Scaling up: fulfilling the promise of X-ray microprobe for biogeochemical research
Brandy M. Toner A D , Sarah L. Nicholas A and Jill K. Coleman Wasik B CA Land and Atmospheric Science Program, Department of Soil, Water, and Climate, University of Minnesota – Twin Cities, St Paul, MN 55108, USA.
B Water Resources Science Department, University of Minnesota – Twin Cities, St Paul, MN 55108, USA.
C St Croix Watershed Research Station, Science Museum of Minnesota, Marine on St Croix, MN 55047, USA.
D Corresponding author. Email: toner@umn.edu
Environmental Chemistry 11(1) 4-9 https://doi.org/10.1071/EN13162
Submitted: 16 May 2013 Accepted: 18 December 2013 Published: 19 February 2014
Environmental context. Although biogeochemical processes in the environment are often considered on large spatial scales, critical processes can occur at fine-spatial scales. Quantifying these processes is a challenge, but significant recent developments in microprobe X-ray absorption spectroscopy in terms of data collection and analysis greatly facilitate micro-scale observations at the sample-level. These mapping methods create datasets that can be integrated with bulk observations with the potential for widespread application to biogeochemical research.
Abstract. Biogeochemists measure and model fluxes of materials among environmental compartments, often considering large spatial-scales within and among ecosystems. However, critical biogeochemical processes occur at fine-spatial scales, and quantifying these processes is a challenge. Recent developments in microprobe X-ray absorption spectroscopy (XAS) data collection and analysis allow for micro-scale observations and quantification of chemical species at the sample-level. These speciation mapping methods create datasets that can be integrated with bulk observations through empirical and theoretical modelling. Speciation mapping approaches are possible with existing instrumentation, but the widespread application to biogeochemical research is hindered by the small number of instruments currently available.
Introduction
Scientists in the field of biogeochemistry measure and model the fluxes of materials among environmental compartments, often considering large spatial-scales within and among ecosystems. Despite this large spatial-scale perspective many critical biogeochemical processes occur at the molecular-, nano- and micrometre spatial-scales: a clay mineral adsorbs an organic molecule from soil solution, a microorganism reduces a molecule of sulfate to hydrogen sulfide. As such, biogeochemists are also interested in identifying the biological and chemical processes underlying fluxes. Recent reviews by Lombi and Susini[1] and Lombi et al.[2] highlight the profusion of biogeochemical studies over the last decade that make use of X-ray absorption spectroscopy (XAS) to describe chemical speciation of various analytes in environmental samples. The recent development of methods for measuring chemical speciation of elements in natural materials using micro- and nanoprobe synchrotron radiation instruments promises to extend researchers’ ability to decipher the microscale processes underlying large-scale biogeochemical cycles.
XAS as a mechanistic tool
Biogeochemical investigations attempt to develop a mechanistic understanding of the processes behind element cycling that apply broadly at ecosystem, regional and global scales. Assumptions are often made based on theoretical or experimentally derived constants and many chemical analyses are operationally defined. Because natural conditions are dynamic, heterogeneous and theoretically non-ideal, it is important to understand chemical speciation in situ as a means of clarifying mechanisms. XAS can define the chemical species within element pools and identify mechanisms that drive fluxes. There is currently an unmet need to develop analytical and statistical methods that allow XAS approaches to fully integrate into a biogeochemical research approach. Recent developments in microprobe XAS methods are discussed here as one opportunity to provide chemical speciation data in a form that can be used in statistical and modelling efforts to bridge gaps in spatial scales, or ‘scale-up’, from particle grains (µm) to whole ecosystems (km).
Bulk XAS approaches measure the average chemical speciation signature of a sample. Relative abundances of species can then be calculated using linear combination fitting (LCF) with reference spectra. This approach is commonly used by practitioners of XAS, and provides high quality information if the database of references is well matched to the samples. Bulk XAS is the method of choice for samples with low heterogeneity, but is poorly suited for highly heterogeneous materials and systems where minor or trace species are the reactive component. In contrast to bulk XAS, microprobe XAS (μXAS) with point-of-interest XAS or point-XAS analysis has proven particularly useful in describing chemical speciation of heterogeneous environmental samples. In practice, the bulk and μXAS approaches are often successfully combined. A μXAS approach describes the diversity of species present, and when the species of interest are abundant, bulk XAS can then be used to quantify them. Broadly speaking, bulk XAS fails and μXAS excels when samples are highly heterogeneous – physical heterogeneity and diversity of chemical species – and the reactive components are in low abundance.
