Source analysis of perfluorocarboxylates in Tokyo Bay during dry weather and wet weather using sewage markers
Michio Murakami A , Chiaki Morita B , Takuya Morimoto B and Hideshige Takada B CA ‘Wisdom of Water’ (Suntory), Corporate Sponsored Research Program, Organization for Interdisciplinary Research Projects, The University of Tokyo, 7-3-1 Hongo, Bunkyo, Tokyo 113-8656, Japan.
B Laboratory of Organic Geochemistry (LOG), Institute of Symbiotic Science and Technology, Tokyo University of Agriculture and Technology, Fuchu, Tokyo 183-8509, Japan.
C Corresponding author. Email: shige@cc.tuat.ac.jp
Environmental Chemistry 8(4) 355-362 https://doi.org/10.1071/EN10130
Submitted: 1 December 2010 Accepted: 24 January 2011 Published: 19 August 2011
Journal Compilation © CSIRO Publishing 2011 Open Access CC BY-NC-ND
Environmental context. As perfluorocarboxylates can be carried by surface runoff to waters and cause adverse effects to aquatic organisms, we evaluated the contributions of wastewater and surface runoff to the concentrations of these compounds in Tokyo Bay during dry and wet weather. Sewage markers revealed that the surface runoff was a significant source of perfluorocarboxylates in the bay during wet weather. This finding leads to a greater understanding of sources and pathways of perfluorocarboxylates in waters.
Abstract. We investigated the occurrence of perfluorocarboxylates (PFCAs) in Tokyo Bay during dry and wet weather and evaluated the contributions of wastewater effluent, untreated wastewater, and surface runoff by using two sewage markers, caffeine and crotamiton. ∑8PFCAs ranged from 11 to 185 ng L–1. Perfluorononanoate (PFNA) was the major species, followed by perfluorooctanoate (PFOA) and perfluoroheptanoate (PFHpA). Principal component analysis followed by multiple linear regression revealed that the PFCAs were derived mainly from wastewater effluent during dry weather, and jointly from wastewater effluent (59%) and combined sewer overflow (41%) during wet weather. We used caffeine-to-crotamiton ratios to evaluate the contributions of untreated wastewater and wastewater effluent. Estimated concentrations of wastewater-derived PFCAs were much lower than observed concentrations during wet weather, indicating the contribution of surface runoff to contamination. During a combined sewer overflow, surface runoff had a significant effect on contamination in the bay.
Additional keywords: combined sewer overflow (CSO), diffuse pollution, non-point source, perfluorinated surfactants (PFSs), perfluorochemicals (PFCs), pharmaceuticals and personal care products (PPCPs), wastewater tracer.
Introduction
After 3M began manufacturing perfluorinated surfactants (PFSs), such as perfluorooctane sulfonate (PFOS; C8F17SO3–) and perfluorooctanoate (PFOA; C7F15COO–), in the USA in the late 1940s, PFSs and their precursors have been widely used by various industries in products such as waxes, carpet cleaners, fire retardants, apparel, and fluoropolymer manufacture, owing to their water repellency and oil repellency.[1,2] PFSs are water soluble (e.g. potassium salt of PFOS, 570 mg L–1 at 24–25°C; PFOA, 9500 mg L–1 at 25°C),[3,4] bioaccumulative,[5,6] and environmentally persistent. Owing to these features, PFSs have been ubiquitously detected in surface water,[7–9] groundwater,[10] seawater,[11–14] drinking water,[15] aquatic organisms,[16,17] and humans.[18,19] As PFSs are suspected of being carcinogenic, of being chronic and reproductive toxicants, and of having adverse effects on humans and other animals,[20–22] concerns about PFSs are increasing. Thus, an understanding of the sources and pathways of PFSs to waters is required.
PFSs in waters derive from both direct and indirect sources.[1,7] Direct sources include the discharge of contaminated water such as untreated wastewater, wastewater effluent and surface runoff.[7,8,23–25] Indirect sources are related to the formation of PFSs from precursors in the atmosphere.[26]
Past studies showed that wastewater effluent is an important source of PFSs in waters.[7,8,23,25] Loadings of PFSs in rivers during dry weather were comparable to those in effluent from upstream wastewater treatment plants.[23] Effluent from semiconductor factories and other manufacturers was the primary source of PFSs in downstream rivers in Taiwan.[25] Concentrations of PFOS, perfluoroheptanoate (PFHpA; C6F13COO–), and perfluorononanoate (PFNA; C8F17COO–) in Japanese rivers were strongly correlated with those of crotamiton, a sewage marker, indicating that these pollutants were derived from wastewater effluent.[8]
PFSs are diffusely present in aerosols and street dust,[27,28] probably owing to the presence of PFSs and their precursors in waxes and windshield washer fluid, and of the atmospheric deposition of volatile precursors.[1,26,29] Consequently, PFSs are present in precipitation and surface runoff.[24,30,31] Surface runoff contributed to contamination by PFOA in urban lakes in the USA.[30] PFS fluxes in the Tsurumi and Hayabuchi rivers, Japan, were greater during wet weather than during dry weather.[23,32] Unlike PFOS, PFOA concentrations in wastewater influent in Singapore during the wet season were not lower than those during the dry season, also suggesting that surface runoff contributed to contamination by PFOA.[33] Murakami et al. surveyed sources of perfluorocarboxylates (PFCAs) in groundwater in Tokyo using differences in PFS compositions among sources and suggested that surface runoff contributed to between 16 and 46% of PFCAs.[10] Consistent findings show that non-point sources play important roles in contamination by PFCAs, but not by perfluoroalkyl sulfonates (PFASs).[24,32,33] The entry of PFSs, especially PFCAs, from surface runoff into waters is a matter of concern.
