Questions of size and numbers in environmental research on microplastics: methodological and conceptual aspects
Montserrat FilellaA Institute F.-A. Forel, University of Geneva, Route de Suisse 10, CH-1290 Versoix, Switzerland. Email: montserrat.filella@unige.ch
B SCHEMA, Rue Principale 92, L-6990 Rameldange, Luxembourg.

Montserrat Filella is a chemist and teaches Environmental Chemistry at the University of Geneva, Switzerland. Her main research interests focus on the understanding of the physicochemical processes regulating the behaviour of chemical elements in environmental compartments, mainly by combining field and laboratory measurements with physicochemical calculations. The three main axes of her research concern the study of natural colloids, natural organic matter (quantification and interaction with trace elements) and ‘less-studied’ elements. She is particularly interested in the exploration and use of critical appraisal and evidence-based methods. |
Environmental Chemistry 12(5) 527-538 https://doi.org/10.1071/EN15012
Submitted: 15 January 2015 Accepted: 21 May 2015 Published: 14 August 2015
Journal Compilation © CSIRO 2015 Open Access CC BY-NC-ND
Environmental context. Microplastics, either purposefully manufactured or formed by fragmentation of discarded ‘end-of-life’ macroplastic items, are accumulating in environmental compartments. As more and more data are collected on microplastics in the environment, discussion of two issues has become indispensable: (i) how reliable are the results in terms of the inherent capabilities and limitations of current methods used for sampling, counting and measuring microplastic particles; and (ii) how can the fate of microplastics be understood in the context of natural particles and colloids?
Abstract. A first important step in evaluating the impact of microplastic pollution in natural systems is assessing the reliability of the results obtained according to the inherent capabilities and limitations of the methods used for sampling, counting and measuring microplastic particles. This study, based on the critical reading of 55 studies containing quantitative microplastic data in waters and sediments, is an attempt to analyse these issues in the light of existing knowledge in the field of natural colloid studies. Existing results are highly dependent on the sampling and methodological procedure chosen and are essentially descriptive. Moreover, often they lack standardisation and adequate reporting of basic information such as the meaning of the size parameter measured. Colloid theory may provide the theoretical background needed to explain microplastic behaviour or, at least, to identify the parameters (e.g. density, surface characteristics, shape) that need to be known in order to gain a predictive knowledge of the subject. They are introduced and discussed. Finally, microplastics are not alone in environmental compartments. For this reason, when possible, published microplastic particle size distributions in natural waters have been quantitatively situated in the context of natural particles.
Introduction
Plastics are one of the most used materials in the world. They are broadly integrated into today’s lifestyle and are present in almost all product areas. Unfortunately, one the characteristics of plastics that make them so useful – durability – also enables them to persist in the environment for very long periods of time. Additionally, because of their low cost, many plastic objects have long been perceived as disposable. The result is the ubiquitous presence of plastic debris all over the planet. Close attention has been paid along the years to the increasing amount of plastics in the oceans and to their noxious effects on marine fauna. The earliest discoveries of plastic debris inside dead marine birds as well as the first attempts to quantify floating plastic debris in the western North Atlantic Ocean date back more than 40 years.[1–5] Since then, a large number of studies have documented their increasing prevalence and undesired effects. The issue has long had a high profile in the media.
For the past several years, increasing attention has been paid to the presence of small pieces of plastic, ‘including those not visible to the naked eye’,[6] in surface waters, mainly in the world’s oceans. These have increasingly been described as microplastics. The defining criterion of what constitutes a ‘microplastic’ is exclusively size, but the threshold beyond which a plastic qualifies as a microplastic remains somewhat fuzzy (this issue is discussed in the next section). The first use of the term microplastics is attributed to a widely cited paper published in 2004[7]; it was first used in the title of an article in 2006.[8] However, although the use of the term itself is relatively recent, studies about a category of plastic objects in this size range – plastic pellets – have been carried out for many years[2,3] and, as early as 1992, the US Environmental Protection Agency (EPA) published a widely cited report listing a significant number of observations.[9]
The aim of the present article is to discuss some issues that are rarely addressed explicitly in published studies and reviews on microplastics. It describes the approach of an environmental chemist with much experience in the field of natural colloids and particles (though a novice in microplastic research) who tries to understand some observations relatively surprising at first glance (e.g. why have smaller microplastics been found in sediments rather than at the water surface (see fig. 2 in Hidalgo-Ruz et al.[10])? Why have smaller plastic particles not been found in natural waters? Why do microplastics not seem to interact with natural particles?). The study is structured in three parts. First, specific analytical aspects are discussed in order to understand the meaning of published results. Then, the parameters needed to explain and predict microplastic behaviour in natural systems, according to the usual framework of colloid science, are introduced and discussed. Finally, existing data on microplastic particle size distributions in natural waters are situated in the context of natural particles. Though the discussion that follows is based on a thorough review of existing literature, its aim is not to produce a narrative review of microplastics (the reader is referred to Andrady,[6] Browne et al.,[11] Moore,[12] Barnes et al.,[13] Cole et al.,[14] Hammer et al.,[15] Wright et al.,[16] and Ivar do Sul and Costa[17] for this type of article), but rather to evaluate the reliability of existing information and to guide future research.
Methodology
Existing literature has been critically reviewed in order to evaluate the extent to which key aspects related to size and number measurement have been addressed in published studies and how. Various specialised search engines were used (Web of Science, SciFinder; keywords: microplastic, microdebris, ocean, sea, lake, river, freshwater). Careful reading of published papers led to other references. Only studies published in peer-reviewed journals have been included. Grey literature (i.e. documentary material that is not commercially published, typical examples being technical reports and conference proceedings) was not included. Studies that reported on only a single type of plastic, usually pellets[2,18–25] but in some cases also fibres,[26,27] were not taken into consideration. Lastly, studies where microplastics were isolated either to perform further experiments (for instance sorption and desorption of metals or persistent organic pollutants)[28] or for observation purposes,[29] but where samples were not sampled quantitatively, have not been included. All the articles evaluated (23 for sediments and 32 for waters) are listed in the Supplementary material file, which sets out the information in tables. This set of articles can be considered comprehensive and representative, but probably not exhaustive, particularly considering the steady publication of new studies.
Definitions
Microplastics are a category of plastic particles defined as a function of size alone. Occasional mention is made of the fact that they cannot be seen (e.g. ‘barely visible particles,‘[30] ‘those not visible to the naked eye’,[6] ‘the invisible fraction’[31]). Most studies give a precise upper size limit but there is no consensus on the value. Whereas, based on the conclusions of a 2008 workshop, the National Oceanic and Atmospheric Administration (NOAA) considers microplastics to be plastic particles that are <5 mm,[32] for other authors (e.g. Browne et al.[33]) particles should be smaller than 1 mm to be considered microplastics. Other sizes have also been given and even a highly idiosyncratic definition (<1 cm) can be found in a recently published paper in a prestigious journal.[34] A review of the literature shows that in recent years, most authors have favoured the 5-mm limit. Some working groups consider further subdivisions and terms. For instance, the Technical Subgroup on Marine Litter for the implementation of the Marine Strategy Framework Directive (MSFD) recommends further subdivision of the ‘visible size fraction’ (>1 mm) into large microplastics (1–5 mm), mesoplastics (5–25 mm) and macroplastics (>25 mm), whereas the ‘invisible fraction’ (<1 mm) is referred to as ‘small microplastics’.[31] The lack of a lower size limit in microplastic definition has rarely been addressed; it is discussed in the Size range section.
Origin and types
The general term microplastics gathers particles of different origin and chemical composition. We can distinguish two broad categories: so-called primary microplastics that are manufactured in the size range of microplastics (e.g. plastic pellets, scrubbers), and secondary microplastics that are formed by degradation of macroplastics or other materials (e.g. fibres, plastic fragments). All have been widely described in existing reviews, reports and article introductions and will be only briefly introduced here. Information available about the relative contribution of primary versus secondary microplastics varies widely and is system-dependent but, based on the observation that the amount of macroplastic accumulating in the marine environment is increasing[35] in conjunction with recent antipollution measurements concerning primary microplastics, it is reasonable to believe that secondary sources of microplastics dominate, or will dominate, microplastic environmental occurrence.
Plastic pellets
Plastic (resin) pellets are the raw materials that are melted and moulded to create plastic products. Plastic may be formed into pellets of various shapes (e.g. spherical, ovoid, cylindrical), sizes (1- to 5-mm diameter) and colours (colourless, translucent or coloured). They are most commonly polyethylene (PE) or polypropylene (PP). Resin pellets are unintentionally released to the environment during both manufacturing and transport. They were the first type of microplastic detected and quantified in the oceans[9] and have been found on beaches and water surfaces all over the world. Aging of pellets is usually accompanied by discolouration (e.g. yellowing), abrasion, cracking, fouling, tarring and encrustation by precipitates,[21] but it is not clear whether they easily break down to produce smaller particles. Because of their capacity to sorb organic pollutants such as polychlorinated biphenyls (PCB) and polycyclic aromatic hydrocarbons (PAH), they are used as sentinels for the presence of these micropollutants in the framework of a world-wide project, ‘The International Pellet Watch’.[36]
Scrubbers
Scrubbers are present in hand cleansers, cosmetic products and airblast cleaning media. In the case of cosmetics, their presence has been known for long time[37,38] but they were long considered a minor source of plastic pollution whereas nowadays, their presence has become prevalent because, on the one hand, microplastics have now replaced natural exfoliating materials (e.g. pumice) in facial cleansers and, on the other hand, the average consumer uses them more often than before, i.e. on a daily, or at least weekly, basis.[39] Recently, the per-capita consumption of microplastic used in personal care products for the USA population was estimated to be ~2.4 mg per person per day.[40] Many facial scrubbers contain PE, PP and polystyrene (PS) granules. These vary in shape and size depending on the product. Public awareness of the problem has recently led some cosmetic manufacturers to announce their elimination (www.beatthemicrobead.org/en/industry, accessed 15 July 2015). Mainly acrylic and polyester scrubbers are used in air-blasting to remove rust and paint from machinery and boat hulls[14] but industrial blasting agents can contain a broad variety of ingredients, including melamine, thermoset polyester polymer, PS, polyallyl diglycol carbonate, and amino thermoset plastic.[41]
Paint degradation
Degradation of ship paints may contribute to introducing different types of polymers to the ocean. Alkyd resins have been found in several studies[42–44]; alkyd is a typical polymer binder in industrial paints.[45] Also, large quantities of paint chips are produced during boat maintenance and cleaning (e.g. paint-chip scraping).[46]
Fibres
Fibres of different origins have been found in surface waters. Browne et al. showed that disposal of domestic wastewater contaminated with fibres from washing clothes was a major source of plastic fibres in the UK.[26] Ropes and fishing gear, when left behind, lost by fishermen or even during normal use, will degrade in the environment and release threads and fragments small enough to qualify as microplastics.[47,48] Vessels themselves have also been identified as possible sources of fibrous plastic particles[8,49,50] owing to the release of polymers from the fibre-reinforced plastic matrices used nowadays in ships.[44,51] A predominance of microfibres has been found in a recent study on deep-sea microplastics.[52]
Fragmentation and degradation of larger plastic materials
Macroplastics and plastic fragments gradually degrade into smaller pieces through a combination of mechanical erosion (combined effect of wave action and abrasion), photodegradation and the possible action of bacteria or fungi. During photodegradation, sunlight oxidises the chemical structure of the plastic, causing bond cleavage that reduces the average molecular mass of polymers. As a result, plastics become brittle and disintegrate, producing smaller fragments. In fact, UV-B radiation simply triggers an autocatalytic degradation sequence that progresses thermo-oxidatively, even in the absence of UV radiation, as long as O2 is available. According to Andrady, who has discussed plastic degradation in depth,[6] light-induced oxidation is orders of magnitude faster than other types of degradation but plastic floating in seawater degrades much more slowly than in air or on a beach surface. Fragmentation into small particles in water may take longer because the very high thermal stability of plastic particles of nanometre-scale size.[53]
The effect of degradation on microplastics has been reported in marine and freshwater samples through scanning electron microscopy (SEM) observations of changes in the surface texture.[22,29,54–57] Grooves, gauges, pits, adhering particles and flakes are commonly observed features. Not all plastics degrade similarly. For instance, the surface of beached PP pellets is not as rough as that of beached PE pellets and looks more cracked than altered.[58] Differences between marine and freshwater samples may be the result of varying weathering rates in different water chemistries (salt water versus fresh water).[59] Chemical weathering has been monitored by following the level of oxidation by Fourier-transform infrared (FTIR) spectroscopy.[21,56,58] The main parameter measured is the carbonyl index (i.e. the absorbance of carbonyl moieties relative to the absorbance of reference peaks) but other IR information has also been used.[58]
However, these observations have remained purely descriptive. There has been no theoretical or experimental systematic study leading to a quantitative prediction of macroplastic fragmentation under natural water conditions. Even if the breaking up of particulate matter into smaller fragments is a ubiquitous process present in many natural and technological processes, and there is a vast body of literature covering fracture and fragmentation processes, it is not a simple subject even in well-defined systems. The data available suggest that, for single fragmentation events, cumulative fragment mass distributions exhibit a power-law decay, independently of the energy input, the relevant length scales or the dominant microscopic interactions involved. What is more, the break-up of solids cannot be understood as a generic stochastic process because the precise mechanism of crack initiation and growth, i.e. the dominance of shear or tensile stresses, can govern fragmentation.[60] However, plastic fragmentation in natural ecosystems is not a single event but rather a continuously evolving process taking place in extremely dynamic media. Thus, citing results on single-impact fragmentation of plastics as being directly applicable to ocean microplastics as in Cozar et al.[34] is disputable. Dedicated fundamental research on macroplastic fragmentation under natural water and soil conditions over long periods of time leading to quantitative predictive tools should be a priority.