Early applications of point-XAS analysis to natural materials were primarily used to understand the chemistry of metal contaminants in soils and sediments[3–7] and soon found applications in fields such as microbiology,[8–10] atmospheric science,[11–13] oceanography[14–18] and geochemistry.[19,20]
Although there is variety among these studies in the specifics of the experiment, there are common components to the approach, and those new to the technique will find Manceau et al.[21] a rich source of information. As a brief introduction, the point-XAS approach usually begins with an elemental map that describes the micrometre-level distribution of material and its elemental composition. The elemental map is generated by X-ray fluorescence (XRF) and is often referred to as an ‘XRF map’. The XRF map alone does not describe speciation but is a navigational tool for collecting point-XAS data. Point-XAS data are then collected at specific locations within the area of the sample described by the XRF map. Point-XAS locations are typically selected to investigate elemental patterns and associations, such as alteration rinds or mineral surfaces in contact with microbial biofilms. Like bulk XAS, point-XAS data can be fit to linear combinations of reference spectra to calculate a relative abundance of species at that point in the sample. At present, it is a challenge to design point-XAS experiments that extend the chemical species observed in a small number of (often non-randomly selected) particles to a statistically robust calculation of species within a whole sample.
Limitations of point-XAS
Point-XAS analysis using X-ray microprobe instruments has enhanced our understanding of a suite of processes, especially those involving mineral-surface mediated and oxidation–reduction reactions. One challenge for point-XAS in the context of biogeochemical research is that the actual distribution of species within the whole sample cannot be calculated using data from the standard point-XAS approach – a small number of non-random points. There are two major consequences of this challenge for biogeochemical research: (1) observed changes in speciation among samples or over time cannot be statistically verified and (2) rare and low abundance species, especially those distributed between very concentrated particles, are easily missed by point observations. Therefore, the ability of biogeochemists to use X-ray microprobe techniques to quantify changes and measure high reactivity, low abundance species is limited and below the potential utility of the instruments. Methods for improved experimental design and data analysis are needed to allow biogeochemists to ‘scale-up’ the micrometre-level observations to larger spatial scales (such as a whole sample).
Speciation mapping to aid point-XAS: discovering diffuse, rare and dynamic species
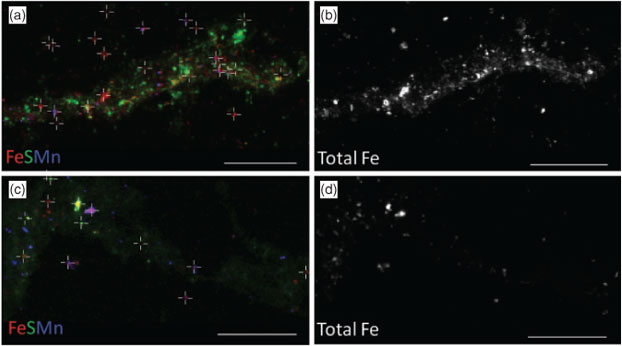
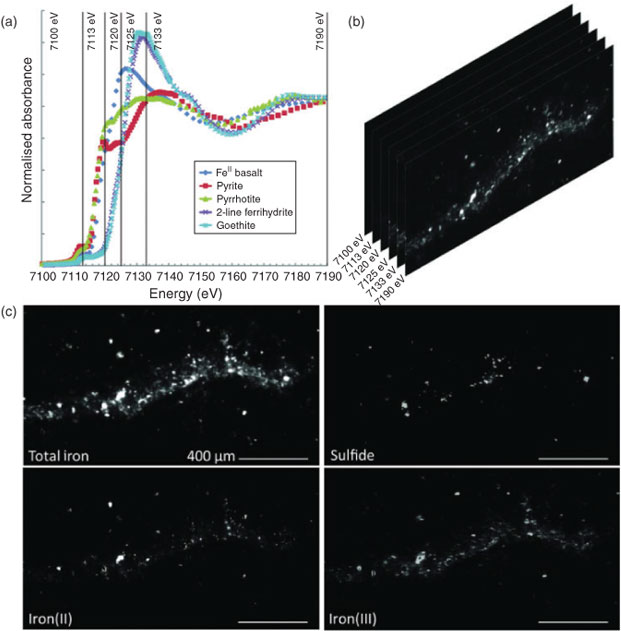