We investigated the occurrence of PFCAs in Tokyo Bay during dry and wet weather. As a combined sewer overflow (CSO) can occur during wet weather, PFCAs might be derived from wastewater effluent, untreated wastewater, or surface runoff. So to evaluate the effect of a CSO, we used two pharmaceutical and personal care products (PPCPs), caffeine and crotamiton, as sewage markers. Caffeine is present at high concentrations in untreated wastewater, but >99% is typically removed during wastewater treatment.[34,35] Caffeine is therefore regarded as a labile marker and can be used as an indicator of a CSO.[36] In contrast, crotamiton is not well removed during conventional wastewater treatment processes and is therefore regarded as a conservative marker.[35,37] Combining these two sewage markers can identify the contribution of untreated wastewater to waters.[38] Using these sewage markers, we evaluated the contributions of PFCAs from wastewater effluent, untreated wastewater, and surface runoff to Tokyo Bay during wet weather. We studied eight PFCAs: PFHpA; PFOA; PFNA; perfluorodecanoate (PFDA; C9F19COO–); perfluoroundecanoate (PFUA; C10F21COO–); perfluorododecanoate (PFDoDA; C11F23COO–); perfluorotridecanoate (PFTrDA; C12F25COO–); and perfluorotetradecanoate (PFTeDA; C13F27COO–).
Experimental
Sample collection
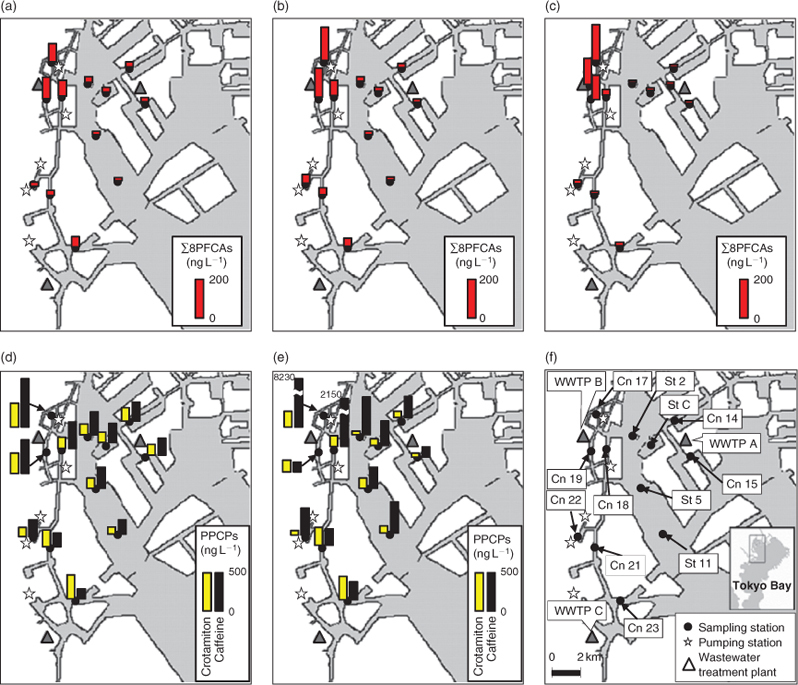
We collected grab water samples at several locations from the surface 30 cm of Tokyo Bay on 16 May, 15 October and 22 December 2009 in a stainless steel bucket (Fig. 1f). Sample IDs are the same as expressed in Environment of Tokyo (see http://www.kankyo.metro.tokyo.jp/en/index.html, accessed 19 April 2011), where St and Cn represent the samples from bay and canals respectively. As heavy rain (total 16 mm; maximum intensity 7.5 mm in 10 min) fell from 2000 to 2250 hours on 14 October, we regarded the samples collected on 15 October as wet-weather samples. As >8 days of dry weather preceded 16 May and 22 December, we regarded the samples collected on those dates as dry-weather samples. Water salinity was measured on site with an electrode. The samples were then transported to the laboratory and filtered through pre-baked glass fibre filters (Whatman GF/F, 0.7-µm pore size). The samples for PPCP analysis were acidified with 4-M HCl to pH ~2. All samples were stored at 5°C before analysis. PFCAs were measured in the samples collected on all three dates. PPCPs were measured in the samples collected on 16 May and 15 October only.
Analyses
Details of analyses of PFCAs are given in Murakami et al.[8] Briefly, 13C-labelled PFOA and 13C-labelled PFDA were spiked into the filtrates (1 L), and the samples were then passed through Sep-Pak Plus tC18 cartridges (Waters) preconditioned with 20 mL of methanol and then 10 mL of distilled water. A flow rate of <10 mL min–1 was maintained. The cartridges were washed with 7 mL of 30% (v/v) methanol in distilled water, and then 7 mL of 55% (v/v) methanol in distilled water that was acidified with 4-M HCl to pH 2.0–2.5. The target compounds were eluted with 20 mL of methanol. The eluate was concentrated to 0.5 or 1 mL, and PFCAs were analysed by liquid chromatography–tandem mass spectrometry (LC-MS/MS: Agilent 1100 and TSQ Quantum) with a Zorbax Rx-C8 column (4.6 × 150 mm, 5 µm, Agilent) in electrospray negative ionisation mode. Ions were monitored in selected reaction monitoring mode. PFHpA, PFOA, and PFNA were label-recovery corrected with 13C-PFOA, as were PFDA and PFUA with 13C-PFDA. PFDoDA, PFTrDA, and PFTeDA were not label-recovery corrected.