Open analytical questions
Recently, the analytical methods used have been comprehensively reviewed by Hidalgo et al.[10] Although many studies have been published since then, most follow the same working approaches. Therefore, only the basic principles will be commented on here. Tables S1 and S2 (Supplementary material) contain published results along with the corresponding methodological information. Table S1 includes location, type of sample, sampling procedure, initial separation and sample treatment methods, and identity verification. Table S2 shows size definition, counting method and results.
The methodological approach differs depending on whether the matrix is a sediment (including beaches and bottom sediments) or water (either surface or column water). In sediments, sample separation is based on an initial sieving step followed by a density-based separation. Flotation is usually achieved by using a concentrated NaCl solution[7] but, in order to obtain better separation gradients, other salts have also been used (i.e. NaI,[61–64] ZnCl2,[57,65] polytungstate).[29] In waters, microplastics are nearly always collected by using techniques initially developed for plankton. Flotation is less used or not mentioned. After collection, both in sediment and water samples, identification and quantification are done visually, normally with the help of an optical microscope. In some cases, initial plastic identification is confirmed by a spectroscopic technique (mainly FTIR but also Raman). In some studies, microplastics are also classified by size (Table S3, Supplementary material).
Several methodological and interpretation problems are associated with the methodology used. Some of them have been discussed in the past[10,63,66] but, apparently, others have gone unnoticed. Methodological obstacles that inhibit interpretation and comparison among studies, along with suggestions for improvements are discussed in the next two sections.
Open methodological questions
Representativeness of the samples
Existing studies point to a strong spatial and temporal heterogeneity of microplastics in surface waters. Over ocean basins, spatial patterns of debris are influenced by large-scale atmospheric and oceanic circulation patterns leading to particularly high accumulation of floating debris in the subtropical gyres.[14] However, what is worrying from the methodological point of view is not this large-scale heterogeneity but the existence of a strong spatial heterogeneity of marine plastic debris at small spatial scales.[67] If this were to be confirmed, it would call the validity of all published results into question. In the case of surface waters, the wind has long been recognised as a main driver of the observed heterogeneity.[68] Kukulka et al.[69] found an inverse relationship between wind speed and concentration of plastic particles on the sea surface in the North Atlantic Subtropical Gyre; higher concentrations are measured at low winds.[67,70] Based on a one-dimensional column model, these authors estimated that, under average wind conditions, 54 % of plastic particles are below surface tow depths.[69] The situation can be even more complex if, as found in Browne et al.,[71] low-density macrodebris move with the wind, but high-density microdebris do not.
Beaches are also very heterogeneous temporal and spatial systems. High intrabeach differences have repeatedly been reported,[27,61,72,73] with big differences observed between low- and high-water marks[61,74] and higher concentrations being found at the high-water zone[61] or in protected tidal mudflats.[65] Comparison of results can also be hindered by other sources of variability such as the fact that the depth of sand being sampled is not always the same (Table S1). However, the main problem is that microplastics are often collected in areas where plastic concentrations are visually higher (i.e. drift lines, strandlines). It is a general fact that authors rarely choose to study systems ‘where nothing happens’ and that this has the automatic consequence of producing a bias in existing results and in the corresponding accepted belief. Although it is inherent to this type of problem that it is impossible to prove that it exists, it is important to mention that such behaviour probably affects the subject considered here, leading to an overestimation of microplastic concentrations in the environment. One study[67] explicitly justified the very high concentrations found as being ‘due to a sampling scheme that deliberately targeted high-plastic areas’.
A sampling strategy based on a priori analysis of the relevant length and time scales of the water body to study would be an important step towards a better assessment of microplastic impact in natural systems.
Sample contamination
In the case of fibres, there is a high risk of procedural contamination because fibres are ubiquitous and will thus be present from sampling to the laboratory. The use of compatible tools and clothing (e.g. steel tweezers, polymer-free gloves, cotton laboratory coats) is rarely mentioned in published studies and running procedural blanks or checks remains rare, albeit with a recent upward trend.[27,42,50,52,64,75,76] Where this has been taken into consideration, results vary. Although in the case of Dekiff et al.,[64] fibres were considered separately and excluded from the quantitative analysis of beach sediments owing to substantial procedural background contamination, Mathalon and Hill[27] and McCormick et al.[77] found evidence of microplastic fibre contamination, and Frias et al.,[42] Woodall et al.[52] and Vianello et al.[75] reported no contamination. Only in a few cases was checking for possible contamination by bottles (PE)[44] or plastic bags (PE)[57] used for sampling mentioned.
Problems related to relying on visual inspection
The identification, enumeration and counting of microplastics in published studies usually rely on visual inspection of the samples. This means that there is a high risk of missing or misidentifying particles. For instance, a dependency on the observer was recently reported by Dekiff et al.[64] These authors reported that three independent observers resulted in different quantitative statements for the same sample extract.
Not all authors confirm the identity of the particles using spectroscopic techniques (57 % in sediments and 41 % in waters do so) and, when done, often only a subset of particles is used (Table S1). When identity is checked, the number of misidentifications is not always quoted. It is thus difficult to evaluate the reliability of the results obtained.
The need to find alternatives to the initial visual inspection has often been mentioned. Using μ-FTIR mapping has been proposed,[78] although some experimental aspects still remain controversial, such as reaching a suitable signal/noise ratio and adequate spatial resolution.
Loss of particles in water samples
In the case of water studies, where microplastics are sampled by using plankton-sampling devices, the concentration of microplastics found is often lower as their size approaches the lower sampling limit. This effect was already noticed in 1994[79] and attributed to a preferential removal of this size of particles by marine organisms. An alternative and plausible reason might be that, as suggested by Isobe et al.,[80] if size is defined as the longest length of each fragment, fragments with irregular shapes easily slip through the net. This issue is discussed in the section Measuring irregular objects in the more general context of size definition when using sieves and meshes.
Lack of certificate reference materials
In the absence of certificate reference materials, it is impossible to check the accuracy of the methods applied. In a few cases, recovery efficiencies have been ascertained by spiking samples with known amounts of particles. In the case of waters, this procedure was followed in only one study and not in real water: Ng and Obbard[8] separately spiked a concentrated saline solution (density 1.2 kg L–1, representing artificial seawater) with 3.45-μm reverse-phase HPLC-grade PE and chromatographic-grade PP. Precision and method recovery were monitored by comparing IR spectra. In sediments, Claessens et al.[72] checked particle recovery by spiking known concentrations of microplastics ‘of similar dimensions as those encountered in the field’ in clean sediments with particle recoveries ranging from 68.8 to 97.5 %, depending on sediment and particle type; Ng and Obbard[8] followed the same procedure described above for waters; and Nor and Obbard[81] spiked 250 g sediment with 25 ppm (equivalent to 71 particles) of Cospheric orange PE spheres of 500–600-μm diameter before twice subjecting the sample to the extraction procedure; particle recovery ranged from 54.9 to 71.8 %. It is difficult to extract general conclusions from such a limited number of experiments but recovery yields do not seem to be excellent. Moreover, sample spiking does not reproduce real conditions (e.g. spiking does not take into account interactions with natural organic matter, effects of biofilm formation, irregular plastic shapes resulting from degradation).
Open questions directly related to size and ‘numbers’
When counting particles in heterodisperse systems, two different parameters need to be measured: size and number.
Size range
Although it is well known that the results obtained (i.e. concentration of microplastics in a given sample) depend on the size range considered and this fact is often acknowledged, all its implications are rarely adequately taken into account in published studies and reviews. For instance:
-
Upper size range limit. No consensus exists about the size that defines microplastics. This clearly has practical implications because the number of objects found will depend on the limit considered. In practice, however, another rarely mentioned but more insidious problem exists: as the evaluation of the studies published shows (Table S2), very often there is a lack of clear-cut limit of the higher particle size (and this independently of the theoretical size considered), i.e. the upper limit is left open (‘higher than’). In these cases, the meaning of the results given obviously remains unclear.
