The concentration-dependent behaviour of nanoparticles
Mohammed Baalousha A B D , Mithun Sikder A B , Ashwini Prasad C , Jamie Lead A B , Ruth Merrifield A and G. Thomas Chandler BA South Carolina SmartState Center for Environmental Nanoscience and Risk (CENR), Arnold School of Public Health, University of South Carolina, Columbia, SC 29208, USA.
B Department of Environmental Health Sciences, Arnold School of Public Health, University of South Carolina, Columbia, SC 29208, USA.
C School of Geography, Earth and Environmental Sciences, University of Birmingham, B15 2TT, UK.
D Corresponding author. Email: mbaalous@mailbox.sc.edu
Environmental Chemistry 13(1) 1-3 https://doi.org/10.1071/EN15142
Submitted: 8 July 2015 Accepted: 31 August 2015 Published: 8 October 2015
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Environmental context. Studies of manufactured nanoparticles (NPs) in the environment have been performed almost exclusively at high NP concentrations. These data lead to misunderstandings related to NP fate and effects at relevant environmental concentrations, which are expected to be low. A better understanding of the concentration-dependent behaviour of NPs will improve our understanding of their fate and effects under environmentally realistic conditions.
Abstract. This rapid communication highlights the importance of nanoparticle concentration in determining their environmental fate and behaviour. Notably, two fate processes have been considered: dissolution and aggregation. The decrease in nanoparticle concentration results in increased dissolution and decreased aggregate sizes, inferring higher potential for environmental transport of nanoparticles.
The behaviour (e.g, dissolution, aggregation, disaggregation) and fate (e.g. mobility, fugacity, non-transient (sink) or transient source) of nanoparticles (NPs) in environmental and toxicological media have been investigated for over a decade, typically at high NP concentrations (e.g. milligram per litre range) which are not relevant to the environment,[1] resulting in some potentially misleading assumptions that (i) NP behaviour is dominated by aggregation and thus their fate is dominated by sedimentation and removal from the water column, or, in porous media, deposition and removal from the aqueous phase[2]; (ii) NP dissolution is limited for many NPs and rarely are all NPs dissolved fully in environmental and biological media over relevant timescales[1] and (iii) many NPs therefore impart little or no toxic risk to pelagic organisms as a result of limited NP dissolution and NP removal by aggregation and sedimentation.[3] Several NP groups (e.g. Ag NPs, Cu NPs, Cd NPs, ZnO) may undergo dissolution and release ions with well known toxic effects.[4] These various issues complicate NP risk characterisation and are exacerbated by the general use of high NP concentrations in NP fate, behaviour and ecotoxicological studies.[2,5] Use of high NP concentrations has been motivated by poor detection limits of available analytical techniques (e.g. dynamic light scattering, laser Doppler electrophoresis, UV-Vis spectroscopy) together with enhanced likelihood of observing more pronounced changes and effects at high NP concentrations.[6] Furthermore, most published nano-ecotoxicological data are acute exposure studies, which also drive the high concentration selection bias in order to generate measurable biological responses. Many NPs tested for toxicity to aquatic organisms have been non-toxic on acute time scales until they reach unrealistically high exposure concentrations. Clear predictive linkages between unrealistic high acute exposures and more realistic low chronic exposures have not been established for aquatic systems, and are likely to be further complicated by differing concentration-dependent behaviours of NPs.