Details of analyses of PPCPs are given in Nakada et al.[37,38] Briefly, acidified filtrates were neutralised with 4-M NaOH to pH 6–8. Crotamiton-d7 and caffeine-d9 were spiked into the neutralised filtrates (500 mL), and the samples were then passed through Oasis HLB cartridges (Waters) preconditioned with 10 mL of methanol and then 10 mL of distilled water. A flow rate of <10 mL min–1 was maintained. The target compounds were eluted with 20 mL of methanol. The eluate was rotary-evaporated to dryness, redissolved in n-hexane, and then purified by 5% H2O-deactivated silica gel column chromatography. The column (1-cm internal diameter × 9 cm) was eluted with 20 mL of n-hexane/dichloromethane (DCM; 75:25, v/v), 40 mL of DCM, 30 mL of DCM/acetone (70:30, v/v), and 40 mL of DCM/acetone (50:50, v/v). The two DCM/acetone fractions were individually reduced in volume, supplemented with injection internal standards (anthracene-d10, benz[a]anthracene-d12 in isooctane), and directly analysed by gas chromatography–mass spectrometry (GC-MS: Agilent, HP5973) with an HP-5MS capillary column (30 m × 0.25-mm internal diameter, Agilent) in selected-ion monitoring mode.
The reproducibility and recovery rates were confirmed by using secondary effluent from a wastewater treatment plant (Table A1 of the Accessory publication, see http://www.publish.csiro.au/?act=view_file&file_id=EN10130_AC.pdf). The relative standard deviation (RSD) was ≤21% for all PFCAs (n = 3) and ≤4% for PPCPs (n = 4). We spiked each PFCA and PPCP standard into the filtrate of each wastewater sample to confirm the recovery rates. Recovery rates (n = 4) ranged from 85 to 114%.
Results and discussion
Occurrence of PFCAs and PPCPs during dry and wet weather
∑8PFCAs ranged from 11 to 185 ng L–1 (Fig. 1a–c). The distributions of PFCAs were similar between the two dry-weather periods (Fig. 1a, c). PFCAs were heavily contaminated at the stations near WWTP B (Cn 17, 18, 19). This result is consistent with previous findings that wastewater effluent is a major source of PFCAs.[8,23] PFCA concentrations during the wet-weather period were comparable to those during the two dry-weather periods. During dry weather, PFHpA represented 11% of ∑8PFCAs (arithmetic mean composition), PFOA 23%, PFNA 51%, PFDA 3.5%, PFUA 6.3%, PFDoDA 2.4%, PFTrDA 2.9%, and PFTeDA 0.1%. During wet weather, PFHpA represented 7% of ∑8PFCAs, PFOA 24%, PFNA 59%, PFDA 3.3%, PFUA 5.3%, and PFDoDA 1.4%. Neither PFTrDA nor PFTeDA was detected during wet weather. During both dry and wet weather, PFNA was greatest, followed by PFOA and PFHpA. The heavy contamination by PFNA was probably related to primary manufacturing of ammonium perfluorononanoate in Japan.[8] The ratio of short-chain to long-chain PFCAs (i.e. (PFHpA + PFOA + PFNA)/(PFDA + PFUA + PFDoDA + PFTrDA + PFTeDA)) was significantly higher during wet weather than dry weather (paired t-test: P < 0.05), indicating that there were differences in sources or behaviour (e.g. partition to solids) of PFCAs between dry and wet weather.
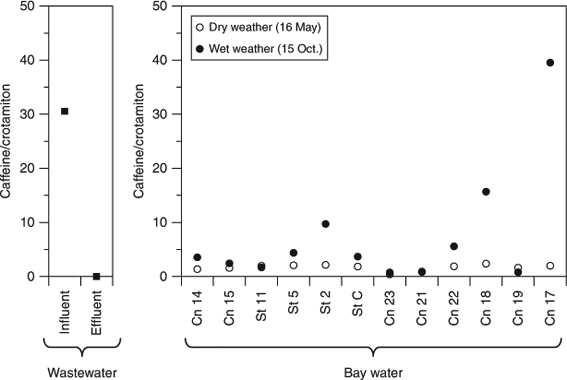
Of the PPCPs, caffeine was detected at high concentrations at stations Cn 17 and 18, near pumping stations, during wet weather, approximately one order of magnitude higher than during dry weather (Fig. 1d, e), indicating a CSO during the wet weather.
Chemical concentrations in the bay are largely affected by the tides. Therefore, to correct for the effects of the tides, we investigated the relationships between chemical concentrations and freshwater ratios, defined as:
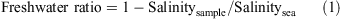
where Salinitysea is Pacific Ocean salinity (35‰).[12]
There was a significant relationship between ∑8PFCAs concentration and freshwater ratio (dry weather, n = 24: R2 = 0.80, P < 0.001; Fig. 2a), indicating that the PFCAs were derived from freshwater inputs into the bay and that PFCA concentrations in the bay depended on dilution by seawater. This is consistent with a previous finding that PFOS and PFOA concentrations were correlated with freshwater ratios in Tokyo Bay.[12] Ratios of crotamiton concentrations to freshwater ratios were significantly lower during wet weather (arithmetic mean ± standard error: 253 ± 33 ng L–1) than during dry weather (395 ± 26 ng L–1; paired t-test, P = 0.001). This difference indicates that crotamiton was diluted by surface runoff during wet weather. In comparison, the caffeine/freshwater ratios during wet weather (1730 ± 820 ng L–1) tended to be higher than those during dry weather (625 ± 74 ng L–1), reflecting the effect of untreated wastewater via a CSO. There were no large differences in the ∑8PFCAs/freshwater ratios among the three dates (16 May, 85 ± 14 ng L–1; 15 October, 90 ± 17 ng L–1; 22 December, 92 ± 20 ng L–1), showing that PFCA concentrations were not decreased during wet weather despite the entry of surface runoff into the bay. Unlike caffeine, PFCA concentrations in wastewater influent were lower than those in wastewater effluent, possibly because of biodegradation of their precursors.[8,39] Therefore, the entry of untreated wastewater via a CSO into the bay did not contribute to the increase of PFCA concentrations in the bay. Thus, surface runoff had a non-negligible effect on PFCAs in the bay during wet weather.