-
Lower size range limit. In the case of waters, the lower range limit is fixed by the mesh of the sampling device. The dependence of the number of particles found on the mesh used is not a new observation; Colton et al. already discussed the issue in 1974[82] and it has been neatly confirmed in various studies since then, for instance, in Norén[83] where plastic particle concentrations in Swedish waters were found to be up to 100 000 times greater when sampled with a 80-μm rather than with a 450-μm mesh or in Song et al.[44] when comparing hand-net (50-μm mesh), manta trawl net (330-μm) and surface microlayer sampling methods. Clearly, another important consequence is that the mesh size used (usually 333 μm) makes it impossible to detect smaller microplastic particles. In sediment studies, the lower limit is often unclear (see Tables S1 and S2) because, even if a final filtration step is included, the filter pore size is much smaller than the particle size that can reasonably be seen and counted.
Concerning the lower range limit, it is important to remember that there is an intrinsic instrumental size limitation associated with the detection and quantification of particles by visual inspection using a microscope. Microscopes are diffraction-limited systems and both lateral resolution and depth resolution depend on the light wavelength and the numerical aperture of the optical system. At 550 nm, where the human eye is most sensitive, a high-performance objective (50× with a numerical aperture = 0.075) resolves 0.42 μm[84] but these conditions are probably far from the ones usually applied in microplastic research (see Table S2, though the information is not always given!). Because FTIR and Raman are often used to validate the composition of microplastics, another limit of detection that needs to be considered is related to the wavelength of IR light (from 2.5 to 18 μm), with Raman offering slightly better detection limits.[66] The smallest size detected by pyrolysis gas chromatography–mass spectrometry (Pyr-GC-MS) has been reported to be 100 μm.[64]
It is common practice in the scientific literature to compare results obtained in a given study with previously published ones. This can be done either by citing some studies (cherry-picking approach) or by including tables systematically reviewing previous studies.[61,68,70,72,85] Tables are usually also included in review papers.[16] However, the use of either different size ranges or unreported or open ones confounds interpretation of comparisons reported in previous research. This point has so far not been adequately acknowledged in this field and such comparisons continue to appear in nearly all published papers. Unfortunately, it is still extremely rare for authors to take into account even the mesh used when sampling waters in their comparisons.[76,77] None consider the other size-related limitations mentioned above.
Measuring irregular objects
Particle size, in the sense commonly used, is a linear length measure. The usual parameter used to characterise the size of a particle is its diameter. However, the diameter unequivocally defines the size of a spherical particle but not of particles of any other shape. For all other shapes, particle size is defined as a function of the measuring method. Unfortunately, although counting is nearly always mentioned to be done by visual inspection, it is not always clear in microplastic research which method is used to measure the size of the objects (Table S2). The two most widely used methods seem to be measurements based on visual observation and sieving, and often a mixture of both (i.e. initial separation made using nets or by sieving but other sizes measured visually).
In the case of visual or microscope-based observations of irregularly shaped objects, parameters like area, minimum and maximum diameter, Feret diameter and perimeter, need to be recorded, and then secondary parameters, such as mean diameter, centre of gravity, radius of gyration, shape factor (SF = 4π × area/perimeter2) calculated (Fig. S1). When the shape factor SF << 1 (e.g. fibrillar material), the calculation of a mean diameter has no physical meaning and alternative parameters need to be used. In the case of microplastics, where many particles have irregular shapes and some are fibrillar, none of these recommended parameters seem to have ever been considered. It is rarely explicitly mentioned which parameter is actually being measured (it is not mentioned in 83 % of sediment and in 73 % of water studies). When stated, the use of the ‘longest dimension’ predominates in water studies (2/3 of the cases where it is mentioned). It is difficult to classify objects intrinsically defined by their size, as is the case for microplastics, when the parameter measured is either not given or it is not sufficient to unequivocally define the particle size.
Particle size obtained by sieving is different from that obtained through visual inspection. A sieve diameter is defined as the width of the minimum square aperture through which the particle will pass, i.e. as the equivalent diameter corresponding to the diameter of a sphere passing through a sieve of defined mesh size with square or circular apertures (Fig. S2). The common sieves are made of woven wire cloth and have square apertures. Inadequate understanding of the meaning of size is probably behind the observations mentioned in the section Loss of particles in water samples.
Statistics of counting
Quantitative applications of microscopy-based methods have traditionally been blamed for the poor statistics produced by applying these techniques. Although it is generally assumed that a high number of particles need to be analysed to produce reliable results, accurate results can still be obtained from counting a much smaller number of particles if adequate statistical analysis is carried out in order to obtain optimal sampling schemes.[86] Moreover, because it is generally accepted that counting processes follow a Poisson distribution in which the mean and the variance have the same value, it is easy to estimate the number of objects that must be counted in order to obtain a certain coefficient of variation (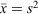 gives
gives 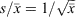 ).[87] An interesting implication of the Poisson distribution is that the expected coefficient of variation does not decrease dramatically once a certain number of objects has been counted (e.g. the coefficient of variation drops from 14 to 10 % as the number of objects counted is increased from 50 to 100, but only from 10 to 7 % if the number of objects is increased again by 100 %, from 100 to 200).
).[87] An interesting implication of the Poisson distribution is that the expected coefficient of variation does not decrease dramatically once a certain number of objects has been counted (e.g. the coefficient of variation drops from 14 to 10 % as the number of objects counted is increased from 50 to 100, but only from 10 to 7 % if the number of objects is increased again by 100 %, from 100 to 200).
No mention of the question of the statistics of counting seems to have ever been made in published studies on microplastics in the environment. Moreover, the number of particles that have actually been counted is often not reported in microplastic studies, which makes it difficult to assess the reliability of the results obtained. For instance, of the 23 studies on microplastics in sediments, this information is only provided in 11 publications (Table S2). The number of particles counted is very variable, ranging from very low, and probably insufficient, numbers (11,[88] 25[8] or 59[64]) to extremely high (12 637[73] or 19 100[74]). The situation is similar in water studies, with only 53 % of studies giving the total number of counted particles.
A power analysis carried out to estimate sample sizes required to detect changes in microplastic abundance[67] showed that the number of samples that need to be counted to detect increases or decreases in microplastics with reasonable probability is very high owing to their high variability.
Dealing with censored data
Another aspect that needs to be taken into consideration is that when authors give a mean value of microplastic concentrations, they ignore the fact that microplastics have sometimes not been found in all the samples. Inspection of Table S2 shows that not all samples contained microplastics. This was the case in 50 % of water studies (and there is a high likelihood that a further 25 % where this information is not given should be added to this). The proportion is lower in sediments, 26 % (plus 35 % of studies with unclear information). This lower proportion is probably due to the fact that it is easier to choose systems where microplastics are present in beaches than in waters (see the section Representativeness of the samples). When systems without microplastics are excluded from the calculation of a mean, the results obtained overestimate the real situation. It is well known that mean values cannot be calculated from measured values by ignoring samples with below-the-detection-limit values (so-called ‘censored’ data sets in statistics). Several data-treatment strategies exist for assigning values to non-detected or non-quantified values[89–91] but none has ever been applied, or at least mentioned, in microplastic research. Editors of the journals that published these studies also seem to be unaware of the problem as well as of the fact that it is not correct to calculate mean values when data have not been proved to be normally distributed.
Particle size distributions
Natural particles in environmental systems (e.g. atmosphere, waters, soils) are not homodisperse but highly polydisperse systems. For this reason, particle concentrations are often expressed as particle size distributions (PSDs), i.e. concentration of particles in a given size class as a function of the class size. Particle size distributions have the advantage of allowing the direct comparison of results for different systems, without depending on the size range considered. However, for this to be possible, several methodological aspects need to be taken into consideration. These have mostly been absent in environmental microplastic research.
First, when the widths of the size classes are not uniform, it is necessary to normalise particle concentrations by dividing them by the size class width. This is also necessary when comparing PSDs for different systems or among different studies. Even if this is a common practice in environmental particle research, only in two cases have PSDs been normalised when dealing with microplastics. The two studies are separated by 20 years.[34,79] Without previous size class normalisation, PSDs cannot be compared, as they have been, for instance, in fig. 3 of Hidalgo-Ruz et al.[10]
Second, it is necessary to consider that PSDs are different depending on whether number, surface or volume (≈mass) particle concentrations are represented. An example is shown in Fig. S3 for illustration. Not all sizing techniques provide the same type of PSD. For instance, size distributions are related to the number, mass and surface area of the particles as described by their number-, weight- or z-averages, depending on the size-measuring technique. Microscope-based counting gives number distributions whereas filtration or sieving followed by weighing provide mass (thus, volume) distributions but number-based if followed by visual counting, as is often the case with microplastics. Different definitions of mean diameters as a function of measuring technique can be found in the literature.[92,93] Mixing up different types of PSDs, as done in fig. 3 of Hidalgo-Ruz et al.[10] is incorrect: in this figure, most distributions are number-based but not Carson’s,[94] which is mass-based (as clearly confirmed by its shape).
Third, if one or several classes are underestimated because of the sampling strategy, the PSD will be inaccurate. Considerations discussed in Statistics of counting apply to each size class.
In the case of microplastics, not all studies provide PSDs. Table S3 collates the cases where they do (5 out of 23 studies in sediments, 20 out of 32 in waters). Besides the lack of standardisation mentioned above, other problems hinder published PSDs and reduce their utility: (i) PSDs are often open-ended; it is important to stress that including open size classes in PSD representations, as is the case in histograms shown in fig. 3 in Hidalgo-Ruz et al.[10] has no meaning. (ii) Results are often expressed as percentages of measured items (always in sediments except in one case)[73]; this does not allow systems to be compared except when it is possible to recalculate the actual concentrations. (iii) Microplastic concentrations are not always expressed in the same units (e.g. number of particles per kilogram or per metre squared in sediments, number of particles per surface or per volume units in waters); this is a general problem affecting not only PSDs but also total microplastic values.
Microplastic fate in aquatic systems
Any particle introduced or created in a fluid (e.g. air, water) can remain either in the fluid or sediment. It can also interact with other (similar or different) particles (i.e. coagulate), which eventually leads to further sedimentation. Microplastics will behave similarly.
The main properties that drive the fate of particles are size, shape and density; these are the properties driving buoyancy and sedimentation; in conjunction with particle surface characteristics, they govern coagulation properties. Particle surface characteristics can also control buoyancy through bubble sorption. Coagulation and sedimentation of natural particles, particularly in the colloidal size range, is a well-studied subject. Theory predicts that small particles will coagulate quickly, medium ones will remain longer in the system, and larger particles will sediment.[95] The actual threshold size for sedimentation depends on the system hydrodynamics and is variable. These properties are discussed below in the case of microplastics in environmental media.
Density
Particle density is a key parameter explaining and predicting particle behaviour in waters. Depending on their density, microplastics will either sediment or remain in the water column and be entrained in currents and travel long distances far from their source. Furthermore, because many studies rely on density-based separation methods, the question of microplastic density is an essential one also from the methodological point of view.