Despite these concerns, little attention has been paid to the characterisation of concentration-dependent environmental behaviour of NPs. This limits our understanding of NP behaviour and risk under realistic environmental conditions. For example, if aggregation is reduced at low concentrations, are NPs relatively more mobile at low, environmentally relevant concentrations compared to the more commonly used test conditions presented in the literature? Could NPs be more toxic at low concentrations because of the increased release of toxic ions? We argue that at low, environmentally relevant concentrations (e.g. nano- to microgram per litre range in surface waters and wastewater effluent) the fate, behaviour and toxicity of NPs will be significantly different and worthy of further research.[7] An important rider to this argument is the need to lower the detection limits of future and currently available analytical techniques to ensure robust NP concentration and size determination. For instance, the recent development of single particle–inductively coupled plasma–mass spectrometry (sp-ICP-MS) enables measuring NPs at environmentally relevant concentrations; however, the poor lower size detection limit (~10–400 nm depending on NP composition) is likely to be a limiting factor when investigating small NPs.[8]
While the environmental fate and behaviour of NPs may depend on solution media characteristics and the physicochemical properties of NPs such as size, shape and crystal structure,[9] we focus here on NP concentration because it is the parameter that has been widely overlooked in the literature because of multiple analytical challenges. Several studies have illustrated the dominant role of NP aggregation and dissolution in controlling their environmental behaviour, but, to our knowledge, few published studies have investigated the effect of NP concentration on dissolution and aggregate size in the milligram per litre NP concentration range. For instance, one study suggested that iron oxide NPs (10–200 mg L–1) form smaller aggregates (micrometre size range) at lower concentrations.[5] Another study demonstrated the decrease in the solubility of NPs (e.g. CuO, ZnO and NiO) in a bacterial exposure medium with increased NP concentrations, although experiments were performed at unrealistically high NP concentrations of 25–200 mg L–1. The observed concentration-dependent solubility was attributed to increased NP aggregation at higher concentrations.[10] A third study suggested an increased dissolution of Tween coated-Ag NPs after dilution to near environmentally realistic Ag NP concentrations (~400 μg L–1) in ultra-high purity water.[11]
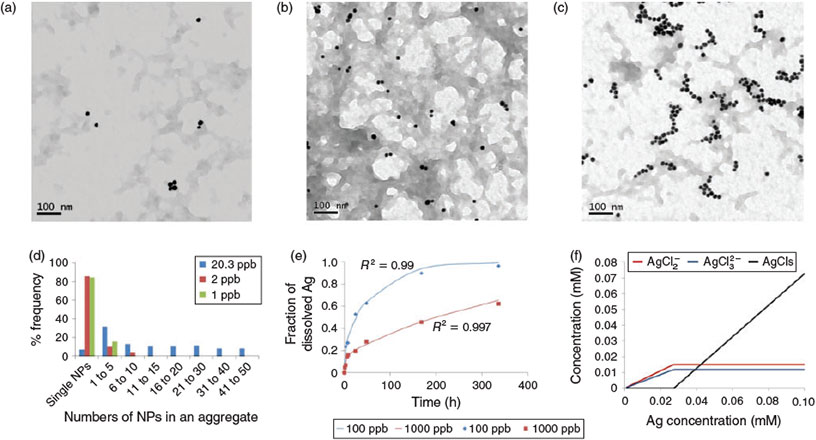
Here, following advances in sample preparation for microscopy analysis in order to lower concentration detection limits,[12,13] and the use of long path UV-Vis cell cuvettes to monitor poly(vinylpyrrolidinone) (PVP)-coated Ag NPs at near-environmentally relevant concentrations (100–1000 μg L–1), we highlight that these concentration-dependent behaviours of nanomaterials become even more pronounced at the microgram per litre levels. This kind of behaviour should be thoroughly considered in any assessment of NP fate and effects for materials with potential for dissolution. Fig. 1a–c shows the aggregation behaviour of citrate-coated Au NPs at environmentally relevant concentrations (1 to 20 μg L–1) suggesting that aggregate sizes decrease with the decrease in NP concentration. The number of NPs forming aggregates – and thus aggregate size – decreases with decreases in NP concentration (Fig. 1d), suggesting that homoaggregation becomes less important at low concentrations due to the lower probability of NP collisions. Similarly, Fig. 1e illustrates that the dissolution (percentage of total Ag concentration) of PVP-coated Ag NPs in 30-ppt sea water increases with the decrease in NP concentration; that is, soluble NPs become even more soluble at lower concentrations and their behaviour may approach that of fully dissolved species. Taking silver as an example, dissolution in seawater is higher at lower concentrations for two reasons: (1) Ag+ binding ligands, such as chloride, bind the aqueous silver ions forming complexes of silver and this drives further dissolution from the NP[14] and (2) smaller aggregate size likely results in the maintenance of higher specific surface area and greater dissolution.[10] Furthermore, the speciation of dissolved silver released from NPs is also concentration-dependent. Fig. 1f illustrates the modelled concentration-dependent speciation of dissolved Ag where at high Ag concentrations (>2 mg L–1) dissolved Ag forms AgCl precipitates, whereas at lower Ag concentrations the speciation of dissolved Ag is dominated by dissolved AgCl complexes (AgCl2– and AgCl32–). Similar arguments apply to other partially soluble NPs such as quantum dots (CdSe), copper, ZnO, etc. Other researchers have investigated the concentration-dependent behaviour of NPs. For instance, Oskolkova et al. illustrated an increased particle attraction between polyethylene glycol-bound (PEGylated) NPs at higher NP concentrations. The concentration dependence was attributed to a decreased solvation of PEG chains because of an increased ratio of ethylene oxide segments to water.[15] Sun et al. demonstrated lower retention of graphene oxides in porous media at high NP concentrations, which was attributed to a blocking effect at high concentrations, where attachment sites are filled more rapidly at the higher NP concentrations.[16] Many other processes such as interaction with buffers, ligands and organic molecules, along with light-induced transformations of NPs are likely to depend on NP concentration, and need in-depth investigation.