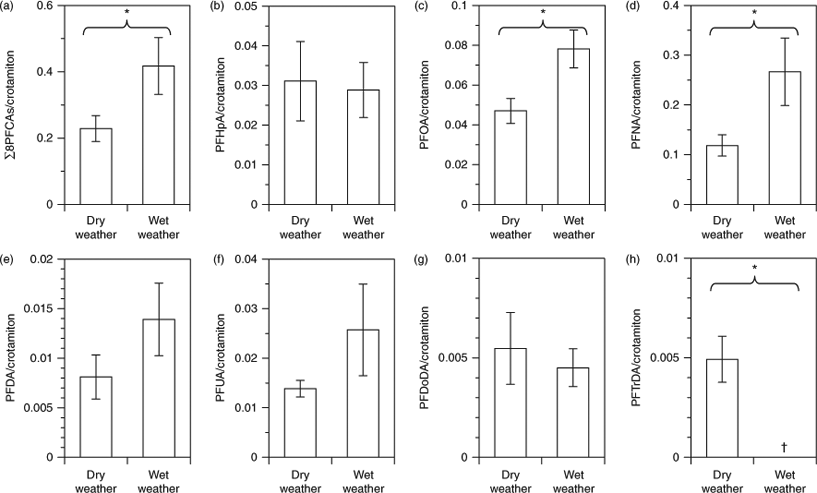
Although crotamiton comes mainly from wastewater,[37,38] PFCAs come from both wastewater and surface runoff.[7,8,23–25] Therefore, we compared the ratios of PFCAs to crotamiton between dry weather (16 May) and wet weather (15 October) to evaluate the effect of surface runoff (Fig. 3). PFTeDA/crotamiton is not shown because PFTeDA was not detected on either date. Ratios during dry weather in the bay were comparable to those in wastewater effluent (e.g. PFOA/crotamiton during dry weather in the bay, 0.047 ± 0.006; in wastewater effluent, 0.064 ± 0.032),[8] indicating again that PFCAs during dry weather were mainly derived from wastewater effluent. This is consistent with the previous findings.[8,23] ∑8PFCAs/crotamiton during wet weather was significantly higher than that during dry weather (paired t-test: P < 0.05, see Fig. 3a). PFOA/crotamiton and PFNA/crotamiton were each significantly higher during wet weather than during dry weather (paired t-test: P < 0.05, see Fig. 3c, d). This difference indicates that not only wastewater effluent but also other additional sources contributed these chemicals during wet weather. In contrast, PFTrDA/crotamiton during wet weather was lower than that during dry weather. Owing to wash-off of surface deposits and suspension of sediments and sewer pipe deposits, suspended solids concentrations are typically higher during CSO events than during dry weather. Long-chain PFCAs are preferentially partitioned to solids.[14,40] The decrease of PFTrDA/crotamiton during wet weather may be attributed to its preferential partitioning to solids.
Source apportionment of PFCAs by principal component analysis followed by multiple linear regression
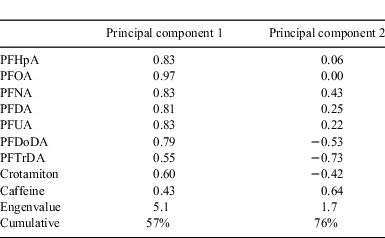
To analyse the sources of PFCAs, we performed principal component analysis (PCA) followed by multiple linear regression using Excel Statistics 2008 software (Social Survey Research Information Co., Ltd). This technique is often used to apportion the sources of air pollutants such as polycyclic aromatic hydrocarbons.[41,42] The purpose of PCA is to represent the total variability of the original data in a minimum number of principal components (PCs). We used concentrations of seven PFCAs (except PFTeDA) and two PPCPs as the original dataset. PFTeDA was not detected on either 16 May or 15 October. PCA was performed to extract the PCs with an eigenvalue >1. Together, PCs 1 and 2 were responsible for 76% of the total variance (Table 1). PC1 had high loadings on all PFCAs and crotamiton, whereas PC2 had a high loading on caffeine and low loadings on PFDoDA and PFTrDA. PC1 can be regarded as an indicator of wastewater effluent, which contain high levels of all PFCAs and crotamiton.[8,37] In contrast, PC2 can be regarded as an indicator of a CSO, because caffeine is present mainly in a CSO.[36] The low loadings on PFDoDA and PFTrDA in PC2 can be explained by preferential partitioning to solids during the CSO event, as described above. The PC2 scores during wet weather were significantly higher than those during dry weather (paired t-test: P < 0.05), supporting the idea that PC2 indicates a CSO (Fig. A1).
|
The contribution of each source group to PFCAs in the waters was then quantitatively assessed by multiple linear regression. The analysis treated the ∑7PFCA concentration in the samples as the criterion variable and the PC scores as the explanatory variables. Stepwise regression was used to add or remove explanatory variables (Fin = Fout = 2.0). Multiple linear regressions were performed separately for the dry-weather and the wet-weather data:
Dry weather (16 May):
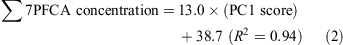
Wet weather (15 October):
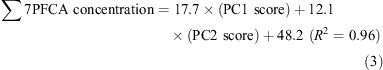
The selection of the PC1 score alone as the explanatory variable for dry weather indicates that the major source of PFCAs during dry weather was wastewater effluent alone. The selection of both PCs for wet weather indicates that the water samples were polluted by both wastewater effluent and a CSO. The average contribution of each source in wet weather was estimated from the ratio of each regression coefficient (PC1 or PC2) to the sum of regression coefficients: wastewater effluent contributed 59% (17.7/(17.7 + 12.1)) and CSO 41% (12.1/(17.7 + 12.1)). Thus, CSOs are a pathway to seawater contamination by PFCAs.