As a result of many studies in seawater quantifying floating plastic debris in surface waters (generally using surface-water collection of debris with Neuston nets), plastics are commonly perceived as being mostly positively buoyant in seawater. This is not strictly true. Although some have densities below or close to seawater (i.e. virgin resins of LDPE, HDPE, PP, PS), many others have not (e.g. PVC, PET, PC, polyurethane, nylon) (see List of Abbreviations for meaning). Moreover, plastics in products are often mixed with fillers and other additives that increase their density. Nevertheless, the density of the most widely used plastics does not differ much from that of seawater and the amount of turbulence necessary for resuspension of debris remains small. On the basis of density considerations alone, the fate of microplastics in freshwaters can be expected to be different from seawater, with increased sedimentation predicted, but the freshwater studies needed to confirm this hypothesis are lacking.
A factor that has been mentioned to significantly decrease the buoyancy of particles and increase their sinking capacity is colonisation by organisms. Fouling by organisms and biofilm formation is a widely described phenomena in macroplastics.[96–99] But, even though already mentioned in the 1972 seminal paper by Carpenter and Smith[3]: ‘At present, the only known biological effect of these particles is that they act as a surface for the growth of hydroids, diatoms, and probably bacteria’, and repeatedly observed[41,100] or at least mentioned since then, its effect on the fate of microplastics has rarely been quantified.[101] The effect of development of microbial films and fouling might not be simple to predict. For instance, it has been suggested that de-fouling in the water column due to foraging by organisms or other mechanisms (e.g. dissolution of carbonates and opal with changing pH conditions) can decrease microparticle density, causing them to return to the surface. A slow cyclic ‘bobbing’ motion of floating plastic debris attributed to this cyclic change in density has been proposed.[102] Furthermore, because seawater density gradually increases with depth, slowly sinking microplastics, slightly exceeding the surface seawater density, may remain suspended at a depth where their density equals that of the medium and not sediment further.
Microplastics have been detected in the seafloor[52,62] and in coastal subsediments (Table S1), which confirms their sedimentation but the number of studies is limited and, as noted by Law et al.,[103] plastic has not been documented so far as a significant component of the material collected in sediment traps. Many aspects of microplastic settling remain unknown, for example, the effect of the form factor of individual particles (i.e. fibres and microplastics originating from macroplastic degradation are largely non-spherical and the settling velocity of non-spherical particles is less than that of a sphere having the same volume and density),[104] bubble sorption or the interaction with similar or natural particles. Some of these aspects are discussed in the following sections.
Surface properties
Solution chemistry as well as particle surface properties control the colloid stability of particles, i.e. their attachment efficiency. Therefore, microplastic surface characteristics will play a key role in their environmental behaviour. According to the DLVO (Derjaguin–Landau–Verwey–Overbeek) theory, particle repulsion due to electrostatic interactions is counteracted by attraction due to van der Waals interactions. Values for the parameters needed to estimate total DLVO interaction energies (i.e. Hamaker constants and surface potentials) can be found in handbooks on colloids and polymers.[105] However, results from DLVO-based calculations have, in general, not been supported by experimental observations in environmental systems. Some reasons are the heterogeneity of such systems and the fact that solid surfaces are made homogeneous by being coated by natural organic matter. This is supported by abundant electrophoretic data that show a negative surface charge for almost all aquatic particles irrespective of their origin.[106] Although data are lacking, there is no reason to expect that this will not also be the case for microplastics.
As mentioned in the Fragmentation and degradation of larger plastic materials section, surface characteristics of microplastics change with time spent in the water, both physically and chemically. These changes can be complex and dependent on type of compound. For instance, Fotopoulos et al.[58] showed that virgin plastic pellets had homogeneous smooth surfaces with no acid–base behaviour but that eroded PE showed an altered surface that at seawater pH acquired a negative charge. However, beached eroded PP was only mechanically eroded (cracked) and although its surface area increased, it remained neutral and did not acquire charged functional groups on its surface. This means that surface properties of microplastics need to be determined under environmentally relevant conditions and for each type of material.
A further point to consider in the case of microplastics is the strong hydrophobic character of plastics. Because of this, plastics have been used in fundamental studies in order to understand the interaction between hydrophobic objects in the absence and in the presence of electrolytes and surfactants. Classical DLVO theory does not entirely explain their behaviour. The presence of an additional non-DLVO interaction was first recognised by Laskowski and Kitchener in 1969.[107] Later, Blake and Kitchener[108] suggested the presence of a ‘hydrophobic force’ in wetting films between bubble-particles. The term ‘hydrophobic force’ is now widely used to describe the long-range, non-DLVO attractive forces measured between macroscopic hydrophobic solid surfaces immersed in water. This theoretical knowledge has been applied to the separation of mixed plastics by flotation or of minerals by froth flotation in the mining industry. A better understanding of the wettability of the different types of plastics in natural water conditions, where their surfaces are probably not ‘naked’ but, as mentioned above, covered by organic matter or biofilms, is needed in order to predict microplastic behaviour and fate.
Microplastic coagulation/interaction with other particles
In published studies, microplastics are usually considered as isolated entities that do not significantly interact between themselves or with natural particles. When mentioned, the effects of the interaction with natural inorganic particles or organic macromolecules are described in an essentially phenomenological way. This is the case of binding with natural organic matter or of other processes such as, for instance, ‘tarring and encrustation by precipitates’.[21]
Natural organic matter is mentioned in some studies as a nuisance for observing and counting microplastics and, on occasion, methods have been applied to destroy it; treatment with H2O2 is the most common both in sediment[27,65] and water[50,76,85] samples but hot acid digestion has also been used.[109] However, the interaction of microplastics with natural organic matter is rarely considered with the aim of understanding microplastic fate. Its possible role is mentioned in only a few cases. For instance, Browne et al.[71] mention that flocculation may contribute to ‘movements of microplastics in the water column’ or Cauwenberge et al.[62] largely discuss marine snow formation to justify the presence of microplastics in seawater sediments.
Homocoagulation has not been specifically studied in the case of microplastics under surface water conditions. Different types of polystyrene (latex) microspheres have been extensively used by the ‘colloid community’ to mimic the behaviour of natural and biological colloidal particles. One such study[110] has even been mentioned[71] to explain that PS aggregates may settle faster than expected for equivalent-sized spherical particles because of their fractal character, forgetting that the particle concentration (25 g L–1!) and coagulation conditions (strong agitation, high ionic strength) used in Johnson et al.[110] are completely different from those of microplastics in seawater. Extrapolation of colloid laboratory studies to natural conditions is often not straightforward because of the many factors that need to be taken into account.
Heterocoagulation with inorganic particles might be worth considering when trying to understand microplastic fate in surface waters. In particular, it is also worth mentioning that rapid delivery of particulate organic matter to the deep ocean is well documented, even if this material is almost neutrally buoyant in seawater.[111] To explain this observation, it has been suggested that detrital particles may acts as ballast, providing an ‘abiotic boost’.[112,113] A similar mechanism could be envisaged for microplastics. In a given aquatic ecosystem, microplastics probably participate in the formation of aggregates where, as described for natural particles,[114] three general classes of natural colloids participate: compact inorganic colloids, large rigid biopolymers, and humic-like substances, each type playing a different role. The formation of miniflocs, flocs and large aggregates by colloid- and particle-based interactions determines the functioning of many biogeochemical processes on a planetary scale and there is no reason to think that microplastics will not be part of them; however, research would be needed to confirm this. The lack of familiarity with colloid research of the microplastic community is, in fact, surprising. For instance, an example can be found in a recent study published in a well-known journal[34]: when the authors try to explain the differences observed between their measured and theoretical PSDs calculated from a simple fragmentation model, they invoke shore deposition, nanofragmentation, biofouling and ingestion as possible sinks, but fail to consider coagulation.
Microplastics in the context of natural particles: the case of surface waters
The size distribution of suspended particles is a function of several variables, including the source and nature of the particles, physical and biological processes of aggregation, and the ‘age’ of suspension. As far as natural particles are concerned, it has been commonly accepted for many years that the number and concentration of particles increase logarithmically with decreasing size, implying a continuum of particulate matter ranging from nanometre-size material to the large particulate matter settling out of the water column. This power-law distribution paradigm arose from initial observations by aerosol physicists and oceanographers for particles in the >1-mm size range. Most of the observed PSDs in ocean waters in the 1970s and early 1980s were such that they showed equal particle volumes in logarithmically increasing size ranges. It was suggested that these flat distributions resulted from the combination of individual components that were log-normal by number and from aggregation processes. This would result in quasistationary distributions being observed. In practice, at proximity to a source, size distributions might not be flat but show peaks. Peaks may also be related to aggregation, for example the formation of peaks related to faster collision by Brownian motion as compared with elimination by shear. In practice, PSDs determined in natural waters have been quantified in terms of a power-law dependence with very similar slopes (3–4).[106] The limits are given by experimental limitations on one end – the measured PSDs rarely contain data below 60 nm – and by sedimentation on the other. This limit will thus depend on the hydrodynamics of the system (i.e. smaller particles will sediment in a quiescent system but not in a turbulent river) and on the density of the particles (i.e. big, loose aggregates essentially formed by organic matter remain in aquatic systems for a long time in the form of so-called marine and lake snow).
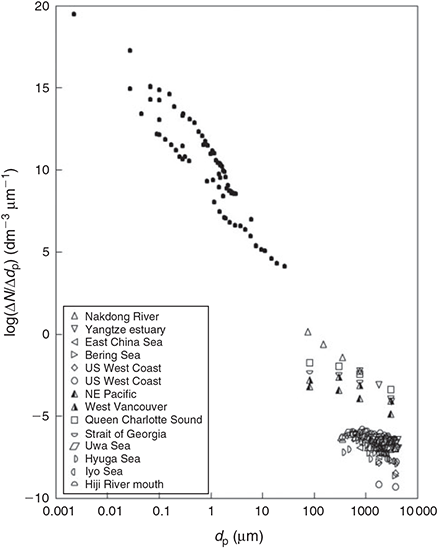
Do microplastics follow similar behaviour? It is difficult to answer this question from the existing data (Table S3) because of the methodological limitations discussed in the present paper. In particular, the low number of well-defined size classes and unit problems drastically reduce the number of studies containing useful data. When the limited number of appropriate results (14 PSDs from five studies) are plotted in a normalised way, together with typical results from natural particles (Fig. 1; natural particles data from Town and Filella[115] in black), microplastic PSDs appear situated in the expected zone, with some data even approximately following the trends given by natural colloids. It is impossible to extract conclusions from such a limited data set, but it is important to stress that this approach is the correct one to take in order to compare microplastic results among themselves and to situate them in the framework of natural particle distributions.
|
Conclusions
The main conclusions that can be drawn from the present study are the following:
-
Procedures and results are often not reported in a complete or understandable way in microplastic studies. There is a need to establish standardised procedures, not only for measurement, but also for reporting.
-
Existing results are very much dependent on the sampling and methodological procedure chosen. In particular, the meaning of the parameter used to define size is often unclear and the changing and undefined size ranges used make it impossible to compare results. Some type of normalisation concerning units is also needed.
-
Fundamental analytical issues, such as how to check for accuracy and precision, need to be solved.
-
Current methods exclude the possibility of exploring smaller size domains.
-
Existing studies are essentially descriptive. Very little attention has been paid so far to the study of processes involving microplastics (ranging from fragmentation processes of macroplastics in natural media to interaction with natural particles and organic macromolecules) and to their role in understanding microplastic fate in the environment.