Data collection for X-ray microprobe speciation mapping begins in a manner identical to the point-XAS data collection described above and in detail elsewhere.[21] Briefly, XRF maps are used to determine the distribution of elements within a sample (Fig. 1a–c), and point-XAS data are collected to identify the suite of species present for the element of interest (Figs 1e, 2a, c, 3a). This established mode of point-XAS data collection is called two-stage cluster sampling (Fig. 1e) – a series of contiguous pixels are sampled by XRF, and then a sub-set of those pixels are selected for point-XAS analysis.[22] Speciation mapping extends this approach by using the point-XAS data to select a series of incident energies spanning the X-ray absorption edge of the element of interest at energies that maximise the detectable differences among species. The incident energy selection process is accomplished using custom beamline software called chem map error estimator. This publically available software was written by Matthew Marcus and is available through beamline 10.3.2 of the Advanced Light Source. An application of the software program to speciation mapping is described by Zeng et al.[23] In concept, it simulates a speciation map dataset using a given family of spectroscopic signatures (reference or experimental spectra) and calculates the error associated with linear combination fitting. The selected area of the sample is then scanned at each of the selected incident energies to create a composite speciation map (Fig. 4a, b). Speciation mapping yields an XAS profile (like a spectrum, but with fewer incident energies) at every pixel within the sampled area (Fig. 1d). This is in contrast to the full absorption spectra per pixel obtained using the Maia detector system.[24] The speciation map is then analysed using linear combination fitting with reference or experimental spectra at each (Fig. 4c) pixel. This mode of data collection is called single-stage cluster sampling.[22] In practice, species having spectra with distinct spectroscopic features and main resonance peak position are more easily distinguished by this method than species with similar spectroscopic features. For example, the approach was successfully applied to sulfur valence states in lake sediments with seven incident energies.[23] In contrast, this approach would not work in its current form to distinguish between spectroscopically similar species, such as FeIII-bearing minerals ferrihydrite and goethite. Ultimately, the quality of the pixel-by-pixel fit to the speciation map data is tested by collecting point-XAS data to ground-truth the fit.
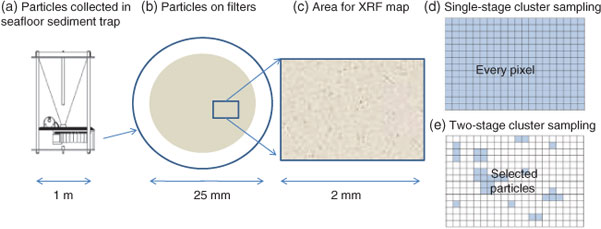
|
|

|
|
|
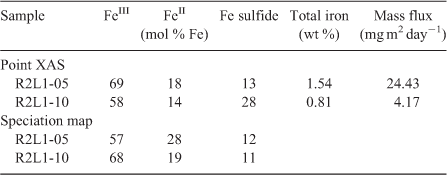
Speciation mapping has the benefit of querying all materials within a chosen area of interest, even particles that are too small to have their shape resolved by the probe or areas low in concentration relative to element-rich particles. The discovery and quantification of diffuse species, such as elements associated with organic-rich flocs between mineral particles, has been demonstrated for iron in marine particulates using speciation mapping.[25] Iron(II) in association with particulate organic carbon was observed for deep-sea hydrothermal plume particles using scanning transmission X-ray microscopy (STXM).[18] However, microprobe point-XAS data collection for the same samples was not able to verify the presence of iron(II)-rich materials within particle aggregates until point-XAS analysis was guided by speciation mapping. Through the development and application of speciation mapping iron(II) was verified and quantified (Fig. 4). The data presented in Fig. 3b are point-XAS observations guided by speciation mapping. Far from being a minor species, the iron(II) is 19–28 ± 0.11 mol-% and the first attempts at using a point-XAS approach failed to detect it.