Source analyses of PFCAs by using caffeine/crotamiton ratios
PFCAs during a CSO can originate from both untreated wastewater and surface runoff, because both include PFCAs.[24] However, PCA followed by multiple linear regression using only two events could not discriminate between these sources. To evaluate the contributions from wastewater effluent, untreated wastewater, and surface runoff to PFCAs in the bay, we calculated caffeine/crotamiton ratios in the bay water samples, wastewater influent, and effluent.
First, we evaluated the contribution of untreated wastewater volume to total wastewater (untreated wastewater + wastewater effluent) volume, assuming that the crotamiton concentration is not changed during wastewater treatment and that both crotamiton and caffeine come from wastewater alone:
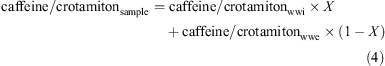
where caffeine/crotamitonwwi is the ratio in wastewater influent (wwi) (30.6 in Japan),[35] caffeine/crotamitonwwe is the ratio in wastewater effluent (wwe) (0.03 in Japan),[35] and X is the fraction of untreated wastewater volume to total wastewater volume.
The caffeine/crotamiton ratios in the bay, especially at stations near pumping stations (Cn 17 and 18), during wet weather were higher than those during dry weather (Fig. 4). Untreated wastewater accounted for ~100% of the total wastewater volume at Cn 17 and 51% at Cn 18.
|
Concentrations of 6PFCAs (PFOA, PFNA, PFDA, PFUA, PFDoDA and PFTrDA) from wastewater were then estimated, for which data in the Kanto region were available[8,24]:
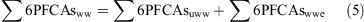


where ∑6PFCAsww is the ∑6PFCA concentration in the sample from wastewater (ng L–1), ∑6PFCAsuww is the ∑6PFCA concentration in the sample from untreated wastewater (ng L–1), ∑6PFCAswwe is the ∑6PFCA concentration in the sample from wastewater effluent (ng L–1), crotamitonuww is the crotamiton concentration in the sample from untreated wastewater (ng L–1), crotamitonwwe is the crotamiton concentration in the sample from wastewater effluent (ng L–1), ∑6PFCAs/crotamitonwwi is the ratio of ∑6PFCA to crotamiton in wastewater influent (0.035–0.24),[8,24] ∑6PFCAs/crotamitonwwe is the ratio of ∑6PFCA to crotamiton in wastewater effluent (0.035–0.40),[8] and crotamitonsample is the crotamiton concentration in the sample (ng L–1).
The estimated ∑6PFCA concentrations derived from wastewater agreed well with the observed values during dry weather (Fig. 5a). This result confirms again that wastewater effluent contributed dominantly to the 6 PFCAs in the bay water samples. In contrast, during wet weather, untreated wastewater also contributed to the 6 PFCAs at the stations near pumping stations (Cn 17, 18), when the estimated concentrations of ∑6PFCAs were much lower than observed, owing to the contribution from surface runoff (Fig. 5b). The shortfalls of ∑6PFCA concentrations were higher than or comparable to the estimated ∑6PFCA concentrations derived from untreated wastewater, suggesting that surface runoff contributed more than half of PFCA contamination via the CSO. During the CSO, surface runoff had a significant effect on the contamination of the bay water.

|
Our results show that surface runoff was a non-negligible source of PFCAs in the bay. The effects of surface runoff vary widely depending on the weather.[24] Comprehensive investigations of PFCAs and sewage markers on several occasions will lead to a greater understanding of the sources.
Acknowledgements
This study was partially supported by a Grant-in-Aid for Scientific Research from the Japan Society for Promotion of Science (No. 19310039) and Kurita Water and Environment Foundation (2009FY).
References
[1] K. Prevedouros, I. T. Cousins, R. C. Buck, S. H. Korzeniowski, Sources, fate and transport of perfluorocarboxylates. Environ. Sci. Technol. 2006, 40, 32.| Sources, fate and transport of perfluorocarboxylates.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXht1Gru7zK&md5=14e91a9069cf17a00b47907f105e54b6CAS |
[2] A. G. Paul, K. C. Jones, A. J. Sweetman, A first global production, emission, and environmental inventory for perfluorooctane sulfonate. Environ. Sci. Technol. 2009, 43, 386.
| A first global production, emission, and environmental inventory for perfluorooctane sulfonate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVyks7%2FJ&md5=63238d1759e406591a922fb0ed3a709cCAS |
[3] Co-operation on existing chemicals – Hazard assessment of perfluorooctane sulfonate (PFOS) and its salts, ENV/JM/RD(2002)17/FINAL, JT00135607 2002 (Organisation for Economic Co-operation and Development).