-
Microplastic research would benefit from including principles from the well-developed field of natural colloids and particles.
On the basis of the current state of knowledge on microplastics in the environment and the methods used to acquire it, three main areas of research arise:
-
Methodological improvements along two main lines: increasing the reliability of the results obtained and expanding the measured size range towards smaller particle sizes.
-
Continuation of environmental data collection, particularly in freshwaters, with the addition of process measurement (e.g. flotation and sedimentation) and recording of ancillary parameters. Beginning data collection in soils.
-
Fundamental research on processes, mainly fragmentation and homo- and heterocoagulation under environmentally relevant conditions.
List of abbreviations
-
DLVO, Derjaguin–Landau–Verwey–Overbeek
-
FTIR, Fourier-transform infrared
-
HDPE, high-density polyethylene
-
HPLC, high-performance liquid chromatography
-
IR, infrared
-
LDPE, low-density polyethylene
-
PAH, polycyclic aromatic hydrocarbon
-
PC, polycarbonate
-
PCB, polychlorinated biphenyls
-
PE, polyethylene
-
PET, polyethylene terephthalate
-
PS, polystyrene
-
PP, polypropylene
-
PSD, particle size distribution
-
PVC, polyvinyl chloride
-
Pyr-GC-MS, thermal desorption pyrolysis gas chromatography–mass spectrometry
-
SEM, scanning electron microscopy
References
[1] K. W. Kenyon, E. Kridler, Laysan albatrosses swallow indigestible matter. Auk 1969, 86, 339.| Laysan albatrosses swallow indigestible matter.Crossref | GoogleScholarGoogle Scholar |
[2] E. J. Carpenter, S. J. Anderson, G. R. Harvey, H. P. Miklas, B. B. Peck, Polystyrene spherules in coastal water. Science 1972, 178, 749.
| Polystyrene spherules in coastal water.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE3sXkslGjsQ%3D%3D&md5=c89e0dc0d37775628878a6476877a376CAS | 4628343PubMed |
[3] E. J. Carpenter, K. L. J. Smith, Plastics on the Sargasso Sea surface. Science 1972, 175, 1240.
| Plastics on the Sargasso Sea surface.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE387hvVaqsQ%3D%3D&md5=9d232b4421a4163cd798f076fbe3e0c6CAS | 5061243PubMed |
[4] S. Rothstein, Plastic particle pollution of the surface of the Atlantic Ocean: evidence from a seabird. Condor 1973, 75, 344.
| Plastic particle pollution of the surface of the Atlantic Ocean: evidence from a seabird.Crossref | GoogleScholarGoogle Scholar |
[5] E. L. Venrick, T. W. Backman, W. C. Bartram, C. J. Platt, M. S. Thornhill, Man-made objects on the surface of the central North Pacific Ocean. Nature 1973, 241, 271.
| Man-made objects on the surface of the central North Pacific Ocean.Crossref | GoogleScholarGoogle Scholar |
[6] A. L. Andrady, Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596.
| Microplastics in the marine environment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXovFKrt74%3D&md5=023b7d5ac18234cb7abff5171550439aCAS | 21742351PubMed |
[7] R. C. Thompson, Y. Olsen, R. P. Mitchell, A. Davis, S. J. Rowland, A. W. G. John, D. McGonigle, A. E. Russell, Lost at sea: where is all the plastic? Science 2004, 304, 838.
| Lost at sea: where is all the plastic?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjvVSntbg%3D&md5=155a0087d37591eee7bfac1da847674bCAS | 15131299PubMed |
[8] K. L. Ng, J. P. Obbard, Prevalence of microplastics in Singapore’s coastal marine environment. Mar. Pollut. Bull. 2006, 52, 761.
| Prevalence of microplastics in Singapore’s coastal marine environment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xns1KhsLw%3D&md5=5ba4d3b8f3ecd9529cd981403989c47aCAS | 16388828PubMed |
[9] EPA Oceans and Coastal Protection Division, Plastic pellets in the aquatic environment: sources and recommendations. Final Report EPA 842/B-92/010 1992 (United States Environmental Protection Agency, Office of Water: Washington, DC).
[10] V. Hidalgo-Ruz, L. Gutow, R. C. Thompson, M. Thiel, Microplastics in the marine environment: a review of the methods used for identification and quantification. Environ. Sci. Technol. 2012, 46, 3060.
| Microplastics in the marine environment: a review of the methods used for identification and quantification.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XitVGhurY%3D&md5=2acb9685cd991453468574d7dc04148eCAS | 22321064PubMed |
[11] M. A. Browne, T. Galloway, R. Thompson, Microplastic – an emerging contaminant of potential concern? Integr. Environ. Assess. Manag. 2007, 3, 559.
| Microplastic – an emerging contaminant of potential concern?Crossref | GoogleScholarGoogle Scholar | 18046805PubMed |
[12] C. J. Moore, Synthetic polymers in the marine environment: a rapidly increasing, long-term threat. Environ. Res. 2008, 108, 131.
| Synthetic polymers in the marine environment: a rapidly increasing, long-term threat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXht1Snt7jK&md5=63c2702657d7296b380640352f76f55aCAS | 18949831PubMed |
[13] D. K. A. Barnes, F. Galgani, R. C. Thompson, M. Barlaz, Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 1985.
| Accumulation and fragmentation of plastic debris in global environments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXpt1Skt7w%3D&md5=c29927e7454ccee2ea68a6c4ffedea9bCAS |
[14] M. Cole, P. Lindeque, C. Halsband, T. S. Galloway, Microplastics as contaminants in the marine environment: a review. Mar. Pollut. Bull. 2011, 62, 2588.
| Microplastics as contaminants in the marine environment: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsV2gsrfM&md5=fcc64c8a0c712ffd04c42c522f208e69CAS | 22001295PubMed |
[15] J. Hammer, M. H. S. Kraak, J. R. Parsons, Plastics in the marine environment: the dark side of a modern gift. Rev. Environ. Contam. Toxicol. 2012, 220, 1.
| Plastics in the marine environment: the dark side of a modern gift.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhtlWqtLc%3D&md5=2ff0712dd5c70d7754fb07bb11d5e71bCAS | 22610295PubMed |
[16] S. L. Wright, R. C. Thompson, T. S. Galloway, The physical impacts of microplastics on marine organisms: a review. Environ. Pollut. 2013, 178, 483.
| The physical impacts of microplastics on marine organisms: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXltVCrtLc%3D&md5=e8d21e474857816bde6b082a00007f7bCAS | 23545014PubMed |
[17] J. A. Ivar do Sul, M. F. Costa, The present and future of microplastic pollution in the marine environment. Environ. Pollut. 2014, 185, 352.
| The present and future of microplastic pollution in the marine environment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhvVKgsLfE&md5=24560c55d83fd778f6f2cfa10272e53aCAS | 24275078PubMed |
[18] C. S. Wong, D. R. Green, W. J. Cretney, Quantitative tar and plastic waste distributions in the Pacific Ocean. Nature 1974, 247, 30.
| Quantitative tar and plastic waste distributions in the Pacific Ocean.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE2cXhtFanurg%3D&md5=96af558e501a3b1cc296d97cb9ee55ebCAS |
[19] A. W. Morris, E. I. Hamilton, Polystyrene spherules in the Bristol Channel. Mar. Pollut. Bull. 1974, 5, 26.
| Polystyrene spherules in the Bristol Channel.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE2cXksFCjtrY%3D&md5=adebf7f45f7b81c223bc216a0baac7a8CAS |
[20] R. J. Morris, Plastic debris in the surface waters of the South Atlantic. Mar. Pollut. Bull. 1980, 11, 164.
| Plastic debris in the surface waters of the South Atlantic.Crossref | GoogleScholarGoogle Scholar |
[21] S. Endo, R. Takizawa, K. Okuda, H. Takada, K. Chiba, H. Kanehiro, H. Ogi, R. Yamashita, T. Date, Concentration of polychlorinated biphenyls (PCBs) in beached resin pellets: variability among individual particles and regional differences. Mar. Pollut. Bull. 2005, 50, 1103.
| Concentration of polychlorinated biphenyls (PCBs) in beached resin pellets: variability among individual particles and regional differences.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtVKhu7nN&md5=1898f1e21a30865aa485c73e6b485aa5CAS | 15896813PubMed |
[22] K. Ashton, L. Holmes, A. Turner, Association of metals with plastic production pellets in the marine environment. Mar. Pollut. Bull. 2010, 60, 2050.
| Association of metals with plastic production pellets in the marine environment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtlKmt7fE&md5=3096b25556e5ed4273b859d9da2e8e9aCAS | 20696443PubMed |
[23] H. K. Karapanagioti, S. Endo, Y. Ogata, H. Takada, Diffuse pollution by persistent organic pollutants as measurements in plastic pellets sampled from various beaches in Greece. Mar. Pollut. Bull. 2011, 62, 312.
| Diffuse pollution by persistent organic pollutants as measurements in plastic pellets sampled from various beaches in Greece.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhs1ertr8%3D&md5=ea82d2127c9d8b00cf0916cc004635e0CAS | 21092999PubMed |
[24] A. Turner, L. Holmes, Occurrence, distribution and characteristics of beached plastic production pellets on the island of Malta (central Mediterranean). Mar. Pollut. Bull. 2011, 62, 377.
| Occurrence, distribution and characteristics of beached plastic production pellets on the island of Malta (central Mediterranean).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhs1ertrY%3D&md5=432da880d622a5e92a7ab38494db1a62CAS | 21030052PubMed |
[25] K. Mizukawa, H. Takada, M. Ito, Y. B. Geok, J. Hosoda, R. Yamashita, M. Saha, S. Suzuki, C. Miguez, J. Frias, J. Cepeda Antunes, P. Sobral, I. Santos, C. Micaelo, A. M. Ferreira, Monitoring of a wide range of organic micropollutants on the Portuguese coast using plastic resin pellets. Mar. Pollut. Bull. 2013, 70, 296.
| Monitoring of a wide range of organic micropollutants on the Portuguese coast using plastic resin pellets.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXktFChtbY%3D&md5=241fcb5b500f002e26d506d5d999896aCAS | 23499535PubMed |
[26] M. A. Browne, P. Crump, S. J. Niven, E. Teuten, A. Tonkin, T. Galloway, R. Thompson, Accumulation of microplastic on shorelines worldwide: sources and sinks. Environ. Sci. Technol. 2011, 45, 9175.
| Accumulation of microplastic on shorelines worldwide: sources and sinks.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXht1Gks7bN&md5=0004f85a816e01343ea841b1c13ca432CAS | 21894925PubMed |
[27] A. Mathalon, P. Hill, Microplastic fibers in the intertidal ecosystem surrounding Halifax Harbor, Nova Scotia. Mar. Pollut. Bull. 2014, 81, 69.
| Microplastic fibers in the intertidal ecosystem surrounding Halifax Harbor, Nova Scotia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXks1Wrsr0%3D&md5=13bc61b2e7068812a2e9ee092018493fCAS | 24650540PubMed |
[28] J. P. G. L. Frias, P. Sobral, A. M. Ferreira, Organic pollutants in microplastics from two beaches of the Portuguese coast. Mar. Pollut. Bull. 2010, 60, 1988.
| Organic pollutants in microplastics from two beaches of the Portuguese coast.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtlKmt7fO&md5=a9f55b6b21e16c5ed5c9049cbab4a14aCAS |
[29] P. L. Corcoran, M. C. Biesinger, M. Grifi, Plastics and beaches: a degrading relationship. Mar. Pollut. Bull. 2009, 58, 80.
| Plastics and beaches: a degrading relationship.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXksVSiug%3D%3D&md5=2c91d26eb302e402126455012cadca89CAS | 18834997PubMed |
[30] M. R. Gregory, A. L. Andrady, Plastics in the marine environment, in Plastics and the Environment (Ed. A. L. Andrady) 2003, pp. 379–402 (Wiley: Hoboken, NJ, USA).