A large majority of environmental fate and effects studies of NPs have been performed at high NP concentrations.[17] Extrapolation of results performed at high NP concentration may lead to inaccuracies in interpretation for environmentally relevant conditions. For NP environmental fate and behaviour studies, smaller aggregate sizes and higher dissolution rates at lower NP concentrations suggest longer distance transport of NPs and their dissolved moieties than that perceived from studies performed at high NP concentrations. For ecotoxicological assays, the nature of the toxicant (e.g. dissolved, number of individual NPs and their aggregated and aggregate size) is different at different concentrations as a result of processes such as dissolution and aggregation. Smaller aggregate sizes and higher dissolution rates at low concentrations suggest that NPs and their dissolved components are likely to be bioavailable for longer periods than have been assessed toxicologically in most cases. Therefore, because of the concentration-dependent behaviour of NPs in environmental and biological media, we suggest that: (1) environmental fate and effects studies of NPs, and the characterisation of NPs underpinning these studies, should be performed at environmentally relevant NP concentrations and (2) hazard assessment of NPs should be performed under chronic exposure conditions at low NP concentrations, which is the most likely exposure scenario in the natural environment. Other confounding factors of real-life exposure media such as complexity and poorly defined media, presence of other ligands, temperature variability and presence of light should also be investigated further to improve our understanding of NP hazards and risks.
Supplementary material
The supplementary material provides full details of the transmission electron microscopy (TEM) sample preparation approach, the dissolution analysis by UV-Vis together with the data fitting, and the silver speciation analysis.
Acknowledgements
The authors acknowledge funding from the National Science Foundation (NSF1437307), the UK Natural Environmental Research Council (NE/H018514/1) along with funding from the South Carolina SmartState Center for Environmental Nanoscience and Risk (CENR).
References
[1] K. Loza, J. Diendorf, C. Sengstock, L. Ruiz-Gonzalez, J. M. Gonzalez-Calbet, M. Vallet-Regi, M. Koller, M. Epple, The dissolution and biological effects of silver nanoparticles in biological media. J. Mater. Chem. B Mater. Biol. Med. 2014, 2, 1634.| The dissolution and biological effects of silver nanoparticles in biological media.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXjt1yksrw%3D&md5=a8a9b10d6938fff8c983d582decffe6dCAS |
[2] I. Velzeboer, J. T. K. Quik, D. van de Meent, A. A. Koelmans, Rapid settling of nanoparticles due to heteroaggregation with suspended sediment. Environ. Toxicol. Chem. 2014, 33, 1766.
| Rapid settling of nanoparticles due to heteroaggregation with suspended sediment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhtFKlsbnJ&md5=b85a462089073043766ae98a9d0699ebCAS | 24753080PubMed |
[3] E. Navarro, A. Baun, R. Behra, N. B. Hartmann, J. Filser, A.-J. Miao, A. Quigg, P. H. Santschi, L. Sigg, Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicol. 2008, 17, 372.
| Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXmsVKrsLc%3D&md5=bd0c43cd3b40073839874f44ea37a2a8CAS |
[4] S. K. Misra, S. Nuseibeh, A. Dybowska, D. Berhanu, T. D. Tetley, E. Valsami-Jones, Comparative study using spheres, rods and spindle-shaped nanoplatelets on dispersion stability, dissolution and toxicity of CuO nanomaterials. Nanotoxicol. 2014, 8, 422.
| Comparative study using spheres, rods and spindle-shaped nanoplatelets on dispersion stability, dissolution and toxicity of CuO nanomaterials.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhvFemtLnM&md5=e264f94edc38b05bd67030f381239296CAS |
[5] M. Baalousha, Aggregation and disaggregation of iron oxide nanoparticles: influence of particle concentration, pH and natural organic matter. Sci. Total Environ. 2009, 407, 2093.
| Aggregation and disaggregation of iron oxide nanoparticles: influence of particle concentration, pH and natural organic matter.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXit1Sjtb0%3D&md5=32f133c3be48c0f29c7a7b5352a58088CAS | 19059631PubMed |
[6] H. Hyung, J. H. Kim, Natural organic matter (NOM) adsorption to multi-walled carbon nanotubes: effect of NOM characteristics and water quality parameters. Environ. Sci. Technol. 2008, 42, 4416.
| Natural organic matter (NOM) adsorption to multi-walled carbon nanotubes: effect of NOM characteristics and water quality parameters.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXlsVKms74%3D&md5=fa139de1c43434c38e16885032e97daaCAS | 18605564PubMed |
[7] F. Gottschalk, E. Kost, B. Nowack, Engineered nanomaterials in water and soils: a risk quantification based on probabilistic exposure and effect modeling. Environ. Toxicol. Chem. 2013, 32, 1278.