[4] E. A. Kauck, A. R. Diesslin, Some properties of perfluorocarboxylic acids. Ind. Eng. Chem. 1951, 43, 2332.
| Some properties of perfluorocarboxylic acids.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaG38Xis1GjsQ%3D%3D&md5=a0e88ce77720c8e368d3e6590c1aa910CAS |
[5] J. P. Giesy, K. Kannan, Global distribution of perfluorooctane sulfonate in wildlife. Environ. Sci. Technol. 2001, 35, 1339.
| Global distribution of perfluorooctane sulfonate in wildlife.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXhsVGnurg%3D&md5=e5a5453b4d2a3d83f723af878a2c9adbCAS |
[6] J. M. Conder, R. A. Hoke, W. de Wolf, M. H. Russell, R. C. Buck, Are PFCAs bioaccumulative? A critical review and comparison with regulatory criteria and persistent lipophilic compounds. Environ. Sci. Technol. 2008, 42, 995.
| Are PFCAs bioaccumulative? A critical review and comparison with regulatory criteria and persistent lipophilic compounds.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXltFWmsw%3D%3D&md5=a5859b58e71eafefd64bade38f26a3c1CAS |
[7] M. F. Simcik, K. J. Dorweiler, Ratio of perfluorochemical concentrations as a tracer of atmospheric deposition to surface waters. Environ. Sci. Technol. 2005, 39, 8678.
| Ratio of perfluorochemical concentrations as a tracer of atmospheric deposition to surface waters.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtV2nurzF&md5=0004684106f9403ddce92c816f4fece2CAS |
[8] M. Murakami, E. Imamura, H. Shinohara, K. Kiri, Y. Muramatsu, A. Harada, H. Takada, Occurrence and sources of perfluorinated surfactants in rivers in Japan. Environ. Sci. Technol. 2008, 42, 6566.
| Occurrence and sources of perfluorinated surfactants in rivers in Japan.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXpt1ynsr4%3D&md5=1b2f6eb003e3b49e796ee8947ab771c8CAS |
[9] A. Pistocchi, R. Loos, A map of European emissions and concentrations of PFOS and PFOA. Environ. Sci. Technol. 2009, 43, 9237.
| A map of European emissions and concentrations of PFOS and PFOA.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtlOlu7fE&md5=c7756dd22525e7201f82d4056757cfaaCAS |
[10] M. Murakami, K. Kuroda, N. Sato, T. Fukushi, S. Takizawa, H. Takada, Groundwater pollution by perfluorinated surfactants in Tokyo. Environ. Sci. Technol. 2009, 43, 3480.
| Groundwater pollution by perfluorinated surfactants in Tokyo.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXkvFertLs%3D&md5=d922b3bdc85bc896b2bbe6b75e466d5aCAS |
[11] N. Yamashita, K. Kannan, S. Taniyasu, Y. Horii, G. Petrick, T. Gamo, A global survey of perfluorinated acids in oceans. Mar. Pollut. Bull. 2005, 51, 658.
| A global survey of perfluorinated acids in oceans.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXht1Sjs7vI&md5=f41d0068b593598fe34002e3ebae727fCAS |
[12] T. Sakurai, S. Serizawa, T. Isobe, J. Kobayashi, K. Kodama, G. Kume, J. H. Lee, H. Maki, Y. Imaizumi, N. Suzuki, T. Horiguchi, M. Morita, H. Shiraishi, Spatial, phase, and temporal distributions of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in Tokyo Bay, Japan. Environ. Sci. Technol. 2010, 44, 4110.
| Spatial, phase, and temporal distributions of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in Tokyo Bay, Japan.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXlsVWntbs%3D&md5=8b4a8fe2d501ac3b20274193df6e8a52CAS |
[13] T. Kirchgeorg, I. Weinberg, A. Dreyer, R. Ebinghaus, Perfluorinated compounds in marine surface waters: data from the Baltic Sea and methodological challenges for future studies. Environ. Chem. 2010, 7, 429.
| Perfluorinated compounds in marine surface waters: data from the Baltic Sea and methodological challenges for future studies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsFChs77M&md5=1a93fb743905f37dc21f4974ecbb2eebCAS |
[14] L. Ahrens, S. Taniyasu, L. W. Y. Yeung, N. Yamashita, P. K. S. Lam, R. Ebinghaus, Distribution of polyfluoroalkyl compounds in water, suspended particulate matter and sediment from Tokyo Bay, Japan. Chemosphere 2010, 79, 266.
| Distribution of polyfluoroalkyl compounds in water, suspended particulate matter and sediment from Tokyo Bay, Japan.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXjtVCmtrc%3D&md5=7a5bd6d15ddd9f51d1aa7c64826e6801CAS |
[15] S. Takagi, F. Adachi, K. Miyano, Y. Koizumi, H. Tanaka, M. Mimura, I. Watanabe, S. Tanabe, K. Kannan, Perfluorooctanesulfonate and perfluorooctanoate in raw and treated tap water from Osaka, Japan. Chemosphere 2008, 72, 1409.
| Perfluorooctanesulfonate and perfluorooctanoate in raw and treated tap water from Osaka, Japan.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXoslGmsL0%3D&md5=8e29b2b02c07bf34d65a1e45c02da500CAS |
[16] K. Kannan, L. Tao, E. Sinclair, S. D. Pastva, D. J. Jude, J. P. Giesy, Perfluorinated compounds in aquatic organisms at various trophic levels in a Great Lakes food chain. Arch. Environ. Contam. Toxicol. 2005, 48, 559.
| Perfluorinated compounds in aquatic organisms at various trophic levels in a Great Lakes food chain.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXksVaisrY%3D&md5=ecd3e1f119232a9f9cfe5f2aba150f1fCAS |
[17] L. Ahrens, N. Marusczak, J. Rubarth, A. Dommergue, R. Nedjai, C. Ferrari, R. Ebinghaus, Distribution of perfluoroalkyl compounds and mercury in fish liver from high-mountain lakes in France originating from atmospheric deposition. Environ. Chem. 2010, 7, 422.