[31] F. Galgani, G. Hanke, S. Werner, L. De Vrees, Marine litter within the European Marine Strategy Framework Directive. ICES J. Mar. Sci. 2013, 70, 1055.
| Marine litter within the European Marine Strategy Framework Directive.Crossref | GoogleScholarGoogle Scholar |
[32] C. Arthur, J. Baker, H. Bamford, (Eds) Proceedings of the International Research Workshop on the Occurrence, Effects and Fate of Microplastic Marine Debris, 9–11 September 2008. NOAA Technical Memorandum NOS-OR&R-30 2009 (US Department of Commerce). Available at http://marinedebris.noaa.gov/sites/default/files/publications-files/TM_NOS-ORR_30.pdf [Verified 29 July 2015].
[33] M. A. Browne, A. Dissanyake, T. S. Galloway, D. Lowe, R. C. Thompson, Ingested microplastic translocates to the circulatory system of the mussel, Mytilus edulis (L.). Environ. Sci. Technol. 2008, 42, 5026.
| Ingested microplastic translocates to the circulatory system of the mussel, Mytilus edulis (L.).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXmsVKhtb8%3D&md5=b4626ca3c3e4bda09b7035ddf2d396e4CAS | 18678044PubMed |
[34] A. Cozar, F. Echevarria, J. I. Gonzalez-Gordillo, X. Irigoien, B. Ubeda, S. Hernandez-Leon, A. T. Palma, S. Navarro, J. García-de-Lomas, A. Ruiz, M. L. Fernández-de-Puelles, C. M. Duarte, Plastic debris in the open ocean. Proc. Natl. Acad. Sci. USA 2014, 111, 10239.
| Plastic debris in the open ocean.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhtVOitL%2FE&md5=51eb383c1c6feeec3795de715aae99dcCAS | 24982135PubMed |
[35] J. R. Jambeck, R. Geyer, C. Wilcox, T. R. Siegler, M. Perryman, A. Andrady, R. Narayan, K. L. Law, Plastic waste inputs from land into the ocean. Science 2015, 347, 768.
| Plastic waste inputs from land into the ocean.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXitlKktrs%3D&md5=17b4697bb04f6e2b241c0460682889d6CAS | 25678662PubMed |
[36] Y. Ogata, H. Takada, K. Mizukawa, H. Hirai, S. Iwasa, S. Endo, Y. Mato, M. Saha, K. Okuda, A. Nakashima, M. Murakami, N. Zurcher, R. Booyatumanondo, M. Pauzi Zakaria, L. Q. Dung, M. Gordon, C. Miguez, S. Suzuki, C. Moore, H. K. Karapanagioti, S. Weerts, T. McClurg, E. Burres, W. Smith, M. Van Velkenburg, J. Selby Lang, R. C. Lang, D. Laursen, B. Danner, N. Stewardson, R. C. Thompson, International pellet watch: global monitoring of persistent organic pollutants (POPs) in coastal waters. 1. Initial phase data on PCBs, DDTs, and HCHs. Mar. Pollut. Bull. 2009, 58, 1437.
| International pellet watch: global monitoring of persistent organic pollutants (POPs) in coastal waters. 1. Initial phase data on PCBs, DDTs, and HCHs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFygs7zK&md5=7b92670f2441ead79b7c9cdd199c924aCAS | 19635625PubMed |
[37] V. Zitko, M. Hanlon, Another source of pollution by plastics: skin cleansers with plastic scrubbers. Mar. Pollut. Bull. 1991, 22, 41.
| Another source of pollution by plastics: skin cleansers with plastic scrubbers.Crossref | GoogleScholarGoogle Scholar |
[38] M. R. Gregory, Plastic ‘scrubbers’ in hand cleansers: a further (and minor) source for marine pollution identified. Mar. Pollut. Bull. 1996, 32, 867.
| Plastic ‘scrubbers’ in hand cleansers: a further (and minor) source for marine pollution identified.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXnvVCqtA%3D%3D&md5=573a3ce45eefd96e38231502c2ef06a6CAS |
[39] L. S. Fendall, M. A. Sewell, Contributing to marine pollution by washing your face: microplastics in facial cleansers. Mar. Pollut. Bull. 2009, 58, 1225.
| Contributing to marine pollution by washing your face: microplastics in facial cleansers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXoslamt78%3D&md5=5caad496b86d56a356408aa80b54e07cCAS | 19481226PubMed |
[40] T. Gouin, N. Roche, R. Lohmann, G. Hodges, A thermodynamic approach for assessing the environmental exposure of chemicals absorbed to microplastic. Environ. Sci. Technol. 2011, 45, 1466.
| A thermodynamic approach for assessing the environmental exposure of chemicals absorbed to microplastic.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtVKns7c%3D&md5=d5bf914bcf7ef4f6604592e3a069308eCAS | 21268630PubMed |
[41] M. Eriksen, S. Mason, S. Wilson, C. Box, A. Zellers, W. Edwards, H. Farley, S. Amato, Microplastic pollution in the surface waters of the Laurentian Great Lakes. Mar. Pollut. Bull. 2013, 77, 177.
| Microplastic pollution in the surface waters of the Laurentian Great Lakes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhs1yrtbrO&md5=e7ba2955d1cea1d616864efbc09ba802CAS | 24449922PubMed |
[42] J. P. G. L. Frias, V. Otero, P. Sobral, Evidence of microplastics in samples of zooplankton from Portuguese coastal waters. Mar. Environ. Res. 2014, 95, 89.
| Evidence of microplastics in samples of zooplankton from Portuguese coastal waters.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXht1Gmsrg%3D&md5=d5b6d9e5275e020ec58147c5b9d90611CAS |
[43] A. R. A. Lima, M. F. Costa, M. Barletta, Distribution patterns of microplastics within the plankton of a tropical estuary. Environ. Res. 2014, 132, 146.
| Distribution patterns of microplastics within the plankton of a tropical estuary.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXpvFWjt7o%3D&md5=dc553279b522dc66fa25057836ea2a27CAS |
[44] Y. K. Song, S. H. Hong, M. Jang, J. H. Kang, O. Y. Kwon, G. M. Han, W. J. Shim, Large accumulation of micro-sized synthetic polymer particles in the sea surface microlayer. Environ. Sci. Technol. 2014, 48, 9014.
| Large accumulation of micro-sized synthetic polymer particles in the sea surface microlayer.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXht1SmtLjM&md5=d5d585afdebcf1df6ae03cc7174027e5CAS | 25059595PubMed |
[45] L. Shtykova, D. Ostrovskii, P. Jacobsson, M. Nydén, Interaction between medetomidine and alkyd resins: NMR and FTIR investigation of antifouling marine paint model systems. J. Appl. Polym. Sci. 2006, 99, 2797.
| Interaction between medetomidine and alkyd resins: NMR and FTIR investigation of antifouling marine paint model systems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhsVGksr8%3D&md5=b390d601450c74db622befa396e53c96CAS |
[46] A. Turner, Marine pollution from antifouling paint particles. Mar. Pollut. Bull. 2010, 60, 159.
| Marine pollution from antifouling paint particles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhvFKisLY%3D&md5=a6803f92a13a573ac9a3d3a999676b84CAS | 20060546PubMed |
[47] F. E. Possatto, M. Barletta, M. F. Costa, J. A. Ivar do Sul, D. V. Dantas, Plastic debris ingestion by marine catfish: an unexpected fisheries impact. Mar. Pollut. Bull. 2011, 62, 1098.
| Plastic debris ingestion by marine catfish: an unexpected fisheries impact.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXlvVahs7o%3D&md5=10dd2c346b65635796033006d2bf7f67CAS | 21354578PubMed |
[48] J. A. A. Ramos, M. Barletta, M. F. Costa, Ingestion of nylon threads by Gerreidae while using a tropical estuary as foraging grounds. Aquat. Biol. 2012, 17, 29.
| Ingestion of nylon threads by Gerreidae while using a tropical estuary as foraging grounds.Crossref | GoogleScholarGoogle Scholar |
[49] I. A. Hinojosa, M. Thiel, Floating marine debris in fjords, gulfs and channels of southern Chile. Mar. Pollut. Bull. 2009, 58, 341.
| Floating marine debris in fjords, gulfs and channels of southern Chile.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXisFaisbk%3D&md5=334b4c082a9ebb0f46c3aebbe578d841CAS | 19124136PubMed |
[50] F. Dubaish, G. Liebezeit, Suspended microplastics and black carbon particles in the Jade System, southern North Sea. Water Air Soil Pollut. 2013, 224, 1352.
| Suspended microplastics and black carbon particles in the Jade System, southern North Sea.Crossref | GoogleScholarGoogle Scholar |
[51] P. K. Mallick, Fiber-Reinforced Composites Materials, Manufacturing, and Design 1993 (Marcel Dekker: New York).
[52] L. C. Woodall, A. Sanchez-Vidal, M. Canals, G. L. J. Paterson, R. Coppock, V. Sleight, A. Calafat, A. D. Rogers, B. E. Narayanaswamy, R. C. Thompson, The deep sea is a major sink for microplastic debris. Roy. Soc. Open Sci. 2014, 1, 140317.