XAS speciation mapping as a biogeochemical tool
When the goal of the research is to quantify changes in elemental speciation in heterogeneous materials, speciation mapping may provide a way forward. This method results in an increase in the number of observations (n) from n = ~5–20 (point-XAS) to n > 400 000 (pixels in a single speciation map). Point-XAS data are used to validate or ground-truth the fits to speciation maps. The mole fraction of species are then summed over the entire speciation map area or investigated for correlations among species with other sample components. With error estimates for the LCF procedure, samples can be compared to one another using statistical approaches. Presently, methods have been published or proposed for iron, sulfur and arsenic at the 1s (or K-) absorption edge. These studies examine changes in elemental speciation in plant tissues,[26] ultra-mafic rocks,[27] marine particles,[25,28] lake sediments,[23] peatland soils[29] and aquifer sediments.[30]
Integration of point-XAS data into larger datasets
Synchrotron-based approaches can be integrated into larger studies and are ready to contribute to major advances in the field of biogeochemistry. Zeng et al.[23] detected large changes in sulfur biogeochemistry in prairie pothole lake sediments with μXAS speciation mapping. Over the course of a season the total (solid) sediment sulfur remained essentially constant despite changing oxidation–reduction conditions in the sediments. However, microprobe XAS speciation mapping demonstrated seasonal changes in sediment sulfur speciation. During the spring-to-summer transition the reduced organic sulfur pool decreased from 55 to 15 mol-% with a corresponding increase in the oxidised sulfur pool. These speciation shifts have implications for methylmercury production by sulfate-reduction processes, degradation of pesticide and fertiliser runoff from adjacent farmland and methane release events. The static total sulfur concentrations were masking significant changes in sulfur speciation as a function of season, and microprobe XAS speciation mapping was the essential tool for revealing these hidden processes.
Conclusions
The community of biogeochemists using synchrotron tools face several challenges in extrapolating microscale observations to processes at the relevant spatial scales. Although there are many technical issues worthy of discussion – soft X-ray energy ranges for elements such as phosphorus, high efficiency detectors for low abundance elements such as mercury and massive parallel detectors for XANES imaging[24] – the largest challenge at this time is capacity: the availability of instruments. In the USA, X-ray microprobe instruments suffer from the uniqueness concept: the idea that only one of each type of synchrotron instrument is needed nationally. The current US capacity for X-ray microprobe instruments with speciation-mapping capabilities is low. An increase in capacity would allow biogeochemists to design studies with greater representative sampling. Studies with few total samples that lack replication are not amenable to statistical analysis and modelling activities, and prevent biogeochemists from ‘scaling-up’ microprobe observations.
Through the development of methods and data analysis protocols for speciation mapping, biogeochemists are poised to address critical environmental processes occurring at micrometre and sub-micrometre spatial scales. The capacity of US X-ray microprobe instruments must expand to accommodate environmentally relevant and statistically robust study designs to fulfil the promise of synchrotron X-ray microprobe approaches for biogeochemical research.
Acknowledgements
The authors thank the anonymous reviewers for contributing to the article. They thank the National Science Foundation (OCE 1038055; BMT) and Gordon and Betty Moore Foundation (BMT) for supporting the development iron and sulfur X-ray microprobe speciation mapping methods for deep-sea hydrothermal plume systems; the Water Resources Center, the USGS and the Center for Urban and Regional Affairs for supporting Sarah Nicholas’ dissertation research (BMT) and Daniel Engstrom for support and guidance of Jill Coleman Wasik. For the East Pacific Rise (EPR) sediment trap samples, the authors thank research collaborators Christopher German, Katrina Edwards and Olivier Rouxel; the cruise principal investigators Jim Cowen, Karen Von Damm and Lauren Mullineaux for access to the EPR; Steve Manganini, Maureen Raymo, Matthew Marcus, Sirine Fakra and Jeffry Sorensen for research support and Breea Govenar, Stace Beaulieu, Susan Mills, Tim Shank and Dan Fornari for trap deployments and recovery. All of the data displayed in this article were collected at the X-ray microprobe beamline 10.3.2 of the Advanced Light Source, and all data analysis tools were produced by beamline scientist Matthew Marcus. The Advanced Light Source is supported by the Director, Office of Science, Office of Basic Energy Sciences, of the US Department of Energy under contract DE-AC02-05CH11231.