| Distribution of perfluoroalkyl compounds and mercury in fish liver from high-mountain lakes in France originating from atmospheric deposition.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsFChsrfF&md5=8c51fe1d823ea2cdc7b40316c40d4942CAS |
[18] K. Kannan, S. Corsolini, J. Falandysz, G. Fillmann, K. S. Kumar, B. G. Loganathan, M. A. Mohd, J. Olivero, N. V. Wouwe, J. H. Yang, K. M. Aldous, Perfluorooctanesulfonate and related fluorochemicals in human blood from several countries. Environ. Sci. Technol. 2004, 38, 4489.
| Perfluorooctanesulfonate and related fluorochemicals in human blood from several countries.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXlvV2qsrY%3D&md5=1602d18b34305713bf175b2ead37f2a7CAS |
[19] K. Harada, A. Koizumi, N. Saito, K. Inoue, T. Yoshinaga, C. Date, S. Fujii, N. Hachiya, I. Hirosawa, S. Koda, Y. Kusaka, K. Murata, K. Omae, S. Shimbo, K. Takenaka, T. Takeshita, H. Todoriki, Y. Wada, T. Watanabe, M. Ikeda, Historical and geographical aspects of the increasing perfluorooctanoate and perfluorooctane sulfonate contamination in human serum in Japan. Chemosphere 2007, 66, 293.
| Historical and geographical aspects of the increasing perfluorooctanoate and perfluorooctane sulfonate contamination in human serum in Japan.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1Cqt7rF&md5=95f9752002f017f86dd57ceac577ad08CAS |
[20] L. B. Biegel, M. E. Hurtt, S. R. Frame, J. C. O'Connor, J. C. Cook, Mechanisms of extrahepatic tumor induction by peroxisome proliferators in male CD rats. Toxicol. Sci. 2001, 60, 44.
| Mechanisms of extrahepatic tumor induction by peroxisome proliferators in male CD rats.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXhslGktro%3D&md5=bec804c6ae9e0a278a3474a4dd8569a1CAS |
[21] M. M. MacDonald, A. L. Warne, N. L. Stock, S. A. Mabury, K. R. Solomon, P. K. Sibley, Toxicity of perfluorooctane sulfonic acid and perfluorooctanoic acid to Chironomus tentans. Environ. Toxicol. Chem. 2004, 23, 2116.
| Toxicity of perfluorooctane sulfonic acid and perfluorooctanoic acid to Chironomus tentans.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXms1GhsLw%3D&md5=c5b1ff3727bd7b0ddd3a96379fa2c6f9CAS |
[22] B. J. Apelberg, F. R. Witter, J. B. Herbstman, A. M. Calafat, R. U. Halden, L. L. Needham, L. R. Goldman, Cord serum concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in relation to weight and size at birth. Environ. Health Perspect. 2007, 115, 1670.
| Cord serum concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in relation to weight and size at birth.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtl2ns7zI&md5=86c4ce6a542008c5aa5645950b8af027CAS |
[23] Y. Zushi, T. Takeda, S. Masunaga, Existence of nonpoint source of perfluorinated compounds and their loads in the Tsurumi River basin, Japan. Chemosphere 2008, 71, 1566.
| Existence of nonpoint source of perfluorinated compounds and their loads in the Tsurumi River basin, Japan.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXktFCmtbg%3D&md5=a8f300244698b6c3342e5ff8c5723b41CAS |
[24] M. Murakami, H. Shinohara, H. Takada, Evaluation of wastewater and street runoff as sources of perfluorinated surfactants (PFSs). Chemosphere 2009, 74, 487.
| Evaluation of wastewater and street runoff as sources of perfluorinated surfactants (PFSs).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFSmsQ%3D%3D&md5=11f36c84b26498cdd012979f76786930CAS |
[25] A. Y. C. Lin, S. C. Panchangam, C. C. Lo, The impact of semiconductor, electronics and optoelectronic industries on downstream perfluorinated chemical contamination in Taiwanese rivers. Environ. Pollut. 2009, 157, 1365.
| The impact of semiconductor, electronics and optoelectronic industries on downstream perfluorinated chemical contamination in Taiwanese rivers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXis1ektrs%3D&md5=0bfe64caead2b417529547bf3fd2765bCAS |
[26] D. A. Ellis, J. W. Martin, A. O. De Silva, S. A. Mabury, M. D. Hurley, M. P. S. Andersen, T. J. Wallington, Degradation of fluorotelomer alcohols: a likely atmospheric source of perfluorinated carboxylic acids. Environ. Sci. Technol. 2004, 38, 3316.
| Degradation of fluorotelomer alcohols: a likely atmospheric source of perfluorinated carboxylic acids.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjvVShsb0%3D&md5=1a2a8158f069618f177c7efa759abbccCAS |
[27] K. Sasaki, K. Harada, N. Saito, T. Tsutsui, S. Nakanishi, H. Tsuzuki, A. Koizumi, Impacts of air-borne perfluorooctane sulfonate on the human body burden and the ecological system. Bull. Environ. Contam. Toxicol. 2003, 71, 408.
| Impacts of air-borne perfluorooctane sulfonate on the human body burden and the ecological system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXmsFGmtLk%3D&md5=d576ce92ab8f48e29a261a85e42dbc1eCAS |
[28] M. Murakami, H. Takada, Perfluorinated surfactants (PFSs) in size-fractionated street dust in Tokyo. Chemosphere 2008, 73, 1172.