[53] P. Paik, K. K. Kar, Kinetics of thermal degradation and estimation of lifetime for polypropylene particles: effects of particle size. Polym. Degrad. Stabil. 2008, 93, 24.
| Kinetics of thermal degradation and estimation of lifetime for polypropylene particles: effects of particle size.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXotl2ltQ%3D%3D&md5=69e3638d7e291decf5105fcbd61c9613CAS |
[54] M. R. Gregory, Plastic pellets on New Zealand beaches. Mar. Pollut. Bull. 1977, 8, 82.
| Plastic pellets on New Zealand beaches.Crossref | GoogleScholarGoogle Scholar |
[55] D. Cooper, P. L. Corcoran, Effects of mechanical and chemical processes on the degradation of plastic beach debris on the island of Kauai, Hawaii. Mar. Pollut. Bull. 2010, 60, 650.
| Effects of mechanical and chemical processes on the degradation of plastic beach debris on the island of Kauai, Hawaii.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmsVehsrw%3D&md5=58b54c7067a737d0bfc89cd9d932b673CAS | 20106491PubMed |
[56] M. Zbyszewski, P. Corcoran, Distribution and degradation of fresh-water plastic particles along the beaches of Lake Huron, Canada. Water Air Soil Pollut. 2011, 220, 365.
| Distribution and degradation of fresh-water plastic particles along the beaches of Lake Huron, Canada.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXpvFCksbc%3D&md5=71b22e25862ad5d71914d1bb5551d1f7CAS |
[57] H. K. Imhof, N. P. Ivleva, J. Schmid, R. Niessner, C. Laforsch, Contamination of beach sediments of a subalpine lake with microplastic particles. Curr. Biol. 2013, 23, R867.
| Contamination of beach sediments of a subalpine lake with microplastic particles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsF2gtbfI&md5=6e11592ca247c7597cdffb6b3d2e79b6CAS | 24112978PubMed |
[58] K. N. Fotopoulou, H. K. Karapanagioti, Surface properties of beached plastic pellets. Mar. Environ. Res. 2012, 81, 70.
| Surface properties of beached plastic pellets.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsVOhs7zP&md5=ce0c980a83ca4084b451464a2298dbc9CAS | 23013745PubMed |
[59] M. C. Biesinger, P. L. Corcoran, M. J. Walzak, Developing ToF-SIMS methods for investigating the degradation of plastic debris on beaches. Surf. Interface Anal. 2011, 43, 443.
| Developing ToF-SIMS methods for investigating the degradation of plastic debris on beaches.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXptVylsg%3D%3D&md5=29a99e8f14b0bb0cf1ce8bca4a7c5787CAS |
[60] G. Timár, J. Blömer, F. Kun, H. J. Hermann, New universality class for fragmentation of plastic materials. Phys. Rev. Lett. 2010, 104, 095502.
| New universality class for fragmentation of plastic materials.Crossref | GoogleScholarGoogle Scholar | 20366993PubMed |
[61] L. Van Cauwenberghe, M. Claessens, M. B. Vandegehuchte, J. Mees, C. R. Janssen, Assessment of marine debris on the Belgian Continental Shelf. Mar. Pollut. Bull. 2013, 73, 161.
| Assessment of marine debris on the Belgian Continental Shelf.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXpvVeqsrg%3D&md5=97a4eadb84f8f3a7253b1c130c5694e1CAS | 23790460PubMed |
[62] L. Van Cauwenberghe, A. Vanreusel, J. Mees, C. R. Janssen, Microplastic pollution in deep-sea sediments. Environ. Pollut. 2013, 182, 495.
| Microplastic pollution in deep-sea sediments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsVejurbN&md5=fcbe5832343a67e12a491ee56c24eb7aCAS | 24035457PubMed |
[63] M.-T. Nuelle, J. H. Dekiff, D. Remy, E. Fries, A new analytical approach for monitoring microplastics in marine sediments. Environ. Pollut. 2014, 184, 161.
| A new analytical approach for monitoring microplastics in marine sediments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhvVWlt7%2FL&md5=6c46200a245f87066cf6d3f148a5a9beCAS | 24051349PubMed |
[64] J. H. Dekiff, D. Remy, J. Klasmeier, E. Fries, Occurrence and spatial distribution of microplastics in sediments from Norderney. Environ. Pollut. 2014, 186, 248.
| Occurrence and spatial distribution of microplastics in sediments from Norderney.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhvVWmsbs%3D&md5=d575caebf98a45bf17a1c8caca7af3c9CAS | 24448461PubMed |
[65] G. Liebezeit, F. Dubaish, Microplastics in beaches of the East Frisian Islands Spiekeroog and Kachelotplate. Bull. Environ. Contam. Toxicol. 2012, 89, 213.
| Microplastics in beaches of the East Frisian Islands Spiekeroog and Kachelotplate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XosVKjsLY%3D&md5=5be4d015000451edd208b4ea7bda1b68CAS | 22526995PubMed |
[66] H. K. Imhof, J. Schmid, R. Niessner, N. P. Ivleva, C. Laforsch, A novel, highly efficient method for the separation and quantification of plastic particles in sediments of aquatic environments. Limnol. Oceanogr. Methods 2012, 10, 524.
| 1:CAS:528:DC%2BC38XhtlyntrfN&md5=b2457dab3b418e16f52c1327551bf523CAS |
[67] M. C. Goldstein, A. J. Titmus, M. Ford, Scales of spatial heterogeneity of plastic marine debris in the north-east Pacific Ocean. PLoS One 2013, 8, e80020.
| Scales of spatial heterogeneity of plastic marine debris in the north-east Pacific Ocean.Crossref | GoogleScholarGoogle Scholar | 24278233PubMed |
[68] G. L. Lattin, C. J. Moore, A. F. Zellers, S. L. Moore, S. B. Weisberg, A comparison of neustonic plastic and zooplankton at different depths near the southern California shore. Mar. Pollut. Bull. 2004, 49, 291.
| A comparison of neustonic plastic and zooplankton at different depths near the southern California shore.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXmsVeru78%3D&md5=e4f5ffaa4578688214e9813830e23ec5CAS | 15341821PubMed |
[69] T. Kukulka, G. Proskurowski, S. Moret-Ferguson, D. W. Meyer, K. L. Law, The effect of wind mixing on the vertical distribution of buoyant plastic debris. Geophys. Res. Lett. 2012, 39, L07601.
| The effect of wind mixing on the vertical distribution of buoyant plastic debris.Crossref | GoogleScholarGoogle Scholar |
[70] A. Collignon, J.-H. Hecq, F. Galgani, P. Voisin, F. Collard, A. Goffart, Neustonic microplastic and zooplankton in the north-western Mediterranean Sea. Mar. Pollut. Bull. 2012, 64, 861.
| Neustonic microplastic and zooplankton in the north-western Mediterranean Sea.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XkvFaht74%3D&md5=1099effbf47ac2616704a807fe89b6dcCAS | 22325448PubMed |
[71] M. A. Browne, T. S. Galloway, R. C. Thompson, Spatial patterns of plastic debris along estuarine shorelines. Environ. Sci. Technol. 2010, 44, 3404.
| Spatial patterns of plastic debris along estuarine shorelines.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXktlyjtb4%3D&md5=6c6adb982561be02195c26f040eba178CAS | 20377170PubMed |
[72] M. Claessens, S. De Meester, L. Van Landuyt, K. De Clerck, C. R. Janssen, Occurrence and distribution of microplastics in marine sediments along the Belgian coast. Mar. Pollut. Bull. 2011, 62, 2199.
| Occurrence and distribution of microplastics in marine sediments along the Belgian coast.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXht1ajt7jF&md5=edeae5137c179ce9b5ad26009fc27ca5CAS | 21802098PubMed |
[73] J. Martins, P. Sobral, Plastic marine debris on the Portuguese coastline: a matter of size? Mar. Pollut. Bull. 2011, 62, 2649.
| Plastic marine debris on the Portuguese coastline: a matter of size?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsV2gsrfL&md5=5488ee01487b266bdb39722c916a0c12CAS | 22019196PubMed |
[74] K. J. McDermid, T. L. McMullen, Quantitative analysis of small-plastic debris on beaches in the Hawaiian archipelago. Mar. Pollut. Bull. 2004, 48, 790.
| Quantitative analysis of small-plastic debris on beaches in the Hawaiian archipelago.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXisVejsLc%3D&md5=6a0a2443d77be03d6836f4b650b0ab7bCAS | 15041436PubMed |
[75] A. Vianello, A. Boldrin, P. Guerriero, V. Moschino, R. Rella, A. Sturaro, L. Da Ros, Microplastic particles in sediments of Lagoon of Venice, Italy: first observations on occurrence, spatial patterns and identification. Estuar. Coast. Shelf Sci. 2013, 130, 54.
| Microplastic particles in sediments of Lagoon of Venice, Italy: first observations on occurrence, spatial patterns and identification.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXmt1Cmtrk%3D&md5=5beb1db8bfbf0b781d73c7228555c8fbCAS |
[76] S. Zhao, L. Zhu, T. Wang, D. Li, Suspended microplastics in the surface water of the Yangtze Estuary System, China: first observations on occurrence, distribution. Mar. Pollut. Bull. 2014, 86, 562.
| Suspended microplastics in the surface water of the Yangtze Estuary System, China: first observations on occurrence, distribution.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhtFCmtLbI&md5=a33f4971ecfd16fd3be0707d14166f85CAS | 25023438PubMed |
[77] A. McCormick, T. Hoellein, S. A. Mason, J. Schluep, J. J. Kelly, Microplastic is an abundant and distinct microbial habitat in an urban river. Environ. Sci. Technol. 2014, 48, 11863.
| Microplastic is an abundant and distinct microbial habitat in an urban river.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhsFGlsrjL&md5=ee63405e95eefec83cf1456e8717c442CAS | 25230146PubMed |
[78] J. P. Harrison, J. J. Ojeda, M. E. Romero-Gonzalez, The applicability of reflectance micro-Fourier-transform infrared spectroscopy for the detection of synthetic microplastics in marine sediments. Sci. Total Environ. 2012, 416, 455.
| The applicability of reflectance micro-Fourier-transform infrared spectroscopy for the detection of synthetic microplastics in marine sediments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsV2jtrc%3D&md5=55d8aa753fc7204320bf0e7c0bfb7becCAS | 22221871PubMed |
[79] D. G. Shaw, R. H. Day, Colour- and form-dependent loss of plastic micro-debris from the North Pacific Ocean. Mar. Pollut. Bull. 1994, 28, 39.
| Colour- and form-dependent loss of plastic micro-debris from the North Pacific Ocean.Crossref | GoogleScholarGoogle Scholar |
[80] A. Isobe, K. Kubo, Y. Kamura, S. i. Kako, E. Nakashima, N. Fujii, Selective transport of microplastics and mesoplastics by drifting in coastal waters. Mar. Pollut. Bull. 2014, 89, 324.
| Selective transport of microplastics and mesoplastics by drifting in coastal waters.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhs1KmtLvN&md5=e5193590b46354aecafcb16d723aa274CAS | 25287228PubMed |
[81] N. H. M. Nor, J. P. Obbard, Microplastics in Singapore’s coastal mangrove ecosystems. Mar. Pollut. Bull. 2014, 79, 278.
| Microplastics in Singapore’s coastal mangrove ecosystems.Crossref | GoogleScholarGoogle Scholar |
[82] J. B. J. Colton, F. D. Knapp, B. R. Burns, Plastic particles in surface waters of the north-west Atlantic. Science 1974, 185, 491.
| Plastic particles in surface waters of the north-west Atlantic.Crossref | GoogleScholarGoogle Scholar |
[83] F. Norén, Small plastic particles in Coastal Swedish waters 2007 (KIMO Sweden, N Research: Lysekil, Sweden).
[84] R. Heilbronner, S. Barrett, Image Analysis in Earth Sciences: Microstructures and Textures of Earth Materials 2013 (Springer Science & Business Media: Berlin).