References
[1] E. Lombi, J. Susini, Synchrotron-based techniques for plant and soil science: opportunities, challenges and future perspectives. Plant Soil 2009, 320, 1.| Synchrotron-based techniques for plant and soil science: opportunities, challenges and future perspectives.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXmvFGjtLo%3D&md5=620f8be30a63879688ab9f76568d105eCAS |
[2] E. Lombi, G. M. Hettiararchchi, K. G. Scheckel, Advanced in situ spectroscopic techniques and their applications in environmental biogeochemistry: introduction to the special section. J. Environ. Qual. 2011, 40, 659.
| Advanced in situ spectroscopic techniques and their applications in environmental biogeochemistry: introduction to the special section.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXmt1OqsLc%3D&md5=9c350d1965c5155df2467023fe7f2253CAS | 21546653PubMed |
[3] M. C. Duff, M. Newville, D. B. Hunter, P. M. Bertsch, S. R. Sutton, I. R. Triay, D. T. Vaniman, P. Eng, M. L. Rivers, Micro-XAS studies with sorbed plutonium on tuff. J. Synchrotron Radiat. 1999, 6, 350.
| Micro-XAS studies with sorbed plutonium on tuff.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXksFCnsr4%3D&md5=b45af3f7e48c797d39668e4a5cab90daCAS | 15263304PubMed |
[4] D. B. Hunter, P. M. Bertsch, In situ examination of uranium contaminated soil particles by micro-X-ray absorption and micro-fluorescence spectroscopies. J. Radioanal. Nucl. Chem. 1998, 234, 237.
| In situ examination of uranium contaminated soil particles by micro-X-ray absorption and micro-fluorescence spectroscopies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXmvV2nsrc%3D&md5=9a6467c2de565bec14bde2e2398dac18CAS |
[5] A. Manceau, B. Lanson, M. L. Schlegel, J. C. Harge, M. Musso, L. Eybert-Berard, J.-L. Hazemann, D. Chateigner, G. M. Lamble, Quantitative Zn speciation in smelter-contaminated soils by EXAFS spectroscopy. Am. J. Sci. 2000, 300, 289.
| Quantitative Zn speciation in smelter-contaminated soils by EXAFS spectroscopy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXltFOgt7o%3D&md5=177a2990e0d46a1fcb673a1e2fd5b787CAS |
[6] R. J. Reeder, M. Nugent, C. D. Tait, D. E. Morris, S. M. Heald, K. M. Beck, W. P. Hess, A. Lanzirotti, Coprecipitation of uranium(VI) with calcite: XAFS, micro-XAS, and luminescence characterization. Geochim. Cosmochim. Acta 2001, 65, 3491.
| Coprecipitation of uranium(VI) with calcite: XAFS, micro-XAS, and luminescence characterization.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXotVOrurY%3D&md5=8c68801d452c73f8e5cdaa3a38c8174cCAS |
[7] D. Strawn, H. Doner, M. Zavarin, S. McHugo, Microscale investigation into the geochemistry of arsenic, selenium, and iron in soil developed in pyritic shale materials. Geoderma 2002, 108, 237.
| Microscale investigation into the geochemistry of arsenic, selenium, and iron in soil developed in pyritic shale materials.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XkvFGqt70%3D&md5=f590dfa75cf828432a70f41f50c0b3fdCAS |
[8] C. S. Chan, S. C. Fakra, D. C. Edwards, D. Emerson, J. F. Banfield, Iron oxyhydroxide mineralization on microbial extracellular polysaccharides. Geochim. Cosmochim. Acta 2009, 73, 3807.
| Iron oxyhydroxide mineralization on microbial extracellular polysaccharides.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXmvVyktbo%3D&md5=625bb9d83a2cb416ce50fae8c09773ccCAS |
[9] C. M. Hansel, C. A. Zeiner, C. M. Santelli, S. M. Webb, MnII oxidation by an ascomycete fungus is linked to superoxide production during asexual reproduction. Proc. Natl. Acad. Sci. USA 2012, 109, 12621.
| MnII oxidation by an ascomycete fungus is linked to superoxide production during asexual reproduction.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhtleqs77F&md5=fbad9229c1f2f96987bb63e87cd3ae11CAS | 22802654PubMed |
[10] B. N. Orcutt, W. Bach, K. Becker, A. T. Fisher, M. Hentscher, B. M. Toner, C. G. Wheat, K. J. Edwards, Colonization of subsurface microbial observatories deployed in young ocean crust. ISME J. 2010, 4, 1.