| Engineered nanomaterials in water and soils: a risk quantification based on probabilistic exposure and effect modeling.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXntVCnsrg%3D&md5=ff00559600974d4d4819d61928a46a20CAS | 23418073PubMed |
[8] S. Lee, X. Bi, R. B. Reed, J. F. Ranville, P. Herckes, P. Westerhoff, Nanoparticle size detection limits by single particle ICP-MS for 40 elements. Environ. Sci. Technol. 2014, 48, 10 291.
| Nanoparticle size detection limits by single particle ICP-MS for 40 elements.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhtlGgsrvP&md5=a8bfa52ecba4cdfe62281776ec5cfbf9CAS |
[9] W. J. Peijnenburg, M. Baalousha, J. Chen, Q. Chaudry, F. von der Kammer, T. A. Kuhlbusch, J. Lead, C. Nickel, J. T. Quik, M. Renker, A review of the properties and processes determining the fate of engineered nanomaterials in the aquatic environment. Crit. Rev. Environ. Sci. Technol. 2015, 45, 2084.
| A review of the properties and processes determining the fate of engineered nanomaterials in the aquatic environment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhtFCgt73N&md5=f9ac078927ec2d4ef2271660d87ff796CAS |
[10] Y. W. Baek, Y. J. An, Microbial toxicity of metal oxide nanoparticles (CuO, NiO, ZnO, and Sb2O3) to Escherichia coli, Bacillus subtilis, and Streptococcus aureus. Sci. Total Environ. 2011, 409, 1603.
| Microbial toxicity of metal oxide nanoparticles (CuO, NiO, ZnO, and Sb2O3) to Escherichia coli, Bacillus subtilis, and Streptococcus aureus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXisFWrsLg%3D&md5=278aa5c024752805936e5af1d1f8440bCAS | 21310463PubMed |
[11] M. Baalousha, K. P. Arkill, I. Romer, R. E. Palmer, J. R. Lead, Transformations of citrate and tween coated silver nanoparticles reacted with Na2S. Sci. Total Environ. 2014,
| 25432129PubMed |
[12] M. Baalousha, A. Prasad, J. R. Lead, Quantitative measurement of the nanoparticle size and number concentration from liquid suspensions by atomic force microscopy. Environ. Sci. Process. Impacts 2014, 16, 1338.
| Quantitative measurement of the nanoparticle size and number concentration from liquid suspensions by atomic force microscopy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXovVKns7s%3D&md5=df235d9e804e5f3eb05a9e73210af9e2CAS | 24668140PubMed |
[13] A. Prasad, M. Baalousha, J. R. Lead, An electron microscopy based method for the detection and quantification of nanomaterial number concentration in environmentally relevant media. Sci. Total Environ. 2015, 537, 479.
| An electron microscopy based method for the detection and quantification of nanomaterial number concentration in environmentally relevant media.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC287ntlKnug%3D%3D&md5=e808d779ea4aa6aaf3ffaab353c4f168CAS | 26322596PubMed |
[14] C. Levard, S. Mitra, T. Yang, A. D. Jew, A. R. Badireddy, G. V. Lowry, G. E. Brown, Effect of chloride on the dissolution rate of silver nanoparticles and toxicity to E. coli. Environ. Sci. Technol. 2013, 47, 5738.
| Effect of chloride on the dissolution rate of silver nanoparticles and toxicity to E. coli.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXmvF2js70%3D&md5=948f6546229a8c03371c6e157f9838f6CAS | 23641814PubMed |
[15] M. Z. Oskolkova, A. Stradner, J. Ulama, J. Bergenholtz, Concentration-dependent effective attractions between PEGylated nanoparticles. RSC Advances 2015, 5, 25 149.
| Concentration-dependent effective attractions between PEGylated nanoparticles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXjs1Sjsrw%3D&md5=21d96f85c3ba5a765b81617c1f6deef3CAS |
[16] Y. Sun, B. Gao, S. A. Bradford, L. Wu, H. Chen, X. Shi, J. Wu, Transport, retention, and size perturbation of graphene oxide in saturated porous media: effects of input concentration and grain size. Water Res. 2015, 68, 24.
| Transport, retention, and size perturbation of graphene oxide in saturated porous media: effects of input concentration and grain size.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhslajtLvK&md5=4ff3a0e0fd445fafb0e94fe9075c0330CAS | 25462714PubMed |
[17] J. T. K. Quik, I. Velzeboer, M. Wouterse, A. A. Koelmans, D. van de Meent, Heteroaggregation and sedimentation rates for nanomaterials in natural waters. Water Res. 2014, 48, 269.
| Heteroaggregation and sedimentation rates for nanomaterials in natural waters.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsF2ntL%2FM&md5=00726910eca65e3f2236eb55a4ceebbdCAS |