[85] C. M. Free, O. P. Jensen, S. A. Mason, M. Eriksen, N. J. Williamson, B. Boldgiv, High levels of microplastic pollution in a large, remote, mountain lake. Mar. Pollut. Bull. 2014, 85, 156.
| High levels of microplastic pollution in a large, remote, mountain lake.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhtlarurbJ&md5=3e7a706e1c1c4479f8eef370965c083bCAS | 24973278PubMed |
[86] D. Kirchman, J. Sigda, R. Kapuscinski, R. Mitchell, Statistical analysis of the direct count method for enumerating bacteria. Appl. Environ. Microbiol. 1982, 44, 376.
| 1:STN:280:DyaL3s%2FitFarsg%3D%3D&md5=dea86fad69c3cfb59b530b2721108072CAS | 6751231PubMed |
[87] M. R. Spiegel, Theory and Problems of Probability and Statistics 1992 (McGraw-Hill: New York).
[88] F. Faure, M. Corbaz, H. Baecher, L. F. De Alencastro, Pollution due to plastics and microplastics in Lake Geneva and in the Mediterranean Sea. Arch. Sci. 2012, 65, 157.
| 1:CAS:528:DC%2BC3sXhsVWjsrzP&md5=4af1b656e55fa2982694c00c59b802d4CAS |
[89] D. R. Helsel, Less than obvious: statistical treatment of data below the detection limit. Environ. Sci. Technol. 1990, 24, 1766.
| Less than obvious: statistical treatment of data below the detection limit.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXmtVequr8%3D&md5=81da3ba41478ec630ca8e63362160154CAS |
[90] R. W. Hornung, L. D. Reed, Estimation of average concentration in the presence of non-detectable values. Appl. Occup. Environ. Hyg. 1990, 5, 46.
| Estimation of average concentration in the presence of non-detectable values.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXltVyitr4%3D&md5=70ca6eeca66e78013a6132b17ab1f74aCAS |
[91] C. C. Travis, M. L. Land, Estimating the mean of data sets with non-detectable values. Environ. Sci. Technol. 1990, 24, 961.
| Estimating the mean of data sets with non-detectable values.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXltVaqsrw%3D&md5=e25d2302a92092ee5f80bfb07e846cd8CAS |
[92] M. Filella, J. Zhang, M. E. Newman, J. Buffle, Analytical applications of photon correlation spectroscopy for size distribution measurements of natural colloidal suspensions: capabilities and limitations. Colloids Surf. A Physicochem. Eng. Asp. 1997, 120, 27.
| Analytical applications of photon correlation spectroscopy for size distribution measurements of natural colloidal suspensions: capabilities and limitations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXht1Kgsro%3D&md5=9c4034ccecdc0867c5dd4e7b608471e0CAS |
[93] J. R. Lead, K. J. Wilkinson, Environmental colloids and particles: current knowledge and future developments, in Environmental Colloids and Particles: Behaviour, Separation and Characterisation (Eds K. J. Wilkinson, J. R. Lead) 2007, pp. 1–15 (Wiley: Chichester, UK).
[94] H. S. Carson, S. L. Colbert, M. J. Kaylor, K. J. McDermid, Small plastic debris changes water movement and heat transfer through beach sediments. Mar. Pollut. Bull. 2011, 62, 1708.
| Small plastic debris changes water movement and heat transfer through beach sediments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXovFKrtbg%3D&md5=83803e301d4f18e72dea8ef84d0c1dcbCAS | 21700298PubMed |
[95] M. Filella, J. Buffle, Factors controlling the stability of submicron colloids in natural waters. Colloids Surf. A Physicochem. Eng. Asp. 1993, 73, 255.
| Factors controlling the stability of submicron colloids in natural waters.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXmslyisL8%3D&md5=12c83c34e42fd2b94376877a22d2d828CAS |
[96] D. K. A. Barnes, Invasions by marine life on plastic debris. Nature 2002, 416, 808.
| Invasions by marine life on plastic debris.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XjtFyksL0%3D&md5=9db3409751e07f8d3c77a171755ee40aCAS |
[97] M. R. Gregory, Environmental implications of plastic debris in marine settings – entanglement, ingestion, smothering, hangers-on, hitch-hiking and alien invasions. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2013.
| Environmental implications of plastic debris in marine settings – entanglement, ingestion, smothering, hangers-on, hitch-hiking and alien invasions.Crossref | GoogleScholarGoogle Scholar | 19528053PubMed |
[98] D. Lobelle, M. Cunliffe, Early microbial biofilm formation on marine plastic debris. Mar. Pollut. Bull. 2011, 62, 197.
| Early microbial biofilm formation on marine plastic debris.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtVSqsbg%3D&md5=62d54491530c037738811891dd06c3edCAS | 21093883PubMed |
[99] E. R. Zettler, T. J. Mincer, L. A. Amaral-Zettler, Life in the ‘Plastisphere’: microbial communities on plastic marine debris. Environ. Sci. Technol. 2013, 47, 7137.
| 1:CAS:528:DC%2BC3sXptFeht7w%3D&md5=ba4d5cb31e1d289133f39f3ee5b73b6dCAS | 23745679PubMed |
[100] A. Collignon, J.-H. Hecq, F. Galgani, F. Collard, A. Goffart, Annual variation in neustonic micro- and meso-plastic particles and zooplankton in the Bay of Calvi (Mediterranean–Corsica). Mar. Pollut. Bull. 2014, 79, 293.
| Annual variation in neustonic micro- and meso-plastic particles and zooplankton in the Bay of Calvi (Mediterranean–Corsica).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhvFOit73N&md5=8b1f7b587c0f3f64fa1a2074b0ac77edCAS | 24360334PubMed |
[101] S. Morét-Ferguson, K. L. Law, G. Proskurowski, E. K. Murphy, E. E. Peacock, C. M. Reddy, The size, mass, and composition of plastic debris in the western North Atlantic Ocean. Mar. Pollut. Bull. 2010, 60, 1873.
| The size, mass, and composition of plastic debris in the western North Atlantic Ocean.Crossref | GoogleScholarGoogle Scholar | 20709339PubMed |
[102] S. Ye, A. L. Andrady, Fouling of floating plastic debris under Biscayne Bay exposure conditions. Mar. Pollut. Bull. 1991, 22, 608.
| Fouling of floating plastic debris under Biscayne Bay exposure conditions.Crossref | GoogleScholarGoogle Scholar |
[103] K. L. Law, S. Morét-Ferguson, N. A. Maximenko, G. Proskurowski, E. E. Peacock, J. Hafner, C. M. Reddy, Plastic accumulation in the North Atlantic Subtropical Gyre. Science 2010, 329, 1185.
| Plastic accumulation in the North Atlantic Subtropical Gyre.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtV2hsr7J&md5=c95c0fb23e15170a4a77516fa330f602CAS | 20724586PubMed |
[104] W. E. Dietrich, Settling velocity of natural particles. Water Resour. Res. 1982, 18, 1615.
| Settling velocity of natural particles.Crossref | GoogleScholarGoogle Scholar |
[105] J. Lyklema, Fundamentals of Interface and Colloid Science. Volume I: Fundamentals 1991 (Academic Press: London).
[106] M. Filella, Colloidal properties of submicron particles in natural waters, in Environmental Colloids and Particles: Behaviour, Separation and Characterisation (Eds K. J. Wilkinson, J. R. Lead) 2007, pp. 17–93 (Wiley: Chichester, UK).
[107] J. Laskowski, J. A. Kitchener, The hydrophilic–hydrophobic transition on silica. J. Colloid Interface Sci. 1969, 29, 670.
| The hydrophilic–hydrophobic transition on silica.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF1MXpvF2ltA%3D%3D&md5=61afd5c67f8945facc0736ee066459c1CAS |
[108] T. D. Blake, J. A. Kitchener, Stability of aqueous films on hydrophobic methylated silica. J. Chem. Soc., Faraday Trans. 1972, 68, 1435.
| Stability of aqueous films on hydrophobic methylated silica.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE38Xksl2qsr4%3D&md5=c53f2bfaba16dbfd0043018e6c1ed368CAS |
[109] J.-P. W. Desforges, M. Galbraith, N. Dangerfield, P. S. Ross, Widespread distribution of microplastics in subsurface seawater in the NE Pacific Ocean. Mar. Pollut. Bull. 2014, 79, 94.
| Widespread distribution of microplastics in subsurface seawater in the NE Pacific Ocean.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXislyqsQ%3D%3D&md5=6789734222ad295a1b73d720bff6beffCAS |
[110] C. P. Johnson, X. Li, B. E. Logan, Settling velocities of fractal aggregates. Environ. Sci. Technol. 1996, 30, 1911.
| Settling velocities of fractal aggregates.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XisFagtr0%3D&md5=e152a18665a9f532d5a3b652d08e8fd7CAS |
[111] B. E. Logan, J. R. Hunt, Advantages to microbes of growth in permeable aggregates in marine system. Limnol. Oceanogr. 1987, 32, 1034.
| Advantages to microbes of growth in permeable aggregates in marine system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2sXmsVaitb8%3D&md5=c6008c73a3daf5d11581170a3daee381CAS |
[112] V. Ittekkot, The abiotically driven biological pump in the ocean and short-term fluctuations in atmospheric CO2 contents. Global Planet. Change 1993, 8, 17.
| The abiotically driven biological pump in the ocean and short-term fluctuations in atmospheric CO2 contents.Crossref | GoogleScholarGoogle Scholar |
[113] R. Thunell, C. Benitez-Nelson, R. Varela, Y. Astor, F. Muller-Karger, Particulate organic carbon fluxes along upwelling-dominated continental margins: rates and mechanism. Global Biogeochem. Cycles 2007, 21, GB1022.
| Particulate organic carbon fluxes along upwelling-dominated continental margins: rates and mechanism.Crossref | GoogleScholarGoogle Scholar |
[114] J. Buffle, K. J. Wilkinson, S. Stoll, M. Filella, J. Zhang, A generalized description of aquatic colloidal interactions: the three-colloidal component approach. Environ. Sci. Technol. 1998, 32, 2887.
| A generalized description of aquatic colloidal interactions: the three-colloidal component approach.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXlsVyqt7w%3D&md5=1a87abb1f5b8b9fb68049b2e326d68e7CAS |
[115] R. M. Town, M. Filella, Size fractionation of trace metal species in freshwaters: implications for understanding their behaviour and fate. Rev. Environ. Sci. Biotechnol. 2002, 1, 277.
| Size fractionation of trace metal species in freshwaters: implications for understanding their behaviour and fate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXhtVSqtLbM&md5=a9b4f1b527fd4ec22c3be159e62ecbc6CAS |
[116] M. J. Doyle, W. Watson, N. M. Bowlin, S. B. Sheavly, Plastic particles in coastal pelagic ecosystems of the north-east Pacific ocean. Mar. Environ. Res. 2011, 71, 41.
| Plastic particles in coastal pelagic ecosystems of the north-east Pacific ocean.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXnsVemsg%3D%3D&md5=43413eba44fce91c7516703af800aedeCAS | 21093039PubMed |


