Gattiker, S. Duvaud, M. R. Wilkins, R. D. Appel, A. Bairoch, Protein identification and analysis tools on the ExPASy server, in The Proteomics Protocols Handbook (Ed. J. M. Walker) 2005, pp. 571–607 (Humana Press: Totowa, NJ).