[11] S. Datta, A. M. Rule, J. N. Mihalic, S. N. Chillrud, B. C. Bostick, J. P. Ramos-Bonilla, I. Han, L. M. Polyak, A. S. Geyh, P. N. Breysse, Use of X-ray absorption spectroscopy to speciate manganese in airborne particulate matter from five counties across the United States. Environ. Sci. Technol. 2012, 46, 3101.
| Use of X-ray absorption spectroscopy to speciate manganese in airborne particulate matter from five counties across the United States.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvVSqsbk%3D&md5=de8fca9cb034f86709213827d2c91609CAS | 22309075PubMed |
[12] E. J. Elzinga, Y. Gao, J. P. Fitts, R. Tappero, Iron speciation in urban dust. Atmos. Environ. 2011, 45, 4528.
| Iron speciation in urban dust.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXotlCgu7Y%3D&md5=97ef55f023df81a26fc51fb4a81166faCAS |
[13] M. M. Shafer, B. M. Toner, J. Overdier, J. J. Schauer, S. C. Fakra, S. Hu, J. D. Herner, A. Ayala, Chemical speciation of vanadium in particulate matter emitted from diesel vehicles and urban atmospheric aerosols. Environ. Sci. Technol. 2012, 46, 189.
| Chemical speciation of vanadium in particulate matter emitted from diesel vehicles and urban atmospheric aerosols.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsVSqsbvL&md5=c2293d085cec5dcfb020e3287f7907a6CAS | 22050708PubMed |
[14] J. A. Breier, B. M. Toner, S. C. Fakra, M. A. Marcus, S. N. White, A. M. Thurnherr, C. R. German, Sulfur, sulfides, oxides and organic matter aggregated in submarine hydrothermal plumes at 9°50′N East Pacific Rise. Geochim. Cosmochim. Acta 2012, 88, 216.
| Sulfur, sulfides, oxides and organic matter aggregated in submarine hydrothermal plumes at 9°50′N East Pacific Rise.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XnvVOrurw%3D&md5=f64a2a8efbbeb2e6d453cf883b26824fCAS |
[15] P. J. Lam, J. K. B. Bishop, C. C. Henning, M. A. Marcus, G. A. Waychunas, I. Y. Fung, Wintertime phytoplankton bloom in the subarctic Pacific supported by continental margin iron. Global Biogeochem. Cycles 2006, 20, GB1006.
| Wintertime phytoplankton bloom in the subarctic Pacific supported by continental margin iron.Crossref | GoogleScholarGoogle Scholar |
[16] C. H. Lamborg, K. O. Buesseler, P. J. Lam, Sinking fluxes of minor and trace elements in the North Pacific Ocean measured during the VERTIGO program. Deep Sea Res. Part II Top. Stud. Oceanogr. 2008, 55, 1564.
| Sinking fluxes of minor and trace elements in the North Pacific Ocean measured during the VERTIGO program.Crossref | GoogleScholarGoogle Scholar |
[17] M. A. Marcus, A. Manceau, M. Kersten, Mn, Fe, Zn and As speciation in a fast-growing ferromanganese marine nodule. Geochim. Cosmochim. Acta 2004, 68, 3125.
| Mn, Fe, Zn and As speciation in a fast-growing ferromanganese marine nodule.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXltlyitbo%3D&md5=92138c09ec21a8a8c7fe251b2a6b43a0CAS |
[18] B. M. Toner, S. C. Fakra, S. J. Manganini, C. M. Santelli, M. A. Marcus, J. W. Moffett, O. Rouxel, C. R. German, K. J. Edwards, Preservation of iron(II) by carbon-rich matrices in hydrothermal plumes. Nat. Geosci. 2009, 2, 197.
| Preservation of iron(II) by carbon-rich matrices in hydrothermal plumes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXisVCgt7Y%3D&md5=d87f7104e9f85ec8683465d4e0302ea8CAS |
[19] A. S. Templeton, E. J. Knowles, D. L. Eldridge, B. W. Arey, A. C. Dohnalkova, S. M. Webb, J. V. Bailey, B. M. Tebo, H. Staudigel, A seafloor microbial biome hosted within incipient ferromanganese crusts. Nat. Geosci. 2009, 2, 872.
| A seafloor microbial biome hosted within incipient ferromanganese crusts.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsVyqsb%2FL&md5=25af5bdf8683a02829dd90d99e053839CAS |
[20] C. L. Peacock, E. M. Moon, Oxidative scavenging of thallium by birnessite: explanation for thallium enrichment and stable isotope fractionation in marine ferromanganese precipitates. Geochim. Cosmochim. Acta 2012, 84, 297.