| Perfluorinated surfactants (PFSs) in size-fractionated street dust in Tokyo.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtlSgs7fL&md5=aca5f38f20d9277c978a28b02d3a3481CAS |
[29] M. J. A. Dinglasan-Panlilio, S. A. Mabury, Significant residual fluorinated alcohols present in various fluorinated materials. Environ. Sci. Technol. 2006, 40, 1447.
| Significant residual fluorinated alcohols present in various fluorinated materials.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XntFGhtA%3D%3D&md5=ee67f970941016f80117418a0f0f64aeCAS |
[30] S. K. Kim, K. Kannan, Perfluorinated acids in air, rain, snow, surface runoff, and lakes: relative importance of pathways to contamination of urban lakes. Environ. Sci. Technol. 2007, 41, 8328.
| Perfluorinated acids in air, rain, snow, surface runoff, and lakes: relative importance of pathways to contamination of urban lakes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXht12gsrbL&md5=731c781add6621ccc7b9d72f4f8c4a32CAS |
[31] K. Y. Kwok, S. Taniyasu, L. W. Y. Yeung, M. B. Murphy, P. K. S. Lam, Y. Horii, K. Kannan, G. Petrick, R. K. Sinha, N. Yamashita, Flux of perfluorinated chemicals through wet deposition in Japan, the United States, and several other countries. Environ. Sci. Technol. 2010, 44, 7043.
| Flux of perfluorinated chemicals through wet deposition in Japan, the United States, and several other countries.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtVKrtrjJ&md5=19f5cf88d24893adf305961af067f707CAS |
[32] Y. Zushi, S. Masunaga, First-flush loads of perfluorinated compounds in stormwater runoff from Hayabuchi River basin, Japan served by separated sewerage system. Chemosphere 2009, 76, 833.
| First-flush loads of perfluorinated compounds in stormwater runoff from Hayabuchi River basin, Japan served by separated sewerage system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXos12ks7w%3D&md5=6a25b2eca6e422eb3bdc58b58891ba9aCAS |
[33] J. Yu, J. Y. Hu, S. Tanaka, S. Fujii, Perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) in sewage treatment plants. Water Res. 2009, 43, 2399.
| Perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) in sewage treatment plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXls1eltbY%3D&md5=aea70e7a805edf616c0f4b2a6f5fe517CAS |
[34] T. Heberer, Tracking persistent pharmaceutical residues from municipal sewage to drinking water. J. Hydrol. (Amst.) 2002, 266, 175.
| Tracking persistent pharmaceutical residues from municipal sewage to drinking water.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XnvFyjur0%3D&md5=1c9316f5cdefc7300309a6e4c150c1c8CAS |
[35] M. Narumiya, T. Okuda, N. Nakada, N. Yamashita, H. Tanaka, K. Sato, M. Sueoka, T. Oiwa, Occurrence and fate of pharmaceuticals and personal care products during wastewater treatments. Environ. Eng. Res. 2009, 46, 175. [in Japanese].
[36] A. Musolff, S. Leschik, F. Reinstorf, G. Strauch, M. Schirmer, Micropollutant loads in the urban water cycle. Environ. Sci. Technol. 2010, 44, 4877.
| Micropollutant loads in the urban water cycle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmslelsrg%3D&md5=65a6d6be42b03772b566489b417a3bdbCAS |
[37] N. Nakada, T. Tanishima, H. Shinohara, K. Kiri, H. Takada, Pharmaceutical chemicals and endocrine disrupters in municipal wastewater in Tokyo and their removal during activated sludge treatment. Water Res. 2006, 40, 3297.
| Pharmaceutical chemicals and endocrine disrupters in municipal wastewater in Tokyo and their removal during activated sludge treatment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xpt1OktLo%3D&md5=5c00c339fe7efc01764f464dc49b6779CAS |
[38] N. Nakada, K. Kiri, H. Shinohara, A. Harada, K. Kuroda, S. Takizawa, H. Takada, Evaluation of pharmaceuticals and personal care products as water-soluble molecular markers of sewage. Environ. Sci. Technol. 2008, 42, 6347.
| Evaluation of pharmaceuticals and personal care products as water-soluble molecular markers of sewage.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXksFyktrY%3D&md5=04497a51706da629cf47614db5ccc240CAS |
[39] E. Sinclair, K. Kannan, Mass loading and fate of perfluoroalkyl surfactants in wastewater treatment plants. Environ. Sci. Technol. 2006, 40, 1408.
| Mass loading and fate of perfluoroalkyl surfactants in wastewater treatment plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XlvFSruw%3D%3D&md5=e0221a9ac39ad5bf269980597b7befbdCAS |
[40] C. P. Higgins, R. G. Luthy, Sorption of perfluorinated surfactants on sediments. Environ. Sci. Technol. 2006, 40, 7251.
| Sorption of perfluorinated surfactants on sediments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtVChtrjJ&md5=1436f5eabf068622e6df6b5b5f74d81aCAS |
[41] R. M. Harrison, D. J. T. Smith, L. Luhana, Source apportionment of atmospheric polycyclic aromatic hydrocarbons collected from an urban location in Birmingham, UK. Environ. Sci. Technol. 1996, 30, 825.
| Source apportionment of atmospheric polycyclic aromatic hydrocarbons collected from an urban location in Birmingham, UK.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XmsV2jsg%3D%3D&md5=20ab0090f6f5931d9c116c627db39676CAS |
[42] R. K. Larsen, J. E. Baker, Source apportionment of polycyclic aromatic hydrocarbons in the urban atmosphere: a comparison of three methods. Environ. Sci. Technol. 2003, 37, 1873.
| Source apportionment of polycyclic aromatic hydrocarbons in the urban atmosphere: a comparison of three methods.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXit1OjsL0%3D&md5=02e18af373dece7d3434dc74682314eaCAS |