| Oxidative scavenging of thallium by birnessite: explanation for thallium enrichment and stable isotope fractionation in marine ferromanganese precipitates.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XltFyqsLs%3D&md5=f31dcff228511ac1c9a0a50fc72f0ac4CAS |
[21] A. Manceau, M. A. Marcus, N. Tamura, Quantitative speciation of heavy metals in soils and sediments by synchrotron x-ray techniques, in Applications of Synchrotron Radiation in Low-Temperature Geochemistry and Environmental Science (Eds P. A. Fenter, M. L. Rivers, N. C. Sturchio, S. R. Sutton) 2002, pp. 341–428 (Mineralogical Society of America: Washington, DC).
[22] S. L. Lohr, Cluster sampling with equal probabilities, in Sampling: Design and Analysis 1999, pp. 131–178. (Duxbury Press: Boston, MA).
[23] T. Zeng, W. A. Arnold, B. M. Toner, Microscale characterization of sulfur speciation in lake sediments. Environ. Sci. Technol. 2013, 47, 1287.
| 1:CAS:528:DC%2BC3sXhslGguw%3D%3D&md5=8d74231f6a03b400ed7bd5d8d76c932eCAS | 23282039PubMed |
[24] B. E. Etschmann, C. G. Ryan, J. Brugger, R. Kirkham, R. M. Hough, G. Moorhead, D. P. Siddons, G. De Geronimo, A. Kuczewski, P. Dunn, D. Paterson, M. D. de Jonge, D. L. Howard, P. Davey, M. Jensen, Reduced As components in highly oxidized environments: evidence from full spectrum imaging using the Maia massively parallel detector. Am. Mineral. 2010, 95, 884.
| Reduced As components in highly oxidized environments: evidence from full spectrum imaging using the Maia massively parallel detector.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmslKksLg%3D&md5=04d5b580c649f0654432b1f7518f3caaCAS |
[25] B. M. Toner, M. A. Marcus, K. J. Edwards, O. Rouxel, C. R. German, Measuring the form of iron in hydrothermal plume particles. Oceanography 2012, 25, 209.
| Measuring the form of iron in hydrothermal plume particles.Crossref | GoogleScholarGoogle Scholar |
[26] I. J. Pickering, E. Y. Sneeden, R. C. Prince, E. Block, H. H. Harris, G. Hirsch, G. N. George, Localizing the chemical forms of sulfur in vivo using X-ray fluorescence spectroscopic imaging: application to onion (Allium cepa) tissues. Biochemistry 2009, 48, 6846.
| Localizing the chemical forms of sulfur in vivo using X-ray fluorescence spectroscopic imaging: application to onion (Allium cepa) tissues.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXotFKmsLo%3D&md5=b6ad9f33ec650a8ab460a75788c6872eCAS | 19463015PubMed |
[27] L. E. Mayhew, S. M. Webb, A. S. Templeton, Microscale imaging and identification of Fe oxidation state, speciation, and distribution in complex geological media. Environ. Sci. Technol. 2011, 45, 4468.
| Microscale imaging and identification of Fe oxidation state, speciation, and distribution in complex geological media.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXltFajtLw%3D&md5=42ba32ddf8efc3df136ad89a2cee0811CAS | 21517061PubMed |
[28] P. J. Lam, D. C. Ohnemus, M. A. Marcus, The speciation of marine particulate iron adjacent to active and passive continental margins. Geochim. Cosmochim. Acta 2012, 80, 108.
| The speciation of marine particulate iron adjacent to active and passive continental margins.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhsleqtb8%3D&md5=93afe1c035b11cecfd3b6b0ff9c258a8CAS |
[29] J. K. Coleman Wasik, B. M. Toner, D. R. Engstrom, P. E. Drevnick, M. A. Marcus, Investigating the effects of hydrologic fluctuations on organic sulfur speciation in boreal peatlands. Mineral. Mag. 2011, 75, A3043.
[30] S. L. Nicholas, B. M. Toner, M. L. Erickson, A. R. Knaeble, Identifying the arsenic source in glacial aquifer sediments, west-central Minnesota, USA. Mineral. Mag. 2012, 76, A1706.


